- Department of Medicinal Chemistry, Virginia Commonwealth University, Richmond, VA, United States
The NLRP3 inflammasome is a multiprotein complex that plays a pivotal role in regulating the innate immune system and inflammatory signaling. Upon activation by PAMPs and DAMPs, NLRP3 oligomerizes and activates caspase-1 which initiates the processing and release of pro-inflammatory cytokines IL-1β and IL-18. NLRP3 is the most extensively studied inflammasome to date due to its array of activators and aberrant activation in several inflammatory diseases. Studies using small molecules and biologics targeting the NLRP3 inflammasome pathway have shown positive outcomes in treating various disease pathologies by blocking chronic inflammation. In this review, we discuss the recent advances in understanding the NLRP3 mechanism, its role in disease pathology, and provide a broad review of therapeutics discovered to target the NLRP3 pathway and their challenges.
Introduction
Inflammation is a defense mechanism characterized by a cascade of signaling that activates the innate immune system in response to pathogens, dead cells, traumas, or chemically induced damage. The innate immune system involves immune cells that are derived from multipotent hematopoietic stem cells (i.e., hemocytoblasts) which are found in the peripheral blood and bone marrow and undergo hematopoiesis to give rise to myeloid and lymphoid progenitors (Kim S. et al., 2019). Myeloid progenitors differentiate into eosinophils, neutrophils, basophils, monocytes, and macrophages; whereas lymphoid progenitors give rise to cells of the lymphatic system such as natural killer (NK) cells, T-cells, and B-cells (Kim S. et al., 2019). While all immune cells play some role in inflammatory responses, the leading players are macrophages, neutrophils, NK, and T-cells during infections and injury. Immune cells, including neutrophils and monocytes, are recruited to the site of injury by chemotaxis (Kolaczkowska and Kubes, 2013; Chen L. et al., 2018). Neutrophils respond quickly and play important roles in the early stages of inflammation by eliminating pathogens through various mechanisms which subsequently leads them to become apoptotic and die. Once inflammation takes place, monocytes differentiate into macrophages and phagocytose the apoptotic neutrophils (Kolaczkowska and Kubes, 2013; Ortega-Gómez et al., 2013; Epelman et al., 2014). This process is tightly regulated, as an inadequate inflammatory response can result in a persistent infection of pathogens and an excessive inflammatory response may lead to chronic inflammation.
The recognition of pro-inflammatory stimuli by the germline-encoded pattern recognition receptors (PRRs) plays a pivotal role in initiating the innate immune response. PRRs can be classified as transmembrane or cytosolic receptors. Transmembrane proteins include Toll-like receptors (TLRs) and the C-type lectin receptors (CLRs) while cytosolic proteins include the nucleotide-binding oligomerization domain (NOD)- Leucine Rich Repeats (LRR)-containing receptors (NLRs), the Retinoic Acid-Inducible Gene 1 (RIG-1)-like receptors (RLRs), and Absence in Melanoma 2 (AIM2)-like receptors (ALRs) (Unterholzner et al., 2010; Lamkanfi and Dixit, 2014; Amarante-Mendes et al., 2018). These sensing receptors can recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) (Chen and Nuñez, 2010). PAMPs represent the conserved structural moieties that are commonly found in microorganisms such as lipopolysaccharide (LPS) found on the membranes of Gram-negative bacteria, bacterial or viral nucleic acids, bacterial peptides such as flagellin, and polysaccharides such as β-glucans (Mahla et al., 2013). DAMPs are endogenous molecules which are released under cellular stress or damage such as chromatin-associated proteins, heat shock proteins, uric acid, and extracellular matrix fragments (Chen and Nuñez, 2010; Lamkanfi and Dixit, 2014). The activation of PRRs by PAMPs or DAMPs triggers a signaling cascade that causes cytosolic PRRs such as NLRs, ALRs, and tripartite motif-containing proteins such as pyrin to form the multimeric protein complex termed “the inflammasome” (Lamkanfi and Dixit, 2014). The inflammasomes play an intricate and vital role in the activation and release of pro-inflammatory cytokines. In the past two decades, the molecular components and mechanism of activation of different inflammasomes have been widely studied; however, the NLRP3 inflammasome is the most studied and characterized at present due to its ability to be activated by a diverse array of stimuli and its implication in several inflammatory diseases.
NLRP3 Inflammasome Assembly and Activation
Assembly
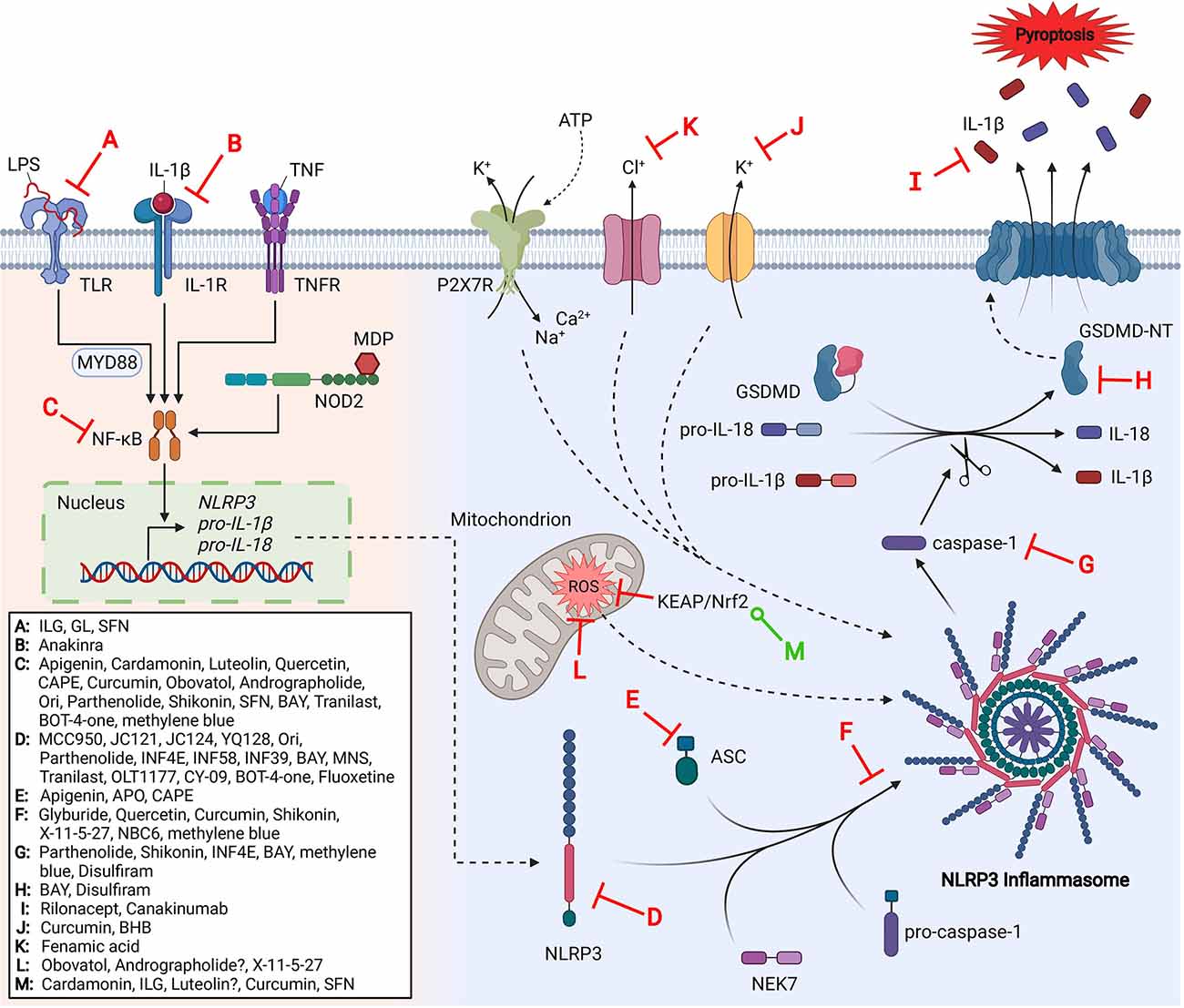
NLRP3 is a 118 kDa cytosolic PRR protein expressed by a variety of cells including neutrophils, macrophages, microglia, lymphocytes, epithelial cells, osteoblasts, neurons, and dendritic cells (Rada et al., 2014; Zahid et al., 2019). The NLRP3 protein contains a C-terminal leucine-rich repeat (LRR) domain, a central ATPase-containing NACHT (present in NAIP, CIITA, HET-E, and TP1) domain that mediates oligomerization, and an N-terminal pyrin (PYD) domain which recruits proteins for inflammasome complex formation (Kelley et al., 2019). Like other inflammasomes, the NLRP3 inflammasome complex consists of a sensor (NLRP3 protein), an adaptor (apoptosis-associated speck-like protein, ASC), and an effector (caspase-1) (de Zoete et al., 2014; Mamantopoulos et al., 2017). Formation of the NLRP3 inflammasome occurs in two stages: priming and activation. Priming is responsible for the transcriptional upregulation of NLRP3 and pro-inflammatory cytokines, pro-interleukin (IL)-1β and pro-IL-18, through TLR, NOD2, IL-1R, or tumor necrosis factor receptor (TNFR) ligand-mediated signaling upon stimulation with PAMPs and DAMPs such as LPS or cytokines such as tumor necrosis factor (TNF) and IL-1β. The detection of PAMPs and DAMPs results in the activation of proteins and nuclear factors such as myeloid differentiation primary response protein (MyD88), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and the activator protein 1 (AP-1) to upregulate NLRP3 and pro-inflammatory cytokines (Liu et al., 2017). The effect of priming on transcriptional factors and protein expression has been widely accepted; however, recent studies have suggested that there is also a non-transcriptional role for priming (Lin et al., 2014). Priming also controls the post-translational modifications of NLRP3 such as ubiquitination and phosphorylation, which plays a major role in regulating the activation of NLRP3 (Yang et al., 2017). In its inactive form, ADP-bound NLRP3 can exist as a monomer or oligomer. Monomeric NLRP3 localizes to membranes which act as a scaffold to form an oligomeric, double-ring structure with 5–8 sets of dimers interlocking LRR domains and arranged back-to-back forming a circular cage (Andreeva et al., 2021; Hochheiser et al., 2021). Cryo-EM studies suggest the PYD domain sits inside the cage to protect it from aberrant activation (Andreeva et al., 2021).
A secondary stimulus initiates the activation step to form the active inflammasome complex. Contrary to most PRRs, NLRP3 is activated by a plethora of stimuli such as particulate matter (e.g., uric acid crystals, silica, asbestos), extracellular ATP, and toxins as well as viral, bacterial, fungal, and protozoan pathogens (Latz et al., 2013; Jo et al., 2016). Although it is unclear how NLRP3 can recognize such diverse signals, it’s suggested that NLRP3 senses a common cellular event caused by all stimuli rather than directly binding to them (Kelley et al., 2019). Chen and Chen (2018) found that disassembly of the trans-Golgi network (TGN) by multiple NLRP3 stimuli can recruit and activate NLRP3 and is now one of the proposed mechanisms for the detection of various diverse stimuli. Upon activation, NIMA-related kinase 7 (NEK7), an essential modulator of NLRP3 inflammasome activation and assembly, binds to NLRP3 (He et al., 2016). Recent studies suggest disassembly of the TGN causes the NLRP3 cage to localize on the dispersed TGN vesicle membranes which are then transported to the microtubule-organizing center where NEK7 resides (Magupalli et al., 2020; Andreeva et al., 2021). It is further hypothesized that the binding of NEK7 to NLRP3 disrupts the NLRP3 double-ring structure and causes structural rearrangement, exposing PYD domains, and allowing for NACHT domain oligomerization (Andreeva et al., 2021). Previous studies have placed the NEK7 binding site at NLRP3’s LRR domain; however, recent studies have shown that the LRR domain is dispensable for NLRP3 inflammasome activation and that NEK7 may have at least one additional binding site (Hafner-Bratkovič et al., 2018; He et al., 2018). Upon NACHT domain activation by ATP-exchange and NEK7 binding, the PYD domain then recruits the adaptor molecule ASC forming a filamentous complex referred to as ASC pyroptosome or “speck” clustering through PYD-PYD domain interactions. The caspase recruitment domain (CARD) of ASC binds to pro-caspase-1 which also contains a CARD domain (CARD-CARD interaction) and pro-caspase-1 is then converted into active caspase-1 by proximity-induced autoproteolysis (Lu et al., 2014; Lu and Wu, 2015; Malik and Kanneganti, 2017). Activated caspase-1 processes the biologically inactive peptides pro-IL-1β and pro-IL-18 into their active forms, IL-1β and IL-18, respectively. It also cleaves and activates the membrane pore-forming gasdermin D (GSDMD), a protein involved in the programmed cell death known as pyroptosis (Shi et al., 2015; Malik and Kanneganti, 2017). GSDMDs N-terminal domain (GSDMD-NT) shows high affinity to plasma membrane lipids cardiolipin and phosphoinositides. Upon cleavage, GSDMD-NT oligomerizes to form pores in the cell membrane. The subsequent disruption of the cell’s osmotic potential leads to pyroptosis and the release of intracellular contents, including inflammatory cytokines IL-1β and IL-18 (Ding et al., 2016; Evavold et al., 2018).
Activation of the NLRP3 inflammasome through canonical and non-canonical pathways has the same consequences, however, through different mechanisms. The non-canonical pathway results in inflammasome formation mediated by human caspase-4, caspase-5, and mouse caspase-11 (also called caspase-4/11) as a result of Gram-negative bacterial infection (Kayagaki et al., 2015). Extracellular LPS found on the membrane of Gram-negative bacteria is detected by TLR4 in conjunction with the adaptor protein TRIF (Rathinam et al., 2012). Similar to the canonical pathway, TLR4-MyD88 begins a downstream signaling cascade that results in the translocation of NF-κB into the nucleus to upregulate the expression of NLRP3, pro-IL-1β, pro-IL-18, and other inflammatory mediators (Liu et al., 2017). TRIF induces interferon regulatory factors (IRF) which then upregulates the expression of interferon (IFN)-α/β. IFN-α/β binds to IFN-α/β receptors which elicit caspase-4/11 expression through JAK/STAT signaling (Gurung et al., 2012; Rathinam et al., 2012). Another study reported that the protease Carboxypeptidase B1 is critical for caspase-11 expression and amplifies p38 MAPK signaling downstream of TLR4 and type I IFN signaling (Napier et al., 2016). Detection of extracellular LPS through TLR4 is not required for non-canonical inflammasome activation, however (Kayagaki et al., 2013). Type I IFN signaling also induces the expression of critical guanylate binding proteins (GBPs) which lyse intracellular bacteria and release LPS into the cytosol (Meunier et al., 2014). Intracellular LPS can be detected by caspase-4/11 which causes oligomerization and autoproteolytic cleavage to activate caspase-4/11 (Shi et al., 2014; Lee B. L. et al., 2018). Activated caspase-4/11 then facilitates pyroptosis through the cleavage of proteins such as GSDMD and regulating the release of IL-1α (Kayagaki et al., 2015; Shi et al., 2015; Wiggins et al., 2019); however, many of caspase-4/11 substrates have yet to be elucidated. Previous studies suggest caspase-4/11 may not have the ability to cleave pro-IL-1β and pro-IL-18 into their biologically active forms, unlike caspase-1, but still promotes NLRP3-induced caspase-1 activation and cytokine release through alternative pathways (Kayagaki et al., 2011; Py et al., 2014; Agnew et al., 2021).
Activators
Ion Fluxes
Ion fluxes (K+, Ca2+, Cl−, and Na+) are suggested as one of the major mechanisms that trigger the activation of the NLRP3 inflammasome. Inflammasome activators such as extracellular ATP (via the non-selective cation channel receptor P2X7), particulate matter (aluminum hydroxide, silica, and calcium pyrophosphate crystals), and nigericin (ionophore) can induce K+ efflux, a necessary signal for NLRP3 activation (Muñoz-Planillo et al., 2013; Katsnelson et al., 2015). Monosodium urate (MSU) crystals cause cellular swelling from water influx resulting in an intracellular decrease of K+, leading to NLRP3 inflammasome activation (Schorn et al., 2011). Quinine, an inhibitor of two-pore domain K+ channels can prevent caspase-1 activity in a dose-dependent manner, highlighting the role of this family of ionic channels in inflammasome activation (Di et al., 2018). Conversely, K+ channel inhibitors Ba2+ (inhibitor of inward-rectifier K+ channels), tetraethylammonium (inhibitor of voltage-gated K+ channels), and iberiotoxin (inhibitor of large-conductance calcium-activated K+ channels) fail to prevent caspase-1 activation in macrophages primed with LPS (Di et al., 2018). Combined, these results leave questions and uncertainty about the role that potassium may have on inflammasome activation. The second messenger inositol 1,4,5-triphosphate (IP3) appeared to promote inflammasome activation by inducing efflux of Ca2+ from the endoplasmic reticulum (ER) through a ligand-gated ion channel, subsequently increasing intracellular concentrations (Murakami et al., 2012). The same study found that either depletion of ER Ca2+ by incubation with an inhibitor of sarcoplasmic/ER Ca2+-ATPase pump or incubation with Ca2+ free media to prevent extracellular Ca2+ entry attenuated NLRP3 inflammasome activation in response to ATP, suggesting mobilization of both extracellular and ER Ca2+ is required for inflammasome activation. Moreover, blockers of volume-activated Cl− channels that are inactive against K+ channels are able to inhibit inflammasome activation, demonstrating the role of Cl− efflux in NLRP3 inflammasome assembly (Compan et al., 2012; Daniels et al., 2016). It’s suggested that Na+ influx also plays a regulatory role in NLRP3 inflammasome activation in conjunction with K+ efflux induced by different stimuli. Na+ influx caused by ionophores cannot activate the NLRP3 inflammasome, however, lysosomal delivery of MSU crystals causes an increase in intracellular Na+ and water influx, subsequently lowering intracellular K+ concentration and activating the NLRP3 inflammasome (Muñoz-Planillo et al., 2013). More recently, the use of amiloride, a common epithelial Na+ channel blocker, can reduce cytokine secretion and caspase-1 activity in primary monocytes (Scambler et al., 2019).
Reactive Oxygen Species and Mitochondrial Dysfunction
Mitochondrial reactive oxygen species (mtROS) is one of the first discovered activators of the NLRP3 inflammasome and is produced during mitochondrial damage, stress, or dysfunction. Early studies suggested the role of mtROS in NLRP3 inflammasome activation when they noted the inhibition of NADPH oxidase-dependent mtROS prevents ATP-induced caspase-1 activation and IL-1β production in macrophages (Cruz et al., 2007). In addition, downregulation of voltage-dependent anion channels (VDAC), which are responsible for regulating mtROS homeostasis, results in decreased mtROS, caspase-1 activation, and IL-1β release in THP-1 cells (Zhou et al., 2011). Palmitate, a fatty acid commonly found in palm oil, is shown to lead to NLRP3 inflammasome activation in a ROS-dependent manner since the use of the ROS inhibitor APDC blocks palmitate-dependent IL-1β production (Wen et al., 2011). The complete mechanism by which NLRP3 senses mtROS is not well understood. Some studies suggest NEK7 may detect mtROS since it is an NLRP3 modulator (Shi et al., 2016). Supporting this idea, imiquimod, a TLR7 agonist, can activate the NLRP3 pathway and induce IL-1β release in a ROS-dependent manner, but fails to induce IL-1β production in cells deficient in NEK7 (Groß et al., 2016). Other studies have suggested mtROS’s involvement in the priming step, not activation, since some ROS inhibitors are able to block NLRP3 expression (Bauernfeind et al., 2011). Furthermore, mtROS is also implicated to be involved in the deubiquitination of NLRP3, subsequently promoting its activation (Juliana et al., 2012).
After the discovery of mtROS and its potential involvement in the inflammasome pathway, mitochondria in general have shown to play an intricate and important role in the activation of inflammasomes through various mechanisms. Mitophagy, the clearance of damaged mitochondria, is negatively correlated with NLRP3 activation, and deficiency causes the accumulation of dysfunctional mitochondria and the release of mtROS and mitochondrial DNA (mtDNA), both known activators of NLRP3 (Nakahira et al., 2011; Zhou et al., 2011). Further investigation revealed that other NLRP3 inflammasome activators, such as alum and nigericin, cause mitochondrial dysfunction and that oxidized mtDNA directly binds to NLRP3 (Shimada et al., 2012). Mitochondrial fission is also implicated in NLRP3 activation through LPS signaling; although the mechanism remains unclear, it’s suspected that fission facilitates NLRP3 localization and complex assembly (Park et al., 2015). The mitochondrial-specific lipid cardiolipin also plays a direct role in NLRP3 activation by binding to NLRP3 in a ROS-dependent and independent manner (Iyer et al., 2013). In addition, hexokinase, the first enzyme in glycolysis, dissociates from the mitochondrial membrane upon inhibition with bacterial peptidoglycans from Gram-positive bacterial cell walls and consequently activates NLRP3 (Wolf et al., 2016). Mitochondrial dysfunction and stress play a significant role in inflammation in vitro and in vivo (Gong et al., 2018; Mahalanobish et al., 2020; Ko et al., 2021); however, some studies dispute the requirement of mitochondrial dysfunction and the mtROS in NLRP3 activation (Muñoz-Planillo et al., 2013; Allam et al., 2014; Lawlor and Vince, 2014).
Lysosomal Damage
Lysosomes are responsible for degrading extracellular material by endocytosis or phagocytosis and degrading or recycling intracellular material via autophagy. Lysosomal damage is considered to play a significant role in NLRP3 activation, and direct evidence of this is shown when the lysosomal damage-inducing dipeptide, L-leucyl-L-leucine methyl ester (Leu-Leu-OMe), is able to activate the NLRP3 inflammasome (Hornung et al., 2008). In addition, NLRP3 activators such as particulate matter (e.g., cholesterol crystals, silica, aluminum salts, uric acid crystals, and asbestos) and misfolded proteins (e.g., amyloid-β) can induce lysosomal damage, indicating an interesting mechanism of NLRP3 activation (Emmerson et al., 1990; Martinon et al., 2006; Halle et al., 2008; Hornung et al., 2008; Duewell et al., 2010; Ito et al., 2020). There are several theories for how lysosomal damage activates NLRP3, one of the most popular being the release of cathepsin proteases, the proteins that mediate lysosomal degradation, into the cytosol where they can have apoptotic or apoptotic-like consequences (Wang F. et al., 2018) In support, genetic and pharmacological inhibition of cathepsin B impairs caspase-1 activation and IL-1β release in response to amyloid-β, Leu-Leu-OMe, and palmitate (Halle et al., 2008; Hornung et al., 2008; Weber and Schilling, 2014). However, no difference in caspase-1 cleavage or IL-1β secretion is observed with genetic or pharmacological inhibition of cathepsin B in response to MSU, hemozoin, alum, or ATP, suggesting a distinct mechanism for activators or cathepsin B (Halle et al., 2008; Dostert et al., 2009). In addition to this, cathepsins L, C, S, and X are also implicated to play a role in inflammasome activation (Orlowski et al., 2015). Other theories on how lysosomal damage induces NLRP3 activation include lysosomal acidification and influencing ion fluxes. Blocking the vacuolar H+-ATPase, a proton pump responsible for lysosomal acidification, prevents IL-1β release in response to silica, suggesting acidification is required (Hornung et al., 2008). In addition, lysosomal damage induced by Leu-Leu-OMe can affect K+ efflux and Ca2+ mobilization, subsequently activating the NLRP3 inflammasome (Murakami et al., 2012; Katsnelson et al., 2016). This also highlights the capability of NLRP3 activation pathways to work in conjunction.
NLRP3 Inflammasome Dysregulation in Diseases
The pivotal defensive role of inflammasomes in response to pathogens and other danger signals also suggests inflammasome dysregulation in inflammation-mediated human diseases (Schroder and Tschopp, 2010; Liu and Chan, 2014; Moossavi et al., 2018; Boxberger et al., 2019; Eren and Özören, 2019). Aberrant NLRP3 activation has been shown to exacerbate disease pathology in several inflammation-driven diseases, and ongoing research is investigating the distinct role that NLRP3 may play in different disease states.
Cryopyrin-Associated Periodic Syndromes
Cryopyrin-associated periodic syndromes (CAPS) are a group of diseases that are caused by a gain-of-function mutation(s) in the nlrp3 gene and was the first autoinflammatory disorder to be directly linked to NLRP3 inflammasome dysregulation (Hoffman et al., 2001). Aberrant activation of NLRP3 causes recurrent episodes of fever, hive-like rashes, inflamed eyes, joint pain, swelling, headaches, and, if left untreated, deafness and amyloidosis (Kuemmerle-Deschner et al., 2017). CAPS include familial cold autoinflammatory syndrome (FCAS), Muckle–Wells syndrome (MWS), and neonatal onset multi-systemic inflammatory disease (NOMID) with symptoms and severity increasing respectively. Mortimer and colleagues found Ser295 in human NLRP3 is essential for negatively regulating NLRP3 activation via phosphorylation by protein kinase A, and mutations in adjacent residues interfere with regulation, rendering NLRP3 aberrantly active (Mortimer et al., 2016). A recent study showed that treatment of a knock-in mouse model expressing the N475K mutation of NLRP3 (in correspondence with the human N477K mutation) with the proton-pump inhibitor (PPI) esomeprazole is able to inhibit IL-1β secretion, reduce amyloid deposition, increase IL-1 receptor antagonist (IL-1Ra) production and survival rates (Bertoni et al., 2020). CAPS are incredibly rare genetic diseases and therefore have limited treatment options. Current treatment options consist of IL-1 inhibitors, however, the development of inhibitors that target the NLRP3 inflammasome is of great interest for these diseases.
Diseases of the CNS
Alzheimer’s disease (AD) is characterized by the accumulation of amyloid-β (Aβ) and hyperphosphorylated tau tangles. Inflammation has been implicated to encourage the progression of AD, and activated inflammatory mediators and high levels of IL-1β are found in the serum, cerebrospinal fluid, and brain of patients with AD where it exerts neurotoxic effects against microglia and astrocytes (Rubio-Perez and Morillas-Ruiz, 2012; Parajuli et al., 2013; Saresella et al., 2016; Italiani et al., 2018; Ng et al., 2018). The accumulation of the Aβ peptide in lysosomes after phagocytosis by microglial cells leads to lysosomal swelling and destabilization causing the release of lysosomal contents, including cathepsin B, and activation of NLRP3 (Halle et al., 2008). The link between NLRP3 and AD pathology has been demonstrated in other studies as well, where genetic deficiency and pharmacological inhibition of NLRP3 in mice over-expressing human amyloid precursor protein (APP) and presenilin 1 (PS1) reduces Aβ deposition and improves cognitive functions (Heneka et al., 2013; Dempsey et al., 2017; Yin J. et al., 2018). Furthermore, microglia treated with Aβ causes ASC specks, a critical component of the NLRP3 inflammasome, but Aβ treatment failed to produce specks in cells with mutations in the PYD domain of ASC (Venegas et al., 2017). In addition, activation of NLRP3 causes hyperphosphorylation of tau in an IL-1β-dependent manner in Tau22 mice, suggesting NLRP3 works upstream (Ising et al., 2019). Clinical trials with drugs targeting Aβ and tau tangles have been unsuccessful, but NLRP3 has accumulated significant interest as a new target for AD.
NLRP3 inflammasome has also been suggested to play an important role in Parkinson’s disease (PD), a neurodegenerative disease characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta, where high levels of IL-1β have been detected in patients suffering from PD (Mogi et al., 1994; Antony et al., 2013; Tan et al., 2020). The disturbed proteostasis and intraneuronal aggregation of fibrillar α-synuclein, known as Lewy bodies, interferes with neurotransmitter release and is a common hallmark of PD. These entities are able to activate the NLRP3 inflammasome through TLR2 and mitochondrial damage (Wang et al., 2020; Trudler et al., 2021). In addition, monocytes and microglia are able to clear α-synuclein which causes a robust release of pro-inflammatory cytokines, including IFN-γ, TNF, and IL-1β which results in neurodegeneration (Tan et al., 2020). Several studies have supported the link between NLRP3 and PD pathology. NLRP3 deficient mice are resistant to the loss of dopaminergic neurons in the substantia nigra after treatment with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a neurotoxin that induces a PD-like phenotype (Yan et al., 2015; Lee E. et al., 2019). In conjunction with this, pharmacological inhibition of NLRP3 improved PD pathology (Gordon et al., 2018). Prolonged exposure of IL-1Ra knockout mice to cytokines IL-1β/IL-1α results in PD-like outcomes, including impaired motor skills (Stojakovic et al., 2017). Together, this highlights the role of NLRP3 and cytokines in PD.
Multiple sclerosis (MS) is an autoimmune neurodegenerative disorder caused by the demyelination of neurons presumably by reactive T-cells that infiltrate the CNS upon weakening of the blood-brain barrier (BBB). NLRP3 and IL-1β have long been implicated to play a role in MS through encouraging immune cell infiltration and promoting excessive inflammation. Caspase-1 and IL-1β are found in MS plaques and are upregulated in peripheral blood mononuclear cells of MS patients (Ming et al., 2002; Cao et al., 2015). In experimental autoimmune encephalomyelitis (EAE), an animal model for MS, NLRP3 inflammasome expressing antigen-presenting cells assist in T-cell migration to the CNS through upregulation of chemotaxis-related proteins (Inoue et al., 2012). In addition, NLRP3 expression is upregulated in the spinal cords of mice with EAE, and NLRP3 and ASC knockout mice are resistant to EAE, highlighting their potential roles in MS (Gris et al., 2010; Inoue et al., 2012). IFNβ, one of the few therapeutic options for MS, can suppress NLRP3 activation and in NLRP3-dependent EAE (Inoue et al., 2016).
Neuroinflammation is the standard response to traumatic brain injury (TBI), and several studies have supported the upregulation of inflammatory mediators, including NLRP3, in the brain hours to days after a TBI (Liu et al., 2013; Wallisch et al., 2017). Cytokines IL-1β and IL-1α’s RNA are elevated as soon as 3 h after TBI in rats, and inhibiting the two cytokines with antibodies prior to TBI significantly reduces the loss of hippocampal neurons (Lu et al., 2005). NLRP3 and other inflammatory mediators are also upregulated in the brain of patients after suffering severe TBI, and recent studies reveal pharmacological inhibition of NLRP3 decreases cell death and prevents neurological deficits in TBI mouse models, highlighting the druggable potential of NLRP3 (Chen et al., 2019; Kuwar et al., 2019; Yan et al., 2020). TBI’s link to AD and PD is well established, and other studies support that patients with a history of TBI are at increased risk for neurodegenerative diseases in general (Gardner and Yaffe, 2015; Hayes et al., 2017; Ramos-Cejudo et al., 2018; Delic et al., 2020). These increased risks are likely contributed to neuroinflammation, as neuroinflammation after TBI shows increased hyperphosphorylation of tau protein and Aβ plaques, two hallmarks of AD (Johnson et al., 2010; Edwards et al., 2020).
Peripheral Inflammatory Diseases
Dysregulation of NLRP3 inflammasome has also been suggested in the pathogenesis of rheumatoid arthritis (RA), a chronic autoimmune disease characterized by persistent synovial inflammation in small diarthrodial joints, progressive cartilage, bone destruction, and autoantibody production (McInnes and Schett, 2011). Numerous studies support the involvement of NLRP3 in the development of RA, however, the mechanism is somewhat elusive. One study suggests pentaxin 3, a biomarker of RA, and its ligand C1q can activate NLRP3 (Wu et al., 2020). Significant NLRP3 expression is observed in the synovial proliferation and subchondral vasculitis areas in the paws of collagen-induced arthritis (CIA) mice compared to healthy mice (Zhang et al., 2016). Similarly, pharmacological inhibition of the NLRP3 inflammasome with different inhibitors reduces RA pathology and secretion of IL-1β and TNF-α, supporting the involvement of NLRP3 in RA (Yan et al., 2012; Voon et al., 2017; Guo et al., 2018; Marchetti et al., 2018b).
Gout has many of the same symptoms as RA, however, the pathology of these two diseases is different. While RA is an autoimmune disease, gout is caused by elevated levels of uric acid in the bloodstream. Eventually, these uric acid crystals accumulate in the joints where they are phagocytosed by synoviocytes (Richette and Bardin, 2010). Upon phagocytosis, uric acid crystals cause lysosomal destabilization which subsequently activates NLRP3, cytokine release and causes inflammation and pain (Martinon et al., 2006). In addition, soluble uric acid was able to activate NLRP3 in a ROS-dependent manner, suggesting inflammation is initiated before crystal formation (Braga et al., 2017). Several NLRP3 inhibitors have been shown to inhibit inflammation caused by gouty arthritis, emphasizing the importance of NLRP3 as a target in treatments for gout (Lee H. G. et al., 2016; Ruiz-Miyazawa et al., 2017; Huang et al., 2018; Marchetti et al., 2018b; Lee H. E. et al., 2019; Deng et al., 2020).
Diabetes mellitus is another disease in which NLRP3 dysregulation has been suggested to play a pathological role (Chausmer, 1998; Tang et al., 2019). High levels of IL-1β have been detected in patients with diabetes and other studies show IL-1β can affect insulin sensitivity through the TNF pathway (Dinarello et al., 2010; Wen et al., 2011). Transgenic mice lacking NLRP3 fed with a high-fat diet, which has previously been shown to activate NLRP3, have lower IL-1β and are protected from high-fat diet-induced insulin resistance, further supporting inflammatory mediators’ involvement in metabolic inflammation and insulin resistance (Vandanmagsar et al., 2011; Wen et al., 2011). Recently, it was reported that NLRP3 inflammasome inhibition could be one of the mechanisms underlying metformin’s effects to inhibit diabetes-accelerated atherosclerosis (Tang et al., 2019). Consistent with this observation, the blockade of IL-1 receptor using anakinra, a drug used for RA, leads to significant improvements in diabetic patients (Larsen et al., 2009).
At least 20% of all cancers arise from infection or chronic inflammation (Grivennikov and Karin, 2011). Chronic inflammation is led by the release of pro-inflammatory cytokines; however, while NLRP3 is an important mediator in inflammation, reports show that NLRP3 can have both a destructive and protective role in different types of cancer, making NLRP3’s role in cancer complex (Hamarsheh and Zeiser, 2020). Several studies have shown that NLRP3 and components of the NLRP3 inflammasome pathway can play a destructive role through upregulation, overactivation, and polymorphisms (Hamarsheh and Zeiser, 2020). One example of this is IL-1 and TNF’s ability to recruit neutrophils, encouraging ROS production and subsequent inflammation as well as inducing adhesion molecules and metalloproteases which encourage tumor invasion (Dinarello, 2006). Polymorphisms and mutations in the NLRP3 gene are also implicated in cancer. Poor survival rate is correlated with invasive colorectal cancer patients that have the Q705K NLRP3 mutation, a mutation that is also prominent in pancreatic cancer patients (Ungerbäck et al., 2012; Miskiewicz et al., 2015). Conversely, NLRP3 is also demonstrated to have anti-tumorigenic effects in colorectal cancer. One study found that mice deficient in NLRP3 or caspase-1 exhibit high sensitivity to azoxymethane (AOM) dextran sodium sulfate (DSS)-induced inflammation and suffer tumor burdens as a result of decreased IL-18 and lack of tumor suppressing cytokines, IFN-γ and single transducer and activator of transcription-1 (STAT-1) (Zaki et al., 2010b). Along those same lines, inflammasome component deficiency also correlates with a worse disease state in colitis-associated cancer (CAC) as a result of decreased IL-18 (Zaki et al., 2010a). The exact role of NLRP3 in different types of cancer is multiplex, but therapies that target inflammation may be of value to some diagnoses.
NLRP3 Inflammasome Pathway Inhibitors
The link between NLRP3 inflammasome dysregulation and a variety of human diseases has suggested the value of NLRP3 inhibitors for therapeutic interventions. Several NLRP3 inflammasome inhibitors have recently emerged and been investigated in a variety of disease states; some of which have entered clinical trials. Herein, we summarize the mechanisms of inhibition and relevant SAR studies that have been conducted for direct and indirect inhibitors of the NLRP3 inflammasome (Figure 1).
Sulfonylurea and Sulfonamide Analogs
Inflammasome inhibitors containing the sulfonylurea and sulfonamide moieties have been developed and studied to improve potency, selectivity, and solubility (Figure 2).
Glyburide®
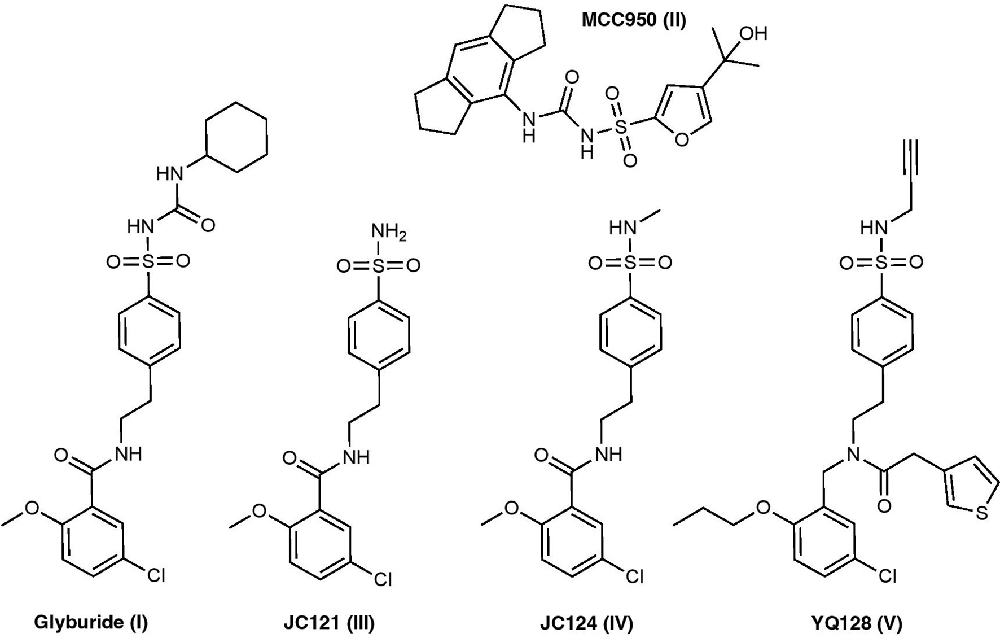
Glyburide is an FDA-approved, anti-diabetic drug that falls into the sulfonylurea class of medications. Glyburide treats diabetes by blocking ATP sensitive potassium (KATP) channels in pancreatic β cells (Ashcroft, 2005). Studies have shown that glyburide inhibits NLRP3 activation and IL-1β secretion induced by LPS/ATP in bone marrow-derived macrophages (BMDMs) with an IC50 of 10–20 μM (Lamkanfi et al., 2009; Hill et al., 2017). Lamkanfi et al. (2009) demonstrated that glyburide inhibits caspase-1 activation and IL-1β secretion independently of KATP channels and later concluded that glyburide likely targets downstream of the purinergic 2X7 receptor (P2X7R) and upstream of inflammasome formation. Pilot structure-function studies revealed that the cyclohexylurea group is responsible for insulin secretion, whereas the benzamide and sulfonyl moiety are critical for NLRP3 inflammasome inhibition (Hill et al., 2017). Unfortunately, the concentration that glyburide is able to elicit its anti-inflammatory properties can induce hypoglycemia which limits its further development as an NLRP3 inflammasome inhibitor (Coll et al., 2015).
MCC950
MCC950 is a sulfonylurea compound initially discovered in 2001 under the name CP-424, 174 (Perregaux et al., 2001). The term CRID3 is also used synonymously with MCC950. Recent studies have demonstrated that MCC950 is a potent and selective NLRP3 inflammasome inhibitor that can inhibit IL-1β release in BMDM cells with an IC50 of 7.5 nM (Coll et al., 2015; O’Neill et al., 2016). However, it should be noted that in a 2011 article by Coll et al. (2011), a different compound was incorrectly termed CRID3 and was described to be nonselective and inhibiting both NLRP3 and AIM2 inflammasomes. Mechanistic studies revealed that MCC950 is a direct NLRP3 inhibitor that binds to the Walker B motif of the ATPase binding pocket in the central NACHT domain subsequently inhibiting ATPase activity, a critical step in inflammasome activation (Coll et al., 2019). Further docking studies supported this binding site (Tapia-Abellan et al., 2019; Andreeva et al., 2021; Hochheiser et al., 2021). MCC950 fails to stimulate insulin secretion in vitro; however, a later study found it’s able to increase insulin sensitivity in an in vivo diabetic mouse model, although this was attributed to MCC950’s effects at NLRP3 (Hill et al., 2017; Zhai et al., 2018). In 2017, Hill et al. (2017) synthesized a series of MCC950, sulfonylurea derivatives that can inhibit NLRP3 inflammasome with nanomolar potencies and also inhibit insulin secretion. Since the discovery of MCC950 as an NLRP3 inhibitor, it’s been employed as a chemical tool to understand the pathological roles of the NLRP3 inflammasome in a variety of disease models including AD, atherosclerosis, asthma, allergic airway inflammation, inflammatory bowel disease (IBD), and many more (Dempsey et al., 2017; van der Heijden et al., 2017; van Hout et al., 2017; Ismael et al., 2018; Perera et al., 2018; Robinson et al., 2018; Xu et al., 2018; Zhai et al., 2018; Theofani et al., 2019; Fu et al., 2020; Ren et al., 2020). The commonly used dose in these studies was 10 mg/kg, relatively high compared to its low nanomolar potency. Recent studies of radio-labeled MCC950 PET tracer demonstrate its low CNS penetration, which may limit its development and use as a CNS agent (Hill et al., 2020).
JC121, JC124, and YQ128
Formerly known as 16673-34-0, JC121 was developed as a sulfonamide derivative of glyburide. JC121 is able to inhibit ASC aggregation, caspase-1 activity, IL-1β secretion, and inflammatory cell death in cardiomyocytes in response to ATP and nigericin (Marchetti et al., 2014). Furthermore, JC121 inhibits the activity of constitutively active NLRP3 in BMDMs from genetically modified mice, suggesting that this compound inhibits downstream inflammasome activation rather than upstream targets (Marchetti et al., 2015). Notably, JC121 does not show hypoglycemia effects in vivo, a concern for its scaffold predecessor glyburide, even at doses as high as 500 mg/kg. This compound exhibits in vivo protective activity in mouse acute myocardial infarction models and preserves cardiac function after ischemic injury (Marchetti et al., 2014, 2015). To overcome the observed solubility issues of JC121, another analog, JC124, was designed that incorporates a methylated sulfonamide into the structure. The results demonstrated that, like JC121, JC124 is also a selective inhibitor of the NLRP3 inflammasome that inhibits IL-1β release with an IC50 of 3.25 μM without significant inhibition on NLRC4 or AIM2 inflammasomes (Fulp et al., 2018). Mechanistic studies employing a photo-affinity probe suggested that JC124 directly interacts with the NLRP3 protein; however, unlike MCC950, JC124 can bind to NLRP3 without affecting the ATPase activity, suggesting a distinct mode of binding for these sulfonamide derivatives (Kuwar et al., 2019). In vivo, JC124 can actively reduce disease pathology and improve functional performance in animal models of TBI, AD, and acute myocardial infarction by engaging the NLRP3 inflammasome (Fulp et al., 2018; Yin et al., 2018; Kuwar et al., 2019). Structure-activity relationship (SAR) studies of JC124 revealed that the sulfonamide moiety can be modified to improve inhibitory potency.
To increase the space for structural optimization, a new chemical scaffold was created based on the structure of JC124 and further medicinal chemistry campaign led to the design of YQ-II-128 (YQ128) as a novel NLRP3 inhibitor (Jiang et al., 2019). YQ128 significantly inhibits NLRP3 mediated IL-1β production in vitro with an IC50 of 0.30 μM in mouse macrophages. Further in vitro studies demonstrated YQ128 as a selective inhibitor of the NLRP3 inflammasome, since it did not inhibit IL-1β production mediated by NLRC4 or AIM2 inflammasomes. In vivo studies also confirmed its selective inhibition on IL-1β production upon LPS challenge in mice. A YQ128 PET tracer showed rapid BBB penetrance with moderate brain uptake; however, preliminary pharmacokinetic studies revealed that YQ128 displays poor oral bioavailability (Jiang et al., 2019; Xu et al., 2021). Ongoing SAR studies are currently underway to improve the potency and pharmacokinetics of these compounds.
Natural Products
Numerous natural products possess anti-inflammatory activity with mechanisms and targets that go far beyond the breadth of this review. However, there are several natural products that have received considerable attention for their anti-inflammatory activity being attributed to the interference of inflammasomes and their pathways (Figure 3).
Flavonoids
Flavonoids are a group of phytonutrients found in a variety of fruits, vegetables, grains, and plants and are well recognized for their neuroprotective, anti-inflammatory, antimutagenic, and antioxidant properties.
Apigenin
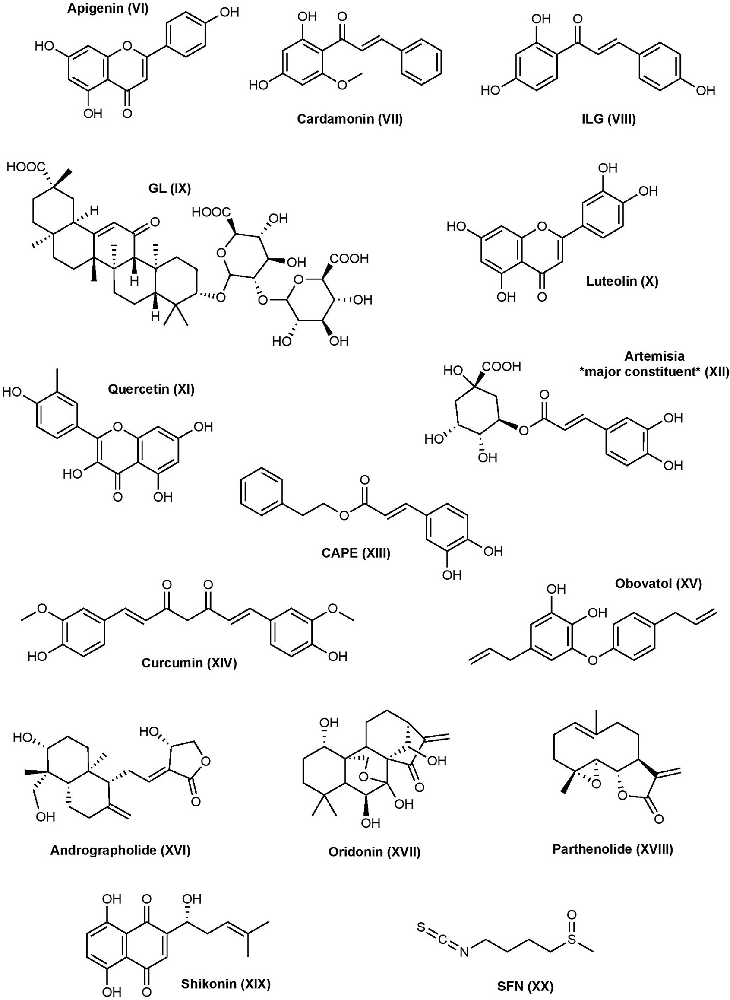
Apigenin is a flavone that inhibits NLRP3-mediated inflammation through a variety of different mechanisms (Zhang et al., 2014). The expression of pro-inflammatory cytokines IL-6, IL-1β, and TNF-α are reduced by apigenin in LPS-primed THP-1 derived macrophages and J774A.1 mouse macrophages. Apigenin shows inhibitory activity on the ERK1/2 and NF-κB pathways. Despite apigenin inhibiting NF-κB activation, it does not affect NLRP3 mRNA or protein levels or ASC protein levels in response to LPS; however, apigenin inhibits ASC speck formation and further studies suggested this happens through specifically targeting ASC (Zhang et al., 2014). A recent study demonstrated that apigenin can inhibit the phosphorylation of spleen tyrosine kinase (Syk) and protein tyrosine kinase 2 (Pyk2), two tyrosine kinases that are key players in the phosphorylation of ASC (Lim et al., 2018). The same study found apigenin can inhibit AIM2-mediated IL-1β production, but not NLRC4-mediated, in THP-1 cells, making apigenin a non-selective inflammasome inhibitor. Apigenin presents therapeutic potential in several disease states, but more research is needed (Salehi et al., 2019).
Cardamonin
Cardamonin is a natural chalcone found in the plant Alpinia katsumadai Hayata. Cardamonin can inhibit the NF-κB pathway through suppression of nitric oxide (NO) and prostaglandin-E2 in IFN-γ- and LPS-induced RAW 264.7 cells (Israf et al., 2007). This results in decreased phosphorylation and degradation of Iκ-Bα and subsequent activation of NF-κB. Other potential mechanisms of cardamonin include upregulating AhR/Nrf2/NQO1 signaling, which is known to negatively regulate NLRP3 (Wang K. et al., 2018). So far, cardamonin has mainly been investigated in cancer, but it has also been studied in IBD, RA, and more (Voon et al., 2017; Wang K. et al., 2018; Jin et al., 2019; Liao et al., 2019).
Isoliquiritigenin and Glycyrrhizin
Isoliquiritigenin (ILG) and Glycyrrhizin (GL) are both extracts from the plant Glycyrrhiza uralensis (licorice) which have been used throughout time to treat a variety of disorders mainly due to their abundance in flavonoids, chalcones, and other phytonutrients. ILG is a flavonoid that resembles a chalcone, while GL is a triterpene saponin. Both ILG and GL inhibit the NF-κB and MAPK activation by suppressing TLR4/MD-2 complex (Honda et al., 2012). ILG has also been recognized to promote the Nrf2 pathway and suppress ROS (Zeng et al., 2017). Further studies show that GL and ILG inhibit NLRP3 inflammasome formation, activation of caspase-1, and production of IL-1β when added during the activation step (Honda et al., 2014). Collectively, the results suggest that ILG and GL can inhibit both the priming and activation steps of the inflammasome pathway. Furthermore, ILG is a selective inhibitor of the NLRP3 inflammasome, as it did not inhibit AIM2 inflammasome formation or IL-1β production in response to poly (dA:dT). Contrarily, GL shows inhibitory activity to both NLRP3 and AIM2 inflammasome mediated IL-1β production (Honda et al., 2014). Because ILG has selectivity for NLRP3, and ASC oligomerization is a shared mechanism between NLRP3 and AIM2, ILG may target NLRP3 directly or somewhere upstream to inhibit inflammation. Because GL is not selective, GL may target ASC or somewhere upstream.
Luteolin
Luteolin is a flavone that can be identified in plants including pepper, broccoli, thyme, and celery (Imran et al., 2019). Mechanistically, luteolin can reduce ROS and expression of NLRP3 and other inflammatory mediators through the NF-κB pathway; however, it is a nonspecific inflammasome modulator as it also affects the expression of AIM2 (Chen et al., 2007; Zhang et al., 2018; Yu et al., 2019). One of these studies showed that luteolin potently inhibits NLRP3 expression as low as 2 μM in RAW267.4 cells and attributed this effect to its ability to enhance M2 macrophage polarization which is known to stimulate immunoregulation (Zhang et al., 2018). Further research suggested the potential involvement of transcription factor Nrf2 in the observed inhibition of inflammasome activation (Hennig et al., 2018). Luteolin and some derivatives have been considered as a treatment for several diseases including ulcerative colitis (inflammatory bowel disease), several types of cancer, and myocardial injury (Ning et al., 2017; Imran et al., 2019; Li B. et al., 2021).
Quercetin
Quercetin is a flavonol that is found in many fruits, vegetables, leaves, grains, and more (Bentz, 2009). Quercetin is a non-selective inflammasome inhibitor that inhibits IL-1β secretion during NLRP3 and AIM2 challenging (Domiciano et al., 2017). During the priming step, quercetin can inhibit NF-κB and MAPK as well as JAK/STAT pathways under AIM2 challenging (Hämäläinen et al., 2007; Lee K. M. et al., 2018). Other studies have indicated that quercetin inhibits the activation step of the NLRP3 inflammasome by interfering with ASC-speck oligomerization, which may explain why it cannot inhibit NLRC4 since ASC is dispensable for NLRC4 activation (Domiciano et al., 2017). Quercetin has been investigated in neurodegenerative disorders, cancer, diabetes, and more (Chen et al., 2016; Amanzadeh et al., 2019; Reyes-Farias and Carrasco-Pozo, 2019; Zaplatic et al., 2019).
Phenols
Artemisia
The genus Artemisia contains a variety of species, some of which have been used in traditional Asian medicines for their anti-inflammatory properties. Extract of Artemisia princeps (APO) contains several phenolic compounds such as caffeoylquinic acids, chlorogenic acid, and neochlorogenic acid; however, the anti-inflammatory properties are mainly attributed to chlorogenic acid which is one of the major constituents in the APO extract. APO can inhibit ASC speck formation upon activation of the NLRP3 or AIM2 inflammasomes, but not NLRC4, in BMDM cells (Kwak et al., 2018). The same study demonstrated that APO interferes with the phosphorylation of a tyrosine residue (Y144) of ASC, which is critical for speck formation. This is consistent with its dual inhibition on the NLRP3 and AIM2 inflammasomes since ASC is not required for the assembly and activation of the NLRC4 inflammasome (Kwak et al., 2018). Artemisia and their extracts have become an attractive area of natural product medicine and several other species have been investigated for their anti-inflammatory properties and suspected roles in the inflammasome pathways (Chen et al., 2021; Manayi et al., 2021; Wang Q. et al., 2021).
Caffeic Acid Phenethyl Ester
Caffeic acid phenethyl ester (CAPE) is a phenolic compound found in propolis from honeybee hives. Similar to the other phenolic compounds, CAPE can suppress the activation of transcription factors, specifically NF-κB, that leads to a decrease in the production of pro-inflammatory mediators (Natarajan et al., 1996; Juman et al., 2012). CAPE has recently been shown to inhibit the activation of NLRP3 and AIM2 inflammasomes by binding to ASC and blocking NLRP3-ASC interactions (Lee H. E. et al., 2016). It was later revealed that CAPE binds to the ASC-PYD domain, but not to the ASC-CARD or NLRP3-PYD domain. Because NLRP3 and AIM2 both require ASC, CAPE likely has a similar mechanism toward AIM2. CAPE has been investigated in gout, cancer, Lou Gehrig’s disease, neuroprotection in status epilepticus, and diabetes (Fontanilla et al., 2012; Yis et al., 2013; Fraser et al., 2016; Lee H. E. et al., 2016; Nie J. et al., 2017).
Curcumin
Curcumin is a widely used polyphenol compound known for its anti-inflammatory and antioxidant effects. A 2016 study found that treatment with curcumin decreases the expression of NLRP3, activation of caspase-1, and cleavage and secretion of IL-1β in PMA-induced macrophages (Kong et al., 2016). The reduced expression of NLRP3 is likely due to its inhibition of IKK phosphorylation, which is required for NF-κB activation; however, the expression of several other pro-inflammatory mediators was also reduced such as TLR4 and MyD88, suggesting multiple targets which is consistent with the promiscuous nature of this compound. Curcumin is also able to reverse P2X7R activation in PMA-induced macrophages, subsequently decreasing the expression of NLRP3 (Kong et al., 2016). Later studies found that curcumin is selective to the NLRP3 pathway, as it does not inhibit NLRC4- or AIM2-mediated caspase-1 activation in LPS-primed BMDMs (Yin H. et al., 2018). Other than priming, curcumin also suppresses ASC speck formation, caspase-1 activation, and IL-1β secretion in LPS-primed BMDMs, suggesting it affects both the priming and activation steps. This study also found that curcumin can inhibit potassium efflux in macrophages and inflammasome NLRP3 complex formation. Despite ROS being one of the activators of NLRP3, curcumin’s antioxidant effects are not the primary mechanism responsible for its inhibitory effect on the NLRP3 inflammasome pathway, however, it is recognized to upregulate the Nrf2 pathway (Yin H. et al., 2018; Rahban et al., 2020). Curcumin itself has been investigated in a variety of different diseases including, but not limited to, AD, osteoarthritis, depression and anxiety, cancer, and many more (Lopresti and Drummond, 2017; Sun et al., 2017; Reddy et al., 2018; Giordano and Tommonaro, 2019).
Obovatol
Obovatol is phenol isolated from the bark of Magnolia obovata. Like other natural biphenolic compounds, obovatol is traditionally used for its anti-inflammatory, anxiolytic, and nootropic characteristics. Obovatol can inhibit NF-κB, JNK, and ERK pathways, resulting in the suppression of inflammatory mediators (Ock et al., 2010). In addition, obovatol can also block the production of mitochondrial ROS and the formation of ASC inflammasome in response to nigericin and dsDNA, suggesting it inhibits both NLRP3 and AIM2 inflammasome activation (Kim J. et al., 2019). Further studies are required to understand the mechanism of action and binding. Obovatol has been investigated for use in AD and cancer (Choi et al., 2012; Duan et al., 2018).
Terpenes
Andrographolide
Andrographolide is a diterpenoid extracted from Andrographis paniculate and is commonly prescribed as a treatment for RA, asthma, laryngitis, and other upper respiratory tract infections (Tan et al., 2017). It is known to suppress the NF-κB and MAPK pathways and may also act as a ROS scavenger in ovalbumin-induced lung injury (Peng et al., 2016; Nie X. et al., 2017). Andrographolide can also inhibit NLRP3 activation by stimulating mitophagy, a process that is negatively correlated with the inflammasome, in colitis-associated cancer (Guo et al., 2014). Recently, andrographolide has been shown to also inhibit the AIM2 inflammasome by preventing AIM2 from translocating into the nucleus to sense DNA damage during the development of radiation fibrosis in BMDM cells (Gao et al., 2019). Andrographolide has been investigated in several diseases, however, due to its effects on AIM2 it is been closely investigated for its antiviral properties (Gupta et al., 2017; Reshi and Chi-Yong, 2020; Shi et al., 2020).
Oridonin
Oridonin (Ori) is a natural diterpenoid from the plant Rabdosia rubescens that is commonly used in East Asia as a natural supplement for its anti-inflammatory, anti-tumor, antimicrobial, and neuroprotective effects (Xu et al., 2018). Studies have shown that Ori can suppress the level of several pro-inflammatory mediators by inhibiting NF-κB signaling, nuclear translocation, and DNA binding (Wang et al., 2014; Cummins et al., 2018). Contrarily, increased expression of pro-TNF-α and increased phosphorylation of IκB in L929 cells have also been observed by Ori treatment, which may contribute to pro-inflammatory effects (Huang et al., 2005). Recent studies showed that Ori is a direct, irreversible, and selective inhibitor of NLRP3 and inhibits IL-1β production with an IC50 of ~0.5 μM (He et al., 2018). Mechanistically, Ori is able to disrupt NLRP3-NEK7 interactions, a crucial interaction for NLRP3 activation and inflammasome assembly, by covalently modifying the cysteine residue C279 in the NACHT domain of NLRP3 protein by Michael addition. Further studies showed that Ori failed to inhibit NEK7-NEK9 interactions, suggesting Ori inhibits the NLRP3-NEK7 interaction by binding to NLRP3. Ori shows no effect on the ATPase activity of NLRP3, NLRP3-NLRP3 interactions, or recruitment of ASC (He et al., 2018). Ori has demonstrated in vitro and in vivo success and has been investigated in a number of disease models including TBI, AD, cardiac hypertrophy, methicillin-resistant Staphylococcus aureus (MRSA), pulmonary fibrosis, cancer, and more (Wang et al., 2016; Fu et al., 2018; Xu et al., 2019; Yuan et al., 2019; Zhang et al., 2019; Yan et al., 2020). Despite the observed in vivo activities of Ori from preclinical studies, there is concern about the toxicity and adverse pharmacological effects of Ori (Li X. et al., 2021). More research on Ori analogs is required to improve pharmacological activity and bioavailability.
Parthenolide
Parthenolide is a natural product found in Tanacetum parthenium and has commonly been used as a preventative for migraine headaches. Its anti-inflammatory activity has been attributed to its ability to inhibit the NF-κB pathway, however, it also shows anti-inflammatory effects independent of the NF-κB mechanism (Saadane et al., 2007). One study has demonstrated that parthenolide inhibits multiple inflammasome pathways including NLRP3, NLRP1, and NLRC4 and directly inhibits caspase-1, but does not inhibit the AIM2 pathway (Juliana et al., 2010; Coll et al., 2011). Conversely, one study demonstrated that parthenolide does not inhibit NLRC4 (Coll et al., 2015). Mass spectrometry results show that parthenolide irreversibly inhibits caspase-1 by alkylating C285 of the p20 subunit, blocking activity and cleavage of downstream events. It is suspected that parthenolide block NLRP3 in a similar manner due to its ability to inhibit ATPase activity in a dose-dependent manner (Juliana et al., 2010). Parthenolide can inhibit IL-1β secretion with an IC50 of ~5 μM in response to NLRP3 challenging and 5–10 μM in response to AIM2 and NLRC4 challenging in BMDM cells (Jiang H. et al., 2017). Naturally occurring parthenolide’s poor aqueous solubility and bioavailability limits its further clinical development, but due to its reactive species it is still being investigated in different disease, states including, cancer and analogs continue to be made in an effort to improve pharmacological properties (Guzman et al., 2007; Alwaseem et al., 2018; Li et al., 2020; Liu et al., 2020; Hotta et al., 2021).
Other
Shikonin
Shikonin is a naphthoquinone compound isolated from the roots of Lithospermum erythrorhizon and is used in traditional Chinese herbal medicine for its variety of medicinal properties. Shikonin inhibits the secretion and cleavage of pro-caspase-1 through suppression of NF-κB as well as directly inhibits caspase-1 itself, likely by targeting a cysteine residue in the active site (Zorman et al., 2016). Furthermore, shikonin was able to inhibit NLRP3-mediated IL-1β secretion with an IC50 of 1.4–2 μM in LPS primed iBMDMs. Another study demonstrated that shikonin prevents ASC pyroptosome formation and NLRP3- and AIM2-mediated IL-1β and IL-18 release in BMDM and THP-1 cells through inhibition of PKM2, a kinase that promotes the activation of NLRP3 and AIM2 inflammasomes (Xie et al., 2016). Shikonin has been investigated in the treatment of AIDS and cancer (Chen et al., 2003; Wang et al., 2017, 2019; Zhao et al., 2018).
Sulforaphane
Sulforaphane (SFN) is a widely used dietary supplement that is found in broccoli extracts and cruciferous vegetables. SFN is known to have anti-inflammatory properties that are attributed to activating the Nrf2 transcription factor, a protein that regulates the antioxidant responses upon oxidative damage caused by ROS, and NF-κB by employing its ability to modify cysteine residues (Heiss et al., 2001; Jiao et al., 2017). SFN can also inhibit TLR activation through covalent modification of a cysteine residue in the hydrophobic pocket of myeloid differentiation 2 (MD2), which complexes with TLR (Koo et al., 2013). However, recent research has shown that SFN has anti-inflammatory properties independent of the priming step and is a non-selective inflammasome inhibitor of the NLRP3, NAIP5/NLRC4, NLRP1b, and AIM2 pathways (Greaney et al., 2016). SFN can inhibit NLRP3-mediated IL-1β production with an IC50 of 5 μM and NLRC4- and AIM2-mediated IL-1β production with an IC50 of ~10 μM (Jiang H. et al., 2017). It’s not clear whether SFN acts in a direct or indirect manner; however, the non-selective inhibition on multiple inflammasomes may suggest that SFN targets a shared mechanism by the inflammasomes during the activation process. Greaney et al. (2016) also showed that the SFN inhibits caspase-1 autoproteolytic cleavage but does not modify cysteine residues in the caspase-1 active site, as incubation with SFN in the presence of active caspase-1 does not inhibit the IL-1β processing. SFN itself has been investigated for the treatment of retinal ischemic/reperfusion injury, cancer, neurodegenerative diseases, and many more diseases (Kan et al., 2018; Gong et al., 2019; Kim, 2021). There is abundant research on the dietary benefits of broccoli in preventing diseases, and SFN is suggested to play a significant role (Subedi et al., 2019; Nandini et al., 2020; Liebman and Le, 2021).
Other Inhibitors
Several synthetic small molecules and biologics have been investigated to inhibit the NLRP3 inflammasome pathway as well, some of which have successfully made it to the market (Figure 4).
X-11-5-27
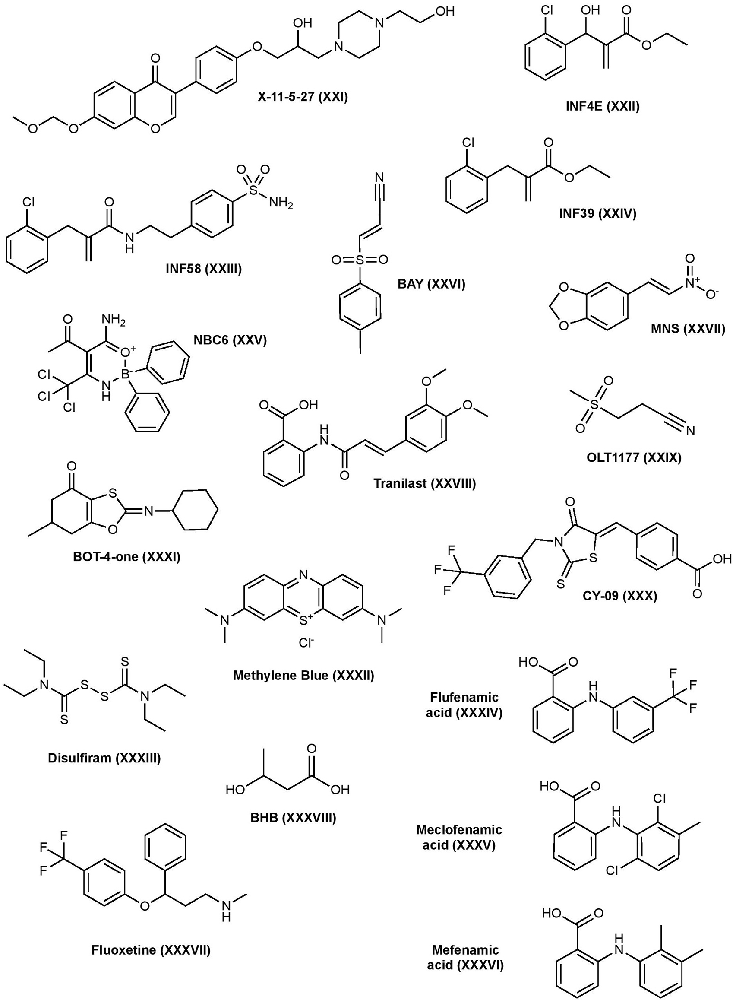
X-11-5-27 is a synthetic derivative of daidzein, a natural isoflavonoid found in soybeans, with antioxidant and anti-inflammatory properties. Daidzein has been shown to suppress the secretion of pro-inflammatory cytokines in vitro and it’s suspected that these effects are the result of a variety of targets in the MAPK pathway (Hämäläinen et al., 2007; Liu et al., 2009; Sakamoto et al., 2016). X-11-5-27 inhibits NLRP3 expression and activation, ASC speck formation, and subsequent caspase-1 activation and IL-1β secretion in BMDMs and THP-1 cells (Zhou et al., 2017). It was also observed that X-11-5-27 inhibits NLRP3 through the induction of autophagy, a process that is negatively correlated with NLRP3. Further studies suggested that the inhibitory activity of X-11-5-27 in these studies may be through antioxidant effects by reducing ROS production and protecting mitochondrial function (Zhou et al., 2017).
INF Analogs
In 2014, a series of α, β-unsaturated warheads from an electrophilic fragment-based library were reported to irreversibly inhibit the NLRP3 inflammasome (Cocco et al., 2014). Further characterization of one of the compounds, INF4E (compound 9), demonstrated its irreversible inhibition of the NLRP3 protein via its Michael acceptor to form a covalent bond with NLRP3, which subsequently inhibits IL-1β secretion in ATP-induced THP-1 cells. INF4E also inhibits NLRP3’s ATPase and caspase-1 activity, suggesting those as targets. Furthermore, INF4E prevents caspase-1 dependent pyroptosis and attenuates ATP- and nigericin-induced cell death (Cocco et al., 2014). To decrease toxicity associated with these types of compounds, further structural derivatization led to a series of acrylamide analogs using the ligand-merging strategy to combine the Michael acceptor moiety and a sulfonamide moiety. One of the analogs, INF58 (compound 14), irreversibly inhibits NLRP3 ATPase with improved potency (Cocco et al., 2016). Further optimization led to the identification of an ethyl acrylate derivative, INF39 (compound 11), that possesses decreased cytotoxicity and reactivity compared to both INF4E and INF58. Mechanistic studies suggest its direct interaction with the NLRP3 ATPase domain and can inhibit IL-1β production with an IC50 of 10 μM (Cocco et al., 2017). The same study found that INF39 can attenuate NEK7-NLRP3 interactions in HEK293 cells expressing NLRP3 and NEK7, confirming that INF39 blocks NLRP3 inflammasome assembly. INF39 continues to be investigated in the inflammasome pathway, a recent study has further verified INF39’s selective interaction with NLRP3, as it does not suppress AIM2 or NLRC4 inflammasomes, and showed the inhibition of ASC speck formation and the cleavage of caspase-1, IL-1β, and GSDMD proteins in THP-1 cells (Shi et al., 2021). Other studies have continued to derivatize INF39 and investigate the mechanism of binding (Gastaldi et al., 2021).
Novel Boron Compounds (NBC)
2-aminoethyldiphenyl borate (2APB), a boron containing compound, is known to inhibit IP3-mediated calcium release, store-operated calcium entry, and disrupt other calcium-dependent pathways in HeLa cells and cardiac myocytes, which may affect the ion flux balance required to activate NLRP3 (Peppiatt et al., 2003). Further studies demonstrated that 2APB can inhibit nigericin- and ATP-induced NLRP3 inflammasome signaling cascade independently of its effect on calcium flux (Katsnelson et al., 2015). However, 2APB’s effects on calcium flux limits its drug development. Further optimization of 2APB to weaken its effects at calcium flux while strengthening the effects on NLRP3 resulted in compound NBC6 with significantly enhanced potency (IC50 = 574 nM) (Baldwin et al., 2017). NBC6 can inhibit ASC oligomerization and was found to be a potent inhibitor of NLRP3; however, it’s also able to inhibit NLRC4 and AIM2 pathways at higher concentrations. Interestingly and unlike 2APB, NBC6 retained activity after washing away free drug, suggesting it may act as a covalent inhibitor. NBC13, a similar analog to NBC6 with better solubility, inhibited IL-1β release in vivo (Baldwin et al., 2017). NBC compounds have yet to be investigated in disease states.
BAY 11-7082
BAY 11-7082 (BAY) is a phenyl vinyl sulfone and is known for its inhibition of TNF-α induced IKK phosphorylation which subsequently inhibits activation of NF-κB and expression of NLRP3 (García et al., 2005). However, due to its non-specific modification of cysteine residues, BAY shows polypharmacological properties with multiple mechanisms suggested (Lee et al., 2012). In 2010, a study found that BAY reduces NLRP3’s ATPase activity and inhibits ASC oligomerization (Juliana et al., 2010). Upon further investigation, this study found that BAY inhibits NLRP3 activity by irreversibly alkylating cysteine residues in the ATPase region of NLRP3 through a Michael addition mechanism and SAR studies of BAY revealed the importance of the vinyl sulfone in the observed inhibitory activity. BAY can inhibit IL-1β production in BMDM’s with an IC50 of ~5 μM (Jiang H. et al., 2017). Increased ubiquitination of NLRP3 by BAY was also observed which may contribute to its inhibition of the NLRP3 protein by interfering with NLRP3-ASC interactions (Shim et al., 2017). A recent study demonstrated that BAY inhibits pore-formation of GSDMD by covalently modifying the critical cysteine residue C191 which is involved in GSDMD pore-formation (Hu et al., 2018). This study also found that BAY can inhibit canonical and non-canonical caspases. BAY has been investigated in a number of disease states and injuries including psoriasis, diabetic nephropathy, spinal cord injury, and extensively in cancer for its effects on the NF-κB pathway (White and Burchill, 2008; Kolati et al., 2015; Irrera et al., 2017; Jiang W. et al., 2017).
3,4-Methylenedioxy-β-Nitrostyrene
3,4-Methylenedioxy-β-nitrostyrene (MNS) is a well-known Syk and Src tyrosine-kinase inhibitor; however, it is also shown to be a direct, selective inhibitor of the NLRP3 inflammasome (He et al., 2014). MNS can block IL-1β production with an IC50 of 2 μM in BMDMs. Pull-down studies suggest that MNS binds to the LRR and NACHT domains of the NLRP3 protein, and further investigation showed that the MNS inhibits NLRP3 ATPase activity. MNS has no inhibitory activity towards AIM2 or NLRC4 inflammasomes. Moreover, SAR studies of MNS indicated that the nitrovinyl group is essential for its inhibition of NLRP3, whereas the dioxole is dispensable. It’s suspected that the vinyl group may interact with the cysteine side chains in NLRP3, possibly in the ATPase region, making it an irreversible inhibitor (He et al., 2014). A recent study found that MNS induced ubiquitination of NLRP3, which may contribute to its inhibitory mechanism (Shim et al., 2017). MNS has been studied in wound healing and in osteosarcoma tumors (Messerschmitt et al., 2012; Xiao et al., 2016).
Tranilast
Tranilast has historically been approved to treat bronchial asthma in Japan, China, and South Korea. Tranilast was initially found to inhibit NF-κB by interfering with NF-κB and CREB-binding protein (CBP) association, but recent studies demonstrated that tranilast is a selective inhibitor that directly binds to the NLRP3 protein and inhibits IL-1β production with an IC50 of 25–50 μM (Spiecker et al., 2002; Huang et al., 2018). Mechanistically, tranilast binds to the NACHT domain of NLRP3, consequently inhibiting NLRP3-NLRP3 and NLRP3-ASC interactions, but not NLRP3-NEK7, inhibiting inflammasome formation and activation of caspase-1. Unlike other direct NLRP3 inhibitors, tranilast does not inhibit ATPase activity (Huang et al., 2018). Recently, tranilast has been investigated for the treatment of neuropathic pain, gouty arthritis, CAPS, and cancer, and has been suggested to be a potential therapy for vitiligo (Huang et al., 2018; Saito et al., 2018; Moore et al., 2019; Zhuang et al., 2020).
OLT1177 (Dapansutrile®)
OLT1177 is a β-sulfonyl nitrile compound that selectively blocks the NLRP3 inflammasome by inhibiting its ATPase activity, preventing NLRP3-ASC interactions and IL-1β release in J774A.1 macrophages with an IC50 of ~1 nM. OLT1177 has demonstrated to be a highly potent and selective inhibitor of NLRP3 since it failed to inhibit IL-1β secretion upon activation of NLRC4 or AIM2 inflammasomes (Marchetti et al., 2018a). OLT1177 has shown both in vitro and in vivo success and has recently been developed into an oral tablet and topical gel, Dapansutrile, that has proven to be safe in humans and completed phase II clinical trial for osteoarthritis of the knee by Olatec Therapeutics LLC (Marchetti et al., 2018a). Aside from clinical trials, OLT1177 has recently been tested as a potential treatment for numerous disease states including AD, autoimmune encephalomyelitis, myocardial ischemia, arthritis, and more (Marchetti et al., 2018b; Sánchez-Fernández et al., 2019; Toldo et al., 2019; Lonnemann et al., 2020).
CY-09
CY-09 has recently been reported to selectively and directly bind to the ATPase domain of NLRP3 and inhibit IL-1β release in BMDM cells with an IC50 of ~6 μM (Jiang H. et al., 2017; El-Sharkawy et al., 2020). Mutating the Walker A motif of NLRP3’s ATPase pocket abolishes the activity of CY-09, whereas mutating the Walker B motif shows no effect, suggesting the Walker A motif of the ATPase pocket as the binding site of CY-09 where it interferes with ATP binding. This is contrary to MCC950 which binds to the Walker B motif of NLRP3. ATP also competes with CY-09 to bind to NLRP3 which further confirms this interaction. Using microscale thermophoresis (MST), CY-09 binds to recombinant NLRP3 with a KD of 500 nM (Jiang H. et al., 2017). CY-09 has shown to be effective in ex vivo and in vivo models for CAPS, type 2 diabetes, gout, non-alcoholic fatty liver disease (NAFLD), pain, and more (Jiang H. et al., 2017; Fan et al., 2021; Wang X. et al., 2021).
BOT-4-One
BOT-4-one is a benzoxathiole derivative that exhibits anti-inflammatory activities through several different mechanisms of action due to its ability to irreversibly alkylate its targets. A 2016 study found that BOT-4-one inhibits the NF-κB pathway by alkylating IKKβ, subsequently inhibiting NLRP3 expression (Lee H. G. et al., 2016). Recent studies demonstrated that BOT-4-one inhibited NLRP3 ATPase activity through alkylation (Shim et al., 2017). Furthermore, alkylation of NLRP3 by BOT-4-one can increase ubiquitination of NLRP3, which could contribute to its inhibitory mechanism. While it’s not clear whether alkylation induces ubiquitination or inhibits deubiquitination, other NLRP3 alkylators BAY and MNS also increase NLRP3 ubiquitination. BOT-4-one is able to inhibit IL-1β secretion induced by ATP with an IC50 of 1.28 μM and by nigericin with an IC50 of 0.67 μM. This compound has been investigated in arthritis, pathogenic skin inflammation, and cancer, particularly Hodgkin’s lymphoma, in animal models (Kim et al., 2011, 2016; Lee H. G. et al., 2016).
Methylene Blue
Methylene blue has a long history of use in medicine, from treatment for methemoglobinemia and septic shock to being used as a dye in surgeries for tissue labeling. Methylene blue is known to have anti-inflammatory, antioxidant, and neuroprotective effects through a variety of mechanisms including its non-selective inhibition of canonical and non-canonical NLRP3, NLRC4, and AIM2 inflammasomes (Ahn et al., 2017). Studies have shown that methylene blue decreases NLRP3, pro-IL-1β, and iNOS expression by interrupting transcription factors NF-κB and STAT1. Methylene blue exhibits no effects on the nuclear transportation of NF-κB and STAT1 but markedly decreases their binding to DNA, thus suggesting methylene blue’s potential interference at binding (Huang et al., 2015). The attenuation of IL-1β and caspase-1 secretion as well as ASC oligomerization when methylene blue is added after LPS suggests that methylene blue inhibits both the priming and activation step of the NLRP3 pathway. In addition, methylene blue also inhibits the activity of recombinant capase-1 (Ahn et al., 2017). Methylene blue has been investigated in inflammation in spinal cord injury, cognitive impairment from neuroinflammation, diabetic retinopathy, AD, and more (Lin et al., 2017; Hao et al., 2019; Soeda et al., 2019; Zhou et al., 2019).
Disulfiram (Antabuse®)
Disulfiram is an FDA-approved drug to treat chronic alcohol dependence through an irreversible cysteine residue modification on aldehyde dehydrogenase. However, it’s recently been discovered that disulfiram contains anti-inflammatory properties. Deng and colleagues found that disulfiram provides lysosomal protection and regulates ROS production that is independent of mitochondria, inhibiting NLRP3-dependent IL-1β secretion with an IC50 of ~5 μM under LPS/ATP conditions in mouse macrophages (Deng et al., 2020). In addition, disulfiram can directly inhibit canonical and non-canonical caspases (Hu et al., 2018). Recent studies demonstrated that disulfiram inhibits GSDMD pore formation by modifying Cys191 of GSDMD and, as a result, suppressing IL-1β secretion and the subsequent pyroptosis (Hu et al., 2020). Others have investigated the potential to repurpose disulfiram to treat several diseases including cancer and bacterial infections and recently has been a suggested as a potential therapy for COVID-19 infection (Viola-Rhenals et al., 2018; Frazier et al., 2019; Fillmore et al., 2021).
Fenamic Acids
Fenamic acid derivatives are known non-steroidal anti-inflammatory (NSAID) agents. While the primary role of NSAIDs is inhibiting prostaglandin synthesis through cyclooxygenase (COX) enzymes, recent studies have shown that fenamic acid derivatives also exhibit anti-inflammatory effects by inhibiting the NLRP3 inflammasome pathway via interference with the Cl− volume-regulated anion channel (VRAC), a regulator of NLRP3 (Daniels et al., 2016). While flufenamic, meclofenamic, and mefenamic acids were able to inhibit IL-1β release in iBMDMs under challenging for the NLRP3 pathway, flufenamic, and mefenamic acid were unable to inhibit IL-1β under NLRC4 and AIM2 challenge, suggesting these acid derivatives are specific to the NLRP3 pathway. In addition, other COX enzyme inhibitors such as Ibuprofen and celecoxib did not affect IL-1β release (Daniels et al., 2016). These fenamic acid derivatives have been studied in a variety of disease models including AD, prostate cancer, and more (Delgado-Enciso et al., 2015; Mangan et al., 2018).
Fluoxetine (Prozac®)
Fluoxetine, also known as Prozac, is an FDA-approved drug used to treat clinical depression. Recently, fluoxetine was found to directly inhibit the NLRP3 protein, stopping inflammasome formation and subsequent IL-1β release in retinal pigmented epithelium (RPE) and macrophage cells (Ambati et al., 2021). In addition, the study demonstrated the physical interaction of fluoxetine with the NLRP3 protein by pull-down. Further binding studies found that a CY-09 probe could also pull-down NLRP3, and that excess fluoxetine was able to compete with the binding of CY-09, an NLRP3 ATPase inhibitor, suggesting these compounds share a similar mode of binding. Both CY-09 and fluoxetine contain a (trifluoromethyl)phenyl moiety and SAR studies suggest that this moiety may assist in the interaction with NLRP3’s ATPase domain. Unlike other antidepressants, fluoxetine reduced RPE degeneration in vivo caused by activating the NLRP3 inflammasome (Ambati et al., 2021). This may open new avenues for the use of fluoxetine; however, more research is needed to prove its potential in different disease states.
β-Hydroxybutyrate
β-Hydroxybutyrate (BHB) is an endogenous ligand produced in the liver and functions as an energy source during nutrient deprivation and low-carb diets. During long fasting periods or ketogenic diets, circulating concentrations of BHB can increase which is shown to be correlated with decreased immune response. Youm and colleagues showed that BHB can inhibit the NLRP3 pathway by blocking potassium efflux, an activator of NLRP3 (Youm et al., 2015). The concentrations of BHB that produced these anti-inflammatory effects in vitro were similar to its endogenous level after strenuous exercise or 2 days of fasting. Unfortunately, in vivo administration of BHB alone resulted in rapid clearance; however, when complexed with nanolipogels, BHB retained its anti-inflammatory activity, reducing NLRP3-driven neutrophil infiltration and decreasing IL-1β release at the injection site of MSU crystals (Youm et al., 2015). In LPS-primed BMDMs, BHB can inhibit NLRP3-mediated IL-1β with an IC50 of ~5 μM (Jiang H. et al., 2017). BHB has been studied in stress-related mood disorders, hypertension, seizures, and gout (Goldberg et al., 2017; Yamanashi et al., 2017; Chakraborty et al., 2018; Rho et al., 2019).
MicroRNA as Post-transcriptional Regulators of NLRP3 Expression
MicroRNA are small, non-coding single-stranded RNA molecules that regulate gene expression post-transcriptionally by RNA silencing. These molecules represent an attractive strategy for drug discovery due to their high specificity. In addition, miRNAs have multiple binding sites, also known as seeding regions, which allows them to be involved in multiple pathways. While there are several miRNAs that influence different targets in the NLRP3 inflammasome pathway, there are several miRNAs that target the NLRP3 protein (Boxberger et al., 2019). The first miRNA discovered to target NLRP3 is miRNA (miR)-223-3p which was found to negatively regulate the NLRP3 protein (Bauernfeind et al., 2012; Haneklaus et al., 2012). It’s been proposed that miR-223-3p is important for mediating protein expression in different cell types since differentiation of monocytes to macrophages resulted in a decreased expression of miR-223-3p and increased levels of NLRP3 protein. This could potentially explain why some cell lines have low responses to inflammatory NLRP3 stimuli. MiR-7-5p and miR-30e-5p were also found to negatively regulate NLRP3 protein expression in vitro and in vivo (Zhou et al., 2016; Li et al., 2018). In addition, delivery of miR-7-5p and miR-30-5p mimics were found to protect dopaminergic neurons from degeneration and decrease inflammatory cytokines through inflammasome suppression in an MPTP-induced PD mouse model. Other miRNA’s that negatively regulate NLRP3 protein expression are miR-22-3p, miR-133b-3p, miR-186-5p, and miR-495-3p which have been studied in a variety of disease states including asthma, AD, allergic inflammation, cardiac microvascular endothelial cell injury (CMEC) and more (Xiao et al., 2017; Chen M. L. et al., 2018; Zhou et al., 2018; Han et al., 2020; Guo et al., 2021). There are currently extensive efforts underway to explore the therapeutic potentials of miRNA. However, the major limitation of these molecules is their delivery and membrane permeability. Nanoparticles show promising results in delivering peptides and could provide hope for the future of miRNAs as a therapy to treat numerous diseases (Wang et al., 2015; Sezlev Bilecen et al., 2017).
Anakinra (Kineret®), Rilonacept (Arcalyst®), and Canakinumab (Ilaris®)
There are currently three FDA-approved biologics that target the NLRP3 pathway. Anakinra was the first to be developed and is a modified IL-1Ra that blocks the binding of IL-1β and IL-1α, inhibiting their signaling pathways and further inflammation. Anakinra was approved for the treatment of RA in 2001 and was later approved for the treatment of CAPS in 2013 (Calabrese, 2002; Jesus and Goldbach-Mansky, 2014). The short plasma half-life of anakinra (4–6 h) is a pitfall among patients due to required daily injections (Granowitz et al., 1992). Rilonacept is a dimeric fusion protein with decoy receptors containing extracellular residues of the two IL-1R subunits, IL-1R1 and IL-1R accessory protein (IL-1RAcP), which can bind to and neutralize IL-1β and IL-1α (Jesus and Goldbach-Mansky, 2014). With an improved half-life (7 days) compared to anakinra, rilonacept was approved for the treatment of CAPS in 2008 and was recently approved for recurrent pericarditis in 2021 (Hoffman, 2009; Fava et al., 2022). Canakinumab is an anti-IL-1β monoclonal antibody that selectively binds to and neutralizes IL-1β. With a half-life of 26 days, canakinumab is a preferred treatment option and has been approved for CAPS, juvenile idiopathic arthritis, rare periodic fever syndromes, and adult-onset Still’s disease (AOSD) (Curran, 2012; Orrock and Ilowite, 2016; Malcova et al., 2020; Sfriso et al., 2020). In addition, a number of clinical trials have recently investigated anakinra and canakinumab as a therapeutic treatment for patients with COVID-19 (Kooistra et al., 2020; Landi et al., 2020; Kyriazopoulou et al., 2021; Kharazmi et al., 2022). These therapies have proven to be safe and effective in treating inflammation-driven diseases, however, some issues remain. All three biologics require administration by injection, a route that is not preferred by patients and is particularly problematic for anakinra due to its short half-life. In addition, due to the nature of biologics and protein-based therapies, it’s likely that these therapeutics have poor BBB penetrance, potentially limiting their applications to diseases outside of the CNS. Furthermore, increased infection is a concern for therapies blocking all IL-1β signaling regardless of the source, an issue that was observed with canakinumab (Dinarello et al., 2012; Mangan et al., 2018). These problems emphasize the necessity for small-molecule inhibitors of NLRP3 to overcome pharmacokinetic and selectivity issues.
Conclusion
The NLRP3 inflammasome plays an intricate and important role in the innate immune system in response to a variety of stimuli. Upon the formation of the NLRP3 inflammasome, caspase-1 is released and activates the pro-inflammatory cytokines IL-1β and IL-18. These cytokines continue the inflammatory signaling cascade and recruit immune cells to fight infections or heal injuries. Aberrant activation of NLRP3 leads to chronic inflammation, a common denominator of several diseases that causes and/or encourages disease pathology. NLRP3 is activated and IL-1β and IL-18 levels are upregulated in diseases where pathology is accompanied by inflammation. Thus, NLRP3 has become an attractive target in the drug discovery field and inhibitors possess high therapeutic value for the treatment of many diseases. Several hurdles remain to finding a potent and selective NLRP3 inhibitor with adequate pharmacokinetic properties, particularly BBB penetrance for CNS diseases. More research is required to further the understanding of the binding and mechanism of compounds targeting this pathway.
Author Contributions
HB and SZ contributed to conceptualization, writing, and editing. SB and YX contributed to editing. All authors contributed to the article and approved the submitted version.
Funding
The work was supported in part by the National Institute on Aging (NIA) of the NIH under award number R01AG058673 (SZ), Commonwealth of Virginia’s Alzheimer’s and Related Disease Research Award Fund administered by the Center on Aging, School of Allied Health Professions, Virginia Commonwealth University (SZ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Figure 1 was created with BioRender.com.
References
Agnew, A., Nulty, C., and Creagh, E. M. (2021). Regulation, activation and function of caspase-11 during health and disease. Int. J. Mol. Sci. 22:1506. doi: 10.3390/ijms22041506
Ahn, H., Kang, S. G., Yoon, S. I., Ko, H. J., Kim, P. H., Hong, E. J., et al. (2017). Methylene blue inhibits NLRP3, NLRC4, AIM2 and non-canonical inflammasome activation. Sci. Rep. 7:12409. doi: 10.1038/s41598-017-12635-6
Allam, R., Lawlor, K. E., Yu, E. C., Mildenhall, A. L., Moujalled, D. M., Lewis, R. S., et al. (2014). Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 15, 982–990. doi: 10.15252/embr.201438463
Alwaseem, H., Frisch, B. J., and Fasan, R. (2018). Anticancer activity profiling of parthenolide analogs generated via P450-mediated chemoenzymatic synthesis. Bioorg. Med. Chem. 26, 1365–1373. doi: 10.1016/j.bmc.2017.08.009
Amanzadeh, E., Esmaeili, A., Rahgozar, S., and Nourbakhshnia, M. (2019). Application of quercetin in neurological disorders: from nutrition to nanomedicine. Rev. Neurosci. 30, 555–572. doi: 10.1515/revneuro-2018-0080
Amarante-Mendes, G. P., Adjemian, S., Branco, L. M., Zanetti, L. C., Weinlich, R., and Bortoluci, K. R. (2018). Pattern recognition receptors and the host cell death molecular machinery. Front. Immunol. 9:2379. doi: 10.3389/fimmu.2018.02379
Ambati, M., Apicella, I., Wang, S. B., Narendran, S., Leung, H., Pereira, F., et al. (2021). Identification of fluoxetine as a direct NLRP3 inhibitor to treat atrophic macular degeneration. Proc. Natl. Acad. Sci. U S A 118:e2102975118. doi: 10.1073/pnas.2102975118
Andreeva, L., David, L., Rawson, S., Shen, C., Pasricha, T., Pelegrin, P., et al. (2021). NLRP3 cages revealed by full-length mouse NLRP3 structure control pathway activation. Cell 184, 6299–6312.e22. doi: 10.1016/j.cell.2021.11.011
Antony, P. M., Diederich, N. J., Kruger, R., and Balling, R. (2013). The hallmarks of Parkinson’s disease. FEBS J. 280, 5981–5993. doi: 10.1111/febs.12335
Ashcroft, F. M. (2005). ATP-sensitive potassium channelopathies: focus on insulin secretion. J. Clin. Invest. 115, 2047–2058. doi: 10.1172/JCI25495
Baldwin, A. G., Rivers-Auty, J., Daniels, M. J. D., White, C. S., Schwalbe, C. H., Schilling, T., et al. (2017). Boron-Based Inhibitors of the NLRP3 Inflammasome. Cell Chem. Biol. 24, 1321–1335.e5. doi: 10.1016/j.chembiol.2017.08.011
Bauernfeind, F., Bartok, E., Rieger, A., Franchi, L., Nuñez, G., and Hornung, V. (2011). Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 187, 613–617. doi: 10.4049/jimmunol.1100613
Bauernfeind, F., Rieger, A., Schildberg, F. A., Knolle, P. A., Schmid-Burgk, J. L., and Hornung, V. (2012). NLRP3 inflammasome activity is negatively controlled by miR-223. J. Immunol. 189, 4175–4181. doi: 10.4049/jimmunol.1201516
Bentz, A. B. (2009). A review of quercetin: chemistry, antioxidant properties and bioavailability. J. Young Investig. 19, 1–14. Available online at: https://www.jyi.org/2009-april/2017/10/15/a-review-of-quercetin-chemistry-antioxidant-properties-and-bioavailability.
Bertoni, A., Carta, S., Baldovini, C., Penco, F., Balza, E., Borghini, S., et al. (2020). A novel knock-in mouse model of cryopyrin-associated periodic syndromes with development of amyloidosis: therapeutic efficacy of proton pump inhibitors. J. Allergy Clin. Immunol. 145, 368–378.e13. doi: 10.1016/j.jaci.2019.05.034
Boxberger, N., Hecker, M., and Zettl, U. K. (2019). Dysregulation of inflammasome priming and activation by MicroRNAs in human immune-mediated diseases. J. Immunol. 202, 2177–2187. doi: 10.4049/jimmunol.1801416
Braga, T. T., Forni, M. F., Correa-Costa, M., Ramos, R. N., Barbuto, J. A., Branco, P., et al. (2017). Soluble uric acid activates the NLRP3 inflammasome. Sci. Rep. 7:39884. doi: 10.1038/srep39884
Calabrese, L. H. (2002). Anakinra treatment of patients with rheumatoid arthritis. Ann. Pharmacother. 36, 1204–1209. doi: 10.1345/aph.1A396
Cao, Y., Goods, B. A., Raddassi, K., Nepom, G. T., Kwok, W. W., Love, J. C., et al. (2015). Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci. Transl. Med. 7:287ra274. doi: 10.1126/scitranslmed.aaa8038
Chakraborty, S., Galla, S., Cheng, X., Yeo, J. Y., Mell, B., Singh, V., et al. (2018). Salt-responsive metabolite, beta-hydroxybutyrate, attenuates hypertension. Cell Rep. 25, 677–689.e4. doi: 10.1016/j.celrep.2018.09.058
Chausmer, A. B. (1998). Zinc, insulin and diabetes. J. Am. Coll. Nutr. 17, 109–115. doi: 10.1080/07315724.1998.10718735
Chen, P., Bai, Q., Wu, Y., Zeng, Q., Song, X., Guo, Y., et al. (2021). The essential oil of artemisia argyi H. Lev. and vaniot attenuates NLRP3 inflammasome activation in THP-1 Cells. Front. Pharmacol. 12:712907. doi: 10.3389/fphar.2021.712907
Chen, J., and Chen, Z. J. (2018). PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature 564, 71–76. doi: 10.1038/s41586-018-0761-3
Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., et al. (2018). Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9, 7204–7218. doi: 10.18632/oncotarget.23208
Chen, S., Jiang, H., Wu, X., and Fang, J. (2016). Therapeutic effects of quercetin on inflammation, obesity and type 2 diabetes. Mediators Inflamm. 2016:9340637. doi: 10.1155/2016/9340637
Chen, M. L., Lin, K., and Lin, S. K. (2018). NLRP3 inflammasome signaling as an early molecular response is negatively controlled by miR-186 in CFA-induced prosopalgia mice. Braz. J. Med. Biol. Res. 51:e7602. doi: 10.1590/1414-431X20187602
Chen, Y., Meng, J., Xu, Q., Long, T., Bi, F., Chang, C., et al. (2019). Rapamycin improves the neuroprotection effect of inhibition of NLRP3 inflammasome activation after TBI. Brain Res. 1710, 163–172. doi: 10.1016/j.brainres.2019.01.005
Chen, G. Y., and Nuñez, G. (2010). Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837. doi: 10.1038/nri2873
Chen, C. Y., Peng, W. H., Tsai, K. D., and Hsu, S. L. (2007). Luteolin suppresses inflammation-associated gene expression by blocking NF-kappaB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 81, 1602–1614. doi: 10.1016/j.lfs.2007.09.028
Chen, X., Yang, L., Zhang, N., Turpin, J. A., Buckheit, R. W., Osterling, C., et al. (2003). Shikonin, a component of chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 47, 2810–2816. doi: 10.1128/AAC.47.9.2810-2816.2003
Choi, D. Y., Lee, J. W., Peng, J., Lee, Y. J., Han, J. Y., Lee, Y. H., et al. (2012). Obovatol improves cognitive functions in animal models for Alzheimer’s disease. J. Neurochem. 120, 1048–1059. doi: 10.1111/j.1471-4159.2011.07642.x
Cocco, M., Garella, D., Di Stilo, A., Borretto, E., Stevanato, L., Giorgis, M., et al. (2014). Electrophilic warhead-based design of compounds preventing NLRP3 inflammasome-dependent pyroptosis. J. Med. Chem. 57, 10366–10382. doi: 10.1021/jm501072b
Cocco, M., Miglio, G., Giorgis, M., Garella, D., Marini, E., Costale, A., et al. (2016). Design, synthesis and evaluation of acrylamide derivatives as direct NLRP3 inflammasome inhibitors. ChemMedChem 11, 1790–1803. doi: 10.1002/cmdc.201600055
Cocco, M., Pellegrini, C., Martinez-Banaclocha, H., Giorgis, M., Marini, E., Costale, A., et al. (2017). Development of an acrylate derivative targeting the NLRP3 inflammasome for the treatment of inflammatory bowel disease. J. Med. Chem. 60, 3656–3671. doi: 10.1021/acs.jmedchem.6b01624
Coll, R. C., Hill, J. R., Day, C. J., Zamoshnikova, A., Boucher, D., Massey, N. L., et al. (2019). MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat. Chem. Biol. 15, 556–559. doi: 10.1038/s41589-019-0277-7
Coll, R. C., Robertson, A., Butler, M., Cooper, M., and O’Neill, L. A. (2011). The cytokine release inhibitory drug CRID3 targets ASC oligomerisation in the NLRP3 and AIM2 inflammasomes. PLoS One 6:e29539. doi: 10.1371/journal.pone.0029539
Coll, R. C., Robertson, A. A., Chae, J. J., Higgins, S. C., Munoz-Planillo, R., Inserra, M. C., et al. (2015). A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255. doi: 10.1038/nm.3806
Compan, V., Baroja-Mazo, A., Lopez-Castejon, G., Gomez, A. I., Martinez, C. M., Angosto, D., et al. (2012). Cell volume regulation modulates NLRP3 inflammasome activation. Immunity 37, 487–500. doi: 10.1016/j.immuni.2012.06.013
Cruz, C. M., Rinna, A., Forman, H. J., Ventura, A. L., Persechini, P. M., and Ojcius, D. M. (2007). ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 282, 2871–2879. doi: 10.1074/jbc.M608083200
Cummins, C. B., Wang, X., Sommerhalder, C., Bohanon, F. J., Nunez Lopez, O., Tie, H. Y., et al. (2018). Natural compound oridonin inhibits endotoxin-induced inflammatory response of activated hepatic stellate cells. Biomed. Res. Int. 2018:6137420. doi: 10.1155/2018/6137420
Curran, M. P. (2012). Canakinumab: in patients with cryopyrin-associated periodic syndromes. BioDrugs 26, 53–59. doi: 10.2165/11208450-000000000-00000
Daniels, M. J., Rivers-Auty, J., Schilling, T., Spencer, N. G., Watremez, W., Fasolino, V., et al. (2016). Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer’s disease in rodent models. Nat. Commun. 7:12504. doi: 10.1038/ncomms12504
de Zoete, M. R., Palm, N. W., Zhu, S., and Flavell, R. A. (2014). Inflammasomes. Cold Spring Harb. Perspect. Biol. 6:a016287. doi: 10.1101/cshperspect.a016287
Delgado-Enciso, I., Soriano-Hernandez, A. D., Rodriguez-Hernandez, A., Galvan-Salazar, H. R., Montes-Galindo, D. A., Martinez-Martinez, R., et al. (2015). Histological changes caused by meclofenamic acid in androgen-independent prostate cancer tumors: evaluation in a mouse model. Int. Braz. J. Urol. 41, 1002–1007. doi: 10.1590/S1677-5538.IBJU.2013.00186
Delic, V., Beck, K. D., Pang, K. C.H., and Citron, B. A. (2020). Biological links between traumatic brain injury and Parkinson’s disease. Acta Neuropathol. Commun. 8:45. doi: 10.1186/s40478-020-00924-7
Dempsey, C., Rubio Araiz, A., Bryson, K. J., Finucane, O., Larkin, C., Mills, E. L., et al. (2017). Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain Behav. Immun. 61, 306–316. doi: 10.1016/j.bbi.2016.12.014
Deng, W., Yang, Z., Yue, H., Ou, Y., Hu, W., and Sun, P. (2020). Disulfiram suppresses NLRP3 inflammasome activation to treat peritoneal and gouty inflammation. Free Radic. Biol. Med. 152, 8–17. doi: 10.1016/j.freeradbiomed.2020.03.007
Di, A., Xiong, S., Ye, Z., Malireddi, R. K. S., Kometani, S., Zhong, M., et al. (2018). The TWIK2 potassium efflux channel in macrophages mediates NLRP3 inflammasome-induced inflammation. Immunity 49, 56–65.e4. doi: 10.1016/j.immuni.2018.04.032
Dinarello, C. A. (2006). The paradox of pro-inflammatory cytokines in cancer. Cancer Metastasis Rev. 25, 307–313. doi: 10.1007/s10555-006-9000-8
Dinarello, C. A., Donath, M. Y., and Mandrup-Poulsen, T. (2010). Role of IL-1beta in type 2 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 17, 314–321. doi: 10.1097/MED.0b013e32833bf6dc
Dinarello, C. A., Simon, A., and van der Meer, J. W. (2012). Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug. Discov. 11, 633–652. doi: 10.1038/nrd3800
Ding, J., Wang, K., Liu, W., She, Y., Sun, Q., Shi, J., et al. (2016). Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116. doi: 10.1038/nature18590
Domiciano, T. P., Wakita, D., Jones, H. D., Crother, T. R., Verri, W. A., Jr., Arditi, M., et al. (2017). Quercetin inhibits inflammasome activation by interfering with ASC oligomerization and prevents Interleukin-1 mediated mouse vasculitis. Sci. Rep. 7:41539. doi: 10.1038/srep41539
Dostert, C., Guarda, G., Romero, J. F., Menu, P., Gross, O., Tardivel, A., et al. (2009). Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One 4:e6510. doi: 10.1371/journal.pone.0006510
Duan, M., Du, X., Ren, G., Zhang, Y., Zheng, Y., Sun, S., et al. (2018). Obovatol inhibits the growth and aggressiveness of tongue squamous cell carcinoma through regulation of the EGF-mediated JAKSTAT signaling pathway. Mol. Med. Rep. 18, 1651–1659. doi: 10.3892/mmr.2018.9078
Duewell, P., Kono, H., Rayner, K. J., Sirois, C. M., Vladimer, G., Bauernfeind, F. G., et al. (2010). NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361. doi: 10.1038/nature08938
Edwards, G., 3rd, Zhao, J., Dash, P. K., Soto, C., and Moreno-Gonzalez, I. (2020). Traumatic brain injury induces tau aggregation and spreading. J. Neurotrauma 37, 80–92. doi: 10.1089/neu.2018.6348
El-Sharkawy, L. Y., Brough, D., and Freeman, S. (2020). Inhibiting the NLRP3 inflammasome. Molecules 25:5533. doi: 10.3390/molecules25235533
Emmerson, B. T., Cross, M., Osborne, J. M., and Axelsen, R. A. (1990). Reaction of MDCK cells to crystals of monosodium urate monohydrate and uric acid. Kidney Int. 37, 36–43. doi: 10.1038/ki.1990.5
Epelman, S., Lavine, K. J., and Randolph, G. J. (2014). Origin and functions of tissue macrophages. Immunity 41, 21–35. doi: 10.1016/j.immuni.2014.06.013
Eren, E., and Özören, N. (2019). The NLRP3 inflammasome: a new player in neurological diseases. Turk J. Biol. 43, 349–359. doi: 10.3906/biy-1909-31
Evavold, C. L., Ruan, J., Tan, Y., Xia, S., Wu, H., and Kagan, J. C. (2018). The pore-forming protein gasdermin D regulates Interleukin-1 secretion from living macrophages. Immunity 48, 35–44.e6. doi: 10.1016/j.immuni.2017.11.013
Fan, Y., Xue, G., Chen, Q., Lu, Y., Dong, R., and Yuan, H. (2021). CY-09 inhibits NLRP3 inflammasome activation to relieve pain via TRPA1. Comput. Math. Methods Med. 2021: 9806690. doi: 10.1155/2021/9806690
Fava, A. M., Reyaldeen, R., Lo Presti, S., Goyal, A., Akintoye, E., Hughes, D., et al. (2022). Rilonacept for the treatment of recurrent pericarditis. Expert Opin. Biol. Ther. 22, 7–16. doi: 10.1080/14712598.2022.2005024
Fillmore, N., Bell, S., Shen, C., Nguyen, V., La, J., Dubreuil, M., et al. (2021). Disulfiram use is associated with lower risk of COVID-19: a retrospective cohort study. PLoS One 16:e0259061. doi: 10.1371/journal.pone.0259061
Fontanilla, C. V., Wei, X., Zhao, L., Johnstone, B., Pascuzzi, R. M., Farlow, M. R., et al. (2012). Caffeic acid phenethyl ester extends survival of a mouse model of amyotrophic lateral sclerosis. Neuroscience 205, 185–193. doi: 10.1016/j.neuroscience.2011.12.025
Fraser, S. P., Hemsley, F., and Djamgoz, M. B. A. (2016). Caffeic acid phenethyl ester: inhibition of metastatic cell behaviours via voltage-gated sodium channel in human breast cancer in vitro. Int. J. Biochem. Cell Biol. 71, 111–118. doi: 10.1016/j.biocel.2015.12.012
Frazier, K. R., Moore, J. A., and Long, T. E. (2019). Antibacterial activity of disulfiram and its metabolites. J. Appl. Microbiol. 126, 79–86. doi: 10.1111/jam.14094
Fu, Q., Li, J., Qiu, L., Ruan, J., Mao, M., Li, S., et al. (2020). Inhibiting NLRP3 inflammasome with MCC950 ameliorates perioperative neurocognitive disorders, suppressing neuroinflammation in the hippocampus in aged mice. Int. Immunopharmacol. 82:106317. doi: 10.1016/j.intimp.2020.106317
Fu, Y., Zhao, P., Xie, Z., Wang, L., and Chen, S. (2018). Oridonin inhibits myofibroblast differentiation and bleomycin-induced pulmonary fibrosis by regulating transforming growth factor β (TGFβ)/Smad pathway. Med. Sci. Monit. 24, 7548–7555. doi: 10.12659/MSM.912740
Fulp, J., He, L., Toldo, S., Jiang, Y., Boice, A., Guo, C., et al. (2018). Structural insights of benzenesulfonamide analogues as NLRP3 inflammasome inhibitors: design, synthesis and biological characterization. J. Med. Chem. 61, 5412–5423. doi: 10.1021/acs.jmedchem.8b00733
Gao, J., Peng, S., Shan, X., Deng, G., Shen, L., Sun, J., et al. (2019). Inhibition of AIM2 inflammasome-mediated pyroptosis by Andrographolide contributes to amelioration of radiation-induced lung inflammation and fibrosis. Cell Death Dis. 10:957. doi: 10.1038/s41419-019-2195-8
García, M. G., Alaniz, L., Lopes, E. C., Blanco, G., Hajos, S. E., and Alvarez, E. (2005). Inhibition of NF-κB activity by BAY 11-7082 increases apoptosis in multidrug resistant leukemic T-cell lines. Leuk Res. 29, 1425–1434. doi: 10.1016/j.leukres.2005.05.004
Gardner, R. C., and Yaffe, K. (2015). Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol. Cell Neurosci. 66, 75–80. doi: 10.1016/j.mcn.2015.03.001
Gastaldi, S., Boscaro, V., Gianquinto, E., Sandall, C. F., Giorgis, M., Marini, E., et al. (2021). Chemical Modulation of the 1-(Piperidin-4-yl)-1,3-dihydro-2H-benzo[d]imidazole-2-one Scaffold as a Novel NLRP3 Inhibitor. Molecules 26:3975. doi: 10.3390/molecules26133975
Giordano, A., and Tommonaro, G. (2019). Curcumin and cancer. Nutrients 11:2376. doi: 10.3390/nu11102376
Goldberg, E. L., Asher, J. L., Molony, R. D., Shaw, A. C., Zeiss, C. J., Wang, C., et al. (2017). beta-hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Rep. 18, 2077–2087. doi: 10.1016/j.celrep.2017.02.004
Gong, Y., Cao, X., Gong, L., and Li, W. (2019). Sulforaphane alleviates retinal ganglion cell death and inflammation by suppressing NLRP3 inflammasome activation in a rat model of retinal ischemia/reperfusion injury. Int. J. Immunopathol. Pharmacol. 33:2058738419861777. doi: 10.1177/2058738419861777
Gong, Z., Pan, J., Shen, Q., Li, M., and Peng, Y. (2018). Mitochondrial dysfunction induces NLRP3 inflammasome activation during cerebral ischemia/reperfusion injury. J. Neuroinflammation 15:242. doi: 10.1186/s12974-018-1282-6
Gordon, R. A., Christie, E. A., Langley, D. C., Kumar, M. R., Mantovani, V., Robertson, S., et al. (2018). Inflammasome inhibition prevents a-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 10:eaah4066. doi: 10.1126/scitranslmed.aah4066
Granowitz, E. V. P., Mier, R., Pribble, J. W., Stiles, J. P., Bloedow, D. M., Catalano, D. C., et al. (1992). Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin-1 receptor antagonist in healthy humans. Cytokine 4, 353–360. doi: 10.1016/1043-4666(92)90078-6
Greaney, A. J., Maier, N. K., Leppla, S. H., and Moayeri, M. (2016). Sulforaphane inhibits multiple inflammasomes through an Nrf2-independent mechanism. J. Leukoc. Biol. 99, 189–199. doi: 10.1189/jlb.3A0415-155RR
Gris, D., Ye, Z., Iocca, H. A., Wen, H., Craven, R. R., Gris, P., et al. (2010). NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J. Immunol. 185, 974–981. doi: 10.4049/jimmunol.0904145
Grivennikov, S. I., and Karin, M. (2011). Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann. Rheum. Dis. 70, i104–i108. doi: 10.1136/ard.2010.140145
Groß, C. J., Mishra, R., Schneider, K. S., Médard, G., Wettmarshausen, J., Dittlein, D. C., et al. (2016). K+ efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity 45, 761–773. doi: 10.1016/j.immuni.2016.08.010
Guo, S., Chen, R., Zhang, L., Wu, M., Wei, Y., Dai, W., et al. (2021). microRNA-22-3p plays a protective role in a murine asthma model through the inhibition of the NLRP3-caspase-1-IL-1beta axis. Exp. Physiol. 106, 1829–1838. doi: 10.1113/EP089575
Guo, C., Fu, R., Wang, S., Huang, Y., Li, X., Zhou, M., et al. (2018). NLRP3 inflammasome activation contributes to the pathogenesis of rheumatoid arthritis. Clin. Exp. Immunol. 194, 231–243. doi: 10.1111/cei.13167
Guo, W., Sun, Y., Liu, W., Wu, X., Guo, L., Cai, P., et al. (2014). Small molecule-driven mitophagy-mediated NLRP3 inflammasome inhibition is responsible for the prevention of colitis-associated cancer. Autophagy 10, 972–985. doi: 10.4161/auto.28374
Gupta, S., Mishra, K. P., and Ganju, L. (2017). Broad-spectrum antiviral properties of andrographolide. Arch. Virol. 162, 611–623. doi: 10.1007/s00705-016-3166-3
Gurung, P., Malireddi, R. K., Anand, P. K., Demon, D., Vande Walle, L., Liu, Z., et al. (2012). Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-β (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J. Biol. Chem. 287, 34474–34483. doi: 10.1074/jbc.M112.401406
Guzman, M. L., Rossi, R. M., Neelakantan, S., Li, X., Corbett, C. A., Hassane, D. C., et al. (2007). An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood 110, 4427–4435. doi: 10.1182/blood-2007-05-090621
Hafner-Bratkovič, I., Sušjan, P., Lainšček, D., Tapia-Abellán, A., Cerović, K., Kadunc, L., et al. (2018). NLRP3 lacking the leucine-rich repeat domain can be fully activated via the canonical inflammasome pathway. Nat. Commun. 9:5182. doi: 10.1038/s41467-018-07573-4
Halle, A., Hornung, V., Petzold, G. C., Stewart, C. R., Monks, B. G., Reinheckel, T., et al. (2008). The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 9, 857–865. doi: 10.1038/ni.1636
Hämäläinen, M., Nieminen, R., Vuorela, P., Heinonen, M., and Moilanen, E. (2007). Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007:45673. doi: 10.1155/2007/45673
Hamarsheh, S., and Zeiser, R. (2020). NLRP3 inflammasome activation in cancer: a double-edged sword. Front. Immunol. 11:1444. doi: 10.3389/fimmu.2020.01444
Han, C., Guo, L., Yang, Y., Guan, Q., Shen, H., Sheng, Y., et al. (2020). Mechanism of microRNA-22 in regulating neuroinflammation in Alzheimer’s disease. Brain Behav. 10:e01627. doi: 10.1002/brb3.1627
Haneklaus, M., Gerlic, M., Kurowska-Stolarska, M., Rainey, A. A., Pich, D., McInnes, I. B., et al. (2012). Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1β production. J. Immunol. 189, 3795–3799. doi: 10.4049/jimmunol.1200312
Hao, J., Zhang, H., Yu, J., Chen, X., and Yang, L. (2019). Methylene blue attenuates diabetic retinopathy by inhibiting NLRP3 inflammasome activation in STZ-Induced diabetic rats. Ocul. Immunol. Inflamm. 27, 836–843. doi: 10.1080/09273948.2018.1450516
Hayes, J. P., Logue, M. W., Sadeh, N., Spielberg, J. M., Verfaellie, M., Hayes, S. M., et al. (2017). Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer’s disease. Brain 140, 813–825. doi: 10.1093/brain/aww344
He, H., Jiang, H., Chen, Y., Ye, J., Wang, A., Wang, C., et al. (2018). Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat. Commun. 9:2550. doi: 10.1038/s41467-018-04947-6
He, Y., Varadarajan, S., Munoz-Planillo, R., Burberry, A., Nakamura, Y., and Nunez, G. (2014). 3,4-methylenedioxy-β-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J. Biol. Chem. 289, 1142–1150. doi: 10.1074/jbc.M113.515080
He, Y., Zeng, M. Y., Yang, D., Motro, B., and Nunez, G. (2016). NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530, 354–357. doi: 10.1038/nature16959
Heiss, E., Herhaus, C., Klimo, K., Bartsch, H., and Gerhauser, C. (2001). Nuclear factor κB is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J. Biol. Chem. 276, 32008–32015. doi: 10.1074/jbc.M104794200
Heneka, M. T., Kummer, M. P., Stutz, A., Delekate, A., Schwartz, S., Vieira-Saecker, A., et al. (2013). NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678. doi: 10.1038/nature11729
Hennig, P., Garstkiewicz, M., Grossi, S., Di Filippo, M., French, L. E., and Beer, H. D. (2018). The crosstalk between Nrf2 and inflammasomes. Int. J. Mol. Sci. 19:562. doi: 10.3390/ijms19020562
Hill, J. R., Coll, R. C., Sue, N., Reid, J. C., Dou, J., Holley, C. L., et al. (2017). Sulfonylureas as concomitant insulin secretagogues and NLRP3 inflammasome inhibitors. ChemMedChem 12, 1449–1457. doi: 10.1002/cmdc.201700270
Hill, J. R., Shao, X., Massey, N. L., Stauff, J., Sherman, P. S., Robertson, A. A.B., et al. (2020). Synthesis and evaluation of NLRP3-inhibitory sulfonylurea [C]MCC950 in healthy animals. Bioorg. Med. Chem. Lett. 30:127186. doi: 10.1016/j.bmcl.2020.127186
Hochheiser, I. V., Pilsl, M., Hagelueken, G., Moecking, J., Marleaux, M., Brinkschulte, R., et al. (2021). Cryo-EM structure of the NLRP3 decamer bound to the cytokine release inhibitory drug CRID3. Nature 604, 184–189. doi: 10.1038/s41586-022-04467-w
Hoffman, H. M. (2009). Rilonacept for the treatment of cryopyrin-associated periodic syndromes (CAPS). Expert. Opin. Biol. Ther. 9, 519–531. doi: 10.1517/14712590902875518
Hoffman, H. M., Mueller, J. L., Broide, D. H., Wanderer, A. A., and Kolodner, R. D. (2001). Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 29, 301–305. doi: 10.1038/ng756
Honda, H., Nagai, Y., Matsunaga, T., Okamoto, N., Watanabe, Y., Tsuneyama, K., et al. (2014). Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-induced adipose tissue inflammation. J. Leukoc Biol. 96, 1087–1100. doi: 10.1189/jlb.3A0114-005RR
Honda, H., Nagai, Y., Matsunaga, T., Saitoh, S., Akashi-Takamura, S., Hayashi, H., et al. (2012). Glycyrrhizin and isoliquiritigenin suppress the LPS sensor toll-like receptor 4/MD-2 complex signaling in a different manner. J. Leukoc Biol. 91, 967–976. doi: 10.1189/jlb.0112038
Hornung, V., Bauernfeind, F., Halle, A., Samstad, E. O., Kono, H., Rock, K. L., et al. (2008). Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856. doi: 10.1038/ni.1631
Hotta, T., Haynes, S. E., Blasius, T. L., Gebbie, M., Eberhardt, E. L., Sept, D., et al. (2021). Parthenolide destabilizes microtubules by covalently modifying tubulin. Curr. Biol. 31, 900–907.e6. doi: 10.1016/j.cub.2020.11.055
Hu, J. J., Liu, X., Xia, S., Zhang, Z., Zhang, Y., Zhao, J., et al. (2020). FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 21, 736–745. doi: 10.1038/s41590-020-0669-6
Hu, J. J., Liu, X., Zhao, J., Xia, S., Ruan, J., Luo, X., et al. (2018). Identification of pyroptosis inhibitors that target a reactive cysteine in gasdermin D. bioRxiv [Preprint]. doi: 10.1038/s41590-020-0669-6
Huang, Y., Jiang, H., Chen, Y., Wang, X., Yang, Y., Tao, J., et al. (2018). Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol. Med. 10:e8689. doi: 10.15252/emmm.201708689
Huang, J. W., Tashiro, L., Onodera, S., and Ikejima, T. (2005). A comparison of the signal pathways between the TNFα- and oridonin-induced murine L929 fibrosarcoma cell death. Acta Med. Okayama 59, 261–270. doi: 10.18926/AMO/31960
Huang, C., Tong, L., Lu, X., Wang, J., Yao, W., Jiang, B., et al. (2015). Methylene blue attenuates iNOS induction through suppression of transcriptional factor binding Amid iNOS mRNA transcription. J. Cell Biochem. 116, 1730–1740. doi: 10.1002/jcb.25132
Imran, M., Rauf, A., Abu-Izneid, T., Nadeem, M., Shariati, M. A., Khan, I. A., et al. (2019). Luteolin, a flavonoid, as an anticancer agent: a review. Biomed. Pharmacother 112:108612. doi: 10.1016/j.biopha.2019.108612
Inoue, M., Chen, P. H., Siecinski, S., Li, Q. J., Liu, C., Steinman, L., et al. (2016). An interferon-beta-resistant and NLRP3 inflammasome-independent subtype of EAE with neuronal damage. Nat. Neurosci. 19, 1599–1609. doi: 10.1038/nn.4421
Inoue, M., Williams, K. L., Gunn, M. D., and Shinohara, M. L. (2012). NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U S A 109, 10480–10485. doi: 10.1073/pnas.1201836109
Irrera, N., Vaccaro, M., Bitto, A., Pallio, G., Pizzino, G., Lentini, M., et al. (2017). BAY 11-7082 inhibits the NF-κB and NLRP3 inflammasome pathways and protects against IMQ-induced psoriasis. Clin. Sci. (Lond) 131, 487–498. doi: 10.1042/CS20160645
Ising, C., Venegas, C., Zhang, S., Scheiblich, H., Schmidt, S. V., Vieira-Saecker, A., et al. (2019). NLRP3 inflammasome activation drives tau pathology. Nature 575, 669–673. doi: 10.1038/s41586-019-1769-z
Ismael, S., Nasoohi, S., and Ishrat, T. (2018). MCC950, the selective inhibitor of nucleotide oligomerization domain-like receptor Protein-3 inflammasome, protects mice against traumatic brain injury. J. Neurotrauma 35, 1294–1303. doi: 10.1089/neu.2017.5344
Israf, D. A., Khaizurin, T. A., Syahida, A., Lajis, N. H., and Khozirah, S. (2007). Cardamonin inhibits COX and iNOS expression via inhibition of p65NF-κB nuclear translocation and Iκ-B phosphorylation in RAW 264.7 macrophage cells. Mol. Immunol. 44, 673–679. doi: 10.1016/j.molimm.2006.04.025
Italiani, P., Puxeddu, I., Napoletano, S., Scala, E., Melillo, D., Manocchio, S., et al. (2018). Circulating levels of IL-1 family cytokines and receptors in Alzheimer’s disease: new markers of disease progression? J. Neuroinflammation 15:342. doi: 10.1186/s12974-018-1376-1
Ito, F., Yanatori, I., Maeda, Y., Nimura, K., Ito, S., Hirayama, T., et al. (2020). Asbestos conceives Fe(II)-dependent mutagenic stromal milieu through ceaseless macrophage ferroptosis and beta-catenin induction in mesothelium. Redox Biol. 36:101616. doi: 10.1016/j.redox.2020.101616
Iyer, S. S., He, Q., Janczy, J. R., Elliott, E. I., Zhong, Z., Olivier, A. K., et al. (2013). Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39, 311–323. doi: 10.1016/j.immuni.2013.08.001
Jesus, A. A., and Goldbach-Mansky, R. (2014). IL-1 blockade in autoinflammatory syndromes. Annu. Rev. Med. 65, 223–244. doi: 10.1146/annurev-med-061512-150641
Jiang, H., He, H., Chen, Y., Huang, W., Cheng, J., Ye, J., et al. (2017). Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 214, 3219–3238. doi: 10.1084/jem.20171419
Jiang, Y., He, L., Green, J., Blevins, H., Guo, C., Patel, S. H., et al. (2019). Discovery of second-generation NLRP3 inflammasome inhibitors: design, synthesis and biological characterization. J. Med. Chem. 62, 9718–9731. doi: 10.1021/acs.jmedchem.9b01155
Jiang, W., Li, M., He, F., Zhou, S., and Zhu, L. (2017). Targeting the NLRP3 inflammasome to attenuate spinal cord injury in mice. J. Neuroinflammation 14:207. doi: 10.1186/s12974-017-0980-9
Jiao, Z., Chang, J., Li, J., Nie, D., Cui, H., and Guo, D. (2017). Sulforaphane increases Nrf2 expression and protects alveolar epithelial cells against injury caused by cigarette smoke extract. Mol. Med. Rep. 16, 1241–1247. doi: 10.3892/mmr.2017.6700
Jin, J., Qiu, S., Wang, P., Liang, X., Huang, F., Wu, H., et al. (2019). Cardamonin inhibits breast cancer growth by repressing HIF-1α-dependent metabolic reprogramming. J. Exp. Clin. Cancer Res. 38:377. doi: 10.1186/s13046-019-1351-4
Jo, E. K., Kim, J. K., Shin, D. M., and Sasakawa, C. (2016). Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol. Immunol. 13, 148–159. doi: 10.1038/cmi.2015.95
Johnson, V. E., Stewart, W., and Smith, D. H. (2010). Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer’s disease. Nat. Rev. Neurosci. 11, 361–370. doi: 10.1038/nrn2808
Juliana, C., Fernandes-Alnemri, T., Kang, S., Farias, A., Qin, F., and Alnemri, E. S. (2012). Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 287, 36617–36622. doi: 10.1074/jbc.M112.407130
Juliana, C., Fernandes-Alnemri, T., Wu, J., Datta, P., Solorzano, L., Yu, J. W., et al. (2010). Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 285, 9792–9802. doi: 10.1074/jbc.M109.082305
Juman, S., Yasui, N., Ikeda, K., Ueda, A., Sakanaka, M., Negishi, H., et al. (2012). Caffeic acid phenethyl ester suppresses the production of pro-inflammatory cytokines in hypertrophic adipocytes through lipopolysaccharide-stimulated macrophages. Biol. Pharm. Bull. 35, 1941–1946. doi: 10.1248/bpb.b12-00317
Kan, S. F., Wang, J., and Sun, G. X. (2018). Sulforaphane regulates apoptosis- and proliferationrelated signaling pathways and synergizes with cisplatin to suppress human ovarian cancer. Int. J. Mol. Med. 42, 2447–2458. doi: 10.3892/ijmm.2018.3860
Katsnelson, M. A., Lozada-Soto, K. M., Russo, H. M., Miller, B. A., and Dubyak, G. R. (2016). NLRP3 inflammasome signaling is activated by low-level lysosome disruption but inhibited by extensive lysosome disruption: roles for K+ efflux and Ca2+ influx. Am. J. Physiol. Cell Physiol. 311, C83–C100. doi: 10.1152/ajpcell.00298.2015
Katsnelson, M. A., Rucker, L. G., Russo, H. M., and Dubyak, G. R. (2015). K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J. Immunol. 194, 3937–3952. doi: 10.4049/jimmunol.1402658
Kayagaki, N., Stowe, I. B., Lee, B. L., O’Rourke, K., Anderson, K., Warming, S., et al. (2015). Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671. doi: 10.1038/nature15541
Kayagaki, N., Warming, S., Lamkanfi, M., Vande Walle, L., Louie, S., Dong, J., et al. (2011). Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121. doi: 10.1038/nature10558
Kayagaki, N., Wong, M. T., Stowe, I. B., Ramani, S. R., Gonzalez, L. C., Akashi-Takamura, S., et al. (2013). Noncanonical inflammasome activation by intracelular LPS independent of TLR4. Science 341, 1246–1249. doi: 10.1126/science.1240248
Kelley, N., Jeltema, D., Duan, Y., and He, Y. (2019). The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 20:3328. doi: 10.3390/ijms20133328
Kharazmi, A. B., Moradi, O., Haghighi, M., Kouchek, M., Manafi-Rasi, A., Raoufi, M., et al. (2022). A randomized controlled clinical trial on efficacy and safety of anakinra in patients with severe COVID-19. Immun. Inflamm. Dis. 10, 201–208. doi: 10.1002/iid3.563
Kim, J. (2021). Pre-Clinical neuroprotective evidences and plausible mechanisms of sulforaphane in Alzheimer’s disease. Int. J. Mol. Sci. 22:2929. doi: 10.3390/ijms22062929
Kim, J., Ahn, H., Han, B. C., Shin, H., Kim, J. C., Jung, E. M., et al. (2019). Obovatol inhibits NLRP3, AIM2 and non-canonical inflammasome activation. Phytomedicine 63:153019. doi: 10.1016/j.phymed.2019.153019
Kim, B. H., Min, Y. S., Choi, J. S., Baeg, G. H., Kim, Y. S., Shin, J. W., et al. (2011). Benzoxathiol derivative BOT-4-one suppresses L540 lymphoma cell survival and proliferation via inhibition of JAK3/STAT3 signaling. Exp. Mol. Med. 43, 313–321. doi: 10.3858/emm.2011.43.5.035
Kim, S., Shah, S. B., Graney, P. L., and Singh, A. (2019). Multiscale engineering of immune cells and lymphoid organs. Nat. Rev. Mater. 4, 355–378. doi: 10.1038/s41578-019-0100-9
Kim, B. H., Yoon, B. R., Kim, E. K., Noh, K. H., Kwon, S. H., Yi, E. H., et al. (2016). Alleviation of collagen-induced arthritis by the benzoxathiole derivative BOT-4-one in mice: implication of the Th1- and Th17-cell-mediated immune responses. Biochem. Pharmacol. 110–111, 47–57. doi: 10.1016/j.bcp.2016.03.018
Ko, M. S., Yun, J. Y., Baek, I. J., Jang, J. E., Hwang, J. J., Lee, S. E., et al. (2021). Mitophagy deficiency increases NLRP3 to induce brown fat dysfunction in mice. Autophagy 17, 1205–1221. doi: 10.1080/15548627.2020.1753002
Kolaczkowska, E., and Kubes, P. (2013). Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175. doi: 10.1038/nri3399
Kolati, S. R., Kasala, E. R., Bodduluru, L. N., Mahareddy, J. R., Uppulapu, S. K., Gogoi, R., et al. (2015). BAY 11-7082 ameliorates diabetic nephropathy by attenuating hyperglycemia-mediated oxidative stress and renal inflammation via NF-κB pathway. Environ. Toxicol. Pharmacol. 39, 690–699. doi: 10.1016/j.etap.2015.01.019
Kong, F., Ye, B., Cao, J., Cai, X., Lin, L., Huang, S., et al. (2016). Curcumin represses NLRP3 inflammasome activation via TLR4/MyD88/NF-κB and P2X7R signaling in PMA-induced macrophages. Front. Pharmacol. 7:369. doi: 10.3389/fphar.2016.00369
Koo, J. E., Park, Z. Y., Kim, N. D., and Lee, J. Y. (2013). Sulforaphane inhibits the engagement of LPS with TLR4/MD2 complex by preferential binding to Cys133 in MD2. Biochem. Biophys. Res. Commun. 434, 600–605. doi: 10.1016/j.bbrc.2013.03.123
Kooistra, E. J., Waalders, N. J.B., Grondman, I., Janssen, N. A. F., de Nooijer, A. H., Netea, M. G., et al. (2020). Anakinra treatment in critically ill COVID-19 patients: a prospective cohort study. Crit. Care 24:688. doi: 10.1186/s13054-020-03364-w
Kuemmerle-Deschner, J. B., Ozen, S., Tyrrell, P. N., Kone-Paut, I., Goldbach-Mansky, R., Lachmann, H., et al. (2017). Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann. Rheum. Dis. 76, 942–947. doi: 10.1136/annrheumdis-2016-209686
Kuwar, R., Rolfe, A., Di, L., Xu, H., He, L., Jiang, Y., et al. (2019). A novel small molecular NLRP3 inflammasome inhibitor alleviates neuroinflammatory response following traumatic brain injury. J. Neuroinflammation 16:81. doi: 10.1186/s12974-019-1471-y
Kwak, S. B., Koppula, S., In, E. J., Sun, X., Kim, Y. K., Kim, M. K., et al. (2018). Artemisia extract suppresses NLRP3 and AIM2 inflammasome activation by inhibition of ASC phosphorylation. Mediators Inflamm. 2018:6054069. doi: 10.1155/2018/6054069
Kyriazopoulou, E., Panagopoulos, P., Metallidis, S., Dalekos, G. N., Poulakou, G., Gatselis, N., et al. (2021). An open label trial of anakinra to prevent respiratory failure in COVID-19. eLife 10:e66125. doi: 10.7554/eLife.66125
Lamkanfi, M., and Dixit, V. M. (2014). Mechanisms and functions of inflammasomes. Cell 157, 1013–1022. doi: 10.1016/j.cell.2014.04.007
Lamkanfi, M., Mueller, J. L., Vitari, A. C., Misaghi, S., Fedorova, A., Deshayes, K., et al. (2009). Glyburide inhibits the cryopyrin/Nalp3 inflammasome. J. Cell Biol. 187, 61–70. doi: 10.1083/jcb.200903124
Landi, L., Ravaglia, C., Russo, E., Cataleta, P., Fusari, M., Boschi, A., et al. (2020). Blockage of interleukin-1β with canakinumab in patients with Covid-19. Sci. Rep. 10:21775. doi: 10.1038/s41598-020-78492-y
Larsen, C. M., Faulenbach, M., Vaag, A., Ehses, J. A., Donath, M. Y., and Mandrup-Poulsen, T. (2009). Sustained effects of interleukin-1-receptor antagonist treatment in type 2 diabetes mellitus. Diabetes Care 32, 1663–1668. doi: 10.2337/dc09-0533
Latz, E., Xiao, T. S., and Stutz, A. (2013). Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411. doi: 10.1038/nri3452
Lawlor, K. E., and Vince, J. E. (2014). Ambiguities in NLRP3 inflammasome regulation: is there a role for mitochondria? Biochim. Biophys. Acta 1840, 1433–1440. doi: 10.1016/j.bbagen.2013.08.014
Lee, H. G., Cho, N. C., Jeong, A. J., Li, Y. C., Rhie, S. J., Choi, J. S., et al. (2016). Immunomodulatory activities of the benzoxathiole derivative BOT-4-One ameliorate pathogenic skin inflammation in mice. J. Invest. Dermatol. 136, 107–116. doi: 10.1038/JID.2015.384
Lee, E., Hwang, I., Park, S., Hong, S., Hwang, B., Cho, Y. (2019). MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration. Cell Death Differ. 26, 213–228. doi: 10.1038/s41418-018-0124-5
Lee, K. M., Kang, J. H., Yun, M., and Lee, S. B. (2018). Quercetin inhibits the poly(dA:dT)-induced secretion of IL-18 via down-regulation of the expressions of AIM2 and pro-caspase-1 by inhibiting the JAK2/STAT1 pathway in IFN-gamma-primed human keratinocytes. Biochem. Biophys. Res. Commun. 503, 116–122. doi: 10.1016/j.bbrc.2018.05.191
Lee, J., Rhee, M. H., Kim, E., and Cho, J. Y. (2012). BAY 11-7082 is a broad-spectrum inhibitor with anti-inflammatory activity against multiple targets. Mediators Inflamm. 2012:416036. doi: 10.1155/2012/416036
Lee, B. L., Stowe, I. B., Gupta, A., Kornfeld, O. S., Roose-Girma, M., Anderson, K., et al. (2018). Caspase-11 auto-proteolysis is crucial for noncanonical inflammasome activation. J. Exp. Med. 215, 2279–2288. doi: 10.1084/jem.20180589
Lee, H. E., Yang, G., Kim, N. D., Jeong, S., Jung, Y., Choi, J. Y., et al. (2016). Targeting ASC in NLRP3 inflammasome by caffeic acid phenethyl ester: a novel strategy to treat acute gout. Sci. Rep. 6:38622. doi: 10.1038/srep38622
Lee, H. E., Yang, G., Park, Y. B., Kang, H. C., Cho, Y. Y., Lee, H. S., et al. (2019). Epigallocatechin-3-gallate prevents acute gout by suppressing NLRP3 inflammasome activation and mitochondrial DNA synthesis. Molecules 24:2138. doi: 10.3390/molecules24112138
Li, B., Du, P., Du, Y., Zhao, D., Cai, Y., Yang, Q., et al. (2021). Luteolin alleviates inflammation and modulates gut microbiota in ulcerative colitis rats. Life Sci. 269:119008. doi: 10.1016/j.lfs.2020.119008
Li, X., Huang, R., Li, M., Zhu, Z., Chen, Z., Cui, L., et al. (2020). Parthenolide inhibits the growth of non-small cell lung cancer by targeting epidermal growth factor receptor. Cancer Cell Int. 20:561. doi: 10.1186/s12935-020-01658-1
Li, D., Yang, H., Ma, J., Luo, S., Chen, S., and Gu, Q. (2018). MicroRNA-30e regulates neuroinflammation in MPTP model of Parkinson’s disease by targeting Nlrp3. Hum. Cell 31, 106–115. doi: 10.1007/s13577-017-0187-5
Li, X., Zhang, C. T., Ma, W., Xie, X., and Huang, Q. (2021). Oridonin: a review of its pharmacology, pharmacokinetics and toxicity. Front. Pharmacol. 12:645824. doi: 10.3389/fphar.2021.645824
Liao, N. C., Shih, Y. L., Chou, J. S., Chen, K. W., Chen, Y. L., Lee, M. H., et al. (2019). Cardamonin induces cell cycle arrest, apoptosis and alters apoptosis associated gene expression in WEHI-3 mouse leukemia cells. Am. J. Chin. Med. 47, 635–656. doi: 10.1142/S0192415X19500332
Liebman, S. E., and Le, T. H. (2021). Eat your broccoli: oxidative stress, NRF2 and sulforaphane in chronic kidney disease. Nutrients 13:266. doi: 10.3390/nu13010266
Lim, H., Min, D. S., Park, H., and Kim, H. P. (2018). Flavonoids interfere with NLRP3 inflammasome activation. Toxicol. Appl. Pharmacol. 355, 93–102. doi: 10.1016/j.taap.2018.06.022
Lin, K. M., Hu, W., Troutman, T. D., Jennings, M., Brewer, T., Li, X., et al. (2014). IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. U S A 111, 775–780. doi: 10.1073/pnas.1320294111
Lin, Z. H., Wang, S. Y., Chen, L. L., Zhuang, J. Y., Ke, Q. F., Xiao, D. R., et al. (2017). Methylene blue mitigates acute neuroinflammation after spinal cord injury through inhibiting NLRP3 inflammasome activation in microglia. Front. Cell Neurosci. 11:391. doi: 10.3389/fncel.2017.00391
Liu, L., and Chan, C. (2014). The role of inflammasome in Alzheimer’s disease. Ageing Res. Rev. 15, 6–15. doi: 10.1016/j.arr.2013.12.007
Liu, H. D., Li, W., Chen, Z. R., Hu, Y. C., Zhang, D. D., Shen, W., et al. (2013). Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochem. Res. 38, 2072–2083. doi: 10.1007/s11064-013-1115-z
Liu, M. H., Lin, Y. S., Sheu, S. Y., and Sun, J. S. (2009). Anti-inflammatory effects of daidzein on primary astroglial cell culture. Nutr. Neurosci. 12, 123–134. doi: 10.1179/147683009X423274
Liu, Y. J., Tang, B., Wang, F. C., Tang, L., Lei, Y. Y., Luo, Y., et al. (2020). Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics 10, 5225–5241. doi: 10.7150/thno.43716
Liu, T., Zhang, L., Joo, D., and Sun, S. C. (2017). NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2:17023. doi: 10.1038/sigtrans.2017.23
Lonnemann, N., Hosseini, S., Marchetti, C., Skouras, D. B., Stefanoni, D., D’Alessandro, A., et al. (2020). The NLRP3 inflammasome inhibitor OLT1177 rescues cognitive impairment in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U S A 117, 32145–32154. doi: 10.1073/pnas.2009680117
Lopresti, A. L., and Drummond, P. D. (2017). Efficacy of curcumin and a saffron/curcumin combination for the treatment of major depression: a randomised, double-blind, placebo-controlled study. J. Affect. Disord. 207, 188–196. doi: 10.1016/j.jad.2016.09.047
Lu, A., Magupalli, V. G., Ruan, J., Yin, Q., Atianand, M. K., Vos, M. R., et al. (2014). Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156, 1193–1206. doi: 10.1016/j.cell.2014.02.008
Lu, K. T., Wang, Y. W., Yang, J. T., Yang, Y. L., and Chen, H. I. (2005). Effect of interleukin-1 on traumatic brain injury-induced damage to hippocampal neurons. J. Neurotrauma 22, 885–895. doi: 10.1089/neu.2005.22.885
Lu, A., and Wu, H. (2015). Structural mechanisms of inflammasome assembly. FEBS J. 282, 435–444. doi: 10.1111/febs.13133
Magupalli, V. G., Negro, R., Tian, Y., Hauenstein, A. V., Di Caprio, G., Skillern, W., et al. (2020). HDAC6 mediates an aggresome-like mechanism for NLRP3 and pyrin inflammasome activation. Science 369:eaas8995. doi: 10.1126/science.aas8995
Mahalanobish, S., Dutta, S., Saha, S., and Sil, P. C. (2020). Melatonin induced suppression of ER stress and mitochondrial dysfunction inhibited NLRP3 inflammasome activation in COPD mice. Food Chem. Toxicol. 144:111588. doi: 10.1016/j.fct.2020.111588
Mahla, R. S., Reddy, M. C., Prasad, D. V., and Kumar, H. (2013). Sweeten PAMPs: role of sugar complexed PAMPs in innate immunity and vaccine biology. Front. Immunol. 4:248. doi: 10.3389/fimmu.2013.00248
Malcova, H., Strizova, Z., Milota, T., Striz, I., Sediva, A., Cebecauerova, D., et al. (2020). IL-1 inhibitors in the treatment of monogenic periodic fever syndromes: from the past to the future perspectives. Front. Immunol. 11:619257. doi: 10.3389/fimmu.2020.619257
Malik, A., and Kanneganti, T. D. (2017). Inflammasome activation and assembly at a glance. J. Cell Sci. 130, 3955–3963. doi: 10.1242/jcs.207365
Mamantopoulos, M., Ronchi, F., Van Hauwermeiren, F., Vieira-Silva, S., Yilmaz, B., Martens, L., et al. (2017). Nlrp6- and ASC-dependent inflammasomes do not shape the commensal gut microbiota composition. Immunity 47, e339–e348. doi: 10.1016/j.immuni.2017.07.011
Manayi, A., Nabavi, S. M., Khayatkashani, M., Habtemariam, S., and Khayat Kashani, H. R. (2021). Arglabin could target inflammasome-induced ARDS and cytokine storm associated with COVID-19. Mol. Biol. Rep. 48, 8221–8225. doi: 10.1007/s11033-021-06827-7
Mangan, M. S. J., Olhava, E. J., Roush, W. R., Seidel, H. M., Glick, G. D., and Latz, E. (2018). Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 17, 588–606. doi: 10.1038/nrd.2018.97
Marchetti, C., Chojnacki, J., Toldo, S., Mezzaroma, E., Tranchida, N., Rose, S. W., et al. (2014). A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injruy after ischemia-reperfusion in the mouse. J. Cardiovasc. Pharmacol. 63, 316–322. doi: 10.1097/FJC.0000000000000053
Marchetti, C., Swartzwelter, B., Gamboni, F., Neff, C. P., Richter, K., Azam, T., et al. (2018a). OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl. Acad. Sci. U S A 115, E1530–E1539. doi: 10.1073/pnas.1716095115
Marchetti, C., Swartzwelter, B., Koenders, M. I., Azam, T., Tengesdal, I. W., Powers, N., et al. (2018b). NLRP3 inflammasome inhibitor OLT1177 suppresses joint inflammation in murine models of acute arthritis. Arthritis Res. Ther. 20:169. doi: 10.1186/s13075-018-1664-2
Marchetti, C., Toldo, S., Chojnacki, J., Mezzaroma, E., Liu, K., Salloum, F. N., et al. (2015). Pharmacologic inhibition of the NLRP3 inflammasome preserves cardiac function after ischemic and nonischemic injury in the mouse. J. Cardiovasc. Pharmacol. 66, 1–8. doi: 10.1097/FJC.0000000000000247
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A., and Tschopp, J. (2006). Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241. doi: 10.1038/nature04516
McInnes, I. B., and Schett, G. (2011). The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219. doi: 10.1056/NEJMra1004965
Messerschmitt, P. J., Rettew, A. N., Schroeder, N. O., Brookover, R. E., Jakatdar, A. P., Getty, P. J., et al. (2012). Osteosarcoma phenotype is inhibited by 3,4-methylenedioxy-beta-nitrostyrene. Sarcoma 2012:479712. doi: 10.1155/2012/479712
Meunier, E., Dick, M. S., Dreier, R. F., Schurmann, N., Kenzelmann Broz, D., Warming, S., et al. (2014). Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature 509, 366–370. doi: 10.1038/nature13157
Ming, X. L., Maeda, W. Y., Blumberg, B., Raval, S., Cook, S. D., Dowling, P. C., et al. (2002). Caspase-1 expression in multiple sclerosis plaques and cultured glial cells. J. Neurol. Sci. 197, 9–18. doi: 10.1016/s0022-510x(02)00030-8
Miskiewicz, A., Szparecki, G., Durlik, M., Rydzewska, G., Ziobrowski, I., and Górska, R. (2015). The Q705K and F359L single-nucleotide polymorphisms of NOD-like receptor signaling pathway: association with chronic pancreatitis, pancreatic cancer and periodontitis. Arch. Immunol. Ther. Exp. (Warsz) 63, 485–494. doi: 10.1007/s00005-015-0355-9
Mogi, M., Harada, M., Kondo, T., Riederer, P., Inagaki, H., Minami, M., et al. (1994). Interleukin-1β, interleukin-6, epidermal growth factor and transforming growth factor-α are elevated in the brain from Parkinsonian patients. Neurosci. Lett. 180, 147–150. doi: 10.1016/0304-3940(94)90508-8
Moore, B. J. R., Islam, B., Ward, S., Jackson, O., Armitage, R., Blackburn, J., et al. (2019). Repurposing of tranilast for potential neuropathic pain treatment by inhibition of sepiapterin reductase in the BH4 pathway. ACS Omega 4, 11960–11972. doi: 10.1021/acsomega.9b01228
Moossavi, M., Parsamanesh, N., Bahrami, A., Atkin, S. L., and Sahebkar, A. (2018). Role of the NLRP3 inflammasome in cancer. Mol. Cancer 17:158. doi: 10.1186/s12943-018-0900-3
Mortimer, L., Moreau, F., MacDonald, J. A., and Chadee, K. (2016). NLRP3 inflammasome inhibition is disrupted in a group of auto-inflammatory disease CAPS mutations. Nat. Immunol. 17, 1176–1186. doi: 10.1038/ni.3538
Muñoz-Planillo, R., Kuffa, P., Martínez-Colón, G., Smith, B. L., Rajendiran, T. M., and Núñez, G. (2013). K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153. doi: 10.1016/j.immuni.2013.05.016
Murakami, T., Ockinger, J., Yu, J., Byles, V., McColl, A., Hofer, A. M., et al. (2012). Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc. Natl. Acad. Sci. U S A 109, 11282–11287. doi: 10.1073/pnas.1117765109
Nakahira, K., Haspel, J. A., Rathinam, V. A., Lee, S. J., Dolinay, T., Lam, H. C., et al. (2011). Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 12, 222–230. doi: 10.1038/ni.1980
Nandini, D. B., Rao, R. S., Deepak, B. S., and Reddy, P. B. (2020). Sulforaphane in broccoli: the green chemoprevention!! Role in cancer prevention and therapy. J. Oral Maxillofac. Pathol. 24:405. doi: 10.4103/jomfp.JOMFP_126_19
Napier, B. A., Brubaker, S. W., Sweeney, T. E., Monette, P., Rothmeier, G. H., Gertsvolf, N. A., et al. (2016). Complement pathway amplifies caspase-11-dependent cell death and endotoxin-induced sepsis severity. J. Exp. Med. 213, 2365–2382. doi: 10.1084/jem.20160027
Natarajan, K., Singh, S., Burke, T. R., Grunberger, D., and Aggarwal, B. B. (1996). Caffeic acid phenethyl ester is a potent and specific inhibitor of activatio of nuclear transcription factor NF-κB. Proc. Natl. Acad. Sci. U S A 93, 9090–9095. doi: 10.1073/pnas.93.17.9090
Ng, A., Tam, W. W., Zhang, M. W., Ho, C. S., Husain, S. F., McIntyre, R. S., et al. (2018). IL-1β, IL-6, TNF-α and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and meta-analysis. Sci. Rep. 8:12050. doi: 10.1038/s41598-018-30487-6
Nie, J., Chang, Y., Li, Y., Zhou, Y., Qin, J., Sun, Z., et al. (2017). Caffeic acid phenethyl ester (propolis extract) ameliorates insulin resistance by inhibiting JNK and NF-κB inflammatory pathways in diabetic mice and HepG2 cell models. J. Agric. Food Chem. 65, 9041–9053. doi: 10.1021/acs.jafc.7b02880
Nie, X., Chen, S. R., Wang, K., Peng, Y., Wang, Y. T., Wang, D., et al. (2017). Attenuation of innate immunity by andrographolide derivatives through NF-kappaB signaling pathway. Sci. Rep. 7:4738. doi: 10.1038/s41598-017-04673-x
Ning, B. B., Zhang, Y., Wu, D. D., Cui, J. G., Liu, L., Wang, P. W., et al. (2017). Luteolin-7-diglucuronide attenuates isoproterenol-induced myocardial injury and fibrosis in mice. Acta Pharmacol. Sin. 38, 331–341. doi: 10.1038/aps.2016.142
O’Neill, L., Coll, R., Cooper, R. M., Robertson, A., and Schroder, K. (2016). Sulfonylureas and related compounds and use of same. AU IE Patent Application. Patent No. PCT/AU2016/050103). Available online at: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2016131098.
Ock, J., Han, H. S., Hong, S. H., Lee, S. Y., Han, Y. M., Kwon, B. M., et al. (2010). Obovatol attenuates microglia-mediated neuroinflammation by modulating redox regulation. Br. J. Pharmacol. 159, 1646–1662. doi: 10.1111/j.1476-5381.2010.00659.x
Orlowski, G. M., Colbert, J. D., Sharma, S., Bogyo, M., Robertson, S. A., and Rock, K. L. (2015). Multiple cathepsins promote pro-IL-1β synthesis and NLRP3-mediated IL-1β activation. J. Immunol. 195, 1685–1697. doi: 10.4049/jimmunol.1500509
Orrock, J. E., and Ilowite, N. T. (2016). Canakinumab for the treatment of active systemic juvenile idiopathic arthritis. Expert Rev. Clin. Pharmacol. 9, 1015–1024. doi: 10.1080/17512433.2016.1204910
Ortega-Gómez, A., Perretti, M., and Soehnlein, O. (2013). Resolution of inflammation: an integrated view. EMBO. Mol. Med. 5, 661–674. doi: 10.1002/emmm.201202382
Parajuli, B., Sonobe, Y., Horiuchi, H., Takeuchi, H., Mizuno, T., and Suzumura, A. (2013). Oligomeric amyloid β induces IL-1β processing via production of ROS: implication in Alzheimer’s disease. Cell Death Dis. 4:e975. doi: 10.1038/cddis.2013.503
Park, S., Won, J. H., Hwang, I., Hong, S., Lee, H. K., and Yu, J. W. (2015). Defective mitochondrial fission augments NLRP3 inflammasome activation. Sci. Rep. 5:15489. doi: 10.1038/srep15489
Peng, S., Gao, J., Liu, W., Jiang, C., Yang, X., Sun, Y., et al. (2016). Andrographolide ameliorates OVA-induced lung injury in mice by suppressing ROS-mediated NF-κB signaling and NLRP3 inflammasome activation. Oncotarget 7, 80262–80274. doi: 10.18632/oncotarget.12918
Peppiatt, C. M., Collins, T. J., Mackenzie, L., Conway, S. J., Holmes, A. B., Bootman, M. D., et al. (2003). 2-Aminoethoxydiphenyl borate (2-APB) antagonises inositol 1,4,5-trisphosphate-induced calcium release, inhibits calcium pumps and has a use-dependent and slowly reversible action on store-operated calcium entry channels. Cell Calcium 34, 97–108. doi: 10.1016/s0143-4160(03)00026-5
Perera, A. P., Fernando, R., Shinde, T., Gundamaraju, R., Southam, B., Sohal, S. S., et al. (2018). MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci. Rep. 8:8618. doi: 10.1038/s41598-018-26775-w
Perregaux, D. G. M., Laliberte, R. P., Hawryluk, N., Peurano, H., Stam, E., Eggler, J., et al. (2001). Identification and characterization of a novel class of interleukin-1 post-translational processing inhibitors. J. Pharmacol. Exp. Ther. 299, 187–197. Available online at: https://jpet.aspetjournals.org/content/299/1/187.long.
Py, B. F., Jin, M., Desai, B. N., Penumaka, A., Zhu, H., Kober, M., et al. (2014). Caspase-11 controls interleukin-1β release through degradation of TRPC1. Cell Rep. 6, 1122–1128. doi: 10.1016/j.celrep.2014.02.015
Rada, B., Park, J. J., Sil, P., Geiszt, M., and Leto, T. L. (2014). NLRP3 inflammasome activation and interleukin-1β release in macrophages require calcium but are independent of calcium-activated NADPH oxidases. Inflamm. Res. 63, 821–830. doi: 10.1007/s00011-014-0756-y
Rahban, M., Habibi-Rezaei, M., Mazaheri, M., Saso, L., and Moosavi-Movahedi, A. A. (2020). Anti-viral potential and modulation of Nrf2 by curcumin: pharmacological implications. Antioxidants (Basel) 9:1228. doi: 10.3390/antiox9121228
Ramos-Cejudo, J., Wisniewski, T., Marmar, C., Zetterberg, H., Blennow, K., de Leon, M. J., et al. (2018). Traumatic brain injury and Alzheimer’s disease: the cerebrovascular link. EBioMed. 28, 21–30. doi: 10.1016/j.ebiom.2018.01.021
Rathinam, V. A., Vanaja, S. K., Waggoner, L., Sokolovska, A., Becker, C., Stuart, L. M., et al. (2012). TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150, 606–619. doi: 10.1016/j.cell.2012.07.007
Reddy, P. H., Manczak, M., Yin, X., Grady, M. C., Mitchell, A., Tonk, S., et al. (2018). Protective effects of indian spice curcumin against amyloid-beta in Alzheimer’s disease. J. Alzheimers Dis. 61, 843–866. doi: 10.3233/JAD-170512
Ren, P., Wu, D., Appel, R., Zhang, L., Zhang, C., Luo, W., et al. (2020). Targeting the NLRP3 inflammasome with inhibitor MCC950 prevents aortic aneurysms and dissections in mice. J. Am. Heart Assoc. 9:e014044. doi: 10.1161/JAHA.119.014044
Reshi, L., and Chi-Yong, W. (2020). Andrographolide as a potent and promising antiviral agent. Chin. J. Nat. Med. 18, 760–769. doi: 10.1016/S1875-5364(20)60016-4
Reyes-Farias, M., and Carrasco-Pozo, C. (2019). The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int. J. Mol. Sci. 20, 3177–3196. doi: 10.3390/ijms20133177
Rho, J. M., Shao, L. R., and Stafstrom, C. E. (2019). 2-Deoxyglucose and beta-hydroxybutyrate: metabolic agents for seizure control. Front. Cell Neurosci. 13:172. doi: 10.3389/fncel.2019.00172
Robinson, K. M., Ramanan, K., Clay, M. E., McHugh, K. J., Pilewski, M. J., Nickolich, K. L., et al. (2018). The inflammasome potentiates influenza/Staphylococcus aureus superinfection in mice. JCI Insight 3:e97470. doi: 10.1172/jci.insight.97470
Rubio-Perez, J. M., and Morillas-Ruiz, J. M. (2012). A review: inflammatory process in Alzheimer’s disease, role of cytokines. ScientificWorldJournal 2012:756357. doi: 10.1100/2012/756357
Ruiz-Miyazawa, K. W., Staurengo-Ferrari, L., Mizokami, S. S., Domiciano, T. P., Vicentini, F., Camilios-Neto, D., et al. (2017). Quercetin inhibits gout arthritis in mice: induction of an opioid-dependent regulation of inflammasome. Inflammopharmacology 25, 555–570. doi: 10.1007/s10787-017-0356-x
Sánchez-Fernández, A., Skouras, D. B., Dinarello, C. A., and López-Vales, R. (2019). OLT1177 (Dapansutrile), a selective NLRP3 inflammasome inhibitor, ameliorates experimental autoimmune encephalomyelitis pathogenesis. Front. Immunol. 10:2578. doi: 10.3389/fimmu.2019.02578
Saadane, A., Masters, S., DiDonato, J., Li, J., and Berger, M. (2007). Parthenolide inhibits IκB kinase, NF-κB activation and inflammatory response in cystic fibrosis cells and mice. Am. J. Respir. Cell Mol. Biol. 36, 728–736. doi: 10.1165/rcmb.2006-0323OC
Saito, H., Fushida, S., Harada, S., Miyashita, T., Oyama, K., Yamaguchi, T., et al. (2018). Importance of human peritoneal mesothelial cells in the progression, fibrosis and control of gastric cancer: inhibition of growth and fibrosis by tranilast. Gastric Cancer 21, 55–67. doi: 10.1007/s10120-017-0726-5
Sakamoto, Y., Kanatsu, J., Toh, M., Naka, A., Kondo, K., and Iida, K. (2016). The dietary isoflavone daidzein reduces expression of pro-inflammatory genes through PPARα/γ and JNK pathways in adipocyte and macrophage co-cultures. PLoS One 11:e0149676. doi: 10.1371/journal.pone.0149676
Salehi, B., Venditti, A., Sharifi-Rad, M., Kregiel, D., Sharifi-Rad, J., Durazzo, A., et al. (2019). The therapeutic potential of apigenin. Int. J. Mol. Sci. 20:1305. doi: 10.3390/ijms20061305
Saresella, M., La Rosa, F., Piancone, F., Zoppis, M., Marventano, I., Calabrese, E., et al. (2016). The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer’s disease. Mol. Neurodegener. 11:23. doi: 10.1186/s13024-016-0088-1
Scambler, T., Jarosz-Griffiths, H. H., Lara-Reyna, S., Pathak, S., Wong, C., Holbrook, J., et al. (2019). ENaC-mediated sodium influx exacerbates NLRP3-dependent inflammation in cystic fibrosis. eLife 8:e49248. doi: 10.7554/eLife.49248
Schorn, C., Frey, B., Lauber, K., Janko, C., Strysio, M., Keppeler, H., et al. (2011). Sodium overload and water influx activate the NALP3 inflammasome. J. Biol. Chem. 286, 35–41. doi: 10.1074/jbc.M110.139048
Schroder, K., and Tschopp, J. (2010). The inflammasomes. Cell 140, 821–832. doi: 10.1016/j.cell.2010.01.040
Sezlev Bilecen, D., Rodriguez-Cabello, J. C., Uludag, H., and Hasirci, V. (2017). Construction of a PLGA based, targeted siRNA delivery system for treatment of osteoporosis. J. Biomater. Sci. Polym. Ed. 28, 1859–1873. doi: 10.1080/09205063.2017.1354675
Sfriso, P., Bindoli, S., Doria, A., Feist, E., and Galozzi, P. (2020). Canakinumab for the treatment of adult-onset Still’s disease. Expert. Rev. Clin. Immunol. 16, 129–138. doi: 10.1080/1744666X.2019.1707664
Shi, T. H., Huang, Y. L., Chen, C. C., Pi, W. C., Hsu, Y. L., Lo, L. C., et al. (2020). Andrographolide and its fluorescent derivative inhibit the main proteases of 2019-nCoV and SARS-CoV through covalent linkage. Biochem. Biophys. Res. Commun. 533, 467–473. doi: 10.1016/j.bbrc.2020.08.086
Shi, Y., Lv, Q., Zheng, M., Sun, H., and Shi, F. (2021). NLRP3 inflammasome inhibitor INF39 attenuated NLRP3 assembly in macrophages. Int. Immunopharmacol. 92:107358. doi: 10.1016/j.intimp.2020.107358
Shi, H., Wang, Y., Li, X., Zhan, X., Tang, M., Fina, M., et al. (2016). NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat. Immunol. 17, 250–258. doi: 10.1038/ni.3333
Shi, J., Zhao, Y., Wang, Y., Gao, W., Ding, J., Li, P., et al. (2014). Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192. doi: 10.1038/nature13683
Shi, J., Zhao, Y., Wang, K., Shi, X., Wang, Y., Huang, H., et al. (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665. doi: 10.1038/nature15514
Shim, D. W., Shin, W. Y., Yu, S. H., Kim, B. H., Ye, S. K., Koppula, S., et al. (2017). BOT-4-one attenuates NLRP3 inflammasome activation: NLRP3 alkylation leading to the regulation of its ATPase activity and ubiquitination. Sci. Rep. 7:15020. doi: 10.1038/s41598-017-15314-8
Shimada, K., Crother, T. R., Karlin, J., Dagvadorj, J., Chiba, N., Chen, S., et al. (2012). Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414. doi: 10.1016/j.immuni.2012.01.009
Soeda, Y., Saito, M., Maeda, S., Ishida, K., Nakamura, A., Kojima, S., et al. (2019). Methylene blue inhibits formation of tau fibrils but not of granular tau oligomers: a plausible key to understanding failure of a clinical trial for Alzheimer’s disease. J. Alzheimers Dis. 68, 1677–1686. doi: 10.3233/JAD-181001
Spiecker, M. L., Marx, N. I., and Darius, H. (2002). Tranilast inhibits cytokine-induced nuclear factor kb activation in vascular endothelial cells. Mol. Pharmacol. 62, 856–863. doi: 10.1124/mol.62.4.856
Stojakovic, A., Paz-Filho, G., Arcos-Burgos, M., Licinio, J., Wong, M. L., and Mastronardi, C. A. (2017). Role of the IL-1 pathway in dopaminergic neurodegeneration and decreased voluntary movement. Mol. Neurobiol. 54, 4486–4495. doi: 10.1007/s12035-016-9988-x
Subedi, L., Cho, K., Park, Y. U., Choi, H. J., and Kim, S. Y. (2019). Sulforaphane-enriched broccoli sprouts pretreated by pulsed electric fields reduces neuroinflammation and ameliorates scopolamine-induced amnesia in mouse brain through its antioxidant ability via Nrf2-HO-1 activation. Oxid. Med. Cell Longev. 2019:3549274. doi: 10.1155/2019/3549274
Sun, Y., Liu, W., Zhang, H., Li, H., Liu, J., Zhang, F., et al. (2017). Curcumin prevents osteoarthritis by inhibiting the activation of inflammasome NLRP3. J. Interferon Cytokine Res. 37, 449–455. doi: 10.1089/jir.2017.0069
Tan, E. K., Chao, Y. X., West, A., Chan, L. L., Poewe, W., and Jankovic, J. (2020). Parkinson disease and the immune system - associations, mechanisms and therapeutics. Nat. Rev. Neurol. 16, 303–318. doi: 10.1038/s41582-020-0344-4
Tan, W. S. D., Liao, W., Zhou, S., and Wong, W. S. F. (2017). Is there a future for andrographolide to be an anti-inflammatory drug? Deciphering its major mechanisms of action. Biochem. Pharmacol. 139, 71–81. doi: 10.1016/j.bcp.2017.03.024
Tang, G., Duan, F., Li, W., Wang, Y., Zeng, C., Hu, J., et al. (2019). Metformin inhibited Nod-like receptor protein 3 inflammasomes activation and suppressed diabetes-accelerated atherosclerosis in apoE−/− mice. Biomed. Pharmacother. 119:109410. doi: 10.1016/j.biopha.2019.109410
Tapia-Abellan, A., Angosto-Bazarra, D., Martinez-Banaclocha, H., de Torre-Minguela, C., Ceron-Carrasco, J. P., Perez-Sanchez, H., et al. (2019). MCC950 closes the active conformation of NLRP3 to an inactive state. Nat. Chem. Biol. 15, 560–564. doi: 10.1038/s41589-019-0278-6
Theofani, E., Semitekolou, M., Morianos, I., Samitas, K., and Xanthou, G. (2019). Targeting NLRP3 inflammasome activation in severe asthma. J. Clin. Med. 8:1615. doi: 10.3390/jcm8101615
Toldo, S., Mauro, A. G., Cutter, Z., Van Tassell, B. W., Messaroma, E., Del Buono, M. G., et al. (2019). The NLRP3 inflammasome inhibitor, OLT1177 (Dapansutrile), reduces infarct size and preserves contractile function after ischemia reperfusion injury in the mouse. J. Cardiovasc. Pharmacol. 73, 215–222. doi: 10.1097/FJC.0000000000000658
Trudler, D., Nazor, K. L., Eisele, Y. S., Grabauskas, T., Dolatabadi, N., Parker, J., et al. (2021). Soluble alpha-synuclein-antibody complexes activate the NLRP3 inflammasome in hiPSC-derived microglia. Proc. Natl. Acad. Sci. U S A 118:e2025847118. doi: 10.1073/pnas.2025847118
Ungerbäck, J., Belenki, D., Jawad ul-Hassan, A., Fredrikson, M., Fransén, K., Elander, N., et al. (2012). Genetic variation and alterations of genes involved in NFκB/TNFAIP3- and NLRP3-inflammasome signaling affect susceptibility and outcome of colorectal cancer. Carcinogenesis 33, 2126–2134. doi: 10.1093/carcin/bgs256
Unterholzner, L., Keating, S. E., Baran, M., Horan, K. A., Jensen, S. B., Sharma, S., et al. (2010). IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 11, 997–1004. doi: 10.1038/ni.1932
van der Heijden, T., Kritikou, E., Venema, W., van Duijn, J., van Santbrink, P. J., Slutter, B., et al. (2017). NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein e–deficient mice—brief report. Arterioscler. Thromb. Vasc. Biol. 37, 1457–1461. doi: 10.1161/ATVBAHA.117.309575
van Hout, G. P., Bosch, L., Ellenbroek, G. H., de Haan, J. J., van Solinge, W. W., Cooper, M. A., et al. (2017). The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur. Heart J. 38, 828–836. doi: 10.1093/eurheartj/ehw247
Vandanmagsar, B., Youm, Y. H., Ravussin, A., Galgani, J. E., Stadler, K., Mynatt, R. L., et al. (2011). The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17, 179–188. doi: 10.1038/nm.2279
Venegas, C., Kumar, S., Franklin, B. S., Dierkes, T., Brinkschulte, R., Tejera, D., et al. (2017). Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer’s disease. Nature 552, 355–361. doi: 10.1038/nature25158
Viola-Rhenals, M., Patel, K. R., Jaimes-Santamaria, L., Wu, G., Liu, J., and Dou, Q. P. (2018). Recent advances in antabuse (disulfiram): the importance of its metal-binding ability to its anticancer activity. Curr. Med. Chem. 25, 506–524. doi: 10.2174/0929867324666171023161121
Voon, F. L., Sulaiman, M. R., Akhtar, M. N., Idris, M. F., Akira, A., Perimal, E. K., et al. (2017). Cardamonin (2’,4’-dihydroxy-6’-methoxychalcone) isolated from Boesenbergia rotunda (L.) Mansf. inhibits CFA-induced rheumatoid arthritis in rats. Eur. J. Pharmacol. 794, 127–134. doi: 10.1016/j.ejphar.2016.11.009
Wallisch, J. S., Simon, D. W., Bayir, H., Bell, M. J., Kochanek, P. M., and Clark, R. S. B. (2017). Cerebrospinal fluid NLRP3 is increased after severe traumatic brain injury in infants and children. Neurocrit. Care 27, 44–50. doi: 10.1007/s12028-017-0378-7
Wang, X., Chi, J., Huang, D., Ding, L., Zhao, X., Jiang, L., et al. (2020). Alpha-synuclein promotes progression of Parkinson’s disease by upregulating autophagy signaling pathway to activate NLRP3 inflammasome. Exp. Ther. Med. 19, 931–938. doi: 10.3892/etm.2019.8297
Wang, F., Gomez-Sintes, R., and Boya, P. (2018). Lysosomal membrane permeabilization and cell death. Traffic 19, 918–931. doi: 10.1111/tra.12613
Wang, Y., Jin, F., Wang, Q., and Suo, Z. (2017). Long-term survival of AIDS patients treated with only traditional chinese medicine. Altern. Complement. Ther. 33, 90–92. doi: 10.1089/act.2017.29106.ywa
Wang, K., Lv, Q., Miao, Y. M., Qiao, S. M., Dai, Y., and Wei, Z. F. (2018). Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem. Pharmacol. 155, 494–509. doi: 10.1016/j.bcp.2018.07.039
Wang, Y., Miao, L., Satterlee, A., and Huang, L. (2015). Delivery of oligonucleotides with lipid nanoparticles. Adv. Drug Deliv. Rev. 87, 68–80. doi: 10.1016/j.addr.2015.02.007
Wang, X., Sun, K., Zhou, Y., Wang, H., Zhou, Y., Liu, S., et al. (2021). NLRP3 inflammasome inhibitor CY-09 reduces hepatic steatosis in experimental NAFLD mice. Biochem. Biophys. Res. Commun. 534, 734–739. doi: 10.1016/j.bbrc.2020.11.009
Wang, S., Yang, H., Yu, L., Jin, J., Qian, L., Zhao, H., et al. (2014). Oridonin attenuates Aβ 1-42-induced neuroinflammation and inhibits NF-κB pathway. PLoS One 9:e104745. doi: 10.1371/journal.pone.0104745
Wang, F., Yao, X., Zhang, Y., and Tang, J. (2019). Synthesis, biological function and evaluation of Shikonin in cancer therapy. Fitoterapia 134, 329–339. doi: 10.1016/j.fitote.2019.03.005
Wang, S., Yu, L., Yang, H., Li, C., Hui, Z., Xu, Y., et al. (2016). Oridonin attenuates synaptic loss and cognitive deficits in an Aβ1-42-induced mouse model of Alzheimer’s disease. PLoS One 11:e0151397. doi: 10.1371/journal.pone.0151397
Wang, Q., Zhang, T., Ke, C. Q., Tang, C., Yao, S., Lin, L., et al. (2021). Guaianolides from Artemisia codonocephala suppress interleukine-1β secretion in macrophages. Phytochemistry 192:112955. doi: 10.1016/j.phytochem.2021.112955
Weber, K., and Schilling, J. D. (2014). Lysosomes integrate metabolic-inflammatory cross-talk in primary macrophage inflammasome activation. J. Biol. Chem. 289, 9158–9171. doi: 10.1074/jbc.M113.531202
Wen, H., Gris, D., Lei, Y., Jha, S., Zhang, L., Huang, M. T., et al. (2011). Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12, 408–415. doi: 10.1038/ni.2022
White, D. E., and Burchill, S. A. (2008). BAY 11–7082 induces cell death through NF-κB-independent mechanisms in the Ewing’s sarcoma family of tumours. Cancer Lett. 268, 212–224. doi: 10.1016/j.canlet.2008.03.045
Wiggins, K. A., Parry, A. J., Cassidy, L. D., Humphry, M., Webster, S. J., Goodall, J. C., et al. (2019). IL-1α cleavage by inflammatory caspases of the noncanonical inflammasome controls the senescence-associated secretory phenotype. Aging Cell 18:e12946. doi: 10.1111/acel.12946
Wolf, A. J., Reyes, C. N., Liang, W., Becker, C., Shimada, K., Wheeler, M. L., et al. (2016). Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell 166, 624–636. doi: 10.1016/j.cell.2016.05.076
Wu, X. Y., Li, K. T., Yang, H. X., Yang, B., Lu, X., Zhao, L. D., et al. (2020). Complement C1q synergizes with PTX3 in promoting NLRP3 inflammasome over-activation and pyroptosis in rheumatoid arthritis. J. Autoimmun. 106:102336. doi: 10.1016/j.jaut.2019.102336
Xiao, L., Jiang, L., Hu, Q., and Li, Y. (2017). MicroRNA-133b ameliorates allergic inflammation and symptom in murine model of allergic rhinitis by targeting Nlrp3. Cell Physiol. Biochem. 42, 901–912. doi: 10.1159/000478645
Xiao, M., Li, L., Li, C., Liu, L., Yu, Y., and Ma, L. (2016). 3,4-Methylenedioxy-β-nitrostyrene ameliorates experimental burn wound progression by inhibiting the NLRP3 inflammasome activation. Plast. Reconstr. Surg. 137, e566–e575. doi: 10.1097/01.prs.0000479972.06934.83
Xie, M., Yu, Y., Kang, R., Zhu, S., Yang, L., Zeng, L., et al. (2016). PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat. Commun. 7:13280. doi: 10.1038/ncomms13280
Xu, M., Wan, C. X., Huang, S. H., Wang, H. B., Fan, D., Wu, H. M., et al. (2019). Oridonin protects against cardiac hypertrophy by promoting P21-related autophagy. Cell Death Dis. 10:403. doi: 10.1038/s41419-019-1617-y
Xu, J., Wold, E. A., Ding, Y., Shen, Q., and Zhou, J. (2018). Therapeutic potential of oridonin and its analogs: from anticancer and antiinflammation to neuroprotection. Molecules 23:474. doi: 10.3390/molecules23020474
Xu, Y., Xu, Y., Blevins, H., Lan, Y., Liu, Y., Yuan, G., et al. (2021). Discovery of carbon-11 labeled sulfonamide derivative: a PET tracer for imaging brain NLRP3 inflammasome. Bioorg. Med. Chem. Lett. 34:127777. doi: 10.1016/j.bmcl.2021.127777
Yamanashi, T., Iwata, M., Kamiya, N., Tsunetomi, K., Kajitani, N., Wada, N., et al. (2017). Beta-hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress-induced behavioral and inflammatory responses. Sci. Rep. 7:7677. doi: 10.1038/s41598-017-08055-1
Yan, J., Chen, Y., He, C., Yang, Z. Z., Lu, C., and Chen, X. S. (2012). Andrographolide induces cell cycle arrest and apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes. Cell Biol. Toxicol. 28, 47–56. doi: 10.1007/s10565-011-9204-8
Yan, Y., Jiang, W., Liu, L., Wang, X., Ding, C., Tian, Z., et al. (2015). Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160, 62–73. doi: 10.1016/j.cell.2014.11.047
Yan, C., Yan, H., Mao, J., Liu, Y., Xu, L., Zhao, H., et al. (2020). Neuroprotective effect of oridonin on traumatic brain injury via inhibiting NLRP3 inflammasome in experimental mice. Front. Neurosci. 14:557170. doi: 10.3389/fnins.2020.557170
Yang, J., Liu, Z., and Xiao, T. S. (2017). Post-translational regulation of inflammasomes. Cell Mol. Immunol. 14, 65–79. doi: 10.1038/cmi.2016.29
Yin, H., Guo, Q., Li, X., Tang, T., Li, C., Wang, H., et al. (2018). Curcumin suppresses IL-1β secretion and prevents inflammation through inhibition of the NLRP3 Inflammasome. J. Immunol. 200, 2835–2846. doi: 10.4049/jimmunol.1701495
Yin, J., Zhao, F., Chojnacki, J. E., Fulp, J., Klein, W. L., Zhang, S., et al. (2018). NLRP3 inflammasome inhibitor ameliorates amyloid pathology in a mouse model of Alzheimer’s disease. Mol. Neurobiol. 55, 1977–1987. doi: 10.1007/s12035-017-0467-9
Yis, U., Topcu, Y., Ozbal, S., Tugyan, K., Bayram, E., Karakaya, P., et al. (2013). Caffeic acid phenethyl ester prevents apoptotic cell death in the developing rat brain after pentylenetetrazole-induced status epilepticus. Epilepsy Behav. 29, 275–280. doi: 10.1016/j.yebeh.2013.08.002
Youm, Y. H., Nguyen, K. Y., Grant, R. W., Goldberg, E. L., Bodogai, M., Kim, D., et al. (2015). The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 21, 263–269. doi: 10.1038/nm.3804
Yu, Q., Zhang, M., Ying, Q., Xie, X., Yue, S., Tong, B., et al. (2019). Decrease of AIM2 mediated by luteolin contributes to non-small cell lung cancer treatment. Cell Death Dis. 10:218. doi: 10.1038/s41419-019-1447-y
Yuan, Z., Ouyang, P., Gu, K., Rehman, T., Zhang, T., Yin, Z., et al. (2019). The antibacterial mechanism of oridonin against methicillin-resistant Staphylococcus aureus (MRSA). Pharm. Biol. 57, 710–716. doi: 10.1080/13880209.2019.1674342
Zahid, A., Li, B., Kombe, A. J.K., Jin, T., and Tao, J. (2019). Pharmacological inhibitors of the NLRP3 Inflammasome. Front. Immunol. 10:2538. doi: 10.3389/fimmu.2019.02538
Zaki, M. H., Boyd, K. L., Vogel, P., Kastan, M. B., Lamkanfi, M., and Kanneganti, T. D. (2010a). The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32, 379–391. doi: 10.1016/j.immuni.2010.03.003
Zaki, M. H., Vogel, P., Body-Malapel, M., Lamkanfi, M., and Kanneganti, T. D. (2010b). IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. J. Immunol. 185, 4912–4920. doi: 10.4049/jimmunol.1002046
Zaplatic, E., Bule, M., Shah, S. Z. A., Uddin, M. S., and Niaz, K. (2019). Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sci. 224, 109–119. doi: 10.1016/j.lfs.2019.03.055
Zeng, J., Chen, Y., Ding, R., Feng, L., Fu, Z., Yang, S., et al. (2017). Isoliquiritigenin alleviates early brain injury after experimental intracerebral hemorrhage via suppressing ROS- and/or NF-κB-mediated NLRP3 inflammasome activation by promoting Nrf2 antioxidant pathway. J. Neuroinflammation 14:119. doi: 10.1186/s12974-017-0895-5
Zhai, Y., Meng, X., Ye, T., Xie, W., Sun, G., and Sun, X. (2018). Inhibiting the NLRP3 inflammasome activation with MCC950 ameliorates diabetic encephalopathy in db/db mice. Molecules 23:522. doi: 10.3390/molecules23030522
Zhang, B., Li, Z., Xu, W., Xiang, C., and Ma, Y. (2018). Luteolin alleviates NLRP3 inflammasome activation and directs macrophage polarizaiton in lipopolysaccharide-stimulated RAW264.7 cells. Am. J. Transl. Res. 10, 265–273. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5801364/.
Zhang, X., Wang, G., Gurley, E. C., and Zhou, H. (2014). Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS One 9:e107072. doi: 10.1371/journal.pone.0107072
Zhang, Y., Zheng, Y., and Li, H. (2016). NLRP3 inflammasome plays an important role in the pathogenesis of collagen-induced arthritis. Mediators Inflamm. 2016:9656270. doi: 10.1155/2016/9656270
Zhang, D., Zhou, Q., Huang, D., He, L., Zhang, H., Hu, B., et al. (2019). ROS/JNK/c-Jun axis is involved in oridonin-induced caspase-dependent apoptosis in human colorectal cancer cells. Biochem. Biophys. Res. Commun. 513, 594–601. doi: 10.1016/j.bbrc.2019.04.011
Zhao, X., Zhu, Y., Hu, J., Jiang, L., Li, L., Jia, S., et al. (2018). Shikonin inhibits tumor growth in mice by suppressing pyruvate kinase M2-mediated aerobic glycolysis. Sci. Rep. 8:14517. doi: 10.1038/s41598-018-31615-y
Zhou, L., Flores, J., Noel, A., Beauchet, O., Sjostrom, P. J., and LeBlanc, A. C. (2019). Methylene blue inhibits Caspase-6 activity and reverses Caspase-6-induced cognitive impairment and neuroinflammation in aged mice. Acta Neuropathol. Commun. 7:210. doi: 10.1186/s40478-019-0856-6
Zhou, W., Liu, X., Cheng, K., Zhang, X., Lu, J., and Hu, R. (2017). X-11-5–27, a daidzein derivative, inhibits NLRP3 inflammasome activity via promoting autophagy. Exp. Cell Res. 360, 320–327. doi: 10.1016/j.yexcr.2017.09.022
Zhou, Y., Lu, M., Du, R. H., Qiao, C., Jiang, C. Y., Zhang, K. Z., et al. (2016). MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol. Neurodegener. 11:28. doi: 10.1186/s13024-016-0094-3
Zhou, T., Xiang, D. K., Li, S. N., Yang, L. H., Gao, L. F., and Feng, C. (2018). MicroRNA-495 ameliorates cardiac microvascular endothelial cell injury and inflammatory reaction by suppressing the NLRP3 inflammasome signaling pathway. Cell Physiol. Biochem. 49, 798–815. doi: 10.1159/000493042
Zhou, R., Yazdi, A. S., Menu, P., and Tschopp, J. (2011). A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225. doi: 10.1038/nature09663
Zhuang, T., Li, S., Yi, X., Guo, S., Wang, Y., Chen, J., et al. (2020). Tranilast directly targets NLRP3 to protect melanocytes from keratinocyte-derived IL-1β under oxidative stress. Front. Cell Dev. Biol. 8:588. doi: 10.3389/fcell.2020.00588
Keywords: NLRP3, inflammasome, NOD-like receptor, innate immune system, inhibitors, inflammatory diseases
Citation: Blevins HM, Xu Y, Biby S and Zhang S (2022) The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 14:879021. doi: 10.3389/fnagi.2022.879021
Received: 18 February 2022; Accepted: 12 May 2022;
Published: 10 June 2022.
Edited by:
Evandro Fei Fang, University of Oslo, NorwayReviewed by:
Yinxia Chao, National Neuroscience Institute (NNI), SingaporeSwati Sudhakar More, University of Minnesota Twin Cities, United States
Copyright © 2022 Blevins, Xu, Biby and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shijun Zhang, c3poYW5nMkB2Y3UuZWR1
 Hallie M. Blevins
Hallie M. Blevins Yiming Xu
Yiming Xu Savannah Biby
Savannah Biby Shijun Zhang
Shijun Zhang