
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 14 April 2022
Sec. Neuroinflammation and Neuropathy
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.860571
This article is part of the Research Topic Insights in Neuroinflammation and Neuropathy View all 8 articles
 Yan Wan1†
Yan Wan1† Hongxiu Guo1†
Hongxiu Guo1† Rentang Bi1†
Rentang Bi1† Shaoli Chen1
Shaoli Chen1 Jing Shen1
Jing Shen1 Man Li1
Man Li1 Yuanpeng Xia1
Yuanpeng Xia1 Lei Zhang1
Lei Zhang1 Zhou Sun1
Zhou Sun1 Xiaolu Chen1
Xiaolu Chen1 Zhuoyuan Cai1
Zhuoyuan Cai1 Zhaowei Wang2
Zhaowei Wang2 Daokai Gong3
Daokai Gong3 Jingwen Xu4
Jingwen Xu4 Dongya Zhu5
Dongya Zhu5 Bo Hu1*
Bo Hu1* Quanwei He1*
Quanwei He1*
This study aimed to compare clinical and prognostic characteristics between recurrent and first-ever ICH. Four thousand twelve patients entered the study, and 64% of them were male. The median age is 62 years (interquartile range, 55–71). Among them, 3,750 (93.5%) patients had no experience of previous ICH, and 262 (6.5%) patients were considered as recurrent ICH. We compared demographic data, baseline clinical characteristics, imaging information, hematological parameters, and clinical outcomes between recurrent and first-ever ICH. We found that recurrent ICH was significantly associated with older age, more frequent history of ischemic heart disease, ischemic stroke, hypertension, and hyperlipidemia, while patients with recurrent ICH had previously received more antihypertensive therapy, and showed lower admission blood pressure (median, 160 vs. 167 mmHg) and higher baseline of National Institute of Health stroke scale (NIHSS) score (median, 10 vs. 9). We also demonstrated that recurrent ICH was an independent risk factor of 3-month function dependence after adjusting for many potentially competitive risk factors.
Intracerebral hemorrhage (ICH) is a more devastating disease than ischemic stroke, features a high mortality rate of 25–50% within a month (Lozano et al., 2012; Li et al., 2021), and the survivors remain at high risk for recurrence. Currently, ICH recurrence rates have been reported up to 2% in 1 year, and 9.6% in 5 years (Hill et al., 2000; Huhtakangas et al., 2013), and recurrent ICH seems to be more disabling or fatal than first-ever ICHs (Pennlert et al., 2014; Skajaa et al., 2021).
Despite various studies that have been conducted to search for candidate risk factors of ICH recurrence (Biffi et al., 2020, 2021; Miki et al., 2020; de Courson et al., 2021; Park et al., 2021a,b; Pinho et al., 2021), the clinical and prognostic characteristics of recurrent ICH have only received limited attention. Indeed, a reliable analysis of recurrent ICH is urgently required to guide the management strategies for secondary prevention and assess the cost-effectiveness of treatment, thereby improving the prognosis of patients. Importantly, controversy still surrounds whether recurrent ICH should be considered as an independent risk factor for adverse outcomes, because recurrent ICH may exhibit a distinguished baseline patient characteristics from first-ever ICH.
To this end, we conducted a population-based cohort study using a nationwide representative sample from the Chinese cerebral hemorrhage: mechanism and intervention (CHERRY) study, to compare clinical and prognostic characteristics between recurrent and first-ever ICH.
We use a representative sample from the CHERRY study. This study included 4,012 patients in 31 medical institutions from December 2018 to March 2021. The ethics of the study is in line with the principles expressed in the Declaration of Helsinki. The local institutional review board approved all aspects of the study (ethical approval number: 2018-S485).
Patients were included according to the following criteria: (1) clinically confirmed spontaneous ICH, which is defined as non-traumatic bleeding into the brain parenchyma confirmed by CT scan (de Oliveira Manoel et al., 2016); (2) 18 years or older; (3) the onset to admission time within 7 days. Exclusion criteria constituted: (1) traumatic ICH; (2) ICH colocalized with primary subdural/epidural/subarachnoid hemorrhage; (3) post-infarct hemorrhagic transformation; (4) hemorrhage after thrombolysis.
The following data were collected: (1) demographic data, including age, gender, medical history, medication history (prior use of antithrombotic and antihypertensive agents); (2) admission data, including baseline blood pressure, the modified Rankin Scale (mRS), the National Institute of Health stroke scale (NIHSS), and the Glasgow Coma Score (GCS), imaging data, such as hematoma location, hematoma volume, and intraventricular hemorrhage (IVH); (3) hematological parameters; (4) the structural lesions, medication, amyloid angiopathy, systemic disease, hypertension, and undetermined (SMASH-U) etiology of ICH. Recurrent ICH was defined as any ICH ≥ 24 h after the first incident event (Coulland Rothwell, 2004). All recurrent ICHs were symptomatic with a newly occurred focal neurologic deficit or decreased level of consciousness, vomiting, headache, etc. The CT scan was performed to confirm the diagnosis of recurrent ICH. Medication history was defined as taking antithrombotic (antiplatelet or anticoagulation) or antihypertensive agents within 30 days before hospitalization for ICH. Alcohol drinking refers to patients who drink regularly, with more than one unit of alcohol (equals to 360 ml of beer, or 50 ml of white wine, or 120 ml of red wine) a week. Experienced neurologists performed the imaging analyses based on the initial CT scan, and hematoma volume was calculated using the ABC/2 formula.
The primary outcome is death and functional dependence referred to the mRS score of 3–6 at 90 days. The second outcomes are 30-day functional dependence, and in-hospital, 30-day and 90-day death.
The continuous or discrete variables were presented as median with interquartile, and categorical variables were presented as percentages. Univariate analysis was analyzed using the χ2 test and Mann–Whitney U test for categorical variables and continuous variables, respectively. Non-normally distributed continuous variables were categorized based on clinical and statistical significance in the subgroup analysis. All variables of univariate analysis with a P-value < 0.1 were included in the multivariate regression model. All tests were two-tailed and a P-value < 0.05 was considered significant. Statistical analyses were performed using SPSS software (version 27.0) and R software (version 4.1.2).
After exclusion for ineligible patients, a total of 4,012 patients were entered into the study (see the patient enrollment flowchart in Figure 1). Among them, 64% of patients were male and the median age is 62 years (interquartile range, 55–71). Three thousand seven fifty (93.5%) patients had no experience of previous ICH, and 262 (6.5%) patients were considered as recurrent ICH. The baseline clinical characteristics of patients grouped by recurrent and first-ever ICH are summarized in Table 1.
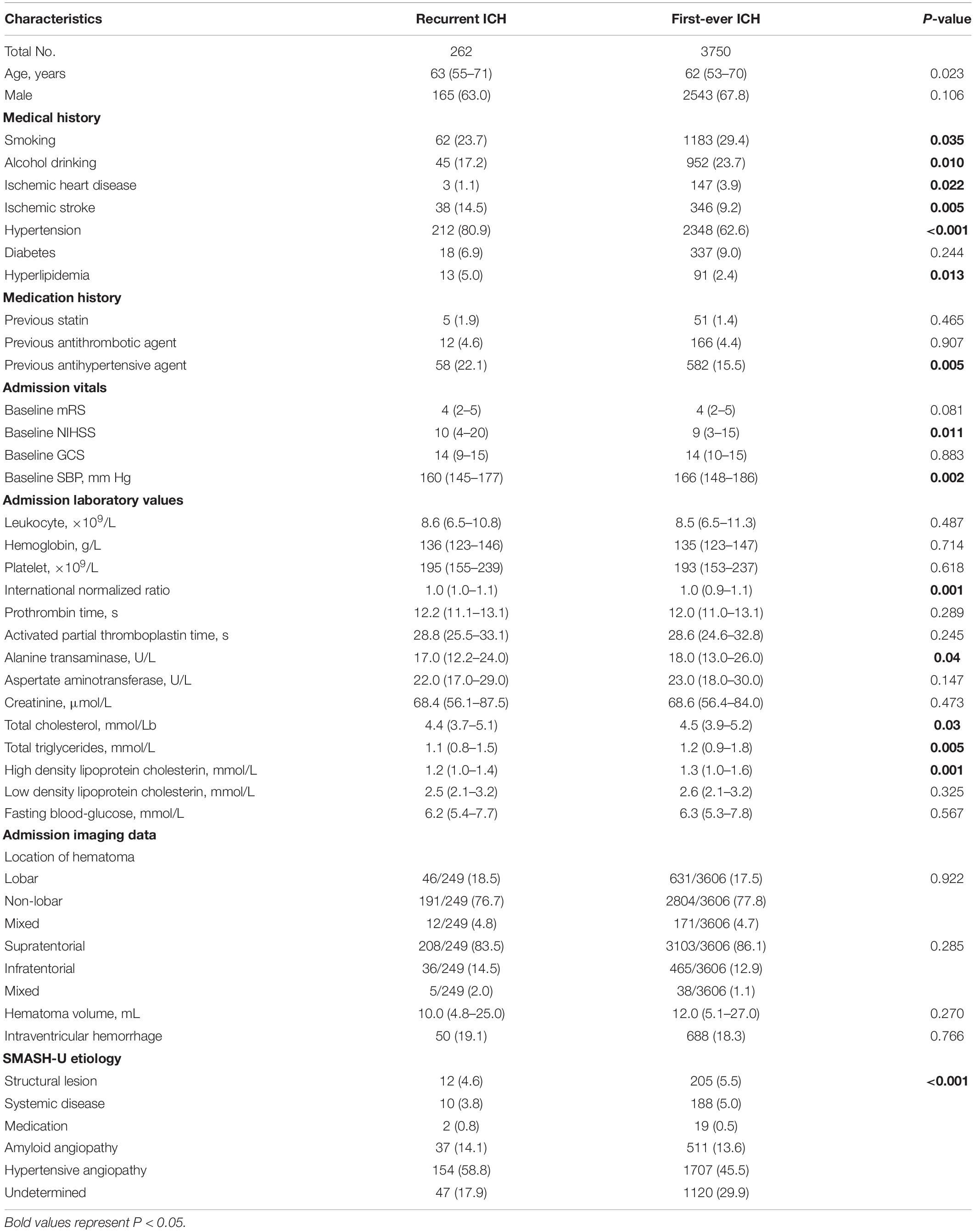
Table 1. Comparisons of demographics and clinical characteristics between patients with first-ever ICH and recurrent ICH.
In contrast to the first-ever ICH group, recurrent ICH patients were in older median age, having a more frequent history of smoking, alcohol drinking, ischemic stroke, ischemic heart disease, hypertension, and hyperlipidemia, accompanied with higher baseline NIHSS score. Meanwhile, recurrent ICH patients had previously received more antihypertensive therapy and featured lower admission blood pressure (median, 160 vs. 166 mmHg). Among admission laboratory tests, recurrent ICH was found related to lower alanine transaminase (ALT), total cholesterol (TC), total triglycerides (TG), high-density lipoprotein cholesterin (HDL-C), and international normalized ratio (INR). Moreover, ICH etiologies of amyloid angiopathy and hypertension were obsevered more frequently in recurrent ICH than first-ever ICH. However, there was no significant difference in the volume and location of a hematoma between the two groups.
When clinical outcomes are compared between recurrent and first-ever ICH, recurrent ICH was associated with more 3-month functional dependence (Table 2), with the distribution of 3-month mRS, as provided in Figure 2. After adjusting for these competitive risk factors, including age, smoking, alcohol drinking, history of ischemic heart disease, ischemic stroke, hypertension, and hyperlipidemia, the previous antihypertensive agent, baseline mRS, and NIHSS, SMASH-U etiology, admission systolic blood pressure (SBP), ALT, TC, TG, HDL-C, and INR, the odds ratio (OR) of recurrent ICH was 1.545 (95% CI, 1.029-2.319) for functional dependence at 3 months.
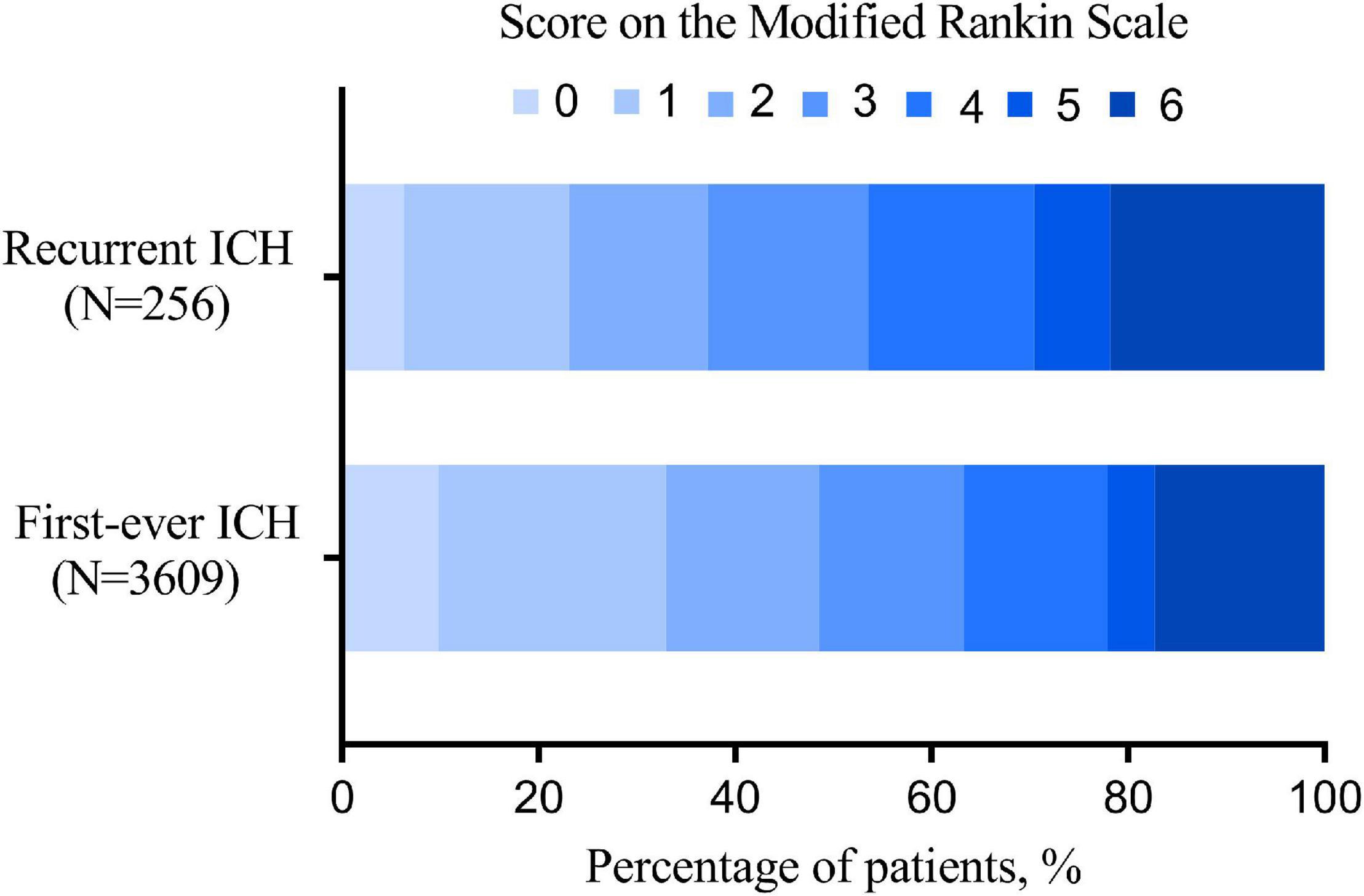
Figure 2. The distribution of 3-month modified Rankin Scale (mRS) of recurrent and first-ever intracerebral hemorrhage (ICH).
We further conducted a subgroup analysis on the above logistic regression results. No statistically significant interaction between recurrent ICH and these interesting factors was observed (all P-values of interaction are greater than 0.05; Table 3).
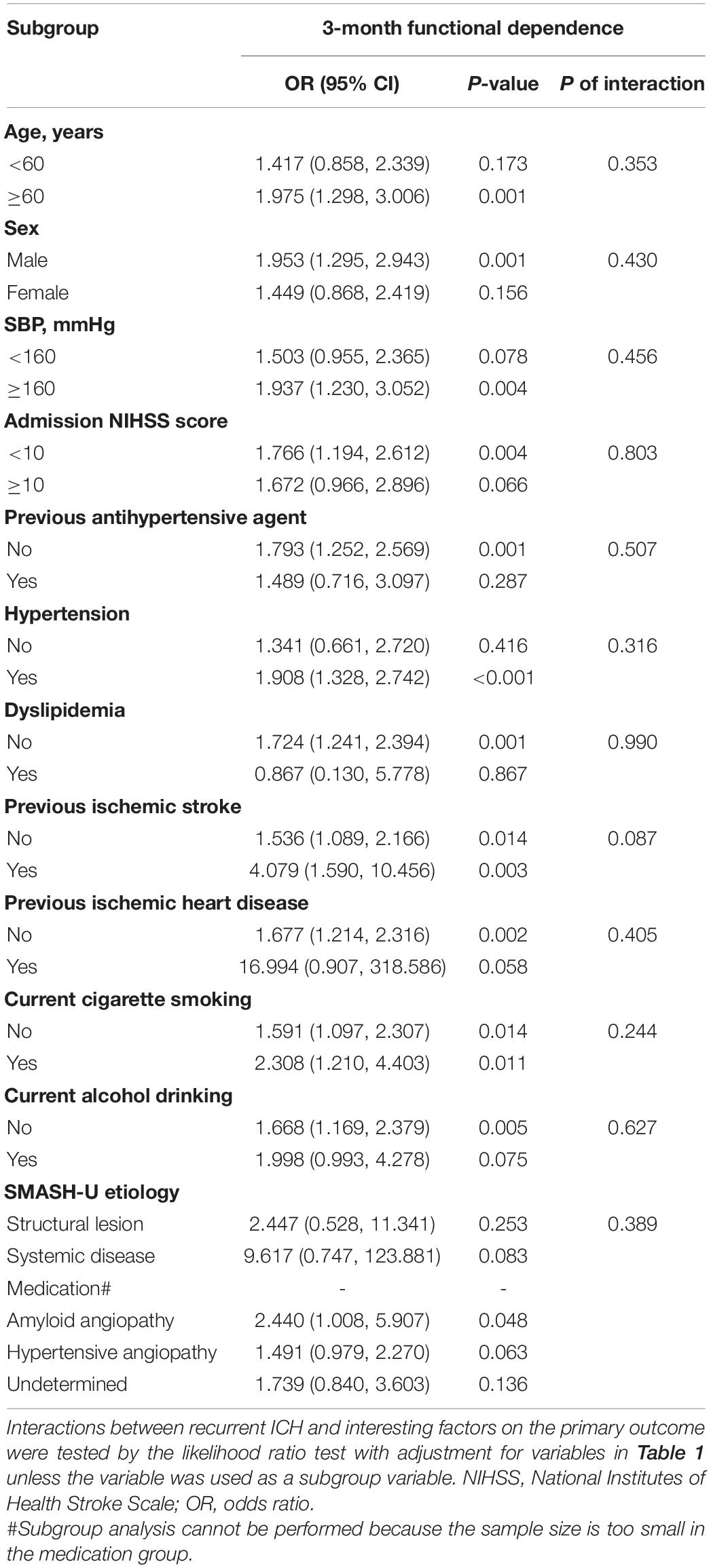
Table 3. Subgroup analysis of the association between recurrent ICH and 3-month functional dependence.
Our study showed that: (1) recurrent ICH was significantly associated with older age, more frequent history of ischemic heart disease, ischemic stroke, hypertension, hyperlipidemia, and higher baseline NIHSS score; (2) patients with recurrent ICH had previously received more antihypertensive therapy and showed lower admission blood pressure; (3) recurrent ICH was independently associated with poor 3-month functional outcomes.
Our analysis revealed that age, previous ischemic stroke, hypertension, and hyperlipidemia show significant group differences between recurrent and first-ever ICH patients. These are all the examined risk factors of ICH recurrence (Raffeld et al., 2015; Biffi et al., 2021; Pinho et al., 2021). Although age and medical history cannot be reversed, the risk of ICH recurrence may be reduced by preferable management strategies.
Intriguingly, patients with recurrent ICH exhibited even lower admission blood pressure, which may be attributable to the previous applications of the antihypertensive agents. Also, it is noteworthy that the admission SBP of patients with recurrent ICH was still as high as 160 (quartile, 145–177) mmHg, although it may be affected by an acute hypertensive response (Hawkesand Rabinstein, 2021). It is unclear whether this difference in blood pressure affects prognosis, as the recent, large, randomized, and controlled trials have not shown any benefit of intensive hypotension (≤160 mmHg) after ICH (Anderson and Qureshi, 2015; Qureshi et al., 2020). It may be speculated that blood pressure in patients, after the first ICH, has still not received enough attention. Effective management of hypertension should be pursued for the secondary prevention of ICH, especially in the Chinese population, which is characterized by suboptimal blood pressure control.
Moreover, our results revealed that patients with recurrent ICH showed lower total serum cholesterol, which has been considered inversely associated with ICH recurrence in previous studies (Koch, 2011; Baang and Sheth, 2021), although lipid control is often necessary for patients with ICH, concerns should remain whether intensive lipid-lowering is necessary for patients with recurrent ICH (Gurevitz et al., 2022). Meanwhile, the patients with recurrent ICH showed statistically lower ALT (17 vs. 18 U/L, P = 0.04), but ALT was almost within the normal range in both groups. This may be due to the improvement in liver function caused by the patient’s lifestyle optimizations after the cerebral hemorrhage, such as avoiding alcohol and high-fat diets.
Most importantly, the patients with recurrent ICH showed a higher risk of functional disability compared with first-ever ICH (62.9% vs. 51.5%, P < 0.001). Most of the previous articles only studied the recurrence of ICH as one of the clinical outcomes, while few studies specifically investigated the prognosis of recurrent ICH (Miki et al., 2020; Park et al., 2021a,b; Pinho et al., 2021; Skajaa et al., 2021), and the proportion of patients with ICH with 90-day adverse outcomes that they reported was 40–56% (Anderson et al., 2013; An et al., 2017; Fukuda-Doi et al., 2021). We firstly demonstrated that recurrent ICH is an independent risk factor for 3-month poor outcome after adjusting for potentially competitive risk factors, and this result showed consistency among SMASH-U etiological subtypes. This means that even if the risk factors, such as blood pressure and blood lipid, are perfectly controlled after the first ICH, the risk of ICH recurrence is still higher than that of the healthy population. However, the specific mechanism remains to be further explored.
Our study is original and has several strengths: (1) we conducted a national multi-center and large-sample study; (2) we strictly reviewed previous disease history for decades; (3) numerous confounders were adjusted to determine the association between ICH recurrence and clinical outcomes. After all, the limitations of our study lie in that: (1) lack of continuous monitoring to clinical characteristics, such as blood pressure and plasma glucose; (2) The enrolled patients were all Chinese.
The raw data supporting the conclusions of this article will be made available by the corresponding authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of Huazhong University of Science and Technology. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YW, HG, and RB conducted the data analysis and wrote the manuscript. BH together with QH designed this study and directed the writing of the manuscript. SC, JS, ML, YX, LZ, ZS, XC, ZC, ZW, DG, JX, and DZ helped with the data collection and literature search. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Nos. 82071335 to QH, 81820108010 to BH, and 81901214 to YW) and the National Key Research and Development Program of China (No. 2018YFC1312200 to BH).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We indeed appreciate the patients and medical institutions that generously provided the clinical data.
An, S. J., Kim, T., and Yoon, B. W. (2017). Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J. Stroke 19, 3–10. doi: 10.5853/jos.2016.00864
Anderson, C. S., and Qureshi, A. I. (2015). Implications of INTERACT2 and other clinical trials: blood pressure management in acute intracerebral hemorrhage. Stroke 46, 291-295.
Anderson, C. S., Heeley, E., Huang, Y., Wang, J., Stapf, C., Delcourt, C., et al. (2013). Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N. Engl. J. Med. 368, 2355–2365. doi: 10.1056/NEJMoa1214609
Baang, H. Y., and Sheth, K. N. (2021). Stroke Prevention After Intracerebral Hemorrhage: Where Are We Now? Curr. Cardiol. Rep. 23:162. doi: 10.1007/s11886-021-01594-0
Biffi, A., Teo, K. C., Castello, J. P., Abramson, J. R., Leung, I. Y. H., Leung, W. C. Y., et al. (2021). Impact of uncontrolled hypertension at 3 months after intracerebral hemorrhage. J. Am. Heart Assoc. 10:e020392. doi: 10.1161/JAHA.120.020392
Biffi, A., Urday, S., Kubiszewski, P., Gilkerson, L., Sekar, P., Rodriguez-Torres, A., et al. (2020). Combining imaging and genetics to predict recurrence of anticoagulation-associated intracerebral hemorrhage. Stroke 51, 2153–2160. doi: 10.1161/STROKEAHA.120.028310
Coull, A. J., and Rothwell, P. M. (2004). Underestimation of the early risk of recurrent stroke: evidence of the need for a standard definition. Stroke 35, 1925–1929. doi: 10.1161/01.STR.0000133129.58126.67
de Courson, H., Ferrer, L., Barbieri, A., Tully, P. J., Woodward, M., Chalmers, J., et al. (2021). Impact of model choice when studying the relationship between blood pressure variability and risk of stroke recurrence. Hypertension 78, 1520–1526. doi: 10.1161/HYPERTENSIONAHA.120.16807
de Oliveira Manoel, A., Goffi, A., Zampieri, F. G., Turkel-Parrella, D., Duggal, A., Marotta, T. R., et al. (2016). The critical care management of spontaneous intracranial hemorrhage: a contemporary review. Crit. care (London, England) 20:272. doi: 10.1186/s13054-016-1432-0
Fukuda-Doi, M., Yamamoto, H., Koga, M., Doi, Y., Qureshi, A. I, Yoshimura, S., et al. (2021). Impact of renal impairment on intensive blood-pressure-lowering therapy and outcomes in intracerebral hemorrhage: results from ATACH-2. Neurology 97, e913–e921. doi: 10.1212/WNL.0000000000012442
Gurevitz, C., Auriel, E., Elis, A., and Kornowski, R. (2022). The association between low levels of low density lipoprotein cholesterol and intracerebral hemorrhage: cause for concern? J. Clin. Med. 11:536. doi: 10.3390/jcm11030536
Hawkes, M. A., and Rabinstein, A. A. (2021). Acute hypertensive response in patients with acute intracerebral hemorrhage: a narrative review. Neurology 97, 316–329. doi: 10.1212/WNL.0000000000012276
Hill, M. D., Silver, F. L., Austin, P. C., and Tu, J. V. (2000). Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke 31, 123–127. doi: 10.1161/01.str.31.1.123
Huhtakangas, J., Löppönen, P., Tetri, S., Juvela, S., Saloheimo, P., Bode, M. K., et al. (2013). Predictors for recurrent primary intracerebral hemorrhage: a retrospective population-based study. Stroke 44, 585–590. doi: 10.1161/STROKEAHA.112.671230
Koch, S. (2011). Intracerebral hemorrhage: Preventing recurrence of ICH-should statins be avoided? Nat. Rev. Neurol. 7, 193–194. doi: 10.1038/nrneurol.2011.36
Li, L., Zuurbier, S. M., Kuker, W., Warlow, C. P., and Rothwell, P. M. (2021). Blood pressure control and recurrent stroke after intracerebral hemorrhage in 2002 to 2018 versus 1981 to 1986: population-based study. Stroke 52, 3243–3248. doi: 10.1161/STROKEAHA.121.034432
Lozano, R., Naghavi, M., Foreman, K., Lim, S., Shibuya, K., Aboyans, V., et al. (2012). Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128. doi: 10.1016/S0140-6736(12)61728-0
Miki, K., Natori, Y., Kai, Y., Yamada, T., Mori, M., Noguchi, N., et al. (2020). Absence of microbleeds reduces the risk for recurrent intracerebral hemorrhage. J. Stroke Cerebrovasc. Dis. 29:104585. doi: 10.1016/j.jstrokecerebrovasdis.2019.104585
Park, J. H., Kwon, S. U., Kwon, H. S., and Heo, S. H. (2021b). Prior intracerebral hemorrhage and white matter hyperintensity burden on recurrent stroke risk. Sci. Rep. 11:17406. doi: 10.1038/s41598-021-96809-3
Park, J. H., Lee, J., Kwon, S. U., Sung Kwon, H., Hwan Lee, M., and Kang, D. W. (2021a). Elevated pulse pressure and recurrent hemorrhagic stroke risk in stroke with cerebral microbleeds or intracerebral hemorrhage. J. Am. Heart Assoc. 11:e022317. doi: 10.1161/JAHA.121.022317
Pennlert, J., Eriksson, M., Carlberg, B., and Wiklund, P. G. (2014). Long-term risk and predictors of recurrent stroke beyond the acute phase. Stroke 45, 1839–1841. doi: 10.1161/STROKEAHA.114.005060
Pinho, J., Araújo, J. M., Costa, A. S., Silva, F., Francisco, A., Quintas-Neves, M., et al. (2021). Intracerebral hemorrhage recurrence in patients with and without cerebral amyloid angiopathy. Cerebrovasc. Dis. Extra 11, 15–21. doi: 10.1159/000513503
Qureshi, A. I., Huang, W., Lobanova, I., Barsan, W. G., Hanley, D. F., Hsu, C. Y., et al. (2020). Outcomes of intensive systolic blood pressure reduction in patients with intracerebral hemorrhage and excessively high initial systolic blood pressure: post hoc analysis of a randomized clinical trial. JAMA Neurol. 77, 1355–1365. doi: 10.1001/jamaneurol.2020.3075
Raffeld, M. R., Biffi, A., Battey, T. W., Ayres, A. M., Viswanathan, A., Greenberg, S. M., et al. (2015). APOE epsilon4 and lipid levels affect risk of recurrent nonlobar intracerebral hemorrhage. Neurology 85, 349–356. doi: 10.1212/WNL.0000000000001790
Keywords: intracerebral hemorrhage, recurrence, clinical characteristics, prognosis, contrast
Citation: Wan Y, Guo H, Bi R, Chen S, Shen J, Li M, Xia Y, Zhang L, Sun Z, Chen X, Cai Z, Wang Z, Gong D, Xu J, Zhu D, Hu B and He Q (2022) Clinical and Prognostic Characteristics of Recurrent Intracerebral Hemorrhage: A Contrast to First-Ever ICH. Front. Aging Neurosci. 14:860571. doi: 10.3389/fnagi.2022.860571
Received: 23 January 2022; Accepted: 28 February 2022;
Published: 14 April 2022.
Edited by:
Aurel Popa-Wagner, University of Medicine and Pharmacy of Craiova, RomaniaReviewed by:
Archana Hinduja, The Ohio State University, United StatesCopyright © 2022 Wan, Guo, Bi, Chen, Shen, Li, Xia, Zhang, Sun, Chen, Cai, Wang, Gong, Xu, Zhu, Hu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quanwei He, aGVxdWFud2VpMjAwOEAxMjYuY29t; Bo Hu, aHVib0BtYWlsLmh1c3QuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.