
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 16 June 2023
Sec. Neuroinflammation and Neuropathy
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1192681
 Xiaohui Li1†
Xiaohui Li1† Xiaodan Qin2†
Xiaodan Qin2† Chengfang Liu1†
Chengfang Liu1† Lin Zhu1
Lin Zhu1 Meng Wang1
Meng Wang1 Teng Jiang1
Teng Jiang1 Yukai Liu1
Yukai Liu1 Shuo Li1
Shuo Li1 Hongchao Shi1
Hongchao Shi1 Huiling Sun2*
Huiling Sun2* Qiwen Deng1,3*
Qiwen Deng1,3* Junshan Zhou1*
Junshan Zhou1*Background: Symptomatic intracranial atherosclerotic stenosis (sICAS) is one of the common causes of ischemic stroke. However, the treatment of sICAS remains a challenge in the past with unfavorable findings. The purpose of this study was to explore the effect of stenting versus aggressive medical management on preventing recurrent stroke in patients with sICAS.
Methods: We prospectively collected the clinical information of patients with sICAS who underwent percutaneous angioplasty and/or stenting (PTAS) or aggressive medical therapy from March 2020 to February 2022. Propensity score matching (PSM) was employed to ensure well-balanced characteristics of two groups. The primary outcome endpoint was defined as recurrent stroke or transient ischemic attack (TIA) within 1 year.
Results: We enrolled 207 patients (51 in the PTAS and 156 in the aggressive medical groups) with sICAS. No significant difference was found between PTAS group and aggressive medical group for the risk of stroke or TIA in the same territory beyond 30 days through 6 months (P = 0.570) and beyond 30 days through 1 year (P = 0.739) except for within 30 days (P = 0.003). Furthermore, none showed a significant difference for disabling stroke, death and intracranial hemorrhage within 1 year. These results remain stable after adjustment. After PSM, all the outcomes have no significant difference between these two groups.
Conclusion: The PTAS has similar treatment outcomes compared with aggressive medical therapy in patients with sICAS across 1-year follow-up.
Stroke is the second leading cause of mortality globally (Feigin, 2007). Intracranial atherosclerotic stenosis (ICAS) is one of the main causes of ischemic stroke and is closely associated with a high incidence and mortality of stroke (Gutierrez et al., 2022). Patients with symptomatic intracranial atherosclerotic stenosis (sICAS) are particularly at high risk for recurrent stroke (Wang et al., 2014). Therefore, effective treatment and secondary prevention are of great significance in reducing the high incidence of cerebrovascular events (Qureshi and Caplan, 2014).
For patients with sICAS who underwent ischemic stroke or transient ischemic attack (TIA), aggressive medical treatment should be given first. Percutaneous angioplasty and/or stenting (PTAS) can be used in sICAS patients with ineffective medical therapy (Powers et al., 2019; Turan et al., 2022). A series of studies (Derdeyn et al., 2014; Zaidat et al., 2015; Gao et al., 2022) have shown no significant benefit from PTAS compared with medical therapy alone. Another study have shown that the 30-day stroke or mortality of stenting is significantly higher than that of medical therapy, suggesting that aggressive medical therapy is superior to PTAS in patients with sICAS (Derdeyn et al., 2014). However, a number of subsequent prospective and retrospective studies have reported the benefits of endovascular therapy (Yu et al., 2012; Miao et al., 2015a,b; Gao et al., 2016; Maier et al., 2018), and individualized endovascular therapy may be effective and safe in patients with sICAS (Ma et al., 2018; Mao et al., 2022). Therefore, the optimal treatment of patients with sICAS remains elusive.
In this study, we aimed to prospectively analyze the effects of PTAS and aggressive medical therapy on sICAS patients within 1 year, and further explore their influence on ischemic events through propensity score matching (PSM) analysis.
This observational prospective cohort study was conducted at the Stroke Center of Nanjing First Hospital, Nanjing Medical University, and aimed to investigate the efficacy of PTAS vs. aggressive medical therapy in patients with sICAS. Consecutive patients were collected from an observational study of the AISRNA study (1NCT04175691) between March 2020 and February 2022. Our inclusion criteria were: (1) patients with ≥70% stenosis of the main trunk of the middle cerebral artery (MCA), internal carotid artery (ICA), vertebral artery (VA), or basilar artery (BA); (2) TIA or stroke in the territory of the target lesion area; and (3) modified Rankin Scale (mRS) ≤ 2. Patients with (1) a potential source of cardiac embolism, (2) mRS > 3, (3) extracranial vascular stenosis > 50%, (4) other known causes of stroke subtypes, and (5) emergency stenting were excluded. The study enrolled 207 eligible patients. The baseline characteristics, including age, sex, body mass index (BMI), medical history, low-density lipoprotein-cholesterol (LDL-C), systolic blood pressure (SBS), symptomatic qualifying artery, the degree of stenosis and National Institute of Health Stroke Scale (NIHSS) score were collected.
The patients were divided into the PTAS group and aggressive medical group both receiving the guidelines for conventional treatment, which included aspirin 100 mg plus clopidogrel 75 mg daily for 90 days (aspirin or clopidogrel alone daily thereafter) and control of stroke risk factors. The primary outcome was the recurrence of stroke or TIA in the same territory within 1 year. Adverse events included disabling stroke, symptomatic intracranial hemorrhage, or death within 1 year. All enrolled individuals were followed up through telephone or outpatient visit. They were admitted to hospital for corresponding imaging examination, included an assessment of the mRS, and establishing whether the patient had any adverse events if necessary. Disabling stroke was defined as an mRS score of 3 or more on a scale of 0 to 6. The primary outcome endpoint was as follows: (1) stroke or TIA in the same territory within 30 days; (2) stroke or TIA in the same territory beyond 30 days through 6 months; (3) stroke or TIA in the same territory beyond 30 days through 1 year.
Continuous variables were presented as mean ± standard deviation, and continuous variables with non-normal distribution were summarized as medians (interquartile range). Quantitative data were compared using Student’s t test or the Mann–Whitney U test. Categorical data were presented as numbers with percentages and were analyzed using the chi-squared test or Fisher exact tests. The event rates (stroke or TIA in the same territory within 30 days, beyond 30 days through 6 months, and beyond 30 days through 1 year) were compared between the two groups. Univariate logistic analysis was used to analyze the influence of the two groups on each outcome node. Multivariate logistic regression analysis was used to remove the risk factors associated with outcomes and calculate 95% confidence intervals (CI). PSM was conducted to balance the baseline characteristics between the PTAS and aggressive medical therapy group. Pairs were matched without replacement on the logit of the propensity score, and a nearest-neighbor 1:1 matching scheme with a caliper size of 0.2 was applied for all matched pairs. Bivariate and multivariable logistic analyses were used to compare outcomes. A p-value < 0.05 was considered statistically significant. The statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version27.0 (SPSS Inc., Chicago, IL, USA).
From March 2020 to February 2022, a total of 310 patients with sICAS were included in this study. Among them, 35 received emergency PTAS, 10 had atrial fibrillation, 42 were mRS > 2 and 20 lacked information. A total of 107 ineligible patients were excluded. Finally, 207 patients were enrolled in the study (51 in the PTAS group and 156 in the aggressive medical group) (Figure 1). The baseline characteristics of the patients were nearly balanced between the two groups. The average age of the cohort was 63.8 ± 11.8 years, and 70.5% of patients were male. The stenotic arteries involved the MCA in 133 patients (64.3%), ICA in 38 (18.4%), VA in 21 (10.1%), and BA in 15 (7.2%). As the study population consisted of patients with minor stroke and TIA, the median NIHSS score was 2 (IQR 0–3) (Table 1).
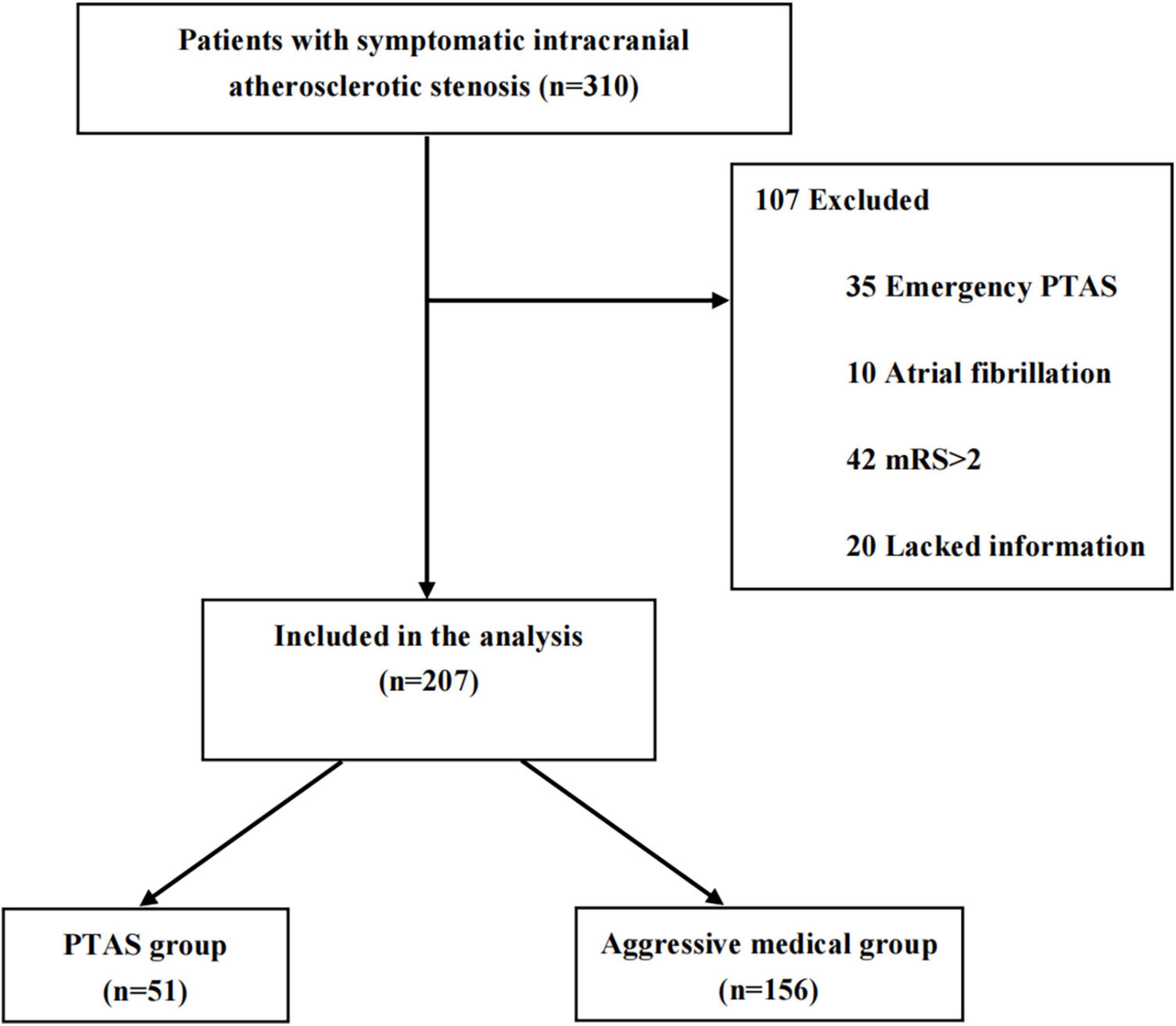
Figure 1. Study flow diagram. PTAS, percutaneous angioplasty and/or stenting; mRS, modified Rankin Scale.
The occurrence of primary endpoints in the PTAS group vs. the aggressive medical group was 8/51 (15.7%) vs. 6/156 (3.8%) within 30 days (P = 0.003), 1/51 (2.0%) vs. 8/156 (5.1%) beyond 30 days through 6 months (P = 0.570), 2/50 (4.0%) vs. 11/155 (7.1%) beyond 30 days through 1 year (P = 0.739) (Table 2). The risk of stroke or TIA in the same territory within 30 days was 8/51 (15.7%) vs. 6/156 (3.8%) (P = 0.003). No significant difference was found between the groups for the risk of stroke or TIA in the same territory beyond 30 days through 6 months and beyond 30 days through 1 year. Intracranial hemorrhage occurred in 1 patient (0.6%) in the aggressive medical group and none in the PTAS group (P = 1.000). There was no significant difference in the rate of 12-month risk of death (P = 0.990). For the incidence of disabling stroke (mRS > 2) within 1 year, 9 patients (18.0%) were in the PTAS group and 16 patients (10.3%) were in the aggressive medical group (P = 0.149). In multivariate logistic regression, adjusting for age, sex, SBS, LDL-C, and the history of stroke or TIA, there was still no significant difference between the PTAS and aggressive medical groups for clinical outcomes except for the recurrence of stroke or TIA in the same territory within 30 days (P = 0.010). Subsequently, PSM analysis was performed to balance the baseline characteristics between the PTAS and aggressive medical groups. The results showed that PSM yielded 88 patients with sICAS who underwent PTAS or aggressive medical therapy. After PSM, there was no significant difference in all outcomes (P > 0.05, Table 3). For the PTAS group, all patients underwent balloon dilated stenting (BMS) except for one of the 51 patients who received self-expanding stenting (SES) (Supplementary Table 1). Due to the small number of cases, subgroup analysis could not be conducted.
This observational prospective study showed no significant difference in stroke or TIA risk over 30 days to 1 year in the same territory after PTAS compared to aggressive medical therapy. There was also no significant difference for secondary outcomes.
However, a significant difference was observed in the occurrence of ischemic events within 30 days (P = 0.003). This finding is consistent with the SAMMPRIS (Derdeyn et al., 2014) and VISSIT (Zaidat et al., 2015) trials, which demonstrated that stenting carries a higher risk than medication for sICAS patients. At day 30, the incidence of the primary endpoint (any stroke or death) was 14.7% vs. 5.8% (P = 0.0016) and 24.1% vs. 9.4% (P = 0.050) in the stenting and medical groups, respectively. This difference may be attributed to the perioperative risk of surgery (Turan et al., 2022), the timing of stenting (Derdeyn et al., 2014; Zaidat et al., 2015; Gao et al., 2022) and difference in stent selection, such as BMS, SES, and simple balloon dilation angioplasty (BDA) (Mao et al., 2022; Ueda et al., 2022) as well as the plaque detachment or reperfusion injury (Yaghi et al., 2019). The CASSISS (Gao et al., 2022) trial demonstrated that minimizing perioperative complications did not result in a significant difference in the risk of stroke within 30 days or beyond 30 days to 1 year (8.0% vs. 7.2%, P = 0.820). Meanwhile, after PSM analysis, no significant difference was also observed for any stroke risk between these two groups.
Mounting evidence indicates that most recurrent cerebral ischemic events occur shortly after the initial ischemic event, and early stent implantation may increase surgical risks (Zhang et al., 2020). Multiple studies (Kasner et al., 2006; Yaghi et al., 2019; Zhang et al., 2020) have shown that endovascular therapy at least 3 weeks after the initial event appeared to be safer than that at less than 3 weeks. When endovascular treatment was performed over 3 weeks, the incidence of short-term death or stroke was 4.3% and 2%, respectively (Miao et al., 2015a; Wang et al., 2020). The CASSISS trial (median time, 35 days) (Gao et al., 2022), WEAVE trial (median time, 22 days) (Alexander et al., 2019) and a retrospective study trial (median time, 21 days) (Mao et al., 2022) had significantly longer interval from onset to endovascular treatment than the SAMMPRIS trial (median time, 7 days) (Derdeyn et al., 2014) and VISSIT trial (median time, 9 days) (Zaidat et al., 2015), thus avoiding the highest risk period of stroke recurrence. This approach also reduced the incidence of perioperative complications after stenting.
Despite the use of aggressive medical therapy, some patients remain at a high risk for stroke, particularly those with impaired hemodynamics (Amin-Hanjani et al., 2016; Wabnitz et al., 2018). sICAS is believed to be associated with plaque rupture, intraplaque hemorrhage, and thrombosis through various mechanisms such as impaired distal perfusion, arterial-arterial embolism, or perforator disease (Li et al., 2023). Aggressive medical therapy is more effective for patients whose only mechanism is arterial-arterial embolism or perforator disease than for patients whose mechanism is distal perfusion impairment (Bodle et al., 2013; Yaghi et al., 2019). In patients with distal flow impairment, medical therapy may help stabilize atherosclerotic plaques and intracranial thrombi, and reduce the risk of worsening embolism and stenosis, but it is unlikely to rapidly improve flow tissue that is at risk of early recurrence and may benefit from revascularization. Recurrent stroke is linked to no improvement in cerebral perfusion during the perioperative period (Yan et al., 2022). The importance of intracranial hemodynamic impairment as a risk factor for stroke has been demonstrated in both the carotid (Wabnitz et al., 2018) and posterior (Maier et al., 2018) circulation. Although many studies have suggested that stenting is less beneficial than medical therapy alone, these studies have not stratified patients by perfusion status. As such, exploring the safety and feasibility of stenting in high-risk patients with impaired blood flow in sICAS is necessary (Stapleton et al., 2020). The primary endpoint of the sICAS primary treatment trial was focused on clinical stroke or TIA, while subclinical infarction was often omitted from the primary analysis (Moftakhar et al., 2005; Levine et al., 2015).
Currently, there is no consensus on the optimal endovascular treatment strategy for sICAS. Nonetheless, previous studies and our study have shown no significant difference between PTAS and aggressive medical therapy. Randomized controlled trials should remain the gold standard to guide treatment, but biomarkers that predict the risk of recurrent stroke may be used to aid in clinical decision-making and lead to more targeted and personalized treatment (Lee et al., 2011; Ballout and Liebeskind, 2022). Nowadays, with the development of endovascular therapy, there are many interventions, such as drug-coated balloons, drug-eluting stents and other self-expanding stents, or combinations (Gruber et al., 2018, 2019; Han et al., 2019; Kadooka et al., 2020; Remonda et al., 2021; Jia et al., 2022; Si et al., 2022). In order to select the best treatment, the location of the narrowed artery, lesion shape, and lesion pathway must be considered. A comprehensive risk assessment of the treatment procedure should be performed, taking into account other risk factors such as the patient’s lifestyle, clinical factors, and the time interval between the final ischemic attacks (Ueda et al., 2022). Future studies should aim to establish clinical, serological, and imaging biomarkers that can identify high-risk patients (Banerjee and Chimowitz, 2017).
It was a single-center observational study that might have been influenced by selection bias. However, the results of our study provided valuable insights and need to be further confirmed by multicenter large-sample randomized controlled studies that reduce the effect of individual selection on the equivalence of results and enhance the generalizability of the findings. Apart from the crude comparison of the baseline disease events in the anterior vs. posterior circulation, our analysis did not evaluate submaximal angioplasty by specific lesion location and the degree of difference in stenosis before and after intervention. Additionally, the effectiveness of antiplatelet drugs was not supported by the test results in our study, and future studies need to compensate for the influence of drug selection such as aspirin and clopidogrel. Our study was limited to the inclusion of BMS treatment and did not evaluate other types of interventions mentioned above. We used routine medical history, NIHSS, and mRS scores at follow-up without systematic brain imaging to identify high-risk potential recurrent strokes, such as hypoperfusion, and a vascular remodeling index. Future studies should aim to overcome these limitations and provide more comprehensive and detailed analyses to improve patient care and outcomes.
For patients with TIA or ischemic stroke caused by symptomatic severe intracranial atherosclerotic stenosis, PTAS had a similar effect on preventing stroke or TIA risk as aggressive medical therapy within 1 year compared to the latter. Future RCT trials should be performed to confirm these findings.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee of Nanjing Medical University [No. (2019)695]. The patients/participants provided their written informed consent to participate in this study.
XL, QD, and JZ contributed to conceptualization. XL, XQ, CL, and HSun contributed to formal analysis and the first draft of the manuscript. All authors made substantial contribution to the design, implementation of the study, reviewed, and approved the final manuscript.
This study was supported by the Medical Scientific Research Project of Jiangsu Commission of Health (ZDA2020019); the Health China BuChang ZhiYuan Public welfare projects for Heart and brain health (HIGHER202102); the National Science and Technology Innovation 2030–Major Program of Brain Science and Brain-Inspired Intelligence Research (2021ZD0201807); and the Postgraduate Research and Practice Innovation Program of Jiangsu Province (JX12013964 and JX12013960).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1192681/full#supplementary-material
Alexander, M., Zauner, A., Chaloupka, J., Baxter, B., Callison, R., Gupta, R., et al. (2019). WEAVE trial: Final results in 152 on-label patients. Stroke 50, 889–894.
Amin-Hanjani, S., Pandey, D., Rose-Finnell, L., Du, X., Richardson, D., Thulborn, K., et al. (2016). Effect of hemodynamics on stroke risk in symptomatic atherosclerotic vertebrobasilar occlusive disease. JAMA Neurol. 73, 178–185. doi: 10.1001/jamaneurol.2015.3772
Ballout, A., and Liebeskind, D. (2022). Recurrent stroke risk in intracranial atherosclerotic disease. Front. Neurol. 13:1001609. doi: 10.3389/fneur.2022.1001609
Banerjee, C., and Chimowitz, M. (2017). Stroke caused by atherosclerosis of the major intracranial arteries. Circ. Res. 120, 502–513. doi: 10.1161/CIRCRESAHA.116.308441
Bodle, J., Feldmann, E., Swartz, R., Rumboldt, Z., Brown, T., and Turan, T. (2013). High-resolution magnetic resonance imaging: An emerging tool for evaluating intracranial arterial disease. Stroke 44, 287–292. doi: 10.1161/STROKEAHA.112.664680
Derdeyn, C., Chimowitz, M., Lynn, M., Fiorella, D., Turan, T., Janis, L., et al. (2014). Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): The final results of a randomised trial. Lancet 383, 333–341. doi: 10.1016/S0140-6736(13)62038-3
Feigin, V. (2007). Stroke in developing countries: Can the epidemic be stopped and outcomes improved? Lancet Neurol. 6, 94–97. doi: 10.1016/S1474-4422(07)70007-8
Gao, P., Wang, D., Zhao, Z., Cai, Y., Li, T., Shi, H., et al. (2016). Multicenter prospective trial of stent placement in patients with symptomatic high-grade intracranial stenosis. AJNR Am. J. Neuroradiol. 37, 1275–1280.
Gao, P., Wang, T., Wang, D., Liebeskind, D., Shi, H., Li, T., et al. (2022). Effect of stenting plus medical therapy vs. medical therapy alone on risk of stroke and death in patients with symptomatic intracranial stenosis: The CASSISS Randomized Clinical Trial. JAMA 328, 534–542. doi: 10.1001/jama.2022.12000
Gruber, P., Braun, C., Kahles, T., Hlavica, M., Anon, J., Diepers, M., et al. (2019). Percutaneous transluminal angioplasty using the novel drug-coated balloon catheter SeQuent Please NEO for the treatment of symptomatic intracranial severe stenosis: Feasibility and safety study. J. Neurointerv. Surg. 11, 719–722. doi: 10.1136/neurintsurg-2018-014378
Gruber, P., Garcia-Esperon, C., Berberat, J., Kahles, T., Hlavica, M., Anon, J., et al. (2018). Neuro Elutax SV drug-eluting balloon versus Wingspan stent system in symptomatic intracranial high-grade stenosis: A single-center experience. J. Neurointerv. Surg. 10, e32. doi: 10.1136/neurintsurg-2017-013699
Gutierrez, J., Turan, T., Hoh, B., and Chimowitz, M. (2022). Intracranial atherosclerotic stenosis: Risk factors, diagnosis, and treatment. Lancet Neurol. 21, 355–368.
Han, J., Zhang, J., Zhang, X., Zhang, J., Song, Y., Zhao, W., et al. (2019). Drug-coated balloons for the treatment of symptomatic intracranial atherosclerosis: Initial experience and follow-up outcome. J. Neurointerv. Surg. 11, 569–573. doi: 10.1136/neurintsurg-2018-014237
Jia, B., Zhang, X., Ma, N., Mo, D., Gao, F., Sun, X., et al. (2022). Comparison of drug-eluting stent with bare-metal stent in patients with symptomatic high-grade intracranial atherosclerotic stenosis: A randomized clinical trial. JAMA Neurol. 79, 176–184. doi: 10.1001/jamaneurol.2021.4804
Kadooka, K., Hagenbuch, N., Anagnostakou, V., Valavanis, A., and Kulcsár, Z. (2020). Safety and efficacy of balloon angioplasty in symptomatic intracranial stenosis: A systematic review and meta-analysis. J. Neuroradiol. 47, 27–32. doi: 10.1016/j.neurad.2019.02.007
Kasner, S., Chimowitz, M., Lynn, M., Howlett-Smith, H., Stern, B., Hertzberg, V., et al. (2006). Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 113, 555–563. doi: 10.1161/CIRCULATIONAHA.105.578229
Lee, J., Im, D., An, Y., Hong, J., Gwag, B., and Joo, I. (2011). Chronic cerebral hypoperfusion in a mouse model of Alzheimer’s disease: An additional contributing factor of cognitive impairment. Neurosci. Lett. 489, 84–88. doi: 10.1016/j.neulet.2010.11.071
Levine, D., Galecki, A., Langa, K., Unverzagt, F., Kabeto, M., Giordani, B., et al. (2015). Trajectory of cognitive decline after incident stroke. JAMA 314, 41–51.
Li, X., Liu, C., Zhu, L., Wang, M., Liu, Y., Li, S., et al. (2023). The role of high-resolution magnetic resonance imaging in cerebrovascular disease: A narrative review. Brain Sci. 13:677. doi: 10.3390/brainsci13040677
Ma, N., Zhang, Y., Shuai, J., Jiang, C., Zhu, Q., Chen, K., et al. (2018). Stenting for symptomatic intracranial arterial stenosis in China: 1-year outcome of a multicentre registry study. Stroke Vasc. Neurol. 3, 176–184.
Maier, I., Karch, A., Lipke, C., Behme, D., Mpotsaris, A., Kabbasch, C., et al. (2018). Transluminal angioplasty and stenting versus conservative treatment in patients with symptomatic basilar artery stenosis: Perspective for future clinical trials. Clin. Neuroradiol. 28, 33–38. doi: 10.1007/s00062-016-0528-x
Mao, L., Ma, A., Liu, Z., Zhang, J., Xu, Y., Chen, W., et al. (2022). A retrospective study of individualized endovascular treatment for symptomatic intracranial atherosclerotic stenosis in patients with ischemic stroke/transient ischemic attack. Front. Neurol. 13:1057935. doi: 10.3389/fneur.2022.1057935
Miao, Z., Song, L., Liebeskind, D., Liu, L., Ma, N., Wang, Y., et al. (2015a). Outcomes of tailored angioplasty and/or stenting for symptomatic intracranial atherosclerosis: A prospective cohort study after SAMMPRIS. J. Neurointerv. Surg. 7, 331–335.
Miao, Z., Zhang, Y., Shuai, J., Jiang, C., Zhu, Q., Chen, K., et al. (2015b). Thirty-day outcome of a multicenter registry study of stenting for symptomatic intracranial artery stenosis in China. Stroke 46, 2822–2829. doi: 10.1161/STROKEAHA.115.010549
Moftakhar, R., Turk, A., Niemann, D., Hussain, S., Rajpal, S., Cook, T., et al. (2005). Effects of carotid or vertebrobasilar stent placement on cerebral perfusion and cognition. AJNR Am. J. Neuroradiol. 26, 1772–1780.
Powers, W., Rabinstein, A., Ackerson, T., Adeoye, O., Bambakidis, N., Becker, K., et al. (2019). Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the american heart association/American Stroke Association. Stroke 50, e344–e418. doi: 10.1161/STR.0000000000000215
Qureshi, A., and Caplan, L. (2014). Intracranial atherosclerosis. Lancet 383, 984–998. doi: 10.1016/S0140-6736(13)61088-0
Remonda, L., Diepers, M., Berberat, J., Kahles, T., Anon, J., Nedeltchev, K., et al. (2021). Drug-coated balloon treatment in symptomatic intracranial high grade stenosis: A retrospective study of 33 patients. Clin. Neuroradiol. 31, 45–49. doi: 10.1007/s00062-020-00936-9
Si, J., Ma, N., Gao, F., Mo, D., Luo, G., and Miao, Z. (2022). Effect of a drug-eluting stent vs. bare metal stent for the treatment of symptomatic intracranial and vertebral artery stenosis. Front. Neurol. 13:854226. doi: 10.3389/fneur.2022.854226
Stapleton, C., Chen, Y., Shallwani, H., Vakharia, K., Turan, T., Woo, H., et al. (2020). Submaximal angioplasty for symptomatic intracranial atherosclerotic disease: A meta-analysis of peri-procedural and long-term risk. Neurosurgery 86, 755–762. doi: 10.1093/neuros/nyz337
Turan, T., Zaidat, O., Gronseth, G., Chimowitz, M., Culebras, A., Furlan, A., et al. (2022). Stroke prevention in symptomatic large artery intracranial atherosclerosis practice advisory: Report of the AAN guideline subcommittee. Neurology 98, 486–498. doi: 10.1212/WNL.0000000000200030
Ueda, T., Takaishi, S., Yoshie, T., Usuki, N., Tatsuno, K., Ohtsubo, H., et al. (2022). Long-term outcome and factors associated with restenosis after combination therapy of balloon angioplasty and stenting for symptomatic intracranial stenosis. BMC Neurol. 22:477. doi: 10.1186/s12883-022-03009-1
Wabnitz, A., Derdeyn, C., Fiorella, D., Lynn, M., Cotsonis, G., Liebeskind, D., et al. (2018). Hemodynamic markers in the anterior circulation as predictors of recurrent stroke in patients with intracranial stenosis. Stroke. 50, 143–147. doi: 10.1161/STROKEAHA.118.020840
Wang, T., Luo, J., Wang, X., Yang, K., Jadhav, V., Gao, P., et al. (2020). Endovascular therapy versus medical treatment for symptomatic intracranial artery stenosis. Cochrane Database Syst. Rev. 8:Cd013267. doi: 10.1002/14651858.CD013267.pub2
Wang, Y., Zhao, X., Liu, L., Soo, Y., Pu, Y., Pan, Y., et al. (2014). Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: The Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke 45, 663–669. doi: 10.1161/STROKEAHA.113.003508
Yaghi, S., Prabhakaran, S., Khatri, P., and Liebeskind, D. (2019). Intracranial atherosclerotic disease. Stroke 50, 1286–1293. doi: 10.1161/STROKEAHA.118.024147
Yan, L., Hou, Z., Fu, W., Yu, Y., Cui, R., Miao, Z., et al. (2022). Association of periprocedural perfusion non-improvement with recurrent stroke after endovascular treatment for Intracranial Atherosclerotic Stenosis. Ther. Adv. Neurol. Disord. 15:17562864221143178. doi: 10.1177/17562864221143178
Yu, S., Leung, T., Hung, E., Lee, K., and Wong, L. (2012). Angioplasty and stenting for intracranial atherosclerotic stenosis with nitinol stent: Factors affecting technical success and patient safety. Neurosurgery 70(1 Suppl Operative), 104–113. doi: 10.1227/NEU.0b013e3182320bb0
Zaidat, O., Fitzsimmons, B., Woodward, B., Wang, Z., Killer-Oberpfalzer, M., Wakhloo, A., et al. (2015). Effect of a balloon-expandable intracranial stent vs. medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: The VISSIT randomized clinical trial. JAMA 313, 1240–1248. doi: 10.1001/jama.2015.1693
Keywords: ischemic stroke, symptomatic intracranial atherosclerotic stenosis, percutaneous transluminal angioplasty and stenting, propensity score matching, recurrent stroke
Citation: Li X, Qin X, Liu C, Zhu L, Wang M, Jiang T, Liu Y, Li S, Shi H, Sun H, Deng Q and Zhou J (2023) Percutaneous angioplasty and/or stenting versus aggressive medical therapy in patients with symptomatic intracranial atherosclerotic stenosis: a 1-year follow-up study. Front. Aging Neurosci. 15:1192681. doi: 10.3389/fnagi.2023.1192681
Received: 23 March 2023; Accepted: 30 May 2023;
Published: 16 June 2023.
Edited by:
Rubem C. A. Guedes, Federal University of Pernambuco, BrazilReviewed by:
Hormozd Bozorgchami, Baylor College of Medicine, United StatesCopyright © 2023 Li, Qin, Liu, Zhu, Wang, Jiang, Liu, Li, Shi, Sun, Deng and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junshan Zhou, emhqc2gzMzNAMTI2LmNvbQ==; Qiwen Deng, cWl3X2RlbmdAbmptdS5lZHUuY24=; Huiling Sun, c3VuaHVpbGluZzE5ODhAeWVhaC5uZXQ=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.