
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 29 September 2022
Sec. Alzheimer's Disease and Related Dementias
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.985406
This article is part of the Research Topic Early Detection of Neurodegenerative Disorders Using Behavioral Markers and New Technologies: New Methods and Perspectives View all 10 articles
Objective: Preoperative levels of cognition-related biomarkers and intraoperative cerebral ischemia and hypoxia might cause postoperative neurocognitive dysfunction (PND). The aim of this study was to evaluate the predictive ability of preoperative plasma biomarkers along with cerebral oxygen saturation (SctO2) for the incidence of PND in elderly patients with mild cognitive impairment (MCI).
Methods: A total of 210 patients aged 65–80 years undergoing spinal surgery were randomly assigned to three groups (n = 70 each): propofol, sevoflurane, and propofol/sevoflurane as anesthesia maintenance protocols. Propofol was administrated target-controlled infusion of 4 μg/ml (group P), the minimum alveolar concentration (MAC) of inhalation anesthetic sevoflurane was 1.3 (group S), and propofol was injected with a target-controlled plasma concentration of 1.2 μg/ml, accompanied by sevoflurane inhalation 0.7 MAC (group PS). Cognitive function was evaluated 1 day preoperatively and on the 7th day postoperatively. Preoperative levels of amyloidβ-40 (Aβ-40), Aβ-42, total tau protein (T-tau), phosphorylated tau protein (P-tau), and triggering receptors on myeloid cells-2 (TREM2) were investigated. SctO2 was monitored intraoperatively.
Results: Aβ-42 had the strongest significant correlation with preoperative MoCA score. The value of Aβ-42 associated with a high risk of PND was 28.34 pg/ml, and the area under the curve (AUC) was predicted to be 0.711. When the preoperative level of Aβ-42 was 28.34 pg/ml, SctO2max% was 9.92%. The AUC was predicted to be 0.872, and the sensitivity and specificity were 0.833 and 0.841, respectively.
Conclusion: Under the conditions of preoperative Aβ-42 less than 28.34 pg/ml, the intraoperative fluctuation range of cerebral oxygen saturation should be maintained within 9.92% to reduce the occurrence of PND in geriatric patients with MCI.
Postoperative neurocognitive dysfunction (PND) is an objectively measured decline in cognition compared with that of the preoperative state (Evered and Silbert, 2018) and it adversely affects the quality of life and rehabilitation of patients (Lin et al., 2020). The clinical diagnosis of PND is a long process that requires the application of various cognitive tests and postoperative follow-up (Miniksar et al., 2021). Preoperative cognitive function is an important factor that cannot be ignored. PND leads to neurological complications due to multifactorial causes, with a high rate of morbidity, especially in geriatric patients with mild cognitive impairment (MCI; Bekker et al., 2010; Kline et al., 2012; Xin et al., 2021). A retrospective analysis of 2014 patients aged above 65 years who underwent surgery found that the incidence of postoperative delirium in patients with MCI before surgery was significantly higher than in patients of the same age with normal cognitive function before surgery (8.7% vs. 2.6%; Sprung et al., 2017). Preoperative plasma biomarkers are closely correlated with MCI and subsequent cognitive dysfunction. Identification of the most optimal-related biomarkers and their proper concentration could enhance PND prevention and treatment practices.
MCI is defined as the clinical stage between the expected cognitive decline of normal healthy aging and a more serious decline characterizing dementia (Ng et al., 2021). The incidence of MCI is 10%–20% of adults above 65 years of age, and approximately 20%–40% of patients with MCI progress to dementia every year (Skolariki et al., 2021). As the population ages, surgery is being performed more frequently, and in progressively older adults with MCI. The inappropriate perioperative management of patients with MCI might increase the incidence of PND and accelerate the progression of MCI to Alzheimer’s disease (AD; Racine et al., 2018; Xin et al., 2021). So the choice of appropriate anesthetic drugs is important to reduce the incidence of PND in patients with MCI (Xin et al., 2021). We previously conducted preliminary research on the types, dosages, and compatibility of general anesthetics for patients with MCI and confirmed that the combination of 0.7 minimum alveolar concentration (MAC) sevoflurane with 1.2 μg/ml propofol (plasma target concentration) is safe for MCI patients undergoing surgery with general anesthesia. Closely related biomarkers contributed uniquely to predict the occurrence of PND.
Intraoperative cerebral ischemia and cerebral oxygen desaturation have been proposed as possible mechanisms of PND (Lei et al., 2007). Optimizing cerebral perfusion may allow for reducing the risk of PND. Cerebral oxygen saturation (SctO2) based on near-infrared spectroscopy monitoring depends on the balance of cerebral oxygen supply and consumption. During noncardiac surgery, a well-maintained level of SctO2 can help reduce the incidence of intraoperative cerebral ischemia in elderly patients (Casati et al., 2005). Studies have shown that the change in intraoperative SctO2 has important implications in PND (Ni et al., 2015). Intraoperative SctO2 less than 50% or a greater than 20% decline from baseline is an independent risk factor for PND (Kim et al., 2016). However, the available literature regarding the relationship between SctO2 and PND in elderly patients with MCI undergoing major surgeries is minimal. Further, whether the combined assessment of preoperative plasma biomarkers and SctO2 is also effective to clinically reduce the incidence of PND is still unclear.
Given these limitations, we hypothesized that screening biomarkers closely correlated to MCI, and limiting the fluctuation in SctO2 based on near-infrared spectroscopy monitoring might further prevent PND in elderly patients with MCI. Hence, the present study was designed to elucidate the predictive capability of preoperative biomarkers and SctO2 for PND in elderly patients with MCI undergoing surgery.
The randomized clinical study was conducted with the approval of the Institutional Human Research Ethics Committee of the Third Central Clinical College of Tianjin Medical University (IRB2019-011-01) and is in compliance with the Helsinki Declaration. The study was registered at the Chinese Clinical Trials Registry Center before patient enrollment (Registration No.: ChiCTR2000038307). Written informed consent for participation in this study was obtained from all patients.
Patients aged 65–80 years and scheduled for spinal surgery were enrolled between June 2019 and July 2020. Sex, body mass index (BMI), and American Society of Anesthesiologists (ASA) physical status II or III were recorded. MCI was diagnosed clinically based on the following criteria (Radtke et al., 2013): subjective memory loss stated by self or family members preoperatively, Montreal Cognitive Assessment (MoCA) score in the range of 15–24, mini-mental state scale (MMSE) score less than 27 points (it depends on the level of education), Clinical Dementia Rating (CDR) of 0.5 points, and activities of daily living (ADL) score less than 26 points. Patients were excluded from the study if they met any of the following criteria: preoperative neurological diseases (such as vascular dementia), severe liver and renal insufficiency, autoimmune diseases, recent use of sedatives, antidepressants, or immunosuppressive drugs, traumatic brain injury or history of alcoholism, and previous participation in the study. In all, 224 patients were enrolled and randomly divided into three groups (n = 70): propofol group (group P), sevoflurane group (group S), and propofol/sevoflurane group (group PS; Figure 1).
Heart rate (HR), blood oxygen saturation (SpO2), invasive arterial pressure, electrocardiogram (ECG), bispectral index (BIS), and SctO2 were continuously monitored in all patients during the perioperative period. Two sensors of a cerebral oximeter were placed on the left and right sides of the forehead for continuous SctO2 monitoring until the end of administration of anesthesia. When SctO2 is a greater than 20% oxygen saturation reduction from baseline or an absolute value of less than 50%, the patient’s head position was checked, and the mean arterial pressure (MAP) was optimized. Surgeons and anesthesiologists were blinded to the patients’ group allocations and the measurement of SctO2 to exclude subjective bias.
All patients received intravenous anesthesia following the same induction protocol with 0.05–0.2 mg/kg midazolam, 0.3–0.6 μg/kg sufentanil, 0.3 mg/kg etomidate, and 0.2 mg/kg cisatracurium. Mechanical ventilation was initiated after endotracheal intubation, and the ventilator parameters were adjusted: inhaled oxygen concentration 60%, oxygen flow rate 1 L/min, respiratory rate 12–14 /min, tidal volume 8–10 mL/kg, inhalation and exhalation ratio 1:2, and maintenance of partial expiratory carbon dioxide pressure between 35 and 45 mmHg (1 mmHg = 0.133 kPa). Group P was administrated target-controlled infusion of propofol, and the plasma target-controlled concentration was 4 μg/ml. Group S was administered 1.3 MAC of sevoflurane by inhalation. In the PS group, propofol was injected with a target-controlled plasma concentration of 1.2 μg/ml, accompanied by sevoflurane inhalation of 0.7 MAC. Remifentanil (0.2–0.5 g·kg−1·min−1) was continuously administered to all patients, and cisatracurium was intermittently administered to maintain muscle relaxation. During the surgery, the anesthetic was adjusted according to the BIS value (controlling BIS 40–60) and hemodynamic parameters, and symptomatic treatment was used to maintain the vital signs within the normal range by administering vasoactive medications if necessary.
Physiologic variables including HR, MAP, SpO2, BIS, and SctO2 were recorded in the three groups. Data were analyzed at six time points: induction of anesthesia (T0), 10 min after anesthesia induction (T1), 20 min after anesthesia (T2), 30 min after the start of surgery (T3), one hour after the start of surgery (T4), at the end of surgery (T5), and before leaving the post-anesthesia care unit (PACU; T6). SctO2 was the average of left and right monitoring data. The duration of surgery, anesthesia, and PACU stay was recorded.
Blood samples were collected in ethylenediaminetetraacetic acid tubes 1 day preoperatively. The levels of amyloidβ-40 (Aβ-40), Aβ-42, total tau protein (T-tau), phosphorylated tau protein (P-tau), and triggering receptors on myeloid cells-2 (TREM2) were measured by commercial enzyme-linked immunosorbent assay (ELISA) kits in accordance with the manufacturer’s instructions (BioVendor, Brno, Czechia). The absorbance was measured using VICTOR Nivo (PerkinElmer, USA) at 420 nm wavelength. Each plasma sample was double-blinded, resulting in an average concentration of two measurements.
MoCA test was used to assess the cognitive function on the day before surgery and on the 7th postoperative day by a trained senior anesthesiologist who was blinded to the clinical information. MoCA test includes attention and concentration, executive function, memory, language, visual structure skills, abstract thinking, calculation, and orientation; it comprises a total of 11 items in eight cognitive fields, with a total score of 30, plus one point if the participant has 12 years or less of education.
The patients were grouped to calculate the standard deviation (SD) in the preoperative MoCA score, and the difference between the postoperative scores and preoperative scores was compared with the SD. If the score was reduced by ≥1 SD, it was considered that the patients had developed PND (He et al., 2019).
The incidence of PND in elderly patients with MCI (above 65 years old) has been reported as 33.3% in non-cardiac surgery on the 7th postoperative day (Tang et al., 2014). The appropriate depth of anesthesia can reduce the incidence of PND by 22% 7 days after surgery according to the results of the pre-experimental study. With the significance set at 0.05 and the power set at 90%, the sample size required to detect differences was 60 patients. Considering that the rate of loss to follow-up is about 10%–20%, a minimum sample size of 70 patients was assigned to each group for the randomized controlled study.
Statistical analyses were performed using IBM SPSS Statistic 21.0 (SPSS Inc., Chicago, IL). The normality of the continuous variables was tested with the Shapiro–Wilk test. Differences in the continuous variables were determined by the independent t-test or Mann-Whitney U test and expressed as mean ± standard deviation, or the median (interquartile range). Categorical variables were analyzed through the χ2 test or Fisher exact test. All reported P values are 2-tailed. A p < 0.05 was considered statistically significant, and all p-values were two-tailed. A single factor linear regression analysis was performed to evaluate the correlation between the biomarkers and MoCA score. The predictive value of SctO2 for PND was evaluated using the receiver operating characteristic (ROC) curve and the area under the curve (AUC).
The flow diagram is shown in Figure 1. A total of 257 patients were screened for eligibility, and 224 patients were ultimately enrolled and randomized. Five patients were excluded from data analysis for the following reasons: operation time not up to standard, and a secondary operation. Nine patients were lost to follow-up. Finally, 210 patients were randomly assigned to the three groups.
There were no statistically significant differences in gender, age, BMI, ASA classification, and years of education among the groups (Table 1). No differences in anesthesia duration, surgery duration, and PACU duration were found among the three groups. The incidence of PND in the P, S, and PS groups was 14% (10/70), 33% (23/70), and 7% (5/70), respectively, with statistically significant differences (χ2 = 16.643, p < 0.05).
Univariate linear regression analysis showed that Aβ-42 had the strongest significant correlation with preoperative MoCA score (R = 0.697, Table 2). The preoperative value of Aβ-42 predicted PND byROC curve analysis, and the critical cutoff value of Aβ-42 predictive of PND was 28.34 pg/ml. The predicted area under the PND curve was 0.711 (95% CI, 0.644–0.777), and the sensitivity and specificity were 0.599 and 0.947, respectively (Figure 2).
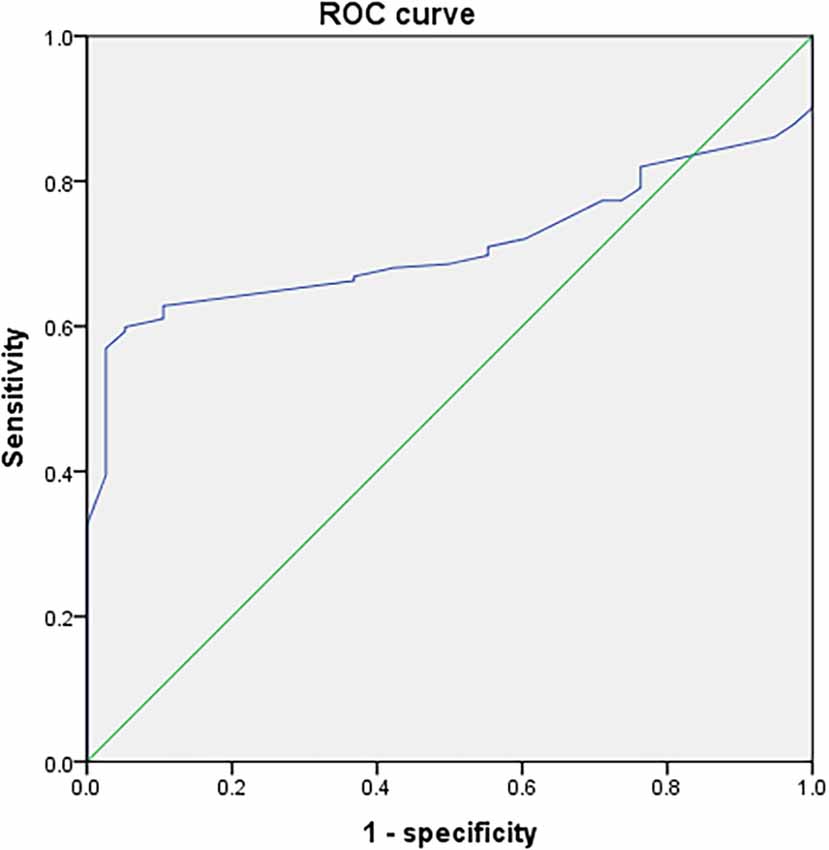
Figure 2. Receiver operating characteristic (ROC) curve analysis of preoperative measurement values of Aβ-42 for postoperative neurocognitive dysfunction (PND).
Based on the cognitive assessment, all patients with a preoperative Aβ-42 value below 28.34 pg/ml were divided into the PND and non-PND groups based on the MoCA score. The differences were found to be significant in age and education years between the group PND and group non-PND (p < 0.05). The duration of PACU stay of the group PND was longer than the group non-PND (p < 0.05), and the difference in surgery and anesthesia time between the groups was not statistically significant. There were no significant differences in SpO2, BIS, HR, and MAP between the two groups (Table 3).
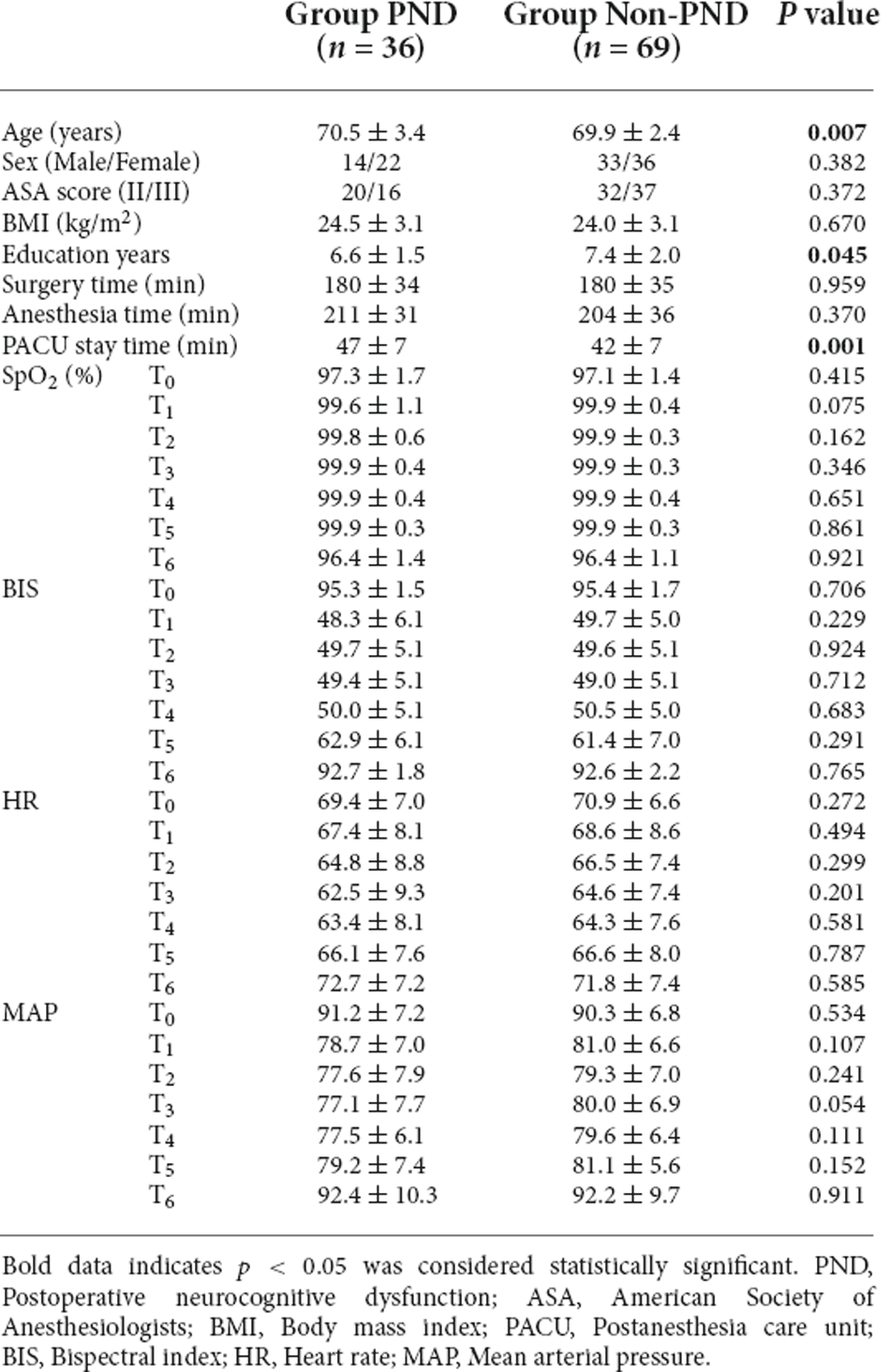
Table 3. Characteristics of the patients, surgery, and anesthesia in the group PND and group Non-PND.
SctO2 in the group PND was lower than that in the group non-PND at time points T3, T4, T5, and T6 (Figure 3). The percentage decrease of SctO2 at T3–6 compared with the baseline value is predictive of PND. When the preoperative value of Aβ-42 was less than the critical value of 28.34 pg/ml, the cutoff value of change in SctO2 was determined to be 9.92% according to AUC and Jordon index. The combination predicted AUC for PND was 0.872 (95% CI: 0.797–0.947), and the sensitivity and specificity were 0.833 and 0.841 respectively (Figure 4).
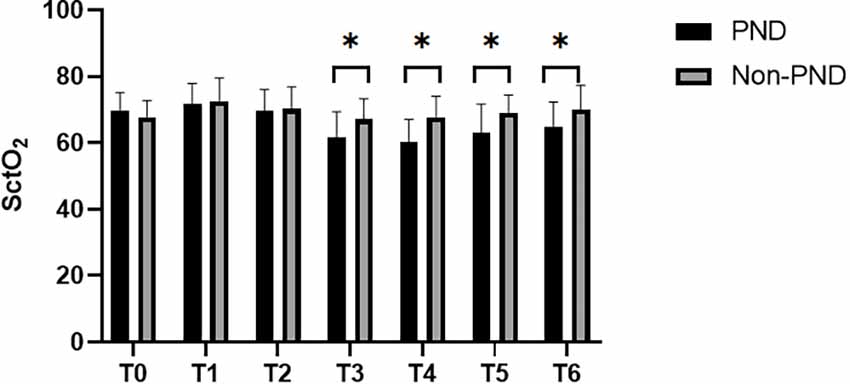
Figure 3. Comparison of SctO2 at different time points between group PND and group non-PND. *p < 0.05.
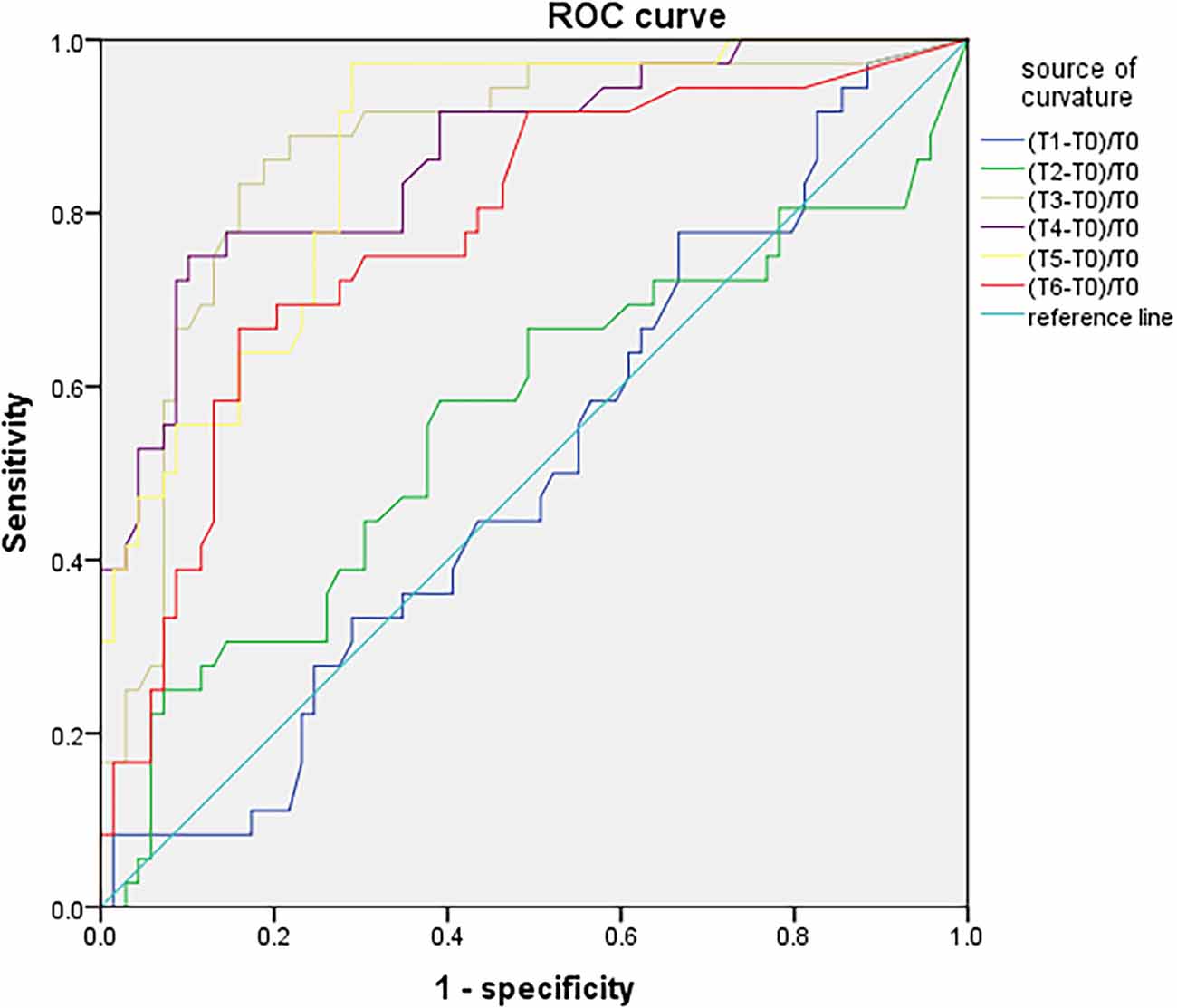
Figure 4. ROC curve analysis of PND predicted by the maximum percentage decline of SctO2 at each time point.
There was no significant difference in SctO2max% of male and female in the group PND and group non-PND (Table 4). When the preoperative Aβ-42 value was less than 28.34 pg/ml, the cutoff value of SctO2 change in male was 11.94% according to the AUC and Jordon index. The AUC of combined prediction of PND was 0.917 (95% CI: 0.833–1.000), and the sensitivity and specificity were 0.929 and 0.879, respectively (Figure 5). The cutoff value of SctO2 change in females was 6.79% according to the AUC and Jordon index. The AUC of the combined prediction of PND was 0.884 (95% CI: 0.797–0.970), and the sensitivity and specificity were 0.864 and 0.955, respectively (Figure 6).
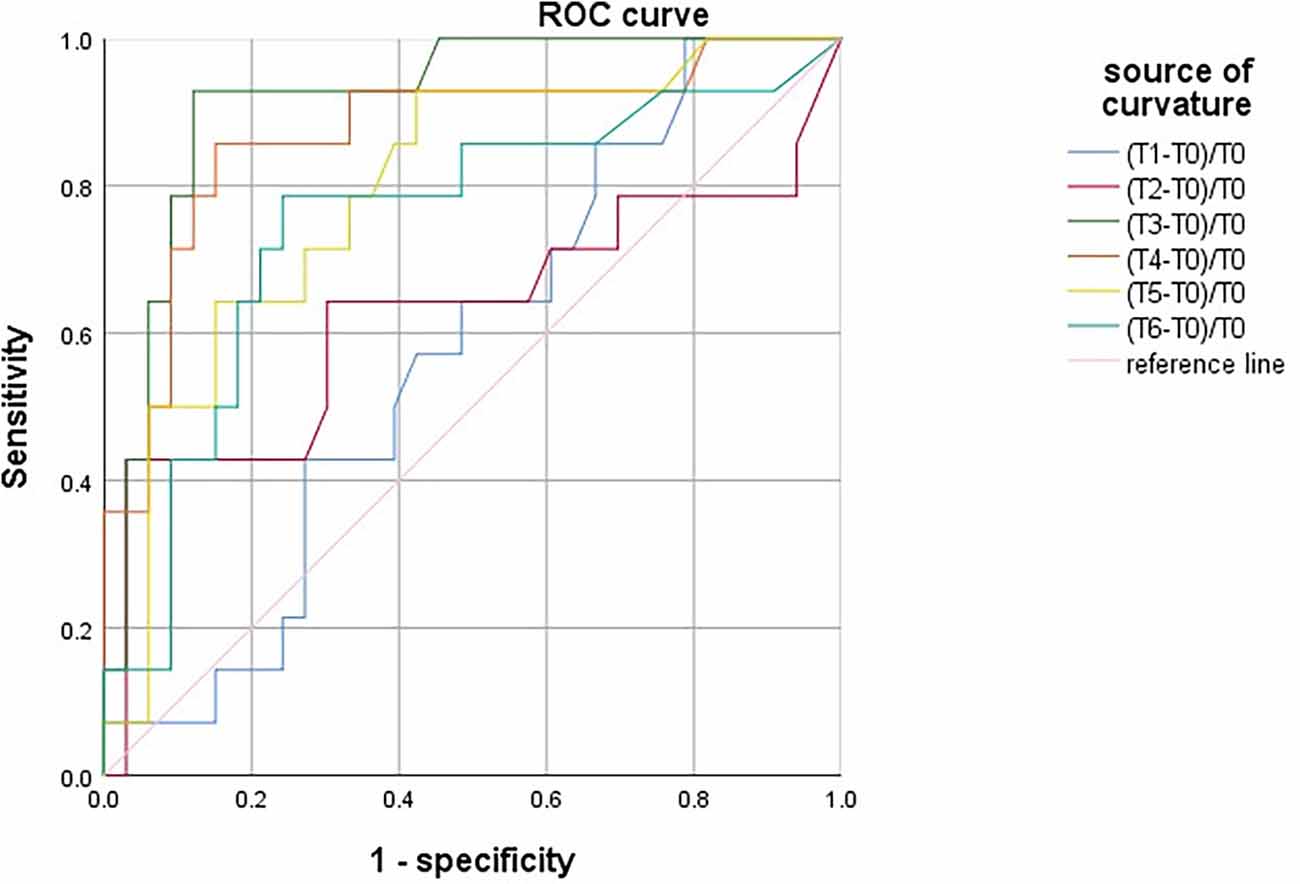
Figure 5. ROC curve analysis of PND predicted by the maximum percentage decline of SctO2 in males at each time point.
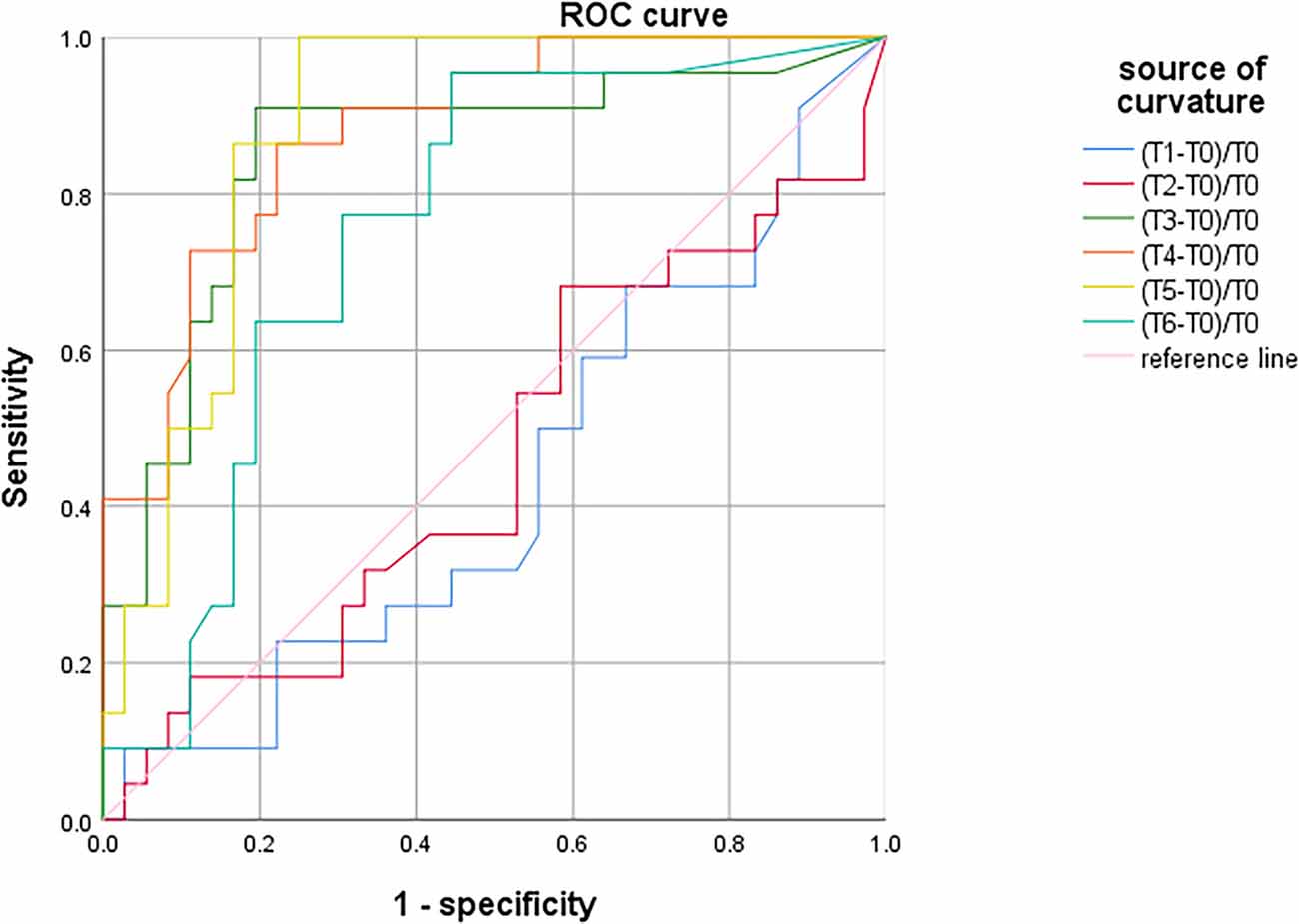
Figure 6. ROC curve analysis of PND predicted by the maximum percentage decline of SctO2 in females at each time point.
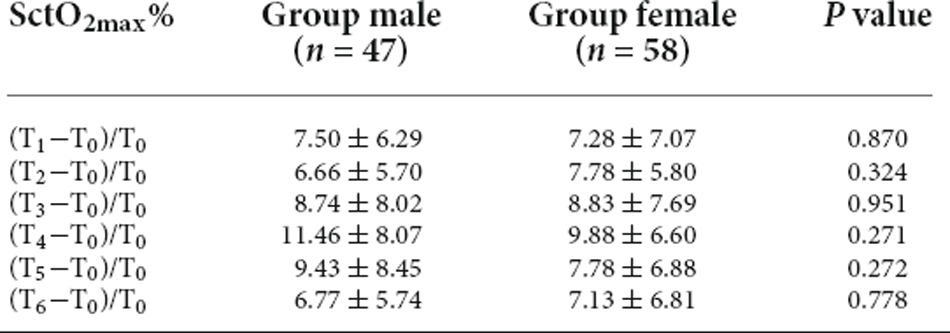
Table 4. Comparison of SctO2max% between males and females at different time points in the group PND and group Non-PND.
The essential finding of the prospective randomized study was that when preoperative Aβ-42 was less than 28.34 pg/ml, the intraoperative fluctuation in SctO2 not exceeding 9.92% could reduce the incidence of PND in elderly patients with MCI. Thus, we confirmed the predictive ability of preoperative plasma biomarkers and SctO2 for patients with MCI.
PND is a common complication of perioperative neurocognition in elderly patients undergoing major surgery. Spinal surgery is performed in the prone position accompany a variety of hemodynamic and respiratory alterations. Deiner and colleagues indicated that elderly spine surgery patients in the prone position were more than twice as likely to experience mild cerebral desaturation as patients in the supine position (Deiner et al., 2014). Notably, it is essential to monitor SctO2 in the prone spinal surgery. In our study, elderly patients undergoing spinal surgery were selected as subjects.
Sevoflurane and propofol are both commonly used anesthetics during general anesthesia. Anesthetics could theoretically also contribute to cognitive deficits by altering central cholinergic transmission through nicotinic and muscarinic receptors. The effective chamber concentration of propofol for general anesthesia is 4 μg/ml (Mahli et al., 2011), and the maintenance concentration of sevoflurane alone is 1.3 MAC (Hu et al., 2014). Studies have shown that sevoflurane anesthesia alone can aggravate PND in patients with MCI (Liu et al., 2013), while propofol with neuroprotection can improve postoperative cognitive function (Kalimefis et al., 2013). The use of compatible anesthetic drugs may reduce the dosage and the adverse reaction of a single drug. Hence, we divided the subjects into three groups for observation. Additionally, there is evidence suggesting that depth of anesthesia may be related to the risk of cognitive impairment (Radtke et al., 2013), and the intraoperative BIS value was maintained at 40–60 to eliminate the interference. In the study, the incidence of PND in the group PS significantly decreased compared with group S and group P. Thus, it is extremely necessary to optimize the anesthesia strategy for elderly patients with MCI.
The pathophysiology of PND is still unknown, although various pathophysiological mechanisms have been suggested in different situations. Preoperative cognitive impairment has been previously shown to negatively affect surgical outcomes. Adogwa found patients with preoperative cognitive impairment were at a greater than a two-fold risk of developing postoperative delirium (Adogwa et al., 2018). MCI, characterized by a transitional state on the continuum of cognitive function between normal aging and dementia, has been associated with biomarkers for AD. Identification of optimal-related biomarkers and development of monitoring strategies could enhance PND prevention and treatment practices.
Aβ has neuronal toxicity and induces neuronal apoptosis, which is a potential sign of nerve damage and persistent neuroinflammation (Wilczyńska and Waszkiewicz, 2020). Aβ-42, the fragment with 42 amino acid residues, is the main component of amyloid plaques found in the brain of patients with AD (Wilczyńska and Waszkiewicz, 2020). A second dominant isoform of Aβ is a peptide with a length of 40 amino acid residues. Neurofibrillary tangles (NFTs) formed by tau protein hyperphosphorylation and senile plaques formed by the accumulation of Aβ in the brain are the initial pathological changes in patients with AD. MCI, the early stage of AD, shows similar pathophysiological changes. Phosphorylated tau protein (P-tau) aggregates in the neurons to form NFTs, which eventually lead to neurodegeneration (Zhao et al., 2020). The elevated level of abnormal P-tau suggests the formation of NFTs in the brain parenchyma. Studies have shown that the level of P-tau protein in the CSF of patients with AD is significantly higher than that in the normal control group, and similar changes are found in patients with MCI (Ahmad et al., 2020; van Maurik et al., 2021). What’s more, high levels of plasma Aβ-42 and T-tau in MCI are associated with cognitive decline (Chen et al., 2019). Triggering receptors on myeloid cell-2 (TREM2) can stimulate the production of inflammatory cytokines during an immune response and chronic inflammation (Shi and Holtzman, 2018; Katsel and Haroutunian, 2019). Therefore, the study explored the association of these biomarkers with MCI preoperatively.
In the univariate linear regression analysis, Aβ-42 had the strongest significant correlation with the preoperative MoCA score. Studies have found a relationship between SctO2 and cerebral hypoxia-ischemia, resulting in cognitive decline (Mailhot et al., 2016). Optimizing SctO2 would potentially improve neurologic outcomes. In our study, patients were divided into the PND and non-PND groups based on the critical cutoff value of Aβ-42. SctO2 in the group PND was lower than that in the group non-PND at time points T3–6. This result was in line with that of a previous research (Colak et al., 2015), indicating that minimizing the SctO2 fluctuation to maintain normal cerebral oxygen supply might reduce the occurrence of PND. However, the scope of intraoperative SctO2 in elderly patients with MCI lacks guidance. This study enrolled geriatric patients with MCI, preoperative screening of the valuable markers and intraoperative dynamically monitoring SctO2 were used to determine the predictive value for PND. In this study, the maximum decrease of SctO2 was 9.92%, in the case of Aβ-42 less than 28.34 pg/ml. This finding provided guidance for the application of SctO2 to reduce PND in elderly patients with MCI.
The study demonstrated that patients in the group PND were older and had less education than the group non-PND. Consistent with previous studies, advanced age is a risk factor for PND (Scott et al., 2014), because of the decline in the central nervous system function reserve. Xu stated that the intraoperative SctO2 value could increase with a MAP elevation (Xu et al., 2020). In the study, there were no significant differences in MAP between the group PND and group non-PND. Thus, there are potential factors affecting SctO2.
With aging, the brain undergoes important structural and physiological changes. Cerebral blood flow significantly declines with age which is most prominent in the prefrontal and frontal cortex. A prospective study indicated that advanced age negatively influences baseline SctO2 which is otherwise influenced by sex, with women showing significantly lower values (Robu et al., 2020). Similar to previous reports, we found that males and females had similar rates of postoperative cognitive decline (Hogue et al., 2003). In addition, there was no significant difference in SctO2max% between males and females at different time points. Interestingly, we found that the cutoff value of change in SctO2 was different in gender. When the preoperative Aβ-42 value was less than 28.34 pg/ml, the cutoff value of SctO2 change was 11.94% and 6.79% in males and females respectively. These data suggest that, although the frequency of PND of geriatric patients with MCI is similar for males and females, females appear more likely to suffer injury to intraoperative change of SctO2. Gender differences in SctO2max% are important for the development and implementation of individualized therapeutic interventions to improve the prognosis of elderly with MCI.
MMSE scores are a widely used tool for the assessment of cognitive status in elderly subjects (Zhu et al., 2020). MoCA score is a comprehensive evaluation (including short-term memory, attention, language, time and space orientation, etc.) and can identify a wide range of changes in cognitive function with high sensitivity and specificity. Recent studies have shown that the MoCA score is the best assessment to identify MCI (Smith et al., 2007) and can sensitively identify PND (Tsoi et al., 2015). Therefore, the evaluation for PND was performed on the seventh day after surgery in this study by MoCA score.
There are limitations to the study. The sample size is relatively small, which can lead to random errors impacting the results. Due to individual and clinical scenarios differences in SctO2, a larger study is required to validate the relationship between the intraoperative thres hold of SctO2 with PND and determine the optimal SctO2 value that has a prognostically important relationship with PND. We only observed the cognitive function 7 days after surgery, and long-term rehabilitation was not monitored. It is recommended to evaluate all perioperative neurocognitive disorders until 12 months after surgery. The follow-up time should be extended to at least 1 year after surgery to verify less risk of group non-PND in conversion from mild cognitive impairment to AD than the group PND. Moreover, we measured the plasma levels of the biomarkers; however, the biomarkers in cerebrospinal fluid may be more representative. Nevertheless, the study has clinical implications for the prevention of PND.
In summary, the randomized controlled study showed that under the conditions of preoperative Aβ-42 less than 28.34 pg/ml, the intraoperative fluctuation range of cerebral oxygen saturation should be maintained within 9.92% to reduce the occurrence of PND in geriatric patients with MCI. Preoperative plasma biomarkers along with SctO2 is a novel predictive strategy for the occurrence of PND in elderly patients with MCI. Biomarkers closely correlated to MCI are a predictive factor for the rapid progression of dementia. It is necessary to strengthen the monitoring of SctO2 undergoing surgery, especially for the elderly with preoperative cognitive decline. At the same time, multicenter studies with larger samples are needed to further accurately confirm the safety range of SctO2 and its relationship with PND.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Institutional Human Research Ethics Committee of The Third Central Hospital of Tianjin. The patients/participants provided their written informed consent to participate in this study.
YL and XX equally contributed to recruitment and initial screening of participants, data collection, and the first draft of the manuscript. HoW was responsible for data acquisition, data interpretation, and blood parameters analysis. WH contributed to study design, and revising the manuscript. XW and YW contributed to statistical analysis and revising the manuscript. PL and TZ were in charge of perioperative management, data collection, and critical manuscript revision. HaW contributed to study design, supervision, critical manuscript revision, and final approval of the version to be published. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation of China (82071220), Natural Science Foundation of Tianjin (20JCYBJC01290), the Science and Technology Foundation of Tianjin Health Commission (MS20013), and Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-072C).
We would like to thank Qingkai Tang and Mingshu Zhao for their advice and support on the design and analysis of this project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
PND, postoperative neurocognitive dysfunction; MCI, mild cognitive impairment; MoCA, Montreal Cognitive Assessment; Aβ-40, amyloidβ-40; Aβ-42, amyloidβ-42; SctO2, cerebral oxygen saturation; AUC, the area under the curve; AD, Alzheimer’s disease; MAC, minimum alveolar concentration; POD, postoperative delirium; PACU, post-anesthesia care unit; MMSE, Mini-Mental State Examination; NFTs, neurofibrillary tangles; TREM2, Triggering receptors on myeloid cell-2; BMI, body mass index; ASA, American Society of Anesthesiologists’ physical; CDR, Clinical Dementia Rating; ADL, activities of daily living.
Adogwa, O., Elsamadicy, A. A., Vuong, V. D., Fialkoff, J., Cheng, J., Karikari, I. O., et al. (2018). Association between baseline cognitive impairment and postoperative delirium in elderly patients undergoing surgery for adult spinal deformity. J. Neurosurg. Spine 28, 103–108. doi: 10.3171/2017.5.SPINE161244
Ahmad, S., Orellana, A., Kohler, I., Frölich, L., and de Rojas, I. (2020). Association of lysophosphatidic acids with cerebrospinal fluid biomarkers and progression to Alzheimer’s disease. Alzheimers Res. Ther. 12:124. doi: 10.1186/s13195-020-00680-9
Bekker, A., Lee, C., de Santi, S., Pirraglia, E., Zaslavsky, A., Farber, S., et al. (2010). Does mild cognitive impairment increase the risk of developing postoperative cognitive dysfunction? Am. J. Surg. 199, 782–788. doi: 10.1016/j.amjsurg.2009.07.042
Casati, A., Fanelli, G., Pietropaoli, P., Proietti, R., Tufano, R., Danelli, G., et al. (2005). Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. Anesth. Analg. 101, 740–747. doi: 10.1213/01.ane.0000166974.96219.cd
Chen, T. B., Lee, Y. J., Lin, S. Y., Chen, J. P., Hu, C. J., Wang, P. N., et al. (2019). Plasma Aβ42 and total Tau predict cognitive decline in amnestic mild cognitive impairment. Sci. Rep. 9:13984. doi: 10.1038/s41598-019-50315-9
Colak, Z., Borojevic, M., Bogovic, A., Ivancan, V., Biocina, B., Majeric-Kogler, V., et al. (2015). Influence of intraoperative cerebral oximetry monitoring on neurocognitive function after coronary artery bypass surgery: a randomized, prospective study. Eur. J. Cardiothorac. Surg. 47, 447–454. doi: 10.1093/ejcts/ezu193
Deiner, S., Chu, I., Mahanian, M., Lin, H. M., Hecht, A. C., Silverstein, J. H., et al. (2014). Prone position is associated with mild cerebral oxygen desaturation in elderly surgical patients. PLoS One 9:e106387. doi: 10.1371/journal.pone.0106387
Evered, L. A., and Silbert, B. S. (2018). Postoperative cognitive dysfunction and noncardiac surgery. Anesth. Analg. 127, 496–505. doi: 10.1213/ANE.0000000000003514
He, X., Long, G., Quan, C., Zhang, B., Chen, J., Ouyang, W., et al. (2019). Insulin resistance predicts postoperative cognitive dysfunction in elderly gastrointestinal patients. Front. Aging Neurosci. 11:197. doi: 10.3389/fnagi.2019.00197
Hogue, C. W., Lillie, R., Hershey, T., Birge, S., Nassief, A. M., Thomas, B., et al. (2003). Gender influence on cognitive function after cardiac operation. Ann. Thorac. Surg. 76, 1119–1125. doi: 10.1016/s0003-4975(03)00817-8
Hu, N., Guo, D., Wang, H., Xie, K., Wang, C., Li, Y., et al. (2014). Involvement of the blood-brain barrier opening in cognitive decline in aged rats following orthopedic surgery and high concentration of sevoflurane inhalation. Brain Res. 1551, 13–24. doi: 10.1016/j.brainres.2014.01.015
Kalimefis, K., Kouni, S., Kostopanagiotou, G., Nomikos, T., Fragopoulou, E., Kakisis, J., et al. (2013). Cognitive function and oxidative stress after carotid endarterectomy: comparison of propofol to sevoflurane anesthesia. J. Cardiothorac. Vasc. Anesth. 27, 1246–1252. doi: 10.1053/j.jvca.2012.12.009
Katsel, P., and Haroutunian, V. (2019). Is Alzheimer disease a failure of mobilizing immune defense? lessons from cognitively fit oldest-old. Dialogues Clin. Neurosci. 21, 7–19. doi: 10.31887/DCNS.2019.21.1/vharoutunian
Kim, J., Shim, J. K., Song, J. W., Kim, E. K., and Kwak, Y. L. (2016). Postoperative cognitive dysfunction and the change of regional cerebral oxygen saturation in elderly patients undergoing spinal surgery. Anesth. Analg. 123, 436–444. doi: 10.1213/ANE.0000000000001352
Kline, R. P., Pirraglia, E., Cheng, H., De Santi, S., Li, Y., Haile, M., et al. (2012). Surgery and brain atrophy in cognitively normal elderly subjects and subjects diagnosed with mild cognitive impairment. Anesthesiology 116, 603–612. doi: 10.1097/ALN.0b013e318246ec0b
Lei, L., Katznelson, R., Fedorko, L., Carroll, J., Poonawala, H., Machina, M., et al. (2007). Cerebral oximetry and postoperative delirium after cardiac surgery: a randomised, controlled trial. Anaesthesia 72, 1456–1466. doi: 10.1111/anae.14056
Lin, X., Chen, Y., Zhang, P., Chen, G., Zhou, Y., Yu, X., et al. (2020). The potential mechanism of postoperative cognitive dysfunction in older people. Exp. Gerontol. 130:110791. doi: 10.1016/j.exger.2019.110791
Liu, Y., Pan, N., Ma, Y., Zhang, S., Guo, W., Li, H., et al. (2013). Inhaled sevoflurane may promote progression of amnestic mild cognitive impairment: a prospective, randomized parallel-group study. Am. J. Med. Sci. 345, 355–360. doi: 10.1097/MAJ.0b013e31825a674d
Mahli, A., Coskun, D., Karaca, G. I., Akcali, D. T., Karabiyik, L., Karadenizli, Y., et al. (2011). Target-controlled infusion of remifentanil with propofol or desflurane under bispectral index guidance: quality of anesthesia and recovery profile. J. Res. Med. Sci. 16, 611–620.
Mailhot, T., Cossette, S., Lambert, J., Cournoyer, A., and Denault, A. Y. (2016). Cerebral oximetry as a biomarker of postoperative delirium in cardiac surgery patients. J. Crit. Care 34, 17–23. doi: 10.1016/j.jcrc.2016.02.024
Miniksar, Ö. H., Çiçekçioglu, F., Kılıç, M., Honca, M., Miniksar, D. Y., Cocmen, A. Y., et al. (2021). Decreased brain-derived neurotrophic factor levels may predict early perioperative neurocognitive disorder in patients undergoing coronary artery bypass surgery. J. Clin. Anesth. 71:110235. doi: 10.1016/j.jclinane.2021.110235
Ng, T. K. S., Tagawa, A., Ho, R. C., Larbi, A., Kua, E. H., Mahendran, R., et al. (2021). Commonalities in biomarkers and phenotypes between mild cognitive impairment and cerebral palsy: a pilot exploratory study. Aging (Albany NY) 13, 1773–1816. doi: 10.18632/aging.202563
Ni, C., Xu, T., Li, N., Tian, Y., Han, Y., Xue, Q., et al. (2015). Cerebral oxygen saturation after multiple perioperative influential factors predicts the occurrence of postoperative cognitive dysfunction. BMC Anesthesiol. 15:156. doi: 10.1186/s12871-015-0117-6
Racine, A. M., Fong, T. G., Gou, Y., Travison, T. G., Tommet, D., Erickson, K., et al. (2018). Clinical outcomes in older surgical patients with mild cognitive impairment. Alzheimers Dement. 14, 590–600. doi: 10.1016/j.jalz.2017.10.010
Radtke, F. M., Franck, M., Lendner, J., Krüger, S., Wernecke, K. D., Spies, C. D., et al. (2013). Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br. J. Anaesth. 110, i98–i105. doi: 10.1093/bja/aet055
Robu, C. B., Koninckx, A., Docquier, M. A., Grosu, I., De Kerchove, L., Mastrobuoni, S., et al. (2020). Advanced age and sex influence baseline regional cerebral oxygen saturation as measured by near-infrared spectroscopy: subanalysis of a prospective study. J. Cardiothorac. Vasc. Anesth. 34, 3282–3289. doi: 10.1053/j.jvca.2020.06.025
Scott, D. A., Evered, L. A., and Silbert, B. S. (2014). Cardiac surgery, the brain and inflammation. J. Extra Corpor. Technol. 46, 15–22.
Shi, Y., and Holtzman, D. M. (2018). Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat. Rev. Immunol. 18, 759–772. doi: 10.1038/s41577-018-0051-1
Skolariki, K., Terrera, G. M., and Danso, S. O. (2021). Predictive models for mild cognitive impairment to Alzheimer’s disease conversion. Neural Regen. Res. 16, 1766–1767. doi: 10.4103/1673-5374.306071
Smith, T., Gildeh, N., and Holmes, C. (2007). The Montreal cognitive assessment: validity and utility in a memory clinic setting. Can. J. Psychiatry 52, 329–332. doi: 10.1177/070674370705200508
Sprung, J., Roberts, R. O., Weingarten, T. N., Nunes Cavalcante, A., Knopman, D. S., Petersen, R. C., et al. (2017). Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br. J. Anaesth. 119, 316–323. doi: 10.1093/bja/aex130
Tang, N., Ou, C., Liu, Y., Zuo, Y., and Bai, Y. (2014). Effect of inhalational anaesthetic on postoperative cognitive dysfunction following radical rectal resection in elderly patients with mild cognitive impairment. J. Int. Med. Res. 42, 1252–1261. doi: 10.1177/0300060514549781
Tsoi, K. K., Chan, J. Y., Hirai, H. W., Wong, S. Y., and Kwok, T. C. (2015). Cognitive tests to detect dementia: a systematic review and meta-analysis. JAMA Intern. Med. 175, 1450–1458. doi: 10.1001/jamainternmed.2015.2152
van Maurik, I. S., Rhodius-Meester, H. F. M., Teunissen, C. E., Scheltens, P., Barkhof, F., Palmqvist, S., et al. (2021). Biomarker testing in MCI patients-deciding who to test. Alzheimers Res. Ther. 13:14. doi: 10.1186/s13195-020-00763-7
Wilczyńska, K., and Waszkiewicz, N. (2020). Diagnostic utility of selected serum dementia biomarkers: Amyloid β-40, Amyloid β-42, Tau protein and YKL-40. J. Clin. Med. 9:3452. doi: 10.3390/jcm9113452
Xin, X., Chen, J., Hua, W., and Wang, H. Y. (2021). Intraoperative dexmedetomidine for prevention of postoperative delirium in elderly patients with mild cognitive impairment. Int. J. Geriatr. Psychiatry 36, 143–151. doi: 10.1002/gps.5406
Xu, X. M., Hu, X. W., Wu, Y., Li, Y., Zhang, Y., Zhang, M. C., et al. (2020). Effects of different BP management strategies on postoperative delirium in elderly patients undergoing hip replacement: a single center randomized controlled trial. J. Clin. Anesth. 62:109730. doi: 10.1016/j.jclinane.2020.109730
Zhao, A., Li, Y., and Deng, Y. (2020). TNF receptors are associated with tau pathology and conversion to Alzheimer’s dementia in subjects with mild cognitive impairment. Neurosci. Lett. 738:135392. doi: 10.1016/j.neulet.2020.135392
Keywords: elderly, mild cognitive impairment, postoperative neurocognitive dysfunction, cerebral oxygen saturation, plasma amyloid β-42
Citation: Liang Y, Xin X, Wang H, Hua W, Wu Y, Wang X, Li P, Zhou T and Wang H (2022) A novel predictive strategy for the incidence of postoperative neurocognitive dysfunction in elderly patients with mild cognitive impairment. Front. Aging Neurosci. 14:985406. doi: 10.3389/fnagi.2022.985406
Received: 03 July 2022; Accepted: 12 September 2022;
Published: 29 September 2022
Edited by:
Valeria Manera, Université Côte d’Azur, FranceReviewed by:
Livia Stocco Sanches Valentin, University of São Paulo, BrazilCopyright © 2022 Liang, Xin, Wang, Hua, Wu, Wang, Li, Zhou and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyun Wang, d2h5ODE5QDEyNi5jb20=
† These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.