- 1Department of Neurology, Second Affiliated Hospital of Nanchang University, Nanchang, China
- 2Department of Anesthesiology, Changsha Hospital for Maternal and Child Health Care Affiliated to Hunan Normal University, Changsha, China
Cognitive impairment in patients with Parkinson’s disease (PD) worsens the prognosis of PD and increases caregivers’ burden and economic consequences. Recently, subjective cognitive decline (SCD), which refers to self-reported cognitive decline without detectable objective cognitive dysfunction, has been regarded as an at-risk state of mild cognitive impairment (MCI) and a prodromal stage for dementia in Alzheimer’s disease (AD). However, studies on PD-SCD have thus far been scarce, and at present there is no consensus regarding the definition of SCD nor a gold standard as an evaluation tool. The present review aimed to look for an association between PD-SCD and objective cognitive function and found that PD with SCD occurred with brain metabolic changes, which were consistent with early aberrant pathological changes in PD. Moreover, PD patients with SCD were likely to progress to future cognitive impairment. It is necessary to establish a guideline for the definition and evaluation of SCD in PD. A larger sample size and more longitudinal investigations are needed to verify the predictive effectiveness of PD-SCD and to detect earlier subtle cognitive decline before MCI.
Introduction
Cognitive dysfunction, one of the most common non-motor symptoms (NMSs) in Parkinson’s disease (PD), is up to six times more common in PD than in the healthy aging population, worsens the prognosis of PD, and increases caregivers’ burden and economic consequences (Aarsland et al., 2021). It was estimated that 40–50% of PD presents with mild cognitive impairment (MCI) at baseline, and 75–80% of MCI progresses to dementia in a longitudinal study (Hely et al., 2005; Stuart et al., 2016; Obeso et al., 2017). Recently, the process of cognitive decline in PD patients have received growing interest.
Subjective cognitive decline (SCD) refers to decreases in cognitive capacity without detectable impairment on neuropsychological tests, indicating intact cognitive functions, accompanied by pathological changes, and was believed to be an at-risk state of MCI and a prodromal stage for dementia in Alzheimer’s disease (AD) (Reisberg et al., 2008; Jessen, 2010; Scheef et al., 2012; Jessen et al., 2014; Jack et al., 2018; Wirth et al., 2018). A study showed β-amyloid deposition and atrophy as well as brain activation in people with SCD, suggesting a compensatory mechanism, which might reflect early neuronal dysfunction together with memory performance preserved (Saykin et al., 2006; Rodda et al., 2009; Perrotin et al., 2012). Thus, the state of SCD was recognized as an essential course of AD pathology and a risk factor for cognitive decline (Jessen et al., 2020). Similar to AD, SCD may be an intermediate state between cognitive normality and MCI in PD. Thus, a possible three-stage clinical performance related to cognition might be applicable to patients with PD, with SCD as the prodromal phase, followed by MCI, finally leading to dementia (Erro et al., 2012; Kjeldsen and Damholdt, 2019; Jones et al., 2021; Yoo et al., 2021; Ophey et al., 2022). Neuroimaging study focused on PD patients with SCD (PD-SCD) demonstrated reduced FDG metabolism in the middle frontal, middle temporal, and occipital areas and the angular gyrus of the cortex, which suggested there may be early pathological changes in PD-SCD (Ophey et al., 2022). Follow-up studies showed a significantly higher risk of developing PD-MCI and dementia for patients with PD-SCD compared to PD without SCD at baseline (Erro et al., 2014; Hong et al., 2014b; Galtier et al., 2019; Purri et al., 2020; Jones et al., 2021). However, there are inconsistent results, results just shown a correlation between SCD and depression, anxiety and other related mood features rather than cognitive dysfunction (Lehrner et al., 2014; Santangelo et al., 2014; Baschi et al., 2018; Barbosa et al., 2019). For example, in Barbosa’s study, he regarded SCD as subjective cognitive complaints (SCC), and found SCD severity was related to depression (p = 0.026) rather than Montreal Cognitive Assessment (MoCA) scores (p = 0.141) in PD with normal cognition (PD-NC), which suggested clinician to alert affective disorder in PD-SCD (Barbosa et al., 2019). Study led by Baschi defined subjective memory complaints (SMC) as SCD, and results showed PD-SCD was significantly associated with anxiety (OR = 3.93) when compared to PD without SCD (Baschi et al., 2018).
Overall, cognitive impairment in PD is a huge financial burden to society as well as caregivers, and it is time to highlight the importance of identifying cognitive decline as early as possible (Aarsland et al., 2021). However, studies on PD-SCD have thus far been scarce, and there is no consensus regarding the definition of SCD nor is there a gold standard as an evaluation tool. Little is known about whether there is a clear association between SCD and cognitive dysfunction or later cognitive decline in PD.
The review aimed to outline how SCD has been used as a diagnostic criterion in studies on PD as well as to describe the possible correlation related to objective cognitive impairment, help recognize SCD as an at-risk state early indicator and allow clinicians to predict conversion to PD-D more accurately.
Methods
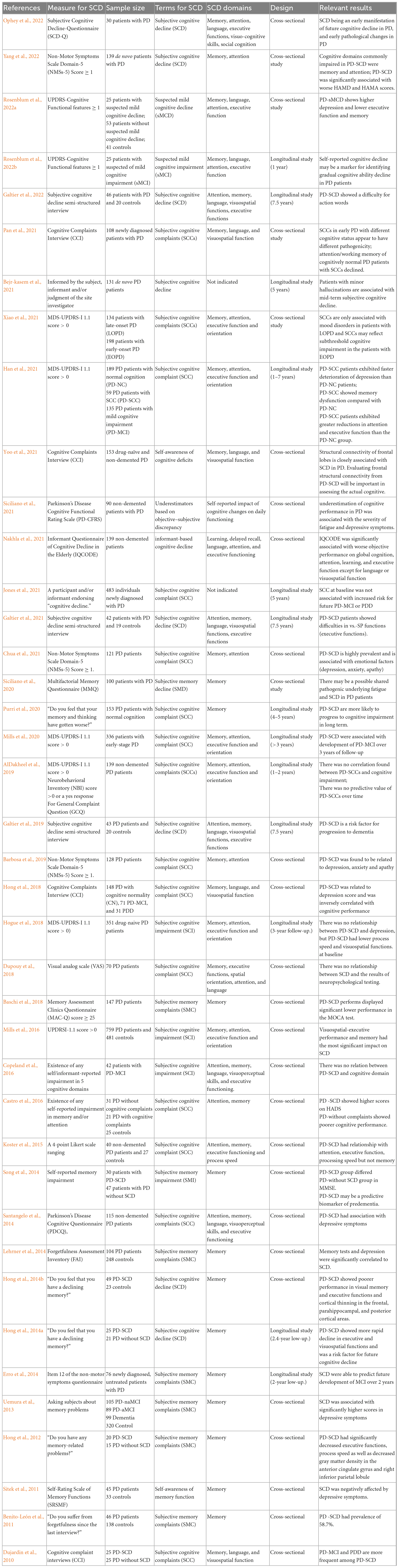
Our aim was to summarize the empirical literature on SCD in patients with PD. We used the following key words: “Parkinson’s disease” (PD) and subjective complaints (“subjective cognitive decline” (SCD), “subjective cognitive impairment” (SCI), “subjective memory complaint(s)” (SMC), “subjective ‘memory impairment” (SMI), or “subjective cognitive complaint(s)” (SCC) to search related literature in the PubMed, Web of Science, and Embase databases. All study designs and articles written in English from 1 January 1970 to 30 April 2023 were included; additionally, related reference lists were carried expand the literature results. Table 1 show the detailed summary of included studies.
Results
Definition
Terminologies for SCD
The current available definition of SCD mainly focused on memory in the context of AD (Jessen et al., 2020). Unfortunately, at present there are no uniform definitions of SCD in PD, and researchers have also used the terms SMD (Siciliano et al., 2020), SMI (Song et al., 2014), SMC (Benito-León et al., 2011; Erro et al., 2012; Hong et al., 2012; Uemura et al., 2013; Lehrner et al., 2014; Baschi et al., 2018), SCI (Copeland et al., 2016; Mills et al., 2016; Hogue et al., 2018), or SCC (Dujardin et al., 2010; Santangelo et al., 2014; Koster et al., 2015; Castro et al., 2016; Mills et al., 2016; Dupouy et al., 2018; Hong et al., 2018; AlDakheel et al., 2019; Barbosa et al., 2019; Purri et al., 2020; Chua et al., 2021; Han et al., 2021; Jones et al., 2021; Pan et al., 2021; Xiao et al., 2021) as descriptions.
Assessment tools for SCD
Although patients with PD mainly exhibit severe deficits in executive function, attention and visuospatial function rather than memory (Kehagia et al., 2010), studies have been focused on memory-related questions. “Do you have any memory-related problems?,” “Do you feel that your memory and thinking have gotten worse” or “Have you suffered from forgetfulness since the last interview? “were the questions adopted frequently to define SCD (Benito-León et al., 2011; Erro et al., 2012; Hong et al., 2012; Uemura et al., 2013; Hong et al., 2014a,b; Purri et al., 2020; Lee et al., 2020; Jones et al., 2021). Similarly, some studies applied the UPDRSI 1.1 [(1.1) cognitive impairment] to assess SCD (Mills et al., 2016; Hogue et al., 2018; Mills et al., 2020; Han et al., 2021; Xiao et al., 2021; Rosenblum et al., 2022a,b). Song and Castro defined the decreased self-awareness of attention/memory as SCD (Song et al., 2014; Castro et al., 2016). Siciliano adopted Multifactorial Memory Questionnaire (MMQ) to identify patients with SCD (Siciliano et al., 2020). In Rosenblum’s study, he classified patients as suspected mild cognitive decline (sMCD) based on UPDRS-Cognitive Functional features score ≥ 1 [the mean score of seven MDS-UPDRS items chosen: (1.1) cognitive impairment, (2.1) speech, (2.4) eating, (2.5) dressing, (2.6) hygiene, (2.7) handwriting, and (2.8) doing hobbies], and regarded sMCD as SCD (Rosenblum et al., 2022a,b). Apart from the above studies measuring SCD by simple questions, other studies utilized questionnaires such as the subjective cognitive decline questionnaire (SCD-Q) (Ophey et al., 2022), Cognitive Complaints Interview (CCI) (Dujardin et al., 2010; Hong et al., 2018; Pan et al., 2021; Yoo et al., 2021), Parkinson’s Disease Cognitive Function Rating Scale (PD-CFRS) (Siciliano et al., 2021), Parkinson’s Disease Cognitive Questionnaire (PD-CQ) (Santangelo et al., 2014), Forgetfulness Assessment Inventory (FAI) (Lehrner et al., 2014), Non-Motor Symptoms Scale Domain-5 Score (NMSS-5) (Barbosa et al., 2019; Chua et al., 2021; Yang et al., 2022), Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) (Nakhla et al., 2021), and Self-Rating Scale of Memory Functions (SRMF) (Sitek et al., 2011). Galtier formulated a subjective cognitive decline semi-structured interview that included seven items (Galtier et al., 2019, 2021, 2022). A visual analog scale was applied by Dupouy et al. (2018) to assess five cognitive domains and helped establish a link between SCD and executive function, language and attention. Koster et al. developed a four-point Likert scale and obtained similar results to those of Koster et al. (2015). Copeland and colleagues took patients as well as their caregivers into account in five cognitive domains, the results showed little agreement between cognitive domains and patients/care partner with subjective reports (Copeland et al., 2016). Bejr-kasem defined patients with SCD according to information informed by the subject, informant and/or judgment of the site investigator, he showed patients with minor hallucinations were associated with mid-term subjective cognitive decline (Bejr-kasem et al., 2021). In order to clarify the relationship between PD-SCD and cognitive performance, AlDakheel adopted 4 methods to measure SCD, MDS-UPDRS-I 1.1 score > 0, Neurobehavioral Inventory (NBI)-subject score >0, NBI-contact score >0, a yes response for General complaint question (GCQ), and he found there were little agreement between SCD and different methods, and no SCD method was associated with cognitive decline (AlDakheel et al., 2019).
Given that patients with PD manifest major impairments in executive function, attention and visuospatial function, not just memory (Kehagia et al., 2010), it is wise for evaluators to assess SCD with questionnaires/problems/interviews including five domains (memory, attention, executive functions, visuospatial functions, language). The definition of SCD should take the functions of these five domains into account, at the same time, the information provided by informant may be contribute to identify PD-SCD. Further research are required to help exploring the application and value of PD-SCD.
Prevalence
Since there are no guidelines for PD-SCD, researchers have classified PD-SCD differently, and some studies have classified PD-SCD as coexisting with objective impairment. For example, among PD patients in the Dujardin cross-sectional study, 32.22% matched a classification of PD-SCD, of these patients with SCD, 44.83% of PD-SCD met the dementia criteria, of these patients without SCD, just 25.41% met the dementia criteria, suggesting that PD-SCD has a higher probability of manifesting cognitive dysfunction than PD without SCD (Dujardin et al., 2010). Erro reported that approximately 25% of patients with PD underwent SCD, and PD-SCD at baseline may be a risk factor for PD-MCI (Erro et al., 2014). Lehrner studied PD-SCD with FAI and found that 31% of the PD patients reported SCD. Pan showed 30.3% and 12.1% of SCD in PD-MCI, PD-NC, respectively, he found SCD in PD-NC exhibited declined attention/working memory and thought there might be different pathogenicity in SCD with different cognitive status (Pan et al., 2021). Xiao classified patients into early-onset PD (EOPD) and late-onset PD (LOPD), there were 18.66% of EOPD and 24.74% of LOPD reported SCD (Xiao et al., 2021). Siciliano focused on PD with fatigue, and he found the prevalence of SCD was higher in fatigued PD patients when comparing with those with no fatigued (35 vs. 9%) (Siciliano et al., 2020). The PD-SCD evaluated by other studies demonstrated proportions of 32.6% (Baschi et al., 2018), 44.74% (Hogue et al., 2018), 85% (Barbosa et al., 2019), 29.7% (Lee et al., 2020), 23% (Jones et al., 2021), 32.3% (Siciliano et al., 2021), 28.6%, and 30.2% (Galtier et al., 2019, 2021).
Some researchers defined SCD as subjective cognitive impairment without objective cognitive decline. Purri described a 53% incidence of PD-SCD among PD patients with normal cognition and thought PD-SCD may be an indicator of subsequent cognitive impairment (Purri et al., 2020). Hong showed a proportion of 54.3% and found that PD-SCD can predict cognitive decline later (Hong et al., 2014a). Yang found the prevalence of PD-SCD was 28.1% (Yang et al., 2022). Galtier exhibited 30.5% were diagnosed with PD-SCD according to subjective cognitive decline semi-structured interview, and the longitudinal study result showed poor performance in verb naming test in PD-SCD, which suggested the role of linguistic impairment in PD-SCD (Galtier et al., 2022). Other studies demonstrated rates of 16.3% (Lehrner et al., 2014), 22.36% (Hogue et al., 2018), and 27.2% (Baschi et al., 2018).
Above all, there were some variations in the prevalence of PD-SCD among those studies, and the following reasons may be responsible for this. First, different demographic characteristics may contribute to the discrepancy; for example, an older cohort is prone to SCD since cognition declines with age. Second, the tool chosen to assess SCD may also be a factor, and a complete questionnaire may be more accurate than a single item. Third, the definition of SCD used in studies also influenced the results greatly, since PD may have normal memory but impaired planning ability, leading to some people being missed if the focus is only on memory. Finally, a state of anxiety, depression, and apathy in the PD group also accounted for the higher existence of SCD.
Neuroimaging
Although studies on neuroimaging in PD-SCD have been scarce, the existing results are encouraging. In a study of FDG-PET in PD with normal cognition (n = 18 as PD-SCD, n = 12 as PD control), results revealed reduced FDG metabolism in the middle frontal, middle temporal, occipital areas, and angular gyrus of the cortex. These regions may be neural correlates of PD-SCD, and could demonstrate an early pathological change in PD-SCD (Ophey et al., 2022), consistent with the finding of hypometabolism in the middle frontal gyrus and inferior parietal lobule in PD-MCI (Huang et al., 2008). Yoo classified PD patients had both CCI score >3 and intact cognition as underestimation of cognitive function, and the group here means to PD-SCD. MRI suggested a close association between the frontal lobes and PD-SCD and thought it important to measure frontal structural connectivity in the early stages of PD (Yoo et al., 2021). Song and colleagues found reduced perfusion in the frontal and inferior temporal cortical regions as well as the anterior cingulate gyrus and thalamus in PD-SCD patients compared to PD patients without SCD, which may provide a potential biomarker of predementia. Other studies by Hong found a decreased gray matter density and cortical thinning in the anterior cingulate gyrus, right inferior parietal lobule and parahippocampal cortices in PD-SCD (Hong et al., 2012, 2014b), suggesting an aberrant PD-related pathology.
The frontal lobe is responsible for executive functions, the temporal area is associated with semantic memory, the anterior cingulate gyrus seems to be relevant to verbal fluency, attention is dominated by the parietal lobe as well as the anterior cingulate gyrus, and the occipital lobe plays a significant role in visuospatial ability (Mohanty et al., 2007; Hong et al., 2012, 2014b; Song et al., 2014; Ophey et al., 2022). The neuroimaging studies above revealed potential neural correlates that underlie PD-SCD, making PD-SCD a promising group to be emphasized in clinician. However, samples from neuroimaging studies to date are relatively small, and larger sample sizes and longitudinal studies are needed for further validation.
Association with emotion
Because SCD was mainly defined through several subjective questions, the mood features in patients with PD should be taken into account, as we mentioned before. Currently, the relationship between PD-SCD and emotional symptoms was debated, as some studies have described a marked correlation between anxiety, depression or apathy and PD-SCD, while other studies have not shown this. For example, Siciliano et al. (2021) defined underestimators as patients with subjective cognitive complaints but no objective cognitive impairment, they regarded PD-SCD as an underestimator actually and found a positive correlation between underestimator scores and fatigue, depression, and anxiety, suggesting a greater focus on mood features in patients with early PD in the clinical setting. In Yang et al.’s (2022) study, they defined PD-SCD according to NMSs-5 ≥ 1, subsequently, they found PD-SCD was associated with worse Hamilton Depression Scale (HAMD) and Hamilton Anxiety Scale (HAMA) scores. Rosenblum et al. (2022a) regarded PD patients with sMCD as PD-SCD, and he found PD-sMCD showed higher depression when compared to those with no suspected. Chua also obtained the association between PD-SCD and emotional factors (depression, anxiety, apathy) (Chua et al., 2021), and other studies also showed association between PD-SCD and emotional factors (Dujardin et al., 2010; Lehrner et al., 2014; Santangelo et al., 2014; Koster et al., 2015; Castro et al., 2016; Baschi et al., 2018; Barbosa et al., 2019; Purri et al., 2020; Han et al., 2021). At the same time, there still remains unclear between PD-SCD and emotions, since some studies showed no relation between these factors (Hong et al., 2012; Dupouy et al., 2018). According to Baschi, emotional disorders may be both a cause and a consequence of PD-SCD; for example, anxiety in individuals with PD-SCD may be caused by their awareness of cognitive function loss (Baschi et al., 2018). This data reinforced the point that when we encounter PD patients with emotional disorders, it is necessary to incorporate this information to further assess objective cognitive functions. Of course, the discrepancy of the results may be related to the methodology, as age, education, duration and severity of PD influenced moods and should be adjusted.
Assessment tools for objective cognitive performance
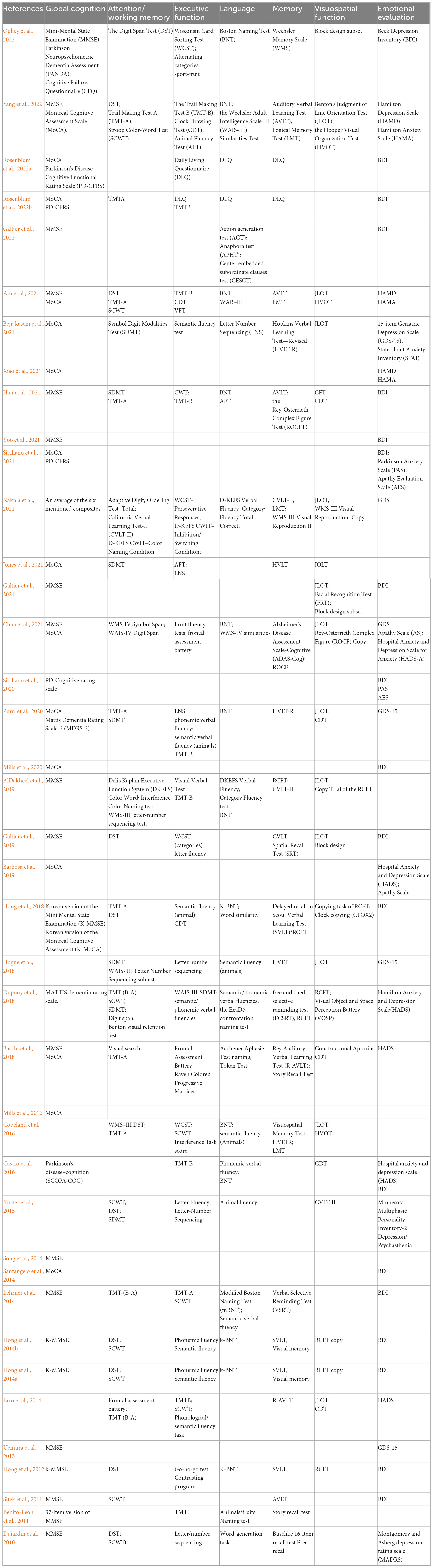
Studies have chosen complete neuropsychological tests for patients with PD, such as the MoCA/Mini Mental State Examination (MMSE) for global cognition, the semantic fluency/trail making test (TMT) for executive function, the word list verbal learning test for memory, the digit span test (DST)/symbol digits modalities test (SDMT) for attention, the naming test for language, and the Rey-Osterrieth complex figure test (ROCFT) for visuospatial ability. Table 2 listed detailed information about neuropsychological tests.
Association with objective cognitive functions in cross-sectional study
Lehrner was interested in the relationship between cognitive complaints and cognitive impairment, he found significant correlations between PD-SCD and worse MMSE performance (Lehrner et al., 2014). Hong et al. (2014b) focused PD-SCD on memory impairment, he investigated the cognitive performance and cortical thickness (n = 49 PD-SCD, n = 23 controls), results showed PD-SCD have poorer performance in visual memory and executive functions, which was consistent with cortical thinning in frontal and posterior cortical areas, since frontal and posterior regions responsible for executive function and visual function, respectively. In Song et al.’s (2014) study, PD-SCD group (n = 30) differed PD-without SCD (n = 47) in global cognition measured by MMSE and suggested PD-SCD may be a predictive biomarker on predementia. Nakhla et al. (2021) took informant-based responses into account and measured them by IQCODE and ultimately found that higher scale scores were negatively correlated with objective performance, including attention, executive function, memory and global cognition. Hong et al. (2018) assessed PD-SCD by CCI and suggested that an increasing score was strongly correlated with poorer objective cognitive functions (global cognition and all five cognitive domains) after adjusting for a depressive score. Baschi found a lower MoCA score in PD-SCD patients than in PD patients without SCD (Baschi et al., 2018). Regression models analyzed by Mills et al. (2016) suggested lower scores on memory, executive and visuospatial functions in PD-SCD. Koster et al. (2015) showed that patients with PD have higher proportion of self-reported difficulties in attention and executive functions but not memory. Dujardin reported more objective cognitive dysfunction among PD patients with cognitive complaints than among PD patients without SCD (Dujardin et al., 2010). In a word, studies referred above showed a relationship between SCD and decreased cognitive manifestation, while the remaining reported mixed results (Castro et al., 2016; Copeland et al., 2016; Dupouy et al., 2018). Castro obtained a contradictory conclusion with the above results. In this study, PD with cognitive complaints performed at a higher level, suggesting better cognitive status (Castro et al., 2016). Additionally, Dupouy and Copeland found no association between neither the patient’s nor the caregiver’s complaints and the patient’s objective cognitive manifestation (Copeland et al., 2016; Dupouy et al., 2018).
Attention and executive functions, which have neural correlates with the parietal and frontal lobes, were the most reported to have a strong correlation with the existence of PD-SCD, and these findings were consistent with the pathological changes in the early stage of PD. Some studies also found that PD-SCD was related to memory, and we think the results were affected by the assessed tools used as well as the targeted population. On the one hand, studies defined SCD as self-reported memory complaints, and the association investigated may be memory rather than other domains. On the other hand, studies comparing PD-SCD and PD-MCI concurrently may increase the correlation with one another. Dupouy and Copeland found no association between SCD and objective cognitive performance, which may be due to the assessment used or the psychiatric symptoms among their cohort. Overall, the association between SCD and objective cognitive performance remains unclear now. SCD are subjective and may be influenced by countless factors and the conflicting results obtained can be associated with measurements of cognitive status, definition of SCD, sample size, study design and so on. Thus, it is difficult to show a clear relationship between the two, especially when the methods applied are not precise or extensive enough to evaluate SCD or objective cognitive functions.
Association with objective cognitive functions in longitudinal study
In Jones et al.’s (2021) study (n = 483 PD patients), a single item was used to assess PD-SCD, and the results revealed no relation between PD-SCD at baseline and PD-MCI/PDD 5 years later. A longitudinal study (n = 139 non-demented PD patients) adopted 4 methods to elicit PD-SCD, it explored the link between PD-SCD and cognitive performance subsequently, results here showed there was no correlation between PD-SCD and cognitive decline and no predictive value of PD-SCD over time (1–2 years) (AlDakheel et al., 2019). Conversely, a follow-up study by Galtier suggested that PD-SCD patients (36.4%) are at higher risk of converting to dementia than PD without SCD (14.3%) 7.5 years later, and the PD-SCD group showed impairments in visuospatial and visuoperceptual functions, which was linked to thinning of posterior cortices (Galtier et al., 2021), at the same time, PD-SCD showed poor performance in verb naming test in the same longitudinal study (n = 46 PD, n = 20 controls), which suggested the possible linguistic dysfunction in PD-SCD (Galtier et al., 2022). Rosenblum followed up PD-sMCD (n = 25) and found self-reported cognitive decline may be a marker for predicting cognitive ability decline after 1 year (Rosenblum et al., 2022b). Han and colleagues regarded PD-SCD as an intermediate status of PD-NC and PD-MCI, they divided people into three groups: PD-NC (n = 189), PD-SCD (n = 59), PD-MCI (n = 135), the longitudinal study (1–7.5 years) found PD-SCD showed memory impairment and greater reductions in attention and executive function compared with PD-NC, which suggested PD-SCD may be high risk individuals progression to cognitive decline later (Han et al., 2021), and the results were similar to Hong’s and Purri’s study (Hong et al., 2014a; Purri et al., 2020). In Purri’s study (n = 153 PD patients with normal cognition), they defined PS-SCD as subjective cognitive complaints with objective cognitive normality and found that the PD-SCD group performed worse on global cognition, executive function and processing speed than the PD without SCD group. Additionally, PD-SCD more easily converted cognitive impairment longitudinally, indicating that PD-SCD may be a warning of subtle cognitive decline (Purri et al., 2020). A larger longitudinal study (n = 336 patients with PD) led by Mills et al. (2020) showed that PD-SCD patients were more likely to develop PD-MCI over 3 years of follow-up and can be used to predict future cognitive impairment, which was similar to other studies (Hong et al., 2014a; Galtier et al., 2019). In Hong et al.’s (2014a) study, the findings were consistent with the conclusion that PD-SCD may be a risk factor for future cognitive dysfunction, since their results showed that PD-SCD could predict progression to decreased cognition 1–4 years later. Erro et al. (2014) also focused on the power of PD-SCD to predict further cognitive decline, he found patients with subjective memory decline at baseline (n = 76) were independent predictor, which can predict the progression to MCI with 2 years longitudinal study, Hogue et al.’s (2018) conclusion was similar to Erro, he created a regression model included SCD in baseline, and the model can predict future cognitive decline, which showed encouraging predictive value of PD-SCD.
In summary, the majority of the above studies considered PD-SCD to be risk factor for impaired objective cognition, and PD-SCD may be a potential way to identify early cognitive decline. However, some problems should be noted. Most studies evaluated PD-SCD with a single item partly concerned with memory, which could eventually influence the results, thus, assessing PD-SCD according to more comprehensive cognitive domains may contribute to the consistency of subjects selected. Secondly, emotional factors should be emphasized and adjusted when exploring the correlation between cognitive performance and PD-SCD, because emotional factors influenced cognitive function and may be linked with PD-SCD, which interfered the results. Thirdly, it may be potential way to associate longitudinal study with more neuroimaging researches in PD-SCD.
Conclusion
Subjective cognitive decline has been gaining increasing interest in recent years. Most of the results reported that patients with PD-SCD had a correlation with objective cognitive decline, and longitudinal studies also revealed a predictive risk for future cognitive dysfunction. Neuroimaging supported the above studies in terms of neural correlates as well as brain metabolism.
However, there are still some problems that need to be solved as soon as possible. First, it is necessary to establish a consensus on PD with SCD. According to the SCD consensus on patients with AD, we recommend that the definition of PD-SCD should be purely self/informant-reported cognitive decline, and this definition may contribute to proving a pre-MCI stage of subtle cognitive decline. Next, studies used different methods to evaluate SCD, and assessments of SCD ranged from a single item to several face-to-face questions and a complete questionnaire. Given the specific characteristics of cognitive impairment in PD, a questionnaire including all five domains should be adopted. Additionally, information derived from caregivers may be useful to detect PD-SCD. Finally, the sample size should be larger, and more longitudinal investigations are needed to verify the predictive effectiveness of PD-SCD.
Overall, a clear definition of PD-SCD would help identify earlier stages of cognitive impairment before PD-MCI, explore more risk factors associated with the existence of PD-SCD and allow for early attention and intervention in subtle cognitive decline stages, which may ultimately reduce the burden of cognitive impairment on society and caregivers.
Author contributions
JH had the idea for the manuscript. WH critically revised the work. All authors contributed to the study conception and design, performed the literature search, data analysis, and drafted the manuscript.
Funding
This work was supported by the Clinical Research Project of the Second Affiliated Hospital of Nanchang University, 2021efyC03.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aarsland, D., Batzu, L., Halliday, G. M., Geurtsen, G. J., Ballard, C., Ray Chaudhuri, K., et al. (2021). Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Primers 7:47. doi: 10.1038/s41572-021-00280-3
AlDakheel, A., Gasca-Salas, C., Armstrong, M. J., Duff-Canning, S., and Marras, C. (2019). Cognitive complaints in nondemented Parkinson’s disease patients and their close contacts do not predict worse cognitive outcome. Alzheimer Dis. Assoc. Disord. 33, 147–153. doi: 10.1097/WAD.0000000000000301
Barbosa, R. P., Mendonça, M. D., Caetano, A. P., Lampreia, T. M., Miguel, R., and Bugalho, P. M. (2019). Cognitive complaints in Parkinson’s disease patients: from subjective cognitive complaints to dementia and affective disorders. J. Neural Transm. 126, 1329–1335. doi: 10.1007/s00702-019-02042-8
Baschi, R., Nicoletti, A., Restivo, V., Recca, D., Zappia, M., and Monastero, R. (2018). Frequency and correlates of subjective memory complaints in Parkinson’s disease with and without mild cognitive impairment: data from the Parkinson’s disease cognitive impairment study. J. Alzheimers Dis. 63, 1015–1024. doi: 10.3233/JAD-171172
Bejr-kasem, H., Sampedro, F., Marín-Lahoz, J., Martínez-Horta, S., Pagonabarraga, J., and Kulisevsky, J. (2021). Minor hallucinations reflect early gray matter loss and predict subjective cognitive decline in Parkinson’s disease. Eur. J. Neurol. 28, 438–447. doi: 10.1111/ene.14576
Benito-León, J., Louis, E. D., Posada, I. J., Sánchez-Ferro, Á, Trincado, R., Villarejo, A., et al. (2011). Population-based case–control study of cognitive function in early Parkinson’s disease (NEDICES). J. Neurol. Sci. 310, 176–182. doi: 10.1016/j.jns.2011.06.054
Castro, P. C. F., Aquino, C. C., Felício, A. C., Doná, F., Medeiros, L. M. I., Silva, S. M. C. A., et al. (2016). Presence or absence of cognitive complaints in Parkinson’s disease: mood disorder or anosognosia? Arq. Neuropsiquiatr. 74, 439–444. doi: 10.1590/0004-282x20160060
Chua, C. Y., Koh, M. R. E., Chia, N. S.-Y., Ng, S. Y.-E., Saffari, S. E., Wen, M.-C., et al. (2021). Subjective cognitive Complaints in early Parkinson’s disease patients with normal cognition are associated with affective symptoms. Parkinsonism Relat. Disord. 82, 24–28. doi: 10.1016/j.parkreldis.2020.11.013
Copeland, J. N., Lieberman, A., Oravivattanakul, S., and Tröster, A. I. (2016). Accuracy of patient and care partner identification of cognitive impairments in Parkinson’s disease-mild cognitive impairment: accuracy of patient and care partner identification. Mov. Disord. 31, 693–698. doi: 10.1002/mds.26619
Dujardin, K., Duhamel, A., Delliaux, M., Thomas-Antérion, C., Destée, A., and Defebvre, L. (2010). Cognitive complaints in Parkinson’s disease: its relationship with objective cognitive decline. J. Neurol. 257, 79–84. doi: 10.1007/s00415-009-5268-2
Dupouy, J., Ory-Magne, F., Mekies, C., Rousseau, V., Puel, M., Rerat, K., et al. (2018). Cognitive complaint in early Parkinson’s disease: a pilot study. Acta Neurol. Scand. 137, 59–66. doi: 10.1111/ane.12808
Erro, R., Santangelo, G., Barone, P., Picillo, M., Amboni, M., Longo, K., et al. (2014). Do subjective memory complaints herald the onset of mild cognitive impairment in Parkinson disease? J. Geriatr. Psychiatry Neurol. 27, 276–281. doi: 10.1177/0891988714532015
Erro, R., Santangelo, G., Picillo, M., Vitale, C., Amboni, M., Longo, K., et al. (2012). Link between non-motor symptoms and cognitive dysfunctions in de novo, drug-naive PD patients. J. Neurol. 259, 1808–1813. doi: 10.1007/s00415-011-6407-0
Galtier, I., Nieto, A., Lorenzo, J. N., and Barroso, J. (2019). Subjective cognitive decline and progression to dementia in Parkinson’s disease: a long-term follow-up study. J. Neurol. 266, 745–754. doi: 10.1007/s00415-019-09197-0
Galtier, I., Nieto, A., Mata, M., Lorenzo, J. N., and Barroso, J. (2021). Analyses of visuospatial and visuoperceptual errors as predictors of dementia in Parkinson’s disease patients with subjective cognitive decline and mild cognitive impairment. J. Int. Neuropsychol. Soc. 27, 722–732. doi: 10.1017/S1355617720001216
Galtier, I., Nieto, A., Mata, M., Lorenzo, J. N., and Barroso, J. (2022). Specific pattern of linguistic impairment in Parkinson’s disease patients with subjective cognitive decline and mild cognitive impairment predicts dementia. J. Int. Neuropsychol. Soc. 13, 1–9. doi: 10.1017/S1355617722000571
Han, L., Wang, L., Xu, Z., Liang, X., Zhang, M., Fan, Y., et al. (2021). Disease progression in Parkinson‘s disease patients with subjective cognitive complaint. Ann. Clin. Transl. Neurol. 8, 2096–2104. doi: 10.1002/acn3.51461
Hely, M. A., Morris, J. G. L., Reid, W. G. J., and Trafficante, R. (2005). Sydney multicenter study of Parkinson’s disease: non- L -dopa–responsive problems dominate at 15 years. Mov. Disord. 20, 190–199. doi: 10.1002/mds.20324
Hogue, O., Fernandez, H. H., and Floden, D. P. (2018). Predicting early cognitive decline in newly-diagnosed Parkinson’s patients: a practical model. Parkinsonism Relat. Disord. 56, 70–75. doi: 10.1016/j.parkreldis.2018.06.031
Hong, J. Y., Lee, J. E., Sohn, Y. H., and Lee, P. H. (2012). Neurocognitive and atrophic patterns in Parkinson’s disease based on subjective memory complaints. J. Neurol. 259, 1706–1712. doi: 10.1007/s00415-011-6404-3
Hong, J. Y., Lee, Y., Sunwoo, M. K., Sohn, Y. H., and Lee, P. H. (2018). Subjective cognitive complaints and objective cognitive impairment in Parkinson’s disease. J. Clin. Neurol. Seoul Korea 14, 16–21. doi: 10.3988/jcn.2018.14.1.16
Hong, J. Y., Yun, H. J., Sunwoo, M. K., Ham, J. H., Lee, J.-M., Sohn, Y. H., et al. (2014b). Cognitive and cortical thinning patterns of subjective cognitive decline in patients with and without Parkinson’s disease. Parkinsonism Relat. Disord. 20, 999–1003. doi: 10.1016/j.parkreldis.2014.06.011
Hong, J. Y., Sunwoo, M. K., Chung, S. J., Ham, J. H., Lee, J. E., Sohn, Y. H., et al. (2014a). Subjective cognitive decline predicts future deterioration in cognitively normal patients with Parkinson’s disease. Neurobiol. Aging 35, 1739–1743. doi: 10.1016/j.neurobiolaging.2013.11.017
Huang, C., Mattis, P., Perrine, K., Brown, N., Dhawan, V., and Eidelberg, D. (2008). Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology 70, 1470–1477. doi: 10.1212/01.wnl.0000304050.05332.9c
Jack, C. R., Bennett, D. A., Blennow, K., Carrillo, M. C., Dunn, B., Haeberlein, S. B., et al. (2018). NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. J. Alzheimers Assoc. 14, 535–562. doi: 10.1016/j.jalz.2018.02.018
Jessen, F. (2010). Prediction of dementia by subjective memory impairment effects of severity and temporal association with cognitive impairment dementia and subjective memory impairment. Arch. Gen. Psychiatry 67:414. doi: 10.1001/archgenpsychiatry.2010.30
Jessen, F., Amariglio, R. E., Buckley, R. F., van der Flier, W. M., Han, Y., Molinuevo, J. L., et al. (2020). The characterisation of subjective cognitive decline. Lancet Neurol. 19, 271–278. doi: 10.1016/S1474-4422(19)30368-0
Jessen, F., Wolfsgruber, S., Wiese, B., Bickel, H., Mösch, E., Kaduszkiewicz, H., et al. (2014). AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimers Dement. 10, 76–83. doi: 10.1016/j.jalz.2012.09.017
Jones, J. D., Uribe, C., Bunch, J., and Thomas, K. R. (2021). Beyond PD-MCI: objectively defined subtle cognitive decline predicts future cognitive and functional changes. J. Neurol. 268, 337–345. doi: 10.1007/s00415-020-10163-4
Kehagia, A. A., Barker, R. A., and Robbins, T. W. (2010). Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 9, 1200–1213. doi: 10.1016/S1474-4422(10)70212-X
Kjeldsen, P. L., and Damholdt, M. F. (2019). Subjective cognitive complaints in patients with Parkinson’s disease. Acta Neurol. Scand. 140, 375–389. doi: 10.1111/ane.13158
Koster, D. P., Higginson, C. I., MacDougall, E. E., Wheelock, V. L., and Sigvardt, K. A. (2015). Subjective cognitive complaints in parkinson disease without dementia: a preliminary study. Appl. Neuropsychol. Adult 22, 287–292. doi: 10.1080/23279095.2014.925902
Lee, J. E., Ju, Y. J., Chun, K. H., and Lee, S. Y. (2020). The frequency of sleep medication use and the risk of subjective cognitive decline (SCD) or SCD with functional difficulties in elderly individuals without dementia. J. Gerontol. Ser. A 75, 1693–1698. doi: 10.1093/gerona/glz269
Lehrner, J., Moser, D., Klug, S., Gleiß, A., Auff, E., Pirker, W., et al. (2014). Subjective memory complaints, depressive symptoms and cognition in Parkinson’s disease patients. Eur. J. Neurol. 21, 1276–e77. doi: 10.1111/ene.12470
Mills, K. A., Mari, Z., Pontone, G. M., Pantelyat, A., Zhang, A., Yoritomo, N., et al. (2016). Cognitive impairment in Parkinson’s disease: association between patient-reported and clinically measured outcomes. Parkinsonism Relat. Disord. 33, 107–114. doi: 10.1016/j.parkreldis.2016.09.025
Mills, K. A., Schneider, R. B., Saint-Hilaire, M., Ross, G. W., Hauser, R. A., Lang, A. E., et al. (2020). Cognitive impairment in Parkinson’s disease: associations between subjective and objective cognitive decline in a large longitudinal study. Parkinsonism Relat. Disord. 80, 127–132. doi: 10.1016/j.parkreldis.2020.09.028
Mohanty, A., Engels, A. S., Herrington, J. D., Heller, W., Ringo Ho, M.-H., Banich, M. T., et al. (2007). Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology 44, 343–351. doi: 10.1111/j.1469-8986.2007.00515.x
Nakhla, M. Z., Holiday, K. A., Filoteo, J. V., Zlatar, Z. Z., Malcarne, V. L., Lessig, S., et al. (2021). Informant-reported cognitive decline is associated with objective cognitive performance in Parkinson’s disease. J. Int. Neuropsychol. Soc. 27, 439–449. doi: 10.1017/S1355617720001137
Obeso, J. A., Stamelou, M., Goetz, C. G., Poewe, W., Lang, A. E., Weintraub, D., et al. (2017). Past, present, and future of Parkinson’s disease: a special essay on the 200th anniversary of the shaking palsy. Mov. Disord. Off. J. Mov. Disord. Soc. 32, 1264–1310. doi: 10.1002/mds.27115
Ophey, A., Krohm, F., Kalbe, E., Greuel, A., Drzezga, A., Tittgemeyer, M., et al. (2022). Neural correlates and predictors of subjective cognitive decline in patients with Parkinson’s disease. Neurol. Sci. 43, 3153–3163. doi: 10.1007/s10072-021-05734-w
Pan, C., Ren, J., Hua, P., Yan, L., Yu, M., Wang, Y., et al. (2021). Subjective cognitive complaints in newly-diagnosed Parkinson’s disease with and without mild cognitive impairment. Front. Neurosci. 15:761817. doi: 10.3389/fnins.2021.761817
Perrotin, A., Mormino, E. C., Madison, C. M., Hayenga, A. O., and Jagust, W. J. (2012). Subjective cognition and amyloid deposition imaging: a Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch. Neurol. 69, 223–229. doi: 10.1001/archneurol.2011.666
Purri, R., Brennan, L., Rick, J., Xie, S. X., Deck, B. L., Chahine, L. M., et al. (2020). Subjective cognitive complaint in Parkinson disease patients with normal cognition: canary in the coal mine? Mov. Disord. Off. J. Mov. Disord. Soc. 35, 1618–1625. doi: 10.1002/mds.28115
Reisberg, B., Prichep, L., Mosconi, L., John, E. R., Glodzik-Sobanska, L., Boksay, I., et al. (2008). The pre–mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimers Dement. 4, S98–S108. doi: 10.1016/j.jalz.2007.11.017
Rodda, J. E., Dannhauser, T. M., Cutinha, D. J., Shergill, S. S., and Walker, Z. (2009). Subjective cognitive impairment: increased prefrontal cortex activation compared to controls during an encoding task. Int. J. Geriatr. Psychiatry 24, 865–874. doi: 10.1002/gps.2207
Rosenblum, S., Meyer, S., Richardson, A., and Hassin-Baer, S. (2022a). Capturing subjective mild cognitive decline in Parkinson’s disease. Brain Sci. 12:741. doi: 10.3390/brainsci12060741
Rosenblum, S., Meyer, S., Richardson, A., and Hassin-Baer, S. (2022b). Early identification of subjective cognitive functional decline among patients with Parkinson’s disease: a longitudinal pilot study. Sci. Rep. 12:22242. doi: 10.1038/s41598-022-26280-1
Santangelo, G., Vitale, C., Trojano, L., Angrisano, M. G., Picillo, M., Errico, D., et al. (2014). Subthreshold depression and subjective cognitive complaints in Parkinson’s disease. Eur. J. Neurol. 21, 541–544. doi: 10.1111/ene.12219
Saykin, A. J., Wishart, H. A., Rabin, L. A., Santulli, R. B., Flashman, L. A., West, J. D., et al. (2006). Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 67, 834–842. doi: 10.1212/01.wnl.0000234032.77541.a2
Scheef, L., Spottke, A., Daerr, M., Joe, A., Striepens, N., Kolsch, H., et al. (2012). Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology 79, 1332–1339. doi: 10.1212/WNL.0b013e31826c1a8d
Siciliano, M., Trojano, L., De Micco, R., Russo, A., Tedeschi, G., and Tessitore, A. (2020). Subjective memory decline in Parkinson’s disease patients with and without fatigue. Parkinsonism Relat. Disord. 70, 15–19. doi: 10.1016/j.parkreldis.2019.11.017
Siciliano, M., Trojano, L., De Micco, R., Sant’Elia, V., Giordano, A., Russo, A., et al. (2021). Correlates of the discrepancy between objective and subjective cognitive functioning in non-demented patients with Parkinson’s disease. J. Neurol. 268, 3444–3455. doi: 10.1007/s00415-021-10519-4
Sitek, E. J., Sołtan, W., Wieczorek, D., Robowski, P., and Sławek, J. (2011). Self-awareness of memory function in Parkinson’s disease in relation to mood and symptom severity. Aging Ment. Health 15, 150–156. doi: 10.1080/13607863.2010.508773
Song, I.-U., Kim, J.-S., Chung, S.-W., Lee, K.-S., Oh, J.-K., and Chung, Y.-A. (2014). Early detection of subjective memory impairment in Parkinson’s disease using cerebral perfusion SPECT. Biomed. Mater. Eng. 24, 3405–3410. doi: 10.3233/BME-141164
Stuart, S., Lord, S., Hill, E., and Rochester, L. (2016). Gait in Parkinson’s disease: a visuo-cognitive challenge. Neurosci. Biobehav. Rev. 62, 76–88. doi: 10.1016/j.neubiorev.2016.01.002
Uemura, Y., Wada-Isoe, K., Nakashita, S., and Nakashima, K. (2013). Depression and cognitive impairment in patients with mild parkinsonian signs. Acta Neurol. Scand. 128, 153–159. doi: 10.1111/ane.12089
Wirth, M., Bejanin, A., La Joie, R., Arenaza-Urquijo, E. M., Gonneaud, J., Landeau, B., et al. (2018). Regional patterns of gray matter volume, hypometabolism, and beta-amyloid in groups at risk of Alzheimer’s disease. Neurobiol. Aging 63, 140–151. doi: 10.1016/j.neurobiolaging.2017.10.023
Xiao, Y., Ou, R., Yang, T., Liu, K., Wei, Q., Hou, Y., et al. (2021). Different associated factors of subjective cognitive complaints in patients with early- and late-onset Parkinson’s disease. Front. Neurol. 12:749471. doi: 10.3389/fneur.2021.749471
Yang, N., Ju, Y., Ren, J., Wang, H., Li, P., Ning, H., et al. (2022). Prevalence and affective correlates of subjective cognitive decline in patients with de novo Parkinson’s disease. Acta Neurol. Scand. 146, 276–282. doi: 10.1111/ane.13662
Keywords: subjective cognitive decline (SCD), Parkinson’s disease, cognitive impairment, objective cognitive function, mild cognitive impairment
Citation: Huang J, Yuan X, Chen L, Hu B, Jiang L, Shi T, Wang H and Huang W (2023) Subjective cognitive decline in patients with Parkinson’s disease: an updated review. Front. Aging Neurosci. 15:1117068. doi: 10.3389/fnagi.2023.1117068
Received: 06 December 2022; Accepted: 08 May 2023;
Published: 26 May 2023.
Edited by:
Jiehui Jiang, Shanghai University, ChinaReviewed by:
Richard Dodel, University of Duisburg-Essen, GermanyTobias Blum, University of Duisburg-Essen, Germany, in collaboration with reviewer RD
Copyright © 2023 Huang, Yuan, Chen, Hu, Jiang, Shi, Wang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Huang, MTM2NzcwODAxOThAMTYzLmNvbQ==
 Juan Huang
Juan Huang Xingxing Yuan2
Xingxing Yuan2 Lijuan Jiang
Lijuan Jiang Ting Shi
Ting Shi Wei Huang
Wei Huang