- 1Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
- 2Department of Radiology, Karolinska University Hospital, Stockholm, Sweden
- 3Division of Neuroradiology, Department of Radiology, Stanford University Hospital, Stanford, CA, United States
- 4Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden
- 5Division of Clinical Geriatrics, Karolinska University Hospital, Stockholm, Sweden
Objective: The apolipoprotein E (APOE) ε4 allele is the main genetic risk factor for dementia and Alzheimer's disease (AD), but the underlying mechanism for the increased risk is not well understood. Cerebral small vessel disease (SVD) is prevalent among patients with cognitive impairment and is thought to play an important role in the pathophysiology of dementia. We aimed to investigate the association between the APOE ε genotype and magnetic resonance imaging (MRI) markers of SVD in a memory clinic population.
Material and Methods: This is a cross-sectional study with a total of 520 patients undergoing dementia investigation, including an MRI brain scan and APOE genotyping in all patients enrolled, and cerebrospinal fluid (CSF) analysis for routine AD biomarkers in 399 patients. MR images were assessed for markers of SVD: cerebral microbleeds (CMBs), cortical superficial siderosis, intracerebral hemorrhage, white matter hyperintensities, lacunar infarcts, and enlarged perivascular spaces.
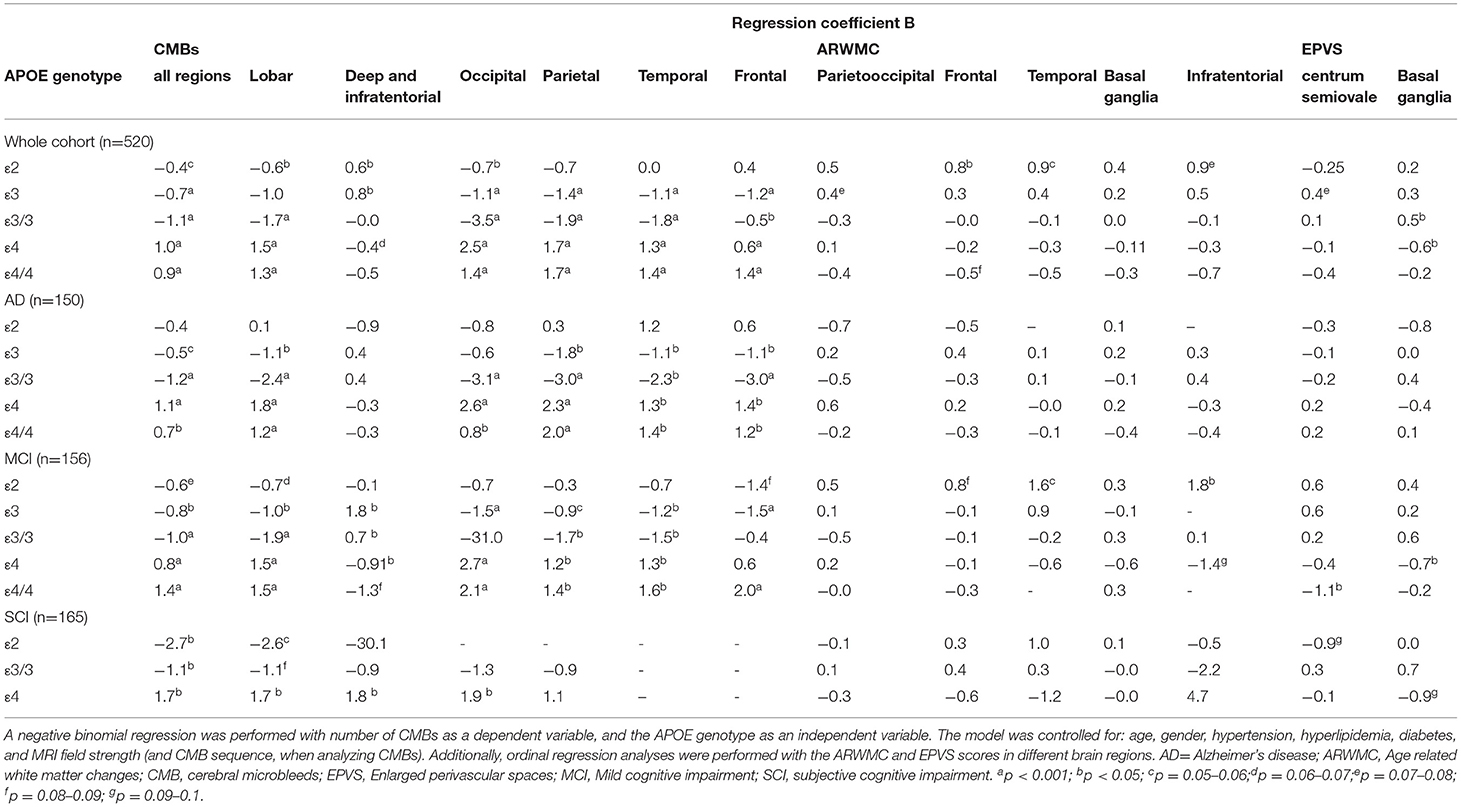
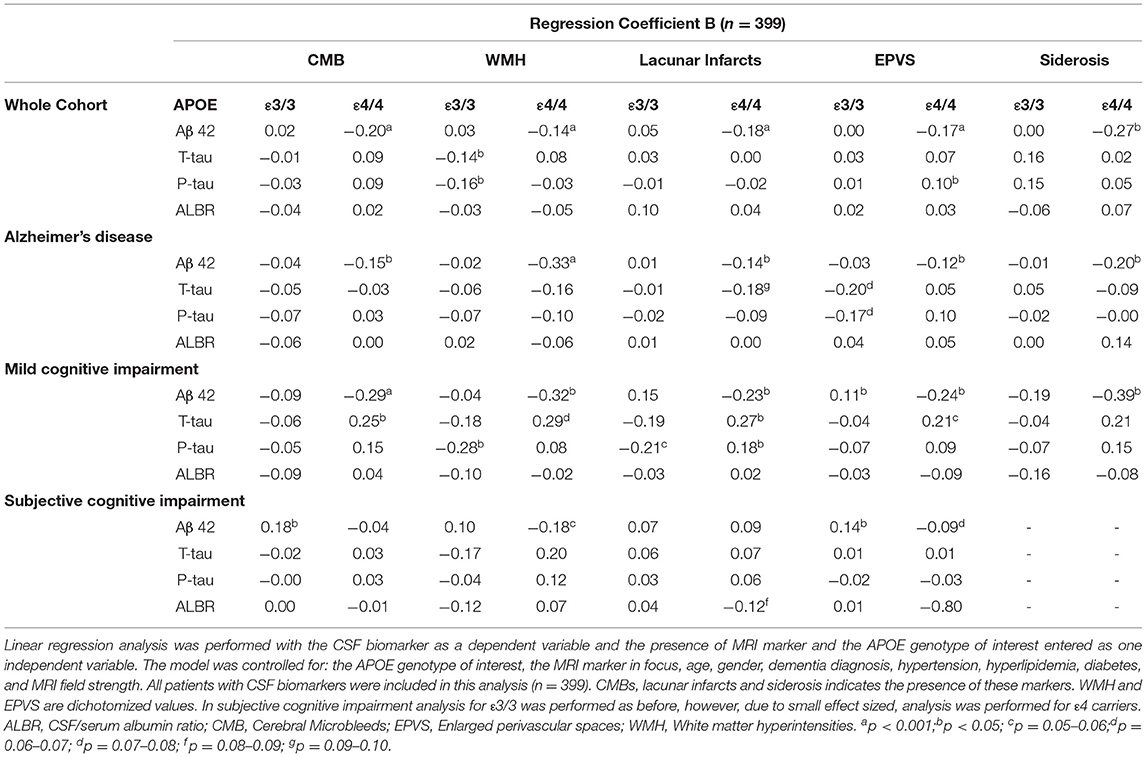
Results: Apolipoprotein E carriers with AD had a higher number of CMBs when looking at all brain regions and lobar brain regions (p < 0.001). A lower number of CMBs were seen in APOE ε2 (p < 0.05), ε3 and ε3/3 carriers (p < 0.001) when looking at all brain regions. A higher number of CMBs in deep and infratentorial regions were seen in APOE ε2 and ε3 (p < 0.05). In APOE ε4/4 carriers, CMBs, cortical superficial siderosis, white matter hyperintensities, and enlarged perivascular spaces were associated with lower levels of CSF amyloid β (Aβ) 42 in the whole cohort, and in individuals with AD and mild cognitive impairment (p < 0.05).
Conclusion: Apolipoprotein E ε4 is associated with MRI markers of SVD related to amyloid pathology, specifically CMBs and Aβ42 plaque formation in the brain, as reflected by decreased CSF Aβ42 levels, whereas APOE ε3 and ε2 are associated with the markers of hypertensive arteriopathy, as reflected by the association with CMBs in deep and infratentorial brain regions.
Introduction
Cerebral microbleeds (CMBs), cortical superficial siderosis, white matter hyperintensities, enlarged perivascular spaces, and lacunar infarcts are all seen as markers of small vessel disease (SVD) on magnetic resonance imaging (MRI) (Feldman et al., 2008; Doubal et al., 2010; Braun and Schreiber, 2013; Charidimou et al., 2013). CMBs have been specifically hypothesized to play an important role in the Alzheimer's pathophysiology (Cordonnier and van der Flier, 2011), are common in a memory clinic population (Cordonnier et al., 2006), and can be seen as a hypointense dots on hemosiderin sensitive MRI sequences and histopathologically as foci of hemosiderin deposits in the brain parenchyma (Werring et al., 2010). The main causes of CMBs are hypertensive arteriopathy, causing deep and infratentorial CMBs, and cerebral amyloid angiopathy, causing lobar CMBs (Werring, 2011). The topography of CMBs may thus help in understanding the underlying pathology. Cortical superficial siderosis is a subpial deposition of hemosiderin and has been linked to cerebral amyloid angiopathy (Feldman et al., 2008). White matter hyperintensities are seen as hyperintensities on T2-weighted and fluid attenuated inversion recovery (FLAIR) MRI sequences and are thought to be an expression of chronic white matter hypoperfusion (Brun and Englund, 1986; Pantoni, 2010). White matter hyperintensities have shown to predict the rate of cognitive decline in patients with Alzheimer's disease (AD) (Brickman et al., 2008). Enlarged perivascular spaces (EPVS) are perivascular cavities, which are fluid filled invaginations of the subarachnoid space, surrounding the penetrating vessels as they pass along and penetrate the subarachnoid space through the brain parenchyma (Braffman et al., 1988; Doubal et al., 2010).
All the abovementioned markers are demonstrated in patients with dementia (Werring, 2011; Zonneveld et al., 2014). MRI markers of SVD have shown to be especially prevalent in patients with vascular dementia and AD, and in AD, almost all patients are thought to suffer from cerebral amyloid angiopathy (Jellinger, 2002; Smith and Greenberg, 2009; Shams et al., 2014). However, although SVD MRI markers have been suggested to play an important role in dementia, their respective roles in dementia pathophysiology still remain unclear (Roher et al., 2003; Cordonnier and van der Flier, 2011).
The apolipoprotein E (APOE) allele is of importance in the development of sporadic and late AD (Verghese et al., 2011). The risk of AD in APOE allele carriers has been reported in the following order: ε4>ε3>ε2. Heterozygous or homozygous carriers for the APOE ε4 allele have an increased risk for late onset AD by 3- or 12-fold, respectively (Verghese et al., 2011). In the world of SVD, the ε4 allele is a risk factor for cerebral amyloid angiopathy and predisposes to intracerebral hemorrhage (Greenberg et al., 1995; Verghese et al., 2011; Schilling et al., 2013). Nevertheless, the role of the APOE ε4 allele in SVD and dementia is still not well understood.
We aimed to increase the understanding of SVD and APOE genotype in dementia by studying a large memory clinic population, focusing on groups with a clinical continuum of increasing AD pathology, from subjective cognitive impairment (SCI) to mild cognitive impairment (MCI) and AD. We hypothesized that ε4 carriers, in contrast to ε3 and ε2 carriers, would have accentuated markers of SVD related with cerebral amyloid angiopathy, especially patients with AD.
Materials and Methods
Study Population
This study is part of the Karolinska Imaging Dementia Study (KIDS), a memory clinic based cross-sectional study on SVD in cognitive impairment. In total, 521 consecutive patients were enrolled, and all patients had been undergoing dementia investigation with accompanying APOE allele analysis and MRI scans at the memory clinic and radiology department, Karolinska University Hospital, between 01/01/2006 and 01/01/2012. Exclusion criteria for all patients were insufficient scan quality on the MRI and a history of traumatic brain injury. In our study, one patient was excluded due to poor scan quality on MRI, leading to a final cohort of 520 patients with 5 different diagnostic categories. The diagnostic category, “Other Disorders” (n = 38), was, however, discarded due to the heterogeneous nature of this group of patients, which limited statistical analysis. The diagnosis was set based on the ICD-10 criteria by an experienced memory clinic team consisting of geriatricians, neuropsychologists, neurophysiologists, and neuroradiologists after the entire clinical picture had been considered. The ICD-10 code used for MCI was G31.84. SCI was used as a diagnosis when the patients had subjective symptoms without objective clinical findings, using ICD code Z03.3. Patient demographics have been outlined in Table 1 and a flow diagram of the participants enrolled in the study can be seen in Figure 1. The presence of hypertension, hyperlipidemia, and diabetes were determined based on self-report/prior medical diagnosis and treatment for patients.
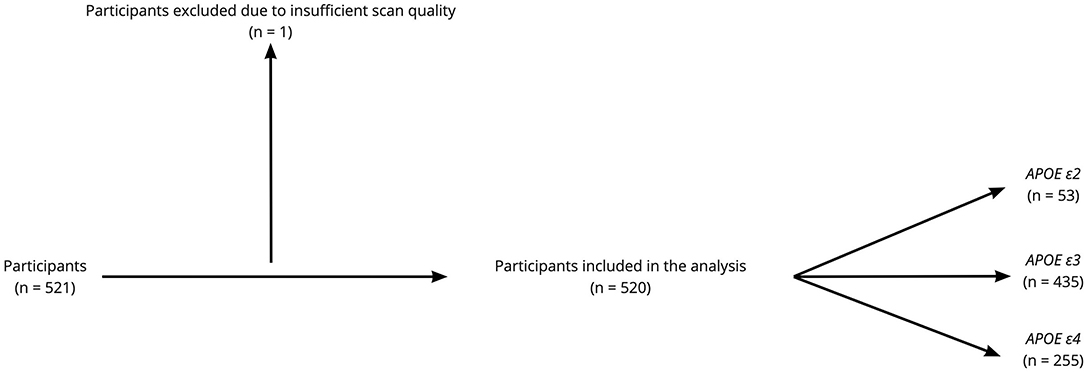
Figure 1. Flow diagram of the participants enrolled in the study. Among the 520 participants included in the analysis, 399 participants had cerebrospinal fluid (CSF) analysis for routine Alzheimer's disease (AD) biomarkers.
Informed consent was obtained for all patients according to the Declaration of Helsinki, and ethical approval was obtained from the regional ethical board in Stockholm, Sweden.
MRI Protocol
Patients were scanned on three MRI scanners (Siemens Medical Systems, Erlangen, Germany) at the radiology department, Karolinska University Hospital, Huddinge. Axial SWI and/or T2* sequences, as well as conventional MRI sequences, such as T1, T2, FLAIR (axial), and diffusion- weighted imaging (DWI), were obtained for all patients. Patients were randomly assigned to the different MRI scanners based on clinical availability, as well as the T2* and SWI sequences. Furthermore, 155 patients were scanned on the 1.5T Siemens Magnetom Symphony, 212 patients on the 1.5T Siemens Magnetom Avanto, 153 patients were scanned on the Siemens Magnetom Trio 3.0T. In the whole cohort, the distribution of patients scanned on the 3T and with SWI sequences included were as follow: AD (3T: 27% and SWI: 19%), MCI (3T: 32% and SWI: 16%), SCI (3T: 28% and SWI: 17%), VaD (3T: 27% and SWI: 18%), and other dementias (3T: 32% and SWI: 24%).
Image Analysis
All MRI images were jointly analyzed by a senior consultant neuroradiologist and an MD/PhD student with 3 years of training and experience in neuroradiology at the time of rating, with both being fully blinded to all patient data during rating.
CMBs were rated on axial T2* and/or SWI according to the microbleed anatomical rating scale (Gregoire et al., 2009), with minor modifications to ensure increased accuracy of CMBs ratings as outlined previously (Shams et al., 2014). Modifications of the rating scale were as follows: CMBs were not rated as probable, and to reduce the number of CMB mimics, hypointensities in the globus pallidus, which may represent calcifications or physiologic iron deposits, were not rated, and similarly, images in which patients had a deep venous anomaly in the vicinity of CMBs were not rated as deep venous anomalies increase the risk of adjacent cavernomas, which can mimic a CMBs. Last, hemorrhagic sensitive sequences were analyzed together with T2-weighted images to better distinguish between vessels and flow voids, which also may mimic CMBs (Shams et al., 2014).
White matter hyperintensities were rated on axial FLAIR images according to the Fazekas scale (0 = none or single, 1 = punctate, 2 = early confluating, and 3 = confluating) (Fazekas et al., 1987) and the age-related white matter changes scale (Wahlund et al., 2001) (0 = none, 1 = punctate, 2 = early confluating, and 3 = confluating. Rated in the following brain regions: infratentorial, parieto-occipital, frontal, temporal, and the basal ganglia.). EPVSs were rated on an axial T2 according to the enlarged perivascular rating scale (0 = none, 1 = 1–10, 2 = 11–20, 3 = 21–40, and 4 = >40) (Maclullich et al., 2004; Doubal et al., 2010). EPVSs were defined as <3 mm in size and thus distinguished from lacunar infarctions that were defined as 3–15 mm in size, with cerebrospinal fluid (CSF) signal on FLAIR, T2 and T1. Cortical superficial siderosis was defined as a linear gyriform pattern of hypointense signal on T2* and/or SWI (Feldman et al., 2008; Vernooij et al., 2009).
CSF Analysis
Cerebrospinal fluid samples were obtained by lumbar puncture in a total of 399 patients and collected in 10 ml polypropylene tubes at the department of clinical chemistry, Karolinska University Hospital, Huddinge. All CSF samples were centrifuged within 2 h, at 1,900 g for 10 min and then frozen until analysis. A small amount of CSF was used for routine analysis of total cells, total protein, and glucose levels. Biomarkers were measured with a sandwich type enzyme linked immunosorbent assay; amyloid β 42 (Aβ42) was measured with Innotest β-Amyloid (1–42), total tau (T-tau) with Innotest hTau-Ag, and tau phosphorylated at threonine 181 (P-tau) with Innotest Phospho-tau(181P) (Innogenetics, Ghent, Belgium). The unit used for biomarkers is ng/L. Corresponding blood samples were collected at the same visit as the lumbar puncture to quantify CSF/serum albumin ratios (Tibbling et al., 1977). The team involved in CSF and blood analysis were unaware of the dementia diagnosis and MRI images.
APOE Genotyping
The APOE genotyping was performed on all patients (n = 520) on coded genomic DNA samples. All analyses were performed at the department of clinical chemistry, Karolinska University Hospital. The team involved in the genotype analysis were unaware of the dementia diagnosis and the neuroimaging data.
Statistics
Generalized linear models were used to determine the association between APOE genotype and MRI markers of SVD. Multiple binary logistic regression analyses were performed with the APOE genotype as an independent variable and dichotomized MRI markers as dependent variables. The APOE genotype was stratified into ε2 (such as, ε2/2, ε2/3, and ε2/4 alleles) and ε4 (ε4/4, ε4/3, and ε2/4 alleles). Separate analysis was done for ε4/4 when effect sizes were sufficient. APOE ε3/3 was used as a reference for all APOE genotypes in our regression models. White matter hyperintensities were dichotomized by separating high scores (2 and 3) from low scores (0 and 1) on the rating scales. Similarly, EPVSs were dichotomized by separating high scores (3 and 4) from low scores (0, 1, and 2) as per the enlarged perivascular scale. Multiple CMBs were defined as having more than 1 CMBs. Negative binomial regression analysis was performed to assess the association between APOE genotype and CMB topography. The number of CMBs in the different topographies was used as a dependent variable and the APOE genotype as an independent variable. Ordinal regression models were used with age related white matter changes and enlarged perivascular scores in different locations as dependent variables and the APOE genotype as an independent variable. All the above models were adjusted for age, gender, hypertension, hyperlipidemia, diabetes, and MRI field strength (and CMB sequence in the negative binomial regression analysis) due to the statistical significance of these markers in multivariable regression models. Linear regression models were used to study the cumulative effect of the presence of APOE ε4/4 or ε3/3 and the MRI marker in focus, on CSF biomarkers. Log transformed CSF biomarkers were put as dependent variables. The presence of APOE homozygote carriership and MRI marker was defined as a separate independent variable, and other independent adjusting variables in the model were: APOE genotype in focus, MRI marker of interest, age, gender, hypertension, hyperlipidemia, diabetes, dementia diagnosis, and MRI field strength. Due to the larger group size, statistical testing was focused on AD, MCI, and SCI. SPSS was used for the statistical analysis and p < 0.05 was set as the threshold of significance.
Results
Patient demographics are presented in Table 1 and imaging markers of SVD analyzed can be seen in Figure 2. In the whole cohort, APOE ε4, ε4/4, and ε2 carriers showed a higher number of CMBs (p < 0.05). A higher number of lacunar infarcts were seen in APOE ε4/4 carriers with MCI (p = 0.01) and APOE ε2 carriers with SCI (p = 0.01). Table 2 shows the odds ratios (ORs) for the SVD markers on MRI depending on the APOE genotype. Only two patients with SCI had APOE ε2/2 and both had the lowest dichotomized white matter hyperintensity and enlarged perivascular score, had no CMBs, large or lacunar infarctions, intracerebral hemorrhage, or cortical superficial siderosis.
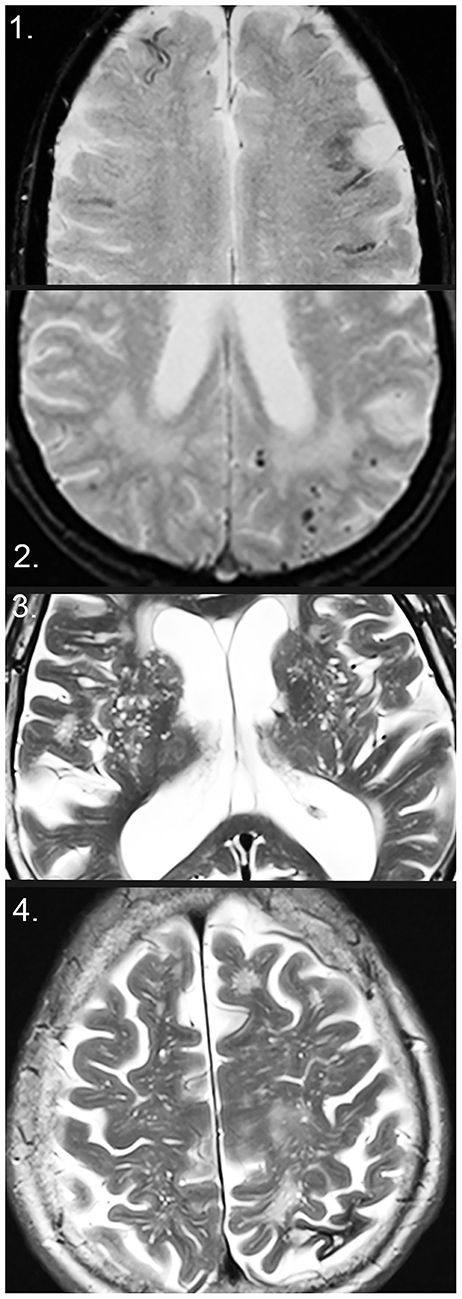
Figure 2. Markers of small vessel disease on MRI. (1) Disseminated superficial siderosis on T2*. (2) Cerebral microbleeds (CMBs), as well as white matter hyperintensities on T2*. (3) Enlarged perivascular spaces (EPVS) in the basal ganglia on T2. (4) EPVS in the centrum semiovale on T2 and intracerebral macrohemorrhage with local atrophy in the left parietal lobe.
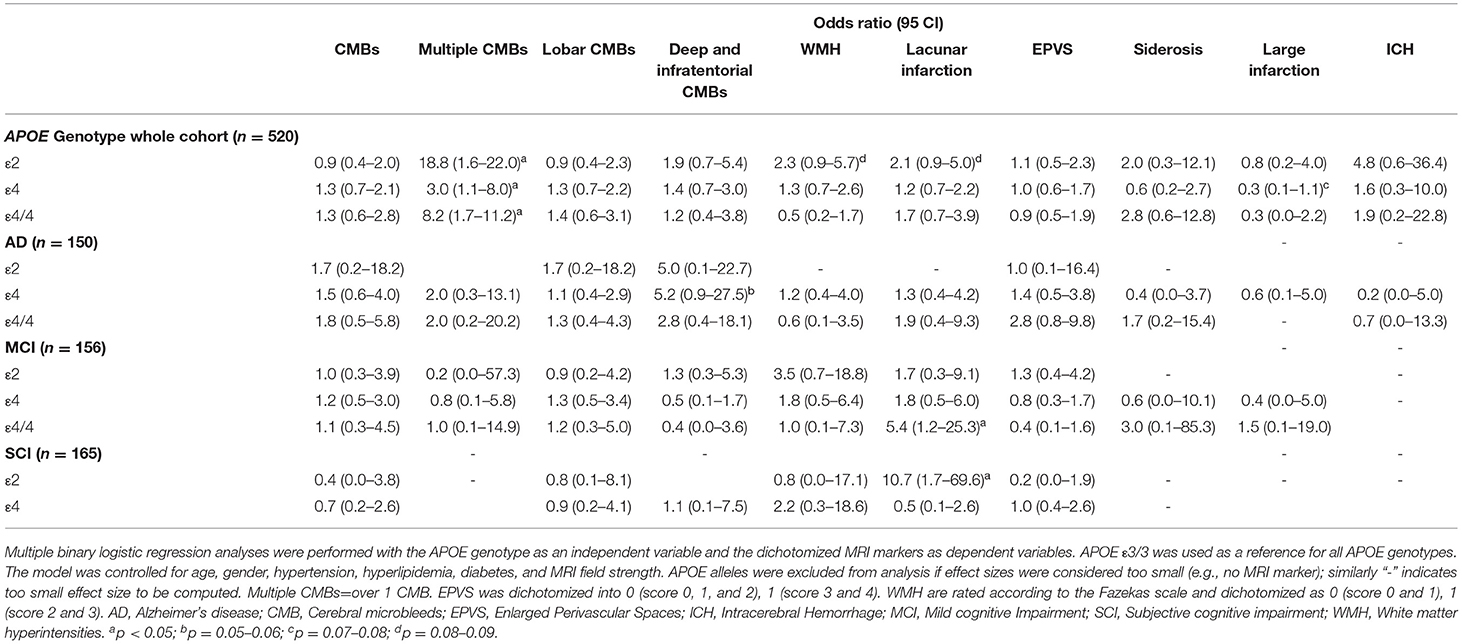
Table 2. Odds ratios (ORs) and 95% confidence interval (CI) for markers of small vessel disease by APOE alleles.
APOE Alleles and Cerebral Microbleeds
The association between the APOE allele and the number of MRI markers of SVD can be seen in Table 3.
APOE ε4
In the whole cohort, APOE ε4 and ε4/4 carriers showed a higher number of CMBs, especially in lobar brain regions (p < 0.001). A separate analysis of each brain lobe in ε4 and ε4/4 carriers showed a significantly higher number of CMBs (p < 0.001). Patients with AD and MCI who were APOE ε4 carriers had a higher number of CMBs in the lobar brain region. Patients with SCI who were APOE ε4 carriers had a higher number of CMBs in lobar, deep, infratentorial, and occipital brain regions (p < 0.05).
APOE ε2 and ε3
The ε2, ε3, and ε3/3 carriers had a lower number of CMBs (p < 0.001). Topographical analysis showed that CMBs were lower in the brain lobes in ε2 and ε3/3 carriers, lower in the occipital lobe in ε2, ε3, and ε3/3 carriers, and lower in the parietal lobe in ε3 and ε3/3 carriers (p < 0.001). The number of CMBs in deep and infratentorial regions were higher in APOE ε2 and ε3 carriers (p < 0.05). This pattern was also seen when looking into the separate diagnostic groups. Patients with AD or MCI who were ε3 carriers had a lower number of CMBs in lobar regions (p < 0.001).
APOE Alleles, MRI Markers of SVD, and CSF Biomarkers
Table 4 shows the relationship between CSF biomarkers and SVD markers in APOE carriers.
APOE ε4
In the whole cohort, there was a negative association between the level of CSF Aβ42 and the number of CMBs, white matter hyperintensities, lacunar infarcts, enlarged perivascular spaces, and cortical superficial siderosis in APOE ε4/4 carriers (p < 0.001). This held true in AD as well as MCI. In the whole cohort, an association was seen in APOE ε4/4 carriers with the higher levels of CSF P-Tau and a higher enlarged perivascular space score (p < 0.05). APOE ε4/4 carriers with MCI demonstrated an association between higher T-tau and P-tau levels with lacunar infarctions and higher T-tau with CMBs (p < 0.05).
APOE ε3
Apolipoprotein E ε3/3 carriers showed lower levels of CSF T-tau and P-tau with increasing white matter hyperintensity (p < 0.05). In APOE ε3/3, a lower level of P-tau was observed with white matter hyperintensity (p < 0.05). APOE ε3/3 carriers with SCI showed a higher CSF Aβ42 concentration with CMBs and EPVSs (p < 0.05).
APOE Alleles and MRI Markers of SVD in Vascular Dementia
All patients with vascular dementia were APOE ε3 carriers (7 patients homozygous for the ε3 allele and 4 patients being heterozygous for the ε3 allele). Comparing these two groups, we found that among ε3 carriers, 2/4 had CMBs, 3/4 had high white matter hyperintensity score, 2/4 had high enlarged perivascular space score, 3/4 had lacunar infarcts, 2/4 had a large infarction, no one had intracerebral hemorrhage, and 1/4 had cortical superficial siderosis. In patients homozygous for the ε3 allele, 3/7 had CMBs, 3/7 had high white matter hyperintensity score, 2/7 had high enlarged perivascular space score, 6/7 had lacunar infarcts, 3/7 had large infarcts, 1/7 had a large bleeding, and no one had siderosis.
Discussion
We show that APOE ε4 carriers have more pronounced SVD MRI markers associated with amyloid pathology, whereas ε3 and ε2 carriers demonstrate MRI markers related with hypertensive arteriopathy. CSF profiles with the presence of SVD in APOE ε4 carriers indicate the importance of SVD in the clinical continuum of AD pathology, from MCI to AD.
Previous studies investigating markers of SVD and APOE genotype in dementia are scarce. In healthy populations, a higher number of lobar CMBs with the APOE ε4 allele, compared with carriers of ε3/3, have been seen (Poels et al., 2012), in line with our results. The association between possession of the APOE ε4 or ε2 genotype and lobar CMBs has also been shown in a stroke population (Kim et al., 2013). However, in the Framingham study, no relationship between the APOE allele and CMBs was found (Jeerakathil et al., 2004).
White matter hyperintensities, studied in general populations, have been shown to increase with APOE ε4, ε4/4, and ε2 (Schilling et al., 2013). Increased white matter hyperintensity volume in the parietal lobe has been shown to predict incident AD and increased parietal white matter hyperintensity volume has been shown to be linked with APOE ε4 (Brickman et al., 2012, 2014). We demonstrated an association between APOE ε2 and higher white matter hyperintensity burden in frontal brain regions. No other relationships between white matter hyperintensity burden, globally or in different brain regions, and the APOE genotype was found. No relationship between APOE and lacunar infarcts has been shown (Kim et al., 2013).
To the best of our knowledge, EPVSs and APOE genotype in dementia have not been investigated previously. Cortical superficial siderosis has been reported to be associated with a APOE ε4 in a memory clinic population (Zonneveld et al., 2014), but this was not demonstrated in our cohort. Intracerebral hemorrhage has been associated with APOE ε4 (Brickman et al., 2014), however, no such relationship was seen in our cohort.
The association between SVD with APOE ε2 are of special interest, as APOE ε2, in contrast to the ε4 allele, is considered to be a protective allele with lower risk of AD related neurodegeneration (Suri et al., 2013). The presence of the APOE ε2 allele has been shown to decrease the risk for AD by a factor of 4 (Corder et al., 1994; Suri et al., 2013). When looking at the APOE ε2 allele, we could see a lower number of lobar CMBs, and a higher number of deep and infratentorial CMBs. The pattern of CMBs seen in APOE ε2 carriers support that deep and infratentorial CMBs are of a different pathogenesis than lobar CMBs, and presumably have little implication in the pathophysiology of AD. However, the positive association between cortical superficial siderosis with APOE ε2, but not APOE ε4, shown in a study in cerebral amyloid angiopathy patients is interesting and may suggest that the underlying pathophysiologic mechanism giving rise to cortical superficial siderosis differs from that giving rise to CMBs in cerebral amyloid angiopathy (Shoamanesh et al., 2014). Moreover, a higher white matter hyperintensity burden was seen in frontal and infratentorial brain regions, in the whole cohort and MCI, respectively, with APOE ε2. A higher number of lacunar infarcts were seen in APOE ε2 carriers in SCI. Our results imply that APOE ε2 is associated with hypertensive arteriopathy, as reflected through the association with deep and infratentorial CMBs, white matter hyperintensity, and lacunar infarcts, and thus hypertensive arteriopathy may cause cortical superficial siderosis and in part explain the increased association of siderosis with APOE ε2.
The APOE genotype, imaging markers of SVD (such as, CMBs, white matter hyperintensities, and lacunar infarcts), and CSF biomarkers in dementia have previously been investigated and have shown lower CSF Aβ42 levels with the presence of CMBs and white matter hyperintensities in APOE ε4 carriers, reflecting increased deposition of Aβ42 in the brain parenchyma (Shoamanesh et al., 2014).
We included additional markers of SVD and demonstrated decreased Aβ42 levels with all included SVD markers in APOE ε4/4 carriers. Moreover, we found higher T-tau and P-tau levels with some SVD markers in APOE ε4/4 carriers in patients with MCI, reflecting the importance of SVD markers in an early stage of dementia, possibly contributing to the dementia pathophysiology. As this relationship was only seen in MCI, it may imply that SVD markers in the early stages of dementia contribute to neuronal damage and facilitates the formation of neurofibrillary tangles as represented by T-tau and P-tau, respectively, ultimately contributing to the final picture of AD. Further on, this implies that the pathophysiology of dementia may partly be mediated through SVD. Patients with SCI and APOE ε4, however, demonstrated minor association between CSF biomarkers and SVD markers, although tendencies toward lower Aβ42 levels with EPVSs and white matter hyperintensities were seen. This may be explained by the fact that SCI represents a heterogeneous group of individuals with cognitive complaints, and that only heterozygous carriers for the ε4 allele were analyzed due to the scarcity of homozygous ε4 carriers in SCI.
To further investigate our hypothesis on the importance of SVD in the early stages of dementia, prospective, longitudinal studies on patients with SCI and MCI should be conducted to see if patients with SVD develop dementia more rapidly.
The stratification of SVD markers in hypertensive arteriopathy with APOE ε3 and ε2, and an amyloid-based pattern with the APOE ε4 genotype, is of interest. In our cohort, all patients with vascular dementia were ε3 carriers and had an overweight of vascular SVD markers, with high baseline CSF Aβ42 levels. However, the synergism between vascular and amyloid pathology, and their overlap in MRI SVD expression is of importance to keep in mind. Amyloid has been shown to impair vessel function and lead to small vessel ischemia (Kester et al., 2014), which may lead to the development of markers caused by vascular pathology, such as lacunar infarctions, cerebral microinfarcts, and white matter hyperintensities. Furthermore, the association of APOE ε2 with markers of hypertensive arteriopathy may not be direct, but rather due to the fact that APOE ε2 carriers tend to have a lower amyloid burden and thus require a greater severity of hypertensive SVD for cognitive impairment to develop. The same alternate explanation may hold true for APOE ε3 carriers, where an association with hypertensive arteriopathy was seen.
The strengths of our study include a large cohort, with patients undergoing thorough dementia investigation as well as neuroradiological analysis. Limitations include a small cohort size when analyzing the separate diagnostic groups, such as vascular dementia, and analyses were excluded if effect sizes in the separate diagnoses were too small. Another limitation is that the dementia diagnosis was set after considering both the clinical and radiological picture, which may have affected the final designation of diagnosis.
In conclusion, our study emphasizes the possible importance of SVD in a continuum of cognitive impairment. APOE ε4 is mainly associated with markers of the amyloid pathology, whereas APOE ε3 and ε2 demonstrate associations with hypertensive arteriopathy.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the regional ethical board in Stockholm, Sweden. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MS: collection of data, establishing database, statistical analysis, and drafting of manuscript. SS: study concept and design, data acquisition, establishing database, and image analysis. JM and LC: image analysis. TG: data acquisition and power calculations for the KIDS. MK: project supervision, study design, critical analysis, and revision of the manuscript. MW: project supervision, critical analysis, and revision of the manuscript. EW: critical analysis and revision of the manuscript. PA, MKW, and L-OW: project supervision, study and planning, patient inclusion and recruitment, and as well as clinical and MRI logistics. All authors designed and conceptualized the study, engaged in data analysis and subsequent critical analysis, and revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study is funded by the Stockholm County Council, Karolinska Institutet, and the Swedish Dementia Association.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to acknowledge Seyed-Mohammad Fereshtehnejad for his engagement in this project.
Abbreviations
MRI, Magnetic resonance imaging; FLAIR, Fluid attenuated inversion recovery; KIDS, Karolinska imaging dementia study; SVD, Small vessel disease; APOE, Apolipoprotein E; CSF, Cerebrospinal fluid; AD, Alzheimer's disease; MCI, Mild cognitive impairment; SCI, Subjective cognitive impairment.
References
Braffman, B. H., Zimmerman, R. A., Trojanowski, J. Q., Gonatas, N. K., Hickey, W. F., and Schlaepfer, W. W. (1988). Brain MR: pathologic correlation with gross and histopathology. 1. Lacunar infarction and Virchow-Robin spaces. AJR Am. J. Roentgenol. 151, 551–558. doi: 10.2214/ajr.151.3.551
Braun, H., and Schreiber, S. (2013). Microbleeds in cerebral small vessel disease. Lancet Neurol. 12, 735–736. doi: 10.1016/S1474-4422(13)70148-0
Brickman, A. M., Honig, L. S., Scarmeas, N., Tatarina, O., Sanders, L., Albert, M. S., et al. (2008). Measuring cerebral atrophy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer disease. Arch. Neurol. 65, 1202–1208. doi: 10.1001/archneur.65.9.1202
Brickman, A. M., Provenzano, F. A., Muraskin, J., Manly, J. J., Blum, S., Apa, Z., et al. (2012). Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident Alzheimer disease in the community. Arch. Neurol. 69, 1621–1627. doi: 10.1001/archneurol.2012.1527
Brickman, A. M., Schupf, N., Manly, J. J., Stern, Y., Luchsinger, J. A., Provenzano, F. A., et al. (2014). APOE ε4 and risk for Alzheimer's disease: Do regionally distributed white matter hyperintensities play a role? Alzheimers Dement J Alzheimers Assoc 10, 619–629. doi: 10.1016/j.jalz.2014.07.155
Brun, A., and Englund, E. (1986). A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann. Neurol. 19, 253–262. doi: 10.1002/ana.410190306
Charidimou, A., Jäger, R. H., Fox, Z., Peeters, A., Vandermeeren, Y., Laloux, P., et al. (2013). Prevalence and mechanisms of cortical superficial siderosis in cerebral amyloid angiopathy. Neurology 81, 626–632. doi: 10.1212/WNL.0b013e3182a08f2c
Corder, E. H., Saunders, A. M., Risch, N. J., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., et al. (1994). Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat. Genet. 7, 180–184. doi: 10.1038/ng0694-180
Cordonnier, C., and van der Flier, W. M. (2011). Brain microbleeds and Alzheimer's disease: innocent observation or key player? Brain. doi: 10.1017/CBO9780511974892.016
Cordonnier, C., van der Flier, W. M., Sluimer, J. D., Leys, D., Barkhof, F., and Scheltens, P. (2006). Prevalence and severity of microbleeds in a memory clinic setting. Neurology 66, 1356–1360. doi: 10.1212/01.wnl.0000210535.20297.ae
Doubal, F. N., MacLullich, A. M. J., Ferguson, K. J., Dennis, M. S., and Wardlaw, J. M. (2010). Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke J. Cereb. Circ. 41, 450–454. doi: 10.1161/STROKEAHA.109.564914
Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I., and Zimmerman, R. A. (1987). MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am. J. Roentgenol. 149, 351–356. doi: 10.2214/ajr.149.2.351
Feldman, H. H., Maia, L. F., Mackenzie, I. R. A., Forster, B. B., Martzke, J., and Woolfenden, A. (2008). Superficial siderosis: a potential diagnostic marker of cerebral amyloid angiopathy in Alzheimer disease. Stroke J. Cereb. Circ. 39, 2894–2897. doi: 10.1161/STROKEAHA.107.510826
Greenberg, S. M., Rebeck, G. W., Vonsattel, J. P., Gomez-Isla, T., and Hyman, B. T. (1995). Apolipoprotein E epsilon 4 and cerebral hemorrhage associated with amyloid angiopathy. Ann. Neurol. 38, 254–259. doi: 10.1002/ana.410380219
Gregoire, S. M., Chaudhary, U. J., Brown, M. M., Yousry, T. A., Kallis, C., Jäger, H. R., et al. (2009). The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology 73, 1759–1766. doi: 10.1212/WNL.0b013e3181c34a7d
Jeerakathil, T., Wolf, P. A., Beiser, A., Hald, J. K., Au, R., Kase, C. S., et al. (2004). Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke J. Cereb Circ. 35, 1831–1835. doi: 10.1161/01.STR.0000131809.35202.1b
Jellinger, K. A. (2002). Alzheimer disease and cerebrovascular pathology: an update. J. Neural. Transm. 109, 813–836. doi: 10.1007/s007020200068
Kester, M. I., Goos, J. D. C., Teunissen, C. E., Benedictus, M. R., Bouwman, F. H., Wattjes, M. P., et al. (2014). Associations between cerebral small-vessel disease and Alzheimer disease pathology as measured by cerebrospinal fluid biomarkers. JAMA Neurol. 71, 855–862. doi: 10.1001/jamaneurol.2014.754
Kim, H. J., Ye, B. S., Yoon, C. W., Cho, H., Noh, Y., Kim, G. H., et al. (2013). Effects of APOE ε4 on brain amyloid, lacunar infarcts, and white matter lesions: a study among patients with subcortical vascular cognitive impairment. Neurobiol. Aging 34, 2482–2487. doi: 10.1016/j.neurobiolaging.2013.05.009
Maclullich, A. M. J., Wardlaw, J. M., Ferguson, K. J., Starr, J. M., Seckl, J. R., and Deary, I. J. (2004). Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J. Neurol. Neurosurg. Psychiatry 75, 1519–1523. doi: 10.1136/jnnp.2003.030858
Pantoni, L. (2010). Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 9, 689–701. doi: 10.1016/S1474-4422(10)70104-6
Poels, M. M. F., Ikram, M. A., van der Lugt, A., Hofman, A., Niessen, W. J., Krestin, G. P., et al. (2012). Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology 78, 326–333. doi: 10.1212/WNL.0b013e3182452928
Roher, A. E., Kuo, Y. M., Esh, C., Knebel, C., Weiss, N., Kalback, W., et al. (2003). Cortical and Leptomeningeal Cerebrovascular Amyloid and White Matter Pathology in Alzheimer's Disease. Mol. Med. 9, 112–122. doi: 10.1007/BF03402043
Schilling, S., DeStefano, A. L., Sachdev, P. S., Choi, S. H., Mather, K. A., DeCarli, C. D., et al. (2013). APOE genotype and MRI markers of cerebrovascular disease: systematic review and meta-analysis. Neurology 81, 292–300. doi: 10.1212/WNL.0b013e31829bfda4
Shams, S., Martola, J., Granberg, T., Li, X., Shams, M., Fereshtehnejad, S. M., et al. (2014). Cerebral microbleeds: different prevalence, topography, and risk factors depending on dementia diagnosis—the karolinska imaging dementia study. Am J Neuroradiol. doi: 10.3174/ajnr.A4176
Shoamanesh, A., Martinez-Ramirez, S., Oliveira-Filho, J., Reijmer, Y., Falcone, G. J., Ayres, A., et al. (2014). Interrelationship of superficial siderosis and microbleeds in cerebral amyloid angiopathy. Neurology 83, 1838–1843. doi: 10.1212/WNL.0000000000000984
Smith, E. E., and Greenberg, S. M. (2009). Beta-amyloid, blood vessels, and brain function. Stroke J. Cereb. Circ. 40, 2601–2606. doi: 10.1161/STROKEAHA.108.536839
Suri, S., Heise, V., Trachtenberg, A. J., and Mackay, C. E. (2013). The forgotten APOE allele: a review of the evidence and suggested mechanisms for the protective effect of APOE ε2. Neurosci. Biobehav. Rev. 37, 2878–2886. doi: 10.1016/j.neubiorev.2013.10.010
Tibbling, G., Link, H., and Ohman, S. (1977). Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand. J. Clin. Lab. Invest. 37, 385–390. doi: 10.3109/00365517709091496
Verghese, P. B., Castellano, J. M., and Holtzman, D. M. (2011). Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 10, 241–252. doi: 10.1016/S1474-4422(10)70325-2
Vernooij, M. W., Ikram, M. A., Hofman, A., Krestin, G. P., Breteler, M. M. B., and van der Lugt, A. (2009). Superficial siderosis in the general population. Neurology 73, 202–205. doi: 10.1212/WNL.0b013e3181ae7c5e
Wahlund, L. O., Barkhof, F., Fazekas, F., Bronge, L., Augustin, M., Sjögren, M., et al. (2001). A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke J. Cereb. Circ. 32, 1318–1322. doi: 10.1161/01.STR.32.6.1318
Werring, D. J. (2011). Cerebral Microbleeds: Pathophysiology to Clinical Practice, 1st ed. Cambridge, MA: Cambridge University Press.
Werring, D. J., Gregoire, S. M., and Cipolotti, L. (2010). Cerebral microbleeds and vascular cognitive impairment. J. Neurol. Sci. 299, 131–135. doi: 10.1016/j.jns.2010.08.034
Keywords: cerebral small vessel disease, cerebral amyloid angiopathy, hypertensive vasculopathy, apolipoprotein E, dementia, Alzheimer's disease, magnetic resonance imaging
Citation: Shams M, Shams S, Martola J, Cavallin L, Granberg T, Kaijser M, Wintermark M, Westman E, Aspelin P, Kristoffersen Wiberg M and Wahlund L-O (2022) MRI Markers of Small Vessel Disease and the APOE Allele in Cognitive Impairment. Front. Aging Neurosci. 14:897674. doi: 10.3389/fnagi.2022.897674
Received: 16 March 2022; Accepted: 17 June 2022;
Published: 13 July 2022.
Edited by:
Nibaldo C. Inestrosa, Pontificia Universidad Católica de Chile, ChileReviewed by:
Matteo De Marco, Brunel University London, United KingdomQian Wang, Guangzhou Women and Children's Medical Center, China
Copyright © 2022 Shams, Shams, Martola, Cavallin, Granberg, Kaijser, Wintermark, Westman, Aspelin, Kristoffersen Wiberg and Wahlund. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mana Shams, bWFuYS5zaGFtc0BraS5zZQ==
 Mana Shams
Mana Shams Sara Shams1,2
Sara Shams1,2 Tobias Granberg
Tobias Granberg Magnus Kaijser
Magnus Kaijser Eric Westman
Eric Westman Maria Kristoffersen Wiberg
Maria Kristoffersen Wiberg Lars-Olof Wahlund
Lars-Olof Wahlund