
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 17 June 2022
Sec. Neurocognitive Aging and Behavior
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.870326
This article is part of the Research TopicModel Organisms in Neuroinflammation and Neuropathy: Drosophila melanogasterView all 5 articles
 Qiushi Zhao1
Qiushi Zhao1 Ying Liu2
Ying Liu2 Siyu Zhang2
Siyu Zhang2 Yuchu Zhao2
Yuchu Zhao2 Chenxi Wang2
Chenxi Wang2 Keqiang Li3
Keqiang Li3 Zecheng Jin3
Zecheng Jin3 Juhui Qiao2*
Juhui Qiao2* Meichen Liu2*
Meichen Liu2*In an increasingly aged global population, achieving healthy life expectancy through natural and safe drug interventions is highly desirable. Here we show that total ginsenosides (TGGR), the main active components in the traditional Chinese medicine, ginseng, promote longevity across species. In Drosophila, an intriguing effect of TGGR on lifespan was the relatively narrow treatment window to elicit long-term benefits. TGGR administration during early adulthood, and especially during midlife, was sufficient to extend lifespan in both sexes. TGGR did not increase lifespan by reducing food intake or reproductive capacity; rather, TGGR increased the fertility of male Drosophila. TGGR augmented healthspan readouts associated with youth and with healthy aging, such as motility, intestinal barrier integrity, and biorhythm homeostasis. TGGR treatment also improved some types of stress resistance in both sexes, including increased tolerance to starvation and oxidation, and shifting “aged” gene expression patterns toward “healthy” patterns seen in the young. Gene expression, pharmacological and genetic epistatic analyses demonstrated that TGGR effects require normal expression of genes involved in insulin, TOR and MAPK signaling. The positive effects of TGGR on both healthspan and lifespan, coupled with its mechanism of action via evolutionarily conserved signaling pathways, demonstrate it to be a promising anti-aging drug.
The population of the world is becoming increasingly aged. It is predicted that the world’s older adult population will reach a peak of 2.1 billion in 2050, which will pose a threat to social and economic stability (Seals et al., 2016). Given this projection, it is of the upmost importance to understand the aging process and to be able to intervene in diseases for which aging is the main risk factor. Aging is a process in which the physiological functions of various systems and different structural levels of an organism decline with increasing age. There are many theories about the mechanisms that underlie aging, but few there are effective treatment options that affect aging. In recent years, plant compounds that have anti-aging effects have been intensively studied (Wu et al., 2022; Zhang et al., 2022). Such compounds are derived from the natural environment and have low toxicity and few side effects and, therefore, have great promise in the development of drugs to treat age-related diseases.
The anti-aging activities of Panax ginseng C. A. Meyer (ginseng) have been documented in the “Huang Di Nei Jing,” the earliest compilation of Chinese medicine written more than 2,000 years ago. Many studies have shown that ginsenosides, the main active components in ginseng, have a positive effect on delaying aging and can effectively improve age-related cardiovascular disease, neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease, as well as tumors (Tangrodchanapong et al., 2020). These actions are closely related to their anti-inflammatory and anti-oxidant effects and effects on mitochondrial homeostasis (Hyun et al., 2022). Therefore, ginsenosides have the potential to be used as an alternative natural medicine for anti-aging and life-prolonging effects. A recent study reported that total ginsenoside extract can prolong the lifespan and healthy lifespan of Caenorhabditis elegans (C. elegans) by activating anti-oxidation signaling pathways (Wang et al., 2021). However, the use of ginsenoside extract for delaying human aging, its anti-aging activities, optimal dosage and means of administration in different species need further investigation, and its multi-target mechanism of action needs to be elucidated.
Here, we used different systems, including Drosophila melanogaster (Drosophila), C. elegans, and human diploid fibroblasts (MRC-5 cells) to systematically evaluate the anti-aging effect of total ginsenosides of ginseng root (TGGR). We established a comprehensive system to characterize Drosophila health status to evaluate the concentration and optimal intervention period and to understand the effects of TGGR on aging-related systemic changes and stress resistance. Transcriptome sequencing and bioinformatics analysis was used to predict the multi-action targets of TGGR and RNA interference was used to identify genes and processes involved in Drosophila lifespan extension by TGGR. These data indicate an evolutionarily conserved anti-aging effect of TGGR and provide a comprehensive dataset for investigating the underlying mechanisms of ginseng in modulating lifespan.
MRC-5 cells were obtained from the American Type Culture Collection (Rockville, United States) and cultured in MEM complete medium (supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin) at 37°C in a 5% CO2 atmosphere. Cell density was 4.0 × 105/mL when the cells were cultured, and each culture flask was inoculated with 1 mL. Drug intervention has been carried out since the PDL7 generation. If the cells cannot grow to the bottom of the bottle within 3 weeks, these cells are defined as the last generation of cells.
Take PDL7 MRC-5 cells (2.0 × 105 cells/mL) for assay. TGGR stock solution (1 mg/mL in PBS), diluted with complete medium to a final concentration of 40, 80, 120, 160, 200, 240, 280 μg/mL. According to the CCK-8 kit (Boster Bioengineering Co., Ltd., Wuhan, China) operating instructions, add 20 μL of detection solution to each well, and place it in the dark at 37°C for a period of time, and then measure the absorbance value (450 nm) of each well.
Take MRC-5 cells of PDL28 (4.0 × 105 cells/mL) for assay. Follow the SA-β-gal staining kit (Biyuntian Biotechnology Co., Ltd., Shanghai, China) for cell fixation and cell staining, observe and take pictures under a microscope, and count the proportion of positive cells (cells are stained blue).
Use NGM solid medium to carry out the life test, and refer to for the specific method (Admasu et al., 2018). Use a sterile self-made insect picker to pick 120 L4 nematodes into NGM petri dishes (with or without drugs) and record the day as day 0. The nematodes were transferred to a new NGM petri dish every day afterward, and the nematodes deaths were counted every other day until the late reproductive period (usually the 4th day). The criteria for judging the death of nematodes are no response to mechanical stimulation and no swallowing.
The wild-type stock Canston-S was collected from Dr. Yufeng Yang (Institute of Life Sciences, Fuzhou University, Fuzhou, China). 4E-BP-RNAi (CG8846), and S6K-RNAi (CG10539) were obtained from the Bloomington Drosophila Stock Center. Akt-RNAi (CG4006) was obtained from the Vienna Drosophila RNAi Center. To eliminate genetic background, RNAi lines were backcrossed into Canton-S for six generations. The males of all RNAi flies were crossed with da-GS-GAL4 virgin females, F1 generation females were collected, transferred to food containing 20 μM RU486 for activation. RU486 (20 μM) was added into the food after cooling it to 50°C. TGGR (Total Ginsenoside Ginseng Root, Shanghai Yuanye Biotechnology Co., Ltd., Shanghai, China) in ddH2O were added to sugar-yeast-agar (SYA) medium to a final concentration of 0.5, 2.5, and 5 mg/mL. Equivalent volumes and concentrations of vehicle were added to SYA medium for control treatments. Female and male flies were sorted onto SYA medium or contains drugs medium that was replaced every 1–2 days throughout life. Flies were cultured in a humidified (60% relative humidity), temperature (26–27°C) controlled incubator with a 12 h on/off light cycle.
Food intake were measured as previously described (Staats et al., 2019). Fecundity was quantified as number of eggs laid within 24 h, and triglyceride measurements were performed as previously described (Niraula et al., 2018).
In order to investigate TGGR effects on such a phenomenon, flies were aged on SYA medium supplemented or not with TGGR until the day of the “Smurf” assay, in which dyed medium was prepared using standard medium with blue dye #1 (Soleibao Technology Co., Ltd., Beijing, China) added at a concentration of 2.5% (w/v). Flies were kept on dyed medium overnight. A fly was counted as a Smurf when dye coloration could be observed outside of the digestive tract.
On the day of assay, flies were transferred to new fly glass pipette (5 × 65 mm) that contain fresh diets. Then, the pipette was placed into the DAM2 Drosophila Locomotor Activity Monitor (Trikinetics, United States) and data were acquired and processed with DAM System 310 software (Trikinetics, United States). The recording signal interval to record once every 5 min, and the light cycle is 12 h on/off for 24 h. The circadian activity rhythm behavior curve is drawn with Excel.
On the day of assay, flies were transferred with CO2 anesthesia into glass pipette (height 22 cm, diameter 1.0 cm, capacity 25 mL) capped with cotton plug, and after 15 min of awakening, the glass pipette is placed vertically for measurement. In the experiment, all flies were shaken down to the bottom of the tube, and the data was recorded by taking pictures at 5 s. Climbing index evaluation method: reserve 18 cm from the bottom of the test tube to the lower end of the tampon as the flies movement area, which was divided into 1, 2, 3, 4, 5, 6, 7, 8, 9 from the bottom to the top (the corresponding score for the area was 1, 2, 3, 4, 5, 6, 7, 8, 9 points) There were 9 areas in total, and the climbing index is the sum of the points of each area.
Sorted flies were fed on SYA or SYA supplemented with TGGR until the day of assay, files were transferred to medium made of 1% agar (starvation assay) or to empty vials (desiccation assay) or to supplemented with SYA diets contain 5% (w/v) H2O2 (H2O2 stress assay) or to supplemented with SYA diets contain 6 mg/mL SDS (SDS stress assay). Construct survival curves after recording dead flies every 12 h (starvation and SDS assays) or every 2 h (H2O2 stress assay) or every 1 h (desiccation assay). Replace flies daily with fresh starvation or SYA/SDS supplemented medium and eliminate dead flies.
Keep the flies in a pre-weighed centrifuge tubes and the total weight was measured at the day of weight assay. Final weight of flies was calculated by subtracting pre-measured weight of centrifuge tubes.
Frozen Drosophila samples (pre-weighed) were homogenized in physiological saline and centrifuged at 3,000 rpm for 10 min at 4°C. Superoxide dismutase (SOD) and catalase (CAT) were detected according to the instructions of the assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Reference substance: Ginsenoside Rg1, Re, Rf, Rg2, Rb1, Ro, Rc, Rb2, Rb3, Rd, purity: 98.10, 98.40, 98.00, 97.80, 98.81, 97.90, 98.00, 97.80, 97.60, and 92.10%, the batch numbers were 22427-39-0, 52286-59-6, 52286-58-5, 52286-74-5, 41753-43-9, 34367-04-9, 11021-14-0, 11021-13-9, 68406-26-8, 52705-93-8, all purchased from Le Mei Tian Pharmaceutical Co., Ltd.
Use HLPC to analyze the content of monomeric saponins contained in TGGR. The analysis method refers to the literature (Yao et al., 2016). The specific operation is as follows:
Chromatographic conditions: Agilent EC-C18 chromatographic column (4.6 mm × 150 mm, 2.7 μm); mobile phase acetonitrile (A) −0.1% phosphoric acid aqueous solution (B); column temperature: 40°C; flow rate 1 mL/min; injection volume: 10 μL; detection wavelength: 203 nm; gradient elution: (0–23 min, 18–21%A; 23–35 min, 21–28%A; 35–80 min, 28–32%A).
Preparation of mixed reference solution: precision weighing reference, add methanol to make ginsenoside, Rg1, Re, Rf, Rg2, Rb1, Ro, Rc, Rb2, Rb3, Rd, the mass concentrations were 1.38, 1.46, 1.50, 0.81, 0.69, 1.33, 1.38, 1.36, 1.60, 1.54 mg/mL mixed reference substance stock solution.
Preparation of test solution: precisely weigh the total ginsenoside sample and add 70% methanol, the concentration was 2.95 mg/mL, ultrasonic extraction (500 Hz) 30 min. Make up the weight after letting it cool, and pass through a 0.22 μm filter membrane.
Transcriptome analysis was performed using six samples of both sexes from young control flies (7 days), aged model (40 days), and aged TGGR-treated flies (40 days).
To extract the genes whose expression was reduced with aging and recovered by TGGR administration, we selected the genes for which the expression in the aged model flies less than that in the young control flies, and those for which the expression in the aged TGGR-treated flies was greater than that in the aged models. In addition, to extract the genes whose expression increased with aging and was reduced by TGGR administration, we selected the genes for which the expression in the aged models was greater than that in the young control flies, and those for which the expression in the aged TGGR-treated flies was less than that in the aged models. All the differential genes of the fold changes were not less than 2.0 [| log2(Fold Change)| > 1] and with an adjusted Q-value less than 0.001. The princomp function in R software was used for PCA analysis. KEGG pathway enrichment analysis of differential genes was used to calculate biologically significant pathways using phyper function in R software,1 and the results of KEGG enrichment analysis were displayed in scatter plots drawn by Origin software (Version No. 9.1, OriginLab corporation, Massachusetts, United States). KEGG enrichment was measured by Rich Factor, Q-value and the number of genes enriched in this pathway. All sequence data were submitted to the Sequence Read Archive (accession number: PRJNA806668) and are freely available at the NCBI.2
Flies were starved for 2 h and stored at −80°C. Total RNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA, United States) and then synthesized into cDNA. Rp49 expression was used as the internal control. Gene expression was calculated using the 2–ΔΔCT method (Dong et al., 2019). The average of at least three repeats was used for each biological data point, and the sequences of all the primers are listed in Supplementary Table 1.
Drosophila were harvested and snap-frozen after aging and drug treatment, and tissue was homogenized and normalized for protein content using lysate (Ratliff et al., 2016). Antibodies used were as follows: Anti-phospho-Thr398-S6K (Cell Signaling #9209, United States); anti-S6K (Cell Signaling #9202, United States); anti-phospho-Thr308-Akt (Thermo Fisher Scientific 44-602G, United States); anti-Akt (Cell Signaling #9272, United States); anti-phospho-Thr37/46-4E-BP1 (Cell Signaling #2855, United States);anti-4E-BP1 (Cell Signaling #9452, United States); anti-ERK1/2 (Cell Signaling #4695, United States); anti-phospho-Thr202/Tyr204-ERK1/2 (Cell Signaling #9101, United States); anti-beta Tublin (abcam ab179513, Britain); horseradish peroxidase conjugated anti-mouse IgG (Cell Signaling #7076, United States) or horseradish peroxidase-conjugated anti-rabbit IgG (ProteinTech SA00001-2, United States). Nitrocellulose filter membranes with attached protein bands were imaged using the iBright FL1000 Imaging System (Invitrogen; Thermo Fisher Scientific, Inc., United States). Image J software 1.53a (National Institutes of Health, United States) was used to quantify the gray value of western blot bands and protein expression level was normalized as the gray value of the target protein/the loading control protein.
Data were expressed as the means ± standard error of mean (SEM). Statistical analysis was performed with GraphPad Prism (Version No. 6, GraphPad Software, La Jolla, CA, United States). Statistical significance was established using one-way ANOVA followed by Dunnett’s t-test except survival. Survival among groups were compared and tested for significance with a log-rank test. p ≤ 0.05 was considered to indicate a statistically significant difference.
We first examined whether TGGR can act as an anti-aging compound in two multicellular organisms, namely, C. elegans and Drosophila. TGGR treatment (125 μg/mL for nematodes and 2.5 mg/mL for flies) prolonged the median lifespan of both model organisms. In nematodes, chronic TGGR treatment extended median lifespan by 11.49% and maximum lifespan by 9.47% (Figures 1A,B). In female flies chronic TGGR treatment extended median lifespan by 9.01% and maximum lifespan by 14.05% (Figures 1C,D), while in male flies median lifespan was extended by 10.43% and maximum lifespan by 13.34% (Figures 1E,F). We also showed that TGGR extended the lifespan of flies to a greater degree compared with ginseng polysaccharide and ginseng total protein (Supplementary Figure 1).
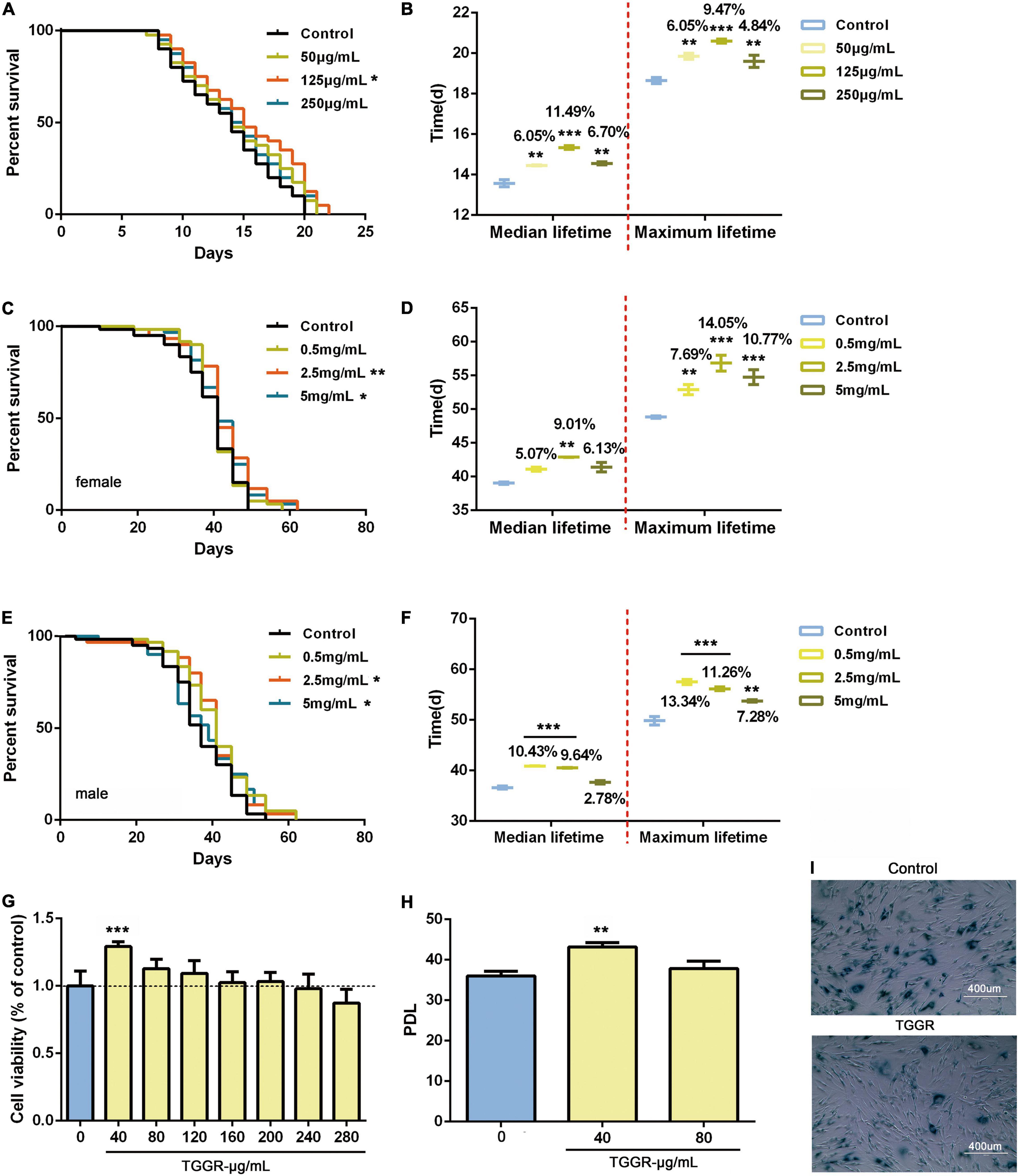
Figure 1. TGGR promotes longevity in C. elegans, Drosophila and human cells. Survival of nematodes (A,B), flies (C–F), and MRC-5 cells (G–I) during aging with supplementation of medium with TGGR. Representative survival curve (A) and median and maximal lifespan (B) in nematodes (N = 80 nematodes, one-way ANOVA). Survival curve and associated pairwise log-rank tests in female (C) and male flies (E). The median and maximal lifespan in female (D) and male flies (F) (N = 120 flies, two-tailed unpaired t-test). (G) CCK-8 assays to detect the effect of TGGR on the viability of MRC-5 cells (N = 6, one-way ANOVA). (H) Effect of TGGR on the population doubling level (PDL) of MRC-5 cells [PDL = 3.32 (logN2-logN1) + X, where “N2” is the number of harvested cells of this generation, “N1” is the number of inoculated cells of the previous generation, and “X” is the PDL of the previous generation of cells] (N = 3, one-way ANOVA). (I) Aging-related galactosidase staining (SA-β-gal). Senescent cells are stained dark blue-green, and have increased cell volume and irregular shapes. *p < 0.05, **p < 0.01, ***p < 0.001 vs. the control.
To confirm and extend our results, we tested the effects of TGGR on human MRC-5 cells at population doubling level (PDL) 7. TGGR (40 μg/mL) had a significant effect on cell proliferation without toxicity in the range 0–240 μg/mL (Figure 1G). TGGR significantly increased the number of passages of MRC-5 cells (Figure 1H). Old cells were defined as PDL 28 (Gao et al., 2019), and aging-related galactosidase staining showed that the proportion of stained cells decreased after TGGR intervention (Figure 1I). These data indicate that TGGR augments the lifespans of evolutionarily diverse species from different phyla.
A lifetime of continuous drug use is not realistic; therefore, we investigated the optimum duration and age for TGGR administration for extension of lifespan in flies. Ten days of TGGR treatment did not extended lifespan (Figures 2A,B), whereas 20 days (Figures 2C,D), 30 days (Figures 2E,F), and 40 days (Figures 2G,H) of TGGR treatment did. Lifespan extension was slightly reduced by TGGR treatment for 40 days compared with that for 20 and 30 days. Therefore, 20 days of continuous TGGR intervention was chosen as the optimum duration.
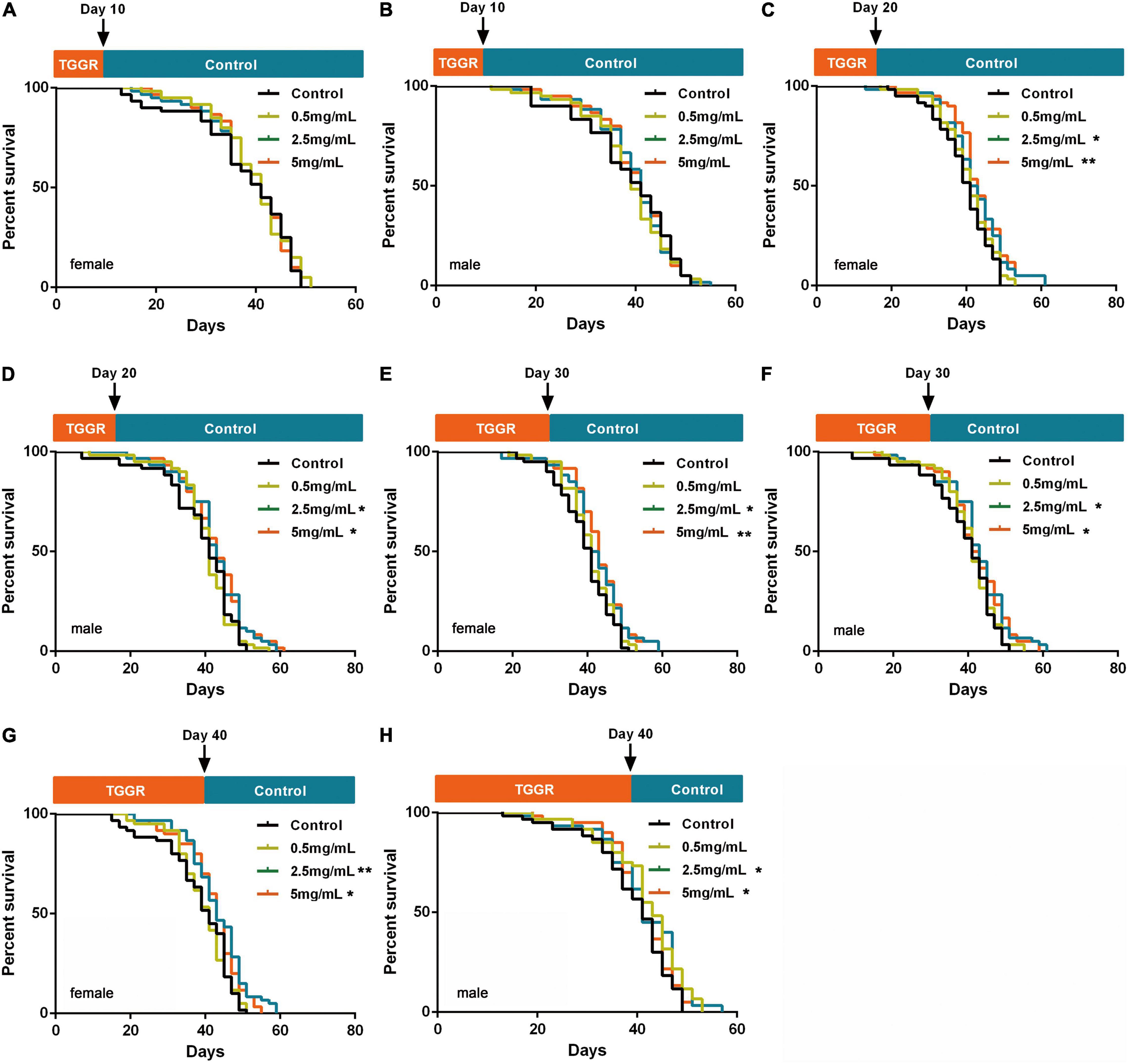
Figure 2. Screening of TGGR treatment duration. Short-term treatment with TGGR for 10 days early in adulthood of female (A) and male (B) flies. Medium-term treatment with TGGR for 20 days in female (C) and male (D) flies. Treatment with TGGR for 30 days in female (E) and male (F) flies. Long-term treatment with TGGR for 40 days in female (G) and male (H) flies. N = 120 flies per condition, Log-rank (Mantel-Cox) test. *p < 0.05, **p < 0.01 vs. the control.
We next continuously exposed flies to TGGR for 20 days at different age stages: early (0–20 days), middle (20–40 days), and late (40–60 days) adulthood. Treatment of early and middle-aged flies with 5 mg/mL TGGR had a significant life-prolonging effect, but low-dose (0.5 mg/mL) TGGR did not. These two administration phases showed similar dose-dependent effects. Early TGGR treatment (5 mg/mL) extended median lifespan by 10.14% in female flies and by 7.64% in male flies (Figures 3A,B). Middle TGGR treatment (5 mg/mL) extended median lifespan by 15.24% in female flies and by 16.59% in male flies (Figures 3C,D). Late TGGR treatment did not extend lifespan (Figures 3E,F) and middle TGGR treatment extended lifespan the most (Figures 3G,H). Based on these results, there appears to be a duration and age specific window in which TGGR can exert its beneficial effects.
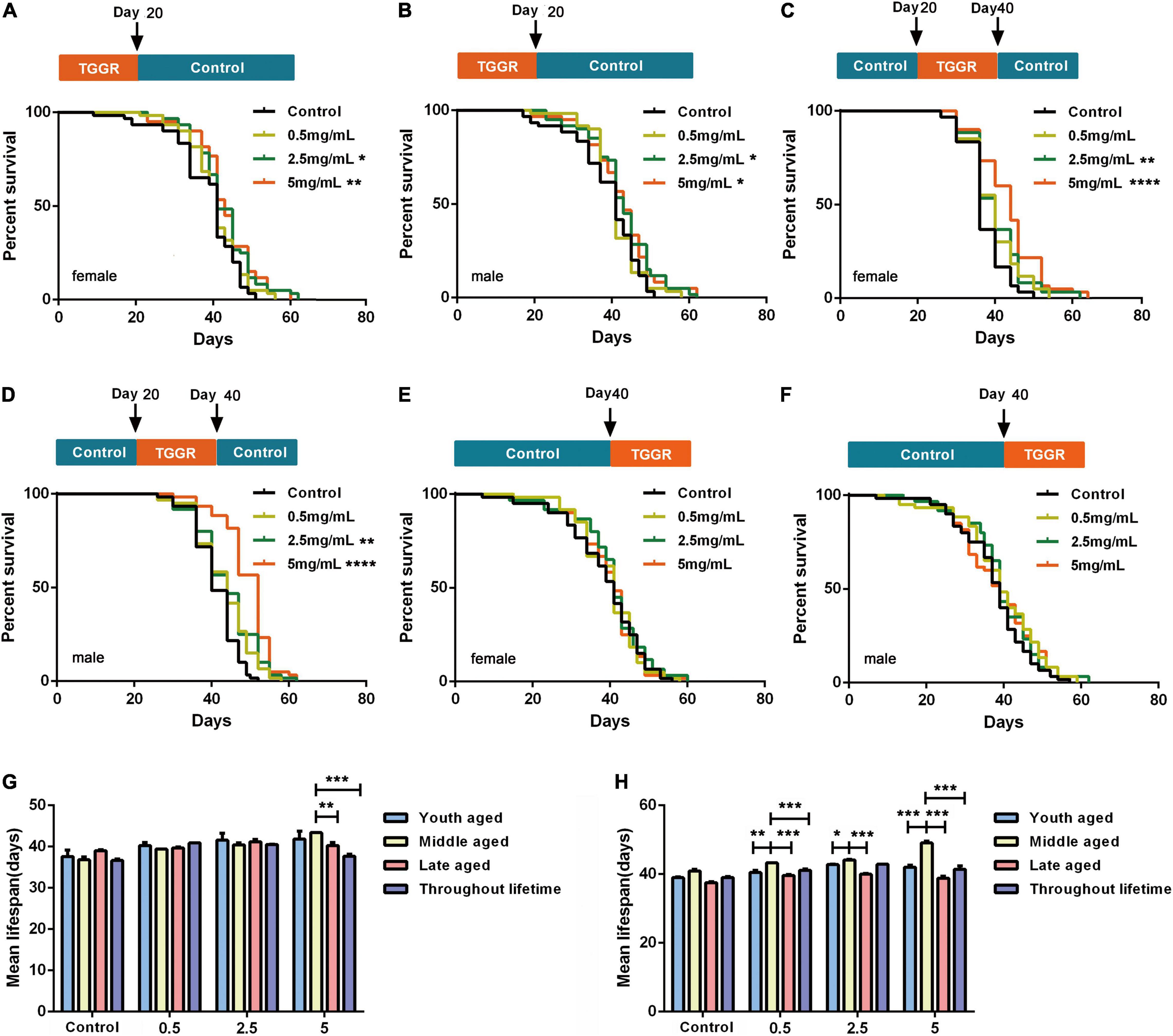
Figure 3. TGGR effect on Drosophila longevity. Brief treatment with TGGR for 20 days early in adulthood extended the lifespan of female (A) and male (B) flies; TGGR extended the lifespan of 20-day-old female (C) and male (D) flies at concentrations from 0.5 to 5 mg/mL (at 40 days, medium was changed to sugar-yeast-agar); Treatment with TGGR for 20 days in late-aged flies (TGGR medium at 40–60 days) did not significantly extend the lifespan of female (E) or male (F) flies [N = 120 flies per condition, Log-rank (Mantel-Cox) test]. Comparative analysis of the median lifespan of the four methods of administration in female (G) and male flies (H); between 0 and 20-day-old (young-aged) flies, between 20 and 40-day-old (middle-aged) flies, and between 40 to 60 days (late-aged) flies (N = 120 flies per condition, two-way ANOVA). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. the control.
To investigate whether TGGR-mediated lifespan extension was related to enhanced healthspan, we examined various healthspan parameters in aged flies (40 day-old) following 20 days of TGGR treatment (continuously exposed from day 20 to 40). TGGR had no effect on body weight (Figure 4A). Reduced food intake can prolong longevity (Song et al., 2020); therefore, we tested whether TGGR treatment affects feeding behavior. We observed no significant change in this behavior with TGGR treatment (Figure 4B). Several interventions that promote longevity are frequently related to reproductive tradeoffs (He et al., 2021). Nevertheless, TGGR increased fecundity in aged male flies (Figure 4C). TGGR treatment produced a significant improvement in circadian rhythm (Figure 4D). Importantly, increased physical activity in the day-time eliminated restlessness at night time. Monitoring of circadian rhythm behavior found that TGGR regulated the biological rhythm of flies. The expression of cyc in female flies did not change, but per, tim, clk, and cry all showed an upward trend in expression in aging flies. TGGR ameliorated disrupted biological rhythm caused by aging to varying degrees (Figures 4E,F). Moreover, it delayed climbing decline at two concentrations that extend lifespan (Figure 4G).
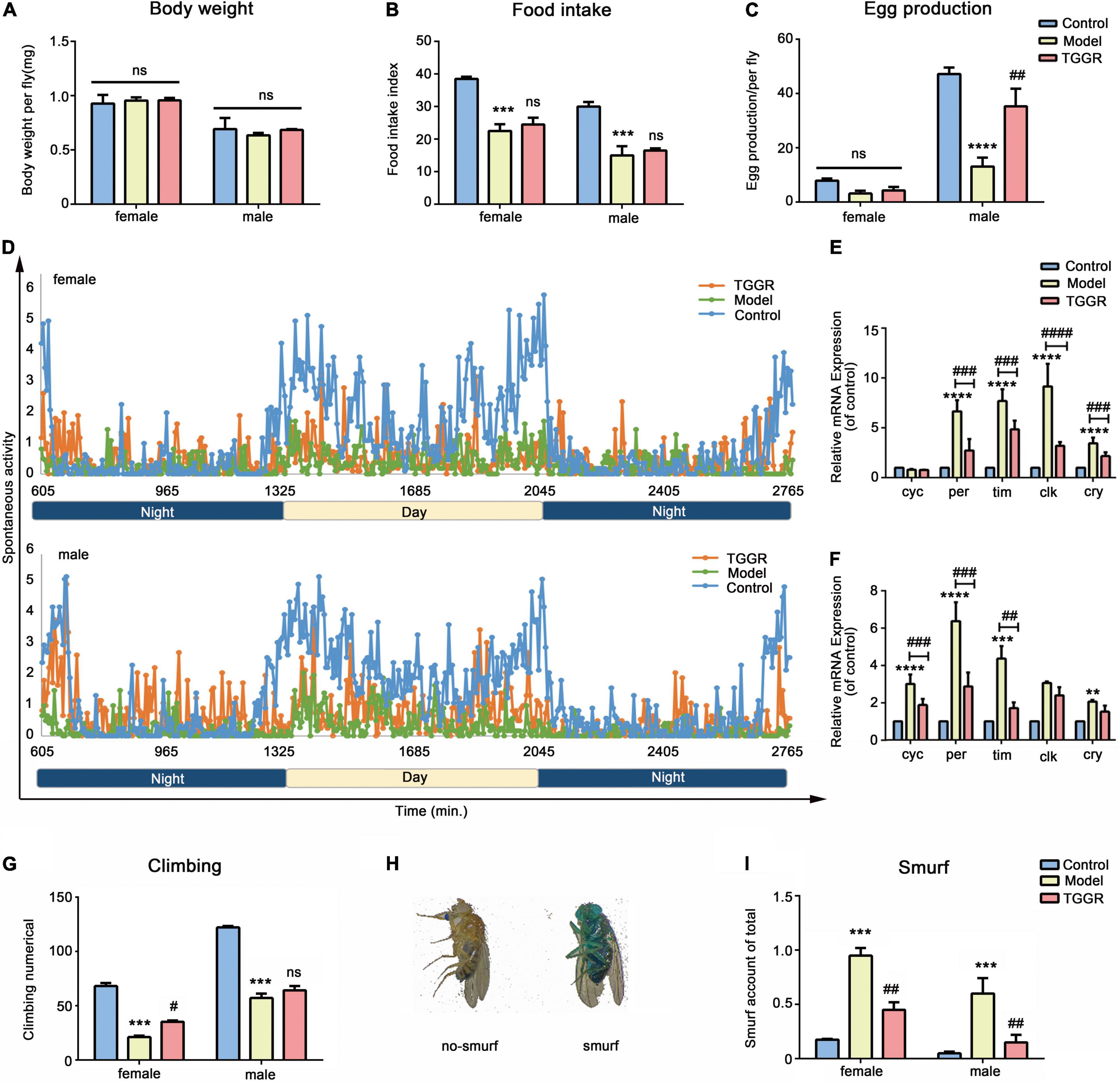
Figure 4. TGGR treatment prolongs healthspan. Body weight (A, N = 90 flies), feeding assay (B, N = 60 flies), fecundity (C, N = 60 flies), climbing numbers (G, N = 90 flies), intestinal integrity (I, N = 90 flies) with or without TGGR treatment. (D) Circadian rhythm of 40-day-old flies with or without TGGR treatment (N = 16 vials of one fly per condition). mRNA levels of biorhythm genes were analyzed in flies with or without TGGR treatment for 40 days (E: female; F: male). (N = 90 flies per condition, two-way ANOVA). (H) Representative images of ‘Smurf’ and ‘no Smurf’ in Drosophila “Control” was 7-day-old flies, “Model” was 40-day-old flies, and “TGGR” was 40-day-old flies with TGGR (5 mg/mL) treatment. ***p < 0.001, ****p < 0.0001 vs. the control; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 vs. the model; ns means p > 0.05.
Organ dysfunction is driven by aging, for example, intestinal barrier function, an evolutionary conserved biomarker (Rodriguez-Fernandez et al., 2019). To determine whether TGGR contributes to the restoration of gut function, we considered the presence of unabsorbed dye outside the Drosophila digestive tract as an indicator of loss of gut barrier function. There was significantly fewer “Smurf” (whole body dyed blue) in TGGR-treated (5 mg/mL) flies than in untreated flies, confirming enhanced barrier integrity in TGGR-treated flies (Figures 4H,I). In addition, TGGR can prolong the life span of flies that were acutely injured by sodium dodecyl sulfate (SDS) (Supplementary Figure 2). Collectively, these data demonstrate that TGGR treatment improves healthspan and modulates tissue homeostasis associated with aging.
The ability to withstand extrinsic stress is a marker of organismal health that declines with age. The effect of TGGR on various stresses, including, starvation, oxidative and heat shock stress, was analyzed in flies.
Resistance to starvation stress was significantly increased by TGGR (Figures 5A,B, female, 10.84% increase; male, 15.60% increase). The storage of fat correlates with survival under starvation conditions; therefore, we examined the quantity of the lipid-storage molecule, triglyceride (TAG), in Drosophila. Aging reduced TAG levels compared with young flies (Figure 5C, female, 28.86% decrease; male, 27.90% decrease); however, no decrease in TAG levels was found with TGGR treatment in females or males. Under H2O2 oxidative stress conditions, TGGR enhanced survival in both sexes (Figure 5D, female, 21.94% increase; Figure 5G, male, 18.57% increase). Next, we examined anti-oxidant enzyme activities and related gene expression levels to confirm the anti-oxidant benefits of TGGR. The enzyme activities of catalase (Cat) (Figure 5E) and superoxide dismutase (SOD) (Figure 5F) were elevated after TGGR treatment (except for SOD activity in male flies). Likewise, the mRNA levels of Cat, Mn-SOD (Sod2), kelch-like ECH-associated protein 1 (keap1) and gstd1 were not significantly increased by TGGR treatment (Figure 5H, except for gstd1 in male flies). No effect of TGGR on heat stress resistance (heat-shock) in females or males was observed (Figures 5I,J).
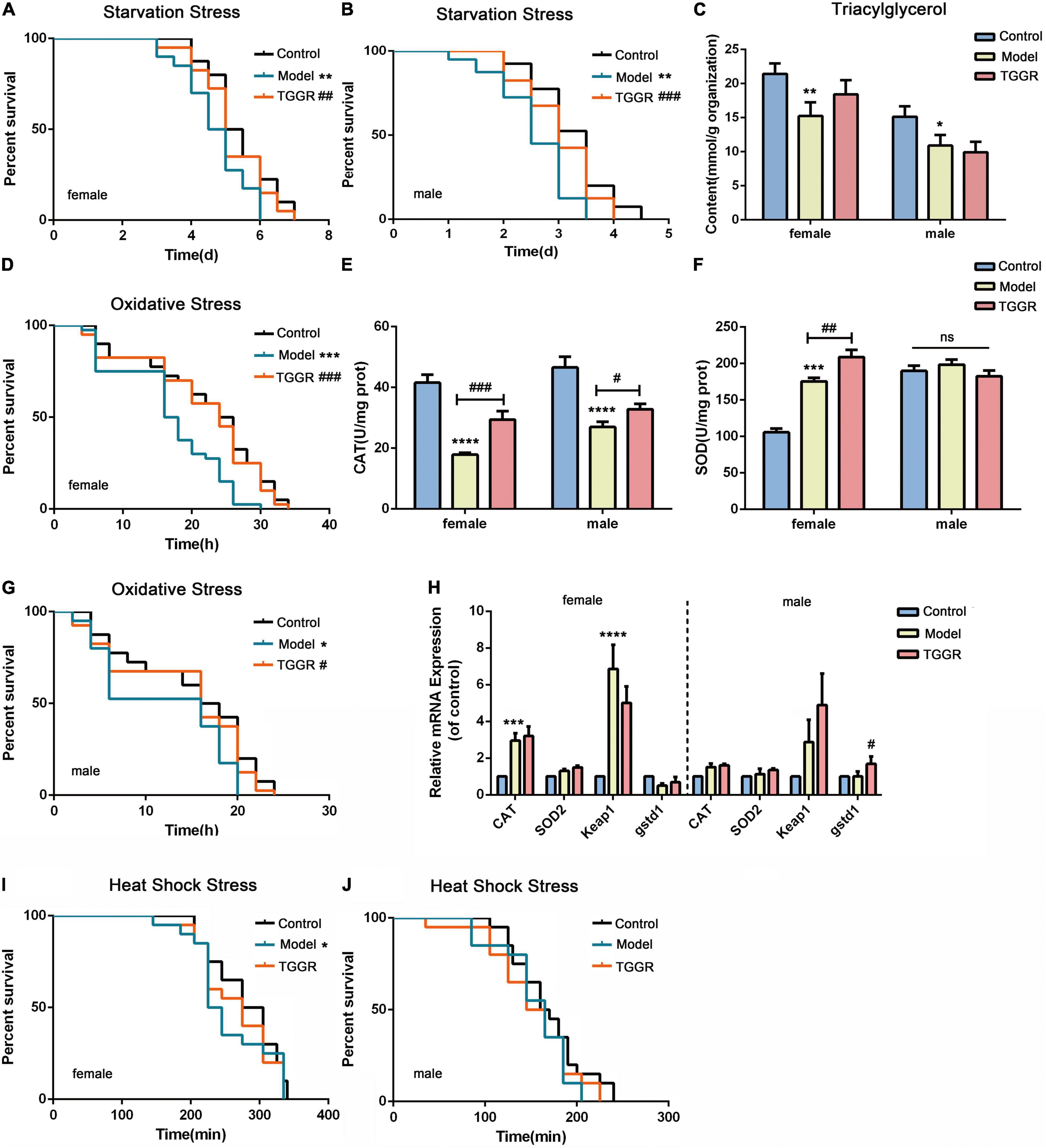
Figure 5. Effect of TGGR on stress resistance in Drosophila. Survival of flies under starvation stress (A, female; B, male), oxidative stress (D, female; G, male), and heat-shock stress (I, female; J, male) with or without TGGR treatment (N = 30 flies in per condition, two-tailed unpaired t-test). TAG levels in flies with or without TGGR treatment (C); Enzyme activity of Cat (E) or SOD (F) with or without TGGR treatment; Analysis of anti-oxidant gene expression in flies by qRT-PCR with or without TGGR treatment (H: the left side of the dotted line indicates females and the right side, males) (N = 60 flies, two-tailed unpaired t-test). “Control” was 7-day-old flies, “Model” was 40-day-old flies, and “TGGR” was 40-day-old flies with TGGR (5 mg/mL) treatment. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. the control; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. the model.
To determine how TGGR extends lifespan and healthspan, we used RNA sequencing (RNA-Seq) to compare transcription in whole flies at 7 days old (control), 40 days old (model), and 40 days old with TGGR treatment for 20 days (TGGR). Principal component analysis (PCA) demonstrated close agreement between replicate samples as well as the expression differences that could largely be attributed to aging or TGGR treatment (X-axis, 51.1%) (Supplementary Figure 3A). Interestingly, compared with the untreated flies, the transcriptome in the TGGR-treated flies showed a significant trend toward a younger pattern. The average reads per kilobase of transcript per million reads mapped (RPKM) values of expressed transcripts of each treatment group were checked by hierarchical cluster analysis, which further emphasized the similarity between duplicate samples and the influence of aging and TGGR treatment on global transcriptome trends (Supplementary Figure 3B).
Genes with a more than a 2.0-fold change in expression (with statistical significance) in response to aging or TGGR treatment are presented in Venn diagrams (Figures 6C–F) and shown in a table (Supplementary Figure 3C). In total, for aging, 705 genes (658 induced; 47 repressed) were altered in males and 1,334 genes (992 induced; 342 repressed) were altered in females. Following TGGR exposure, 29 repressed genes in females and 23 in males were up-regulated, while 162 induced genes in females and 232 in males were down-regulated. The differential expression of these genes is represented in a heatmap (Figures 6A,B,G,H). Genes expressed differentially in response to TGGR also showed a significant shift toward more youthful expression patterns.
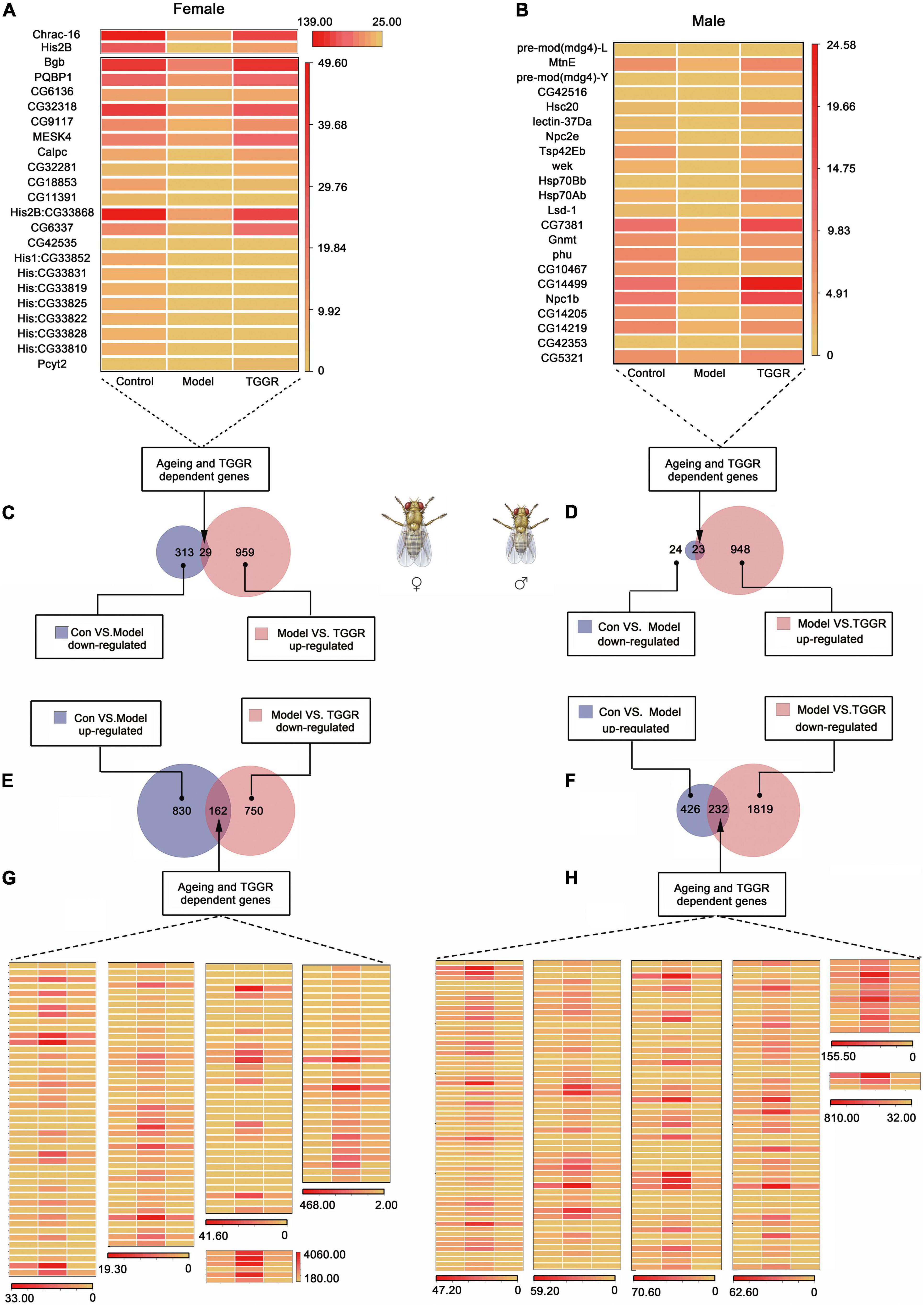
Figure 6. Analysis of differential gene expression. (A,B) and (G,H) Genes with aging- or TGGR-induced fold changes in expression are represented by heatmaps. (C–F) Venn diagrams of aging and TGGR regulation of differentially expressed genes (female, A,C,E,G; male, B,D,F,H). “Control” was 7-day-old flies, “Model” was 40-day-old flies, and “TGGR” was 40-day-old flies with TGGR (5 mg/mL) treatment.
Functional characteristics of differentially expressed genes were discovered using KEGG pathway enrichment analyses. Among the molecular pathways stimulated by TGGR, many are associated with longevity, including those of AKT, MAPK, TOR, FOXO, and insulin signaling, cellular senescence, apoptosis, oxidative phosphorylation, regulation of circadian rhythms, synaptic plasticity, and circadian entrainment (Figure 7).
To validate the results of the RNA-Seq analysis, we used qRT-PCR to analyze the expression of the most representative genes, including protein kinase B (Akt), phosphatidylinositol 3 kinase (PI3K), target of complex (TORC), 4E binding protein (4E-BP), and protein s6 kinase (S6K) (Supplementary Figure 4). Changes in these genes were similar to those seen by RNA-Seq analysis.
The KEGG pathway enrichment analyses prompted us to examine the relationship between TGGR and longevity-regulating signaling pathways, especially insulin, AKT, MAPK, and TOR signaling pathways. To assess the link between the effect of TGGR on longevity and insulin, phosphorylation of the signaling intermediate, Akt, was first examined. Compared with the aging group, TGGR treatment decreased levels of p-Akt to those of the control (Figure 8A). We next tested whether TGGR-mediated lifespan extension was affected by this reduction in insulin and Akt knockdown flies. We found a marked increase in starvation resistance (Figures 8B,C; except in male starvation-resistant flies) and lifespan (Figures 8D,E). TGGR treatment was ineffective in Akt knockdown flies.
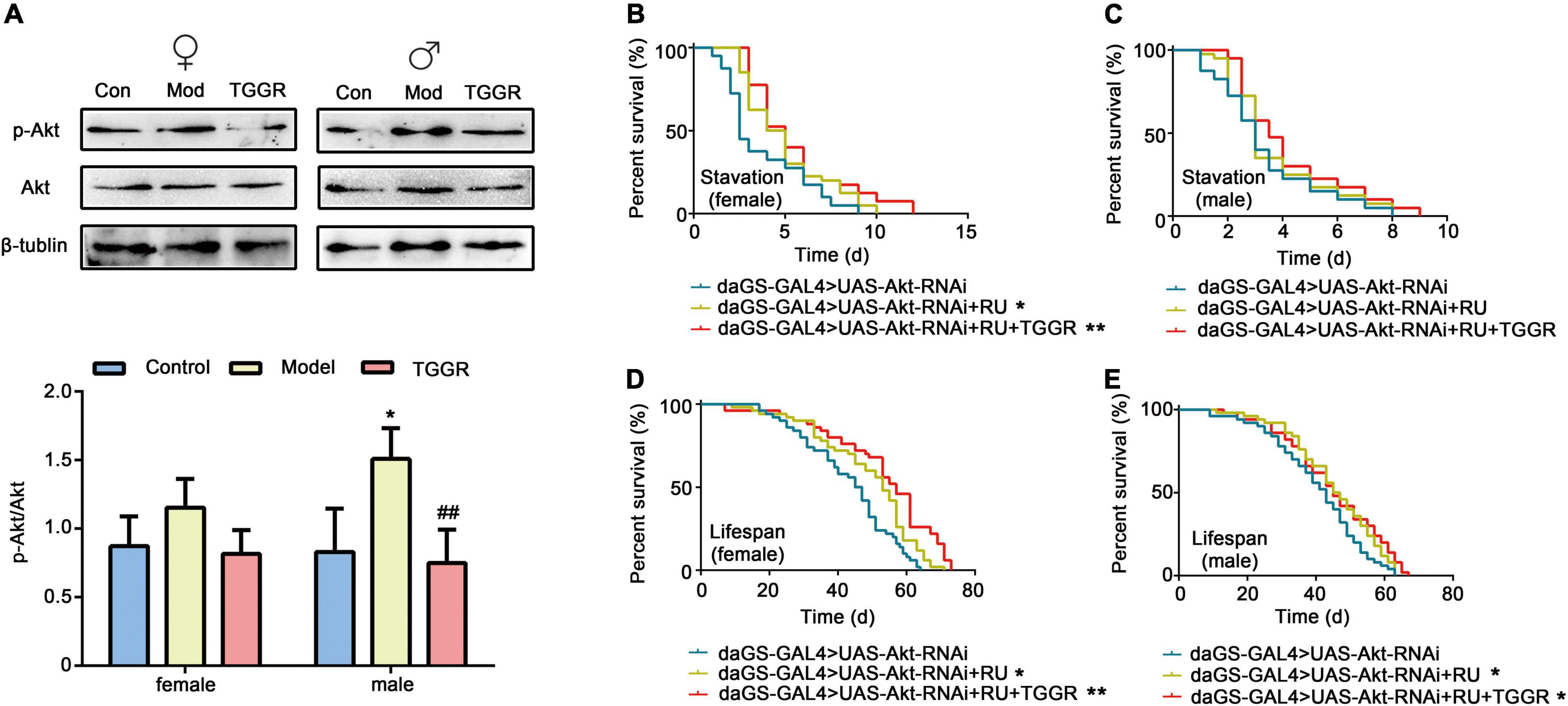
Figure 8. TGGR-mediated longevity is influenced by Akt, a component of the insulin pathway. (A) TGGR decreased the level of p-Akt (N = 50 flies per condition, two-tailed unpaired t-test). (B,C) Effects of Akt knockdown on starvation resistance effects in flies; (D,E) changes in lifespan of Akt knockdown flies [N = 60 flies per condition, Log-rank (Mantel-Cox) test]. “Control” was 7-day-old flies, “Model” was 40-day-old flies, and “TGGR” was 40-day-old flies with TGGR (5 mg/mL) treatment. *p < 0.05, **p < 0.01 vs. the control; ##p < 0.01 vs. the model.
Akt is a key effector of insulin and TOR signal transduction. Therefore, we next examined the effect of TGGR on the TOR pathway. Phosphorylation of ribosomal S6K, which lies downstream of TOR, was decreased in TGGR-treated flies (Figure 9A). To explore the target of TOR affected by TGGR treatment, we down-regulated S6K using RNAi, and starvation resistance of flies was not affected by TGGR treatment (Figures 9B,C). Otherwise, knockdown of S6K increased fly lifespan in non-starvation conditions and TGGR failed to extend it further (Figures 9D,E).
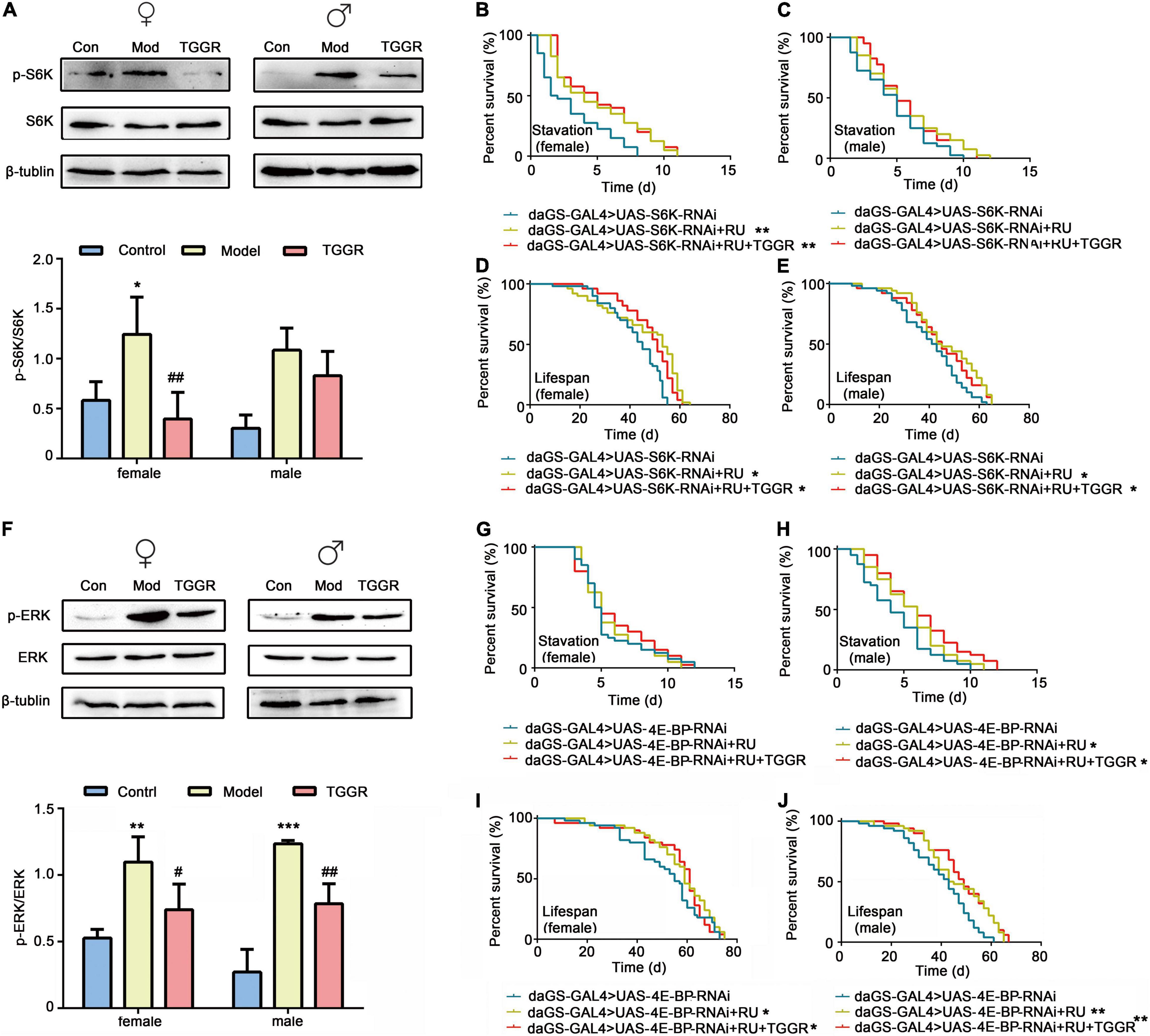
Figure 9. TGGR regulates TOR and MAPK pathways by affecting the major components, S6K, 4E-BP and ERK. (A) Phosphorylation levels of S6K were analyzed by western blotting (N = 50 flies per condition, two-tailed unpaired t-test). (B,C) Starvation resistance in S6K-knockdown flies. (D,E) Lifespan changes in S6K knockdown flies [N = 50 flies per condition, Log-rank (Mantel-Cox) test]. (F) Western blot analysis of ERK phosphorylation levels (N = 50 flies per condition, two-tailed unpaired t-test). (G,H) 4E-BP downregulates starvation resistance in flies. (I,J) Lifespan changes in 4E-BP knockdown flies [N = 50 flies per condition, Log-rank (Mantel-Cox) test]. “Control” was 7-day-old flies, “Model” was 40-day-old flies, and “TGGR” was 40-day-old flies with TGGR (5 mg/mL) treatment. *p < 0.05, **p < 0.01, ***p < 0.001 vs. the control; #p < 0.05, ##p < 0.01 vs. the model.
RNAi of 4E-BP in female flies had no significant effect on starvation resistance, but prevented starvation resistance exerted by TGGR treatment (Figures 9G,H). Similarly, TGGR did not prolong the lifespan of 4E-BP knockdown flies (Figures 9I,J). Taken together, normal output of the TOR pathway is required for TGGR to combat starvation tolerance and longevity.
ERK1/ERK2 is part of the MAPK pathway involved in the stress response and is known to regulate TOR signaling through phosphorylation in mammalian models. We therefore examined phosphorylation levels of ERK1/ERK2. TGGR did not significantly alter the total protein level of ERK but reduced the pERK/ERK ratio relative to controls (Figure 9F). These findings indicate that TGGR reduces MAPK signaling, which may in turn reduce TOR signaling.
To identify the main ginsenosides in the TGGR used, high performance liquid chromatography (HPLC) was applied (Supplementary Figure 5). We quantified 10 ginsenosides (Rg1, Re, Rf, Rg2, Rb1, Ro, Rc, Rb2, Rb3, Rd), of which Rg1, Re, Rb1, Ro, Rb2, and Rd were the most abundant (Supplementary Table 2). The main components of TGGR that affect longevity in Drosophila were explored by assessing the effects of different ginsenoside monomers on lifespan. Re and Rg1 had significant longevity effects second only to TGGR during the optimal intervention window (female: Figures 10A,B; male: Figures 10C,D). We speculate that the main active ingredients in aging delay were Rg1 and Re.
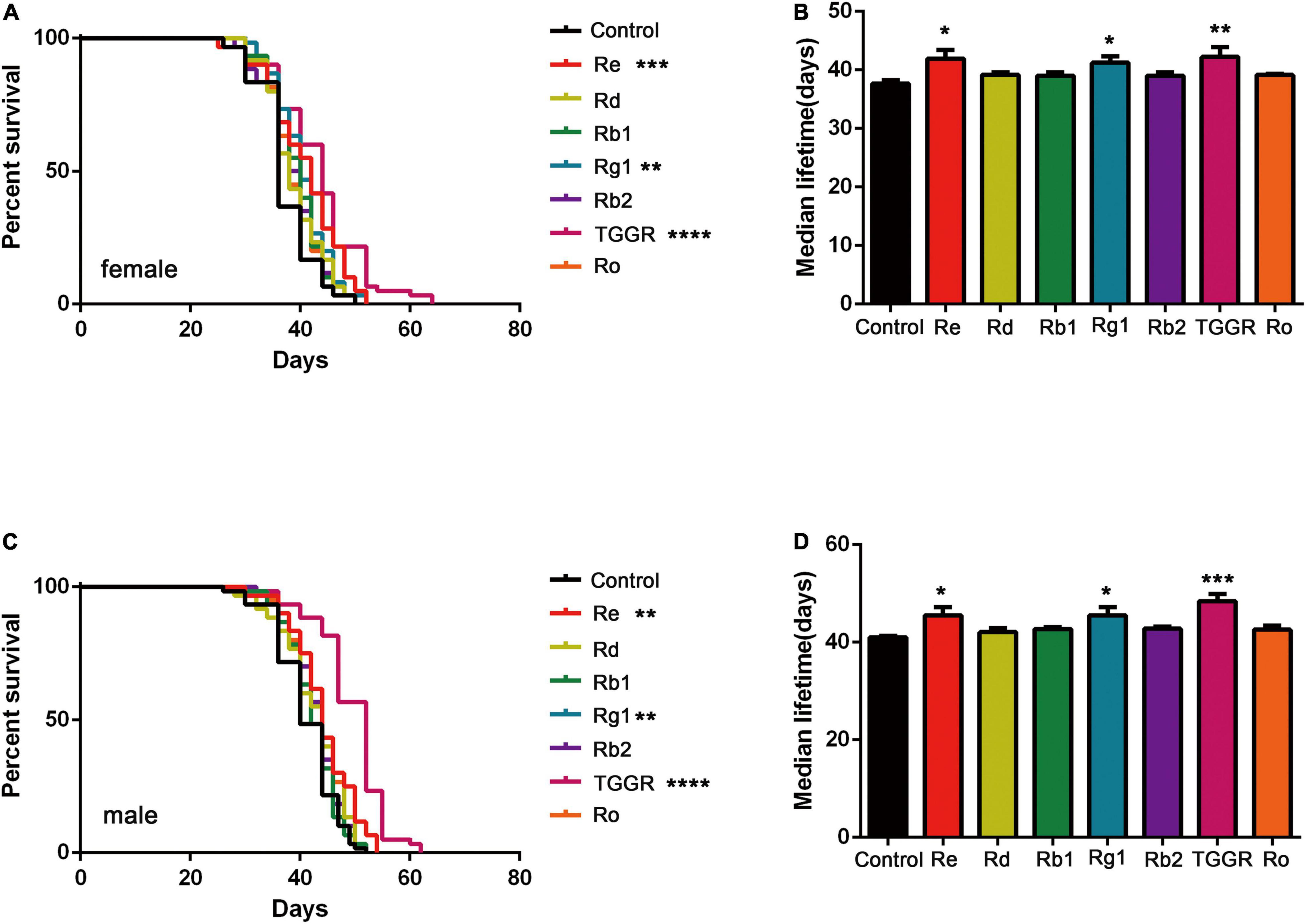
Figure 10. The effect of TGGR monomer components on longevity in Drosophila. Survival of female (A) or male (C) flies treated with TGGR or different ginsenosides [N = 60 flies per condition, Log-rank (Mantel-Cox) test]; The median lifespan of female (B) or male (D) flies treated with different ginsenosides (N = 60 flies per condition, One-way ANOVA). The flies were treated with the following drug concentrations: Re (300 μg/mL), Rd (500 μg/mL), Rb1 (300 μg/mL), Rg1 (500 μg/mL), Rb2 (300 μg/mL), TGGR (5 mg/mL), Ro (300 μg/mL). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. the control.
Aging is the most important risk factor for all major chronic ailments, including cancer, cardiovascular and neurodegenerative diseases (Ma et al., 2022). However, behavioral and pharmacological interventions that promote health upon aging remain rare. Here we report anti-aging properties of natural TGGR from ginseng. TGGR administration extends the lifespan of Drosophila and C. elegans, and reduces the senescence of human cell cultures. The present data together with previous data from C. elegans shows that TGGR has the potential to promote longevity across species. Interestingly, TGGR did not improve lifespan by reducing reproductive capacity; in fact TGGR increased the fertility of male flies. TGGR augmented health span readouts associated with youth and healthy aging, such as motility, intestinal barrier integrity, and biorhythm homeostasis. TGGR treatment also improved resistance to certain kinds of stress in both sexes, such as tolerance to starvation and oxidation. Furthermore, TGGR shifted gene expression in aged flies toward “healthy” patterns seen in young flies. Importantly, in no assay did we find any detrimental side-effects. Thus, TGGR can increase both lifespan and healthspan in Drosophila.
A particularly interesting feature of the effect of TGGR on longevity is the relatively small therapeutic window for long-term benefits. TGGR administration during early adulthood, and especially during midlife, was sufficient to extend lifespan in both male and female flies, indicating a “memory” effect. If TGGR reduces the risk of acute and reversible mortality at a given age, early life treatment with TGGR is expected to have effects similar to dietary restriction (Kebbe et al., 2021). Conversely, early life TGGR treatment slowed the underlying aging process, and flies had progressively longer life with TGGR treatment. Importantly, this effect was maintained when treatment was initiated in midlife, indicating meaningful therapeutic properties, a phenomenon that should be subject to further investigation in mammals.
The increased resistance to stress is also potentially a direct consequence of TGGR administration. Intriguingly, male flies show the same resistance to starvation stress compared with females but have lower TAG levels, indicating a mechanism other than increased fat storage. In addition, female flies showed better resistance to acute oxidative stress than male flies after TGGR intervention. TGGR can significantly increase the activity of both CAT and SOD in female flies but only CAT in male flies. Also, TGGR only increased Cat mRNA expression in female flies. The Keap1-Nrf2 signal transduction pathway plays a highly conserved role in regulating oxidative stress (Yao et al., 2022). Under normal physiological conditions, Nrf2 binds to the cytoplasmic inhibitor, Keap1, and exists in the cytoplasm, maintaining low transcriptional activity (Luo et al., 2021). However, under oxidative stress, Nrf2 translocates to the nucleus, binds to Marf proteins to form a heterodimer, and then binds to anti-oxidant response elements (AREs) to activate the expression of anti-oxidation genes (Matsumaru and Motohashi, 2021). Oxidative stress increased the mRNA level of the oxidation-promoting factor, keap1, which was inhibited by TGGR intervention. This inhibition was also more pronounced in female flies. These results indicated that there may be gender differences in anti-oxidant capacity induced by TGGR. However, there was no significant difference in the effects of TGGR on the healthy lifespan of both sexes, indicating that there must be other regulatory pathways in addition to oxidative stress involved in the response.
Restoring disrupted gene expression and the normal activities of signaling pathways through drug intervention is the most promising way to improve healthy aging (Roichman et al., 2021). The transcription profile of TGGR-treated flies revealed that age- and TGGR-dependent expression in flies involved many processes already known to be associated with aging, mainly involving the insulin, TOR and MAPK pathways. In yeast, C. elegans, Drosophila, and mice, these three pathways regulate lifespan (Tang et al., 2019). In the present study, pharmacological and genetic epistatic analysis in Drosophila demonstrated that the effect of TGGR on aging required normal expression of genes involved in insulin, TOR and MAPK signaling. The phosphorylation of AKT in insulin-like signaling, S6 kinase (S6K) in TOR signaling, and ERK1/2 in MAP kinase (MAPK) signaling was reduced by TGGR treatment relative to controls. These findings were corroborated by knockdown of Akt and S6K, which increased fly lifespan in both starvation and non-starvation conditions; increases that TGGR was not able to extend. It should be mentioned that no significant changes were observed in the protein expression levels of Akt and S6K compared to the mRNA levels. It is speculated that the possible reasons are the difference between sensitivity of the two assays (qRT-PCR and WB) (Sroka et al., 2016), the decrease of mRNA stability due to aging and the possible indirect regulatory effect of TGGR at the transcriptional level. Knockdown of 4E-BP blocked TGGR treatment-induced starvation resistance and also did not alter the lifespan of 4E-BP knockdown flies. The positive effects of ginseng in coping with aging-related diseases have been reported to be mediated by insulin (Hahn et al., 2010). For example, total saponin from Korean red ginseng has life-extending effects, in addition to reducing thrombin-induced phosphorylation of PI3K and Akt (Hou et al., 2020). Finally, to elucidate the components of TGGR that might be responsible for the pro-longevity effects, we used HPLC to identify and quantify the ginsenoside compounds in TGGR. The ginsenosides Rb1, Rg1, Rd, Rb2, Ro, and Re were abundant in TGGR. Rg1 and Re exhibited similar anti-aging effects as TGGR in Drosophila. Consistent with other studies, Rg1 and Re are the major components of Panax ginseng, which prolongs the lifespan of C. elegans (Wang et al., 2021), and Rg1 prevents cognitive impairment and hippocampal senescence in a rat model of aging (Zhou et al., 2020). In future studies, specific anti-aging effects of single saponins should be clarified, and synergistic studies of each saponin based on clear molecular mechanisms should be examined.
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
QZ, JQ, and ML: conceptualization. KL and ZJ: methodology. YL and SZ: formal analysis. YZ and CW: data curation. QZ: writing– original draft preparation. JQ and ML: writing – review and editing. ML: project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript.
This work was performed with the financial support of the Science and Technology Project of Jilin Provincial Education Department (JJKH20210966KJ), the Natural Science Foundation of China (U19A2013), the National College Students Innovation and Entrepreneurship Training Program (202110199032X, S202110199112X).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Jeremy Allen, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing a draft of this manuscript. We would also like to thank Yufeng Yang for providing Drosophila for this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.870326/full#supplementary-material
Admasu, T. D., Chaithanya Batchu, K., Barardo, D., Ng, L. F., Lam, V. Y. M., Xiao, L., et al. (2018). Drug synergy slows aging and improves healthspan through IGF and SREBP lipid signaling. Dev. Cell. 47, 67.e5–79.e5. doi: 10.1016/j.devcel.2018.09.001
Dong, Y., Sun, H., Yang, W., Ma, S., Du, B., and Xu, H. (2019). The effect of inulin on lifespan, related gene expression and gut microbiota in InR(p5545)/TM3 mutant Drosophila melanogaster: a preliminary study. Nutrients 11:636. doi: 10.3390/nu11030636
Gao, J., Lai, C., Zhang, W., and Yang, X. (2019). A study on alterations in mitochondrial biological characteristics during cellular senescence of human embryonic lung fibroblasts. Zhonghua Yu Fang Yi Xue Za Zhi 53, 309–315. doi: 10.3760/cma.j.issn.0253-9624.2019.03.014
Hahn, K., Miranda, M., Francis, V. A., Vendrell, J., Zorzano, A., and Teleman, A. A. (2010). PP2A regulatory subunit PP2A-B’ counteracts S6K phosphorylation. Cell Metab. 11, 438–444. doi: 10.1016/j.cmet.2010.03.015
He, J., Li, X., Yang, S., Li, Y., Lin, X., Xiu, M., et al. (2021). Gastrodin extends the lifespan and protects against neurodegeneration in the Drosophila PINK1 model of Parkinson’s disease. Food Funct. 12, 7816–7824. doi: 10.1039/d1fo00847a
Hou, W., Pei, J., Wang, Y., Zhang, J., Zheng, H., and Cui, R. (2020). Anti-ageing effects of red ginseng on female Drosophila melanogaster. J. Cell. Mol. Med. 24, 3751–3755. doi: 10.1111/jcmm.15029
Hyun, S. H., Bhilare, K. D., In, G., Park, C. K., and Kim, J. H. (2022). Effects of Panax ginseng and ginsenosides on oxidative stress and cardiovascular diseases: pharmacological and therapeutic roles. J. Ginseng. Res. 46, 33–38. doi: 10.1016/j.jgr.2021.07.007
Kebbe, M., Sparks, J. R., Flanagan, E. W., and Redman, L. M. (2021). Beyond weight loss: current perspectives on the impact of calorie restriction on healthspan and lifespan. Expert Rev. Endocrinol. Metab. 16, 95–108. doi: 10.1080/17446651.2021.1922077
Luo, L. P., Suo, P., Ren, L. L., Liu, H. J., Zhang, Y., and Zhao, Y. Y. (2021). Shenkang injection and its three anthraquinones ameliorates renal fibrosis by simultaneous targeting IƙB/NF-ƙB and Keap1/Nrf2 signaling pathways. Front. Pharmacol. 12:800522. doi: 10.3389/fphar.2021.800522
Ma, R., Zhao, L., Zhao, Y., and Li, Y. (2022). Puerarin action on stem cell proliferation, differentiation and apoptosis: therapeutic implications for geriatric diseases. Phytomedicine 96:153915. doi: 10.1016/j.phymed.2021.153915
Matsumaru, D., and Motohashi, H. (2021). The KEAP1-NRF2 system in healthy aging and longevity. Antioxidants 10:1929. doi: 10.3390/antiox10121929
Niraula, P., Ghimire, S., Lee, H., and Kim, M. S. (2018). Ilex paraguariensis extends lifespan and increases an ability to resist environmental stresses in Drosophila. Rejuvenation Res. 21, 497–505. doi: 10.1089/rej.2017.2023
Ratliff, E., Kotzebue, R., Molina, B., Mauntz, R., Gonzalez, A., Barekat, A., et al. (2016). Assessing basal and acute autophagic responses in the adult DrosophiIla nervous system: the impact of gender, genetics and diet on endogenous pathway profiles. PLoS One 11:e0164239. doi: 10.1371/journal.pone.0164239
Rodriguez-Fernandez, I. A., Qi, Y., and Jasper, H. (2019). Loss of a proteostatic checkpoint in intestinal stem cells contributes to age-related epithelial dysfunction. Nat. Commun. 10:1050. doi: 10.1038/s41467-019-08982-9
Roichman, A., Elhanati, S., Aon, M. A., Abramovich, I., Di Francesco, A., Shahar, Y., et al. (2021). Restoration of energy homeostasis by SIRT6 extends healthy lifespan. Nat. Commun. 12:3208. doi: 10.1038/s41467-021-23545-7
Seals, D. R., Justice, J. N., and LaRocca, T. J. (2016). Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J. Physiol. 594, 2001–2024. doi: 10.1113/jphysiol.2014.282665
Song, L., Zhou, Y., Ni, S., Wang, X., Yuan, J., Zhang, Y., et al. (2020). Dietary intake of β-glucans can prolong lifespan and exert an antioxidant action on aged fish Nothobranchius guentheri. Rejuvenation Res. 23, 293–301. doi: 10.1089/rej.2019.2223
Sroka, A., Ufir, K., Salamon, D., Bulanda, M., and Gosiewski, T. (2016). Nested-PCR real time as alternative molecular tool for detection of Borrelia burgdorferi compared to the classical serological diagnosis of the blood. Med. Dosw. Mikrobiol. 68, 47–56.
Staats, S., Wagner, A. E., Lüersen, K., Künstner, A., Meyer, T., Kahns, A. K., et al. (2019). Dietary ursolic acid improves health span and life span in male Drosophila melanogaster. Biofactors 45, 169–186. doi: 10.1002/biof.1467
Tang, R., Chen, X., Dang, T., Deng, Y., Zou, Z., Liu, Q., et al. (2019). Lycium barbarum polysaccharides extend the mean lifespan of Drosophila melanogaster. Food Funct. 10, 4231–4241. doi: 10.1039/c8fo01751d
Tangrodchanapong, T., Sobhon, P., and Meemon, K. (2020). Frondoside a attenuates amyloid-β proteotoxicity in transgenic caenorhabditis elegans by suppressing its formation. Front. Pharmacol. 11:553579. doi: 10.3389/fphar.2020.553579
Wang, H., Zhang, S., Zhai, L., Sun, L., Zhao, D., Wang, Z., et al. (2021). Ginsenoside extract from ginseng extends lifespan and health span in Caenorhabditis elegans. Food Funct. 12, 6793–6808. doi: 10.1039/d1fo00576f
Wu, L., Liu, X., Hu, R., Chen, Y., Xiao, M., Liu, B., et al. (2022). Prebiotic Agrocybe cylindracea crude polysaccharides combined with Lactobacillus rhamnosus GG postpone aging-related oxidative stress in mice. Food Funct. 13, 1218–1231. doi: 10.1039/d1fo02079j
Yao, H., Li, X., Liu, Y., Wu, Q., and Jin, Y. (2016). An optimized microwave-assisted extraction method for increasing yields of rare ginsenosides from Panax quinquefolius L. J. Ginseng. Res. 40, 415–422. doi: 10.1016/j.jgr.2016.06.007
Yao, H., Zhang, W., Yang, F., Ai, F., Du, D., and Li, Y. (2022). Discovery of caffeoylisocitric acid as a Keap1-dependent Nrf2 activator and its effects in mesangial cells under high glucose. J. Enzyme Inhib. Med. Chem. 37, 178–188. doi: 10.1080/14756366.2021.1998025
Zhang, S., Ma, Y. T., Feng, Y. C., Wang, C. Y., and Zhang, D. J. (2022). Potential effects of mung bean protein and a mung bean protein-polyphenol complex on oxidative stress levels and intestinal microflora in aging mice. Food Funct. 13, 186–197. doi: 10.1039/d1fo03058b
Zhou, Y., Wang, Y. P., He, Y. H., and Ding, J. C. (2020). Ginsenoside Rg1 performs anti-aging functions by suppressing mitochondrial pathway-mediated apoptosis and activating sirtuin 3 (SIRT3)/superoxide dismutase 2 (SOD2) pathway in Sca-1+ HSC/HPC cells of an aging rat model. Med. Sci. Monit. 26:e920666. doi: 10.12659/msm.920666
Keywords: total ginsenosides, aging, healthy lifespan, transcriptome analysis, mechanism
Citation: Zhao Q, Liu Y, Zhang S, Zhao Y, Wang C, Li K, Jin Z, Qiao J and Liu M (2022) Studies on the Regulation and Molecular Mechanism of Panax Ginseng Saponins on Senescence and Related Behaviors of Drosophila melanogaster. Front. Aging Neurosci. 14:870326. doi: 10.3389/fnagi.2022.870326
Received: 06 February 2022; Accepted: 30 May 2022;
Published: 17 June 2022.
Edited by:
Suzana Makpol, National University of Malaysia, MalaysiaReviewed by:
Yongfu Wang, Stowers Institute for Medical Research, United StatesCopyright © 2022 Zhao, Liu, Zhang, Zhao, Wang, Li, Jin, Qiao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juhui Qiao, c3VucWlhbzE1MDUwOUAxNjMuY29t; Meichen Liu, bGl1bWMwMzY3QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.