
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Aging Neurosci. , 08 September 2022
Sec. Cellular and Molecular Mechanisms of Brain-aging
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.986443
This article is part of the Research Topic Advances in Neural Reprogramming, Disease Modeling and Therapeutic Insights View all 13 articles
 Yiyi Li1,2
Yiyi Li1,2 Fang Li1,2
Fang Li1,2 Dongdong Qin1,2
Dongdong Qin1,2 Hongyu Chen1,2
Hongyu Chen1,2 Jianhao Wang1,2
Jianhao Wang1,2 Jiabei Wang1,2
Jiabei Wang1,2 Shafei Song1,2
Shafei Song1,2 Chao Wang1,2
Chao Wang1,2 Yamei Wang1,2
Yamei Wang1,2 Songyan Liu1,2
Songyan Liu1,2 Dandan Gao1,2
Dandan Gao1,2 Zhi-Hao Wang1,2*
Zhi-Hao Wang1,2*Brain derived neurotrophic factor (BDNF) has multiple biological functions which are mediated by the activation of two receptors, tropomyosin receptor kinase B (TrkB) receptor and the p75 neurotrophin receptor, involving in physiological and pathological processes throughout life. The diverse presence and activity of BDNF indicate its potential role in the pathogenesis, progression and treatment of both neurological and psychiatric disorders. This review is to provide a comprehensive assessment of the current knowledge and future directions in BDNF-associated research in the central nervous system (CNS), with an emphasis on the physiological and pathological functions of BDNF as well as its potential treatment effects in CNS diseases, including depression, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, multiple sclerosis, and cerebral ischemic stroke.
Brain derived neurotrophic factor, a member of the neurotrophin family, plays a critical role not only in the growth and development of the nervous system but also in supporting neurons survival and facilitating neurogenesis. In addition, it participates in glutamatergic and gamma-aminobutyric acid (GABA)-ergic synaptic plasticity and affects serotonergic and dopaminergic neurotransmission (Colucci-D’Amato et al., 2020). It is widely distributed in the cortical region, hippocampus, and visual cortex, as well as other parts of CNS such as substantia nigra, striatum, retrorubral region, and ventral tegmental area. The expression level of BDNF in brain tissues is determined by transcription of BDNF mRNA and translation of BDNF protein.
Brain derived neurotrophic factor signals are mediated by TrkB receptor and p75 neurotrophin receptor. The former is the key receptor for BDNF in adult brain since its wide range of expression and a higher binding affinity for BDNF than the latter. TrkB receptor has tyrosine residues in its kinase domain, and phosphorylated-TrkB triggers downstream signaling cascades such as phospholipase C (PLC), mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K), exerting neuronal protective effects. However, truncated TrkB without intracellular kinase domain possesses inhibitory effect against mature BDNF signaling which activates full length TrkB. Furthermore, pro-BDNF is preferential to bind with p75 neurotrophin receptor, which has a low affinity to mature BDNF and leads to negative effects such as neuronal cell death (Teng and Hempstead, 2004).
It was reported that BDNF is highly active in cortex, hippocampus, and basal forebrain (Mirowska-Guzel et al., 2013). The diverse presence of BDNF leads to its important involvements in multiple neurological and psychiatric disorders. In this review, we briefly summarize the current state of knowledge regarding the physiological and pathological functions of BDNF as well as its potential treatment effects in CNS diseases.
Brain derived neurotrophic factor is mainly produced by neurons and glial cells and serves as a pleiotropic role in CNS (Lessmann and Brigadski, 2009). It is essential for neuronal genesis, differentiation, survival and growth, and acts as a mediator, modulator, or instructor of synaptic plasticity, viability and transmission (Kowiański et al., 2018). There are two forms of BDNF in human brain, the BDNF precursor (proBDNF) and mature BDNF (mBDNF). The proBDNF stored in either dendrites or axons (Lessmann et al., 2003), is synthesized as pre-proBDNF in endoplasmic reticulum followed by removing signal peptide in golgi apparatus to form proBDNF eventually (Wang M. et al., 2021). The mBDNF is converted from proBDNF which undergoes cleavage and strips off the pro-domain (BDNF pro-peptide, pBDNF) intracellularly (by furin or PC1/3/7) or extracellularly (by tPA/plasmin system or matrix metallopeptidases) (Lee et al., 2001; Mowla et al., 2001; Wang M. et al., 2021).
Brain derived neurotrophic factor was released both by presynaptic and postsynaptic terminals (Matsuda et al., 2009) as a mixture of pro- and mature forms in an activity dependent manner (Pang et al., 2004). Two types of BDNF maintain a dynamic balance and the ratio between them varies at different stages of brain development. During early postnatal period, proBDNF reaches a high concentration, while mBDNF is more dominant in adulthood (Yang et al., 2009, 2014). mBDNF takes effect by binding with two kinds of plasma membrane receptors, the TrkB receptor (Martin-Zanca et al., 1986) and the p75 neurotrophin receptor (Dechant and Barde, 2002). mBDNF, as a member of the neurotrophin family, has a high affinity (dissociation constant ∼1011 M) to TrkB receptor (Rodriguez-Tébar and Barde, 1988), which promotes cell survival (Volosin et al., 2006), facilitates long term potentiation (LTP) and increases spine complexity (Zagrebelsky et al., 2005). However, mBDNF has a low affinity (dissociation constant ∼109 M) to p75 neurotrophin receptor (Meeker and Williams, 2015), which preferentially binds to proBDNF, and the latter signal involves in brain development, facilitates long term depression (LTD) and induces apoptosis (Woo et al., 2005; Friedman, 2010). Due to its critical role in LTP, BDNF has been assumed to be an essential part of supporting memory formation and maintenance by promoting synaptic integration (Bramham and Messaoudi, 2005). Furthermore, BDNF increases neurogenesis by promoting cell survival and proliferation (Katoh-Semba et al., 2002; Lee S. H. et al., 2007).
As is mentioned above, BDNF takes actions mainly by binding to TrkB, which is abundantly expressed in hippocampal neurons. After binding, the BDNF/TrkB complex is then internalized into the neuron and serves as a docking site for diverse signaling cascades, protein phosphorylation cascades, and secondary signaling systems (Huang and Reichardt, 2003; Nykjaer et al., 2005; Yoshii and Constantine-Paton, 2010). The binding of BDNF to TrkB receptor leads to phosphorylation of TrkB, thus activating several important intracellular downstream signaling cascades, including PLC, phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT), mitogen-activated protein kinase/extracellular signal-related kinase (MAPK/ERK) and guanosine triphosphate hydrolases (GTP-ases) of the Ras homolog (Rho) gene family pathways (Minichiello, 2009; Mohammadi et al., 2018). The BDNF/TrkB/PKC signaling is essential for synaptic function and maintenance (Lanuza et al., 2019), and it links pre- and post-synaptic activity to maintain neuromuscular function, which can be affected by a decrease in neuromuscular activity such as occurring neuromuscular disorders (Hurtado et al., 2017). BDNF/TrkB/PI3K/Akt pathway has an antiapoptotic effect and suppresses autophagy by which further inhibits the degradation of three important postsynaptic proteins (PSD-95, PICK1, and SHANK3) that is essential for N-methyl-D-aspartate receptor (NMDAR)-dependent synaptic plasticity (Nikoletopoulou et al., 2017). Synaptic plasticity is a dynamic process that involves in competition between Ca2+/calmodulin-dependent protein kinase II (CaMKII) and PSD-95 for binding to the NR2A NMDAR subunit, while LTP is associated with an increase of αCaMKII binding with the NR2A/B subunit of the NMDA receptor and a concomitant decrease binding with PSD-95 (Stachowicz, 2022). In addition, BDNF also has direct effect on NMDA receptor subunits but with different level. Acute stimulation of hippocampal neurons with BDNF rapidly upregulated the protein levels and delivery to the plasma membrane of the NR1 and NR2B NMDA receptor subunits, while with a delayed increase of NR2A subunit (Caldeira et al., 2007). Another study revealed that BDNF increased Girdin phosphorylation through the TrkB/PI3K/Akt pathway leading to phosphorylation of the NR2B subunit and NMDA receptor activation, which is crucial for synaptic plasticity and memory (Nakai et al., 2014). The PI3K/Akt/mTOR pathway promotes dendritic growing and branching through regulation of protein synthesis and cytoskeleton development (Jaworski et al., 2005; Kumar et al., 2005). The MAPK/ERK pathway is pivotal not only for early response gene expression such as c-Fos but also for cytoskeleton protein synthesis such as Arc and cypin (Gonzalez et al., 2016). Moreover, it plays a critical role in dendritic growth and branching of hippocampal neurons (Kwon et al., 2011). In addition, through BDNF/AMPAR/mTOR signaling, ketamine induces structural plasticity in mouse mesencephalic and human induced pluripotent stem cells-derived dopaminergic neurons (Cavalleri et al., 2018). The BDNF/TrkB/GTP-ases pathway promotes actin and microtubule synthesis, resulting in growth of neuronal fibers (Gonzalez et al., 2016). The pro-BDNF/p75 neurotrophin receptor/sortilin binding complex initiates signaling cascades leading to activation of c-Jun amino terminal kinase (JNK), ras homolog gene family member A (RhoA), and nuclear factor kappa B (NF-kB) downstream, which involves in neuronal apoptosis (Anastasia et al., 2013), neuronal growth cone development and motility (Meeker and Williams, 2015), and neuronal survival (Lee et al., 2001; Figure 1).
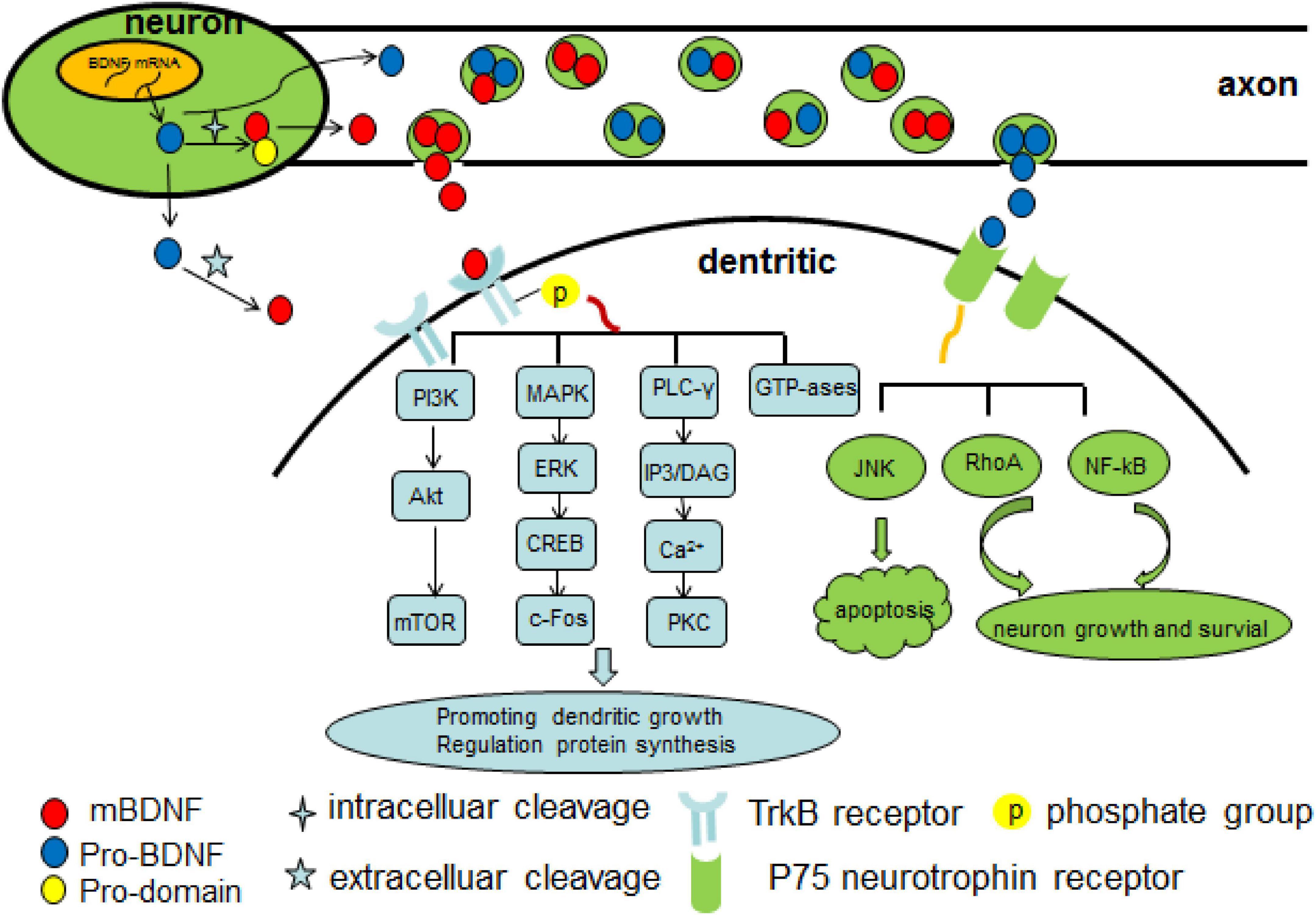
Figure 1. Overview of Brain derived neurotrophic factor (BDNF) signaling cascade. There are two forms of BDNF–proBDNF and mBDNF. The proBDNF is initially synthesized and then cleaved intra or extracellularly into mBDNF. Two types of BDNF activate several important intracellular downstream signaling cascades as above shown by mediating TrkB receptor and p75 neurotrophin receptor. Pro-domain BNDF propeptide, PI3K phosphatidylinositol 3-kinase, MAPK mitogen-activated protein kinase, ERK extracellular-signal-regulated kinase, CREB Camp response element-binding protein, c-Fos transcription factor c-Fos, PLC-γ phospholipase C-γ, IP3 inositol 1,4,5-trisphosphate, DAG 1,2-diacylglycerol, PKC protein kinase C, GTP-ases guanosine triphosphate hydrolases, JNK c-Jun amino terminal kinase, RhoA Ras homolog gene family member, NF-kB nuclear factor kappa B.
The change of BDNF level in both peripheral blood and CNS and the imbalance or insufficient of pro-BDNF transformation into mBDNF have been found to be involved in the pathogenesis of multiple diseases such as depression, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, multiple sclerosis, and cerebral ischemic stroke (Eyileten et al., 2021; Wang M. et al., 2021). In these diseases, failure of the switch of pro-BDNF to mBDNF is due to the abnormal proteolytic cleavage. For example, altered expression and/or activities of components in the tPA/plasmin system (extracellular cleavage proteases) participated in pathological processes related to depression and anxiety (Wang M. et al., 2021). Details will be summarized as below (Figure 2 and Table 1) and introduced in the following seven sections.
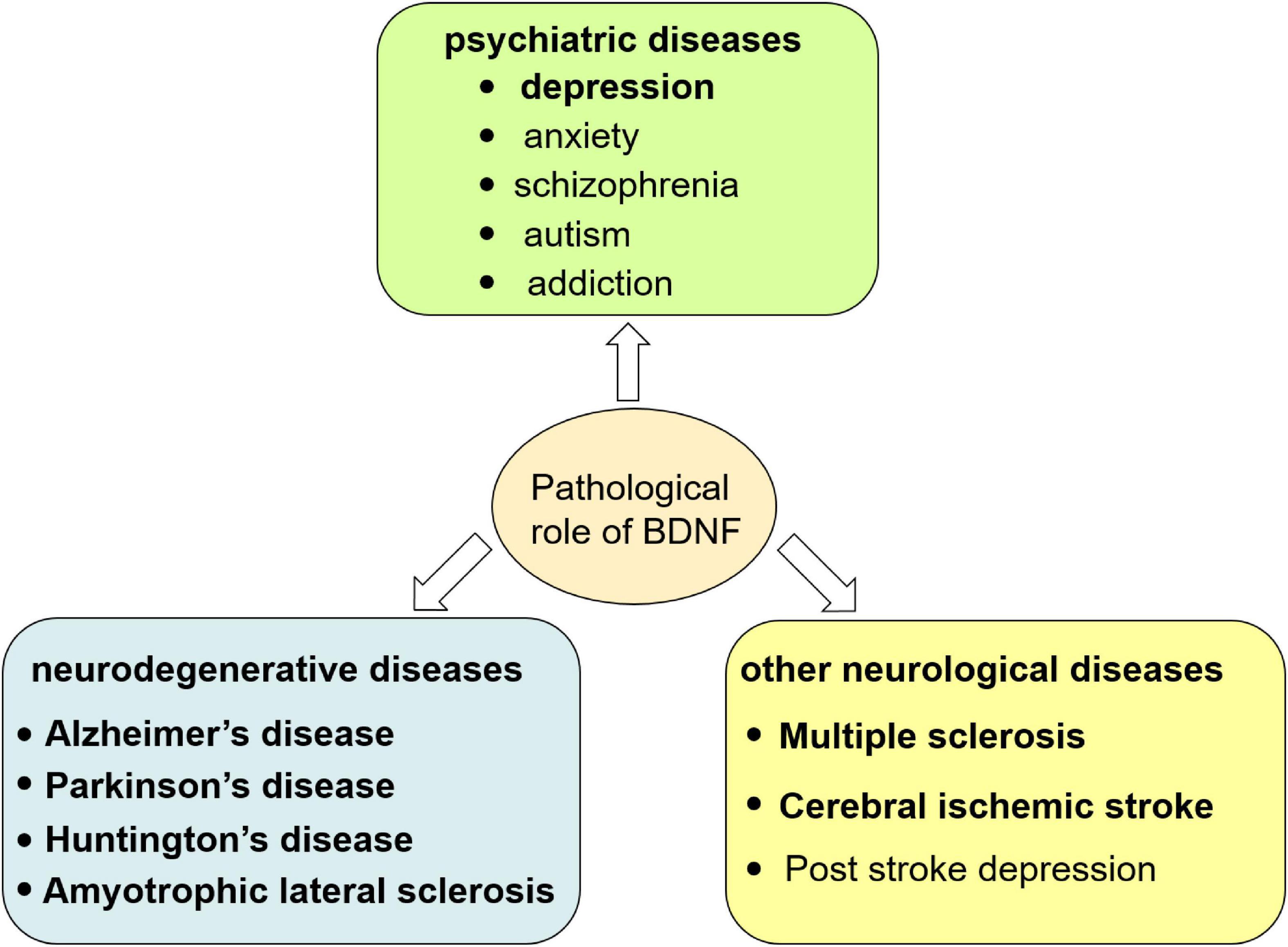
Figure 2. Pathological role of BDNF in central nervous system. The dysfunction of BDNF signaling causes neurological disorders including psychiatric, neurodegenerative diseases and other neurological diseases.
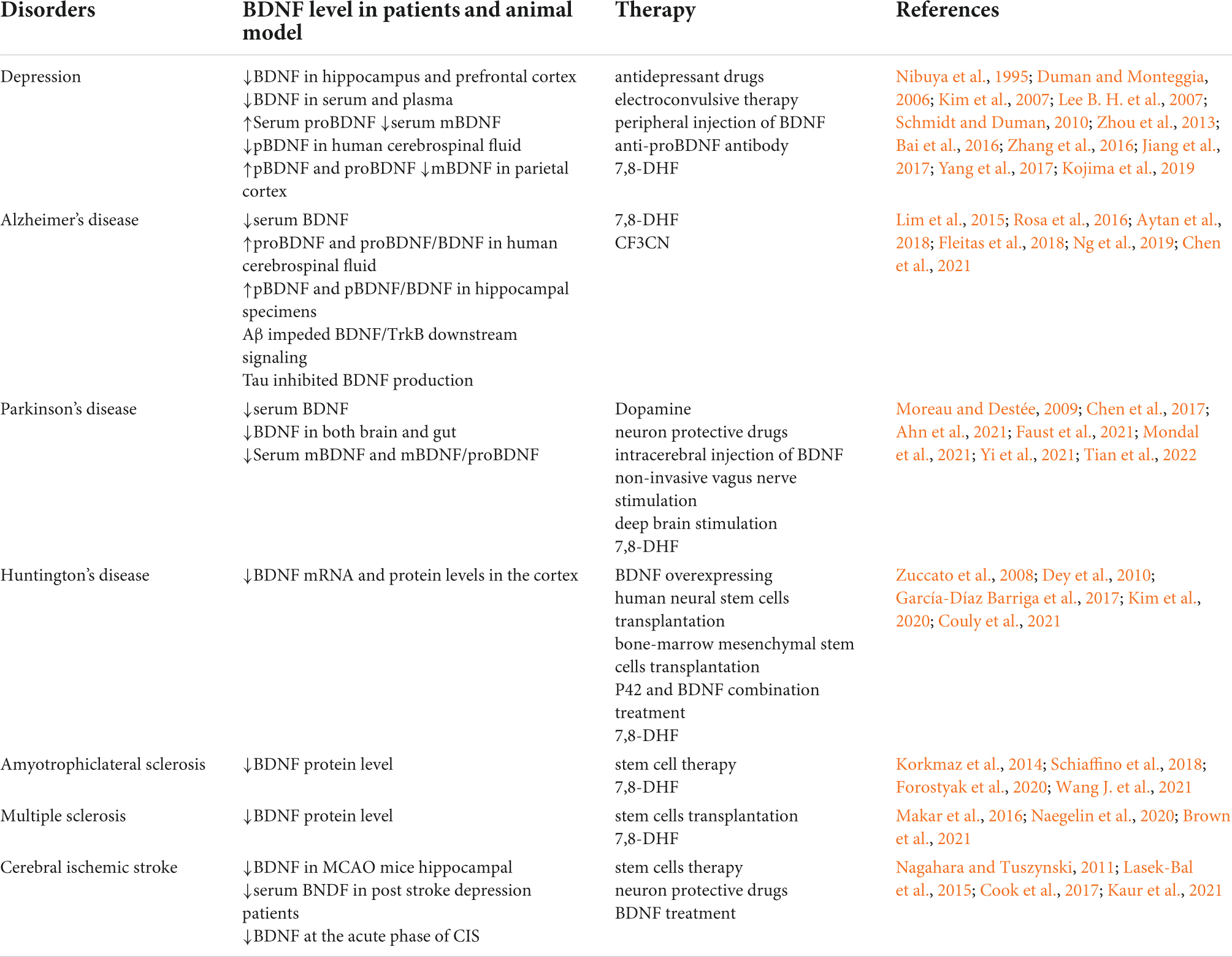
Table 1. Brain derived neurotrophic factor (BDNF) level in different brain disorders and BDNF associated therapy.
Depression is not an unusual psychiatric disease at present. Major depressive disorder is characterized by emotional, motivational, cognitive and physiological domain symptoms, making it a complicated disease to treat. BDNF/TrkB signaling is involved in various psychiatric diseases and is widely studied in the context of depression, anxiety disorders, schizophrenia, autism, and addiction (Autry and Monteggia, 2012). Here, we mainly discuss the role of BDNF in depression. Clinical studies have shown that patients with severe depression have a low level of BDNF in the hippocampus and prefrontal cortex, accompanied by cerebral hippocampus atrophy, neuronal apoptosis and synaptic loss (Duman and Monteggia, 2006). In addition, the serum BDNF level was also found to be decreased in depression patients (Lee B. H. et al., 2007), and the level of plasma BDNF seems to be lower in patients with severe depression compared to mild depression patients (Kim et al., 2007). However, treatment with conventional antidepressant drugs as well as electroconvulsive therapy enhanced the mRNA expressions of BDNF and TrkB in the hippocampal and cortical regions (Nibuya et al., 1995). Several intracellular signaling pathways such as mammalian target of rapamycin (mTOR) and glycogen synthase kinase 3 (GSK-3) have been implicated in the antidepressant effects of ketamine (Li et al., 2010; Beurel et al., 2011). Whereas, previous works examining the antidepressant effects of ketamine demonstrated that mTOR was a downstream target of BDNF (Autry et al., 2011). Animal model of depression revealed that BDNF signaling was rescued in the hippocampus after peripheral injection of BDNF for 14 consecutive days, and its mRNA level in the CA3 area of the hippocampus was also increased, suggesting the antidepressant-effect of BDNF (Schmidt and Duman, 2010). All these mentioned indicate that up-regulating BDNF level may have positive effect to the treatment of depression. Some clinical studies revealed that the serum proBDNF level in severe depression patients was apparently higher than that in healthy subjects, while the level of mBDNF shows the opposite trend. What’s more, the ratio of mBDNF/proBDNF was much lower in depression patients compare to healthy control, while was increased after antidepressant drugs treatment (Zhou et al., 2013; Jiang et al., 2017). Another study showed that, in human cerebrospinal fluid, levels of pro-peptide BDNF (pro-domain of BDNF, pBDNF) were significantly lower in patients with major depressive disorder than in controls (Kojima et al., 2019). In addition, autopsy results of depression patients showed that the expressions of pBDNF and proBDNF increased in parietal cortex while mBDNF decreased (Yang et al., 2017). Furthermore, intraperitoneal or intraventricular injection of anti-proBDNF antibody can neutralize proBDNF and alleviate depression-like behavior in rats (Bai et al., 2016). A research demonstrated that chronic 7,8-DHF (7,8-Dihydroxyflavone, a TrkB agonist) treatment rescued the depressive-like behaviors in sucrose preference test and novelty suppressed feeding test by regulating TrkB signaling, increasing BDNF levels and promoting synaptic protein expression (Zhang et al., 2016). In summary, overall consideration of the dynamic equilibrium and mutual interaction between proBDNF and mBDNF as well as pBDNF is of more significant than merely focusing on mBDNF.
Alzheimer’s disease (AD) is the most common form of dementia, which was pathologically characterized by extracellular senile plaque (SP) formed by the deposition of β-amyloid (Aβ), and intracellular neurofibrillary tangles (NFTs) formed by the abnormally phosphorylated Tau protein aggregation as well as vascular amyloidosis and loss of neurons in the cortex and hippocampus (Edler et al., 2017; Szaruga et al., 2017). Multiple studies showed that BDNF, proBDNF and pBDNF played important roles in the occurrence and development of AD. A meta-analysis indicated that AD patients, rather than mild cognitive impairment patients, have significantly lower serum BDNF levels compared to age-matched healthy controls, and a significant decrease in peripheral BDNF levels can only be detected at the late stage of dementia (Ng et al., 2019). A study on AD patients showed a negative correlation between cognitive decline and BDNF mRNA level in the dorsal lateral prefrontal cortex (Buchman et al., 2016). Furthermore, significant increases in proBDNF expression and the ratio of proBDNF/BDNF in AD patients’ cerebrospinal fluid were detected, which led to an increase of pathogenicity and a decrease of trophic effect and caused neuronal apoptosis through p75 neurotrophin receptor (Fleitas et al., 2018). In hippocampal specimens of AD patients, the level of pBDNF was 16 times higher than that in healthy individuals, and the ratio of pBDNF/BDNF was 30 times higher compared to control. What’s more, cell death rate increased when treating cells with pBDNF and Aβ simultaneously compared to giving pBDNF solely, which indicated that they may cause synergistic toxicity in AD (Lim et al., 2015). In addition, Aβ promoted the production of shortened TrkB receptor mRNA and the decomposition of the full-length TrkB receptor, which impeded BDNF/TrkB downstream signaling (Jerónimo-Santos et al., 2015). Interestingly, we have found that deficiency in BDNF/TrkB neurotrophic signaling can increase CCAAT/enhancer binding protein β (C/EBPβ) expression and then activate δ-secretase which leads to APP and Tau fragmentation and causes AD eventually (Wang et al., 2019). On the other hand, excessive or pathologically altered Tau can inhibit BDNF production and neurotoxicity without Tau gene mutation or neurofibrillary tangles formation (Rosa et al., 2016). Reversely, BDNF can also impede phosphorylation of Tau (Jiao et al., 2016).
In all, the interactions between BDNF and Aβ or Tau need further investigation to find an effective therapy for AD. In 5xFAD mice models, researchers found that 2 months’ treatment of 7,8-DHF decreased cortical Aβ plaque deposition and protected dendritic arbor complexity in cortical neurons but without huge impact on the density of dendritic spines (Aytan et al., 2018). In addition, CF3CN, an optimized synthetic 7,8-DHF chemical, has the ability to activate TrkB neurotrophic signaling and inhibits the activation of delta-secretase, which attenuates AD pathologies and alleviates cognitive dysfunctions in 5xFAD mice. All these suggest that 7,8-DHF or CF3CN may be potential therapeutic agents for the treatment of AD (Chen et al., 2021).
Parkinson’s disease (PD), the second most prevalent neurodegenerative disease in the world, is characterized by progressive degeneration of nigrostriatal dopaminergic neurons and the development of intracellular proteinaceous aggregates. It is a combination of motor and non-motor symptoms. The former which weights most contains tremor, bradykinesia, rigidity, and postural instability, and the latter involves in hyposmia, sleep disorders, autonomic nervous dysfunction, and mental disorders. Study shows that the non-motor symptoms not only precede but often are accompanied by PD, of which neuropsychiatric symptoms including depression, anxiety, apathy, hallucinations and impulse control disorders are up to 60% in PD patients, in which almost 20-40% suffer from depression (Song et al., 2015). Many studies have shown that the mRNA and protein levels of serum BDNF in patients with depression and PD were significantly reduced. What’s more, BDNF gene polymorphism, especially the Met allele, is associated with a higher neuropsychiatric burden in PD (Ramezani et al., 2020). An interesting clinical study has found that PD patients with higher self-rating Depression Scale (SDS) score show lower serum BDNF level (Chen et al., 2017). On the other hand, the decrease of BDNF in PD patients may also affect neuronal function in hippocampus, prefrontal cortex and amygdala and eventually leads to depressive symptoms (Enomoto et al., 2016). Therefore, some researchers recognized serum BDNF as a clinical biomarker for motor severity in depressed PD patients, especially in female (Huang et al., 2021).
Except for these neuropsychiatric symptoms, BDNF also takes effect in motor symptoms and other non-motor symptoms. BDNF supports the midbrain dopaminergic neurons surviving, promotes differentiation, and protects dopamine neurons from neurotoxins (Minichiello et al., 2002). BDNF overexpression alleviated motor deficits and cognitive impairment in MPTP-induced PD mice through mitigating mitochondrial damage (Chang and Wang, 2021). What’s more, BDNF level was significantly decreased in the colon of MPTP-treated group compared to the vehicle-treated group, indicating a role of BDNF in the gut-brain axis in PD (Choi et al., 2021). In accordance with this, BDNF and Netrin-1 are strongly decreased in both brain and gut of PD patients, and conditionally knocking out of these trophic factors in gut leads to dopaminergic neuronal loss, constipation and motor deficits (Ahn et al., 2021). A clinical study included one hundred and fifty-six patients with limb tremor and/or bradykinesia who meet the MDS Parkinson’s diagnostic criteria and analyzed their serum level of proBDNF and mBDNF. They found that serum levels of mBDNF and mBDNF/proBDNF were significantly lower in the PD group compared with non-PD group, while the proBDNF showed opposite result. Thus, they supposed that mBDNF/proBDNF can be used as biomarker for early stage of PD and the combination of mBDNF and proBDNF has better diagnostic value than mBDNF alone in PD diagnosis (Yi et al., 2021).
Over the past decades, most of drugs for PD treatment mainly focus on modulating dopamine concentrations in the basal ganglia. Recently, studies of neurotrophic factors such as glial cell line-derived neurotrophic growth factor and BDNF, which are considered the primary factors for neuroprotection in PD, have gained a great attention. Intracerebral injection of BDNF markedly improves levodopa-induced dyskinesia in PD rat model without affecting the therapeutic effect of levodopa (Moreau and Destée, 2009). BDNF is being developed as a neuron protective drug, and the underlying mechanisms − include promoting neuronal regeneration, repairing the damaged neurons, promoting functional recovery, and enhancing the activity of antioxidant enzymes. However, exogenous BDNF cannot cross the blood-brain barrier, therefore, the treatment of PD is still under research. A recent study has shown that non-invasive vagus nerve stimulation, which is an established neurostimulation therapy used in the treatment of epilepsy, migraine and cluster headache, is demonstrated to improve gait and motor function in PD patients, and it can significantly increase BDNF level (Mondal et al., 2021). What’s more, the level of BDNF in the motor cortex increased after deep brain stimulation (DBS) in the subthalamic nucleus (STN), which is a powerful therapeutic alternative for the treatment of PD (Faust et al., 2021).
The α-Synuclein (α-Syn) is a major component of Lewy body which is a pathological marker of PD. Physiologically, α-Syn takes effect in intercellular dopamine storage, synaptic membrane biogenesis, and lipid transport (Lotharius and Brundin, 2002). Wild-type α-Syn induces BDNF expression, while α-Syn mutant (A30P and A53T) cannot induce BDNF expression (Kohno et al., 2004). These α-Syn mutants tend to form protofibrils and further aggregate into larger inclusion bodies associated with PD, which significantly inhibit axonal transport of BDNF/TrkB (Papapetropoulos and Mash, 2005; Volpicelli-Daley et al., 2014). However, BDNF reduces the degradation of TrkB through inhibiting the formation of the α-Syn –TrkB complex (Kang et al., 2017). Further studies are needed to clarify the crosstalk between BDNF/TrkB and the biomarkers of PD such as α-Syn and find effective treatment for neuronal degeneration. Recently, a study found that 7,8-DHF could ameliorate α-Syn 1-103-induced mitochondrial impairment and motor dysfunction, indicating a novel oral bioactive therapeutic agent for the treatment of PD (Tian et al., 2022).
All in all, BDNF plays an important role in the diagnosis and the treatment of PD, as well as involving in the development of PD through affecting PD pathology.
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disease caused by an expanded CAG repeat sequence in the huntingtin gene which leads to an abnormal expansion of the polyglutamine tract in the N-terminus of the protein huntingtin. This mutant huntingtin protein misfolds, aggregates, and disrupts proteostasis, which leads to degeneration of striatal medium spiny neurons (Rosas et al., 2003). Clinically, it is a progressive motor, cognitive, and mental dysfunction disease, whose mean onset age is 35 to 44 years and median survival time is 15 to 18 years once onset (Caron et al., 1993), and unfortunately, it affects the quality of life of their offspring and their partners.
The relationship between serum (or plasma) and cerebrospinal fluid levels of BDNF and HD has been controversial. A recent report concluded that levels of BDNF in plasma and cerebrospinal fluid are not a biomarker for HD (Ou et al., 2021). It is reported that the striatum cannot produce BDNF, and it maintains its function depending on BDNF from the cortex (about 95%), thalamus and mesencephalon (Bawari et al., 2019). BDNF, which is synthesized in cortical neurons and then delivered into striatum by anterograde transportation, is essential for the cortico-striatal synaptic activity and the survival of GABAergic neurons. Depletion of BDNF in the cortex of HD patients may make striatal neurons more vulnerable to injury (Zuccato et al., 2001). A study showed significant reduction of BDNF mRNA and protein levels in the cortex of HD patients through a systematic and quantitative assessment (Zuccato et al., 2008). Additionally, it is found that the delivery of BDNF is attenuated while transferring from the cortex to the striatum in an animal model of HD. Therefore, the reduction of cortical BDNF delivery would result in decreased activity of the cortex and striatum synaptic activity and synaptic loss (Yu et al., 2018). BDNF pro-domain knockout mouse showed impaired righting reflex, abnormal motor behaviors, obvious weight loss and short lifespan, which displayed a Huntington’s disease-like phenotype, supporting the BDNF hypothesis in the pathogenesis of HD (Li et al., 2020). Furthermore, low expression of BDNF in HD pathogenesis is potentially mediated by cAMP, MAPK and Ras signaling pathways (Zhou et al., 2021).
The astrocyte constitutes the largest population of brain cells and plays multiple roles, one of which is that it involves in neuronal dysfunction of HD. Expression mutant huntingtin with 160 polyQ specifically in astrocytes causes age-dependent neurological symptoms in transgenic mice, indicating a role of mutant huntingtin in exacerbating HD neuropathology (Bradford et al., 2009). What’s more, the mutation of huntingtin gene affects the processing and secretion of the BDNF in astrocytes, leads to progressive degeneration of striatal GABAergic medium spiny neurons and decreases BDNF level in the brain of HD patients (Wang et al., 2012; Bawari et al., 2019). In return, BDNF modulates striatal astrocyte function by inducing astrocyte to secrete soluble neuroprotective factors that selectively protect neurons expressing mutant huntingtin (Saba et al., 2020).
The potential therapeutic effects of BDNF for HD have been reported in various rodent models. In a rat model of HD, researchers transplanted BDNF-overexpressing human neural stem cells (HB1.F3.BDNF) into the contra-lateral side of unilateral quinolinic acid-lesioned striatum, and found that BDNF can promote migration, differentiation and functional restoration of HD rat (Kim et al., 2020). Another research showed that transplantation bone-marrow mesenchymal stem cells with BDNF over-expressed into striatum slowed neurodegenerative processes and alleviated behavioral disorder in the YAC 128 mouse model of HD (Dey et al., 2010). However, the long-term safety and efficacy of this approach still need further research. A dual therapy that combines P42 treatment which increases TrkB expression in striatum, with BDNF-enhancing therapy such as environmental enrichment efficiently delayed HD pathology in R6/2 mice (Couly et al., 2021). Furthermore, bilateral expression of BDNF by adeno-associated virus1/2 in the striatum alleviated motor and cognitive dysfunction in transgenic HD rats, accompanied by increased volume of the striatum and number of neural cells (Connor et al., 2016). In a more practical way, researchers found that oral 7,8-DHF ameliorated cognitive and motor deficits in a mouse model of HD through specific activation of the PLCγ1 pathway (García-Díaz Barriga et al., 2017). These results suggest that BDNF has a compensating effect to HD and might be a promising treatment for HD.
Amyotrophic lateral sclerosis (ALS) is an adult-onset neurodegenerative disorder, characterized by the loss and degeneration of both lower and upper motor neurons. The etiopathogenesis of ALS is still unknown, but the possible hypotheses include oxidative stress, inflammation, protein aggregation and miss-folding, as well as glutamate excitotoxicity, RNA processing and epigenetic dysregulation (Jankovic et al., 2021). Therefore, the treatments of ALS are of multi-level but with limited therapeutic effects.
As a member of the neurotrophin family, BDNF promotes cell regeneration and survival. A study conducted on spinal cord tissue in SOD1 G93A mice (a model of ALS) showed that the expression level of BDNF decreased significantly compared to control mice (Schiaffino et al., 2018). In addition, another study showed that physical exercise such as running and swimming can improve the BDNF/TrkB neurotrophic signaling at the neuromuscular junction and reduce the impact of ALS in mice (Just-Borràs et al., 2020). Based on the above evidence, recent studies have recognized the critical role of BDNF in maintaining motoneurons survival, and take BDNF as a potential treatment for ALS. BDNF have been used in ALS human clinical trials, but the results are disappointing due to the poor pharmacokinetics and pharmacodynamics of BDNF. Deletion of the BDNF receptor TrkB.T1 alleviated muscle weakness and motoneuron cell death of spinal cord in the G93A SOD1 animal model of ALS, indicating TrkB.T1 may limit the neuroprotective BDNF signaling to motoneurons via a non-cell autonomous mechanism, which providing new understanding of the reasons for past clinical failures (Yanpallewar et al., 2021). In a transgenic rat model, intraspinal transplantation of pluripotent stem cells might rescue or replace dying motoneurons and these cells display neuroprotective effects by regulating local gene expression and extracellular matrix plasticity of central nervous system, which means that stem cell therapy is a promising therapeutic strategy for ALS (Forostyak et al., 2020). Additionally, transplantation of BDNF-overexpressing human umbilical cord mesenchymal stem cell-derived motor neurons was capable of increasing motor ability and prolonging lifespan of hSOD1 G93A mice (Wang J. et al., 2021). What’s more, chronic administration of 7,8-DHF significantly improved motor deficits, and preserved spinal motor neurons count and dendritic spines in SOD1 G93A mice (Korkmaz et al., 2014). In summary, BDNF and its equivalent drugs may be a promising therapeutic strategy for ALS, but further researches are needed to overcome the difficulties of poor pharmacokinetics and pharmacodynamics of BDNF.
Multiple sclerosis (MS) is a chronic autoimmune disease characterized by neuronal inflammation, degeneration and demyelinating lesions within brain and spinal cord (primarily in the white matter of the brain), which leads to motor dysfunction and cognitive decline. The mechanisms underlying the pathogenesis and progression of MS are not fully understood and current treatments have limited efficacy. Several molecules such as BDNF, IL-1β, PDGF, and CB1Rs, are involved in functional recovery of MS from the acute phase and are thus taken as potential therapeutic targets (Ksiazek-Winiarek et al., 2015). Recently, a novel drug, 7,8-DHF was found to reduce the clinical and pathological severity of MS in a murine model through activation of TrkB/AKT/STAT3 signaling and reduction of inflammation and demyelination (Makar et al., 2016), which brought good news to this disease.
The versatile BDNF acts as a neuroprotective factor in the process of inflammation, degeneration and demyelination of MS. BDNF is required to drive the endogenous repair of regeneration and remyelination after demyelinating inflammatory injury in MS, which is crucial for neuronal preservation and prevention of clinical progression (Brod, 2022). Study showed that neural stem cells transplantation rescued the progression of MS by inducing anti-inflammation and promoting neurogenesis and myelination, possibly by modulating BDNF and FGF signaling pathways in an experimental autoimmune encephalomyelitis mouse model of MS (Brown et al., 2021). Another study revealed that BDNF is initially presented in T cells and macrophages in MS lesions to confirm the capability of producing BDNF in human immune system. However, high levels of cytokines tend to negatively regulate circulating BDNF levels indicating BNDF may act as an immune-mediated defense of neurons in MS lesions (Sorenson et al., 2014). To determine whether BDNF can serve as a biomarker for MS, a comparatively large cohort research found that the level of BDNF can be detected to have a difference between MS patients and healthy controls, with mean BDNF levels in the former were lower by 8% than the latter. However, the difference is minor and thus it is unlikely to effectively evaluate and inform decision-making processes at an individual patient level. Therefore, it is hard to claim that serum BDNF is a biomarker for MS although it has multifunction in this disease (Naegelin et al., 2020).
Cerebral stroke is one of the leading causes of death and long-term disability worldwide, of which cerebral ischemic stroke (CIS) takes up the overwhelming majority. Recently, BDNF has been focused on in CIS, especially its relationship with post-stroke mobility. A study found that the expression of BDNF and TrkB decreased both in oxygen glucose deprivation cells and in mice hippocampal experiencing surgery of middle cerebral artery occlusion (MCAO). Shuxuening injection reverses it and promotes the recovery of post-stroke cognitive and motor deficiencies via BDNF-mediated Neurotrophin/Trk Signaling (Li et al., 2021). Furthermore, intra-arterially mesenchymal stem cells therapy in post-stroke facilitates neuroprotection and regulates ER stress-mediated apoptosis via the BDNF/TrkB signaling pathway (Kaur et al., 2021). Additionally, BDNF is also involved in post stroke depression (PSD). PSD patients show lower level of serum BNDF compared to those without PSD. Furthermore, antidepressants could improve the expression of BDNF in brain, which further alleviates depression symptoms. However, the mechanisms of BDNF in the development of PSD are still unknown (Zhang and Liao, 2020).
Except for post-stroke, it has been reported that BDNF level was significantly decreased at the acute phase of CIS and it can act as a factor warning poor prognosis for the functional status of patients on the 90th day after onset (Lasek-Bal et al., 2015). In addition, when exogenous BDNF is given immediately (within hours) after CIS, it can act as a neuroprotective agent. Surprisingly, when it is given several days after ischemic injury, it can promote axons protruding budding and synapses formation (Nagahara and Tuszynski, 2011). However, BDNF cannot penetrate the undamaged blood-brain barrier, which makes peripheral BDNF injection ineffective. What’s more, transcranial BDNF treatment makes the time of tissue distribution much too short. Therefore, the way of delivering BDNF need further research to achieve the optimal treatment (Cook et al., 2017).
In this review, we introduced the physiological and pathological functions of BDNF as well as its potential treatment effect in CNS diseases including depression, Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis, amyotrophic lateral sclerosis and cerebral ischemic stroke. We discussed the alteration of BDNF level and BDNF-based therapies in different disease models. Apart from the disorders above mentioned, the role of BDNF was also investigated in many other diseases such as diabetes and cancer (Eyileten et al., 2017; Guzel et al., 2021). Understanding the role of BDNF and its signaling as well as how they regulate repair or regrowth of CNS will be useful. Myriad compelling studies reveal that enhancing BDNF levels or restoring its receptor’s signaling cascades can improve the phenotype of animal models in different diseases. However, there are still many questions to be answered. Firstly, BDNF has a large molecular weight and is difficult to pass through the blood-brain barrier, so it cannot reach the CNS and play a role when intravenous administration. Intraventricular injection has high technical requirements for operators and is prone to cause huge damage, which leads to it difficult to popularize. Using BDNF agonists to increase the release of BDNF in vivo can solve this problem, but what agonists we should use is another problem. Recently, more and more researchers take their emphasis on BDNF-overexpressed stem cells transplantation in animal model, which might be a promising therapeutic strategy but it is on the try phase and further studies are needed. Secondly, at present, BDNF is still limited to animal experiments and cannot be applied in clinical practice on a large scale. Thirdly, there is no consensus on when and at what dose BDNF should be administered to specific diseases.
Z-HW conceived and revised the manuscript. YL, FL, DQ, and HC found the associated documents. JHW, JBW, SS, CW, YW, SL, and DG helped to draw the figures. YL wrote the manuscript with input from all authors. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (No. 82101479) to Z-HW and National Key Research Projects of China (No. 2021YFA1302400) to Z-HW.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahn, E. H., Kang, S. S., Liu, X., Cao, X., Choi, S. Y., Musazzi, L., et al. (2021). BDNF and Netrin-1 repression by C/EBPβ in the gut triggers Parkinson’s disease pathologies, associated with constipation and motor dysfunctions. Prog. Neurobiol. 198:101905. doi: 10.1016/j.pneurobio.2020.101905
Anastasia, A., Deinhardt, K., Chao, M. V., Will, N. E., Irmady, K., Lee, F. S., et al. (2013). Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nat. Commun. 4:2490. doi: 10.1038/ncomms3490
Autry, A. E., Adachi, M., Nosyreva, E., Na, E. S., Los, M. F., Cheng, P. F., et al. (2011). NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475, 91–95. doi: 10.1038/nature10130
Autry, A. E., and Monteggia, L. M. (2012). Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 64, 238–258. doi: 10.1124/pr.111.005108
Aytan, N., Choi, J. K., Carreras, I., Crabtree, L., Nguyen, B., Lehar, M., et al. (2018). Protective effects of 7,8-dihydroxyflavone on neuropathological and neurochemical changes in a mouse model of Alzheimer’s disease. Eur. J. Pharmacol. 828, 9–17. doi: 10.1016/j.ejphar.2018.02.045
Bai, Y. Y., Ruan, C. S., Yang, C. R., Li, J. Y., Kang, Z. L., Zhou, L., et al. (2016). ProBDNF Signaling Regulates Depression-Like Behaviors in Rodents under Chronic Stress. Neuropsychopharmacology 41, 2882–2892. doi: 10.1038/npp.2016.100
Bawari, S., Tewari, D., Argüelles, S., Sah, A. N., Nabavi, S. F., Xu, S., et al. (2019). Targeting BDNF signaling by natural products: Novel synaptic repair therapeutics for neurodegeneration and behavior disorders. Pharmacol. Res. 148:104458. doi: 10.1016/j.phrs.2019.104458
Beurel, E., Song, L., and Jope, R. S. (2011). Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol. Psychiatry 16, 1068–1070. doi: 10.1038/mp.2011.47
Bradford, J., Shin, J. Y., Roberts, M., Wang, C. E., Li, X. J., and Li, S. (2009). Expression of mutant huntingtin in mouse brain astrocytes causes age-dependent neurological symptoms. Proc. Natl. Acad. Sci. U.S.A. 106, 22480–22485. doi: 10.1073/pnas.0911503106
Bramham, C. R., and Messaoudi, E. (2005). BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 76, 99–125. doi: 10.1016/j.pneurobio.2005.06.003
Brod, S. A. (2022). A proposal: How to study pro-myelinating proteins in MS. Autoimmun. Rev. 21:102924. doi: 10.1016/j.autrev.2021.102924
Brown, C., McKee, C., Halassy, S., Kojan, S., Feinstein, D. L., and Chaudhry, G. R. (2021). Neural stem cells derived from primitive mesenchymal stem cells reversed disease symptoms and promoted neurogenesis in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Stem. Cell Res. Ther. 12:499. doi: 10.1186/s13287-021-02563-8
Buchman, A. S., Yu, L., Boyle, P. A., Schneider, J. A., De Jager, P. L., and Bennett, D. A. (2016). Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology 86, 735–741. doi: 10.1212/wnl.0000000000002387
Caldeira, M. V., Melo, C. V., Pereira, D. B., Carvalho, R. F., Carvalho, A. L., and Duarte, C. B. (2007). BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol. Cell Neurosci. 35, 208–219. doi: 10.1016/j.mcn.2007.02.019
Caron, N. S., Wright, G. E. B., and Hayden, M. R. (1993). “Huntington disease,” in Gene reviews® [Internet]. eds M. P. Adam, G. M. Mirzaa, R. A. Pagon, S. E. Wallace, L. J. H. Bean, K. W. Gripp, et al. (Seattle, WA: University of Washington).
Cavalleri, L., Merlo Pich, E., Millan, M. J., Chiamulera, C., Kunath, T., Spano, P. F., et al. (2018). Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol. Psychiatry 23, 812–823. doi: 10.1038/mp.2017.241
Chang, E., and Wang, J. (2021). Brain-derived neurotrophic factor attenuates cognitive impairment and motor deficits in a mouse model of Parkinson’s disease. Brain Behav. 11:e2251. doi: 10.1002/brb3.2251
Chen, C., Ahn, E. H., Liu, X., Wang, Z. H., Luo, S., Liao, J., et al. (2021). Optimized TrkB Agonist Ameliorates Alzheimer’s Disease Pathologies and Improves Cognitive Functions via Inhibiting Delta-Secretase. ACS Chem. Neurosci. 12, 2448–2461. doi: 10.1021/acschemneuro.1c00181
Chen, H., Lombès, M., and Le Menuet, D. (2017). Glucocorticoid receptor represses brain-derived neurotrophic factor expression in neuron-like cells. Mol. Brain 10:12. doi: 10.1186/s13041-017-0295-x
Choi, J. G., Jeong, M., Joo, B. R., Ahn, J. H., Woo, J. H., Kim, D. H., et al. (2021). Reduced Levels of Intestinal Neuropeptides and Neurotrophins in Neurotoxin-Induced Parkinson Disease Mouse Models. J. Neuropathol. Exp. Neurol. 80, 15–20. doi: 10.1093/jnen/nlaa113
Colucci-D’Amato, L., Speranza, L., and Volpicelli, F. (2020). Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 21:7777. doi: 10.3390/ijms21207777
Connor, B., Sun, Y., von Hieber, D., Tang, S. K., Jones, K. S., and Maucksch, C. (2016). AAV1/2-mediated BDNF gene therapy in a transgenic rat model of Huntington’s disease. Gene. Ther. 23, 283–295. doi: 10.1038/gt.2015.113
Cook, D. J., Nguyen, C., Chun, H. N., Chiu, A. S., Machnicki, M., Zarembinski, T. I., et al. (2017). Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J. Cereb. Blood Flow Metab. 37, 1030–1045. doi: 10.1177/0271678x16649964
Couly, S., Carles, A., Denus, M., Benigno-Anton, L., Maschat, F., and Maurice, T. (2021). Exposure of R6/2 mice in an enriched environment augments P42 therapy efficacy on Huntington’s disease progression. Neuropharmacology 186:108467. doi: 10.1016/j.neuropharm.2021.108467
Dechant, G., and Barde, Y. A. (2002). The neurotrophin receptor p75(NTR): Novel functions and implications for diseases of the nervous system. Nat. Neurosci. 5, 1131–1136. doi: 10.1038/nn1102-1131
Dey, N. D., Bombard, M. C., Roland, B. P., Davidson, S., Lu, M., Rossignol, J., et al. (2010). Genetically engineered mesenchymal stem cells reduce behavioral deficits in the YAC 128 mouse model of Huntington’s disease. Behav. Brain Res. 214, 193–200. doi: 10.1016/j.bbr.2010.05.023
Duman, R. S., and Monteggia, L. M. (2006). A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 59, 1116–1127. doi: 10.1016/j.biopsych.2006.02.013
Edler, M. K., Sherwood, C. C., Meindl, R. S., Hopkins, W. D., Ely, J. J., Erwin, J. M., et al. (2017). Aged chimpanzees exhibit pathologic hallmarks of Alzheimer’s disease. Neurobiol. Aging 59, 107–120. doi: 10.1016/j.neurobiolaging.2017.07.006
Enomoto, S., Shimizu, K., Nibuya, M., Toda, H., Yoshino, A., Suzuki, E., et al. (2016). Increased expression of endocytosis-Related proteins in rat hippocampus following 10-day electroconvulsive seizure treatment. Neurosci. Lett. 624, 85–91. doi: 10.1016/j.neulet.2016.05.015
Eyileten, C., Kaplon-Cieslicka, A., Mirowska-Guzel, D., Malek, L., and Postula, M. (2017). Antidiabetic Effect of Brain-Derived Neurotrophic Factor and Its Association with Inflammation in Type 2 Diabetes Mellitus. J. Diabetes Res. 2017:2823671. doi: 10.1155/2017/2823671
Eyileten, C., Sharif, L., Wicik, Z., Jakubik, D., Jarosz-Popek, J., Soplinska, A., et al. (2021). The Relation of the Brain-Derived Neurotrophic Factor with MicroRNAs in Neurodegenerative Diseases and Ischemic Stroke. Mol. Neurobiol. 58, 329–347. doi: 10.1007/s12035-020-02101-2
Faust, K., Vajkoczy, P., Xi, B., and Harnack, D. (2021). The Effects of Deep Brain Stimulation of the Subthalamic Nucleus on Vascular Endothelial Growth Factor, Brain-Derived Neurotrophic Factor, and Glial Cell Line-Derived Neurotrophic Factor in a Rat Model of Parkinson’s Disease. Stereotact. Funct. Neurosurg. 99, 256–266. doi: 10.1159/000511121
Fleitas, C., Piñol-Ripoll, G., Marfull, P., Rocandio, D., Ferrer, I., Rampon, C., et al. (2018). proBDNF is modified by advanced glycation end products in Alzheimer’s disease and causes neuronal apoptosis by inducing p75 neurotrophin receptor processing. Mol. Brain 11:68. doi: 10.1186/s13041-018-0411-6
Forostyak, S., Forostyak, O., Kwok, J. C. F., Romanyuk, N., Rehorova, M., Kriska, J., et al. (2020). Transplantation of Neural Precursors Derived from Induced Pluripotent Cells Preserve Perineuronal Nets and Stimulate Neural Plasticity in ALS Rats. Int. J. Mol. Sci. 21:9593. doi: 10.3390/ijms21249593
Friedman, W. J. (2010). Proneurotrophins, seizures, and neuronal apoptosis. Neuroscientist 16, 244–252. doi: 10.1177/1073858409349903
García-Díaz Barriga, G., Giralt, A., Anglada-Huguet, M., Gaja-Capdevila, N., Orlandi, J. G., Soriano, J., et al. (2017). 7,8-dihydroxyflavone ameliorates cognitive and motor deficits in a Huntington’s disease mouse model through specific activation of the PLCγ1 pathway. Hum. Mol. Genet. 26, 3144–3160. doi: 10.1093/hmg/ddx198
Gonzalez, A., Moya-Alvarado, G., Gonzalez-Billaut, C., and Bronfman, F. C. (2016). Cellular and molecular mechanisms regulating neuronal growth by brain-derived neurotrophic factor. Cytoskeleton 73, 612–628. doi: 10.1002/cm.21312
Guzel, T. A., Mech, K., Wroński, M., Gerkowicz, K., Bednarczyk, A., Adamczyk, W., et al. (2021). Brain-derived neurotrophic factor in gastroenterology oncology – short review of current literature. Ann. Agric. Environ. Med. 28, 367–371. doi: 10.26444/aaem/122628
Huang, E. J., and Reichardt, L. F. (2003). Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 72, 609–642. doi: 10.1146/annurev.biochem.72.121801.161629
Huang, Y., Huang, C., Zhang, Q., Wu, W., and Sun, J. (2021). Serum BDNF discriminates Parkinson’s disease patients with depression from without depression and reflect motor severity and gender differences. J. Neurol. 268, 1411–1418. doi: 10.1007/s00415-020-10299-3
Hurtado, E., Cilleros, V., Nadal, L., Simó, A., Obis, T., Garcia, N., et al. (2017). Muscle Contraction Regulates BDNF/TrkB Signaling to Modulate Synaptic Function through Presynaptic cPKCα and cPKCβI. Front. Mol. Neurosci. 10:147. doi: 10.3389/fnmol.2017.00147
Jankovic, M., Novakovic, I., Gamil Anwar Dawod, P., Gamil Anwar Dawod, A., Drinic, A., Abdel Motaleb, F. I., et al. (2021). Current Concepts on Genetic Aspects of Mitochondrial Dysfunction in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 22:9832. doi: 10.3390/ijms22189832
Jaworski, J., Spangler, S., Seeburg, D. P., Hoogenraad, C. C., and Sheng, M. (2005). Control of dendritic arborization by the phosphoinositide-3’-kinase-Akt-mammalian target of rapamycin pathway. J. Neurosci. 25, 11300–11312. doi: 10.1523/jneurosci.2270-05.2005
Jerónimo-Santos, A., Fonseca-Gomes, J., Guimarães, D. A., Tanqueiro, S. R., Ramalho, R. M., Ribeiro, J. A., et al. (2015). Brain-derived neurotrophic factor mediates neuroprotection against Aβ-induced toxicity through a mechanism independent on adenosine 2A receptor activation. Growth Factors 33, 298–308. doi: 10.3109/08977194.2015.1080696
Jiang, H., Chen, S., Li, C., Lu, N., Yue, Y., Yin, Y., et al. (2017). The serum protein levels of the tPA-BDNF pathway are implicated in depression and antidepressant treatment. Transl. Psychiatry 7:e1079. doi: 10.1038/tp.2017.43
Jiao, S. S., Shen, L. L., Zhu, C., Bu, X. L., Liu, Y. H., Liu, C. H., et al. (2016). Brain-derived neurotrophic factor protects against tau-related neurodegeneration of Alzheimer’s disease. Transl. Psychiatry 6:e907. doi: 10.1038/tp.2016.186
Just-Borràs, L., Hurtado, E., Cilleros-Mañé, V., Biondi, O., Charbonnier, F., Tomàs, M., et al. (2020). Running and swimming prevent the deregulation of the BDNF/TrkB neurotrophic signalling at the neuromuscular junction in mice with amyotrophic lateral sclerosis. Cell Mol. Life Sci. 77, 3027–3040. doi: 10.1007/s00018-019-03337-5
Kang, S. S., Zhang, Z., Liu, X., Manfredsson, F. P., Benskey, M. J., Cao, X., et al. (2017). TrkB neurotrophic activities are blocked by α-synuclein, triggering dopaminergic cell death in Parkinson’s disease. Proc. Natl. Acad. Sci. U.S.A. 114, 10773–10778. doi: 10.1073/pnas.1713969114
Katoh-Semba, R., Asano, T., Ueda, H., Morishita, R., Takeuchi, I. K., Inaguma, Y., et al. (2002). Riluzole enhances expression of brain-derived neurotrophic factor with consequent proliferation of granule precursor cells in the rat hippocampus. Faseb. J. 16, 1328–1330. doi: 10.1096/fj.02-0143fje
Kaur, H., Sarmah, D., Veeresh, P., Datta, A., Kalia, K., Borah, A., et al. (2021). Endovascular Stem Cell Therapy Post Stroke Rescues Neurons from Endoplasmic Reticulum Stress-Induced Apoptosis by Modulating Brain-Derived Neurotrophic Factor/Tropomyosin Receptor Kinase B Signaling. ACS Chem. Neurosci. 12, 3745–3759. doi: 10.1021/acschemneuro.1c00506
Kim, H. S., Jeon, I., Noh, J. E., Lee, H., Hong, K. S., Lee, N., et al. (2020). Intracerebral Transplantation of BDNF-overexpressing Human Neural Stem Cells (HB1.F3.BDNF) Promotes Migration, Differentiation and Functional Recovery in a Rodent Model of Huntington’s Disease. Exp. Neurobiol. 29, 130–137. doi: 10.5607/en20011
Kim, Y. K., Lee, H. P., Won, S. D., Park, E. Y., Lee, H. Y., Lee, B. H., et al. (2007). Low plasma BDNF is associated with suicidal behavior in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 31, 78–85. doi: 10.1016/j.pnpbp.2006.06.024
Kohno, R., Sawada, H., Kawamoto, Y., Uemura, K., Shibasaki, H., and Shimohama, S. (2004). BDNF is induced by wild-type alpha-synuclein but not by the two mutants, A30P or A53T, in glioma cell line. Biochem. Biophys. Res. Commun. 318, 113–118. doi: 10.1016/j.bbrc.2004.04.012
Kojima, M., Matsui, K., and Mizui, T. (2019). BDNF pro-peptide: Physiological mechanisms and implications for depression. Cell Tissue Res. 377, 73–79. doi: 10.1007/s00441-019-03034-6
Korkmaz, O. T., Aytan, N., Carreras, I., Choi, J. K., Kowall, N. W., Jenkins, B. G., et al. (2014). 7,8-Dihydroxyflavone improves motor performance and enhances lower motor neuronal survival in a mouse model of amyotrophic lateral sclerosis. Neurosci. Lett. 566, 286–291. doi: 10.1016/j.neulet.2014.02.058
Kowiański, P., Lietzau, G., Czuba, E., Waśkow, M., Steliga, A., and Moryś, J. (2018). BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell Mol. Neurobiol. 38, 579–593. doi: 10.1007/s10571-017-0510-4
Ksiazek-Winiarek, D. J., Szpakowski, P., and Glabinski, A. (2015). Neural Plasticity in Multiple Sclerosis: The Functional and Molecular Background. Neural. Plast. 2015:307175. doi: 10.1155/2015/307175
Kumar, V., Zhang, M. X., Swank, M. W., Kunz, J., and Wu, G. Y. (2005). Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J. Neurosci. 25, 11288–11299. doi: 10.1523/jneurosci.2284-05.2005
Kwon, M., Fernández, J. R., Zegarek, G. F., Lo, S. B., and Firestein, B. L. (2011). BDNF-promoted increases in proximal dendrites occur via CREB-dependent transcriptional regulation of cypin. J. Neurosci. 31, 9735–9745. doi: 10.1523/jneurosci.6785-10.2011
Lanuza, M. A., Just-Borràs, L., Hurtado, E., Cilleros-Mañé, V., Tomàs, M., Garcia, N., et al. (2019). The Impact of Kinases in Amyotrophic Lateral Sclerosis at the Neuromuscular Synapse: Insights into BDNF/TrkB and PKC Signaling. Cells 8:1578. doi: 10.3390/cells8121578
Lasek-Bal, A., Jędrzejowska-Szypułka, H., Różycka, J., Bal, W., Holecki, M., Duława, J., et al. (2015). Low Concentration of BDNF in the Acute Phase of Ischemic Stroke as a Factor in Poor Prognosis in Terms of Functional Status of Patients. Med. Sci. Monit. 21, 3900–3905. doi: 10.12659/msm.895358
Lee, B. H., Kim, H., Park, S. H., and Kim, Y. K. (2007). Decreased plasma BDNF level in depressive patients. J. Affect. Disord. 101, 239–244. doi: 10.1016/j.jad.2006.11.005
Lee, S. H., Kim, Y. J., Lee, K. M., Ryu, S., and Yoon, B. W. (2007). Ischemic preconditioning enhances neurogenesis in the subventricular zone. Neuroscience 146, 1020–1031. doi: 10.1016/j.neuroscience.2007.02.058
Lee, R., Kermani, P., Teng, K. K., and Hempstead, B. L. (2001). Regulation of cell survival by secreted proneurotrophins. Science 294, 1945–1948. doi: 10.1126/science.1065057
Lessmann, V., and Brigadski, T. (2009). Mechanisms, locations, and kinetics of synaptic BDNF secretion: An update. Neurosci. Res. 65, 11–22. doi: 10.1016/j.neures.2009.06.004
Lessmann, V., Gottmann, K., and Malcangio, M. (2003). Neurotrophin secretion: Current facts and future prospects. Prog. Neurobiol. 69, 341–374. doi: 10.1016/s0301-0082(03)00019-4
Li, H., Lin, L. Y., Zhang, Y., Lim, Y., Rahman, M., Beck, A., et al. (2020). Pro-BDNF Knockout Causes Abnormal Motor Behaviours and Early Death in Mice. Neuroscience 438, 145–157. doi: 10.1016/j.neuroscience.2020.05.007
Li, N., Lee, B., Liu, R. J., Banasr, M., Dwyer, J. M., Iwata, M., et al. (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329, 959–964. doi: 10.1126/science.1190287
Li, Z., Wang, H., Xiao, G., Du, H., He, S., Feng, Y., et al. (2021). Recovery of post-stroke cognitive and motor deficiencies by Shuxuening injection via regulating hippocampal BDNF-mediated Neurotrophin/Trk Signaling. Biomed. Pharmacother. 141:111828. doi: 10.1016/j.biopha.2021.111828
Lim, J. Y., Reighard, C. P., and Crowther, D. C. (2015). The pro-domains of neurotrophins, including BDNF, are linked to Alzheimer’s disease through a toxic synergy with Aβ. Hum. Mol. Genet. 24, 3929–3938. doi: 10.1093/hmg/ddv130
Lotharius, J., and Brundin, P. (2002). Pathogenesis of Parkinson’s disease: Dopamine, vesicles and alpha-synuclein. Nat. Rev. Neurosci. 3, 932–942. doi: 10.1038/nrn983
Makar, T. K., Nimmagadda, V. K., Singh, I. S., Lam, K., Mubariz, F., Judge, S. I., et al. (2016). TrkB agonist, 7,8-dihydroxyflavone, reduces the clinical and pathological severity of a murine model of multiple sclerosis. J. Neuroimmunol. 292, 9–20. doi: 10.1016/j.jneuroim.2016.01.002
Martin-Zanca, D., Hughes, S. H., and Barbacid, M. (1986). A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature 319, 743–748. doi: 10.1038/319743a0
Matsuda, N., Lu, H., Fukata, Y., Noritake, J., Gao, H., Mukherjee, S., et al. (2009). Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. J. Neurosci. 29, 14185–14198. doi: 10.1523/jneurosci.1863-09.2009
Meeker, R. B., and Williams, K. S. (2015). The p75 neurotrophin receptor: At the crossroad of neural repair and death. Neural. Regen. Res. 10, 721–725. doi: 10.4103/1673-5374.156967
Minichiello, L. (2009). TrkB signalling pathways in LTP and learning. Nat. Rev. Neurosci. 10, 850–860. doi: 10.1038/nrn2738
Minichiello, L., Calella, A. M., Medina, D. L., Bonhoeffer, T., Klein, R., and Korte, M. (2002). Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron 36, 121–137. doi: 10.1016/s0896-6273(02)00942-x
Mirowska-Guzel, D., Litwin, T., Gromadzka, G., Czlonkowski, A., and Czlonkowska, A. (2013). Influence of BDNF polymorphisms on Wilson’s disease susceptibility and clinical course. Metab. Brain Dis. 28, 447–453. doi: 10.1007/s11011-013-9399-x
Mohammadi, A., Amooeian, V. G., and Rashidi, E. (2018). Dysfunction in Brain-Derived Neurotrophic Factor Signaling Pathway and Susceptibility to Schizophrenia. Parkinson’s and Alzheimer’s Diseases. Curr. Gene. Ther. 18, 45–63. doi: 10.2174/1566523218666180302163029
Mondal, B., Choudhury, S., Banerjee, R., Roy, A., Chatterjee, K., Basu, P., et al. (2021). Non-invasive vagus nerve stimulation improves clinical and molecular biomarkers of Parkinson’s disease in patients with freezing of gait. NPJ Parkinsons Dis. 7:46. doi: 10.1038/s41531-021-00190-x
Moreau, C., and Destée, A. (2009). A new perspective on brain derived neurotrophin factor polymorphism in L-dopa induced dyskinesia. J. Neurol. Neurosurg. Psychiatry 80:129. doi: 10.1136/jnnp.2008.163568
Mowla, S. J., Farhadi, H. F., Pareek, S., Atwal, J. K., Morris, S. J., Seidah, N. G., et al. (2001). Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J. Biol. Chem. 276, 12660–12666. doi: 10.1074/jbc.M008104200
Naegelin, Y., Saeuberli, K., Schaedelin, S., Dingsdale, H., Magon, S., Baranzini, S., et al. (2020). Levels of brain-derived neurotrophic factor in patients with multiple sclerosis. Ann. Clin. Transl. Neurol. 7, 2251–2261. doi: 10.1002/acn3.51215
Nagahara, A. H., and Tuszynski, M. H. (2011). Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug. Discov. 10, 209–219. doi: 10.1038/nrd3366
Nakai, T., Nagai, T., Tanaka, M., Itoh, N., Asai, N., Enomoto, A., et al. (2014). Girdin phosphorylation is crucial for synaptic plasticity and memory: A potential role in the interaction of BDNF/TrkB/Akt signaling with NMDA receptor. J. Neurosci. 34, 14995–15008. doi: 10.1523/jneurosci.2228-14.2014
Ng, T. K. S., Ho, C. S. H., Tam, W. W. S., Kua, E. H., and Ho, R. C. (2019). Decreased Serum Brain-Derived Neurotrophic Factor (BDNF) Levels in Patients with Alzheimer’s Disease (AD): A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 20:257. doi: 10.3390/ijms20020257
Nibuya, M., Morinobu, S., and Duman, R. S. (1995). Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 15, 7539–7547. doi: 10.1523/jneurosci.15-11-07539.1995
Nikoletopoulou, V., Sidiropoulou, K., Kallergi, E., Dalezios, Y., and Tavernarakis, N. (2017). Modulation of Autophagy by BDNF Underlies Synaptic Plasticity. Cell Metab. 26:230–242.e235. doi: 10.1016/j.cmet.2017.06.005
Nykjaer, A., Willnow, T. E., and Petersen, C. M. (2005). p75NTR–live or let die. Curr. Opin. Neurobiol. 15, 49–57. doi: 10.1016/j.conb.2005.01.004
Ou, Z. A., Byrne, L. M., Rodrigues, F. B., Tortelli, R., Johnson, E. B., Foiani, M. S., et al. (2021). Brain-derived neurotrophic factor in cerebrospinal fluid and plasma is not a biomarker for Huntington’s disease. Sci. Rep. 11:3481. doi: 10.1038/s41598-021-83000-x
Pang, P. T., Teng, H. K., Zaitsev, E., Woo, N. T., Sakata, K., Zhen, S., et al. (2004). Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science 306, 487–491. doi: 10.1126/science.1100135
Papapetropoulos, S., and Mash, D. C. (2005). Alpha-synuclein aggregation and its relation to neurodegenerative diseases. Ann. Neurol. 57, 605;authorrely605–606. doi: 10.1002/ana.20414
Ramezani, M., Ruskey, J. A., Martens, K., Kibreab, M., Javer, Z., Kathol, I., et al. (2020). Association Between BDNF Val66Met Polymorphism and Mild Behavioral Impairment in Patients With Parkinson’s Disease. Front. Neurol. 11:587992. doi: 10.3389/fneur.2020.587992
Rodriguez-Tébar, A., and Barde, Y. A. (1988). Binding characteristics of brain-derived neurotrophic factor to its receptors on neurons from the chick embryo. J. Neurosci. 8, 3337–3342. doi: 10.1523/jneurosci.08-09-03337.1988
Rosa, E., Mahendram, S., Ke, Y. D., Ittner, L. M., Ginsberg, S. D., and Fahnestock, M. (2016). Tau downregulates BDNF expression in animal and cellular models of Alzheimer’s disease. Neurobiol. Aging 48, 135–142. doi: 10.1016/j.neurobiolaging.2016.08.020
Rosas, H. D., Koroshetz, W. J., Chen, Y. I., Skeuse, C., Vangel, M., Cudkowicz, M. E., et al. (2003). Evidence for more widespread cerebral pathology in early HD: An MRI-based morphometric analysis. Neurology 60, 1615–1620. doi: 10.1212/01.wnl.0000065888.88988.6e
Saba, J., López Couselo, F., Turati, J., Carniglia, L., Durand, D., de Laurentiis, A., et al. (2020). Astrocytes from cortex and striatum show differential responses to mitochondrial toxin and BDNF: Implications for protection of striatal neurons expressing mutant huntingtin. J. Neuroinflammation 17:290. doi: 10.1186/s12974-020-01965-4
Schiaffino, L., Bonafede, R., Scambi, I., Parrella, E., Pizzi, M., and Mariotti, R. (2018). Acetylation state of RelA modulated by epigenetic drugs prolongs survival and induces a neuroprotective effect on ALS murine model. Sci. Rep. 8:12875. doi: 10.1038/s41598-018-30659-4
Schmidt, H. D., and Duman, R. S. (2010). Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology 35, 2378–2391. doi: 10.1038/npp.2010.114
Song, X., Hu, X., Zhou, S., Xu, Y., Zhang, Y., Yuan, Y., et al. (2015). Association of specific frequency bands of functional MRI signal oscillations with motor symptoms and depression in Parkinson’s disease. Sci. Rep. 5:16376. doi: 10.1038/srep16376
Sorenson, M., Jason, L., Peterson, J., Herrington, J., and Mathews, H. (2014). Brain derived neurotrophic factor is decreased in chronic fatigue syndrome and multiple sclerosis. J. Neurol. Neurophysiol. s12:S12–S013.
Stachowicz, K. (2022). Is PSD-95 entangled in the side effects of antidepressants? Neurochem. Int. 159:105391. doi: 10.1016/j.neuint.2022.105391
Szaruga, M., Munteanu, B., Lismont, S., Veugelen, S., Horré, K., Mercken, M., et al. (2017). Alzheimer’s-Causing Mutations Shift Aβ Length by Destabilizing γ-Secretase-Aβn Interactions. Cell 170, 443–456.e414. doi: 10.1016/j.cell.2017.07.004
Teng, K. K., and Hempstead, B. L. (2004). Neurotrophins and their receptors: Signaling trios in complex biological systems. Cell Mol. Life Sci. 61, 35–48. doi: 10.1007/s00018-003-3099-3
Tian, Y., Pan, L., Yuan, X., Xiong, M., Zhang, Z., Meng, L., et al. (2022). 7,8-Dihydroxyflavone ameliorates mitochondrial impairment and motor dysfunction in the α-synuclein 1-103 transgenic mice. Neurobiol. Dis. 169:105736. doi: 10.1016/j.nbd.2022.105736
Volosin, M., Song, W., Almeida, R. D., Kaplan, D. R., Hempstead, B. L., and Friedman, W. J. (2006). Interaction of survival and death signaling in basal forebrain neurons: Roles of neurotrophins and proneurotrophins. J. Neurosci. 26, 7756–7766. doi: 10.1523/jneurosci.1560-06.2006
Volpicelli-Daley, L. A., Gamble, K. L., Schultheiss, C. E., Riddle, D. M., West, A. B., and Lee, V. M. (2014). Formation of α-synuclein Lewy neurite-like aggregates in axons impedes the transport of distinct endosomes. Mol. Biol. Cell 25, 4010–4023. doi: 10.1091/mbc.E14-02-0741
Wang, J., Hu, W., Feng, Z., and Feng, M. (2021). BDNF-overexpressing human umbilical cord mesenchymal stem cell-derived motor neurons improve motor function and prolong survival in amyotrophic lateral sclerosis mice. Neurol. Res. 43, 199–209. doi: 10.1080/01616412.2020.1834775
Wang, M., Xie, Y., and Qin, D. (2021). Proteolytic cleavage of proBDNF to mBDNF in neuropsychiatric and neurodegenerative diseases. Brain Res. Bull. 166, 172–184. doi: 10.1016/j.brainresbull.2020.11.005
Wang, L., Lin, F., Wang, J., Wu, J., Han, R., Zhu, L., et al. (2012). Expression of mutant N-terminal huntingtin fragment (htt552-100Q) in astrocytes suppresses the secretion of BDNF. Brain Res. 1449, 69–82. doi: 10.1016/j.brainres.2012.01.077
Wang, Z. H., Xiang, J., Liu, X., Yu, S. P., Manfredsson, F. P., Sandoval, I. M., et al. (2019). Deficiency in BDNF/TrkB Neurotrophic Activity Stimulates δ-Secretase by Upregulating C/EBPβ in Alzheimer’s Disease. Cell Rep. 28:655–669.e655. doi: 10.1016/j.celrep.2019.06.054
Woo, N. H., Teng, H. K., Siao, C. J., Chiaruttini, C., Pang, P. T., Milner, T. A., et al. (2005). Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat. Neurosci. 8, 1069–1077. doi: 10.1038/nn1510
Yang, B., Ren, Q., Zhang, J. C., Chen, Q. X., and Hashimoto, K. (2017). Altered expression of BDNF, BDNF pro-peptide and their precursor proBDNF in brain and liver tissues from psychiatric disorders: Rethinking the brain-liver axis. Transl. Psychiatry 7:e1128. doi: 10.1038/tp.2017.95
Yang, J., Harte-Hargrove, L. C., Siao, C. J., Marinic, T., Clarke, R., Ma, Q., et al. (2014). proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus. Cell Rep. 7, 796–806. doi: 10.1016/j.celrep.2014.03.040
Yang, J., Siao, C. J., Nagappan, G., Marinic, T., Jing, D., McGrath, K., et al. (2009). Neuronal release of proBDNF. Nat. Neurosci. 12, 113–115. doi: 10.1038/nn.2244
Yanpallewar, S., Fulgenzi, G., Tomassoni-Ardori, F., Barrick, C., and Tessarollo, L. (2021). Delayed onset of inherited ALS by deletion of the BDNF receptor TrkB.T1 is non-cell autonomous. Exp. Neurol. 337:113576. doi: 10.1016/j.expneurol.2020.113576
Yi, X., Yang, Y., Zhao, Z., Xu, M., Zhang, Y., Sheng, Y., et al. (2021). Serum mBDNF and ProBDNF Expression Levels as Diagnosis Clue for Early Stage Parkinson’s Disease. Front. Neurol. 12:680765. doi: 10.3389/fneur.2021.680765
Yoshii, A., and Constantine-Paton, M. (2010). Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev. Neurobiol. 70, 304–322. doi: 10.1002/dneu.20765
Yu, C., Li, C. H., Chen, S., Yoo, H., Qin, X., and Park, H. (2018). Decreased BDNF Release in Cortical Neurons of a Knock-in Mouse Model of Huntington’s Disease. Sci. Rep. 8:16976. doi: 10.1038/s41598-018-34883-w
Zagrebelsky, M., Holz, A., Dechant, G., Barde, Y. A., Bonhoeffer, T., and Korte, M. (2005). The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J. Neurosci. 25, 9989–9999. doi: 10.1523/jneurosci.2492-05.2005
Zhang, E., and Liao, P. (2020). Brain-derived neurotrophic factor and post-stroke depression. J. Neurosci. Res. 98, 537–548. doi: 10.1002/jnr.24510
Zhang, M. W., Zhang, S. F., Li, Z. H., and Han, F. (2016). 7,8-Dihydroxyflavone reverses the depressive symptoms in mouse chronic mild stress. Neurosci. Lett. 635, 33–38. doi: 10.1016/j.neulet.2016.10.035
Zhou, L., Xiong, J., Lim, Y., Ruan, Y., Huang, C., Zhu, Y., et al. (2013). Upregulation of blood proBDNF and its receptors in major depression. J. Affect. Disord. 150, 776–784. doi: 10.1016/j.jad.2013.03.002
Zhou, Z., Zhong, S., Zhang, R., Kang, K., Zhang, X., Xu, Y., et al. (2021). Functional analysis of brain derived neurotrophic factor (BDNF) in Huntington’s disease. Aging 13, 6103–6114. doi: 10.18632/aging.202603
Zuccato, C., Ciammola, A., Rigamonti, D., Leavitt, B. R., Goffredo, D., Conti, L., et al. (2001). Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science 293, 493–498. doi: 10.1126/science.1059581
Keywords: brain derived neurotrophic factor, physiological, pathological, treatment, central nervous system
Citation: Li Y, Li F, Qin D, Chen H, Wang J, Wang J, Song S, Wang C, Wang Y, Liu S, Gao D and Wang Z-H (2022) The role of brain derived neurotrophic factor in central nervous system. Front. Aging Neurosci. 14:986443. doi: 10.3389/fnagi.2022.986443
Received: 05 July 2022; Accepted: 23 August 2022;
Published: 08 September 2022.
Edited by:
Shong Lau, Salk Institute for Biological Studies, United StatesReviewed by:
Xavier Xifró, University of Girona, SpainCopyright © 2022 Li, Li, Qin, Chen, Wang, Wang, Song, Wang, Wang, Liu, Gao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Hao Wang, d2FuZ3poODZAd2h1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.