
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 22 July 2022
Sec. Alzheimer's Disease and Related Dementias
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.912477
 Olivier Beauchet1,2,3,4*
Olivier Beauchet1,2,3,4* Jacqueline Matskiv2
Jacqueline Matskiv2 Cyrille P. Launay3
Cyrille P. Launay3 Yves Rolland5
Yves Rolland5 Anne-Marie Schott6
Anne-Marie Schott6 Gilles Allali7
Gilles Allali7Background: “Emergency Room Evaluation and Recommendations” (ER2) risk levels (i.e., low, moderate and high) may be used to screen for major neurocognitive disorders (MNCD) in older emergency department users, as a high ER2 risk level is associated with MNCD diagnosis. This study aims to examine the association of ER2 risk levels with incident MNCD in community-dwelling older adults.
Methods: A total of 709 participants of the EPIDémiologie de l’OStéoporose (EPIDOS) study—an observational population-based cohort study—were recruited in Toulouse (France). ER2 low, moderate and high risk levels were determined at baseline. Incident MNCD and their type (i.e., Alzheimer’s disease (AD) vs. non-AD) were diagnosed after a 7-year follow-up period.
Results: The overall incidence of MNCD was 29.1%. A low ER2 risk level was associated with low incidence of MNCD [Hazard ratio (HR) = 0.71 with P = 0.018] and AD (HR = 0.56 with P = 0.003), whereas a high risk level, both individually and when combined with a moderate risk level, was associated with high incidence of MNCD (HR ≥ 1.40 with P ≤0.018) and AD (HR ≥ 1.80 with P ≤ 0.003). No association was found with incident non-AD.
Conclusion: ER2 risk levels were positively associated with incident MNCD in EPIDOS participants, suggesting that ER2 may be used for risk screening of MNCD in the older population.
Major neurocognitive disorders (MNCD) are highly prevalent in the older population (Alzheimer’s Association, 2021). Even if age-specific incidence rates are lower than previous decades, the number of people with MNCD is still rising (Livingston et al., 2020). Today, 55 million people live with MNCD worldwide, and this figure is expected to triple over the next decade (Alzheimer’s Association, 2021). MNCD cause adverse outcomes in physical, psychological and social domains for patients, as well as their caregivers and society at large (Livingston et al., 2020; Alzheimer’s Association, 2021). Care strategies for MNCD have gradually shifted from curative to preventive interventions which emphasize the role of modifiable risk factors (MRFs) (Curran et al., 2021). But for prevention to occur, those at risk must be identified (Curran et al., 2021). Screening people at risk of MNCD is the first step of an effective strategy to delay or reduce the incidence of MNCD (Livingston et al., 2020; Curran et al., 2021).
“Emergency Room Evaluation and Recommendations” (ER2) is a simple clinical assessment tool stratifying risks of adverse outcomes into three levels (i.e., low, moderate and high) in older emergency department (ED) users (Launay et al., 2021b). The prevalence of MNCD in older ED users is around 30%. This population is more prone to a high risk of adverse outcomes compared to ED visitors without MNCD, in part because they are underdiagnosed (Launay et al., 2021b; Beauchet et al., 2022). Recently, we showed that a high ER2 risk level successfully screened older ED users with MNCD upon their arrival to the ED (Beauchet et al., 2022). Generally, MNCD and risk of MNCD remain under-screened in the community (Boustani et al., 2003; Haubois et al., 2011). The narrow window for assessment may in part account for this (Boustani et al., 2003). There is a need for simple and efficient MNCD screening tests in community-dwelling older adults. ER2 is a simple clinical tool which can be easily performed in primary care. An association between ER2 risk levels and incident MNCD has not yet been reported. We hypothesized that ER2 risk levels could be used to screen community-dwelling older adults at risk of incident MNCD. Given that Alzheimer’s disease (AD) is the most common cause of MNCD in the older population, the probability of detecting individuals at risk of AD with a test like the ER2 may be greater when compared to non-AD detection (Livingston et al., 2020; Alzheimer’s Association, 2021). This study thus aims to examine the association of ER2 risk levels with incident MNCD, with an emphasis on AD, in this population.
We had the opportunity to use the database of the “EPIDémiologie de l’OStéoporose” (EPIDOS) study, which is an observational population-based cohort study (Dargent-Molina et al., 1996). This study was originally designed to examine risk factors for hip fracture in older French women. Participants recruited in Toulouse (France) between January 1992 and January 1994 were assessed by mail and/or phone questionnaires every 4 months, over an initial follow-up period of 4 years (like the other EPIDOS participants). Following this initial period (i.e., between 1996 and 1998), this subset of participants were invited to take part in an additional 3-year follow-up. Only one visit was planned at the end of this second follow-up period (i.e., between 1999 and 2002). This final visit took place at the University Hospital of Toulouse or at the participants’ home. Thus, the Toulouse EPIDOS participants had two exhaustive assessments: at baseline, when they were enrolled in the study, and at the end of the 7-year follow-up (Figure 1).
7,598 women aged ≥ 75 and living in one of five French cities (Amiens, Lyon, Montpellier, Paris and Toulouse) were recruited at baseline. 1,462 (19.2%) participants among this initial EPIDOS set were recruited in Toulouse and agreed to take part in an additional 3 years of follow-up. From this subset, we excluded participants with a suspicion of MNCD at the initial baseline assessment of the EPIDOS cohort (i.e., 1992–1994) using the threshold value of ≥ 3 incorrect answers on the Short Portable Mental Status Questionnaire (SPMSQ), as well as those without information on their cognitive status at the end of follow-up (i.e., no MNCD vs. MNCD and its etiology coded as Alzheimer’s Disease (AD) vs. non-AD) (Pfeiffer, 1975). At the end of the 7-year follow-up, the cognitive status of 714 women was known (48.8% of the initial Toulouse cohort). Among this subset, 5 participants were excluded because information about ER2 was missing. Finally, 709 (48.5%) participants were selected for the study.
Information on age, living situation (in residence or not, with or without someone), frequency of contacts with others over the past week, education level, number of drugs taken daily, weight (in kg), height (in cm), regular physical activity (i.e., ≥ 1 h a week during the past month), use of a walking aid regardless of its type, history of falls in the past 6 months, and inability to name the date (an item of SPMSQ) were recorded during a standardized face-to-face baseline assessment (Pfeiffer, 1975). Body mass index (BMI) was calculated and overweight and/or obesity were considered present if BMI was ≥ 25 kg/m2. Polypharmacy was defined as ≥ 5 drugs taken daily. A high education level was defined as high school or above.
Six close-ended questions (i.e., yes vs. no) comprise the ER2 (Launay et al., 2021b; Beauchet et al., 2022). They include age (≥85), sex (i.e., male vs. female), polypharmacy, use of formal (i.e., healthcare and/or social services) and/or informal (i.e., family and/or friends) home support, use of a walking aid regardless of type and/or history of falls in the past 6 months, temporal disorientation (i.e., inability to name the current month and/or year). For the present study, two ER2 items adapted. First, “use of formal and/or informal home support” was changed to “lives with someone and/or had contact with someone over the past week.” We made this changed because the targeted population in the EPIDOS study was community-dwelling older adults in a relatively good health condition at baseline, which differs from the older populations used in studies for the development of ER2. Formal or informal home support are not only a criterion of functional decline but also of the individual’s social network (Launay et al., 2021b; Beauchet et al., 2022). Thus, we made the decision to use this component for the adaptation of this ER2 criterion. Second, temporal disorientation was defined as the inability to name the date (an item of SPMSQ). The ER2 item used in previous studies was an inability to name the month and/or the year. This level of information was not accessible in the EPIDOS database. Thus, a surrogate measure was to consider the date. A score of 5 points was assigned to items “use of a walking aid” and “temporal disorientation,” while a score of one point was assigned to the other items as defined and validated in previous studies (Launay et al., 2021a,b; Beauchet et al., 2022). The ER2 score ranged from 0 (lowest risk) to 14 (highest risk) and stratified risk of adverse outcomes into low (score 0–3), moderate (score 4–5) and high (score ≥6) risk (Launay et al., 2021a,b; Beauchet et al., 2022).
A cognitive assessment was performed at the seventh year of follow-up using standardized tests including the SPMSQ (Pfeiffer, 1975), the Mini Mental State Examination (MMSE) (Folstein et al., 1975), and the Grober and Buschke test (i.e., Free and cued selective reminding test) (Grober et al., 1988). The SPMSQ and MMSE assess global cognitive functioning. The SPMSQ score ranges from 0 (no cognitive impairment) to 10 (severe cognitive impairment). A score between 0 and 2 means no cognitive impairment. The MMSE score ranges from 0 (severe cognitive impairment) to 30 (no cognitive impairment). A MMSE score below 26 means cognitive impairment. The Grober and Buschke test is a verbal episodic memory test which controls for attention and acquisition deficit, by providing category cues in the learning process. Its score ranges from 0 (severe memory impairment) to 48 (no impairment), with a total recall cut-score of ≤ 46/48. In addition, results of brain imaging reports and/or the images themselves were reviewed to contribute to the diagnosis of MNCD subtypes [i.e., cortical and subcortical atrophy vs. brain abnormalities like microvascular ischemic lesion, infarcts, intracerebral lesions (tumors or hematomas] and enlarged ventricles]. The results of all tests were analyzed by a geriatrician and a neurologist in a double-blind manner. DSM-IV criteria were used for the diagnosis of MNCD (American Psychiatric Association, 1994). AD was diagnosed using the criteria of the NINCDS-ADRDA Work Group (McKhann et al., 1984; Tucker, 1999; Hogervorst et al., 2000). Participants who satisfied DSM-IV criteria but not NINCDS-ADRDA criteria were classified with a diagnosis of non-AD. Participants were separated in 2 groups: No MNCD and MNCD with two subsets (i.e., AD and non-AD).
This study was conducted in accordance with the ethical standards set forth in the Helsinki Declaration (1983). The Research Ethics Board (REB) of Toulouse University Hospital approved the EPIDOS protocol. Written informed consent for research was obtained for all recruited EPIDOS participants.
Means, SD, and percentages described the participants’ characteristics. Participants were divided according to their cognitive status: with or without MNCD. Group comparisons were performed using unpaired t-tests or Chi square tests, as appropriate. Cox regressions were performed to examine the association of ER2 risks (independent variable; separated model for each risk level) and incident MNCD (all categories, non-AD and AD; dependent variable; separated model for each type of MNCD). All models are adjusted by place of living, high education level, abnormal body mass index (i.e., ≥ 25 kg/m2) and regular physical activity. P-values < 0.05 were considered statistically significant. All statistics were performed using SPSS (version 28.0; SPSS, Inc., Chicago, IL).
The incidence of MNCD was 29.1% (Table 1). Participants who developed MNCD were older (P ≤0.001), lived more frequently in residence (P = 0.005), had a lower education level (P ≤ 0.001) and lower physical activity levels (P = 0.035), and took more medications (P = 0.017) compared to those without MNCD at the end of follow-up. The mean ER2 score at baseline was higher in participants who had incident MNCD than in those who stayed cognitively healthy (P ≤ 0.001). The prevalence of older participants (i.e., > 85) (P = 0.002), home support (P = 0.010) and inability to name the date (P ≤ 0.001) were higher in the group with incident MNCD compared to participants who stayed cognitively healthy over the 7 years. The prevalence of participants with a low ER2 risk level was lower in the group that developed MNCD than in those who stayed cognitively healthy (P = 0.001), whereas there were more participants with a high ER2 risk level in the group with incident MNCD when compared to their MNCD-free counterparts (P ≤ 0.001).
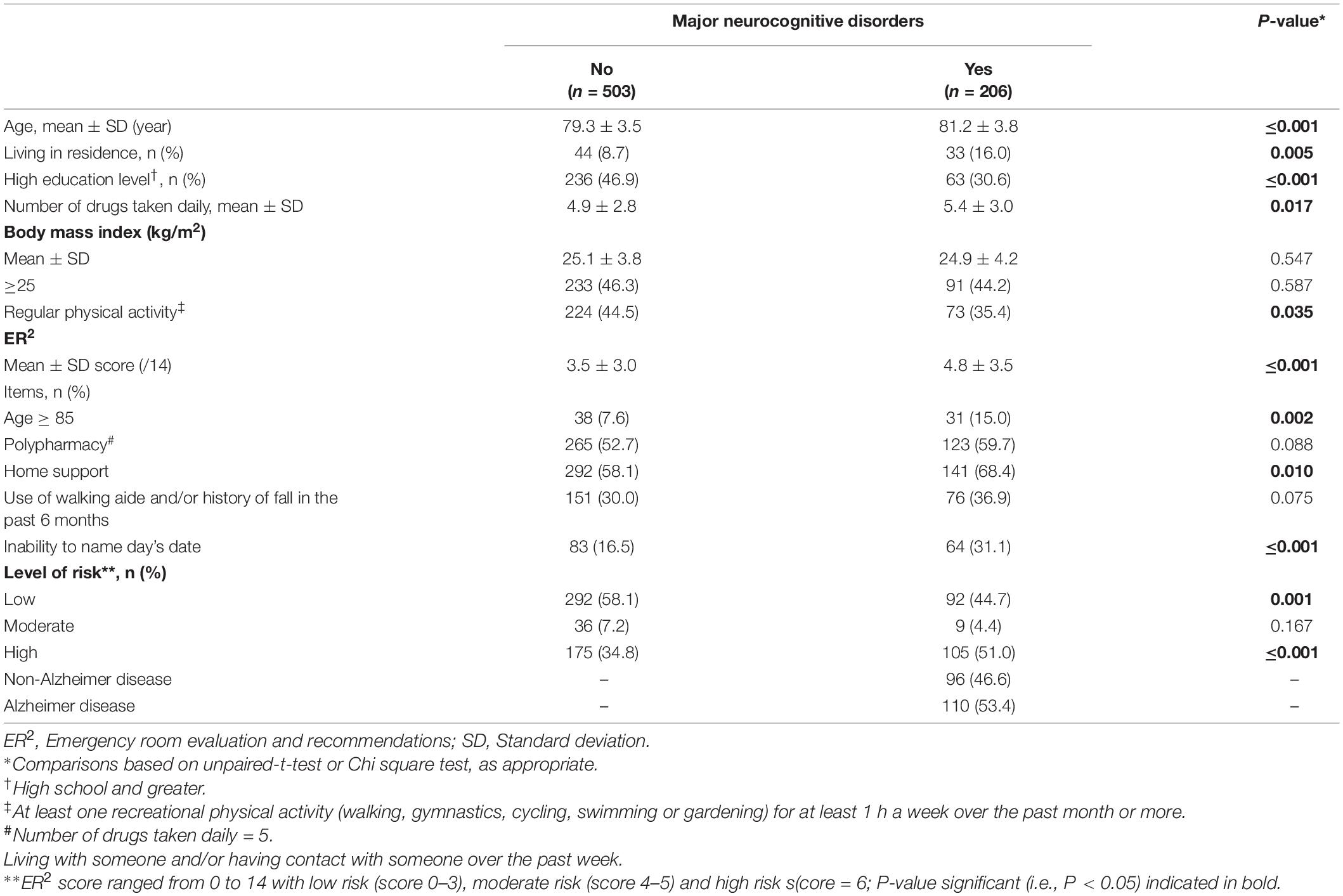
Table 1. Comparisons of participant’s baseline characteristics and incident major neurocognitive disorders according to their cognitive status at the end of the 7-year follow-up (n = 709).
Cox regressions revealed that a low ER2 risk level was significantly associated with low incidence of MNCD and AD [Hazard ratio (HR) = 0.71 with P = 0.018 and HR = 0.56 with P = 0.003, respectively], whereas the ER2 high risk level, both individually and merged with the moderate level, was significantly associated with high incidence of MNCD and AD (HR ≥ 1.40 with P ≤ 0.018 and HR ≥1.80 with P ≤ 0.003, respectively Table 2). When participants with a low ER2 risk level were used as the reference group, only the ER2 high risk level was associated with high incidence of MNCD (HR = 1.50 with P = 0.006) and AD (HR = 1.94 with P = 0.001).
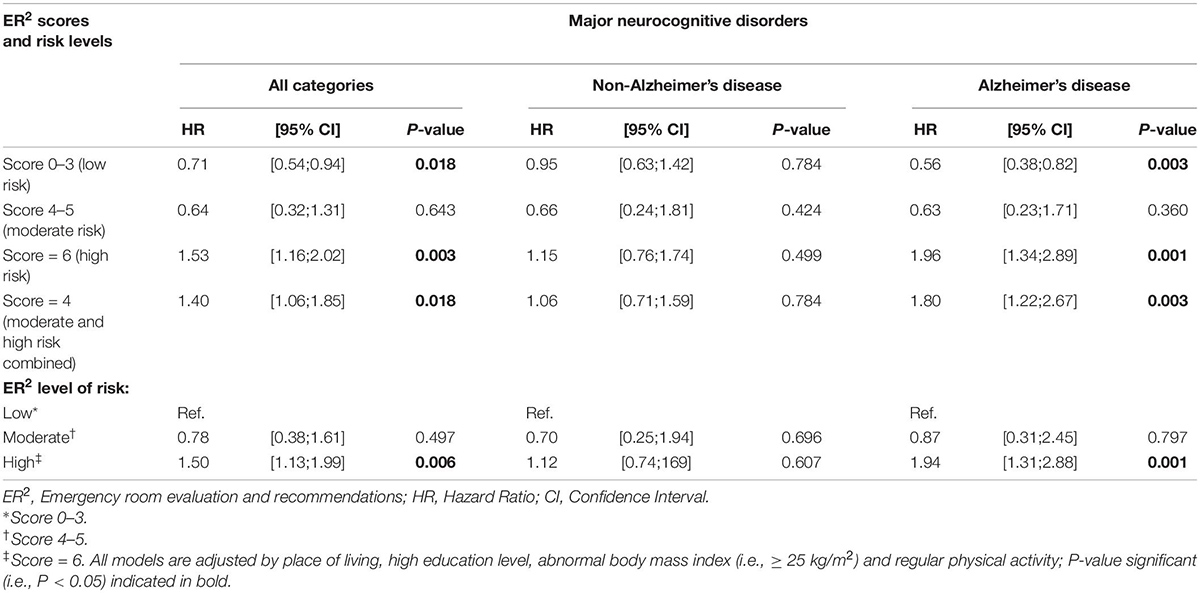
Table 2. Cox regressions showing the association between ER2 risk levels (independent variable; separated model for each risk level) and incident major neurocognitive disorders (all categories, non-Alzheimer’s disease, Alzheimer’s disease; dependent variable; separated model for each variable) in EPIDOS participants (n = 709).
The findings show a positive association between ER2 risk levels and overall incidence of MNCD and AD, with a low risk level associated with low incidence and high risk level with high incidence. No association was found with incidence of non-AD.
The profile of association between ER2 risk levels and incidence of MNCD observed in our study is consistent with previous studies which examined incidence of adverse outcomes in older ED users (Launay et al., 2021a,b; Beauchet et al., 2022). These studies showed that a high ER2 risk level was associated with hospital admission and a long length of stay in ED, while a low risk level was associated with a short length of stay in ED (Launay et al., 2021a,b; Beauchet et al., 2022). The novelty of the results of the present study lies in the population, which was composed of community-dwellers rather than ED users, and its screening of a morbidity (i.e., MNCD) rather than surrogate measures like long length of stay in ED and hospital admissions.
An explanation of this positive association between ER2 risk levels and MNCD may be related to frailty. Frailty is defined as an individual’s health state characterized by vulnerability to stressors due to decreased physiological reserves (Blodgett et al., 2015). The ER2 tool may be assimilated as a short assessment of frailty using the deficit accumulation frailty model proposed by de Vries et al. (2011) and Dent et al. (2016). This model combines clinical information such as symptoms, signs, diseases and disability, and is based on the idea that a greater number of deficits indicates a higher frailty state (de Vries et al., 2011; Blodgett et al., 2015; Dent et al., 2016). We observed that the greatest association with incident MNCD was shown with the ER2 high risk level. Given that frailty is associated with an increased incidence of MNCD, our results confirm the rationale to integrate the ER2 into frailty assessments (Beauchet et al., 2022).
An operative primary prevention strategy is based on two successive steps: first, screening individuals at-risk and second, addressing their MRFs (Livingston et al., 2020; Alzheimer’s Association, 2021). MRFs are chronic morbidities like cardio-vascular risk factors (Livingston et al., 2020). Thus, an effective prevention of MNCD needs to not only consider MRFs individually, but also their accumulation and its adverse consequence. A significant adverse consequence of chronic morbidity accumulation is frailty (de Vries et al., 2011; Dent et al., 2016). Frailty is associated with an increased risk of MNCD (Chu et al., 2021). Frailty may be reversed and, thus, assimilated as a MRF of ADRD (de Vries et al., 2011; Dent et al., 2016; Chu et al., 2021). Addressing frailty might be a key intervention to prevent ADRD.
No significant association between ER2 risk levels and incident non-AD was reported, whereas it was with AD. This non-conclusive result for non-AD is difficult to explain. Incidence of AD and non-AD were both high (53.4 vs. 46.6%). This may be related to the various phenotypes included in non-AD dementia: non-AD dementia is caused by different brain lesions including vascular dementia, Lewy bodies dementia, or other neurodegenerative conditions.
MNCD disease-modifying treatments remain elusive, which explains the emphasis on preventive models targeting MRFs (Livingston et al., 2020; Alzheimer’s Association, 2021). A recent review of the literature shows that up to 40% of late-onset MNCD could potentially be prevented or delayed by addressing MRFs (Livingston et al., 2020). Screening people at-risk for MNCD and addressing their MRFs may thus be an effective strategy to delay or reduce incidence of MNCD. Hence, there is a need to optimize and increase accessibility to clinical risk assessment of MNCDin community-dwelling older populations. The results of the study showed that ER2 is simple and easy to use in clinical practice and, thus, may be incorporated as screening test for MNCD in the older population (both community-dwellers and ED visitors).
We used the term “MNCD” rather than dementia because the former is consensual and considered less stigmatizing. Dementia was replaced in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) for this reason. In fact, the word “dementia” is related to a Latin word for “mad,” or “insane.” Because of this, the introduction of the term “neurocognitive disorder” aimed to reduce the stigma associated with both the word dementia and the conditions that it refers to. When the DSM-5 was published in May 2013, the American Psychiatric Association gave a year’s grace period for the world to absorb the changes before they took effect. Acknowledging that old habits die hard, however, DSM-5 also states that use of the term “dementia” is not precluded “where that term is standard.”
The EPIDOS study design (i.e., observational population-based cohort study) and its long prospective follow-up period (7 years) are the two main strengths of the present study. However, some limitations need to be considered. First, the sample of participants was composed of only females, which limits the generalizability of findings to an older population composed of both sexes. Females have a higher risk for dementia than men (Sindi et al., 2021). It has been suggested that this difference is related to the greater life expectancy and lower cognitive reserves of females compared to males. Thus, our results cannot be generalized to address dementia risks in males. Second, participants that were cognitively impaired were excluded. We used the SPMSQ score with a threshold exclusion value ≥ 3 incorrect answers, to be sure to exclude participants with mild MNCD. This exclusion of participants with minor neurocognitive disorders, which is a risk factor for MNCD, may influenced the incidence of MNCD in our results. Third, the cognitive status of participants was determined only at the end of the 7-year follow-up. Fourth, Cox models were adjusted for baseline characteristics, but residual confounders may still be present and modify the association between ER2 risk stratification and incident MNCD. Additionally, comparisons between groups were not adjusted for multiple comparisons. It should also be noted that there are less people in the ER2 moderate risk group compared to the other groups, which may reduce the significance of the association in this group of participants. Finally, EPIDOS data were collected before 2003 and it is thus possible, though unproven, that participants’ profiles, and thus their incident risks, have changed since then.
ER2 risk levels were associated with incident MNCD, with a high risk level predicting MNCD occurrence in EPIDOS participants. The ER2 tool is simple and easy to use in clinical practice. This demonstrates the potential of using ER2 for screening older adults at-risk of MNCD.
The data analyzed in this study is subject to the following licenses/restrictions: Access to EPIDOS Database can be obtain by the Contacting Corresponding author. Requests to access these datasets should be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Research Ethics Boards (REB) of Toulouse University Hospital approved the EPIDOS protocol. The patients/participants provided their written informed consent to participate in this study.
OB and GA conceived and designed the experiments. A-MS and YR contributed to the cohort data collection. OB, CL, and GA analyzed, interpreted the data, and wrote the manuscript. OB contributed to the reagents, materials, analysis tools, and data. A-MS, YR, and JM contributed to the revision of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the French Ministry of Health.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The main investigators of EPIDOS study were G. Breart, P. Dargent-Molina, P.J. Meunier, A.M. Schott, D. Hans, and P.D. Delmas and the co-investigators were C. Baudoin and J.L. Sebert (Amiens), M.C. Chapuy and A.M. Schott (Lyon), F. Favier and C. Marcelli (Montpellier), C.J. Menkes, C. Cormier, and E. Hausherr (Paris), and H. Grandjean and C. Ribot (Toulouse).
American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders, 4th Edn. Washington DC: American Psychiatric Association.
Beauchet, O., Cooper-Brown, L. A., Lubov, J., Allali, G., Afilalo, M., and Launay, C. P. (2022). “Emergency room evaluation and recommendations” (ER2) tool for the screening of older emergency department visitors with major neurocognitive disorders: results from the ER2 database. Front. Neurol. 12:767285. doi: 10.3389/fneur.2021.767285
Blodgett, J., Theou, O., Kirkland, S., Andreou, P., and Rockwood, K. (2015). Frailty in NHANES: Comparing the frailty index and phenotype. Arch. Gerontol. Geriatr. 60, 464–470. doi: 10.1016/j.archger.2015.01.016
Boustani, M., Peterson, B., Hanson, L., Harris, R., and Lohr, K. N. (2003). U.S. Preventive Services Task Force. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 138, 927–937. doi: 10.7326/0003-4819-138-11-200306030-00015
Chu, W., Chang, S. F., and Ho, H. Y. (2021). Adverse Health Effects of Frailty: Systematic Review and Meta-Analysis of Middle-Aged and Older Adults With Implications for Evidence-Based Practice. Worldviews Evid. Based Nurs. 18, 282–289. doi: 10.1111/wvn.12508
Curran, E., Chong, T. W. H., Godbee, K., Abraham, C., Lautenschlager, N. T., and Palmer, V. J. (2021). General population perspectives of dementia risk reduction and the implications for intervention: A systematic review and thematic synthesis of qualitative evidence. PLoS One 16:e0257540. doi: 10.1371/journal.pone.0257540
Dargent-Molina, P., Favier, F., Grandjean, H., Baudoin, C., Schott, A. M., Hausherr, E., et al. (1996). Fall-related factors and risk of hip fracture: the EPIDOS prospective study. Lancet 348, 145–149. doi: 10.1016/S0140-6736(96)01440-7
de Vries, N. M., Staal, J. B., van Ravensberg, C. D., Hobbelen, J. S., Olde Rikkert, M. G., and Nijhuis-van der Sanden, M. W. (2011). Outcome instruments to measure frailty: a systematic review. Ageing Res. Rev. 10, 104–114. doi: 10.1016/j.arr.2010.09.001
Dent, E., Kowal, P., and Hoogendijk, E. O. (2016). Frailty measurement in research and clinical practice: A review. Eur. J. Intern. Med. 31, 3–10. doi: 10.1016/j.ejim.2016.03.007
Folstein, M., Folstein, S., and McHugh, P. (1975). Mini mental state. a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Grober, E., Buschke, H., Crystal, H., Bang, S., and Dresner, R. (1988). Screening for dementia by memory testing. Neurology 38, 900–903. doi: 10.1212/WNL.38.6.900
Haubois, G., Annweiler, C., Launay, C., Fantino, B., de Decker, L., Allali, G., et al. (2011). Development of a short form of Mini-Mental State Examination for the screening of dementia in older adults with a memory complaint: a case control study. BMC Geriatr. 11:59. doi: 10.1186/1471-2318-11-59
Hogervorst, E., Barnetson, L., Jbst, K. A., Nagy, Z., Combrinck, M., and Smith, A. D. (2000). Diagnosing dementia: interrater reliability assessment and accuracy of the NINCDS/ADRDA criteria versus CERAD histopathological criteria for Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 11, 107–113. doi: 10.1159/000017222
Launay, C. P., Lubov, J., Galery, K., Vilcocq, C., Maubert, É, Afilalo, M., et al. (2021b). Prognosis tools for short-term adverse events in older emergency department users: result of a Québec observational prospective cohort. BMC Geriatr. 21:73. doi: 10.1186/s12877-020-01999-6
Launay, C. P., Galery, K., Vilcocq, C., Afilalo, M., and Beauchet, O. (2021a). Risk for short-term undesirable outcomes in older emergency department users: Results of the ER2 observational cohort study. PLoS One 16:e0249882. doi: 10.1371/journal.pone.0249882
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446.
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., and Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944. doi: 10.1212/wnl.34.7.939
Alzheimer’s Association (2021). 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 17, 327–406.
Pfeiffer, E. (1975). A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc. 23, 433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x
Sindi, S., Kåreholt, I., Ngandu, T., Rosenberg, A., Kulmala, J., Johansson, L., et al. (2021). Sex differences in dementia and response to a lifestyle intervention: Evidence from Nordic population-based studies and a prevention trial. Alzheimers Dement. 17, 1166–1178. doi: 10.1002/alz.12279
Keywords: older adults, epidemiology, cohort study, dementia, incidence
Citation: Beauchet O, Matskiv J, Launay CP, Rolland Y, Schott A-M and Allali G (2022) Emergency Room Evaluation and Recommendations and Risk Screening of Incident Major Neurocognitive Disorders in Older Females: Results of an Observational Population-Based Cohort Study. Front. Aging Neurosci. 14:912477. doi: 10.3389/fnagi.2022.912477
Received: 04 April 2022; Accepted: 23 June 2022;
Published: 22 July 2022.
Edited by:
Stephen D. Ginsberg, Nathan Kline Institute for Psychiatric Research, United StatesReviewed by:
Zhang-Yu Zou, Fujian Medical University Union Hospital, ChinaCopyright © 2022 Beauchet, Matskiv, Launay, Rolland, Schott and Allali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olivier Beauchet, b2xpdmllci5iZWF1Y2hldEB1bW9udHJlYWwuY2E=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.