
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Aging Neurosci., 28 April 2022
Sec. Alzheimer's Disease and Related Dementias
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.866313
This article is part of the Research TopicVascular Inflammation in Aging and NeurodegenerationView all 13 articles
 Phillip A. Bonney1*
Phillip A. Bonney1* Robert G. Briggs1
Robert G. Briggs1 Kevin Wu2
Kevin Wu2 Wooseong Choi2
Wooseong Choi2 Anadjeet Khahera1
Anadjeet Khahera1 Brandon Ojogho3,4
Brandon Ojogho3,4 Xingfeng Shao3
Xingfeng Shao3 Zhen Zhao5
Zhen Zhao5 Matthew Borzage2,6
Matthew Borzage2,6 Danny J. J. Wang3
Danny J. J. Wang3 Charles Liu1,4
Charles Liu1,4 Darrin J. Lee1,4
Darrin J. Lee1,4The pathophysiologic mechanisms underpinning idiopathic normal pressure hydrocephalus (iNPH), a clinically diagnosed dementia-causing disorder, continue to be explored. An increasing body of evidence implicates multiple systems in the pathogenesis of this condition, though a unifying causative etiology remains elusive. Increased knowledge of the aberrations involved has shed light on the iNPH phenotype and has helped to guide prognostication for treatment with cerebrospinal fluid diversion. In this review, we highlight the central role of the cerebrovasculature in pathogenesis, from hydrocephalus formation to cerebral blood flow derangements, blood-brain barrier breakdown, and glymphatic pathway dysfunction. We offer potential avenues for increasing our understanding of how this disease occurs.
Idiopathic normal pressure hydrocephalus (iNPH) is a common dementia-causing neurological disorder seen in the elderly, with 120 new cases per year per 100,000 population greater than 70 years (Iseki et al., 2014; Martín-Láez et al., 2015). iNPH classically presents with the clinical triad of gait disturbance, urinary incontinence, and dementia, with gait disturbance typically presenting first and cognitive manifestations arising later. The hallmark of the disease is an enlarged ventricular system without an increase in intracranial pressure (ICP). Despite progress in characterizing iNPH and its natural history, its pathophysiology has not been clearly defined.
Treatment for iNPH is centered around cerebrospinal fluid (CSF) shunting, which leads to improvement in symptoms, including dementia, in many patients (Toma et al., 2013). This distinguishes iNPH from other causes of dementia which are largely irreversible and hence represents an opportunity to characterize mechanisms contributing to cognitive impairment. Ultimately, clinical responses to shunting are varied, and long-term outcomes indicate that while shunting improves the natural history, the durability of treatment is less than with other etiologies of hydrocephalus (Junkkari et al., 2021). Taken together, these data suggest that ventriculomegaly alone does not account for the natural history of iNPH, challenging the traditional role of CSF dynamics and appealing for a better understanding of iNPH’s underlying pathogenesis.
iNPH has been examined from many view points, ranging from intracranial pressure dynamics, traditional radiographic parameters, advanced neuroimaging modalities, analysis of regional and global cerebral perfusion, and changes at the cellular and molecular levels, including the activity of the glymphatic pathways and the blood-brain barrier. Conclusions to be drawn from this disparate body of work are unsettled. In this manuscript, we review recent evidence related to the pathophysiology of iNPH in the context of current theories, noting areas of interest for future study. In particular, we focus on changes involving the cerebrovasculature, which may be central to pathogenesis.
The diagnosis of iNPH involves a combination of clinical symptoms, radiologic findings, and results of diagnostic evaluations (Table 1; Relkin et al., 2005; Nakajima et al., 2021). The criteria for probable iNPH differ somewhat between American/European and Japanese guidelines and are listed separately in Table 1. While both guidelines are accepted and used in practice, the broader radiographic criteria in the American/European guidelines may lead to a greater proportion of patients classified as probable iNPH (Andersson et al., 2017). Confounding the work-up of iNPH is the lack of specificity in diagnostic criteria and overlapping features shared with other neurodegenerative conditions including Alzheimer’s disease and other movement disorders including Parkinsonism. Gait disturbance is the most common feature and typically is the first symptom to arise (Hebb and Cusimano, 2001).
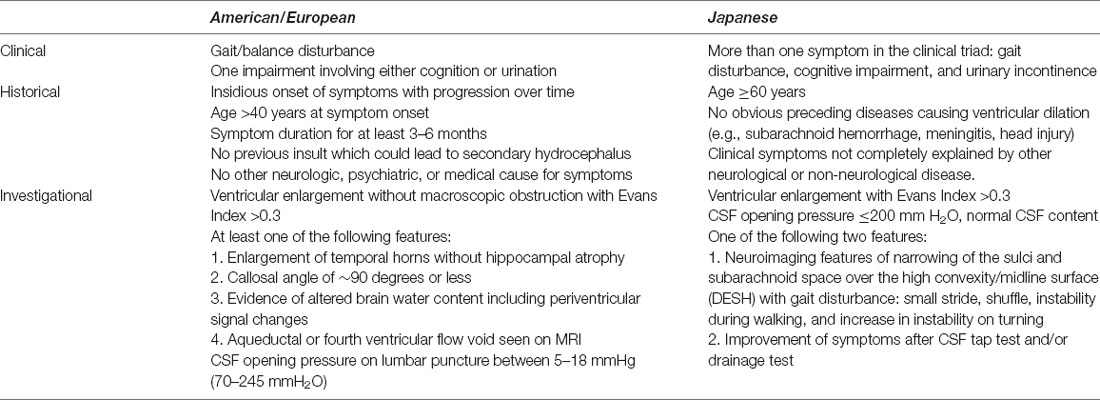
Table 1. American/European (Relkin et al., 2005) and Japanese (Nakajima et al., 2021) criteria for probable idiopathic normal pressure hydrocephalus.
Diagnostic radiographic features include ventricular enlargement with an Evans index of 0.3 or greater (Figure 1; Jacobs and Kinkel, 1976; George et al., 1995). Other common findings on brain imaging include a callosal angle of 90 degrees or less (Borzage et al., 2021), periventricular hyperintensities, and enlargement of the temporal horns (Relkin et al., 2005). A trial of CSF drainage is often undertaken to aid in the diagnosis, commonly via the lumbar subarachnoid space. Clinical benefit after temporary CSF drainage is strongly predictive of improvement in at least one symptom after shunting (Marmarou et al., 2005; Toma et al., 2013). In patients unable or unwilling to receive a shunt, serial lumbar punctures may be a treatment option (Isik et al., 2019).
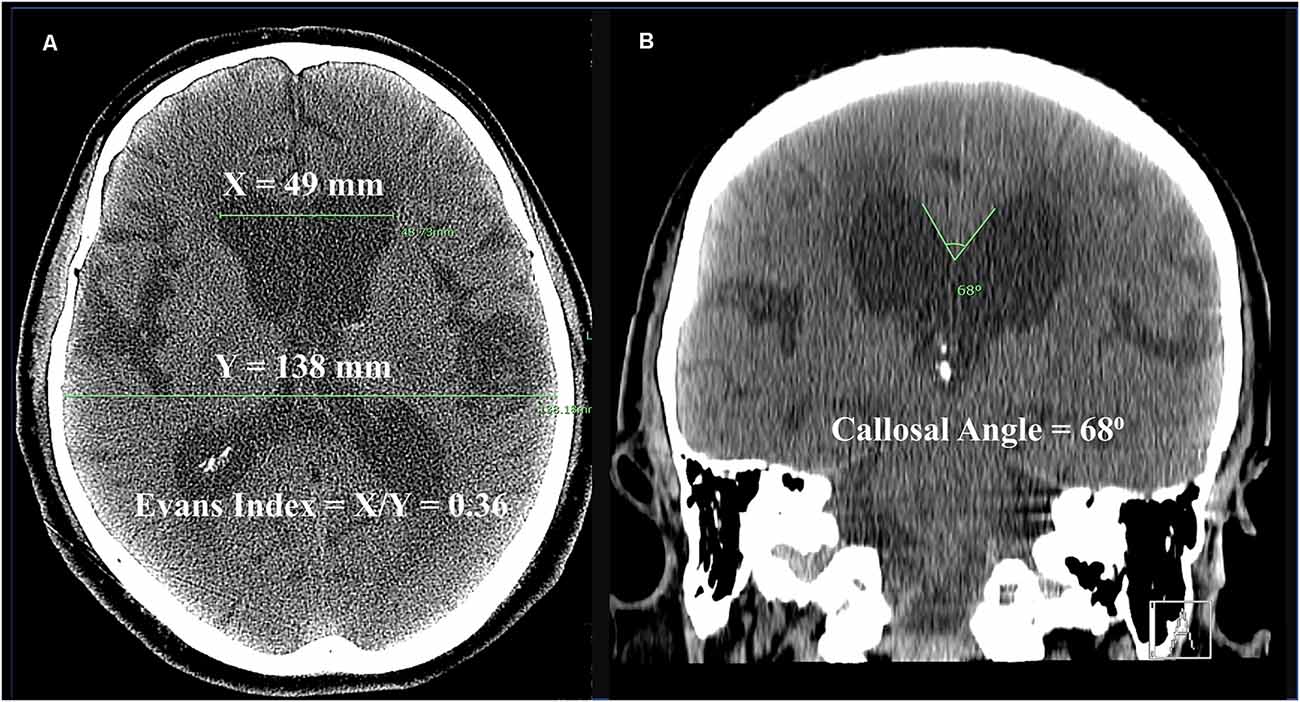
Figure 1. Computed tomography (CT) scan from a 70-year-old man with iNPH. (A) Axial image demonstrating Evans Index, which is the ratio of the maximum bifrontal horn width (X) to the maximum biparietal internal diameter of the skull (Y). An Evans Index >0.3 is present in iNPH. (B) Coronal image demonstrating callosal angle, here measured to 68 degrees. Normal callosal angles are greater than 90 degrees, while acute callosal angles occur in iNPH. Disproportionately enlarged subarachnoid space hydrocephalus (DESH) is apparent, as the Sylvian fissures are dilated out of proportion to sulci near the convexity.
Frustrating the iNPH clinical picture, there is not a clear neuroanatomic basis for the condition’s manifestations. It was originally held that stretching of white matter tracts from ventricular dilation led to iNPH’s clinical findings (Hakim and Adams, 1965), but there are several limitations with this hypothesis. First, it is not immediately obvious which white matter tracts would be causative. While the corticospinal tracts are clearly at risk for stretch, corticospinal tract-related gait disorders tend to differ from the hypokinesia and disequilibrium typical of iNPH (Rubino, 2002; Bugalho and Guimarães, 2007; Baker, 2018). Second, ventricular volume prior to CSF diversion-a surrogate for white matter tract stretching-does not correlate with the degree of symptomatology (Neikter et al., 2020). Third, other causes of ventriculomegaly both pathological (e.g., obstructive hydrocephalus) and physiological (e.g., ex vacuo hydrocephalus), do not typically cause gait disturbance as a primary manifestation. Fourth, the actual decrease in ventricular size after shunting is fairly modest in clinical responders, typically resulting in less than a 10% reduction in Evan’s index (Neikter et al., 2020). Thus, while changes in the corticospinal tracts and corpus callosum correlating with iNPH have been noted in some studies using tractography and transcranial magnetic stimulation (Röricht et al., 1998; Mataró et al., 2007; Siasios et al., 2016; Sarica et al., 2021), it is difficult to correlate symptomatic improvement following CSF drainage with decreased stretching of white matter tracts alone, and these changes may ultimately be epiphenomena.
Some authors have hypothesized that dysfunction of the cortico-basal ganglia-thalamo-cortical (CBGTC) is involved in iNPH (Curran and Lang, 1994; Lenfeldt et al., 2008), though the CBGTC loop may not explain dementia and urinary incontinence. Recent investigations have implicated striatal dopamine reuptake transporter density in iNPH gait impairment, offering a potential mechanism for basal ganglia dysfunction (Pozzi et al., 2021; Todisco et al., 2021). Other research has implicated frontal lobe dysfunction in not only the cognitive and urinary disturbances, but also gait disorder as well (Bugalho and Guimarães, 2007). As multiple lesional effects are suggested in iNPH, a fruitful approach to understanding the neuroanatomic basis may involve network-level investigations of functional connectivity (Griffa et al., 2020).
Given shared features with other neurodegenerative conditions, iNPH is defined as a unique entity by ventricular enlargement. Our efforts to understand the etiology of the disease are thus inextricably tied to understanding how hydrocephalus develops in iNPH patients.
Generally speaking, communicating hydrocephalus in iNPH and other etiologies results from an imbalance between CSF formation and removal. This is thought to be due to impairment in the return of CSF to the circulation in most cases, through scarring or obstruction of the arachnoid granulations (Chen et al., 2017). A compelling anecdote to challenge this trad itional model is the observation that subarachnoid spaces are not universally dilated in communicating hydrocephalus (Egnor et al., 2002; Greitz, 2004). It warrants mention that in chronic states of hydrocephalus, CSF absorption is coupled to production; that is, while hydrocephalus may initially occur because of impaired absorption of CSF, the imbalance is a transient phenomenon, and the compensated state implies that a new equilibrium between absorption and production is reached. Theoretically, the new equilibrium may be reached through increased CSF absorption through other means, or by decreased CSF production.
Unlike other types of communicating hydrocephalus, such as subarachnoid hemorrhage, a lesion causing impaired CSF outflow is not apparent in iNPH. An alternative explanation of communicating hydrocephalus, however, invokes arterial pulsations. In this theory, homeostasis of the CSF spaces including the ventricles relies on the normal propagation through the cerebrovasculature of pulsations delivered through the cardiac cycle. In cases in which cerebral arteries lose compliance, the additional pulse pressure is delivered distally to the capillaries and veins, which may alter CSF dynamics in such a way to produce ventriculomegaly (Egnor et al., 2002; Greitz, 2004). Preliminary evidence suggests that CSF drainage may improve vascular compliance and, subsequently, CBF (Bateman, 2000). Hence, as there is not a lesion to block CSF egress at the level of the arachnoid granulations in the traditional model of communicating hydrocephalus, impaired vascular compliance may be sufficient to produce iNPH.
Pulsations transferred through the elastic arterial system cause movement of CSF back and forth through the aqueduct during the cardiac cycle (Marmarou et al., 1978; Linninger et al., 2005; Kahlon et al., 2007; Scollato et al., 2008; Ringstad et al., 2015; Yamada et al., 2020). In healthy adults the net movement of ventricular CSF is craniocaudal, however, in iNPH patients, the net movement is typically reversed, towards the third and lateral ventricles (Kim et al., 1999; Penn et al., 2011; Ringstad et al., 2016), which results in transependymal flow of ventricular CSF into the interstitial space (Ringstad et al., 2017). The flow pattern often reverts to anterograde flow after shunting (Ringstad et al., 2016). Clinical study of CSF dynamics in iNPH indicates elevated resistance to CSF outflow and increased CSF pulsatility. These features predict treatment response after CSF diversion, indicating normalization of CSF dynamics and a more physiologic state (Eide and Sorteberg, 2008, 2010; Malm et al., 2011; Qvarlander et al., 2013; Jacobsson et al., 2018).
That iNPH may fundamentally represent a vascular disorder is intriguing, given the high incidence of vascular risk factors including hypertension and diabetes in iNPH patients (Eide and Pripp, 2014; Jaraj et al., 2016; Israelsson et al., 2017). Supporting this notion is the near-ubiquitous finding of deep white matter and periventricular lesions in iNPH (Krauss et al., 1997), hallmarks of small vessel disease. Variations in regional hypoperfusion and degree of hypoxic changes may help explain the clinical heterogeneity of iNPH and poor responses to shunting. Our view is that iNPH is fundamentally a cerebrovasculature disorder. Impaired compliance triggers a cascade of events culminating in the development of hydrocephalus, which subsequently begins a cycle that unchecked eventually progresses to irreversible dementia and neurologic injury. Shunting reverses some of the clinical manifestations, although even with treatment the disease is associated with progressive morbidity, which suggests a component of irreversible small vessel disease. Below we discuss three interrelated systems that may be central to the development and progression of iNPH: cerebral blood flow, the glymphatic system, and the blood-brain barrier. A flow diagram demonstrating possible pathogenic relationships is depicted in Figure 2.
Our understanding of the role of cerebral blood flow (CBF) in iNPH has evolved considerably in the last two decades. In early work, consistent patterns could not be drawn between the association between various CBF changes and: (1) the diagnosis of iNPH, (2) disease severity, or (3) improvement after shunting, which may have been due in part to disparate imaging protocols, inconsistent diagnostic criteria, and small sample sizes (Owler and Pickard, 2001). More recently, several studies reported regional hypoperfusion in critical areas, suggesting that vascular insufficiency is relevant to iNPH.
A number of studies have found regional CBF deficits in iNPH patients compared to age-matched healthy controls, including deficits in the periventricular white matter (Momjian et al., 2004; Ziegelitz et al., 2014, 2016; Virhammar et al., 2017), lentiform nucleus (Owler et al., 2004a; Ziegelitz et al., 2014, 2016; Virhammar et al., 2017), thalamus (Owler et al., 2004a; Virhammar et al., 2017), caudate (Owler et al., 2004a), and basal medial frontal cortex (Ziegelitz et al., 2014). Global reductions have been identified as well (Momjian et al., 2004; Owler et al., 2004a; Ziegelitz et al., 2014, 2016). One study noted inverse correlations between thalamic and putaminal CBF and severity of iNPH (Owler et al., 2004a), but most studies found no association between the magnitude of global or regional CBF values and severity of iNPH.
Clinical improvements after CSF drainage have been associated with improvements in CBF in the lateral and frontal white matter regions (Virhammar et al., 2014), periventricular white matter (Ziegelitz et al., 2015, 2016; Satow et al., 2017), periventricular thalamus (Ziegelitz et al., 2015), medial frontal region (Klinge et al., 2008), supplemental motor area (Lenfeldt et al., 2008), brainstem (Agerskov et al., 2020), and globally (Chang et al., 2009). Relating to prognostic variables prior to treatment, one study found decreased preoperative CBF in basal frontal lobes and anterior cingulate region in iNPH patients who responded to shunting compared to non-responders (Murakami et al., 2007). However, in most studies, no associations were found between preoperative regional CBF values and clinical response to shunting.
In light of this body of work, it is useful to consider the clinical findings in iNPH as the result of hypoperfusion. Depending on the extent of the CBF deficit, hypoperfusion may account fo rany or all findings of iNPH. Bladder dysfunction in iNPH is typically referable to detrusor overactivity, which may occur through effects on the frontal lobe or basal ganglia (Andersson, 2004; Sakakibara et al., 2008, 2012). Similarly, both the frontal lobe and basal ganglia have been implicated in gait disturbances that characterize iNPH (Bugalho and Guimarães, 2007). While cognitive impairment may be considered a diffuse lesion, some evidence suggests early frontal involvement in iNPH, consisting of psychomotor slowing and impaired attention rather than memory deficits, before progressing to more profound impairment (Iddon et al., 1999; Ogino et al., 2006; Picascia et al., 2015). With improved perfusion after shunting, symptoms may regress unless infarcts have already occurred.
The mechanism of impaired perfusion of deep gray matter and periventricular white matter is a subject of debate. Hypoperfusion may result from compression of the deep vascularity, compression of superficial venous outflow, impaired autoregulation, changes related to transependymal flow, or some combination of these factors (Momjian et al., 2004; Owler et al., 2004b; Bateman, 2008; Scollato et al., 2008; Chang et al., 2009). While a shunt would potentially improve any of these factors, the manner by which improvement in CBF occurs after shunting has not been well characterized. The effect of CSF drainage on cerebral perfusion pressure (CPP) through decreased ICP is limited as the ICP is not elevated, raising questions as to whether improved CPP alone may explain CBF changes (Eide and Sorteberg, 2010). One explanation may involve the CSF pulsatility curve, in which a relatively small change in ICP leads to decreased CSF pulsatility which may have downstream effects on CBF (Qvarlander et al., 2013).
The glymphatic (glial-lymphatic) system is a recently discovered homeostatic mechanism by which fluid moves through the brain parenchyma, acting as a waste disposal mechanism for brain tissue (Figure 3; Iliff et al., 2012; Nedergaard, 2013). In brief, subarachnoid CSF is pumped along periarterial channels by arterial pulsations (Iliff et al., 2013; Mestre et al., 2018) and enters the interstitial compartment through aquaporin-4 transporters within astrocyte endfeet (Plog and Nedergaard, 2018). CSF joins interstitial fluid and moves through the interstitial space towards perivenous and perineural channels through which it is removed from the brain. These efflux pathways include perisinusal lymphatic vessels that drain into extracranial lymphatics (Louveau et al., 2015; Ahn et al., 2019). Glymphatic circulation constitutes a primary role in the clearance of toxoids and waste products from the brain parenchyma, akin to the lymphatic function of other organs (Jessen et al., 2015), and is most active during sleep (Kang et al., 2009; Shokri-Kojori et al., 2018).
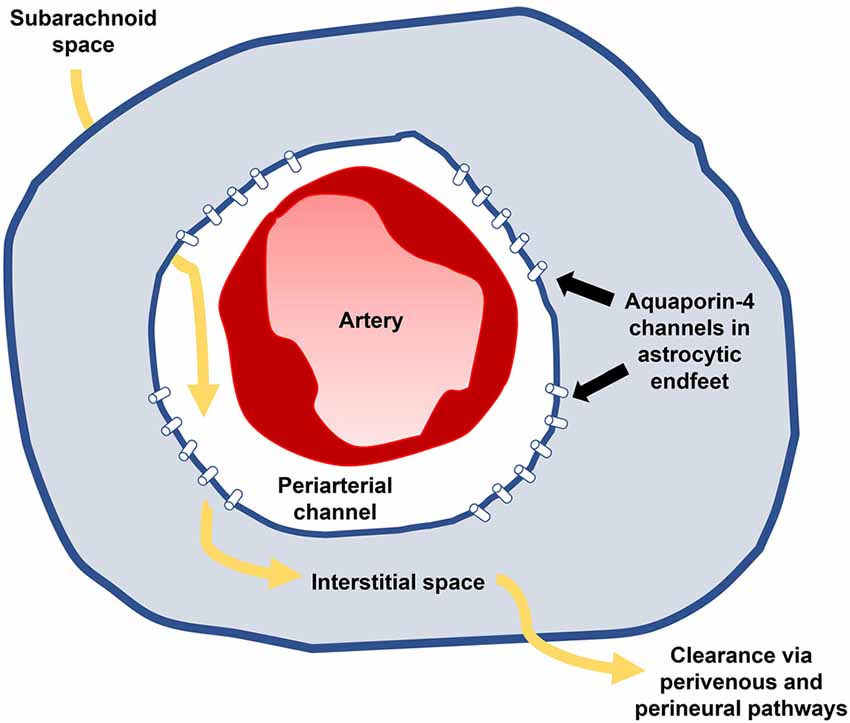
Figure 3. Glymphatic influx (yellow arrows) occurs along periarterial channels within subarachnoid spaces (white) and enters the parenchyma (blue) through aquaporin-4 transporters on astrocytic endfeet. Subarachnoid CSF joins interstitial fluid and passes through the brain, delivering substances to and from the parenchyma before being absorbed along perivenous and perineural channels. Impaired glymphatic circulation may result in part from impaired influx through poor arterial compliance and results in progressive neurotoxicity contributing to iNPH’s clinical manifestations.
Mounting evidence suggests impairment of the glymphatic system by multiple mechanisms contributes to neurodegenerative diseases (Rasmussen et al., 2018). Much of the advances in understanding these pathways hail from the recognition that dysfunction of the glymphatic system contributes to amyloid-beta buildup in Alzheimer’s disease (Rasmussen et al., 2018; Mestre et al., 2020). Other conditions associated with glymphatic impairment relevant to iNPH include aging (Zhou et al., 2020), diabetes (Jiang et al., 2017), and hypertension (Mestre et al., 2018).
Clinical evaluation of iNPH patients demonstrates sluggish glymphatic flow (Ringstad et al., 2017; Eide and Ringstad, 2019; Bae et al., 2021). Lumbar intrathecal gadobutrol injection in iNPH patients resulted in delayed enhancement of subarachnoid spaces and cortical surfaces, compared to younger patients receiving gadobutrol for workup of intracranial hypotension (Ringstad et al., 2017). The age difference between the two patient groups somewhat limits the study’s conclusions, as even healthy older people have impaired glymphatic function (Zhou et al., 2020). Given that glymphatic dysfunction also is involved in Alzheimer’s disease (Tarasoff-Conway et al., 2015), this may represent a common pathway for cognitive decline in the two conditions (Reeves et al., 2020), but may not necessarily be related to urinary incontinence and gait in iNPH.
One theory posited to explain glymphatic impairment in iNPH is loss of arterial compliance. As vessels become increasingly stiff, the pump driving glymphatic influx is weakened, resulting in the buildup of waste substances in the interstitial fluid. This may in part explain the retrograde movement of subarachnoid CSF into the ventricular system, as the outflow resistance increases along glymphatic pathways. It is possible that the improvement in CSF dynamics after shunting (Ringstad et al., 2016) improves the glymphatic flow and thus cognition. However, that this could represent a primary insult in iNPH has been called into question (Gallina et al., 2020).
Another potential mechanism for glymphatic impairment is through reduced expression of aquaporin-4 channels, which has been demonstrated in iNPH patients (Hasan-Olive et al., 2019). In Alzheimer’s disease, decreased expression of aquaporin-4 leads to impaired clearance of misfolded proteins, which results in neurotoxicity and cognitive decline (Xu et al., 2015; Zeppenfeld et al., 2017). Glymphatic impairment in iNPH may lead to a similar buildup of waste products with resultant neurotoxicity that leads to cognitive dysfunction. Improvement in the glymphatic flow after shunting iNPH and subsequent clearance of accumulated interstitial substances may improve neuronal function and hence cognition after shunting, though this is untested.
The blood-brain barrier (BBB), the brain’s unique microvascular interface consisting of endothelial tight junctions, pericytes, and astrocytic endfeet, plays a critical role in maintaining the optimal conditions for proper neuronal functioning by acting as a selective barrier between the blood and brain (Figure 4; Bradbury et al., 1963; Bernacki et al., 2008; Abbott et al., 2010). The BBB prevents the entry of toxins while facilitating the transportation of metabolites and nutrients into the CNS. Given its role in homeostasis and neuroprotection, the BBB has been investigated in a host of neurological disorders (Sweeney et al., 2018; Profaci et al., 2020).
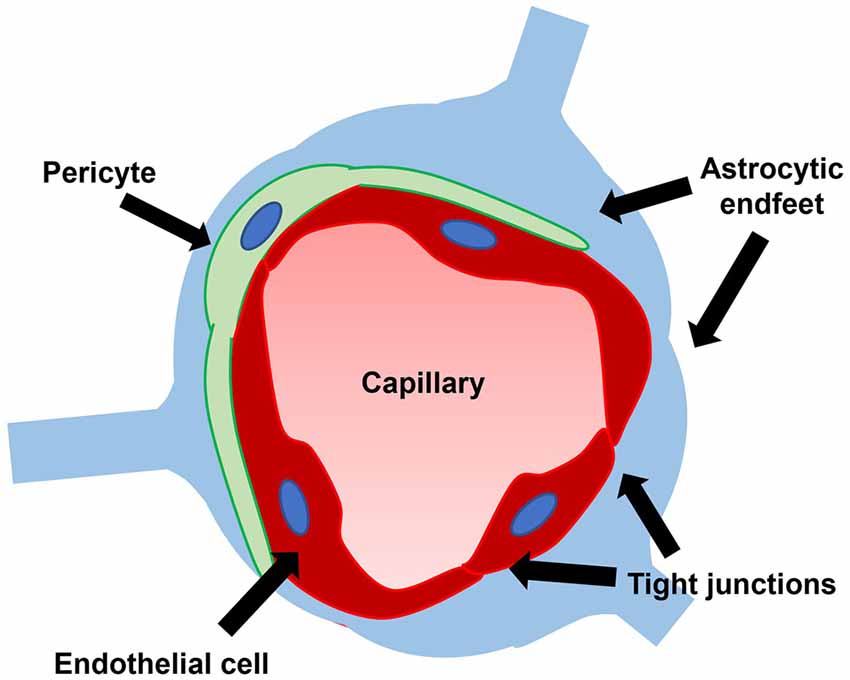
Figure 4. The blood-brain barrier consists of endothelial tight junctions, pericytes, and astrocytic endfeet. A leaky blood-brain barrier through dysfunction of one or multiple of these constituent parts has been demonstrated in iNPH.
Several investigations have demonstrated pathology related to the BBB in iNPH patients. Distorted and thickened basement membranes and degenerated pericyte processes were demonstrated in biopsy specimens from iNPH patients (Eidsvaag et al., 2017; Eide and Hansson, 2020). Pericytes are essential components of the BBB and play important roles in induction, maintenance, and selective permeability (Abbott, 2002; Armulik et al., 2010). Pericyte degeneration has been shown to cause increased permeability to water and both low- and high-molecular-weight tracers, creating a leaky BBB (Armulik et al., 2010).
Additional evidence for compromise of the BBB was identified in iNPH patients through the extravasation of fibrin, a blood coagulation protein, in frontal biopsy specimens (Eide and Hansson, 2020). In this study, scattered fibrin staining around capillaries within cortical layers was seen in all iNPH patients compared to fewer than 30% of patients with other neurological diseases (Eide and Hansson, 2020). Increased fibrin deposition in the brain parenchyma in NPH biopsies correlated with increased levels of glial fibrillary acidic protein (GFAP), a marker of reactive astrogliosis (Eide and Hansson, 2020). Astrogliosis decreases compliance, which may in turn contribute to altered CSF dynamics in NPH (Lu et al., 2011; Fattahi et al., 2016). Both the degree of fibrin extravasation and astrogliosis correlated with reduced expression of aquaporin-4 transporters on perivascular astrocytic endfeet in biopsies of NPH patients, suggesting a link with glymphatic function (Eide and Hansson, 2018, 2020).
BBB dysfunction in iNPH may be related to deficient CBF. In a murine model of chronic cerebral hypoperfusion, hypoxia-induced injury to pericytes lead to BBB disruption (Liu et al., 2019). Though not tested in iNPH, a similar mechanism may explain pericyte injury and subsequent loss of BBB integrity. Pericyte dysfunction and other BBB insults have been demonstrated through means such as inflammation, hyperglycemia, and ischemia in Alzheimer’s disease, traumatic brain injury, and other disorders (Erickson and Banks, 2013).
From the initial insult leading to hydrocephalus and onset of clinical manifestations to the irreversible changes occurring later in untreated cases, the cerebrovasculature is closely tied to the pathogenesis of iNPH. Relevant mechanisms include diminished CBF, glymphatic disruption, and changes to the BBB. Additional work is needed to further characterize how these pathophysiologic mechanisms inter-relate. Further, future studies should address how these pathologic features are reversed with shunting, which will provide insights into both iNPH and other neurodegenerative conditions. Answers to these questions will shed light on improving clinical responses and enhancing the durability of shunting.
PB, RB, KW, WC, and AK: investigation, manuscript writing, and editing. BO and XS: manuscript editing. ZZ, MB, and DW: conception and manuscript editing. CL and DL: conception, manuscript editing, and supervision. All authors contributed to the article and approved the submitted version.
This work was partially supported by a USC CTSI Team Science Grant (Southern California Clinical and Translational Science Institute) and the USC Neurorestoration Center.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbott, N. J. (2002). Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 200, 629–638. doi: 10.1046/j.1469-7580.2002.00064.x
Abbott, N. J., Patabendige, A. A. K., Dolman, D. E. M., Yusof, S. R., and Begley, D. J. (2010). Structure and function of the blood-brain barrier. Neurobiol. Dis. 37, 13–25. doi: 10.1016/j.nbd.2009.07.030
Agerskov, S., Arvidsson, J., Ziegelitz, D., Lagerstrand, K., Starck, G., Björkman-Burtscher, I. M., et al. (2020). MRI diffusion and perfusion alterations in the mesencephalon and pons as markers of disease and symptom reversibility in idiopathic normal pressure hydrocephalus. PLoS One 15:e0240327. doi: 10.1371/journal.pone.0240327
Ahn, J. H., Cho, H., Kim, J.-H., Kim, S. H., Ham, J.-S., Park, I., et al. (2019). Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572, 62–66. doi: 10.1038/s41586-019-1419-5
Andersson, K.-E. (2004). Mechanisms of disease: central nervous system involvement in overactive bladder syndrome. Nat. Clin. Pract. Urol. 1, 103–108. doi: 10.1038/ncpuro0021
Andersson, J., Rosell, M., Kockum, K., Söderström, L., and Laurell, K. (2017). Challenges in diagnosing normal pressure hydrocephalus: evaluation of the diagnostic guidelines. eNeurologicalScience 7, 27–31. doi: 10.1016/j.ensci.2017.04.002
Armulik, A., Genové, G., Mäe, M., Nisancioglu, M. H., Wallgard, E., Niaudet, C., et al. (2010). Pericytes regulate the blood-brain barrier. Nature 468, 557–561. doi: 10.1038/nature09522
Bae, Y. J., Choi, B. S., Kim, J.-M., Choi, J.-H., Cho, S. J., and Kim, J. H. (2021). Altered glymphatic system in idiopathic normal pressure hydrocephalus. Parkinsonism Relat. Disord. 82, 56–60. doi: 10.1016/j.parkreldis.2020.11.009
Bateman, G. A. (2000). Vascular compliance in normal pressure hydrocephalus. Am. J. Neuroradiol. 21, 1574–1585.
Bateman, G. A. (2008). The pathophysiology of idiopathic normal pressure hydrocephalus: cerebral ischemia or altered venous hemodynamics? Am. J. Neuroradiol. 29, 198–203. doi: 10.3174/ajnr.A0739
Bernacki, J., Dobrowolska, A., Nierwińska, K., and Małecki, A. (2008). Physiology and pharmacological role of the blood-brain barrier. Pharmacol. Rep. 60, 600–622.
Borzage, M., Saunders, A., Hughes, J., McComb, J. G., Blüml, S., and King, K. S. (2021). The first examination of diagnostic performance of automated measurement of the callosal angle in 1856 elderly patients and volunteers indicates that 12.4% of exams met the criteria for possible normal pressure hydrocephalus. Am. J. Neuroradiol. 42, 1942–1948. doi: 10.3174/ajnr.A7294
Bradbury, M. W., Stubbs, J., Hughes, I. E., and Parker, P. (1963). The distribution of potassium, sodium, chloride and urea between lumbar cerebrospinal fluid and blood serum in human subjects. Clin. Sci. 25, 97–105.
Bugalho, P., and Guimarães, J. (2007). Gait disturbance in normal pressure hydrocephalus: a clinical study. Parkinsonism Relat. Disord. 13, 434–437. doi: 10.1016/j.parkreldis.2006.08.007
Chang, C.-C., Asada, H., Mimura, T., and Suzuki, S. (2009). A prospective study of cerebral blood flow and cerebrovascular reactivity to acetazolamide in 162 patients with idiopathic normal-pressure hydrocephalus. J. Neurosurg. 111, 610–617. doi: 10.3171/2008.10.17676
Chen, S., Luo, J., Reis, C., Manaenko, A., and Zhang, J. (2017). Hydrocephalus after subarachnoid hemorrhage: pathophysiology, diagnosis and treatment. Biomed Res. Int. 2017:8584753. doi: 10.1155/2017/8584753
Curran, T., and Lang, A. E. (1994). Parkinsonian syndromes associated with hydrocephalus: case reports, a review of the literature and pathophysiological hypotheses. Mov. Disord. 9, 508–520. doi: 10.1002/mds.870090503
Egnor, M., Zheng, L., Rosiello, A., Gutman, F., and Davis, R. (2002). A model of pulsations in communicating hydrocephalus. Pediatr. Neurosurg. 36, 281–303. doi: 10.1159/000063533
Eide, P. K., and Hansson, H.-A. (2018). Astrogliosis and impaired aquaporin-4 and dystrophin systems in idiopathic normal pressure hydrocephalus. Neuropathol. Appl. Neurobiol. 44, 474–490. doi: 10.1111/nan.12420
Eide, P. K., and Hansson, H.-A. (2020). Blood-brain barrier leakage of blood proteins in idiopathic normal pressure hydrocephalus. Brain Res. 1727:146547. doi: 10.1016/j.brainres.2019.146547
Eide, P. K., and Pripp, A. H. (2014). Increased prevalence of cardiovascular disease in idiopathic normal pressure hydrocephalus patients compared to a population-based cohort from the HUNT3 survey. Fluids Barriers CNS 11:19. doi: 10.1186/2045-8118-11-19
Eide, P. K., and Ringstad, G. (2019). Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: a glymphatic magnetic resonance imaging study. J. Cereb. Blood Flow Metab. 39, 1355–1368. doi: 10.1177/0271678X18760974
Eide, P. K., and Sorteberg, W. (2008). Changes in intracranial pulse pressure amplitudes after shunt implantation and adjustment of shunt valve opening pressure in normal pressure hydrocephalus. Acta Neurochir. (Wien) 150, 1141–1147. doi: 10.1007/s00701-008-0138-8
Eide, P. K., and Sorteberg, W. (2010). Diagnostic intracranial pressure monitoring and surgical management in idiopathic normal pressure hydrocephalus: a 6-year review of 214 patients. Neurosurgery 66, 80–91. doi: 10.1227/01.NEU.0000363408.69856.B8
Eidsvaag, V. A., Hansson, H.-A., Heuser, K., Nagelhus, E. A., and Eide, P. K. (2017). Brain capillary ultrastructure in idiopathic normal pressure hydrocephalus: relationship with static and pulsatile intracranial pressure. J. Neuropathol. Exp. Neurol. 76, 1034–1045. doi: 10.1093/jnen/nlx091
Erickson, M. A., and Banks, W. A. (2013). Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J. Cereb. Blood Flow Metab. 33, 1500–1513. doi: 10.1038/jcbfm.2013.135
Fattahi, N., Arani, A., Perry, A., Meyer, F., Manduca, A., Glaser, K., et al. (2016). MR elastography demonstrates increased brain stiffness in normal pressure hydrocephalus. Am. J. Neuroradiol. 37, 462–467. doi: 10.3174/ajnr.A4560
Gallina, P., Porfirio, B., and Lolli, F. (2020). iNPH as a ‘2-hit’ intracranial hydrodynamic derangement disease. Trends Mol. Med. 26, 531–532. doi: 10.1016/j.molmed.2020.04.002
George, A. E., Holodny, A., Golomb, J., and de Leon, M. J. (1995). The differential diagnosis of Alzheimer’s disease. Cerebral atrophy versus normal pressure hydrocephalus. Neuroimaging Clin. N. Am. 5, 19–31.
Greitz, D. (2004). Radiological assessment of hydrocephalus: new theories and implications for therapy. Neurosurg. Rev. 27, 145–165. doi: 10.1007/s10143-004-0326-9
Griffa, A., Van De Ville, D., Herrmann, F. R., and Allali, G. (2020). Neural circuits of idiopathic Normal Pressure Hydrocephalus: a perspective review of brain connectivity and symptoms meta-analysis. Neurosci. Biobehav. Rev. 112, 452–471. doi: 10.1016/j.neubiorev.2020.02.023
Hakim, S., and Adams, R. D. (1965). The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure: observations on cerebrospinal fluid hydrodynamics. J. Neurol. Sci. 2, 307–327. doi: 10.1016/0022-510x(65)90016-x
Hasan-Olive, M. M., Enger, R., Hansson, H.-A., Nagelhus, E. A., and Eide, P. K. (2019). Loss of perivascular aquaporin-4 in idiopathic normal pressure hydrocephalus. Glia 67, 91–100. doi: 10.1002/glia.23528
Hebb, A. O., and Cusimano, M. D. (2001). Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery 49, 1166–1184. doi: 10.1097/00006123-200111000-00028
Iddon, J. L., Pickard, J. D., Cross, J. J. L., Griffiths, P. D., Czosnyka, M., and Sahakian, B. J. (1999). Specific patterns of cognitive impairment in patients with idiopathic normal pressure hydrocephalus and Alzheimer’s disease: a pilot study. J. Neurol. Neurosurg. Psychiatry 67, 723–732. doi: 10.1136/jnnp.67.6.723
Iliff, J. J., Wang, M., Liao, Y., Plogg, B. A., Peng, W., Gundersen, G. A., et al. (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4:147ra111. doi: 10.1126/scitranslmed.3003748
Iliff, J. J., Wang, M., Zeppenfeld, D. M., Venkataraman, A., Plog, B. A., Liao, Y., et al. (2013). Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 33, 18190–18199. doi: 10.1523/JNEUROSCI.1592-13.2013
Iseki, C., Takahashi, Y., Wada, M., Kawanami, T., Adachi, M., and Kato, T. (2014). Incidence of idiopathic normal pressure hydrocephalus (iNPH): A 10-year follow-up study of a rural community in Japan. J. Neurol. Sci. 339, 108–112. doi: 10.1016/j.jns.2014.01.033
Isik, A. T., Kaya, D., Ates Bulut, E., Dokuzlar, O., and Soysal, P. (2019). The outcomes of serial cerebrospinal fluid removal in elderly patients with idiopathic normal pressure hydrocephalus. Clin. Interv. Aging 14, 2063–2069. doi: 10.2147/CIA.S228257
Israelsson, H., Carlberg, B., Wikkelsö, C., Laurell, K., Kahlon, B., Leijon, G., et al. (2017). Vascular risk factors in INPH: a prospective case-control study (the INPH-CRasH study). Neurology 88, 577–585. doi: 10.1212/WNL.0000000000003583
Jacobs, L., and Kinkel, W. (1976). Computerized axial transverse tomography in normal pressure hydrocephalus. Neurology 26, 501–507. doi: 10.1212/wnl.26.6.501
Jacobsson, J., Qvarlander, S., Eklund, A., and Malm, J. (2018). Comparison of the CSF dynamics between patients with idiopathic normal pressure hydrocephalus and healthy volunteers. J. Neurosurg. 1–6. [Online ahead of print] doi: 10.3171/2018.5.JNS173170
Jaraj, D., Agerskov, S., Rabiei, K., Marlow, T., Jensen, C., Guo, X., et al. (2016). Vascular factors in suspected normal pressure hydrocephalus: a population-based study. Neurology 86, 592–599. doi: 10.1212/WNL.0000000000002369
Jessen, N. A., Munk, A. S. F., Lundgaard, I., and Nedergaard, M. (2015). The glymphatic system: a beginner’s guide. Neurochem. Res. 40, 2583–2599. doi: 10.1007/s11064-015-1581-6
Jiang, Q., Zhang, L., Ding, G., Davoodi-Bojd, E., Li, Q., Li, L., et al. (2017). Impairment of the glymphatic system after diabetes. J. Cereb. Blood Flow Metab. 37, 1326–1337. doi: 10.1177/0271678X16654702
Junkkari, A., Sintonen, H., Danner, N., Jyrkkänen, H. K., Rauramaa, T., Luikku, A. J., et al. (2021). 5-Year health-related quality of life outcome in patients with idiopathic normal pressure hydrocephalus. J. Neurol. 268, 3283–3293. doi: 10.1007/s00415-021-10477-x
Kahlon, B., Annertz, M., Ståhlberg, F., and Rehncrona, S. (2007). Is aqueductal stroke volume, measured with cine phase-contrast magnetic resonance imaging scans useful in predicting outcome of shunt surgery in suspected normal pressure hydrocephalus? Neurosurgery 60, 124–129. doi: 10.1227/01.NEU.0000249208.04344.A3
Kang, J.-E., Lim, M. M., Bateman, R. J., Lee, J. J., Smyth, L. P., Cirrito, J. R., et al. (2009). Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007. doi: 10.1126/science.1180962
Kim, D. S., Choi, J. U., Huh, R., Yun, P. H., and Kim, D. I. (1999). Quantitative assessment of cerebrospinal fluid hydrodynamics using a phase-contrast cine MR image in hydrocephalus. Childs Nerv. Syst. 15, 461–467. doi: 10.1007/s003810050440
Klinge, P. M., Brooks, D. J., Samii, A., Weckesser, E., van den Hoff, J., Fricke, H., et al. (2008). Correlates of local cerebral blood flow (CBF) in normal pressure hydrocephalus patients before and after shunting—A retrospective analysis of [15O]H2O PET-CBF studies in 65 patients. Clin. Neurol. Neurosurg. 110, 369–375. doi: 10.1016/j.clineuro.2007.12.019
Krauss, J. K., Regel, J. P., Vach, W., Orszagh, M., Jüngling, F. D., Bohus, M., et al. (1997). White matter lesions in patients with idiopathic normal pressure hydrocephalus and in an age-matched control group: a comparative study. Neurosurgery 40, 491–496. doi: 10.1097/00006123-199703000-00011
Lenfeldt, N., Larsson, A., Nyberg, L., Andersson, M., Birgander, R., Eklund, A., et al. (2008). Idiopathic normal pressure hydrocephalus: increased supplementary motor activity accounts for improvement after CSF drainage. Brain 131, 2904–2912. doi: 10.1093/brain/awn232
Linninger, A. A., Tsakiris, C., Zhu, D. C., Xenos, M., Roycewicz, P., Danziger, Z., et al. (2005). Pulsatile cerebrospinal fluid dynamics in the human brain. IEEE Trans. Biomed. Eng. 52, 557–565. doi: 10.1109/TBME.2005.844021
Liu, Q., Radwanski, R., Babadjouni, R., Patel, A., Hodis, D. M., Baumbacher, P., et al. (2019). Experimental chronic cerebral hypoperfusion results in decreased pericyte coverage and increased blood-brain barrier permeability in the corpus callosum. J. Cereb. Blood Flow Metab. 39, 240–250. doi: 10.1177/0271678X17743670
Louveau, A., Smirnov, I., Keyes, T. J., Eccles, J. D., Rouhani, S. J., Peske, J. D., et al. (2015). Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341. doi: 10.1038/nature14432
Lu, Y.-B., Iandiev, I., Hollborn, M., Körber, N., Ulbricht, E., Hirrlinger, P. G., et al. (2011). Reactive glial cells: increased stiffness correlates with increased intermediate filament expression. FASEB J. 25, 624–631. doi: 10.1096/fj.10-163790
Malm, J., Jacobsson, J., Birgander, R., and Eklund, A. (2011). Reference values for CSF outflow resistance and intracranial pressure in healthy elderly. Neurology 76, 903–909. doi: 10.1212/WNL.0b013e31820f2dd0
Marmarou, A., Bergsneider, M., Klinge, P., Relkin, N., and Black, P. M. (2005). The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 57, S17–S28. doi: 10.1227/01.neu.0000168184.01002.60
Marmarou, A., Shulman, K., and Rosende, R. M. (1978). A nonlinear analysis of the cerebrospinal fluid system and intracranial pressure dynamics. J. Neurosurg. 48, 332–344. doi: 10.3171/jns.1978.48.3.0332
Martín-Láez, R., Caballero-Arzapalo, H., López-Menéndez, L. Á., Arango-Lasprilla, J. C., and Vázquez-Barquero, A. (2015). Epidemiology of idiopathic normal pressure hydrocephalus: a systematic review of the literature. World Neurosurg. 84, 2002–2009. doi: 10.1016/j.wneu.2015.07.005
Mataró, M., Matarín, M., Poca, M. A., Pueyo, R., Sahuquillo, J., Barrios, M., et al. (2007). Functional and magnetic resonance imaging correlates of corpus callosum in normal pressure hydrocephalus before and after shunting. J. Neurol. Neurosurg. Psychiatry 78, 395–398. doi: 10.1136/jnnp.2006.096164
Mestre, H., Mori, Y., and Nedergaard, M. (2020). The brain’s glymphatic system: current controversies. Trends Neurosci. 43, 458–466. doi: 10.1016/j.tins.2020.04.003
Mestre, H., Tithof, J., Du, T., Song, W., Peng, W., Sweeney, A. M., et al. (2018). Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. 9:4878. doi: 10.1038/s41467-018-07318-3
Momjian, S., Owler, B. K., Czosnyka, Z., Czosnyka, M., Pena, A., and Pickard, J. D. (2004). Pattern of white matter regional cerebral blood flow and autoregulation in normal pressure hydrocephalus. Brain 127, 965–972. doi: 10.1093/brain/awh131
Murakami, M., Hirata, Y., and Kuratsu, J.-I. (2007). Predictive assessment of shunt effectiveness in patients with idiopathic normal pressure hydrocephalus by determining regional cerebral blood flow on 3D stereotactic surface projections. Acta Neurochir. (Wien) 149, 991–997. doi: 10.1007/s00701-007-1259-1
Nakajima, M., Yamada, S., Miyajima, M., Ishii, K., Kuriyama, N., Kazui, H., et al. (2021). Guidelines for management of idiopathic normal pressure hydrocephalus (third edition): endorsed by the Japanese society of normal pressure hydrocephalus. Neurol. Med. Chir. (Tokyo) 61, 63–97. doi: 10.2176/nmc.st.2020-0292
Nedergaard, M. (2013). Neuroscience. Garbage truck of the brain. Science 340, 1529–1530. doi: 10.1126/science.1240514
Neikter, J., Agerskov, S., Hellström, P., Tullberg, M., Starck, G., Ziegelitz, D., et al. (2020). Ventricular volume is more strongly associated with clinical improvement than the evans index after shunting in idiopathic normal pressure hydrocephalus. Am. J. Neuroradiol. 41, 1187–1192. doi: 10.3174/ajnr.A6620
Ogino, A., Kazui, H., Miyoshi, N., Hashimoto, M., Ohkawa, S., Tokunaga, H., et al. (2006). Cognitive impairment in patients with idiopathic normal pressure hydrocephalus. Dement. Geriatr. Cogn. Disord. 21, 113–119. doi: 10.1159/000090510
Owler, B. K., Momjian, S., Czosnyka, Z., Czosnyka, M., Péna, A., Harris, N. G., et al. (2004a). Normal pressure hydrocephalus and cerebral blood flow: a PET study of baseline values. J. Cereb. Blood Flow Metab. 24, 17–23. doi: 10.1097/01.WCB.0000093326.88757.49
Owler, B. K., Pena, A., Momjian, S., Czosnyka, Z., Czosnyka, M., Harris, N. G., et al. (2004b). Changes in cerebral blood flow during cerebrospinal fluid pressure manipulation in patients with normal pressure hydrocephalus: a methodological study. J. Cereb. Blood Flow Metab. 24, 579–587. doi: 10.1097/00004647-200405000-00012
Owler, B. K., and Pickard, J. D. (2001). Normal pressure hydrocephalus and cerebral blood flow: a review. Acta Neurol. Scand. 104, 325–342. doi: 10.1034/j.1600-0404.2001.00092.x
Penn, R. D., Basati, S., Sweetman, B., Guo, X., and Linninger, A. (2011). Ventricle wall movements and cerebrospinal fluid flow in hydrocephalus: clinical article. J. Neurosurg. 115, 159–164. doi: 10.3171/2010.12.JNS10926
Picascia, M., Zangaglia, R., Bernini, S., Minafra, B., Sinforiani, E., and Pacchetti, C. (2015). A review of cognitive impairment and differential diagnosis in idiopathic normal pressure hydrocephalus. Funct. Neurol. 30, 217–228. doi: 10.11138/fneur/2015.30.4.217
Plog, B. A., and Nedergaard, M. (2018). The glymphatic system in central nervous system health and disease: past, present and future. Annu. Rev. Pathol. 13, 379–394. doi: 10.1146/annurev-pathol-051217-111018
Pozzi, N. G., Brumberg, J., Todisco, M., Minafra, B., Zangaglia, R., Bossert, I., et al. (2021). Striatal dopamine deficit and motor impairment in idiopathic normal pressure hydrocephalus. Mov. Disord. 36, 124–132. doi: 10.1002/mds.28366
Profaci, C. P., Munji, R. N., Pulido, R. S., and Daneman, R. (2020). The blood-brain barrier in health and disease: important unanswered questions. J. Exp. Med. 217:e20190062. doi: 10.1084/jem.20190062
Qvarlander, S., Lundkvist, B., Koskinen, L.-O. D., Malm, J., and Eklund, A. (2013). Pulsatility in CSF dynamics: pathophysiology of idiopathic normal pressure hydrocephalus. J. Neurol. Neurosurg. Psychiatry 84, 735–741. doi: 10.1136/jnnp-2012-302924
Rasmussen, M. K., Mestre, H., and Nedergaard, M. (2018). The glymphatic pathway in neurological disorders. Lancet Neurol. 17, 1016–1024. doi: 10.1016/S1474-4422(18)30318-1
Reeves, B. C., Karimy, J. K., Kundishora, A. J., Mestre, H., Cerci, H. M., Matouk, C., et al. (2020). Glymphatic system impairment in Alzheimer’s disease and idiopathic normal pressure hydrocephalus. Trends Mol. Med. 26, 285–295. doi: 10.1016/j.molmed.2019.11.008
Relkin, N., Marmarou, A., Klinge, P., Bergsneider, M., and Black, P. M. (2005). Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery 57, S4–S16. doi: 10.1227/01.neu.0000168185.29659.c5
Ringstad, G., Emblem, K. E., and Eide, P. K. (2016). Phase-contrast magnetic resonance imaging reveals net retrograde aqueductal flow in idiopathic normal pressure hydrocephalus. J. Neurosurg. 124, 1850–1857. doi: 10.3171/2015.6.JNS15496
Ringstad, G., Emblem, K. E., Geier, O., Alperin, N., and Eide, P. K. (2015). Aqueductal stroke volume: comparisons with intracranial pressure scores in idiopathic normal pressure hydrocephalus. Am. J. Neuroradiol. 36, 1623–1630. doi: 10.3174/ajnr.A4340
Ringstad, G., Vatnehol, S. A. S., and Eide, P. K. (2017). Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 140, 2691–2705. doi: 10.1093/brain/awx191
Röricht, S., Meyer, B. U., Woiciechowsky, C., and Lehmann, R. (1998). Callosal and corticospinal tract function in patients with hydrocephalus: a morphometric and transcranial magnetic stimulation study. J. Neurol. 245, 280–288. doi: 10.1007/s004150050219
Sakakibara, R., Kanda, T., Sekido, T., Uchiyama, T., Awa, Y., Ito, T., et al. (2008). Mechanism of bladder dysfunction in idiopathic normal pressure hydrocephalus. Neurourol. Urodyn. 27, 507–510. doi: 10.1002/nau.20547
Sakakibara, R., Uchida, Y., Ishii, K., Kazui, H., Hashimoto, M., Ishikawa, M., et al. (2012). Correlation of right frontal hypoperfusion and urinary dysfunction in iNPH: a SPECT study. Neurourol. Urodyn. 31, 50–55. doi: 10.1002/nau.21222
Sarica, A., Quattrone, A., Mechelli, A., Vaccaro, M. G., Morelli, M., and Quattrone, A. (2021). Corticospinal tract abnormalities and ventricular dilatation: a transdiagnostic comparative tractography study. Neuroimage Clin. 32:102862. doi: 10.1016/j.nicl.2021.102862
Satow, T., Aso, T., Nishida, S., Komuro, T., Ueno, T., Oishi, N., et al. (2017). Alteration of venous drainage route in idiopathic normal pressure hydrocephalus and normal aging. Front. Aging Neurosci. 9:387. doi: 10.3389/fnagi.2017.00387
Scollato, A., Tenenbaum, R., Bahl, G., Celerini, M., Salani, B., and Di Lorenzo, N. (2008). Changes in aqueductal CSF stroke volume and progression of symptoms in patients with unshunted idiopathic normal pressure hydrocephalus. Am. J. Neuroradiol. 29, 192–197. doi: 10.3174/ajnr.A0785
Shokri-Kojori, E., Wang, G.-J., Wiers, C. E., Demiral, S. B., Guo, M., Kim, S. W., et al. (2018). β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. U S A 115, 4483–4488. doi: 10.1073/pnas.1721694115
Siasios, I., Kapsalaki, E. Z., Fountas, K. N., Fotiadou, A., Dorsch, A., Vakharia, K., et al. (2016). The role of diffusion tensor imaging and fractional anisotropy in the evaluation of patients with idiopathic normal pressure hydrocephalus: a literature review. Neurosurg. Focus 41:E12. doi: 10.3171/2016.6.FOCUS16192
Sweeney, M. D., Sagare, A. P., and Zlokovic, B. V. (2018). Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133–150. doi: 10.1038/nrneurol.2017.188
Tarasoff-Conway, J. M., Carare, R. O., Osorio, R. S., Glodzik, L., Butler, T., Fieremans, E., et al. (2015). Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 11, 457–470. doi: 10.1038/nrneurol.2015.119
Todisco, M., Zangaglia, R., Minafra, B., Pisano, P., Trifirò, G., Bossert, I., et al. (2021). Clinical outcome and striatal dopaminergic function after shunt surgery in patients with idiopathic normal pressure hydrocephalus. Neurology 96, e2861–e2873. doi: 10.1212/WNL.0000000000012064
Toma, A. K., Papadopoulos, M. C., Stapleton, S., Kitchen, N. D., and Watkins, L. D. (2013). Systematic review of the outcome of shunt surgery in idiopathic normal-pressure hydrocephalus. Acta Neurochir. (Wien) 155, 1977–1980. doi: 10.1007/s00701-013-1835-5
Virhammar, J., Laurell, K., Ahlgren, A., Cesarini, K. G., and Larsson, E.-M. (2014). Idiopathic normal pressure hydrocephalus: cerebral perfusion measured with pCASL before and repeatedly after CSF removal. J. Cereb. Blood Flow Metab. 34, 1771–1778. doi: 10.1038/jcbfm.2014.138
Virhammar, J., Laurell, K., Ahlgren, A., and Larsson, E.-M. (2017). Arterial spin-labeling perfusion MR imaging demonstrates Regional CBF decrease in idiopathic normal pressure hydrocephalus. Am. J. Neuroradiol. 38, 2081–2088. doi: 10.3174/ajnr.A5347
Xu, Z., Xiao, N., Chen, Y., Huang, H., Marshall, C., Gao, J., et al. (2015). Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol. Neurodegener. 10:58. doi: 10.1186/s13024-015-0056-1
Yamada, S., Ishikawa, M., Ito, H., Yamamoto, K., Yamaguchi, M., Oshima, M., et al. (2020). Cerebrospinal fluid dynamics in idiopathic normal pressure hydrocephalus on four-dimensional flow imaging. Eur. Radiol. 30, 4454–4465. doi: 10.1007/s00330-020-06825-6
Zeppenfeld, D. M., Simon, M., Haswell, J. D., D’Abreo, D., Murchison, C., Quinn, J. F., et al. (2017). Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains. JAMA Neurol. 74, 91–99. doi: 10.1001/jamaneurol.2016.4370
Zhou, Y., Cai, J., Zhang, W., Gong, X., Yan, S., Zhang, K., et al. (2020). Impairment of the glymphatic pathway and putative meningeal lymphatic vessels in the aging human. Ann. Neurol. 87, 357–369. doi: 10.1002/ana.25670
Ziegelitz, D., Arvidsson, J., Hellström, P., Tullberg, M., Wikkelsø, C., and Starck, G. (2015). In patients with idiopathic normal pressure hydrocephalus postoperative cerebral perfusion changes measured by dynamic susceptibility contrast magnetic resonance imaging correlate with clinical improvement. J. Comput. Assist. Tomogr. 39, 531–540. doi: 10.1097/RCT.0000000000000254
Ziegelitz, D., Arvidsson, J., Hellström, P., Tullberg, M., Wikkelsø, C., and Starck, G. (2016). Pre-and postoperative cerebral blood flow changes in patients with idiopathic normal pressure hydrocephalus measured by computed tomography (CT)-perfusion. J. Cereb. Blood Flow Metab. 36, 1755–1766. doi: 10.1177/0271678X15608521
Ziegelitz, D., Starck, G., Kristiansen, D., Jakobsson, M., Hultenmo, M., Mikkelsen, I. K., et al. (2014). Cerebral perfusion measured by dynamic susceptibility contrast MRI is reduced in patients with idiopathic normal pressure hydrocephalus. J. Magn. Reson. Imaging 39, 1533–1542. doi: 10.1002/jmri.24292
Keywords: idiopathic normal pressure hydrocephalus (iNPH), glymphatic circulation, ventriculoperitoneal (VP) shunt, cerebral blood flow, dementia, communicating hydrocephalus, blood brain barrier (BBB) breakdown
Citation: Bonney PA, Briggs RG, Wu K, Choi W, Khahera A, Ojogho B, Shao X, Zhao Z, Borzage M, Wang DJJ, Liu C and Lee DJ (2022) Pathophysiological Mechanisms Underlying Idiopathic Normal Pressure Hydrocephalus: A Review of Recent Insights. Front. Aging Neurosci. 14:866313. doi: 10.3389/fnagi.2022.866313
Received: 31 January 2022; Accepted: 28 March 2022;
Published: 28 April 2022.
Edited by:
Mark Stecker, Independent Practitioner, Fresno, United StatesReviewed by:
Per Kristian Eide, University of Oslo, NorwayCopyright © 2022 Bonney, Briggs, Wu, Choi, Khahera, Ojogho, Shao, Zhao, Borzage, Wang, Liu and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Phillip A. Bonney, cGhpbC5hbGFuLmJvbm5leUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.