
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 18 July 2023
Sec. Parkinson’s Disease and Aging-related Movement Disorders
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1213977
This article is part of the Research Topic Insights in Parkinson’s Disease and Aging-related Movement Disorders: 2022 View all 12 articles
Introduction: Hyposmia is a common prodrome in patients with Parkinson’s disease (PD). This study investigates whether olfactory changes in PD differ according to the degree of olfactory dysfunction and whether there are changes in motor and non-motor symptoms.
Methods: The 129 subjects with PD were divided into two groups: anosmia and non-anosmia. All cases were reassessed within 1–3 years after the initial assessment. The assessment included the MDS-Unified PD Rating Scale (MDS-UPDRS), the University of Pennsylvania Smell Identification Test (UPSIT), Beck’s Depression Inventory-II (BDI-II), Montreal Cognitive Assessment (MoCA), and equivalence dose of daily levodopa (LEDD). The generalized estimating equation (GEE) model with an exchangeable correlation structure was used to analyze the change in baseline and follow-up tracking and the disparity in change between these two groups.
Results: The anosmia group was older and had a longer disease duration than the non-anosmia group. There was a significant decrease in UPSIT after follow-up in the non-anosmia group (β = −3.62, p < 0.001) and a significant difference in the change between the two groups (group-by-time effect, β = 4.03, p < 0.001). In the third part of the UPDRS motor scores, there was a tendency to increase the score in the non-anosmia group compared to the anosmia group (group-by-time effect, β = −4.2, p < 0.038). There was no significant difference in the group-by-time effect for UPDRS total score, LEDD, BDI-II, and MoCA scores.
Discussion: In conclusion, this study found that olfactory sensation may still regress in PD with a shorter disease course without anosmia, but it remains stable in the anosmia group. Such a decline in olfaction may not be related to cognitive status but may be associated with motor progression.
Parkinson’s disease (PD) is the second most common neurodegenerative disease, with a prevalence of around 1.4–3.0 per thousand in Taiwan which increases with aging (Liu et al., 2016a,b). In addition to motor symptoms such as bradykinesia, tremor, and rigidity, non-motor symptoms contribute to poor quality of life in patients with PD (Rodríguez-Violante et al., 2015; Tibar et al., 2018; Santos Garcia et al., 2019). Some non-motor symptoms appear before motor symptoms, known as prodromal non-motor symptoms of PD (Poewe et al., 2017). Olfactory dysfunction, constipation, depression, and rapid eye movement (REM) sleep behavior disorder (RBD) can represent prodromal symptoms.
The Braak staging system explains prodromal symptoms because alpha-synuclein aggregates, a pathological hallmark of PD, are initially found in the olfactory bulb and the dorsal motor nucleus of the vagus (Braak et al., 2003). One route of propagation of alpha-synuclein inclusion in the dual-hit hypothesis starts from the enteric nervous system with the gut to brain spreading. This route is suggested to be associated with the involvement of the autonomic nervous system and premotor RBD, naming the body-first subtype. The other route of alpha-synuclein pathology starts from the olfactory bulb and anterior olfactory nucleus and spreads to adjacent areas such as the olfactory tubercle, piriform cortex, periamygdaloid cortex, and entorhinal cortex. However, the evidence of entry via the olfactory pathway is still controversial because no advanced lesions are found in non-olfactory cortical areas (Braak et al., 2003; Horsager et al., 2020). In a PD mouse model, RBD-like behavior occurred earlier than hyposmia, which correlates with the finding in humans that PD patients with RBD were more hyposmic than PD patients without RBD (Taguchi et al., 2020). These findings suggest that the ascending pathway of the brainstem may predominate in the spread of alpha-synuclein, despite the initial deposition in the olfactory bulb (Braak et al., 2003; Horsager et al., 2020).
However, hyposmia is still one of the common non-motor symptoms in PD related to Lewy body pathology in the olfactory system (Haehner et al., 2009; Rodríguez-Violante et al., 2017). As the disease progresses, Lewy body pathology increases in the olfactory system, but most studies show inconsistent results in the relationship between hyposmia and disease severity (Berendse et al., 2011; Yoo et al., 2020). Few studies discuss the association between the duration of the disease and olfactory dysfunction, and most of them did not show an obvious correlation, and even the results of some longitudinal studies are inconsistent (Ercoli et al., 2022). Due to the ambiguous relationship between olfactory dysfunction and disease duration, this study aims to investigate the longitudinal change of olfactory function in PD patients based on their degree of olfactory dysfunction. Given this uncertainty, we also conducted a comprehensive analysis of disease severity, medication usage, cognitive function, and depression during the longitudinal follow-up period to provide a more comprehensive comparison.
Participants were recruited from the outpatient clinic at Taichung Veteran General Hospital from 2017. Subjects were selected on the basis of International Parkinson and MDS Clinical Diagnostic Criteria for Parkinson’s disease. At the first visit (T0), all subjects received a complete survey that included the MDS-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), the University of Pennsylvania Smell Identification Test (UPSIT), Beck’s Depression Inventory-II (BDI-II), and Montreal’s Cognitive Assessment (MoCA). Follow-up (T1) was conducted for these patients with PD in 1–3 years after the first visit, and a comprehensive survey was also conducted including MDS-UPDRS, UPDIT, BDI, and MoCA. The equivalent dose of daily levodopa was also calculated on the date of the first visit and the follow-up, respectively. Individuals were excluded if they did not meet the MDS clinical diagnostic criteria for Parkinson’s disease during follow-up or could not complete the questionnaire. Those who had upper respiratory tract infection and sinonasal disease which might affect olfaction were also excluded. Written informed consent was obtained from all participants. This study was approved by Taichung Veterans General Hospital Institutional Review Board/Ethics Committee (No. CE22189B). All methods were performed in accordance with the Declaration of Helsinki guidelines and hospital regulations.
The olfactory function was evaluated with the validated Taiwanese version of UPSIT, an odor identification (Jiang et al., 2010). The total score was 40 in this test and the cutoff value of total anosmia was less than 19. Considering that the mean UPSIT score is 17–20 in PD patients which is close to the cutoff value 19 of anomia in UPSIT, we divided subjects into two groups, anosmia and non-anosmia based on the UPSIT score at the first visit to represent the characteristics of profound olfactory deficit or milder symptom in PD, respectively (Doty, 2001, 2012; Picillo et al., 2014; Lawton et al., 2016). Non-motor symptoms of PD were also assessed. For cognition, we used MoCA due to its validation for assessing global cognitive abilities in PD (Litvan et al., 2012). BDI-II was used for mood investigation (Beck et al., 1996). Regarding the severity of motor symptoms in PD, the part 3 score of MDS-UPDRS (UPDRS 3) and the equivalent dose of daily levodopa (LEDD) were used to determine the severity of motor symptoms (Goetz et al., 2008). The total score of MDS-UPDRS (UPDRS T) was used to represent the disease burden of PD. Scores of MDS-UPDRS Part 1 and Part 2 were used to represent the non-motor and motor experiences of daily living, and Part 4 was used for motor complication. To determine the motor subtypes, we utilized 11 items (2.10, 3.15–3.18) for tremor and five items (2.12, 2.13, 3.10–3.12) for postural instability/gait difficulty (PIGD) from the MDS-UPDRS. The ratio of mean tremor scores to the mean PIGD scores was employed to define the following subtypes: (1) tremor subtype with a ratio ≥ 1.15 and (2) PIGD subtype with a ratio ≤ 0.90 (Stebbins et al., 2013).
Baseline clinical characteristics between the anosmia and non-anosmia groups were compared by using chi-square test for binary variables. UPSIT, MoCA, BDI-II, MDS UPDRS scores, and LEDD scores were analyzed as continuous variables. For continuous variables that follow a normal distribution, Student t-tests were used for analysis. For variables that do not follow a normal distribution, non-parametric Mann–Whitney U tests were used for analysis. Multiple linear regression adjusted for age, gender and disease was carried out to analyze the relationship between UPSIT and each variable including MoCA, BDI-II, UPDRS 3, UPDRS T, and LEDD scores at baseline. Generalized estimating equation (GEE) model with an exchangeable correlation structure, was used to assess the change of longitudinal data, including MoCA, BDI-II, UPDRS 3, UPDRS T, and LEDD, between the anosmia and non-anosmia groups at T1 compared with T0.
All tests were with a statistical significance level of p < 0.05 and were reported with 95% confidence intervals (CIs). Data analysis was performed with SPSS software (IBM Corporation, Armonk, New York, NY, USA).
A total of 129 participants were enrolled in this study. Table 1 shows that the anosmia and non-anosmia groups comprised 73 and 56 subjects, respectively. At baseline, the anosmia group was older than the non-anosmia group (66.65 vs. 63.21, p = 0.032) and had a longer disease duration (4.89 years vs. 3.27, p = 0.033). The group with anosmia also demonstrated higher scores on UPDRST and UPDRS3, but exhibited lower scores on the MoCA. However, no significant differences were found between the two groups regarding gender, follow-up interval, motor subtypes, scores of UPDRS1, 2, and 4, LEDD and BDI scores. After the follow-up for UPSIT re-evaluation, it was observed that 20 patients from the non-anosmia group at the first visit had developed anosmia, accounting for 35.7% of the non-anosmia group. Conversely, seven patients from the anosmia group had transitioned to non-anosmia. Eventually, the anosmia and non-anosmia groups comprised 86 and 43 subjects, respectively.
The UPSIT scores of all participants at baseline were significantly correlated with MoCA (β = 0.14, p = 0.015), UPDRS 3 (β = −0.67, p = 0.001), and UPDRS T (β = −0.84, p = 0.007), after adjusting for age, gender, and disease duration. However, no significant correlations were found between UPSIT and BDI or LEDD.
In the GEE analysis (Table 2), a significant group effect revealed a lower UPSIT score in the anosmia group (β = −10.58, p < 0.001). The time effect was significant in the non-anosmia group (β = −3.62, p < 0.001) but not in the anosmia group. The group-by-time effect was also significant (β = 4.03, p < 0.001), indicating that the UPSIT score remained stable in the anosmia group but decreased significantly in the non-anosmia group (Figure 1A). These results remained significant after adjusting for age, gender, and disease duration (Table 3).
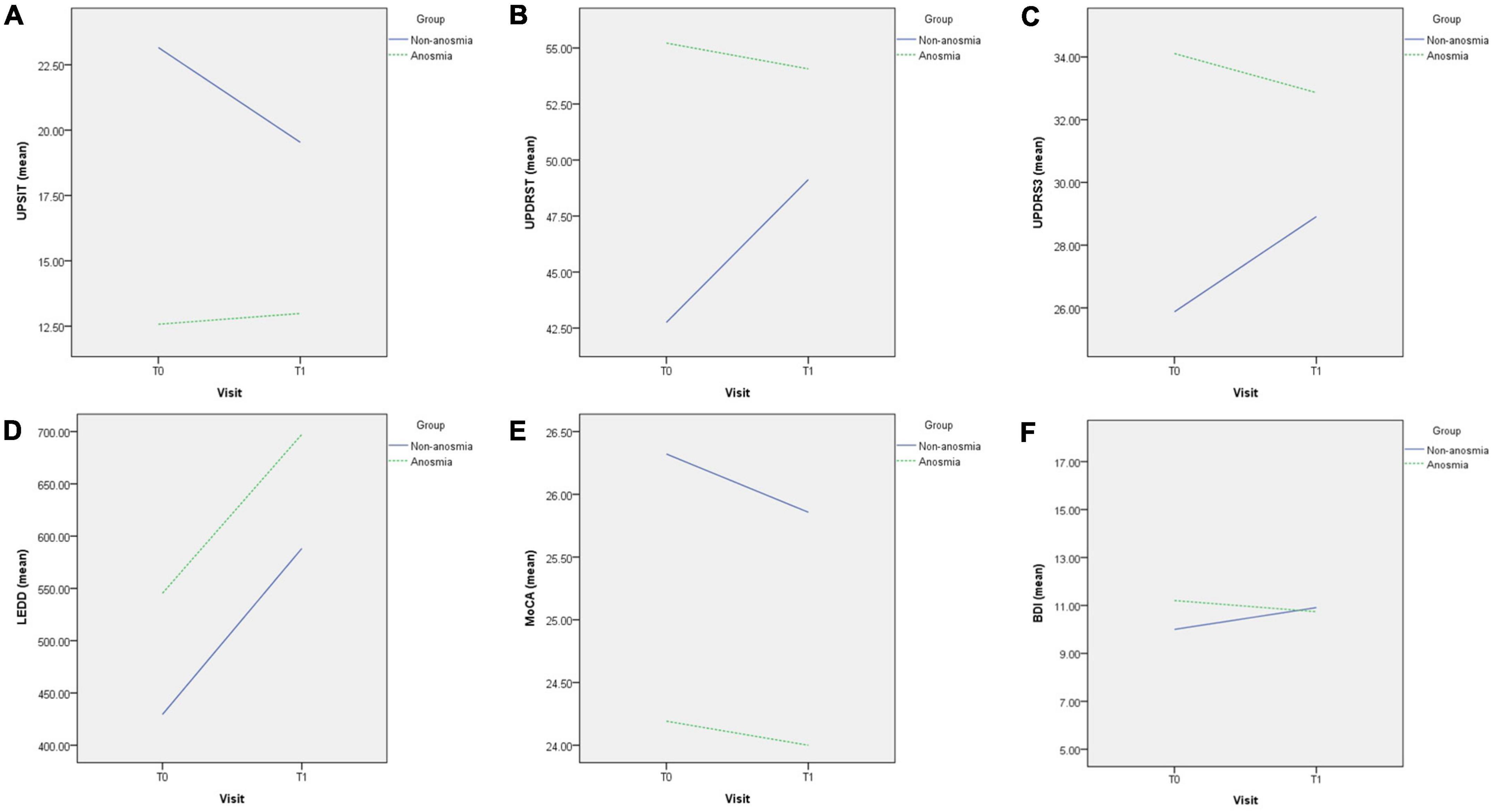
Figure 1. Difference in changes between non-anosmia and anosmia group in UPSIT (A), UPDRST (B), UPDRS3 (C), LEDD (D), MoCA (E), and BDI-II (F) during the follow-up. T0, first visit; T1, follow-up visit; UPSIT, University of Pennsylvania Smell Identification Test; UPDRST, total score of MDS-UPDRS; UPDRS3, part 3 score of MDS-UPDRS; LEDD, equivalent dose of daily levodopa; MoCA, Montreal’s Cognitive Assessment; BDI-II, Beck’s Depression Inventory-II.
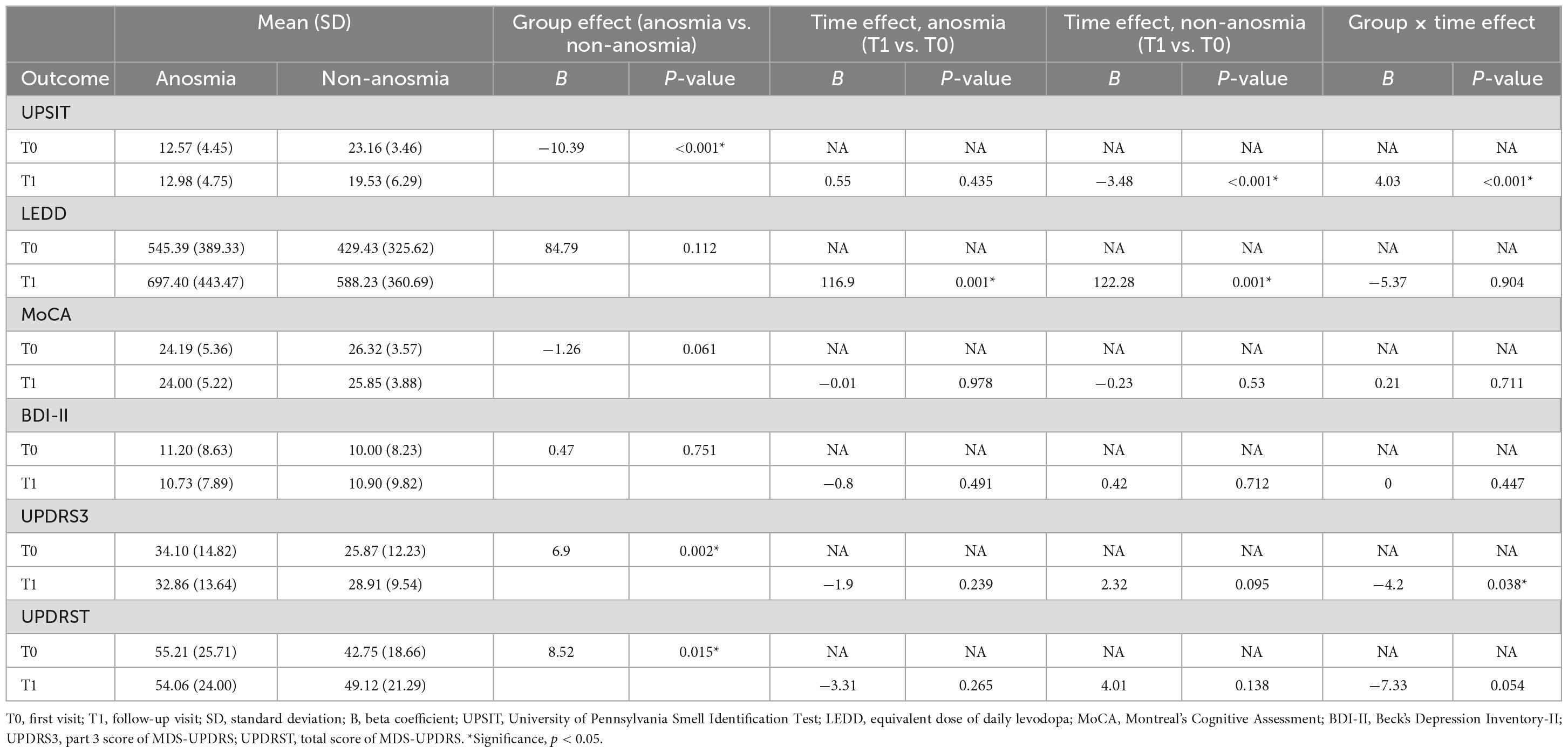
Table 3. Generalized estimating equation analysis for the comparison of outcomes, adjusted for age, gender, and disease duration.
Significant group effects were found for UPDRS 3 and UPDRS T (Table 3), indicating higher scores in the anosmia group at baseline. Although there were trends of increasing UPDRS T and UPDRS 3 scores in the non-anosmia group at follow-up (Figures 1B, C and Table 2), the time effect lost its significance after adjustment (Table 3). However, the group-by-time effect for UPDRS 3 remained significant (β = −4.2, p < 0.038) after adjusting for age, gender, and disease duration (Table 3). The increase in UPDRS 3 score was much more pronounced in the non-anosmia group (Figure 1C).
Regarding LEDD, the time effects for both the anosmia group (β = 116.90, p = 0.001) and the non-anosmia group (β = 122.28, p = 0.001) were significant, but there was no significance in either group effect or group-by-time effect (Figure 1D and Table 3).
The MoCA score was lower in the anosmia group with a significant group effect (β = −2.13, p = 0.007) (Table 2), but the significance disappeared after adjusting for age, gender, and disease duration (Table 3). No significant effects for MoCA were found for time or group-by-time effects (Figure 1E and Table 3). Likewise, no significant effects were found for BDI in terms of group effect, time effect, or group-by-time effect (Figure 1F and Table 3).
The present study demonstrated that the UPSIT score regressed in the non-anosmia group while remaining stable in the anosmia group. Notably, the non-anosmia group had a relatively short course of the disease in this study. Our findings are consistent with those of other longitudinal studies. For instance, Lewis et al. (2020) analyzed PD patients annually and found that UPSIT significantly decreased in early and middle-stage PD but not in later-stage PD with disease duration exceeding 5 years. Domellof et al. (2017) explored the UPSIT outcome with the interaction effect between the group (hyposmic/normosmic) and time, revealing that UPSIT deteriorated over time in the normosmic group while remaining stable in the hyposmic group. Meusel et al. (2010) showed a larger olfactory decline in the subgroup of patients with no severe initial olfactory deficit over 5 years of tracking. The patients with marked olfactory regression had an average disease duration of 2.3 years at the beginning of the visit.
Our results support these findings by indicating that the rate of olfactory decline with disease progression is more pronounced in patients without severe initial olfactory deficits, whereas the olfactory deficit remains relatively stable in patients with profound olfactory deficits. While olfactory impairment is considered a premotor feature of Parkinson’s disease (PD), it is important to note that the olfactory impairment may continue to progress even after motor symptoms have emerged until it reaches a point known as the “floor effect” in the current olfactory test (Fullard et al., 2017). This corresponds to the hypothesis proposed by Huisman et al. (2004) suggesting that dopaminergic neurons in the olfactory bulb, which act as possible suppressors in olfactory transmission, increase as a compensatory mechanism to the dopamine deficit in the basal ganglia. With disease progression, the decrease in olfactory bulb volume and the deposition of Lewy bodies in the olfactory bulb may neutralize such inhibitory changes, resulting in less significant olfactory degeneration (Herting et al., 2008). However, olfactory loss in PD may not be simply explained by imbalance of dopamine projection because the olfactory function involves several neurotransmitters such as acetylcholine, norepinephrine, serotonin and GABA (Doty, 2017). As olfactory dysfunction appears to be more closely associated with the body-first type of alpha-synuclein propagation, the pathology primarily affecting the dorsal motor nucleus of the vagus or brainstem may impact olfactory function through the development of alpha-synucleinopathy in the bilateral olfactory bulbs or other brainstem nuclei that project to the olfactory system (Borghammer, 2021). Some cross-sectional studies have shown that olfactory degeneration is unrelated to the disease’s course (Cavaco et al., 2015; Masala et al., 2018). Other longitudinal studies have also shown no significant change in olfaction over time in patients with PD (Doty et al., 1988; Muller et al., 2002; Herting et al., 2008; Campabadal et al., 2017; Fujio et al., 2020). Such different results may be related to different study designs, such as the number of patients enrolled, the characteristics of different patient groups, and so on. In our study, patients were divided into two groups, anosmia and non-anosmia, and the course of the disease differed between the two groups. Therefore, grouping patients according to the degree or duration of olfactory abnormalities may explain the discrepancies between the results of these studies.
Olfactory deterioration in patients with PD is thought to be associated with cognitive decline, and in particular, the accuracy of olfactory identification tests is often affected by cognitive decline (Laing and Doty, 2003). However, the results of this study showed that although the UPSIT scores of the non-anosmia group decreased after follow-up, there was no significant difference in the MoCA scores for the cognitive function component. This may suggest that while there is a significant association between hyposmia in PD patients and cognitive decline, the initial regression in olfactory identification is not solely attributed to cognitive decline. Other factors, such as Lewy body-related pathology in the peripheral and central olfactory organs or change in the balance of neurotransmitters, may play a role.
Regarding disease severity, although the association with olfactory abnormalities remains inconclusive, our study found a significant association between UPSIT and UPDRS T score and UPDRS 3 scores, in line with the results of other studies (Roos et al., 2019). Unlike the longitudinal study by He et al. (2020) which showed that olfactory abnormalities were predictive of disease progression, our study found no change in UPDRS T and UPDRS 3 score in the anosmia group during short-term follow-up, but there was a tendency for symptoms to progress in the non-anosmia group. These different results may be due to differences in the length of follow-up, patient subgroups, and analysis methods.
In addition, the worsening of Parkinson’s symptoms and olfaction in the non-anosmia group during the follow-up period may indirectly support the theory of Lewy body pathology between the brainstem and olfactory organs, as well as the influence of neurotransmitters such as dopamine. In the Braak staging system, Lewy body pathology was initially found in the olfactory bulb, but this lesion did not progress further, suggesting that a cascade of pathological changes from the brainstem upward is the main pathway (Braak et al., 2003). Horsager et al. (2020) proposed a body-first and brain-first model for the progression of PD pathology based on the presence or absence of RBD and the results of 123I-metaiodobenzylguanidine (MIBG) scintigraphy. The body-first model corresponds to the spreading pathway of the Braak staging system. In addition to autonomic-related prodrome and RBD, the body-first model has a faster progression of motor symptoms and earlier olfactory abnormalities than the brain-first model (Borghammer et al., 2021). These features of the body-first model may reflect the association between olfactory Lewy pathology and the caudo-rostral progression of Lewy pathology. However, olfactory Lewy pathology is not only related to caudo-rostral progression. Kok et al. (2021) found two features of olfactory Lewy pathology in the Vantaa85 + cohort: caudo-rostral progression and amygdala-based progression, corresponding to the body-first and brain-first models, respectively. This may also explain why not all patients in the non-anosmia group in our study turned to anosmia during follow-up and indicates that the severity and pathological changes of olfaction in PD are not a single pattern of progression. Further research with larger, more definitive patient classification, longer follow-up studies, and the inclusion of pathology and imaging is required to elucidate the relationship between olfaction and PD.
This study has some limitations. First, the follow-up period of 1–3 years and the single follow-up session may not have been sufficient to detect changes in clinical data over a longer period. However, changes in olfaction in patients with shorter disease duration and non-anosmia progressed within 3 years, while the severity of significant motor symptoms and cognitive function may require a longer follow-up period to observe a difference. Second, although we tried to exclude the possibility that olfactory tests were affected by diseases such as sinonasal disease or upper respiratory tract infection, which commonly affect the sense of smell, there are many other causes of olfactory abnormalities, including idiopathic causes (which may account for 18% of patients with olfactory abnormalities), that may affect test results (Temmel et al., 2002). Thirdly, for safety and the subjects’ preference, we used the On status UPDRS score for the assessment of motor symptoms and disease severity, and therefore, the assessment may be influenced by medication. Nevertheless, these patients are regularly followed up in the outpatient clinic, and the physician ensures that the patient’s medication dosage is adequate. We also analyzed LEDD, which showed that the non-anosmia group had a lower LEDD than the anosmia group, but there was no significant difference between the two. This indirectly implies that the non-anosmia group was not using fewer medications despite having a lower UPDRS score. Therefore, the effect of insufficient dosage of medication on the increasing UPDRS score in the non-anosmia group in this study may be subtle.
In conclusion, this study shows that olfactory sensation may still regress in Parkinson’s patients with a shorter course of the disease without anosmia, while it remains stable in the anosmia group. Such a decline in olfaction may not be related to cognitive status but may be associated with disease progression. Larger, long-term follow-up studies incorporating pathology and imaging analysis are needed to elucidate the underlying mechanisms.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Taichung Veterans General Hospital Institutional Review Board/Ethics Committee (No. CE22189B). The patients/participants provided their written informed consent to participate in this study.
T-CF and M-HC conceptualized the project. T-CF and Y-ST performed the data acquisition and analysis. T-CF wrote the first draft of the manuscript. M-HC critically reviewed the manuscript. All authors contributed to writing and revising the manuscript.
This study was funded by the Taichung Veterans General Hospital TCVGH 1123402C and 1123401C.
We thank the staffs at the Department of Neurology in Taichung Veteran General Hospital for participant recruitment.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Beck, A., Steer, R., and Brown, G. (1996). BDI-II: Beck Depression Inventory Manual, 2nd Edn. San Antonio: Psychological Corporation.
Berendse, H. W., Roos, D. S., Raijmakers, P., and Doty, R. L. (2011). Motor and non-motor correlates of olfactory dysfunction in Parkinson’s disease. J. Neurol. Sci. 310, 21–24. doi: 10.1016/j.jns.2011.06.020
Borghammer, P. (2021). The alpha-Synuclein Origin and Connectome Model (SOC Model) of Parkinson’s Disease: Explaining motor asymmetry, non-motor phenotypes, and cognitive decline. J. Parkinsons Dis. 11, 455–474. doi: 10.3233/JPD-202481
Borghammer, P., Horsager, J., Andersen, K., Van Den Berge, N., Raunio, A., Murayama, S., et al. (2021). Neuropathological evidence of body-first vs. brain-first Lewy body disease. Neurobiol. Dis. 161:105557. doi: 10.1016/j.nbd.2021.105557
Braak, H., Del Tredici, K., Rub, U., de Vos, R. A., Jansen Steur, E. N., and Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211. doi: 10.1016/s0197-4580(02)00065-9
Campabadal, A., Uribe, C., Segura, B., Baggio, H. C., Abos, A., Garcia-Diaz, A. I., et al. (2017). Brain correlates of progressive olfactory loss in Parkinson’s disease. Parkinsonism Relat. Disord. 41, 44–50. doi: 10.1016/j.parkreldis.2017.05.005
Cavaco, S., Goncalves, A., Mendes, A., Vila-Cha, N., Moreira, I., Fernandes, J., et al. (2015). Abnormal Olfaction in Parkinson’s Disease Is Related to Faster Disease Progression. Behav. Neurol. 2015:976589. doi: 10.1155/2015/976589
Domellof, M. E., Lundin, K. F., Edstrom, M., and Forsgren, L. (2017). Olfactory dysfunction and dementia in newly diagnosed patients with Parkinson’s disease. Parkinsonism Relat. Disord. 38, 41–47. doi: 10.1016/j.parkreldis.2017.02.017
Doty, R. L. (2001). The Smell Identification Test Administration Manual. Philadelphia, PA: Sensonics, Inc.
Doty, R. L. (2012). Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol. 8, 329–339. doi: 10.1038/nrneurol.2012.80
Doty, R. L. (2017). Olfactory dysfunction in neurodegenerative diseases: is there a common pathological substrate? Lancet Neurol. 16, 478–488. doi: 10.1016/S1474-4422(17)30123-0
Doty, R. L., Deems, D. A., and Stellar, S. (1988). Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology 38, 1237–1244. doi: 10.1212/wnl.38.8.1237
Ercoli, T., Masala, C., Cadeddu, G., Mascia, M. M., Orofino, G., Gigante, A. F., et al. (2022). Does Olfactory Dysfunction Correlate with Disease Progression in Parkinson’s Disease? A Systematic Review of the Current Literature. Brain Sci. 12, 513. doi: 10.3390/brainsci12050513
Fujio, H., Inokuchi, G., Kuroki, S., Tatehara, S., Katsunuma, S., Kowa, H., et al. (2020). Three-year prospective study on olfaction of patients with Parkinson’s disease. Auris. Nasus Larynx 47, 899–904. doi: 10.1016/j.anl.2019.08.008
Fullard, M. E., Morley, J. F., and Duda, J. E. (2017). Olfactory Dysfunction as an Early Biomarker in Parkinson’s Disease. Neurosci. Bull. 33, 515–525. doi: 10.1007/s12264-017-0170-x
Goetz, C. G., Tilley, B. C., Shaftman, S. R., Stebbins, G. T., Fahn, S., Martinez-Martin, P., et al. (2008). Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170. doi: 10.1002/mds.22340
Haehner, A., Boesveldt, S., Berendse, H. W., Mackay-Sim, A., Fleischmann, J., Silburn, P. A., et al. (2009). Prevalence of smell loss in Parkinson’s disease–a multicenter study. Parkinsonism Relat. Disord. 15, 490–494. doi: 10.1016/j.parkreldis.2008.12.005
He, R., Zhao, Y., He, Y., Zhou, Y., Yang, J., Zhou, X., et al. (2020). Olfactory dysfunction predicts disease progression in Parkinson’s Disease: A longitudinal study. Front. Neurosci. 14:569777. doi: 10.3389/fnins.2020.569777
Herting, B., Schulze, S., Reichmann, H., Haehner, A., and Hummel, T. (2008). A longitudinal study of olfactory function in patients with idiopathic Parkinson’s disease. J. Neurol. 255, 367–370. doi: 10.1007/s00415-008-0665-5
Horsager, J., Andersen, K. B., Knudsen, K., Skjaerbaek, C., Fedorova, T. D., Okkels, N., et al. (2020). Brain-first versus body-first Parkinson’s disease: a multimodal imaging case-control study. Brain 143, 3077–3088. doi: 10.1093/brain/awaa238
Huisman, E., Uylings, H. B., and Hoogland, P. V. (2004). A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson’s disease. Mov. Disord. 19, 687–692. doi: 10.1002/mds.10713
Jiang, R. S., Su, M. C., Liang, K. L., Shiao, J. Y., Wu, S. H., and Hsin, C. H. (2010). A pilot study of a traditional Chinese version of the University of Pennsylvania Smell Identification Test for application in Taiwan. Am. J. Rhinol. Allergy 24, 45–50. doi: 10.2500/ajra.2010.24.3388
Kok, E. H., Savola, S., Raunio, A., Oinas, M., Tuimala, J., Polvikoski, T., et al. (2021). Alpha-synuclein pathology of olfactory bulbs/peduncles in the Vantaa85+ cohort exhibit two divergent patterns: a population-based study. Acta Neuropathol. 142, 777–780. doi: 10.1007/s00401-021-02364-6
Laing, D. G. D. G., and Doty, R. L. (2003). Psychophysical measurement of human olfactory function, including odorant mixture assessment. New York City, NY: Marcel Dekker.
Lawton, M., Hu, M. T., Baig, F., Ruffmann, C., Barron, E., Swallow, D. M., et al. (2016). Equating scores of the University of Pennsylvania smell identification test and sniffin’ sticks test in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 33, 96–101. doi: 10.1016/j.parkreldis.2016.09.023
Lewis, M. M., Harkins, E., Lee, E. Y., Stetter, C., Snyder, B., Corson, T., et al. (2020). Clinical Progression of Parkinson’s Disease: Insights from the NINDS Common Data Elements. J. Parkinsons Dis. 10, 1075–1085. doi: 10.3233/JPD-201932
Litvan, I., Goldman, J. G., Troster, A. I., Schmand, B. A., Weintraub, D., Petersen, R. C., et al. (2012). Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 27, 349–356. doi: 10.1002/mds.24893
Liu, C. C., Li, C. Y., Lee, P. C., and Sun, Y. (2016a). Variations in incidence and prevalence of parkinson’s disease in Taiwan: A population-based nationwide study. Parkinsons. Dis. 2016:8756359. doi: 10.1155/2016/8756359
Liu, W. M., Wu, R. M., Lin, J. W., Liu, Y. C., Chang, C. H., and Lin, C. H. (2016b). Time trends in the prevalence and incidence of Parkinson’s disease in Taiwan: A nationwide, population-based study. J. Formos Med. Assoc. 115, 531–538. doi: 10.1016/j.jfma.2015.05.014
Masala, C., Solla, P., Liscia, A., Defazio, G., Saba, L., Cannas, A., et al. (2018). Correlation among olfactory function, motors’ symptoms, cognitive impairment, apathy, and fatigue in patients with Parkinson’s disease. J. Neurol. 265, 1764–1771. doi: 10.1007/s00415-018-8913-9
Meusel, T., Westermann, B., Fuhr, P., Hummel, T., and Welge-Lussen, A. (2010). The course of olfactory deficits in patients with Parkinson’s disease–a study based on psychophysical and electrophysiological measures. Neurosci. Lett. 486, 166–170. doi: 10.1016/j.neulet.2010.09.044
Muller, A., Reichmann, H., Livermore, A., and Hummel, T. (2002). Olfactory function in idiopathic Parkinson’s disease (IPD): results from cross-sectional studies in IPD patients and long-term follow-up of de-novo IPD patients. J. Neural Transm. 109, 805–811. doi: 10.1007/s007020200067
Picillo, M., Pellecchia, M. T., Erro, R., Amboni, M., Vitale, C., Iavarone, A., et al. (2014). The use of University of Pennsylvania smell identification test in the diagnosis of Parkinson’s disease in Italy. Neurol. Sci. 35, 379–383. doi: 10.1007/s10072-013-1522-6
Poewe, W., Seppi, K., Tanner, C. M., Halliday, G. M., Brundin, P., Volkmann, J., et al. (2017). Parkinson disease. Nat. Rev. Dis. Primers 3:17013. doi: 10.1038/nrdp.2017.13
Rodríguez-Violante, M., Camacho-Ordoñez, A., Cervantes-Arriaga, A., González-Latapí, P., and Velázquez-Osuna, S. (2015). Factors associated with the quality of life of subjects with Parkinson’s disease and burden on their caregivers. Neurología 30, 257–263. doi: 10.1016/j.nrleng.2014.01.002
Rodríguez-Violante, M., Ospina-García, N., Pérez-Lohman, C., and Cervantes-Arriaga, A. (2017). Spotlight on olfactory dysfunction in Parkinson’s disease. J. Parkinson. Restless Legs Syndr. 7, 33–41. doi: 10.2147/jprls.S125390
Roos, D. S., Twisk, J. W. R., Raijmakers, P., Doty, R. L., and Berendse, H. W. (2019). Hyposmia as a marker of (non-)motor disease severity in Parkinson’s disease. J. Neural Transm. 126, 1471–1478. doi: 10.1007/s00702-019-02074-0
Santos Garcia, D., de Deus Fonticoba, T., Suarez Castro, E., Borrue, C., Mata, M., Solano Vila, B., et al. (2019). Non-motor symptoms burden, mood, and gait problems are the most significant factors contributing to a poor quality of life in non-demented Parkinson’s disease patients: Results from the COPPADIS Study Cohort. Parkinsonism Relat. Disord. 66, 151–157. doi: 10.1016/j.parkreldis.2019.07.031
Stebbins, G. T., Goetz, C. G., Burn, D. J., Jankovic, J., Khoo, T. K., and Tilley, B. C. (2013). How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov. Disord. 28, 668–670. doi: 10.1002/mds.25383
Taguchi, T., Ikuno, M., Hondo, M., Parajuli, L. K., Taguchi, K., Ueda, J., et al. (2020). alpha-Synuclein BAC transgenic mice exhibit RBD-like behaviour and hyposmia: a prodromal Parkinson’s disease model. Brain 143, 249–265. doi: 10.1093/brain/awz380
Temmel, A. F., Quint, C., Schickinger-Fischer, B., Klimek, L., Stoller, E., and Hummel, T. (2002). Characteristics of olfactory disorders in relation to major causes of olfactory loss. Arch. Otolaryngol. Head Neck Surg. 128, 635–641. doi: 10.1001/archotol.128.6.635
Tibar, H., El Bayad, K., Bouhouche, A., Ait Ben Haddou, E. H., Benomar, A., Yahyaoui, M., et al. (2018). Non-motor symptoms of Parkinson’s disease and their impact on quality of life in a cohort of moroccan patients. Front. Neurol. 9:170. doi: 10.3389/fneur.2018.00170
Keywords: Parkinson’s disease, olfactory dysfunction, UPSIT, MDS-UPDRS, equivalence dose of daily levodopa, cognition, depression
Citation: Fang T-C, Tsai Y-S and Chang M-H (2023) Sequential change in olfaction and (non) motor symptoms: the difference between anosmia and non-anosmia in Parkinson’s disease. Front. Aging Neurosci. 15:1213977. doi: 10.3389/fnagi.2023.1213977
Received: 28 April 2023; Accepted: 04 July 2023;
Published: 18 July 2023.
Edited by:
Muthuraman Muthuraman, University Hospital Würzburg, GermanyReviewed by:
Wooyoung Jang, Gangneung Asan Hospital, Republic of KoreaCopyright © 2023 Fang, Tsai and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-Hong Chang, Y21oNTAwODA5QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.