
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Aging Neurosci. , 31 May 2023
Sec. Alzheimer's Disease and Related Dementias
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1095996
This article is part of the Research Topic Behavior Support for People with Dementia View all 9 articles
Objective: This network meta-analysis aimed to compare and rank the efficacy of animal-assisted therapy (AAT) and pet-robotic therapy (PRT) in the management of dementia.
Methods: Relevant studies were identified by searching PubMed, EMBASE, the Cochrane Library, SCOPUS, and Web of Science (WoS) until October 13, 2022. Traditional meta-analysis was first conducted based on the random-effects model, then random network meta-analysis was conducted to determine the relative efficacy and rank probability of AAT and PRT.
Results: Nineteen randomized controlled trials (RCTs) were included in this network meta-analysis. Network meta-analysis revealed that PRT marginally benefited agitation alleviation compared with control (standard mean difference [SMD]: −0.37, 95% confidence interval [95%CI]: −0.72 to −0.01) although both AAT and PRT did not improve cognitive function, reduce depression, and improve Quality of Life (QoL). The SUCRA probabilities indicated that PRT ranked better than AAT in agitation, cognitive function, and QoL, although there were no differences between the two therapies.
Conclusion: The present network meta-analysis reveals that PRT may help alleviate agitated behaviors in people with dementia. However, future studies are warranted to establish evidence of the effectiveness of PRT and further evaluate the differences between different robot types in managing dementia.
Dementia is a chronic degenerative encephalopathy condition characterized by cognitive and consciousness disorders, personality changes, abnormal behavior, and a decreased capacity for performing daily activities (Prince et al., 2013; Gallaway et al., 2017; Kishita et al., 2020). It is usually a subsequent condition in other diseases, such as Alzheimer's or language cortex tumors (Gale et al., 2018). According to the World Health Organization (WHO), global dementia cases will grow to 152 million by 2050 (2022). Undoubtedly, an increase in dementia cases will lead to a rise in the total cost of healthcare each year (Skaria, 2022). According to estimates, the current yearly cost of treating dementia might reach $1 trillion; by 2030, this expense will have doubled (Wimo et al., 2017).
Behavioral and psychological symptoms of dementia (BPSD), consisting of apathy, depression, agitation, aggression, anxiety, and display of behavioral deficits or excesses (Tible et al., 2017; Kang et al., 2020), are pretty prevalent in patients diagnosed with dementia (Kales et al., 2015). With the progression of their dementia, more than 90% of patients will suffer from one or more BPSD (Kales et al., 2019). Notably, BPSD has a poor impact on dementia's prognosis, referrals, hospitalization, expenditures, and quality of life (QoL), and it will also raise the burden on caregivers (Prince et al., 2013; Tible et al., 2017; Sado et al., 2018). Since there are no better therapies to slow the neurocognitive deterioration process, effective management and treatment of BPSD are crucial.
It is recommended to begin with a thorough assessment of the cause of dementia in treating cognitive degeneration (Herrmann, 2001). Non-pharmacological therapies have been recommended for the first-line treatment of BPSD in dementia in recent decades (Dyer et al., 2016). Its non-invasive nature, fewer side effects, and more affordable cost have outweighed pharmaceutical intervention (Quintavalla et al., 2021). Except for the widespread concern of overdose (Herrmann, 2001), repeated use of the drugs may also raise the risk of falls (Porsteinsson et al., 2014; Masopust et al., 2018), produce gastrointestinal problems (Tan et al., 2014; Dyer et al., 2018), and accelerate cognitive decline (Ma et al., 2014; Masopust et al., 2018).
Among numerous non-pharmacological treatments available, animal-assisted therapy (AAT) and pet-robotic therapy (PRT) have been extensively used in the management of dementia due to their significant impact on the lives of people of all ages and socioeconomic backgrounds (Hughes et al., 2020; Yu et al., 2022). Currently, several meta-analyses have evaluated the efficacy of AAT (Zafra-Tanaka et al., 2019; Batubara et al., 2022; Chen et al., 2022) and PRT (Leng et al., 2019; Lu et al., 2021; Ong et al., 2021) on BPSD in dementia, but reported conflicting results. Additionally, the difference between AAT and PRT in managing BPSD remains controversial, as only one study involving seven studies used a network meta-analysis to assess the comparative efficacy of these two therapies in reducing agitation (Leng et al., 2020). Therefore, we conducted the current network meta-analysis to comprehensively evaluate the effectiveness of AAT vs. PRT in managing BPSD in dementia by pooling direct and indirect evidence.
We conducted this network meta-analysis following the guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for reporting network meta-analysis (Hutton et al., 2015). Institutional review board ethical approval and patient informed consent were unnecessary for this study because all statistical analyses were completed based on the published data. The formal protocol of this network meta-analysis was not registered on any public platform.
Two independent authors (Hongdi Du and Lin Bo) systematically searched PubMed, EMBASE, the Cochrane Library, SCOPUS, and Web of Science (WoS) for retrieving potentially eligible studies from their inception until October 13, 2020. We developed search strategies using the MeSH terms and their synonyms, including “dementia,” “Alzheimer's disease,” “Animal-assisted therapy,” “robotics/therapy,” “robotics/therapeutic use,” and “random.” The detailed search strategies of target databases are documented in Supplementary Table 1. The search was only limited to English publications. Additionally, we manually checked references of the included studies and topic-related meta-analyses to identify those eligible studies missing from the electronic search. Any divergences between the two authors (Xiaopeng Huo and Xiaoxing Lai) were resolved by discussion until a consensus was reached.
Based on the previous meta-analyses (Leng et al., 2020; Ong et al., 2021; Chen et al., 2022), the following inclusion criteria, which were developed based on the PICOS acronym, were used for guiding the selection of potentially eligible studies: (a) adult patients were diagnosed with dementia, (b) patients in the experimental group received AAT or PRT, (c) patients in the control group received usual care or one of AAT and PRT, (d) studies reported at least one of agitation, cognitive function, depression, and the QoL, and (e) randomized controlled trials published with full texts.
Studies were excluded if they met the following criteria: (a) repeated reports of the same study; (b) studies assessing the robot's acceptability to participants, (c) studies were conducted to evaluate companion robot's development and usability, (d) lack of data to perform the network meta-analysis; and (e) studies using ineligible designs such as review articles, conference abstracts, qualitative studies, contemplated articles, or animal studies.
We used EndNote X9.2 (Clarivate Analytics) to manage records retrieved from electronic retrieval. EndNote software was first used to exclude duplicates before evaluating the eligibility of these records. Two independent authors (Hongdi Du and Xiaoxing Lai) then evaluated the eligibility of all unique records according to the eligibility criteria by preliminarily screening their titles and abstracts. For those studies retained after screening the titles and abstracts, their eligibility was evaluated by screening full texts. Any divergences between the two authors (Xiaopeng Huo and Hongwei Zhu) were resolved by discussion until a consensus was reached.
Two independent authors (Lin Bo and Hongwei Zhu) used a pre-designed data extraction sheet to complete data extraction. Specifically, the following information was extracted from the included studies: the first author's surname, country, study design, setting, sample size, participants' mean age, dementia stage, details of interventions, outcomes of interest, and detailed information of quality assessment. We extracted the data of the final follow-up for meta-analysis. We used the recognized formula to estimate mean and standard deviation according to median and range, standard error, or interquartile range (Wan et al., 2014). Any divergences between the two authors (Hongdi Du and Xiaopeng Huo) were resolved by discussion until a consensus was reached.
We considered agitation the primary outcome in this network meta-analysis, while cognitive function, depression, and QoL were secondary outcomes. The instruments used to measure the outcomes were not limited but must have acceptable reliability and validity.
The evidence structures of all outcomes were constructed using a network plot. The network plot had two essential elements: a node and a line. The node represented the intervention and was weighted using the accumulated sample size; however, the line represented the comparison that directly compared two interventions, and the width of the line was weighted by using the number of eligible studies (Salanti et al., 2011).
Two authors (Hongdi Du and Lin Bo) independently assessed the methodological quality of the included studies using the Cochrane risk of bias assessment tool (Higgins et al., 2011). Specifically, the methodological quality of each study was determined by evaluating the following seven items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessor, incomplete outcome data, selective reporting, and other bias. For each item, one of three labels would be assigned, including “low,” “high,” or “unclear” risk. The overall level of methodological quality of each study might be rated as “high” level if all items were labeled with “low” risk, as “low” level if at least one of the seven items were labeled with “high” risk, or “moderate” level if at least one the seven items were labeled with “unclear” risk but no item was labeled with “high” risk. Any divergences between the two authors (Xiaopeng Huo and Xiaoxing Lai) were resolved by discussion until a consensus was reached.
Standard mean difference (SMD) with the corresponding 95% confidence interval (CI) was used to express the pooled estimates because all outcomes were continuous variables in this study but were measured using various tools. We first conducted a traditional meta-analysis to evaluate the efficacy of AAT or PRT in the management of participants with dementia (DerSimonian and Laird, 1986). Statistical heterogeneity between the included studies was assessed using the Cochrane Q statistic (Bowden et al., 2011) and Higgins' inconsistency factor (I2) (Higgins and Thompson, 2002). Statistical heterogeneity was considered significant if P < 0.1 and I2 > 50%. Nevertheless, meta-analysis was conducted based on the random-effects model because it was not rational to deny the variations between studies (Biggerstaff and Tweedie, 1997).
Then, a network meta-analysis based on a random-effects model was conducted to determine the difference between AAT and PRT in terms of all outcomes of interest (Lu and Ades, 2004; Dias and Caldwell, 2019). First, the transitivity examination was first conducted to determine whether it is rational to conduct a network meta-analysis based on the major factors (Salanti, 2012; Cipriani et al., 2013), including publication year, origin, sample size, mean age, gender ratio, and dementia stage. Then, global inconsistency was examined using the design-by-treatment interaction method (Tu, 2015), and local inconsistency was tested using the node-splitting method when both direct and indirect evidence were available (Higgins et al., 2012). Third, we used the node-splitting method to assess the closed-loop inconsistency (Lu and Ades, 2006; Yu-Kang, 2016). Fourth, we calculated the surface under the cumulative ranking curve (SUCRA) probabilities to rank AAT and PRT, and a higher SUCRA value indicated a better ranking (Mbuagbaw et al., 2017). Finally, a comparison-adjusted funnel plot was used to assess the small-sample effect (Sterne et al., 2001). Additionally, Egger's and Begg's tests were used to evaluate publication bias quantitatively (Palma Perez and Delgado Rodriguez, 2006; Page et al., 2018). We conducted all analyses using STATA 14.0 (StataCorp LP, College Station, Texas, USA) (Chaimani et al., 2013; White, 2017).
A total of 1,122 relevant studies were retrieved from the databases and the published meta-analyses. Before conducting the eligibility evaluation, 288 duplicate studies and 26 registries were excluded. After excluding 752 ineligible studies based on title and abstract screening, 56 were screened by checking the full texts. Finally, 19 studies (Moyle et al., 2013, 2017, 2019; Robinson et al., 2013; Travers et al., 2013; Bono et al., 2015; Friedmann et al., 2015; Jøranson et al., 2015, 2016; Valenti Soler et al., 2015; Pope et al., 2016; Olsen et al., 2016a,b; Liang et al., 2017; Petersen et al., 2017; Pu et al., 2020; Briones et al., 2021; Quintavalla et al., 2021; Vegue Parra et al., 2021) were included in this network meta-analysis after excluding 37 ineligible studies due to ineligible patients (n = 1), unrelated to the topic (n = 7), lack of outcomes (n = 5), ineligible study designs (n = 15), lack of essential data (n = 4), ineligible language (n = 1), and ineligible aims (n = 4). The process of study selection is depicted in Figure 1.
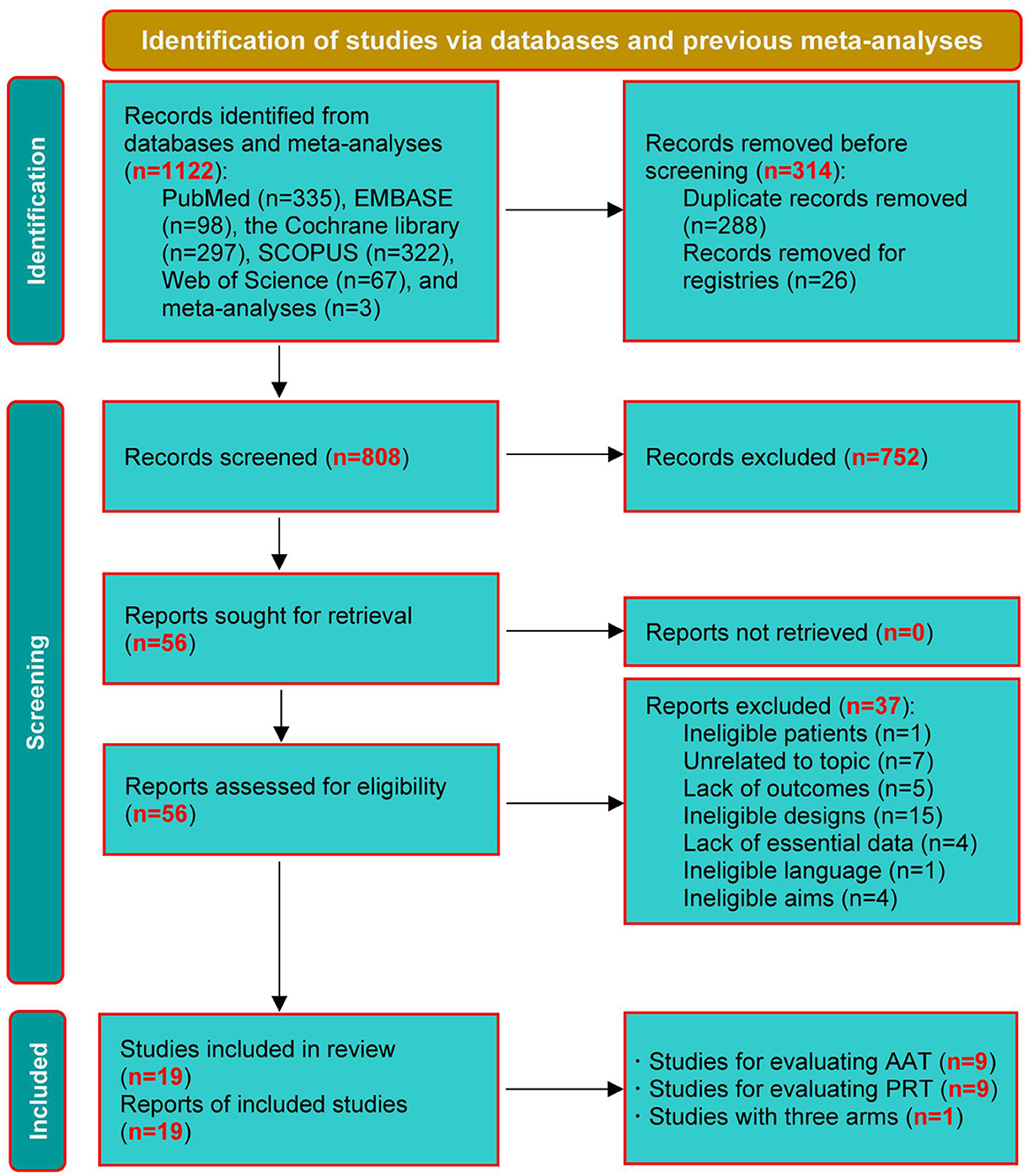
Figure 1. PRISMA diagram of study retrieval and selection. AAT, animal-assisted therapy; PRT, per-robot therapy.
We summarized the basic characteristics of the included studies in Table 1, and listed the detailed information on AAT and PRT of the included studies in Supplementary Table 2. The outcomes and measurements of the included studies are documented in Supplementary Table 3. Overall, 18 studies (Moyle et al., 2013, 2017, 2019; Robinson et al., 2013; Travers et al., 2013; Bono et al., 2015; Friedmann et al., 2015; Jøranson et al., 2015, 2016; Pope et al., 2016; Olsen et al., 2016a,b; Liang et al., 2017; Petersen et al., 2017; Pu et al., 2020; Briones et al., 2021; Quintavalla et al., 2021; Vegue Parra et al., 2021) were two-arm design and one study (Valenti Soler et al., 2015) was three-arm design, and all studies were published between 2013 and 2021. The sample size of the included studies ranged from 24 to 334, accumulating a total sample size of 1464. Nine studies (Moyle et al., 2013, 2019; Friedmann et al., 2015; Jøranson et al., 2015; Pope et al., 2016; Olsen et al., 2016b; Liang et al., 2017; Pu et al., 2020) reported the data of agitation. Five studies (Bono et al., 2015; Jøranson et al., 2016; Liang et al., 2017; Quintavalla et al., 2021; Vegue Parra et al., 2021) reported the data on cognitive function, and eleven studies (Moyle et al., 2013; Robinson et al., 2013; Travers et al., 2013; Bono et al., 2015; Friedmann et al., 2015; Jøranson et al., 2015; Olsen et al., 2016b; Liang et al., 2017; Petersen et al., 2017; Pu et al., 2020; Vegue Parra et al., 2021) reported the data of depression. Eight studies (Moyle et al., 2013; Robinson et al., 2013; Travers et al., 2013; Valenti Soler et al., 2015; Jøranson et al., 2016; Olsen et al., 2016a,b; Briones et al., 2021) reported the data of QoL. The evidence structures of all outcomes are depicted in Supplementary Figure 1.
All included studies correctly generated random sequences, but only five (Jøranson et al., 2015, 2016; Liang et al., 2017; Moyle et al., 2017, 2019) adequately reported the information on allocation concealment. A total of16 studies (Moyle et al., 2013; Travers et al., 2013; Bono et al., 2015; Friedmann et al., 2015; Jøranson et al., 2015, 2016; Valenti Soler et al., 2015; Pope et al., 2016; Olsen et al., 2016a,b; Liang et al., 2017; Petersen et al., 2017; Pu et al., 2020; Briones et al., 2021; Quintavalla et al., 2021; Vegue Parra et al., 2021) did not blind participants and personnel, and 12 studies (Robinson et al., 2013; Friedmann et al., 2015; Jøranson et al., 2015, 2016; Pope et al., 2016; Olsen et al., 2016a,b; Liang et al., 2017; Moyle et al., 2017, 2019; Petersen et al., 2017; Pu et al., 2020) did not blind outcome assessors. Six studies (Moyle et al., 2013; Robinson et al., 2013; Travers et al., 2013; Bono et al., 2015; Olsen et al., 2016a) had incomplete data but did not use an appropriate approach to conduct data analysis. Five studies (Moyle et al., 2013; Travers et al., 2013; Jøranson et al., 2015, 2016; Valenti Soler et al., 2015) were labeled with high risk due to selective outcome reporting. Four studies (Pope et al., 2016; Olsen et al., 2016b; Moyle et al., 2019; Briones et al., 2021) had high risk in other bias sources. The risk-of-bias assessment of the included studies is displayed in Supplementary Figure 2. Overall, the methodological quality of the included studies was low.
Transitivity between the included studies was assessed based on six major factors: origin, publication year, sample size, the mean age of participants, gender ratio, and dementia stage. As shown in Supplementary Table 4, the distributions of these six factors were not significantly different in all comparisons.
As shown in Supplementary Figure 3, the results indicated non-significant global consistency for cognitive function (P = 0.441) and depression (P = 0.608) but significant global inconsistency for agitation (P = 0.045). Nevertheless, we selected an inconsistency model for network meta-analysis of these three outcomes because no direct evidence was available for cognitive function, agitation, and depression. For QoL, the global inconsistency examination was not statistically significant (P = 0.940). Meanwhile, the local inconsistency examination for QoL was insignificant, reporting a P of 0.749 for comparing AAT with control, a P of 0.730 for comparing PRT with control, and a P of 0.686 for the comparison of AAT with PRT. Therefore, the consistency model was used for the network meta-analysis of QoL.
Three (Friedmann et al., 2015; Pope et al., 2016; Olsen et al., 2016b) and six (Moyle et al., 2013, 2017, 2019; Jøranson et al., 2015; Liang et al., 2017; Pu et al., 2020) studies compared AAT and PRT with control, respectively. Traditional meta-analysis revealed that, as shown in Figure 2A, AAT (SMD: −0.31, 95%CI: −0.70 to 0.08, P=0.114) and PRT (SMD: −0.35, 95%CI: −0.73 to 0.04, P = 0.078) did not significantly alleviate agitated behaviors of participants of dementia. As shown in Figure 3A, the network meta-analysis results revealed that AAT did not considerably alleviate agitation (SMD: −0.32; 95% CI: −0.79 to 0.15), but PRT marginally benefited agitation alleviation (SMD: −0.37; 95% CI: −0.72 to −0.01). Nevertheless, network meta-analysis revealed that AAT and PRT did not differ significantly in agitation (SMD: −0.04, 95% CI: −0.64 to 0.55).
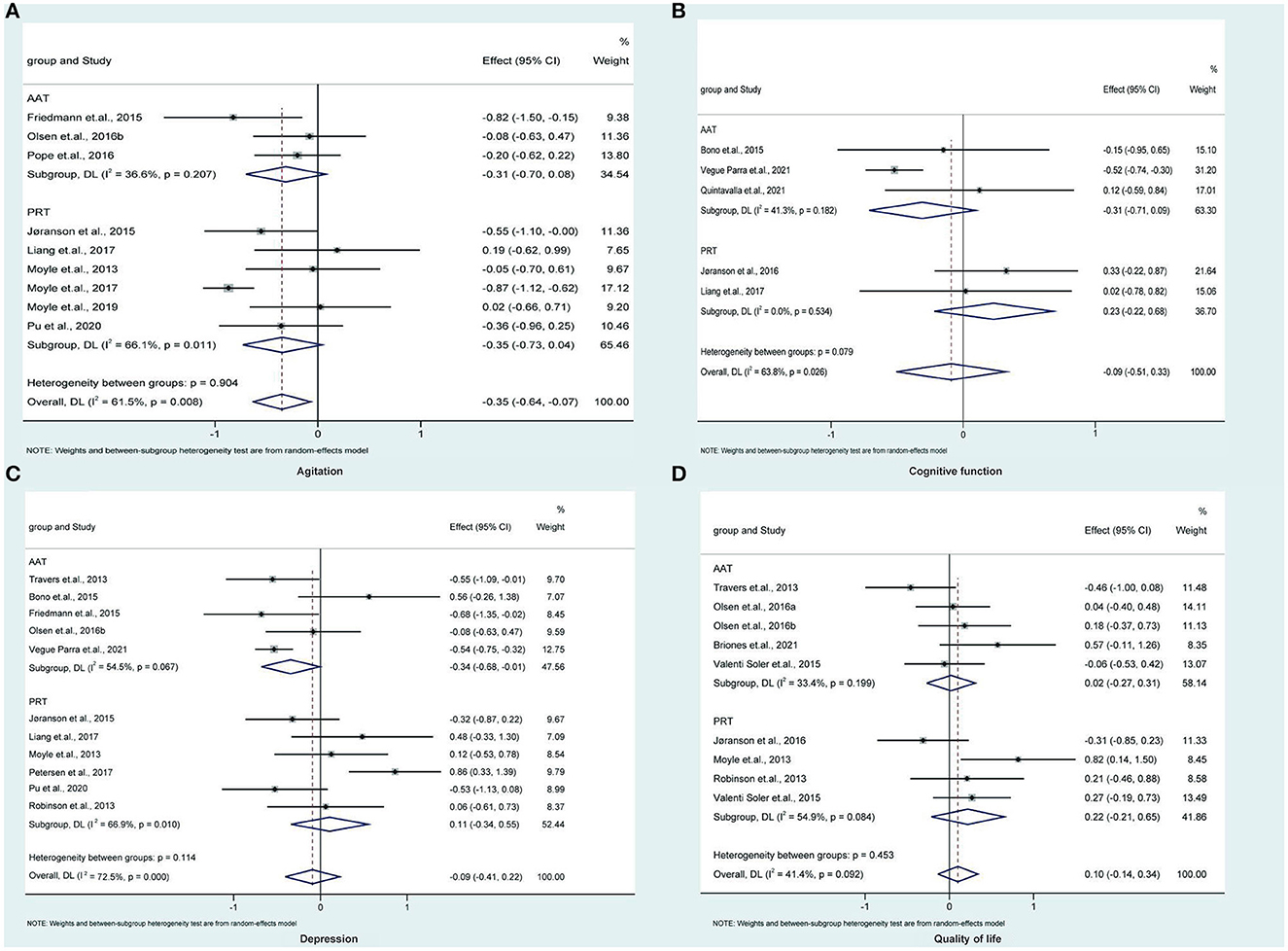
Figure 2. Traditional direct meta-analysis of AAT and PRT in terms of agitation (A), cognitive function (B), depression (C), and quality of life (D). AAT, animal-assisted therapy; PRT, per-robot therapy; CI, confidence interval; DL, Dersimonian-Laird.
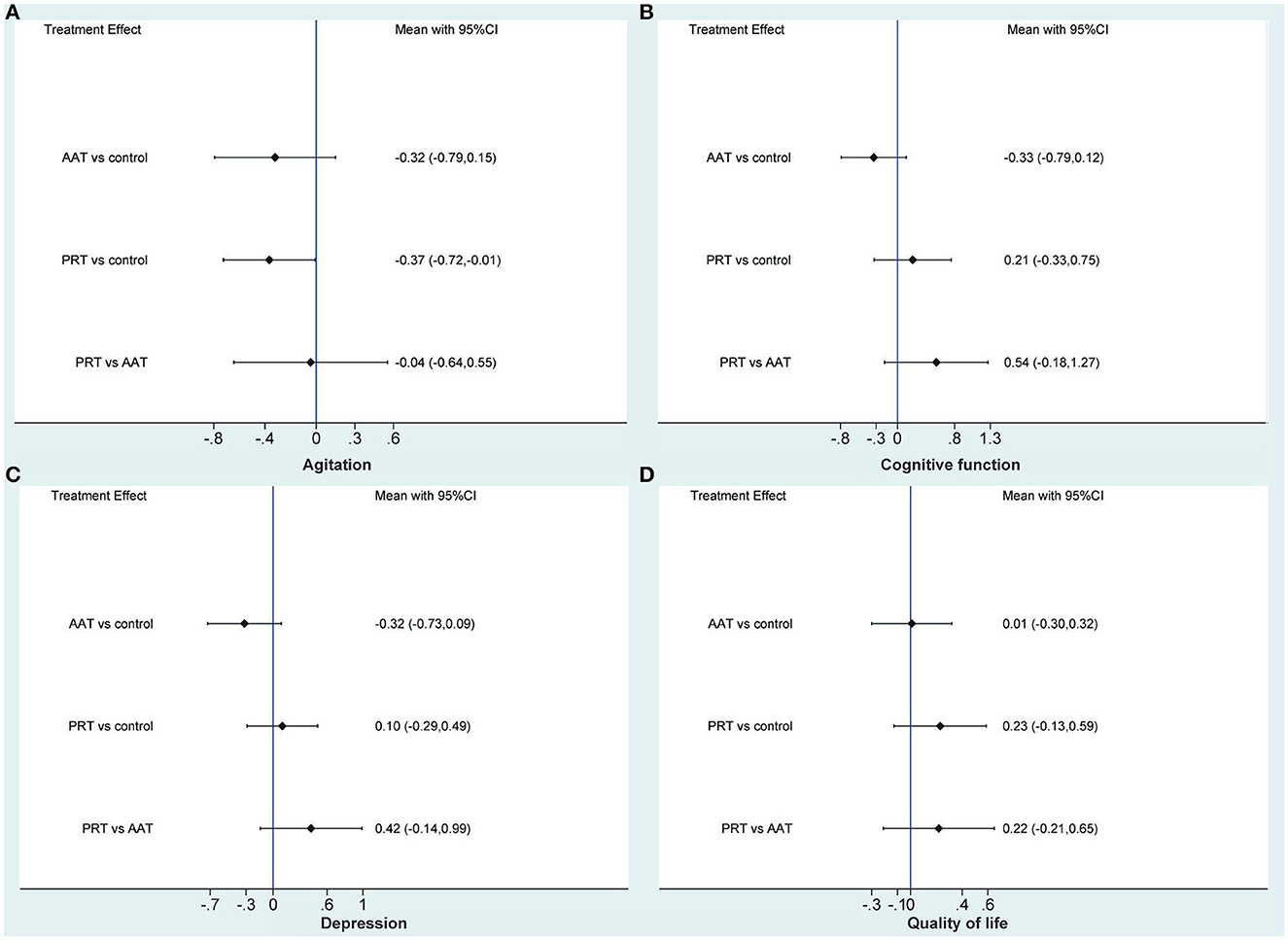
Figure 3. Network meta-analysis of AAT and PRT in terms of agitation (A), cognitive function (B), depression (C), and quality of life (D). AAT, animal-assisted therapy; PRT, per-robot therapy; CI, confidence interval.
Three (Bono et al., 2015; Quintavalla et al., 2021; Vegue Parra et al., 2021) and two (Jøranson et al., 2016; Liang et al., 2017) studies compared AAT and PRT with control, respectively. Traditional meta-analysis revealed that, as shown in Figure 2B, AAT (SMD: −0.31, 95%CI: −0.71 to 0.09, P = 0.130) and PRT (SMD: 0.23, 95%CI: −0.22 to 0.68, P = 0.316) did not significantly improve cognitive function of participants of dementia. As shown in Figure 3B, the results of the traditional meta-analysis were further confirmed by network meta-analysis. Furthermore, network meta-analysis revealed that AAT and PRT did not differ significantly in cognitive function (SMD: 0.54, 95% CI: −0.18 to 1.27).
Five (Travers et al., 2013; Bono et al., 2015; Friedmann et al., 2015; Olsen et al., 2016b; Vegue Parra et al., 2021) and six (Moyle et al., 2013; Robinson et al., 2013; Jøranson et al., 2015; Liang et al., 2017; Petersen et al., 2017; Pu et al., 2020) studies compared AAT and PRT with control, respectively. Traditional meta-analysis revealed that, as shown in Figure 2C, AAT (SMD: −0.34, 95%CI: −0.68 to −0.01, P = 0.045) rather than PRT (SMD: 0.11, 95%CI: −0.34 to 0.55, P = 0.641) marginally benefited depression reduction. As shown in Figure 3C, however, network meta-analysis revealed that both AAT (SMD: −0.32; 95% CI: −0.73 to 0.09) and PRT (SMD: 0.10; 95% CI: −0.29 to 0.49) did not significantly reduce depression. Furthermore, network meta-analysis also revealed that AAT and PRT did not differ significantly in terms of agitation (SMD: 0.42, 95% CI: −0.14 to 0.99).
Five (Travers et al., 2013; Valenti Soler et al., 2015; Olsen et al., 2016a,b; Briones et al., 2021) and four (Moyle et al., 2013; Robinson et al., 2013; Valenti Soler et al., 2015; Jøranson et al., 2016) studies compared AAT and PRT with control, respectively. Traditional meta-analysis revealed that, as shown in Figure 2D, both AAT (SMD: 0.02, 95% CI: −0.27 to 0.31, P = 0.894) and PRT (SMD: 0.22, 95%CI:−0.21 to 0.65, P = 0.322) did not significantly improve QoL. As shown in Figure 3D, the results of the traditional meta-analysis were further confirmed by network meta-analysis. Furthermore, network meta-analysis revealed that AAT and PRT did not differ significantly in QoL (SMD: 0.22, 95% CI: −0.21 to 0.65).
As shown in Supplementary Figure 4, the SUCRA probability of PRT was 77.7% in agitation, 85.4% in cognitive function, 18.4% in depression, and 87.1% in QoL; however, the SUCRA probability of AAT was 66.7% in agitation, 7.4% in cognitive function, 93.4% in depression, and 34.3% in QoL. Overall, the PRT was probably a better choice than the AAT because it ranks better in most outcomes, but more studies should validate it.
For AAT, the local statistical heterogeneity for meta-analysis of agitation, cognitive function, depression, and QoL was 36.6, 41.3, 54.5, and 33.4%, respectively. However, for PRT, the local statistical heterogeneity for meta-analysis of agitation, cognitive function, depression, and QoL was 66.1, 0.0, 66.9, and 54.9%, respectively. The global statistical heterogeneity was 61.5, 63.8, 72.5, and 41.4% for agitation, cognitive function, depression, and QoL, respectively.
As shown in Supplementary Figure 5, the assumption of publication bias was not detected for all outcomes because the outlines of all funnel plots were symmetric. However, Begg's and Egger's tests revealed publication bias for agitation (P = 0.013 for Beeg's test and P < 0.000 for Egger's test) and cognitive function (P = 1.000 for Beeg's test and P = 0.008 for Egger's test) although revealed no publication bias for depression (P = 0.297 for Beeg's test and P = 0.225 for Egger's test), and QoL (P = 0.233 for Beeg's test and P = 0.270 for Egger's test).
Dementia has emerged as a major global public health problem and will continue to be a public health burden in the future (Frankish and Horton, 2017). Therefore, it is crucial to improve the management and treatment of BPSD. AAT and PRT have been widely used to manage dementia; however, the difference in efficacy between the two therapies in managing BPSD remains controversial. In this network meta-analysis, 19 eligible RCTs were included to estimate the relative efficacy of AAT vs. PRT. The results revealed that compared with control, PRTsignificantly alleviated agitation in dementia, although AAT and PRT did not improve cognitive function, reduce depression, or improve QoL. The SUCRA probabilities indicated that PRT ranked better than AAT in terms of agitation, cognitive function, and QoL, although the two therapies did not differ significantly in these outcomes.
To date, several meta-analyses (Leng et al., 2019, 2020; Zafra-Tanaka et al., 2019; Park et al., 2020; Lu et al., 2021; Ong et al., 2021; Batubara et al., 2022; Chen et al., 2022) have evaluated the efficacy of AAT and PRT on BPSD in dementia. Of these meta-analyses, three (Zafra-Tanaka et al., 2019; Batubara et al., 2022; Chen et al., 2022) focused on the role of AAT, three (Leng et al., 2019; Lu et al., 2021; Ong et al., 2021) focused on the role of PRT, and one (Park et al., 2020) simultaneously focused on roles of AAT and PRT. Three meta-analyses (Zafra-Tanaka et al., 2019; Batubara et al., 2022; Chen et al., 2022) focusing on AAT revealed that AAT had no effect on cognitive function, agitation and QoL but had a positive effect on depression in patients with dementia. It is important to note that these meta-analyses did not include all eligible studies, and some even simultaneously pooled the results of RCTs and non-RCTs. Therefore, the results of these meta-analyses should be interpreted with caution. In this study, the traditional meta-analysis revealed a positive effect of AAT on depression; however, network meta-analysis significantly changed the results of the traditional meta-analysis. So, it is speculated that insufficient statistical power might be the major contributor to the false positive result. Another three meta-analyses focusing on PRT (Leng et al., 2019; Lu et al., 2021; Ong et al., 2021) consistently revealed that PRT significantly alleviated agitation in dementia. Additionally, of these three meta-analyses, two (Lu et al., 2021; Ong et al., 2021) showed no positive effect of PRT on depression and QoL, but one (Leng et al., 2019) showed a positive effect of PRT on depression. Similarly, some eligible studies have been missed from these meta-analyses, thus significantly decreasing the robustness and reliability of their findings.
To determine the relative efficacy of different non-pharmacological interventions in managing agitation in dementia, Leng et al. did a Bayesian network meta-analysis in 2020 (Leng et al., 2020). Their network meta-analysis included four studies focusing on AAT and three studies focusing on PRT to calculate the relative efficacy of AAT versus PRT. The pooled result showed that AAT was not significantly different from PRT in reducing agitation (SMD: 0.41, 95%CI: −6.32 to 7.40), which was consistent with the results of this network meta-analysis. However, more outcomes were assessed in the present network meta-analysis than in previous network meta-analyses, including cognitive function, depression, and QoL, thus inevitably increasing the reference value of our network meta-analysis. In addition, it cannot be ignored that more eligible studies were included in this network meta-analysis, thus significantly increasing statistical power. Therefore, our findings should be prioritized for decision-making.
The current network meta-analysis yields some promising findings because it has unique strengths. First, direct and indirect evidence were pooled simultaneously to increase the statistical power significantly. Seconds, all eligible studies included in this network meta-analysis were RCTs, which benefited from decreasing the negative impact of confounding factors on the pooled results. Third, traditional and network meta-analyses were simultaneously conducted to estimate the efficacy of AAT and PRT, which benefited in determining whether the results from the traditional meta-analysis were false positive or negative, resulting from insufficient statistical power. Finally, we calculated SUCRA probabilities for AAT and PRT in all outcomes to detect subtle differences between these two therapies, which benefited in determining which might be the preferred strategy for a particular outcome.
This network meta-analysis has some limitations. First, Egger's and Begg's tests confirmed publication bias for meta-analyses of cognitive function and agitation, so it is impossible to deny that a small sample effect may negatively affect the reliability of the pooled results. Seconds, although 19 eligible studies were included in this network meta-analysis, it is unavoidable that insufficient sample size may compromise the robustness of our findings. Third, although the 19 eligible studies included in this network meta-analysis were RCTs, the overall methodological quality of these included RCTs was low according to the risk of bias assessment. Therefore, poor methodological quality will inevitably challenge the reliability of our findings. Fourth, details of AAT or PRT varied among the included studies. Although the efficacy of these two therapies was closely related to the frequency and duration of treatment, the limited number of included studies made it inappropriate to divide the two therapies into groups based on frequency and duration of treatment. Smaller groups meant limited data, which would seriously compromise the accuracy and reliability of results. Fifth, we estimated the relative efficacy of AAT and PRT in most outcomes based only on indirect evidence, which inevitably compromised the reliability of pooled results. Therefore, more studies directly comparing AAT and PRT are needed to determine how these two therapies differ in managing dementia. Finally, the cultural and architectural environment may also affect the effects of AAT and PRT on dementia. However, all eligible studies did not provide this information; therefore, it is necessary to evaluate the effects of the information on the effectiveness of AAT and PRT in managing dementia in future studies.
We conducted an exhaustive comparison of the efficacy of AAT and PRT on the common BPSD in dementia. Network meta-analysis shows that PRT alleviates agitation in patients diagnosed with dementia compared to control, although neither AAT nor PRT does not improve cognitive function, reduces depression, and improves QoL. The SUCRA probabilities indicate that PRT ranks better than AAT regarding agitation, cognitive function, and QoL, although the two therapies do not differ significantly in these outcomes. Therefore, in clinical practice, PRT may be a potential strategy for managing agitated behaviors in dementia to reduce the burden on healthcare systems and caregivers. In addition, more studies directly comparing AAT with PRT are needed to establish evidence of effectiveness, and further studies are also warranted to determine the difference between different robot types in the management of dementia.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
HD and LB carried out the studies, participated in collecting data, and drafted the manuscript. XL and HZ performed the statistical analysis and participated in its design. HD and XH participated in acquiring, analyzing, interpreting data, and drafting the manuscript. All authors read and approved the final manuscript.
This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (Grant number. 2020-I2M-C&T-B-008), National High-Level Hospital Clinical Research Funding (Grant number. 2022-PUMCH-B-031), and Nursing Research Funding of Peking Union Medical College Hospital (Grant number. XHHLKY202116).
We would like to deeply appreciate all authors who performed all eligible studies which have been included in the present network meta-analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1095996/full#supplementary-material
Batubara, S. O., Tonapa, S. I., Saragih, I. D., Mulyadi, M., and Lee, B. O. (2022). Effects of animal-assisted interventions for people with dementia: a systematic review and meta-analysis. Geriatr Nurs 43, 26–37. doi: 10.1016/j.gerinurse.2021.10.016
Biggerstaff, B. J., and Tweedie, R. L. (1997). Incorporating variability in estimates of heterogeneity in the random effects model in meta-analysis. Stat. Med. 16, 753–768.
Bono, A. V., Benvenuti, C., Buzzi, M., Ciatti, R., Chiarelli, V., Chiambretto, P., et al. (2015). Effects of animal assisted therapy (AAT) carried out with dogs on the evolution of mild cognitive impairment. Giornale di Gerontol. 63, 32–36.
Bowden, J., Tierney, J. F., Copas, A. J., and Burdett, S. (2011). Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med. Res. Methodol. 11, 41. doi: 10.1186/1471-2288-11-41
Briones, M. Á., Pardo-García, I., and Escribano-Sotos, F. (2021). Effectiveness of a dog-assisted therapy program to enhance quality of life in institutionalized dementia patients. Clin. Nurs. Res. 30, 89–97. doi: 10.1177/1054773819867250
Chaimani, A., Higgins, J. P., Mavridis, D., Spyridonos, P., and Salanti, G. (2013). Graphical tools for network meta-analysis in STATA. PLoS ONE 8, e76654. doi: 10.1371/journal.pone.0076654
Chen, H., Wang, Y., Zhang, M., Wang, N., Li, Y., Liu, Y., et al. (2022). Effects of animal-assisted therapy on patients with dementia: a systematic review and meta-analysis of randomized controlled trials. Psychiatry Res. 314. doi: 10.1016/j.psychres.2022.114619
Cipriani, A., Higgins, J. P., Geddes, J. R., and Salanti, G. (2013). Conceptual and technical challenges in network meta-analysis. Ann. Intern. Med. 159, 130–137. doi: 10.7326/0003-4819-159-2-201307160-00008
DerSimonian, R., and Laird, N. (1986). Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188. doi: 10.1016/0197-2456(86)90046-2
Dias, S., and Caldwell, D. M. (2019). Network meta-analysis explained. Arch. Dis. Child. Fetal Neonatal Ed. 104, F8–f12. doi: 10.1136/archdischild-2018-315224
Dyer, S. M., Harrison, S. L., Laver, K., Whitehead, C., and Crotty, M. (2018). An overview of systematic reviews of pharmacological and non-pharmacological interventions for the treatment of behavioral and psychological symptoms of dementia. Int. Psychogeriatr. 30, 295–309. doi: 10.1017/S1041610217002344
Dyer, S. M., Laver, K., Pond, C. D., Cumming, R. G., Whitehead, C., Crotty, M., et al. (2016). Clinical practice guidelines and principles of care for people with dementia in Australia. Aust. Fam. Physician 45, 884–889.
Frankish, H., and Horton, R. (2017). Prevention and management of dementia: a priority for public health. Lancet 390, 2614–2615. doi: 10.1016/S0140-6736(17)31756-7
Friedmann, E., Galik, E., Thomas, S. A., Hall, P. S., Chung, S. Y., McCune, S., et al. (2015). Evaluation of a pet-assisted living intervention for improving functional status in assisted living residents with mild to moderate cognitive impairment: a pilot study. Am. J. Alzheimer's Dis. Other Dementias 30, 276–289. doi: 10.1177/1533317514545477
Gale, S. A., Acar, D., and Daffner, K. R. (2018). Dementia. Am. J. Med. 131, 1161–1169. doi: 10.1016/j.amjmed.2018.01.022
Gallaway, P. J., Miyake, H., Buchowski, M. S., Shimada, M., Yoshitake, Y., Kim, A. S., et al. (2017). Physical activity: a viable way to reduce the risks of mild cognitive impairment, Alzheimer's disease, and vascular dementia in older adults. Brain Sci. 7, 22. doi: 10.3390/brainsci7020022
Herrmann, N. (2001). Recommendations for the management of behavioral and psychological symptoms of dementia. Can. J. Neurol. Sci. 28, S96–107. doi: 10.1017/S0317167100001268
Higgins, J. P., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi: 10.1136/bmj.d5928
Higgins, J. P., Jackson, D., Barrett, J. K., Lu, G., Ades, A. E., White, I. R., et al. (2012). Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res. Synth. Methods 3, 98–110. doi: 10.1002/jrsm.1044
Higgins, J. P., and Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558. doi: 10.1002/sim.1186
Hughes, M. J., Verreynne, M. L., Harpur, P., and Pachana, N. A. (2020). Companion animals and health in older populations: a systematic review. Clin. Gerontol. 43, 365–377. doi: 10.1080/07317115.2019.1650863
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162, 777–784. doi: 10.7326/M14-2385
Jøranson, N., Pedersen, I., Rokstad, A. M., and Ihlebæk, C. (2015). Effects on symptoms of agitation and depression in persons with dementia participating in robot-assisted activity: a cluster-randomized controlled trial. J. Am. Med. Directors Associat.16, 867–873. doi: 10.1016/j.jamda.2015.05.002
Jøranson, N., Pedersen, I., Rokstad, A. M., and Ihlebaek, C. (2016). Change in quality of life in older people with dementia participating in Paro-activity: a cluster-randomized controlled trial. J. Adv. Nursing 72, 3020–3033. doi: 10.1111/jan.13076
Kales, H. C., Gitlin, L. N., and Lyketsos, C. G. (2015). Assessment and management of behavioral and psychological symptoms of dementia. BMJ 350, h369. doi: 10.1136/bmj.h369
Kales, H. C., Lyketsos, C. G., Miller, E. M., and Ballard, C. (2019). Management of behavioral and psychological symptoms in people with Alzheimer's disease: an international Delphi consensus. Int. Psychogeriatr. 31, 83–90. doi: 10.1017/S1041610218000534
Kang, H. S., Makimoto, K., Konno, R., and Koh, I. S. (2020). Review of outcome measures in PARO robot intervention studies for dementia care. Geriatr Nurs 41, 207–214. doi: 10.1016/j.gerinurse.2019.09.003
Kishita, N., Backhouse, T., and Mioshi, E. (2020). Nonpharmacological interventions to improve depression, anxiety, and quality of life (QoL) in people with dementia: an overview of systematic reviews. J. Geriatr. Psychiatry Neurol. 33, 28–41. doi: 10.1177/0891988719856690
Leng, M., Liu, P., Zhang, P., Hu, M., Zhou, H., Li, G., et al. (2019). Pet robot intervention for people with dementia: a systematic review and meta-analysis of randomized controlled trials. Psychiatry Res. 271, 516–525. doi: 10.1016/j.psychres.2018.12.032
Leng, M., Zhao, Y., and Wang, Z. (2020). Comparative efficacy of non-pharmacological interventions on agitation in people with dementia: a systematic review and Bayesian network meta-analysis. Int. J. Nurs. Stud. 102, 103489. doi: 10.1016/j.ijnurstu.2019.103489
Liang, A., Piroth, I., Robinson, H., MacDonald, B., Fisher, M., Nater, U. M., et al. (2017). A pilot randomized trial of a companion robot for people with dementia living in the community. J. Am. Med. Direct. Associat. 18, 871–878. doi: 10.1016/j.jamda.2017.05.019
Lu, G., and Ades, A. E. (2004). Combination of direct and indirect evidence in mixed treatment comparisons. Stat. Med. 23, 3105–3124. doi: 10.1002/sim.1875
Lu, G., and Ades, A. E. (2006). Assessing evidence inconsistency in mixed treatment comparisons. J. Am. Stat. Assoc. 101, 447–459. doi: 10.1198/016214505000001302
Lu, L. C., Lan, S. H., Hsieh, Y. P., Lin, L. Y., Lan, S. J., Chen, J. C., et al. (2021). Effectiveness of companion robot care for dementia: a systematic review and meta-analysis. Innov Aging 5, igab013. doi: 10.1093/geroni/igab013
Ma, H., Huang, Y., Cong, Z., Wang, Y., Jiang, W., Gao, S., et al. (2014). The efficacy and safety of atypical antipsychotics for the treatment of dementia: a meta-analysis of randomized placebo-controlled trials. J. Alzheimers. Dis. 42, 915–937. doi: 10.3233/JAD-140579
Masopust, J., Protopopová, D., Vališ, M., Pavelek, Z., and Klímová, B. (2018). Treatment of behavioral and psychological symptoms of dementias with psychopharmaceuticals: a review. Neuropsychiatr. Dis. Treat. 14, 1211–1220. doi: 10.2147/NDT.S163842
Mbuagbaw, L., Rochwerg, B., Jaeschke, R., Heels-Andsell, D., Alhazzani, W., Thabane, L., et al. (2017). Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst. Rev. 6, 79. doi: 10.1186/s13643-017-0473-z
Moyle, W., Cooke, M., Beattie, E., Jones, C., Klein, B., Cook, G., et al. (2013). Exploring the effect of companion robots on emotional expression in older adults with dementia: a pilot randomized controlled trial. J. Gerontol. Nursing 39, 46–53. doi: 10.3928/00989134-20130313-03
Moyle, W., Jones, C. J., Murfield, J. E., Thalib, L., Beattie, E. R. A., Shum, D. K. H., et al. (2017). Use of a robotic seal as a therapeutic tool to improve dementia symptoms: a cluster-randomized controlled trial. J. Am. Med. Directors Associat. 18, 766–773. doi: 10.1016/j.jamda.2017.03.018
Moyle, W., Murfield, J., Jones, C., Beattie, E., Draper, B., Ownsworth, T., et al. (2019). Can lifelike baby dolls reduce symptoms of anxiety, agitation, or aggression for people with dementia in long-term care? Findings from a pilot randomised controlled trial. Aging Ment. Health 23, 1442–1450. doi: 10.1080/13607863.2018.1498447
Olsen, C., Pedersen, I., Bergland, A., Enders-Slegers, M. J., and Ihlebæk, C. (2016a). Effect of animal-assisted activity on balance and quality of life in home-dwelling persons with dementia. Geriatric Nursing 37, 284–291. doi: 10.1016/j.gerinurse.2016.04.002
Olsen, C., Pedersen, I., Bergland, A., Enders-Slegers, M. J., Patil, G., Ihlebaek, C., et al. (2016b). Effect of animal-assisted interventions on depression, agitation and quality of life in nursing home residents suffering from cognitive impairment or dementia: a cluster randomized controlled trial. Int. J. Geriatr. Psychiatry 31, 1312–1321. doi: 10.1002/gps.4436
Ong, Y. C., Tang, A., and Tam, W. (2021). Effectiveness of robot therapy in the management of behavioural and psychological symptoms for individuals with dementia: a systematic review and meta-analysis. J. Psychiatr. Res. 140, 381–394. doi: 10.1016/j.jpsychires.2021.05.077
Page, M. J., McKenzie, J. E., and Higgins, J. P. T. (2018). Tools for assessing risk of reporting biases in studies and syntheses of studies: a systematic review. BMJ Open 8, e019703. doi: 10.1136/bmjopen-2017-019703
Palma Perez, S., and Delgado Rodriguez, M. (2006). Practical considerations on detection of publication bias. Gac. Sanit. 20, 10–16. doi: 10.1157/13101085
Park, S., Bak, A., Kim, S., Nam, Y., Kim, H. S., Yoo, D. H., et al. (2020). Animal-assisted and pet-robot interventions for ameliorating behavioral and psychological symptoms of dementia: a systematic review and meta-analysis. Biomedicines 8, 150. doi: 10.3390/biomedicines8060150
Petersen, S., Houston, S., Qin, H., Tague, C., and Studley, J. (2017). The utilization of robotic pets in dementia care. J. Alzheimer's Dis. 55, 569–574. doi: 10.3233/JAD-160703
Pope, W., Hunt, C., and Ellison, K. (2016). Animal assisted therapy for elderly residents of a skilled nursing facility. J. Nursing Edu. Practice 6, 56–62. doi: 10.5430/jnep.v6n9p56
Porsteinsson, A. P., Drye, L. T., Pollock, B. G., Devanand, D. P., Frangakis, C., Ismail, Z., et al. (2014). Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA 311, 682–691. doi: 10.1001/jama.2014.93
Prince, M., Bryce, R., Albanese, E., Wimo, A., Ribeiro, W., Ferri, C. P., et al. (2013). The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer's Dement 9, 63–75.e62. doi: 10.1016/j.jalz.2012.11.007
Pu, L., Moyle, W., Jones, C., and Todorovic, M. (2020). The effect of using PARO for people living with dementia and chronic pain: a pilot randomized controlled trial. J. Am. Med. Directors Associat. 21, 1079–1085. doi: 10.1016/j.jamda.2020.01.014
Quintavalla, F., Cao, S., Spinelli, D., Caffarra, P., Rossi, F. M., Basini, G., et al. (2021). Effects of dog-assisted therapies on cognitive mnemonic capabilities in people affected by Alzheimer's disease. Animals 11, 1356. doi: 10.3390/ani11051366
Robinson, H., MacDonald, B. A., Kerse, N., and Broadbent, E. (2013). Suitability of healthcare robots for a dementia unit and suggested improvements. J. Am. Med. Dir. Assoc. 14, 34–40. doi: 10.1016/j.jamda.2012.09.006
Sado, M., Ninomiya, A., Shikimoto, R., Ikeda, B., Baba, T., Yoshimura, K., et al. (2018). The estimated cost of dementia in Japan, the most aged society in the world. PLoS ONE 13, e0206508. doi: 10.1371/journal.pone.0206508
Salanti, G. (2012). Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res. Synth. Methods 3, 80–97. doi: 10.1002/jrsm.1037
Salanti, G., Ades, A. E., and Ioannidis, J. P. (2011). Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J. Clin. Epidemiol. 64, 163–171. doi: 10.1016/j.jclinepi.2010.03.016
Skaria, A. P. (2022). The economic and societal burden of Alzheimer disease: managed care considerations. Am. J. Manag. Care 28, S188–s196. doi: 10.37765/ajmc.2022.89236
Sterne, J. A., Egger, M., and Smith, G. D. (2001). Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ 323, 101–105. doi: 10.1136/bmj.323.7304.101
Tan, C. C., Yu, J. T., Wang, H. F., Tan, M. S., Meng, X. F., Wang, C., et al. (2014). Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer's disease: a systematic review and meta-analysis. J. Alzheimers. Dis. 41, 615–631. doi: 10.3233/JAD-132690
Tible, O. P., Riese, F., Savaskan, E., and von Gunten, A. (2017). Best practice in the management of behavioural and psychological symptoms of dementia. Ther. Adv. Neurol. Disord. 10, 297–309. doi: 10.1177/1756285617712979
Travers, C., Perkins, J., Rand, J., Bartlett, H., and Morton, J. (2013). An evaluation of dog-assisted therapy for residents of aged care facilities with dementia. Anthrozoös 26, 213–225. doi: 10.2752/175303713X13636846944169
Tu, Y. K. (2015). Using generalized linear mixed models to evaluate inconsistency within a network meta-analysis. Value Health. 18, 1120–1125. doi: 10.1016/j.jval.2015.10.002
Valenti Soler, M., Aguera-Ortiz, L., Olazaran Rodriguez, J., Mendoza Rebolledo, C., Perez Munoz, A., Rodriguez Perez, I., et al. (2015). Social robots in advanced dementia. Front. Aging Neurosci. 7, 133. doi: 10.3389/fnagi.2015.00133
Vegue Parra, E., Hernández Garre, J. M., and Echevarría Pérez, P. (2021). Benefits of dog-assisted therapy in patients with dementia residing in aged care centers in spain. Int. J. Environ. Res. Public Health 18, 1471. doi: 10.3390/ijerph18041471
Wan, X., Wang, W., Liu, J., and Tong, T. (2014). Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14, 135. doi: 10.1186/1471-2288-14-135
White, I. (2017). Network: stata module to perform network meta-analysis. Stat. Software Components. Available online at: https://econpapers.repec.org/software/bocbocode/s458319.htm
Wimo, A., Guerchet, M., Ali, G. C., Wu, Y. T., Prina, A. M., Winblad, B., et al. (2017). The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers. Dement. 13, 1–7. doi: 10.1016/j.jalz.2016.07.150
Yu, C., Sommerlad, A., Sakure, L., and Livingston, G. (2022). Socially assistive robots for people with dementia: systematic review and meta-analysis of feasibility, acceptability and the effect on cognition, neuropsychiatric symptoms and quality of life. Ageing Res. Rev. 78, 101633. doi: 10.1016/j.arr.2022.101633
Yu-Kang, T. (2016). Node-splitting generalized linear mixed models for evaluation of inconsistency in network meta-analysis. Value Health 19, 957–963. doi: 10.1016/j.jval.2016.07.005
Keywords: dementia, animal-assisted therapy, pet-robot therapy, agitation, network meta-analysis
Citation: Du H, Bo L, Lai X, Zhu H and Huo X (2023) Network meta-analysis of comparative efficacy of animal-assisted therapy vs. pet-robot therapy in the management of dementia. Front. Aging Neurosci. 15:1095996. doi: 10.3389/fnagi.2023.1095996
Received: 11 November 2022; Accepted: 21 April 2023;
Published: 31 May 2023.
Edited by:
Stephen Macfarlane, Dementia Centre, HammondCare, AustraliaReviewed by:
Chariklia Tziraki-Segal, Hellenic Mediterranean University, GreeceCopyright © 2023 Du, Bo, Lai, Zhu and Huo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongdi Du, dm9zdW5ueUAxMjYuY29t; Xiaopeng Huo, dm9odW94cDAwMUAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.