- 1Department of Psychiatry, Chang Gung Memorial Hospital, College of Medicine, Chang Gung University, Taoyuan City, Taiwan
- 2Department of Nuclear Medicine, Center for Advanced Molecular Imaging and Translation, Chang Gung Memorial Hospital, Taoyuan City, Taiwan
- 3Department of Medical Imaging and Radiological Sciences, College of Medicine and Healthy Aging Research Center, Chang Gung University, Taoyuan City, Taiwan
- 4Neuroscience Research Center, Chang Gung Memorial Hospital, Linkou Medical Center, Taoyuan City, Taiwan
- 5Department of Radiology, Chang Gung Memorial Hospital, Taoyuan City, Taiwan
- 6Department of Psychiatry, Kaohsiung Medical University Hospital, College of Medicine, Kaohsiung Medical University, Kaohsiung City, Taiwan
Cerebral amyloid-β (Aβ) depositions in depression in old age are controversial. A substantial proportion of individuals with late-life major depressive disorder (MDD) could be classified as having suspected non-Alzheimer’s disease pathophysiology (SNAP) by a negative test for the biomarker amyloid-β (Aβ−) but positive neurodegeneration (ND+). This study aimed to evaluate subthreshold Aβ loads in amyloid-negative MDD, particularly in SNAP MDD patients. This study included 46 amyloid-negative MDD patients: 23 SNAP (Aβ−/ND+) MDD and 23 Aβ−/ND− MDD, and 22 Aβ−/ND− control subjects. All subjects underwent 18F-florbetapir PET, FDG-PET, and MRI. Regions of interest (ROIs) and voxel-wise group comparisons were performed with adjustment for age, gender, and level of education. The SNAP MDD patients exhibited significantly deceased 18F-florbetapir uptakes in most cortical regions but not the parietal and precuneus cortex, as compared with the Aβ−/ND− MDD and control subjects (FDR correction, p < 0.05). No correlations of neuropsychological tests or depression characteristics with global cortical uptakes, but significant positive correlations between cognitive functions and adjusted hippocampal volumes among different groups were observed. The reduced Aβ depositions in the amyloid-negative MDD patients might be attributed mainly to the SNAP MDD patients. Our results indicated that meaningfully low amounts of subclinical Aβ might contain critical information on the non-amyloid-mediated pathogenesis.
Introduction
Converging evidence from multiple meta-analyses (Jorm, 2001; Ownby et al., 2006; Diniz et al., 2013) suggests that depression approximately doubles an individual’s risk of developing dementia later in life. Brain amyloid-β (Aβ) deposition serves as a gold-standard hallmark of pathogenesis in Alzheimer’s disease (AD). Early autopsy studies (Rapp et al., 2006) have shown more pronounced Aβ plaque in AD patients who have lifetime major depressive disorder (MDD) as compared with those without MDD. Depression in old age has been increasingly investigated in terms of the relationship with in vivo cerebral Aβ via validated amyloid imaging in the past decade. However, the results regarding cerebral Aβ amounts in depression have been inconsistent and controversial. Butters et al. (2008) and Wu et al. (2014) found that MDD patients have increased cortical Aβ in comparison with non-depressed healthy controls; however, Madsen et al. (2012) found no difference between midlife MDD and cognitively normal individuals. Unexpectedly, a recent study by Mackin et al. (2021) showed an even more reduced cortical Aβ level in depressed elderly patients as compared with non-depressed cognitively normal subjects. Therefore, to date, related studies have yielded variable and conflicting results.
Depression in old age, not surprisingly, might represent an etiological entity with both clinical and pathophysiological heterogeneity. Our previous study (Wu et al., 2018) provided evidence of the diversity of involved neurodegenerative processes in elderly depressed individuals. We found that some depressed elderly patients entered the AD prodrome; others might be subject to a neurodegenerative pathway completely distinct from AD. In the past, we have always paid more attention to amyloid positivity for an accurate diagnosis of AD; however, the impact of subthreshold Aβ is gradually being explored (Bischof and Jacobs, 2019). Recent studies reported subthreshold Aβ and Aβ accumulation rate could predict early tau deposition in those who were nominally amyloid negative (Leal et al., 2018). Additionally, non-amyloid-mediated neurodegeneration could be associated with subthreshold Aβ changes (Jack et al., 2013). Subthreshold Aβ might provide clinically meaningful and useful information that may reflect various individual neurodegenerative processes. To date, a subthreshold Aβ condition among amyloid-negative MDD patients remains largely unclear. In order to make group comparisons on the same basis of amyloid negativity status, both samples of amyloid-negative MDD and control subjects were included in this study.
Among the amyloid-negative individuals, a suspected non-Alzheimer disease pathophysiology (SNAP) can be indicated by a negative test for β-amyloid (Aβ−) but a positive test for neurodegeneration (ND+). Hippocampal atrophy and glucose hypometabolism within AD-vulnerable regions have been widely adopted as ND biomarkers (Jack et al., 2012; Caroli et al., 2015; Mormino et al., 2016). In this study, amyloid-negative MDD patients were further classified into SNAP (Aβ−/ND+) MDD and Aβ−/ND− MDD groups. This study aimed to investigate the subthreshold Aβ characteristics in amyloid-negative MDD subjects, particularly in SNAP MDD patients, utilizing 18F-florbetapir PET imaging.
Materials and Methods
Subjects
This prospective, cross-sectional study, performed at Chang Gung Memorial Hospital Geriatric Psychiatry Center from July 2015 to June 2017, enrolled 50 MDD patients and 12 non-depressed control subjects. Of the enrolled subjects, 4 MDD patients and 1 control subject were excluded due to amyloid-positive results on 18F-florbetapir PET scanning. To increase the control sample size, another 11 control subjects were recruited from the Taiwan-Alzheimer’s Disease Neuroimaging Initiative (T-ADNI) study cohort (Lin et al., 2016) owing to the availability of complete information with regards to the Aβ−/ND− profile in that cohort. Thus, a total of 46 amyloid-negative MDD and 22 Aβ−/ND− control subjects were included in the study.
Each MDD patient was assessed for the presence of lifetime major depressive episodes according to the DSM-IV [DSM-5 (American Psychiatric Association, 2013) after 2016] via a clinical structured interview and retrospective medical chart review. The lifetime course of major depression was also clarified, including age at onset of major depression, number of major depressive episodes, late-onset major depression (cut-off set at 60 years) and time since onset of first depression. Control subjects were confirmed as having an absence of lifetime psychiatric illnesses, and were deemed cognitively normal (MMSE ≥ 27 and CDR = 0). All subjects were aged > 50 years; had no definite neurologic diseases affecting brain structure (e.g., completed stroke, traumatic head injury or epilepsy); suffered no unstable medical diseases involving the heart, lungs, liver or kidneys; and did not have alcohol or other substance abuse currently or in the past 1 year. None of the subjects met the NIA-AA criteria for dementia due to AD (Jack et al., 2018), the IWG criteria for typical/atypical AD dementia (Dubois et al., 2014), or the DSM-5 criteria for any type of dementia (American Psychiatric Association, 2013).
All eligible subjects underwent scans of 18F-florbetapir PET, FDG-PET, and brain MRI. Apolipoprotein E (APOE) genotypes were determined by polymerase chain reaction (PCR) study, and vascular risk factors as defined by the Framingham Stroke Risk Score (FSRS) were also identified. Except for the 11 control subjects recruited from the T-ADNI study cohort, comprehensive neuropsychological tests were performed by all subjects as per our previous study, and the results are presented as standard z-scores transformed using regression-based norms, adjusted for age and level of education (Wu et al., 2016). Written informed consent was obtained from all subjects, and the study protocol was approved by the Institutional Review Boards of the Ministry of Health and Welfare and Chang Gung Medical Center.
Amyloid-negative results were evaluated according to visual rating criteria from 18F-florbetapir PET scans (Sabri et al., 2015), which were confirmed by the same experienced nuclear physician who was blind to the clinical data and imaging analysis of each subject. The adjusted hippocampal volume (HVa) atrophy, a cut-off value described previously (Wu et al., 2018), and glucose hypometabolism within AD-vulnerable regions, as defined by the FDG t-sum score (Herholz et al., 2002), were adopted as ND biomarkers. Subjects were classified as neurodegeneration-positive (ND+) when positive for one of HVa atrophy or glucose hypometabolism (cut-off value of 6,879 mm3 for HVa and 11,089.681 for FDG t-sum score). All control subjects met the Aβ−/ND− profile.
Magnetic Resonance Imaging Acquisition and Data Preprocessing
T1-weighted MRI was performed for all subjects using a 3T Siemens Magnetom TIM Trio scanner on PET/MR (Siemens Medical Solutions, Malvern, PA, United States). An acquisition protocol using a sagittal T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence was applied with the following acquisition parameters: Repetition Time (TR)/Echo Time (TE) = 2,600/3.12 ms, TI = 900 ms, flip angle = 13°, voxel size = 0.5 mm × 0.5 mm × 1.1 mm. Structural scans were processed using FreeSurfer version 5.3 image analysis software1 for total bilateral hippocampal and intracranial volumes (Dale et al., 1999). A linear-regression normalization method was applied (Voevodskaya et al., 2014), with the total bilateral hippocampal volume adjusted by the estimated total intracranial volume to obtain the adjusted hippocampal volume (HVa), as described in our previous study (Wu et al., 2018), to reduce inter-subject variability.
Positron Emission Tomography Imaging Acquisition and Data Preprocessing
Radiosynthesis of 18F-florbetapir (Yao et al., 2010) and amyloid PET data acquisition followed the same procedures as previously described (Lin et al., 2010). During the study, each 18F-florbetapir PET scan (380 ± 5 MBq) at 50–60 min post-injection was obtained using a Biograph mMR PET/MR System (Siemens Medical Solutions). The 3-D OSEM PET reconstruction algorithm (three iterations, 21 subsets; Gaussian filter: 2 mm; zoom: 3) with MR-based attenuation correction, scatter and random corrections was applied to obtain PET images with a matrix size of 344 × 344 × 127 and a voxel size of 0.83 mm3 × 0.83 mm3 × 2.03 mm3.
18F-FDG data were acquired at 30–50 min post-injection with a dose of 374 ± 13 MBq using a Biograph mCT PET/CT system (Siemens Medical Solutions). PET images were reconstructed using the 3D ordered subsets expectation maximization reconstruction algorithm with the parameters of four iterations, 24 subsets, Gaussian filter 2 mm, Zoom 3. In addition, CT-based attenuation correction, scatter and random corrections were performed using the correction methods provided by the manufacturer. The resulting reconstructed images were of a matrix size of 400 × 400 × 109 and a voxel size of 0.68 mm3 × 0.68 mm3 × 2.03 mm3.
Subsequent image quantitation analysis was performed using PMOD image analysis software (version 3.7, PMOD Technologies Ltd., Zurich, Switzerland). PET images were spatially normalized to the Montreal Neurological Institute (MNI) MRI template (Hsiao et al., 2013) using an MR-based method. Standardized uptake value ratio (SUVR) images for 18F-florbetapir were generated using the whole cerebellum as the reference region for subsequent analysis.
Statistical Analysis
Demographic and clinical data are expressed as means ± SD or absolute numbers with proportions for descriptive statistics. Continuous variables were analyzed by non-parametric statistics using the Kruskal-Wallis test with Dunn’s post hoc multiple comparison. Categorical data were analyzed using the X2 test (Fisher’s exact test for APOE4, given the small numbers). Regions of interest (ROIs) comparisons of 18F-florbetapir SUVRs were conducted using the Kruskal-Wallis test with Dunn’s post hoc analysis. In addition, analyses of covariance (ANCOVA) were conducted to compare regional 18F-florbetapir SUVRs among the three groups, with adjustment for age and years of education; pairwise differences among the adjusted means were further evaluated with Bonferroni correction. Partial correlations between global 18F-florbetapir SUVRs and the neuropsychological test data or depression characteristics were evaluated using Pearson correlation analysis, with adjustment for age, years of education and HVa. Statistical analyses were performed using IBM SPSS version 25.0 (IBM Corp., Armonk, NY, United States), and p < 0.05 was considered to indicate statistical significance.
Voxel-wise group comparisons were performed in Statistical Parametric Mapping 12 (SPM 122) using an ANCOVA model with age, gender and level of education as covariates. Pairwise group contrasts were performed between the SNAP MDD, Aβ−/ND− MDD and control groups. To reduce the likelihood of volume effects on the results, both ROIs and voxel-wise analyses in the study were conducted with atrophy-corrected data using partial volume correction (PVC) (Gonzalez-Escamilla et al., 2017) unless otherwise specified. Furthermore, comparison results were corrected for multiple comparisons using a false discovery rate (FDR) correction at p values (pFDR) < 0.05 at the voxel level.
Results
Subjects
The demographic and clinical characteristics of each group are shown in Table 1. The groups did not differ significantly in age, gender, ApoEε4 carriers, and vascular risk factors. The SNAP MDD and Aβ−/ND− MDD patients had similar HAM-D scores, in addition to similar clinical depressive features (age at onset, disease duration, depressive episodes, and late-onset depression).
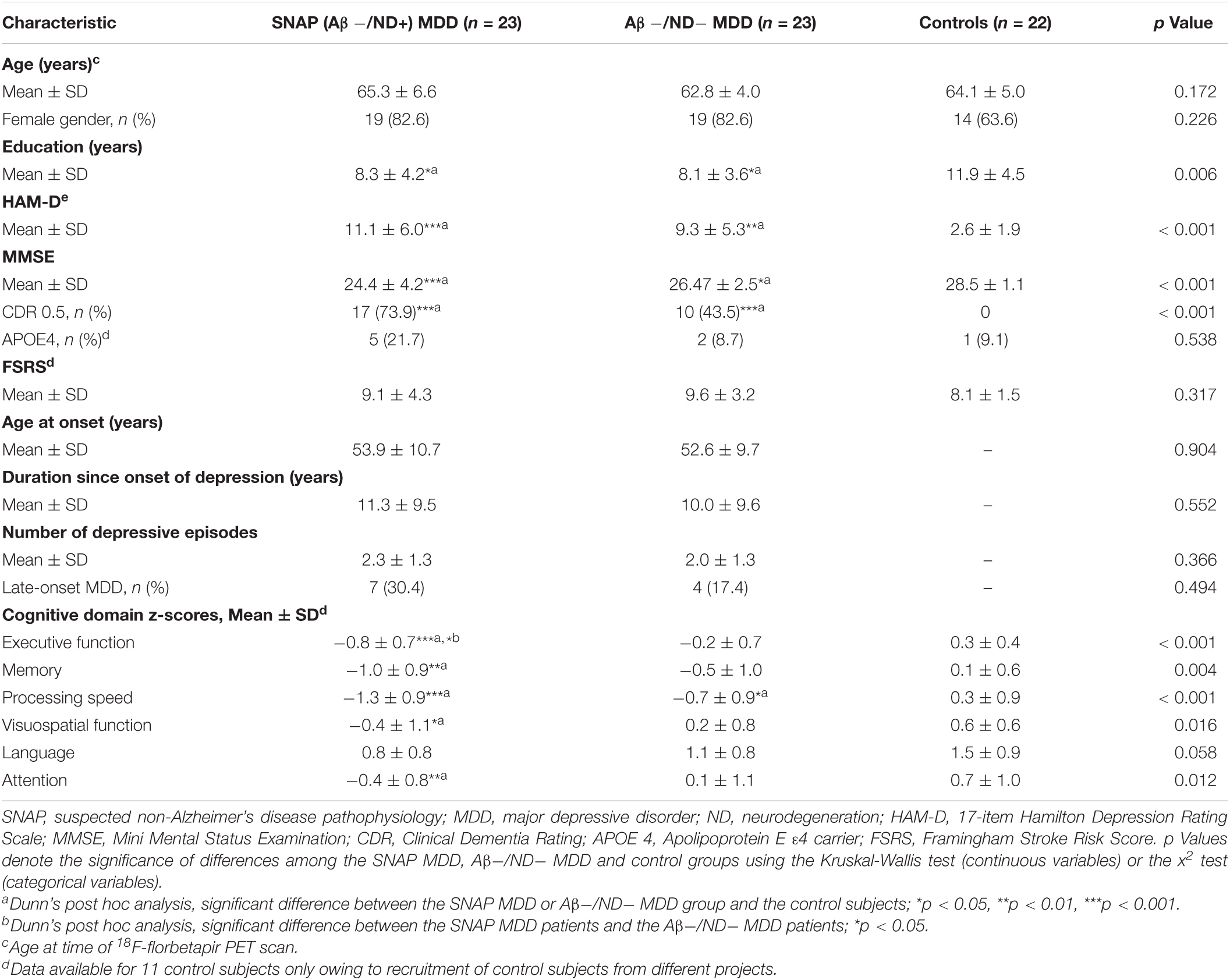
Table 1. Demographic and clinical characteristics of the SNAP MDD patients, Aβ−/ND− MDD patients, and control subjects.
In terms of neuropsychological testing, the SNAP MDD patients had greater cognitive deficits than the control subjects in all neuropsychological tests after post hoc analyses; the most severe deficits occurred in the executive function (p < 0.001) and processing speed domains (p < 0.001).
Of the 23 SNAP MDD patients, there were five subjects with only hippocampal atrophy, and 11 subjects with only hypometabolism, and seven subjects with the presence of both. The neurodegeneration biomarker distributions are displayed in the Supplementary Figure 1.
Regions of Interest Group Comparisons
The 46 amyloid-negative MDD patients showed significantly decreased 18F-florbetapir SUVRs as compared with the controls in most cortices except the parietal and precuneus cortex (Supplementary Table 1). The three-group comparisons among the SNAP MDD, Aβ−/ND− MDD and control subjects are shown in Table 2. There were significant differences among the three groups in all ROIs assessed except the precuneus cortex. Post hoc analyses showed that, as compared with the controls, the SNAP MDD patients exhibited significantly decreased 18F-florbetapir uptakes in the frontal, anterior and posterior cingulate, occipital, temporal, hippocampus, basal ganglia and global cortex. Compared with the Aβ−/ND− MDD patients, the SNAP MDD patients showed 18F-florbetapir regions with greater decreases in the parietal cortex in addition to the ROIs observed in a post hoc comparison of the SNAP MDD and control subjects. No differences in 18F-florbetapir uptake were observed between the Aβ−/ND− MDD and control subjects in the post hoc ROI analyses. The differences among the three groups are shown in Figure 1. In order to confirm our findings, analyses were repeated using non-PVC original data; furthermore, ANCOVA analyses were conducted using PVC data with age and level of education as covariates. Comparable results were found, and are shown in Supplementary Tables 2, 3.
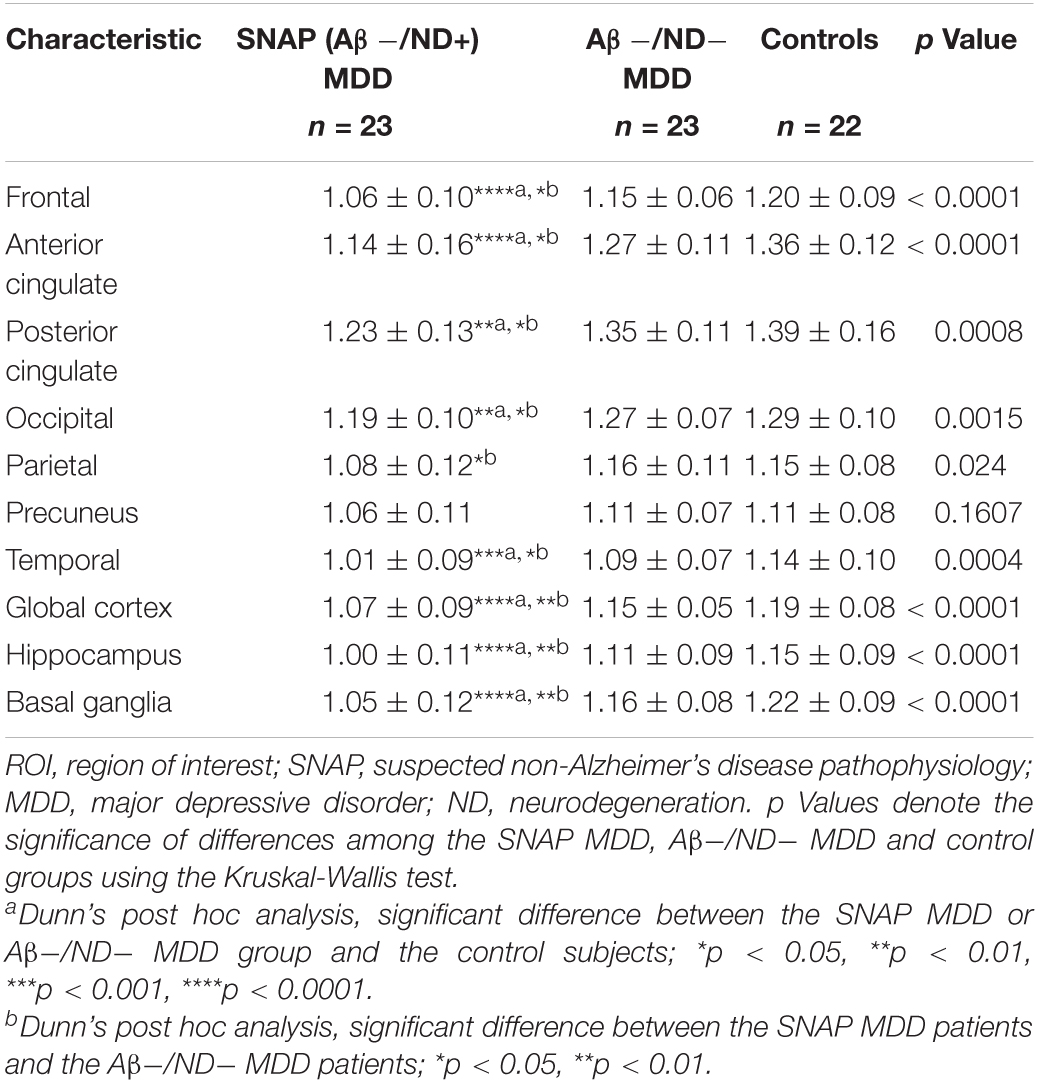
Table 2. Region of interest (ROI) group comparisons among the SNAP MDD, Aβ−/ND− MDD, and control groups.
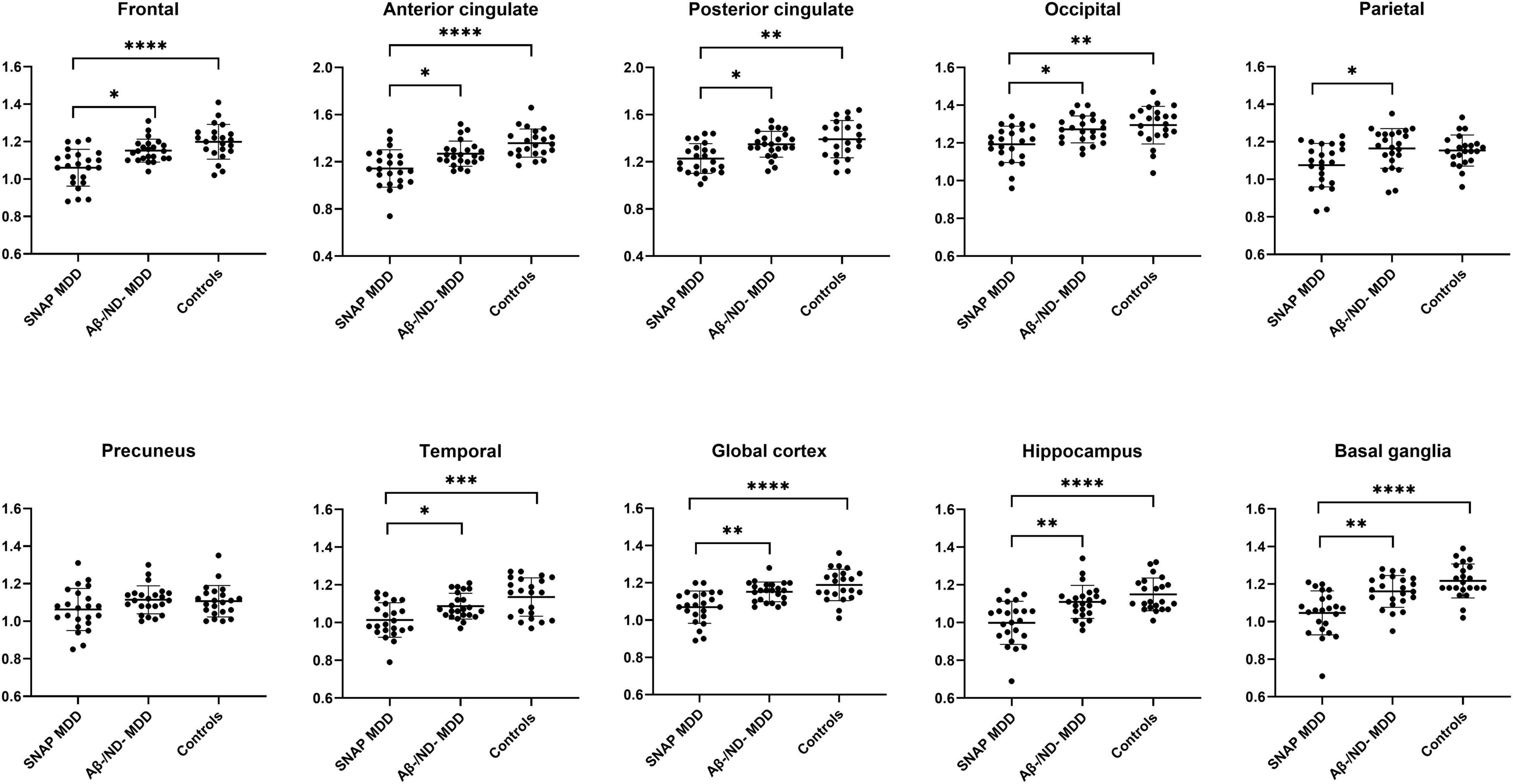
Figure 1. Regions of interest comparisons of 18F-florbetapir SUVRs in the SNAP (n = 23) and Aβ–/ND– MDD (n = 23) patients and controls (n = 22) using data of partial volume correction (PVC). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 using the Kruskal-Wallis test with Dunn’s post hoc analysis.
Voxel-Wise Group Comparisons
The SNAP MDD patients exhibited much lower 18F-florbetapir uptakes than the control subjects in the lateral and medial frontal, anterior and posterior cingulate, temporoparietal junction, and occipital cortices, but this was not the case in the superior parietal and precuneus areas. The most prominent decreases in 18F-florbetapir SUVR were observed in the bilateral mesial temporal cortex and hippocampus (Figure 2, pFDR < 0.05, adjusted for age, gender and level of education). Moreover, the SNAP MDD patients showed significantly decreased 18F-florbetapir retention as compared with the Aβ−/ND− MDD patients in similar areas to varying degrees, involving the frontal, anterior and posterior cingulate, occipital and mesial temporal cortices (Figure 2, pFDR < 0.05, adjusted for age and years of education). Regions of decreased retention were overall symmetrical.
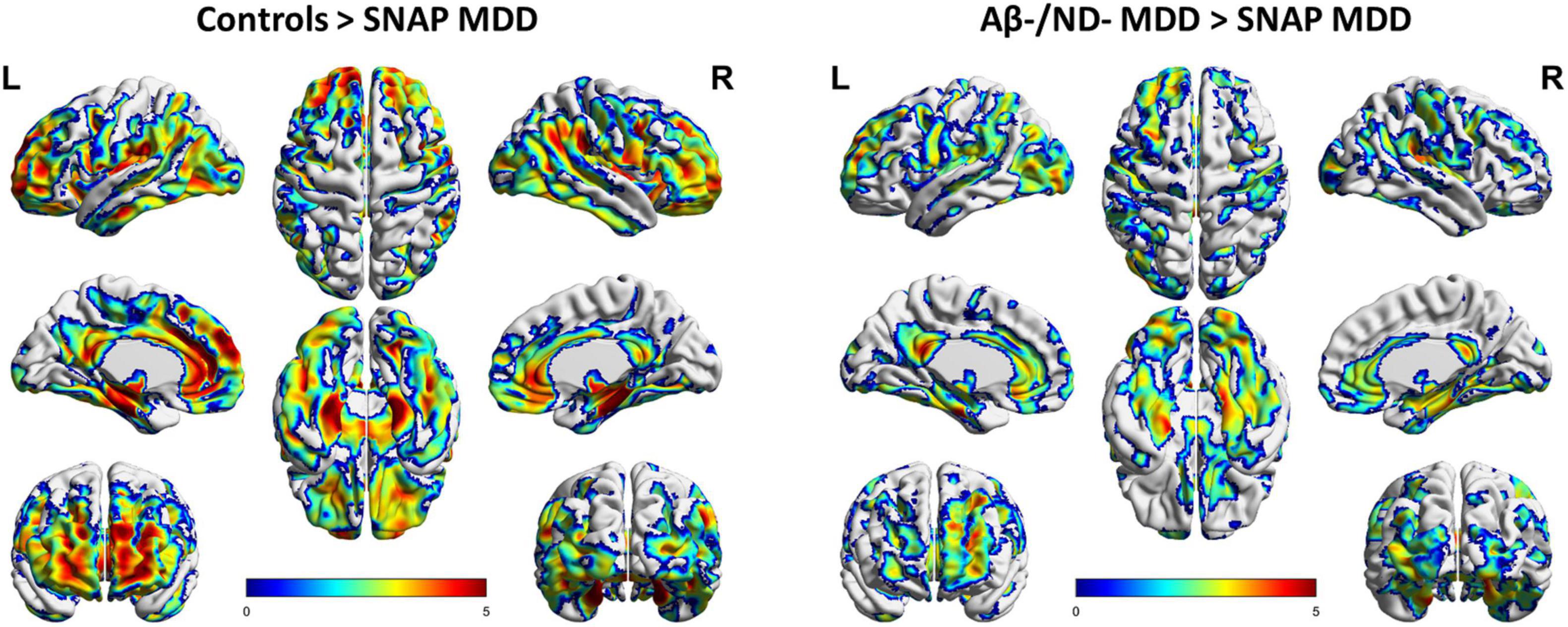
Figure 2. Voxel-wise comparisons of 18F-florbetapir uptakes in the SNAP (n = 23) and Aβ–/ND– MDD (n = 23) patients and controls (n = 22) using data of partial volume correction (PVC). Voxel-wise analyses were performed, including age, gender and level of education as covariates. Shown are T-maps using false discovery rate (FDR) correction for multiple comparisons (pFDR < 0.05), with 3D brain renderings of 18F-florbetapir PET analysis on the ch2 template brain. Renders were created using BrainNet Viewer (https://www.nitrc.org/projects/bnv/).
However, in contrast, no areas of differing 18F-florbetapir retention were found between the Aβ−/ND− MDD patients and controls. Besides, reverse contrast revealed no areas of increased 18F-florbetapir retention in the SNAP MDD patients as compared with the Aβ−/ND− MDD and control groups.
Correlation of 18F-Florbetapir Retention and Cognition, and Characteristics of Depression
Given Aβ differences among the groups, separate partial correlation analyses were conducted in the SNAP MDD, Aβ−/ND− MDD, all MDD, and the whole MDD and control subject groups. After controlling for age, level of education, and HVa, there were no correlations between the global cortical 18F-florbetapir retention and cognitive functions, including the Mini Mental State Examination (MMSE) results and each domain-specific cognitive score in each group subset. However, significant positive correlations between HVa and cognitive functions were observed in different sample groups after controlling for age, level of education, and global cortical 18F-florbetapir retention. Details of partial correlation coefficients are shown in Table 3.
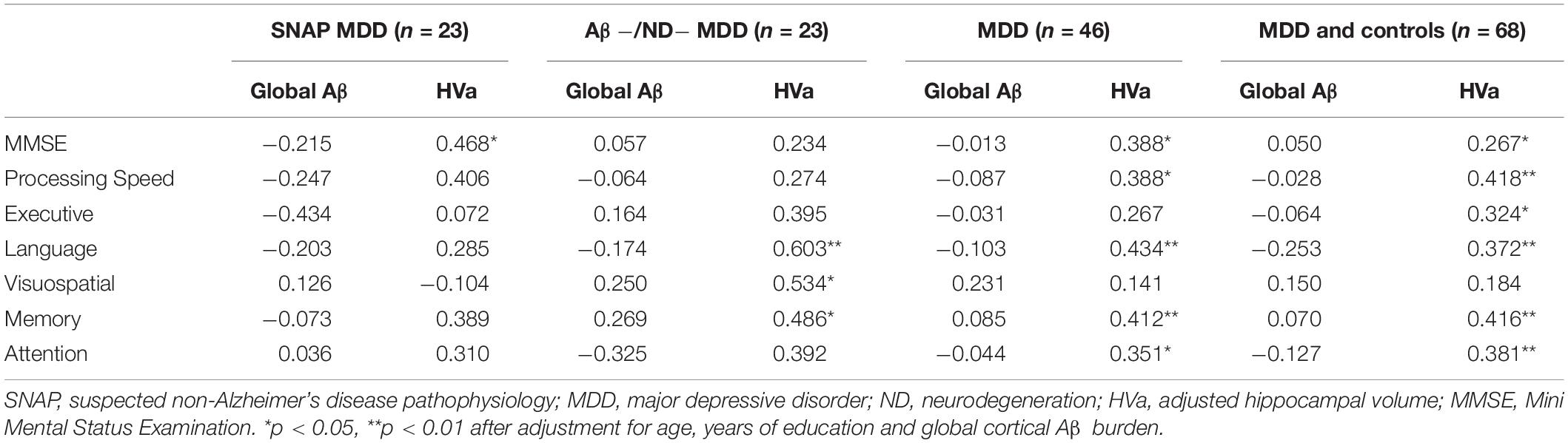
Table 3. Correlations of cognitive functions with global cortical Aβ depositions and HVa in the different sample groups.
The global 18F-florbetapir retention was not significantly correlated with HAM-D (r = −0.149, p = 0.336), age at onset of depression (r = −0.084, p = 0.587), number of major depressive episodes (r = 0.037, p = 0.812), or time since onset of depression (r = 0.064, p = 0.682), after controlling for age and level of education.
Discussion
Unexpectedly, the SANP MDD group not only met the predefined criteria of an amyloid-negative status, but even exhibited a significantly reduced cerebral Aβ burden relative to the control and Aβ−/ND− MDD subjects in several brain regions. The most prominent decrease emerged in the bilateral mesial temporal cortex. The lack of differences of Aβ burden in the parietal and precuneus cortex between the SNAP MDD and control groups supported that the SNAP MDD patients had no preexisting early AD pathophysiology into the amyloid pathway. HVa atrophy, but not Aβ burden, had significant correlations with cognitive deficits in the total MDD and Aβ−/ND− MDD samples. However, in the SNAP MDD patients, neither HVa nor Aβ deposition were correlated with cognitive functions.
In the context of depression increasing the risk of incident dementia, early evidence connecting Aβ to depression came from postmortem studies of AD dementia patients, revealing an association between Aβ plaque and a lifetime history of MDD (Rapp et al., 2006). The advances of validated amyloid PET imaging enabled study of Aβ in vivo. In our previous studies (Wu et al., 2014, 2016), elderly patients with lifetime MDD, especially those with amnestic mild cognitive impairment, carried a greater Aβ burden in the parietal and precuneus cortex as compared with the controls. However, among subjects with midlife MDD, Madsen et al. (2012) demonstrated no global Aβ difference between cognitively normal MDD patients and control subjects. A population-based longitudinal study performed in Rotterdam (Mirza et al., 2016) provided some insight into the inconsistent results regarding Aβ in depression in old age, as that study uncovered that a substantially higher risk of dementia appeared in elderly depressed groups with an increasing depression trajectory, suggesting that depression might be a prodrome of dementia. In response to the postulation regarding depression as an AD prodrome, longitudinal studies have identified incident depressive symptoms corresponding to the baseline Aβ burden in cognitively normal older adults (Harrington et al., 2017; Donovan et al., 2018; Gatchel et al., 2019). Taken together, current evidence indicates that depression, especially late-onset depression, appears more likely to be a marker of incipient dementia than a true risk factor.
Challenging existing assumptions, Mackin et al. (2021) presented a shocking finding contrary to expectations, in that depressed elderly patients showed less Aβ accumulation than non-depressed subjects. Compared with the non-depressed group, which included individuals with a matched proportion of MCI, the depressed group exhibited decreased global Aβ accumulation and a lower proportion of Aβ positivity. They performed a second similar analysis restricting the sample to subsets of cognitively normal participants both in the depressed and non-depressed groups, which yielded results indicating a significant difference with regards to Aβ positivity, but not for Aβ burden.
In the present study, we observed that the amyloid-negative MDD patients had a significantly lesser Aβ burden than the control subjects. Moreover, the SNAP MDD population was the group that contributed most strongly to the result of reduced Aβ deposition. Corresponding to the reports by Mackin et al. (2021), our findings might provide a possibility as well as evidence to explain that other non-amyloid-mediated pathways may be associated with potential cortical Aβ reduction in depressed older adults. Nevertheless, we did not agree with the interpretation speculated by Mackin et al. that reduced cerebral blood flow or hypometabolism in depression may limit regional Aβ uptake (Mackin et al., 2021). Regional cerebral hypometabolism in SNAP by definition would mimic the metabolism pattern in AD; however, AD patients present with a typical abundant Aβ burden, and therefore decreased Aβ accumulation in the SNAP MDD group might be related to their own pathology-specific factors. To date, no study has investigated the characteristic Aβ patterns showing reduced Aβ depositions in SNAP patients with or without depression (Jack et al., 2012; Burnham et al., 2016).
Even though our findings demonstrated that other non-amyloid-mediated pathways were likely associated with reduced Aβ in the SNAP MDD patients, we did not refute the possibility that an increased Aβ burden is associated with the AD pathway in depression in old age. We very much agree with the comments of Christopher et al. (Van Dyck et al., 2021) that these diverse results should remind us of the tremendous heterogeneity regarding neurodegenerative pathophysiology in depression in old age. We should not expect most of depression in old age to have a uniform relationship with dementia pathogenesis. Some depressed individuals who enter the Aβ cascade in preclinical or prodromal AD stages may carry a greater Aβ burden; other depressed individuals have diverse Aβ depositions when they are on other non-AD pathways. Global cortical Aβ deposition was not found to be associated with any neuropsychological tests, whether in SNAP, Aβ−/ND− MDD, or all amyloid-negative MDD patients. However, HVa was positively associated with several neuropsychological tests in the amyloid-negative MDD sample, and when restricting the MDD sample to the Aβ−/ND− MDD patients. These correlations disappeared when further restricting the sample to the SNAP MDD subjects, the lack of a correlation in the SNAP MDD sample perhaps being associated with unrevealed factors other than Aβ and HVa.
Several limitations of this study must be acknowledged. First, this study was limited to small sample sizes; however, our results remained robust, even when using atrophy-corrected PVC data and a stringent threshold of FDR for multiple comparisons. Second, there are amyloid-positive individuals in the cognitively healthy general population, but we included control subjects with the Aβ−/ND− profile for group comparisons on the same amyloid-negative basis. However, the SNAP MDD patients exhibited much lower Aβ depositions, while amyloid-positive control subjects were also included in the study. Third, although atrophy and hypometabolism are widely recognized as ND biomarkers in relevant SNAP studies (Caroli et al., 2015; Jack et al., 2016; Mormino et al., 2016), an operational standardized approach to measurement remains lacking a consensus (Jack et al., 2012). Individuals may be misclassified due to arbitrary cut-off values. Moreover, the pathophysiology of SNAP should remain heterogeneous. Other non-amyloid neuropathological changes, such as Aβ-independent tauopathy, hippocampal sclerosis with TDP-43, α-synucleinopathy, or argyrophilic grain disease, might contribute to and coexist with each other (Wirth et al., 2013; Mormino et al., 2016; Villeneuve, 2016). Data regarding specific pathologies of the SNAP subjects were unavailable in this study. Finally, interpretation and generalization of the findings to non-depressive subjects must acknowledge the potential limitations of this study. Notably, the finding that the SNAP and Aβ-/ND-MDD groups differed in amyloid burden despite similar psychopathological features between these two depressed groups, supported that SNAP, but not depression, is associated with reduced amyloid burden. Future studies in non-depressed subjects with SNAP are critical to determine the role of isolated SNAP on brain Aβ changes.
Conclusion
In this study, the amyloid-negative MDD patients had a significantly lesser Aβ burden than the control subjects. The SNAP MDD patients were the group that contributed most strongly to the result of reduced Aβ deposition. SNAP MDD subjects represent a distinct study population with ND biomarkers mimicking AD, but pathological biomarkers not doing so. Our findings might provide a possible evidence that other non-amyloid-mediated pathways may be involved in underlying cortical Aβ reduction in depressed older adults. We envisage that the currently labeled entity “amyloid-negative individuals” might be further refined to discern individuals with substantially low amounts of Aβ. Meaningfully low amounts of subclinical Aβ might provide critical information on the pathogenesis of non-AD individuals.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by Chang Gung Medical Foundation Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
K-YW, K-JL, and I-TH designed the study. K-YW, K-JL, I-TH, C-HC, Y-MW, and C-YL acquired the data. K-YW, K-JL, Y-MW, T-CY, C-SC, and I-TH analyzed the data. K-YW, K-JL, T-CY, and I-TH wrote the manuscript. All authors revised and approved the article for publication.
Funding
This study was carried out with financial support from the Ministry of Science and Technology, Taiwan (MOST-108-2314-B-182A-067-MY3, MOST-110-2314-B-182A-039-MY3, MOST-106-2314-B-182-017-MY3, MOST 109-2314-B-182A-043-MY3, and MOST-109-2314-B-182-019-MY3), and grants from the Research Fund of Chang Gung Memorial Hospital (CMRPG5H0041-3, CMRPD1H0391-3, BMRP488, CORPG3J0342, CMRPG3J0371, and CMRPG3J0361). The authors also acknowledge the administrative support of the Chang Gung Memorial Hospital Clinical Trial Center, which was funded by the Ministry of Health and Welfare, Taiwan (MOHW104-TDU-B-212-113003, MOHW105-TDU-B-212-13302, MOHW106-TDU-B-212-113005, MOHW107-TDU-B-212-123005, MOHW108-TDU-B-212-133005, and MOHW109-TDU-B-212-114005).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Avid Radiopharmaceuticals Inc. (Philadelphia, PA, United States) for providing the precursor for the preparation of 18F-florbetapir. We also thank the Center for Advanced Molecular Imaging and Translation, Chang Gung Memorial Hospital, Linkou, for technical support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.857940/full#supplementary-material
Footnotes
References
American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association.
Bischof, G. N., and Jacobs, H. I. L. (2019). Subthreshold amyloid and its biological and clinical meaning: Long way ahead. Neurology 93, 72–79. doi: 10.1212/WNL.0000000000007747
Burnham, S. C., Bourgeat, P., Dore, V., Savage, G., Brown, B., Laws, S., et al. (2016). Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer’s disease pathophysiology (SNAP) or Alzheimer’s disease pathology: a longitudinal study. Lancet Neurol. 15, 1044–1053. doi: 10.1016/S1474-4422(16)30125-9
Butters, M. A., Klunk, W. E., Mathis, C. A., Price, J. C., Ziolko, S. K., and Hoge, J. A. (2008). Imaging Alzheimer pathology in late-life depression with PET and Pittsburgh Compound-B. Alzheimer. Dis. Assoc. Disord. 22, 261–268. doi: 10.1097/WAD.0b013e31816c92bf
Caroli, A., Prestia, A., Galluzzi, S., Ferrari, C., Der Flier, W. M., and Ossenkoppele, R. (2015). Mild cognitive impairment with suspected nonamyloid pathology (SNAP): prediction of progression. Neurology 84, 508–515. doi: 10.1212/WNL.0000000000001209
Dale, A. M., Fischl, B., and Sereno, M. I. (1999). Cortical surface-based analysis. Neuroimage 9, 179–194. doi: 10.1006/nimg.1998.0395
Diniz, B. S., Butters, M. A., Albert, S. M., Dew, M. A., and Reynolds, C. F. III (2013). Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br. J. Psychiatry 202, 329–335. doi: 10.1192/bjp.bp.112.118307
Donovan, N. J., Locascio, J. J., Marshall, G. A., Gatchel, J., Hanseeuw, B. J., Rentz, D. M., et al. (2018). Longitudinal Association of Amyloid Beta and Anxious-Depressive Symptoms in Cognitively Normal Older Adults. Am. J. Psychiatry 175, 530–537. doi: 10.1176/appi.ajp.2017.17040442
Dubois, B., Feldman, H. H., Jacova, C., Hampel, H., Molinuevo, J. L., and Blennow, K. (2014). Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 13, 614–629.
Gatchel, J. R., Rabin, J. S., Buckley, R. F., Locascio, J. J., Quiroz, Y. T., and Yang, H. S. (2019). Longitudinal Association of Depression Symptoms With Cognition and Cortical Amyloid Among Community-Dwelling Older Adults. JAMA Netw. Open. 2:e198964. doi: 10.1001/jamanetworkopen.2019.8964
Gonzalez-Escamilla, G., Lange, C., Teipel, S., Buchert, R., Grothe, M. J., and Initiative, A. S. D. N. (2017). PETPVE12: an SPM toolbox for Partial Volume Effects correction in brain PET - Application to amyloid imaging with AV45-PET. Neuroimage 147, 669–677. doi: 10.1016/j.neuroimage.2016.12.077
Harrington, K. D., Gould, E., Lim, Y. Y., Ames, D., Pietrzak, R. H., and Rembach, A. (2017). Amyloid burden and incident depressive symptoms in cognitively normal older adults. Int. J. Geriatr. Psychiatry 32, 455–463. doi: 10.1002/gps.4489
Herholz, K., Salmon, E., Perani, D., Baron, J. C., Holthoff, V., and Frolich, L. (2002). Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage 17, 302–316. doi: 10.1006/nimg.2002.1208
Hsiao, T., Huang, C.-C., Hsieh, C.-J., Wey, S.-P., Kung, M.-P., Yen, T.-C., et al. (2013). Perfusion-like template and standardized normalization-based brain image analysis using 18F-florbetapir (AV-45/Amyvid) PET. Eur. J. Nucl. Med. Mol. I. 40, 908–920. doi: 10.1007/s00259-013-2350-x
Jack, C. R. Jr., Bennett, D. A., Blennow, K., Carrillo, M. C., Dunn, B., and Haeberlein, S. B. (2018). NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562. doi: 10.1016/j.jalz.2018.02.018
Jack, C. R. Jr., Knopman, D. S., Weigand, S. D., Wiste, H. J., Vemuri, P., Lowe, V., et al. (2012). An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann. Neurol. 71, 765–775. doi: 10.1002/ana.22628
Jack, C. R., Knopman, D. S., Chetelat, G., Dickson, D., Fagan, A. M., Frisoni, G. B., et al. (2016). Suspected non-Alzheimer disease pathophysiology - concept and controversy. Nat. Rev. Neurol. 12, 117–124. doi: 10.1038/nrneurol.2015.251
Jack, C. R., Wiste, H. J., Weigand, S. D., Knopman, D. S., Lowe, V., Vemuri, P., et al. (2013). Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology 81, 1732–1740. doi: 10.1212/01.wnl.0000435556.21319.e4
Jorm, A. F. (2001). History of depression as a risk factor for dementia: an updated review. Aust. N. Z. J. Psychiatry. 35, 776–781. doi: 10.1046/j.1440-1614.2001.00967.x
Leal, S. L., Lockhart, S. N., Maass, A., Bell, R. K., and Jagust, W. J. (2018). Subthreshold amyloid predicts tau deposition in aging. J. Neurosci. 38, 4482–4489. doi: 10.1523/JNEUROSCI.0485-18.2018
Lin, K. J., Hsu, W. C., Hsiao, I. T., Wey, S. P., Jin, L. W., Skovronsky, D., et al. (2010). Whole-body biodistribution and brain PET imaging with [18F]AV-45, a novel amyloid imaging agent–a pilot study. Nucl. Med. Biol. 37, 497–508. doi: 10.1016/j.nucmedbio.2010.02.003
Lin, K.-J., Hsiao, T., Hsu, J.-L., Huang, C.-C., Huang, K.-L., Hsieh, C.-J., et al. (2016). Imaging characteristic of dual-phase 18F-florbetapir (AV-45/Amyvid) PET for the concomitant detection of perfusion deficits and beta-amyloid deposition in Alzheimer’s disease and mild cognitive impairment. Eur. J. Nucl. Med. Mol. I. 43, 1304–1314. doi: 10.1007/s00259-016-3359-8
Mackin, R. S., Insel, P. S., Landau, S., Bickford, D., Morin, R., Rhodes, E., et al. (2021). Late-life depression is associated with reduced cortical amyloid burden: Findings from the Alzheimer’s Disease Neuroimaging Initiative Depression Project. Biol. Psychiatry 89, 757–765. doi: 10.1016/j.biopsych.2020.06.017
Madsen, K., Hasselbalch, B. J., Frederiksen, K. S., Haahr, M. E., Gade, A., Law, I., et al. (2012). Lack of association between prior depressive episodes and cerebral [11C]PiB binding. Neurobiol. Aging 33, 2334–2342. doi: 10.1016/j.neurobiolaging.2011.11.021
Mirza, S. S., Wolters, F. J., Swanson, S. A., Koudstaal, P. J., Hofman, A., Tiemeier, H., et al. (2016). 10-year trajectories of depressive symptoms and risk of dementia: a population-based study. Lancet Psychiat. 3, 628–635. doi: 10.1016/S2215-0366(16)00097-3
Mormino, E. C., Papp, K. V., Rentz, D. M., Schultz, A. P., Lapoint, M., Amariglio, R., et al. (2016). Heterogeneity in Suspected Non-Alzheimer Disease Pathophysiology Among Clinically Normal Older Individuals. JAMA Neurol. 73, 1185–1191. doi: 10.1001/jamaneurol.2016.2237
Ownby, R. L., Crocco, E., Acevedo, A., John, V., and Loewenstein, D. (2006). Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch. Gen. Psychiatry 63, 530–538. doi: 10.1001/archpsyc.63.5.530
Rapp, M. A., Schnaider-Beeri, M., Grossman, H. T., Sano, M., Perl, D. P., Purohit, D. P., et al. (2006). Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch. Gen. Psychiatry 63, 161–167. doi: 10.1001/archpsyc.63.2.161
Sabri, O., Seibyl, J., Rowe, C., and Barthel, H. (2015). Beta-amyloid imaging with florbetaben. Clin. Transl. I. 3, 13–26. doi: 10.1007/s40336-015-0102-6
Van Dyck, C. H., O’dell, R. S., and Mecca, A. P. (2021). Amyloid-Associated Depression-or Not? Biol. Psychiatry 89, 737–738. doi: 10.1016/j.biopsych.2021.02.008
Villeneuve, S. (2016). Cause of Suspected Non-Alzheimer Disease Pathophysiology If Not Tau Pathology. Then What? JAMA Neurol. 73, 1177–1179. doi: 10.1001/jamaneurol.2016.2842
Voevodskaya, O., Simmons, A., Nordenskjold, R., Kullberg, J., Ahlstrom, H., Lind, L., et al. (2014). The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer’s disease. Front. Aging Neurosci. 6:264. doi: 10.3389/fnagi.2014.00264
Wirth, M., Villeneuve, S., Haase, C. M., Madison, C. M., Oh, H., Landau, S. M., et al. (2013). Associations between Alzheimer disease biomarkers, neurodegeneration, and cognition in cognitively normal older people. JAMA Neurol. 70, 1512–1519. doi: 10.1001/jamaneurol.2013.4013
Wu, K. Y., Hsiao, I. T., Chen, C. S., Chen, C. H., Hsieh, C. J., Wai, Y. Y., et al. (2014). Increased brain amyloid deposition in patients with a lifetime history of major depression: evidenced on 18F-florbetapir (AV-45/Amyvid) positron emission tomography. Eur. J. Nucl. Med. Mol. I. 41, 714–722. doi: 10.1007/s00259-013-2627-0
Wu, K. Y., Lin, K. J., Chen, C. H., Chen, C. S., Liu, C. Y., Huang, S. Y., et al. (2018). Diversity of neurodegenerative pathophysiology in nondemented patients with major depressive disorder: Evidence of cerebral amyloidosis and hippocampal atrophy. Brain Behav. 8:e01016. doi: 10.1002/brb3.1016
Wu, K. Y., Liu, C. Y., Chen, C. S., Chen, C. H., Hsiao, I. T., Hsieh, C. J., et al. (2016). Beta-amyloid deposition and cognitive function in patients with major depressive disorder with different subtypes of mild cognitive impairment: (18)F-florbetapir (AV-45/Amyvid) PET study. Eur. J. Nucl. Med. Mol. I. 43, 1067–1076. doi: 10.1007/s00259-015-3291-3
Keywords: suspected non-Alzheimer pathophysiology (SNAP), major depressive disorder (MDD), amyloid-β (Aβ), 18F-florbetapir (AV-45/Amyvid), neurodegeneration, depression in old age
Citation: Wu K-Y, Lin K-J, Chen C-H, Liu C-Y, Wu Y-M, Chen C-S, Yen T-C and Hsiao I-T (2022) Decreased Cerebral Amyloid-β Depositions in Patients With a Lifetime History of Major Depression With Suspected Non-Alzheimer Pathophysiology. Front. Aging Neurosci. 14:857940. doi: 10.3389/fnagi.2022.857940
Received: 19 January 2022; Accepted: 11 April 2022;
Published: 26 May 2022.
Edited by:
Wenjing Zhang, Sichuan University, ChinaReviewed by:
Valeria Isella, University of Milano-Bicocca, ItalyJorge Matias-Guiu, Complutense University of Madrid, Spain
Copyright © 2022 Wu, Lin, Chen, Liu, Wu, Chen, Yen and Hsiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ing-Tsung Hsiao, aWhzaWFvQG1haWwuY2d1LmVkdS50dw==
 Kuan-Yi Wu1
Kuan-Yi Wu1 Chia-Hsiang Chen
Chia-Hsiang Chen Tzu-Chen Yen
Tzu-Chen Yen Ing-Tsung Hsiao
Ing-Tsung Hsiao