
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 11 July 2022
Sec. Alzheimer's Disease and Related Dementias
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.893018
This article is part of the Research TopicThe Alzheimer's Disease Challenge, Volume IIView all 14 articles
 Ghulam Md Ashraf1,2*
Ghulam Md Ashraf1,2* Stylianos Chatzichronis3
Stylianos Chatzichronis3 Athanasios Alexiou3,4*
Athanasios Alexiou3,4* Gazala Firdousi5
Gazala Firdousi5 Mohammad Amjad Kamal6,7,8,9
Mohammad Amjad Kamal6,7,8,9 Magdah Ganash10
Magdah Ganash10Alzheimer’s disease is still an incurable disease with significant social and economic impact globally. Nevertheless, newly FDA-approved drugs and non-pharmacological techniques may offer efficient disease treatments. Furthermore, it is widely accepted that early diagnosis or even prognosis of Alzheimer’s disease using advanced computational tools could offer a compelling alternative way of management. In addition, several studies have presented an insight into the role of mitochondrial dynamics in Alzheimer’s development. In combination with diverse dietary and obesity-related diseases, mitochondrial bioenergetics may be linked to neurodegeneration. Considering the probabilistic expectations of Alzheimer’s disease development or progression due to specific risk factors or biomarkers, we designed a Bayesian model to formulate the impact of diet-induced obesity with an impaired mitochondrial function and altered behavior. The applied probabilities are based on clinical trials globally and are continuously subject to updating and redefinition. The proposed multiparametric model combines various data types based on uniform probabilities. The program simulates all the variables with a uniform distribution in a sample of 1000 patients. First, the program initializes the variable age (30–95) and the four different diet types (“HFO_diet,” “Starvation,” “HL_diet,” “CR”) along with the factors that are related to prodromal or mixed AD (ATP, MFN1, MFN2, DRP1, FIS1, Diabetes, Oxidative_Stress, Hypertension, Obesity, Depression, and Physical_activity). Besides the known proteins related to mitochondrial dynamics, our model includes risk factors like Age, Hypertension, Oxidative Stress, Obesity, Depression, and Physical Activity, which are associated with Prodromal Alzheimer’s. The outcome is the disease progression probability corresponding to a random individual ID related to diet choices and mitochondrial dynamics parameters. The proposed model and the programming code are adjustable to different parameters and values. The program is coded and executed in Python and is fully and freely available for research purposes and testing the correlation between diet type and Alzheimer’s disease progression regarding various risk factors and biomarkers.
Mitochondria are vital organelles across every nucleated cell that generate energy in the form of ATP via the oxidative phosphorylation (OXPHOS) system (Saada, 2014). Mitochondria are defined as dynamic organelles that have been through coordinated cycles of fission and fusion, referred to as mitochondrial dynamics. Mitochondrial dynamics are ongoing processes of mitochondrial fusion, fission, biogenesis, and mitophagy that work together to maintain optimal cellular bioenergetics and reactive oxygen species (ROS) homeostasis (Archer, 2013). These mitochondrial dynamics regulate the number, distribution, and morphology of mitochondria in the cell and thus play critical roles in various mitochondrial functions such as energy production, metabolism, intracellular signaling, and apoptosis. Mitochondrial fusion is carried out by the dynamin-like GTPases regulatory mitofusin proteins mfn1, mfn2, and Opa1 (Itoh et al., 2013; Mishra and Chan, 2016). Mfn1 and Mfn2 are outer membranous proteins found on the outer side of the mitochondrial membrane, whereas OPA1 is a transmembrane protein found within the inner mitochondrial membrane (IMM) (Ni et al., 2015) that plays a role in mitochondrial quality regulation, which is mediated by mitophagy (Natarajan et al., 2020). Mitophagy corresponds to removing damaged or unnecessary mitochondria using autophagic machinery. Mitophagy is critical for mitochondrial quality control and homeostasis maintenance (Liao et al., 2017). Mitochondrial fission is required to form new mitochondria, excluding damaged mitochondria. Mitochondrial fission is essential for mitochondrial replication and the removal of damaged organelles via selective autophagy (Liao et al., 2017). The dynamin-related GTPase dynamin-related protein 1 (Drp1) is recruited by Fis1 protein from the cytosol to the mitochondrial outer membrane during mitochondrial fission (Cho et al., 2013). In contrast, mitochondrial fusion results in tubular or elongated mitochondria, which allow material exchange between mitochondria and can compensate for functional defects. Mfn1 and Mfn2 are responsible for mitochondrial outer membrane (MOM) fusion (Suárez-Rivero et al., 2017). OPA1 is responsible for mitochondrial inner membrane (MIM) fusion (Griparic et al., 2004; Suárez-Rivero et al., 2017). The OPA1 protein is involved in various functions, including respiratory chain and potential membrane maintenance, cristae organization and apoptosis control, and mtDNA maintenance (Griparic et al., 2004; Ni et al., 2015).
Mitochondrial morphology is dynamic and responsive to metabolic changes. Mitochondrial fusion is linked to increased ATP production, whereas fusion inhibition is linked to impaired OXPHOS, mtDNA depletion, and ROS production. OPA1 deficiency causes mitochondrial fragmentation and cell death in pancreatic cells, impairing insulin secretion and systemic glucose homeostasis. Alterations in mitochondrial dynamics can result in disease neuropathies characterized by impaired mitochondrial fusion and transport or significant optic atrophy caused by reduced mitochondrial fusion (Züchner et al., 2004). Mitochondrial dynamics shed new light on the pathophysiology of mitochondrial disorders and other diseases associated with mitochondrial dysfunction, such as diabetes, heart failure, and neurodegenerative diseases like Alzheimer’s disease (AD) (Alexiou et al., 2018a,b).
Additionally, different dietary elements have been suggested to affect AD and mitochondrial function and their dynamic behavior, such as Omega 3 (PUFA), Ketogenic Diet, and saturated fatty acid. Increased fat oxidation, energy expenditure, and reduced-fat deposition are potential effects of omega 3 PUFAs that could help prevent obesity and related metabolic disorders (DeFronzoand Tripathy, 2009). The capacity of omega 3 polyunsaturated fatty acids (PUFAs) to reduce inflammation, a characteristic of obesity and related metabolic disorders is well established (Buckley and Howe, 2010; Lalia and Lanza, 2016; Lepretti et al., 2018). Insulin resistance is closely linked to the emergence of inflammatory pathways, mitochondrial dysfunction, oxidative damage, and ER stress. The positive modulatory effects of omega 3 PUFAs on Mfn2 may explain the improvement of mitochondrial performance and the stimulation of fusion events related to maintaining the mitochondrial-associated endoplasmic reticulum membrane integrity, given Mfn2’s unique position as an ER-mitochondria bridge (Lepretti et al., 2018). Amyloid plaques, including beta-amyloid peptides (A-βeta), are the neuropathological hallmarks of AD (Mattson, 2004). According to recent studies, soluble oligomeric forms of Aβ may play a vital role in the etiology of AD. Amyloid Precursor Protein Processing (APP) is inextricably linked to cellular membranes, and membrane biophysical characteristics are crucial (Chen et al., 2017). PUFAs play an essential role in defining cell membrane fluidity. There is evidence that membrane fluidity influences APP processing regulation in multiple ways (Afshordel et al., 2015; Gammone et al., 2019), while A-βeta reduces membrane fluidity and thus stimulates its production, starting a vicious cycle (Pagani and Eckert, 2011). Recent in vitro findings suggest that the positive effects of docosahexaenoic acid (DHA), found in fish oil, are linked to mitochondria and APP processing modulation and significantly impact mitochondrial membrane phospholipid composition and function (Chen et al., 2017).
Modern biodata is characterized by abundance and diversity, including nucleic acid structures, gene expression levels, molecular interactions maps, gene maps, prediction of proteins’ unfolding, mutants identification, and pattern and motifs recognition mainly based on mathematical and computational models of low complexity and high efficiency. Mathematical Biology includes many interdisciplinary fields applied to modeling a natural phenomenon or mechanism, such as Theory of Algorithms, Data Mining, Genetic Algorithms, Neural Networks, Artificial Intelligence, and Machine Learning, Combined Learning Mathematics, Bayesian Statistics, Stochastic Analysis, Pattern Recognition, and Simulation. Theoretical models are a set of rules and laws that represent this phenomenon. When these rules or laws are expressed in mathematical relations, we refer to a mathematical model, a structure that approaches the properties of a random phenomenon through a simplification process. Mathematical modeling enables the analysis and monitoring of complex biological processes revealing any possible connections or disruptions that may lead, for example, to the development of a hypothesis related to a disease etiology or a drug discovery (Fischer, 2008). However, sometimes the limitations in the accuracy and the complexity of a mathematical model in Biology may lead to insufficient clinical application. Therefore, in this study, we formulate the probabilistic expectations of AD development or progression due to specific risk factors or biomarkers related to the impact of diet-induced obesity with an impaired mitochondrial function and altered behavior. We designed and programmed a Bayesian model to formulate and test diet type and AD progression correlation regarding various risk factors and biomarkers.
In ketogenic diets (KD), fats are in huge quantity, proteins are comparatively low, and carbohydrates are in insufficient quantity, which results in protein and carbohydrates’ limited metabolism and the metabolic rate of fats being high (Allen et al., 2014; Lima et al., 2014). The ketogenic diet is used to treat neurodegenerative diseases such as AD because ketone bodies such as acetoacetate (AA) and -hydroxybutyrate (-OHB) reduce oxidative stress and improve mitochondrial biogenesis and function (Branco et al., 2016). The ketogenic diet makes it a viable alternative energy precursor. Furthermore, this diet may aid in the reduction of amyloid plaque accumulation as well as the reversal of amyloid-beta toxicity (Broom et al., 2019). KD may address these metabolic issues while protecting against A plaques associated with AD. Recent memory loss is associated with amyloid-βeta (Aβ) peptide deposition and hippocampal neuronal death in AD. In vitro studies suggest that the ketogenic diet can help with this, as -OHB has been shown to protect against toxicity in cultured hippocampal neurons (Branco et al., 2016). Evidence suggests that brain ketone uptake is not impaired in AD, unlike glucose.
Different fat sources (high lard diet) in a high-fat diet affected serum levels of metabolites associated with the development of obesity and obesity-related diseases differently. A high-fat, saturated-fatty-acid-rich diet (high lard, HL, diet) resulted in hepatic fat accumulation and insulin resistance and impaired mitochondrial function, increased ROS production, and decreased production of mitochondrial motility proteins (Lionetti et al., 2014; Putti et al., 2015; Chen et al., 2018). Mfn2 expression was reduced in the skeletal muscles of obese Zucker rats and type 2 diabetic patients (Bach et al., 2005; Hernández-Alvarez et al., 2010; Putti et al., 2015). Besides that, saturated fatty acids have been shown in vitro to induce fission processes in differentiated C2C12 skeletal muscle cells, associated with mitochondrial dysfunction (Jheng et al., 2012). In vivo, smaller mitochondria and increased mitochondrial fission machinery have been observed in the skeletal muscles of genetically obese rats and those with diet-induced obesity, as previously described (Jheng et al., 2012). Rats fed high-lard (L rats) and high-fish-oil (F rats) diets presented similar increases in energy intake in comparison with the intake of rats fed standard diets (N rats) (Lionetti et al., 2014). L rats significantly gained additional bodyweight than F rats (Lionetti et al., 2014). Serum TG and ALT levels were significantly higher in L rats than in N rats and both parameters were lower in F rats than in L rats, with TG levels not differing between F and N rats (Lionetti et al., 2014). L and F rats also displayed significantly higher serum glucose levels than N rats. In contrast, the serum insulin level and the HOMA index value were highest in L rats, with TG levels not differing between F and N rats (Lionetti et al., 2014). Compared to high lard feeding, high fish oil feeding was associated with the development of obesity, dyslipidemia, insulin resistance, and a lower degree of liver injury. Protection against ROS damage through mild uncoupling markers of mitochondrial oxidative stress was evaluated (Lionetti et al., 2014). Because once compared to both N and F rats, L rats generated more mitochondrial ROS due to their lower basal/total aconitase activity ratio. Similarly, to their significantly higher H2O2 production in isolated mitochondria. The administration of a high-fat diet to rats increases the activity of AgRP neurons and significantly disrupts systemic energy metabolism (Figlewicz et al., 2013). Mfn2 deletion in these neurons prevents these adverse metabolic effects: fat mass was reduced, insulin and glucose levels were legitimized, and obesity was avoided (Dietrich et al., 2013). A high-fat diet caused mitochondrial fragmentation in AgRP neurons, leading to the hypothesis that reduced mitochondrial fusion contributes to neuronal regulation of whole-body energy metabolism and behavior patterns. A high-fat diet suppresses POMC neurons, which produce various peptide hormones that reduce appetite, food intake, and body weight (Wai and Langer, 2016). Unlike the effects in AgRP neurons, genetic ablation of Mfn2 in POMC neurons results in severe obesity characterized by overeating, low energy expenditure, and endocrine dysregulation. Mfn2 POMC deletion did not disrupt whole-body energy homeostasis, whereas Mfn1 POMC deletion did.
The fusion events are significant because they enable mitochondria to mix their contents, facilitating many vital functions like an equal distribution of metabolites, mtDNA repair, protein complementation, autophagy promotion, and isolation of damaged mitochondrial segments (Twig et al., 2008). On the other hand, the fission events enhance mitochondrial distribution along the cytoskeletal tracks and divide mitochondria equally into two daughter cells. Any malfunctioning in this process may result in programmed cell death. Fusion and fission, motility, transport, and mitophagy consist of the other three essential aspects of mitochondrial dynamics. These points are especially critical in neurons as they require mitochondria at the sites far from the cell body, and they are essential for functioning in cells of smaller size. A significant decrease in the mitochondrial movement has been reported when there are defects in fusion and fission. The decline in brain mitochondria’ normal functioning with increasing age has been associated with increased mitochondrial biogenesis (Grimm and Eckert, 2017).
Neurodegenerative diseases are observed to be associated with mitochondrial dysfunction, illustrating the importance of mitochondrial dynamics for human health. Mitochondria’s fusion and fission process maintain significant mitochondrial from and integrity. So, dysfunctions in mitochondrial fusion or fission may also contribute to neurological disorders. Motor neuron diseases characterized by progressive axonal degeneration have altered mitochondrial transport through the cytoskeleton. Mitochondria must be adequately positioned to meet the demands of the cell. Accordingly, mitochondria are delivered to areas of the axon where metabolic demand is high, such as synapses, active cones, and branches, or areas where axonal protein synthesis occurs (Babic et al., 2015). Alzheimer’s disease is one example of how mitochondrial transport has been linked to pathophysiological alterations (Magrané et al., 2014). In neurons, mitochondrial fission and fusion are essential for mitochondrial transport (Sheng and Cai, 2012). Fission is affected by Drp1 inhibition because it leads to enlarged mitochondria that cannot be adequately localized to dendrites or axons, and it suppresses synaptic formation and function (Chen et al., 2007; Kageyama et al., 2012; Itoh et al., 2013).
Mitophagy defects can cause the mitochondrial respiratory function to be lost in Drp1-null neurons, and Drp1 may be deficient in HeLa cells due to oxidative damage to mitochondrial components. ROS generation can be exacerbated by impaired respiration (Kageyama et al., 2012; Itoh et al., 2013). Although the processes underlying these alterations are unknown, there could be direct consequences of amyloid-beta (Ab), as AB fragments increased in mitochondria and mitochondrial mass decreased in cultured neurons’ neurites. S-nitrosylation-mediated enhancement of Drp1 activity is required for this mechanism (Cho et al., 2009). Mitochondrial morphological abnormalities, displayed as fragmented mitochondria with damaged inner membrane structures, have been progressively observed in neurons from AD patients and AD animal models overexpressed or treated with Aβ or tau (DuBoff et al., 2012; Manczak and Reddy, 2012; Li et al., 2016). Outward deposition of amyloid plaques is followed by accumulation of intraneuronal neurofibrillary tangles of hyperphosphorylated tau on a pathological level. It is linked to the loss of synapses, which leads to neuronal death (Huang and Mucke, 2012).
The lack of mitochondria or fusion and fission regulators in neurites and concurrent abnormalities in axon repair and axonal transport implies that mitochondrial and neuronal dysfunction in AD may be ascribed to mitochondrial trafficking impairment (Gao et al., 2017). Nonetheless, faulty mitochondrial morphology and transport in cell bodies, axons, and synaptic terminals produce localized energy in AD. Deficiency causes or exacerbates neuron malfunction and destruction in AD. As a result, aberrant mitochondrial dynamics could be critical in mitochondrial malfunction and neuronal dysfunction in the AD brain (Huang and Mucke, 2012).
In this research paper, a new mathematical model is designed to formulate the correlation of AD progression with diverse diets and its mitochondrial dynamics side effects. The computational implementation was made in the Python programming language, and the Bayesian inference was applied in simulating data.
Bayesian theory is based on probability theory. Even though many supporters of the Classical approach oppose the Bayesian Inference due to the weak approach of prior distributions, the Markov Chain Monte Carlo theory was provided as a solution for this problem for disease assessments with satisfactory results (Tzoufras, 2009). Bayesian statistics uses random variables to pre-define the prior distribution of the desired model and calculate the posterior distribution f(θ | y). This posterior distribution can be expressed as (Vidakovic, 2011; Højsgaard, 2012):
including both the prior and the observed data by the expression of the prior distribution f(θ) and the likelihood f(y | θ) (Alexiou et al., 2017) as follows:
Considering the probabilistic expectations of AD development or progression due to specific risk factors or biomarkers (Table 1), we designed a Bayesian model to formulate the impact of diet-induced obesity with an impaired mitochondrial function and altered behavior (Putti et al., 2015; Alexiou et al., 2017). The applied probabilities are based on clinical trials globally and are continuously subject to updating and redefinition (Alexiou et al., 2017). Therefore the proposed model and the programming code are adjustable to different parameters and values.
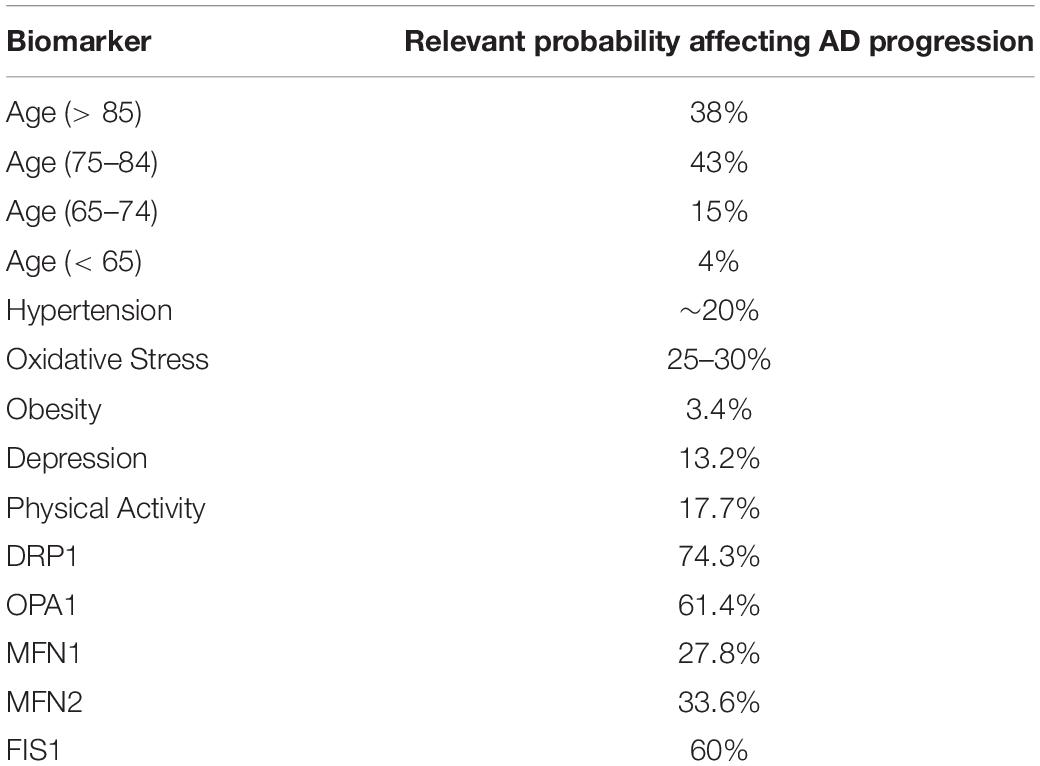
Table 1. The relevant probabilities affecting Alzheimer’s disease progression related to age and factors that influence Obesity and Mitochondrial Dynamics, according to the literature (Christen, 2000; Modrego and Ferrández, 2004; Wang et al., 2009; Israeli-Korn et al., 2010; Barnes and Yaffe, 2011; Alzheimer’s Association, 2015; Alexiou et al., 2017; Mantzavinos and Alexiou, 2017).
The proposed Bayesian model is presented in an acyclic graph (Figure 1), and the related variables are formulated using the uniform distribution:
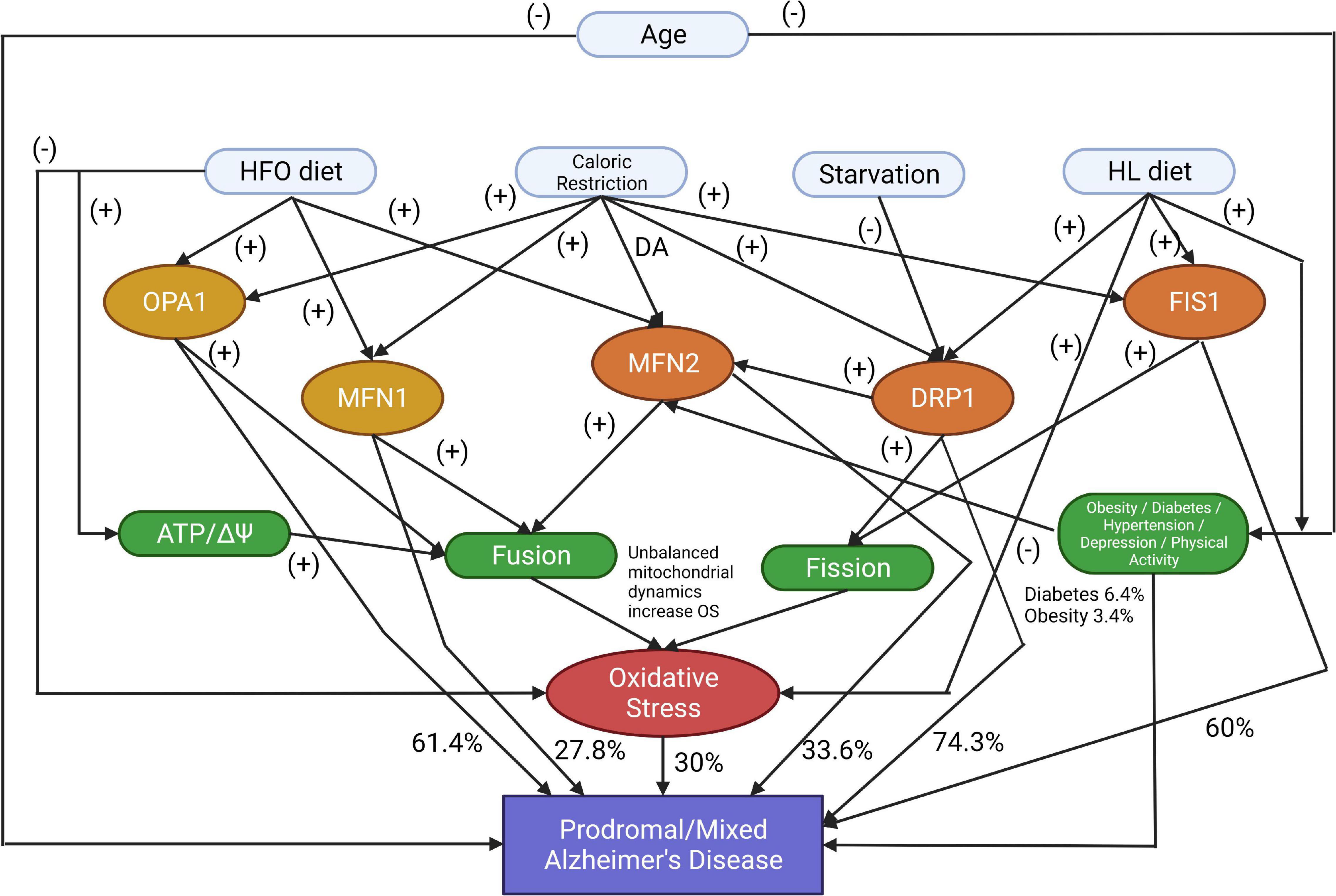
Figure 1. The proposed model identifies the markers that affect Prodromal or Mixed Alzheimer’s disease development or progression (DA, Don’t Affect; HFO diet, a high-fat diet rich in fish oil; HL diet, a high-fat diet rich in saturated fatty acids). The symbol (-) shows that the parent node increases the possibility of the child node occurring. The symbol (+) shows that the current node is a deterrent factor for activating the next node.
For the Bayesian inference, we mainly apply a conjugate prior in medical experiments, where this conjugate prior to the uniform distribution is the Pareto distribution (Tenenbaum, 1998):
The program is coded and executed in Python and is fully and freely available for research purposes and testing the correlation between diet type and AD progression regarding various risk factors and biomarkers. The proposed multiparametric model combines various data types based on uniform probabilities. The calculated error is the Monte Carlo Error that measures the variability of each estimation due to simulation, increasing the model’s accuracy almost to 100% (Alexiou et al., 2017). Furthermore, in this Bayesian model, every biomarker (knots in the graph) can be linked with more than one other biomarker in the form of False | False, False | True, True | False, True | True (Alexiou et al., 2017).
The program simulates all the variables with a uniform distribution in a sample of 1,000 patients. First, the program initializes age (30–95) and the four different diet types (“HFO_diet,” “Starvation,” “HL_diet,” “CR”) along with the factors that are related to prodromal or mixed AD (ATP, MFN1, MFN2, DRP1, FIS1, Diabetes, Oxidative_Stress, Hypertension, Obesity, Depression, and Physical_activity). Then, the program executes the simulation according to the probabilities of Table 1, assigning values 1 or 0 in the case of a positive (abnormal) or not positive biomarker. The outcome is a table with the probability of AD development or progression related to diet choices and mitochondrial dynamics parameters (Table 2).
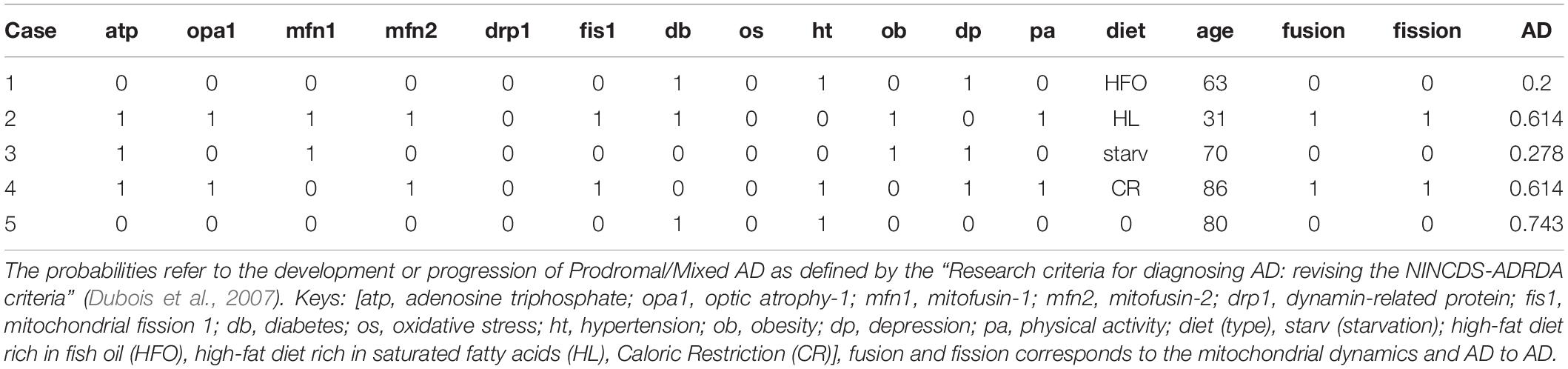
Table 2. Randomly selected results from the simulation of the 1,000 patients, in accordance with the five cases above.
The model represents specific proteins or diets’ negative or positive impact on developing AD. The symbol (-) shows that the parent node increases the possibility of the child node occurring. The symbol (+) shows that the current node is a deterrent factor for activating the next node. In the experimentation, zeros and ones were used as the result of an activation function that checks if the node’s current state is efficient enough to activate the next node. These calculations have included all the factors, with corresponding prior probabilities applied to uniformed distributed data. Results show different combinations of those factors that produce specific patients’ profiles and cases. Besides the known proteins related to mitochondrial dynamics, our model includes risk factors like Age, Hypertension, Oxidative Stress, Obesity, Depression, and Physical Activity, which are associated with Prodromal AD.
Strong evidence suggests the necessity of an optimized bioenergetics balance between mitochondrial fusion and fission (Putti et al., 2015). Therefore we present a few cases generated from our simulation accordingly to the corresponding theory and the relevant probabilities affecting AD progression related to age and other factors (Table 1).
Case 1: Subject with decreased oxidative stress due to HFO diets resulting in increased mfn2 expression and ATP levels and promoting fusion within a risk group due to the age (age > 60):
Case 2: Subject with abnormal mitochondrial fission proteins (fis1 increased) and mfn2 (decreased) associated with high fat diet-induced obesity (HL diet), leading to an increased ROS:
Case 3: Subject with increased mfn1 and opa1 due to starvation-induced mitochondrial fusion, which increases ATP, within a risk group due to the age (age > 60):
Case 4: Subject with an increased mitochondrial population, based on a Caloric Restriction diet type leading to abnormal fis1 and drp1 (increased) and normal mfn1, mfn2, opa1 levels within a risk group due to the age (age > 60):
Case 5: Subject within a risk group due to the age (age > 60), with problems related to a certain quality of life factors (diabetes and hypertension) but with no other evidence of abnormal biomarkers. The model calculates the probability of leading to AD due to these factors:
Mitochondrial dysfunction and oxidative stress are significant causes of neurodegeneration. Both processes produce high levels of ROS, which are detrimental to all cellular macromolecules, including nucleic acid, lipid, and protein damage (Lauritzen et al., 2016). Plenty of studies confirm that mitochondrial dysfunction is the most likely underlying mechanism of cortical contractility, especially in areas of the brain involved in learning and memory, such as the hippocampus (Stefanova et al., 2016). Mitochondrial changes can increase the production and accumulation of amyloid-β, which is directly toxic to mitochondria, and delay the neurodegenerative process. Tau hyperphosphorylation, which causes a concatenation of events, causes neurodegenerative disease, synaptic damage, and neuronal cell death, and is a detrimental feature of AD (Grimm et al., 2016). As a result, KD may provide neuroprotection by improving mitochondrial function through biochemical changes caused by inhibition of glycolysis and increased KB (ketone body) formation (Rusek et al., 2019). In addition, KB can affect mitochondrial homeostasis by altering calcium-induced membrane permeability transitions (MPTs) and preventing pores from opening.
Furthermore, certain polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid, arachidonic acid, and docosahexaenoic acid suppress ROS production, reduce inflammatory mediators, and block voltage-gated sodium and calcium channels. In addition, it can promote neuronal cell membrane stimulation (McDonald and Cervenka, 2018). A high-fat diet is a primary etiology of overweight, obesity, and inflammation, leading to high levels of circulating free fatty acids, which stimulates the formation of amyloid and tau filaments. A high-fat diabetic diet promotes the etiology of AD, and a diet high in docosahexaenoic acid (DHA) prevents AD (Cole et al., 2010; Pugazhenthi et al., 2017).
Several lately published studies provide tools for an efficient early diagnosis or even prognosis of AD development or progression based on the Bayesian Inference or Machine Learning Techniques (Mantzavinos and Alexiou, 2017; Ali et al., 2019; Ashraf and Alexiou, 2019, 2022; De Velasco Oriol et al., 2019; Alexiou et al., 2020; Tan et al., 2021; Mirzaei and Adeli, 2022).
This article presents the first attempt in the literature to simulate the side effects of dietary types in the mitochondrial population, highly correlated to AD and other related neurodegenerative disorders. Furthermore, it provides a freely accessed programming code for reusing and expanding the experimental procedures.
In Table 2, several defined cases reveal the potential presence of Prodromal/Mixed AD due to specific biomarkers. Our base study (Putti et al., 2015) showed that HL is highly correlated to altered mitochondrial dynamics and increased ROS production. On the contrary, HFO positively affects mitochondrial functionality, reduces ROS production, and promotes mitochondrial fusion. In addition, the HFO diet can lead to decreased lipid accumulation, obesity, and insulin sensibility compared to HL (Lionetti et al., 2013; Putti et al., 2015). The results of Table 2 fit the theory of the highly correlated dietary types and mitochondrial dynamics, while mitochondrial fission and fusion seem to be depending on the high-fat diet and starvation (Table 2).
Further analysis and observational studies could also validate the model with patient data. This program is an assistive research tool for AD prognosis, but alternative prognostic tools must not replace the clinician’s diagnosis.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
AA and GF wrote the manuscript. AA and SC performed the analysis and data collection. SC designed the programming code. GA and MG provided the funding. MG, GA, and MK provided critical feedback and edited the manuscript. All authors revised the manuscript and agreed to its final form.
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under the grant No. G: 1440-141-1346. We thank DSR for technical and financial support.
AA holds an unpaid position on the scientific board of the company AFNP Med Austria.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Afshordel, S., Hagl, S., Werner, D., Röhner, N., Kögel, D., Bazan, N. G., et al. (2015). Omega-3 polyunsaturated fatty acids improve mitochondrial dysfunction in brain aging–impact of Bcl-2 and NPD-1 like metabolites. Prostagl. Leukotr. Essent. Fatty Acids 92, 23–31. doi: 10.1016/j.plefa.2014.05.008
Alexiou, A., Chatzichronis, S., and Ashraf, G. M. (2020). “Chapter 23 – The prediction of Alzheimer’s disease,” in Diagnosis and Management in Dementia: The Neuroscience of Dementia, Vol. 1, eds C. R. Martin and V. R. Preedy (Amsterdam: Elsevier), 365–378. doi: 10.1016/B978-0-12-815854-8.00023-9
Alexiou, A., Mantzavinos, V. D., Greig, N. H., and Kamal, M. A. (2017). A Bayesian Model for the Prediction and Early Diagnosis of Alzheimer’s Disease. Front. Aging Neurosci. 9:77. doi: 10.3389/fnagi.2017.00077
Alexiou, A., Nizami, B., Khan, F. I., Soursou, G., Vairaktarakis, C., Chatzichronis, S., et al. (2018a). Mitochondrial Dynamics and Proteins Related to Neurodegenerative Diseases. Curr. Prot. Pept. Sci. 19, 850–857. doi: 10.2174/1389203718666170810150151
Alexiou, A., Soursou, G., Yarla, N. S., and Md Ashraf, G. (2018b). Proteins Commonly Linked to Autism Spectrum Disorder and Alzheimer’s Disease. Curr. Prot. Pept. Sci. 19, 876–880. doi: 10.2174/1389203718666170911145321
Ali, M. A., Alexiou, A., and Ashraf, G. M. (2019). “Biotechnology and Bioinformatics Applications in Alzheimer’s Disease,” in Biological, Diagnostic and Therapeutic Advances in Alzheimer’s Disease, eds G. Ashraf and A. Alexiou (Singapore: Springer), doi: 10.1007/978-981-13-9636-6_12
Allen, B. G., Bhatia, S. K., Anderson, C. M., Eichenberger-Gilmore, J. M., Sibenaller, Z. A., and Mapuskar, K. A. (2014). Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2, 963–970. doi: 10.1016/j.redox.2014.08.002
Alzheimer’s Association (2015). Alzheimer’s Disease Facts and Figures Alzheimer’s & Dementia. Alzheimers Dement 11, 1–88.
Archer, S. L. (2013). Mitochondrial dynamics—mitochondrial fission and fusion in human diseases. New Engl. J. Med. 369, 2236–2251.
Ashraf, G. M., and Alexiou, A. (2019). Biological, Diagnostic and Therapeutic Advances in Alzheimer’s Disease - Non-Pharmacological Therapies for Alzheimer’s Disease. New York, NY: Springer, ISBN: 978-981-13-9635-9 doi: 10.1007/978-981-13-9636-6,
Ashraf, G. M., and Alexiou, A. (2022). Autism Spectrum Disorder and Alzheimer’s Disease - Advances in Research. New York, NY: Springer Nature, doi: 10.1007/978-981-16-4558-7
Babic, M., Russo, G. J., Wellington, A. J., Sangston, R. M., Gonzalez, M., and Zinsmaier, K. E. (2015). Miro’s N-terminal GTPase domain is required for transport of mitochondria into axons and dendrites. J. Neurosci. 35, 5754–5771. doi: 10.1523/JNEUROSCI.1035-14.2015
Bach, D., Naon, D., Pich, S., Soriano, F. X., Vega, N., Rieusset, J., et al. (2005). Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes 54, 2685–2693. doi: 10.2337/diabetes.54.9.2685
Barnes, D. E., and Yaffe, K. (2011). The projected impact of risk factor reduction on Alzheimer’s disease prevalence. Lancet. Neurol. 10, 819–828. doi: 10.1016/S1474-4422(11)70072-2
Branco, A. F., Ferreira, A., Simoes, R. F., Magalhães-Novais, S., Zehowski, C., Cope, E., et al. (2016). Ketogenic diets: from cancer to mitochondrial diseases and beyond. Eur. J. Clin. Investig. 46, 285–298.
Broom, G. M., Shaw, I. C., and Rucklidge, J. J. (2019). The ketogenic diet as a potential treatment and prevention strategy for Alzheimer’s disease. Nutrition 60, 118–121.
Buckley, J. D., and Howe, P. R. (2010). Long-chain omega-3 polyunsaturated fatty acids may be beneficial for reducing obesity—a review. Nutrients 2, 1212–1230. doi: 10.3390/nu2121212
Chen, D., Li, X., Zhang, L., Zhu, M., and Gao, L. (2018). A high-fat diet impairs mitochondrial biogenesis, mitochondrial dynamics, and the respiratory chain complex in rat myocardial tissues. J. Cell. Biochem. 119, 9602–9602. doi: 10.1002/jcb.27068
Chen, G. F., Xu, T. H., Yan, Y., Zhou, Y. R., Jiang, Y., Melcher, K., et al. (2017). Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 38, 1205–1235.
Chen, H., McCaffery, J. M., and Chan, D. C. (2007). Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130, 548–562. doi: 10.1016/j.cell.2007.06.026
Cho, B., Choi, S. Y., Cho, H. M., Kim, H. J., and Sun, W. (2013). Physiological and pathological significance of dynamin-related protein 1 (drp1)-dependent mitochondrial fission in the nervous system. Exp. Neurob. 22:149. doi: 10.5607/en.2013.22.3.149
Cho, D. H., Nakamura, T., Fang, J., Cieplak, P., Godzik, A., Gu, Z., et al. (2009). S-nitrosylation of Drp1 mediates beta-amyloidrelated mitochondrial fission and neuronal injury. Science 324, 102–105.
Christen, Y. (2000). Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 71, 621S–629S. doi: 10.1093/ajcn/71.2.621s
Cole, G. M., Ma, Q. L., and Frautschy, S. A. (2010). Dietary fatty acids and the aging brain. Nutrit. Rev. 68(Suppl._2), S102–S111. doi: 10.1111/j.1753-4887.2010.00345.x
De Velasco Oriol, J., Vallejo, E. E., Estrada, K., and Gerardo, J. (2019). Benchmarking machine learning models for late-onset alzheimer’s disease prediction from genomic data. BMC Bioinform. 20:709. doi: 10.1186/s12859-019-3158-x
DeFronzo, R. A., and Tripathy, D. (2009). Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32, 157–163.
Dietrich, M. O., Liu, Z. W., and Horvath, T. L. (2013). Mitochondrial dynamics controlled by mitofusins regulate Agrp neuronal activity and diet-induced obesity. Cell 155, 188–199. doi: 10.1016/j.cell.2013.09.004
DuBoff, B., Gotz, J., and Feany, M. B. (2012). Tau promotes neurodegeneration via Drp1 mislocalization in vivo. Neuron 75, 618–632. doi: 10.1016/j.neuron.2012.06.026
Dubois, B., Feldman, H. H., Jacova, C., Dekosky, S. T., Barberger-Gateau, P., Cummings, J., et al. (2007). Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 6, 734–746. doi: 10.1016/S1474-4422(07)70178-3
Figlewicz, D. P., Jay, J. L., Acheson, M. A., Magrisso, I. J., West, C. H., Zavosh, A., et al. (2013). Moderate high fat diet increases sucrose self-administration in young rats. Appetite 61, 19–29. doi: 10.1016/j.appet.2012.09.021
Fischer, H. P. (2008). Mathematical modeling of complex biological systems: from parts lists to understanding systems behavior. Alcohol. Res. Health 31, 49–59.
Gammone, M. A., Riccioni, G., Parrinello, G., and D’Orazio, N. (2019). Omega-3 polyunsaturated fatty acids: benefits and endpoints in sport. Nutrients 11:46.
Gao, J., Wang, L., Liu, J., Xie, F., Su, B., and Wang, X. (2017). Abnormalities of mitochondrial dynamics in neurodegenerative diseases. Antioxidants 6:25.
Grimm, A., and Eckert, A. (2017). Brain aging and neurodegeneration: from a mitochondrial point of view. J. Neurochem. 143, 418–431.
Grimm, A., Friedland, K., and Eckert, A. (2016). Mitochondrial dysfunction: the missing link between aging and sporadic Alzheimer’s disease. Biogerontology 17, 281–296.
Griparic, L., van der Wel, N. N., Orozco, I. J., Peters, P. J., and van der Bliek, A. M. (2004). Loss of the intermembrane space protein mgm1/opa1 induces swelling and localized constrictions along the lengths of mitochondria. J. Biol. Chem. 279, 18792–18798. doi: 10.1074/jbc.M400920200
Hernández-Alvarez, M. I., Thabit, H., Burns, N., Shah, S., Brema, I., Hatunic, M., et al. (2010). Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1α/Mitofusin-2 regulatory pathway in response to physical activity. Diabetes Care 33, 645–651. doi: 10.2337/dc09-1305
Højsgaard, S. (2012). Graphical independence networks with the gRain package for R. J Stat Softw. 46, 1–26. doi: 10.18637/jss.v046.i10
Huang, Y., and Mucke, L. (2012). Alzheimer mechanisms and therapeutic strategies. Cell 148, 1204–1222.
Israeli-Korn, S. D., Masarwa, M., Schechtman, E., Abuful, A., Strugatsky, R., Avni, S., et al. (2010). Hypertension increases the probability of Alzheimer’s disease and of mild cognitive impairment in an Arab community in northern Israel. Neuroepidemiology 34, 99–105. doi: 10.1159/000264828
Itoh, K., Nakamura, K., Iijima, M., and Sesaki, H. (2013). Mitochondrial dynamics in neurodegeneration. Trends Cell Biol. 23, 64–71.
Jheng, H. F., Tsai, P. J., Guo, S. M., Kuo, L. H., Chang, C. S., Su, I. J., et al. (2012). Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol. Cell. Biol. 32, 309–319. doi: 10.1128/MCB.05603-11
Kageyama, Y., Zhang, Z., Roda, R., Fukaya, M., Wakabayashi, J., Wakabayashi, N., et al. (2012). Mitochondrial division ensures the survival of postmitotic neurons by suppressing oxidative damage. J. Cell Biol. 197, 535–551. doi: 10.1083/jcb.201110034
Lalia, A. Z., and Lanza, I. R. (2016). Insulin-Sensitizing Effects of Omega-3 Fatty Acids: lost in Translation? Nutrients 8:329. doi: 10.3390/nu8060329
Lauritzen, K. H., Hasan-Olive, M. M., Regnell, C. E., Kleppa, L., Scheibye-Knudsen, M., Gjedde, A., et al. (2016). A ketogenic diet accelerates neurodegeneration in mice with induced mitochondrial DNA toxicity in the forebrain. Neurobiol. Aging 48, 34–47. doi: 10.1016/j.neurobiolaging.2016.08.005
Lepretti, M., Martucciello, S., Burgos Aceves, M. A., Putti, R., and Lionetti, L. (2018). Omega-3 fatty acids and insulin resistance: focus on the regulation of mitochondria and endoplasmic reticulum stress. Nutrients 10:350. doi: 10.3390/nu10030350
Li, X. C., Hu, Y., Wang, Z. H., Luo, Y., Zhang, Y., Liu, X. P., et al. (2016). Human wild-type full-length tau accumulation disrupts mitochondrial dynamics and the functions via increasing mitofusins. Sci. Rep. 6:24756. doi: 10.1038/srep24756
Liao, C., Ashley, N., Diot, A., Morten, K., Phadwal, K., Williams, A., et al. (2017). Dysregulated mitophagy and mitochondrial organization in optic atrophy due to OPA1 mutations. Neurology 88, 131–142. doi: 10.1212/WNL.0000000000003491
Lima, P. A., Sampaio, L. P., and Damasceno, N. R. (2014). Neurobiochemical mechanisms of a ketogenic diet in refractory epilepsy. Clinics 69, 699–705. doi: 10.6061/clinics/2014(10)09
Lionetti, L., Mollica, M. P., Donizzetti, I., Gifuni, G., Sica, R., Pignalosa, A., et al. (2014). High-lard and high-fish-oil diets differ in their effects on function and dynamic behaviour of rat hepatic mitochondria. PLoS One 9:e92753. doi: 10.1371/journal.pone.0092753
Lionetti, L., Sica, R., Mollica, M. P., and Putti, R. (2013). High-lard and high-fish oil diets differ in their effects on insulin resistance development, mitochondrial morphology and dynamic behaviour in rat skeletal muscle. Food Nutr. Sci. 4, 105–112. doi: 10.4236/fns.2013.49A1017
Magrané, J., Cortez, C., Gan, W. B., and Manfredi, G. (2014). Abnormal mitochondrial transport and morphology are common pathological denominators in SOD1 and TDP43 ALS mouse models. Hum. Mole. Genet. 23, 1413–1424. doi: 10.1093/hmg/ddt528
Manczak, M., and Reddy, P. H. (2012). Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer’s disease neurons: implications for mitochondrial dysfunction and neuronal damage. Hum. Mole. Genet. 21, 2538–2547. doi: 10.1093/hmg/dds072
Mantzavinos, V., and Alexiou, A. (2017). Biomarkers for Alzheimer’s Disease Diagnosis. Curr. Alzheimer Res. 14, 1149–1154.
McDonald, T. J., and Cervenka, M. C. (2018). Ketogenic diets for adult neurological disorders. Neurotherapeutics 15, 1018–1031.
Mirzaei, G., and Adeli, H. (2022). Machine learning techniques for diagnosis of alzheimer disease, mild cognitive disorder, and other types of dementia. Biomed. Sign. Proc. Control 72:103293. doi: 10.1016/j.bspc.2021.103293
Mishra, P., and Chan, D. C. (2016). Metabolic regulation of mitochondrial dynamics. J. Cell Biol. 212, 379–387.
Modrego, P. J., and Ferrández, J. (2004). Depression in Patients with Mild cognitive impairment increases the risk of developing dementia of Alzheimer typea prospective cohort study. Arch. Neurol. 61, 1290–1293. doi: 10.1001/archneur.61.8.1290
Natarajan, V., Chawla, R., Mah, T., Vivekanandan, R., Tan, S. Y., Sato, P. Y., et al. (2020). Mitochondrial dysfunction in age-related metabolic disorders. Proteomics 20:1800404.
Ni, H. M., Williams, J. A., and Ding, W. X. (2015). Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 4, 6–13. doi: 10.1016/j.redox.2014.11.006
Pagani, L., and Eckert, A. (2011). Amyloid-Beta interaction with mitochondria. Internat. J. Alzheimer’s Dis. 2011:925050. doi: 10.4061/2011/925050
Pugazhenthi, S., Qin, L., and Reddy, P. H. (2017). Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochimica et biophysica acta 1863, 1037–1045.
Putti, R., Sica, R., Migliaccio, V., and Lionetti, L. (2015). Diet impact on mitochondrial bioenergetics and dynamics. Front. Physiol. 6:109. doi: 10.3389/fphys.2015.00109
Rusek, M., Pluta, R., Ułamek-Kozioł, M., and Czuczwar, S. J. (2019). Ketogenic diet in Alzheimer’s disease. Internat. J. Mole. Sci. 20:3892. doi: 10.3390/ijms20163892
Saada, A. (2014). Mitochondria: mitochondrial OXPHOS (dys) function ex vivo–The use of primary fibroblasts. Internat. J. Biochem. Cell Biol. 48, 60–65. doi: 10.1016/j.biocel.2013.12.010
Sheng, Z. H., and Cai, Q. (2012). Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat. Rev. Neurosci. 13, 77–93.
Stefanova, N. A., Muraleva, N. A., Maksimova, K. Y., Rudnitskaya, E. A., Kiseleva, E., Telegina, D. V., et al. (2016). An antioxidant specifically targeting mitochondria delays progression of Alzheimer’s disease-like pathology. Aging 8:2713. doi: 10.18632/aging.101054
Suárez-Rivero, J. M., Villanueva-Paz, M., la Cruz-Ojeda, D., De la Mata, M., Cotán, D., Oropesa-Avila, M., et al. (2017). Mitochondrial dynamics in mitochondrial diseases. Diseases 5:1.
Tan, M. S., Cheah, P. L., Chin, A. V., Looi, L. M., and Chang, S. W. (2021). A review on omics-based biomarkers discovery for Alzheimer’s disease from the bioinformatics perspectives: statistical approach vs machine learning approach. Comp. Biol. Med. 139:104947. doi: 10.1016/j.compbiomed.2021.104947
Tenenbaum, J. (1998). Bayesian modeling of human concept learning. Adv. Neural. Inform. Proc. Syst. 1998:11.
Twig, G., Hyde, B., and Shirihai, O. S. (2008). Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochimica et Biophysica Acta 1777, 1092–1097. doi: 10.1016/j.bbabio.2008.05.001
Wai, T., and Langer, T. (2016). Mitochondrial dynamics and metabolic regulation. Trends Endocrinol. Metabol. 27, 105–117.
Wang, X., Su, B., Lee, H. G., Li, X., Perry, G., Smith, M. A., et al. (2009). Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J. Neurosci. 29, 9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009
Keywords: Alzheimer’s disease, bayesian inference, dietary, mitochondrial dynamics, Python programming language, simulation
Citation: Ashraf GM, Chatzichronis S, Alexiou A, Firdousi G, Kamal MA and Ganash M (2022) Dietary Alterations in Impaired Mitochondrial Dynamics Due to Neurodegeneration. Front. Aging Neurosci. 14:893018. doi: 10.3389/fnagi.2022.893018
Received: 09 March 2022; Accepted: 20 June 2022;
Published: 11 July 2022.
Edited by:
Wenquan Zou, Case Western Reserve University, United StatesReviewed by:
Dimitra Dafou, Aristotle University of Thessaloniki, GreeceCopyright © 2022 Ashraf, Chatzichronis, Alexiou, Firdousi, Kamal and Ganash. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Athanasios Alexiou, YWxleGlvdUBuZ2NlZi5uZXQ=; Ghulam Md Ashraf, Z2FzaHJhZkBrYXUuZWR1LnNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.