
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Aging Neurosci. , 16 March 2023
Sec. Parkinson’s Disease and Aging-related Movement Disorders
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1109914
A commentary has been posted on this article:
Commentary: “Association between diabetes mellitus, prediabetes and risk, disease progression of Parkinson's disease: a systematic review and meta-analysis”
 Qifan Zhong
Qifan Zhong Shenglong Wang*
Shenglong Wang*Background: Previous studies reported inconsistent results regarding association between diabetes mellitus (DM), prediabetes and risk, disease progression of Parkinson's disease (PD). The meta-analysis was made to investigate association between DM, prediabetes and risk, disease progression of PD.
Methods: Literatures investigating association between DM, prediabetes and risk, disease progression of PD were searched in these databases: PubMed and Web of Science. Included literatures were published before October 2022. STATA 12.0 software was used to compute odds ratios (ORs)/relative risks (RRs) or standard mean differences (SMDs).
Results: DM was associated with a higher risk of PD, compared to non-diabetic participants with a random effects model (OR/RR = 1.23, 95% CI 1.12–1.35, I2 = 90.4%, p < 0.001). PD with DM (PD-DM) was associated with a faster motor progression compared to PD without DM (PD-noDM) with a fixed effects model (RR = 1.85, 95% CI 1.47–2.34, I2 = 47.3%, p = 0.091). However, meta-analysis for comparison in change rate of United Rating Scale (UPDRS) III scores from baseline to follow-up time between PD-DM and PD-noDM reported no difference in motor progression between PD-DM and PD-noDM with a random effects model (SMD = 2.58, 95% CI = −3.11 to 8.27, I2 = 99.9%, p < 0.001). PD-DM was associated with a faster cognitive decline compared to PD-noDM with a fixed effects model (OR/RR = 1.92, 95% CI 1.45–2.55, I2 = 50.3%, p = 0.110).
Conclusions: In conclusion, DM was associated with a higher risk and faster disease decline of PD. More large-scale cohort studies should be adopted to evaluate the association between DM, prediabetes and PD.
Diabetes mellitus (DM) has been deemed as one of the most common and serious chronic diseases worldwide, resulting in disabling, life threatening and costly complications, and eventually shortening life expectancy, reducing the quality of life (Heald et al., 2020). DM has a global prevalence of 9% (463 million adults) in 2019 (Sun et al., 2022). In addition, the global prevalence of DM is rising due to the aging of populations. Prediabetes, a high-risk period for DM, is defined by the blood glucose levels between normal and diabetes thresholds. Five to ten percent of prediabetes patients progress into patients with DM per year (Tabák et al., 2012).
Parkinson's disease (PD) is the second most common neurodegenerative disease and affects more than 1% of the population with the age of > 50 years old (Calabrese, 2007). Some studies (Deischinger et al., 2021; Sánchez-Gómez et al., 2021) reported that DM had a higher risk to be diagnosed with PD compared to non-diabetic participants, whereas some studies (Skeie et al., 2013; De Pablo-Fernandez et al., 2017) did not show any association between prevalence of PD and DM. In addition, some studies (Athauda et al., 2022) found that PD with DM (PD-DM) patients had significantly faster motor symptom progression and were more likely to develop mild cognitive impairment compared with PD without DM (PD-noDM), whereas some studies (Ou et al., 2021) reported no significant association between DM and disease progression of PD. In addition, no sufficient information was supported for association between prediabetes and PD. The present meta-analysis was made to investigate the association between DM, prediabetes and risk, disease progression of PD.
The study was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guideline (Moher et al., 2009). Supplementary Table 1 showed the PRISMA checklist.
Literatures investigating association between DM, prediabetes and risk, disease progression of PD were searched in these databases: PubMed and Web of Science. Included literatures were published before October 2022. We used these search terms: (“diabetes” OR “prediabetes” OR “glucose” OR “hyperglycemia” OR “insulin resistance” OR “HbA1c”) AND (“Parkinson's disease” OR “Parkinson's disease”).
We adopted these inclusion criteria: (1) studies investigated DM or prediabetes; (2) studies investigated PD; (3) studies were published in English. We adopted these exclusion criteria included: (1) reviews, meta-analysis and case-reports were excluded; (2) literatures were excluded if literature did not provide sufficient information for odds ratios (ORs)/relative risks (RRs) and 95% confidence intervals (CIs) regarding association between DM, prediabetes and risk, disease progression of PD.
These data were extracted from included literatures with Excel document: Author and publication year, study location, study type, sample size, age, gender, event for analysis, results and adjusted variables.
We adopted STATA 12.0 software to compute the results. ORs/RRs and 95% CIs were computed to acquire a computed OR/RR and 95% CI. In addition, a computed standard mean difference (SMD) and a 95% CI was acquired using STATA 12.0 software. P-value < 0.05 was considered statistically significant. A random effects model was used for high heterogeneity (p-value for Q-test ≤ 0.05 and I2 ≥ 50%); inversely, a fixed effects model was used for low heterogeneity (p-value for Q-test > 0.05 and I2 <50%). We adopted meta-regression analysis and subgroup studies (for different ethnicities and different study types) to investigate the source of heterogeneity. Sensitivity analysis was employed to assess the study stabilization. We adopted Begg's test, Egger's test and funnel plot to evaluate publication bias. Quality appraisal was conducted using the Cochrane Risk of Bias Tool. Data were analyzed with Review Manager 5.3.
Figure 1 illustrated result of initial search and study selection process. Tables 1, 2 showed characteristics regarding included studies. N = 12 case-control studies (Morano et al., 1994; Leibson et al., 2006; Powers et al., 2006; Scigliano et al., 2006; Becker et al., 2008; D'Amelio et al., 2009; Rugbjerg et al., 2009; Miyake et al., 2010; Schernhammer et al., 2011; Savica et al., 2012; Skeie et al., 2013; De Pablo-Fernandez et al., 2017) [including N = 21,118 PD patients and N = 89,150 healthy controls (HCs)], N = 11 cohort studies (Grandinetti et al., 1994; Hu et al., 2007; Simon et al., 2007; Driver et al., 2008; Palacios et al., 2011; Xu et al., 2011; Sun et al., 2012; Yang et al., 2017; De Pablo-Fernandez et al., 2018; Kizza et al., 2019; Sánchez-Gómez et al., 2021) (including N = 35,939 PD patients and N = 7,062,700 participants) and N = 1 cross-sectional study (Deischinger et al., 2021) (including N = 235,268 PD patients and N = 1,938,173 HCs) were included regarding association between DM and risk of PD. Only N = 1 cohort study (Sánchez-Gómez et al., 2021) (including N = 13,715 PD patients and N = 3,104,460 participants) was included for association between prediabetes and risk of PD. N = 7 cohort studies (Cereda et al., 2012; Malek et al., 2016; Ong et al., 2017; Pagano et al., 2018; De Pablo-Fernandez et al., 2021; Ou et al., 2021; Athauda et al., 2022) (including N = 473 PD-DM patients and N = 4,081 PD-noDM patients) were included regarding association between DM and progression of PD.
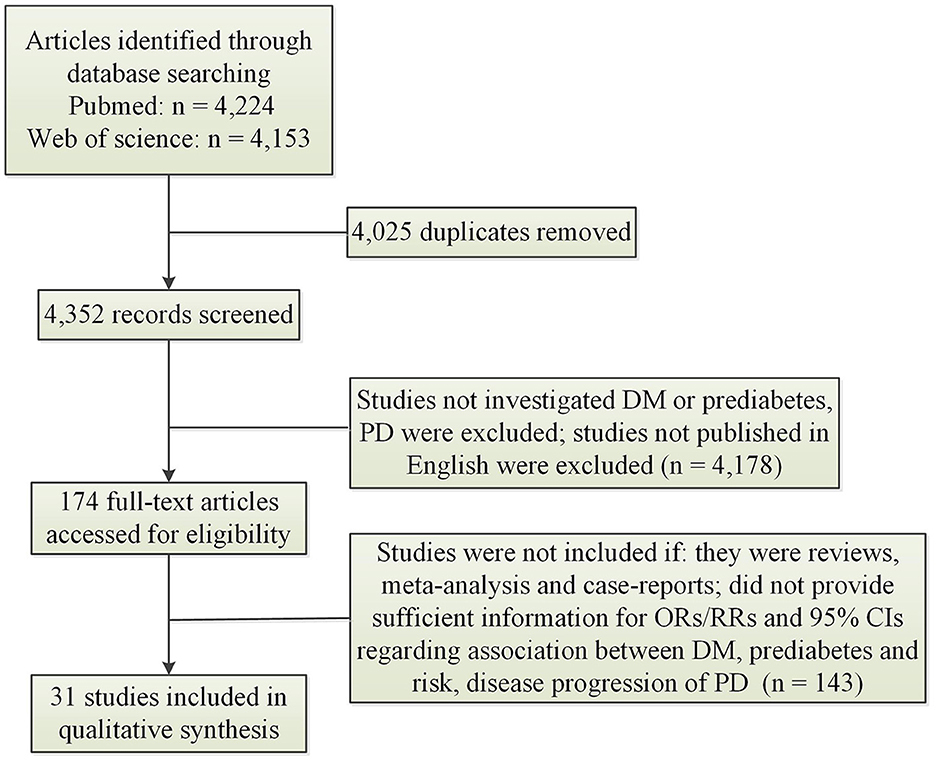
Figure 1. Search results and selection process. CI, confidence interval; DM, diabetes mellitus; OR, odds ratio; PD, Parkinson's disease; RR, relative risk.
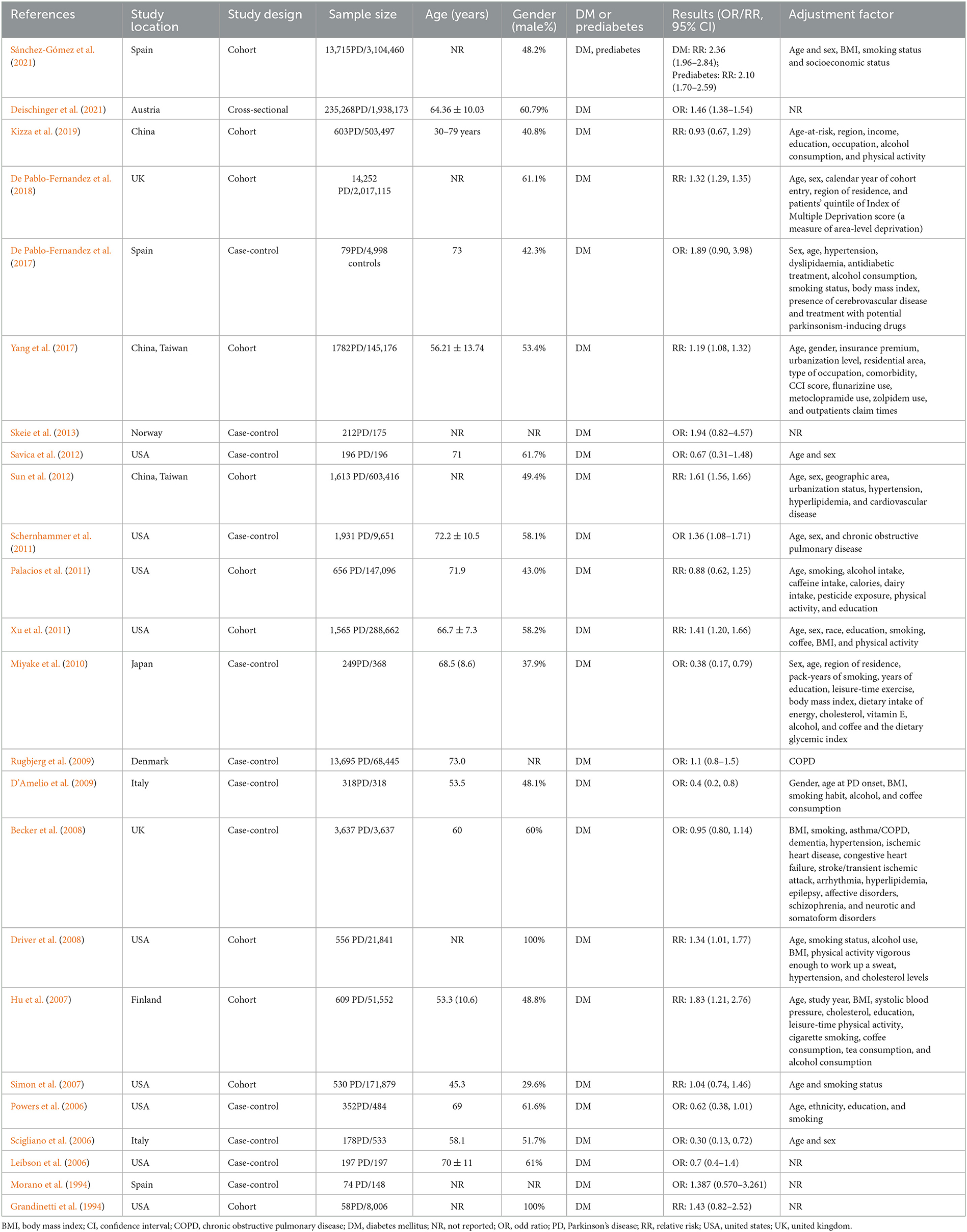
Table 1. Characteristics of included studies exploring association between DM, prediabetes and risk of PD.
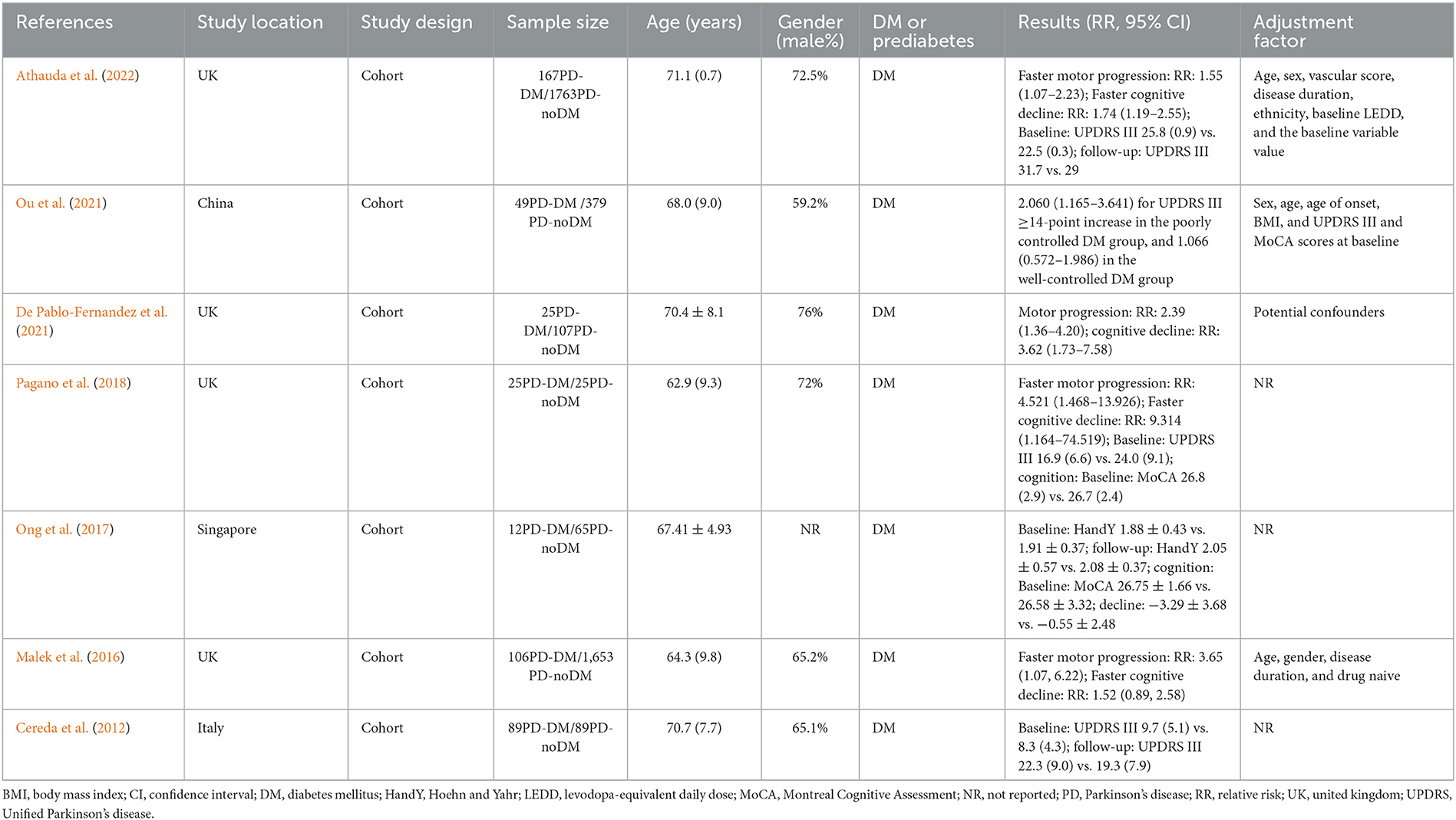
Table 2. Characteristics of included studies exploring association between DM and disease progression of PD.
DM was associated with a higher risk of PD, compared to non-diabetic participants with a random effects model (OR/RR = 1.23, 95% CI 1.12–1.35, p < 0.001, I2 = 90.4%, p-value for Q-test < 0.001; Figure 2). Meta-regression analysis indicated that publication year, age and gender were not responsible for heterogeneity between studies (publication year: p = 0.109; age: p = 0.730; gender: p = 0.878). Subgroup analysis found that DM was associated with a higher risk of PD in Caucasian compared to non-diabetic participants, whereas no significant association was showed between DM and risk of PD in Asian (Caucasian: OR/RR = 1.23, 95% CI 1.10–1.37; Asian: OR/RR = 1.11, 95% CI 0.82–1.48; Supplementary Figure 1). Subgroup analysis found that DM was associated with a higher risk of PD in cohort studies compared to non-diabetic participants, whereas no significant association was showed between DM and risk of PD in case-control studies (cohort: RR = 1.37, 95% CI 1.23–1.54; case-control: OR = 0.86, 95% CI 0.66–1.11; Supplementary Figure 2). Sensitivity analysis indicated no change in the direction of effect while any one study was excluded from the meta-analysis (Supplementary Figure 3). Begg's test, Egger's test and funnel plot showed no significant risk of publication bias (Begg's test p = 0.442; Egger's test: p = 0.120; Supplementary Figure 4).
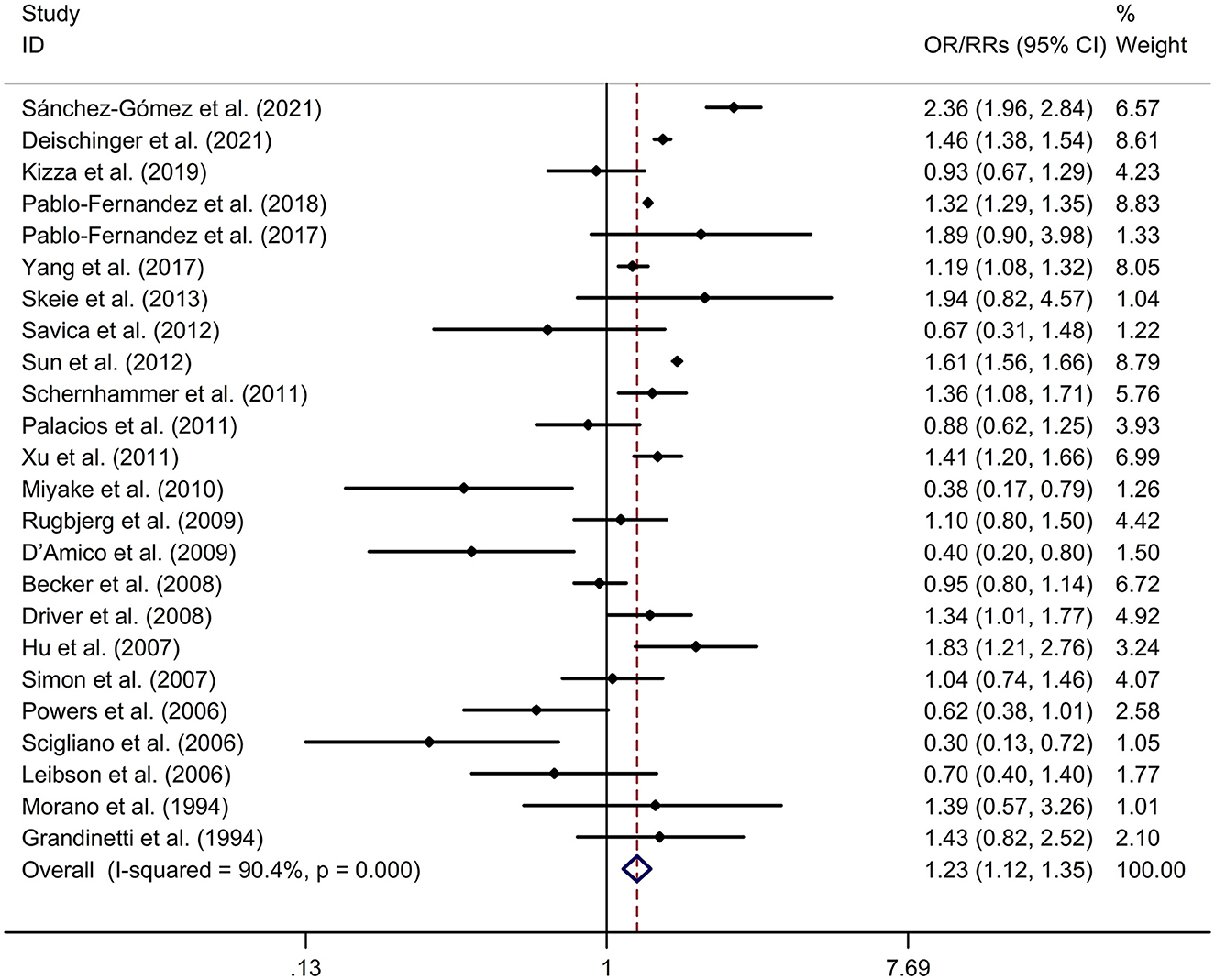
Figure 2. Forest plot for association between DM and risk of PD. CI, confidence interval; DM, diabetes mellitus; OR, odds ratio; PD, Parkinson's disease; RR, relative risk.
PD-DM was associated with a faster motor progression compared to PD-noDM with a fixed effects model (RR = 1.85, 95% CI 1.47–2.34, p < 0.001, I2 = 47.3%, p-value for Q-test = 0.091; Figure 3). Meta-regression analysis indicated that publication year, age and gender were not responsible for heterogeneity between studies (publication year: p = 0.736; age: p = 0.652; gender: p = 0.371). Subgroup analysis found that PD-DM was associated with a faster motor progression in Caucasian compared to PD-noDM (OR/RR = 2.01, 95% CI 1.52–2.67; Supplementary Figure 5). Sensitivity analysis indicated no change in the direction of effect while any one study was excluded from the meta-analysis (Supplementary Figure 6). Begg's test, Egger's test and funnel plot showed no significant risk of publication bias (Begg's test p = 0.260; Egger's test: p = 0.152; Supplementary Figure 7).
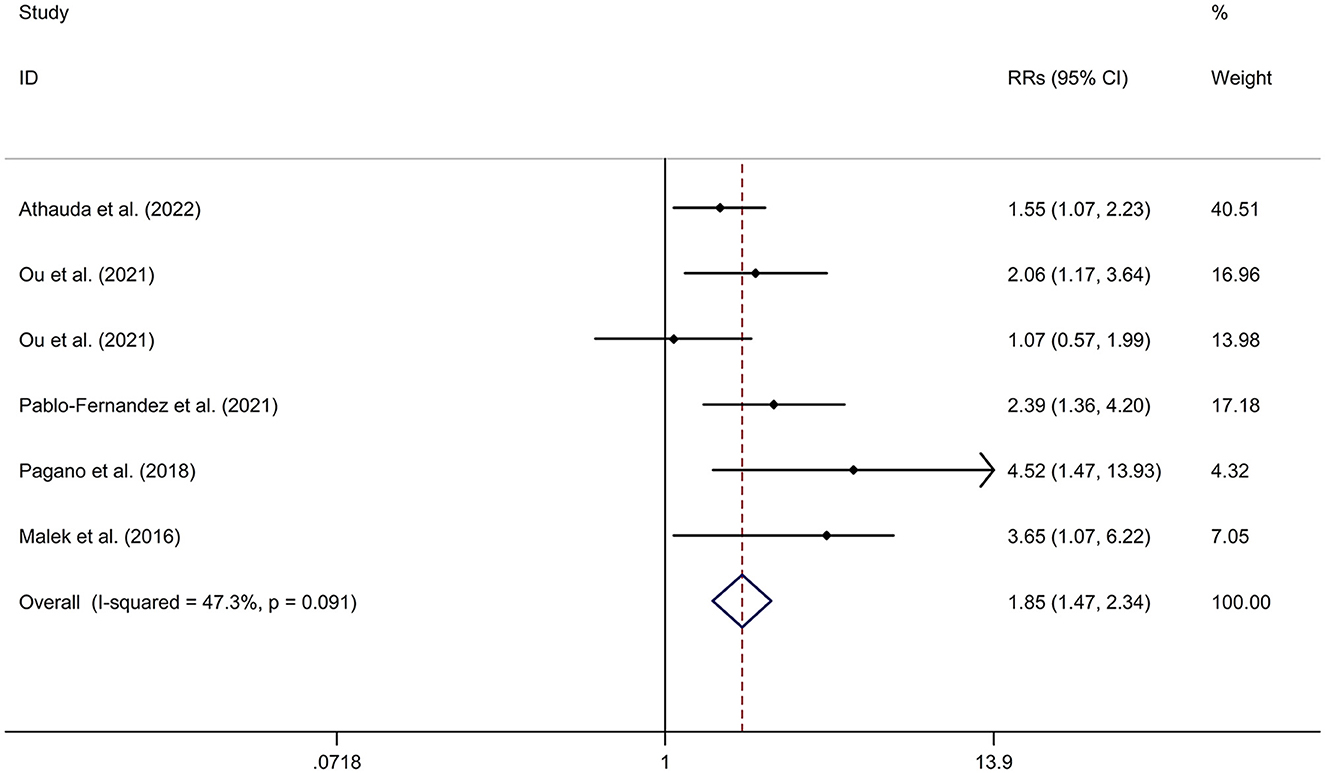
Figure 3. Forest plot for association between DM and motor progression of PD. CI, confidence interval; DM, diabetes mellitus; PD, Parkinson's disease; RR, relative risk.
Mean values and standard deviation (SD) of increase or reduction rate of United Rating Scale (UPDRS) III scores from baseline to follow-up time in PD-DM and PD-noDM were collected from studies. Meta-analysis for comparison in change rate of UPDRS III scores from baseline to follow-up time between PD-DM and PD-noDM reported no difference in motor progression between PD-DM and PD-noDM with a random effects model (SMD = 2.58, 95% CI = −3.11 to 8.27, p = 0.374, I2 = 99.9%, p-value for Q-test < 0.001, Figure 4). Meta-regression analysis indicated that publication year, age and gender were not responsible for heterogeneity between studies (publication year: p = 0.339; age: p = 0.598). Sensitivity analysis indicated no change in the direction of effect while any one study was excluded from the meta-analysis (Supplementary Figure 8). Begg's test, Egger's test and funnel plot showed no significant risk of publication bias (Begg's test p = 0.602; Egger's test: p = 0.792; Supplementary Figure 9).
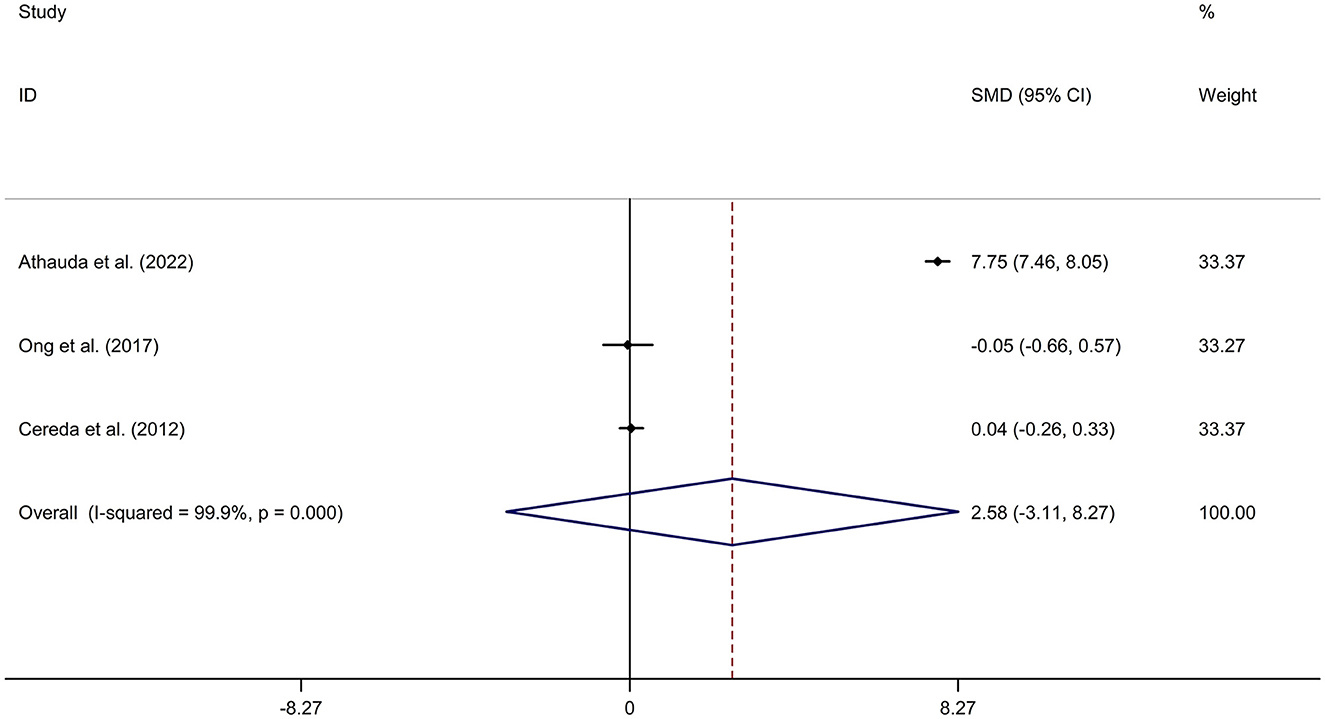
Figure 4. Forest plot for comparison in change of motor function between PD-DM and PD-noDM. CI, confidence interval; DM, diabetes mellitus; PD, Parkinson's disease; PD-DM, PD with DM; PD-noDM, PD without DM; SMD, standard mean difference.
PD-DM was associated with a faster cognitive decline compared to PD-noDM with a fixed effects model (OR/RR = 1.92, 95% CI 1.45–2.55, p < 0.001, I2 = 50.3%, p-value for Q-test = 0.110; Figure 5). Meta-regression analysis indicated that publication year, age and gender were not responsible for heterogeneity between studies (publication year: p = 0.477; age: p = 0.478; gender: p = 0.478). Sensitivity analysis indicated no change in the direction of effect while any one study was excluded from the meta-analysis (Supplementary Figure 10). Begg's test, Egger's test and funnel plot showed no significant risk of publication bias (Begg's test p = 0.497; Egger's test: p = 0.181; Supplementary Figure 11).

Figure 5. Forest plot for association between DM and cognitive decline of PD. CI, confidence interval; DM, diabetes mellitus; PD, Parkinson's disease; RR, relative risk.
Regarding the association between prediabetes and risk of PD, Sánchez-Gómez et al. (2021) found that prediabetes were associated with a higher risk of PD (RR = 1.07, 95% CI 1.00–1.14).
Supplementary Figure 12 illustrated the risk of bias graph. Details of the risk of bias summary were showed in Supplementary Figure 13.
The meta-analysis found that DM was associated with a higher risk of PD, compared to non-diabetic participants. In addition, PD-DM was associated with a faster motor progression and cognitive decline, compared to PD-noDM.
Corresponding to the epidemiological evidence for the association between DM and PD, experimental research supported the common mechanisms in the two diseases. Recent evidence supported the presence of local insulin resistance in brain in neurodegenerative diseases [including PD and Alzheimer's disease (AD)] (Morris et al., 2014; Arnold et al., 2018). Brain insulin resistance refers to reaction failure of brain cells to insulin (Mielke et al., 2005). Brain insulin resistance results in deficits in neurotransmitter release or receptor regulation in neurons, neuroplasticity impairment, abnormal protein deposition and failure of clearance (Sharma et al., 2015). Additionally, systemic insulin resistance may also cause brain impairment (including inflammatory response, microvascular disease, and deficit of the blood brain barrier) through hyperglycaemia and its complications (Santiago and Potashkin, 2013). Genome-wide association studies have showed a network of genes regarding autoimmunity which is shared with PD, AD and diabetes (Menon and Farina, 2011). In addition, antidiabetic drugs have shown some immunomodulatory properties in PD animal models. Pioglitazone, a proliferator-activated receptor (PPAR)-γ agonist, can reduce microglia and astrocyte activation (Breidert et al., 2002); NLY01, a glucagon-like peptide-1 receptor (GLP1R) agonist, inhibited the phenoconversion of astrocytes toward a pro-inflammatory phenotype and protected against the loss of dopamine neurons and behavioral deficits in the model of sporadic PD (Yun et al., 2018). More studies are essential to explore the mechanism regarding association between DM and PD.
The study reported a higher risk of PD in DM. The result was consistent with a recent meta-analysis (Liu and Tang, 2021) (including 7 case-control studies and 9 cohort studies), which reported that DM was associated with an elevated risk of PD (OR/RR = 1.15, 95% CI 1.03–1.28). In addition, subgroup study was corresponding to the previous meta-analysis (Liu and Tang, 2021), which showed that DM was associated with higher risk of PD in cohort studies (RR = 1.29, 95% CI 1.15–1.45), whereas no significant association was indicated between DM and risk of PD in case-control studies (OR = 0.74, 95% CI 0.51–1.09). The different result might derive from the difference in study design. Traditionally, the results of cohort studies are usually more reliable compared to retrospective case-control studies, due to the absence of recall and interviewer bias. Subgroup analysis showed that DM was associated with a higher risk of PD in Caucasian compared to non-diabetic participants, whereas no significant association was showed between DM and risk of PD in Asian. Only N = 4 studies (Miyake et al., 2010; Sun et al., 2012; Yang et al., 2017; Kizza et al., 2019) explored association between DM and risk of PD in Asian. Sun et al. (2012) and Yang et al. (2017) reported that DM was associated with a significantly elevated risk of PD with cohort studies, whereas Miyake et al. (2010) reported that DM was significantly associated with a decreased risk of PD with a case-control study. The different result might derive from the difference in study design. More studies were essential to investigate association between DM and risk of PD in Asian. In addition, the result is also consistent with a recent meta-analysis (Komici et al., 2021), which found that DM patients showed a higher risk of developing PD compared to non-DM, and PD patients with DM showed a greater severity of motor symptoms, with higher motor dysfunction, compared with PD-noDM. The present meta-analysis did not explore the association between DM and disease severity of PD. Thus, more large-scale, cohort studies were needed to investigate the association between the two diseases.
PD-DM was associated with a faster motor progression and cognitive decline, compared to PD-noDM. Motor progression and cognitive decline in PD was associated with progression of dopaminergic deficit in PD (Fereshtehnejad et al., 2017). Dopamine uptake is intensive in the presence of insulin (Shaughness et al., 2020). The mechanism might mediate the association between DM and disease progression of PD. The study showed no difference in change rate of UPDRS III scores from baseline to follow-up time between PD-DM and PD-noDM. The result showed that PD-DM showed a faster motor progression, but not a greater motor progression, compared to PD-noDM. Only N = 3 studies were included for comparison in change rate of UPDRS III scores from baseline to follow-up time between PD-DM and PD-noDM. More cohort studies were essential to explore the association between DM and disease progression of PD.
Regarding the association between prediabetes and risk of PD, only one cohort study (Sánchez-Gómez et al., 2021) found that prediabetes were associated with a higher risk of PD. This is the first study to evaluate association between prediabetes and risk of PD development in a large cohort. A community-based study (Wong et al., 2016) reported that prediabetes was an independent risk factor of pRBD (probable rapid eye movement sleep behavior disorder), which linked prediabetes with PD. More studies are warranted to support the association between prediabetes and PD.
There are some limitations in the meta-analysis. Firstly, high heterogeneity was showed between studies exploring association between DM and risk of PD. The present meta-analysis used meta-regression analysis and subgroup analysis to investigate the source of heterogeneity across included studies. However, the source of heterogeneity still remains unclear. Secondly, the present study did not explore the association between prediabetes and PD.
In conclusion, DM was associated with a higher risk and faster disease decline of PD. More large-scale cohort studies should be adopted to evaluate the association between DM, prediabetes and PD.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
QZ: study design, manuscript writing, data collection, data analysis, and software use. SW: study design, manuscript writing and revision, data collection, data analysis, software use, and supervision. All authors read and approved the final version of the manuscript.
This study was supported by the Jiangsu Shengze Hospital High level Talent Fund Project (No. SYK202102).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1109914/full#supplementary-material
Arnold, S. E., Arvanitakis, Z., Macauley-Rambach, S. L., Koenig, A. M., Wang, H. Y., Ahima, R. S., et al. (2018). Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat. Rev. Neurol. 14, 168–181. doi: 10.1038/nrneurol.2017.185
Athauda, D., Evans, J., Wernick, A., Virdi, G., Choi, M. L., Lawton, M., et al. (2022). The impact of type 2 diabetes in Parkinson's disease. Mov. Disord. 37, 1612–1623. doi: 10.1002/mds.29122
Becker, C., Brobert, G. P., Johansson, S., Jick, S. S., and Meier, C. R. (2008). Diabetes in patients with idiopathic Parkinson's disease. Diabetes Care 31, 1808–1812. doi: 10.2337/dc08-0479
Breidert, T., Callebert, J., Heneka, M. T., Landreth, G., Launay, J. M., and Hirsch, E. C. (2002). Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson's disease. J. Neurochem. 82, 615–624. doi: 10.1046/j.1471-4159.2002.00990.x
Calabrese, V. P. (2007). Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 69, 223–4. author reply 4. doi: 10.1212/01.wnl.0000271777.50910.73
Cereda, E., Barichella, M., Cassani, E., Caccialanza, R., and Pezzoli, G. (2012). Clinical features of Parkinson disease when onset of diabetes came first: a case-control study. Neurology 78, 1507–1511. doi: 10.1212/WNL.0b013e3182553cc9
D'Amelio, M., Ragonese, P., Callari, G., Di Benedetto, N., Palmeri, B., Terruso, V., et al. (2009). Diabetes preceding Parkinson's disease onset. A case-control study. Parkins. Related Disord. 15, 660–664. doi: 10.1016/j.parkreldis.2009.02.013
De Pablo-Fernandez, E., Courtney, R., Rockliffe, A., Gentleman, S., Holton, J. L., and Warner, T. T. (2021). Faster disease progression in Parkinson's disease with type 2 diabetes is not associated with increased alpha-synuclein, tau, amyloid-beta or vascular pathology. Neuropathol. Appl. Neurobiol. 47, 1080–1091. doi: 10.1111/nan.12728
De Pablo-Fernandez, E., Goldacre, R., Pakpoor, J., Noyce, A. J., and Warner, T. T. (2018). Association between diabetes and subsequent Parkinson disease: a record-linkage cohort study. Neurology 91, e139–e42. doi: 10.1212/WNL.0000000000005771
De Pablo-Fernandez, E., Sierra-Hidalgo, F., Benito-León, J., and Bermejo-Pareja, F. (2017). Association between Parkinson's disease and diabetes: data from NEDICES study. Acta Neurol. Scand. 136, 732–736. doi: 10.1111/ane.12793
Deischinger, C., Dervic, E., Kaleta, M., Klimek, P., and Kautzky-Willer, A. (2021). Diabetes mellitus is associated with a higher relative risk for Parkinson's disease in women than in men. J. Parkinson's Dis. 11, 793–800. doi: 10.3233/JPD-202486
Driver, J. A., Smith, A., Buring, J. E., Gaziano, J. M., Kurth, T., and Logroscino, G. (2008). Prospective cohort study of type 2 diabetes and the risk of Parkinson's disease. Diab. Care 31, 2003–2005. doi: 10.2337/dc08-0688
Fereshtehnejad, S. M., Zeighami, Y., Dagher, A., and Postuma, R. B. (2017). Clinical criteria for subtyping Parkinson's disease: biomarkers and longitudinal progression. Brain J. Neurol. 140, 1959–1976. doi: 10.1093/brain/awx118
Grandinetti, A., Morens, D. M., Reed, D., and MacEachern, D. (1994). Prospective study of cigarette smoking and the risk of developing idiopathic Parkinson's disease. Am. J. Epidemiol. 139, 1129–1138. doi: 10.1093/oxfordjournals.aje.a116960
Heald, A. H., Stedman, M., Davies, M., Livingston, M., Alshames, R., Lunt, M., et al. (2020). Estimating life years lost to diabetes: outcomes from analysis of National Diabetes Audit and Office of National Statistics data. Cardiovasc. Endocrinol. Metab. 9, 183–185. doi: 10.1097/XCE.0000000000000210
Hu, G., Jousilahti, P., Bidel, S., Antikainen, R., and Tuomilehto, J. (2007). Type 2 diabetes and the risk of Parkinson's disease. Diab. Care 30, 842–847. doi: 10.2337/dc06-2011
Kizza, J., Lewington, S., Mappin-Kasirer, B., Turnbull, I., Guo, Y., Bian, Z., et al. (2019). Cardiovascular risk factors and Parkinson's disease in 500,000 Chinese adults. Ann. Clin. Transl. Neurol. 6, 624–632. doi: 10.1002/acn3.732
Komici, K., Femminella, G. D., Bencivenga, L., Rengo, G., and Pagano, G. (2021). Diabetes mellitus and Parkinson's disease: a systematic review and meta-analyses. J. Parkinson's Dis. 11, 1585–1596. doi: 10.3233/JPD-212725
Leibson, C. L., Maraganore, D. M., Bower, J. H., Ransom, J. E., O'brien, P. C., and Rocca, W. A. (2006). Comorbid conditions associated with Parkinson's disease: a population-based study. Mov. Disord. 21, 446–455. doi: 10.1002/mds.20685
Liu, W., and Tang, J. (2021). Association between diabetes mellitus and risk of Parkinson's disease: a prisma-compliant meta-analysis. Brain Behav. 11, e02082. doi: 10.1002/brb3.2082
Malek, N., Lawton, M. A., Swallow, D. M., Grosset, K. A., Marrinan, S. L., Bajaj, N., et al. (2016). Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson's disease. Mov. Disord. 31, 1518–1526. doi: 10.1002/mds.26698
Menon, R., and Farina, C. (2011). Shared molecular and functional frameworks among five complex human disorders: a comparative study on interactomes linked to susceptibility genes. PLoS ONE 6, e18660. doi: 10.1371/journal.pone.0018660
Mielke, J. G., Taghibiglou, C., Liu, L., Zhang, Y., Jia, Z., Adeli, K., et al. (2005). A biochemical and functional characterization of diet-induced brain insulin resistance. J. Neurochem. 93, 1568–1578. doi: 10.1111/j.1471-4159.2005.03155.x
Miyake, Y., Tanaka, K., Fukushima, W., Sasaki, S., Kiyohara, C., Tsuboi, Y., et al. (2010). Case-control study of risk of Parkinson's disease in relation to hypertension, hypercholesterolemia, and diabetes in Japan. J. Neurol. Sci. 293, 82–86. doi: 10.1016/j.jns.2010.03.002
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clin. Res. Ed.) 339, b2535. doi: 10.1136/bmj.b2535
Morano, A., Jim Nez-Jim Nez, F. J., Molina, J. A., and Antolin, M. A. (1994). Risk-factors for Parkinson's disease: case-control study in the province of Cáceres, Spain. Acta Neurol. Scand. 89, 164–170. doi: 10.1111/j.1600-0404.1994.tb01655.x
Morris, J. K., Vidoni, E. D., Perea, R. D., Rada, R., Johnson, D. K., Lyons, K., et al. (2014). Insulin resistance and gray matter volume in neurodegenerative disease. Neuroscience 270, 139–147. doi: 10.1016/j.neuroscience.2014.04.006
Ong, M., Foo, H., Chander, R. J., Wen, M. C., Au, W. L., Sitoh, Y. Y., et al. (2017). Influence of diabetes mellitus on longitudinal atrophy and cognition in Parkinson's disease. J. Neurol. Sci. 377, 122–126. doi: 10.1016/j.jns.2017.04.010
Ou, R., Wei, Q., Hou, Y., Zhang, L., Liu, K., Lin, J., et al. (2021). Effect of diabetes control status on the progression of Parkinson's disease: a prospective study. Ann. Clin. Transl. Neurol. 8, 887–897. doi: 10.1002/acn3.51343
Pagano, G., Polychronis, S., Wilson, H., Giordano, B., Ferrara, N., Niccolini, F., et al. (2018). Diabetes mellitus and Parkinson disease. Neurology 90, e1654–e62. doi: 10.1212/WNL.0000000000005475
Palacios, N., Gao, X., Mccullough, M. L., Jacobs, E. J., Patel, A. V., Mayo, T., et al. (2011). Obesity, diabetes, and risk of Parkinson's disease. Mov. Disord. 26, 2253–2259. doi: 10.1002/mds.23855
Powers, K. M., Smith-Weller, T., Franklin, G. M. Jr., Longstreth, W. T, Swanson, P. D., and Checkoway, H. (2006). Diabetes, smoking, and other medical conditions in relation to Parkinson's disease risk. Parkins. Relat. Disord. 12, 185–189. doi: 10.1016/j.parkreldis.2005.09.004
Rugbjerg, K., Friis, S., Ritz, B., Schernhammer, E. S., Korbo, L., and Olsen, J. H. (2009). Autoimmune disease and risk for Parkinson disease: a population-based case-control study. Neurology 73, 1462–1468. doi: 10.1212/WNL.0b013e3181c06635
Sánchez-Gómez, A., Díaz, Y., Duarte-Salles, T., Compta, Y., and Mart,í, M. J. (2021). Prediabetes, type 2 diabetes mellitus and risk of Parkinson's disease: a population-based cohort study. Parkins. Relat. Disord. 89, 22–27. doi: 10.1016/j.parkreldis.2021.06.002
Santiago, J. A., and Potashkin, J. A. (2013). Shared dysregulated pathways lead to Parkinson's disease and diabetes. Trends Mol. Med. 19, 176–186. doi: 10.1016/j.molmed.2013.01.002
Savica, R., Grossardt, B. R., Ahlskog, J. E., and Rocca, W. A. (2012). Metabolic markers or conditions preceding Parkinson's disease: a case-control study. Mov. Disord. 27, 974–979. doi: 10.1002/mds.25016
Schernhammer, E., Hansen, J., Rugbjerg, K., Wermuth, L., and Ritz, B. (2011). Diabetes and the risk of developing Parkinson's disease in Denmark. Diab. Care 34, 1102–1108. doi: 10.2337/dc10-1333
Scigliano, G., Musicco, M., Soliveri, P., Piccolo, I., Ronchetti, G., and Girotti, F. (2006). Reduced risk factors for vascular disorders in Parkinson disease patients: a case-control study. Stroke 37, 1184–1188. doi: 10.1161/01.STR.0000217384.03237.9c
Sharma, A. N., Ligade, S. S., Sharma, J. N., Shukla, P., Elased, K. M., and Lucot, J. B. (2015). GLP-1 receptor agonist liraglutide reverses long-term atypical antipsychotic treatment associated behavioral depression and metabolic abnormalities in rats. Metab. Brain Dis. 30, 519–527. doi: 10.1007/s11011-014-9591-7
Shaughness, M., Acs, D., Brabazon, F., Hockenbury, N., and Byrnes, K. R. (2020). Role of insulin in neurotrauma and neurodegeneration: a review. Front. Neurosci. 14, 547175. doi: 10.3389/fnins.2020.547175
Simon, K. C., Chen, H., Schwarzschild, M., and Ascherio, A. (2007). Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology 69, 1688–1695. doi: 10.1212/01.wnl.0000271883.45010.8a
Skeie, G. O., Muller, B., Haugarvoll, K., Larsen, J. P., and Tysnes, O. B. (2013). Parkinson disease: associated disorders in the Norwegian population based incident ParkWest study. Parkins. Relat. Disord. 19, 53–55. doi: 10.1016/j.parkreldis.2012.07.003
Sun, H., Saeedi, P., Karuranga, S., Pinkepank, M., Ogurtsova, K., Duncan, B. B., et al. (2022). IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diab. Res. Clin. Pract. 183, 109119. doi: 10.1016/j.diabres.2021.109119
Sun, Y., Chang, Y. H., Chen, H. F., Su, Y. H., Su, H. F., and Li, C. Y. (2012). Risk of Parkinson disease onset in patients with diabetes: a 9-year population-based cohort study with age and sex stratifications. Diab. Care 35, 1047–1049. doi: 10.2337/dc11-1511
Tabák, A. G., Herder, C., Rathmann, W., Brunner, E. J., and Kivimäki, M. (2012). Prediabetes: a high-risk state for diabetes development. Lancet 379, 2279–2290. doi: 10.1016/S0140-6736(12)60283-9
Wong, J. C., Li, J., Pavlova, M., Chen, S., Wu, A., Wu, S., et al. (2016). Risk factors for probable REM sleep behavior disorder: a community-based study. Neurology 86, 1306–1312. doi: 10.1212/WNL.0000000000002414
Xu, Q., Park, Y., Huang, X., Hollenbeck, A., Blair, A., Schatzkin, A., et al. (2011). Diabetes and risk of Parkinson's disease. Diab. Care 34, 910–915. doi: 10.2337/dc10-1922
Yang, Y. W., Hsieh, T. F., Li, C. I., Liu, C. S., Lin, W. Y., Chiang, J. H., et al. (2017). Increased risk of Parkinson disease with diabetes mellitus in a population-based study. Medicine 96, e5921. doi: 10.1097/MD.0000000000005921
Keywords: diabetes mellitus, meta-analysis, Parkinson's disease, prediabetes, risk
Citation: Zhong Q and Wang S (2023) Association between diabetes mellitus, prediabetes and risk, disease progression of Parkinson's disease: A systematic review and meta-analysis. Front. Aging Neurosci. 15:1109914. doi: 10.3389/fnagi.2023.1109914
Received: 28 November 2022; Accepted: 17 February 2023;
Published: 16 March 2023.
Edited by:
Roberto Cilia, IRCCS Carlo Besta Neurological Institute Foundation, ItalyReviewed by:
Fabiana Colucci, University of Ferrara, ItalyCopyright © 2023 Zhong and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shenglong Wang, aGRnc2pkazg3MzhAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.