- 1Department of Neurology, The Second Affiliated Hospital, Army Medical University, Chongqing, China
- 2Department of Orthopedic Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 3Department of Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 4College of Life and Environmental Sciences, Minzu University of China, Beijing, China
- 5Key Laboratory of Neuroscience, School of Basic Medical Sciences, Guangzhou Medical University, Guangzhou, China
- 6Chongqing Key Laboratory of Neurobiology, Department of Anatomy, Institute of Neuroscience, Chongqing Medical University, Chongqing, China
Editorial on the Research Topic
The role of astrocyte in vascular aging
Introduction
Aging-induced decline in the function of the cerebrovascular system is one of the most common physiological factors that causes the death of elderly people in the world. Indeed, the incidence of cerebrovascular diseases increases exponentially with age during the later years of people's lives. For people older than 65, hypertension, diabetes, smoking, and aging are the most prominent risk factors for cerebrovascular diseases. Among them, cerebrovascular aging has been largely associated with cellular senescence of the vascular endothelium and the exacerbation of vascular inflammation.
Previous studies have shown that mitochondrial dysfunction, inflammation, loss of proteostasis, genomic instability, epigenetic alterations, progenitor cell exhaustion, and extracellular matrix remodeling are all well-known hallmarks of aging, including cerebrovascular aging. For instance, activation of the ROS-matrix metalloproteases (MMP) axis, an important trigger of tissue aging, could promote the development of cerebral microhemorrhages, which often results in geriatric psychiatric syndromes with cognitive decline and gait disorders. Therefore, better understanding of the cellular mechanisms underlying cerebrovascular aging is of great importance for reducing cerebrovascular-related mortality in an aging population.
This Research Topic aimed at expanding our horizon about how astrocytes contribute to the cerebrovascular aging process and associated diseases. To manage and edit the submitted manuscripts, we invited several guest editors with expertise in cerebrovascular system and/or aging, including Professor Feng-Quan Zhou from Johns Hopkins University School of Medicine, Dr. Jin-Bo Cheng from College of Life and Environmental Sciences at Minzu University of China, Dr. Gui-Qiong He from Chongqing Medical University, Dr. Xiang-Dong Sun from Guangzhou Medical University, and Dr. Sen Lin from Army Medical University. This issue currently includes four papers with various related Research Topics, such as astrocyte reprogramming and a novel method to regulate astrocytes in a neurovascular unit (NVU).
Astrocytes steer cerebrovascular aging
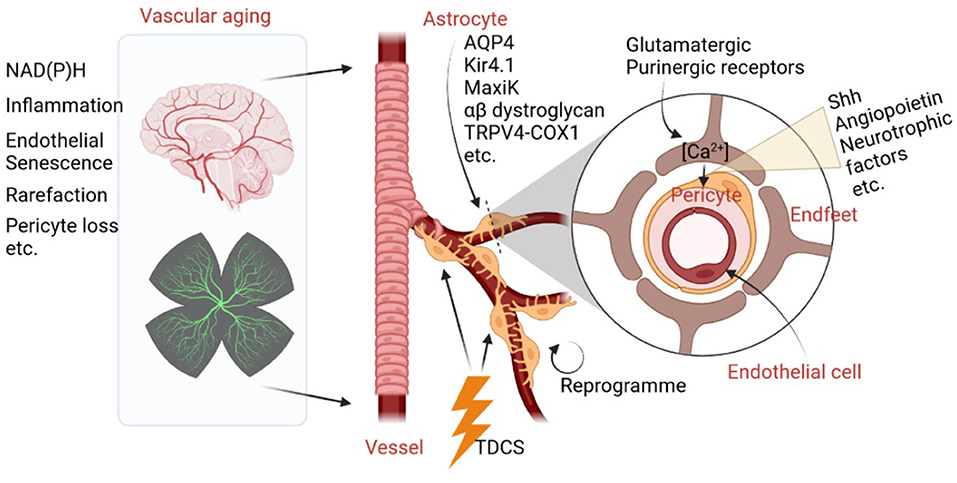
Increased activities of NAD(P)H (Bagi et al., 2022), cerebrovascular inflammation (Jin et al., 2021), endothelial replicative senescence (Yamazaki et al., 2016), microvascular rarefaction, and pericyte loss (Bell et al., 2010) are major negative contributing factors in brain aging. Astrocytes, the major glial cells in the brain, together with cerebrovascular cells, construct a flexible astrocyte-vasculature unit. Therefore, they have the capacity to control and impact cerebrovascular development, degeneration, diseases, and natural aging. What are the physical clues and chemical factors which astrocytes use to control cerebrovascular aging? One well-known fact is that astrocytic endfeet physically interact closely with cerebrovascular cells and regulate key vascular functions. For instance, aquaporin 4 (AQP4) water channel, the inwardly rectifying K+ channel Kir4.1, and the Ca2+-dependent K+ channel MaxiK are all enriched in astrocyte endfeet, which anchor to the vascular basement membranes via the α-β dystroglycan complex (Gondo et al., 2014). Moreover, it has been shown that astrocytes regulate ultra-slow arteriole oscillations via stretch-mediated TRPV4-COX-1 feedback (Haidey et al., 2021), which bidirectionally communicate between arterioles and astrocyte endfeet to regulate oscillatory microvasculature activity, providing an unambiguous physical clue for the physical relationship between astrocytes and the cerebrovascular system. As a result, normal brain aging is marked by vascular deficits induced by astrocytic endfeet retraction or separation from blood vessels.
For chemical factors, astrocytes are capable of sensing neuronal activity changes via glutamatergic and purinergic receptors, which lead to increased Ca2+ levels in endfeet and the release of vasoactive molecules, such as sonic hedgehog (Shh), angiopoietin, and glial-derived neurotrophic factor. These proteins then act on pericytes to modulate blood vessel diameter (Mulligan and MacVicar, 2004; Mishra et al., 2016). In addition, a review article (Han et al.) titled “The Important Double-Edged Role of Astrocytes in Neurovascular Unit After Ischemic Stroke” published in a recent organized topic, “The Role of Astrocyte in Vascular Aging,” summarizes how astrocytes play an important role in the regulation of blood–brain barrier (BBB) permeability. Moreover, interactions between astrocytes and endothelial progenitor cells may mediate neurovascular remodeling after stroke (Han et al.). Thus, astrocytes not only play essential roles in protecting neurons by releasing neurotrophic factors, but can also be detrimental to the pathological process of cerebral ischemia by activating microglia and disrupting the BBB.
Perspective of attenuation of cerebrovascular aging by astrocytes
Because astrocytes play pivotal roles in the process of vascular aging and ischemic stroke, a variety of drugs targeting astrocytes and astrocyte reprogramming methods have been investigated to reduce cerebrovascular-related CNS damage. In the current topic, Han et al. summarized a series of small molecules that could potentially target astrocytes to alleviate cerebral injury and promote neurogenesis and functional recovery. Wen and colleagues reported that in mice with preclinical Alzheimer's Disease, Transcranial Direct Current Stimulation (TDCS) method can be used to alleviate NVU dysfunction (Luo et al.) via detecting different levels of important markers of NVU and BBB integrity. Therefore, TDCS is considered to be a novel method that benefits astrocytes and blood vessels. In addition, Peng et al. have reviewed how astrocytes could be reprogrammed by transcription factors, microRNAs, and small molecules to have beneficial roles in stroke. Therefore, astrocyte reprogramming, either trans-differentiation or de-differentiations, could not only provide newly generated neurons after neural injury, but also contribute to astrocyte-vascular unit reconstitution. As a result, such a strategy may shed light on delaying brain aging and treating ischemia stroke through coordinated angiogenesis and neurogenesis. Collectively, cerebrovascular aging, NVU homeostasis, and dysfunction are tightly linked with astrocytes. The essential roles of astrocytes on vasculature aging are shown in Figure 1.
Author contributions
SL contributed to organize the topic review, invited guest editors, and wrote the manuscript. F-QZ, J-BC, X-DS, and G-QH contributed to invited reviewer and review the manuscripts. F-QZ polished and revised the editorial. All authors contributed to the article and approved the submitted version.
Funding
SL was supported by the National Natural Science Foundation of China (No. 82071392).
Acknowledgments
We thank Biorender.com for providing fantastic scientific modules and icons.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bagi, Z., Kroenke, C. D., Fopiano, K. A., Tian, Y., Filosa, J. A., Sherman, L. S., et al. (2022). Association of cerebral microvascular dysfunction and white matter injury in Alzheimer's disease. Geroscience. doi: 10.1007/s11357-022-00585-5. [Epub ahead of print].
Bell, R. D., Winkler, E. A., Sagare, A. P., Singh, I., LaRue, B., Deane, R., and Zlokovic, B. V. (2010). Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 68, 409–427. doi: 10.1016/j.neuron.2010.09.043
Gondo, A., Shinotsuka, T., Morita, A., Abe, Y., and Yasui, M., and Nuriya M. (2014). Sustained down-regulation of beta-dystroglycan and associated dysfunctions of astrocytic endfeet in epileptic cerebral cortex. J. Biol. Chem. 289, 30279–30288. doi: 10.1074/jbc.M114.588384
Haidey, J. N., Peringod, G., Institoris, A., Gorzo, K. A., Nicola, W., Vandal, M., et al. (2021). Astrocytes regulate ultra-slow arteriole oscillations via stretch-mediated TRPV4-COX-1 feedback. Cell Rep. 36, 109405. doi: 10.1016/j.celrep.2021.109405
Jin, W. N., Shi, K., He, W., Sun, J. H., Van Kaer, L., Shi, F. D., et al. (2021). Neuroblast senescence in the aged brain augments natural killer cell cytotoxicity leading to impaired neurogenesis and cognition. Nat. Neurosci. 24, 61–73. doi: 10.1038/s41593-020-00745-w
Mishra, A., Reynolds, J. P., Chen, Y., Gourine, A. V., and Rusakov, D. A. (2016). Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat. Neurosci. 19, 1619–1627. doi: 10.1038/nn.4428
Mulligan, S. J., and MacVicar, B. A. (2004). Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 431, 195–199. doi: 10.1038/nature02827
Keywords: cerebrovascular aging, BBB, astrocyte, inflammation, astrocyte reprogramming
Citation: Lin S, Zhou F-Q, Cheng J-B, Sun X-D and He G-Q (2022) Editorial: The role of astrocyte in vascular aging. Front. Aging Neurosci. 14:961288. doi: 10.3389/fnagi.2022.961288
Received: 04 June 2022; Accepted: 16 June 2022;
Published: 03 August 2022.
Edited and reviewed by: Jorge Busciglio, University of California, Irvine, United States
Copyright © 2022 Lin, Zhou, Cheng, Sun and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sen Lin, sam.lin@tmmu.edu.cn
 Sen Lin
Sen Lin