
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 30 September 2022
Sec. Neuroinflammation and Neuropathy
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.999568
 Lu Wang1†
Lu Wang1† Hongyun Li1†
Hongyun Li1† Jiheng Hao2†
Jiheng Hao2† Chao Liu2
Chao Liu2 Jiyue Wang2
Jiyue Wang2 Jingjun Feng2
Jingjun Feng2 Zheng Guo3
Zheng Guo3 Yulu Zheng3
Yulu Zheng3 Yanbo Zhang4*
Yanbo Zhang4* Hongxiang Li4*
Hongxiang Li4* Liyong Zhang2*
Liyong Zhang2* Haifeng Hou1,3
Haifeng Hou1,3Background: Stroke patients have to face a high risk of recurrence, especially for those with comorbid T2DM, which usually lead to much more serious neurologic damage and an increased likelihood of death. This study aimed to explore determinants of stroke relapse among patients with comorbid T2DM.
Materials and methods: We conducted this case-control study nested a prospective cohort of ischemic stroke (IS) with comorbid T2DM. During 36-month follow-up, the second stroke occurred in 84 diabetic IS patients who were allocated into the case group, while 613 patients without recurrence were the controls. We collected the demographic data, behaviors and habits, therapies, and family history at baseline, and measured the variables during follow-up. LASSO and Logistic regression analyses were carried out to develop a prediction model of stroke recurrence. The receiver operator characteristic (ROC) curve was employed to evaluate the performance of the prediction model.
Results: Compared to participants without recurrence, the higher levels of pulse rate (78.29 ± 12.79 vs. 74.88 ± 10.93) and hypertension (72.6 vs. 61.2%) were recorded at baseline. Moreover, a lower level of physical activity (77.4 vs. 90.4%), as well as a higher proportion of hypoglycemic therapy (36.9 vs. 23.3%) was also observed during 36-month follow-up. Multivariate logistic regression revealed that higher pulse rate at admission (OR = 1.027, 95 %CI = 1.005–1.049), lacking physical activity (OR = 2.838, 95% CI = 1.418–5.620) and not receiving hypoglycemic therapy (OR = 1.697, 95% CI = 1.013–2.843) during follow-up increased the risk of stroke recurrence. We developed a prediction model using baseline pulse rate, hypoglycemic therapy, and physical activity, which produced an area under ROC curve (AUC) of 0.689.
Conclusion: Physical activity and hypoglycemic therapy play a protective role for IS patients with comorbid diabetes. In addition to targeted therapeutics, the improvement of daily-life habit contributes to slowing the progress of the IS.
Globally, stroke has ranked as the second leading cause of death (Wu Y. et al., 2019; Zheng et al., 2022) and the third leading cause of disability (Cao et al., 2019; Collaborators, 2021). Ischemic stroke (IS), characterized by temporary or permanent cerebrovascular occlusion (Liu et al., 2018), accounts for 82% of acute stroke events (Mehra et al., 2019; Feske, 2021). The incidence and mortality of stroke are significantly higher in China than in developed countries: a study on the Global Burden of Disease in 2019 reported stroke as the leading cause of death in this country (Wu S. et al., 2019).
Stroke patients face a high likelihood of recurrence after the initial onset of IS, ranging from 5.4% at 1 year to 11.3% at 5 years (Khanevski et al., 2019). Patients with stroke recurrence have a higher likelihood to endure more serious brain damage and neurologic dysfunction, leading to a worse functional status and/or higher case fatality rate vs. initial stroke (Lin B. et al., 2021; Wang et al., 2021). Physical activity, antihypertensive treatment, and high fiber intake (i.e., fruits and vegetables) have preventive effects on IS recurrence (Gillman et al., 1995; Zonneveld et al., 2018; Lin Y. et al., 2021). Other modifiable lifestyle characteristics, including obesity, hypertension, diabetes, depression and smoking, usually serve as risk factors (Black et al., 2015; Chen et al., 2019; Huang et al., 2019; Zheng and Yao, 2019; Cao et al., 2020; Szlachetka et al., 2020; Kumral et al., 2021). Despite recent progress in the management and prevention of recurrent stroke, enhanced approaches are needed to further reduce relapse risk.
Diabetes mellitus (DM), a complex disease featuring a deficiency or resistance to insulin, exposes individuals to hyperglycemia (Tang et al., 2014). This state causes pathologic changes in the blood vessels of various organs, including cerebral vessels (Li et al., 2020). The incidence of cardiovascular diseases, such as IS, is apparently higher in patients with DM than in persons without. As one of established risk factors for stroke, the prevalence of DM-induced IS has been increasing in all age groups (Benjamin et al., 2019; Venkat et al., 2021). Acute stroke with comorbid DM has significantly higher risks of aggravated pathology, disability, and death. These consequences mostly arise from extensive unbalance of metabolism, severe vascular damage, deteriorated white matter, and specific inflammatory milieu (Ergul et al., 2016; Venkat et al., 2017, 2021). As understood, diabetic stroke patients face a higher probability of stroke recurrence. Controlling glucose levels and other risk factors has been considered an effective means of preventing subsequent strokes (Lau et al., 2019). However, few studies have focused on precise prevention strategies for stroke recurrence in Chinese patients with comorbid DM. Identifying determinants of stroke recurrence in DM patients will help to alleviate stroke-related deterioration. Findings will also advance personalized healthcare strategies.
This nested case–control study was conducted with a Chinese Han stroke cohort established at the Brain Hospital of Liaocheng People’s Hospital (BHLPH) in Shandong Province, China. This cohort enrolled 1,793 patients with acute IS who were admitted to BHLPH between January 1, 2017, and September 30, 2018. Among these patients, 717 had T2DM. After 36 months of follow-up, patients with recurrent stroke were recruited in the case group; those without recurrence constituted the control group.
Study inclusion criteria were as follows: (1) First-ever stroke; (2) with comorbid T2DM; (3) a diagnosis of acute IS, confirmed via magnetic resonance imaging with reference to the Chinese Guidelines for the Diagnosis and Treatment of Acute Ischemic Stroke (Neurology and Society, 2018); (4) age ≥ 18 years; and (5) complete clinical data were available. The exclusion criteria covered (1) patients with severe mental disorders; (2) pregnant or breastfeeding women; (3) patients with incomplete clinical characteristics; (4) patients with severe somatic diseases (e.g., heart diseases, cancers, and infectious diseases); and (5) patients enrolled in other clinical trials.
The study protocol was reviewed and approved by the ethics committee of BHLPH (No. BHLPH085). All participants provided their written informed consent.
Participants’ demographic data were obtained through face-to-face interviews. Clinical features were collected by trained physicians. According to body mass index (BMI), the participants were classified were classified as (1) underweight (BMI < 18.5), (2) normal weight (BMI of 18.5–23.9), (3) overweight (BMI of 24.0–27.9), or (4) obese (BMI ≥ 28). The National Institutes of Health Stroke Scale (NIHSS), a 15-item neurologic-impairment scale with scores ranging from 0 (no deficit) to 42 (quadriplegia and coma) was used to assess acute stroke deficits. NIHSS frequencies were classified into 5 groups: no measured stroke symptoms (0); minor stroke (1–4); moderate stroke (5–15); moderate to severe stroke (16–20); and severe stroke (21–42) (Saber and Saver, 2020). The Modified Rankin Scale (mRS) is a disability scale ranging from 0 (no symptoms) to 6 (death), by which patients were classified into two groups: good outcome (0–2), and worse outcome (3–6) (Armangue et al., 2018). A fasting venous blood sample was collected for determination of hematologic and biochemical parameters, including high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), total cholesterol (TC), fasting blood glucose (FBG), and total homocysteine (tHcy), were quantified with an automatic analyzer (Hitachi, Tokyo, Japan). Pulse rate was automatically recorded by a finger pulse oximetric device (Yuwell, Jiangsu, China). Blood pressure (BP) was measured by a trained nurse in a sitting position with at least 5 min rest before the first measurement, and the mean of two measurements was recorded.
The 36-month follow-up was performed by a trained clinical neurologist after patients were discharged from the hospital. Parameters including stroke recurrence, age, gender, smoking, alcohol use, systolic blood pressure (SBP), diastolic blood pressure (DBP), FBG, physical activity (often: ≥ 3 sessions per week and ≥ 30 min per session, or moderate-intensity exercise), family history, and medications were obtained when visited annually. The mean of each variable was calculated during the follow-up period.
Patients who take the medicines as prescribed are regarded as receiving the relevant treatment: (1) Antiplatelet therapy (e.g., aspirin, clopidogrel, or ticagrelor), (2) antihypertensive therapy [e.g., angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blocker (ARB)], (3) hypoglycemic therapy (e.g., insulin, pioglitazone), (4) antithrombotic therapy (e.g., aspirin, ozagrel), and (5) lipid-lowering drug therapy (e.g., statins, niacin and its derivatives).
For the purposes of reporting results, continuous variables are expressed herein as means and standard deviations; categorical variables are presented as frequencies and/or percentages. The Kolmogorov–Smirnov test was used to examine the normal distribution of continuous data. If normality was assumed, a Student’s t-test was performed to analyze the difference between groups; otherwise, the data were analyzed using a Mann–Whitney U-test. Meanwhile, between-group differences in categorical data were tested via a chi-square test. To alleviate collinearity between multiple variables, a least absolute shrinkage and selection operator (LASSO) regression was carried out to select variables potentially associated with IS recurrence in patients with comorbid T2DM. A multivariate logistic regression was performed to screen statistically significant variables: The odds ratio (OR) and 95% confidence interval (CI) were calculated for each variable. Then, a multivariate prediction model was developed using these significant variables. To evaluate the model’s performance in predicting stroke recurrence, a receiver operator characteristic (ROC) curve was constructed, and the area under the ROC curve (AUC) was determined. In addition, a nomogram was designed on the basis of the multivariate logistic regression model; the concordance index (C-index) was used to assess the nomogram’s prediction accuracy.
Statistical analyses were performed in SPSS 26.0 (IBM, NY) and R packages 4.1.0 (R Core Team). A two-tailed P-value of less than 0.05 was considered statistically significant.
Twenty individuals were lost to follow-up after 36 months (2.8%, 20/717). Stroke recurred in 84 (12.05%) of participants in the case group, while 613 patients without recurrence were allocated into the control group. Study participants were aged 65.2 ± 11.28 years, of whom 61.4% (428/697) were men. Figure 1 presents a flowchart describing study population selection.
Participants’ baseline data are summarized in Table 1 and Supplementary Table 1. Differences in age, gender, BMI, smoking, alcohol consumption, FBG, triglycerides, TC, LDL-C, HDL-C, tHcy, neck vascular stenosis, Family history of stroke, coronary heart disease and hypertension, mRS score on admission, and NIHSS score on admission were not statistically significant between the case and control groups. Patients with stroke recurrence had a higher prevalence of hypertension at admission than the control group (P < 0.05). In addition, the pulse rate of recurrent cases was significantly higher than the controls (P < 0.05). The results of biochemical tests are shown in Figure 2.
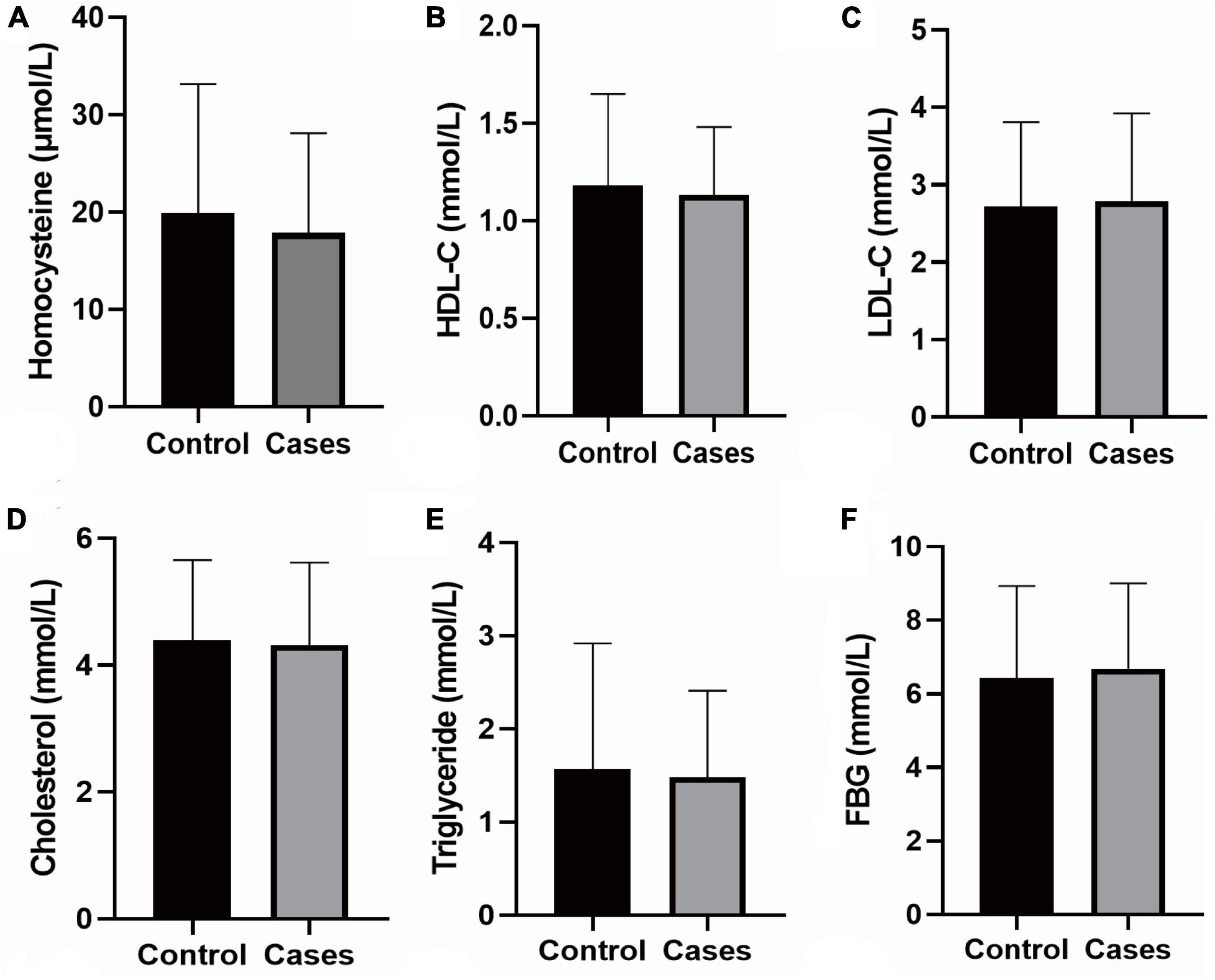
Figure 2. Biochemical tests. (A) The level of Homocysteine; (B) the level of HDL-C; (C) the level of LDL-Cs; (D) the level of Cholesterol; (E) the level of Triglyceride; (F) the level of FBG; the data shown in the graphs represent the mean ± SD. FBG, fasting blood-glucose; LDL-C, low density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; SD, standard deviations.
As shown in Table 2 and Supplementary Table 1, no significant between-group differences were detected for variables including smoking; FBG; mRS score, rehabilitation treatment; and antihypertension, antiplatelet, antithrombosis, and lipid-lowering therapies. The proportions of physical activity and hypoglycemic therapy were statistically different between groups (P < 0.05).
A LASSO model was used to screen potential determinants associated with stroke recurrence in T2DM patients. In Figure 3, red dots denote the target parameter to which each λ corresponds; the two dotted lines refer to two special λ values. Each curve matched the track of a single covariate coefficient. Finally, 38 covariates were selected for this model, with an optimal λ of 0.000584.
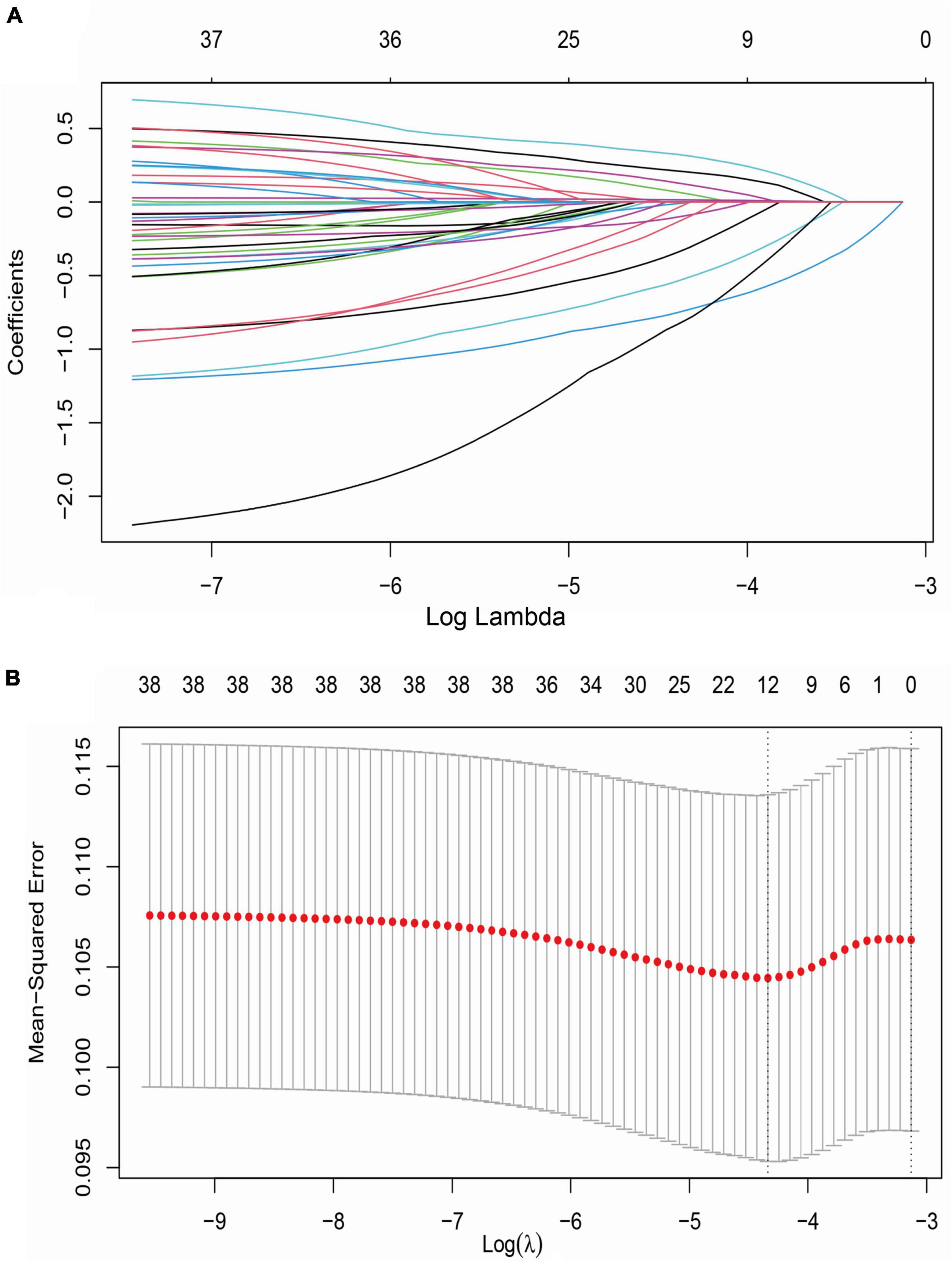
Figure 3. LASSO regression analysis for variable selection. (A) LASSO coefficient of 38 variables; (B) optimal penalty coefficient (λ = 0.000584) in LASSO regression identified with the minimum criterion; LASSO, least absolute shrinkage and selection operator; SE, standard error.
A logistic regression model, along with the LASSO regression, was established to screen the determinants of stroke recurrence. After adjusting for age, sex, BMI, tHcy, LDL-C, HDL-C, cholesterol, smoking and family history of stroke, a higher pulse rate at admission (OR = 1.027, 95% CI = 1.005–1.049) was significantly associated with increased risk for recurrence of stroke. Not receiving Hypoglycemic therapy (OR = 1.697, 95% CI = 1.013–2.843) and lacking physical activity during follow-up (OR = 2.838, 95% CI = 1.418–5.620) correlated with a higher risk for recurrence (Figure 4 and Supplementary Table 2).
The ROC curve analysis (Figure 5) showed a high performance of differentiation between high and low risk for stroke recurrence (AUC = 0.689, 95% CI = 0.628–0.750). According to the ROC curve, our prediction model exerted a relatively high accuracy, with a sensitivity of 61.9% and a specificity of 70.8% at a cut-off point of 0.130.
A nomogram was constructed with the above three predictors to forecast the 36-month risk of stroke recurrence (Figure 6). Internal validation was based on the random 70:30 partitioning of study participants data into training data: Test data sets. The nomogram’s prediction accuracy was evaluated by the C-index: 0.624 (95% CI = 0.553–0.695) for the training set and 0.653 (95% CI = 0.513–0.793) for the test set. As such, the nomogram displayed relatively good performance.
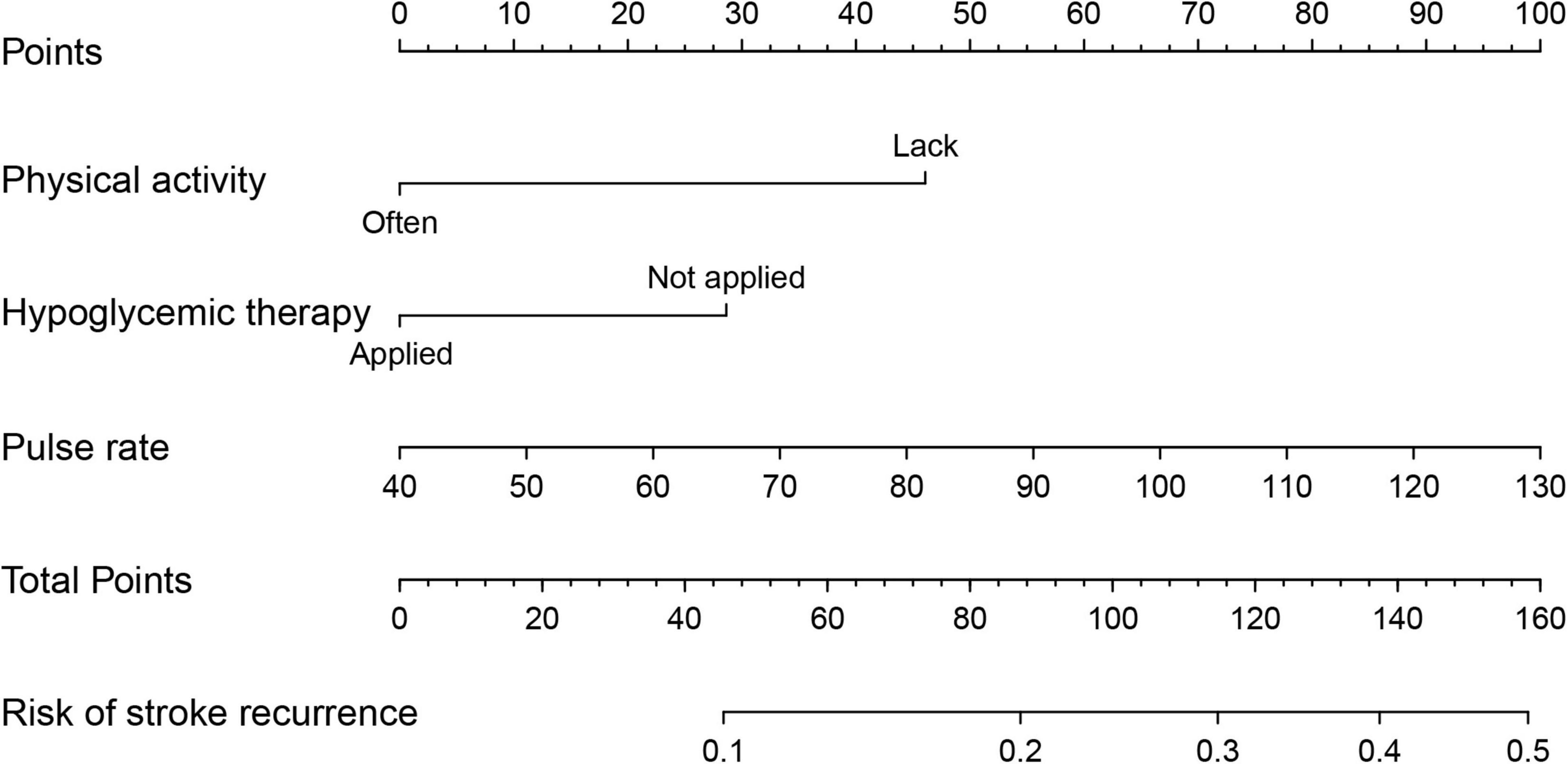
Figure 6. Nomogram to predict 36-month risk of stroke recurrence. Draw a line perpendicular from the corresponding axis of each factor until it reaches the top line labeled “Points”. Sum up the number of points for all factors, then draw a line descending from the axis labeled “Total Points” until it intercepts each of the axes to predict 36-month risk of stroke recurrence.
The recurrence rate of stroke was 12.05% over 36 months of follow-up in this diabetic IS cohort. Higher pulse rate at admission, physical inactivity and not receiving hypoglycemic therapy during follow-up increased the risk of stroke recurrence among patients with comorbid T2DM. Our prediction model based on the three risk factors appeared capable of identifying recurrence risk.
Accurately identifying modifiable risk factors for stroke recurrence is crucial for developing strategies to lower stroke-related mortality and morbidity. Compared with first-ever stroke, patients with recurrence have markedly more severe functional disability and higher mortality (Andreasen et al., 2018). One randomized controlled trial found that DM is associated with a higher risk of new stroke in patients with minor stroke (Chen et al., 2017). Although prevention measures targeting stroke have lessened relapse rates, patients with recurrence account for nearly 30% of all stroke cases (He et al., 2020). Several determinants correlated with stroke recurrence have been acknowledged (Kolmos et al., 2021). However, few studies have been conducted on IS with comorbid T2DM in China. Within the Chinese Han population profiled in this research, we observed 12.05% stroke recurrence during 36 months of follow-up, consistent with previous studies (Andersen et al., 2015; He et al., 2015; Khanevski et al., 2019).
IS patients with comorbid T2DM have poorer clinical outcomes and long-term prognoses than those with normal blood glucose levels (Kang and Park, 2017). Hyperglycemia increases stroke patients’ risk of death by 87% at 30 days, 75% at 1 year, and 41% at 6 years after stroke, respectively (Johnston and Beckman, 2019). In addition to being an independent predictor of stroke-induced death (Geary et al., 2019), hyperglycemia is a determinant of stroke recurrence (Hotter et al., 2019). Hyperglycemia augments brain injury through multiple potential mechanisms, including endothelial dysfunction, oxidative stress, increment of inflammatory response, and impaired fibrinolysis (Garg et al., 2006; MacDougall and Muir, 2011; Nogueira-Machado et al., 2011; Krinock and Singhal, 2021). Compared with patients who have normal blood glucose levels, hyperglycemia leads to a 2.5-fold increase in the risk of 90-day stroke recurrence (Guo et al., 2021). Our between-group comparisons revealed the proportion of hypoglycemic therapy to be lower in patients with recurrent stroke. Multivariate logistic regression contrarily demonstrated that not receiving hypoglycemic therapy was associated with a higher risk of recurrence after adjusting for age, sex, BMI, tHcy, LDL-C, HDL-C, cholesterol, family history, smoking, and pulse rate. This finding corroborates clinical trials and a systematic review (Lee et al., 2017; Spence et al., 2019). Hypoglycemic agents such as pioglitazone reduce the risk of stroke recurrence by activating PPARγ signaling in adipocytes, immune and endothelial cells, and other tissues (Kernan et al., 2016; Yaghi et al., 2018). This process contributes to a lower likelihood of stroke recurrence among patients with insulin resistance, prediabetes, or DM (Yang et al., 2018).
Physical activity can lower venous pressure, elevate venous flow, and reduce thrombosis (Johansson et al., 2019). Physical activity also helps reduce the risk of atherosclerosis by boosting lipid metabolism and raising blood levels of HDL-C (Sun et al., 2021). These effects can prevent relapse in atherosclerotic stroke patients (Waters et al., 2016; Sun et al., 2018; Gardener et al., 2019). Clinical studies have documented that physical activity and exercise enhance neuroplasticity and change brain activity patterns in post-stroke survivors, reducing the risk of recurrence (Kramer et al., 2019). A dose–response relationship has been identified between physical activity duration and stroke recurrence as well (Hou et al., 2021). Regular exercise results in genome-wide epigenetic modifications in human skeletal muscle and adipose tissue, which could affect metabolic phenotypes associated with stroke (Ling and Rönn, 2014). Our study revealed a significant relationship between physical inactivity and stroke relapse, similar to relevant studies (Oza et al., 2017; Turan et al., 2017).
In terms of pulse waves, scholars have taken pulse wave velocity and heart rate as major parameters when assessing arteriosclerosis development (Munakata, 2014; Townsend et al., 2015; Ueki et al., 2017). Increased pulse pressure may expose cerebral small vessels to high pulsatile pressure and flow, which damage the cerebral microvasculature and hinder the restoration of stroke-impaired neurological function (O’Rourke and Safar, 2005; Ishizuka et al., 2016). Pulse rate has been proposed as a surrogate for heart rate (Chang and Chang, 2009). Although the direct connection between stroke and pulse rate has yet to be clearly explained, epidemiological evidence implies that an elevated resting heart rate is associated with cardiovascular morbidity and mortality (Hjalmarson et al., 1990). Experimental and clinical findings suggest that sustained heart rate elevation, independent of the underlying trigger, is associated with the pathogenesis of cardiovascular diseases; pharmacological or interventional heart rate reduction can help to mitigate cardiovascular outcomes (Custodis et al., 2010). Animal studies have illustrated that a high heart rate contributes to upregulation of vascular oxidative stress, endothelial dysfunction, and accelerated atherogenesis (Custodis et al., 2008). Clinical studies point to an association between increased resting heart rate and inflammatory markers (i.e., C-reactive protein [CRP], white blood cell count, and fibrinogen) (Sajadieh et al., 2004; Rogowski et al., 2007). In this study, we illustrated that pulse rate is associated with increased risk of stroke recurrence.
Several limitations of our research require attention. First, this study was conducted with a single cohort from one medical center in northern China. This factor might cause Berkson’s bias, limiting the generalizability of findings. Second, because this study entailed a long-term follow-up among stroke patients, loss to follow-up might underestimate recurrence. Third, potentially significant variables may have been overlooked at baseline due to this study’s limited aims. Information bias could arise as a result.
Our findings indicate that higher pulse rate at admission, physical inactivity and not receiving hypoglycemic therapy during follow-up expose the individuals with comorbid T2DM to higher risk of stroke recurrence. This result underlines the importance of healthy lifestyle behaviors and targeted therapeutics in alleviating the adverse outcomes of stroke.
The data analyzed in this study is subject to the following licenses/restrictions: All data and methods supporting the findings of this study are available from the corresponding author upon reasonable request. Requests to access these datasets should be directed to YBZ, YmJubmJuQDE2My5jb20=.
The studies involving human participants were reviewed and approved by the Ethics Committee of BHLPH (No. BHLPH085). The participants provided their written informed consent to participate in this study.
HH, HXL, LZ, and YBZ designed the study and took responsibility for the integrity of the data and the accuracy of the data analysis. LW, HYL, JH, CL, JW, JF, ZG, and YLZ contributed to data collection. LW, HYL, and JH contributed to statistical analysis and manuscript writing. HXL, LZ, and YBZ revised the manuscript. All authors made a significant intellectual contribution and approved the final version.
This work was supported by grants from the National Natural Science Foundation of China (81773527) and European Commission Horizon 2020 Framework Programme (PRODEMOS-779238).
We thank Jun Wen at Edith Cowan University for English language editing and proofreading to help improve the quality of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.999568/full#supplementary-material
Andersen, S. D., Gorst-Rasmussen, A., Lip, G. Y., Bach, F. W., and Larsen, T. B. (2015). Recurrent stroke: The value of the CHA2DS2VASc score and the essen stroke risk score in a nationwide stroke cohort. Stroke 46, 2491–2497. doi: 10.1161/strokeaha.115.009912
Andreasen, C., Jørgensen, M. E., Gislason, G. H., Martinsson, A., Sanders, R. D., Abdulla, J., et al. (2018). Association of timing of aortic valve replacement surgery after stroke with risk of recurrent stroke and mortality. JAMA Cardiol. 3, 506–513. doi: 10.1001/jamacardio.2018.0899
Armangue, T., Spatola, M., Vlagea, A., Mattozzi, S., Cárceles-Cordon, M., Martinez-Heras, E., et al. (2018). Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: A prospective observational study and retrospective analysis. Lancet Neurol. 17, 760–772. doi: 10.1016/s1474-4422(18)30244-8
Benjamin, E. J., Muntner, P., Alonso, A., Bittencourt, M. S., Callaway, C. W., Carson, A. P., et al. (2019). Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation 139:e56–e528. doi: 10.1161/cir.0000000000000659
Black, M., Wang, W., and Wang, W. (2015). Ischemic stroke: From next generation sequencing and GWAS to community genomics? OMICS 19, 451–460. doi: 10.1089/omi.2015.0083
Cao, W., Li, X., Zhang, X., Zhang, J., Sun, Q., Xu, X., et al. (2019). No causal effect of telomere length on ischemic stroke and its subtypes: A Mendelian randomization study. Cells 8:159. doi: 10.3390/cells8020159
Cao, W., Zheng, D., Zhang, J., Wang, A., Liu, D., Zhang, J., et al. (2020). Association between telomere length in peripheral blood leukocytes and risk of ischemic stroke in a Han Chinese population: A linear and non-linear Mendelian randomization analysis. J. Transl. Med. 18:385. doi: 10.1186/s12967-020-02551-1
Chang, K.-M., and Chang, K.-M. (2009). Pulse rate derivation and its correlation with heart rate. J. Med. Biol. Eng. 29, 132–137.
Chen, J., Li, S., Zheng, K., Wang, H., Xie, Y., Xu, P., et al. (2019). Impact of smoking status on stroke recurrence. J. Am. Heart Assoc. 8:e011696. doi: 10.1161/jaha.118.011696
Chen, W., Pan, Y., Jing, J., Zhao, X., Liu, L., Meng, X., et al. (2017). Recurrent stroke in minor ischemic stroke or transient ischemic attack with metabolic syndrome and/or diabetes mellitus. J. Am. Heart Assoc. 6:e005446. doi: 10.1161/jaha.116.005446
Collaborators, G. B. D. S. (2021). Global, regional, and national burden of stroke and its risk factors, 1990-2019: A systematic analysis for the global burden of disease study 2019. Lancet Neurol. 20, 795–820. doi: 10.1016/S1474-4422(21)00252-0
Custodis, F., Baumhäkel, M., Schlimmer, N., List, F., Gensch, C., Böhm, M., et al. (2008). Heart rate reduction by ivabradine reduces oxidative stress, improves endothelial function, and prevents atherosclerosis in apolipoprotein E-deficient mice. Circulation 117, 2377–2387. doi: 10.1161/circulationaha.107.746537
Custodis, F., Schirmer, S. H., Baumhäkel, M., Heusch, G., Böhm, M., and Laufs, U. (2010). Vascular pathophysiology in response to increased heart rate. J. Am. Coll. Cardiol. 56, 1973–1983. doi: 10.1016/j.jacc.2010.09.014
Ergul, A., Hafez, S., Fouda, A., and Fagan, S. C. (2016). Impact of comorbidities on acute injury and recovery in preclinical stroke research: Focus on hypertension and diabetes. Transl. Stroke Res. 7, 248–260. doi: 10.1007/s12975-016-0464-8
Gardener, H., Leifheit, E. C., Lichtman, J. H., Wang, Y., Wang, K., Gutierrez, C. M., et al. (2019). Racial/ethnic disparities in mortality among medicare beneficiaries in the FL–PR CR eSD study. J. Am. Heart Assoc. 8:e009649. doi: 10.1161/jaha.118.009649
Garg, R., Chaudhuri, A., Munschauer, F., and Dandona, P. (2006). Hyperglycemia, insulin, and acute ischemic stroke: A mechanistic justification for a trial of insulin infusion therapy. Stroke 37, 267–273. doi: 10.1161/01.Str.0000195175.29487.30
Geary, L., Hasselström, J., Carlsson, A. C., Eriksson, I., and von Euler, M. (2019). Secondary prevention after stroke/transient ischemic attack: A randomized audit and feedback trial. Acta Neurol. Scand. 140, 107–115. doi: 10.1111/ane.13109
Gillman, M. W., Cupples, L. A., Gagnon, D., Posner, B. M., and Wolf, P. A. (1995). Protective effect of fruits and vegetables on development of stroke in men. JAMA 273, 1113–1117.
Guo, Y., Wang, G., Jing, J., Wang, A., Zhang, X., Meng, X., et al. (2021). Stress hyperglycemia may have higher risk of stroke recurrence than previously diagnosed diabetes mellitus. Aging 13, 9108–9118. doi: 10.18632/aging.202797
He, V. Y., Condon, J. R., You, J., Zhao, Y., and Burrow, J. N. (2015). Adverse outcome after incident stroke hospitalization for Indigenous and non-Indigenous Australians in the Northern Territory. Int. J. Stroke 10, 89–95. doi: 10.1111/ijs.12600
He, X. F., Zeng, Y. X., Li, G., Feng, Y. K., Wu, C., Liang, F. Y., et al. (2020). Extracellular ASC exacerbated the recurrent ischemic stroke in an NLRP3-dependent manner. J. Cereb. Blood Flow Metab. 40, 1048–1060. doi: 10.1177/0271678x19856226
Hjalmarson, A., Gilpin, E. A., Kjekshus, J., Schieman, G., Nicod, P., Henning, H., et al. (1990). Influence of heart rate on mortality after acute myocardial infarction. Am. J. Cardiol. 65, 547–553. doi: 10.1016/0002-9149(90)91029-6
Hotter, B., Galinovic, I., Kunze, C., Brunecker, P., Jungehulsing, G. J., Villringer, A., et al. (2019). High-resolution diffusion-weighted imaging identifies ischemic lesions in a majority of transient ischemic attack patients. Ann. Neurol. 86, 452–457. doi: 10.1002/ana.25551
Hou, L., Li, M., Wang, J., Li, Y., Zheng, Q., Zhang, L., et al. (2021). Association between physical exercise and stroke recurrence among first-ever ischemic stroke survivors. Sci. Rep. 11:13372. doi: 10.1038/s41598-021-92736-5
Huang, Z. X., Lin, X. L., Lu, H. K., Liang, X. Y., Fan, L. J., and Liu, X. T. (2019). Lifestyles correlate with stroke recurrence in Chinese inpatients with first-ever acute ischemic stroke. J. Neurol. 266, 1194–1202. doi: 10.1007/s00415-019-09249-5
Ishizuka, K., Hoshino, T., Shimizu, S., Shirai, Y., Mizuno, S., Toi, S., et al. (2016). Brachial-ankle pulse wave velocity is associated with 3-month functional prognosis after ischemic stroke. Atherosclerosis 255, 1–5. doi: 10.1016/j.atherosclerosis.2016.08.027
Johansson, M., Johansson, L., Wennberg, P., and Lind, M. (2019). Physical activity and risk of first-time venous thromboembolism. Eur. J. Prev. Cardiol. 26, 1181–1187. doi: 10.1177/2047487319829310
Johnston, F. M., and Beckman, M. (2019). Updates on management of gastric cancer. Curr. Oncol. Rep. 21:67. doi: 10.1007/s11912-019-0820-4
Kang, D. H., and Park, J. (2017). Endovascular stroke therapy focused on stent retriever thrombectomy and direct clot aspiration: Historical review and modern application. J. Korean Neurosurg. Soc. 60, 335–347. doi: 10.3340/jkns.2016.0809.005
Kernan, W. N., Viscoli, C. M., Furie, K. L., Young, L. H., Inzucchi, S. E., Gorman, M., et al. (2016). Pioglitazone after ischemic stroke or transient ischemic attack. N. Engl. J. Med. 374, 1321–1331. doi: 10.1056/NEJMoa1506930
Khanevski, A. N., Bjerkreim, A. T., Novotny, V., Naess, H., Thomassen, L., Logallo, N., et al. (2019). Recurrent ischemic stroke: Incidence, predictors, and impact on mortality. Acta Neurol. Scand. 140, 3–8. doi: 10.1111/ane.13093
Kolmos, M., Christoffersen, L., and Kruuse, C. (2021). Recurrent Ischemic Stroke–a systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 30:105935. doi: 10.1016/j.jstrokecerebrovasdis.2021.105935
Kramer, S. F., Hung, S. H., and Brodtmann, A. (2019). The impact of physical activity before and after stroke on stroke risk and recovery: A narrative review. Curr. Neurol. Neurosci. Rep. 19:28. doi: 10.1007/s11910-019-0949-4
Krinock, M. J., and Singhal, N. S. (2021). Diabetes, stroke, and neuroresilience: Looking beyond hyperglycemia. Ann. N.Y.Acad. Sci. 1495, 78–98. doi: 10.1111/nyas.14583
Kumral, E., Erdoğan, C. E., Arı, A., Bayam, F. E., and Saruhan, G. (2021). Association of obesity with recurrent stroke and cardiovascular events. Rev. Neurol. 177, 414–421. doi: 10.1016/j.neurol.2020.06.019
Lau, L. H., Lew, J., Borschmann, K., Thijs, V., and Ekinci, E. I. (2019). Prevalence of diabetes and its effects on stroke outcomes: A meta-analysis and literature review. J. Diabetes Investig. 10, 780–792. doi: 10.1111/jdi.12932
Lee, M., Saver, J. L., Liao, H. W., Lin, C. H., and Ovbiagele, B. (2017). Pioglitazone for secondary stroke prevention: A systematic review and meta-analysis. Stroke 48, 388–393. doi: 10.1161/strokeaha.116.013977
Li, R. Z., Ding, X. W., Geetha, T., Al-Nakkash, L., Broderick, T. L., and Babu, J. R. (2020). Beneficial effect of genistein on diabetes-induced brain damage in the ob/ob mouse model. Drug Des. Dev. Ther. 14, 3325–3336. doi: 10.2147/dddt.S249608
Lin, B., Zhang, Z., Mei, Y., Wang, C., Xu, H., Liu, L., et al. (2021). Cumulative risk of stroke recurrence over the last 10 years: A systematic review and meta-analysis. Neurol. Sci. 42, 61–71. doi: 10.1007/s10072-020-04797-5
Lin, Y., Zhang, B., Hu, M., Xu, M., Qin, C., and Zhu, C. (2021). [Causal relationship between physical exercise and risk of ischemic stroke recurrence based on the potential outcome theory]. Nan Fang Yi Ke Da Xue Xue Bao 41, 1191–1197. doi: 10.12122/j.issn.1673-4254.2021.08.10
Ling, C., and Rönn, T. (2014). Epigenetic adaptation to regular exercise in humans. Drug Discov. Today 19, 1015–1018. doi: 10.1016/j.drudis.2014.03.006
Liu, D., Zhao, Z., Wang, A., Ge, S., Wang, H., Zhang, X., et al. (2018). Ischemic stroke is associated with the pro-inflammatory potential of N-glycosylated immunoglobulin G. J. Neuroinflammation 15, 123. doi: 10.1186/s12974-018-1161-1
MacDougall, N. J., and Muir, K. W. (2011). Hyperglycaemia and infarct size in animal models of middle cerebral artery occlusion: Systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 31, 807–818. doi: 10.1038/jcbfm.2010.210
Mehra, M. R., Vaduganathan, M., Fu, M., Ferreira, J. P., Anker, S. D., Cleland, J. G. F., et al. (2019). A comprehensive analysis of the effects of rivaroxaban on stroke or transient ischaemic attack in patients with heart failure, coronary artery disease, and sinus rhythm: The COMMANDER HF trial. Eur. Heart J. 40, 3593–3602. doi: 10.1093/eurheartj/ehz427
Munakata, M. (2014). Brachial-ankle pulse wave velocity in the measurement of arterial stiffness: Recent evidence and clinical applications. Curr. Hypertens. Rev. 10, 49–57. doi: 10.2174/157340211001141111160957
Neurology, C., and Society, C. S. (2018). Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chin. J. Neurol. 51, 666–682.
Nogueira-Machado, J. A., Volpe, C. M., Veloso, C. A., and Chaves, M. M. (2011). HMGB1, TLR and RAGE: A functional tripod that leads to diabetic inflammation. Expert Opin. Ther. Targets 15, 1023–1035. doi: 10.1517/14728222.2011.575360
O’Rourke, M. F., and Safar, M. E. (2005). Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension 46, 200–204. doi: 10.1161/01.Hyp.0000168052.00426.65
Oza, R., Rundell, K., and Garcellano, M. (2017). Recurrent ischemic stroke: Strategies for prevention. Am. Fam. Physician 96, 436–440.
Rogowski, O., Shapira, I., Shirom, A., Melamed, S., Toker, S., and Berliner, S. (2007). Heart rate and microinflammation in men: A relevant atherothrombotic link. Heart 93, 940–944. doi: 10.1136/hrt.2006.101949
Saber, H., and Saver, J. L. (2020). Distributional validity and prognostic power of the national institutes of health stroke scale in US administrative claims data. JAMA Neurol. 77, 606–612. doi: 10.1001/jamaneurol.2019.5061
Sajadieh, A., Nielsen, O. W., Rasmussen, V., Hein, H. O., Abedini, S., and Hansen, J. F. (2004). Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur. Heart J. 25, 363–370. doi: 10.1016/j.ehj.2003.12.003
Spence, J. D., Viscoli, C. M., Inzucchi, S. E., Dearborn-Tomazos, J., Ford, G. A., Gorman, M., et al. (2019). Pioglitazone therapy in patients with stroke and prediabetes: A post hoc analysis of the iris randomized clinical trial. JAMA Neurol. 76, 526–535. doi: 10.1001/jamaneurol.2019.0079
Sun, B., Wang, L., Li, X., Zhang, J., Zhang, J., Liu, X., et al. (2021). Intracranial atherosclerotic plaque characteristics and burden associated with recurrent acute stroke: A 3D quantitative vessel wall MRI study. Front. Aging Neurosci. 13:706544. doi: 10.3389/fnagi.2021.706544
Sun, P., Liu, L., Pan, Y., Wang, X., Mi, D., Pu, Y., et al. (2018). Intracranial atherosclerosis burden and stroke recurrence for symptomatic intracranial artery stenosis (sICAS). Aging Dis. 9, 1096–1102. doi: 10.14336/ad.2018.0301
Szlachetka, W. A., Pana, T. A., Tiamkao, S., Clark, A. B., Kongbunkiat, K., Sawanyawisuth, K., et al. (2020). Impact of diabetes on complications, long term mortality and recurrence in 608,890 hospitalised patients with stroke. Glob. Heart 15:2. doi: 10.5334/gh.364
Tang, W. H., Stitham, J., Jin, Y., Liu, R., Lee, S. H., Du, J., et al. (2014). Aldose reductase-mediated phosphorylation of p53 leads to mitochondrial dysfunction and damage in diabetic platelets. Circulation 129, 1598–1609. doi: 10.1161/circulationaha.113.005224
Townsend, R. R., Wilkinson, I. B., Schiffrin, E. L., Avolio, A. P., Chirinos, J. A., Cockcroft, J. R., et al. (2015). Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the american heart association. Hypertension 66, 698–722. doi: 10.1161/hyp.0000000000000033
Turan, T. N., Nizam, A., Lynn, M. J., Egan, B. M., Le, N. A., Lopes-Virella, M. F., et al. (2017). Relationship between risk factor control and vascular events in the SAMMPRIS trial. Neurology 88, 379–385. doi: 10.1212/wnl.0000000000003534
Ueki, Y., Miura, T., Minamisawa, M., Abe, N., Nishimura, H., Hashizume, N., et al. (2017). The usefulness of brachial-ankle pulse wave velocity in predicting long-term cardiovascular events in younger patients. Heart Vessels 32, 660–667. doi: 10.1007/s00380-016-0919-6
Venkat, P., Chopp, M., and Chen, J. (2017). Blood-brain barrier disruption, vascular impairment, and ischemia/reperfusion damage in diabetic stroke. J. Am. Heart Assoc. 6:e005819. doi: 10.1161/jaha.117.005819
Venkat, P., Ning, R., Zacharek, A., Culmone, L., Liang, L., Landschoot-Ward, J., et al. (2021). Treatment with an Angiopoietin-1 mimetic peptide promotes neurological recovery after stroke in diabetic rats. CNS Neurosci. Ther. 27, 48–59. doi: 10.1111/cns.13541
Wang, T. A., Wu, T. H., Pan, S. L., Chen, H. H., and Chiu, S. Y. (2021). Impacts of treatments on recurrence and 28-year survival of ischemic stroke patients. Sci. Rep. 11:15258. doi: 10.1038/s41598-021-94757-6
Waters, M. F., Hoh, B. L., Lynn, M. J., Kwon, H. M., Turan, T. N., Derdeyn, C. P., et al. (2016). Factors associated with recurrent ischemic stroke in the medical group of the SAMMPRIS Trial. JAMA Neurol. 73, 308–315. doi: 10.1001/jamaneurol.2015.4315
Wu, S., Wu, B., Liu, M., Chen, Z., Wang, W., Anderson, C. S., et al. (2019). Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 18, 394–405. doi: 10.1016/s1474-4422(18)30500-3
Wu, Y., Fan, Z., Chen, Y., Ni, J., Liu, J., Han, J., et al. (2019). Determinants of developing stroke among low-income, rural residents: A 27-year population-based, prospective cohort study in Northern China. Front. Neurol. 10:57. doi: 10.3389/fneur.2019.00057
Yaghi, S., Furie, K. L., Viscoli, C. M., Kamel, H., Gorman, M., Dearborn, J., et al. (2018). Pioglitazone prevents stroke in patients with a recent transient ischemic attack or ischemic stroke: A planned secondary analysis of the IRIS Trial (Insulin Resistance Intervention After Stroke). Circulation 137, 455–463. doi: 10.1161/circulationaha.117.030458
Yang, J. L., Mukda, S., and Chen, S. D. (2018). Diverse roles of mitochondria in ischemic stroke. Redox Biol. 16, 263–275. doi: 10.1016/j.redox.2018.03.002
Zheng, S., and Yao, B. (2019). Impact of risk factors for recurrence after the first ischemic stroke in adults: A systematic review and meta-analysis. J. Clin. Neurosci. 60, 24–30. doi: 10.1016/j.jocn.2018.10.026
Zheng, Y., Guo, Z., Zhang, Y., Shang, J., Yu, L., Fu, P., et al. (2022). Rapid triage for ischemic stroke: A machine learning-driven approach in the context of predictive, preventive and personalised medicine. EPMA J. 13, 285–298. doi: 10.1007/s13167-022-00283-4
Zonneveld, T. P., Edo, R., Mervyn, D. V., Nederkoorn, P. J., Rob, D. H., Bwem, R. Y., et al. (2018). Blood pressure-lowering treatment for preventing recurrent stroke, major vascular events, and dementia in patients with a history of stroke or transient ischaemic attack. Cochrane Database Syst. Rev. 7:CD007858.
Keywords: ischemic stroke, diabetes mellitus, recurrence, risk factors, nested case-control study
Citation: Wang L, Li H, Hao J, Liu C, Wang J, Feng J, Guo Z, Zheng Y, Zhang Y, Li H, Zhang L and Hou H (2022) Thirty-six months recurrence after acute ischemic stroke among patients with comorbid type 2 diabetes: A nested case-control study. Front. Aging Neurosci. 14:999568. doi: 10.3389/fnagi.2022.999568
Received: 21 July 2022; Accepted: 09 September 2022;
Published: 30 September 2022.
Edited by:
Rubem C. A. Guedes, Federal University of Pernambuco, BrazilReviewed by:
Rahim Alhamzawi, University of Al-Qadisiyah, IraqCopyright © 2022 Wang, Li, Hao, Liu, Wang, Feng, Guo, Zheng, Zhang, Li, Zhang and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanbo Zhang, YmJubmJuQDE2My5jb20=; Hongxiang Li, ZnlsaWhvbmd4aWFuZ0AxMjYuY29t; Liyong Zhang, MTMzNDYyNTY5MzZAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.