
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 05 May 2023
Sec. Neurocognitive Aging and Behavior
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1120935
 Benedetta Tafuri1,2*
Benedetta Tafuri1,2* Marco Filardi1,2
Marco Filardi1,2 Daniele Urso1,3
Daniele Urso1,3 Valentina Gnoni1,3,4
Valentina Gnoni1,3,4 Roberto De Blasi1,5
Roberto De Blasi1,5 Salvatore Nigro1†
Salvatore Nigro1† Giancarlo Logroscino1,2† and on behalf of the Frontotemporal Lobar Degeneration Neuroimaging Initiative‡
Giancarlo Logroscino1,2† and on behalf of the Frontotemporal Lobar Degeneration Neuroimaging Initiative‡Introduction: Primary Progressive Aphasia (PPA) is a neurological disease characterized by linguistic deficits. Semantic (svPPA) and non-fluent/agrammatic (nfvPPA) variants are the two main clinical subtypes. We applied a novel analytical framework, based on radiomic analysis, to investigate White Matter (WM) asymmetry and to examine whether asymmetry is associated with verbal fluency performance.
Methods: Analyses were performed on T1-weighted images including 56 patients with PPA (31 svPPA and 25 nfvPPA) and 53 age- and sex-matched controls. Asymmetry Index (AI) was computed for 86 radiomics features in 34 white matter regions. The relationships between AI, verbal fluency performance (semantic and phonemic) and Boston Naming Test score (BNT) were explored through Spearman correlation analysis.
Results: Relative to controls, WM asymmetry in svPPA patients involved regions adjacent to middle temporal cortex as part of the inferior longitudinal (ILF), fronto-occipital (IFOF) and superior longitudinal fasciculi. Conversely, nfvPPA patients showed an asymmetry of WM in lateral occipital regions (ILF/IFOF). A higher lateralization involving IFOF, cingulum and forceps minor was found in nfvPPA compared to svPPA patients. In nfvPPA patients, semantic fluency was positively correlated to asymmetry in ILF/IFOF tracts. Performances at BNT were associated with AI values of the middle temporal (ILF/SLF) and parahippocampal (ILF/IFOF) gyri in svPPA patients.
Discussion: Radiomics features depicted distinct pathways of asymmetry in svPPA and nfvPPA involving damage of principal fiber tracts associated with speech and language. Assessing asymmetry of radiomics in PPA allows achieving a deeper insight into the neuroanatomical damage and may represent a candidate severity marker for language impairments in PPA patients.
Primary Progressive Aphasia (PPA) is a rare neurological syndrome, with estimated prevalence of three cases per 100.000 people/persons in the general population (Coyle-Gilchrist et al., 2016). PPA is pathologically associated with Frontotemporal lobe degeneration (FTLD) and clinically characterized by prominent linguistic deficits (Mesulam, 1982; Gorno-Tempini et al., 2011; Leyton et al., 2011; Tee and Gorno-Tempini, 2019). PPA usually arises with subtle speech problems that could progress into an almost total inability to speak.
The semantic (svPPA) and non-fluent/agrammatic (nfvPPA) variants of primary progressive aphasia represent the two main clinical subtypes of PPA (Gorno-Tempini et al., 2011). svPPA and nfvPPA exhibit distinct patterns of cortical atrophy and a discrete underlying pathology (Mesulam, 2001). Over 50% of nfvPPA patients have FTLD tauopathy (including Pick’s disease, Corticobasal Degeneration, Progressive Supranuclear Palsy), and around 20% of patients have TDP-43 proteinopathy (predominantly type A). Conversely, the majority of svPPA has a TDP-43 pathology (Harris and Jones, 2014). Patients with svPPA present loss of word knowledge with difficulties in single-word comprehension and naming, despite relatively preserved grammar and fluency. Conversely, patients with nfvPPA show abnormality of grammar in spoken or written language, and/or apraxia of speech with relatively preserved single-word comprehension (Mesulam, 1982; Ash et al., 2010; Wilson et al., 2010; Gorno-Tempini et al., 2011).
In the past years, several studies have investigated the brain damage associated with PPA, reveling an asymmetric prominence of grey and white matter changes, neuronal loss and disease-specific proteinopathy (Grossman et al., 2008; Mesulam et al., 2008, 2014; Grossman, 2012). Concerning neuroimaging investigations, patients with svPPA show a predominant left-sided temporal patter of gray matter atrophy, with subcortical compromission of the amygdala and hippocampus (Brambati et al., 2009; Collins et al., 2017; Nigro et al., 2021). Conversely, a prominent atrophy of left frontal regions is usually observed in nfvPPA patients (Gorno-Tempini et al., 2011; Mesulam et al., 2014; Mandelli et al., 2016a,b). Moreover, studies that use diffusion tensor imaging (DTI) highlight a severe damage of white matter tracts with a lateralization involving the left inferior longitudinal fasciculus (ILF), uncinate fasciculus (UF) and posterior cingulate in svPPA patients (Galantucci et al., 2011; Agosta et al., 2012; Mahoney et al., 2013; Mesulam et al., 2014; Vandenberghe, 2016; Bouchard et al., 2019; Reyes et al., 2019) while a compromission of the left intrafrontal and frontostriatal fascicules has been reported in nfvPPA patients (Mandelli et al., 2014, 2016a).
In recent years, several studies have highlighted the potentialities of radiomics in the clinical and diagnostic work-up of patients with neurodegenerative diseases as an approach that allows capturing from radiological images non-trivial and complex features (compared to classical morphological approaches) associated with clinical and biological outcomes (Abbasian Ardakani et al., 2022). The radiomic-based analytical framework combines features of global and local regions with machine and deep learning algorithms aiming at unveiling higher-order information underlying specific disorders (Wu et al., 2019; Xiao et al., 2019; Cao et al., 2020; Feng and Ding, 2020; Liu et al., 2020; Jain et al., 2021; Kim et al., 2021; Zhou et al., 2021; Cheung et al., 2022). Studies that used radiomics on gray matter and white matter regions in patients with Alzheimer’s (AD) and Parkinson’s diseases (PD) showed promising results in terms of patients’ characterization by merging the high-throughput extraction of pattern-based information over specific brain regions with clinical data. In the field of FTLD dementias, radiomics has shown excellent performances in distinguishing FTD subtypes (Tafuri et al., 2022).
Given previous evidences of an asymmetrical brain involvement in PPA patients, in this study we applied radiomic analysis to explore white matter laterality pattern in patients with PPA variants and to investigate its relationship with performance at linguistic tasks.
Data used in the preparation of this retrospective study were obtained from the Frontotemporal Lobar Degeneration Neuroimaging Initiative (FTLDNI) database (for up-to-date information on participation and protocol, please visit1). We included 56 patients with PPA (31 svPPA and 25 nfvPPA) and 53 age- and sex-matched healthy control (HC) who had a valid baseline T1-weighted MRI sequence. In order to avoid potential bias derived from different imaging protocols, we selected exclusively images acquired at the University of California, San Francisco (i.e., the largest recruiting center). Approval for the FTLDNI protocol has been granted by institutional review board at the study site. All patients underwent clinical, imaging, language, and neuropsychological examination and fulfilled the current diagnostic criteria for PPA (Gorno-Tempini et al., 2011). The control group consisted of individuals with no previous history of diagnosed neurological or psychiatric disorder and no complaint of memory decline (more information at2).
The Clinical Dementia Rating scale (CDR), with its language subscore (CDR language), was administered to assess the global cognitive status (Morris, 1993; Knopman et al., 2008).
Linguistic abilities were tested with the semantic verbal fluency (animal) and the phonemic verbal fluency (d words) tests (Benton, 1969). Semantic fluency test assesses the ability to verbally generate as many words as possible from a given semantic category. In phonemic task participants are asked to produce as many words as possible beginning with the letter D in 1 minute. Finally, the total Boston Naming Test (BNT) correct score was computed as a measure of general linguistic ability, object naming and word retrieval (Kaplan et al., 1983).
MR images were acquired on a 3 T Siemens Trio Tim system equipped with a 12-channel head coil including whole-brain three-dimensional T1 MPRAGE (TR/TE = 2,300/2.9 ms, matrix = 240 × 256 × 160, isotropic voxels 1 mm3, slice thickness = 1 mm). An experienced neuroradiologist examined the images for brain abnormalities other than atrophy.
The MRI were processed with FreeSurfer 6.0 with the standard cross-sectional pipeline (recon-all). The pre-processing steps comprised removal of non-brain tissue, bias correction, and segmentation into gray matter (GM), white matter (WM), and cerebrospinal fluid. The brain extracted non-uniform intensity corrected image (nu.mgz) was used to compute the radiomics features in WM regions. In particular, the FreeSurfer white matter parcellation approach (Salat et al., 2009) was used to classify the white matter according to the nearest cortical region (Desikan et al., 2006) and obtaining 34 regions of interest (ROIs) for each hemisphere. Detailed information on these procedures have been described in previous publications (Dale et al., 1999; Fischl et al., 2002, 2004).
The python package PyRadiomics (van Griethuysen et al., 2017) was used to extract radiomics features from 68 white matter ROIs (34 regions for each hemisphere). For each ROI, we defined a set of 86 radiomic features, including 16 first-order features to describe voxel intensity distribution within image mask and 70 second-order textural measures to highlight spatial distribution of voxels through five different matrices: 24 features from Gray Level Co-occurrence Matrices (GLCM), 16 from Gray Level Run Length Matrices (GLRLM), 14 measures from Gray Level Dependence Matrices (GLDM) and 16 features from Gray Level Size Zone Matrices (GLSZM) (Zwanenburg et al., 2020).
Given a brain region, the Asymmetry Index (AI) was computed for each radiomics measure as the ratio of the absolute difference between left and right feature values to its sum, multiplied by 100 (Pedraza et al., 2004; Heckemann et al., 2011; Kim et al., 2012; Okada et al., 2016; Sarica et al., 2018):
Data were explored with descriptive statistics (mean ± standard deviation or frequency).
Group differences in demographical, clinical data and performance at linguistic tasks were analyzed with chi-square test and Kruskal-Wallis analysis of variance followed by post-hoc test.
For each radiomics feature, analysis of variance was used to compare asymmetry index values between groups while controlling for age, gender and education, followed by effect size (Cohen’s d) computation (Cohen, 1988). Significance threshold was set at p < 0.05 after Bonferroni correction for multiple comparisons.
Partial correlation (Spearman’s correlation) was used to explore the relationship between linguistic tests performances and AI values while controlling for the influence of age, gender, education and CDR total score. We considered only moderate correlations (Spearman r > |0.4|) (Schober et al., 2018). Statistical analysis was performed by using R software (Version 3.6.3: R Foundation for Statistical Computing, Vienna, Austria).
White matter areas that were statistically significant in pairwise comparisons and correlation analyses were mapped onto the JHU WM tractography atlas (Wakana et al., 2007) to identify white matter tracts involved in PPA neurodegeneration.
Demographic and clinical data are reported in Table 1. No significant group differences emerged in education and gender. Patients with nfvPPA were older than controls and svPPA patients (p < 0.01, Bonferroni corrected). Concerning clinical data, PPA groups had higher CDR scores than controls (value of p < 0.001, Bonferroni corrected). Linguistic tests scores were lower in PPA patients respect to control group (p < 0.001, Bonferroni corrected). Significant differences in phonemic verbal fluency test emerged between patients’ groups, with nfvPPA patients presenting lower scores than svPPA (d words, p = 0.01, Bonferroni corrected) and svPPA patients showing lower scores at BNT test than nfvPPA patients (p < 0.001, Bonferroni corrected).
As reported in Figure 1, svPPA and nfvPPA patients showed an asymmetric pattern of compromission involving ILF and IFOF white matter fasciculi. In particular, radiomics asymmetry in svPPA patients mostly involved white matter regions adjacent to the medial orbitofrontal and middle temporal cortices as part of ILF/IFOF, SLF fasciculi and forceps minor white matter tract (detailed radiomics features are reported in Figure 2A). On the contrary, nfvPPA showed an asymmetrical radiomics in fusiform, isthmus cingulate and lateral occipital regions comprised in ILF, IFOF and forceps major fiber tracts (Figure 2B). Finally, between groups comparison depicted significative lateralized measures in rostral middle frontal, superior parietal and superior frontal cortices, corresponding to SLF, IFOF, cingulum and forceps minor white matter tracts (Figures 1C, 2C).
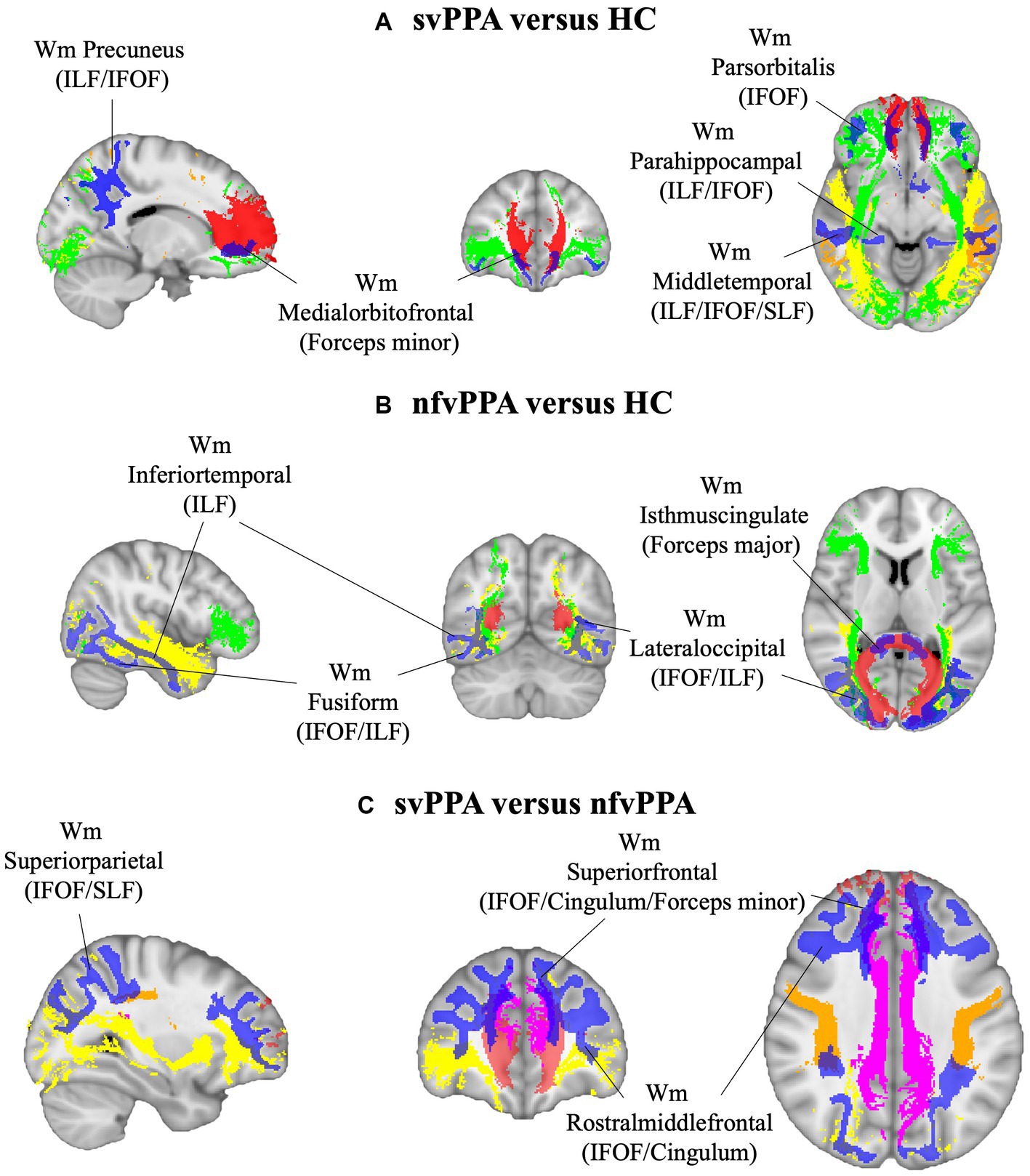
Figure 1. White matter ROIs (in blue) and tracts involved (red, Fornix; green, IFOF; yellow, ILF; orange, SLF; pink, cingulum) for (A) svPPA versus HC, (B) nfvPPA versus HC and (C) svPPA versus nfvPPA comparisons. HC, Healthy Controls; nfvPPA, non-fluent variant of Primary Progressive Aphasia; svPPA, semantic variant of Primary Progressive Aphasia; WM, white matter; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus.
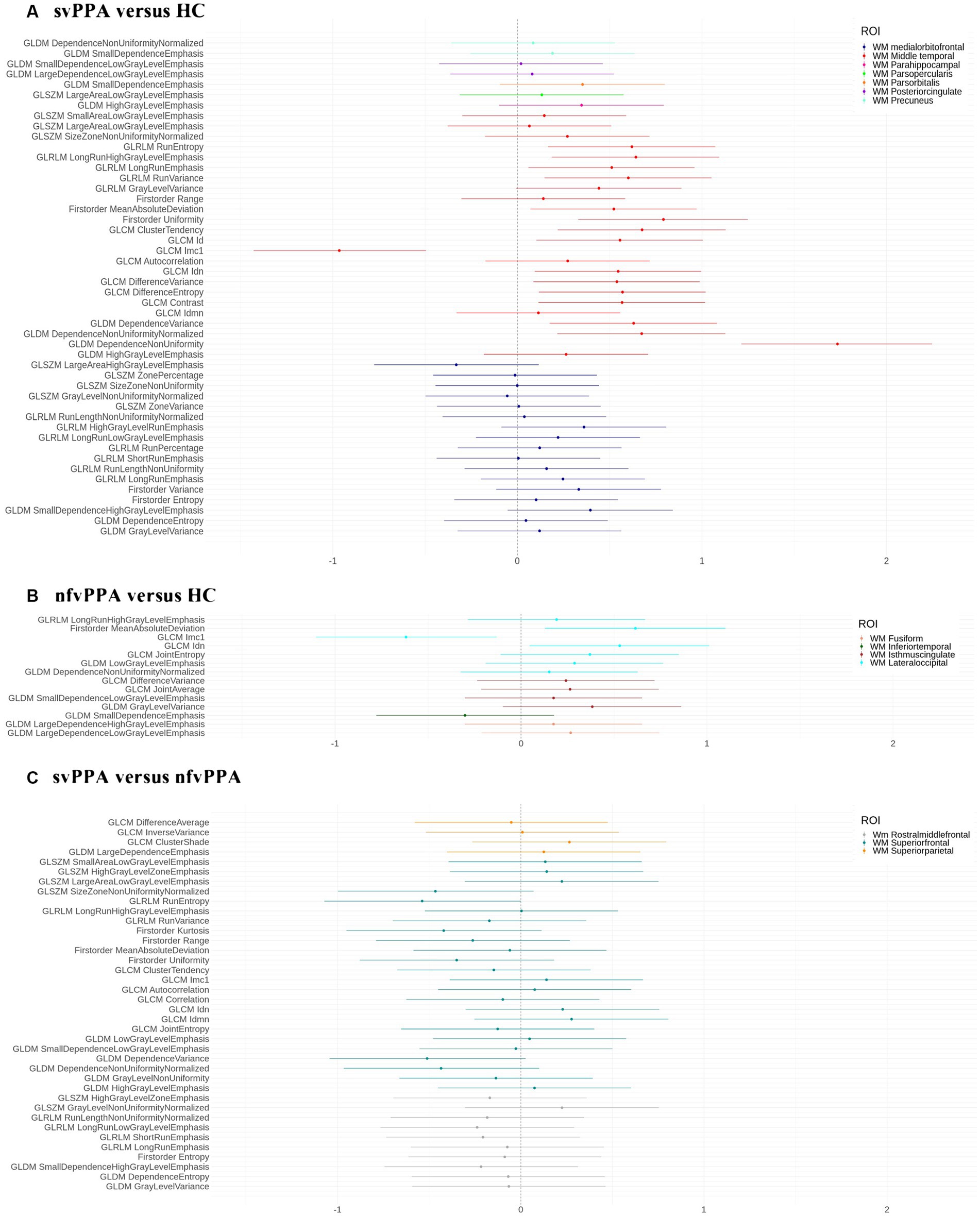
Figure 2. Effect size d for each binary comparison. (A) svPPA versus HC; (B) nfvPPA versus HC; (C) svPPA versus nfvPPA. We report the selected radiomics features (y-axis) for each ROI (white matter regions). HC, Healthy Controls; nfvPPA, non-fluent variant of Primary Progressive Aphasia; svPPA, semantic variant of Primary Progressive Aphasia; WM, white matter; GLCM, Gray Level Co-occurrence Matrices; GLRLM, Gray Level Run Length Matrices; GLDM, Gray Level Dependence Matrices; GLSZM, Gray Level Size Zone Matrices.
Results of correlation analysis are reported in Table 2. In nfvPPA patients, a significant correlation (p < 0.0059, r = 0.592) was found between semantic fluency scores and AI values of GLCM lmc1 (Informational measure of correlation-1, for more details visits3) evaluated in WM region adjacent to lateral occipital gyrus (ILF/IFOF). On the other hand, BNT scores were significantly associated with two AI measures in WM near the middle temporal (ILF/IFOF/SLF) (p = 0.0002, r = 0.654) and parahippocampal gyri (ILF/IFOF) (p = 0.0004, r = −0.631) of svPPA patients, namely a positive correlation with the range of voxel intensities and a negative correlation with GLDM high grey level emphasis values. No significant correlation was found between AI values and phonemic fluency for both PPA groups.
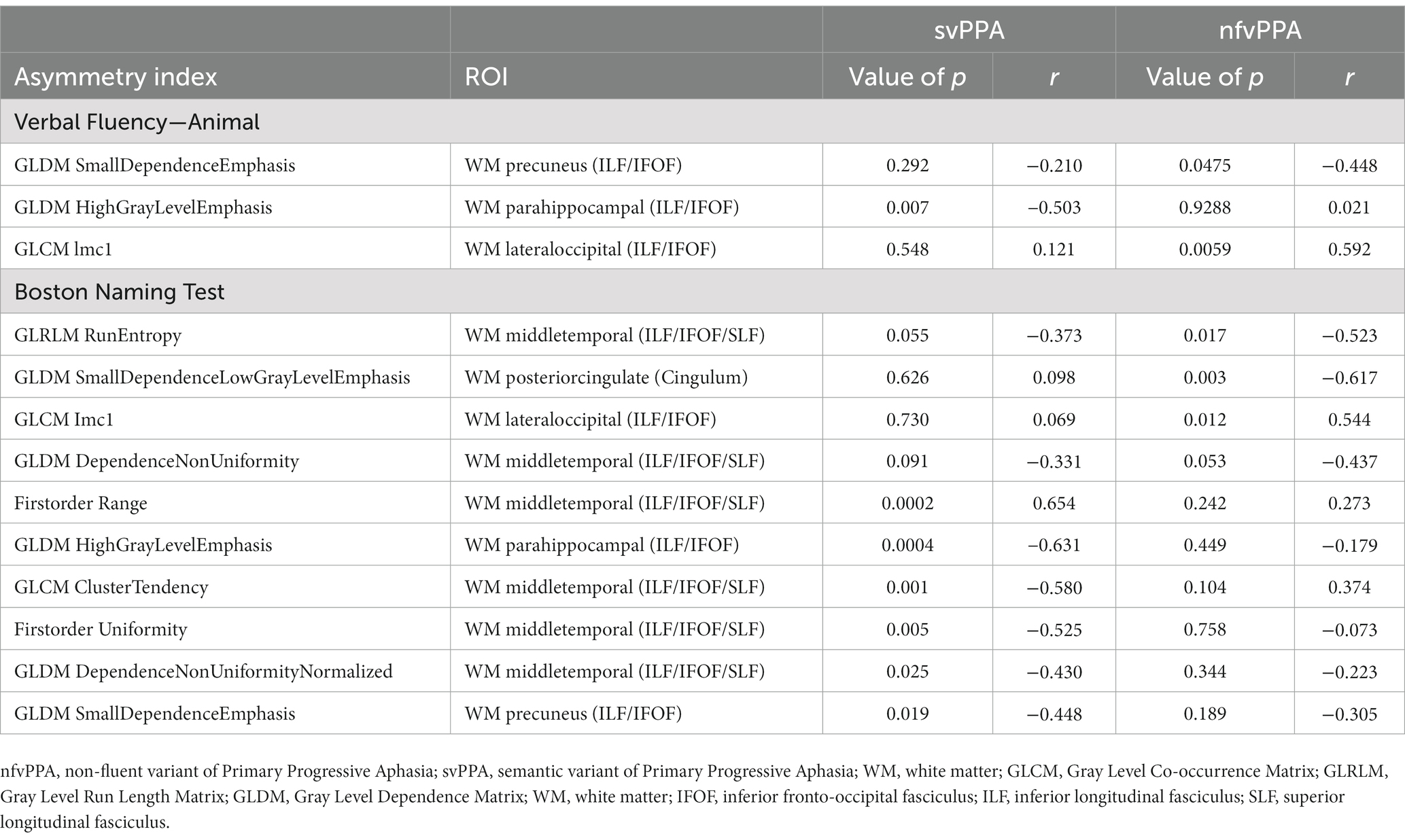
Table 2. Spearman’s correlation coefficients of Verbal Fluency-Animal and Boston Naming test scores with radiomics AI for nfvPPA and svPPA patients.
In the study, we investigated the asymmetry of radiomics features in white matter of svPPA and nfvPPA patients. Compared to controls, PPA patients were characterized by a lateralized structural compromission involving fronto-temporal and occipital white matter areas corresponding to different fiber tracts, namely the inferior longitudinal (ILF) and superior longitudinal (SLF) fasciculi, inferior fronto-occipital fasciculus (IFOF), fornix and cingulum (Routier et al., 2018; Cocquyt et al., 2020). Moreover, an increased asymmetry of specific radiomic measurements in white matter areas adjacent to the lateral occipital gyrus was associated with lower semantic fluency performance in nfvPPA.
Overall, our findings provide further evidence that PPA patients exhibit an asymmetric damage that involved both gray and white matter morphology. Concerning svPPA patients, radiomics findings reported several significative AIs in ILF/IFOF and SLF with a predominance of the white matter near the middle temporal gyrus. Respect to previous study (Lombardi et al., 2021), our finding was in line with the left-sided compromission of this region. Similarly, tractography studies on diffusion-weighted imaging data also confirmed this lateralized damage (Agosta et al., 2012; Routier et al., 2018). In particular, tract analysis showed a focal asymmetric reduction of fractal anisotropy involving the left temporal regions (left SLF) (Bouchard et al., 2019), corresponding to an increased mean diffusivity of ILF, bilaterally. Non fluent/agrammatic variant of PPA, instead, showed an asymmetric white matter damage close to the lateral occipital gyrus, corresponding to ILF and IFOF tracts (Galantucci et al., 2011; Mandelli et al., 2014). Structural compromission of this WM region has been reported in previous voxel-wise study, depicting a lateralized damage of inferior temporal and lateral occipital WM in nfvPPA (Lombardi et al., 2021). This finding was also confirmed by diffusion MRI results observing a fractal anisotropy decreasing in left occipital WM fibers in nfvPPA respect to healthy subjects (Agosta et al., 2012). Noteworthy, the most discriminative WM asymmetry to differentiate svPPA from nfvPPA was found in frontal regions (cingulum and the forceps minor WM tracts). Our result was suggestive of a more pronounced lateralization in frontal WM of nfvPPA respect to svPPA patients (Catani et al., 2013; Spinelli et al., 2017). Furthermore, radiomics analysis assessed the left-sided compromission of the ‘frontal aslant tract’ in nfvPPA patients, as the principal pathway that connect the frontal gyrus with supplementary and pre-supplementary motor areas and playing a crucial role in speech production (Catani et al., 2013; Mandelli et al., 2014; Canu et al., 2019).
Correlation analysis highlighted a strong relationship between the severity of linguistic deficits and AI of radiomics measures particularly between semantic fluency and asymmetry of lateral occipital white matter in nfvPPA patients. This neural correlate confirmed previous findings that demonstrated an association between semantic fluency deficit and a cortical thinning of the left lateral occipital cortex and lingual gyrus (Vonk et al., 2019). Moreover, Li et al. (2017) highlighted the same correlation results with a left-sided compromission of the IFOF tract, posteriorly starting from the lateral occipital white matter (Almairac et al., 2015). On the other hand, our lateralization analysis revealed an association in svPPA patients between WM AIs in the areas close to the parahippocampal/middle temporal and the Boston Naming Test scores. These findings further corroborate the connection of noun production and WM tracts projecting in temporal regions (including the cingulum), underlining the key role of the frontotemporal network in noun production (Ash et al., 2013). Of note, a study of surface stimulation over the parahippocampal gyrus resulted in aphasic reactions, suggesting that it has a role in language comprehension and production (Sharp et al., 2004).
Overall, the strength of our approach raised from the potentiality of radiomics to capture the asymmetric structural deterioration of white matter in PPA patients. Indeed, radiomics was able to highlight the lateralized damage of the principal tracts involved in PPA variants. We demonstrated a focal involvement of the inferior longitudinal and inferior fronto-occipital fasciculi, as part of the speech-production network. Furthermore, correlation analysis disclosed another captivating finding, highlighting the importance of ILF and IFOF tracts as involved in written and spoken language production. Accordingly, radiomics asymmetry may represent a candidate severity marker for language impairments in PPA patients. Overall, all these findings suggest our novel approach extracted from structural MRI makes the proposed study noteworthy and easily reproducible in the clinical practice routine as support to diagnosis and prognosis steps.
The principal limitation of our work is the lack of confirmation of the lateralization of white matter with diffusion-based data. Future studies should merge radiomics approach with diffusion weighted images to investigate the asymmetry of tract damage and to highlight possible association with language deficits in PPA variants. Moreover, the study has a relatively small sample size, which may not be entirely representative of the PPA spectrum, also lacking patients with logopenic variant. Of note, none of our patients had a histopathological diagnostic confirmation. Finally, the lack of clinical interpretation of the selected radiomic measurements makes it difficult to accurately interpret the results. Future studies should points out possible correlations of radiomics with biological data (genomics, transcriptomics, metabolomics, etc.) to give further insights into the clinical relevance of the radiomic findings.
This work provided important findings over structural white matter asymmetry in Primary Progressive Aphasia. Radiomics features depicted a mostly lateralized pattern on semantic variant respect to non-fluent/agrammatic PPA patients involving damage of principal linguistic fiber tracts, respectively the temporal and occipital parts of inferior longitudinal/fronto-occipital fasciculi. Moreover, the evaluation of radiomics asymmetry in association with neuropsychological fluency tasks highlighted novel neuroanatomical correlates with semantic fluency and Boston Naming Test specific for each variant of PPA.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
BT, SN, and GL contributed to conception and design of the study. BT, SN, MF, DU, VG, and RB contributed to analysis and interpretation of data. BT drafted the article. SN, DU, MF, RB, VG, and GL contributed to revise it critically for important intellectual content. BT, SN, MF, DU, RB, VG, and GL provided approval for publication of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
The FTLDNI was funded through the National Institute of Aging and started in 2010. The primary goals of FTLDNI are to identify neuroimaging modalities and methods of analysis for tracking frontotemporal lobar degeneration and to assess the value of imaging vs. other markers in diagnostic roles. The principal investigator of NIFD was Howard Rosen, MD, at the University of California, San Francisco. The data are the result of collaborative efforts at three different sites in North America. Access to the FTLDNI data was approved by the data access committee. This work has been supported with the founding of Regione Puglia and CNR for Tecnopolo per la Medicina di Precisione. D.G.R. n. 2117 of 21.11.2018 (CUPB84I18000540002)—C.I.R.E.M.I.C. (Research Center of Excellence for Neurodegenerative Diseases and Brain Aging)—University of Bari “Aldo Moro.” Data collection and sharing for this project was funded by the Frontotemporal Lobar Degeneration Neuroimaging Initiative (National Institutes of Health Grant R01AG032306). The study is coordinated through the University of California, San Francisco, Memory and Aging Center. FTLDNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^http://memory.ucsf.edu/research
2. ^https://memory.ucsf.edu/research-trials/research/4rtni-2
3. ^https://pyradiomics.readthedocs.io/en/latest/features.html
Abbasian Ardakani, A., Bureau, N. J., Ciaccio, E. J., and Acharya, U. R. (2022). Interpretation of radiomics features-a pictorial review. Comput. Methods Prog. Biomed. 215:106609. doi: 10.1016/j.cmpb.2021.106609
Agosta, F., Scola, E., Canu, E., Marcone, A., Magnani, G., Sarro, L., et al. (2012). White matter damage in frontotemporal lobar degeneration spectrum. Cereb. Cortex N. Y. N 22, 2705–2714. doi: 10.1093/cercor/bhr288
Almairac, F., Herbet, G., Moritz-Gasser, S., de Champfleur, N. M., and Duffau, H. (2015). The left inferior fronto-occipital fasciculus subserves language semantics: a multilevel lesion study. Brain Struct. Funct. 220, 1983–1995. doi: 10.1007/s00429-014-0773-1
Ash, S., Evans, E., O’Shea, J., Powers, J., Boller, A., Weinberg, D., et al. (2013). Differentiating primary progressive aphasias in a brief sample of connected speech. Neurology 81, 329–336. doi: 10.1212/WNL.0b013e31829c5d0e
Ash, S., McMillan, C., Gunawardena, D., Avants, B., Morgan, B., Khan, A., et al. (2010). Speech errors in progressive non-fluent aphasia. Brain Lang. 113, 13–20. doi: 10.1016/j.bandl.2009.12.001
Benton, A. L. (1969). Development of a multilingual aphasia battery. Progress and problems. J. Neurol. Sci. 9, 39–48. doi: 10.1016/0022-510x(69)90057-4
Bouchard, L.-O., Wilson, M. A., Laforce, R., and Duchesne, S. (2019). White matter damage in the semantic variant of primary progressive aphasia. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 46, 373–382. doi: 10.1017/cjn.2019.37
Brambati, S. M., Rankin, K. P., Narvid, J., Seeley, W. W., Dean, D., Rosen, H. J., et al. (2009). Atrophy progression in semantic dementia with asymmetric temporal involvement: a tensor-based morphometry study. Neurobiol. Aging 30, 103–111. doi: 10.1016/j.neurobiolaging.2007.05.014
Canu, E., Agosta, F., Imperiale, F., Fontana, A., Caso, F., Spinelli, E. G., et al. (2019). Added value of multimodal MRI to the clinical diagnosis of primary progressive aphasia variants. Cortex. J. Devoted Study Nerv. Syst. Behav. 113, 58–66. doi: 10.1016/j.cortex.2018.11.025
Cao, X., Wang, X., Xue, C., Zhang, S., Huang, Q., and Liu, W. (2020). A Radiomics approach to predicting Parkinson’s disease by incorporating whole-brain functional activity and Gray matter structure. Front. Neurosci. 14:751. doi: 10.3389/fnins.2020.00751
Catani, M., Mesulam, M. M., Jakobsen, E., Malik, F., Martersteck, A., Wieneke, C., et al. (2013). A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain J. Neurol. 136, 2619–2628. doi: 10.1093/brain/awt163
Cheung, E. Y. W., Chau, A. C. M., and Tang, F. H., On Behalf Of The Alzheimer’s Disease Neuroimaging Initiative, null (2022). Radiomics-based artificial intelligence differentiation of neurodegenerative diseases with reference to the Volumetry. Life Basel Switz. 12:514. doi: 10.3390/life12040514
Cocquyt, E.-M., Lanckmans, E., van Mierlo, P., Duyck, W., Szmalec, A., Santens, P., et al. (2020). The white matter architecture underlying semantic processing: a systematic review. Neuropsychologia 136:107182. doi: 10.1016/j.neuropsychologia.2019.107182
Cohen, J. (1988). Statistical power analysis for the behavioral sciences. 2nd edn. Hillsdale, N.J: L. Erlbaum Associates.
Collins, J. A., Montal, V., Hochberg, D., Quimby, M., Mandelli, M. L., Makris, N., et al. (2017). Focal temporal pole atrophy and network degeneration in semantic variant primary progressive aphasia. Brain J. Neurol. 140, 457–471. doi: 10.1093/brain/aww313
Coyle-Gilchrist, I. T. S., Dick, K. M., Patterson, K., Vázquez Rodríquez, P., Wehmann, E., Wilcox, A., et al. (2016). Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology 86, 1736–1743. doi: 10.1212/WNL.0000000000002638
Dale, A. M., Fischl, B., and Sereno, M. I. (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage 9, 179–194. doi: 10.1006/nimg.1998.0395
Desikan, R. S., Ségonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021
Feng, Q., and Ding, Z. (2020). MRI Radiomics classification and prediction in Alzheimer’s disease and mild cognitive impairment: a review. Curr. Alzheimer Res. 17, 297–309. doi: 10.2174/1567205017666200303105016
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. doi: 10.1016/s0896-6273(02)00569-x
Fischl, B., van der Kouwe, A., Destrieux, C., Halgren, E., Ségonne, F., Salat, D. H., et al. (2004). Automatically parcellating the human cerebral cortex. Cereb. Cortex N. Y. N. 14, 11–22. doi: 10.1093/cercor/bhg087
Galantucci, S., Tartaglia, M. C., Wilson, S. M., Henry, M. L., Filippi, M., Agosta, F., et al. (2011). White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain J. Neurol. 134, 3011–3029. doi: 10.1093/brain/awr099
Gorno-Tempini, M. L., Hillis, A. E., Weintraub, S., Kertesz, A., Mendez, M., Cappa, S. F., et al. (2011). Classification of primary progressive aphasia and its variants. Neurology 76, 1006–1014. doi: 10.1212/WNL.0b013e31821103e6
Grossman, M. (2012). The non-fluent/AGRAMMATIC variant of primary progressive aphasia. Lancet Neurol. 11, 545–555. doi: 10.1016/S1474-4422(12)70099-6
Grossman, M., Xie, S. X., Libon, D. J., Wang, X., Massimo, L., Moore, P., et al. (2008). Longitudinal decline in autopsy-defined frontotemporal lobar degeneration. Neurology 70, 2036–2045. doi: 10.1212/01.wnl.0000303816.25065.bc
Harris, J. M., and Jones, M. (2014). Pathology in primary progressive aphasia syndromes. Curr. Neurol. Neurosci. Rep. 14:466. doi: 10.1007/s11910-014-0466-4
Heckemann, R. A., Keihaninejad, S., Aljabar, P., Gray, K. R., Nielsen, C., Rueckert, D., et al. (2011). Automatic morphometry in Alzheimer’s disease and mild cognitive impairment. NeuroImage 56, 2024–2037. doi: 10.1016/j.neuroimage.2011.03.014
Jain, M., Rai, C. S., and Jain, J. (2021). A novel method for differential prognosis of brain degenerative diseases using Radiomics-based textural analysis and ensemble learning classifiers. Comput. Math. Methods Med. 2021, 7965677–7965613. doi: 10.1155/2021/7965677
Kaplan, E., Goodglass, H., and Weintraub, S. (1983). Boston naming test. Philadelphia: Lea & Febiger.
Kim, J. P., Kim, J., Jang, H., Kim, J., Kang, S. H., Kim, J. S., et al. (2021). Predicting amyloid positivity in patients with mild cognitive impairment using a radiomics approach. Sci. Rep. 11:6954. doi: 10.1038/s41598-021-86114-4
Kim, J. H., Lee, J. W., Kim, G. H., Roh, J. H., Kim, M.-J., Seo, S. W., et al. (2012). Cortical asymmetries in normal, mild cognitive impairment, and Alzheimer’s disease. Neurobiol. Aging 33, 1959–1966. doi: 10.1016/j.neurobiolaging.2011.06.026
Knopman, D. S., Kramer, J. H., Boeve, B. F., Caselli, R. J., Graff-Radford, N. R., Mendez, M. F., et al. (2008). Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain J. Neurol. 131, 2957–2968. doi: 10.1093/brain/awn234
Leyton, C. E., Villemagne, V. L., Savage, S., Pike, K. E., Ballard, K. J., Piguet, O., et al. (2011). Subtypes of progressive aphasia: application of the international consensus criteria and validation using β-amyloid imaging. Brain J. Neurol. 134, 3030–3043. doi: 10.1093/brain/awr216
Li, M., Zhang, Y., Song, L., Huang, R., Ding, J., Fang, Y., et al. (2017). Structural connectivity subserving verbal fluency revealed by lesion-behavior mapping in stroke patients. Neuropsychologia 101, 85–96. doi: 10.1016/j.neuropsychologia.2017.05.008
Liu, P., Wang, H., Zheng, S., Zhang, F., and Zhang, X. (2020). Parkinson’s disease diagnosis using Neostriatum Radiomic features based on T2-weighted magnetic resonance imaging. Front. Neurol. 11:248. doi: 10.3389/fneur.2020.00248
Lombardi, J., Mayer, B., Semler, E., Anderl-Straub, S., Uttner, I., Kassubek, J., et al. (2021). Quantifying progression in primary progressive aphasia with structural neuroimaging. Alzheimers Dement. J. Alzheimers Assoc. 17, 1595–1609. doi: 10.1002/alz.12323
Mahoney, C. J., Malone, I. B., Ridgway, G. R., Buckley, A. H., Downey, L. E., Golden, H. L., et al. (2013). White matter tract signatures of the progressive aphasias. Neurobiol. Aging 34, 1687–1699. doi: 10.1016/j.neurobiolaging.2012.12.002
Mandelli, M. L., Caverzasi, E., Binney, R. J., Henry, M. L., Lobach, I., Block, N., et al. (2014). Frontal white matter tracts sustaining speech production in primary progressive aphasia. J. Neurosci. 34, 9754–9767. doi: 10.1523/JNEUROSCI.3464-13.2014
Mandelli, M. L., Vilaplana, E., Brown, J. A., Hubbard, H. I., Binney, R. J., Attygalle, S., et al. (2016a). Healthy brain connectivity predicts atrophy progression in non-fluent variant of primary progressive aphasia. Brain 139, 2778–2791. doi: 10.1093/brain/aww195
Mandelli, M. L., Vitali, P., Santos, M., Henry, M., Gola, K., Rosenberg, L., et al. (2016b). Two insular regions are differentially involved in behavioral variant FTD and nonfluent/agrammatic variant PPA. Cortex. J. Devoted Study Nerv. Syst. Behav. 74, 149–157. doi: 10.1016/j.cortex.2015.10.012
Mesulam, M. M. (1982). Slowly progressive aphasia without generalized dementia. Ann. Neurol. 11, 592–598. doi: 10.1002/ana.410110607
Mesulam, M.-M., Weintraub, S., Rogalski, E. J., Wieneke, C., Geula, C., and Bigio, E. H. (2014). Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain J. Neurol. 137, 1176–1192. doi: 10.1093/brain/awu024
Mesulam, M., Wicklund, A., Johnson, N., Rogalski, E., Léger, G. C., Rademaker, A., et al. (2008). Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann. Neurol. 63, 709–719. doi: 10.1002/ana.21388
Morris, J. C. (1993). The clinical dementia rating (CDR): current version and scoring rules. Neurology 43, 2412–2414. doi: 10.1212/wnl.43.11.2412-a
Nigro, S., Tafuri, B., Urso, D., De Blasi, R., Cedola, A., Gigli, G., et al. (2021). Altered structural brain networks in linguistic variants of frontotemporal dementia. Brain Imaging Behav. 16, 1113–1122. doi: 10.1007/s11682-021-00560-2
Okada, N., Fukunaga, M., Yamashita, F., Koshiyama, D., Yamamori, H., Ohi, K., et al. (2016). Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol. Psychiatry 21, 1460–1466. doi: 10.1038/mp.2015.209
Pedraza, O., Bowers, D., and Gilmore, R. (2004). Asymmetry of the hippocampus and amygdala in MRI volumetric measurements of normal adults. J. Int. Neuropsychol. Soc. JINS 10, 664–678. doi: 10.1017/S1355617704105080
Reyes, P. A., Rueda, A. D. P., Uriza, F., and Matallana, D. L. (2019). Networks disrupted in linguistic variants of frontotemporal dementia. Front. Neurol. 10:903. doi: 10.3389/fneur.2019.00903
Routier, A., Habert, M.-O., Bertrand, A., Kas, A., Sundqvist, M., Mertz, J., et al. (2018). Structural, microstructural, and metabolic alterations in primary progressive aphasia variants. Front. Neurol. 9:766. doi: 10.3389/fneur.2018.00766
Salat, D., Lee, S., van der Kouwe, A., Greve, D., Fischl, B., and Rosas, H. (2009). Age-associated alterations in cortical Gray and white matter signal intensity and Gray to white matter contrast. NeuroImage 48, 21–28. doi: 10.1016/j.neuroimage.2009.06.074
Sarica, A., Vasta, R., Novellino, F., Vaccaro, M. G., Cerasa, A., Quattrone, A., et al. (2018). MRI asymmetry index of hippocampal subfields increases through the continuum from the mild cognitive impairment to the Alzheimer’s disease. Front. Neurosci. 12:576. doi: 10.3389/fnins.2018.00576
Schober, P., Boer, C., and Schwarte, L. A. (2018). Correlation coefficients: appropriate use and interpretation. Anesth. Analg. 126, 1763–1768. doi: 10.1213/ANE.0000000000002864
Sharp, D. J., Scott, S. K., and Wise, R. J. S. (2004). Retrieving meaning after temporal lobe infarction: the role of the basal language area. Ann. Neurol. 56, 836–846. doi: 10.1002/ana.20294
Spinelli, E. G., Mandelli, M. L., Miller, Z. A., Santos-Santos, M. A., Wilson, S. M., Agosta, F., et al. (2017). Typical and atypical pathology in primary progressive aphasia variants. Ann. Neurol. 81, 430–443. doi: 10.1002/ana.24885
Tafuri, B., Filardi, M., Urso, D., De Blasi, R., Rizzo, G., Nigro, S., et al. (2022). Radiomics model for frontotemporal dementia diagnosis using T1-weighted MRI. Front. Neurosci. 16:828029. doi: 10.3389/fnins.2022.828029
Tee, B. L., and Gorno-Tempini, M. L. (2019). Primary progressive aphasia: a model for neurodegenerative disease. Curr. Opin. Neurol. 32, 255–265. doi: 10.1097/WCO.0000000000000673
van Griethuysen, J. J. M., Fedorov, A., Parmar, C., Hosny, A., Aucoin, N., Narayan, V., et al. (2017). Computational Radiomics system to decode the radiographic phenotype. Cancer Res. 77, e104–e107. doi: 10.1158/0008-5472.CAN-17-0339
Vandenberghe, R. (2016). Classification of the primary progressive aphasias: principles and review of progress since 2011. Alzheimers Res. Ther. 8:16. doi: 10.1186/s13195-016-0185-y
Vonk, J. M. J., Rizvi, B., Lao, P. J., Budge, M., Manly, J. J., Mayeux, R., et al. (2019). Letter and category fluency performance correlates with distinct patterns of cortical thickness in older adults. Cereb. Cortex N. Y. N 29, 2694–2700. doi: 10.1093/cercor/bhy138
Wakana, S., Caprihan, A., Panzenboeck, M. M., Fallon, J. H., Perry, M., Gollub, R. L., et al. (2007). Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage 36, 630–644. doi: 10.1016/j.neuroimage.2007.02.049
Wilson, S. M., Henry, M. L., Besbris, M., Ogar, J. M., Dronkers, N. F., Jarrold, W., et al. (2010). Connected speech production in three variants of primary progressive aphasia. Brain J. Neurol. 133, 2069–2088. doi: 10.1093/brain/awq129
Wu, Y., Jiang, J.-H., Chen, L., Lu, J.-Y., Ge, J.-J., Liu, F.-T., et al. (2019). Use of radiomic features and support vector machine to distinguish Parkinson’s disease cases from normal controls. Ann. Transl. Med. 7:773. doi: 10.21037/atm.2019.11.26
Xiao, B., He, N., Wang, Q., Cheng, Z., Jiao, Y., Haacke, E. M., et al. (2019). Quantitative susceptibility mapping based hybrid feature extraction for diagnosis of Parkinson’s disease. NeuroImage Clin. 24:102070. doi: 10.1016/j.nicl.2019.102070
Zhou, P., Zeng, R., Yu, L., Feng, Y., Chen, C., Li, F., et al. (2021). Deep-learning Radiomics for discrimination conversion of Alzheimer’s disease in patients with mild cognitive impairment: a study based on 18F-FDG PET imaging. Front. Aging Neurosci. 13:764872. doi: 10.3389/fnagi.2021.764872
Keywords: primary progressive aphasia, radiomics, asymmetry, structural white matter, MRI, verbal fluency, neuroimaging
Citation: Tafuri B, Filardi M, Urso D, Gnoni V, De Blasi R, Nigro S, and Logroscino G (2023) Asymmetry of radiomics features in the white matter of patients with primary progressive aphasia. Front. Aging Neurosci. 15:1120935. doi: 10.3389/fnagi.2023.1120935
Received: 10 December 2022; Accepted: 17 April 2023;
Published: 05 May 2023.
Edited by:
Alessandro Martorana, University of Rome Tor Vergata, ItalyReviewed by:
Enrico Premi, University of Brescia, ItalyCopyright © 2023 Tafuri, Filardi, Urso, Gnoni, De Blasi, Nigro, Logroscino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benedetta Tafuri, YmVuZWRldHRhLnRhZnVyaUBnbWFpbC5jb20=
†These authors share last authorship
‡Data used in the preparation of this article were obtained from the Frontotemporal Lobar Degeneration Neuroimaging Initiative (FTLDNI) database (http://4rtni-ftldni.ini.usc.edu/). The investigators at NIFD/FTLDNI contributed to the design and implementation of FTLDNI and/or provided data but did not participate in the analysis or writing of this report (unless otherwise listed)
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.