- 1Shanghai Frontiers Science Center for Chinese Medicine Chemical Biology, Institute of Interdisciplinary Integrative Medicine Research, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Department of Biomedical Sciences, University of Sassari, Sassari, Italy
Background: Neuroinflammation is one of the key factors leading to neuron death and synapse dysfunction in Alzheimer's disease (AD). Amyloid-β (Aβ) is thought to have an association with microglia activation and trigger neuroinflammation in AD. However, inflammation response in brain disorders is heterogenous, and thus, it is necessary to unveil the specific gene module of neuroinflammation caused by Aβ in AD, which might provide novel biomarkers for AD diagnosis and help understand the mechanism of the disease.
Methods: Transcriptomic datasets of brain region tissues from AD patients and the corresponding normal tissues were first used to identify gene modules through the weighted gene co-expression network analysis (WGCNA) method. Then, key modules highly associated with Aβ accumulation and neuroinflammatory response were pinpointed by combining module expression score and functional information. Meanwhile, the relationship of the Aβ-associated module to the neuron and microglia was explored based on snRNA-seq data. Afterward, transcription factor (TF) enrichment and the SCENIC analysis were performed on the Aβ-associated module to discover the related upstream regulators, and then a PPI network proximity method was employed to repurpose the potential approved drugs for AD.
Results: A total of 16 co-expression modules were primarily obtained by the WGCNA method. Among them, the green module was significantly correlated with Aβ accumulation, and its function was mainly involved in neuroinflammation response and neuron death. Thus, the module was termed the amyloid-β induced neuroinflammation module (AIM). Moreover, the module was negatively correlated with neuron percentage and showed a close association with inflammatory microglia. Finally, based on the module, several important TFs were recognized as potential diagnostic biomarkers for AD, and then 20 possible drugs including ibrutinib and ponatinib were picked out for the disease.
Conclusion: In this study, a specific gene module, termed AIM, was identified as a key sub-network of Aβ accumulation and neuroinflammation in AD. Moreover, the module was verified as having an association with neuron degeneration and inflammatory microglia transformation. Moreover, some promising TFs and potential repurposing drugs were presented for AD based on the module. The findings of the study shed new light on the mechanistic investigation of AD and might make benefits the treatment of the disease.
1. Introduction
Alzheimer's disease (AD) is a complex brain disorder that can explain nearly 60%−70% of worldwide dementia (Holtzman et al., 2011). With increasing prevalence and lacking effective treatment, more than 150 million people are estimated to be affected by AD in 2050 (GBD 2019 Dementia Forecasting Collaborators, 2022). Traditional drug research mostly focused on amyloid-β (Aβ), one extracellular hallmark of AD, but all failed except aducanumab that is approved by FDA in 2021, which is still controversial (Doig et al., 2017; Karran and De Strooper, 2022). On the other hand, neuroinflammation has been acknowledged as another important indication of events of AD development. A reduced AD risk is observed in clinics when anti-inflammatory drugs were used in some epidemiological research (Akiyama et al., 2000). As an inflammation response event in the brain of AD patients, Aβ accumulation was thought to be the key trigger of the disease. Some relevant targets and possible drugs have been presented but most of them do not gain the expected results in clinics due to the poor understanding of neuroinflammation caused by Aβ accumulation (Miguel-Álvarez et al., 2015; Fu et al., 2019; Dhapola et al., 2021). Therefore, it is necessary to systematically investigate the underlying network of neuroinflammation induced by Aβ which could facilitate to deeply comprehend the AD pathological mechanism and find a possible therapeutic approach.
Weighted gene co-expression network analysis (WGCNA) is an effective method to infer the trait-specific functional gene regulatory network (GRN) based on transcriptomic data (Langfelder and Horvath, 2008). For example, Feng et al. (2022) identified a cancer-associated fibroblast (CAF)-related module for ovarian cancer through the method. Lin et al. (2021) investigated the calcium signaling pathway-related GRN in ischemic stroke using the method. Additionally, the technology of single-cell RNA sequencing (scRNA-seq) has been developed in recent years to obtain the transcriptomic characteristics of individual cells in a tissue, which greatly contributes to discovering the key cell populations in a specific biological state (Kolodziejczyk et al., 2015; Andrews et al., 2021). For example, Obradovic et al. (2021) explored the cellular atlas of tumor microenvironment in clear cell renal carcinoma and identified the infiltrating macrophage subtype by comparing scRNA-seq data of the tumor tissue and the corresponding adjacent normal tissue. Derived analytic strategies such as cell communication analysis and master regulon inference will be of great benefit to understanding the roles of specific cell populations in a certain disease (Jin et al., 2021; Kumar et al., 2021). Compared to scRNA-seq, single-nucleus RNA-seq (snRNA-seq) is more suitable for frozen or hard-to-dissociate samples, especially brain tissues (Lake et al., 2016). In addition, spatial transcriptome (ST) provides precise transcriptomic heterogeneity of adjacent small spots in tissue (Longo et al., 2021). In this study, module identifying and drug repositioning analysis were conducted to elucidate the underlying mechanism of AD and find potential repurposing drugs for the disease. As the study flowchart showed (Figure 1), we first constructed the gene regulatory networks based on the transcriptomic data of AD patients and normal controls through the WGCNA method. Then, an Aβ-induced neuroinflammation module for AD was picked out based on the expression score and functional information. Afterward, the module was correlated with the neuron and distinct subtype of microglia to reveal its possible roles in the process of neuroinflammation development. Finally, several transcription factors regulating the module were presented as biomarkers for AD diagnosis, and 20 repurposing drugs against AD neuroinflammation were provided.
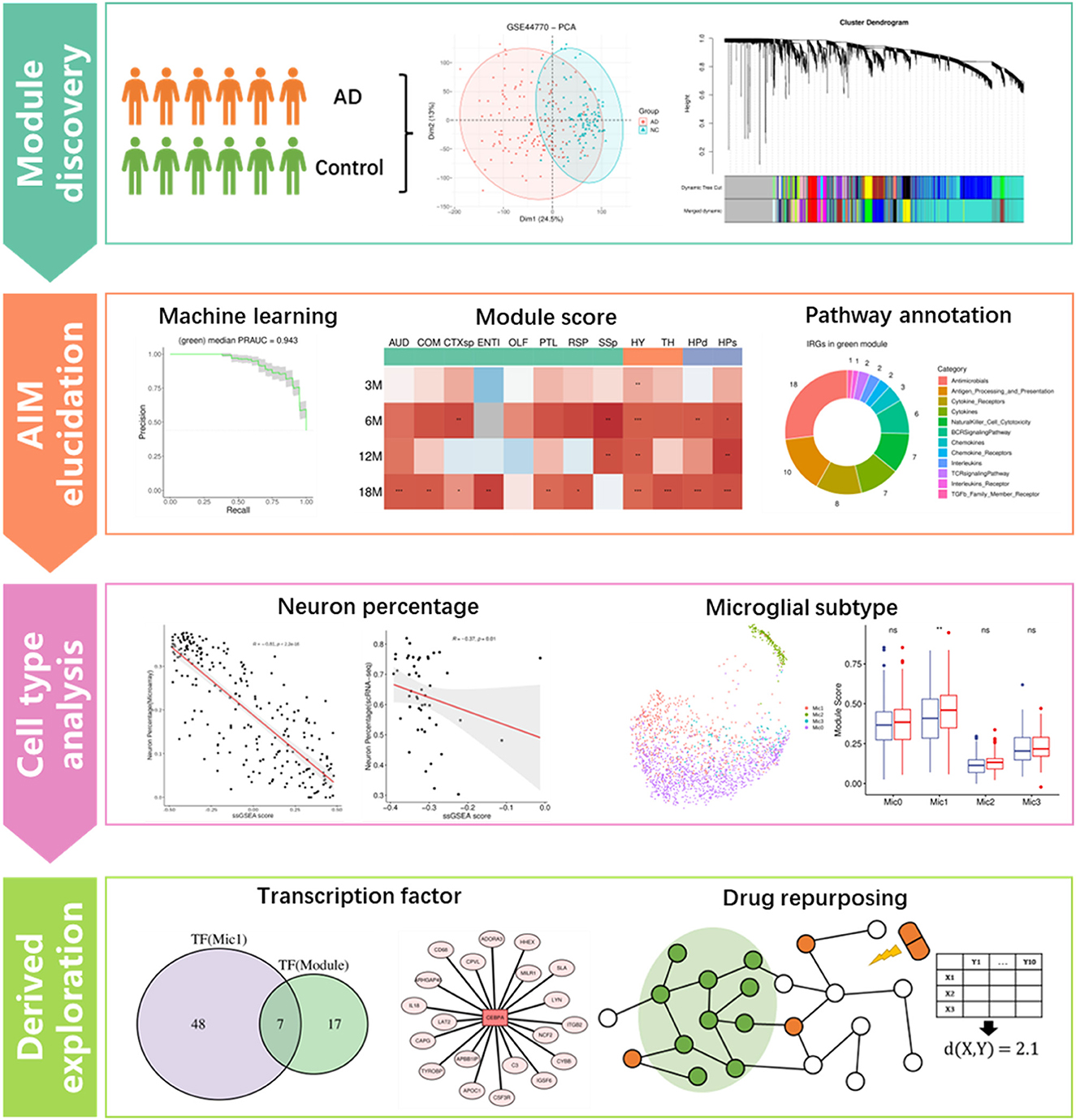
Figure 1. Flowchart of this study, which includes four main analysis steps. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
2. Materials and methods
2.1. Public transcriptomic data collection
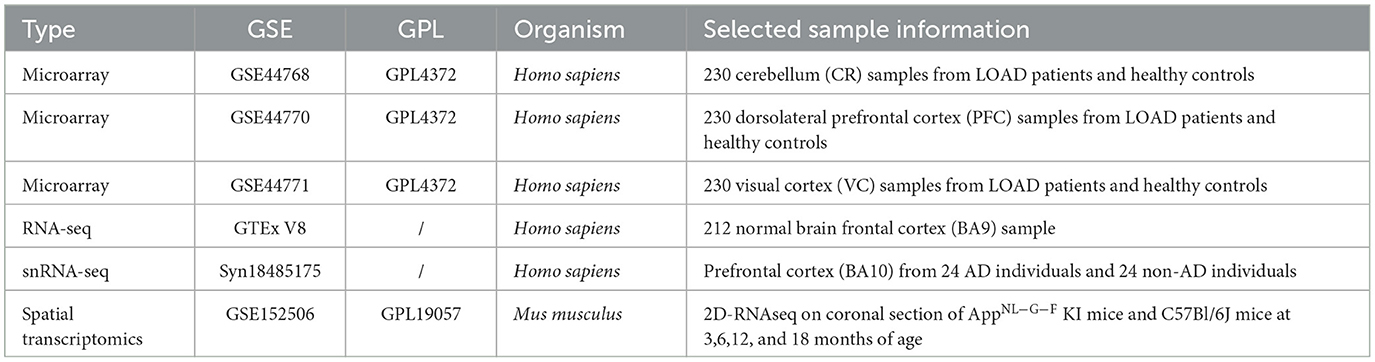
AD-related transcriptomic data (detailed information is shown in Table 1) were mainly downloaded from the Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo) database. In practice, three microarrays (GSE44768, GSE44770, and GSE44771) datasets of the cerebellum (CR), dorsolateral prefrontal cortex (PFC), and visual cortex (VC) were collected, which included 129 late-onset Alzheimer's disease (LOAD) patients and 101 healthy controls. The probe ID was transformed into a gene symbol based on GPL4372 platform annotation information. Moreover, RNA-seq data with TPM normalized of the frontal cortex (BA9) from 120 normal brain samples (age >60) were downloaded from the Genotype-Tissue Expression project (GTEx, https://gtexportal.org/home/datasets). In addition, we obtained spatial transcriptomic data on 12 anatomical brain regions of AppNL−G−F KI and C57Bl/6J mice in 3, 6, 12, and 18 months (GSE152506), where each spot covered tissues with a diameter of 100 μm. Noteworthily, the Aβ plaque condition of these spots was inferred according to immunostaining assays of adjacent brain sections. Furthermore, we collected snRNA-seq data of the PFC region (BA10, containing 24 AD samples and 24 control samples) from the Synapse database (syn18485175).
2.2. Weighted gene co-expression network analysis
Principal component analysis (PCA) was performed to check the difference between the AD and control group samples. Then, the weighted gene co-expression network analysis (WGCNA) was applied according to the recommended pipeline (https://horvath.genetics.ucla.edu/html/CoexpressionNetwork/Rpackages/WGCNA/Tutorials/index.html, WGCNA package of R software, version 1.71). In practice, genes with higher standard deviation among all samples were first filtered. Then, we removed distinct outlier samples by the “cutreeStatic” function, and the threshold of the scale-free topology index was set to 0.9. Afterward, the co-expression modules were identified by the “blockwiseModules” function, where the default parameters were used except for setting “corType” to “bicor” and “mergeCutHeight” to 0.1. Next, we calculated the correlation between each module and sample trait (AD or control) based on module eigengenes.
2.3. Module hub gene identification and logistic regression analysis
The unassigned gray module and modules with a large size (over 1,000 genes) were first dropped from the WGCNA result. Then, for the genes of the remaining modules, their module membership (known as kME) was evaluated by calculating the expression correlation with module eigengenes. The higher kME value (0–1) for a gene module pair means the gene is an important (central) element in the module. Therefore, the top 10 genes with the highest kME of each module were marked as hub genes.
For logical regression analysis, we used module hub gene expression as features and sample grouping as a binary indicator variable. AD patients were labeled “1” and healthy controls were labeled “0.” The R package mlr3 (version 0.13.4) was applied to build the logical regression model. The metric of Area Under the Precision-Recall Curve (AUPRC) was used for robust evaluation, and the median of three replicates of five-fold cross-validation (CV) was calculated to compare the performance of different models.
2.4. Calculation of module expression score
For microarray or RNA-seq datasets, the single-sample Gene Set Enrichment Analysis (ssGSEA) was performed to obtain the relative expression intensity of target modules. In practice, the “gsva” function (“ssgsea” method) of the GSVA package (version 1.42.0) was utilized to calculate the module score based on the expression matrix. For microglia cells in snRNA-seq data and spatial transcriptome datasets, expression count matrixes were first loaded into the Seurat package (version 4.1.1) and then normalized mainly for the library-size effect. Next, the “AddModuleScore” function was applied to calculate the expression score of modules for each cell or spot. As for each sample of the snRNA-seq data, their expression count matrixes were summarized to pseudo-bulk RNA-seq expression matrixes, and then the GSVA package was applied to calculate the overall expression score for the target module.
2.5. Functional annotation for genes of target modules
Seventeen immune-related gene (IRG) lists including 1,793 genes were first downloaded from the ImmPort website (https://www.immport.org/resources). Meanwhile, three classic pathway sets [including Gene Ontology Biological Process (GOBP), Kyoto Encyclopedia of Genes and Genomes (KEGG), and Reactome Pathway] were collected from the MsigDB database (https://www.gsea-msigdb.org/gsea/msigdb/). Then, the clusterProfiler package (version 4.2.2) was utilized to perform functional enrichment analysis based on pathway sets for genes of target modules, where the adjusted p-value threshold was set to 0.05. Additionally, human protein–protein interaction (PPI) information was extracted from the STRING database (https://string-db.org), where the combined score threshold was set to 600 to obtain credible interactions.
2.6. Cell-type enrichment analysis
The xCell package (version 1.1.0) was utilized to perform the cell-type enrichment analysis and predict neuron percentage for each sample of the microarray dataset. Then, the Spearman correlation coefficient between neuron percentage and the module score or expression of hub genes was calculated.
2.7. snRNA-seq data preprocessing and analysis
The snRNA-seq data were processed with the two-dimensional Uniform Manifold Approximation and Projection (UMAP) method implemented in the Seurat package (version 4.1.1) of R software to display cell types. Then, cell types were annotated based on the description of the original manuscript (Mathys et al., 2019), which included eight cell types, i.e., excitatory neurons (Ex), inhibitory neurons (In), microglia (Mic), astrocytes (Ast), oligodendrocytes (Oli), oligodendrocyte progenitors (Opc), endothelial (End), and pericytes (Per). Among them, microglia were further divided into four subtypes, namely, Mic0, Mic1, Mic2, and Mic3. Subsequently, for each sample of the snRNA-seq dataset, the correlation between neuron percentage and the module score was calculated. Then, the module score of cell types and microglia subtypes was compared between AD and control samples. Moreover, marker genes (average log2FC >0.5, adjusted p-value <0.05) and related biological pathways were identified for each microglia subtype. In practice, Fisher's exact test was performed to explore the link between microglia subtype and module genes. Additionally, pseudo-time trajectory among microglia subtypes was inferred using the monocle package (version 2.26.0). Finally, master regulons of microglia subtypes were inferred through the pySCENIC software (version 0.11.2). In detail, the network inference and motif enrichment were first performed to obtain potential regulons and target genes and then the expression activity of each regulon was evaluated through the Aucell function of the software.
2.8. Transcription factor enrichment analysis
A web-based tool, ChEA3 (https://maayanlab.cloud/chea3), was used to infer the relevant regulatory TFs for interested module genes, where the ReMap library was selected as the reference and Fisher's exact test was utilized as the statistical method.
2.9. Information collection for drugs and targets
Approved small molecular drugs were acquired from the TTD website (http://db.idrblab.net/ttd). Protein targets of these drugs were retrieved from the ChEMBL database (https://www.ebi.ac.uk/chembl). When a target's pChEMBL value was over 6, it was considered an effective one. Compound ID transformation between PubChem CID and ChEMBL ID was done through the PubChem Identifier Exchange Service (https://pubchem.ncbi.nlm.nih.gov/idexchange/idexchange.cgi).
2.10. PPI network proximity calculation from drug targets to hub genes of the interested module
A refined network proximity calculation method was adopted to evaluate the relevance between drugs and the interested module according to a previous study (Cheng et al., 2019). Here, X denoted all targets of a drug, Y denoted hub genes of the interested module, and d(x, y) represented the shortest PPI distance from a specific drug target (x) to one hub gene (y) of the interested module, while D(X, Y) reflected the average PPI distance for a drug from its targets to the hub genes of the module. The smaller the value of D(X, Y), the bigger the probability of a drug effect on the module. To evaluate the significance of the D(X, Y) value, background distributions were generated by randomly selecting the same number of pseudo-targets 1,000 times for a specific drug. These distributions were validated to conform to the Gaussian characteristics and μ and σ represent the mean and standard deviation, respectively. Subsequently, the Z score and corresponding p-value were calculated for D(X, Y):
3. Results
3.1. WGCNA identified 16 modules and related hub genes from the PFC region
The PFC region expression data of 129 AD and 101 control brain samples from GSE44770 were used to construct the co-expression network. Based on probe annotation, an expression matrix comprising 230 samples and 19,870 genes was presented. The PCA result indicated that there was an obvious difference in the first principal component between groups (Figure 2A). Then, five outlier samples (over threshold 25 in hierarchical clustering) were dropped, and 5,000 highly variable genes were identified. We opted nine for the best soft-threshold leading to the eligible scale independence (Figure 2B). After the WGCNA analysis, the primary 18 modules were obtained (Figure 2C). Among these modules, the unassigned module (gray module) and module with too many genes (turquoise) were discarded, and thus, 16 modules were kept for further analysis. For these remainder modules, the top 10 hub genes for each module were identified subsequently according to strong eigengene-based connectivity (kME value; Supplementary Table 1).
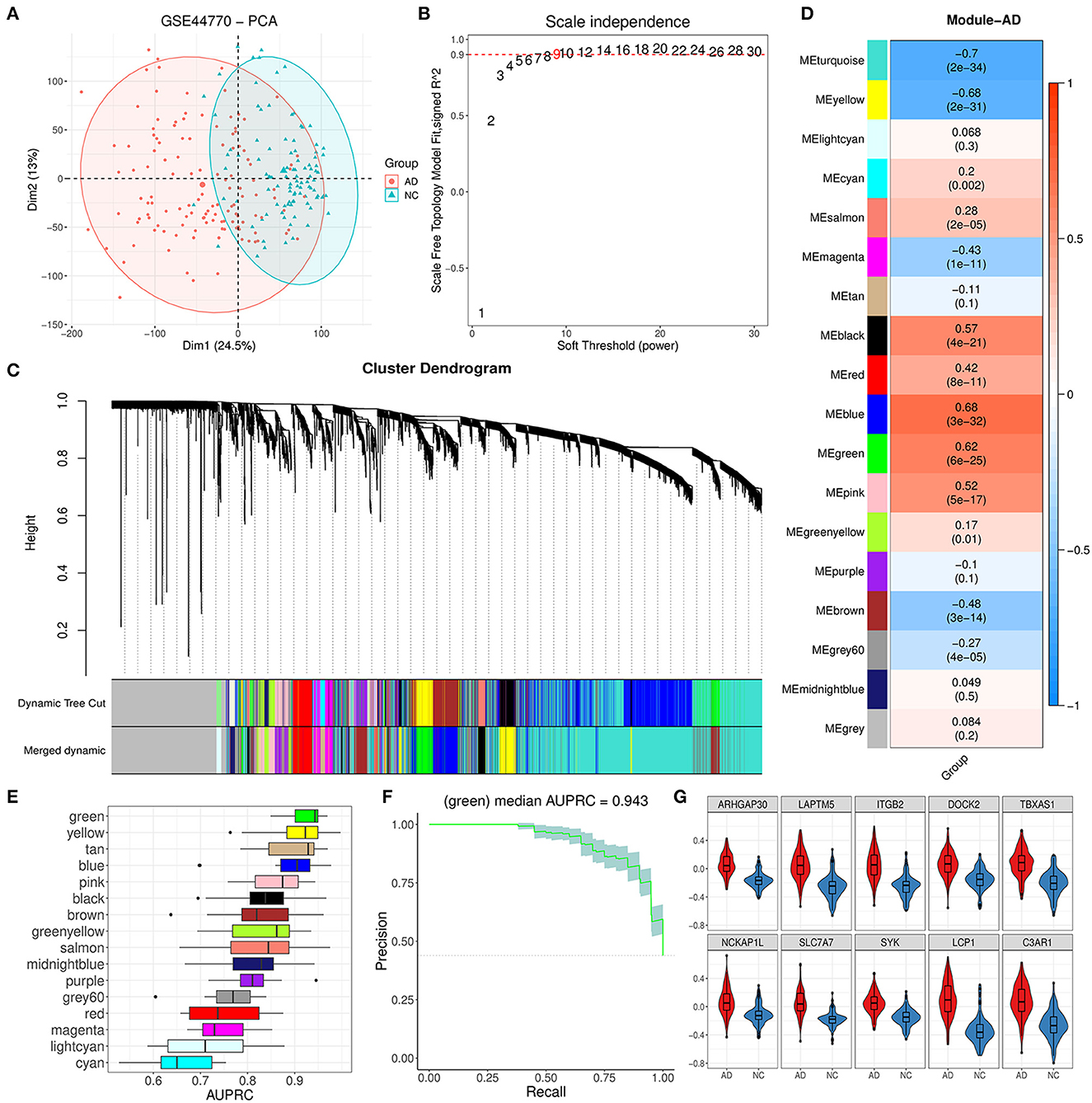
Figure 2. Module identification by WGCNA and primary exploration. (A) PCA dimension reduction plot for the difference between AD and NC samples in GSE44770. (B) Diagram displaying different soft-thresholding power and corresponding scale-free fit index. (C) Cluster dendrogram of genes with dissimilarity based on the topological overlap before and after merging. (D) Correlation heatmap between module eigengene and AD grouping. Each cell denoted the corresponding correlation and p-value for each module. (E) AUPRC distribution of cross-validation (CV) for 16 valid modules. (F) Precision–recall curve for the green module. The gray area represented the confidence interval of the CV. (G) Expression difference for 10 hub genes of the green module between AD and NC.
3.2. Trait analysis found that the green module was abnormal in AD patients
As Figure 2D showed, the module eigengene of blue, green, pink, and black had a significant positive correlation to AD while yellow and brown modules exhibited opposite characteristics. To further reveal the most relevant module to AD, we evaluated the AD discrimination capability for each module based on expression information of their hub genes via the logistic regression model. According to the results of AUPRC assays, we found that the green module was the best one for AD discrimination among 16 modules with a performance median of 0.943 (Figures 2E, F). Afterward, we inspected the expression of the top 10 hub genes (ARHGAP30, LAPTM5, ITGB2, DOCK2, TBXAS1, NCKAP1L, SLC7A7, SYK, LCP1, and C3AP1) of the module and found that they were all upregulated significantly (Figure 2G), comparing the AD group with the control group. These results implied that the green module could play a vital role in AD progression.
3.3. Module scoring revealed that the green module was correlated with Aβ accumulation
To further investigate the relationship between the green module with AD, we calculated the overall activity of the module and found that the ssGSEA score in AD was considerably higher than that of the control group not only in the PFC region but also in CR(GSE44768) and VC(GSE44771) regions (Figure 3A). These findings implied that the green module was activated sharply in multiple brain regions under AD conditions. Moreover, there was no obvious difference in the gender of the disease for the module (Figure 3B). Normal brain data from GTEx also confirmed that the green module was independent of gender (Figure 3B).
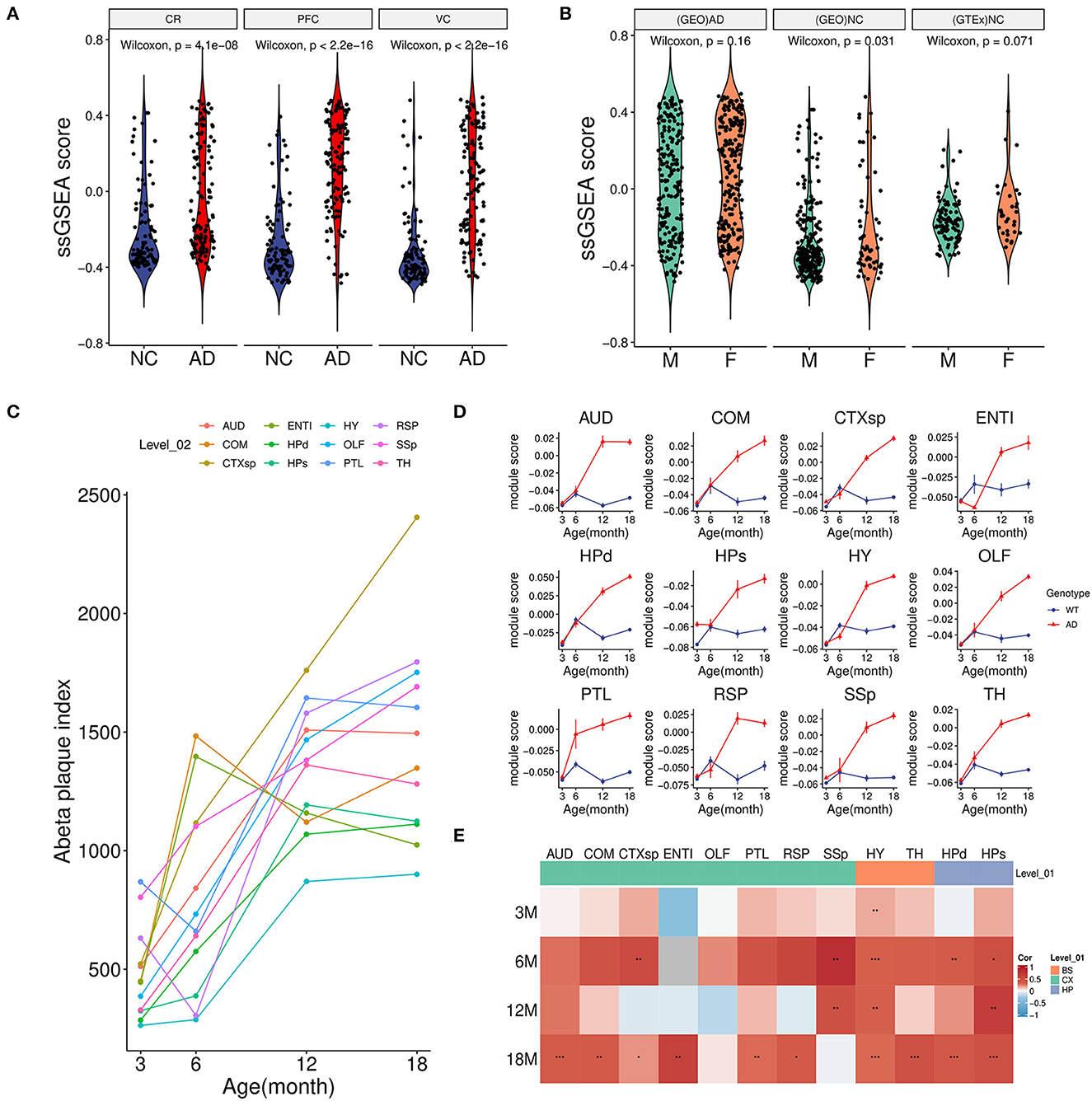
Figure 3. Association between module activity and Aβ. (A) Boxplot of the green module ssGSEA score difference between AD and NC among three brain regions. CR, cerebellum (GSE44768); PFC, dorsolateral prefrontal cortex (GSE44770); VC, visual cortex (GSE44771). (B) Boxplot of the green module ssGSEA score difference between male (M) and female (F) in different groups. (GEO)AD, all AD patients of above microarray data from GEO; (GEO)NC, all healthy controls of above microarray data from GEO; (GTEx)NC, normal brain samples from the GTEx project. (C) Line plot showed an average Aβ plaque index of different age stages in AD model mice among 12 brain regions. For detailed region information, refer the link (https://alzmap.org). (D) Line plots of the green module score difference at different age stages between the AD model and control mice among 12 brain regions. (E) Correlation heatmap of the Aβ plaque loading index and green module score between different age stages and brain regions in AD model mice. *p < 0.05, **p < 0.01, ***p < 0.001.
Combined with mice spatial transcriptome data (GSE152506), the correlation between the module and amyloid plaque was further explored. First of all, increasing amyloid deposition was observed in most brain regions of the AD model mice from 3 to 18 months (Figure 3C). Correspondingly, we found that the expression of the green module in the AD group showed a significantly increasing trend during the period (Figure 3D). However, these regions in the control group maintained relatively low expression activity. It was worth noting that the module score of AD and control groups was very similar in the early stage which implied that long-term and increasing-intensity stimulation from Aβ could be the main reason for the high expression of the module genes across many brain regions. Moreover, in the old model (18-month-old) mice of the AD group, a strongly positive correlation of module activity with the plaque index in most regions further verified that amyloid accumulation might be responsible for gene expression of the green module (Figure 3E).
3.4. Functional annotation showed that the green module was involved in neuroinflammation
To reveal the role of the green module in AD progression, functional annotation was conducted for the genes of the module. According to annotation results, 44 out of the 177 genes in the green module (Figure 4A, Supplementary Table 2) were immune-related genes, and they were involved in antimicrobe, antigen processing and presentation, cytokine signal transduction, and natural killer cell cytotoxicity (Figure 4B). Subsequently, we performed pathway enrichment analysis based on different pathway sets (Supplementary Table 3). Biological process analysis results showed that genes of the module were mainly associated with TNF superfamily cytokine production, toll-like receptor signaling, synapse pruning, neuroinflammation, and neuron death response (Figure 4C). The signal pathway detection result was focused on FC GAMMA-mediated phagocytosis, cell adhesion molecules CAMs, complement, and coagulation cascades (Figure 4D). Moreover, the significant pathway from Reactome showed that the module was related to interleukin signaling, DAP12 signaling, and ROS and RNS production (Figure 4E). Above enriched pathways implied that the gene overexpressed abnormally in the green module could activate unexpected immune responses in the brain and boost neuroinflammation, which was harmful to normal neurons and synapses. Therefore, the green module was termed the Aβ-induced neuroinflammation module (AIM) based on the above analysis results.
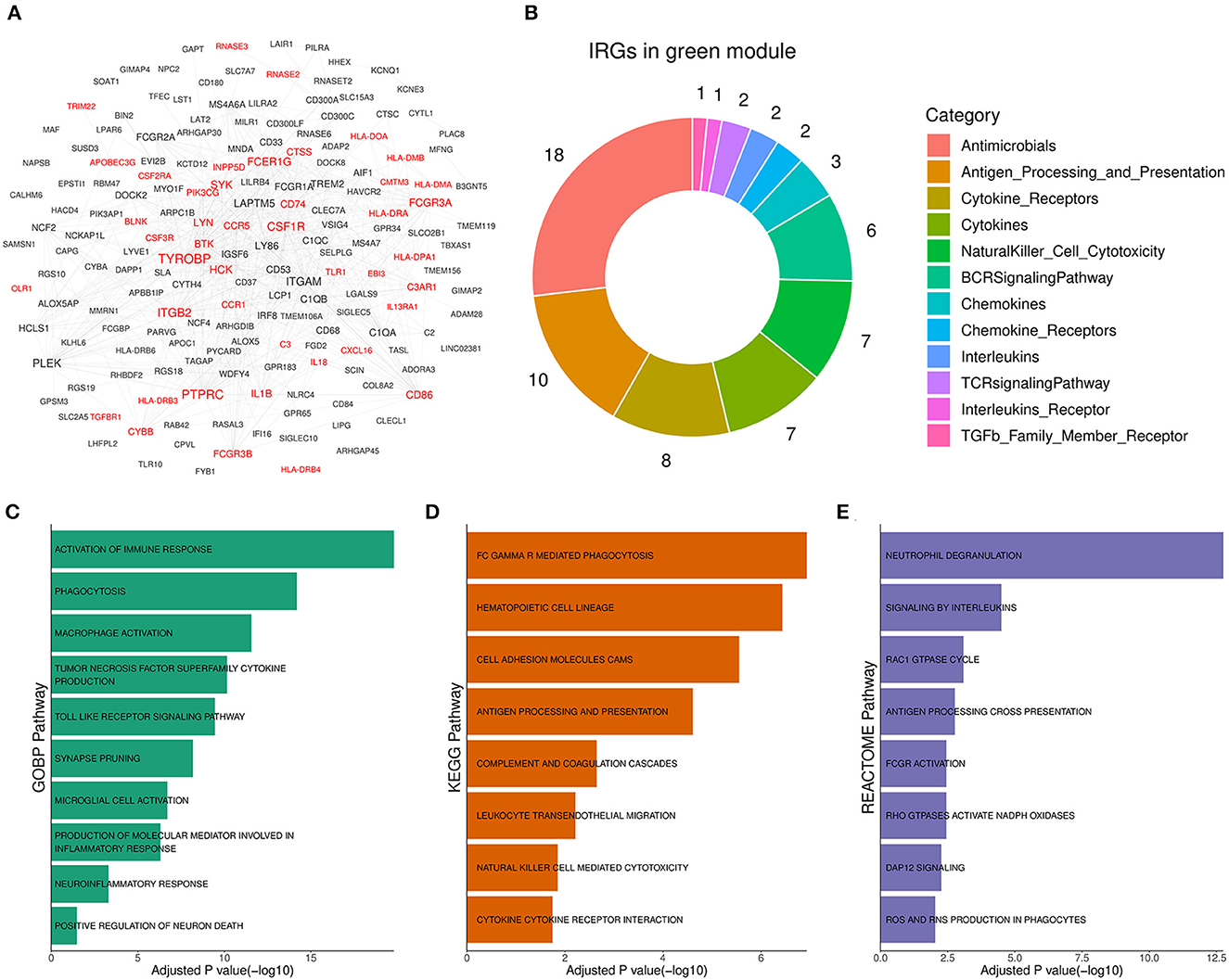
Figure 4. Functional annotation for the green module. (A) Diagram for the PPI network of the green module. Labels with red color represented immune-related genes (IRGs). A combined score above 600 was considered as a reliable PPI link. (B) Donut chart of the immune categories that IRGs in the green module involved. Enrichment analysis for green module genes using (C) GOBP, (D) KEGG, and (E) Reactome pathway set, respectively. The threshold of BH adjusted p-value was set to 0.05.
3.5. Correlation analysis showed neuron and microglia was associated with the AIM
For each sample of the microarray dataset, neuron percentage was first predicted, and then its correlation with the AIM score was calculated. Calculation results showed that there existed a significantly negative correlation between neuron percentage and AIM score (R = −0.81, p < 0.05, Figure 5A). Furthermore, correlation coefficients of the top 10 hub genes of AIM were calculated, respectively, and they were all below −0.6 (Figure 5B). While for samples of the snRNA-seq dataset, a negative correlation with the AIM score was also observed in the overall neuron (Figure 5C), excitatory neuron (Figure 5D), and inhibitory neuron (Figure 5E). These results validated that AIM upregulation could be responsible for neuron degeneration in AD. In order to understand the potential mechanism, we investigated the association between AIM and other cell types. A remarkable AIM score was exclusively observed in microglia (Figures 6A, B). A significantly increased AIM score was identified for the AD group as compared to the control group in oligodendrocytes, microglia, and astrocytes (Figure 6C). These results suggested that AIM was associated with neuron reduction and microglia transition.
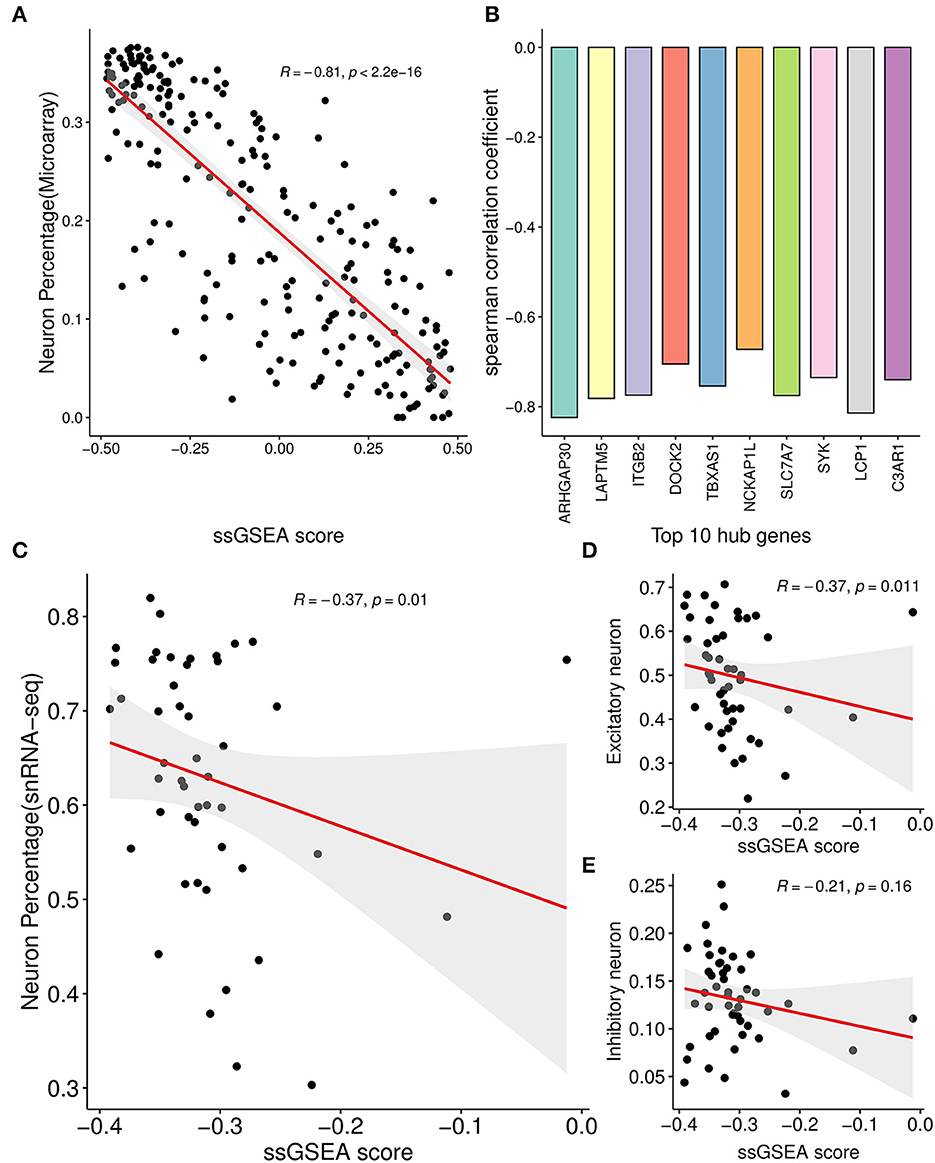
Figure 5. Correlation analysis between AIM and neuron percentage. (A) Scatter plot of the relationship between the module ssGSEA score and neuron percentage predicted by the xCell algorithm in GSE44770 microarray samples. R represented the Spearman correlation coefficient. (B) Bar plot showing the Spearman correlation coefficients between 10 module hub genes and neuron percentage predicted by the xCell algorithm in GSE44770 microarray data. (C) Scatter plot of the relationship between the module ssGSEA score and neuron percentage in syn18485175 snRNA-seq samples. (D, E) were similar and based on excitatory and inhibitory neurons, respectively.
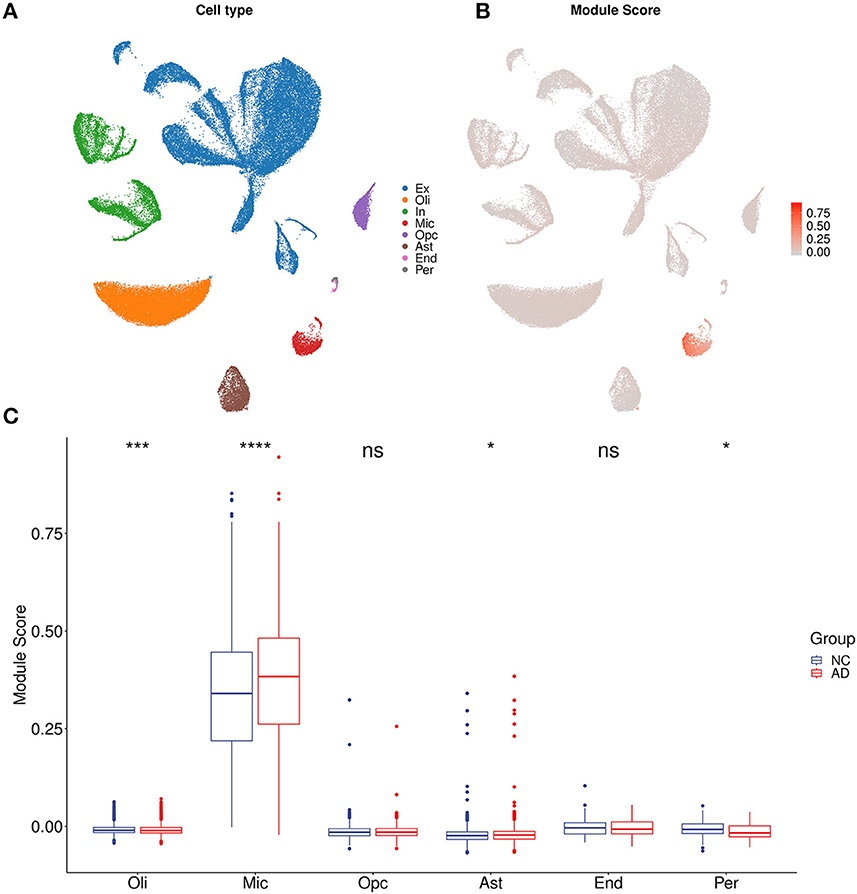
Figure 6. AIM-related cell type analysis. (A) UMAP plot of eight primary cell types in syn18485175 snRNA-seq. Ex, excitatory neurons; In, inhibitory neurons; Mic, microglia; Oli, oligodendrocytes; Opc, oligodendrocyte progenitors; Ast, astrocytes; End, endothelial; Per, pericytes. (B) UMAP plot of snRNA-seq. Each cell was colored according to AIM expression. (C) Bar plot showing AIM expression in different cell types. Blue denotes the control group and red denotes the AD group. The Wilcoxon test method was used to compare the group difference. ns: p > 0.05, *p ≤ 0.05, ***p ≤ 0.001, ****p ≤ 0.0001.
Considering the heterogeneity of microglia, microglia cells were divided into four subtypes (Mic0, Mic1, Mic2, and Mic3) and then the association between subtypes and AIM was explored. Mic0 and Mic1 subtypes showed a higher association score than other subtypes. In addition, Mic1 was the only subtype whose association score in the AD group was significantly greater than that in the control group (Figure 7A). Interestingly, for the sample of Mic1, the percentage of the microglia population was also positively correlated with AIM (R = 0.28, p = 0.056, Figure 7B). Subsequently, marker genes of subtypes were detected, and there were 123, 199, 295, and 106 marker genes in Mic0, Mic1, Mic2, and Mic3, respectively (Supplementary Table 4). Through Fisher's exact test, we found that the marker genes of Mic1 and Mic0 considerably overlapped with AIM genes (Figure 7C). Pathway enrichment analysis showed that marker genes of Mic1 were focused on synapse pruning, neuron death, neuron apoptotic process, regulation of inflammatory response, and positive regulation of NIK NF KAPPB signaling. While marker genes of Mic0 were involved in GTPASE activity regulation, synapse organization, RAS protein signal transduction, and glial cell migration (Figure 7D). Pseudo-time trajectory analysis revealed that Mic1 and partial Mic0 might be advanced subtypes of microglia cells (Figures 7E–G). These results implicated that Mic1 was a distinct subtype associated with AIM, which might provide new clues to neuroinflammation and neuron reduction in AD.
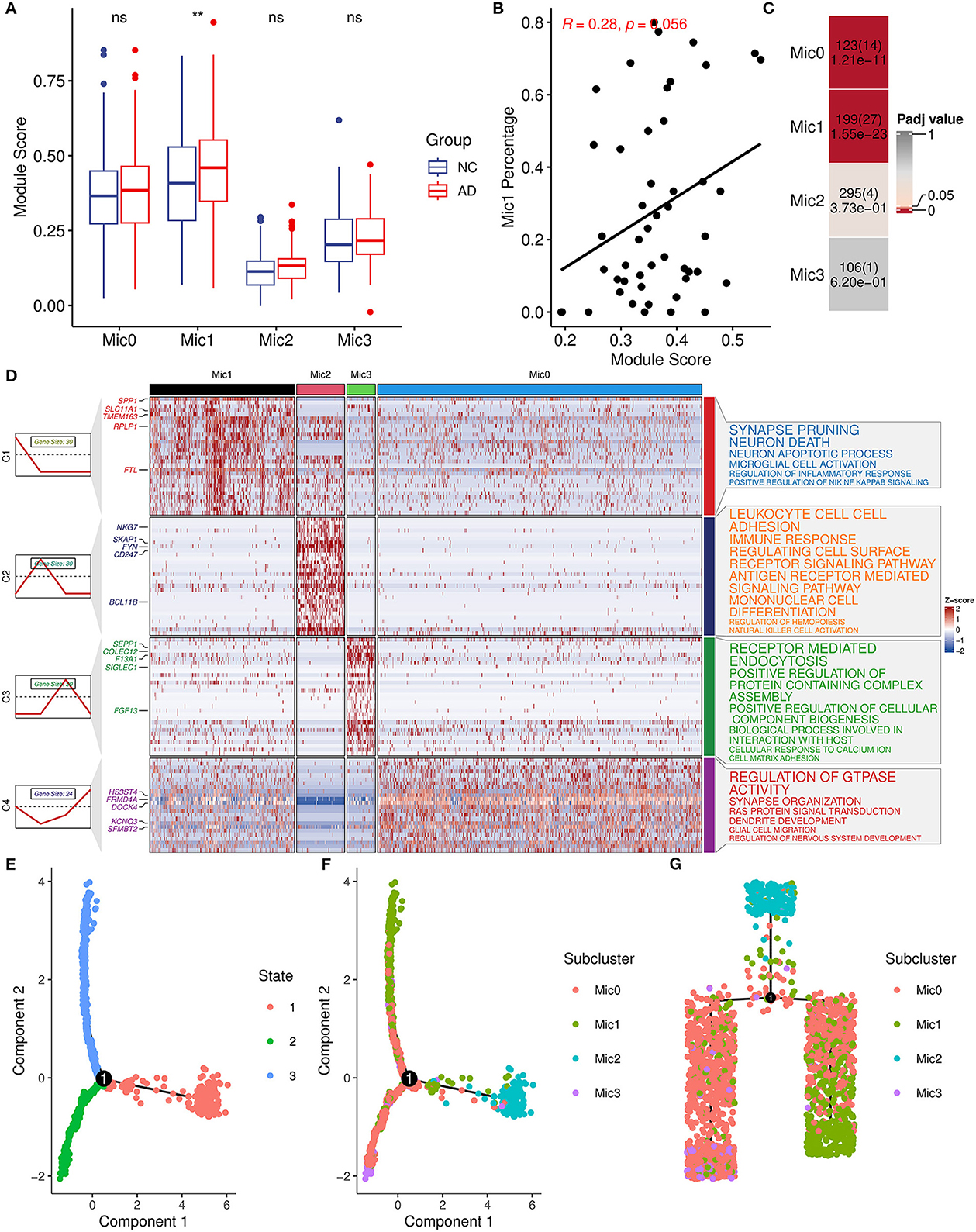
Figure 7. AIM-related microglia subcluster analysis. (A) Bar plot showing AIM expression in different microglia subclusters. Blue denotes the control group and red denotes the AD group. The Wilcoxon test method was used to compare the group difference. ns: p > 0.05, **p ≤ 0.01. (B) Scatter plot of the relationship between the module score and Mic1 percentage in the snRNA-seq microglia population. R represents the Spearman correlation coefficient. (C) Heatmap of Fisher's exact test for the overlapped genes from the AIM to different microglia subtype marker genes. The numbers in each grid denote marker gene size, overlapped gene size, and adjusted p-value. (D) Heatmap of scaled gene expression in different microglia subtypes. C1, C2, C3, and C4 represent marker genes of Mic1, Mic2, Mic3, and Mic0, respectively. The top five marker genes for each subtype were labeled. The right panel represented the enriched GO biological pathways of the corresponding marker gene set. The larger text size represented more significant enrichment results. (E) Trajectory plot of cells in the reduced dimensional space. Each cell was colored by inferred states and (F) was colored by microglia subclusters. (G) Trajectory tree of cells and each cell was colored by microglia subclusters.
3.6. Transcription factor analysis found the vital upstream regulator of the AIM
To unearth the underlying upstream transcription factors (TFs) of AIM genes, TF enrichment analysis was performed based on ReMap library (Keenan et al., 2019), and 24 significant TFs (p value < 0.05) were obtained as a result. Simultaneously, Mic1-associated TFs were detected through the SCENIC analysis, and 55 TF regulons were picked out. Furthermore, seven overlapped TFs (SPI1, IRF4, ETV6, STAT5A, RBPJ, CEBPA, and BCL6) were identified as important upstream regulators (Figures 8A–C). Then, the diagnostic value in AD discrimination of these seven TFs was verified by comparing them with seven random human transcription factors (Figure 8D). Among them, CEBPA expression in the AD group was higher than that in the control group in three brain regions (Figure 8E). Its target genes in AIM included some known pro-inflammatory factors such as C3, IL18, CD68, and ITGB2 (Figure 8F). According to these findings, we speculated that CEBPA could be a crucial TF for AD involved in microglia-mediated neuroinflammation.
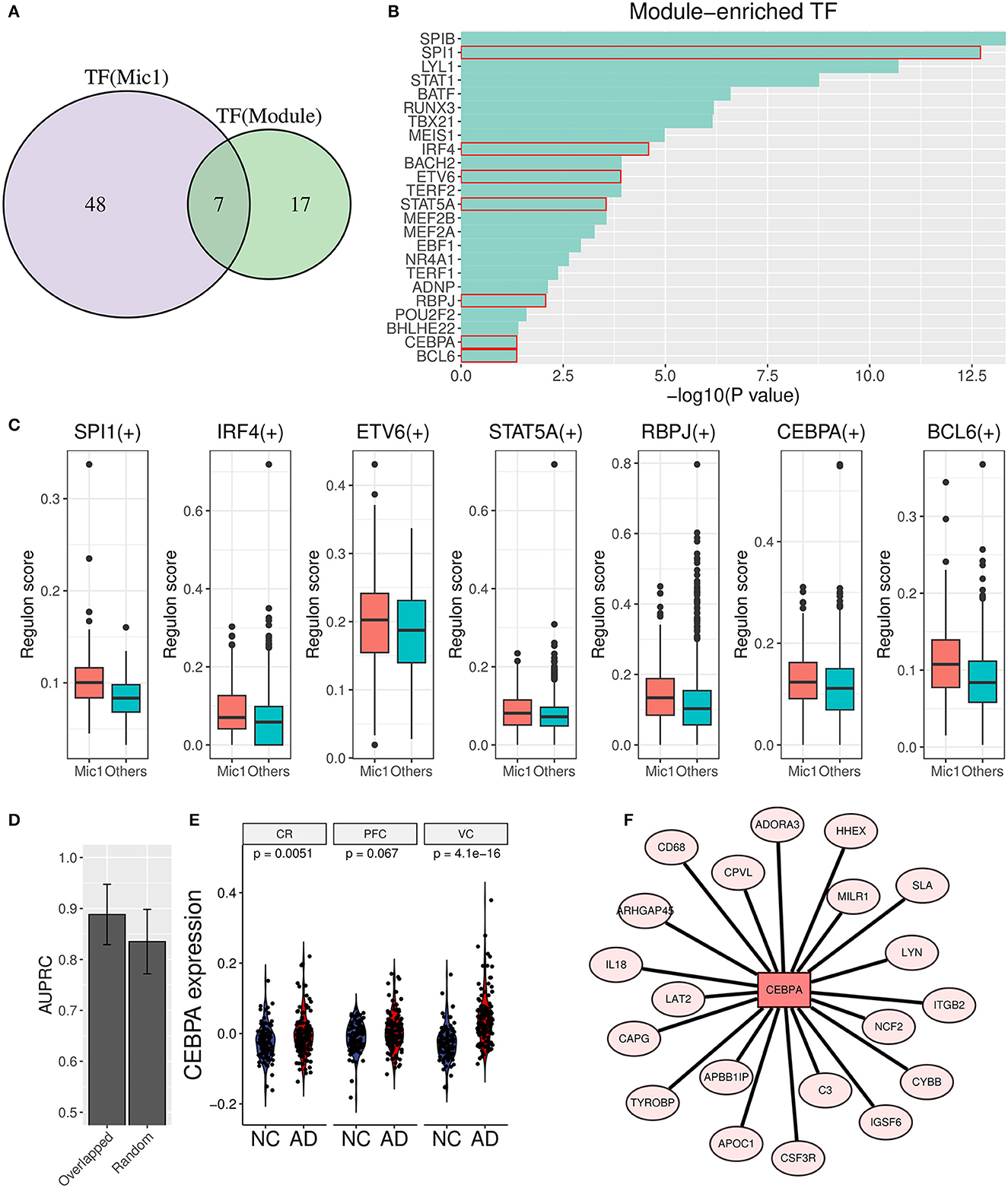
Figure 8. Transcription factor analysis for AIM. (A) Venn diagram displays the overlap between AIM-enriched TFs and Mic1-related TFs. (B) Bar plot of AIM-enriched TFs. The seven shared TFs were marked by a red box. (C) Boxplot of seven overlapped TFs involved regulon activity between the Mic1 subtype and other subtypes. (D) Bar plot of AUPRC distribution of above TFs or random seven human TFs based on GSE44770 microarray data. The model was based on a logistic algorithm. Selected TF expression was used as a feature, and sample grouping (AD or non-AD) was used as diagnostic targets. (E) Violin plot of CEBPA expression difference between NC and AD is based on three microarray datasets (GSE44768, GSE44770, and GSE44771). (F) TF network diagram shows CEBPA and its target genes in the AIM.
3.7. AIM-based repurposing method aided in anti-neuroinflammation drug discovery
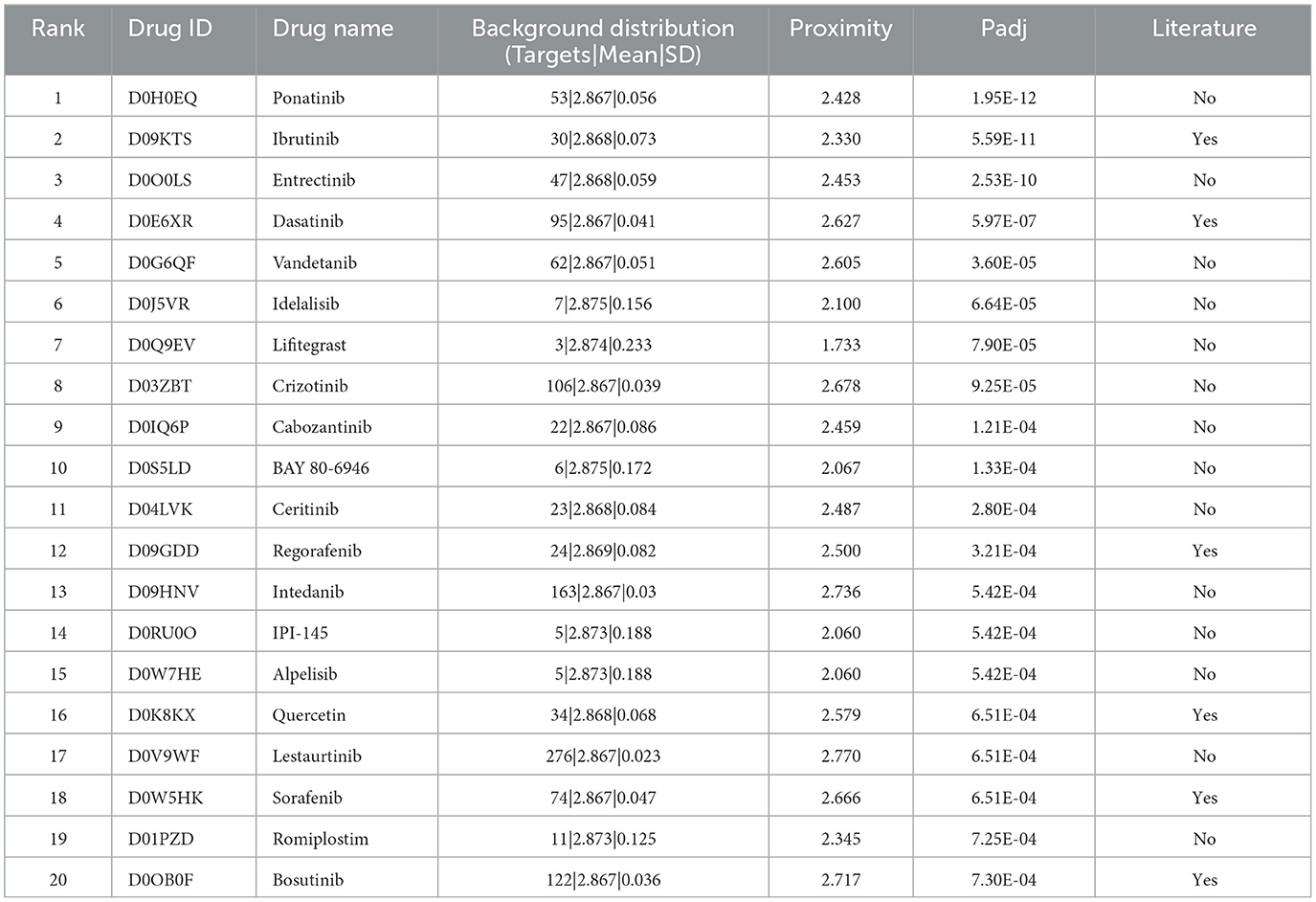
To apply the therapeutic role of the AIM, we adopted a PPI network proximity method to estimate the possible effect of approved drugs for anti-neuroinflammation based on the AIM, which might subsequently benefit the treatment of AD. First, 1,084 approved small molecular drugs, and their literature-based protein targets were retrieved from public databases. Then, the average PPI distance from drug targets to the top 10 hub genes of AIM was calculated (Figure 9A). As for a specific drug, the possibility of anti-neuroinflammation was estimated by the Z-score and a p-value based on the corresponding background Gaussian distribution (Figures 9B, C). Ultimately, the top 20 potential drugs were presented according to the Benjamini–Hochberg (BH) adjusted p-value (Figure 9D, Table 2). Among these drugs, six drugs with an anti-neuroinflammation effect have been reported, which confirmed the validity of our method, while the other 14 drugs could be new potential small molecules for AD treatment.
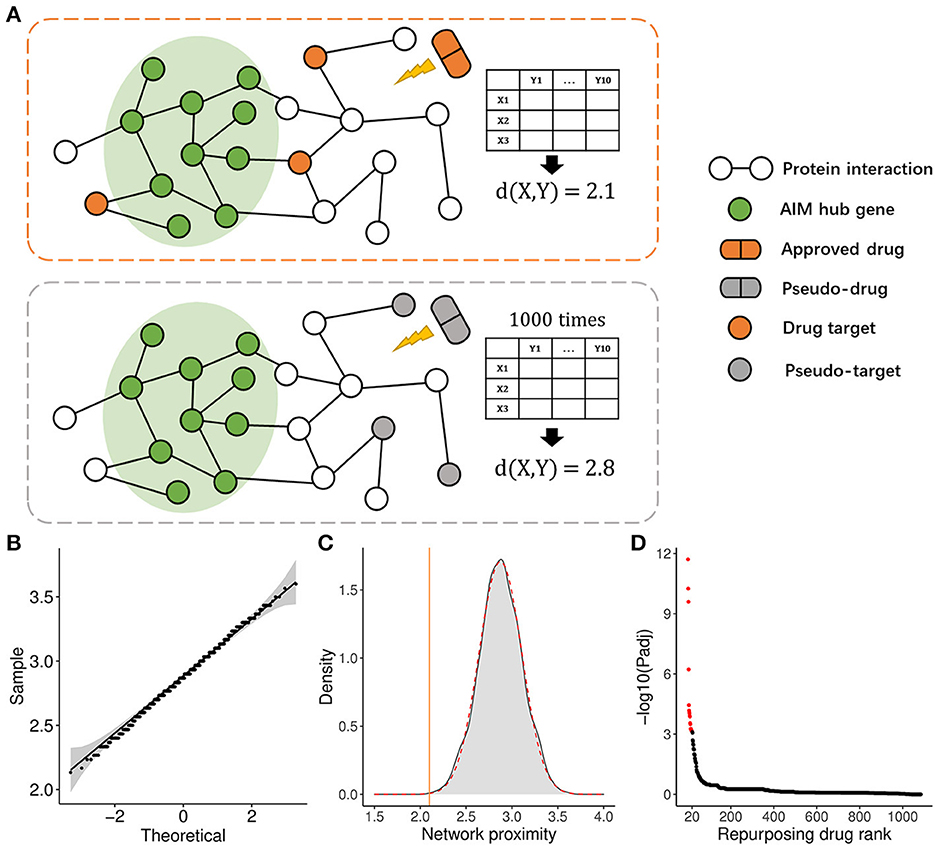
Figure 9. Drug repurposing method based on the AIM. (A) Diagram demonstrated the average PPI network proximity calculation method from all drug targets to module hub genes (e.g., X = 3, Y = 10). (B) Quantile-Quantile plot (QQ plot) indicated background distribution based on random pseudo-targets conformed to Gaussian distribution well. (C) Gray density plot represents background distribution based on random three pseudo-targets and the red dashed curve represents fitted Gaussian distribution. The brown line indicated the actual proximity of the drug. (D) The scatter plot showed the rank of 1,084 approved drugs based on the adjusted p-value.
4. Discussion
Alzheimer's disease is one of the most common neurodegenerative diseases, which is characterized by amyloid-β (Aβ) accumulation and phosphorylated tau (ptau) (Braak and Braak, 1991; Yuksel and Tacal, 2019). Aβ cascade hypothesis supposes amyloid plaque is a major cause of neuron death and synapse dysfunction (Hardy and Higgins, 1992). Although some drugs directly target Aβ that could reduce plaque loading, AD symptoms do not get the expected relief in clinical trials (Lannfelt et al., 2014). On the other hand, it is increasingly recognized that neuroinflammation is a vital event in AD onset and progression (Hammond et al., 2019). GWAS research studies show that some important AD risk genes are closely related to immune function and immune cells (Sims et al., 2017; Jansen et al., 2019). Simultaneously, emerging evidence proposes that amyloid plaque is responsible for microglia activation which can cause neuroinflammation (Cheng et al., 2021; Leng and Edison, 2021). However, due to complex immune signaling and microglia heterogeneity in the brain, immune-related therapies against AD neuroinflammation are limited at present. More systemic research is needed to explore the potential gene network of Aβ-induced neuroinflammation in AD, which might help to uncover novel biomarkers and provide a new treatment for the disease.
In this study, we successfully identified an Aβ-induced neuroinflammation module (AIM) for AD via the WGCNA method combined with module scoring and pathway enrichment analysis. In practice, microarray and snRNA-seq datasets were mainly focused on the PFC region, which was closely related to AD symptoms (Sampath et al., 2017; Sun et al., 2022). Primary WGCNA results based on the PFC microarray dataset indicated that the module comprising 177 genes was one of the modules most relevant to AD. Moreover, we also found amyloid plaque was the major factor that affected gene expression of the AIM in the spatial transcriptome of the AD model of mice. Genes' function of the module was mainly involved in the neuroinflammation-related process discovered by pathway enrichment analysis. Aβ, comprised of short peptides cleaved from amyloid precursor protein (APP), is an extracellular hallmark of AD. As its well-known toxicity of endogenous stimuli to neurons, an innate immune system like microglia in the brain is commonly activated to exert a protective response for Aβ accumulation. However, a long-term immune stimulus would cause a detrimental effect on the neuron and synapse due to the unexpected inflammation response in the brain.
As for genes in AIM, some are pro-inflammatory mediators including cytokines (IL1B and IL18) and chemokines (CXCL16, RNASE2, and CCR1), which can lead to neuronal dysfunction and death (Hanisch, 2002; Micheau and Tschopp, 2003). For example, IL1B is correlated to the loss of synaptic connections in rat hippocampus (Mishra et al., 2012). Some genes (C3, C1QA) about complement cascade play an important role in synapse refinement during brain development (Schafer et al., 2012), but aberrant upregulation and deposition of complement will lead to synapse loss and cognitive impairment. Some AD risk genes are also found in AIM, such as HLA-DRA, TREM2, MS4A6A, CD33, and PIK3CG. Recent studies have revealed that CD33 and TREM2 are the two most potential targets, which play a respective pro-/anti-neuroinflammation role during the AD process. Relevant immunotherapies of CD33 inhibiting and TREM2 elevating have made a little progress in a clinical trial (Griciuc et al., 2019; Griciuc and Tanzi, 2021). Additionally, other genes of AIM might be associated with AD neuroinflammation. For example, three hub genes (ARHGAP30, DOCK2, and NCKAP1L), NCF, and TAGAP are involved in the RAC1 GTPase cycle, which can regulate the production of neurotoxic reactive oxygen species (ROS) (Etienne-Manneville and Hall, 2002). To sum up, the AIM can be used to pinpoint key genes about Aβ-induced AD neuroinflammation and discover possible novel targets for the disease.
Afterward, we investigated AIM-related cell types to further explore their roles in AD. First of all, we found a negative correlation between AIM activity and neuron ratio in both microarray data and snRNA-seq data, which confirmed the potential link of the module to AD symptoms. Furthermore, we found that microglia showed a remarkable AIM score. Microglia is one resident innate immune cell type originating from a yolk sac progenitor and mainly exerts immune surveillance and clearance in the central nervous system (CNS) (Ousman and Kubes, 2012; Gomez Perdiguero et al., 2015). Its activation against endogenous or exogenous stimulation (such as abnormal aggregating of some essential proteins) is an important defensive response to reduce the currency of neurological disease (Heneka et al., 2015). However, microglia often show spatial and morphological heterogeneity which present complex roles and phenotypes in neurological diseases (Plescher et al., 2018; Tan et al., 2020). Moreover, its adverse activation is recognized as a risk event of neuroinflammation in AD (Yu and Ye, 2015; Nguyen et al., 2017). The original manuscript (Mathys et al., 2019) of snRNA-seq data identified four microglia subtypes, and Mic1 was a distinct microglia subtype that presented partial features of DAM, which was derived from the mouse model and reflected phagocytosis phenotype against Aβ particles (Keren-Shaul et al., 2017). In this study, we validated that AIM was considerably associated with Mic1, and then functional exploration revealed that the subtype could mediate neuroinflammation and induce neuron degeneration.
Next, through transcription factor (TF) enrichment analysis and single-cell regulatory network inference and clustering analysis, seven potential TFs related to AD (SPI1, IRF4, ETV6, STAT5A, RBPJ, CEBPA, and BCL6) were identified. Among these TFs, CEBPA (CCAAT/Enhancer-Binding Protein Alpha), containing a basic leucine zipper (bZIP) domain, can recognize the CCAAT motif in the promoters of interested target genes. It was found that CEBPA had a higher expression in AD samples compared to normal ones in three brain regions. It is confirmed that CEBPA plays an important role in the proliferation and differentiation of a myeloid progenitor, and its biallelic mutation is highly related to acute myeloid leukemia (AML) (Leroy et al., 2005; Wilhelmson and Porse, 2020). Emerging evidence has uncovered its role in microglia-associated neuroinflammation. For example, downregulated CEBPA is associated with anti-inflammation microglia (M2) polarization (Yu et al., 2017). Another research demonstrates that CEBPA can coordinate with other two TFs (IRF1 and LXR) to regulate pro-inflammation cytokine production in microglia stimulated by lipopolysaccharide (LPS) (Gao et al., 2019). Notably, they also found that siRNA against CEBPA can significantly inhibit the production of IL6, IL1b, and IL5. In addition, its targets gene in AIM includes inflammatory markers such as IL18, CD68, TYPOBP, and AD risk genes such as ARHGAP45 and APOC1 (Xue et al., 2021; Kulminski et al., 2022). In summary, these seven candidate TFs, especially CEBPA, could be used as potential biomarkers for AD diagnosis.
Finally, 20 potential drugs of anti-neuroinflammation against AD were presented based on significant network proximity. Some of them have been proven to have an anti-neuroinflammation effect, which reflected the validity of our method. For instance, ponatinib is a multi-target tyrosine kinase inhibitor and ranked first among all drugs. Previous studies reported that ponatinib can reduce inflammation of obesity and influenza (Chen et al., 2019; Lin et al., 2022) due to its inhibitory effect on the two isoforms of JAK (JAK1 and JAK2). The JAK/STAT pathway plays important roles in glial activation and neuroinflammation response in many neurodegenerative diseases (Jain et al., 2021). Additionally, ibrutinib is originally known as a bruton tyrosine kinase inhibitor (BTKi) and has bioactivity against other kinases (Cheng et al., 2014). It has been approved by FDA for multiple diseases such as mantle cell lymphoma (MCL) and chronic lymphocytic leukemia (CLL). A total of three genes (HCK, BTK, LYN) out of 30 Ibrutinib targets were found in AIM, and its proximity score ranked second among all drugs. Recent studies show that ibrutinib can attenuate neuroinflammatory responses by inhibiting AKT/STAT3 signaling pathways in the LPS-stimulated BV2 cell line and reduce glia activation and cytokine levels in animal experiments (Nam et al., 2018; Li et al., 2021). Another research further indicates that the anti-neuroinflammation effect of ibrutinib is associated with Aβ accumulation in 5xFAD mouse models (Lee et al., 2021). In addition, regorafenib, ranked 11th, has been proven as having effects on neuroinflammation suppression and dendritic spine formation (Han et al., 2020).
There were also some limitations in our study. For 177 constituent genes in the AIM, it is important to exclude the possible false positive genes and further recognize minor submodules that could participate in Aβ-induced neuroinflammation in different roles during the AD process. Moreover, a prospective study for identified novel biomarkers and screened candidate drugs is worthy of deep investigation through molecular biology experiments.
5. Conclusion
We identified a vital Aβ-induced neuroinflammation module (AIM) made up of 177 genes for AD. The neuron reduction and AIM-related inflammatory microglia subtype were further discovered to elucidate the roles of the module. Moreover, some potential TF biomarkers and some candidate repurposing drugs were presented. In short, our findings provided sights into the gene regulatory network and drug targets of AD neuroinflammation, which might facilitate mechanistic investigation of AD and make benefits to treatment of the disease.
Data availability statement
The public sequencing data presented in the study are deposited in the GEO repository: https://www.ncbi.nlm.nih.gov/geo/. GSE44768: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE44768; GSE44770: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE44770; GSE44771: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE44771; GSE160936: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE160936; GSE152506: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE152506 and Synapse repository: https://www.synapse.org/Syn18485175: https://www.synapse.org/#!Synapse:syn18485175.
Author contributions
SL, DL, and GZ designed the study and wrote the manuscript. SL, CL, and ZZ collected and analyzed the data. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (82104521).
Acknowledgments
We want to appreciate Dr. Jiangming Zeng (University of Macau) for his suggestion for the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1161405/full#supplementary-material
References
Akiyama, H., Barger, S., Barnum, S., Bradt, B., Bauer, J., Cole, G. M., et al. (2000). Inflammation and Alzheimer's disease. Neurobiol. Aging 21, 383–421. doi: 10.1016/S0197-4580(00)00124-X
Andrews, T. S., Kiselev, V. Y., McCarthy, D., and Hemberg, M. (2021). Tutorial: guidelines for the computational analysis of single-cell RNA sequencing data. Nat. Protoc. 16, 1–9. doi: 10.1038/s41596-020-00409-w
Braak, H., and Braak, E. (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259. doi: 10.1007/BF00308809
Chen, S., Liu, G., Chen, J., Hu, A., Zhang, L., Sun, W., et al. (2019). Ponatinib protects mice from lethal influenza infection by suppressing cytokine storm. Front. Immunol. 10, 1393. doi: 10.3389/fimmu.2019.01393
Cheng, F., Kovács, I. A., and Barabási, A.-L. (2019). Network-based prediction of drug combinations. Nat. Commun. 10, 1197. doi: 10.1038/s41467-019-09186-x
Cheng, J., Dong, Y., Ma, J., Pan, R., Liao, Y., Kong, X., et al. (2021). Microglial Calhm2 regulates neuroinflammation and contributes to Alzheimer's disease pathology. Sci. Adv. 7, abe3600. doi: 10.1126/sciadv.abe3600
Cheng, S., Ma, J., Guo, A., Lu, P., Leonard, J. P., Coleman, M., et al. (2014). Btk inhibition targets in vivo CLL proliferation through its effects on B-cell receptor signaling activity. Leukemia 28, 649–657. doi: 10.1038/leu.2013.358
Dhapola, R., Hota, S. S., Sarma, P., Bhattacharyya, A., Medhi, B., Reddy, D. H., et al. (2021). Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer's disease. Inflammopharmacology 29, 1669–1681. doi: 10.1007/s10787-021-00889-6
Doig, A. J., Del Castillo-Frias, M. P., Berthoumieu, O., Tarus, B., Nasica-Labouze, J., Sterpone, F., et al. (2017). Why is research on amyloid-β failing to give new drugs for Alzheimer's disease? ACS Chem. Neurosci. 8, 1435–1437. doi: 10.1021/acschemneuro.7b00188
Etienne-Manneville, S., and Hall, A. (2002). Rho Gtpases in cell biology. Nature 420, 629–635. doi: 10.1038/nature01148
Feng, S., Xu, Y., Dai, Z., Yin, H., Zhang, K., Shen, Y., et al. (2022). Integrative analysis from multicenter studies identifies a WGCNA-derived cancer-associated fibroblast signature for ovarian cancer. Front. Immunol. 13, 951582. doi: 10.3389/fimmu.2022.951582
Fu, W.-Y., Wang, X., and Ip, N. Y. (2019). Targeting neuroinflammation as a therapeutic strategy for Alzheimer's disease: mechanisms, drug candidates, and new opportunities. ACS Chem. Neurosci. 10, 872–879. doi: 10.1021/acschemneuro.8b00402
Gao, T., Jernigan, J., Raza, S. A., Dammer, E. B., Xiao, H., Seyfried, N. T., et al. (2019). Transcriptional regulation of homeostatic and disease-associated-microglial genes by IRF1, LXRβ, and CEBPα. Glia 67, 1958–1975. doi: 10.1002/glia.23678
GBD 2019 Dementia Forecasting Collaborators (2022). Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health 7, e105–e125. doi: 10.1016/S2468-2667(21)00249-8
Gomez Perdiguero, E., Klapproth, K., Schulz, C., Busch, K., Azzoni, E., Crozet, L., et al. (2015). Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518, 547–551. doi: 10.1038/nature13989
Griciuc, A., Patel, S., Federico, A. N., Choi, S. H., Innes, B. J., Oram, M. K., et al. (2019). Trem2 acts downstream of Cd33 in modulating microglial pathology in Alzheimer's disease. Neuron 103, 820–835.e7. doi: 10.1016/j.neuron.2019.06.010
Griciuc, A., and Tanzi, R. E. (2021). The role of innate immune genes in Alzheimer's disease. Curr. Opin. Neurol. 34, 228–236. doi: 10.1097/WCO.0000000000000911
Hammond, T. R., Marsh, S. E., and Stevens, B. (2019). Immune signaling in neurodegeneration. Immunity 50, 955–974. doi: 10.1016/j.immuni.2019.03.016
Han, K.-M., Kang, R. J., Jeon, H., Lee, H.-J., Lee, J.-S., Park, H., et al. (2020). Regorafenib regulates Ad pathology, neuroinflammation, and dendritic spinogenesis in cells and a mouse model of Ad. Cells 9, 1655. doi: 10.3390/cells9071655
Hanisch, U.-K. (2002). Microglia as a source and target of cytokines. Glia 40, 140–155. doi: 10.1002/glia.10161
Hardy, J. A., and Higgins, G. A. (1992). Alzheimer's disease: the amyloid cascade hypothesis. Science 256, 184–185. doi: 10.1126/science.1566067
Heneka, M. T., Carson, M. J., El Khoury, J., Landreth, G. E., Brosseron, F., Feinstein, D. L., et al. (2015). Neuroinflammation in Alzheimer's disease. Lancet Neurol. 14, 388–405. doi: 10.1016/S1474-4422(15)70016-5
Holtzman, D. M., Morris, J. C., and Goate, A. M. (2011). Alzheimer's disease: the challenge of the second century. Sci. Transl. Med. 3, 77sr.1. doi: 10.1126/scitranslmed.3002369
Jain, M., Singh, M. K., Shyam, H., Mishra, A., Kumar, S., Kumar, A., et al. (2021). Role of Jak/Stat in the neuroinflammation and its association with neurological disorders. Ann Neurosci 28, 191–200. doi: 10.1177/09727531211070532
Jansen, I. E., Savage, J. E., Watanabe, K., Bryois, J., Williams, D. M., Steinberg, S., et al. (2019). Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer's disease risk. Nat. Genet. 51, 404–413. doi: 10.1038/s41588-018-0311-9
Jin, S., Guerrero-Juarez, C. F., Zhang, L., Chang, I., Ramos, R., Kuan, C.-H., et al. (2021). Inference and analysis of cell-cell communication using cellchat. Nat. Commun. 12, 1088. doi: 10.1038/s41467-021-21246-9
Karran, E., and De Strooper, B. (2022). The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat. Rev. Drug Discov. 21, 306–318. doi: 10.1038/s41573-022-00391-w
Keenan, A. B., Torre, D., Lachmann, A., Leong, A. K., Wojciechowicz, M. L., Utti, V., et al. (2019). Chea3: transcription factor enrichment analysis by orthogonal omics integration. Nucleic Acids Res. 47, W212–W224. doi: 10.1093/nar/gkz446
Keren-Shaul, H., Spinrad, A., Weiner, A., Matcovitch-Natan, O., Dvir-Szternfeld, R., Ulland, T. K., et al. (2017). A unique microglia type associated with restricting development of Alzheimer's disease. Cell 169, 1276–1290.e17. doi: 10.1016/j.cell.2017.05.018
Kolodziejczyk, A. A., Kim, J. K., Svensson, V., Marioni, J. C., and Teichmann, S. A. (2015). The technology and biology of single-cell RNA sequencing. Mol. Cell 58, 610–620. doi: 10.1016/j.molcel.2015.04.005
Kulminski, A. M., Philipp, I., Shu, L., and Culminskaya, I. (2022). Definitive roles of Tomm40-Apoe-Apoc1 variants in the Alzheimer's risk. Neurobiol. Aging 110, 122–131. doi: 10.1016/j.neurobiolaging.2021.09.009
Kumar, N., Mishra, B., Athar, M., and Mukhtar, S. (2021). Inference of gene regulatory network from single-cell transcriptomic data using pyscenic. Methods Mol. Biol. 2328, 171–182. doi: 10.1007/978-1-0716-1534-8_10
Lake, B. B., Ai, R., Kaeser, G. E., Salathia, N. S., Yung, Y. C., Liu, R., et al. (2016). Neuronal subtypes and diversity revealed by single-nucleus RNA sequencing of the human brain. Science 352, 1586–1590. doi: 10.1126/science.aaf1204
Langfelder, P., and Horvath, S. (2008). Wgcna: an R package for weighted correlation network analysis. BMC Bioinformatics 9, 559. doi: 10.1186/1471-2105-9-559
Lannfelt, L., Relkin, N. R., and Siemers, E. R. (2014). Amyloid-ß-directed immunotherapy for Alzheimer's disease. J. Intern. Med. 275, 284–295. doi: 10.1111/joim.12168
Lee, H.-J., Jeon, S. G., Kim, J., Kang, R. J., Kim, S.-M., Han, K.-M., et al. (2021). Ibrutinib modulates Aβ/Tau pathology, neuroinflammation, and cognitive function in mouse models of Alzheimer's disease. Aging Cell 20, e13332. doi: 10.1111/acel.13332
Leng, F., and Edison, P. (2021). Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat. Rev. Neurol. 17, 157–172. doi: 10.1038/s41582-020-00435-y
Leroy, H., Roumier, C., Huyghe, P., Biggio, V., Fenaux, P., Preudhomme, C., et al. (2005). CEBPA point mutations in hematological malignancies. Leukemia 19, 329–334. doi: 10.1038/sj.leu.2403614
Li, W., Ali, T., He, K., Liu, Z., Shah, F. A., Ren, Q., et al. (2021). Ibrutinib alleviates LPS-induced neuroinflammation and synaptic defects in a mouse model of depression. Brain Behav. Immun. 92, 10–24. doi: 10.1016/j.bbi.2020.11.008
Lin, W., Wang, Y., Chen, Y., Wang, Q., Gu, Z., Zhu, Y., et al. (2021). Role of calcium signaling pathway-related gene regulatory networks in ischemic stroke based on multiple WGCNA and single-cell analysis. Oxid. Med. Cell. Longev. 2021, 8060477. doi: 10.1155/2021/8060477
Lin, Z., Lin, X., Lai, Y., Han, C., Fan, X., Tang, J., et al. (2022). Ponatinib modulates the metabolic profile of obese mice by inhibiting adipose tissue macrophage inflammation. Front. Pharmacol. 13, 1040999. doi: 10.3389/fphar.2022.1040999
Longo, S. K., Guo, M. G., Ji, A. L., and Khavari, P. A. (2021). Integrating single-cell and spatial transcriptomics to elucidate intercellular tissue dynamics. Nat. Rev. Genet. 22, 627–644. doi: 10.1038/s41576-021-00370-8
Mathys, H., Davila-Velderrain, J., Peng, Z., Gao, F., Mohammadi, S., Young, J. Z., et al. (2019). Single-cell transcriptomic analysis of Alzheimer's disease. Nature 570, 332–337. doi: 10.1038/s41586-019-1195-2
Micheau, O., and Tschopp, J. (2003). Induction of Tnf receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190. doi: 10.1016/S0092-8674(03)00521-X
Miguel-Álvarez, M., Santos-Lozano, A., Sanchis-Gomar, F., Fiuza-Luces, C., Pareja-Galeano, H., Garatachea, N., et al. (2015). Non-steroidal anti-inflammatory drugs as a treatment for Alzheimer's disease: a systematic review and meta-analysis of treatment effect. Drugs Aging 32, 139–147. doi: 10.1007/s40266-015-0239-z
Mishra, A., Kim, H. J., Shin, A. H., and Thayer, S. A. (2012). Synapse loss induced by interleukin-1β requires pre- and post-synaptic mechanisms. J. Neuroimmune Pharmacol. 7, 571–578. doi: 10.1007/s11481-012-9342-7
Nam, H. Y., Nam, J. H., Yoon, G., Lee, J.-Y., Nam, Y., Kang, H.-J., et al. (2018). Ibrutinib suppresses LPS-induced neuroinflammatory responses in Bv2 microglial cells and wild-type mice. J. Neuroinflammation 15, 271. doi: 10.1186/s12974-018-1308-0
Nguyen, H. M., Grössinger, E. M., Horiuchi, M., Davis, K. W., Jin, L.-W., Maezawa, I., et al. (2017). Differential Kv1.3, Kca3.1, and Kir2.1 expression in “classically” and “alternatively” activated microglia. Glia 65, 106–121. doi: 10.1002/glia.23078
Obradovic, A., Chowdhury, N., Haake, S. M., Ager, C., Wang, V., Vlahos, L., et al. (2021). Single-cell protein activity analysis identifies recurrence-associated renal tumor macrophages. Cell 184, 2988–3005.e16. doi: 10.1016/j.cell.2021.04.038
Ousman, S. S., and Kubes, P. (2012). Immune surveillance in the central nervous system. Nat. Neurosci. 15, 1096–1101. doi: 10.1038/nn.3161
Plescher, M., Seifert, G., Hansen, J. N., Bedner, P., Steinhäuser, C., Halle, A., et al. (2018). Plaque-dependent morphological and electrophysiological heterogeneity of microglia in an Alzheimer's disease mouse model. Glia 66, 1464–1480. doi: 10.1002/glia.23318
Sampath, D., Sathyanesan, M., and Newton, S. S. (2017). Cognitive dysfunction in major depression and Alzheimer's disease is associated with hippocampal-prefrontal cortex dysconnectivity. Neuropsychiatr. Dis. Treat. 13, 1509–1519. doi: 10.2147/NDT.S136122
Schafer, D. P., Lehrman, E. K., Kautzman, A. G., Koyama, R., Mardinly, A. R., Yamasaki, R., et al. (2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705. doi: 10.1016/j.neuron.2012.03.026
Sims, R., van der Lee, S. J., Naj, A. C., Bellenguez, C., Badarinarayan, N., Jakobsdottir, J., et al. (2017). Rare coding variants in Plcg2, Abi3, and Trem2 implicate microglial-mediated innate immunity in Alzheimer's disease. Nat. Genet. 49, 1373–1384. doi: 10.1038/ng.3916
Sun, Q., Zhang, J., Li, A., Yao, M., Liu, G., Chen, S., et al. (2022). Acetylcholine deficiency disrupts extratelencephalic projection neurons in the prefrontal cortex in a mouse model of Alzheimer's disease. Nat. Commun. 13, 998. doi: 10.1038/s41467-022-28493-4
Tan, Y.-L., Yuan, Y., and Tian, L. (2020). Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry 25, 351–367. doi: 10.1038/s41380-019-0609-8
Wilhelmson, A. S., and Porse, B. T. (2020). Ccaat enhancer binding protein alpha (CEBPA) biallelic acute myeloid leukaemia: cooperating lesions, molecular mechanisms and clinical relevance. Br. J. Haematol. 190, 495–507. doi: 10.1111/bjh.16534
Xue, D., Bush, W. S., Renton, A. E., Marcora, E. A., Bis, J. C., Kunkle, B. W., et al. (2021). Large-scale sequencing studies expand the known genetic architecture of Alzheimer's disease. Alzheimers. Dement. 13, e12255. doi: 10.1002/dad2.12255
Yu, A., Zhang, T., Duan, H., Pan, Y., Zhang, X., Yang, G., et al. (2017). Mir-124 contributes to M2 polarization of microglia and confers brain inflammatory protection via the C/Ebp-α pathway in intracerebral hemorrhage. Immunol. Lett. 182, 1–11. doi: 10.1016/j.imlet.2016.12.003
Yu, Y., and Ye, R. D. (2015). Microglial Aβ receptors in Alzheimer's disease. Cell. Mol. Neurobiol. 35, 71–83. doi: 10.1007/s10571-014-0101-6
Keywords: Alzheimer's disease, neuroinflammation, amyloid-β, data integration, drug repurposing
Citation: Li S, Lu C, Zhao Z, Lu D and Zheng G (2023) Uncovering neuroinflammation-related modules and potential repurposing drugs for Alzheimer's disease through multi-omics data integrative analysis. Front. Aging Neurosci. 15:1161405. doi: 10.3389/fnagi.2023.1161405
Received: 10 February 2023; Accepted: 10 May 2023;
Published: 02 June 2023.
Edited by:
Giulia Abate, University of Brescia, ItalyReviewed by:
Mario Skarica, Yale University, United StatesRaymond Scott Turner, Georgetown University, United States
Copyright © 2023 Li, Lu, Zhao, Lu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Lu, c3pfbHVkb25nQGZveG1haWwuY29t; Guangyong Zheng, Z3l6aGVuZ0BzaHV0Y20uZWR1LmNu
 Shensuo Li
Shensuo Li Changhao Lu2
Changhao Lu2 Dong Lu
Dong Lu Guangyong Zheng
Guangyong Zheng