
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 16 June 2022
Sec. Alzheimer's Disease and Related Dementias
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.911028
This article is part of the Research Topic Protein Aggregation and Propagation in Neurodegenerative Diseases View all 11 articles
 Wei-jiao Zhang1
Wei-jiao Zhang1 Dan-ning Li1
Dan-ning Li1 Teng-hong Lian2
Teng-hong Lian2 Peng Guo2
Peng Guo2 Ya-nan Zhang3
Ya-nan Zhang3 Jing-hui Li1
Jing-hui Li1 Hui-ying Guan1
Hui-ying Guan1 Ming-yue He1
Ming-yue He1 Wen-jing Zhang1
Wen-jing Zhang1 Wei-jia Zhang1
Wei-jia Zhang1 Dong-mei Luo1
Dong-mei Luo1 Xiao-min Wang4
Xiao-min Wang4 Wei Zhang2,5,6,7*
Wei Zhang2,5,6,7*Background: The aim of this study was to explore clinical features and potential mechanisms relating neuropathological biomarkers and blood-brain barrier (BBB) in Alzheimer’s disease (AD) and hearing loss (HL).
Materials and Methods: A total of 65 patients with AD were recruited and auditory function was assessed by threshold of pure tone audiometry (PTA). Patients were divided into AD with HL (AD-HL) and AD with no HL (AD-nHL) groups based on the standard of World Health Organization. Clinical symptoms were assessed by multiple rating scales. The levels of neuropathological biomarkers of β amyloid1-42 (Aβ1–42) and multiple phosphorylated tau (P-tau), and BBB factors of matrix metalloproteinases (MMPs), receptor of advanced glycation end products, glial fibrillary acidic protein, and low-density lipoprotein receptor related protein 1 were measured.
Results: (1) Compared with AD-nHL group, AD-HL group had significantly impaired overall cognitive function and cognitive domains of memory, language, attention, execution, and activities of daily living (ADL) reflected by the scores of rating scales (P < 0.05). PTA threshold was significantly correlated with the impairments of overall cognitive function and cognitive domains of memory and language, and ADL in patients with AD (P < 0.05). (2) P-tau (S199) level was significantly increased in CSF from AD-HL group (P < 0.05), and was significantly and positively correlated with PTA threshold in patients with AD. (3) MMP-3 level was significantly elevated in CSF from AD-HL group (P < 0.05), and was significantly and positively correlated with PTA threshold in patients with AD (P < 0.05). (4) In AD-HL group, P-tau (S199) level was significantly and positively correlated with the levels of MMP-2 and MMP-3 in CSF (P < 0.05).
Conclusion: AD-HL patients have severely compromised overall cognitive function, multiple cognitive domains, and ADL. The potential mechanisms of AD-HL involve elevations of AD neuropathological biomarker of P-tau (S199) and BBB factor of MMP-3, and close correlations between P-tau (S199) and MMP-2/MMP-3 in CSF. Findings from this investigation highly suggest significance of early evaluation of HL for delaying AD progression, and indicate new directions of drug development by inhibiting neuropathological biomarkers of AD and protecting BBB.
Alzheimer’s disease (AD) is the most common form of neurodegenerative disease and ranks the top among all cognitive disorders, contributing to approximately 60–70% of the total cases worldwide (World Health Organization, 2020). The recently published epidemiological data from China showed that 9.83 million of patients with AD aged 60 years or older out of 15.07 million dementia cases (Jia et al., 2020). The neuropathological features of AD include neuritic plaques and neurofibrillary tangles with β amyloid (Aβ) and hyperphosphorylated tau (P-tau) as major components, respectively. AD usually starts insidiously and worsens progressively with clinical symptoms of cognitive impairment, neuropsychiatric symptoms, and compromised activities of daily living (ADL), which brings about heavy economic and caregiver burdens to both families and society.
Hearing loss (HL) is one of the most common symptoms in the elderly population and its prevalence is increasing with age. In the population older than 60 years, over 25% of the total population were affected by disabling HL (World Health Organization, 2021). In the meta-analysis of prospective cohort studies, HL patients had the relative risk of 2.82 of developing mild cognitive impairment (MCI) due to AD and AD dementia, and 4.87 of developing AD dementia (Zheng et al., 2017). Additionally, it was found that hearing aids was helpful in delaying or preventing cognitive decline (Taljaard et al., 2016; Maharani et al., 2018). Hence, HL is one of the modifiable risk factors of AD.
Currently, there are insufficient studies on the clinical features of AD with HL (AD-HL). It was found that AD mice with HL had significantly impaired memory (Kim et al., 2020). However, there is no investigation about the frequency and clinical features of AD patients with HL.
Increasing evidence showed that sensory abnormalities, such as HL (Kim et al., 2020), olfactory (Kim et al., 2020), and visual dysfunctions (Hart et al., 2016) were related to AD. Accordingly, depositions of neuropathological biomarkers of AD, Aβ, and P-tau in the sensory organs have attracted widespread attention. Autopsy studies from AD patients demonstrated that tau pathology in the olfactory epithelium and areas was related to olfactory information processing (Attems and Jellinger, 2006; Murphy, 2019). Aβ and P-tau were found in the retina of AD patients (Chiasseu et al., 2017). However, there is no study reporting AD pathology in peripheral auditory organs, such as cochlea. A previous study reported that central auditory processing reflected by dichotic sentence identification (DSI) right ear advantage (REA, right minus left ear score) was correlated with P-tau and total tau (T-tau) but not Aβ in elderly with normal cognition and family history of AD (Tuwaig et al., 2017). However, few studies pay attention to peripheral auditory processing and its relationship with Aβ and multiple forms of P-tau in AD patients.
Blood-brain barrier (BBB) is a protective structure for brain, and it’s damage can be reflected by the alterations of related factors, including matrix metalloproteinases (MMPs), receptor of advanced glycation end products (RAGE), glial fibrillary acidic protein (GFAP), and low-density lipoprotein receptor related protein 1 (LRP1). In animal experiment on AD and HL, the elevations of MMPs indicated impairment of BBB (Wang et al., 2014; Wu et al., 2017). In patients with AD, MMPs levels were significantly elevated (Wu et al., 2017). For examples, MMP-9 was observed in neuritic plaques, neurofibrillary tangles, cytoplasm of neurons, and vascular walls in hippocampus and cortex; MMP-9 level was even significantly elevated in serum from patients with MCI due to AD compared with control (Lorenzl et al., 2003, 2008). Meanwhile, MMP-9 level was also significantly elevated in cochlea from HL patients (Wu et al., 2017). However, there is no investigation about the relationship between AD-HL and BBB factors.
At present, there is no study on the relationship between the clinical features and potential mechanisms relating neuropathological biomarkers of AD and BBB factors in AD-HL patients. Hence, in this study, demographic variables of patients with AD were collected, and a host of professional rating scales were used to evaluate cognitive impairment, neuropsychiatric symptoms, and ADL. Pure tone audiometry (PTA) threshold was detected by otorhinolaryngology doctors. The levels of neuropathological biomarkers of AD, including Aβ1–42, P-tau (T181), P-tau (S199), P-tau (T231), P-tau (S396), and T-tau, and BBB factors, including MMP-2, MMP-3, MMP-9, RAGE, GFAP, and LRP1 in cerebrospinal fluid (CSF) from patients with AD were measured by enzyme-linked immunosorbent assay (ELISA). The above variables were compared between AD-HL and AD with no HL (AD-nHL) groups, and the correlations among the above variables were analyzed in patients with AD. Results from this study may help understand the clinical features and potential mechanisms of AD-HL, indicate the significance of early identification of HL, and provide potential therapeutic target for the drug development of AD.
This study was approved by the Review Board of Beijing Tiantan Hospital, Capital Medical University, and written informed consents were obtained from all participants and their caregivers.
This study included patients with MCI due to AD and patients with AD dementia according to the National Institute of Aging and Alzheimer’s Association (NIA-AA) criteria (Albert et al., 2011; McKhann et al., 2011).
The exclusion criteria of this study were as follows: (1) the presence of neurological disorders besides AD that might affect cognition, including Parkinson’s disease, multiple sclerosis, epilepsy, etc.; (2) the presence of systemic diseases, including hypothyroidism, severe chronic diseases, and other medical diseases that might affect hearing; (3) histories of alcoholism, carbon monoxide poisoning, etc.; (4) the presence of one or more of following otological diseases, including acoustic nerve dysplasia, common cavity and other serious inner ear malformations, acoustic neuropathy, and acoustic neuroma; and (5) the inability to cooperate with subjective speech tests.
PTA was performed by the doctors using an audiometer (Astera, Conera TM) in a sound-isolated room in the Department of Otorhinolaryngology in our hospital.
PTA threshold refers to the average of hearing level at a set of specified frequencies, which gives a snapshot of hearing level of each ear. The frequencies used for PTA were 500, 1,000, 2,000, and 4,000 Hz, with an intensity range of –10 to 110 dB. PTA threshold was the average of intensities measured at the abovementioned frequencies. World Health Organization defined a PTA ≥ 20 dB, in either ear, as HL (World Health Organization, 2021).
Demographic variables of gender, age, age of onset, disease duration, and years of education were collected.
Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) scales were used to rate the overall cognitive function of patients with AD (Cockrell and Folstein, 1988; Pinto et al., 2019). Patients with illiteracy, primary education, or more than a junior education were identified as having cognitive impairment when the MMSE score was below 17, 20, or 24 points, respectively. MoCA score ≤ 26 indicated potential cognitive impairment. If the educational level of an individual was less than 12 years, 1 point was added. The lower are the scores of the two scales, the severe is the overall cognitive impairment.
A variety of cognitive domains were assessed by the following rating scales:
Auditory Verbal Learning Test (AVLT) was used to assess verbal memory. AVLT N1-3, AVLT N4, and AVLT N5 evaluated immediate recall, short-delayed recall, and long-delayed recall, respectively. AVLT N1-5 reflected the general state of verbal memory. AVLT N6 tested logical memory and AVLT N7 rated ability of recognition (Guo et al., 2009). The Complex Figure Test (CFT)-delayed memory was used to assess visual delayed memory (Zhou et al., 2006). The lower score of this test suggested worse memory.
Visuospatial ability was evaluated by using the CFT-imitation (Zhou et al., 2006). The lower score of this test indicated worse visuospatial ability.
Language function was evaluated by using the Boston Naming Test (BNT) (Lin et al., 2014) and Verbal Fluency Test (VFT), including Animal Fluency Test (AFT), VFT-household items (VFT-H), and VFT-alternating fluency (Sebaldt et al., 2009). The decreased scores of these scales implied compromised language function.
Attention was evaluated by using the Trail Making Test A, B (TMT-A, B) and Symbol Digit Modalities Test (SDMT) (Gong, 1992; Guo et al., 2007). The longer it took to complete the TMT-A and TMT-B, the worse was the attention. The lower TMT-A, TMT-B, and SDMT scores indicated more severe attention deficit.
Executive function was rated by using the Stroop Color-Word Test (SCWT) (Guo et al., 2007). The reduced score of this test revealed an impaired executive function.
Overall neuropsychiatric symptoms were assessed by using the Neuropsychiatric Inventory (NPI). The higher score implied severe overall neuropsychiatric symptoms (Wolinsky et al., 2018).
Individual neuropsychiatric symptoms were then assessed by using the following rating scales.
Depression was evaluated by using the Hamilton Depression Scale (HAMD)-24 items. The higher score indicated more severe depression, and a score of ≥ 8 suggested the presence of depression (Whisman et al., 1989).
Anxiety was evaluated by using the Hamilton Anxiety Scale (HAMA)-14 items. The higher score suggested more severe anxiety, and a score of ≥8 indicated the existence of anxiety (Guy, 1976).
Agitation was rated by using the Cohen-Mansfield Agitation Inventory (CMAI). The elevated CMAI score displayed severe agitation (Lin et al., 2007).
Apathy was rated by using the Modified Apathy Estimate Scale (MAES). The higher was the MAES score, the more severe was the apathy. A score of >14 revealed clinically meaningful apathy (Guercio et al., 2015).
Daytime sleepiness was assessed by using Epworth Sleepiness Scale (ESS). The higher was the ESS score, the more severe was the daytime sleepiness. A score of >10 implied daytime sleepiness (Lee et al., 2007).
ADL was assessed by using the ADL scale, which included the basic ADL (BADL) and the instrumental ADL (IADL) scales. The enhanced score of ADL scale demonstrated the compromised ADL performance (Katz et al., 1963).
Anti-AD drugs were withdrawn for 12–14 h prior to CSF collection. In order to prevent the blood contamination of CSF, the lumber puncture for each patient was performed by a professionally trained neurologist, strictly following the standardized protocol. The first and second tubes might contain blood, as well as tissue fragments and contaminated skin microorganisms; thus, routinely, the third tube of CSF was retained for the measurement. CSF samples from both AD-HL and AD-nHL groups in this study all followed this standardized protocol, trying to avoid this potential bias. A total of 5 ml CSF was taken in a polypropylene tube (Beijing JingkeHongda Biotechnology Co., Ltd.) under a fasting condition.
CSF samples were centrifuged immediately at 2,000 g at 4°C. Approximately 0.5 ml CSF supernatant was aliquoted into separate Nunc cryotubes (Beijing JingkeHongda Biotechnology Co., Ltd.) and kept frozen at −80°C until further assay.
The levels of neuropathological biomarkers of AD, including Aβ1–42, P-tau (T181), P-tau (S199), P-tau (T231), P-tau (S396), and T-tau, in CSF from patients in AD-HL and AD-nHL groups were detected by using ELISA. CSB-E10684h kit and CSBE12011h kit (CUSABIO Company, Wuhan, China) were used for the measurements of Aβ1–42 and T-tau, respectively. KHB7031 kit, KHO0631 kit, KHB7041 kit, and KhB8051 kit (Invitrogen Company, Carlsbad, CA, United States) were used for the measurements of P-tau (T181), P-tau (S199), P-tau (T231), and P-tau (S396), respectively.
The levels of BBB factors, including MMP-2, MMP-3, MMP-9, RAGE, GFAP, and LRP1, in CSF from patients in AD-HL and AD-nHL groups were detected by using ELISA. MMP200 kit, DMP300 kit, DMP900 kit, and DRG00 kit (R&D systems Company, Minneapolis, MN, United States) were used for the measurements of MMP-2, MMP-3, MMP-9, and RAGE, respectively. NS830 kit (Merck Millipore Company, Darmstadt, Germany) was used for the measurement of GFAP. MBS772326 kit (MyBioSource Company, San Diego, CA, United States) was used for the measurement of LRP1.
Statistical analyses were performed by using the SPSS Statistics 23.0 (IBM Corp., Armonk, NY, United States). A value of P < 0.05 was considered statistically significant. Continuous variables, if normally distributed, were presented as means ± SDs, and were compared by using the two-sample t-test. Continuous variables, if they were not normally distributed, were presented as medians (quartiles), and were compared by using a non-parametric test. The bivariate correlation method was used to analyze the relationships among the variables.
Among the 65 patients with AD who were analyzed, 21 cases (32.31%) had no HL, and 44 cases had HL with the frequency up to 67.69%, indicating that HL was very common in AD patients. Among the AD patients with HL, 25 cases (38.46%) had mild HL, 14 cases (21.54%) had moderate HL, 5 cases (7.69%) had moderate to severe HL, and 0 cases (0.00%) had severe HL.
Demographic variables, including sex, age, age of onset, disease duration, and years of education were compared between AD-nHL and AD-HL groups (Table 1). The results showed that demographic variables had no significant differences between the two groups.
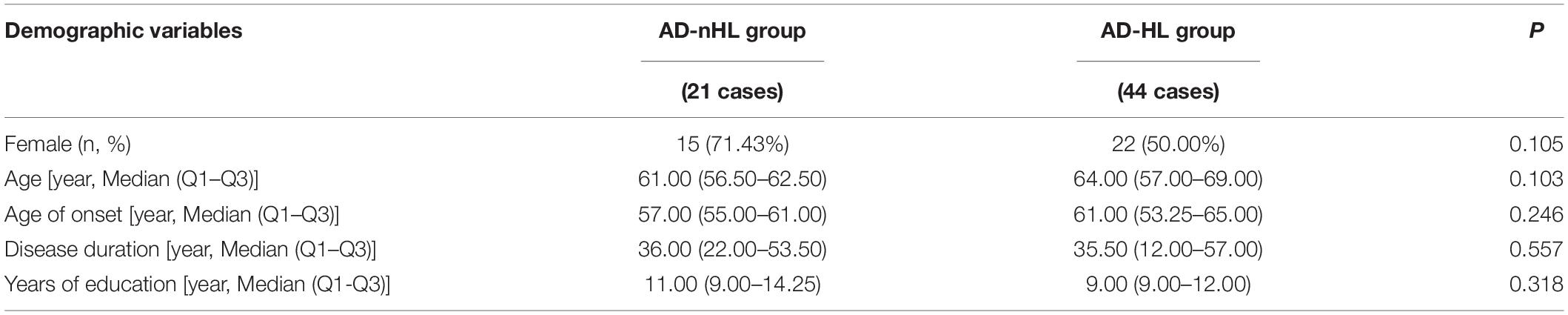
Table 1. Demographic variables of Alzheimer’s disease with no hearing loss (AD-nHL) and Alzheimer’s disease with hearing loss (AD-HL) groups.
The scores of cognitive functions, neuropsychiatry symptoms, and ADL were compared between AD-nHL and AD-HL groups (Table 2). The data displayed that AD-HL group had a significantly lower score of MMSE and MoCA scales, and scores of AVLT, VFT, BNT, TMT-B, SCWT-C, and SDMT than AD-nHL group. There were no significant differences in neuropsychiatry symptoms between the two groups. AD-HL group had a significantly higher ADL score than AD-nHL group (P < 0.05).
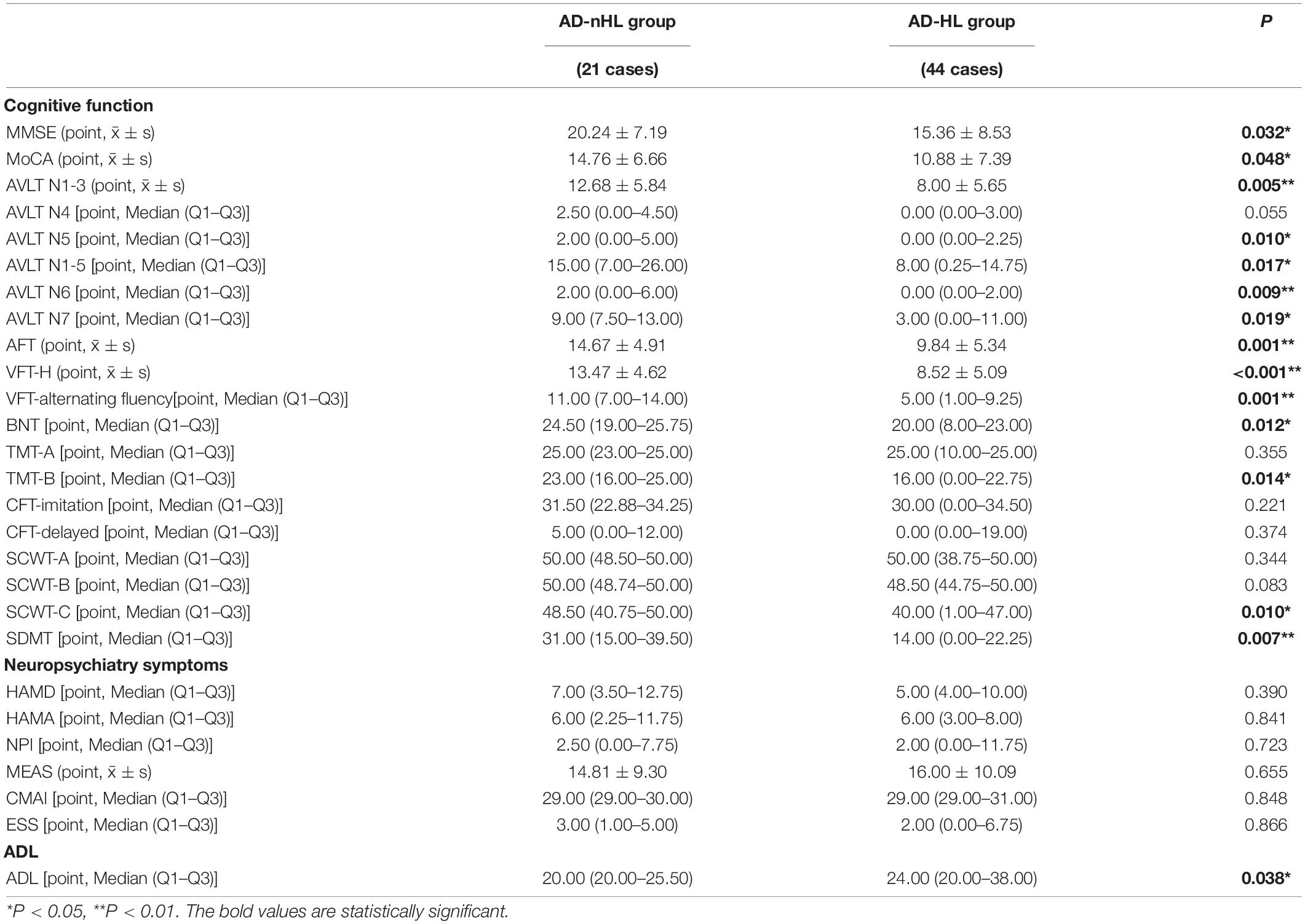
Table 2. Cognitive function, neuropsychiatry symptoms, and activities of daily living (ADL) between AD-nHL and AD-HL groups.
The correlations of PTA threshold with the scores of clinical symptoms in AD patients were analyzed (Table 3). The results suggested that PTA threshold was significantly and negatively correlated with the scores of MMSE, AVLT N1-3, N1-5, N7, VFT, and BNT in AD patients (P < 0.05), and was significantly and positively correlated with the score of ADL (P < 0.05).
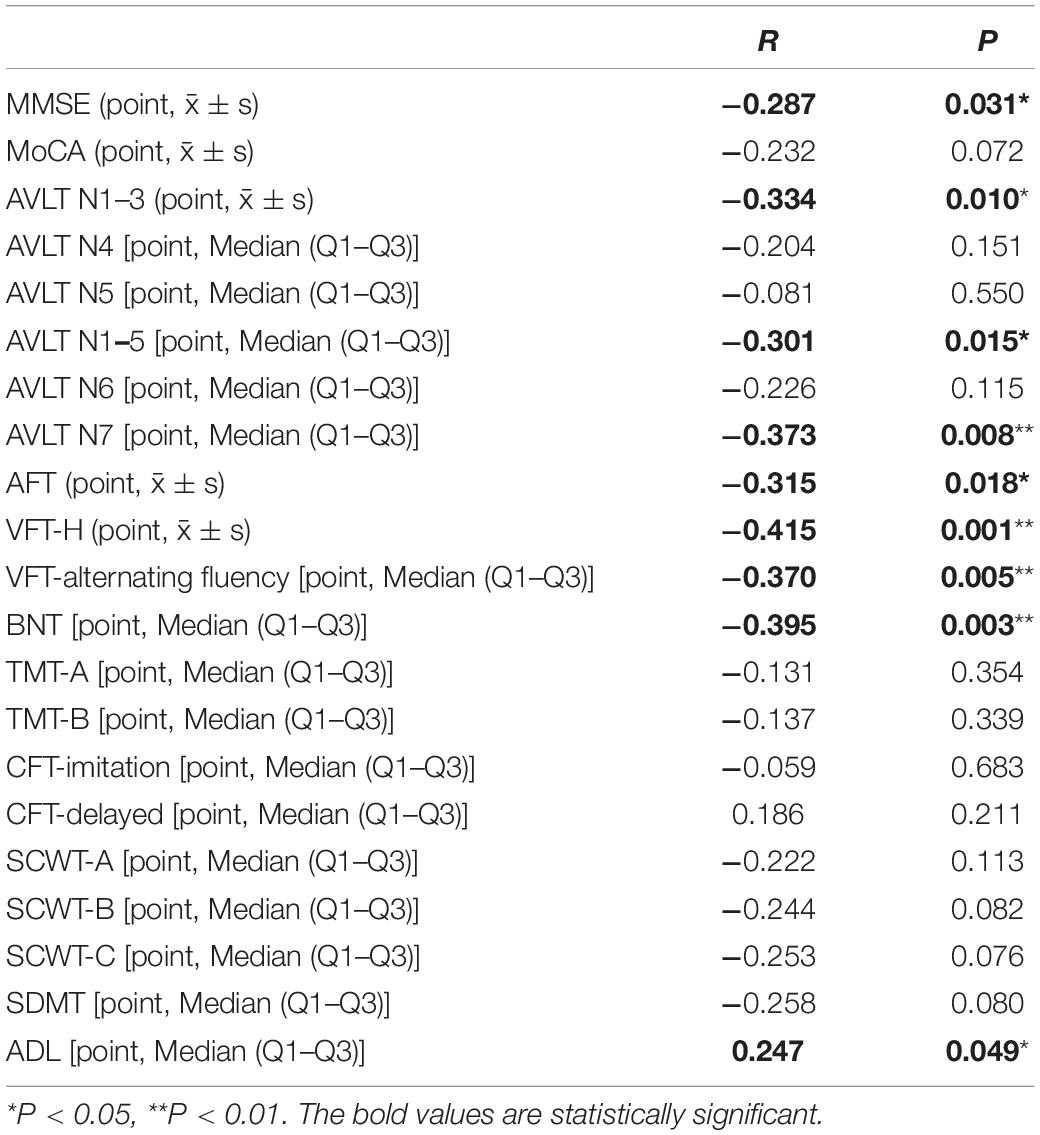
Table 3. Correlations of pure tone audiometry (PTA) threshold with the scores of clinical symptoms from Alzheimer’s disease (AD) patients.
The levels of neuropathological biomarkers of AD, including Aβ1–42, P-tau (T181), P-tau (S199), P-tau (T231), and P-tau (S396) and T-tau in CSF from AD-nHL and AD-HL groups were compared (Table 4). The results showed that the level of P-tau (S199) was significantly increased in CSF from AD-HL group (P < 0.05).
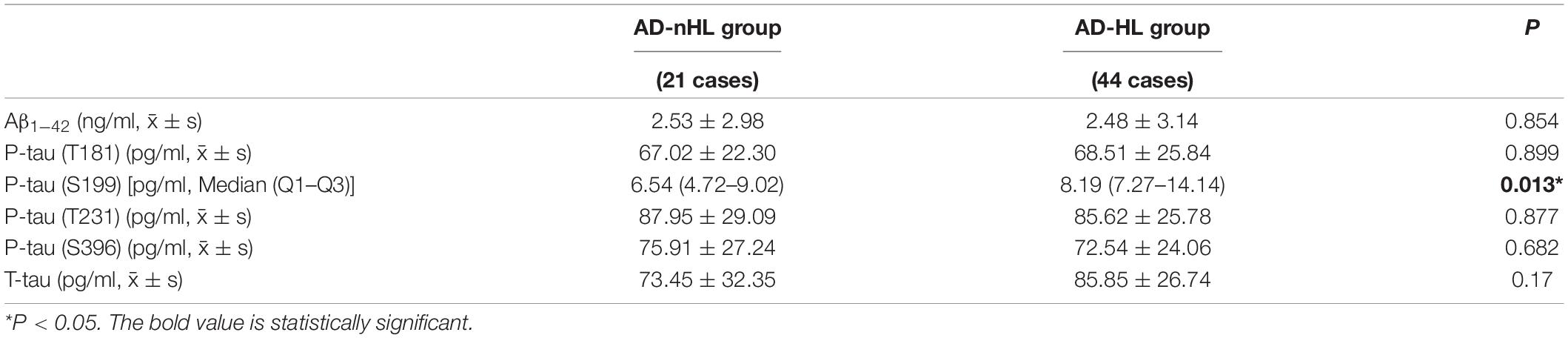
Table 4. Levels of neuropathological biomarkers of AD in cerebrospinal fluid (CSF) from AD-nHL and AD-HL groups.
The correlations of PTA threshold with the levels of Aβ1–42, P-tau (T181), P-tau (S199), P-tau (T231), P-tau (S396), and T-tau in CSF from AD patients were analyzed (Table 5). It was found that the PTA threshold was significantly and positively correlated with P-tau (S199) level in CSF from AD patients (P < 0.05). PTA threshold was not correlated with the levels of Aβ1–42, P-tau (T181), P-tau (T231), P-tau (S396), and T-tau in CSF in AD patients (P > 0.05).
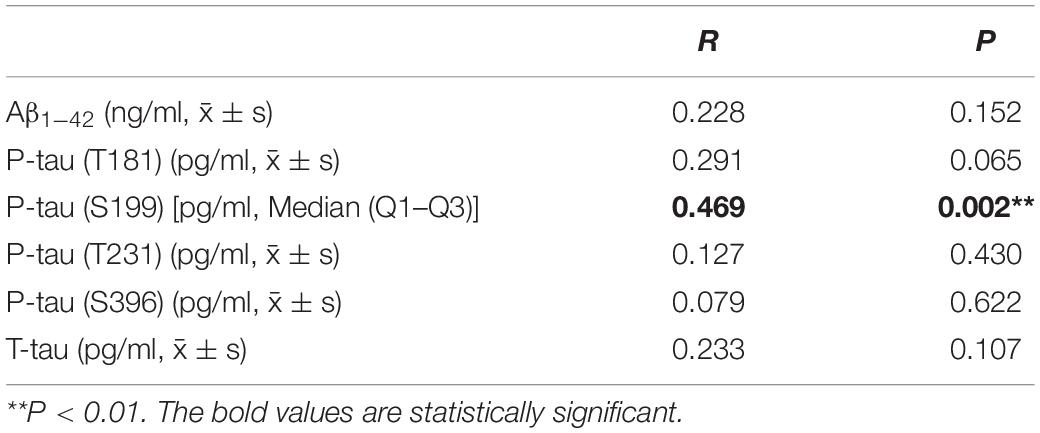
Table 5. Correlations of PTA threshold with the levels of neuropathological biomarkers of AD in CSF from AD patients.
The levels of BBB factors, including MMP-2, MMP-3, MMP-9, RAGE, GFAP, and LRP1, in CSF from AD-nHL and AD-HL groups were compared (Table 6). The results indicated that MMP-3 level was significantly elevated in CSF from AD-HL group compared with that from AD-nHL group (P < 0.05).
The correlations of PTA threshold with the levels of BBB factors, including MMP-2, MMP-3, MMP-9, RAGE, GFAP, and LRP1, in CSF from AD patients were analyzed (Table 7). It was found that PTA threshold had a significant and positive correlation with MMP-3 level in CSF from AD patients (P < 0.05). PTA threshold was not correlated with the levels of MMP 2, MMP 9, RAGE, GFAP, and LRP1 in CSF from AD patients (P > 0.05).
The correlations of the levels of neuropathological biomarkers of AD with the levels of BBB factors in CSF from AD-HL patients were analyzed (Table 8). In AD-HL patients, it was found that the P-tau (S199) level had a significant and positive correlation with the levels of MMP-2 and MMP-3 (P < 0.05).
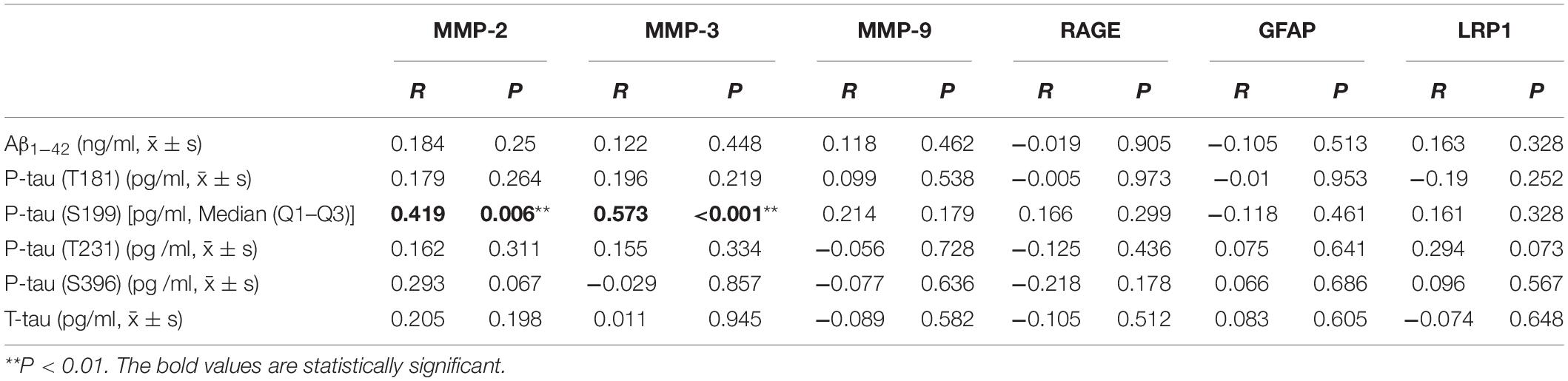
Table 8. Correlations of levels of neuropathological biomarkers of AD with the levels of BBB factors in CSF from AD-HL patients.
Currently, there is no study on the frequency of HL in AD patients. In this study, the frequency of AD-HL was 67.69%, demonstrating that HL is very common in AD patients. HL is one of the risk factors of AD that can be intervened. Therefore, evaluation of auditory function should be routinely performed, and HL should be intervened as early as possible for AD patients (Alzheimer’s Disease International [ADI], 2020). It is very necessary to conduct an in-depth exploration on the clinical features and related mechanisms of AD-HL, providing clinical evidence for finding new targets for intervention and slowing down the progression of the disease.
Demographic variables, including sex, age, age of onset, disease duration, and years of education in AD-nHL and AD-HL groups were compared. The results showed no significant differences in the abovementioned demographic variables between the two groups, indicating that the following variables investigated in this study was comparable (Table 1).
MMSE and MoCA scales are widely used for evaluating the overall cognitive function. In this study, the overall cognitive function was severely impaired in AD-HL group compared with AD-nHL group (Table 2). The score of each cognitive domain was then analyzed.
HL drastically compromised immediate memory and overall auditory memory for AD patients, and prevented AD patients from adopting strategies for memory process, such as classification, and did great harm to the ability of recognition (Tables 2, 3). However, HL exerted no remarkable influence on the delayed recall memory. In the mouse model of auditory deprivation, there were robust microglial activation and oxidative stress, which prominently elicited damage to hippocampal neurogenesis and caused subsequent impairment of immediate memory and learning (Kim et al., 2020; Kurioka et al., 2021). Hence, HL might aggravate memory impairment by suppressing hippocampal neurons via neuroinflammation and oxidative stress as well as AD pathology.
In this study, HL greatly contributed to the serious impairment of language function for patients with AD (Tables 2, 3). Language function was associated with a posterior section of superior and middle temporal gyrus (Yi et al., 2019) and left triangularis in frontal lobe and superior temporal lobe (Obler et al., 2010), which were close to the auditory cortex. Particularly, superior temporal gyrus combined hearing with speech due to its close connection with auditory cortex (Bernstein and Liebenthal, 2014). Therefore, HL might cause damage to language function due to the fact that their correspondent regions in brain were anatomically adjacent.
In this investigation, HL significantly propagated deficit of attention for patients with AD (Tables 2, 3). A previous investigation found that adults with attention deficit or hyperactivity disorders had worse performances in the tests of visual and auditory attention (Taitelbaum-Swead et al., 2019). It might be speculated that more cognitive resources were dedicated to auditory processing under the condition of HL, resulting in the depletion of the resources for multiple cognitive domains, including attention (Lin et al., 2013).
In this exploration, AD-HL group had worse performances in the tests of executive function than AD-nHL group (Tables 2, 3). It was reported that HL and concomitant impairment in auditory cortex increased the recruitment of executive function as well as short-term memory to aid speech perception (Wong et al., 2014), elucidating a redistribution of cognitive resources in the case of HL. It could be speculated that short-term reallocation of cognitive resources might improve performance of cognitive function as a compensation for HL, however, the levels of cognitive domains might decline as a result of exhaustion of overall cognitive resources, as the duration of HL was prolonged.
The impairment of visuospatial function is usually observed in the middle and late stages of AD. Here, patients recruited were in all stages of AD, and those who were in the early stage of the disease might have no visuospatial dysfunction, which might explain that AD-HL and AD-nHL groups were not different in the visuospatial function (Tables 2, 3). We will conduct an in-depth investigation including patients in different stages of AD in the future.
In this study, no significant differences of neuropsychiatric symptoms were observed between AD-HL and AD-nHL groups (Tables 2, 3). There are a body of neuropsychiatric symptoms of AD, among which, some occur in the early stage, like depression and anxiety, and some occur in the late stage, like hallucination and delusion. Patients in all stages recruited might account for the indiscrimination of neuropsychiatric symptoms between the two groups.
The current study showed that AD-HL group had a significantly compromised ADL (Tables 2, 3). It might be because that plenty of cognitive resources were dedicated to the auditory processing, and thus induced severe impairment of cognitive function, which eventually caused poor ADL.
Increasing studies revealed that AD pathology was relevant to HL. P-tau expression in hippocampus of mice with HL was significantly elevated (Omata et al., 2016). Simultaneously expressed Aβ1–42 and tau exerted an obvious synergistic effect, contributing to severe hearing defect (Braak and Braak, 1991). In the rare human studies, it was found that the levels of P-tau (T181) and T-tau but not Aβ1–42 level in CSF were significantly elevated in the HL individuals (Park et al., 2018), indicating that tau pathology played a more important role on HL than Aβ did in humans.
In this study, tau pathology indicated by the elevation of P-tau (S199) was highly associated with HL in patients with AD (Tables 4, 5). In AD, NFTs with the major component of P-tau started from layer II of entorhinal cortex, and finally arrived to hippocampus and neocortex (Braak and Braak, 1991). Although entorhinal cortex and hippocampus were mainly related to memory, they still had wide connections with auditory processing regions (Chen et al., 2013; Aronov et al., 2017; Ahmed et al., 2020). It was observed that HL increased tau phosphorylation via intensified neuroinflammation in mice/rat hippocampus (Cui et al., 2012; Shen et al., 2021). P-tau accumulation promoted neuroinflammation and oxidative stress in brain neurons (Alavi Naini and Soussi-Yanicostas, 2015).
Pathological tau conformers were transferred among cells through several ways and multiple mechanisms (Guo and Lee, 2014). Hence, we supposed that HL might increase deposition of P-tau, which was then spread from cognitive to auditory regions in brain, eventually aggravating the hearing dysfunction.
HL patients need and use up more cognitive reserve for auditory processing. The depletion of cognitive reserve was more vulnerable to AD pathology (Scarmeas and Stern, 2003). Because it is difficult to quantify cognitive reserve, the structural and neurobiological changes of cognitive reserve have not been elucidated so far. However, cognitive reserve and abnormal tau, including P-tau, led to the reduced synapses and shrunk dendrites (Guerrero-Muñoz et al., 2015). Additionally, HL led to social isolation due to the difficulties in communication, and thus served as a critical risk factor of AD (Wilson et al., 2007; Shankar et al., 2013; Boss et al., 2015). In a mouse experiment, isolation promoted AD pathology through neuroinflammation and oxidative stress (Schiavone et al., 2009; Huang et al., 2015; Liu et al., 2017), which might be the potential mechanism of social isolation relevant AD.
This study found that AD-HL was significantly related to the elevated P-tau (S199), but not declined Aβ, indicating that HL was more likely to play a significant role on the progression of AD reflected by the accumulation of P-tau (S199) formation, rather than in the early stage of AD indicated by the formation of Aβ. Thus, early intervention of HL might decrease the progression of tau pathology and thus prevent deterioration of AD.
This study failed to find differences in other forms of P-tau between AD-HL and AD-nHL groups, suggesting that P-tau (S199) might be the important neuropathological biomarker indicating AD aggravation by HL. The detailed mechanisms need to be investigated in the future.
In this study, HL reflected by PTA threshold was not only significantly correlated with AD neuropathological biomarker of P-tau (S199) level in CSF (Tables 4, 5), but also with BBB impairment reflected by the elevated MMP-3 level in CSF from AD patients (Tables 6, 7). In AD-HL patients, a further analysis suggested that P-tau (S199) level was significantly and positively correlated with the levels of MMP-2 and MMP-3 (Table 8).
MMPs was a multigene family of proteinases that played pivotal roles on the disrupted integrity of BBB (Lakhan et al., 2013) via digesting proteins of tight junction and basement membrane. Blood-labyrinth barrier and BBB had similarity in structure; thus, MMPs was inferred to cause damages to both of them. Previous studies in guinea pigs found that the levels of MMP-2 and MMP-9 in healthy vascularis were markedly increased after noise exposure, causing damage to tight junctional proteins, and compromising the instability of cochlear blood-labyrinth barrier (Wu et al., 2017). Accordingly, homeostasis of internal environment of auditory pathway was significantly compromised by MMPs.
MMP-2 and P-tau were co-localized in neurofibrillary tangles and dystrophic neurites under confocal microscopy (Terni and Ferrer, 2015). Furthermore, P-tau stimulated the expression of MMP-2 (Terni and Ferrer, 2015), accordingly, MMP-2 level might be elevated as P-tau level increased in brain. A previous study showed that, at early Braak stage of AD pathology, entorhinal cortex with increased MMP-2 had a wide connection with auditory pathway and cortex (Terni and Ferrer, 2015), proving more direct evidence of close relationships among AD pathology, MMP-2, and AD-HL.
MMP-3 was overexpressed in astrocytes and neurons exposed to Aβ, eliciting microglial activation, and activated microglia in turn propagated accumulations of Aβ and P-tau, which continuously precipitated microglial activation and intensified neuroinflammatory cascade event (Vasto et al., 2007). Thus, AD pathology might trigger the elevation of MMP through neuroinflammation. Moreover, AD and HL, as aging diseases, shared the similar mechanisms of neuroinflammation and oxidative stress, which precipitated P-tau deposition and MMP-3 expression, and both of them in turn promoted oxidative stress and neuroinflammation, producing plenty of free radicals and neuroinflammatory factors (Kim and Hwang, 2011). Particularly, advanced glycation end product, serving as an important initiator of oxidative stress and neuroinflammation, was significantly elevated in both AD and HL patients (Kim and Hwang, 2011; Niihata et al., 2018). The large amount of toxic neuroinflammatory factors and free radicals produced might transfer to the periphery through the disrupted BBB, and thus cause damage to peripheral auditory system and induce deterioration of HL in AD patients.
Socially isolated rats suffered from robust oxidative stress in brain (Schiavone et al., 2009; Colaianna et al., 2013). In a rat experiment, numerous generated neuroinflammatory factors and free radicals mediated BBB disruption through MMPs, such as MMP-2, MMP-3, and MMP-9 (Lehner et al., 2011). AD-HL patients were more prone to loneliness and social isolation, which intensified neuroinflammation and oxidative stress (Li and Xia, 2020), and therefore might elevate the levels of MMPs, like MMP-3, which was observed in this study.
The above findings established the relationships among P-tau (S199), MMP-2/MMP-3 and AD-HL. The mutual promotion between P-tau (S199) and MMP-2/MMP-3 might explain disease progression in AD patients with HL.
This study failed to find differences in other BBB factors between AD-HL and AD-nHL groups, suggesting that MMP-2/MMP-3 might be the important factors indicating BBB damage aggravated by HL in AD patients. The detailed mechanisms need to be studied in the future.
This research was a single-center study, and the results might be biased and need to be interpreted with caution. A multicenter study will be conducted in the future.
In summary, AD patients have a high frequency of HL. AD-HL patients have severely compromised overall cognitive function and multiple cognitive domains of memory, language, attention and executive function, and ADL. The potential mechanisms of AD-HL may involve the elevations of AD pathological biomarker of P-tau (S199) and BBB factor of MMP-3, and the close correlations between P-tau (S199) and MMP-2/ MMP-3 in CSF. Findings from this investigation highly suggest that early evaluation of HL is very pivotal to delay AD progression, and cast a new light for drug development by inhibiting neuropathological biomarkers of AD and protecting BBB in the future.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Review Board of Beijing Tiantan Hospital, Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
Wei-jiaoZ drafted the manuscript, carried out the analysis of data, accepted responsibility for the conduct of the research, final approval for the research, and performed the acquisition of data and the statistical analysis. D-NL, T-HL, PG, and X-MW carried out the analysis of data, accepted responsibility for the conduct of the research, and provided final approval. M-YH, Y-NZ, Wen-jingZ, D-ML, and Wei-jiaZ carried out the acquisition of data, accepted responsibility for the conduct of the research, and provided final approval. J-HL and H-YG drafted the manuscript, accepted responsibility for the conduct of the research, and provided final approval. WZ prepared the study design, carried out the analysis of data, accepted responsibility for the conduct of the research, provided final approval, performed the acquisition of data, the statistical analysis, and study supervision. All authors contributed to the article and approved the submitted version.
This study was supported by the National Key Research and Development Program of China (2016YFC1306300 and 2016YFC1306000); the National Key R&D Program of China-European Commission Horizon 2020 (2017YFE0118800-779238); The National Natural Science Foundation of China (81970992, 81571229, 81071015, and 30770745); Capital’s Funds for Health Improvement and Research (CFH)(2022-2-2048); The Key Technology R&D Program of Beijing Municipal Education Commission (kz201610025030); The Natural Science Foundation of Beijing, China (7082032), The Key Project of Natural Science Foundation of Beijing, China (4161004); Project of Scientific and Technological Development of Traditional Chinese Medicine in Beijing (JJ2018-48); Capital Clinical Characteristic Application Research (Z121107001012161); High Level Technical Personnel Training Project of Beijing Health System, China (2009-3-26); Project of Beijing Institute for Brain Disorders (BIBD-PXM2013_014226_07_000084); Excellent Personnel Training Project of Beijing, China (20071D0300400076); Important National Science and Technology Specific Projects (2011ZX09102-003-01); National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2013BAI09B03); Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges Under Beijing Municipality (IDHT20140514); Beijing Healthcare Research Project, China (JING-15-2); Basic-Clinical Research Cooperation Funding of Capital Medical University, China (2015-JL-PT-X04, 10JL49, and 14JL15); Natural Science Foundation of Capital Medical University, Beijing, China (PYZ2018077); and Youth Research Funding, Beijing Tiantan Hospital, Capital Medical University, China (2015-YQN-14, 2015-YQN-15, and 2015-YQN-17).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor JL, declared a shared parent affiliation with the authors at the time of the review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmed, M. S., Priestley, J. B., Castro, A., Stefanini, F., Solis Canales, A. S., Balough, E. M., et al. (2020). Hippocampal network reorganization underlies the formation of a temporal association memory. Neuron 107, 283–291.e6. doi: 10.1016/j.neuron.2020.04.013
Alavi Naini, S. M., and Soussi-Yanicostas, N. (2015). Tau hyperphosphorylation and oxidative stress, A critical vicious circle in neurodegenerative tauopathies? Oxid. Med. Cell. Longev. 2015:151979. doi: 10.1155/2015/151979
Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279. doi: 10.1016/j.jalz.2011.03.008
Alzheimer’s Disease International [ADI] (2020). World Alzheimer Report 2020. Available online at: https://www.alzint.org/resource/world-alzheimer-report-2020/ (accessed August 31, 2021).
Aronov, D., Nevers, R., and Tank, D. W. (2017). Mapping of a non-spatial dimension by the hippocampal–entorhinal circuit. Nature 543, 719–722. doi: 10.1038/nature21692
Attems, J., and Jellinger, K. A. (2006). Olfactory tau pathology in Alzheimer disease and mild cognitive impairment. Clin. Neuropathol. 25, 265–271.
Bernstein, L. E., and Liebenthal, E. (2014). Neural pathways for visual speech perception. Front. Neurosci. 8:386. doi: 10.3389/fnins.2014.00386
Boss, L., Kang, D. H., and Branson, S. (2015). Loneliness and cognitive function in the older adult: a systematic review. Int. Psychogeriatr. 27, 541–553. doi: 10.1017/s1041610214002749
Braak, H., and Braak, E. (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259. doi: 10.1007/bf00308809
Chen, X., Guo, Y., Feng, J., Liao, Z., Li, X., Wang, H., et al. (2013). Encoding and retrieval of artificial visuoauditory memory traces in the auditory cortex requires the entorhinal cortex. J. Neurosci. 33, 9963–9974. doi: 10.1523/jneurosci.4078-12.2013
Chiasseu, M., Alarcon-Martinez, L., Belforte, N., Quintero, H., Dotigny, F., Destroismaisons, L., et al. (2017). Tau accumulation in the retina promotes early neuronal dysfunction and precedes brain pathology in a mouse model of Alzheimer’s disease. Mol. Neurodegener. 12:58. doi: 10.1186/s13024-017-0199-3
Cockrell, J. R., and Folstein, M. F. (1988). Mini-Mental State Examination (MMSE). Psychopharmacol. Bull. 24, 689–692.
Colaianna, M., Schiavone, S., Zotti, M., Tucci, P., Morgese, M. G., Bäckdahl, L., et al. (2013). Neuroendocrine profile in a rat model of psychosocial stress: relation to oxidative stress. Antioxid. Redox Signal. 18, 1385–1399. doi: 10.1089/ars.2012.4569
Cui, B., Zhu, L., She, X., Wu, M., Ma, Q., Wang, T., et al. (2012). Chronic noise exposure causes persistence of tau hyperphosphorylation and formation of NFT tau in the rat hippocampus and prefrontal cortex. Exp. Neurol. 238, 122–129. doi: 10.1016/j.expneurol.2012.08.028
Gong, Y. (1992). Manual of Wechsler Adult Intelligence Scale-Chinese Version. Changsha: Chinese Map Press.
Guercio, B. J., Donovan, N. J., Munro, C. E., Aghjayan, S. L., Wigman, S. E., Locascio, J. J., et al. (2015). The apathy evaluation scale: a comparison of subject, informant, and clinician report in cognitively normal elderly and mild cognitive impairment. J. Alzheimers Dis. 47, 421–432. doi: 10.3233/jad-150146
Guerrero-Muñoz, M. J., Gerson, J., and Castillo-Carranza, D. L. (2015). Tau oligomers: the toxic player at synapses in Alzheimer’s Disease. Front. Cell. Neurosci. 9:464. doi: 10.3389/fncel.2015.00464
Guo, J. L., and Lee, V. M. Y. (2014). Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat. Med. 20, 130–138. doi: 10.1038/nm.3457
Guo, Q., Sun, Y., Yuan, J., Hong, Z., and Lu, C. (2007). Application of eight executive tests in participants at Shanghai communities. Chin. J. Behav. Med. Sci. 16, 628–631.
Guo, Q., Zhao, Q., Chen, M., Ding, D., and Hong, Z. (2009). A comparison study of mild cognitive impairment with 3 memory tests among Chinese individuals. Alzheimer Dis. Assoc. Disord. 23, 253–259. doi: 10.1097/WAD.0b013e3181999e92
Guy, W. (1976). ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: U.S. National Institute of Health, Psychopharmacology Research Branch.
Hart, N. J., Koronyo, Y., Black, K. L., and Koronyo-Hamaoui, M. (2016). Ocular indicators of Alzheimer’s: exploring disease in the retina. Acta Neuropathol. 132, 767–787. doi: 10.1007/s00401-016-1613-6
Huang, H., Wang, L., Cao, M., Marshall, C., Gao, J., Xiao, N., et al. (2015). Isolation housing exacerbates Alzheimer’s disease-like pathophysiology in aged APP/PS1 mice. Int. J. Neuropsychopharmacol. 18:pyu116. doi: 10.1093/ijnp/pyu116
Jia, L., Du, Y., Chu, L., Zhang, Z., Li, F., Lyu, D., et al. (2020). Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health 5, e661–e671. doi: 10.1016/S2468-2667(20)30185-7
Katz, S., Ford, A. B., Moskowitz, R. W., Jackson, B. A., and Jaffe, M. W. (1963). Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 185, 914–919. doi: 10.1001/jama.1963.03060120024016
Kim, E. M., and Hwang, O. (2011). Role of matrix metalloproteinase-3 in neurodegeneration. J. Neurochem. 116, 22–32. doi: 10.1111/j.1471-4159.2010.07082.x
Kim, J. S., Lee, H. J., Lee, S., Lee, H. S., Jeong, Y. J., Son, Y., et al. (2020). Conductive hearing loss aggravates memory decline in Alzheimer model mice. Front. Neurosci. 14:843. doi: 10.3389/fnins.2020.00843
Kurioka, T., Mogi, S., and Yamashita, T. (2021). Decreasing auditory input induces neurogenesis impairment in the hippocampus. Sci. Rep. 11:423. doi: 10.1038/s41598-020-80218-z
Lakhan, S. E., Kirchgessner, A., Tepper, D., and Leonard, A. (2013). Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front. Neurol. 4:32. doi: 10.3389/fneur.2013.00032
Lee, J. H., Bliwise, D. L., Ansari, F. P., Goldstein, F. C., Cellar, J. S., Lah, J. J., et al. (2007). Daytime sleepiness and functional impairment in Alzheimer disease. Am. J. Geriatr. Psychiatry 15, 620–626. doi: 10.1097/JGP.0b013e3180381521
Lehner, C., Gehwolf, R., Tempfer, H., Krizbai, I., Hennig, B., Bauer, H. C., et al. (2011). Oxidative stress and blood-brain barrier dysfunction under particular consideration of matrix metalloproteinases. Antioxid. Redox Signal. 15, 1305–1323. doi: 10.1089/ars.2011.3923
Li, H., and Xia, N. (2020). The role of oxidative stress in cardiovascular disease caused by social isolation and loneliness. Redox Biol. 37:101585. doi: 10.1016/j.redox.2020.101585
Lin, C. Y., Chen, T. B., Lin, K. N., Yeh, Y. C., Chen, W. T., Wang, K. S., et al. (2014). Confrontation naming errors in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 37, 86–94. doi: 10.1159/000354359
Lin, F. R., Yaffe, K., Xia, J., Xue, Q.-L., Harris, T. B., Purchase-Helzner, E., et al. (2013). Hearing loss and cognitive decline in older adults. JAMA Intern. Med. 173, 293–299. doi: 10.1001/jamainternmed.2013.1868
Lin, L. C., Kao, C. C., Tzeng, Y. L., and Lin, Y. J. (2007). Equivalence of Chinese version of the Cohen-Mansfield Agitation Inventory. J. Adv. Nurs. 59, 178–185. doi: 10.1111/j.1365-2648.2007.04303.x
Liu, Z., Zhou, T., Ziegler, A. C., Dimitrion, P., and Zuo, L. (2017). Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid. Med. Cell. Longev. 2017:2525967. doi: 10.1155/2017/2525967
Lorenzl, S., Albers, D. S., Relkin, N., Ngyuen, T., Hilgenberg, S. L., Chirichigno, J., et al. (2003). Increased plasma levels of matrix metalloproteinase-9 in patients with Alzheimer’s disease. Neurochem. Int. 43, 191–196. doi: 10.1016/s0197-0186(03)00004-4
Lorenzl, S., Buerger, K., Hampel, H., and Beal, M. F. (2008). Profiles of matrix metalloproteinases and their inhibitors in plasma of patients with dementia. Int. Psychogeriatr. 20, 67–76. doi: 10.1017/s1041610207005790
Maharani, A., Dawes, P., Nazroo, J., Tampubolon, G., and Pendleton, N. (2018). Longitudinal relationship between hearing aid use and cognitive function in older Americans. J. Am. Geriatri. Soc. 66, 1130–1136. doi: 10.1111/jgs.15363
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R. Jr., Kawas, C. H., et al. (2011). The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 263–269. doi: 10.1016/j.jalz.2011.03.005
Murphy, C. (2019). Olfactory and other sensory impairments in Alzheimer disease. Nat. Rev. Neurol. 15, 11–24. doi: 10.1038/s41582-018-0097-5
Niihata, K., Takahashi, S., Kurita, N., Yajima, N., Omae, K., Fukuma, S., et al. (2018). Association between accumulation of advanced glycation end-products and hearing impairment in community-dwelling older people: a cross-sectional Sukagawa Study. J. Am. Med. Dir. Assoc. 19, 235–239.e1. doi: 10.1016/j.jamda.2017.09.008
Obler, L. K., Rykhlevskaia, E., Schnyer, D., Clark-Cotton, M. R., Spiro, A. III, Hyun, J., et al. (2010). Bilateral brain regions associated with naming in older adults. Brain Lang. 113, 113–123. doi: 10.1016/j.bandl.2010.03.001
Omata, Y., Tharasegaran, S., Lim, Y. M., Yamasaki, Y., Ishigaki, Y., Tatsuno, T., et al. (2016). Expression of amyloid-β in mouse cochlear hair cells causes an early-onset auditory defect in high-frequency sound perception. Aging 8, 427–439. doi: 10.18632/aging.100899
Park, S. Y., Kim, M. J., Kim, H. L., Kim, D. K., Yeo, S. W., and Park, S. N. (2018). Cognitive decline and increased hippocampal p-tau expression in mice with hearing loss. Behav. Brain Res. 342, 19–26. doi: 10.1016/j.bbr.2018.01.003
Pinto, T. C. C., Machado, L., Bulgacov, T. M., Rodrigues-Júnior, A. L., Costa, M. L. G., Ximenes, R. C. C., et al. (2019). Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int. Psychogeriatr. 31, 491–504. doi: 10.1017/S1041610218001370
Scarmeas, N., and Stern, Y. (2003). Cognitive reserve and lifestyle. J. Clin. Exp. Neuropsychol. 25, 625–633. doi: 10.1076/jcen.25.5.625.14576
Schiavone, S., Sorce, S., Dubois-Dauphin, M., Jaquet, V., Colaianna, M., Zotti, M., et al. (2009). Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol. Psychiatry 66, 384–392. doi: 10.1016/j.biopsych.2009.04.033
Sebaldt, R., Dalziel, W., Massoud, F., Tanguay, A., Ward, R., Thabane, L., et al. (2009). Detection of cognitive impairment and dementia using the animal fluency test: the DECIDE study. Can. J. Neurol. Sci. 36, 599–604. doi: 10.1017/s0317167100008106
Shankar, A., Hamer, M., McMunn, A., and Steptoe, A. (2013). Social isolation and loneliness: relationships with cognitive function during 4 years of follow-up in the English Longitudinal Study of Ageing. Psychosom. Med. 75, 161–170. doi: 10.1097/PSY.0b013e31827f09cd
Shen, Y., Hu, H., Fan, C., Wang, Q., Zou, T., Ye, B., et al. (2021). Sensorineural hearing loss may lead to dementia-related pathological changes in hippocampal neurons. Neurobiol. Dis. 156:105408. doi: 10.1016/j.nbd.2021.105408
Taitelbaum-Swead, R., Kozol, Z., and Fostick, L. (2019). Listening effort among adults with and without attention-deficit/hyperactivity disorder. J. Speech Lang. Hear. Res. 62, 4554–4563. doi: 10.1044/2019_jslhr-h-19-0134
Taljaard, D. S., Olaithe, M., Brennan-Jones, C. G., Eikelboom, R. H., and Bucks, R. S. (2016). The relationship between hearing impairment and cognitive function: a meta-analysis in adults. Clin. Otolaryngol. 41, 718–729. doi: 10.1111/coa.12607
Terni, B., and Ferrer, I. (2015). Abnormal expression and distribution of MMP2 at initial stages of Alzheimer’s disease-related pathology. J. Alzheimers Dis. 46, 461–469. doi: 10.3233/jad-142460
Tuwaig, M., Savard, M., Jutras, B., Poirier, J., Collins, D. L., Rosa-Neto, P., et al. (2017). Deficit in central auditory processing as a biomarker of pre-clinical Alzheimer’s disease. J. Alzheimers Dis. 60, 1589–1600. doi: 10.3233/JAD-170545
Vasto, S., Candore, G., Duro, G., Lio, D., Grimaldi, M. P., and Caruso, C. (2007). Alzheimer’s disease and genetics of inflammation: a pharmacogenomic vision. Pharmacogenomics 8, 1735–1745. doi: 10.2217/14622416.8.12.1735
Wang, X. X., Tan, M. S., Yu, J. T., and Tan, L. (2014). Matrix metalloproteinases and their multiple roles in Alzheimer’s disease. Biomed Res. Int. 2014:908636. doi: 10.1155/2014/908636
Whisman, M. A., Strosahl, K., Fruzzetti, A. E., Schmaling, K. B., Jacobson, N. S., and Miller, D. M. (1989). A structured interview version of the Hamilton Rating Scale for Depression: reliability and validity. Psychol. Assess. 1, 238–241.
Wilson, R. S., Krueger, K. R., Arnold, S. E., Schneider, J. A., Kelly, J. F., Barnes, L. L., et al. (2007). Loneliness and risk of Alzheimer disease. Arch. Gen. Psychiatry 64, 234–240. doi: 10.1001/archpsyc.64.2.234
Wolinsky, D., Drake, K., and Bostwick, J. (2018). Diagnosis and management of neuropsychiatric symptoms in Alzheimer’s disease. Curr. Psychiatry Rep. 20:117. doi: 10.1007/s11920-018-0978-8
Wong, L. L., Yu, J. K., Chan, S. S., and Tong, M. C. (2014). Screening of cognitive function and hearing impairment in older adults: a preliminary study. Biomed Res. Int. 2014:867852. doi: 10.1155/2014/867852
World Health Organization (2020). Dementia Fact Sheet. Available online at: https://www.who.int/en/news-room/fact-sheets/detail/dementia (accessed August 31, 2021).
Wu, J., Han, W., Chen, X., Guo, W., Liu, K., Wang, R., et al. (2017). Matrix metalloproteinase-2 and -9 contribute to functional integrity and noise-induced damage to the blood-labyrinth-barrier. Mol. Med. Rep. 16, 1731–1738. doi: 10.3892/mmr.2017.6784
Yi, H. G., Leonard, M. K., and Chang, E. F. (2019). The encoding of speech sounds in the superior temporal Gyrus. Neuron 102, 1096–1110. doi: 10.1016/j.neuron.2019.04.023
Zheng, Y., Fan, S., Liao, W., Fang, W., Xiao, S., and Liu, J. (2017). Hearing impairment and risk of Alzheimer’s disease: a meta-analysis of prospective cohort studies. Neurol. Sci. 38, 233–239. doi: 10.1007/s10072-016-2779-3
Keywords: Alzheimer’s disease, hearing loss, clinical features, neuropathological biomarkers of AD, blood-brain barrier, cerebrospinal fluid
Citation: Zhang W-j, Li D-n, Lian T-h, Guo P, Zhang Y-n, Li J-h, Guan H-y, He M-y, Zhang W-j, Zhang W-j, Luo D-m, Wang X-m and Zhang W (2022) Clinical Features and Potential Mechanisms Relating Neuropathological Biomarkers and Blood-Brain Barrier in Patients With Alzheimer’s Disease and Hearing Loss. Front. Aging Neurosci. 14:911028. doi: 10.3389/fnagi.2022.911028
Received: 01 April 2022; Accepted: 05 May 2022;
Published: 16 June 2022.
Edited by:
Jia Liu, Capital Medical University, ChinaCopyright © 2022 Zhang, Li, Lian, Guo, Zhang, Li, Guan, He, Zhang, Zhang, Luo, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhang, dHR5eXp3QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.