- 1Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
- 2Alzheimer’s Disease Convergence Research Center, Samsung Medical Center, Seoul, Republic of Korea
- 3Department of Statistics, Chung-Ang University, Seoul, Republic of Korea
- 4Department of Ophthalmology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
- 5Department of Neurology, Yongin Severance Hospital, Yonsei University Health System, Yongin-si, Republic of Korea
- 6Department of Health Sciences and Technology, SAIHST, Sungkyunkwan University, Seoul, Republic of Korea
- 7Department of Public Health Sciences, Seoul National University, Seoul, Republic of Korea
- 8Samsung Advanced Institute for Health Sciences and Technology (SAIHST), Sungkyunkwan University, Seoul, Republic of Korea
Background: We aimed to investigate the incidence of dementia by age and year as well as the population-attributable fractions (PAFs) for known dementia risk factors in Republic of Korea.
Methods: A 12-year, nationwide, population-based, retrospective cohort study was conducted. We used customized health information from the National Health Insurance Service (NHIS) data from 2002 to 2017. We analyzed age- and sex-adjusted incidence rates and PAF of dementia for each risk factor such as depression, diabetes, hemorrhagic stroke, ischemic stroke, hypertension, osteoporosis and physical inactivity using Levin’s formula.
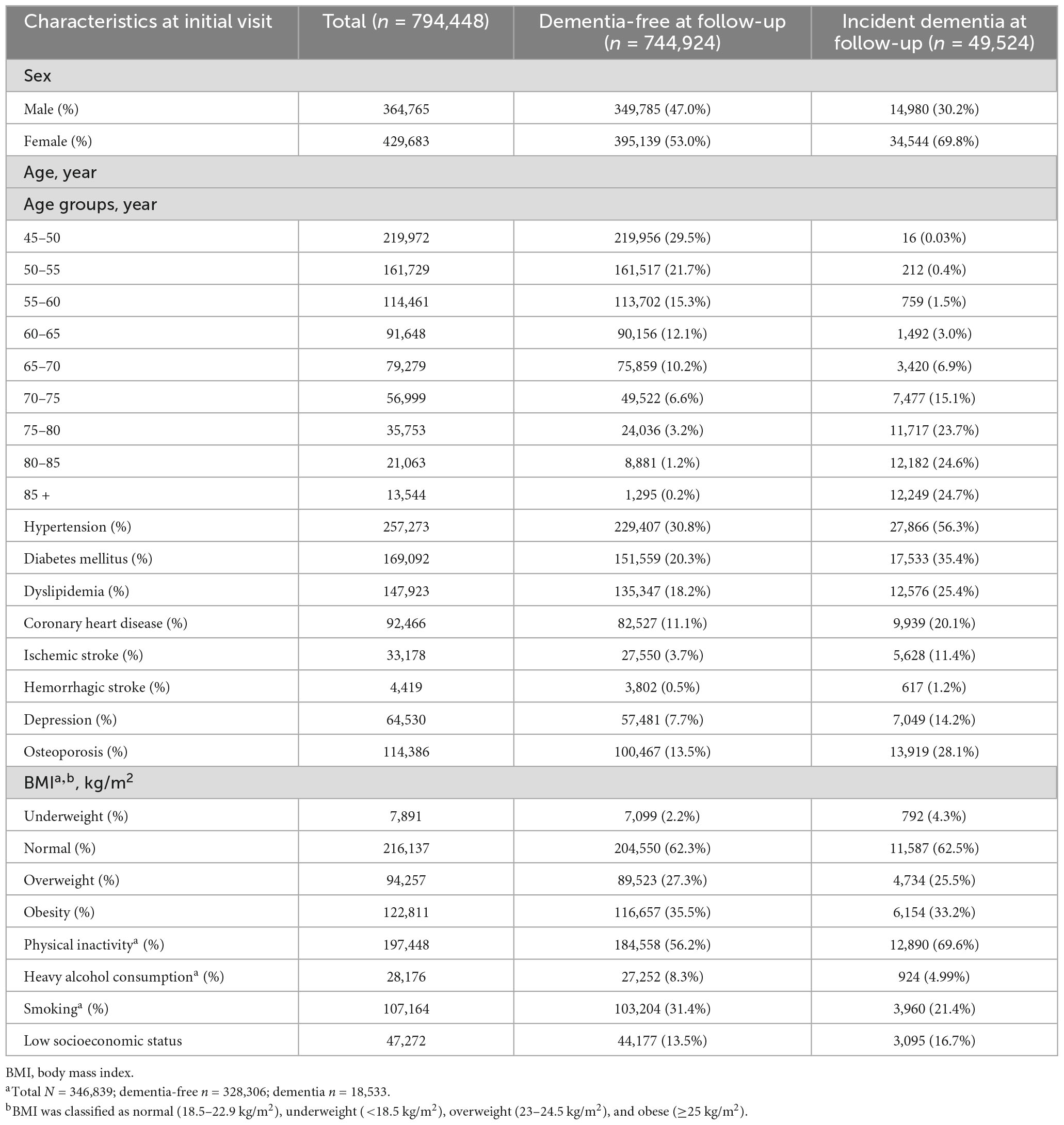
Results: Of the 794,448 subjects in the dementia-free cohort, 49,524 (6.2%) developed dementia. Dementia incidence showed annual growth from 1.56 per 1,000 person-years in 2006 to 6.94 per 1,000 person-years in 2017. Of all dementia cases, 34,544 subjects (69.8%) were female and 2,479 subjects (5.0%) were early onset dementia. AD dementia accounted for 66.5% of the total dementia incidence. Considering relative risk and prevalence, physical inactivity attributed the greatest to dementia (PAF, 8.1%), followed by diabetes (PAF, 4.2%), and hypertension (PAF, 2.9%). Altogether, the significant risk factors increased the risk of dementia by 18.0% (overall PAF).
Conclusion: We provided the incidence of dementia and PAFs for dementia risk factors in Republic of Korea using a 12-year, nationwide cohort. Encouraging lifestyle modifications and more aggressive control of risk factors may effectively prevent dementia.
Introduction
As the world’s population ages, the number of dementia patients gradually increases along with increased medical and societal costs for dementia care (Livingston et al., 2017). Alzheimer’s disease (AD) is the most common type of dementia that currently has no treatment for cure (Fiest et al., 2016). Therefore, identifying the incidence rate of dementia and finding effective strategies to prevent dementia is important in planning for public healthcare policies.
The prevalence and incidence of dementia vary across countries. According to the World Health Organization, approximately 47 million people worldwide suffer from dementia. In the United States, the prevalence of AD and related dementia in people over the age of 65 years was reported to be 11.5% (Matthews et al., 2019). In the Framingham Heart Study, the incidence of AD dementia and vascular dementia in those over the age of 60 was 2.0 per 100 persons over 5 years (Satizabal et al., 2016). In the English population, the incidence of AD dementia in those over the age of 65 was 7.06 per 1,000 person-years (Cadar et al., 2018). In the Dutch population, the incidence of AD dementia in people over the age of 60 was 5.77 per 1,000 person-years (van Bussel et al., 2017). In Japan, the incidence of all-cause dementia and AD dementia in those over 65 years of age was 41.6 and 28.2 per 1,000 person-years (Ohara et al., 2017; Table 1). In the South Korean population, the prevalence of dementia was gradually increasing, and the prevalence in people over the age of 65 was reported to be approximately 10.16% (Korean Dementia Observatory 2019) (Cho et al., 2014). However, there is no detailed data on dementia incidence in the South Korean population.
There are various known risk factors for dementia, and these factors have varying degrees of impact on dementia. Although some risk factors, including age, sex, race, familial history and genetic factors, are not modifiable, there are several modifiable risk factors that can be acted upon (Livingston et al., 2020; Qiu et al., 2022). A recent study suggested that modifiable risk factors, such as less education, hearing loss, traumatic brain injury, hypertension, alcohol misuse, obesity, smoking, depression, social isolation, physical inactivity, diabetes, and air pollution, contribute to around 39% of dementia (Livingston et al., 2020). Because no medication is known to cure dementia, current trends in the management of dementia focus on its prevention (Livingston et al., 2017). Risk factors that have higher prevalence and higher risk for dementia would contribute more to dementia incidence. Thus, when establishing population-level public health strategies for dementia prevention, the prevalence and relative risk of each risk factor in the target population should be considered.
Using National Health Insurance Service data (NHIS), we first aimed to provide dementia incidence by age and year in each sex in Republic of Korea. Second, we evaluated the relative risk of each risk factor for dementia and estimated the attributable fraction for dementia in Republic of Korea. We used customized health information from the National Health Insurance Service (NHIS) data from 2002 to 2017.
Materials and methods
This study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Samsung Medical Center Institutional Review Board (SMC IRB 2018-08-017). The requirement for informed consent from all subjects was waived as we used the national cohort data.
Data source
For this national cohort study, we used customized health information from the National Health Insurance Service (NHIS) data, which includes more than 99% of Koreans (approximately 50 million).1 The NHIS operates national health check-up, and the NHIS database includes personal information, health insurance claim codes (procedures and prescriptions), diagnostic codes from the Korean Standard Classification of Diseases, 7th Revision (KCD-7), which is based on the International Classification of Diseases, 10th Revision (ICD-10), socioeconomic data (residence and income), and health examination data for each participant from 2002 to 2017. Data on body mass index (BMI), and behavioral characteristics, such as smoking status, alcohol consumption, and physical activity, were merged from the general health examinations in the NHIS database which were assessed at baseline (2006). The behavioral characteristics were assessed via standardized questionnaires.
Definition of dementia
Dementia was defined by both ICD-10 diagnosis codes (F00, F01, F02, F03, G30, or G31) and medication prescriptions with donepezil, galantamine, rivastigmine, or memantine. Dementia was subdivided into AD dementia with ICD-10 codes F00 and G30.
Dementia-free cohort
In the NHIS database, a total of 16,389,533 subjects aged 45 years or older were identified in 2006. We excluded 664,878 subjects diagnosed with dementia between January 1, 2002, and December 31, 2005 and identified a total of 15,724,655 subjects without dementia. Then, to meet the size limit for data extraction, we selected 880,000 subjects using simple random sampling method. We additionally excluded subjects who did not have hospital visit records from 2002 to 2017 (18,365 subjects), subjects without follow-up since 2006 (3,120 subjects), subjects who lost their health insurance eligibility from 2002 to 2005 (30,820 subjects), and subjects who died before December 2005 (33,247 subjects). Finally, 794,448 subjects were followed up from 2006 to 2017 (Figure 1).
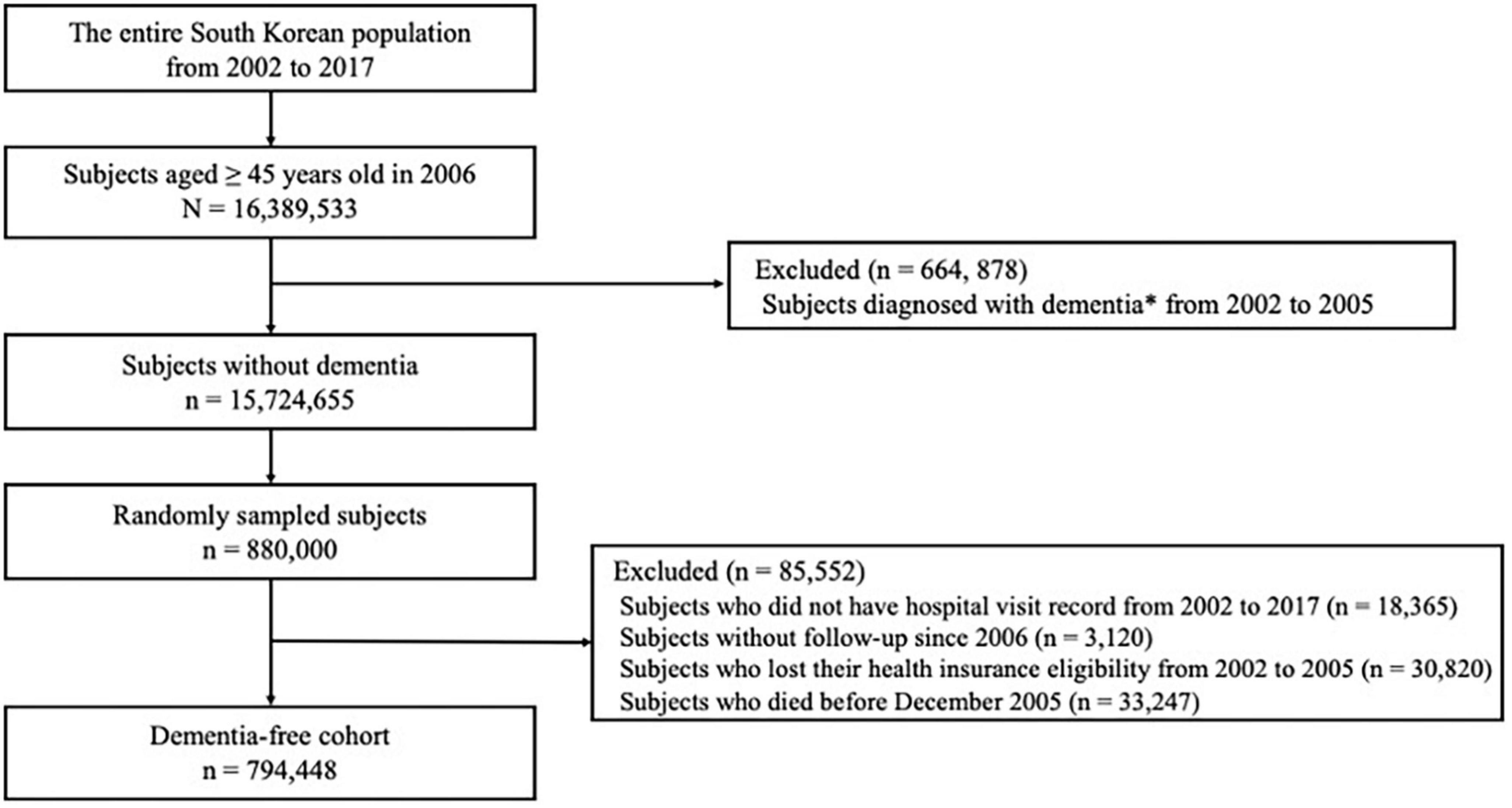
Figure 1. Flow chart of eligible subjects in the dementia-free cohort. *Diagnosis of dementia was based on both International Classification of Diseases-10 diagnosis codes (F00, F01, F02, F03, G30, or G31) and medication prescriptions (donepezil, galantamine, rivastigmine, or memantine).
Risk factors and covariates
With respect to risk factors for dementia, we considered coronary heart disease, depression, diabetes, hemorrhagic stroke, ischemic stroke, hypertension, hyperlipidemia, osteoporosis, physical inactivity, smoking status, heavy alcohol consumption, household income, and BMI. Age and sex were considered as covariates. Among these risk factors, coronary heart disease, depression, diabetes, hemorrhagic stroke, ischemic stroke, hypertension, hyperlipidemia, and osteoporosis were diagnosed using the corresponding ICD-10 codes at baseline. BMI was categorized into three groups: normal (18.5–22.9 kg/m2), underweight (<18.5 kg/m2), overweight (23–24.9 kg/m2), or obesity (≥25 kg/m2) (Seo et al., 2019). Smoking was defined as ever-smoker, indicating person who has smoked at least one hundred cigarettes and cigars in lifetime. Physical inactivity was defined as the absence of moderate- or high-intensity physical activity. Moderate- or high-intensity physical activity was defined as having physical activity for more than 10 min at least once a week. Heavy alcohol consumption was defined as ≥30 g of alcohol consumption per day. Low socioeconomic status was defined as lower 20% of household income.
Statistical analysis
Our cohort was dementia free at baseline and was followed up for dementia incidence. We calculated the crude, age-specific, and sex-specific dementia incidence rates. CIs of incidence rates were obtained under the assumption that the number of events follows a Poisson distribution. Age- and sex-adjusted incidence rates were summed to obtain the standardized Korean incidence rates by weighting them with their proportions in the general Korean population. Furthermore, we calculated the relative risks (RRs) using log-binomial regression to adjust for the effect of covariates (McNutt et al., 2003). The response variables were assumed to follow a binomial distribution, and a logarithm was used as a link function. Univariable and multivariable log-binomial models were adopted to assess the risk factors associated with dementia so that the RR of each risk factor could be calculated. The multivariable model included factors that showed statistical significance in the univariate analysis (Supplementary Table 1). The model was adjusted for age, sex, BMI, smoking status, alcohol consumption, socioeconomic status, and physical activity. We also calculated the population-attributable fraction (PAF) using Levin’s formula:
In our PAF analyses we used a nationwide representative cohort data and RRs were estimated after adjusting the effect of other important covariates by using a generalized linear model (GLM) with a log link function for binomial data. Our GLM analyses included several covariates, and correlations between RR were adjusted. Therefore, we did not need to adjust the communality.
Estimates using the log-binomial model after adjusting for covariates were utilized for RR. The Health Insurance Review & Assessment Service-National Patient Sample (HIRA-NPS) and the Korea National Health and Nutrition Examination Survey data, a representative sample database of the Korean population, were used to investigate the prevalence of risk factors. The prevalence of coronary heart disease, depression, diabetes, hemorrhagic stroke, ischemic stroke, hypertension, hyperlipidemia, and osteoporosis for those aged 45 years and older were estimated using the HIRA-NPS dataset (2018). The prevalence of underweight, overweight, obesity, physical inactivity, smoking, heavy alcohol consumption, and low socioeconomic status among those aged 45 years and older were estimated by the Korea National Health and Nutrition Examination Survey (2018).
All statistical analyses were performed using SAS (version 9.3; SAS Institute, Inc., Cary, NC, USA), R Statistical Software (version 3.5; Foundation for Statistical Computing, Vienna, Austria), and Rex (Version 3.0.3, RexSoft Inc., Seoul, Republic of Korea). The significance level for the statistical analyses was set at 0.05.
Results
Among the 794,448 subjects in the dementia-free cohort, 364,765 (45.9%) were males, and 429,683 subjects (54.1%) were females. The most prevalent risk factors at baseline were physical inactivity (56.9%), followed by hypertension (32.4%), smoking (30.9%), and diabetes (21.3%) (Table 2). Among 794,448 subjects in the dementia-free cohort, 49,524 subjects developed all-cause dementia between 2006 and 2017. Compared to subjects who were dementia-free at follow-up, subjects who developed dementia had a female predominance and had a higher prevalence of hypertension, diabetes, dyslipidemia, coronary heart disease, ischemic stroke, hemorrhagic stroke, depression, osteoporosis, underweight, physical inactivity, and low socioeconomic status.
Dementia incidence by year, age, and sex
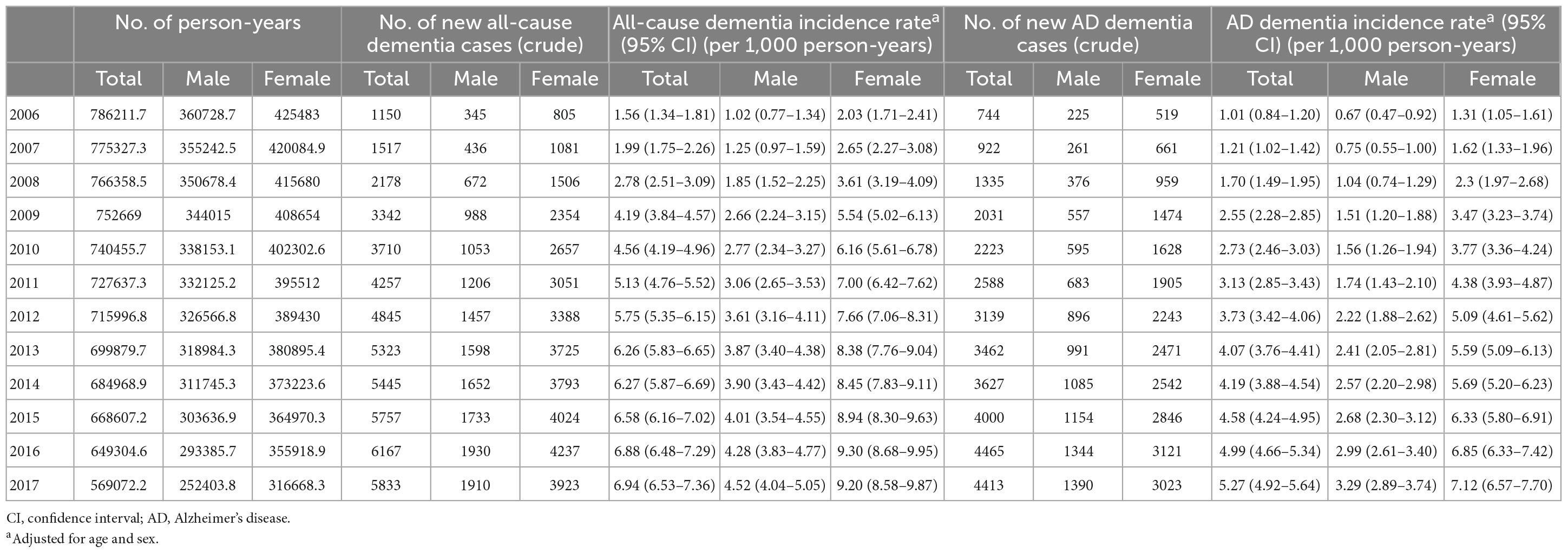
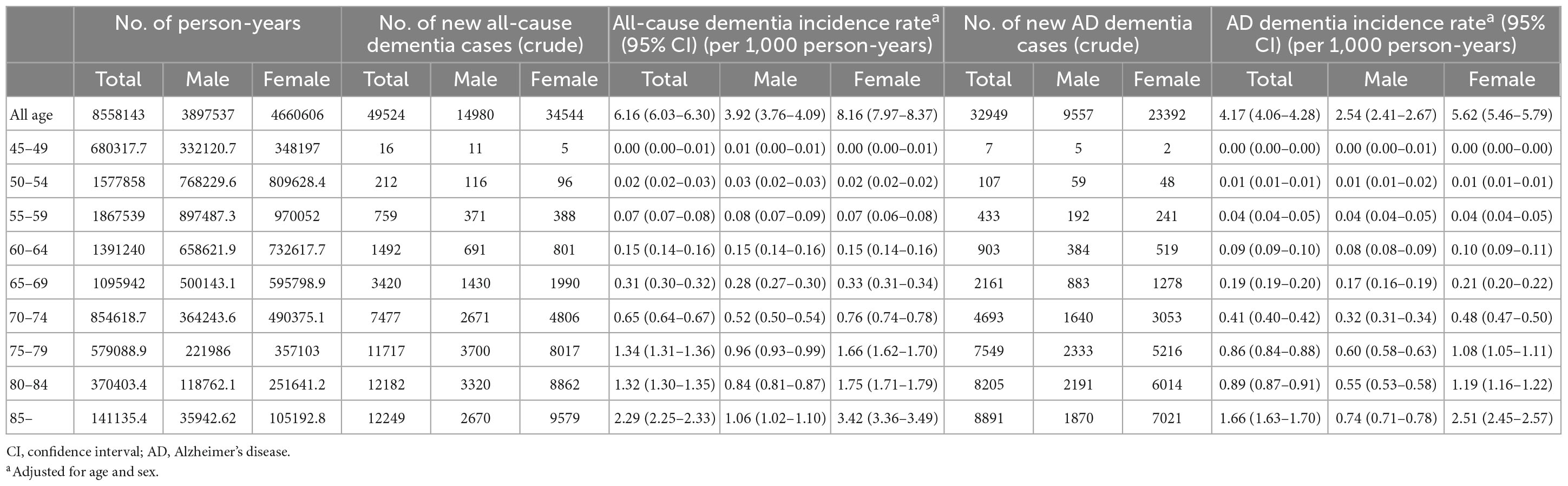
Of all patients with dementia, 66.5% (32,949 subjects) had AD dementia. The incidence rate of all-cause dementia showed annual growth, increasing from 1.56 per 1,000 person-years in 2006 to 6.94 per 1,000 person-years in 2017. The incidence rate of AD dementia also showed annual growth, increasing from 1.01 per 1,000 person-years in 2006 to 5.27 per 1,000 person-years in 2017 (Table 4 and Figures 2A, B).
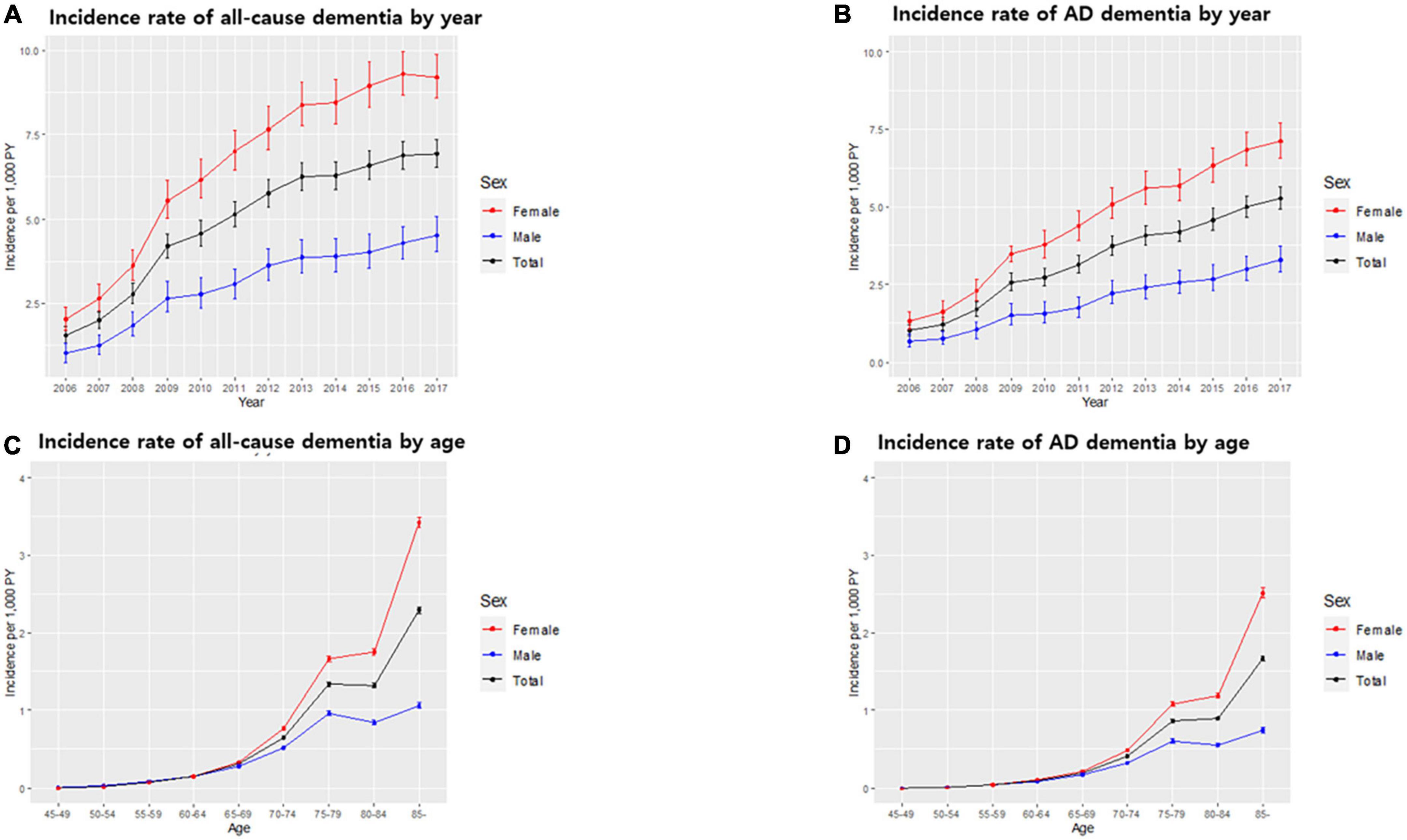
Figure 2. Incidence rate of dementia in the South Korean population. Incidence rate of all-cause dementia (A) and Alzheimer’s disease (AD) dementia (B) by year. Incidence rate of all-cause dementia (C) and AD dementia (D) by age. Bars represent 95% confidence intervals.
The incidence of dementia increases with age. In our cohort, among 49,524 subjects who developed dementia, 2,479 subjects (5.0%) were early onset who were diagnosed with dementia younger than 65 years of age. The incidence rate of early onset dementia (EOD) was 0.24 per 1,000 person-years, while the incidence rate of late-onset dementia was 5.91 per 1,000 person-years. Among dementia subtypes, the incidence rate of early onset AD (EOAD) was 0.14 per 1,000 person-years, while the incidence rate of late-onset AD was 4.01 per 1,000 person-years (Table 3 and Figures 2C, D).
With respect to dementia incidence by sex, of all dementia patients, more females (34,544; 69.8%) than males (14,980; 30.2%) developed dementia. The incidence rates of all-cause dementia and AD dementia in males were lower than those in females over the total follow-up period (Table 3). There was no significant difference in the incidence rate by age between males and females aged <65 years. However, in the age group over 65 years, the incidence rate of all-cause dementia and AD dementia by age in females was higher than that in males, and the gap of the incidence rate between males and females increased with age (Table 3 and Figures 2C, D). The incidence rate of all-cause EOD was 0.27 per 1,000 person-years in males and 0.24 per 1,000 person-years in females. Among dementia subtypes, the incidence rate of EOAD was 0.13 per 1,000 person-years in males and 0.15 per 1,000 person-years in females.
Risk factors for all-cause dementia and AD dementia
In the multivariable log-binomial regression model, depression (RR 1.25, 95% CI 1.21–1.30), diabetes (RR 1.19, 95% CI 1.16–1.22), ischemic stroke (RR 1.19, 95% CI 1.14–1.23), physical inactivity (RR 1.12, 95% CI 1.09–1.15), osteoporosis (RR 1.10, 95% CI 1.07–1.13), and hypertension (RR 1.08, 95% CI 1.04–1.11) increased risk of all-cause dementia (Table 4). In the same analysis depression (RR 1.29, 95% CI 1.24–1.35), hemorrhagic stroke (RR 1.22, 95% CI 1.05–1.42), diabetes (RR 1.18, 95% CI 1.14–1.22), physical inactivity (RR 1.12, 95% CI 1.08–1.16), ischemic stroke (RR 1.12, 95% CI 1.07–1.19), osteoporosis (RR 1.08, 95% CI 1.04–1.12), and hypertension (RR 1.06, 95% CI 1.02–1.10) increased the risk of AD dementia (Table 5).
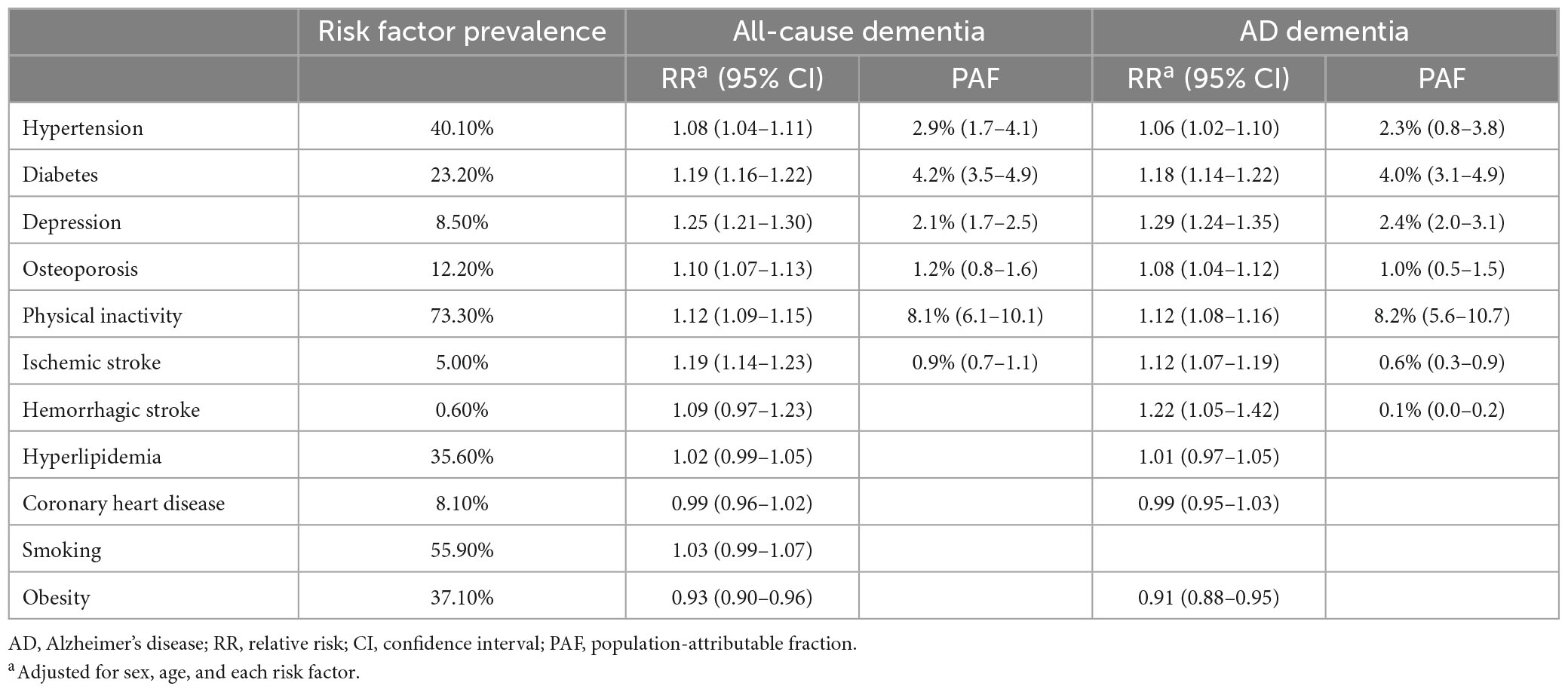
Table 5. Relative risk and population-attributable fraction of risk factors for all-cause dementia and AD dementia.
PAF for all-cause dementia and AD dementia
Among the risk factors, physical inactivity attributed the greatest to all-cause dementia (PAF, 8.1%), followed by diabetes (PAF, 4.2%) and hypertension (PAF, 2.9%). The overall PAF for all-cause dementia was 18.0%. With respect to AD dementia, physical inactivity attributed the greatest (PAF, 8.2%) followed by diabetes (PAF, 4.0%) and depression (PAF, 2.4%). The overall PAF for AD dementia was 17.4%.
Discussion
In this study, we identified dementia incidence and dementia risk factors using the Korean national cohort data. The incidence rate of all-cause dementia continuously increased from 2006 to 2017, reaching 6.94 (4.53 in males, 9.20 in females) per 1,000 person-years in 2017. Of the total incidence of all-cause dementia, 5.0% of cases were EOD. AD dementia accounted for 66.5% of the total dementia incidence. The incidence rate of AD dementia also increased from 2006 to 2017, reaching 5.27 (3.29 in males, 7.20 in females) per 1,000 person-years in 2017. Of several risk factors, physical inactivity and diabetes contributed the highest to the occurrence of dementia.
We identified an increasing trend in the incidence of dementia in Republic of Korea over 10 years. Trends in dementia incidence vary according to study design, population, and time of study. Declining or stable trends were reported in several western high-income countries such as the United States, the UK, Sweden, Netherlands, Canada, and France during the 2000s and the early 2010s (Manton et al., 2005; Schrijvers et al., 2012; Matthews et al., 2013, 2016; Qiu et al., 2013; Satizabal et al., 2016; Derby et al., 2017; Wu et al., 2017; Stephan et al., 2018; Livingston et al., 2020). Multiple factors appear to have a complex influence on these trends. Several previous studies suggested that higher educational level, decreasing smoking prevalence, advances in management of vascular risk factors and cardiovascular diseases, and healthier lifestyles, might explain the decrease in dementia incidence (Larson et al., 2013; de Bruijn et al., 2015; Jørgensen et al., 2015; Grasset et al., 2016; Satizabal et al., 2016; Langa et al., 2017; Livingston et al., 2017, 2020; Wu et al., 2017). Conversely, several studies showed annual growth in the incidence of dementia. In the Danish population aged 65–74 years, the incidence of dementia increased from 0.79 per 1,000 person-years in 2000 to 1.52 per 1,000 person-years in 2009 (Jørgensen et al., 2015). In the Welsh population aged 60 years and older, the incidence of AD dementia increased from 1.4 per 1,000 person-years in 1999 to 1.9 per 1,000 person-years in 2010 (Engelhardt et al., 2002). In addition, increasing trends have been reported in other far-eastern high-income countries such as Japan, Hong Kong, and Taiwan (Wu et al., 2017; Gao et al., 2019; Livingston et al., 2020). The longevity or aging population is the main cause of increasing dementia incidence. In addition, along with economic growth more screening tests for dementia are performed. In Republic of Korea, since the National Institution of Dementia was established in 2013 as a national project, the dementia screening service was initiated nationwide. Consequently, previously undiagnosed patients were diagnosed with dementia, which resulted in an increased dementia incidence.
As expected, the incidence of dementia increased with age. Of the total dementia incidence, EOD accounted for 5.0%, and the incidence rate of EOD was 0.24 per 1,000 person-years in Republic of Korea. In several community-based or nationwide studies, the frequency of EOD among all-cause dementia ranges from 7.3 to 45.3% (Fujihara et al., 2004; Yokota et al., 2005; Garre-Olmo et al., 2010; Noh et al., 2014). According to the results of two recent population-based studies, the incidence rate of EOD was 0.13 per 1000 person-years, and EOD cases were 6.9% of all-cause dementia in Spain (Garre-Olmo et al., 2010; Noh et al., 2014). In the UK, the incidence rate of EOD was 0.12 per 1000 person-years (Mercy et al., 2008; Noh et al., 2014). The incidence rate of AD dementia also increased with age. Of the total AD dementia incidence, EOAD accounted for 4.4%, and the incidence rate of EOAD was 0.14 per 1,000 person-years. According to the results of nationwide studies in several other countries, the incidence rate of EOAD varied from 0.024 to 0.226 per 1000 person-years (Vieira et al., 2013).
We observed that in the age group over 65 years, the incidence rate of all-cause dementia and AD dementia in females was significantly higher than that in males. Females accounted for 69.8% of all patients with dementia. There are several possible reasons for this discrepancy. First, there are risk factors for dementia which differ by sex in frequency and prevalence. For example, a low education level, depression, and limited social activities are more frequent in females (Rocca et al., 2014; Mielke, 2018). Second, since the life expectancy for females is longer than for males, females have greater lifetime risk for dementia. In addition, a physiologic condition such as menopause, a dramatic fall of estrogen and progesterone in late middle age, may increase dementia risk for females (Scheyer et al., 2018).
We found that the contribution of the three highest modifiable risk factors (physical inactivity, diabetes, and hypertension) to all-cause dementia was 15.2%, and the contribution of the three highest modifiable risk factors (physical inactivity, diabetes, and depression) to AD dementia was 15.8%. A Japanese cohort study conducted in 2006 showed similar results to our study, that physical inactivity, diabetes and severe psychological distress are the highest modifiable risk factors for dementia (Kotaki et al., 2019). On the other hand, our results were different from the PAFs reported by Livingston et al. (2017, 2020). In the previous study, the RR and the prevalence of each risk factor were obtained from other reports, whereas in our study, the RR and prevalence of each risk factor were obtained from the Korean national cohort, which we believe better reflects the situation of dementia in Republic of Korea. In addition, the risk factors included in the current study was different from those included in previous studies. In Republic of Korea, the most prevalent modifiable risk factors were physical inactivity (73.3%), hypertension (40.1%), and diabetes (23.2%). There is lack of physical activity in the Korean adult population (Kim et al., 2002). In addition, the prevalence of type 2 diabetes is rapidly increasing in Asian populations (Ramachandran et al., 2010; Kim et al., 2015) including South Korean population. Therefore, more aggressive monitoring and control of these risk factors will enable more effective dementia prevention. Especially, encouraging lifestyle modifications to increase physical activity is critical in dementia prevention. Ultimately, effective dementia prevention strategies may reduce the medical and societal costs of dementia management and burden on the patient’s family or guardians.
The present study has several limitations. First, since the presence of risk factors was evaluated only at baseline, newly developed risk factors during the follow-up period were not considered. Second, because data on the education level, hearing loss, traumatic brain injury, social isolation, and air pollution were not available from the NHIS data, the PAFs of these factors could not be estimated. In addition, the relationships between the risk factors are complex, as one may affect the other. For example, lack of physical activity may lead to hypertension and diabetes, ultimately increasing the risk for dementia. It will be necessary to investigate the contribution of various risk factors to dementia considering their complex relationships in future studies. Third, because dementia occurrence was defined by the first prescription of dementia medicine and diagnosis with dementia codes based on the Korean Standard Classification of Diseases, dementia subtypes other than AD might have been underdiagnosed. Finally, we did not calculate medication effects between risk factors.
Nevertheless, the strength of this study is that it is the first national cohort study in Republic of Korea to investigate dementia incidence by year, age, and sex using a dataset representing the South Korean population. Furthermore, since this study was conducted using data from one single national cohort, high communality was guaranteed. This study could contribute to better understand the current situation of dementia in various countries and predict about future trends in dementia worldwide.
Conclusion
As the incidence of dementia in Republic of Korea is gradually increasing, the social and individual burden of dementia is also growing. It is important to understand the trend of dementia incidence and modifiable risk factors for dementia to establish optimal strategies for dementia prevention in each population.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: the datasets used and/or analyzed during the current study are available from the corresponding authors (DL and HK) on reasonable request. Requests to access these datasets should be directed to DL, bGRobHNlQGdtYWlsLmNvbQ==.
Ethics statement
The studies involving human participants were reviewed and approved by the Samsung Medical Center Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
DL and HK: study design and conduct. SH and JL: data analysis and writing the manuscript. JL, GH, and SW: statistical analysis. MC: acquisition of data. HJ, SS, and DN: interpretation of findings. All authors read and approved the final manuscript.
Funding
This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (HU21C0111), the MSIT (Ministry of Science and ICT), Republic of Korea, under the ICT Creative Consilience program (IITP-2023-2020-0-01821), supervised by the IITP (Institute for Information & Communications Technology Planning & Evaluation), and the National Research Foundation of Korea grant funded by the Korean Government’s Ministry of Education (NRF-2021R1C1C1007795, Seoul, Republic of Korea).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1126587/full#supplementary-material
Abbreviations
AD, Alzheimer’s disease; BMI, body mass index; CI, confidence interval; EOD, early onset dementia; EOAD, early onset AD; HIRA-NPS, health insurance review and assessment service-national patient sample; ICD-10, international classification of diseases, 10th revision; KCD-7, Korean standard classification of diseases, 7th revision; NHIS, national health insurance service data; PAFs, population-attributable fractions; RR, relative risks.
Footnotes
References
Cadar, D., Lassale, C., Davies, H., Llewellyn, D. J., Batty, G. D., and Steptoe, A. (2018). Individual and area-based socioeconomic factors associated with dementia incidence in England: evidence from a 12-year follow-up in the English longitudinal study of ageing. JAMA Psychiatry 75, 723–732. doi: 10.1001/jamapsychiatry.2018.1012
Cho, H., Kim, J.-H., Kim, C., Ye, B. S., Kim, H. J., Yoon, C. W., et al. (2014). Shape changes of the basal ganglia and thalamus in Alzheimer’s disease: a three-year longitudinal study. J. Alzheimer’s Dis. 40, 285–295. doi: 10.3233/JAD-132072
de Bruijn, R. F., Bos, M. J., Portegies, M. L., Hofman, A., Franco, O. H., Koudstaal, P. J., et al. (2015). The potential for prevention of dementia across two decades: the prospective, population-based Rotterdam study. BMC Med. 13:132. doi: 10.1186/s12916-015-0377-5
Derby, C. A., Katz, M. J., Lipton, R. B., and Hall, C. B. (2017). Trends in dementia incidence in a birth cohort analysis of the Einstein Aging Study. JAMA Neurol. 74, 1345–1351. doi: 10.1001/jamaneurol.2017.1964
Engelhardt, E., Laks, J., Dourado, M. C., Mezzasalma, M. A. U., Carvalho-Pinto, M., Chalita-Gomes, A., et al. (2002). Demência pré-senil: impacto psicossocial. Rev. Brasil. Neurol. 38, 5–11.
Fiest, K. M., Roberts, J. I., Maxwell, C. J., Hogan, D. B., Smith, E. E., Frolkis, A., et al. (2016). The prevalence and incidence of dementia due to Alzheimer’s disease: a systematic review and meta-analysis. Can. J. Neurol. Sci. 43, S51–S82.
Fujihara, S., Brucki, S., Rocha, M. S. G., Carvalho, A. A., and Piccolo, A. C. (2004). Prevalence of presenile dementia in a tertiary outpatient clinic. Arq. Neuro Psiquiatr. 62, 592–595. doi: 10.1590/s0004-282x2004000400005
Gao, S., Burney, H. N., Callahan, C. M., Purnell, C. E., and Hendrie, H. C. (2019). Incidence of dementia and Alzheimer disease over time: a meta-analysis. J. Am. Geriatr. Soc. 67, 1361–1369. doi: 10.1111/jgs.16027
Garre-Olmo, J., Batlle, D. G., del Mar Fernández, M., Daniel, F. M., de Eugenio Huélamo, R., Casadevall, T., et al. (2010). Incidence and subtypes of early-onset dementia in a geographically defined general population. Neurology 75, 1249–1255. doi: 10.1212/WNL.0b013e3181f5d4c4
Grasset, L., Brayne, C., Joly, P., Jacqmin-Gadda, H., Peres, K., Foubert-Samier, A., et al. (2016). Trends in dementia incidence: evolution over a 10-year period in France. Alzheimer’s Dement. 12, 272–280. doi: 10.1016/j.jalz.2015.11.001
Jørgensen, T. S. H., Torp-Pedersen, C., Gislason, G. H., Andersson, C., and Holm, E. (2015). Time trend in Alzheimer diagnoses and the association between distance to an Alzheimer clinic and Alzheimer diagnosis. Eur. J. Public Health 25, 522–527. doi: 10.1093/eurpub/cku118
Kim, H. J., Im, K., Kwon, H., Lee, J. M., Kim, C., Kim, Y. J., et al. (2015). Clinical effect of white matter network disruption related to amyloid and small vessel disease. Neurology 85, 63–70. doi: 10.1212/wnl.0000000000001705
Kim, J. K., Kim, S. U., Hahm, B. J., Lee, J. Y., and Cho, M. J. (2002). Three and half year follow-up study on a rural elderly cohort: prevalence, incidence, and service utilization of dementia and depressive disorders. J. Korean Geriatr. Psychiatry 6, 88–96.
Kotaki, Y., Tomata, Y., Tanji, F., Zhang, S., Sugawara, Y., and Tsuji, I. (2019). Joint impact of seven risk factors on incident dementia in elderly Japanese: the Ohsaki Cohort 2006 study. J. Neurol. 266, 1222–1229. doi: 10.1007/s00415-019-09252-w
Langa, K. M., Larson, E. B., Crimmins, E. M., Faul, J. D., Levine, D. A., Kabeto, M. U., et al. (2017). A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Int. Med. 177, 51–58.
Larson, E. B., Yaffe, K., and Langa, K. M. (2013). New insights into the dementia epidemic. N. Engl. J. Med. 369:2275.
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet 396, 413–446.
Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D., et al. (2017). Dementia prevention, intervention, and care. Lancet 390, 2673–2734.
Manton, K. G., Gu, X., and Ukraintseva, S. (2005). Declining prevalence of dementia in the US elderly population. Adv. Gerontol. 16, 30–37.
Matthews, F. E., Arthur, A., Barnes, L. E., Bond, J., Jagger, C., Robinson, L., et al. (2013). A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the cognitive function and ageing Study I and II. Lancet 382, 1405–1412. doi: 10.1016/S0140-6736(13)61570-6
Matthews, F. E., Stephan, B. C., Robinson, L., Jagger, C., Barnes, L. E., Arthur, A., et al. (2016). A two decade dementia incidence comparison from the cognitive function and ageing Studies I and II. Nat. Commun. 7:11398.
Matthews, K. A., Xu, W., Gaglioti, A. H., Holt, J. B., Croft, J. B., Mack, D., et al. (2019). Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged= 65 years. Alzheimer’s Dement. 15, 17–24. doi: 10.1016/j.jalz.2018.06.3063
McNutt, L.-A., Wu, C., Xue, X., and Hafner, J. P. (2003). Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am. J. Epidemiol. 157, 940–943.
Mercy, L., Hodges, J., Dawson, K., Barker, R., and Brayne, C. (2008). Incidence of early-onset dementias in Cambridgeshire. U. K. Neurol. 71, 1496–1499.
Mielke, M. M. (2018). Sex and gender differences in Alzheimer’s disease dementia. Psychiatr. Times 35, 14–17.
Noh, Y., Jeon, S., Lee, J. M., Seo, S. W., Kim, G. H., Cho, H., et al. (2014). Anatomical heterogeneity of Alzheimer disease: based on cortical thickness on MRIs. Neurology 83, 1936–1944. doi: 10.1212/WNL.0000000000001003
Ohara, T., Hata, J., Yoshida, D., Mukai, N., Nagata, M., Iwaki, T., et al. (2017). Trends in dementia prevalence, incidence, and survival rate in a Japanese community. Neurology 88, 1925–1932.
Qiu, C., Kivipelto, M., and Von Strauss, E. (2022). Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci. 11, 111–128.
Qiu, C., von Strauss, E., Bäckman, L., Winblad, B., and Fratiglioni, L. (2013). Twenty-year changes in dementia occurrence suggest decreasing incidence in central Stockholm. Sweden. Neurol. 80, 1888–1894. doi: 10.1212/WNL.0b013e318292a2f9
Ramachandran, A., Ma, R. C., and Snehalatha, C. (2010). Diabetes in Asia. Lancet 375, 408–418. doi: 10.1016/s0140-6736(09)60937-5
Rocca, W. A., Mielke, M. M., Vemuri, P., and Miller, V. M. (2014). Sex and gender differences in the causes of dementia: a narrative review. Maturitas 79, 196–201. doi: 10.1016/j.maturitas.2014.05.008
Satizabal, C. L., Beiser, A. S., Chouraki, V., Chêne, G., Dufouil, C., and Seshadri, S. (2016). Incidence of dementia over three decades in the Framingham heart study. N. Engl. J. Med. 374, 523–532.
Scheyer, O., Rahman, A., Hristov, H., Berkowitz, C., Isaacson, R. S., Diaz Brinton, R., et al. (2018). Female sex and Alzheimer’s risk: the menopause connection. J. Prev. Alzheimers Dis. 5, 225–230. doi: 10.14283/jpad.2018.34
Schrijvers, E. M., Verhaaren, B. F., Koudstaal, P. J., Hofman, A., Ikram, M. A., and Breteler, M. M. (2012). Is dementia incidence declining?: Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology 78, 1456–1463.
Seo, M. H., Lee, W.-Y., Kim, S. S., Kang, J.-H., Kang, J.-H., Kim, K. K., et al. (2019). 2018 Korean society for the study of obesity guideline for the management of obesity in Korea. J. Obes. Metab. Syndr. 28, 40–45.
Stephan, B., Birdi, R., Tang, E. Y. H., Cosco, T. D., Donini, L. M., Licher, S., et al. (2018). Secular trends in dementia prevalence and incidence worldwide: a systematic review. J. Alzheimer’s Dis. 66, 653–680.
van Bussel, E. F., Richard, E., Arts, D. L., Nooyens, A. C., Coloma, P. M., de Waal, M. W., et al. (2017). Dementia incidence trend over 1992-2014 in the Netherlands: analysis of primary care data. PLoS Med. 14:e1002235. doi: 10.1371/journal.pmed.1002235
Vieira, R. T., Caixeta, L., Machado, S., Silva, A. C., Nardi, A. E., Arias-Carrión, O., et al. (2013). Epidemiology of early-onset dementia: a review of the literature. Clin. Pract. Epidemiol. Ment. Health 9, 88–95.
Wu, Y.-T., Beiser, A. S., Breteler, M. M., Fratiglioni, L., Helmer, C., Hendrie, H. C., et al. (2017). The changing prevalence and incidence of dementia over time—current evidence. Nat. Rev. Neurol. 13, 327–339. doi: 10.1038/nrneurol.2017.63
Keywords: dementia, Alzheimer’s disease dementia, national cohort, risk factor, population-attributable fraction
Citation: Hwangbo S, Lee JY, Han G, Chun MY, Jang H, Seo SW, Na DL, Won S, Kim HJ and Lim DH (2023) Dementia incidence and population-attributable fraction for dementia risk factors in Republic of Korea: a 12-year longitudinal follow-up study of a national cohort. Front. Aging Neurosci. 15:1126587. doi: 10.3389/fnagi.2023.1126587
Received: 18 December 2022; Accepted: 23 June 2023;
Published: 13 July 2023.
Edited by:
Allison B. Reiss, New York University, United StatesReviewed by:
Karen Schliep, University of Utah Health Care, United StatesYuhang Wu, Central South University, China
Copyright © 2023 Hwangbo, Lee, Han, Chun, Jang, Seo, Na, Won, Kim and Lim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hee Jin Kim, ZXZla2hqQGdtYWlsLmNvbQ==; Dong Hui Lim, bGRobHNlQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Song Hwangbo
Song Hwangbo Jin Young Lee3†
Jin Young Lee3† Sang Won Seo
Sang Won Seo Sungho Won
Sungho Won Hee Jin Kim
Hee Jin Kim Dong Hui Lim
Dong Hui Lim