- 1Department of Neurology and National Research Center for Aging and Medicine & National Center for Neurological Disorders, State Key Laboratory of Medical Neurobiology, Huashan Hospital, Fudan University, Shanghai, China
- 2Positron Emission Tomography (PET) Center at Huashan Hospital, Institute of Functional and Molecular Medical Imaging, Human Phenome Institute, Fudan University, Shanghai, China
Background: Cognitive dysfunctions have been reported in multiple system atrophy (MSA). However the underlying mechanisms remain to be elucidated. This study aimed to explore the possible cerebral metabolism associated with domain-specific cognitive performances in MSA.
Methods: A total of 84 patients were diagnosed as probable or possible MSA, comprised of 27 patients as MSA with predominant parkinsonism (MSA-P) and 57 patients as MSA with predominant cerebellar ataxia (MSA-C). The comprehensive neuropsychological tests and 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) imaging were performed. Z-score was calculated to non-dimensionalize and unify indicators of different tests in the domains of executive function, attention, language, memory, and visuospatial function. Correlations between specific Z-score and cerebral 18F-FDG uptake were analyzed using statistical parametric mapping. The cognition-related metabolic differences between patients with MSA-P and MSA-C were analyzed using the post-hoc test.
Results: Z-scores of the domains including attention, executive function, and language correlated positively with the metabolism in the superior/inferior frontal gyrus and cerebellum, but negatively with that in the insula and fusiform gyrus (p < 0.001). No significant differences in neuropsychological performances and frontal metabolism were found between patients with MSA-P and MSA-C. Only lower metabolism in the cerebellum was observed in MSA-C.
Conclusion: Metabolic changes in the frontal lobe and cerebellum may participate in the cognitive impairments of patients with MSA. Nevertheless, cognitive and corresponding metabolic differences between the two subtypes of MSA still need more exploration.
Introduction
Multiple system atrophy (MSA) is a sporadic, adult-onset neurodegenerative disorder, with the classical clinical presence of progressive autonomic failure, parkinsonism, and/or cerebellar ataxia (Wenning et al., 1997; Stefanova et al., 2009; Fanciulli and Wenning, 2015). Although dementia is a non-supporting item according to the second consensus statement on the diagnosis of MSA (Gilman et al., 2008), accumulating data showed great evidence for possible cognitive decline in MSA. Specifically, nearly half of the patients with MSA presented with cognitive impairments in some epidemiological data, with a broad spectrum of injury (Brown et al., 2010; Stankovic et al., 2014; Shen et al., 2021). At present, however, the underlying mechanisms of cognitive impairments in MSA are still far from being clearly understood. In the study of patients with MSA with mild dementia and aphasia, atrophy and neuronal cell loss were observed macroscopically and microscopically in the frontal lobe (Konagaya et al., 1999). Subsequent studies have compared the pathological burden degrees of glial cytoplasmic inclusions (GCIs) and neuronal cytoplasmic inclusions (NCIs) between MSA with normal cognition (MSA-NC) and MSA with cognitive impairment (MSA-CI; Cykowski et al., 2015; Homma et al., 2016; Koga et al., 2017; Jellinger, 2020; Miki et al., 2020). However, these results were variable and uncertain, which might be partially attributed to the small sample size.
The 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) imaging can reflect the cerebral metabolic changes and disclose disease-specific alterations (Eckert et al., 2005, 2008). In our previous report, MSA-CI exhibited lower glucose metabolism in the left middle and superior frontal lobe, compared with MSA-NC (Shen et al., 2021). Nevertheless, the cerebral metabolic changes related to detailed cognitive performances in MSA remain to be further explored. In addition, the previous studies on comparisons of the profile and severity of cognitive dysfunction between two MSA subtypes, MSA with predominant parkinsonism (MSA-P) and MSA with predominant cerebellar ataxia (MSA-C; Gilman et al., 2008) remained limited and controversial (Kawai et al., 2008; Chang et al., 2009; Santangelo et al., 2020). Therefore, the comparison of cognition-related cerebral metabolism in different MSA subtypes will be helpful to understand the intrinsic pathological characteristics, which has not been reported earlier.
In this study, we aimed to detect the underlying cerebral metabolic changes by 18F-FDG PET imaging related to domain-specific cognitive dysfunctions in patients with MSA. Furthermore, we compared the neuropsychological performances and cognition-related brain metabolism between the two subtypes of MSA preliminarily.
Participants and Methods
Subjects
A total of 84 patients diagnosed with probable or possible MSA were recruited in the Department of Neurology, Huashan Hospital, Fudan University between February 2012 and May 2019. The clinical diagnosis and categorization between MSA-P and MSA-C were made according to the second consensus statement on the diagnosis of MSA published in 2008 (Gilman et al., 2008). Demographic information including age, age at onset, sex, disease duration, and education degree were systematically collected. All patients included neither had head injury nor had other neurological or psychiatric diseases.
This study was approved by the Human Studies Institutional Review Board, Huashan Hospital, Fudan University. Written informed consents were obtained from the participants before entering this study in accordance with the Declaration of Helsinki.
Motor and Neuropsychological Assessments
Motor disability was evaluated by the Unified Parkinson’s Disease Rating Scale III (UPDRS III) in all participants in a Med-Off state, which means that the patients were off anti-parkinsonism drugs for more than 12 h. Global cognitive status and cognitive dysfunction in five domains covering attention, executive function, memory, visuospatial function, and language were assessed using Mini-Mental State Examination (MMSE) and a series of comprehensive neuropsychological tests, which were as follows: attention by the Symbol Digit Modalities Test (SDMT; Sheridan et al., 2006) and part A of Trail-Making Test (TMT-A; Zhao et al., 2013); executive function by the part C of Stroop Color-Word Test (CWT-C; Steinberg et al., 2005) and part B of Trail-Making Test (TMT-B; Zhao et al., 2013); memory function by the Auditory Verbal Learning Test (AVLT; Guo et al., 2009) and delayed recall task of the Rey-Osterrieth Complex Figure Test (CFT-delay recall) (Caffarra et al., 2002); visuospatial function by copy task of Rey-Osterrieth Complex Figure Test (CFT; Caffarra et al., 2002) and the Clock Drawing Test (CDT; Ricci et al., 2016); and language ability by Animal Verbal Fluency Test (AVFT) and Boston Naming Test (BNT; Lucas et al., 2005). To eliminate dimensional differences in tests and quantify impairments of cognition, neuropsychological results of each test of all patients were transformed into Z-score using the mean and SD of the norm data from healthy controls of similar age and education in the Shanghai area (Wu et al., 2018; Shen et al., 2021): Z-score = (test score - mean score of norm data)/SD of norm data (when data was a score) or Z-score = (mean time of norm data - test time)/SD of norm data (when data were a time metrics) (Wu et al., 2018). Z-score less than −1.5 was defined to be the impairment in each test and Z-score of each cognition domain was calculated using the average Z-score of two tests in the same cognition domain.
18F-Fluorodeoxyglucose Positron Emission Tomography Scan and Image Processing
18F-fluorodeoxyglucose PET imaging was acquired in all patients within 1 month before or after the clinical assessment in the Huashan Hospital. Patients were instructed to be fasting for at least 6 h in an off-drug state before the scan. Forty-five minutes resting after injection of 18F-FDG (185 MBq), a short CT scan, and subsequent 10-min PET scan was performed by a Siemens Biograph 64 PET/CT (Siemens, Munich, Germany) in the three-dimensional (3D) mode in a dimly lit and quiet room. Images were subsequently reconstructed using a 3D ordered-subset expectation-maximization algorithm (4 iterations; 24 subsets; Gaussian filter, 2 mm; zoom, 3). Reconstructed images had a matrix size of 168 × 168 × 148 and a voxel size of 2 × 2 × 1.5 mm3.
Positron emission tomography images were transformed for further processing using the MRIcron tool (output format: hdr/img). Then, images were processed by statistical parametric mapping (SPM5) software operated in MATLAB platform (Mathworks Inc., Sherborn, MA, United States). First, the original images were spatially normalized to normative stereotactic Montreal Neurological Institute (MNI) space using default [15O]-H2O PET template (Della Rosa et al., 2014)1. Second, they were smoothed through a three-dimensional Gaussian filter with 10-mm full-width at half-maximum (FWHM).
To compare the metabolic characteristics between MSA-P and MSA-C, the two-sample t-test was used with age, sex, disease duration, and UPDRS score as covariates according to the general linear model at each voxel in SPM. To assess whether metabolic characteristics in a specific brain region would correlate with Z-score in each cognitive domain, the multiple regression model established by us previously (Ge et al., 2015; Wu et al., 2018) was applied first to detect clusters with peak threshold set at P < 0.001 over whole brain regions and extent threshold set as 120 voxels (960 mm3) and further to highlight clusters survived after a false discovery rate (FDR) correction at p < 0.05. In this step, education degree and UPDRS III score were both included as covariates to eliminate the effect of these factors. The differences in global metabolic values were modeled by analysis of covariance (ANCOVA) to minimize intersubject variability and improve signal-noise ratios. Exact coordinates and corresponding brain regions of detected clusters were ascertained through an MNI-to-Talairach conversion using Talairach–Daemon software (Research Imaging Center, University of Texas Health Science Center, San Antonio, TX, United States). The SPM maps for positive and negative correlations were superpositioned on a standard T1-weighted MRI brain template. Then, the spherical volume of interest (VOI) (4 mm radius) with the center of the circle at the peak voxel of the cluster was constructed, and metabolic value in each VOI was quantified using ScAnVP software (Version 5.9.1; Center for Neuroscience, the Feinstein Institute for Medical Research, Manhasset, NY, United States). To eliminate the individual differences in global metabolic value, the normalized regional cerebral metabolic rate of glucose (rCMRglc) was calculated by the following formula: [VOI value/whole-brain metabolism] × 100%.
Statistical Analysis
Differences in the demographic information, clinical characteristics, and neuropsychological performances between MSA-P and MSA-C were analyzed through Student’s t-test in continuous and normally distributed data. Pearson’s χ2 test was used in categorical data. Mann–Whitney U test was used in continuous and non-parametric data. The post-hoc correlation analysis between Z-score in the specific cognitive domain and normalized rCMRglc in each VOI was performed through Pearson correlation and linear regression analyses. In the post-hoc metabolic comparison between patients with MSA-P and MSA-C, multiple comparisons via the Benjamini–Hochberg procedure were performed to control the FDR. Statistical analyses were performed using SPSS software (SPSS for Windows, version 22.0; SPSS Inc., Chicago, IL, United States). p < 0.05 was considered statistically significant.
Results
Clinical Characteristics and Neuropsychological Performances in Patients With Multiple System Atrophy
Demographic, clinical, and detailed neuropsychological information of the total 84 patients with MSA was depicted in Table 1. The mean age at onset was 54.89 ± 7.97 years, and the mean disease duration was 26.80 ± 20.69 months. Patients with MSA were divided into two subtypes according to their predominant clinical symptoms, including 27 patients with MSA-P and 57 patients with MSA-C. No group differences were found for age at onset and education, but differences were observed in age, sex, disease duration, and UPDRS III score (Table 1). Both subtypes had considerable and extensive cognitive impairments in each domain represented by number and percentage of impairment as follows: attention, MSA-P, 18(67%), MSA-C, 30(53%); executive function, MSA-P, 13(48%), MSA-C, 32(56%); memory, MSA-P, 18(67%), MSA-C, 25(44%); visuospatial function, MSA-P, 12(44%), MSA-C, 22(39%); language, MSA-P, 10(37%), MSA-C, 21(37%). Compared with patients with MSA-C, patients with MSA-P performed worse on the attention domain, judging by a lower Z-score of attention domain and two corresponding tests (SDMT and TMT-A) (p < 0.05). However, this difference in attention performance was not significant after adjusting age, sex, disease duration, and UPDRS score factors.
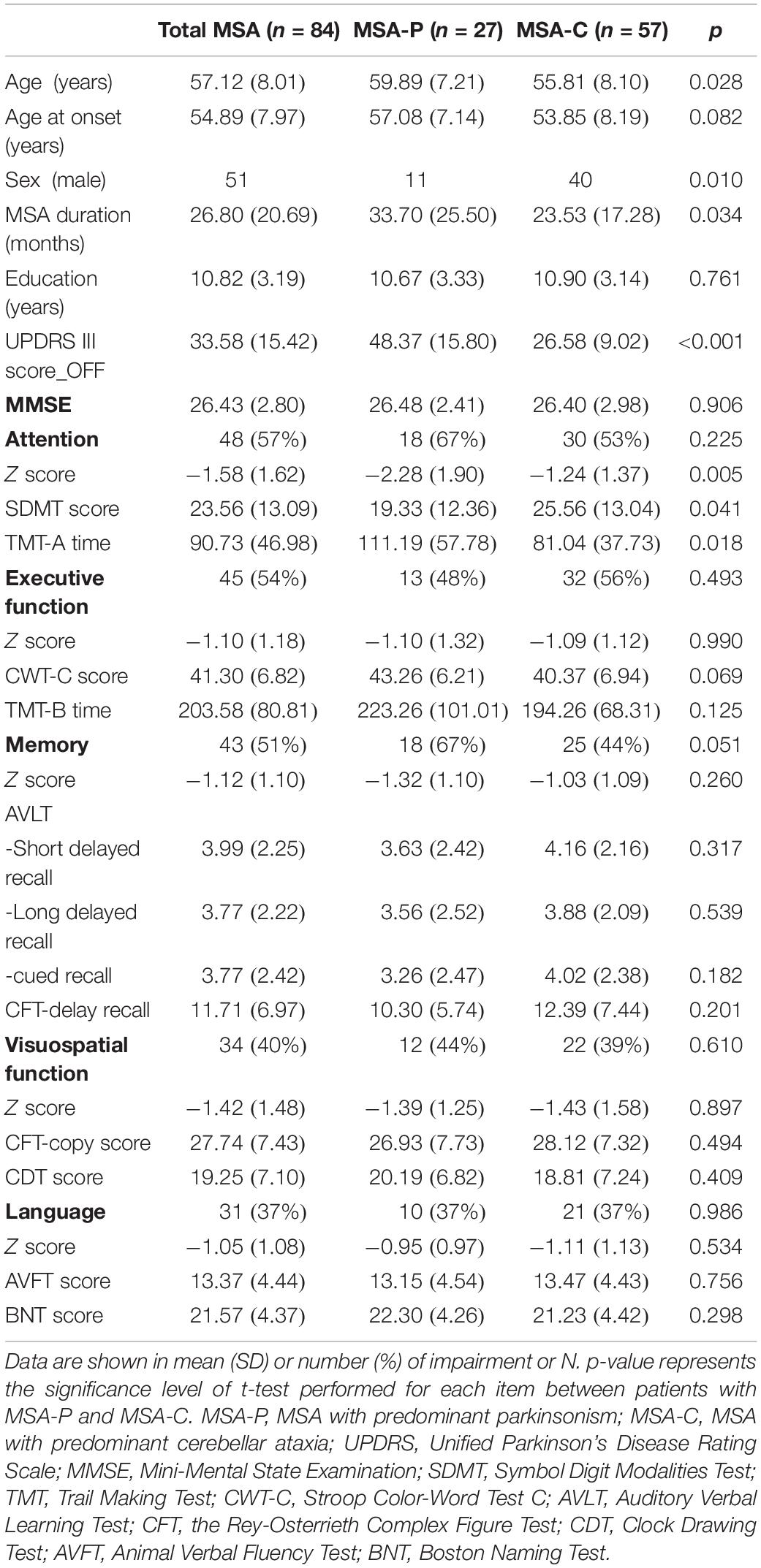
Table 1. Demographic, clinical characteristics, and neuropsychological performance in 84 patients with multiple system atrophy (MSA).
The Regional Metabolism Correlating With Cognitive Declines in Multiple System Atrophy
Global metabolic values did not correlate with any Z-score in five domains (regression analysis: absolute value r ≤ 0.07, p ≥ 0.40). However, the positive and negative correlations between the regional 18F-FDG metabolism and the cognitive Z-score in the five domains were detected (Figure 1).
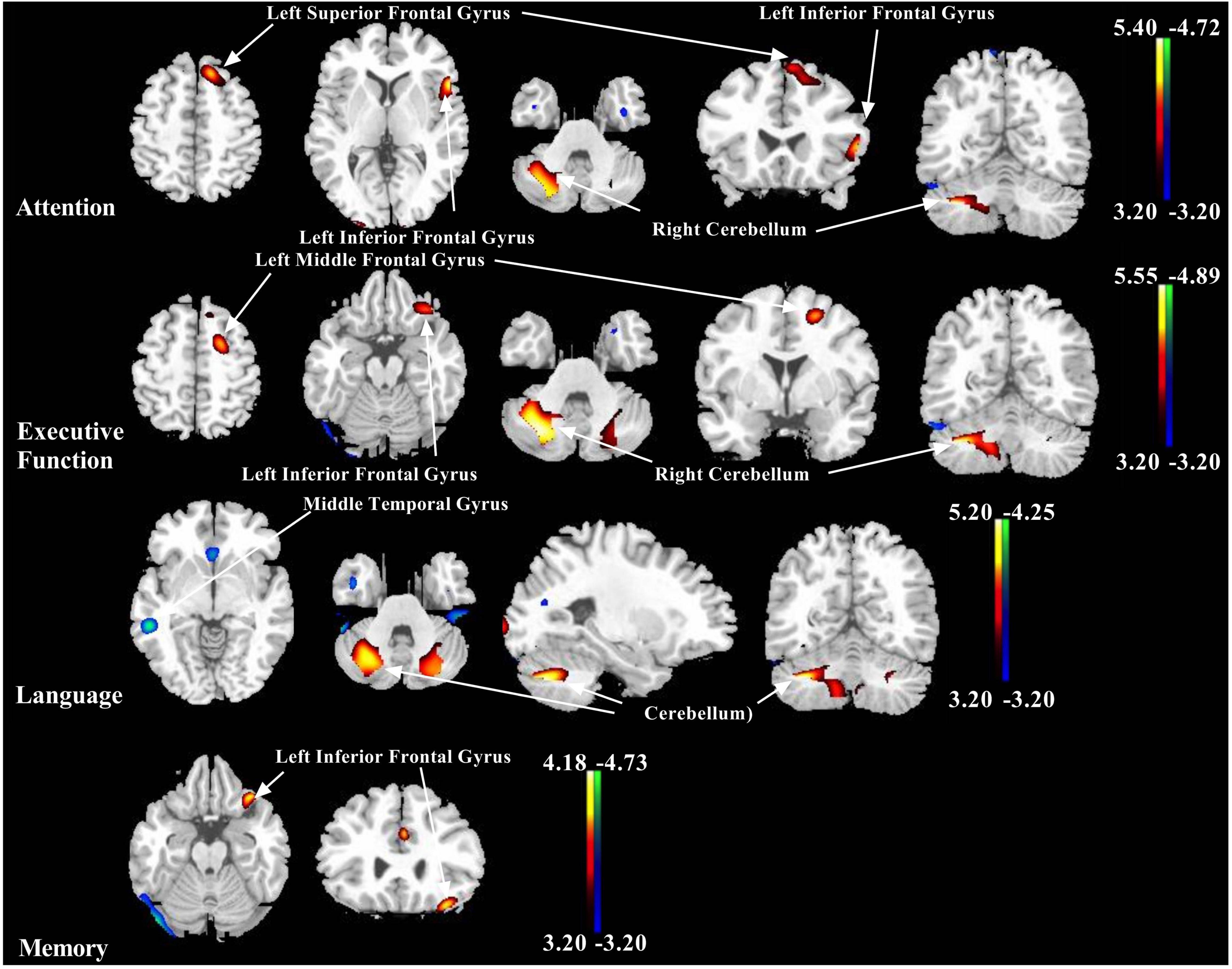
Figure 1. Representative images showing correlations between Z-score of each cognitive domain and 18F-fluorodeoxyglucose (18F-FDG) metabolism in patients with multiple system atrophy (MSA). Positive relationships are displayed with a red-yellow scale while negative relationships are displayed using a blue-green scale. Both are overlaid on a structural magnetic resonance image (MRI) brain template. White arrows show the representative brain regions. The thresholds of the color bars indicate T-values, and voxel threshold was set at p < 0.001.
The brain regions correlated positively to the Z-score of the separate cognitive domain were described as follows, respectively: attention with left inferior frontal gyrus, left superior frontal gyrus, left medial frontal gyrus, and right cerebellum (uvula and cerebellar tonsil); executive function with left inferior frontal gyrus, left middle frontal gyrus, left cerebellum (uvula), and right cerebellum (pyramis and inferior semilunar lobule); language with left superior frontal gyrus, left middle frontal gyrus, left cerebellum (pyramis and uvula), and right cerebellum (tuber, pyramis, cerebellar tonsil, and declive); memory with inferior frontal gyrus; and visuospatial function with left cerebellum (pyramis) (Figure 1 and Table 2).
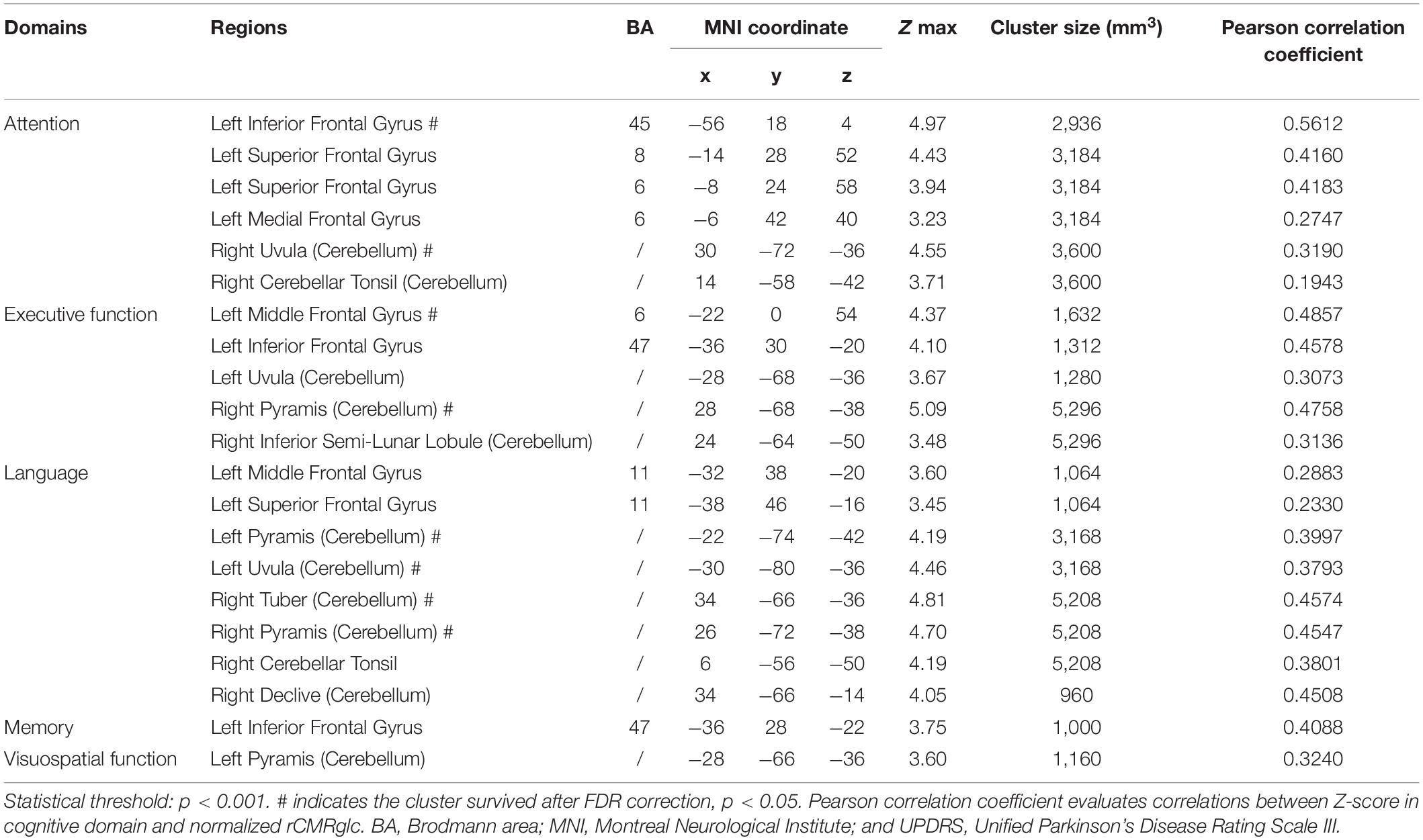
Table 2. Brain regions in the 84 patients with MSA exhibiting positive correlations between the Z-score of cognitive domains and regional brain metabolism adjusting for education and Unified Parkinson’s Disease Rating Scale (UPDRS) score.
However, the brain regions associated negatively with the Z-score of the separate cognitive domain were shown as follows, respectively: attention with right fusiform gyrus and right insula; executive function with right fusiform gyrus and right inferior temporal gyrus; language with left anterior cingulate, right middle temporal gyrus, and right fusiform gyrus; and memory with right fusiform gyrus and right inferior occipital gyrus (Figure 1 and Table 3).
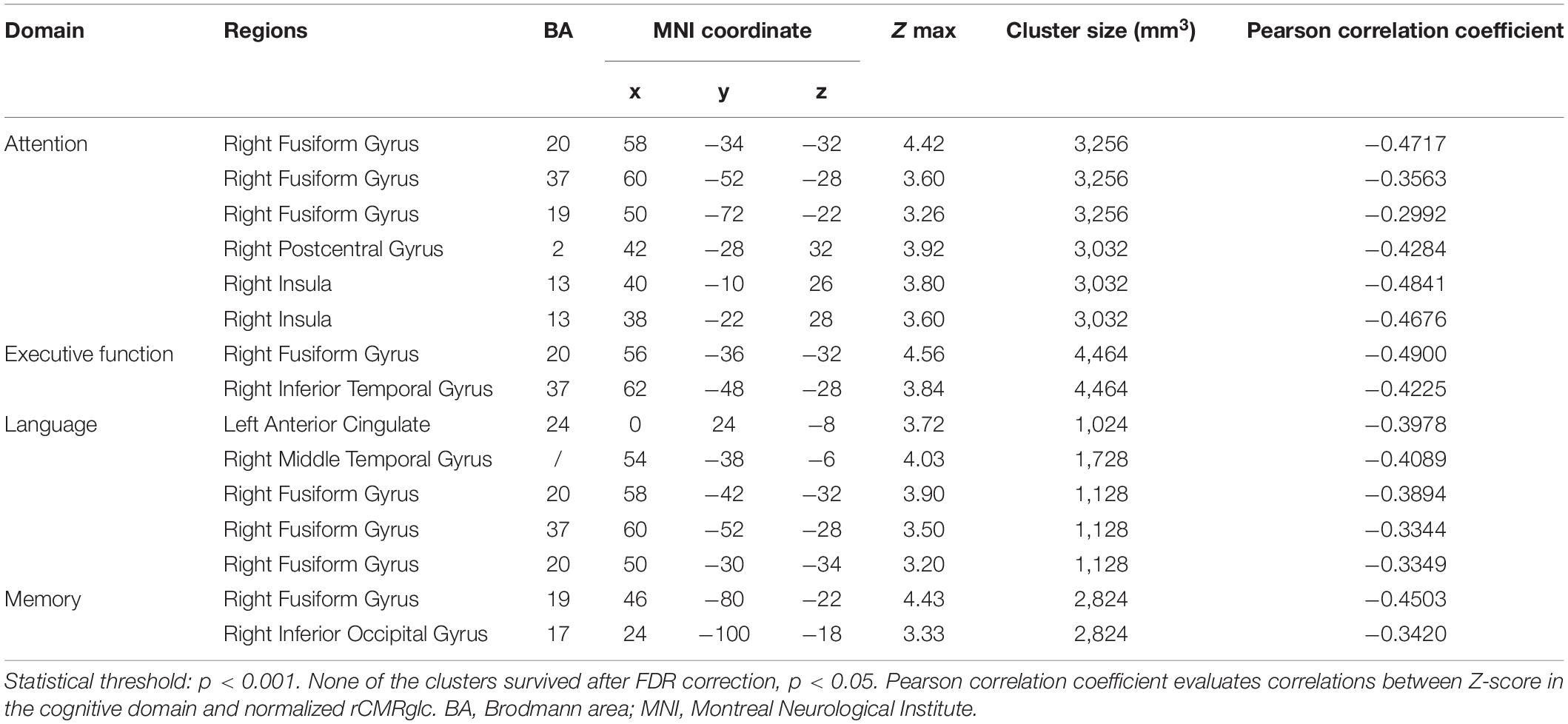
Table 3. Brain regions in the 84 patients with MSA exhibiting negative correlations between the Z-score of cognitive domains and regional brain metabolism adjusting for education and UPDRS score.
Moreover, the post-hoc correlations between the Z-score in separate cognition domain and the normalized rCMRglc in each significant brain region were calculated, with the correlation coefficients listed in Tables 2, 3. The scatterplots and relationships of cognition and metabolic value in 11 representative regions are shown in Figure 2.
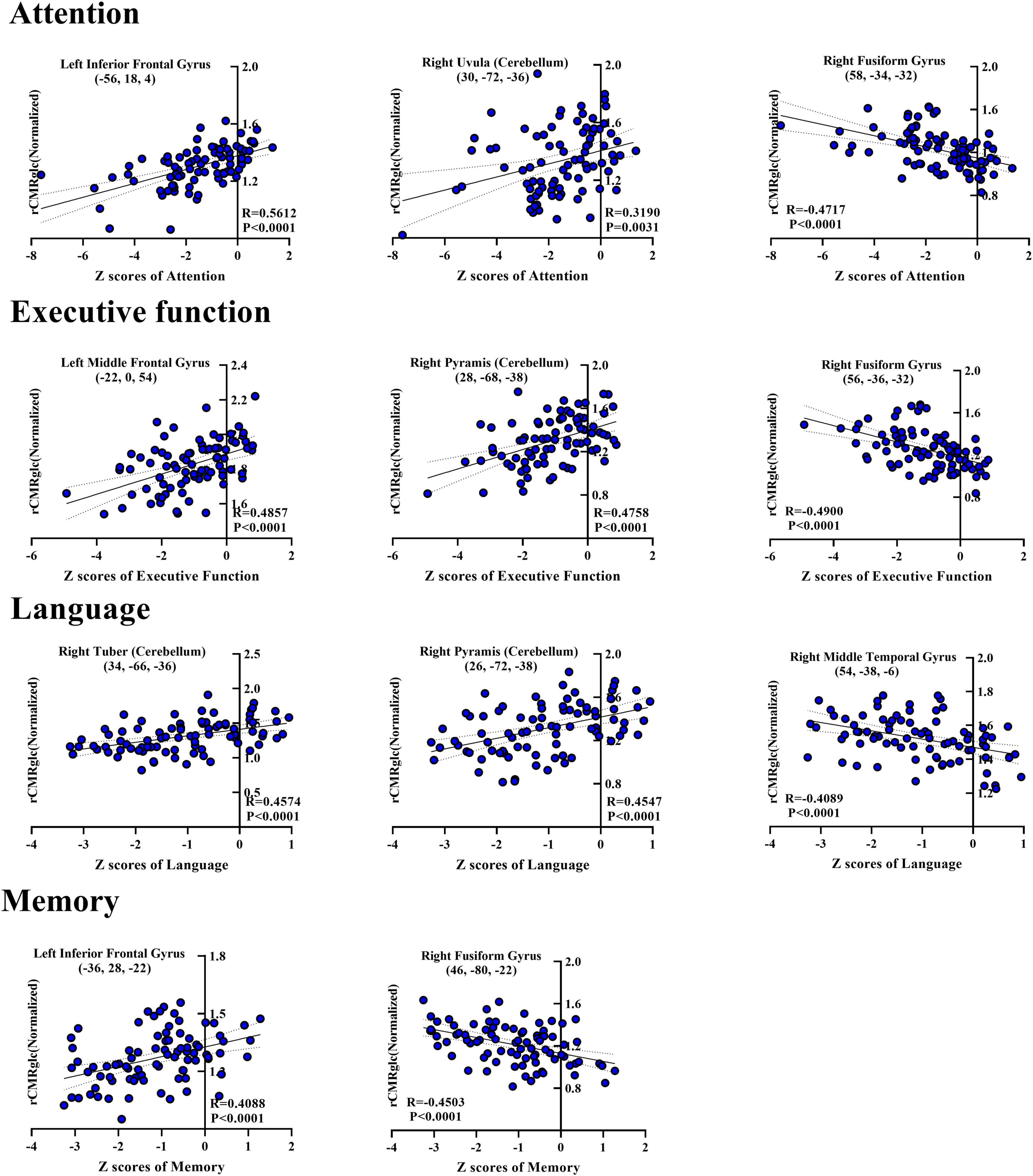
Figure 2. Scatterplots showing correlations between Z-score of each cognitive domain and normalized regional glucose metabolic activities for the significantly correlated regions in the post-hoc analysis. Each blue-filled circle represents one patient with Z-score of specific cognitive domain and regional cerebral metabolic rate of glucose (rCMRglc) in certain brain region. R-value means the correlation coefficients. rCMRglc, regional cerebral metabolic rate of glucose.
Cognition-Related Regional Metabolism in Two Subtypes of Multiple System Atrophy
Compared with patients with MSA-C, increased metabolism mainly in the bilateral cerebellum while decreased metabolism in bilateral putamen was observed in MSA-P, with no difference in frontal gyrus (Supplementary Table 1).
Considering the unadjusted cognitive differences in the attention domain between MSA-P and MSA-C, the comparisons of regional metabolism in attention-related areas mentioned above between two subtypes are presented in Figure 3 and Table 4. However, no significant metabolic intergroup differences were observed in the left inferior frontal gyrus, superior frontal gyrus, medial frontal gyrus, right fusiform gyrus, right postcentral gyrus, and right insula. Only the metabolism in the right cerebellum (including uvula and cerebellar tonsil) was significantly lower in patients with MSA-C than patients with MSA-P after adjustment for confounders and post-hoc correction for multiple comparisons.
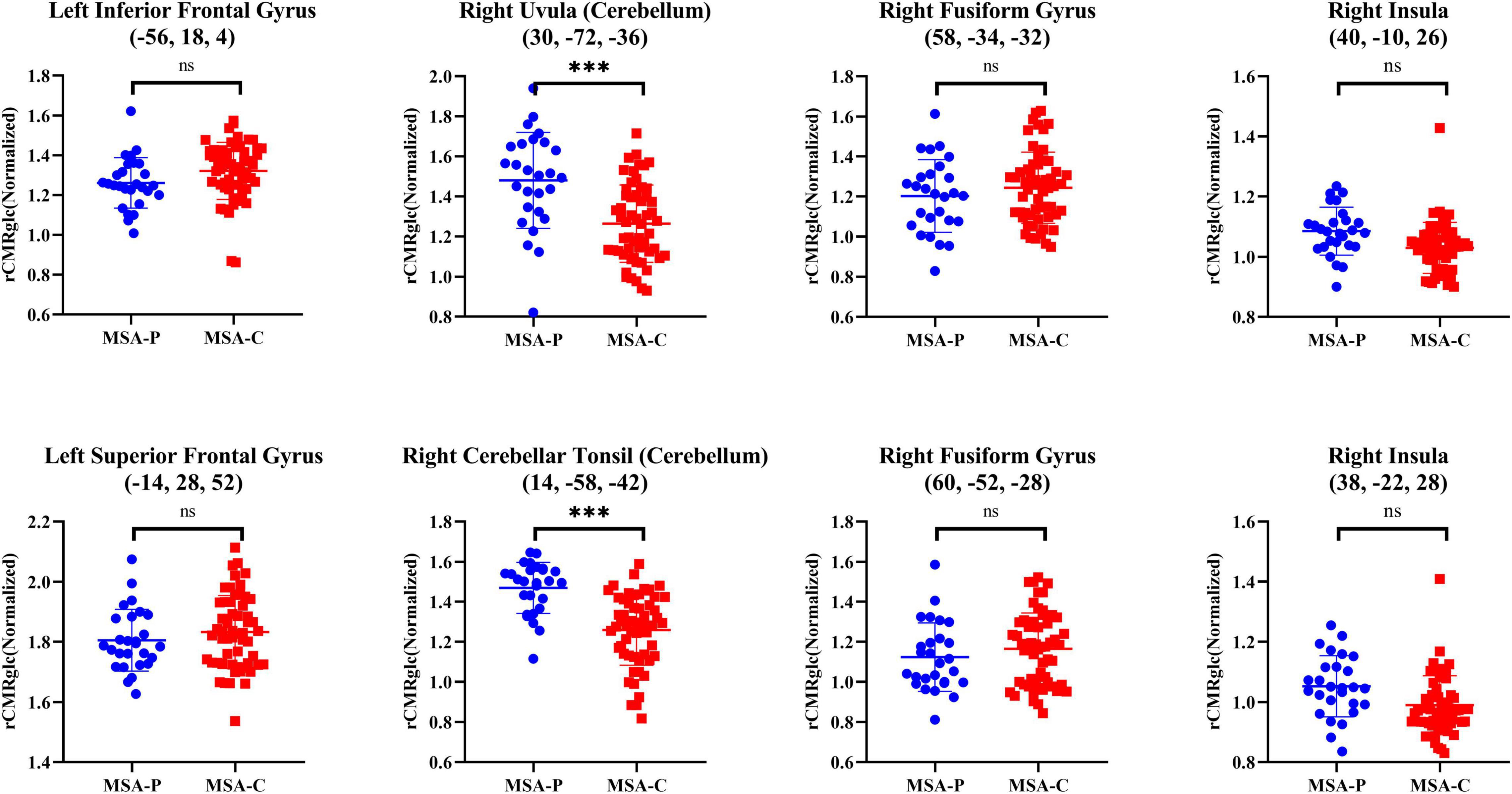
Figure 3. Differences of normalized rCMRglc in representative attention-related regions between two subtypes of patients with MSA. The error bars represent SD. ***p < 0.0001; *p < 0.01; nsp > 0.05. rCMRglc, regional cerebral metabolic rate of glucose; MSA-P, multiple system atrophy with predominant parkinsonism; MSA-C, multiple system atrophy with cerebellar ataxia.
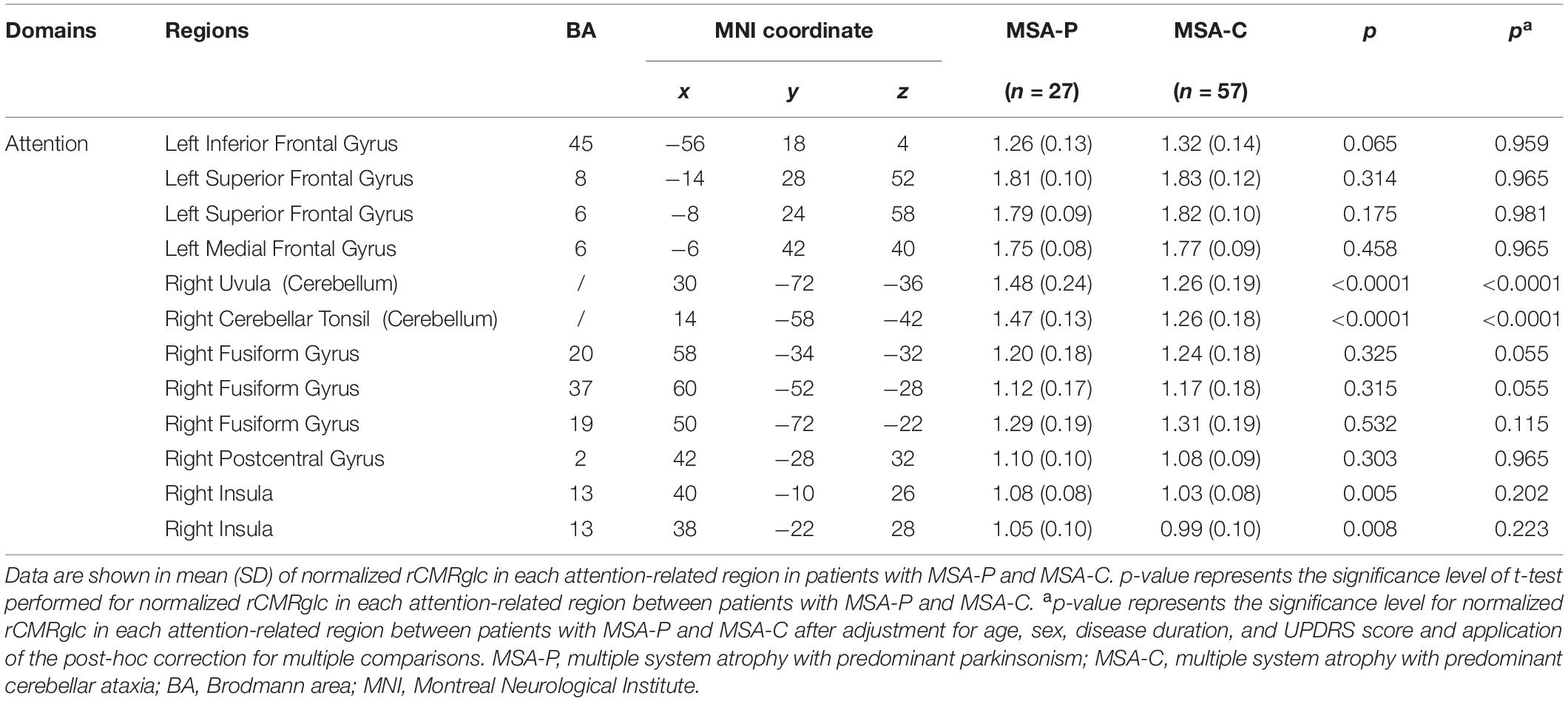
Table 4. Differences of regional brain metabolism in attention-related areas between patients with MSA with predominant parkinsonism (MSA-P) and MSA with predominant cerebellar ataxia (MSA-C).
In addition, correlations between metabolism and the cognitive Z-score in the five domains separately in two subtypes are shown in Supplementary Tables 2, 3. Cognition in patients with MSA-C correlated positively with metabolism in the frontal lobe and cerebellum, negatively with that in the fusiform gyrus and insula, whereas the clusters correlated with cognition in patients with MSA-P were limited to the cerebellum (positively) and temporal lobe (negatively).
Discussion
In this study, there were three main findings. First and most importantly, lesions in the domains of attention, executive function, and language (Z-scores) correlated positively with the metabolism in the frontal gyrus and cerebellum and negatively with the insula and fusiform gyrus. Second, the attention function tended to be more severely impaired in MSA-P than in MSA-C, though significance did not sustain after adjusting confounders. Third, among all the attention-related brain regions, there was only lower metabolism of the cerebellum in MSA-C than in MSA-P.
18F-fluorodeoxyglucose PET imaging can detect cerebral glucose metabolism and offer us some hints for mechanisms in neural hubs or networks behind cognitive impairments in patients with MSA. In our analysis, metabolism in the frontal gyrus and cerebellum showed a great correlation with the severity of cognitive impairments in attention, executive function, and language domains.
The lower metabolism in the frontal gyrus of patients with MSA with worse cognition might suggest the disruption of frontostriatal dopamine signaling. The prefrontal cortex, as a key hub in the executive network, is associated with working memory and selective attention (Elliott, 2003; Gratwicke et al., 2015). Dopamine released from the substantia nigra and ventral tegmental area modulates prefrontal D2 receptors and facilitates cognitive flexibility (Floresco and Magyar, 2006). In previous neuropathology studies, there was a significant loss of neurons in the frontal cortex in patients with MSA with executive dysfunction compared with those with normal executive function (Salvesen et al., 2017). Analogously, (Fiorenzato et al., 2017) found that MSA-CI had focal volume reduction in the left middle frontal lobe compared with MSA-NC through voxel-based morphometry. Particularly, the study by Chang et al. (2009) showed that memory scores were well associated with prefrontal lobe atrophy. In addition, our hypothesis of striatofrontal differentiation in patients with MSA-CI was supported by the finding from FDG study by Kim et al. (2017), in which the glucose metabolism in the striatum was the most powerful determinant to metabolism in the frontal gyrus in the patients with MSA but not in healthy controls. Considering all these studies together, we believed that the frontostriatal network was involved in cognitive dysfunction in patients with MSA.
Besides the well-known motor function, the cerebellum is also involved in maintaining the functions of working memory, language, and social cognition. As previously reported, cerebellar lesions could lead to executive dysfunctions, deficits in visuospatial processing, and linguistic skills, termed cerebellar cognitive affective syndrome (Schmahmann and Sherman, 1998; Buckner, 2013). In study by Kawabata et al. (2019) with resting-state fMRI, it was reported that Addenbrooke’s Cognitive Examination-Revised scores correlated strongly with functional connectivity between the cerebellum and the medial prefrontal/anterior cingulate cortices. All these studies indicated extensive disruptions in fronto-cerebellar networks in patients with MSA. In this study, we found the reduced metabolism in the frontal lobe and cerebellum associated with severer cognitive injury, possibly supported by the role of cerebellum degeneration in cognitive impairments.
Furthermore, the cognitive impairments in MSA-P and MSA-C were evaluated and compared in this study. As previously reported, some studies suggested the patients with MSA-P showed more deteriorated cognitive functions (Kawai et al., 2008; Li et al., 2021), some found patients with MSA-C performing worse on specific cognitive tests (Chang et al., 2009; Barcelos et al., 2018), while the rest showed no difference (Siri et al., 2013; Santangelo et al., 2020). In our study, patients with MSA-P performed poorly in the domain of attention, to a weak degree that did not survive after adjusting confounding factors. Therefore, we could not make any definite conclusion on the differences in cognitive dysfunctions between two MSA subtypes so far.
Among attention-related brain regions in this study, we found regional metabolism in the superior/inferior/medial frontal gyrus showing no difference between MSA-P and MSA-C, but metabolism in the cerebellum was lower in MSA-C. Kawai et al. (2008) found cerebellar hypoperfusion in MSA-C, and hypoperfusion in right putamen in MSA-P when comparing brain perfusion between MSA-P and MSA-C. Given significant differences in motion spectrum between MSA-P and MSA-C, the metabolic pattern in putamen and cerebellum was essentially distinct. Therefore, there is not enough evidence to support whether hypermetabolism in the cerebellum in MSA-P could explain the unadjusted difference of attention performance between two types of MSA. More studies with rigorous design are needed to answer this question.
Our study had several strengths. First, our study sample size was relatively large compared with the previous reports. Second, the neuropsychological tests applied in this study were comprehensive to cover the five domains. However, several limitations should also be admitted. Partial volume effect (PVE) correction was not performed due to the incomplete data of MRI. In future studies, rigorous results performed based on PVE correction in newly recruited patients are warranted. Moreover, the demographic information of patients with MSA-P and MSA-C was mismatched, and the sample size of MSA-P was relatively small in our study. Further study with a more paired and more complete population is needed to confirm the neuropsychological and metabolic comparisons between the two groups. In addition, double-dipping problem causing selective bias was another limitation. It derived from performing MSA-P and MSA-C comparison after extracting metabolic values from statistically significant regions. We hope that another independent data set in the future could confirm our results.
Conclusion
The frontal lobe and cerebellum participated in the pathogenesis of cognitive impairments in MSA in this 18F-FDG PET imaging study. Longitudinal studies with specific radioligands are needed to clarify the unique role of dopaminergic deficiency and misfolded protein burden in the development of cognition decline in MSA.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Human Studies Institutional Review Board, Huashan Hospital, Fudan University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JW and F-TL were involved in the conception and design of the study. CS and Q-SC took part in the acquisition, screening, and analysis of data. CS was responsible for figures and tables. CS, Q-SC, and F-TL wrote the first draft of the manuscript. C-TZ, F-TL, and JW revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Nature Science Foundation of China (Grant Numbers: 81701250, 91949118, 81771372, 81671239, and 81971641), the Ministry of Science and Technology of China (Grant Numbers: 2016YFC1306500 and 2016YFC1306504), the Shanghai Sailing Program (No. 18YF1403100), the Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01) and ZJ Lab, and Project of Chinese Academy of Sciences (SKLN-201904).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to all the patients who took part in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.788166/full#supplementary-material
Footnotes
References
Barcelos, L. B., Saad, F., Giacominelli, C., Saba, R. A., de Carvalho Aguiar, P. M., Silva, S. M. A., et al. (2018). Neuropsychological and clinical heterogeneity of cognitive impairment in patients with multiple system atrophy. Clin. Neurol. Neurosurg. 164, 121–126. doi: 10.1016/j.clineuro.2017.10.039
Brown, R. G., Lacomblez, L., Landwehrmeyer, B. G., Bak, T., Uttner, I., Dubois, B., et al. (2010). Cognitive impairment in patients with multiple system atrophy and progressive supranuclear palsy. Brain 133(Pt 8), 2382–2393. doi: 10.1093/brain/awq158
Buckner, R. L. (2013). The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 80, 807–815. doi: 10.1016/j.neuron.2013.10.044
Caffarra, P., Vezzadini, G., Dieci, F., Zonato, F., and Venneri, A. (2002). Rey-Osterrieth complex figure: normative values in an Italian population sample. Neurol. Sci. 22, 443–447. doi: 10.1007/s100720200003
Chang, C. C., Chang, Y. Y., Chang, W. N., Lee, Y. C., Wang, Y. L., Lui, C. C., et al. (2009). Cognitive deficits in multiple system atrophy correlate with frontal atrophy and disease duration. Eur. J. Neurol. 16, 1144–1150. doi: 10.1111/j.1468-1331.2009.02661.x
Cykowski, M. D., Coon, E. A., Powell, S. Z., Jenkins, S. M., Benarroch, E. E., Low, P. A., et al. (2015). Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain 138(Pt 8), 2293–2309. doi: 10.1093/brain/awv114
Della Rosa, P. A., Cerami, C., Gallivanone, F., Prestia, A., Caroli, A., Castiglioni, I., et al. (2014). A standardized [18F]-xFDG-PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics 12, 575–593. doi: 10.1007/s12021-014-9235-4
Eckert, T., Barnes, A., Dhawan, V., Frucht, S., Gordon, M. F., Feigin, A. S., et al. (2005). FDG PET in the differential diagnosis of parkinsonian disorders. Neuroimage 26, 912–921. doi: 10.1016/j.neuroimage.2005.03.012
Eckert, T., Tang, C., Ma, Y., Brown, N., Lin, T., Frucht, S., et al. (2008). Abnormal metabolic networks in atypical parkinsonism. Mov. Disord. 23, 727–733. doi: 10.1002/mds.21933
Elliott, R. (2003). Executive functions and their disorders. Br. Med. Bull. 65, 49–59. doi: 10.1093/bmb/65.1.49
Fanciulli, A., and Wenning, G. K. (2015). Multiple-system atrophy. N. Engl. J. Med. 372, 249–263. doi: 10.1056/NEJMra1311488
Fiorenzato, E., Weis, L., Seppi, K., Onofrj, M., Cortelli, P., Zanigni, S., et al. (2017). Brain structural profile of multiple system atrophy patients with cognitive impairment. J. Neural Transm. 124, 293–302. doi: 10.1007/s00702-016-1636-0
Floresco, S. B., and Magyar, O. (2006). Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 188, 567–585. doi: 10.1007/s00213-006-0404-5
Ge, J., Wu, P., Peng, S., Yu, H., Zhang, H., Guan, Y., et al. (2015). Assessing cerebral glucose metabolism in patients with idiopathic rapid eye movement sleep behavior disorder. J. Cereb. Blood Flow Metab. 35:1902. doi: 10.1038/jcbfm.2015.208
Gilman, S., Wenning, G. K., Low, P. A., Brooks, D. J., Mathias, C. J., Trojanowski, J. Q., et al. (2008). Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71, 670–676. doi: 10.1212/01.wnl.0000324625.00404.15
Gratwicke, J., Jahanshahi, M., and Foltynie, T. (2015). Parkinson’s disease dementia: a neural networks perspective. Brain 138(Pt 6), 1454–1476. doi: 10.1093/brain/awv104
Guo, Q., Zhao, Q., Chen, M., Ding, D., and Hong, Z. (2009). A comparison study of mild cognitive impairment with 3 memory tests among Chinese individuals. Alzheimer Dis. Assoc. Disord. 23, 253–259. doi: 10.1097/WAD.0b013e3181999e92
Homma, T., Mochizuki, Y., Komori, T., and Isozaki, E. (2016). Frequent globular neuronal cytoplasmic inclusions in the medial temporal region as a possible characteristic feature in multiple system atrophy with dementia. Neuropathology 36, 421–431. doi: 10.1111/neup.12289
Jellinger, K. A. (2020). Neuropathological findings in multiple system atrophy with cognitive impairment. J. Neural Transm. 127, 1031–1039. doi: 10.1007/s00702-020-02201-2
Kawabata, K., Hara, K., Watanabe, H., Bagarinao, E., Ogura, A., Masuda, M., et al. (2019). Alterations in cognition-related cerebello-cerebral networks in multiple system atrophy. Cerebellum 18, 770–780. doi: 10.1007/s12311-019-01031-7
Kawai, Y., Suenaga, M., Takeda, A., Ito, M., Watanabe, H., Tanaka, F., et al. (2008). Cognitive impairments in multiple system atrophy: MSA-C vs MSA-P. Neurology 70(16 Pt 2), 1390–1396. doi: 10.1212/01.wnl.0000310413.04462.6a
Kim, H. W., Oh, M., Oh, J. S., Oh, S. J., Lee, S. J., Chung, S. J., et al. (2017). Striatofrontal deafferentiation in MSA-P: evaluation with [18F]FDG brain PET. PLoS One 12:e0169928. doi: 10.1371/journal.pone.0169928
Koga, S., Parks, A., Uitti, R. J., van Gerpen, J. A., Cheshire, W. P., Wszolek, Z. K., et al. (2017). Profile of cognitive impairment and underlying pathology in multiple system atrophy. Mov. Disord. 32, 405–413. doi: 10.1002/mds.26874
Konagaya, M., Sakai, M., Matsuoka, Y., Konagaya, Y., and Hashizume, Y. (1999). Multiple system atrophy with remarkable frontal lobe atrophy. Acta Neuropathol. 97, 423–428. doi: 10.1007/s004010051008
Li, N., Yang, T., Ran, W., Zhang, X., Wang, Y., Xu, Z., et al. (2021). A study on the characteristics of cognitive function in patients with multiple system atrophy in China. Sci. Rep. 11:4995. doi: 10.1038/s41598-021-84393-5
Lucas, J. A., Ivnik, R. J., Smith, G. E., Ferman, T. J., Willis, F. B., Petersen, R. C., et al. (2005). Mayo’s older African Americans normative studies: norms for boston naming test, controlled oral word association, category fluency, animal naming, token test, WRAT-3 reading, trail making test, stroop test, and judgment of line orientation. Clin. Neuropsychol. 19, 243–269. doi: 10.1080/13854040590945337
Miki, Y., Foti, S. C., Hansen, D., Strand, K. M., Asi, Y. T., Tsushima, E., et al. (2020). Hippocampal alpha-synuclein pathology correlates with memory impairment in multiple system atrophy. Brain 143, 1798–1810. doi: 10.1093/brain/awaa126
Ricci, M., Pigliautile, M., D’Ambrosio, V., Ercolani, S., Bianchini, C., Ruggiero, C., et al. (2016). The clock drawing test as a screening tool in mild cognitive impairment and very mild dementia: a new brief method of scoring and normative data in the elderly. Neurol. Sci. 37, 867–873. doi: 10.1007/s10072-016-2480-6
Salvesen, L., Winge, K., Brudek, T., Agander, T. K., Løkkegaard, A., and Pakkenberg, B. (2017). Neocortical neuronal loss in patients with multiple system atrophy: a stereological study. Cereb. Cortex 27, 400–410. doi: 10.1093/cercor/bhv228
Santangelo, G., Cuoco, S., Picillo, M., Erro, R., Squillante, M., Volpe, G., et al. (2020). Evolution of neuropsychological profile in motor subtypes of multiple system atrophy. Parkinsonism Relat. Disord. 70, 67–73. doi: 10.1016/j.parkreldis.2019.12.010
Schmahmann, J. D., and Sherman, J. C. (1998). The cerebellar cognitive affective syndrome. Brain 121(Pt 4), 561–579. doi: 10.1093/brain/121.4.561
Shen, C., Chen, L., Ge, J. J., Lu, J. Y., Chen, Q. S., He, S. J., et al. (2021). Cerebral metabolism related to cognitive impairments in multiple system atrophy. Front. Neurol. 12:652059. doi: 10.3389/fneur.2021.652059
Sheridan, L. K., Fitzgerald, H. E., Adams, K. M., Nigg, J. T., Martel, M. M., Puttler, L. I., et al. (2006). Normative symbol digit modalities test performance in a community-based sample. Arch. Clin. Neuropsychol. 21, 23–28. doi: 10.1016/j.acn.2005.07.003
Siri, C., Duerr, S., Canesi, M., Delazer, M., Esselink, R., Bloem, B. R., et al. (2013). A cross-sectional multicenter study of cognitive and behavioural features in multiple system atrophy patients of the parkinsonian and cerebellar type. J. Neural Transm. 120, 613–618. doi: 10.1007/s00702-013-0997-x
Stankovic, I., Krismer, F., Jesic, A., Antonini, A., Benke, T., Brown, R. G., et al. (2014). Cognitive impairment in multiple system atrophy: a position statement by the Neuropsychology Task Force of the MDS multiple system atrophy (MODIMSA) study group. Mov. Disord. 29, 857–867. doi: 10.1002/mds.25880
Stefanova, N., Bucke, P., Duerr, S., and Wenning, G. K. (2009). Multiple system atrophy: an update. Lancet Neurol. 8, 1172–1178. doi: 10.1016/S1474-4422(09)70288-1
Steinberg, B. A., Bieliauskas, L. A., Smith, G. E., and Ivnik, R. J. (2005). Mayo’s older americans normative studies: age- and IQ-adjusted norms for the trail-making test, the stroop test, and MAE controlled oral word association test. Clin. Neuropsychol. 19, 329–377. doi: 10.1080/13854040590945210
Wenning, G. K., Tison, F., Ben Shlomo, Y., Daniel, S. E., and Quinn, N. P. (1997). Multiple system atrophy: a review of 203 pathologically proven cases. Mov. Disord. 12, 133–147. doi: 10.1002/mds.870120203
Wu, L., Liu, F. T., Ge, J. J., Zhao, J., Tang, Y. L., Yu, W. B., et al. (2018). Clinical characteristics of cognitive impairment in patients with Parkinson’s disease and its related pattern in (18) F-FDG PET imaging. Hum. Brain Mapp. 39, 4652–4662. doi: 10.1002/hbm.24311
Keywords: cognitive impairment, 18F-fluorodeoxyglucose, fronto-striatal dopamine signaling, cerebellum, multiple system atrophy
Citation: Shen C, Chen Q-S, Zuo C-T, Liu F-T and Wang J (2022) The Frontal and Cerebellar Metabolism Related to Cognitive Dysfunction in Multiple System Atrophy. Front. Aging Neurosci. 14:788166. doi: 10.3389/fnagi.2022.788166
Received: 01 October 2021; Accepted: 11 January 2022;
Published: 10 February 2022.
Edited by:
Kenneth S. Hettie, Stanford University, United StatesReviewed by:
Jungho Cha, Icahn School of Medicine at Mount Sinai, United StatesLiping Fu, China-Japan Friendship Hospital, China
Copyright © 2022 Shen, Chen, Zuo, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng-Tao Liu, bGl1ZmVuZ3Rhb0BmdWRhbi5lZHUuY24=; Jian Wang, d2FuZ2ppYW5faHNAZnVkYW4uZWR1LmNu
†These authors have contributed equally to this work
‡These authors jointly supervised the work
 Cong Shen
Cong Shen Qi-Si Chen
Qi-Si Chen Chuan-Tao Zuo
Chuan-Tao Zuo Feng-Tao Liu
Feng-Tao Liu Jian Wang
Jian Wang