- 1Department of Neurology, Nanjing Yuhua Hospital, Yuhua Branch of Nanjing First Hospital, Nanjing, China
- 2Department of Radiology, Nanjing Maternity and Child Health Care Hospital, Women’s Hospital of Nanjing Medical University, Nanjing, China
- 3Department of Radiology, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
Purpose: Previous research has found that women with second pregnancy may have an increased risk of cognitive dysfunction. This study aims to investigate the intrinsic functional connectivity (FC) pattern of the DMN anchored on posterior cingulate cortex (PCC) in postpartum women, especially the parous women using resting-state functional magnetic resonance imaging (rs-fMRI).
Methods: Twenty parous women, 26 primiparous women, and 30 nulliparous women were included for rs-fMRI scan. They were age and education well matched. A seed based FC method was conducted to reveal FC patterns with other brain regions using a region of interest in the PCC. The relationships between FC patterns and cognitive performance were further detected.
Results: Relative to primiparous women, parous women had significantly decreased FC primarily between the PCC and the right middle frontal gyrus and right parahippocampal gyrus. The decreased FC to the right parahippocampal gyrus in parous women was positively associated with the reduced DST scores (rho = 0.524, p = 0.031). Moreover, parous women compared with nulliparous women showed significantly decreased FC between the PCC and the left superior frontal gyrus and left middle frontal gyrus. The reduced FC to the left superior frontal gyrus in parous women was also positively associated with the lower DST scores (rho = 0.550, p = 0.022).
Conclusion: Our result highlights that women with second pregnancy revealed decreased FC between the DMN regions with the parahippocampal gyrus and prefrontal cortex, which was correlated with specific impaired cognitive function. This study may provide new insights into the neuropathological mechanisms of postpartum cognitive impairment and enhance our understanding of the neurobiological aspects during postpartum period.
Introduction
The universal two-child policy has been conducted in China for a long time since October, 2015 (Zeng and Hesketh, 2016). Women with second pregnancy may face multiple complex psychological challenges owing to the abrupt policy changes (Priya et al., 2018). In addition to postpartum depression, anxiety or other mental disorders, women may show an increased risk of cognitive dysfunction, which primarily manifests as poor memory, forgetfulness, difficulty concentrating, and distractibility (Christensen et al., 2010; Postma et al., 2014; Albin-Brooks et al., 2017). Prior studies have found that postpartum women show cognitive impairment that may occur prior to affective disorder (Christensen et al., 2010; Postma et al., 2014; Meena et al., 2016). However, the etiology of the neurophysiological mechanism of this increased risk remains still unclear.
Multimodal neuroimaging techniques have been used to investigate the brain structural and functional alterations in primiparous women, which are linked with cognitive dysfunction (Kim et al., 2010; Postma et al., 2014; Hoekzema et al., 2017; Zheng et al., 2018, 2020). Hoekzema et al. (2017) confirmed that primiparous women exhibited a symmetrical pattern of extensive gray matter (GM) reductions throughout pregnancy, mainly affecting the bilateral lateral prefrontal and temporal cortex. Nonetheless, no study is reported regarding the brain network alterations in the central nervous system of women with second pregnancy.
Using resting-state functional magnetic resonance imaging (rs-fMRI), low frequency fluctuations (<0.01 Hz) in blood oxygenation level-dependent (BOLD) signal during rest reflect spontaneous neural activity can be conceptualized as a network of anatomically linked regions (Mantini et al., 2007; Lee et al., 2013). Previous studies have used rs-fMRI to examine the abnormal brain activity and network in postpartum depression (Xiao-Juan et al., 2011; Chase et al., 2013; Deligiannidis et al., 2013; Fisher et al., 2016; Zhang et al., 2020). Furthermore, the default mode network (DMN) shows consistently higher BOLD activity during rest in several brain regions, such as the posterior cingulate cortex (PCC), medial prefrontal cortex (mPFC), and anterior cingulate cortex (ACC) (Greicius et al., 2003). The DMN acts a pivotal role in various cognitive functions, including memory, prospection, and self-processing (Spreng and Grady, 2010). Among these regions, the PCC is a key region with strong connectivity with the hippocampal memory system, which is involved in autobiographical memory, and imagining the future as well as spatial navigation (Leech and Sharp, 2014). During cognitive processing, the PCC is functionally linked to the DMN regions, such as the mPFC (Fransson and Marrelec, 2008; Li et al., 2020). Several studies have reported reduced resting-state functional connectivity (FC) in DMN regions involved in social cognition in postpartum women (Xiao-Juan et al., 2011; Chase et al., 2013; Zheng et al., 2020). Moreover, aberrant brain activity of the PCC in postpartum women has also been detected in prior studies (Zheng et al., 2018; Bak and Nah, 2021). However, how DMN dysfunction draws a relation to cognitive symptoms in parous women compared with primiparous women still remains unknown.
To address this problem, this study attempted to explore and analyze the resting-state FC analysis of the PCC in the brain between postpartum women and nulliparous women. We determine whether parous women exhibited aberrant FC pattern of the DMN using a seed-based approach. Moreover, the relationships between abnormal DMN connectivity and cognitive dysfunction were further investigated in parous women compared with primiparous women. We assumed that the parous women might show disrupted brain FC that was associated with cognitive dysfunction compared with primiparous women or nulliparous women. Our findings might provide new insights into neurophysiological mechanisms of cognitive impairment in women with second pregnancy.
Materials and methods
Participants
A total of 76 women (aged between 20 and 40 years, right-handed, ≥9 years of education) made up of 20 parous women, 26 primiparous women, and 30 nulliparous women were included through community health screening, which were age and education-matched. No subject was subsequently excluded because of the exceeded limits for head motion during scanning. All the parous and primiparous women were medication free and had delivered a healthy and full-term infant in the preceding 3 months. None of the women experienced any complications during pregnancy or delivery, such as hypertension, heart disease, eclampsia, diabetes, or postpartum hemorrhage. This study was approved by the Research Ethics Committee of the Nanjing Medical University. All the subjects provided written informed consent before participation.
Subjects were excluded if they suffered from severe smoking, alcoholism, Alzheimer’s disease, Parkinson’s disease, stroke, brain trauma, major depression, epilepsy, and MRI contraindications. None of the women had postnatal depression according to the Edinburgh Postnatal Depression Scale (EPDS, overall scores <12) (Cox et al., 1987).
Blood samples were collected by venepuncture at 8 A.M. to assess the levels of fasting plasma glucose (FPG), triglycerides, total cholesterol, low density lipoprotein (LDL)-cholesterol, and high-density lipoprotein (HDL)-cholesterol, in order to exclude the hyperglycemia and hyperlipidemia at the time of examination.
The neuropsychological status of the participants was established using the Mini Mental State Exam (MMSE), Montreal Cognitive Assessment (MoCA), Complex Figure Test (CFT), Clock-Drawing Test (CDT), Verbal Fluency Test (VFT), Digit Span Test (DST), Auditory Verbal Learning Test (AVLT), Trail-Making Test (TMT) A and B, Digit Symbol Substitution Test (DSST), Self-Rating Depression Scale (SDS), and Self-Rating Anxiety Scale (SAS).
Magnetic resonance imaging acquisition
Magnetic resonance imaging data were acquired using a 3.0 Tesla MRI scanner (Ingenia, Philips Medical Systems, Netherlands) with an 8-channel receiver array head coil. During scanning, the subjects were supposed to lie quietly with their eyes closed and avoid head movement, but not to fall asleep or think about anything special. Structural images were obtained using a three-dimensional turbo fast echo (3D-TFE) T1WI sequence as follows: repetition time (TR) = 8.1 ms; echo time (TE) = 3.7 ms; slices = 170; thickness = 1 mm; gap = 0 mm; flip angle (FA) = 8°; acquisition matrix = 256 × 256; field of view (FOV) = 256 mm × 256 mm. Fluid-attenuated inversion recovery (FLAIR) were also acquired: TR = 7000 ms; TE = 120 ms; slices = 18; thickness = 6 mm; gap = 1.3 mm; FA = 110°; and voxel size = 0.65 mm × 0.95 mm × 6 mm. Functional images were acquired using a gradient echo-planar imaging (EPI) sequence as follows: TR = 2000 ms; TE = 30 ms; slices = 36; thickness = 4 mm; gap = 0 mm; FOV = 240 mm × 240 mm; acquisition matrix = 64 × 64; and FA = 90°.
Functional data analysis
Functional magnetic resonance imaging data preprocessing was performed using Data Processing and Analysis for (Resting-State) Brain Imaging (DPABI_V6.1_220101)1 with the following procedures. Removed the first ten volumes function images, slice timing effects and head motion corrected. The remaining dataset was spatially normalized to the Montreal Neurological Institute (MNI) 152 space (resampling voxel size = 3 mm × 3 mm × 3 mm). Moreover, smoothing with an 8-mm full width at half-maximum Gaussian kernel, detrending and filtering (0.01–0.08 Hz) were performed.
The seed regions of interest (ROI) of the PCC were generated from Brodmann template using the WFU_PickAtlas software (Maldjian et al., 2003). For seed based FC analysis, correlation analysis of time course was performed between the spherical seed region (PCC) and each voxel of the whole brain for each subject using DPABI software. Six head motion parameters and mean time courses of global, WM and CSF signals were included in the regression analysis.
Structural analysis
Structural images were processed using the VBM toolbox in SPM12.2 Briefly, the structural images were normalized and segmented into GM, white matter (WM), and cerebrospinal fluid (CSF) in SPM12. The GM, WM, and brain parenchyma volume were divided by the total intracranial volumes. T1 images were normalized to the MNI template using affine linear registration followed by Gaussian smoothing (FWHM = 8 mm).
Statistical analysis
Differences in demographic information and clinical measures were analyzed using one-way analysis of variance (ANOVA) among the three groups followed by a post-hoc test (t-test for means and χ2-test for proportions). The SPSS software (version 25.0, Chicago, IL, United States) was used for statistical analyses and p < 0.05 was considered significant.
To investigate the between-group FC differences, post-hoc analysis was further conducted by one-way ANOVA. Significant thresholds were corrected using false discovery rate (FDR) criterion and set at p < 0.01. Age, education, and GM volume were used as nuisance covariates to adjust for the effect of these factors. To identify the relationship between aberrant FC and cognitive variables, the mean z-values of each brain region that showed significant group differences were calculated within every subject. Spearman correlation analysis between the mean z-values and each variable were performed using SPSS software. The statistical threshold was set at p < 0.05. Partial correlations were performed after correction for age, education, and GM volume. Bonferroni correction was applied for multiple comparisons in the correlation analyses.
Results
Clinical data analysis
The demographic characteristics and neuropsychological results of the parous women, primiparous women and nulliparous women were shown in Table 1. The three groups did not significantly differ in terms of age, education, FPG, blood lipids and WM hyperintensity (all p > 0.05). The parous women had significantly poorer DST scores than the primiparous women (p < 0.05). The other neuropsychological tests exhibited no significant differences among parous women, primiparous women and nulliparous women.
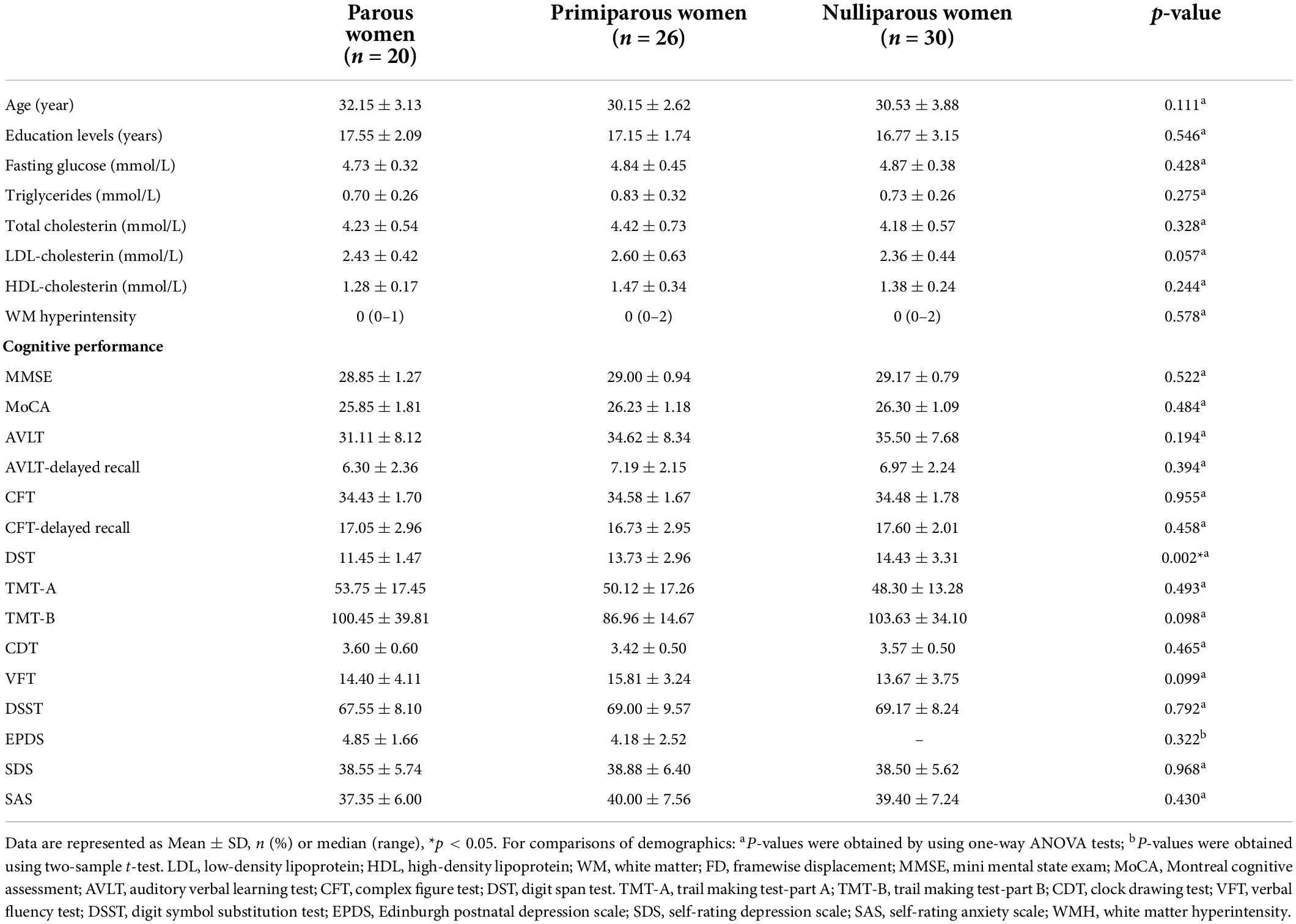
Table 1. Demographics and neurocognitive characteristics of the parous, primiparous, and nulliparous women.
Structural data analysis
Table 2 presents the comparisons of the whole-brain volumes (GM volume, WM volume, and brain parenchyma volume) among parous women, primiparous women and nulliparous women. The results showed that there were no significant changes in GM and WM volumes among the three groups (p > 0.05).
Functional data analysis
Parous women vs. primiparous women
Compared with primiparous women, parous women showed significantly decreased FC between the PCC and the right middle frontal gyrus and right parahippocampal gyrus (Figure 1 and Table 3, p < 0.01, FDR corrected).
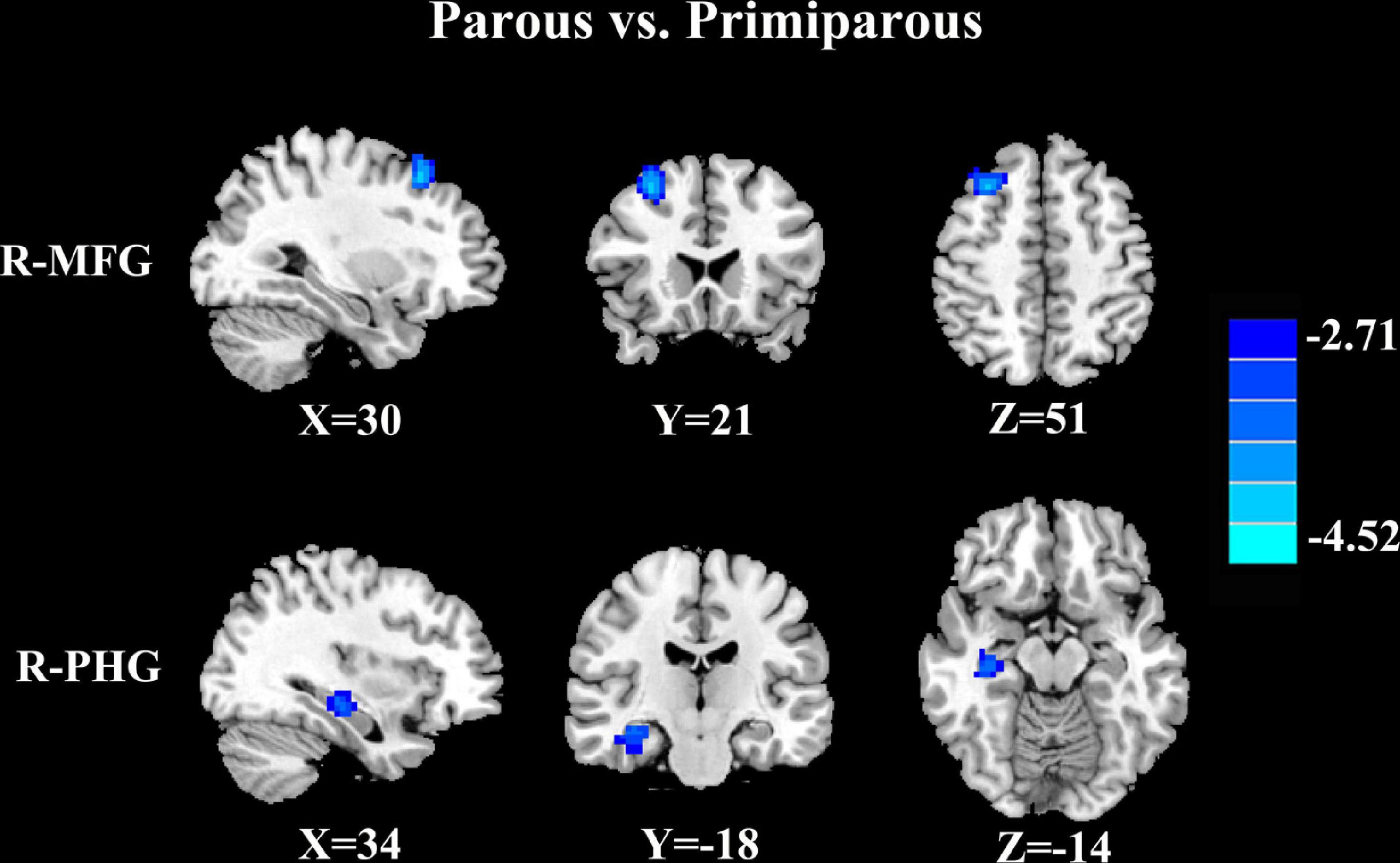
Figure 1. Compared with primiparous women, parous women showed significantly decreased FC between the PCC and the right middle frontal gyrus (R-MFG) and right parahippocampal gyrus (R-PHG) (p < 0.01, FDR corrected).
Parous women vs. nulliparous women
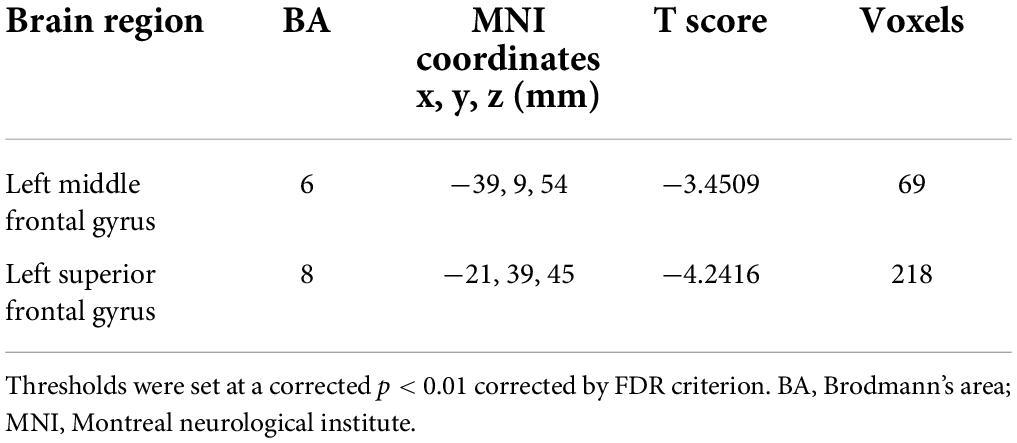
Parous women compared with nulliparous women exhibited significantly reduced FC between the PCC and the left superior frontal gyrus and left middle frontal gyrus (Figure 2 and Table 4, p < 0.01, FDR corrected).
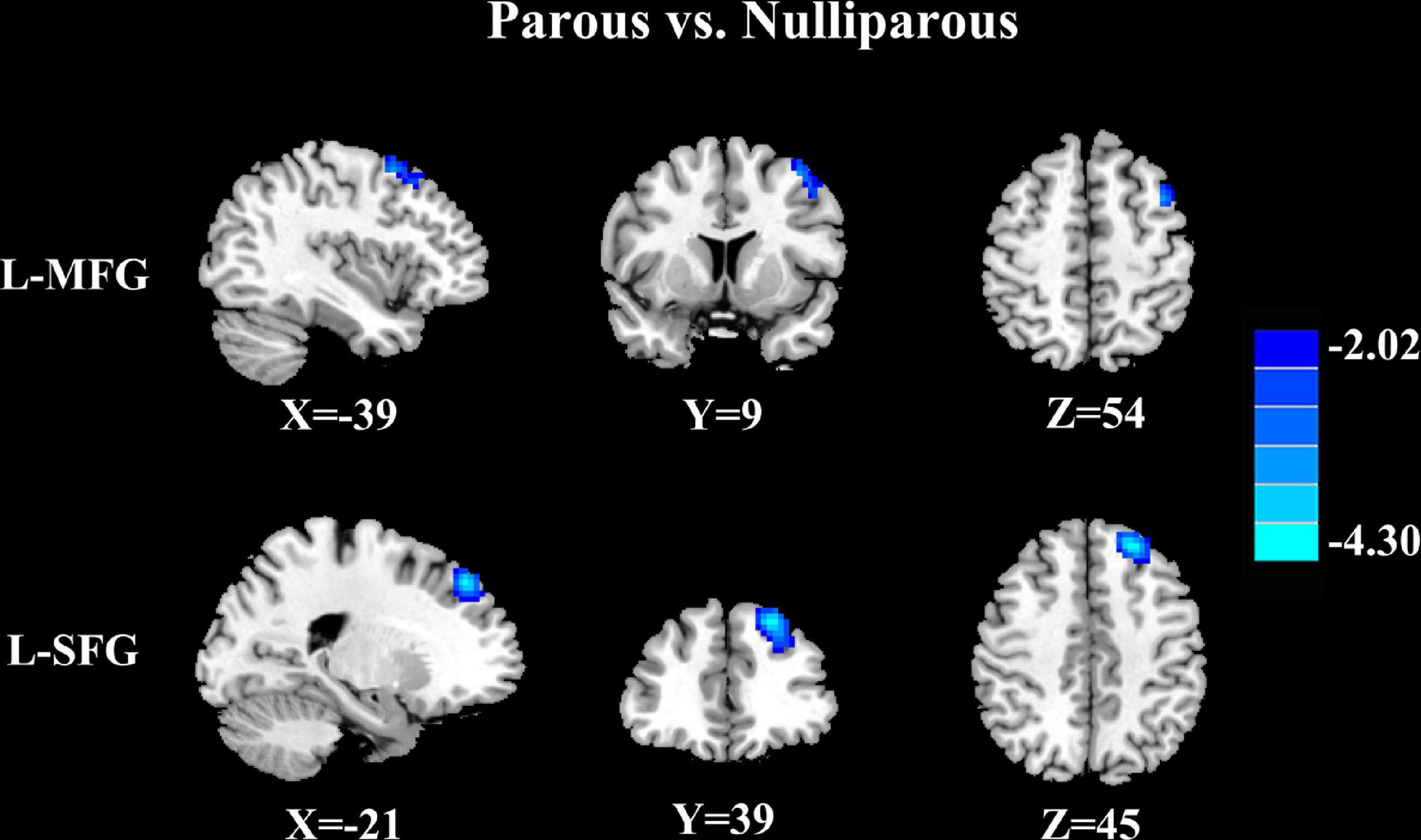
Figure 2. Parous women compared with nulliparous women exhibited significantly reduced FC between the PCC and the left superior frontal gyrus (L-SFG) and left middle frontal gyrus (L-MFG) (p < 0.01, FDR corrected).
Primiparous women vs. nulliparous women
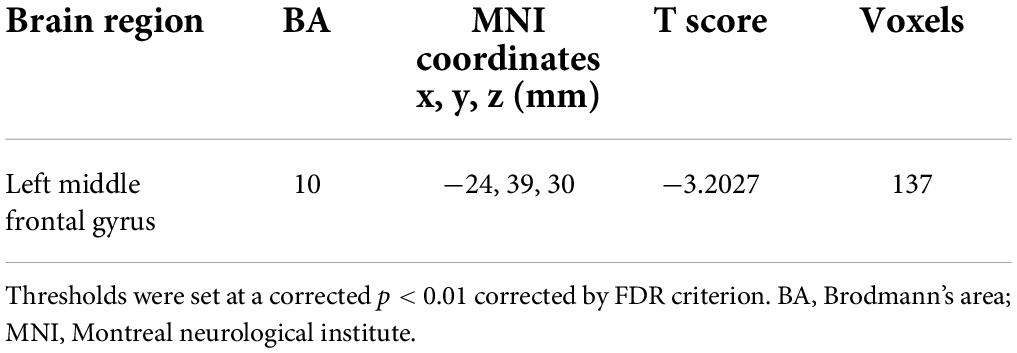
Relative to nulliparous women, primiparous women revealed a significant reduction in FC between the left middle frontal gyrus (Figure 3 and Table 5, p < 0.01, FDR corrected).
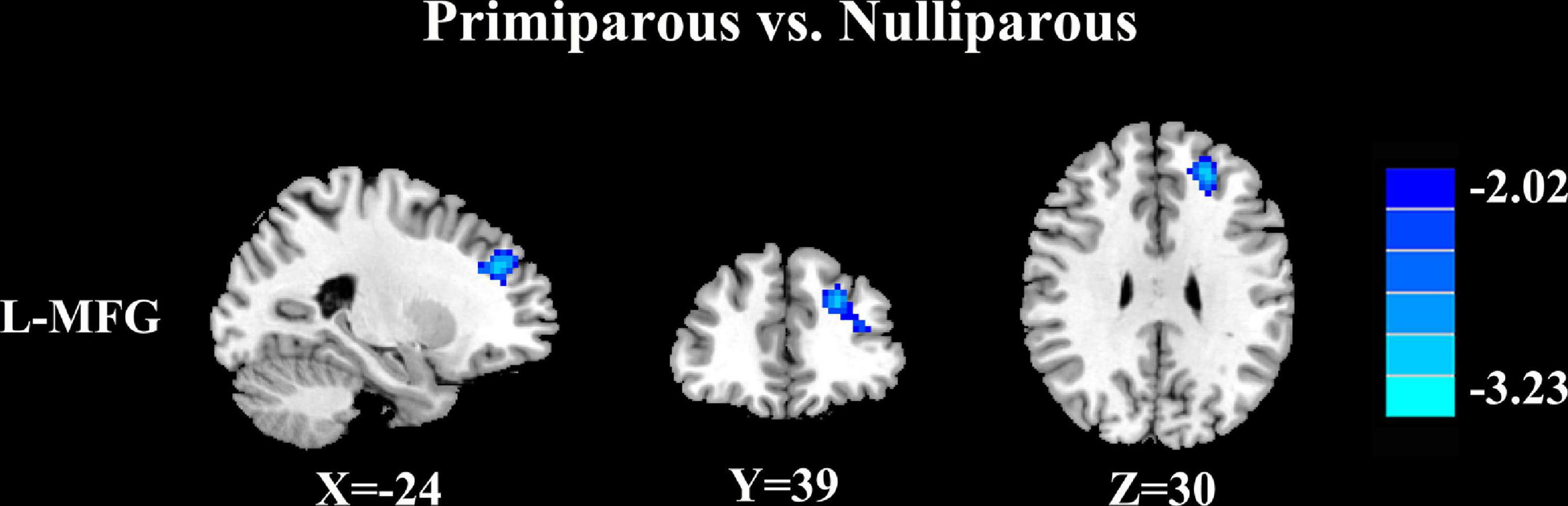
Figure 3. Relative to nulliparous women, primiparous women revealed a significant reduction in FC between the left middle frontal gyrus (L-MFG) (p < 0.01, FDR corrected).
Correlation results
Compared with primiparous women, the decreased FC of the PCC to the right parahippocampal gyrus in parous women was positively associated with the reduced DST scores (rho = 0.524, p = 0.031) (Figure 4A). Moreover, compared with nulliparous women, the decreased FC of the PCC to the left superior frontal gyrus in parous women was also positively associated with the lower DST scores (rho = 0.550, p = 0.022) (Figure 4B). These correlations were corrected for age, education, and GM volume. None of the other decreased FC was correlated with other cognitive performances.
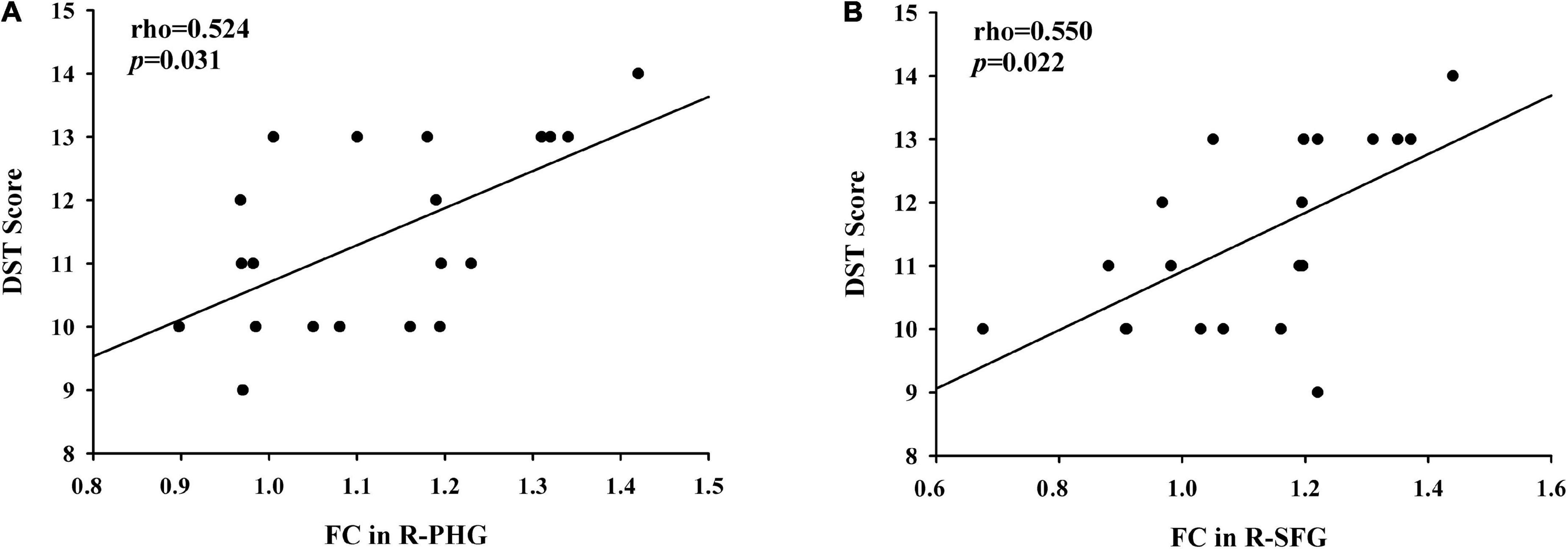
Figure 4. (A) Compared with primiparous women, the decreased FC of the PCC to the right parahippocampal gyrus in parous women was positively associated with the reduced DST scores (rho = 0.524, p = 0.031); (B) compared with nulliparous women, the decreased FC of the PCC to the left superior frontal gyrus in parous women was positively associated with the lower DST scores (rho = 0.550, p = 0.022).
Discussion
As the central region of the DMN, the PCC performs diverse cognitive functions including visuospatial memory and processing of emotional and non-emotional information (Vogt et al., 2006; Leech and Sharp, 2013; Sestieri et al., 2017). Moreover, the DST score was used to assess the immediate memory (Hale et al., 2002). Therefore, the correlation between decreased PCC activity and impaired DST scores may indicate the decline of the short-term memory in postpartum women. The PCC is also recognized for its role in self-referential processing and social cognition (Mars et al., 2012). Alterations in endogenous sex steroid hormone levels during the postpartum period may result in widespread neural changes, including in the PCC (Fisher et al., 2016). Furthermore, our previous study had mainly demonstrated decreased resting-state FC patterns within the DMN regions, especially the PCC, which were associated with impaired cognitive function in primiparous women without depression (Zheng et al., 2020). Therefore, our findings indicate that reduced PCC connectivity pattern within the DMN may be responsible for the impaired short-term memory in parous women, which is different from that in primiparous women.
Furthermore, reduced FC of the PCC to the right parahippocampal gyrus in parous women was positively associated with the impairment DST scores. The parahippocampal area has been hypothesized to play a critical role in memory recollection and transferring information from the hippocampus to the association areas (Diederen et al., 2010). Women with postpartum psychosis showed smaller gray matter volume and decreased surface area in parahippocampal gyrus compared to women without postpartum psychosis (Fusté et al., 2017). Sharma et al. (2021) demonstrated disrupted FC between the posterior DMN and parahippocampal gyrus in older adults with subjective cognitive decline and correlates with subjective memory ability. Our results suggested that the FC of the PCC to the parahippocampal gyrus is another connection route associated with short-term memory. Since PCC–parahippocampal cortical connections provide access to autobiographical and other memories involved in self/other-relevant thought, women with second pregnancy may be less apt to access these cognitive functions.
The prefrontal cortex, especially the superior and middle frontal cortex, is mainly responsible for executive function (Yuan and Raz, 2014). We found decreased FC between PCC and prefrontal cortex were linked to impaired DST performance in parous women, which may indicate the dysfunction of executive abilities. Prior studies have detected disrupted executive function as one of the main cognitive dysfunction in postpartum women (Anderson and Rutherford, 2012; Meena et al., 2016). A fMRI study showed that the prefrontal brain activity during a response inhibition task was decreased throughout the first postpartum weeks in healthy women (Bannbers et al., 2013). Our combined ALFF and ReHo analyses revealed reduced neuronal activity, mainly in the PCC and prefrontal cortex, which was associated with executive dysfunction in primiparous women (Zheng et al., 2018). Furthermore, compared with the nulliparous women, postpartum women had a significantly decreased FC between the PCC and the left mPFC (Zheng et al., 2020). The current study extends the work that aberrant brain connectivity in the prefrontal cortex may play a pivotal role in postpartum cognitive impairment, especially the executive dysfunction.
This study has several limitations. First, the relatively limited sample size may lead to statistical bias and may reduce the ability to detect a relationship between abnormal FC and cognitive decline in parous women. Further studies with larger sample sizes are required in the future. Second, we only selected the PCC as the ROI but did not analyze FC changes in the other regions of the DMN. Therefore, future work should further analyze FC changes within the DMN in postpartum women. Third, the low temporal resolution and hemodynamic response delay of fMRI may affect the connectivity results. Studies on the combination of brain structure at the molecular or cellular level will be helpful to further explore the underlying neural mechanisms of cognitive dysfunction after pregnancy. Finally, physiologic noise, including respiratory, head motion, and cardiac fluctuations, might have compromised our results. These confounding factors should be taken into account in future studies.
Conclusion
Taken together, this study showed that women with second pregnancy may cause FC alterations between the DMN regions with the parahippocampal gyrus and prefrontal cortex. Significant associations were also found between the disrupted FC networks and cognitive dysfunction in parous women. These results may provide new insights into the neuropathological mechanisms of cognitive impairment during postpartum period, especially for the parous women. The second pregnancy is associated with potential brain connectivity network alterations, which may provide more pivotal information for the clinical obstetrics about the early prevention from postpartum cognitive dysfunction.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee of the Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JZ and TZ designed the experiment, collected the data, performed the analysis, and wrote the manuscript. Y-CC, HC, and YF collected the data. W-WT and J-XZ contributed to the discussion and manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the Jiangsu Provincial Maternal and Child Health Research Project (No. F201829) and Nanjing Special Fund for Health Science and Technology Development (No. YKK18162).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Albin-Brooks, C., Nealer, C., Sabihi, S., Haim, A., and Leuner, B. (2017). The influence of offspring, parity, and oxytocin on cognitive flexibility during the postpartum period. Horm. Behav. 89, 130–136. doi: 10.1016/j.yhbeh.2016.12.015
Anderson, M. V., and Rutherford, M. D. (2012). Cognitive reorganization during pregnancy and the postpartum period: an evolutionary perspective. Evol. Psychol. 10, 659–687. doi: 10.1177/147470491201000402
Bak, Y., and Nah, Y. (2021). Neural correlates of empathy for babies in postpartum women: a longitudinal study. Hum. Brain Mapp. 42, 3295–3304. doi: 10.1002/hbm.25435
Bannbers, E., Gingnell, M., Engman, J., Morell, A., Sylvén, S., Skalkidou, A., et al. (2013). Prefrontal activity during response inhibition decreases over time in the postpartum period. Behav. Brain Res. 241, 132–138. doi: 10.1016/j.bbr.2012.12.003
Chase, H. W., Moses-Kolko, E. L., Zevallos, C., Wisner, K. L., and Phillips, M. L. (2013). Disrupted posterior cingulate–amygdala connectivity in postpartum depressed women as measured with resting BOLD fMRI. Soc. Cogn. Affect. Neurosci. 9, 1069–1075. doi: 10.1093/scan/nst083
Christensen, H., Leach, L. S., and Mackinnon, A. (2010). Cognition in pregnancy and motherhood: prospective cohort study. Br. J. Psychiatry 196, 126–132. doi: 10.1192/bjp.bp.109.068635
Cox, J. L., Holden, J. M., and Sagovsky, R. (1987). Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br. J. Psychiatry 150, 782–786. doi: 10.1192/bjp.150.6.782
Deligiannidis, K. M., Sikoglu, E. M., Shaffer, S. A., Frederick, B., Svenson, A. E., Kopoyan, A., et al. (2013). GABAergic neuroactive steroids and resting-state functional connectivity in postpartum depression: a preliminary study. J. Psychiatr. Res. 47, 816–828. doi: 10.1016/j.jpsychires.2013.02.010
Diederen, K. M., Neggers, S. F., Daalman, K., Blom, J. D., Goekoop, R., Kahn, R. S., et al. (2010). Deactivation of the parahippocampal gyrus preceding auditory hallucinations in schizophrenia. Am. J. Psychiatry 167, 427–435. doi: 10.1176/appi.ajp.2009.09040456
Fisher, P. M., Larsen, C. B., Beliveau, V., Henningsson, S., Pinborg, A., Holst, K. K., et al. (2016). Pharmacologically induced sex hormone fluctuation effects on resting-state functional connectivity in a risk model for depression: a randomized trial. Neuropsychopharmacology 42, 446–453. doi: 10.1038/npp.2016.208
Fransson, P., and Marrelec, G. (2008). The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage 42, 1178–1184. doi: 10.1016/j.neuroimage.2008.05.059
Fusté, M., Pauls, A., Worker, A., Reinders, A., Simmons, A., and Williams, S. C. R. (2017). Brain structure in women at risk of postpartum psychosis: an MRI study. Transl. Psychiatry 7:1286. doi: 10.1038/s41398-017-0003-8
Greicius, M. D., Krasnow, B., Reiss, A. L., and Menon, V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U.S.A. 100, 253–258. doi: 10.1073/pnas.0135058100
Hale, J. B., Hoeppner, J.-A. B., and Fiorello, C. A. (2002). Analyzing digit span components for assessment of attention processes. J. Psychoeduc. Assess. 20, 128–143. doi: 10.1177/073428290202000202
Hoekzema, E., Barba-Müller, E., Pozzobon, C., Picado, M., Lucco, F., García-García, D., et al. (2017). Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci. 20, 287–296. doi: 10.1038/nn.4458
Kim, P., Leckman, J. F., Mayes, L. C., Feldman, R., Wang, X., and Swain, J. E. (2010). The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behav. Neurosci. 124, 695–700. doi: 10.1037/a0020884
Lee, M. H., Smyser, C. D., and Shimony, J. S. (2013). Resting-state fMRI: a review of methods and clinical applications. Am. J. Neuroradiol. 34, 1866–1872. doi: 10.3174/ajnr.A3263
Leech, R., and Sharp, D. J. (2013). The role of the posterior cingulate cortex in cognition and disease. Brain 137, 12–32.
Leech, R., and Sharp, D. J. (2014). The role of the posterior cingulate cortex in cognition and disease. Brain 137, 12–32. doi: 10.1093/brain/awt162
Li, W., Xu, X., Jiang, W., Wang, P., and Gao, X. (2020). Functional connectivity network estimation with an inter-similarity prior for mild cognitive impairment classification. Aging (Albany NY) 12, 17328–17342. doi: 10.18632/aging.103719
Maldjian, J. A., Laurienti, P. J., Kraft, R. A., and Burdette, J. H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239. doi: 10.1016/S1053-8119(03)00169-1
Mantini, D., Perrucci, M. G., Del Gratta, C., Romani, G. L., and Corbetta, M. (2007). Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. 104, 13170–13175. doi: 10.1073/pnas.0700668104
Mars, R. B., Neubert, F.-X., Noonan, M. P., Sallet, J., Toni, I., and Rushworth, M. F. (2012). On the relationship between the “default mode network” and the “social brain. Front. Hum. Neurosci. 6:189. doi: 10.3389/fnhum.2012.00189
Meena, P. S., Soni, R., Jain, M., and Jilowa, C. S. (2016). Cognitive dysfunction and associated behaviour problems in postpartum women: a study from North India. East Asian Arch. Psychiatry 26:104.
Postma, I. R., De Groot, J. C., Aukes, A. M., Aarnoudse, J. G., and Zeeman, G. G. (2014). Cerebral white matter lesions and perceived cognitive dysfunction: the role of pregnancy. Am. J. Ostet.Gynecol. 211, .e1–.e5. doi: 10.1016/j.ajog.2014.02.031
Priya, A., Chaturvedi, S., Bhasin, S. K., Bhatia, M. S., and Radhakrishnan, G. (2018). Depression, anxiety and stress among pregnant women: a community-based study. Indian J. Psychiatry 60, 151–152. doi: 10.4103/psychiatry.IndianJPsychiatry_230_17
Sestieri, C., Shulman, G. L., and Corbetta, M. (2017). The contribution of the human posterior parietal cortex to episodic memory. Nat. Rev. Neurosci. 18:183. doi: 10.1038/nrn.2017.6
Sharma, N., Murari, G., Vandermorris, S., Verhoeff, N., Herrmann, N., Chen, J. J., et al. (2021). Functional connectivity between the posterior default mode network and parahippocampal gyrus is disrupted in older adults with subjective cognitive decline and correlates with subjective memory ability. J. Alzheimers Dis. 82, 435–445. doi: 10.3233/JAD-201579
Spreng, R. N., and Grady, C. L. (2010). Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J. Cogn. Neurosci. 22, 1112–1123. doi: 10.1162/jocn.2009.21282
Vogt, B. A., Vogt, L., and Laureys, S. (2006). Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage 29, 452–466. doi: 10.1016/j.neuroimage.2005.07.048
Xiao-Juan, W., Jian, W., Zhi-Hong, L., Yan, M., and Shi-Wei, Z. (2011). Increased posterior cingulate, medial frontal and decreased temporal regional homogeneity in depressed mothers. a resting-state functional magnetic resonance study. Procedia Environ. Sci. 8, 737–743. doi: 10.1016/j.proenv.2011.10.112
Yuan, P., and Raz, N. (2014). Prefrontal cortex and executive functions in healthy adults: a meta-analysis of structural neuroimaging studies. Neurosci. Biobehav. Rev. 42, 180–192. doi: 10.1016/j.neubiorev.2014.02.005
Zeng, Y., and Hesketh, T. (2016). The effects of China’s universal two-child policy. Lancet 388, 1930–1938. doi: 10.1016/S0140-6736(16)31405-2
Zhang, S., Wang, W., Wang, G., Li, B., Chai, L., Guo, J., et al. (2020). Aberrant resting-state interhemispheric functional connectivity in patients with postpartum depression. Behav. Brain Res. 382:112483. doi: 10.1016/j.bbr.2020.112483
Zheng, J. X., Chen, Y. C., Chen, H., Jiang, L., Bo, F., Feng, Y., et al. (2018). Disrupted spontaneous neural activity related to cognitive impairment in postpartum women. Front. Psychol. 9:624. doi: 10.3389/fpsyg.2018.00624
Keywords: parous women, primiparous women, posterior cingulate cortex, resting-state fMRI, cognitive dysfunction
Citation: Zhang J, Zhang T, Chen Y-C, Chen H, Feng Y, Tang W-W and Zheng J-X (2022) Decreased brain functional connectivity associated with cognitive dysfunction in women with second pregnancy. Front. Aging Neurosci. 14:963943. doi: 10.3389/fnagi.2022.963943
Received: 08 June 2022; Accepted: 06 July 2022;
Published: 22 July 2022.
Edited by:
Zhengxia Wang, Hainan University, ChinaReviewed by:
Fei Chen, First Affiliated Hospital of Zhengzhou University, ChinaZhenyu Xiong, The State University of New Jersey, United States
Copyright © 2022 Zhang, Zhang, Chen, Chen, Feng, Tang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Wei Tang, dHd3MzA3N0AxNjMuY29t; Jin-Xia Zheng, emhlbmdqaW54aWE4NzhAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Juan Zhang1†
Juan Zhang1† Huiyou Chen
Huiyou Chen Yuan Feng
Yuan Feng Jin-Xia Zheng
Jin-Xia Zheng