- 1Department of Neurology, Affiliated Lianyungang Hospital of Xuzhou Medical University, Lianyungang, China
- 2School of Biomedical Engineering, Health Science Center, Shenzhen University, Shenzhen, China
- 3Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 4Department of Neurology, Suzhou Hospital, Affiliated Hospital of Medical School, Nanjing University, Suzhou, China
- 5Department of Neurology, Gusu School, Suzhou Science and Technology Town Hospital, Nanjing Medical University, Suzhou, China
- 6Department of Neurology, Guanyun People’s Hospital, Guanyun, China
- 7Department of Neurology, Suzhou TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Suzhou, China
- 8Pengcheng Laboratory, Shenzhen, China
- 9School of Biomedical Engineering, ShanghaiTech University, Shanghai, China
Introduction: Apathy is a prevalent mood disturbance that occurs in a wide range of populations, including those with normal cognitive aging, mental disorders, neurodegenerative disorders and traumatic brain injuries. Recently, neuroimaging technologies have been employed to elucidate the neural substrates underlying brain disorders accompanying apathy. However, the consistent neural correlates of apathy across normal aging and brain disorders are still unclear.
Methods: This paper first provides a brief review of the neural mechanism of apathy in healthy elderly individuals, those with mental disorders, neurodegenerative disorders, and traumatic brain injuries. Further, following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines, the structural and functional neuroimaging meta-analysis using activation likelihood estimation method is performed on the apathy group with brain disorders and the healthy elderly, aiming at exploring the neural correlates of apathy.
Results: The structural neuroimaging meta-analysis showed that gray matter atrophy is associated with apathy in the bilateral precentral gyrus (BA 13/6), bilateral insula (BA 47), bilateral medial frontal gyrus (BA 11), bilateral inferior frontal gyrus, left caudate (putamen) and right anterior cingulate, while the functional neuroimaging meta-analysis suggested that the functional connectivity in putamen and lateral globus pallidus is correlated with apathy.
Discussion: Through the neuroimaging meta-analysis, this study has identified the potential neural locations of apathy in terms of brain structure and function, which may offer valuable pathophysiological insights for developing more effective therapeutic interventions for affected patients.
Highlights
- Structural gray matter atrophy in the precentral gyrus, bilateral insula, medial/inferior frontal gyrus, left caudate, and right anterior cingulate is associated with apathy;
- Functional connectivity in the putamen and lateral globus pallidus is correlated with apathy;
- The identified neural locations may serve as therapeutic targets for individuals with apathy.
1. Introduction
Apathy is commonly defined as a state of reduced feeling, emotion, and interest (Mann, 1990; Marin, 1991), where its association with the aging process (Fazio et al., 2016; Jang et al., 2021), various mental disorders (MD) (Alexopoulos et al., 2013; Yuen et al., 2014), neurodegenerative disorders (NDD) (Reijnders et al., 2010; Wei et al., 2020; Miller et al., 2021; Azocar et al., 2022), and traumatic brain injury (TBI) (Knutson et al., 2014; Hogeveen et al., 2021) suggests that it can serve as an independent risk factor for a range of diseases, including those affecting healthy elderly individuals (Eurelings et al., 2014). In this section, we first provide a concise review of the neural mechanisms underlying apathy, focusing on neuroimaging findings in the context of normal cognitive aging, mental disorder, neurodegenerative disorder, and traumatic brain injury. We also present a summary of key findings related to apathy across the healthy elderly and brain disorders in Table 1.
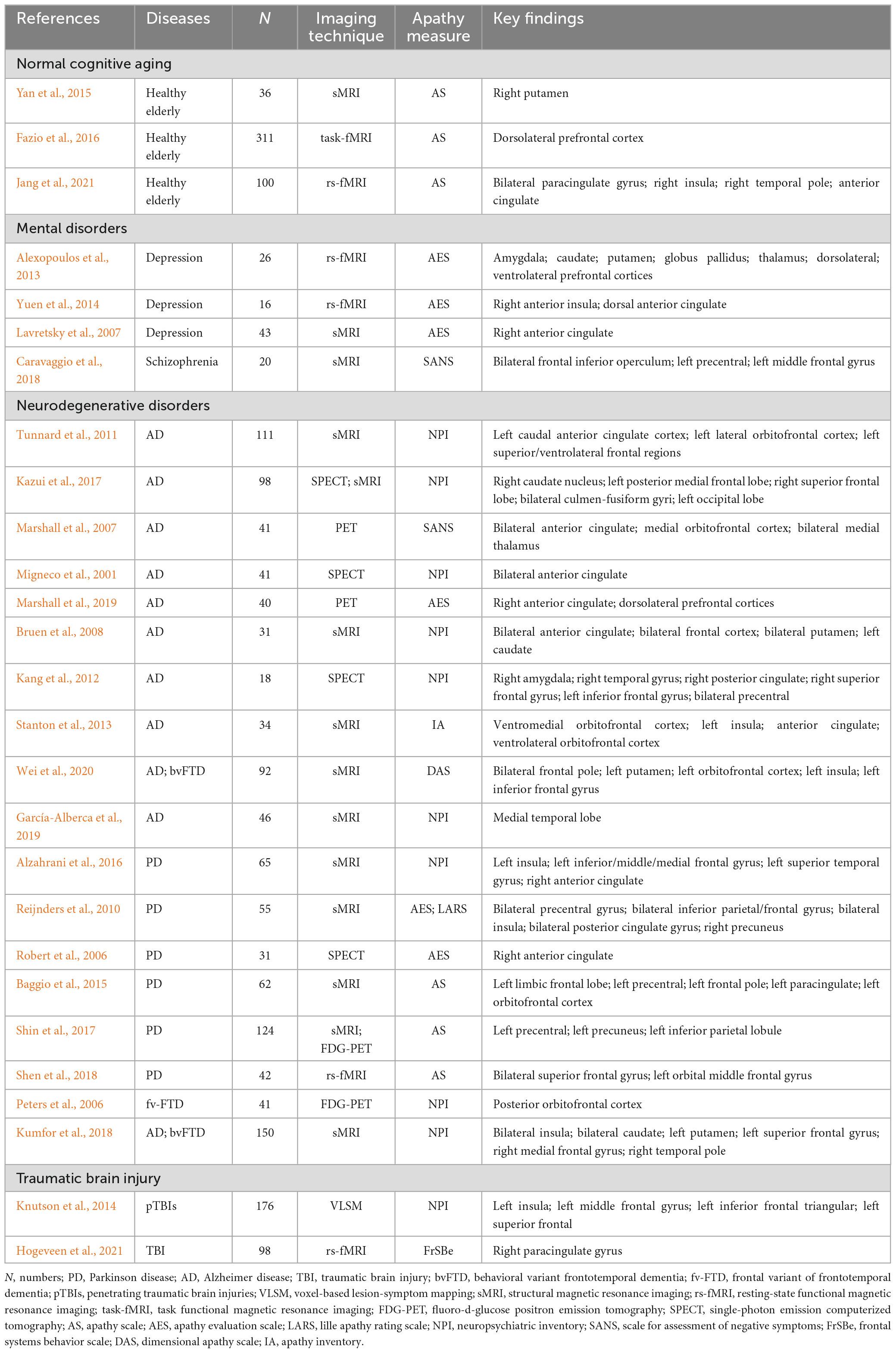
Table 1. A neuroimaging summary of the negative effects of apathy on normal cognitive aging, mental disorders, neurodegenerative disorders, and traumatic brain injury.
As the global elderly population continues to increase rapidly, maintaining a “healthy” aging life has become an important concern. Unfortunately, apathy is a neuropsychiatric symptom with the prevalence rates ranging from 2 to 4.8% among cognitively normal older adults (Onyike et al., 2007; Geda et al., 2008; Lanctôt et al., 2017), which may affect their activities of daily living functioning and quality of life. Moreover, a 6 years follow-up study of 3,427 community-dwelling older people found that apathy was significantly associated with the risk of dementia, especially in individuals without cognitive impairment (van Dalen et al., 2018a). At the neural circuit level, research has revealed that apathy symptoms were associated with the gray matter volume in the prefrontal-basal-ganglia network (Yan et al., 2015), and the integrity of the frontal-subcortical network (Lanctôt et al., 2017). Studies have also demonstrated that healthy elderly individuals with apathy had smaller gray matter volumes in the frontal and temporal regions, parietal white matter volumes, thalamus volumes, and higher numbers of frontal white matter lesions (Grool et al., 2014). Therefore, age-related changes in the prefrontal cortex may make the elderly more vulnerable to apathy (Kawagoe et al., 2017).
Apathy is also a commonly observed symptom across various mental disorders (Steffens et al., 2022). For example, apathy is a negative symptom in schizophrenia, significantly reducing bilateral frontal lobe volumes happened in the high apathy group (Roth et al., 2004). Further, a replication neuroimaging study conducted by Burrer et al. (2020) showed that apathy is not only associated with reduced ventral striatal volume in schizophrenia, which also suggests that functional and structural striatal neuroimaging correlates of apathy can occur independently in schizophrenia (Burrer et al., 2020). Additionally, a negative correlation between the domain of apathy and the resting-state functional connectivity (rsFC) in the default mode network (DMN) has been observed (Forlim et al., 2020). Besides, it has been found that an increased level of apathy symptom mediates the relationship between cognition and depression (Funes et al., 2018). In depressed elderly patients with high levels of apathy, a decrease in rsFC of the nucleus accumbens (NAcc) with the amygdala, caudate, putamen, globus pallidus, and thalamus was observed, along with an increase in rsFC with the dorsomedial prefrontal cortex (dACC), superior frontal cortex, and insula compared to non-apathetic patients (Alexopoulos et al., 2013). Additionally, the apathetic subjects with late-life depression had lower saliency network rsFC and altered network FC patterns in right dorsolateral prefrontal cortex (DLPFC) nodes of the cognitive control network com-pared to older depressive patients without apathy (Yuen et al., 2014).
Moreover, there is growing neuroimaging evidence to suggest that apathy plays a significant role in various neurodegenerative disorders, such as Parkinson’s disease (PD), mild cognitive impairment (MCI), Alzheimer’s disease (AD), etc. Based on resting-state functional magnetic resonance imaging (rs-fMRI) technology, the study by Baggio et al. (2015) showed that the PD patients with apathy had decreased FC between the left striatal and frontal areas, and amongst PD patients’ apathy was inversely correlated with FC between the subdivisions of the left frontal lobe. Apart from PD, the MCI represents a transitional stage between healthy aging and dementia, with the prevalence of MCI estimated between 5.0 and 36.7% in the general older population (Sachdev et al., 2015; Ma, 2020), and apathy prevalence reported to range from 10.7 to 44.8% (Palmer et al., 2010; Richard et al., 2012). In a recent study conducted by Raimo et al. (2019), an activation likelihood estimation (ALE)-based meta-analysis (Eickhoff et al., 2009) was employed to investigate the neural underpinnings of apathy among individuals diagnosed with neurodegenerative disorders such as Fronto-Temporal Dementia (FTD), AD, and PD. The findings revealed a significant association between apathy and both hypometabolism and reduced gray matter volume specifically localized in the left inferior frontal gyrus (Raimo et al., 2019). A systematic review found that the apathy was associated with an approximately 2-fold increased risk of dementia in memory clinic patients (van Dalen et al., 2018b). Robert et al. (2006) and colleagues observed that MCI patients with apathy were more likely to develop into AD more than those without apathy. In the AD population, apathy has been linked to the reduced daily functioning, caregiver distress, and poor outcome. The study by Onyike et al. (2007) suggested that apathy is an early sign of cognitive decline. The emergence of an MCI plus apathy phenotype progresses to dementia (Bruen et al., 2008), and it is also possible that apathy precedes MCI, implying apathy as a potential target for treatment in AD (Mortby et al., 2022).
Besides, apathy is a prevalent symptom that occurs following TBI, which can cause severe cognitive impairment and negative psychosocial outcomes. Estimates of apathy following TBI range widely from 15 to 71% (Skidmore et al., 2015). It can be challenging to differentiate dysexecutive disorders from apathy after TBI because the cognitive aspects of apathy usually include executive functions associated with goal-directed behavior (Green et al., 2022). Alternatively, as the apathy is often characterized by a loss of interest in activities, some authors have suggested that deficits in sustained attention after frontal injury may be the main underlying factor (Daffner et al., 2000). TBI typically results in damage to orbitofrontal regions and often involves ventromedial areas of the prefrontal cortex, which can lead to decision-making deficits, as argued by Bechara (2004). In addition, different apathy profiles may arise from lateral prefrontal and medial prefrontal damage (Knutson et al., 2014).
The brief review presented above suggests that apathy may serve as an independent risk factor for a variety of brain diseases, as well as being observed in healthy elderly individuals. Building upon this literature, it is hypothesized that apathy may share a common neurophysiological mechanism among apathetic healthy elderly individuals and individuals affected by various brain diseases. In order to investigate the neurophysiological basis of apathy further, the present study next aims to conduct a meta-analysis utilizing neuroimaging studies related to apathy.
2. Meta-analyses of neuroimaging apathy
A systematic selection of appropriate peer-reviewed studies was undertaken by searching the databases of PubMed, Google scholar, Web of Science and by checking references cited in each paper according to the standard preferred reporting items for systematic reviews and meta-analyses (PRISMA) procedure (Moher et al., 2009). The keyword combination is “apathy” AND “neuroimaging” OR “magnetic resonance imaging” OR “MRI” OR “functional magnetic resonance imaging” OR “fMRI” AND “Parkinson” OR “Alzheimer” OR “Dementia” OR “Huntington” OR “Mild Cognitive Impairment” OR “Mental disorder” OR “Psychiatric” OR “Depression” OR “Schizophrenia” OR “Traumatic Brain Injury” OR “Healthy Aged People.” The detailed procedure was presented in Figure 1.
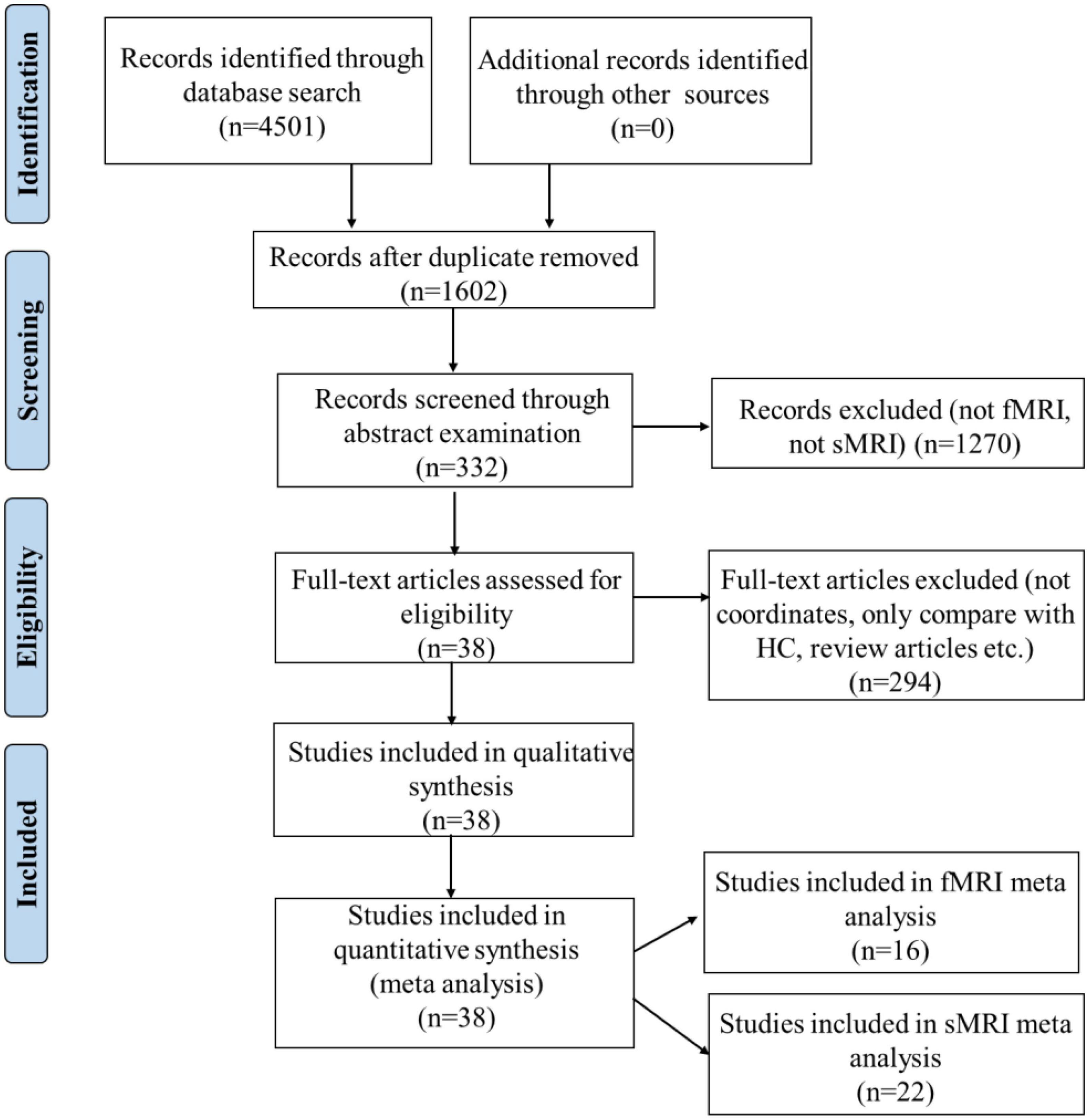
Figure 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram of study selection. HC, healthy controls.
2.1. ALE meta-analysis
Activation likelihood estimation is a widely-used meta-analytic technique in neuroimaging to combine multiple studies, and to identify brain regions that consistently show activation across experiments (Turkeltaub et al., 2002; Laird et al., 2005, 2009a,b). Briefly, the ALE procedure involves the following steps:
1. Data collection: Gather a set of studies that investigate a specific condition using neuroimaging techniques such as magnetic resonance imaging (MRI), functional MRI (fMRI) or positron emission tomography (PET).
2. Coordinate extraction: Identify the peak coordinates of activation reported in each study. These coordinates represent the locations of maximum brain activity associated with the task or condition of interest.
3. Modeling activation: Create three-dimensional Gaussian probability distributions centered on each peak coordinate. The size of these distributions represents the spatial uncertainty associated with the reported activations.
4. Spatial modeling: Combine the individual activation models to create an overall activation likelihood map. This map represents the likelihood of activation at each voxel in the brain across all studies.
5. Activation likelihood estimation calculation: Calculate the ALE score for each voxel by determining the proportion of overlapping activation likelihoods from different studies.
6. Thresholding: Establish a significance threshold to determine which voxels show activation beyond what would be expected by chance. Various statistical methods can be used to determine the threshold, such as permutation testing, false discovery rate correction, etc.
7. Cluster analysis: Identify clusters of significant activation by grouping adjacent activated voxels. This step helps to reduce the likelihood of false-positive findings.
8. Interpretation: Analyze the clusters of significant activation to infer brain regions that consistently exhibit activation across the included studies.
Studies were included in this meta-analysis compared subjects with or without apathy (e.g., healthy elderly with apathy vs. healthy elderly without apathy, AD with apathy vs. AD without apathy, etc.). Studies focused on functional connectivity/gray matter volume alterations associated with apathy. The criterion of apathy diagnosis was clearly reported in the involved studies (listed in Tables 2, 3) in this meta-analysis, which was subject to different apathy evaluation questionnaires such as Apathy Scale (AS) (Starkstein et al., 1992), Apathy Evaluation Scale (AES) (Marin et al., 1991), Neuropsychiatric Inventory (NPI) (Cummings, 2020), etc. We performed an ALE analysis using the GingerALE software (Version 3.0.2) (Eickhoff et al., 2009, 2011, 2012; Turkeltaub et al., 2012). The resulting p-values were threshold at p = 0.05 with 1,000 threshold permutations, cluster-level family-wise error (cFWE) with cluster-forming threshold at p < 0.001. Based on a recent simulation study (Eickhoff et al., 2016), a recommendation was made to include at least 17–20 experiments in ALE meta-analyses in order to have sufficient power to detect smaller effects and to also make sure that results are not driven by single experiments. The ALE mapping results were displayed on the MNI template using Mircron1.
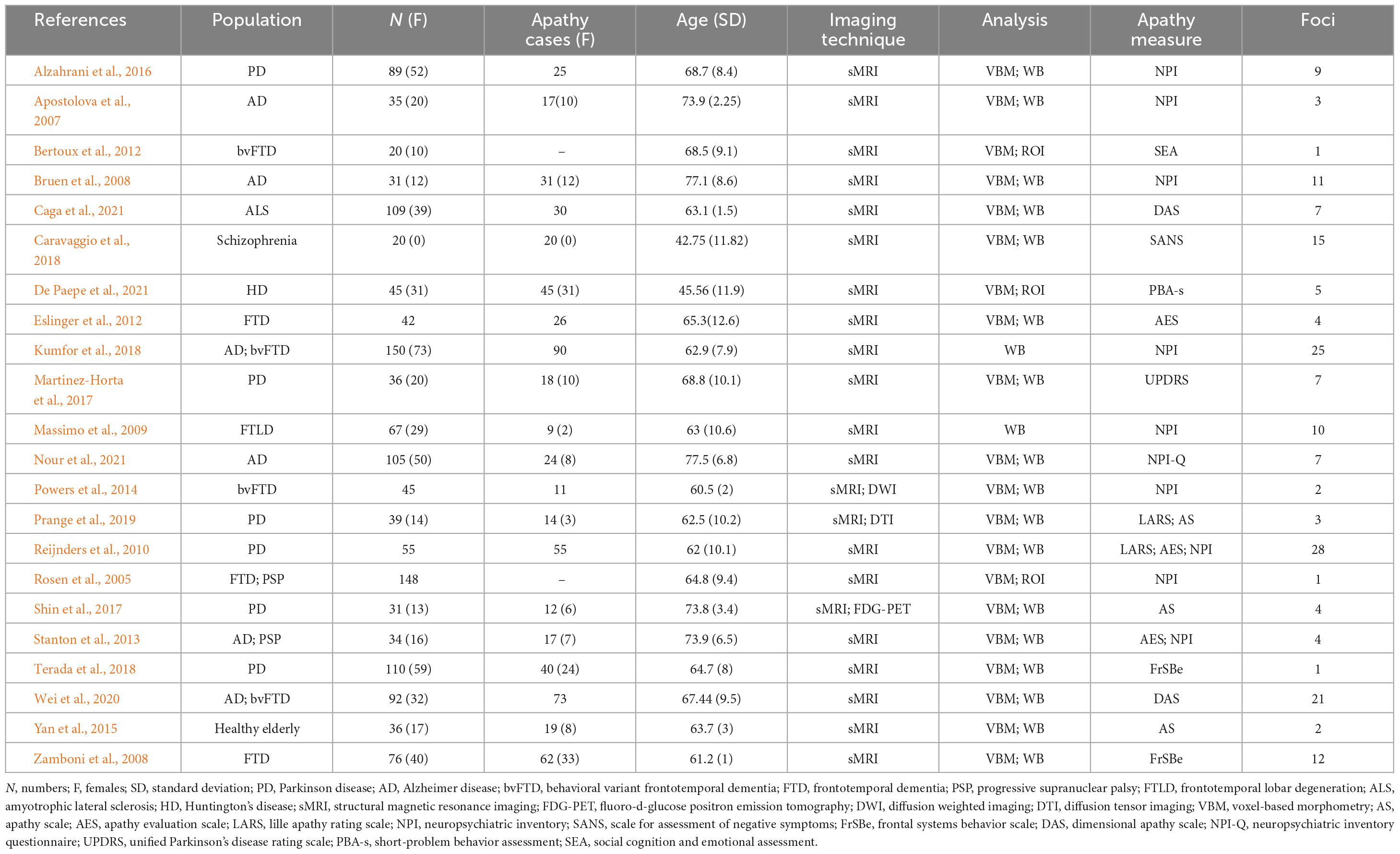
Table 2. Original structural magnetic resonance imaging (MRI) studies included in the structural neuroimaging meta-analysis.
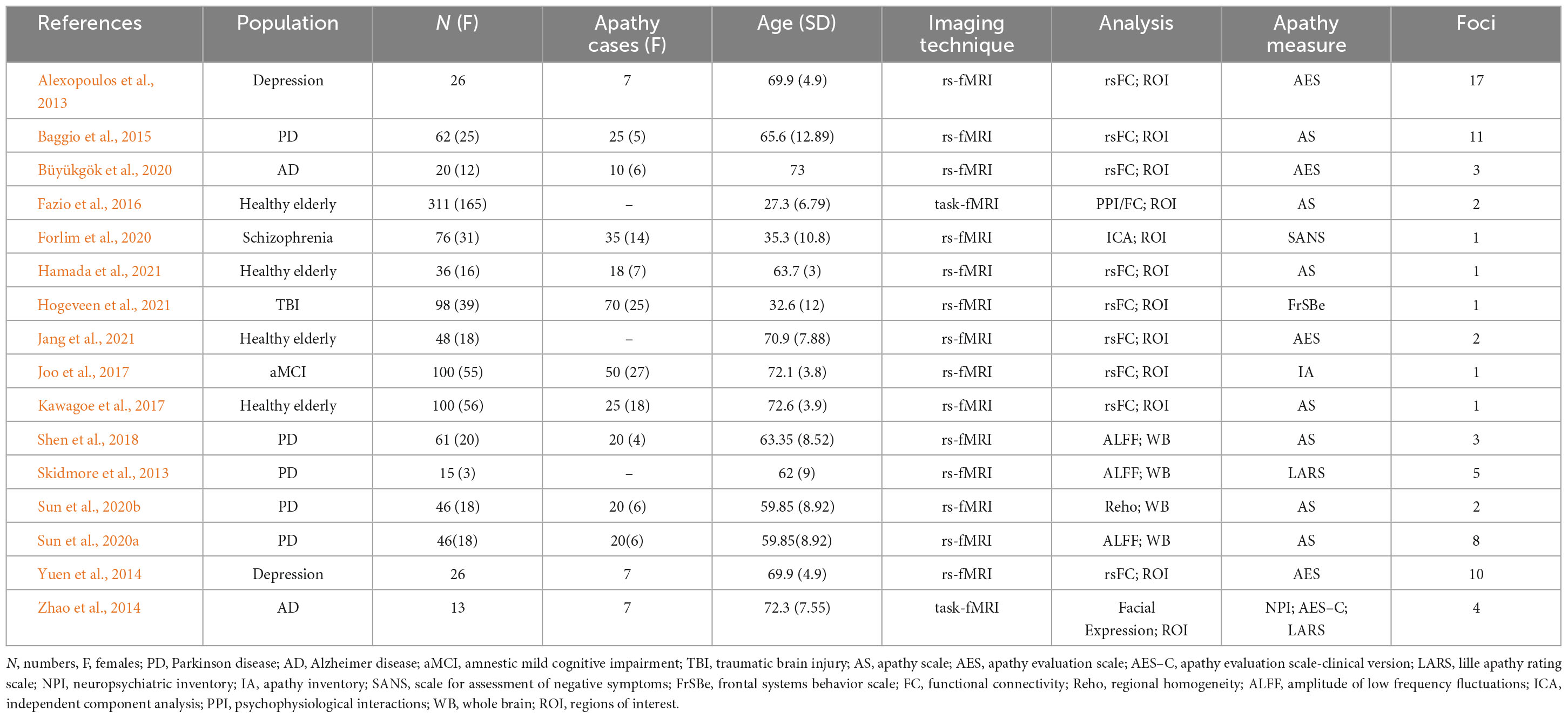
Table 3. Original functional magnetic resonance imaging (MRI) studies included in the functional neuroimaging meta-analysis.
According to the PRISMA flow diagram, there are 22 studies included 31 experiments from 1,578 patients and reported 182 foci of gray matter volume decreases associated with apathy (see Table 2).
According to the PRISMA flow diagram, there are 16 studies included 20 experiments from 706 patients and reported 73 foci of functional connectivity/ALFF decreases associated with apathy (see Table 3).
3. Results
Our meta-analysis of structural MRI studies revealed six cluster of significant convergence between the studies, and they were in left Precentral Gyrus (BA 13/6), right Insula (BA 47), right Medial Frontal Gyrus (BA 11), left Inferior Frontal Gyrus and left Caudate (Putamen), which are shown in Table 4, Figure 2.
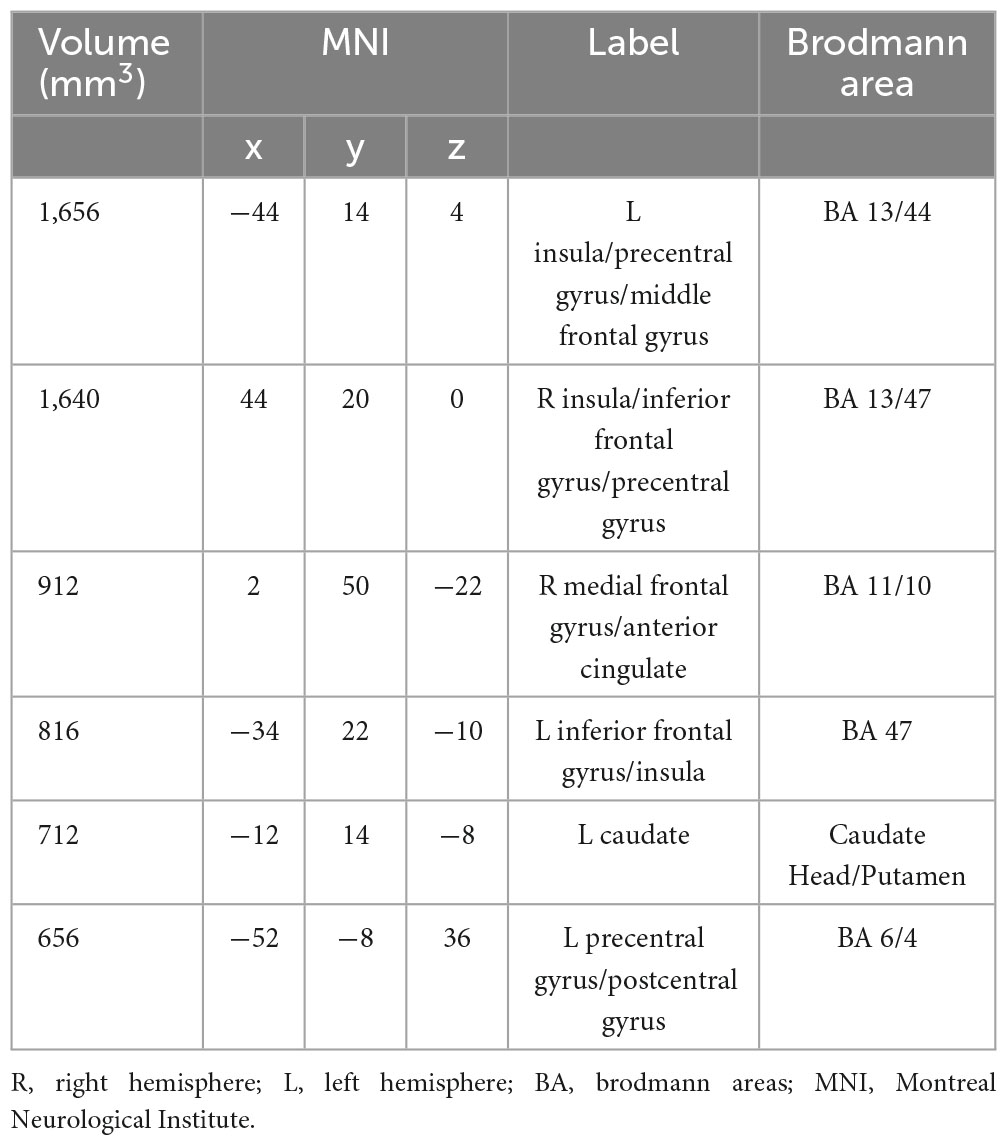
Table 4. Strengthened activation results in the apathy-free group in contrast to the apathy group in structural neuroimaging meta-analysis.
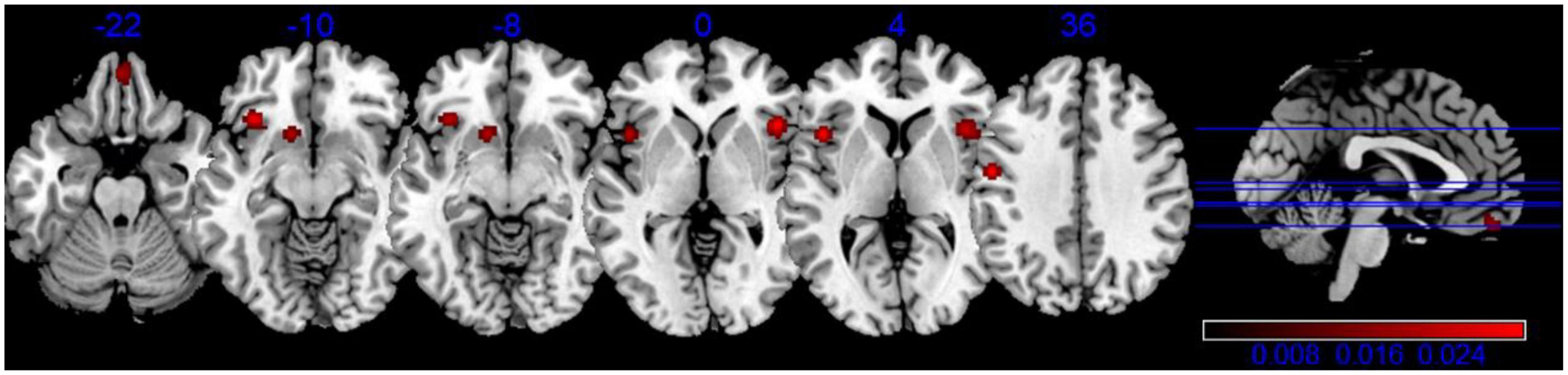
Figure 2. Activation activation likelihood estimation (ALE) map for gray matter atrophy associated with apathy. The ALE map significance was tested by using 1,000 permutations with a cluster-forming threshold of p < 0.001, and was corrected with a cluster-level family-wise error threshold of p < 0.05.
Our analysis of functional neuroimaging techniques revealed that Lentiform (Putamen) was correlated with apathy. The results indicated one significant cluster in this contrast, and they were located in Putamen and Lateral Globus Pallidus, shown in Table 5, Figure 3.
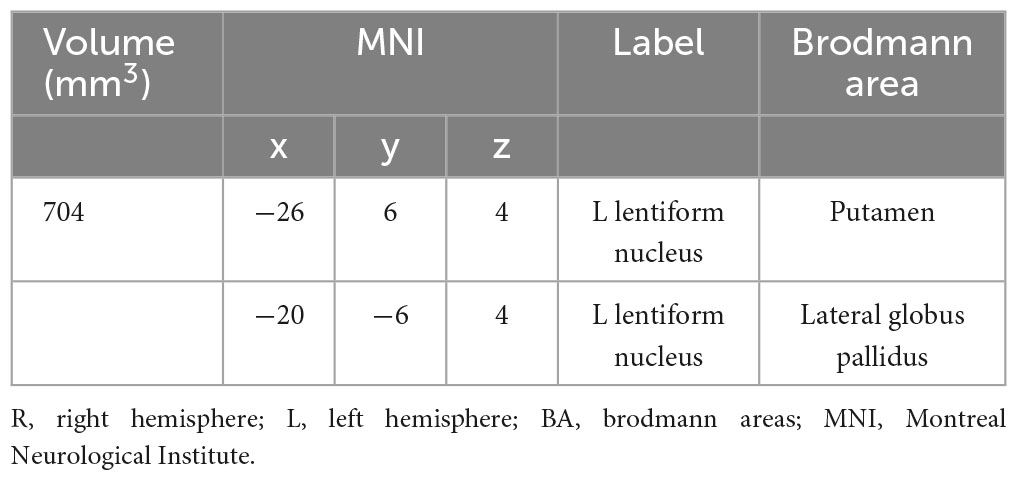
Table 5. Strengthened activation results in the apathy-free group in contrast to the apathy group in functional neuroimaging meta-analysis.
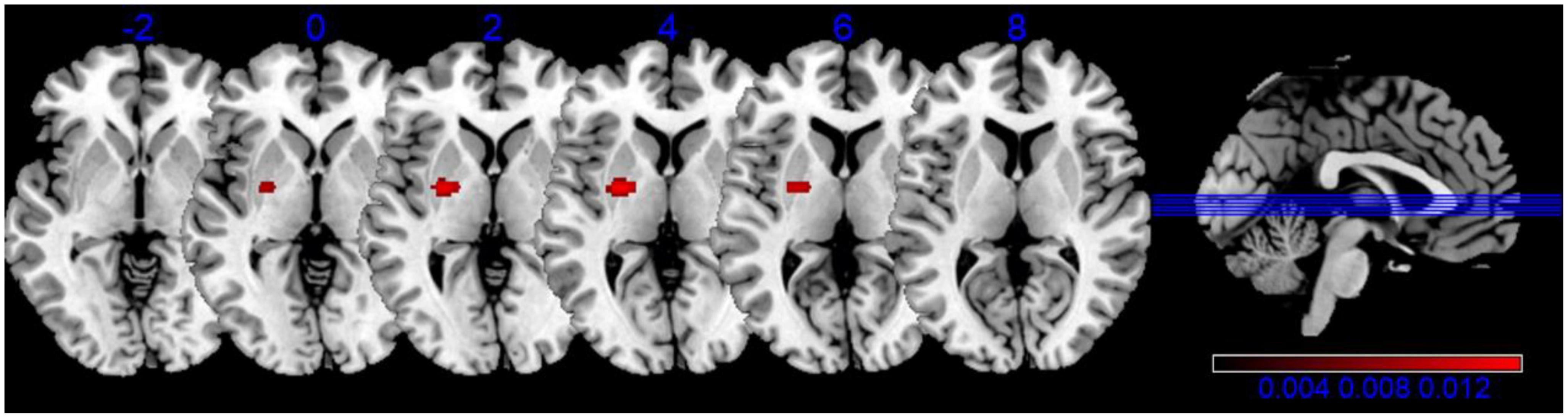
Figure 3. Activation activation likelihood estimation (ALE) map for the decreased brain functional connectivity associated with apathy. The ALE map significance was tested by using 1,000 permutations with a cluster-forming threshold of p < 0.001, and was corrected with a cluster-level family-wise error threshold of p < 0.05.
4. Discussion
Apathy is a prevalent mood disturbance, being widely distributed among the normal aging, mental disorders, neurodegenerative disorders and traumatic brain injuries. The aim of our study was to reveal the neural basis of apathy between the non-apathy and apathy groups across diverse populations, including healthy elderly, mental disorders, neurodegenerative disorders, acquired brain injuries. Despite the differences among these populations, the manifestations of apathy symptoms are generally consistent, and apathy is widely recognized as a common and incapacitating condition in various disorders, including AD, PD, FTD, TBI and so on Kos et al. (2016).
4.1. Altered structural and functional correlation of apathy
In our study, the apathy group exhibited structural atrophy in brain regions including the bilateral insula, precentral gyrus, inferior frontal gyrus, medial frontal gyrus, left caudate, postcentral gyrus, and right anterior cingulate, compared to the non-apathy group. Furthermore, we observed inverse correlations between apathy and brain functional connectivity, specifically in regions such as the left putamen and lateral globus pallidus. These findings suggest significant atrophy and dysfunction in the intrinsic neural activity of these regions in apathetic patients.
A recent ALE meta-analysis investigated the neural correlates of apathy in patients with neurodegenerative disorders, revealing a link between apathy and hypometabolism as well as reduced gray matter volume in the left inferior frontal gyrus (Raimo et al., 2019). Our study expands upon this research in several notable ways. Firstly, we included a larger subgroup of subjects with apathy, encompassing healthy elderly individuals, those with acquired brain injury, mental disorders, in addition to neurodegenerative disorder patients. Secondly, we employed stricter selection criteria by focusing exclusively on MRI/fMRI-based studies for the meta-analysis. Lastly, our study identified additional brain regions associated with apathy, extending beyond the left inferior frontal gyrus, through structural neuroimaging meta-analysis.
The putamen, particularly the caudate head, plays a role in the executive function of the fronto-striatal network (Baggio et al., 2015; Lucas-Jiménez et al., 2018). A SPECT study found dopaminergic neuronal loss in the bilateral putamen of patients with apathy, including those with Alzheimer’s disease and dementia with Lewy bodies (David et al., 2008). Additionally, the anterior cingulate circuit, part of the frontal-striatal circuits described in the model by Tekin and Cummings (2002), consists of a feed-forward loop from frontal cortical areas to the caudate nucleus and putamen (Mesulam, 2000). Another study observed abnormalities in the left putamen/ventral striatum following negative feedback (Waltz et al., 2013). Together, these findings partially support our results and suggest that apathy may arise from aberrant processing of reward stimuli and anticipation.
Our findings also revealed atrophy in the bilateral insula, a frontal lobe region involved in inhibitory control, body representation, and subjective emotional experience (Damasio, 1996). The observed insular damage in our study aligns with previous findings on apathy in neurodegenerative disorders and normal aging (Yuen et al., 2014; Kumfor et al., 2018; Jang et al., 2021). For instance, Moon et al. (2014) identified a negative correlation between apathy and volume of the bilateral anterior insular cortex. Moreover, a task-based fMRI study investigating cognitive and emotional empathy identified the precentral gyrus (BA 4) and right insula (BA 13) as potentially important components of emotional empathy (Kim et al., 2020). Additionally, mutism has been associated with right insula damage in stroke patients (Berthier et al., 1987; Gasquoine, 2014).
Significantly decreased volumes were observed in the bilateral precentral gyrus (more prominently in the left hemisphere), inferior frontal gyrus, and medial frontal gyrus in our study. According to Frijda (2010), emotions play a role in both the generation and execution/control of actions. Furthermore, Reijnders et al. (2010) found a correlation between high apathy scores and decreased gray matter density in the bilateral precentral gyrus. These brain imaging findings suggest the involvement of the precentral gyrus in both emotional and cognitive processes. Raimo et al. (2019) investigated structural and metabolic alterations associated with apathy across AD, FTD, and PD, and demonstrated that apathy was mainly associated with the left inferior frontal gyrus, which is strongly connected to a frontal-subcortical circuit involved in action planning and purpose generation (Levy and Dubois, 2006). The medial frontal gyrus (BA 10) is believed to play a crucial role in linking affect or emotional information with planned or ongoing behaviors (Kringelbach, 2005). The right medial frontal gyrus (BA 11/10) may also have a fundamental role in the development of apathy due to its functional and structural connectivity (Moayedi et al., 2015; Ray et al., 2015). Hence, abnormal changes in these brain areas may be associated with apathy symptoms.
4.2. Target areas for apathy-related neuroplasticity modulation
The structural and functional changes associated with apathy may indicate crucial brain regions for the treatment of apathy-related brain disorders. Our research robustly identified certain brain regions (refer to Figures 2, 3) through meta-analysis that consistently exhibited apathy-related alterations across both healthy elderly individuals and those with brain disorders. These commonly identified regions, displaying structural or functional changes, could be targeted for treatment of apathy-related brain disorders using neuromodulation techniques such as transcranial magnetic stimulation (TMS) (Kobayashi and Pascual-Leone, 2003; Hallett, 2007) and transcranial direct current stimulation (tDCS) (Paulus, 2011). For instance, Reis et al. (2008) conducted a comprehensive review of relevant studies and observed that these stimulation techniques could regulate memory formation and motor learning in healthy individuals. Furthermore, several studies have demonstrated the effectiveness of these stimulation techniques in treating mental disorders such as major depressive disorder and schizophrenia (Reis et al., 2008; Liu et al., 2017; Kennedy et al., 2018; Osoegawa et al., 2018), traumatic brain injury (Dhaliwal et al., 2015; Zhang et al., 2020), and neurodegenerative disorders (Elder and Taylor, 2014; Di Lorenzo et al., 2022). Specifically, Levkovitz et al. (2011) found that deep TMS applied over the prefrontal cortex led to remission of apathy in one-third of depressive patients with moderate apathy. Conversely, Suemoto et al. (2014) found no effect on apathy in elderly patients with moderate Alzheimer’s disease following repeated anodal tDCS over the left dorsolateral prefrontal cortex. Hence, based on previous studies, the brain regions identified (listed in Tables 4 and 5) are likely to be targeted for neural modulation of apathy-related neuroplasticity at the individual or group level in future.
4.3. Other neuroimaging studies of apathy
Other neuroimaging techniques used in studying apathy among the aging population include positron emission tomography (PET) (Marshall et al., 2007), single-photon emission computerized tomography (SPECT) (Benoit et al., 2004; David et al., 2008), and diffusion tensor imaging (DTI) (Prange et al., 2019). Early SPECT studies have shown a correlation between apathy and reduced regional cerebral blood flow in the orbitofrontal cortex (DeKosky and Scheff, 1990) and anterior cingulate (Migneco et al., 2001). Additionally, PET amyloid imaging using (11C) PiB has indicated a link between apathy and the accumulation of fibrillar amyloid in specific subregions of the prefrontal cortex (PFC), including the orbital, ventromedial, and polar PFC, as well as the anterior cingulate (Mori et al., 2014). Moreover, DTI analysis has demonstrated lower fractional anisotropy values in the left anterior cingulate of the apathy group compared to the non-apathy group (Kim et al., 2011; Tighe et al., 2012).
4.4. Limitations and future research
This study focuses exclusively on identifying the shared neural mechanism underlying apathy in normal aging and brain disorders, providing potential neural correlates for apathy. However, further investigations are necessary to address the following aspects: (1) Understanding the interaction between functional and structural changes is essential, as our analysis revealed apathy-related alterations in separate domains. (2) Exploring the evolving mechanism and role of apathy in the aging process and progression of brain disorders is crucial. (3) Although our ALE meta-analysis employed strict criteria for literature selection, the reliability of the findings may still be influenced by factors such as sample size, populations, comorbidities, medications, and number of studies. Therefore, careful validation of these apathy-related findings is required in future research. (4) Future studies should consider exploring the neural correlates of apathy specific to individual brain disorders through meta-analysis, once a sufficient number of related studies have been accumulated.
5. Conclusion
In summary, our meta-analysis study indicates that the neural correlates of apathy exist across normal aging and various brain disorders, suggesting that it may serve as an independent risk factor for brain disorders. Specifically, we noted that the putamen area was significantly activated in both the structural and functional meta-analysis, indicating a close correlation with apathy. Additionally, our structural meta-analysis revealed that gray matter atrophy in the Precentral Gyrus, Insula, Medial Frontal Gyrus, and Inferior Frontal Gyrus was also associated with apathy. These observed changes in structural/functional activation associated with apathy provide promising pathophysiological insights that have the potential to guide the development of more efficacious therapeutic interventions for brain disorders.
Author contributions
HY, HW, and ZC: conceptualization, methodology, validation, formal analysis, and writing–original draft. ZC and HY: funding acquisition. SD and LL: investigation, idea discussion, and writing–review and editing. BX, CC, and NW: conceptualization, resources, writing–review and editing, supervision, funding acquisition, and project administration. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (No. 82001160), “HaiYan Plan” Scientific Research Funding Project of Lianyungang City (No. 2017-QD-009), The First People’s Hospital of Lianyungang–Advanced Technology Support Project (No. XJ1811), Project of Huaguoshan Mountain Talent Plan - Doctors for Innovation and Entrepreneurship, Special Project for Diagnosis and Treatment of Key Clinical Diseases in Suzhou (No. LCZX202029), Suzhou City Medical Device and New Medicine Clinical Trial Institutional Capacity Improvement Project (No. SLT202001), Suzhou Science and Technology Development Plan (No. SS2019048), Scientific research project of Gusu School, Nanjing Medical University (No. GSKY20210240), and Research Projects on Aging Health of Lianyungang City (No. L202201).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Alexopoulos, G. S., Hoptman, M. J., Yuen, G., Kanellopoulos, D., Seirup, J. K., Lim, K. O., et al. (2013). Functional connectivity in apathy of late-life depression: A preliminary study. J. Affect. Disord. 149, 398–405.
Alzahrani, H., Antonini, A., and Venneri, A. (2016). Apathy in mild Parkinson’s disease: Neuropsychological and neuroimaging evidence. J. Parkinsons Dis. 6, 821–832. doi: 10.3233/JPD-160809
Apostolova, L. G., Akopyan, G. G., Partiali, N., Steiner, C. A., Dutton, R. A., Hayashi, K. M., et al. (2007). Structural correlates of apathy in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 24, 91–97.
Azocar, I., Rapaport, P., Burton, A., Meisel, G., and Orgeta, V. (2022). Risk factors for apathy in Alzheimer’s disease: A systematic review of longitudinal evidence. Ageing Res. Rev. 79:101672. doi: 10.1016/j.arr.2022.101672
Baggio, H. C., Segura, B., Garrido-Millan, J., Marti, M., Compta, Y., Valldeoriola, F., et al. (2015). Resting-state frontostriatal functional connectivity in Parkinson’s disease–related apathy. Mov. Disord. 30, 671–679. doi: 10.1002/mds.26137
Bechara, A. (2004). The role of emotion in decision-making: Evidence from neurological patients with orbitofrontal damage. Brain Cogn. 55, 30–40. doi: 10.1016/j.bandc.2003.04.001
Benoit, M., Clairet, S., Koulibaly, P. M., Darcourt, J., and Robert, P. H. (2004). Brain perfusion correlates of the apathy inventory dimensions of Alzheimer’s disease. Int. J. Geriatr. Psychiatry 19, 864–869.
Berthier, M., Starkstein, S., and Leiguarda, R. (1987). Behavioral effects of damage to the right insula and surrounding regions. Cortex 23, 673–678. doi: 10.1016/s0010-9452(87)80057-6
Bertoux, M., Volle, E., Funkiewiez, A., de Souza, L. C., Leclercq, D., and Dubois, B. (2012). Social cognition and emotional assessment (SEA) is a marker of medial and orbital frontal functions: A voxel-based morphometry study in behavioral variant of frontotemporal degeneration. J. Int. Neuropsychol. Soc. 18, 972–985. doi: 10.1017/S1355617712001300
Bruen, P. D., McGeown, W. J., Shanks, M. F., and Venneri, A. (2008). Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer’s disease. Brain 131, 2455–2463.
Burrer, A., Caravaggio, F., Manoliu, A., Plitman, E., Gütter, K., Habermeyer, B., et al. (2020). Apathy is not associated with reduced ventral striatal volume in patients with schizophrenia. Schizoph. Res. 223, 279–288.
Büyükgök, D., Bayraktaroğlu, Z., Buker, H., Kulaksızoğlu, M., and Gurvit, İH. (2020). Resting-state fMRI analysis in apathetic Alzheimer’s disease. Diagnost. Int. Radiol. 26:363.
Caga, J., Tu, S., Dharmadasa, T., Nga, Y. T., Zoing, M. C., Huynh, W., et al. (2021). Apathy is associated with parietal cortical-subcortical dysfunction in ALS. Cortex 145, 341–349. doi: 10.1016/j.cortex.2021.02.029
Caravaggio, F., Fervaha, G., Menon, M., Remington, G., Graff-Guerrero, A., and Gerretsen, P. (2018). The neural correlates of apathy in schizophrenia: An exploratory investigation. Neuropsychologia 118, 34–39. doi: 10.1016/j.neuropsychologia.2017.10.027
Cummings, J. (2020). The neuropsychiatric inventory: Development and applications. J. Geriatr. Psychiatry Neurol. 33, 73–84. doi: 10.1177/0891988719882102
Daffner, K. R., Mesulam, M. M., Holcomb, P. J., Calvo, V., Acar, D., Chabrerie, A., et al. (2000). Disruption of attention to novel events after frontal lobe injury in humans. J. Neurol. Neurosurg. Psychiatry 68, 18–24.
Damasio, A. R. (1996). The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond. Series B Biol. Sci. 351, 1413–1420.
David, R., Koulibaly, M., Benoit, M., Garcia, R., Caci, H., Darcourt, J., et al. (2008). Striatal dopamine transporter levels correlate with apathy in neurodegenerative diseases: A SPECT study with partial volume effect correction. Clin. Neurol. Neurosurg. 110, 19–24. doi: 10.1016/j.clineuro.2007.08.007
De Paepe, A. E., Ara, A., Garcia-Gorro, C., Martinez-Horta, S., Perez-Perez, J., Kulisevsky, J., et al. (2021). Gray matter vulnerabilities predict longitudinal development of apathy in Huntington’s disease. Mov. Disord. 36, 2162–2172. doi: 10.1002/mds.28638
DeKosky, S. T., and Scheff, S. W. (1990). Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Ann. Neurol. 27, 457–464.
Dhaliwal, S. K., Meek, B. P., and Modirrousta, M. M. (2015). Non-invasive brain stimulation for the treatment of symptoms following traumatic brain injury. Front. Psychiatry 6:119. doi: 10.3389/fpsyt.2015.00119
Di Lorenzo, F., Oliviero, A., Guerra, A., and Fried, P. J. (2022). Non-invasive brain stimulation for neurodegenerative disorders: From investigation to therapeutic application. Front. Neurol. 13:820942. doi: 10.3389/fneur.2022.820942
Eickhoff, S. B., Bzdok, D., Laird, A. R., Kurth, F., and Fox, P. T. (2012). Activation likelihood estimation meta-analysis revisited. Neuroimage 59, 2349–2361.
Eickhoff, S. B., Bzdok, D., Laird, A. R., Roski, C., Caspers, S., Zilles, K., et al. (2011). Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage 57, 938–949. doi: 10.1016/j.neuroimage.2011.05.021
Eickhoff, S. B., Laird, A. R., Grefkes, C., Wang, L. E., Zilles, K., and Fox, P. T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 30, 2907–2926. doi: 10.1002/hbm.20718
Eickhoff, S. B., Nichols, T. E., Laird, A. R., Hoffstaedter, F., Amunts, K., Fox, P. T., et al. (2016). Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage 137, 70–85. doi: 10.1016/j.neuroimage.2016.04.072
Elder, G. J., and Taylor, J. P. (2014). Transcranial magnetic stimulation and transcranial direct current stimulation: Treatments for cognitive and neuropsychiatric symptoms in the neurodegenerative dementias? Alzheimers Res. Ther. 6:74. doi: 10.1186/s13195-014-0074-1
Eslinger, P. J., Moore, P., Antani, S., Anderson, C., and Grossman, M. (2012). Apathy in frontotemporal dementia: Behavioral and neuroimaging correlates. Behav. Neurol. 25, 127–136.
Eurelings, L. S., Ligthart, S. A., van Dalen, J. W., Moll van Charante, E. P., van Gool, W. A., and Richard, E. (2014). Apathy is an independent risk factor for incident cardiovascular disease in the older individual: A population-based cohort study. Int. J. Geriatr. Psychiatry 29, 454–463.
Fazio, L., Logroscino, G., Taurisano, P., Amico, G., Quarto, T., Antonucci, L. A., et al. (2016). Prefrontal activity and connectivity with the basal ganglia during performance of complex cognitive tasks is associated with apathy in healthy subjects. PLoS One 11:e0165301. doi: 10.1371/journal.pone.0165301
Forlim, C. G., Klock, L., Bächle, J., Stoll, L., Giemsa, P., Fuchs, M., et al. (2020). Reduced resting-state connectivity in the precuneus is correlated with apathy in patients with schizophrenia. Sci. Rep. 10:2616. doi: 10.1038/s41598-020-59393-6
Funes, C. M., Lavretsky, H., Ercoli, L., Cyr, N. S., and Siddarth, P. (2018). Apathy mediates cognitive difficulties in geriatric depression. Am. J. Geriatr. Psychiatry 26, 100–106.
García-Alberca, J., Florido, M., Cáceres, M., Sánchez-Toro, A., Lara, J. P., and García-Casares, N. (2019). Medial temporal lobe atrophy is independently associated with behavioural and psychological symptoms in Alzheimer’s disease. Psychogeriatrics 19, 46–54.
Gasquoine, P. G. (2014). Contributions of the insula to cognition and emotion. Neuropsychol. Rev. 24, 77–87.
Geda, Y. E., Roberts, R. O., Knopman, D. S., Petersen, R. C., Christianson, T. J., Pankratz, V. S., et al. (2008). Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: Population-based study. Arch. Gene. Psychiatry 65, 1193–1198.
Green, S. L., Gignac, G. E., Watson, P. A., Brosnan, N., Becerra, R., Pestell, C., et al. (2022). Apathy and depression as predictors of activities of daily living following stroke and traumatic brain injuries in adults: A meta-analysis. Neuropsychol. Rev. 32, 51–69.
Grool, A. M., Geerlings, M. I., Sigurdsson, S., Eiriksdottir, G., Jonsson, P. V., Garcia, M. E., et al. (2014). Structural MRI correlates of apathy symptoms in older persons without dementia: Ages-reykjavik study. Neurology 82, 1628–1635. doi: 10.1212/WNL.0000000000000378
Hamada, C., Kawagoe, T., Takamura, M., Nagai, A., Yamaguchi, S., and Onoda, K. (2021). Altered resting-state functional connectivity of the frontal-striatal circuit in elderly with apathy. PLoS One 16:e0261334. doi: 10.1371/journal.pone.0261334
Hogeveen, J., Aragon, D. F., Rogge-Obando, K., Campbell, R. A., Shuttleworth, C. W., Avila-Rieger, R. E., et al. (2021). Ventromedial prefrontal-anterior cingulate hyperconnectivity and resilience to apathy in traumatic brain injury. J. Neurotr. 38, 2264–2274. doi: 10.1089/neu.2020.7363
Jang, J. Y., Han, S. D., Yew, B., Blanken, A. E., Dutt, S., Li, Y., et al. (2021). Resting-state functional connectivity signatures of apathy in community-living older adults. Front. Aging Neurosci. 13:691710.. doi: 10.3389/fnagi.2021.691710
Joo, S. H., Lee, C. U., and Lim, H. K. (2017). Apathy and intrinsic functional connectivity networks in amnestic mild cognitive impairment. Neuropsychiatr. Dis. Treat. 13:61. doi: 10.2147/NDT.S123338
Kang, J. Y., Lee, J. S., Kang, H., Lee, H. W., Kim, Y. K., Jeon, H. J., et al. (2012). Regional cerebral blood flow abnormalities associated with apathy and depression in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 26, 217–224.
Kawagoe, T., Onoda, K., and Yamaguchi, S. (2017). Apathy and executive function in healthy elderly—resting state fMRI study. Front. Aging Neurosci. 9:124. doi: 10.3389/fnagi.2017.00124
Kazui, H., Takahashi, R., Yamamoto, Y., Yoshiyama, K., Kanemoto, H., Suzuki, Y., et al. (2017). Neural basis of apathy in patients with amnestic mild cognitive impairment. J. Alzheimers Dis. 55, 1403–1416. doi: 10.3233/JAD-160223
Kennedy, N. I., Lee, W. H., and Frangou, S. (2018). Efficacy of non-invasive brain stimulation on the symptom dimensions of schizophrenia: A meta-analysis of randomized controlled trials. Eur. Psychiatry 49, 69–77.
Kim, E. J., Son, J. W., Park, S. K., Chung, S., Ghim, H. R., Lee, S., et al. (2020). Cognitive and emotional empathy in young adolescents: An fMRI study. J. Korean Acad. Child Adolesc. Psychiatry 31:121.
Kim, J. W., Lee, D. Y., Choo, I. H., Seo, E. H., Kim, S. G., Park, S. Y., et al. (2011). Microstructural alteration of the anterior cingulum is associated with apathy in Alzheimer disease. Am. J. Geriatr. Psychiatry 19, 644–653.
Knutson, K. M., Dal Monte, O., Raymont, V., Wassermann, E. M., Krueger, F., and Grafman, J. (2014). Neural correlates of apathy revealed by lesion mapping in participants with traumatic brain injuries. Hum. Brain Mapp. 35, 943–953. doi: 10.1002/hbm.22225
Kobayashi, M., and Pascual-Leone, A. (2003). Transcranial magnetic stimulation in neurology. Lancet Neurol. 2, 145–156.
Kos, C., van Tol, M. J., Marsman, J. B. C., Knegtering, H., and Aleman, A. (2016). Neural correlates of apathy in patients with neurodegenerative disorders, acquired brain injury, and psychiatric disorders. Neurosci. Biobehav. Rev. 69, 381–401. doi: 10.1016/j.neubiorev.2016.08.012
Kringelbach, M. L. (2005). The human orbitofrontal cortex: Linking reward to hedonic experience. Nat. Rev. Neurosci. 6, 691–702.
Kumfor, F., Zhen, A., Hodges, J. R., Piguet, O., and Irish, M. (2018). Apathy in Alzheimer’s disease and frontotemporal dementia: Distinct clinical profiles and neural correlates. Cortex 103, 350–359.
Laird, A. R., Eickhoff, S. B., Kurth, F., Fox, P. M., Uecker, A. M., Turner, J. A., et al. (2009a). ALE meta-analysis workflows via the brainmap database: Progress towards a probabilistic functional brain atlas. Front. Neuroinform. 3:23. doi: 10.3389/neuro.11.023.2009
Laird, A. R., Eickhoff, S. B., Li, K., Robin, D. A., Glahn, D. C., and Fox, P. T. (2009b). Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J. Neurosci. 29, 14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009
Laird, A. R., Fox, P. M., Price, C. J., Glahn, D. C., Uecker, A. M., Lancaster, J. L., et al. (2005). ALE meta-analysis: Controlling the false discovery rate and performing statistical contrasts. Hum. Brain Mapp. 25, 155–164. doi: 10.1002/hbm.20136
Lanctôt, K. L., Agüera-Ortiz, L., Brodaty, H., Francis, P. T., Geda, Y. E., Ismail, Z., et al. (2017). Apathy associated with neurocognitive disorders: Recent progress and future directions. Alzheimers Dement. 13, 84–100.
Lavretsky, H., Ballmaier, M., Pham, D., Toga, A., and Kumar, A. (2007). Neuroanatomical characteristics of geriatric apathy and depression: A magnetic resonance imaging study. Am. J. Geriatr. Psychiatry 15, 386–394. doi: 10.1097/JGP.0b013e3180325a16
Levkovitz, Y., Sheer, A., Harel, E. V., Katz, L. N., Most, D., Zangen, A., et al. (2011). Differential effects of deep TMS of the prefrontal cortex on apathy and depression. Brain Stimul. 4, 266–274. doi: 10.1016/j.brs.2010.12.004
Levy, R., and Dubois, B. (2006). Apathy and the functional anatomy of the prefrontal cortex–basal ganglia circuits. Cereb. Cortex 16, 916–928.
Liu, S., Sheng, J., Li, B., and Zhang, X. (2017). Recent advances in non-invasive brain stimulation for major depressive disorder. Front. Hum. Neurosci. 11:526. doi: 10.3389/fnhum.2017.00526
Lucas-Jiménez, O., Ojeda, N., Peña, J., Cabrera-Zubizarreta, A., Díez-Cirarda, M., Gómez-Esteban, J. C., et al. (2018). Apathy and brain alterations in Parkinson’s disease: A multimodal imaging study. Ann. Clin. Transl. Neurol. 5, 803–814.
Ma, L. (2020). Depression, anxiety, and apathy in mild cognitive impairment: Current perspectives. Front. Aging Neurosci. 12:9. doi: 10.3389/fnagi.2020.00009
Mann, R. S. (1990). Differential diagnosis and classification of apathy. Am. J. Psychiatry 147, 22–30.
Marin, R. S. (1991). Apathy: A neuropsychiatric syndrome. J. Neuropsychiatry Clin. Neurosci. 3, 243–254. doi: 10.1176/jnp.3.3.243
Marin, R. S., Biedrzycki, R. C., and Firinciogullari, S. (1991). Reliability and validity of the apathy evaluation scale. Psychiatry Res. 38, 143–162.
Marshall, G. A., Gatchel, J. R., Donovan, N. J., Muniz, M. C., Schultz, A. P., Becker, J. A., et al. (2019). Regional tau correlates of instrumental activities of daily living and apathy in mild cognitive impairment and Alzheimer’s disease dementia. J. Alzheimers Dis. 67, 757–768. doi: 10.3233/JAD-170578
Marshall, G. A., Monserratt, L., Harwood, D., Mandelkern, M., Cummings, J. L., and Sultzer, D. L. (2007). Positron emission tomography metabolic correlates of apathy in Alzheimer disease. Arch. Neurol. 64, 1015–1020.
Martinez-Horta, S., Sampedro, F., Pagonabarraga, J., Fernandez-Bobadilla, R., Marin-Lahoz, J., Riba, J., et al. (2017). Non-demented Parkinson’s disease patients with apathy show decreased grey matter volume in key executive and reward-related nodes. Brain Imaging Behav. 11, 1334–1342. doi: 10.1007/s11682-016-9607-5
Massimo, L., Powers, C., Moore, P., Vesely, L., Avants, B., Gee, J., et al. (2009). Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dement. Geriatr. Cogn. Disord. 27, 96–104. doi: 10.1159/000194658
Mesulam, M. M. (2000). Principles of behavioral and cognitive neurology. Oxford: Oxford University Press.
Migneco, O., Benoit, M., Koulibaly, P. M., Dygai, I., Bertogliati, C., Desvignes, P., et al. (2001). Perfusion brain SPECT and statistical parametric mapping analysis indicate that apathy is a cingulate syndrome: A study in Alzheimer’s disease and nondemented patients. Neuroimage 13, 896–902. doi: 10.1006/nimg.2000.0741
Miller, D. S., Robert, P., Ereshefsky, L., Adler, L., Bateman, D., Cummings, J., et al. (2021). Diagnostic criteria for apathy in neurocognitive disorders. Alzheimers Dement. 17, 1892–1904.
Moayedi, M., Salomons, T. V., Dunlop, K. A. M., Downar, J., and Davis, K. D. (2015). Connectivity-based parcellation of the human frontal polar cortex. Brain Struct. Funct. 220, 2603–2616. doi: 10.1007/s00429-014-0809-6
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Prisma Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 151, 264–269.
Moon, Y., Moon, W. J., Kim, H., and Han, S. H. (2014). Regional atrophy of the insular cortex is associated with neuropsychiatric symptoms in Alzheimer’s disease patients. Eur. Neurol. 71, 223–229.
Mori, T., Shimada, H., Shinotoh, H., Hirano, S., Eguchi, Y., Yamada, M., et al. (2014). Apathy correlates with prefrontal amyloid β deposition in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 85, 449–455.
Mortby, M. E., Adler, L., Agüera-Ortiz, L., Bateman, D. R., Brodaty, H., Cantillon, M., et al. (2022). Apathy as a treatment target in Alzheimer’s disease: Implications for clinical trials. Am. J. Geriatr. Psychiatry 30, 119–147. doi: 10.1016/j.jagp.2021.06.016
Nour, M., Jiao, Y., and Teng, G. J. (2021). Neuroanatomical associations of depression, anxiety and apathy neuropsychiatric symptoms in patients with Alzheimer’s disease. Acta Neurol. Belg. 121, 1469–1480. doi: 10.1007/s13760-020-01349-8
Onyike, C. U., Sheppard, J. M. E., Tschanz, J. T., Norton, M. C., Green, R. C., Steinberg, M., et al. (2007). Epidemiology of apathy in older adults: The cache county study. Am. J. Geriatr. Psychiatry 15, 365–375.
Osoegawa, C., Gomes, J. S., Grigolon, R. B., Brietzke, E., Gadelha, A., Lacerda, A. L., et al. (2018). Non-invasive brain stimulation for negative symptoms in schizophrenia: An updated systematic review and meta-analysis. Schizoph. Res. 197, 34–44. doi: 10.1016/j.schres.2018.01.010
Palmer, K., Di Iulio, F., Varsi, A. E., Gianni, W., Sancesario, G., Caltagirone, C., et al. (2010). Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: The role of depression and apathy. J. Alzheimers Dis. 20, 175–183. doi: 10.3233/JAD-2010-1352
Paulus, W. (2011). Transcranial electrical stimulation (tES–tDCS; tRNS, tACS) methods. Neuropsychol. Rehabil. 21, 602–617. doi: 10.1080/09602011.2011.557292
Peters, F., Perani, D., Herholz, K., Holthoff, V., Beuthien-Baumann, B., Sorbi, S., et al. (2006). Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dement. Geriatr. Cogn. Disord. 21, 373–379.
Powers, J. P., Massimo, L., McMillan, C. T., Yushkevich, P. A., Zhang, H., Gee, J. C., et al. (2014). White matter disease contributes to apathy and disinhibition in behavioral variant frontotemporal dementia. Cogn. Behav. Neurol. 27:206. doi: 10.1097/WNN.0000000000000044
Prange, S., Metereau, E., Maillet, A., Lhommée, E., Klinger, H., Pelissier, P., et al. (2019). Early limbic microstructural alterations in apathy and depression in de novo Parkinson’s disease. Mov. Disord. 34, 1644–1654. doi: 10.1002/mds.27793
Raimo, S., Santangelo, G., D’Iorio, A., Trojano, L., and Grossi, D. (2019). Neural correlates of apathy in patients with neurodegenerative disorders: An activation likelihood estimation (ALE) meta-analysis. Brain Imaging Behav. 13, 1815–1834. doi: 10.1007/s11682-018-9959-0
Ray, K. L., Zald, D. H., Bludau, S., Riedel, M. C., Bzdok, D., Yanes, J., et al. (2015). Co-activation based parcellation of the human frontal pole. Neuroimage 123, 200–211. doi: 10.1016/j.neuroimage.2015.07.072
Reijnders, J. S., Scholtissen, B., Weber, W. E., Aalten, P., Verhey, F. R., and Leentjens, A. F. (2010). Neuroanatomical correlates of apathy in Parkinson’s disease: A magnetic resonance imaging study using voxel-based morphometry. Mov. Disord. 25, 2318–2325.
Reis, J., Robertson, E., Krakauer, J. W., Rothwell, J., Marshall, L., Gerloff, C., et al. (2008). Consensus: Can tDCS and TMS enhance motor learning and memory formation? Brain Stimul. 1, 363–369. doi: 10.1016/j.brs.2008.08.001
Richard, E., Schmand, B., Eikelenboom, P., Yang, S. C., Ligthart, S. A., Van Charante, E. M., et al. (2012). Symptoms of apathy are associated with progression from mild cognitive impairment to Alzheimer’s disease in non-depressed subjects. Dement. Geriatr. Cogn. Disord. 33, 204–209. doi: 10.1159/000338239
Robert, P. H., Berr, C., Volteau, M., Bertogliati, C., Benoit, M., Sarazin, M., et al. (2006). Apathy in patients with mild cognitive impairment and the risk of developing dementia of Alzheimer’s disease: A one-year follow-up study. Clin. Neurol. Neurosurg. 108, 733–736. doi: 10.1016/j.clineuro.2006.02.003
Rosen, H. J., Allison, S. C., Schauer, G. F., Gorno-Tempini, M. L., Weiner, M. W., and Miller, B. L. (2005). Neuroanatomical correlates of behavioural disorders in dementia. Brain 128, 2612–2625.
Roth, R. M., Flashman, L. A., Saykin, A. J., McAllister, T. W., and Vidaver, R. (2004). Apathy in schizophrenia: Reduced frontal lobe volume and neuropsychological deficits. Am. J. Psychiatry 161, 157–159. doi: 10.1176/appi.ajp.161.1.157
Sachdev, P. S., Lipnicki, D. M., Kochan, N. A., Crawford, J. D., Thalamuthu, A., Andrews, G., et al. (2015). The prevalence of mild cognitive impairment in diverse geographical and ethnocultural regions: The COSMIC collaboration. PLoS One 10:e0142388. doi: 10.1371/journal.pone.0142388
Shen, Y. T., Li, J. Y., Yuan, Y. S., Wang, X. X., Wang, M., Wang, J. W., et al. (2018). Disrupted amplitude of low-frequency fluctuations and causal connectivity in Parkinson’s disease with apathy. Neurosci. Lett. 683, 75–81. doi: 10.1016/j.neulet.2018.06.043
Shin, J. H., Shin, S. A., Lee, J. Y., Nam, H., Lim, J. S., and Kim, Y. K. (2017). Precuneus degeneration and isolated apathy in patients with Parkinson’s disease. Neurosci. Lett. 653, 250–257. doi: 10.1016/j.neulet.2017.05.061
Skidmore, E. R., Whyte, E. M., Butters, M. A., Terhorst, L., and Reynolds, I. C. F. (2015). Strategy training during inpatient rehabilitation may prevent apathy symptoms after acute stroke. PMR 7, 562–570. doi: 10.1016/j.pmrj.2014.12.010
Skidmore, F. M., Yang, M., Baxter, L., Von Deneen, K., Collingwood, J., He, G., et al. (2013). Apathy, depression, and motor symptoms have distinct and separable resting activity patterns in idiopathic Parkinson disease. Neuroimage 81, 484–495. doi: 10.1016/j.neuroimage.2011.07.012
Stanton, B. R., Leigh, P. N., Howard, R. J., Barker, G. J., and Brown, R. G. (2013). Behavioural and emotional symptoms of apathy are associated with distinct patterns of brain atrophy in neurodegenerative disorders. J. Neurol. 260, 2481–2490.
Starkstein, S. E., Mayberg, H. S., Preziosi, T., Andrezejewski, P., Leiguarda, R., and Robinson, R. G. (1992). Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 4, 134–139.
Steffens, D. C., Fahed, M., Manning, K. J., and Wang, L. (2022). The neurobiology of apathy in depression and neurocognitive impairment in older adults: A review of epidemiological, clinical, neuropsychological and biological research. Transl. Psychiatry 12, 1–16. doi: 10.1038/s41398-022-02292-3
Suemoto, C. K., Apolinario, D., Nakamura-Palacios, E. M., Lopes, L., Leite, R. E. P., Sales, M. C., et al. (2014). Effects of a non-focal plasticity protocol on apathy in moderate Alzheimer’s disease: A randomized, double-blind, sham-controlled trial. Brain Stimul. 7, 308–313. doi: 10.1016/j.brs.2013.10.003
Sun, H. H., Hu, J. B., Chen, J., Wang, X. Y., Wang, X. L., Pan, P. L., et al. (2020a). Abnormal spontaneous neural activity in Parkinson’s disease with “pure” apathy. Front. Neurosci. 14:830. doi: 10.3389/fnins.2020.00830
Sun, H. H., Pan, P. L., Hu, J. B., Chen, J., Wang, X. Y., and Liu, C. F. (2020b). Alterations of regional homogeneity in Parkinson’s Disease with “pure” apathy: A resting-state fMRI study. J. Affect. Disord. 274, 792–798. doi: 10.1016/j.jad.2020.05.145
Tekin, S., and Cummings, J. L. (2002). Frontal–subcortical neuronal circuits and clinical neuropsychiatry: An update. J. Psychos. Res. 53, 647–654. doi: 10.1016/s0022-3999(02)00428-2
Terada, T., Miyata, J., Obi, T., Kubota, M., Yoshizumi, M., and Murai, T. (2018). Reduced gray matter volume is correlated with frontal cognitive and behavioral impairments in Parkinson’s disease. J. Neurol. Sci. 390, 231–238.
Tighe, S. K., Oishi, K., Mori, S., Smith, G. S., Albert, M., Lyketsos, C. G., et al. (2012). Diffusion tensor imaging of neuropsychiatric symptoms in mild cognitive impairment and Alzheimer’s dementia. J. Neuropsychiatry Clin. Neurosci. 24, 484–488.
Tunnard, C., Whitehead, D., Hurt, C., Wahlund, L. O., Mecocci, P., Tsolaki, M., et al. (2011). Apathy and cortical atrophy in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 26, 741–748.
Turkeltaub, P. E., Eden, G. F., Jones, K. M., and Zeffiro, T. A. (2002). Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. Neuroimage 16, 765–780.
Turkeltaub, P. E., Eickhoff, S. B., Laird, A. R., Fox, M., Wiener, M., and Fox, P. (2012). Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 33, 1–13. doi: 10.1002/hbm.21186
van Dalen, J. W., Van Wanrooij, L. L., van Charante, E. P. M., Richard, E., and van Gool, W. A. (2018a). Apathy is associated with incident dementia in community-dwelling older people. Neurology 90, e82–e89.
van Dalen, J. W., van Wanrooij, L. L., van Charante, E. P. M., Brayne, C., van Gool, W. A., and Richard, E. (2018b). Association of apathy with risk of incident dementia: A systematic review and meta-analysis. JAMA Psychiatry 75, 1012–1021.
Waltz, J. A., Kasanova, Z., Ross, T. J., Salmeron, B. J., McMahon, R. P., Gold, J. M., et al. (2013). The roles of reward, default, and executive control networks in set-shifting impairments in schizophrenia. PLoS One 8:e57257. doi: 10.1371/journal.pone.0057257
Wei, G., Irish, M., Hodges, J. R., Piguet, O., and Kumfor, F. (2020). Disease-specific profiles of apathy in Alzheimer’s disease and behavioural-variant frontotemporal dementia differ across the disease course. J. Neurol. 267, 1086–1096. doi: 10.1007/s00415-019-09679-1
Yan, H., Onoda, K., and Yamaguchi, S. (2015). Gray matter volume changes in the apathetic elderly. Front. Hum. Neurosci. 9:318. doi: 10.3389/fnhum.2015.00318
Yuen, G. S., Gunning-Dixon, F. M., Hoptman, M. J., AbdelMalak, B., McGovern, A. R., Seirup, J. K., et al. (2014). The salience network in the apathy of late-life depression. Int. J. Geriatr. Psychiatry 29, 1116–1124.
Zamboni, G., Huey, E. D., Krueger, F., Nichelli, P. F., and Grafman, J. (2008). Apathy and disinhibition in frontotemporal dementia: Insights into their neural correlates. Neurology 71, 736–742.
Zhang, X., Liu, B., Li, N., Li, Y., Hou, J., Duan, G., et al. (2020). Transcranial direct current stimulation over prefrontal areas improves psychomotor inhibition state in patients with traumatic brain injury: A pilot study. Front. Neurosci. 14:386. doi: 10.3389/fnins.2020.00386
Keywords: apathy, meta-analysis, mental disorder, neurodegenerative disorder, traumatic brain injury, normal cognitive aging
Citation: Yan H, Wu H, Cai Z, Du S, Li L, Xu B, Chang C and Wang N (2023) The neural correlates of apathy in the context of aging and brain disorders: a meta-analysis of neuroimaging studies. Front. Aging Neurosci. 15:1181558. doi: 10.3389/fnagi.2023.1181558
Received: 07 March 2023; Accepted: 05 June 2023;
Published: 16 June 2023.
Edited by:
Amer M. Burhan, Ontario Shores Centre for Mental Health Sciences, CanadaReviewed by:
Corinne E. Fischer, St. Michael’s Hospital, CanadaShankar Tumati, University of Toronto, Canada
Copyright © 2023 Yan, Wu, Cai, Du, Li, Xu, Chang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nizhuan Wang, d2FuZ25pemh1YW4xMTIwQGdtYWlsLmNvbQ==; Chunqi Chang, Y3FjaGFuZ0BzenUuZWR1LmNu; Bingchao Xu, YmluZ2N4QGhvdG1haWwuY29t; Hongjie Yan, eWFuaGpuc0BnbWFpbC5jb20=
†These authors have contributed equally to this work
 Hongjie Yan
Hongjie Yan Huijun Wu
Huijun Wu Zenglin Cai
Zenglin Cai Shouyun Du
Shouyun Du Lejun Li
Lejun Li Bingchao Xu1*
Bingchao Xu1* Chunqi Chang
Chunqi Chang Nizhuan Wang
Nizhuan Wang