
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 23 August 2023
Sec. Alzheimer's Disease and Related Dementias
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1246592
 Ling-Chun Huang1,2,3
Ling-Chun Huang1,2,3 Mei-Yueh Lee4,5
Mei-Yueh Lee4,5 Ching-Fang Chien1,2,3
Ching-Fang Chien1,2,3 Yang-Pei Chang1,2,3
Yang-Pei Chang1,2,3 Kuan-Ying Li1,2,3
Kuan-Ying Li1,2,3 Yuan-Han Yang1,2,3,6*
Yuan-Han Yang1,2,3,6*Introduction: The Apolipoprotein E (APOE) epsilon (ε) 4 allele is a well-established risk factor for late-onset Alzheimer’s disease (AD). Reports on white ancestry populations have showed that age, sex, and ethnicity have different effects on the association between APOE genotype and AD. However, studies on Asian populations such as Taiwan Chinese populations are limited. This study aimed to evaluate the association between APOE genotype and AD in a Taiwan Chinese population, and to explore if the association varies by age and sex.
Methods: We conducted a case-control study in 725 patients with AD and 1,067 age- and sex- matched controls without dementia from a Taiwan Chinese population. Logistic regression models were used to test the association between AD and APOE genotypes. Secondary analyses considered age (<75 or ≥75 years old), and sex stratified models.
Results: The risk of AD was significantly increased for people with at least one copy of APOE ε4 (OR = 2.52, 95% CI = 2.01–3.17, p < 0.001) and in a dose-dependent manner. Our results did not show an statistically significance different in AD risk when women and men carrying APOEε4 were compared. Despite not reaching statistical significance, the risk of APOE ε4 for AD was higher among younger participants (OR = 3.21, 95% CI = 2.26–4.56, p < 0.001) compared to older ones (OR = 2.13, 95% CI = 1.53–2.97, p < 0.001). When considering both sex and age, the risk of AD was higher among older men carrying APOE ε4 (OR = 2.64, 95% CI = 1.51–4.60 in men; OR = 1.90, 95% CI = 1.26–2.86 in women), while women carrying APOE ε4 appeared to have an increased risk at a younger age (OR = 3.29, 95% CI = 2.20–4.93 in women; OR = 2.91, 95% CI = 1.40–6.05 in men).
Discussion: The APOE ε4 allele represents a major risk factor for AD in the Taiwanese population. The effect of APOE ε4 allele on AD risk appeared to be stronger among men aged 75 years or more and among younger women.
Alzheimer’s disease (AD) is the leading cause of dementia in elderly individuals (Alzheimers Dement, 2020). Polymorphism in the apolipoprotein E (APOE) gene is a major risk determinant of late-onset AD (Farrer et al., 1997; Liu et al., 2013). Of the three major APOE allelic variants, epsilon (ε) 2, ε3 and ε4, APOE ε4 is associated with an increased risk, while APOE ε2 has been reported as having a protective effect over the risk of AD (Saunders et al., 1993; Corder et al., 1994; Farrer et al., 1997; Liu et al., 2013). Having a single APOE ε4 allele increases the risk of AD onset 2–4 fold and having two APOE ε4 alleles increases the risk about 8–12 fold (Farrer et al., 1997). Increasing evidence suggests that the effect of APOE ε4 on AD risk is exerted through inhibition of amyloid-β (Aβ) clearance and promotion of Aβ aggregation (Liu et al., 2013; Yamazaki et al., 2019). APOE ε4 also contributes to AD pathogenesis by impairing microglial responsiveness, lipid transport, synaptic integrity and plasticity, glucose metabolism, and cerebrovascular integrity and function (Liu et al., 2013; Yamazaki et al., 2019).
The risk conferred by APOE ε4 varies by age and sex, and these differences in AD risk have important implications for treatment trials, diagnostics, and therapeutics (Ungar et al., 2014). APOE ε4 exerts its maximal effect on AD risk by the early 70’s, with a reduction in risk after age 85 in both sexes (Jarvik et al., 1995; Farrer et al., 1997). Evidence indicates that the APOE ε4 risk for AD is greater in women than men (Payami et al., 1994; Farrer et al., 1997; Altmann et al., 2014; Buckley et al., 2018). Whereas Neu et al. (2017) found that men and women with the APOE ε4 genotype did not show a difference in AD risk across the age span of 55–85 years, but women had an increased risk between the ages of 65 and 75 (Neu et al., 2017).
The effect of ethnic background on the APOE association with AD risk has long been known, with African American and Hispanic APOE ε4 carriers having a lower risk than Caucasian APOE ε4 carriers, and Japanese carriers having the highest odd ratios (ORs) (Farrer et al., 1997; Tang et al., 1998). China has the largest population of patients with dementia in the world (Jia et al., 2020). The associations between APOE genotype and AD risk in the Chinese population were reported (Liu et al., 2014; Wu et al., 2015; Chen et al., 2022), however, the effect of age and sex on APOE ε4 risk in Chinese population is still unknown. In addition, data on this in the Taiwan Chinese population remains limited due to small sample sizes in previous studies (Hong et al., 1996; Liu et al., 1999; Hu et al., 2000; Huang et al., 2002; Lai et al., 2003). Therefore, we conducted the current study to evaluate the association between APOE genotype and AD in a Taiwan Chinese population, and to explore if the association varies by age and sex.
The current study was part of a project by the Taiwan Precision Medicine Initiative (TPMI). The TPMI is a partnership between Academia Sinica and top medical centers in Taiwan to bring genetic information into clinical practice. It is utilizing big data analysis of the genetic and clinical information of a large cohort to accurately predict personal risk for common diseases, and TPMI aims to promote early disease screening, tailored medical treatment, and prevention in Taiwan. This is a case-control study and we recruited subjects at the outpatient departments of Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung Municipal Hsiao-Kang Hospital and Kaohsiung Medical University Hospital. The enrollment period was from November 2019 to December 2021. A total of 725 clinically diagnosed AD patients were recruited. The diagnosis of AD was based on the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association criteria (McKhann et al., 1984). One thousand five hundred thirty-seven non-demented participants who visited the outpatient departments for subjective memory complaints other medical purposes were also recruited. After being age- and sex-matched with the AD group, 1,067 participants were assigned as the control group. All subjects in the control group were screened by an instrument of ascertainment of dementia 8 (AD8) (Yang et al., 2011) and had a score of <2 to exclude individuals with early stage dementia, or they were determined to have a Clinical Dementia Rating® (Morris, 1993) score of zero as conducted by an experienced physician. All subjects enrolled in this study were Taiwanese. Subjects were examined for mutations of the amyloid precursor protein gene, presenilin-1 gene and presenilin-2 gene to exclude familial AD (Cruts et al., 1998). The participants and their relatives were informed of the details of the study. The Kaohsiung Medical University Hospital Institutional Review Board [KMUHIRB-SV(II)-20190059, KMUHIRB-SV(II)-20200034, KMUHIRB-E(II)-20220263 and KMUHIRB-SV(I)-20230025] approved the study protocol and the participants provided written informed consent prior to their inclusion.
The blood DNA samples from all subjects participating in the TPMI project with Academia Sinica were genotyped using the Axiom Genome-Wide TWB 2.0 Array Plate (Thermo Fisher Scientific, Waltham, MA, USA) (Wei et al., 2021). The data for the two APOE Single-Nucleotide Polymorphisms (SNPs) (rs429358 and rs7412) were exported using Axiom Analysis Suite (Thermo Fisher Scientific) and PLINK software (Purcell et al., 2007). The data for the two SNPs from a total of 1,792 samples from the AD and control groups were validated by TaqMan® SNP Genotyping Assays (Thermo Fisher Scientific).
Data were presented as the mean ± standard deviation or proportions. The χ2 test was used to compare categorical data (sex, APOE genotype and allele) and the t-test was used to compare continuous data (age) between AD and control groups. Due to the lower frequency of APOE ε2/ε2 and ε4/ε4 genotypes, we merged ε2/ε2 with the ε2/ε3 to form the ε2 group, and ε3/ε4 with the ε4/ε4 to form the ε4 group to test the effect of APOE genotype using the reference of ε3/ε3.
The association between AD and APOE was tested using logistic regression models, and age and/or sex were adjusted as appropriate. Secondary analyses considered age and sex stratified analyses. Age was dichotomized using 75 years of age as the threshold to define two different groups: younger (<75 years old) and older (≥75 years old) participants. To compare the differences in ORs among different age groups and sex, we analyzed the interactions between APOE and age, and APOE and sex, respectively. All analyses were performed using SPSS 26.0 (SPSS Inc., Chicago, IL, USA). A two-tailed P-value of <0.05 was considered to indicate a statistically significant difference.
Table 1 presents the demographic characteristics and APOE genotype of the participants. A total of 1,792 participants were recruited: 725 AD cases and 1,067 cognitively healthy individuals. The proportion of women was slightly higher among the AD cases compared to cognitively healthy participants (68 versus 66%, respectively). The average age of all participants was 75 years. As expected, the frequency of APOE ε4 was significantly higher among AD cases compared to cognitively healthy individuals (17.9 versus 8.2%, respectively).
As shown in Table 2, the risk of AD was significantly increased for carriers of at least one copy of APOE ε4 allele (OR = 2.52, 95% CI = 2.01–3.17). Homozygous individuals for the ε4 allele had an increased risk of developing AD (OR = 7.46, 95% CI = 3.22–17.30, p < 0.001) when compared to heterozygous carriers (OR = 2.32, 95% CI = 1.84–2.93, p < 0.001). Our findings indicate that APOE ε2 alleles confer a protective effect against the risk of AD, however, the results did not reach statistical significance. Using ε3/ε3 as the reference, the OR for APOE ε2/ε2 carriers is 0.26 (CI = 0.31–2.19, p = 0.216), and for ε2/ε3 carriers, the OR is 0.81 (CI = 0.59–1.12, p = 0.200).
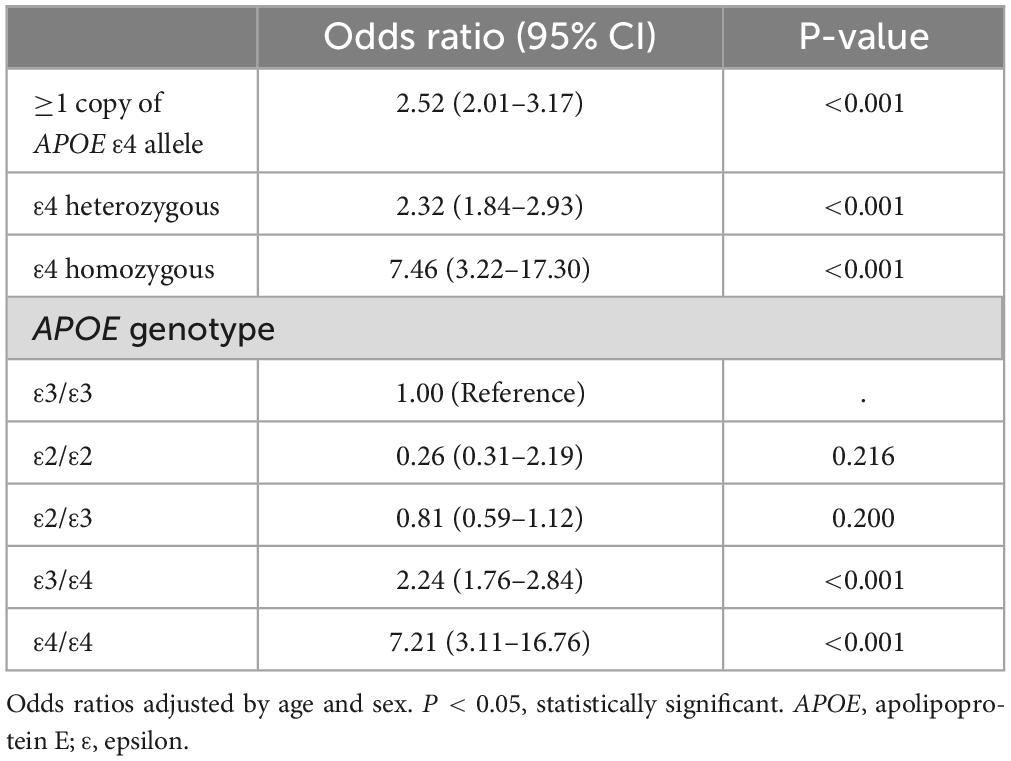
Table 2. The association between Alzheimer’s disease and APOE ε4 allele and genotypes in Taiwanese participants.
Apolipoprotein E ε4 carrier women (OR = 2.38, CI = 1.81–3.14) and men (OR = 2.64, CI = 1.75–3.98) did not show a significant difference in AD risk (APOE-sex interaction P-value = 0.46); however, APOE ε4 homozygous women appear to have an increased risk compared with homozygous men, but the number of ε4 homozygous cases is too small to demonstrate statistical differences between sexes (Table 3).
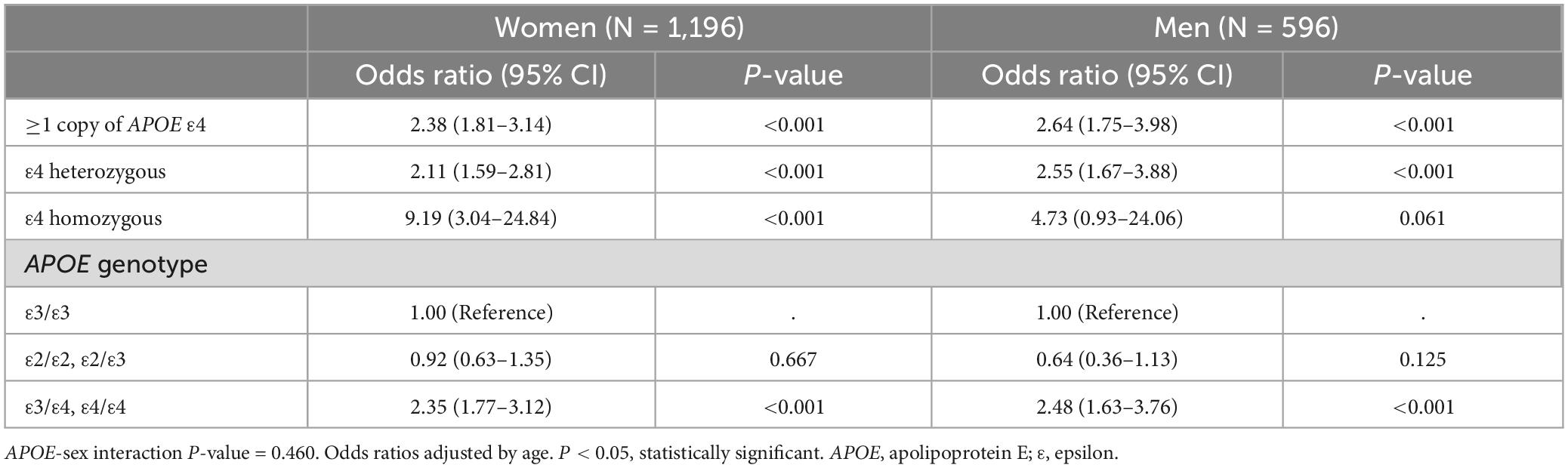
Table 3. The sex stratified association between Alzheimer’s disease and APOE ε4 allele and genotypes.
The study participants were categorized into two different age groups using 75 years of age as the cutoff. As shown in Table 4, the risk of AD was increased among those carrying at least one copy of the APOE ε4 allele in both groups (OR = 2.23, 95% CI = 1.61–3.09 in the older group, OR = 3.33, 95% CI = 2.36–4.69 in the younger group). Despite not reaching statistical significance (APOE-age interaction P-value = 0.265), the APOE ε4 effect seems more evident at a younger age.
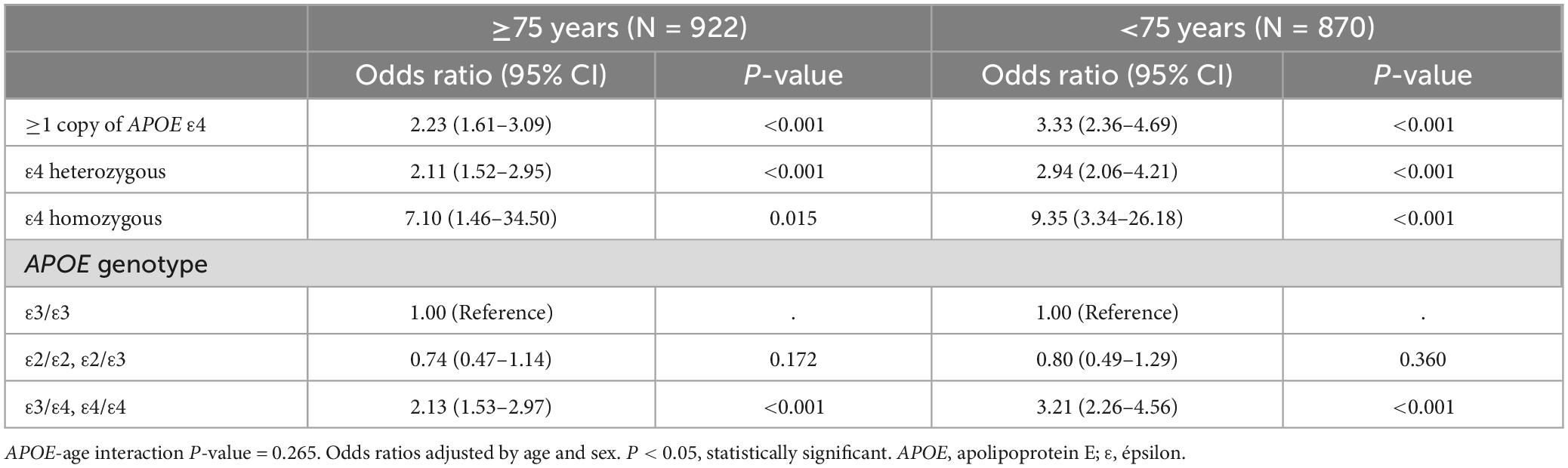
Table 4. The age stratified association between Alzheimer’s disease and APOE ε4 allele and genotypes.
Stratifying by sex and age (Table 5), our results showed that in older age groups, APOE ε4 men carriers had a higher AD risk than women (OR = 2.64, 95% CI = 1.51–4.60 in men; OR = 1.90, 95% CI = 1.26–2.86 in women). Conversely, women carrying APOEε4 had an increased risk at a younger age compared to men (OR = 3.29, 95% CI = 2.20–4.93 in women; OR = 2.91, 95% CI = 1.40–6.05 in men).
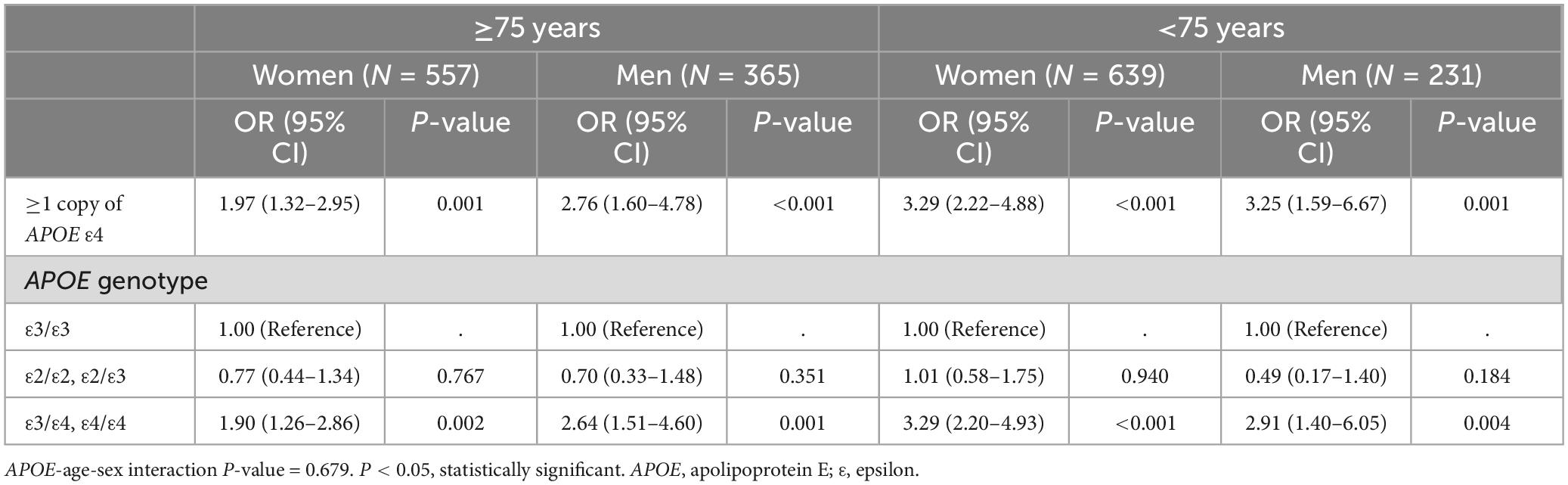
Table 5. The age and sex stratified association between Alzheimer’s disease and APOE ε4 allele and genotypes.
We have demonstrated that the APOE ε4 allele represents a major risk factor for AD in the Taiwanese Chinese population, although its effect is weaker when compared to Caucasian populations. Although the difference did not reach a level of statistical significance, the risk appears to be higher in younger age groups. APOE ε4 carrier women and men did not show a significant difference in AD risk but APOE ε4 carrier men appear to have a higher risk at older age, while APOE ε4 carrier women had an increased risk at a younger age. While the impact of age and sex on the association between APOE genotype and AD is well-established in Caucasians, to the best of our knowledge, this is the first study in the Taiwan Chinese population.
Apolipoprotein E ε4 allele frequency in AD patients and the related risk for AD are different in people of different ethnicities. A previous study showed that APOE ε4 allele frequency in AD patients was highest in Caucasian (36.7%) followed by African American (32.2%), Japanese (27.8%) and Hispanic (19.2%) individuals (Farrer et al., 1997). Although APOE ε4 is considered the most important risk factor for AD, the APOE ε4 allele frequency in AD patients is lower in Taiwanese (17.9%) which was in line with previous studies (Liu et al., 1999; Huang et al., 2002). According to the study conducted by Farrer et al. (1997), the association between APOE ε4 and AD in Japanese people (ε3/ε4: OR 5.6, 95% CI = 3.9–8.0, ε4/ε4: OR 33.1, 95% CI = 13.6–80.5 relative to ε3/ε3) was higher than in Caucasian people (ε3/ε4: OR 2.7, 95% CI = 2.2–3.2, ε4/ε4: OR 12.5, 95% CI = 8.8–17.7), while the association was lower among African Americans (ε3/ε4: OR 1.1, 95% CI = 0.7–1.8, ε4/ε4: OR 5.7, 95% CI = 2.3–14.1) and Hispanics (ε3/ε4: OR 2.2, 95% CI = 1.3–3.4, ε4/ε4: OR 2.2, 95% CI = 0.7–6.7) (Farrer et al., 1997). Our study reports the APOE ε4 related risk for AD in a Taiwan Chinese population (ε3/ε4: OR 2.24, 95% CI = 1.76–2.84, ε4/ε4: OR 7.21, 95% CI = 3.11–16.76). When including our findings, the risk of having AD in APOE ε4 carriers was highest in Japanese, followed by Caucasian, Taiwan Chinese, African Americans and Hispanics. The comparison of APOE ε4 allele frequency in AD patients and the related risk for AD among different ethnicities is summarized in Table 6. This should be verified by more studies in the future.
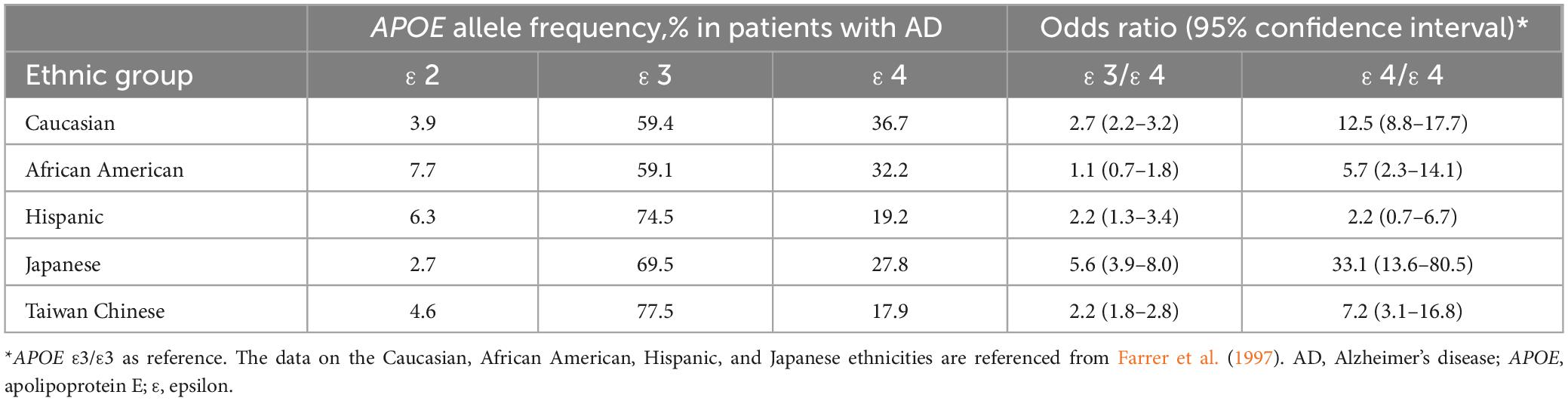
Table 6. The comparison of APOE ε4 allele frequency in patients with Alzheimer’s disease and the related risk for Alzheimer’s disease among different ethnicities.
Unlike previous studies (Payami et al., 1994; Farrer et al., 1997; Altmann et al., 2014), APOE ε4 carrier women and men did not show a significant difference in AD risk. This finding is consistent with a previous study that men and women with one copy of APOE ε4 have nearly the same odds of developing AD across the age span of 55–85 years (Neu et al., 2017). APOEε4 homozygous women appear to have a increased risk compared to homozygous men, which aligns with the study conducted by Farrer et al. (1997). Their study showed that the OR for APOE ε4/ε4 leaps to 10 and above for men and women, but even among the homozygotes there appears to be a slightly greater effect in women (Farrer et al., 1997). However, it is important to interpret the result cautiously due to the limited number of APOE ε4 homozygotes in our study participants.
Our observation of a stronger effect of APOE ε4 in individuals under the age of 75 is consistent with previous reports that APOE ε4 exerts its maximal effect on AD risk by the early 70’s, with a reduction in risk after the age of 85 (Jarvik et al., 1995; Farrer et al., 1997). This finding is in line with epidemiological evidence that APOE ε4 is not only associated with an increased risk of the development of AD but also a lower age of onset (Corder et al., 1993; Rebeck et al., 1993; Farrer et al., 1997; Liu et al., 2013). The mean age of clinical onset is 68 years in ε4 homozygotes, 76 years in ε4 heterozygotes, and 84 years in ε4 non-carriers, indicating that APOE ε4 dramatically increases the risk of AD development with an earlier age of onset in a gene dose-dependent manner (Corder et al., 1993; Rebeck et al., 1993).
In our study, APOE ε4 carrier men showed a non-significant higher risk after the age of 75, whereas APOE ε4 carrier women exhibited an increase in risk before the age of 75. The increased risk of AD in women before the age of 75 years may be associated with the events that occur 15–20 years earlier, possibly coinciding with the period of menopause (Dubal and Rogine, 2017), which on average begins at 51 years of age, and during which physiological changes and estrogen loss occurs (McKinlay et al., 1992). A possible explanation for the higher AD risk in APOE ε4 carrier men at an older age is that the age-adjusted period of prevalence for cardiovascular disease (CVD) related to APOE genotype is higher in men than in women (18.6% in the ε4 group for men and 9.9% for ε4 women) (Lahoz et al., 2001). The prevalence of CVD has also been shown to increase with age (Yazdanyar and Newman, 2009; North and Sinclair, 2012), and individuals with CVD are at a higher risk for AD especially if they carry the APOE4 allele (Stampfer, 2006; Eriksson et al., 2010; de Bruijn and Ikram, 2014). Therefore, older men with APOE ε4 may have a higher risk of AD. This hypothesis needs further validation. It is also important to carefully exclude cognitive impairments caused by stroke or cerebrovascular diseases, as the comorbidity of CVD and AD is high in the elderly.
There were some limitations to the current study. First, we did not adjust for known AD risk factors, such as the number of years of education, family history of AD and vascular risk factors (Barnes and Yaffe, 2011), as they are all known risk factors for developing AD, in addition to APOE ε4. Second, no participants with APOE ε2/ε4 genotyping were recruited in our study. Therefore, whether the protective effect of the ε2 allele overcomes the risk brought on by the ε4 allele is unknown. Third, these findings are based on a population from hospital outpatient clinics and may not be generalized to the general population. Fourth, we did not report the prevalence of CVD in the recruited subjects; future studies with information on the prevalence of CVD are warranted to better understand the association between APOE genotype, CVD, dementia and AD, especially in older men. Even so, our findings warrant further investigation as it is likely a complex set of risk factors associated with AD development, and consideration should be given to age and sex-specific treatments for cognitive decline and AD. For example, if women are at an increased risk of AD at a younger age, it is plausible that treatments for women, especially those who carry an APOE ε4 allele, may need to be initiated earlier. Additionally, it is important to vigorously control modifiable cardiovascular risk factors, particularly in men who are APOE ε4 carriers, due to their higher risks of AD and CVD at an older age.
In conclusion, we reported the association between APOE genotype and AD in a Taiwan Chinese population. At older age (≥75 years), APOE ε4 carrier men have a higher risk of AD, while APOE ε4 carrier women have an increased risk at a younger age. This is important to consider for individual patients in terms of diagnostics, treatment, and genetic counseling.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Kaohsiung Medical University Hospital Institutional Review Board [KMUHIRB-SV(II)-20190059, KMUHIRB-SV(II)-20200034, KMUHIRB-E(II)-20220263, and KMUHIRB-SV(I)-20230025]. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
L-CH and Y-HY contributed to the study conception and design. M-YL, C-FC, Y-PC, K-YL, and Y-HY contributed to the acquisition of data. L-CH, C-FC, Y-PC, and Y-HY analyzed and interpreted the data. L-CH and Y-HY conducted the statistical analyses and involved in writing the initial draft of the manuscript. All authors reviewed and revised the manuscript and approved the submitted version.
This study was supported by the Ministry of Science and Technology (MOST109-2321-B-037-001, MOST110-2321-B-037-003, and MOST111-2321-B-037-003), Kaohsiung Medical University Research Center (KMU-TC111B02), and the Department of Neurology, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung, Taiwan.
The authors thank the Division of Medical Statistics and Bioinformatics and the Department of Medical Research, Kaohsiung Medical University Hospital, Kaohsiung Medical University for their help.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Altmann, A., Tian, L., Henderson, V. W., Greicius, M. D., and Alzheimer’s Disease Neuroimaging Initiative (2014). Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol. 75, 563–573. doi: 10.1002/ana.24135
Alzheimers Dement, (2020). 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. doi: 10.1002/alz.12068 [Epub ahead of print].
Barnes, D. E., and Yaffe, K. (2011). The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 10, 819–828. doi: 10.1016/S1474-4422(11)70072-2
Buckley, R. F., Mormino, E. C., Amariglio, R. E., Properzi, M. J., Rabin, J. S., Lim, Y. Y., et al. (2018). Sex, amyloid, and APOE epsilon4 and risk of cognitive decline in preclinical Alzheimer’s disease: Findings from three well-characterized cohorts. Alzheimers Dement. 14, 1193–1203. doi: 10.1016/j.jalz.2018.04.010
Chen, Q., Wang, T., Kang, D., and Chen, L. (2022). Protective effect of apolipoprotein E epsilon 3 on sporadic Alzheimer’s disease in the Chinese population: A meta-analysis. Sci. Rep. 12:13620. doi: 10.1038/s41598-022-18033-x
Corder, E. H., Saunders, A. M., Risch, N. J., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C. Jr., et al. (1994). Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat. Genet. 7, 180–184. doi: 10.1038/ng0694-180
Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., Small, G. W., et al. (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923. doi: 10.1126/science.8346443
Cruts, M., van Duijn, C. M., Backhovens, H., Van den Broeck, M., Wehnert, A., Serneels, S., et al. (1998). Estimation of the genetic contribution of presenilin-1 and -2 mutations in a population-based study of presenile Alzheimer disease. Hum. Mol. Genet. 7, 43–51. doi: 10.1093/hmg/7.1.43
de Bruijn, R. F. A. G., and Ikram, M. A. (2014). Cardiovascular risk factors and future risk of Alzheimer’s disease. BMC Med. 12:130. doi: 10.1186/s12916-014-0130-5
Dubal, D. B., and Rogine, C. (2017). Apolipoprotein E epsilon4 and risk factors for Alzheimer disease-let’s talk about sex. JAMA Neurol. 74, 1167–1168. doi: 10.1001/jamaneurol.2017.1470
Eriksson, U. K., Bennet, A. M., Gatz, M., Dickman, P. W., and Pedersen, N. L. (2010). Nonstroke cardiovascular disease and risk of Alzheimer disease and dementia. Alzheimer Dis. Assoc. Disord. 24, 213–219. doi: 10.1097/WAD.0b013e3181d1b99b
Farrer, L. A., Cupples, L. A., Haines, J. L., Hyman, B., Kukull, W. A., Mayeux, R., et al. (1997). Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer disease meta-analysis consortium. JAMA 278, 1349–1356.
Hong, C. J., Liu, T. Y., Liu, H. C., Wang, S. J., Fuh, J. L., Chi, C. W., et al. (1996). Epsilon 4 allele of apolipoprotein E increases risk of Alzheimer’s disease in a Chinese population. Neurology 46, 1749–1751. doi: 10.1212/wnl.46.6.1749
Hu, C. J., Sung, S. M., Liu, H. C., Hsu, W. C., Lee, L. S., Lee, C. C., et al. (2000). Genetic risk factors of sporadic Alzheimer’s disease among Chinese in Taiwan. J. Neurol. Sci. 181, 127–131. doi: 10.1016/s0022-510x(00)00443-3
Huang, H. M., Kuo, Y. M., Ou, H. C., Lin, C. C., and Chuo, L. J. (2002). Apolipoprotein E polymorphism in various dementias in Taiwan Chinese population. J. Neural Transm. 109, 1415–1421. doi: 10.1007/s00702-002-0751-2
Jarvik, G. P., Wijsman, E. M., Kukull, W. A., Schellenberg, G. D., Yu, C., and Larson, E. B. (1995). Interactions of apolipoprotein E genotype, total cholesterol level, age, and sex in prediction of Alzheimer’s disease: A case-control study. Neurology 45, 1092–1096. doi: 10.1212/wnl.45.6.1092
Jia, L., Quan, M., Fu, Y., Zhao, T., Li, Y., Wei, C., et al. (2020). Dementia in China: Epidemiology, clinical management, and research advances. Lancet Neurol. 19, 81–92. doi: 10.1016/S1474-4422(19)30290-X
Lahoz, C., Schaefer, E. J., Cupples, L. A., Wilson, P. W., Levy, D., Osgood, D., et al. (2001). Apolipoprotein E genotype and cardiovascular disease in the Framingham Heart Study. Atherosclerosis 154, 529–537. doi: 10.1016/s0021-9150(00)00570-0
Lai, C. L., Tai, C. T., Lin, S. R., Lin, R. T., Yang, Y. H., and Liu, C. K. (2003). Apolipoprotein E in Taiwan Chinese patients with dementia. Dement. Geriatr. Cogn. Disord. 16, 208–211. doi: 10.1159/000072804
Liu, C. C., Liu, C. C., Kanekiyo, T., Xu, H., and Bu, G. (2013). Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 9, 106–118. doi: 10.1038/nrneurol.2012.263
Liu, H. C., Hong, C. J., Wang, S. J., Fuh, J. L., Wang, P. N., Shyu, H. Y., et al. (1999). ApoE genotype in relation to AD and cholesterol: A study of 2,326 Chinese adults. Neurology 53, 962–966. doi: 10.1212/wnl.53.5.962
Liu, M., Bian, C., Zhang, J., and Wen, F. (2014). Apolipoprotein E gene polymorphism and Alzheimer’s disease in Chinese population: A meta-analysis. Sci. Rep. 4:4383. doi: 10.1038/srep04383
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., and Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of health and human services task force on Alzheimer’s disease. Neurology 34, 939–944. doi: 10.1212/wnl.34.7.939
McKinlay, S. M., Brambilla, D. J., and Posner, J. G. (1992). The normal menopause transition. Maturitas 14, 103–115. doi: 10.1016/0378-5122(92)90003-m
Morris, J. C. (1993). The clinical dementia rating (CDR): Current version and scoring rules. Neurology 43, 2412–2414. doi: 10.1212/wnl.43.11.2412-a
Neu, S. C., Pa, J., Kukull, W., Beekly, D., Kuzma, A., Gangadharan, P., et al. (2017). Apolipoprotein E genotype and sex risk factors for Alzheimer disease: A meta-analysis. JAMA Neurol. 74, 1178–1189. doi: 10.1001/jamaneurol.2017.2188
North, B. J., and Sinclair, D. A. (2012). The intersection between aging and cardiovascular disease. Circ. Res. 110, 1097–1108. doi: 10.1161/CIRCRESAHA.111.246876
Payami, H., Montee, K. R., Kaye, J. A., Bird, T. D., Yu, C. E., Wijsman, E. M., et al. (1994). Alzheimer’s disease, apolipoprotein E4, and gender. JAMA 271, 1316–1317.
Purcell, S., Neale, B., Todd-Brown, K., Thomas, L., Ferreira, M. A., Bender, D., et al. (2007). PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575. doi: 10.1086/519795
Rebeck, G. W., Reiter, J. S., Strickland, D. K., and Hyman, B. T. (1993). Apolipoprotein E in sporadic Alzheimer’s disease: Allelic variation and receptor interactions. Neuron 11, 575–580. doi: 10.1016/0896-6273(93)90070-8
Saunders, A. M., Strittmatter, W. J., Schmechel, D., George-Hyslop, P. H., Pericak-Vance, M. A., Joo, S. H., et al. (1993). Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 43, 1467–1472. doi: 10.1212/wnl.43.8.1467
Stampfer, M. J. (2006). Cardiovascular disease and Alzheimer’s disease: Common links. J. Intern. Med. 260, 211–223. doi: 10.1111/j.1365-2796.2006.01687.x
Tang, M. X., Stern, Y., Marder, K., Bell, K., Gurland, B., Lantigua, R., et al. (1998). The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA 279, 751–755. doi: 10.1001/jama.279.10.751
Ungar, L., Altmann, A., and Greicius, M. D. (2014). Apolipoprotein E, gender, and Alzheimer’s disease: An overlooked, but potent and promising interaction. Brain Imaging Behav. 8, 262–273. doi: 10.1007/s11682-013-9272-x
Wei, C. Y., Yang, J. H., Yeh, E. C., Tsai, M. F., Kao, H. J., Lo, C. Z., et al. (2021). Genetic profiles of 103,106 individuals in the Taiwan Biobank provide insights into the health and history of Han Chinese. NPJ Genom. Med. 6:10. doi: 10.1038/s41525-021-00178-9
Wu, P., Li, H. L., Liu, Z. J., Tao, Q. Q., Xu, M., Guo, Q. H., et al. (2015). Associations between apolipoprotein E gene polymorphisms and Alzheimer’s disease risk in a large Chinese Han population. Clin. Interv. Aging 10, 371–378. doi: 10.2147/CIA.S73396
Yamazaki, Y., Zhao, N., Caulfield, T. R., Liu, C. C., and Bu, G. (2019). Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat. Rev. Neurol. 15, 501–518. doi: 10.1038/s41582-019-0228-7
Yang, Y. H., Galvin, J. E., Morris, J. C., Lai, C. L., Chou, M. C., and Liu, C. K. (2011). Application of AD8 questionnaire to screen very mild dementia in Taiwanese. Am. J. Alzheimers Dis. Other Dement. 26, 134–138. doi: 10.1177/1533317510397330
Keywords: Alzheimer’s disease, apolipoprotein E gene, genetic association, sex and age stratified analyses, Taiwan Chinese population
Citation: Huang L-C, Lee M-Y, Chien C-F, Chang Y-P, Li K-Y and Yang Y-H (2023) Age and sex differences in the association between APOE genotype and Alzheimer’s disease in a Taiwan Chinese population. Front. Aging Neurosci. 15:1246592. doi: 10.3389/fnagi.2023.1246592
Received: 24 June 2023; Accepted: 07 August 2023;
Published: 23 August 2023.
Edited by:
Boon-Seng Wong, Singapore Institute of Technology, SingaporeReviewed by:
Eva Bagyinszky, Gachon University, Republic of KoreaCopyright © 2023 Huang, Lee, Chien, Chang, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan-Han Yang, ZW5kbGVzc3loeUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.