- 1Department of Otolaryngology-Head and Neck Surgery, The Medical Center of PLA General Hospital, Beijing, China
- 2National Clinical Research Center for Otolaryngologic Diseases, Beijing, China
- 3State Key Laboratory of Hearing Science, Ministry of Education, Beijing, China
- 4Beijing Key Laboratory of Hearing Impairment Prevention and Treatment, Beijing, China
- 5Academy of Medical Technology and Information Engineering, Zhejiang Chinese Medical University, Hangzhou, China
Background: Vertigo and hearing loss are both prevalent in the elderly. This study retrospectively analyzed hearing test results from elderly patients experiencing vertigo and dizziness at ENT outpatient over a 10-year period, in order to study the patterns of hearing loss in this patient population.
Methods: Nine thousand three hundred eighty four patients over 50 years old underwent retrospective collection and screening of outpatient diagnosis, pure tone audiometry, acoustic immittance measurement (tympanogram) and auditory brainstem response (ABR) test. The patient's audiograms are divided into 7 subtypes according to a set of fixed criteria. Meanwhile, K-Means clustering analysis method was used to classify the audiogram.
Results: The Jerger classification of tympanogram in elderly patients with vertigo and dizziness showed the majority falling under type A. The leading audiogram shapes were flat (27.81% in right ear and 26.89% in left ear), high-frequency gently sloping (25.97% in right ear and 27.34% in left ear), and high-frequency steeply sloping (21.60% in right ear and 22.53% in left ear). Meniere's disease (MD; 30.87%), benign recurrent vertigo (BRV; 19.07%), and benign paroxysmal positional vertigo (BPPV; 15.66%) were the most common etiologies in elderly vestibular diseases. We observed statistically significant differences in hearing thresholds among these vestibular diseases (P < 0.001). K-Means clustering analysis suggested that the optimal number of clusters was three, with sample sizes for the three clusters being 2,747, 2,413, and 4,139, respectively. The ANOVA statistical results of each characteristic value showed P < 0.001.
Conclusion: The elderly patients often have mild to moderate hearing loss as a concomitant symptom with vertigo. Female patients have better hearing thresholds than males. The dominant audiometric shapes in this patient population were flat, high-frequency gently sloping, and high-frequency steeply sloping according to a set of fixed criteria. This study highlights the need for tailored strategies in managing hearing loss in elderly patients with vertigo and dizziness.
1. Introduction
Previous studies indicate that vertigo is prevalent in the elderly population, with estimates of incidence ranging from 20 to 58% (Lasisi and Gureje, 2014; Lindell et al., 2021; Fancello et al., 2023). The pathogenesis of vertigo is multifactorial and primarily characterized by illusions of rotational motion, often accompanied by symptoms such as nystagmus, postural imbalance, falls, and neurovegetative effects (Roque Reis et al., 2016; Du et al., 2022). These symptoms limit daily activities, significantly impacting the physical and mental health and overall quality of life of affected individuals.
The inner ear, with its complex metabolic mechanisms, can be adversely affected by alterations in blood concentrations of glucose and insulin, potentially leading to hearing loss and vestibular disorders (Albernaz, 2016). Approximately 20% of patients with dizziness also experience hearing loss (Sunitha et al., 2019). These patients often show severe cochlear damage and may have extensive or deep ischemia in the inner ear (Kuhn et al., 2011). Notably, the incidence of vertigo can reach 20–60% among individuals with sensorineural hearing loss (Rambold et al., 2005). The co-occurrence of sudden hearing loss (SHL) and vertigo, especially when occurring in close temporal proximity, has been associated with a higher risk of subsequent stroke compared to SHL or vertigo alone (Chang et al., 2018). This indicates that SHL in vertigo patients should not be viewed as merely a benign peripheral vestibular sign.
Given the potential severe consequences of concomitant hearing loss in elderly patients with vertigo and dizziness, this condition merits increased clinical attention. Accordingly, this study retrospectively analyzes hearing examination reports of elderly patients experiencing dizziness from outpatient hearing centers over a 10-year period. Our aim is to characterize the types of hearing loss in this specific population.
2. Materials and methods
2.1. Participants
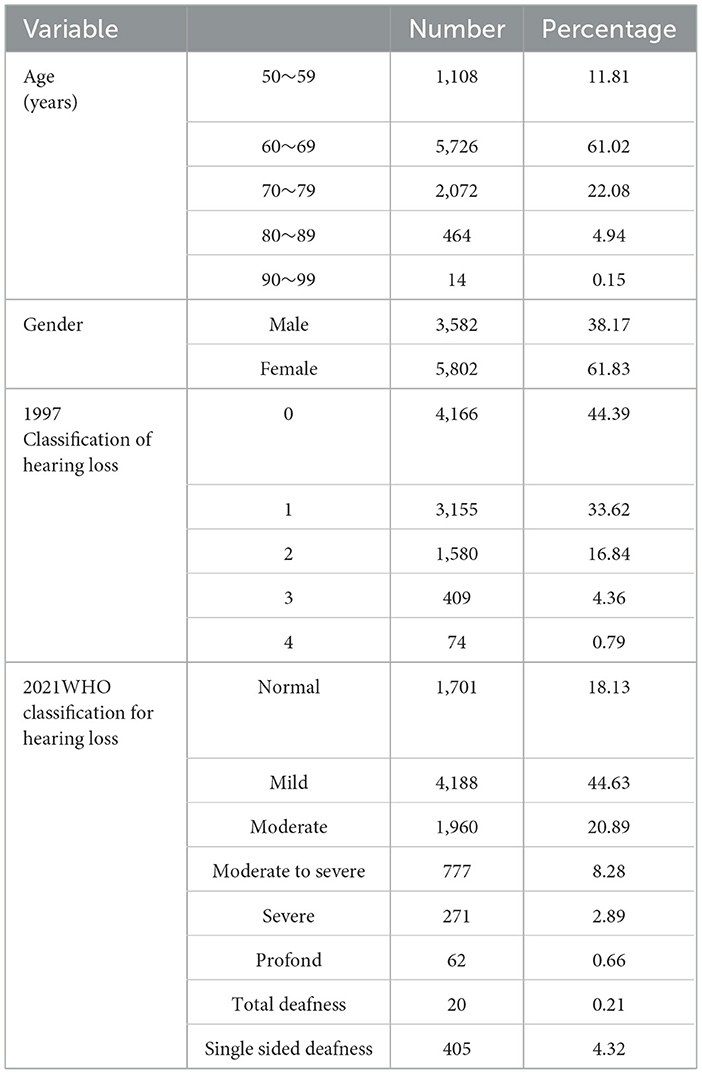
This study involved retrospective collection and examination of outpatient diagnosis reports, pure tone audiometry, acoustic immittance measurement and auditory brainstem response (ABR) tests from January 2010 to December 2021. Participants were patients over 50 years old with vertigo and dizziness, visiting the General Hospital of the People's Liberation Army in the Chinese People's Republic. A total of 9,384 patients (3,582 males and 5,802 females, aged 50–96 years, average age 66.24 ± 7.04 years) with hearing loss were included. The study was approved by the PLA General Hospital's Ethics Committee (No. S2022-673-01), and all procedures complied with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
2.2. Hearing evaluation methods
2.2.1. Pure tone audiometry
Post-routine otolaryngology examinations and history collection, the Astera pure tone audiometry (Natus, US) was used to obtain the hearing threshold. The EAR-3A insert phones were applied (American Speech-Language-Hearing Association, 2005), and frequencies from 250 Hz to 8 kHz were tested using the ascending method (ISO 8253-1: 2010).
2.2.2. Tympanogram
The middle ear's tympanograms were obtained using TympStar clinical tympanometer (Grason-Stadler, US) and Titan tympanometer (Interacoustics, Denmark), with a probe tone of 226 Hz. Tympanograms were classified into five types (A, AD, AS, B, and C) according to the Liden-Jerger classification criteria.
2.2.3. Auditory electrophysiology tests
ABR tests were conducted using the Eclipse EP25 platform (Interacoustics, Denmark) with insert earphones (3A, Etymotic Research, US). Alternating short-duration clicks with a repetition rate of 19.3 Hz were used as stimuli. Parameters for the test are detailed.
2.3. Pure-tone audiogram typing
To facilitate the diagnostic classification of hearing loss in elderly patients, we adopted a typing criterion for pure-tone audiograms based on clinical observations and a review of existing literature (see Figure 1) (Demeester et al., 2009; Lee et al., 2020). We defined 250 and 500 Hz as “low frequency (LF),” 1 and 2 kHz as “middle frequency (MF),” and 4 and 8 kHz as “high frequency (HF).”
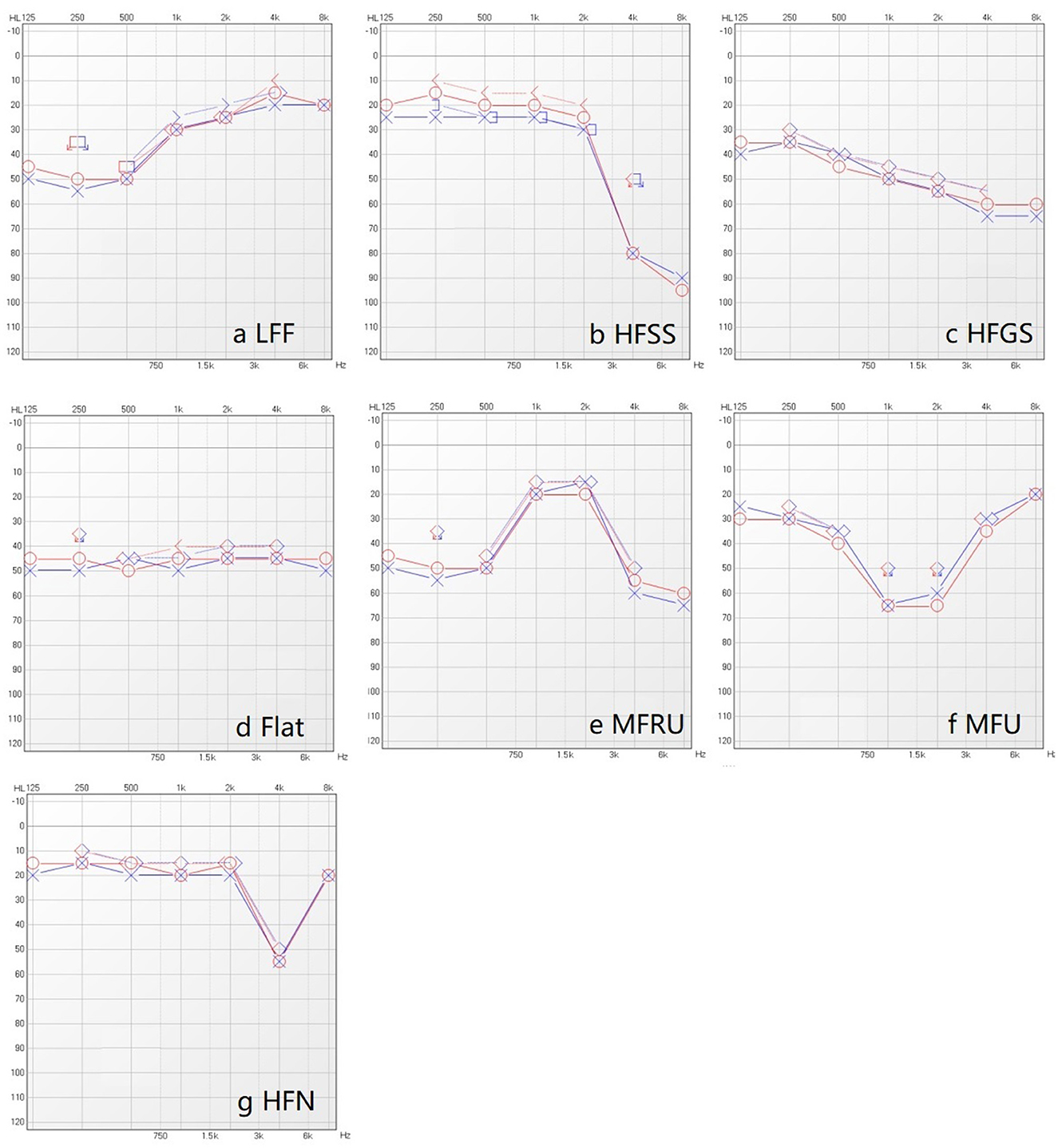
Figure 1. Seven typing of pure-tone audiogram. (a) Low frequency falling (LFF): The difference between the poor low frequency threshold (i.e., the larger listening threshold in 250 Hz and 500 Hz) and the good high frequency threshold (i.e., the smaller listening threshold in 4 kHz and 8 kHz) is greater than 15 dB, and the former is greater than the latter. (b) High frequency steeply sloping (HFSS): The difference between the mean value of 500 Hz and 1 kHz air conduction hearing thresholds and the average values of high frequency (4 kHz and 8 kHz) air conduction hearing thresholds is greater than 30 dB, and the former was smaller than the latter. (c) High frequency gently sloping (HFGS): The difference between the average values of air conduction threshold (500 Hz and 1 kHz) and the average value of high frequency air conduction thresholds (4 kHz and 8 kHz) is greater than 15 dB, meanwhile less than or equal to 29 dB, and the former is less than the latter. (d) Flat: The difference between the average values of the three air conduction hearing thresholds(low-frequency, medium-frequency, high-frequency) is less than 15 dB. (e) Mid frequency Reverse U-shape (MFRU): The difference between the medium frequency optimal threshold (i.e., the smaller threshold in 1 kHz and 2 kHz), the low frequency optimal threshold (i.e., the smaller threshold in 250 Hz and 500 Hz) and the high frequency optimal threshold (i.e., the smaller threshold in 4 kHz and 8 kHz) is more than 15 dB, and the medium frequency is better than (the threshold is less than) the low frequency and high frequency. (f) Mid frequency U-shape (MFU): The difference between the worst listening threshold of medium frequency (the larger threshold of 1 kHz and 2 kHz), the poor listening threshold of low frequency (the larger threshold of 250 Hz and 500 Hz) and the poor threshold of high frequency (the larger listening threshold of 4 kHz and 8 kHz) is more than 15 dB, and the medium frequency less than (the threshold is greater than) low frequency and high frequency. (g) High frequency notching (HFN): The threshold of 4 kHz is the worst, and the difference between 4kHz and other frequency thresholds is greater than 15 dB.
2.4. Inclusion and exclusion criteria
2.4.1. Inclusion criteria
(1) Age ≥ 50 years;
(2) Diagnosed by a careful interview and vestibular function results by an otologist, and another specialist reviewed the clinical notes to confirm the diagnosis. All diagnoses could be divided into benign recurrent vertigo (BRV) (van Leeuwen et al., 2022), MD (Monsell et al., 1995), vestibular neuropathy (VN, the diagnosis was based on the history of acute sustained vertigo or imbalance, positive spontaneous nystagmus or unilateral weakness >25% in vHIT or unilateral VOR gain loss combined with obvious catch-up saccades in vHIT and no additional central lesion signs) (Haeussler et al., 2022), BPPV (Kim et al., 2021), functional and psychiatric vertigo (PV) (Traschütz et al., 2021), vestibular migraine (VM) (García et al., 2021), bilateral vestibular hypofunction (BVH) (Lucieer et al., 2016), delayed endolymphatic hydrops (DEH) (Reynard et al., 2018), others [including vestibular paroxysmia (VP), acoustic neurinoma (AN, radiologically diagnosed and went through vHIT before surgery), traumatic vertigo (TV, diagnosed by imaging), Ramsay Hunt Syndrome (RHS, diagnosed with an ipsilateral herpetic eruption on the auricle and external ear canal, facial palsy, and vertigo) and vascular vertigo, cervicogenic vertigo, tinnitus with vertigo, and complication with MD and VM, complication with MD and BPPV].
(3) The complete binaural (L, R) air conduction (125, 250, 500, 1,000, 2,000, 4,000, 8,000 Hz) and bone conduction (250, 500 Hz, 1, 2, 4 kHz) based on the data of pure-tone threshold;
(4) The results of acoustic immittance test and auditory brainstem response (ABR) test.
2.4.2. Exclusion criteria
Participants were excluded from the study if they:
(1) Had incomplete data from the pure tone audiometry (either not done or if the air bone conduction threshold data was incomplete).
(2) Had missing age, gender, or diagnostic information.
2.5. Statistical analysis
In this study, the descriptive analysis was mainly used. The counting data was expressed as frequency (percentage). Comparison of pure tone hearing threshold and acoustic immittance measurement applied one-way ANOVA (When P < 0.05, there is a statistical difference). Least-significant difference (LSD) was used for Post-hoc Multiple Comparisons.
K-Means clustering was used for the secondary classification of audiograms. We extracted the features of the left and right audiogram curves, including maximum, minimum, mean, variance, slope of each inflection point, and curve distance as the feature values. These variables were standardized to ensure comparability, and the resulting dataset was used as the input for the subsequent K-means clustering. The optimal number of clusters was determined by fitting the K-means unsupervised machine learning algorithm to 3–6 clusters, respectively.
3. Results
3.1. Data overview
As depicted in Table 1, the largest group of patients with vertigo and dizziness was aged between 60 and 69 years old, with a total of 5,726 cases, which accounted for 61.02% of all admissions. There was a higher prevalence of female patients (61.83%) than male patients (38.17%). Male patients generally had a worse hearing threshold than females, with a difference of 10 dB HL observable at 4 k and 8 kHz (see Figure 2A). In the 2021 WHO's hearing classification, the largest category of hearing loss patients, comprising 44.63% (n = 4,188), was classified as “mild.”
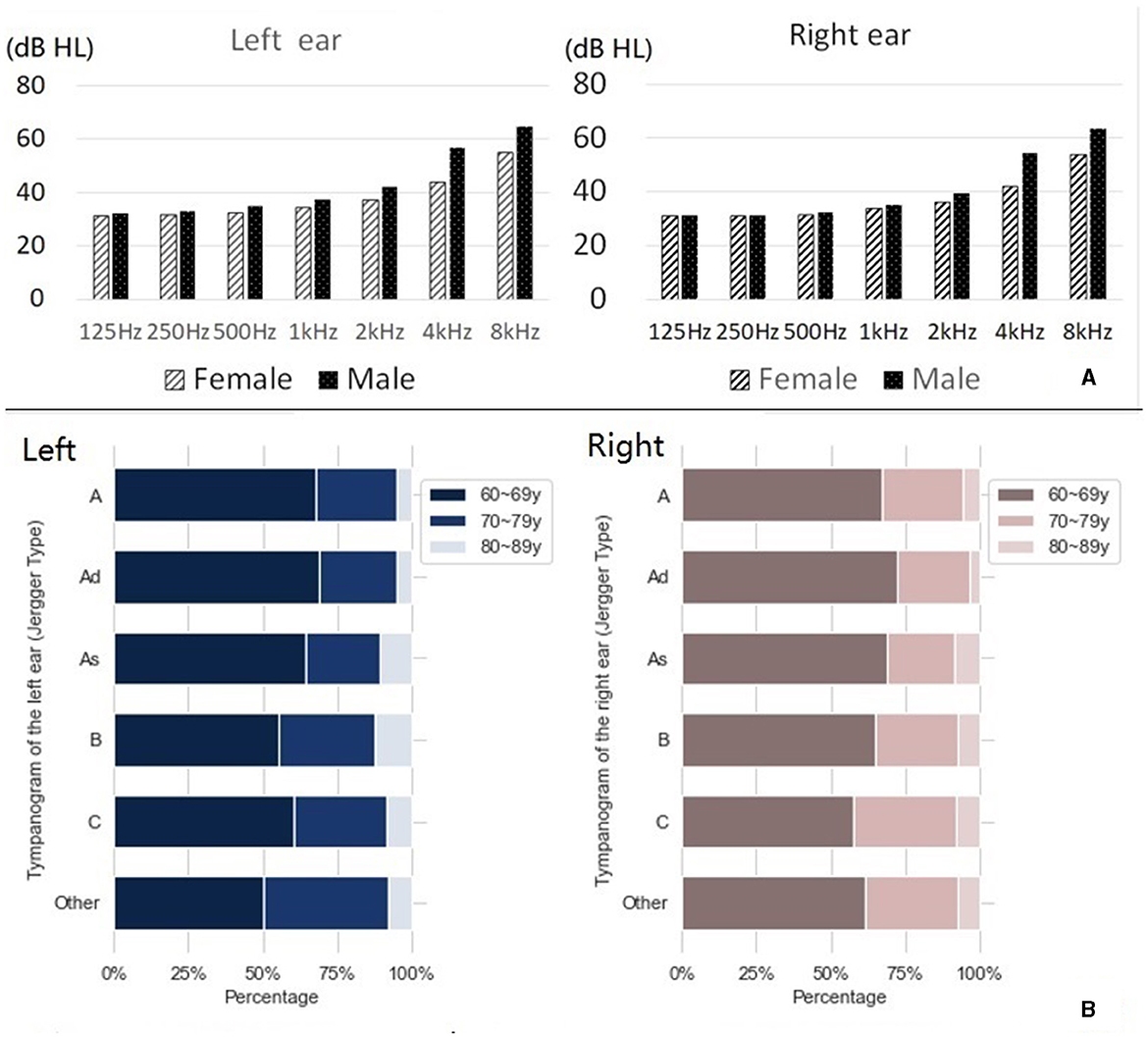
Figure 2. Hearing results of elderly patients with vertigo and dizziness. (A) Hearing thresholds for males and females at each frequency. (B) Jerger classification of tympanic diagram.
3.2. Jerger classification of the tympanic diagram
Figure 2B shows the Jerger classification of tympanograms in patients with vertigo and dizziness (additional details can be found in Supplementary Table 2). There was a near equal distribution between left and right ears, with type A being the most common Jerger classification of tympanogram.
3.3. ABR results
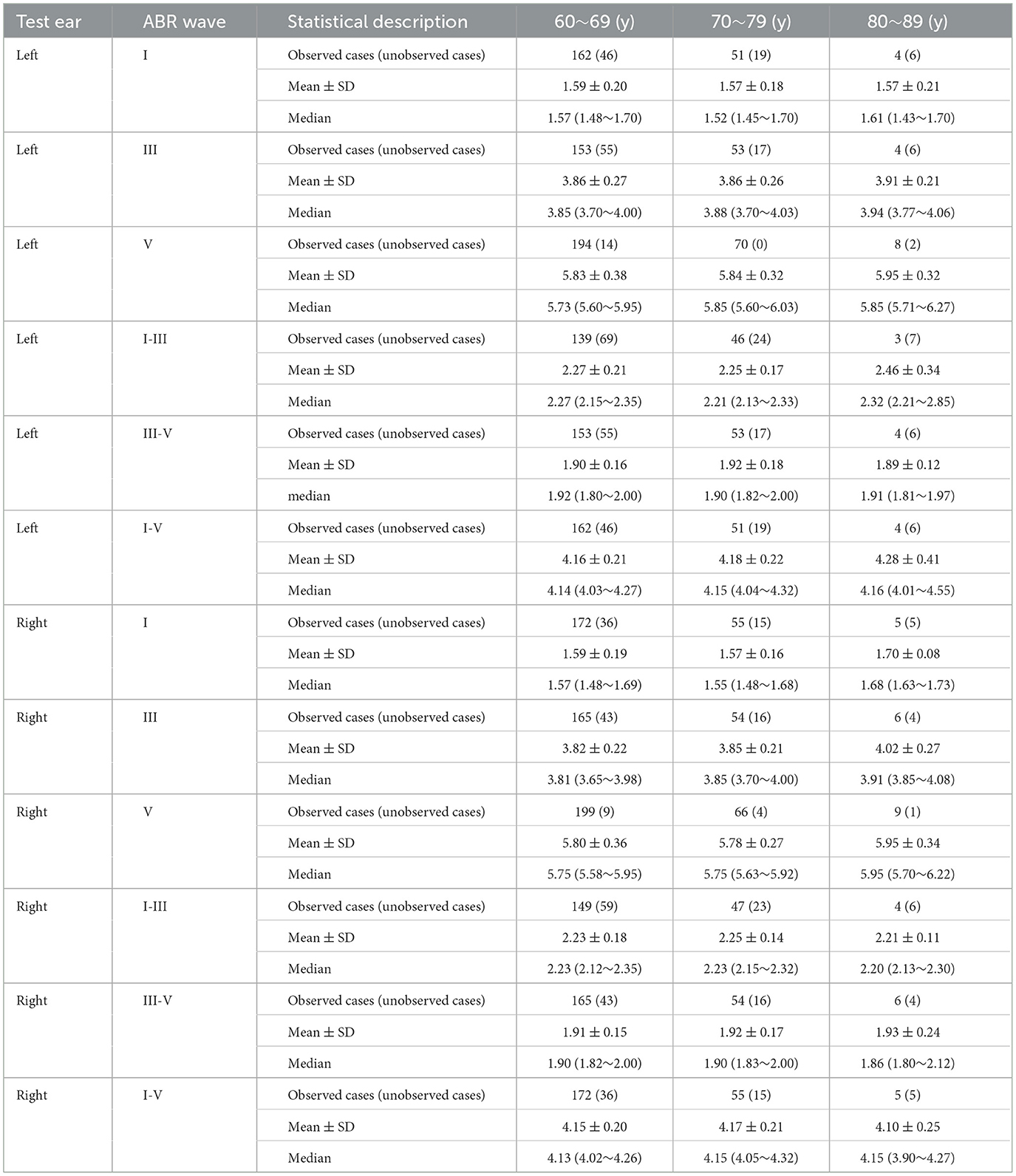
Each wave latency and inter-wave period fell within the normal range, as shown in Table 2.
3.4. Classification of audiogram shapes in elderly patients with vertigo and dizziness
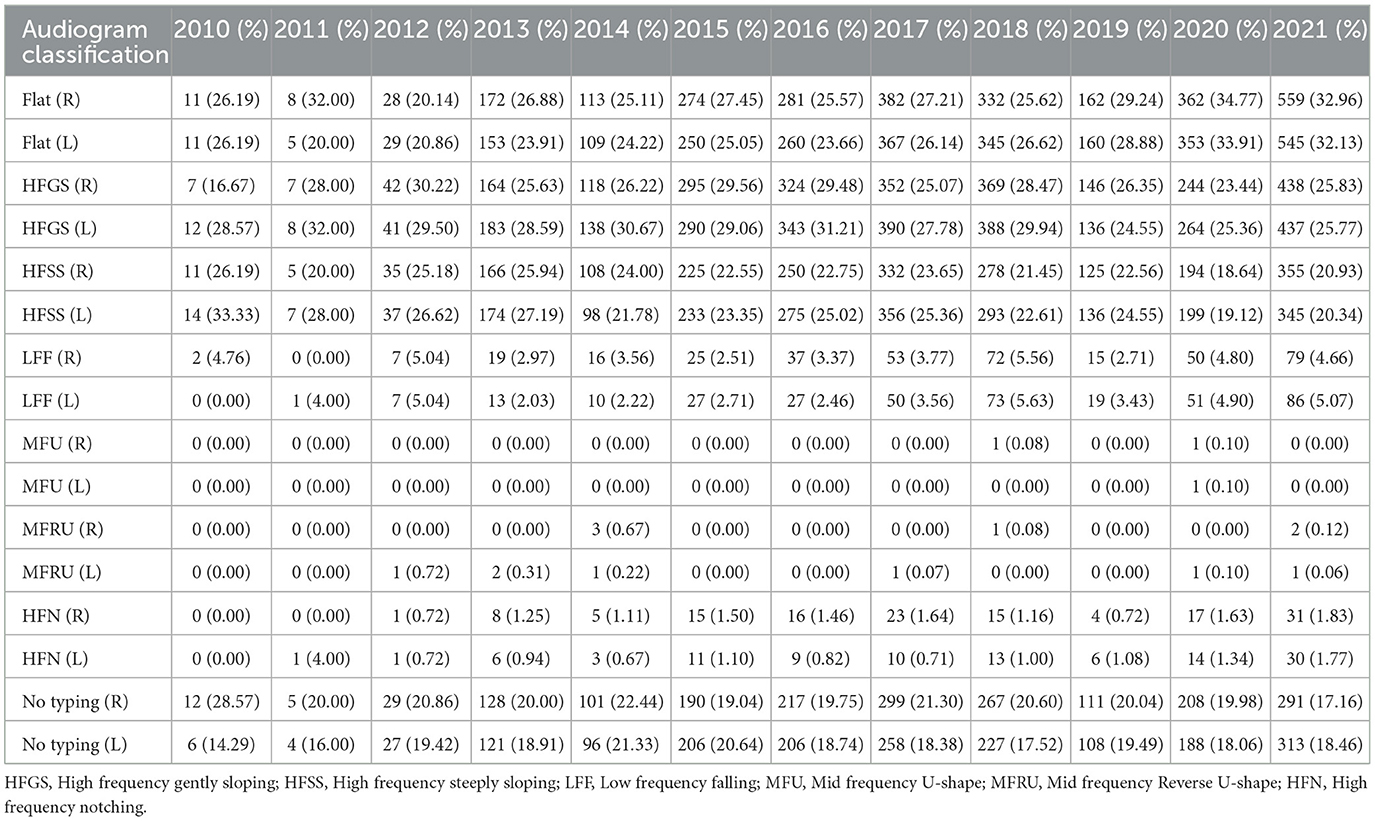
Table 3 outlines the pure-tone audiogram typing and the corresponding proportions of patients with vertigo and dizziness. Similar patterns were observed in both left and right ears. Predominant audiometric shapes included flat (27.81% in the right ear, 26.89% in the left), high-frequency gently sloping (HFGS) (25.97% in the right ear, 27.34% in the left), and high-frequency steeply sloping (HFSS) (21.60% in the right ear, 22.53% in the left) (see Figure 3).
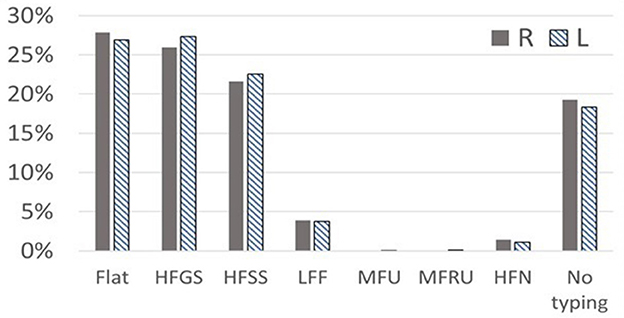
Figure 3. The classification of audiogram shapes in elderly patients with vertigo syndrome. Typing of pure-tone audiogram: Flat, HFGS (high frequency gently sloping), HFSS (high frequency steeply sloping), LFF (low frequency falling), MFU (mid frequency U-shape), MFRU (mid frequency Reverse U-shape), and HFN (high frequency notching).
However, Figure 3 also reveals a significant number of patients categorized as “No typing” (18.3% in the left ear, 19.25% in the right) based on existing criteria, suggesting that current hearing classification standards may not be entirely suitable for elderly vertigo and dizziness patients with hearing loss.
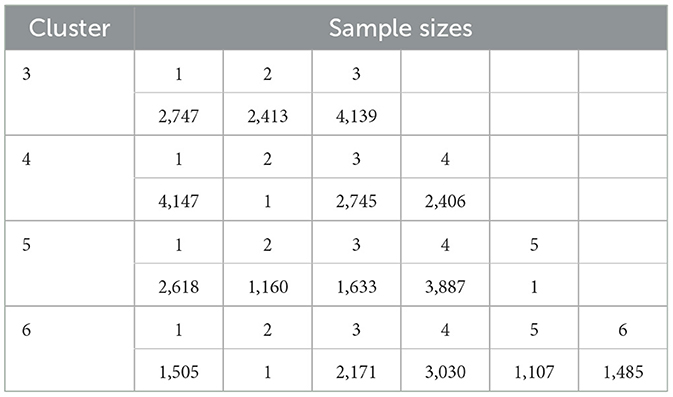
Upon reclassification of the pure tone results (n = 9,299), K-Means clustering analysis suggested that the optimal number of clusters was three (Table 4). The ANOVA statistical results of each characteristic value showed P = 0.000, with sample sizes for the three clusters being 2,747, 2,413, and 4,139, respectively.
3.5. Auditory examination results of patients with definite diagnosis
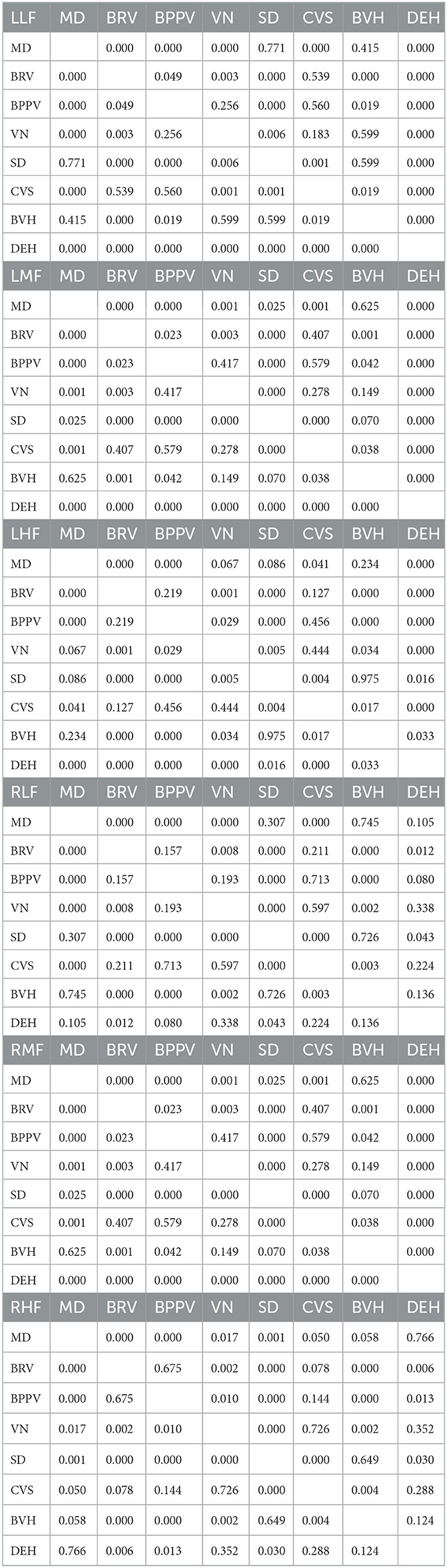
Among the 907 elderly patients with definitively diagnosed vestibular syndrome in this study, the three most prevalent were Ménière's disease (MD, 30.87%), benign recurrent vertigo (BRV, 19.07%), and benign paroxysmal positional vertigo (BBPV, 15.66%). The distribution of these diseases is presented in Figure 4A.
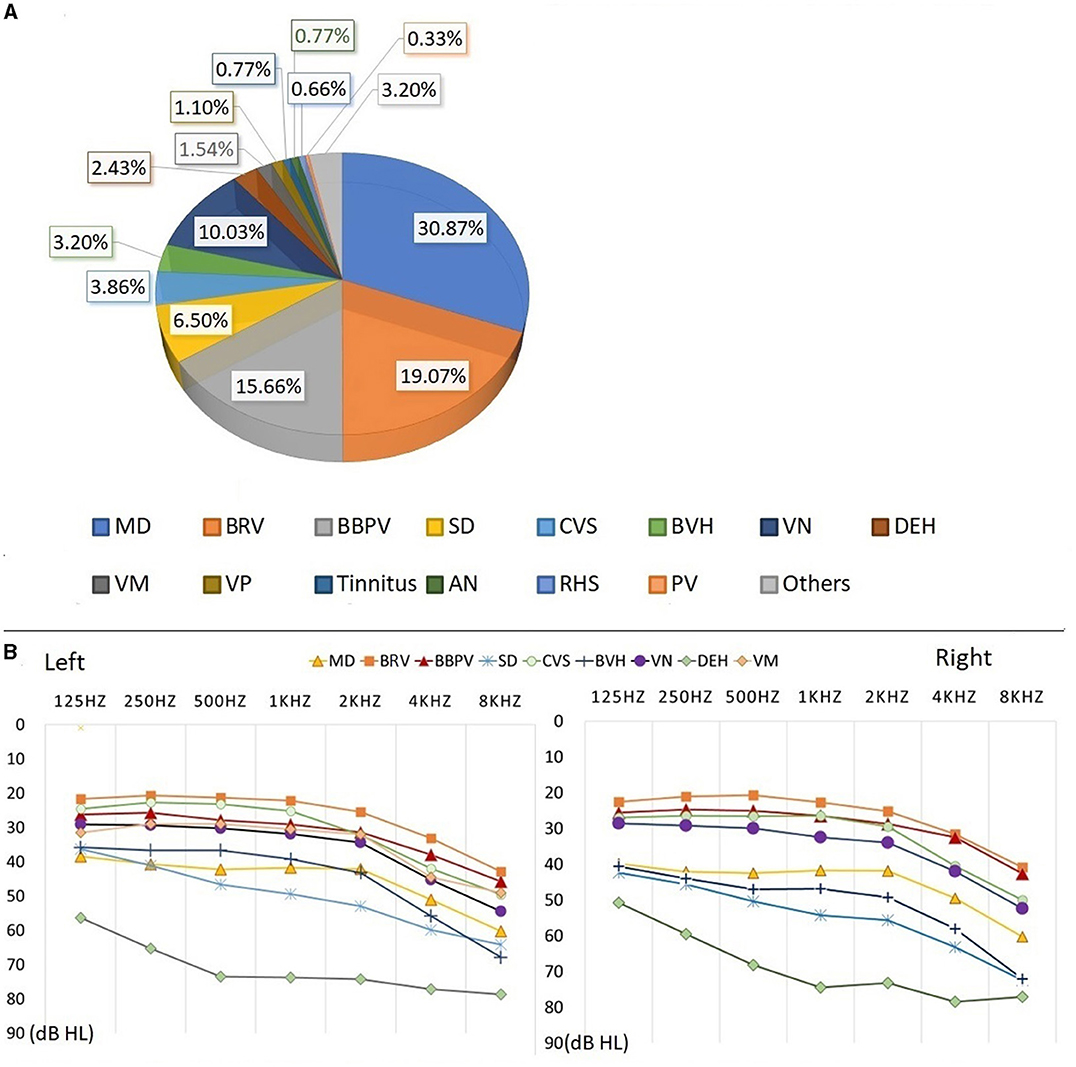
Figure 4. Auditory examination results of patients with definite diagnosis. The percentage of different vestibular syndrome is shown in this figure [Diagnosis types of vestibular syndrome: Meniere disease (MD), Benign recurrent vertigo (BRV), Benign paroxysmal positional vertigo (BPPV), Sudden deafness (SD), Chronic vestibular syndrome (CVS), Bilateral vestibular hypofunction (BVH), Vestibular neuropathy (VN), Delayed endolymphatic hydrops (DEH) Vestibular migraine (VM), Vestibular paroxysmia (VP), Tinnitus, Acoustic neurinoma (AN), Ramsay Hunt Syndrome (RHS), Psychiatric vertigo (PV)]. Eight diseases (MD, BRV, BBPV, VN, SD, CVS, BVH, and DEH) with a higher proportion are analyzed. The percentage of remaining diseases(VM, VP, Tinnitus, AN, RHS, PV and other diseases) is less than 2.00%. (A) Hearing thresholds of MD, BRV, BBPV, VN, SD, CVS, BVH, and DEH are shown in this figure. (B) The patient's hearing threshold gradually increases from 125 to 8,000 Hz. The hearing threshold of 8000 Hz can be 17.03–32.00dB HL higher than that of 125 Hz. The average hearing thresholds (500, 1,000, 2,000, 4,000 Hz) for eight types of vestibular diseases were calculated, and the results show that he hearing threshold of DEH is higher than other vestibular diseases, at around 75 dB HL. The average hearing threshold of other seven vestibular diseases is between 25.04 and 55.86 dB HL.
Auditory examination results from the eight main types of vestibular syndrome (each comprising more than 2.00% of the total) were analyzed, with the corresponding auditory thresholds depicted in Figure 4B. The hearing thresholds in low, medium, and high frequencies for the left and right ears are presented in Table 5. Statistical analysis revealed significant differences in hearing thresholds among the various vestibular diseases (P < 0.001). Detailed results from multiple comparisons of hearing thresholds for different diseases are provided in Table 6.
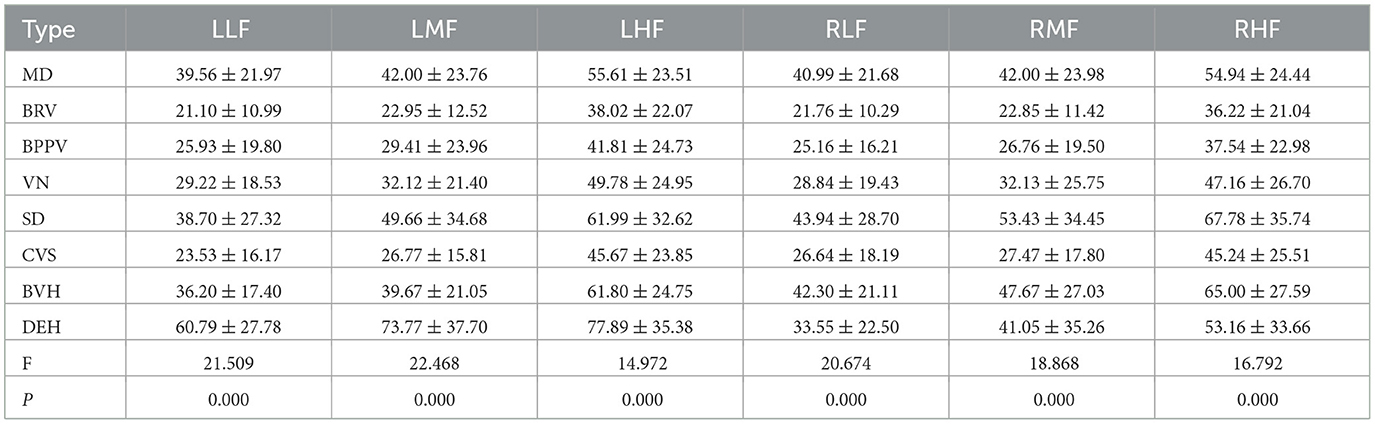
Table 5. Mean and standard deviation of low, medium and high frequency hearing threshold for different vestibular diseases in the left and right ears.
4. Discussion
From the overall result of elderly patients with vertigo and dizziness, 83.10% of them were aged from 60 to 79. Among them, the grading of hearing loss was mainly in level 1 (44.63%) and 2(20.89%), indicating that elderly patients with vertigo and dizziness generally have mild to moderate hearing loss in this age range. Previous studies abroad have shown that caloric test responses depend on several factors that could be affected by age, such as ear canal volume, temporal bone thickness, and blood supply to the temporal bone (Enrietto et al., 1999). Several studies have found that caloric responses tend to increase in middle age with a peak between 50 and 70 years, and then decline modestly thereafter (Fernández et al., 2015). In clinical diagnosis, it's difficult to obtain a complete, meaningful, and treatment-oriented diagnosis in elderly dizzy patients. More than half of elderly patients with balance disorders are vague, inconsistent, or contradictory in describing their symptoms (Newman-Toker et al., 2007). Besides, there is not a single symptom that can predict with specificity the underlying causes of dizziness, and most of the time, elderly patients have more than one cause of dizziness (Fernández et al., 2015). In this study, the incidence of dizziness in females is higher than that in males, but the hearing threshold of females is better.
In this study, the tympanogram of elderly vertigo patients was mainly classified as type A. The wave latency and inter wave period of ABR were within normal range. Analyzing the cause of deafness may be related to blood supply disorders in the inner ear. According to the theory of internal ear blood supply disorder, the labyrinthine artery is the main artery of internal ear blood supply. When the labyrinthine artery has thrombosis, embolism or vasospasm, it will cause labyrinthine artery blood supply disorder, leading to sudden deafness; At the same time, since the labyrinthine artery enters the inner ear and is divided into the common cochlear artery and the vestibular artery, when the blood supply of the labyrinthine artery is impaired, the vestibular function of the patient will also be affected, and vertigo symptoms will appear (Prince and Stucken, 2021). The appearance of vestibular symptoms such as dizziness indicates the severity of the disease and the breadth of the lesion. In previous studies, the wave latency and inter wave period of ABR in elderly patients should be prolonged (Gupta et al., 2014). This phenomenon did not occur in this study, which may be related to the low patient sample size in the age group over 80 years old. For elderly people, it is also necessary to give a special normal reference value for the judgment of each wave latency and wave interval.
In this study, the main audiometric shapes of elderly patients with dizziness were flat, high-frequency gently sloping (HFGS) and high-frequency steeply sloping (HFSS). Due to the large oxygen consumption of the cochlea bottom, the metabolic rate is high. Compared with the cochlea top, the blood supply of the cochlea bottom is poor, and its auditory hair cell is more vulnerable to damage. The cause of deafness in patients with dizziness involves a wide range of surrounding organs, affecting the vestibular area. Moreover, as a result of the bottom of the cochlea near the vestibule, patients with dizziness may have relatively severe cochlear damage, and their inner ear may have a larger or deeper degree of ischemia (Yu and Li, 2018). From Figure 3, it can be seen that a large number of deaf patients are classified as having “No typing” (18.3% in the left ear, and 19.25% in the right ear) based on the current criteria. Presbycusis patients with vertigo and dizziness are often associated with complicated basic diseases such as diabetes, hypertension and coronary heart disease, and the degree of hearing loss is high and cause the diversity of hearing changes in elderly deaf patients. This indicated the possibility of inappropriate classification methods for elderly patients with hearing loss according to the fixed criteria for audiometric classification. Further detailed research is needed to analyze the hearing status of aged patients with different diseases.
5. Conclusion
Our study revealed that elderly patients with vertigo and dizziness primarily experienced mild to moderate hearing loss. Interestingly, we found that the hearing threshold of female patients was generally better than that of their male counterparts. We also discovered that the most common audiometric shapes in these patients were flat, high-frequency gently sloping (HFGS), and high-frequency steeply sloping (HFSS). Importantly, we identified significant differences in hearing thresholds across various vestibular diseases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by PLA General Hospital's Ethics Committee (No. S2022-673-01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QW performed the methodology and writing—original draft. AC performed the data curation. MH performed the formal analysis. XL performed the writing—review and editing. YD performed the visualization. ZW performed the supervision. FJ performed the conceptualization, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by funding of the Open Project National Clinical Research Center for Otolaryngologic Diseases (202200010) and the Capital's Funds for Health Improvement and Research (No. 2022-1-2023).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1225786/full#supplementary-material
References
Albernaz, P. L. (2016). Hearing loss, dizziness, and carbohydrate metabolism. Int. Arch. Otorhinolaryngol. 20, 261–270. doi: 10.1055/s-0035-1558450
American Speech-Language-Hearing Association (2005). Guidelines for manual pure-tone threshold audiometry. Rockville, MD: American Speech-Language-Hearing Association. Available online at: http://www.asha.org/members/deskref-journal/deskref/default
Chang, T. P., Wang, Z., Winnick, A. A., Chuang, H. Y., Urrutia, V. C., Carey, J. P., et al. (2018). Sudden hearing loss with vertigo portends greater stroke risk than sudden hearing loss or vertigo alone. J. Stroke Cerebrovasc. Dis. 27, 472–478. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.033
Demeester, K., van Wieringen, A., Hendrickx, J. J., Topsakal, V., Fransen, E., van Laer, L., et al. (2009). Audiometric shape and presbycusis. Int. J. Audiol. 48, 222–232. doi: 10.1080/14992020802441799
Du, Y., Ren, L., Liu, X., and Wu, Z. (2022). Machine learning method intervention: determine proper screening tests for vestibular disorders. Auris Nasus Larynx. 49, 564–570. doi: 10.1016/j.anl.2021.10.003
Enrietto, J. A., Jacobson, K. M., and Baloh, R. W. (1999). Aging effects on auditory and vestibular responses: a longitudinal study. Am. J. Otolaryngol. 20, 371–378. doi: 10.1016/S0196-0709(99)90076-5
Fancello, V., Hatzopoulos, S., Santopietro, G., Fancello, G., Palma, S., Skarżyński, P. H., et al. (2023). Vertigo in the elderly: a systematic literature review. J. Clin. Med. 12, 2182. doi: 10.3390/jcm12062182
Fernández, L., Breinbauer, H. A., and Delano, P. H. (2015). Vertigo and dizziness in the elderly. Front. Neurol. 6:144. doi: 10.3389/fneur.2015.00144
García, A., Madrigal, J., and Castillo, M. (2021). Vestibular migraine and tinnitus: a challenging narrative. Cureus. 13:e15998. doi: 10.7759/cureus.15998
Gupta, S., Mittal, S., Baweja, P., Kumar, A., Singh, K. D., Sharma, R., et al. (2014). Analysis of gender based differences in auditory evoked potentials among healthy elderly population. Adv. Biomed. Res. 3, 208. doi: 10.4103/2277-9175.143243
Haeussler, S. M., Zabaneh, S. I., Stegemann, M., Olze, H., Böttcher, A., Stölzel, K., et al. (2022). Is Vestibular0020SDNeuropathy rather a neuritis? Cureus. 14:e29959. doi: 10.7759/cureus.29959
Kim, H. J., Park, J., and Kim, J. S. (2021). Update on benign paroxysmal positional vertigo. J. Neurol. 268, 1995–2000. doi: 10.1007/s00415-020-10314-7
Kuhn, M., Heman-Ackah, S. E., Shaikh, J. A., and Roehm, P. C. (2011). Sudden sensorineural hearing loss: a review of diagnosis, treatment, and prognosis. Trends Amplif. 15, 91–105. doi: 10.1177/1084713811408349
Lasisi, A. O., and Gureje, O. (2014). Prevalence and correlates of dizziness in the Ibadan study of ageing. Ear Nose Throat J. 93, E37–44.
Lee, J. H., Bahng, J., Kim, C., and Kim, Y. Y. (2020). Quantitative criteria for age-related hearing loss using audiometric configuration analysis. Eur. Arch. Otorhinolaryngol. 277, 93–102. doi: 10.1007/s00405-019-05689-x
Lindell, E., Kollén, L., Johansson, M., Karlsson, T., Rydén, L., Fässberg, M. M., et al. (2021). Dizziness and health-related quality of life among older adults in an urban population: a cross-sectional study. Health Qual. Life Outcomes. 19, 231. doi: 10.1186/s12955-021-01864-z
Lucieer, F., Vonk, P., Guinand, N., Stokroos, R., Kingma, H., van de Berg, R., et al. (2016). Bilateral Vestibular hypofunction: insights in etiologies, clinical subtypes, and diagnostics. Front. Neurol. 7:26. doi: 10.3389/fneur.2016.00026
Monsell, E. M., Balkany, T. A., Gates, G. A., Goldenberg, R. A., Meyerhoff, W. L., and House, J. W. (1995). Committee on hearing and equilibrium guidelines for the diagnosis and evaluation of therapy in Menière's disease. American Academy of Otolaryngology Head and Neck Foundatiion, Inc. Otolaryngol. Head Neck Surg. 113, 181–185. doi: 10.1016/S0194-5998(95)70102-8
Newman-Toker, D. E., Cannon, L. M., Stofferahn, M. E., Rothman, R. E., Hsieh, Y. H., Zee, D. S., et al. (2007). Imprecision in patient reports of dizziness symptom quality: a crosssectional study conducted in an acute care setting. Mayo Clin. Proc. 82, 1329–1340. doi: 10.4065/82.11.1329
Prince, A. D. P., and Stucken, E. Z. (2021). Sudden sensorineural hearing loss: a diagnostic and therapeutic emergency. J. Am. Board Fam. Med. 34, 216–223. doi: 10.3122/jabfm.2021.01.200199
Rambold, H., Boenki, J., Stritzke, G., Wisst, F., Neppert, B., Helmchen, C., et al. (2005). Differential vestibular dysfunction in sudden unilateral hearing loss. Neurology. 64, 148–151. doi: 10.1212/01.WNL.0000148599.18397.D2
Reynard, P., Karkas, A., Gavid, M., Lelonge, Y., and Bertholon, P. (2018). Delayed endolymphatic hydrops. special emphasis on nystagmus associated with episodes and contribution of chemical labyrinthectomy. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 135, 321–326. doi: 10.1016/j.anorl.2018.08.001
Roque Reis, L., Lameiras, R., Cavilhas, P., and Escada, P. (2016). Epidemiologia da vertigem na urgência hospitalar [epidemiology of vertigo on hospital emergency]. Acta Med. Port. 29, 326–331. doi: 10.20344/amp.6571
Sunitha, M., Asokan, L., and Sambandan, A. P. (2019). Vertigo: incidences, diagnosis and its relations with hearing loss. Indian J. Otolaryngol. Head Neck Surg. 71, 1282–1286. doi: 10.1007/s12070-018-1315-6
Traschütz, A., Cortese, A., Reich, S., Dominik, N., Faber, J., Jacobi, H., et al. (2021). M; RFC1 study group. natural history, phenotypic spectrum, and discriminative features of multisystemic RFC1 disease. Neurology. 96, e1369–e1382. doi: 10.1212/WNL.0000000000011528
van Leeuwen, R. B., Colijn, C., van Esch, B. F., and Schermer, T. R. (2022). Benign recurrent vertigo: the course of vertigo attacks compared to patients with menière's disease and vestibular migraine. Front. Neurol. 13:817812. doi: 10.3389/fneur.2022.817812
Keywords: hearing loss, vertigo, elderly, dizziness, pure tone audiometry, acoustic immittance measurement, auditory brainstem response (ABR)
Citation: Wang Q, Chen A, Hong M, Liu X, Du Y, Wu Z, Cheng W and Ji F (2023) Investigation of hearing loss in elderly vertigo and dizziness patients in the past 10 years. Front. Aging Neurosci. 15:1225786. doi: 10.3389/fnagi.2023.1225786
Received: 19 May 2023; Accepted: 21 August 2023;
Published: 15 September 2023.
Edited by:
Lisheng Yu, Peking University People's Hospital, ChinaReviewed by:
Qing Zhang, Shanghai Jiaotong University School of Medicine, ChinaZhaoli Meng, Sichuan University, China
Copyright © 2023 Wang, Chen, Hong, Liu, Du, Wu, Cheng and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Ji, YXJnZmVpMzAxQDE2My5jb20=
 Qian Wang
Qian Wang Aiting Chen1,2,3,4
Aiting Chen1,2,3,4 Yi Du
Yi Du Ziming Wu
Ziming Wu Fei Ji
Fei Ji