- Department of Ophthalmology, Jiangxi Center of National Ocular Disease Clinical Research Center, The First Affiliated Hospital of Nanchang University, Nanchang, China
Objective: In this study, the regional homogeneity (ReHo) method was used to investigate levels of cerebral homogeneity in individuals with age-related macular degeneration (AMD), with the aim of exploring whether these measures are associated with clinical characteristics.
Materials and Methods: Patients with AMD and healthy controls attending the First Affiliated Hospital of Nanchang University were invited to participate. Resting state functional magnetic resonance images were recorded in each participant and levels of synchronous neural activity were evaluated using ReHo. Receiver operating characteristic (ROC) curves were used to evaluate the sensitivity and specificity of this method.
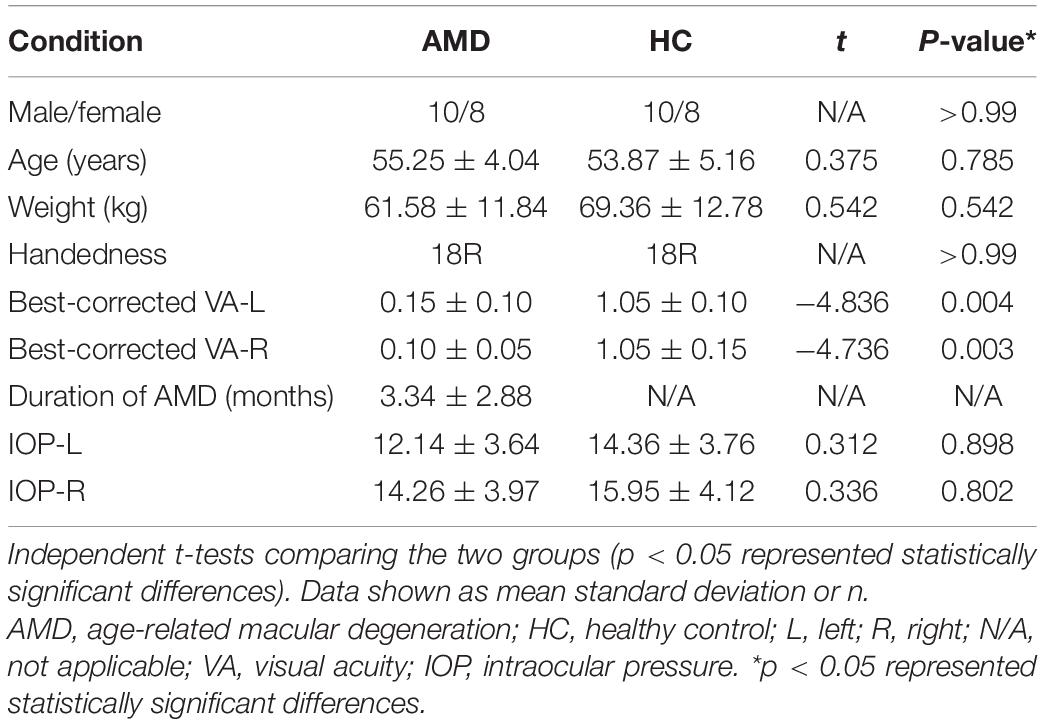
Results: Eighteen patients with AMD (9 males and 9 females) and 15 healthy controls (HCs) were recruited. The two groups were approximately matched in age, gender and weight. Compared with controls, the ReHo values were significantly higher in the AMD group at the limbic lobe and parahippocampal gyrus, and were significantly reduced at the cingulate gyrus, superior frontal gyrus, middle frontal gyrus, inferior parietal lobule, and precentral gyrus. Mean ReHo values at the cingulate gyrus and the superior frontal gyrus were negatively correlated with clinical symptoms.
Conclusion: Brain neural homogeneity dysfunction is a manifestation of visual pathways in AMD patients, and may be one of the pathological mechanisms of chronic vision loss, anxiety and depression in AMD patients. In addition, the ReHo data may be useful for early screening for AMD.
Introduction
Age-related macular degeneration (AMD) is a significant cause of irreversible blindness in the elderly (VanNewkirk et al., 2000). AMD affects over one-quarter of those who aged over 75 and is the world’s third most common blinding eye disease, as well as the most common reason for irreversible blindness among the elderly in Western countries (Pennington and DeAngelis, 2016). Asia has recently witnessed an increase in the incidence of AMD and it has been predicted to evolve into a global disease, with the total number of people affected worldwide reaching 196 million by 2020 and increasing to 288 million in 2040 (Wong et al., 2014). The treatment of retinal angiogenesis and fluid leakage in neovascular AMD is currently treated by blocking vascular endothelial growth factor A (Bakri et al., 2019). The mechanisms by which AMD exerts long-term effects on the human brain and behavior are not clear, and there is no cure for this disease. Few researchers have explored the relationship between AMD and spontaneous brain activity. Previous studies using functional magnetic resonance imaging (fMRI) and electroencephalography (EEG) have suggested that synchronous neuronal activity may also make a difference in numerous neurophysiological events (Ward, 2003; Spencer et al., 2004; Li et al., 2015). Resting state fMRI (rs-fMRI) is one of the most effective ways to detect changes in brain activity. Regional homogeneity (ReHo) is a measurement technique used in rs-fMRI to estimate the consistency of signals related to blood oxygen levels between adjacent voxels throughout the brain at rest (Tononi et al., 1998; Zang et al., 2004). ReHo is a method to evaluate brain activity in its resting state, as well as one of the methods currently available to study the partial synchronization of idiopathic fMRI signs. Our previous research using the ReHo method has assessed neurological status in eye diseases including corneal ulcer (Xu et al., 2019), diabetic retinopathy (Liao et al., 2019), optical neuritis (Shao et al., 2015) and others (Dai et al., 2012; Song et al., 2014; Huang et al., 2016a,b, 2017a,2017b; Li et al., 2016, 2020; Tan et al., 2016a,b; Tang et al., 2018; Ye et al., 2018; Zhu et al., 2018; Shao et al., 2019; Xiang et al., 2019; Zhang et al., 2020).
Resting-state fMRI and ReHo values may be useful indicators of macular degeneration at an early stage. In the present study, correlation analysis was used to calculate the average ReHo signal in different brain regions to explore the relationship between the signal and clinical symptoms in AMD patients.
Materials and Methods
Subjects
Patients with AMD who regularly visited the ophthalmology department of the First Affiliated Hospital of Nanchang University were invited to participate in this study. Inclusion criteria for AMD patients were: (1) age-related macular degeneration diagnosed using fundus fluorescein angiography and confirmed by indocyanine green angiography (Spectralis HRA-OCT; Heidelberg Engineering, Heidelberg, Germany; Figure 1); (2) no eye disease other than AMD; (3) no anti-vascular endothelial growth factor treatment; (4) no dementia (based on an existing diagnosis, or five or more errors in the Short Portable Mental Status Questionnaire); and (5) no history of brain surgery.
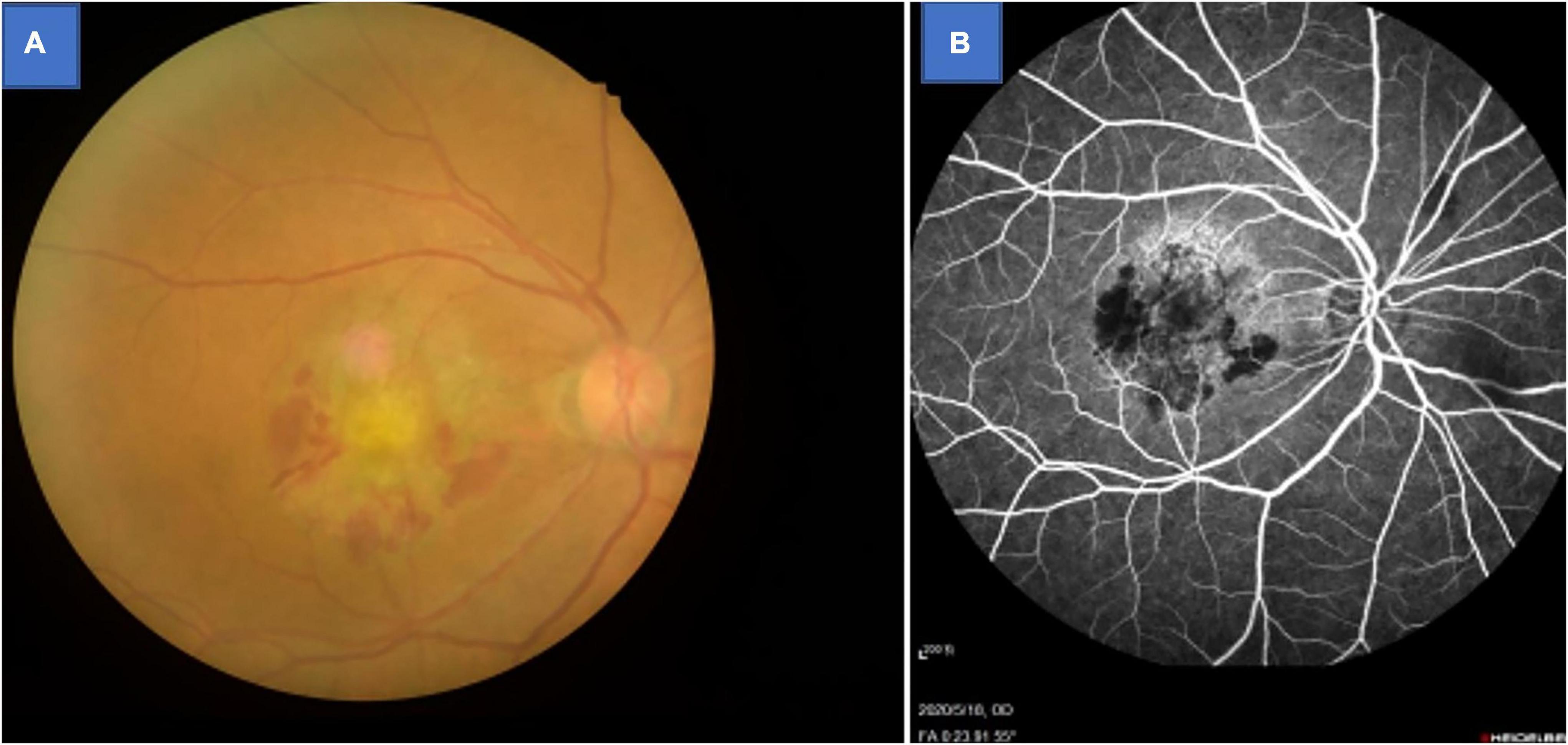
Figure 1. Example of age-related macular degeneration seen on fundus camera (A) and fluorescence fundus angiography (B).
In addition, patients with a history or diagnosis of mild cognitive impairment, Alzheimer’s disease, generalized anxiety disorder, depressive disorder, Parkinson’s disease, proliferative retinopathy, other retinopathy, retinal vein occlusion, neovascular glaucoma, chronic myeloproliferative disease, macular or cystic macular edema were excluded since these conditions may alter the value of the ReHo signals in the brain region associated with macular degeneration.
Healthy controls who met the following criteria were eligible for inclusion: (1) no contraindications to MRI scan (such as implanted metal device); (2) no neurological diseases or psychiatric diseases (such as mania or depression); (3)no prior or present age-related macular degeneration or other retinal or fundus lesions.
The methods used in this study were consistent with the Declaration of Helsinki. The study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University. Research protocols and procedures were fully explained to each subject before obtaining written informed consent.
MRI Parameters
MRI scanning was performed on a 3-Tesla MR scanner (Trio, Siemens, Munich, Germany). T1-weighted images with high resolution were acquired using a tridimensional destruction gradient echo sequence, with repetition time = 190 ms, echo time = 2.26 ms, thickness = 3.0 mm, gap = 0.5 mm, acquisition matrix = 256 × 256, field of view = 250 mm × 250 mm, and flip angle = 9°. Some functional images needed to be corrected at thickness = 4.0 mm, repetition time = 2,000 ms, echo time = 30 ms, gap = 1.2 mm, and field of view = 220 mm × 220 mm, 29 axial.
Data Analysis From Functional Magnetic Resonance Imaging
Using MRIcro1 software [MRIcro software (McCausland Center for Brain Imaging, Columbia, SC, United States)],1 all images were checked and any defective images removed. The first 10 volumes recorded from each subject were discarded to remove any noise associated with movement at the beginning of the procedure. The valid images were processed using SPM82 and Data Processing Assistant for rs-fMRI DPARSFA (Institute of Psychology, CAS., Beijing, People’s Republic of China) software. Following this, slice timing, head motion correction (any of the six parameters within 1.5 mm or 1.5), and spatial normalization were performed on the digital data. The data were then smoothed using a 6 mm full-width at half-maximum Gaussian. Finally, the fMRI image space was normalized to the Montreal Neurological Institute space employing an echo plane imaging template and was resampled at a resolution of 3 mm × 3 mm × 3 mm. To optimize reliability, the data were de-trended and bandpass filtered (0.01–0.08 Hz) to remove low-frequency drift and physiological high-frequency respiratory and cardiac noise.
Statistical Analysis
To compare ReHo values between AMD and HC groups, SPM8 software was used to conduct an independent-samples test after excluding other influencing factors such as age and gender (two-tail, voxel level: P < 0.005 Gaussian random field correction, cluster-level: P < 0.05, cluster: 162).
Brain–Behavior Correlation Analysis
Regions of interest were defined on images from each group using REST software.2 Within each region, the average ReHo value was obtained from the ReHo values of all voxels. Correlation analysis were used to determine whether the ReHo values were associated with clinical manifestations (P < 0.05 was considered statistically significant).
Clinical Data Analysis
Intraocular pressure, best-corrected visual acuity and body weight were measured in each participant, and these plus disease duration were recorded. Demographic and clinical variables were compared between the two groups using SPSS version 20.0 software, and P-values < 0.05 were again considered significant. Receiver operating characteristic (ROC) curves were used to test stability, sensitivity and specificity.
Results
Demographics and Behavioral Results
The two groups were statistically similar in weight (P = 0.542) and age (P = 0.785), but significantly poorer monocular visual acuities were found in the AMD than HC group (right P = 0.003; left P = 0.004) (Table 1).
Regional Homogeneity Value Comparisons Between Groups
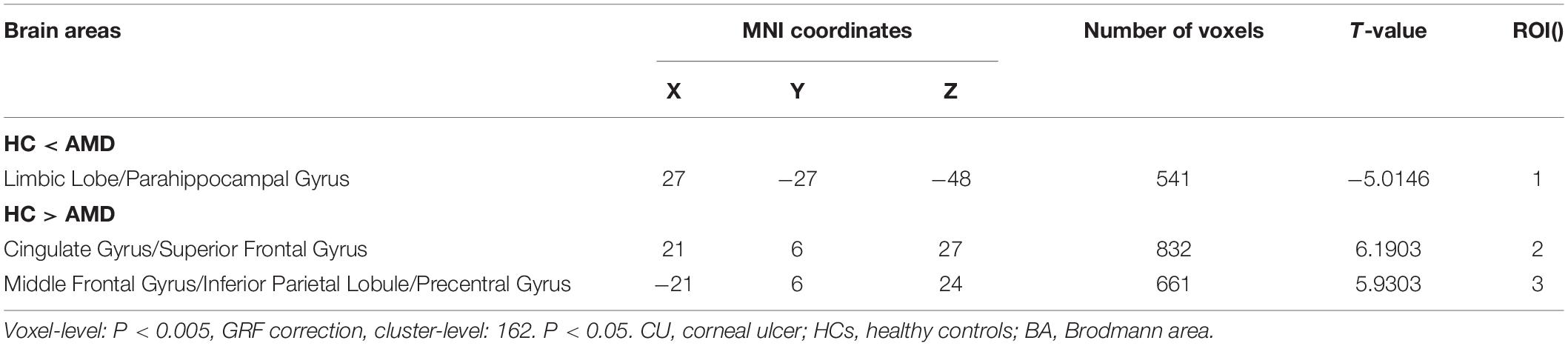
ReHo values in the AMD group were significantly higher than controls at the limbic lobe and parahippocampal gyrus (P < 0.05), and significantly lower at the cingulate gyrus, superior frontal gyrus, middle frontal gyrus, inferior parietal lobule and precentral gyrus (P < 0.05) (Figures 2, 3 and Table 2).
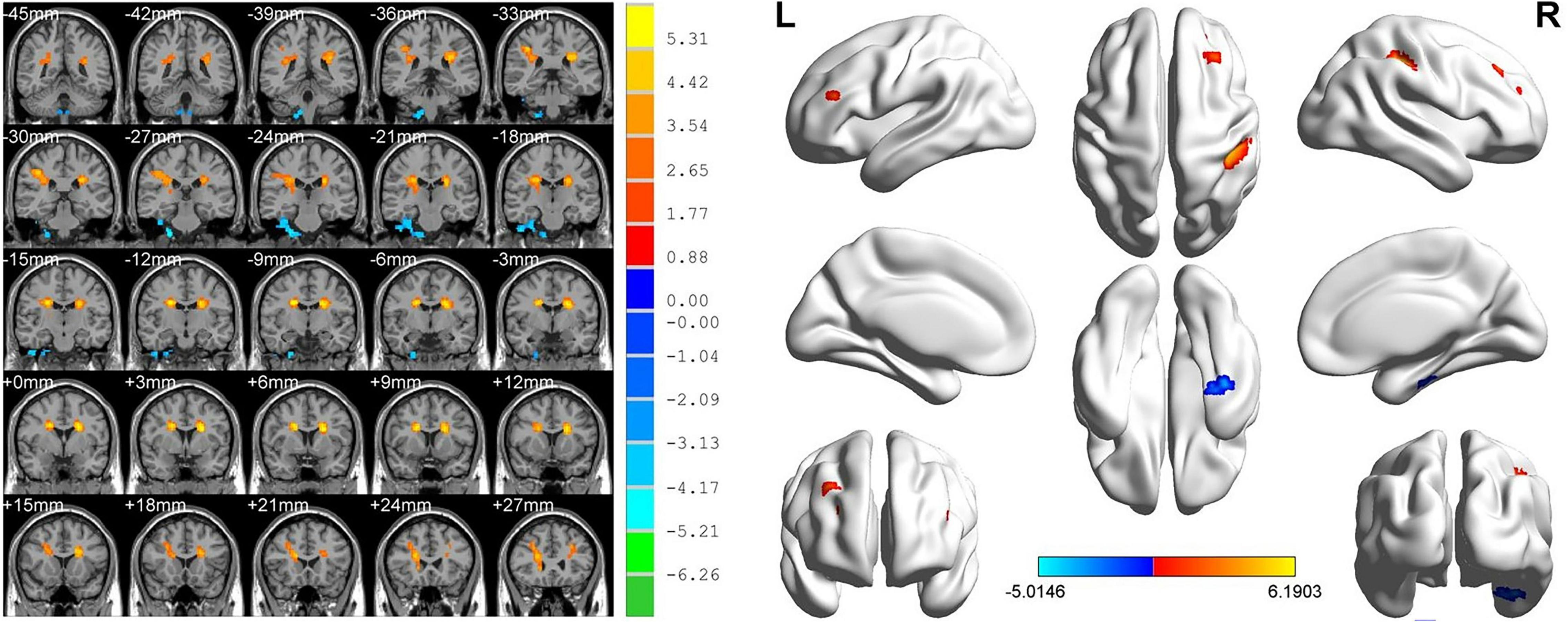
Figure 2. Significant differences in ReHo values between the AMD group and HCs. Blue areas denote significantly reduced ReHo values in the cingulate gyrus, superior frontal gyrus, middle frontal gyrus, inferior parietal lobule, and precentral gyrus, red areas denote significantly increased ReHo values in the limbic lobe and parahippocampal gyrus.
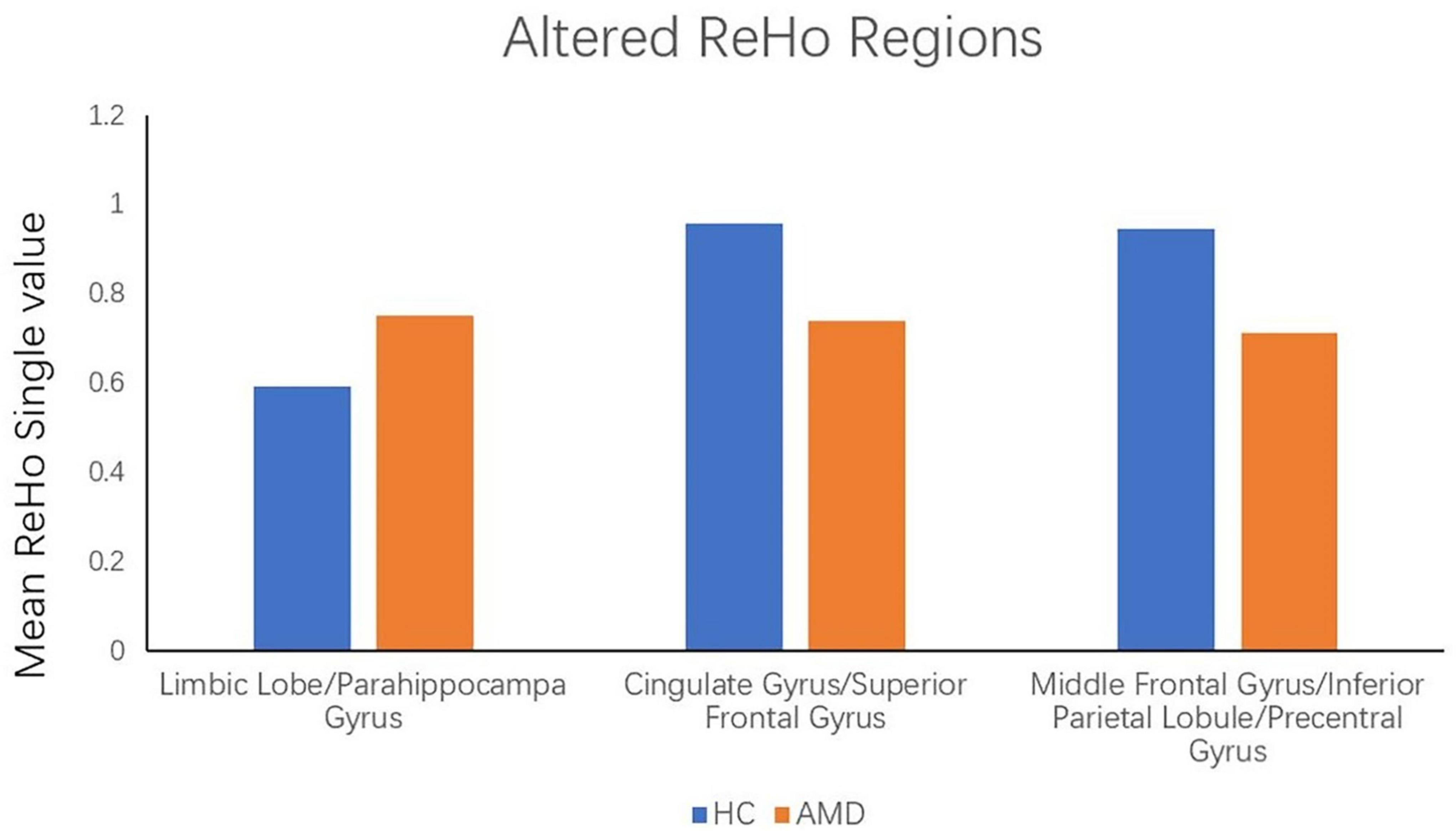
Figure 3. The mean single ReHo value between the AMDs group and HCs. Data presented as mean ± standard deviation. ReHo, regional homogeneity; HCs, healthy controls; N/A, not applicable; AMD, age-related macular degeneration.
Receiver Operating Characteristic Curve
Since ReHo values differed between groups, as explained above, they were further analyzed using ROC curves to assess how well these values distinguish between the two groups. AUC (Area Under Curve) is defined as the area under the ROC curve enclosed by the coordinate axis, The closer the AUC is to 1.0, the higher the authenticity of the detection method AUCfor ReHo values at the cingulate gyrus, superior frontal gyrus, middle frontal gyrus, inferior parietal lobule and precentral gyrus was 0.944 in each case (AMDs > HCs) (Figure 4B). ROC curve can also reflect to some extent that ReHo values have certain advantages in diagnosing AMD (Figure 4A).
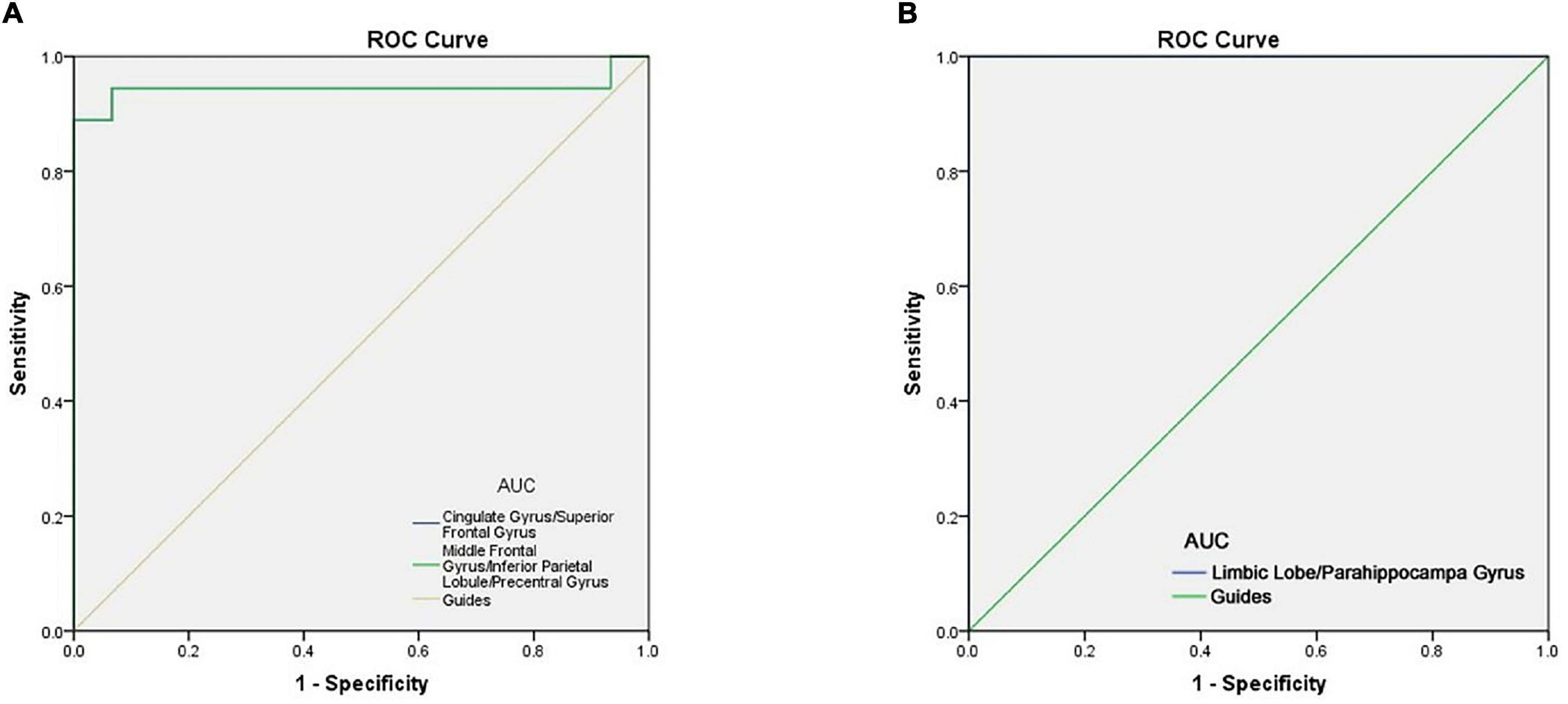
Figure 4. ROC curve analysis of the mean ReHo values for altered brain regions. (A) The area under the ROC curve were 0.944 (p < 0.0001; 95% CI: 0.845–1.000), for Cingulate Gyrus/Superior Frontal Gyrus, Middle Frontal Gyrus/Inferior Parietal Lobule/Precentral Gyrus 0.944 (p < 0.0001; 95% CI: 0.845–1.000). (B) The area under the ROC curve were 1.000 (p < 0.0001; 95% CI: 1.000–1.000), for Limbic Lobe/Parahippocampal Gyrus. AUC, area under the curve; ROC, receiver operating characteristic.
Correlation Analysis
The present study used the Chinese version of the Hospital Anxiety and Depression Scale (HADS). The HADS questionnaire, 10 which involves self-assessment, has been found to be a reliable instrument for determining depression and anxiety status in a hospital outpatient clinic setting. The anxiety and depressive subscales are also valid measures of the severity of emotional disorder. We defined that a score greater than or equal to 8 points was positive. The higher the score, the more serious the depression and anxiety. Within the AMD group, anxiety scores, depression scores and disease duration were all inversely correlated with the ReHo values at the cingulate gyrus and at the superior frontal gyrus (P < 0.05). These data indicate that AMD is associated with all three factors (Figure 5).
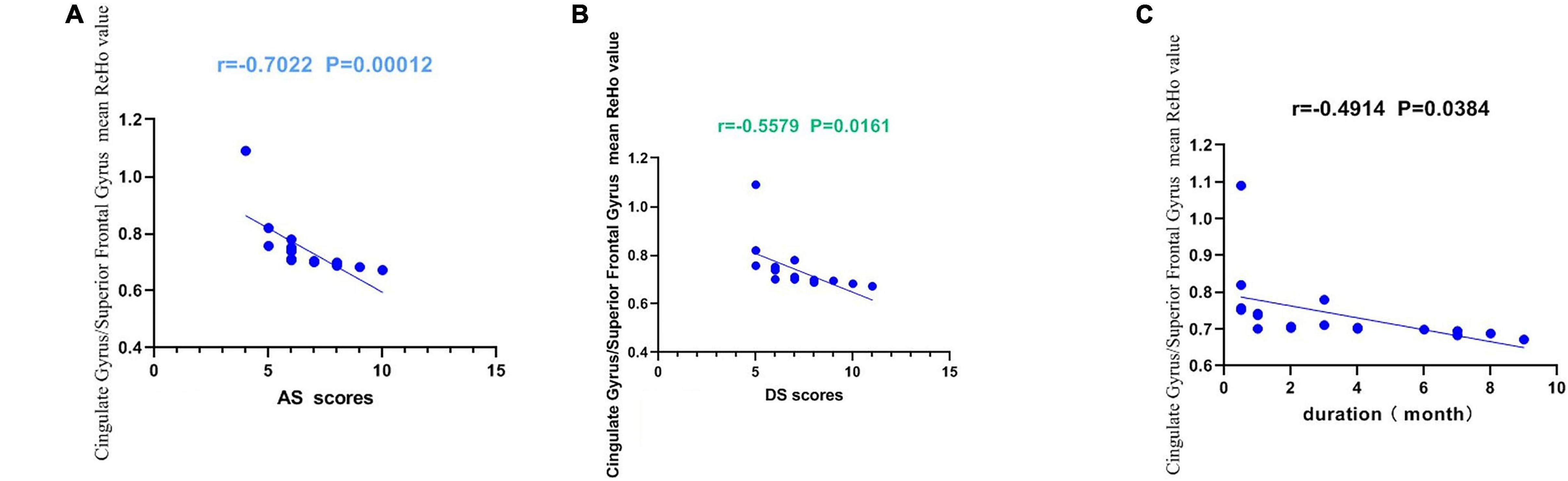
Figure 5. Correlations between the mean ReHo values of the Cingulate Gyrus/Superior Frontal Gyrus and the clinical behaviors. (A) The AS showed a negative correlation with the mean ReHo values of the Cingulate Gyrus/Superior Frontal Gyrus (r = −0.702, p = 0.0001 < 0.001), (B) the DS showed a negative correlation with the mean ReHo values of the Cingulate Gyrus/Superior Frontal Gyrus (r = −0.5579, p = 0.0161 < 0.05). (C) The duration showed a negative correlation with the mean ReHo values of the Cingulate Gyrus/Superior Frontal Gyrus (r = 0.4914, p = 0.0384 < 0.05). AS, anxiety scores; DS, depression scores.
Discussion
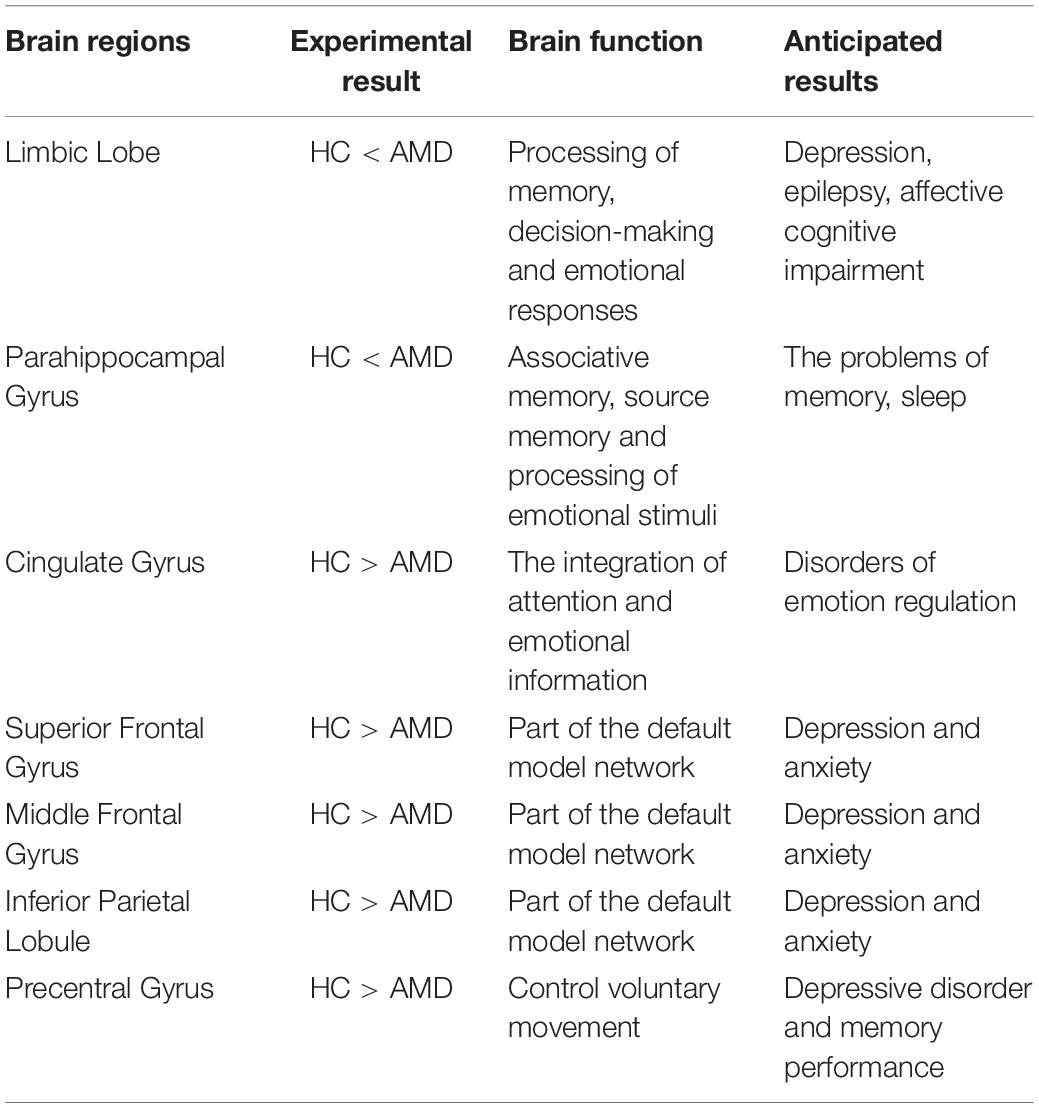
Our previous studies on the ReHo method have demonstrated that it can be applied to a variety of ophthalmic diseases and has broad scope for further development (Table 3). The studies have highlighted regional disease-related changes in brain activity and their potential effects that needed to be further examined (Table 4). So far, there is no consensus on the relationship between ReHo value and resting state of AMD patients, and the present study aimed to fill this gap.
We found that ReHo values were significantly different between AMD patients and controls, being higher in AMD at the limbic lobe and parahippocampal gyrus and lower at the cingulate gyrus, superior frontal gyrus, middle frontal gyrus, inferior parietal lobule and the precentral gyrus (Figure 6).
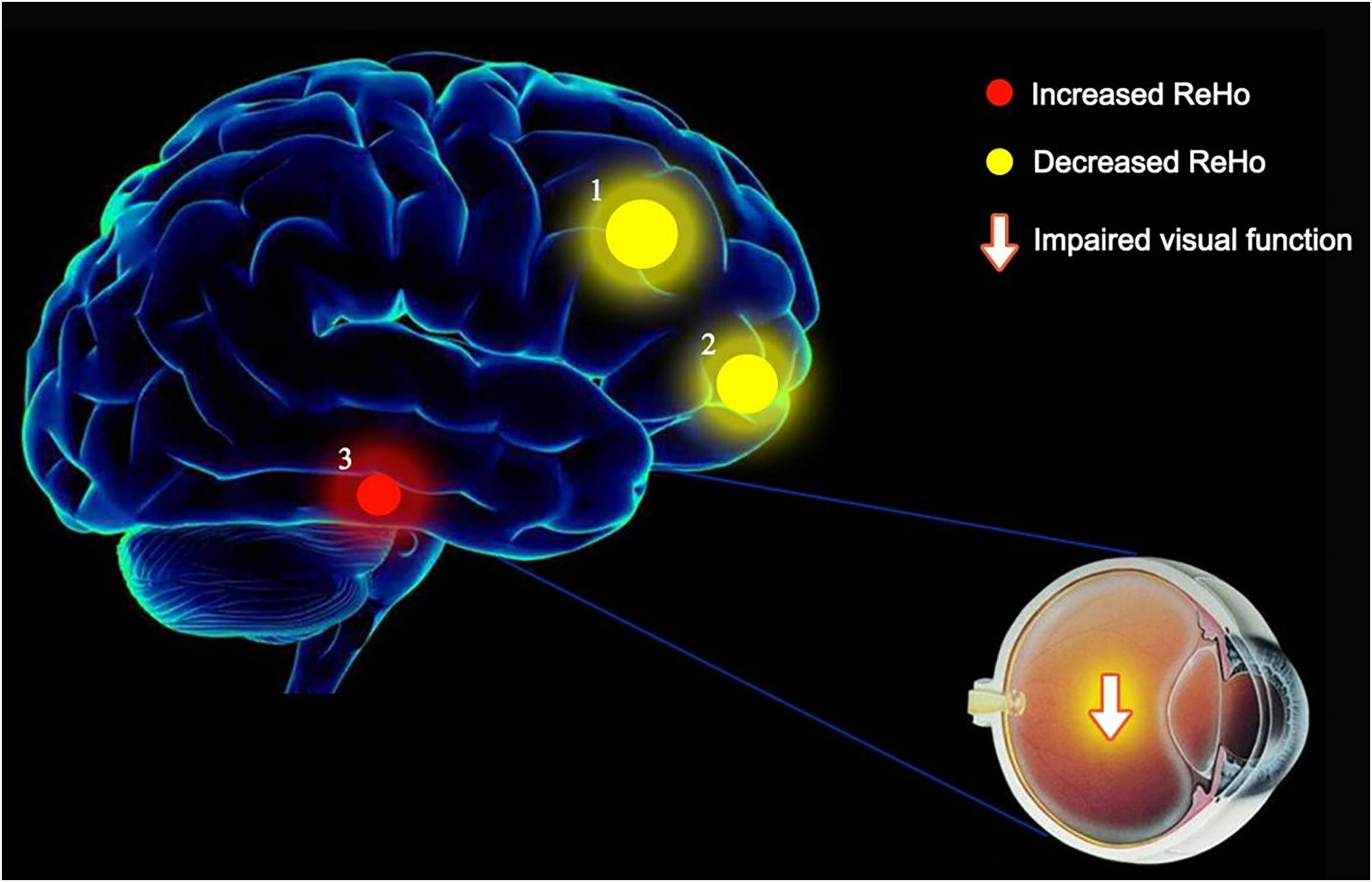
Figure 6. The ReHo results of brain activity in the AMD group. Compared with the HCs, the ReHo values of the following regions were decreased to various extents: 1- Cingulate Gyrus/Superior Frontal Gyrus (BA 32, t = 6.1903), 2- Middle Frontal Gyrus/Inferior Parietal Lobule/Precentral Gyrus (t = 5.9303). Compared with the HCs, the ReHo values of the following regions were increased to various extents: 3- Limbic Lobe/Parahippocampal Gyrus (t = −5.0146). HCs, healthy controls; BA, Brodmann’s area.
Implications of Increased Regional Homogeneity Values in Age-Related Macular Degeneration
The limbic system plays a major part in memory, decision making and emotional feedback. Research has shown that its damage interferes with memories that are enhanced by emotion (Amunts et al., 2005) and that the limbic system is associated with depression and anxiety in the epilepsies (Krishnan, 2020). The brain combines emotion with cognition to produce flexible behavioral output based on its judgments of the environment (Rusbridge, 2020). In addition, the parahippocampal gyrus is associated with episodic memory relating to source memory, associative memory and processing of emotional stimuli (Suthana et al., 2012). For example, Mankin found that deep brain stimulation of hippocampal circuits can modulate human memory (Mankin and Fried, 2020). In addition to its role in memory, the parahippocampal cortex is involved in visuospatial processing related to scene perception and spatial representation of navigation (Aminoff et al., 2013). However, further study is needed to confirm whether an increase in the value of ReHo in the parahippocampal gyrus in AMD has an effect on memory enhancement (Figure 7).
Figure 7. Correlations between AMDs ReHo and behavioral performance, compare with HCs, AMDs may suffer more problems with dealing with emotion, memory and visual disturbances.
Implications of Decreased Regional Homogeneity Values in Age-Related Macular Degeneration
Anatomically, the anterior central gyrus, also called precentral gyrus, is divided into four parts by three contours in the paracentral lobule and gyrus. It is bounded above by the anterior central sulcus and below by the lateral fissure, which is mainly located on the lateral side of the cerebral hemisphere. Study has shown that abnormal or weak connections between them may be risk factors for disease (Nebel et al., 2014). According to previous studies, the paracentral gyrus has also been associated with memory ability and depression disorders (Nebel et al., 2014; Li et al., 2018; Shang et al., 2018).
The structure on the medial side of the cerebral hemisphere between the cingulate sulcus and the corpus callosum sulcus is called the cingulate gyrus. It belongs to the cortical part of the limbic system and is an important region connecting the orbitofrontal cortex, amygdala, insular lobe, septal nucleus, and hypothalamus. The cingulate gyrus is the bridge between attention and emotional processing and is responsible for the integration of attention and emotional information. Burger’s research points out that cingulate gyrus activation was sharply reduced in major depressive disorders (Nebel et al., 2014) potentially indicating impaired bottom-up emotional processing and abnormal automatic emotional regulation. The Fischer’s study suggested that parietal activity may be particularly important for linking long-term memory representation and attention components (Bürger et al., 2017). In other fMRI study, frontal and parietal activation was found in spatial memory-guided attention tasks (Fischer et al., 2021).
To some extent, the decline of memory and depression in AMD patients can be traced to the changes in brain activity (Giesbrecht et al., 2013). The present results showed that the ReHo value of five brain regions were decreased in AMD, with reliability verified by the ROC curve analysis results. AUC values of over 0.7 are considered high, and our analysis showed that the AUC values of ReHo values in the above brain regions were all greater than 0.9, indicating very high accuracy. The abnormality of ReHo values in some brain regions is an important finding relating to the diagnosis of AMD based on imaging data. The results suggest that the ReHo method may be a non-invasive, rapid and sensitive method for early diagnosis of AMD patients in the future.
Conclusion
The present findings suggest that AMD patients have abnormal spontaneous brain activity, which may prove useful for early disease detection. Activity in the cingulate gyrus and superior frontal gyrus was inversely associated with anxiety, depression and disease duration. These findings provide powerful information for further research. However, there are still some limitations in our study. Such as larger sample sizes and detailed grouping of different types of AMDs are needed. Moreover, our study only demonstrated the existence of the correlation between changes of ReHo values in specific brain regions and RVO. But It is unclear whether AMD will cause changes in brain activity or whether patients with brain dysfunction are susceptible to AMD.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the First Affiliated Hospital of Nanchang University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Q-YiL, Y-CP, H-YS, YS, and QZ: study design and manuscript preparation. Q-YiL, Y-CP, H-YS, L-JZ, YS, and QZ: data collection. Q-YiL, Y-CP, H-YS, Q-YuL, YS, and QZ: statistical analysis. Q-YiL, Y-CP, H-YS, Q-MG, YS, and QZ: data interpretation and literature search. All authors read and approved the final manuscript.
Funding
This work was supported by the Central Government Guides Local Science and Technology Development Foundation (No. 20211ZDG02003), the Key Research Foundation of Jiangxi Province (Nos. 20181BBG70004 and 20203BBG73059), the Excellent Talents Development Project of Jiangxi Province (No. 20192BCBL23020), the Natural Science Foundation of Jiangxi Province (No. 20181BAB205034), the Grassroots Health Appropriate Technology “Spark Promotion Plan” Project of Jiangxi Province (No. 20188003), the Health Development Planning Commission Science Foundation of Jiangxi Province (Nos. 20201032 and 202130210), the Health Development Planning Commission Science TCM Foundation of Jiangxi Province (Nos. 2018A060 and 2020A0087), and the Education Department Foundation of Jiangxi Province (Nos. GJJ200157, GJJ200159, and GJJ200169).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Aminoff, E. M., Kveraga, K., and Bar, M. (2013). The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 17, 379–390. doi: 10.1016/j.tics.2013.06.009
Amunts, K., Kedo, O., Kindler, M., Pieperhoff, P., Mohlberg, H., Shah, N. J., et al. (2005). Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. (Berl). 210, 343–352. doi: 10.1007/s00429-005-0025-5
Bakri, S. J., Thorne, J. E., Ho, A. C., Ehlers, J. P., Schoenberger, S. D., Yeh, S., et al. (2019). Safety and efficacy of anti-vascular endothelial growth factor therapies for neovascular age-related macular degeneration: a report by the American academy of ophthalmology. Ophthalmology 126, 55–63. doi: 10.1016/j.ophtha.2018.07.028
Bürger, C., Redlich, R., Grotegerd, D., Meinert, S., Dohm, K., Schneider, I., et al. (2017). Differential abnormal pattern of anterior cingulate gyrus activation in unipolar and bipolar depression: an fMRI and pattern classification approach. Neuropsychopharmacology 42, 1399–1408. doi: 10.1038/npp.2017.36
Dai, X. J., Gong, H. H., Wang, Y. X., Zhou, F. Q., Min, Y. J., Zhao, F., et al. (2012). Gender differences in brain regional homogeneity of healthy subjects after normal sleep and after sleep deprivation: a resting-state fMRI study. Sleep Med. 13, 720–727. doi: 10.1016/j.sleep.2011.09.019
Fischer, M., Moscovitch, M., and Alain, C. (2021). A systematic review and meta-analysis of memory-guided attention: frontal and parietal activation suggests involvement of fronto-parietal networks. Wiley Interdiscip. Rev. Cogn. Sci. 12:e1546. doi: 10.1002/wcs.1546
Giesbrecht, B., Sy, J. L., and Guerin, S. A. (2013). Both memory and attention systems contribute to visual search for targets cued by implicitly learned context. Vis. Res. 85, 80–89. doi: 10.1016/j.visres.2012.10.006
Huang, X., Li, H. J., Ye, L., Zhang, Y., Wei, R., Zhong, Y. L., et al. (2016a). Altered regional homogeneity in patients with unilateral acute open-globe injury: a resting-state functional MRI study. Neuropsychiatr. Dis. Treat. 12, 1901–1906. doi: 10.2147/NDT.S110541
Huang, X., Li, S. H., Zhou, F. Q., Zhang, Y., Zhong, Y. L., Cai, F. Q., et al. (2016b). Altered intrinsic regional brain spontaneous activity in patients with comitant strabismus: a resting-state functional MRI study. Neuropsychiatr. Dis. Treat. 12, 1303–1308. doi: 10.2147/NDT.S105478
Huang, X., Li, D., Li, H. J., Zhong, Y. L., Freeberg, S., Bao, J., et al. (2017a). Abnormal regional spontaneous neural activity in visual pathway in retinal detachment patients: a resting-state functional MRI study. Neuropsychiatr. Dis. Treat. 13, 2849–2854. doi: 10.2147/NDT.S147645
Huang, X., Ye, C. L., Zhong, Y. L., Ye, L., Yang, Q. C., Li, H. J., et al. (2017b). Altered regional homogeneity in patients with late monocular blindness: a resting-state functional MRI study. Neuroreport 28, 1085–1091. doi: 10.1097/WNR.0000000000000855
Krishnan, V. (2020). Depression and anxiety in the epilepsies: from bench to bedside. Curr. Neurol. Neurosci. Rep. 20:41. doi: 10.1007/s11910-020-01065-z
Li, C., Dong, M., Yin, Y., Hua, K., Fu, S., and Jiang, G. (2018). Aberrant effective connectivity of the right anterior insula in primary insomnia. Front. Neurol. 9:317. doi: 10.3389/fneur.2018.00317
Li, H., Li, L., Shao, Y., Gong, H., Zhang, W., Zeng, X., et al. (2016). Abnormal intrinsic functional hubs in severe male obstructive sleep apnea: evidence from a voxel-wise degree centrality analysis. PLoS One. 11:e0164031. doi: 10.1371/journal.pone.0164031
Li, M. G., Liu, T. F., Zhang, T. H., Chen, Z. Y., Nie, B. B., Lou, X., et al. (2020). Alterations of regional homogeneity in Parkinson’s disease with mild cognitive impairment: a preliminary resting-state fMRI study. Neuroradiology 62, 327–334. doi: 10.1007/s00234-019-02333-7
Li, R., Li, Y., An, D., Gong, Q., Zhou, D., and Chen, H. (2015). Altered regional activity and inter-regional functional connectivity in psychogenic non-epileptic seizures. Sci. Rep. 5:11635. doi: 10.1038/srep11635
Liao, X. L., Yuan, Q., Shi, W. Q., Li, B., Su, T., Lin, Q., et al. (2019). Altered brain activity in patients with diabetic retinopathy using regional homogeneity: a resting-state fMRI study. Endocr. Pract. 25, 320–327. doi: 10.4158/EP-2018-0517
Mankin, E. A., and Fried, I. (2020). Modulation of human memory by deep brain stimulation of the entorhinal-hippocampal circuitry. Neuron 106, 218–235. doi: 10.1016/j.neuron.2020.02.024
Nebel, M. B., Eloyan, A., Barber, A. D., and Mostofsky, S. H. (2014). Precentral gyrus functional connectivity signatures of autism. Front. Syst. Neurosci. 8:80. doi: 10.3389/fnsys.2014.00080
Pennington, K. L., and DeAngelis, M. M. (2016). Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors. Eye Vis. (Lond). 3:34. doi: 10.1186/s40662-016-0063-5
Rusbridge, C. (2020). Neurobehavioral disorders: the corticolimbic system in health and disease. Vet. Clin. North Am. Small Anim. Pract. 50, 1157–1181. doi: 10.1016/j.cvsm.2020.06.009
Shang, C. Y., Lin, H. Y., Tseng, W. Y., and Gau, S. S. (2018). A haplotype of the dopamine transporter gene modulates regional homogeneity, gray matter volume, and visual memory in children with attention-deficit/hyperactivity disorder. Psychol. Med. 48, 2530–2540. doi: 10.1017/S0033291718000144
Shao, Y., Cai, F. Q., Zhong, Y. L., Huang, X., Zhang, Y., Hu, P. H., et al. (2015). Altered intrinsic regional spontaneous brain activity in patients with optic neuritis: a resting-state functional magnetic resonance imaging study. Neuropsychiatr. Dis. Treat. 11, 3065–3073. doi: 10.2147/NDT.S92968
Shao, Y., Li, Q. H., Li, B., Lin, Q., Su, T., Shi, W. Q., et al. (2019). Altered brain activity in patients with strabismus and amblyopia detected by analysis of regional homogeneity: a resting-state functional magnetic resonance imaging study. Mol. Med. Rep. 19, 4832–4840. doi: 10.3892/mmr.2019.10147
Song, Y., Mu, K., Wang, J., Lin, F., Chen, Z., Yan, X., et al. (2014). Altered spontaneous brain activity in primary open angle glaucoma: a resting-state functional magnetic resonance imaging study. PLoS One. 9:e89493. doi: 10.1371/journal.pone.0089493
Spencer, K. M., Nestor, P. G., Perlmutter, R., Niznikiewicz, M. A., Klump, M. C., Frumin, M., et al. (2004). Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 101, 17288–17293. doi: 10.1073/pnas.0406074101
Suthana, N., Haneef, Z., Stern, J., Mukamel, R., Behnke, E., Knowlton, B., et al. (2012). Memory enhancement and deep-brain stimulation of the entorhinal area. N. Engl. J. Med. 366, 502–510. doi: 10.1056/NEJMoa1107212
Tan, G., Huang, X., Ye, L., Wu, A. H., He, L. X., Zhong, Y. L., et al. (2016a). Altered spontaneous brain activity patterns in patients with unilateral acute open globe injury using amplitude of low-frequency fluctuation: a functional magnetic resonance imaging study. Neuropsychiatr. Dis. Treat. 12, 2015–2020. doi: 10.2147/NDT.S110539
Tan, G., Huang, X., Zhang, Y., Wu, A. H., Zhong, Y. L., Wu, K., et al. (2016b). A functional MRI study of altered spontaneous brain activity pattern in patients with congenital comitant strabismus using amplitude of low-frequency fluctuation. Neuropsychiatr. Dis. Treat. 12, 1243–1250. doi: 10.2147/NDT.S104756
Tang, L. Y., Li, H. J., Huang, X., Bao, J., Sethi, Z., Ye, L., et al. (2018). Assessment of synchronous neural activities revealed by regional homogeneity in individuals with acute eye pain: a resting-state functional magnetic resonance imaging study. J. Pain Res. 11, 843–850. doi: 10.2147/JPR.S156634
Tononi, G., McIntosh, A. R., Russell, D. P., and Edelman, G. M. (1998). Functional clustering: identifying strongly interactive brain regions in neuroimaging data. Neuroimage 7, 133–149. doi: 10.1006/nimg.1997.0313
VanNewkirk, M. R., Nanjan, M. B., Wang, J. J., Mitchell, P., Taylor, H. R., and McCarty, C. A. (2000). The prevalence of age-related maculopathy: the visual impairment project. Ophthalmology 107, 1593–1600. doi: 10.1016/s0161-6420(00)00175-5
Ward, L. M. (2003). Synchronous neural oscillations and cognitive processes. Trends Cogn. Sci. 7, 553–559. doi: 10.1016/j.tics.2003.10.012
Wong, W. L., Su, X., Li, X., Cheung, C. M., Klein, R., Cheng, C. Y., et al. (2014). Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob, Health 2, e106–e116. doi: 10.1016/S2214-109X(13)70145-1
Xiang, C. Q., Liu, W. F., Xu, Q. H., Su, T., Yong-Qiang, S., Min, Y. L., et al. (2019). Altered spontaneous brain activity in patients with classical trigeminal neuralgia using regional homogeneity: a resting-state functional MRI study. Pain Pract. 19, 397–406. doi: 10.1111/papr.12753
Xu, M. W., Liu, H. M., Tan, G., Su, T., Xiang, C. Q., Wu, W., et al. (2019). Altered regional homogeneity in patients with corneal ulcer: a resting-state functional MRI study. Front. Neurosci. 13:743. doi: 10.3389/fnins.2019.00743
Ye, L., Wei, R., Huang, X., Shi, W. Q., Yang, Q. C., Yuan, Q., et al. (2018). Reduction in interhemispheric functional connectivity in the dorsal visual pathway in unilateral acute open globe injury patients: a resting-state fMRI study. Int. J. Ophthalmol. 11, 1056–1060. doi: 10.18240/ijo.2018.06.26
Zang, Y., Jiang, T., Lu, Y., He, Y., and Tian, L. (2004). Regional homogeneity approach to fMRI data analysis. Neuroimage 22, 394–400. doi: 10.1016/j.neuroimage.2003.12.030
Zhang, B., Li, B., Liu, R. Q., Shu, Y. Q., Min, Y. L., Yuan, Q., et al. (2020). Altered spontaneous brain activity pattern in patients with ophthalmectomy: an resting-state fMRI study. Int. J. Ophthalmol. 13, 263–270. doi: 10.18240/ijo.2020.02.10
Keywords: neural regional homogeneity, resting state, functional magnetic resonance imaging, age-related macular degeneration (AMD), pathogenesis
Citation: Liu Q-Y, Pan Y-C, Shu H-Y, Zhang L-J, Li Q-Y, Ge Q-M, Shao Y and Zhou Q (2022) Brain Activity in Age-Related Macular Degeneration Patients From the Perspective of Regional Homogeneity: A Resting-State Functional Magnetic Resonance Imaging Study. Front. Aging Neurosci. 14:865430. doi: 10.3389/fnagi.2022.865430
Received: 29 January 2022; Accepted: 30 March 2022;
Published: 09 May 2022.
Edited by:
Yuzhen Xu, Tongji University, ChinaReviewed by:
Yuan Liu, University of Miami Health System, United StatesWensi Tao, University of Miami Health System, United States
Copyright © 2022 Liu, Pan, Shu, Zhang, Li, Ge, Shao and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Shao, ZnJlZWJlZTk5QDE2My5jb20=; Qiong Zhou, cWlvbmd6RDA2QDEyNi5jb20=
†These authors have contributed equally to this work
 Qi-Ying Liu
Qi-Ying Liu Yi-Cong Pan
Yi-Cong Pan Hui-Ye Shu
Hui-Ye Shu Li-Juan Zhang
Li-Juan Zhang Qiu-Yu Li
Qiu-Yu Li Qian-Min Ge
Qian-Min Ge Yi Shao
Yi Shao Qiong Zhou*
Qiong Zhou*