
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Aging Neurosci. , 04 February 2022
Sec. Parkinson’s Disease and Aging-related Movement Disorders
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.784314
This article is part of the Research Topic Melatonin in Aging: From Variation in Its Synthesis to Involvement in Age-Associated Diseases View all 4 articles
Objective: The efficacy of melatonin on sleep disorders in Parkinson's disease (PD) is still unclear. The purpose of this study was to investigate the efficacy of melatonin on sleep disorders in PD by summarizing evidence from randomized clinical trials (RCTs).
Methods: PubMed, Cochrane Library, EMBASE, and Web of Science databases were searched for studies published before 20 August 2021. Results were analyzed using Review Manager 5.2 software. We used Trial Sequential Analysis (TSA) software to avoid false-positive results caused by random errors.
Results: We included 7 studies in this systematic review and meta-analysis. The results of the meta-analysis showed that compared with placebo, the subjective sleep quality of patients with PD significantly improved after melatonin treatment (MD = −2.19, 95% CI: −3.53 to −0.86, P = 0.001). In the systematic review, we qualitatively analyzed the efficacy of melatonin on the objective sleep quality of patients with PD, and the results showed that melatonin exerted a positive effect with good safety and tolerability. However, there was no significant improvement in excessive daytime sleepiness assessed by the Epworth Sleepiness Scale (ESS).
Conclusion: We found that melatonin can significantly improve the subjective and objective sleep quality of patients with PD with good safety and tolerability. Melatonin could be considered an effective treatment for insomnia in patients with PD.
Parkinson's disease (PD), the second most common progressive neurodegenerative disorder, is characterized by motor and non-motor symptoms. The prevalence of PD increases with age (Lang and Lozano, 1998a,b; Tysnes and Storstein, 2017; Hayes, 2019). The Global Burden of Disease Study showed that the number of PD cases worldwide will double from approximately 7 million in 2015 to approximately 13 million in 2040, which may bring a tremendous burden to society (GBD 2016 Parkinson's Disease Collaborators, 2018).
Sleep disorders are a common non-motor symptom in patients with PD (Schrempf et al., 2014). The up 88% to 98% of patients with PD have sleep disorders, such as insomnia, daytime sleepiness with sleep attacks, and rapid eye movement sleep behavior disorder (RBD), which has an enormous negative impact on the quality of life of patients with PD (Schrempf et al., 2014; Ahn et al., 2020). Patients with PD had blunted circadian rhythms of melatonin secretion, and both the amplitude of the melatonin rhythm and the 24-h area-under-the-curve for circulating melatonin levels were significantly lower in PD participants, which indicated that circadian dysfunction may underlie excessive daytime sleepiness in PD (Videnovic et al., 2014).
Melatonin, a neurohormone, is synthesized and secreted by the pineal gland at night (Palagini et al., 2021). Melatonin has chronobiotic action, which can entrain the circadian rhythms of several biological functions, including sleep/wake rhythms, and it also has sleep-promoting action (McCall et al., 2017; Geoffroy et al., 2019). Melatonin has positive effects on sleep quality in adults with respiratory diseases, metabolic disorders, and primary sleep disorders (Fatemeh et al., 2021). However, the efficacy of melatonin on sleep disorders in patients with PD is still unclear. Lyashenko et al. concluded that melatonin can be used for RBD treatment in patients with PD (Lyashenko et al., 2015). However, the latest randomized controlled trials showed that melatonin was ineffective for PD-RBD (Ahn et al., 2020; Gilat et al., 2020). Although Zhang et al. performed a meta-analysis to evaluate the effect of exogenous melatonin on sleep disorders in neurodegenerative diseases (Zhang et al., 2016), they did not systematically evaluate the effect of melatonin on other sleep disorders in patients with PD, such as excessive daytime sleepiness. Due to the inconsistency and incompleteness of the existing research results, we planned to perform a meta-analysis of RCTs and a systematic review to comprehensively investigate the efficacy of melatonin in the treatment of sleep disorders in patients with PD, such as subjective and objective sleep quality, excessive daytime sleepiness, and PD-RBD.
Studies published before 20 August 2021 were searched in PubMed, Cochrane Library, EMBASE, and Web of Science. The keywords were entered using a standard search and included “Parkinson's disease,” “melatonin,” and “sleep disorders”. There was no restriction on language or date. Two authors screened the titles and abstracts independently. This protocol was conducted following the Preferred Reporting Items guidelines for Systematic Reviews and Meta-analyses (PRISMA) Protocols. We used PubMed as an example in the detailed search strategy presented in Supplementary Material 1.
The inclusion criteria were as follows: (1) Study type: randomized controlled trials (RCTs); (2) Participants: patients who were clinically diagnosed with PD; (3) Interventions: the experimental group was given melatonin or prolonged-release melatonin (PRM); (4) Control: the control group was given placebo or clonazepam; and (5) Outcome: at least one of the following 4 instruments was employed: Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), RBD questionnaire (RBDQ) and polysomnography (PSG) sleep parameters.
Exclusion criteria were: (1) Non-randomized controlled trial; (2) Repeated publication; and (3) Studies whose outcomes did not meet our meta-analysis requirements.
The following data were collected from each included study: (1) baseline characteristics including the first author's name, year of publication, country, study design, age, sample size, sex ratio, duration of treatment, measuring tools, daily dose of melatonin supplementation, and duration of PD; (2) mean and standard deviation (SD) of PSQI scores at the end of treatment. Two researchers extracted the required data for this meta-analysis and systematic review. The third independent researcher resolved disagreements and differences, if necessary.
The risk of bias of each RCT was assessed independently by two authors, and another author resolved any disagreement. We used the Cochrane Risk of Bias Tool to assess the risk of bias. The assessment tool is composed of seven parts: (1) random sequence generation; (2) allocation consultation; (3) blinding of the participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting; and (7) other bias. We divided the research into three categories, including “low risk of bias,” “high risk of bias,” or “unclear risk of bias”.
We used Review Manager (RevMan 5.2) to conduct this meta-analysis. We calculated the mean difference (MD) among the continuous variable data. The 95% confidence interval (CI) was used to represent each effect size. The p-value of < 0.05 was considered to indicate statistical significance. We estimated the heterogeneity between studies using I2 statistics. When I2 < 50%, there was no significant heterogeneity in the included studies, and a fixed-effects model was applied. When I2 ≥ 50%, there was heterogeneity, and a random-effects model was applied. To avoid false-positive results caused by random errors, we conducted the analysis using Trial Sequential Analysis (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark, https://www.ctu.dk/tsa).
As shown in Figure 1, we identified 821 records from the PubMed, Cochrane Library, EMBASE, and Web of Science databases. After excluding duplicates and irrelevant studies by reading titles and abstracts, the remaining 53 articles required reading of the full text to identify available data. Forty-six articles were excluded. Finally, we included 7 studies in this systematic review and meta-analysis. The characteristics of the included studies are shown in Table 1.
The Cochrane Risk of Bias Tool was used to assess the risk of bias. The results are shown in Figure 2.
Three studies (Medeiros et al., 2007; Ahn et al., 2020; Daneshvar Kakhaki et al., 2020) used the PSQI scale to assess the subjective sleep quality of patients with PD. However, only two studies (Medeiros et al., 2007; Daneshvar Kakhaki et al., 2020) reported the mean and SD of PSQI scores at the end of treatment. One study (4-week, randomized, double-blind, placebo-controlled) showed that compared with baseline and the placebo group, the PSQI score significantly improved in patients with PD treated with 4 weeks of 2 mg prolonged-release melatonin (PRM) (Ahn et al., 2020). These results showed that in the PRM group, the mean change in the PSQI score at the end of treatment was 1.75 (18.4%) (95% CI: 0.33–3.17; p = 0.049; Ahn et al., 2020). We also found that in the PRM group, the PSQI subcomponents improved, including subjective sleep quality (mean difference = 0.38; p = 0.029), sleep latency (mean difference = 0.38; p = 0.029), and sleep disturbance (mean difference = 0.25; p = 0.041; Ahn et al., 2020). However, in the Ahn et al. study, the mean and SD of PSQI scores after treatment could not be extracted. Therefore, we performed a qualitative analysis in our study.
Two studies (Medeiros et al., 2007; Daneshvar Kakhaki et al., 2020) reported the mean and SD of PSQI scores. Our meta-analysis results showed that compared with the placebo group, the subjective sleep quality of patients with PD had a significant improvement after receiving melatonin treatment (2 studies, n = 69, MD = −2.19, 95% CI: −3.53 to −0.86, P = 0.001; Heterogeneity: Chi2 = 1.72, I2 = 42%, P = 0.19; see Figure 3). A fixed-effects model was applied.
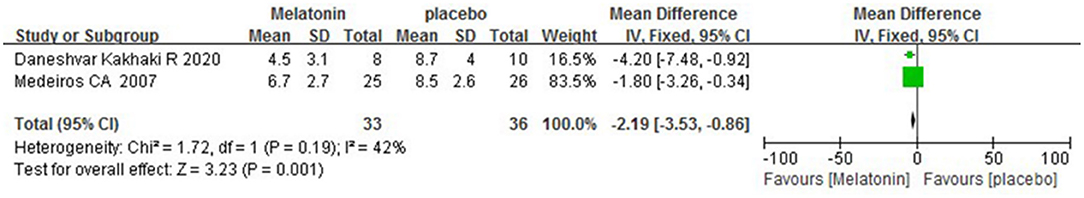
Figure 3. Forest plot of the efficacy of melatonin on the subjective sleep quality of patients with PD.
The results of TSA on the data of PSQI scores at the end of treatment are shown in Figure 4. The accumulated Z value of the meta-analysis crossed both the TSA boundary value and the traditional boundary value before the required information size of 140 was reached.
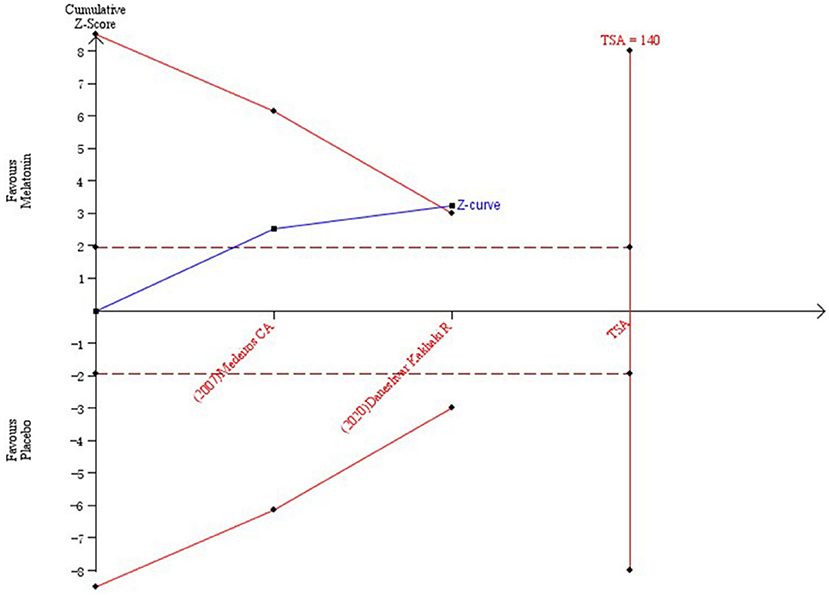
Figure 4. Trial sequential analysis of the cumulative meta-analysis of the efficacy of melatonin vs. placebo on subjective sleep quality in patients with PD.
Two studies (Medeiros et al., 2007; Litvinenko et al., 2012) used PSG sleep parameters to assess the objective sleep quality of patients with PD after melatonin treatment. In a randomized, double-blind, parallel-group, placebo-controlled study, the authors did not observe a significant difference in polysomnographic measures after treatment with 4 weeks of 3 mg melatonin. However, total sleep time (TST) improved in the melatonin-treated group (Medeiros et al., 2007). The sample size and a type II error may be causes of the undetected differences in PSG findings. In another study, Litvinenko et al. found that changes in PSG scores at the end of week 6, compared with the beginning of the trial, were in favor of the group treated with melatonin (Litvinenko et al., 2012). Their results showed that significant changes in total sleep time/time in bed (TST/TIB) and sleep latency (SL) were observed after treatment with 6 weeks of 3 mg melatonin (p = 0.001, p = 0.004, respectively).
Five studies (Dowling et al., 2005; Medeiros et al., 2007; Litvinenko et al., 2012; Ahn et al., 2020; Delgado-Lara et al., 2020) used the Epworth Sleepiness Scale to assess the daytime sleepiness of patients with PD. The study of Medeiros showed that in the melatonin group, the mean change in ESS score at the end of treatment was 0.3; it was 0.2 in the placebo group. However, the difference was not statistically significant. The trial showed that treatment with 4 weeks of 3 mg melatonin did not affect daytime sleepiness in patients with PD. In a 6-week, randomized, clonazepam-controlled study, Litvinenko et al. reported that melatonin and clonazepam increased ESS scores in patients with PD. The results showed that ESS scores significantly increased after treatment with 6 weeks of 2 mg clonazepam (from 3.8 ± 1.2 to 7.3 ± 2.2; p = 0.0002) and were slightly increased after treatment with 6 weeks of 3 mg melatonin (from 4.1 ± 1.4 to 4.7± 1.4; p = 0.06; Litvinenko et al., 2012).
Delgado et al.'s study used 25 mg of melatonin to assess its efficacy on daytime sleepiness in patients with PD. Melatonin was taken at noon and 30 min before bedtime for 3 months. The results showed that the use of high-dose melatonin failed to reduce the presence of excessive daytime sleepiness (Delgado-Lara et al., 2020). Meanwhile, similar results were observed in that ESS scores after treatment with 4 weeks of 2 mg PRM were not different from baseline (Ahn et al., 2020).
In another study, Dowling et al. (2005) chose to compare 5 and 50 mg doses of melatonin vs. placebo over 2 weeks of administration. The results showed that compared with placebo, neither dose of melatonin significantly improved the ESS scores of patients with PD. Dowling et al. also used the general sleep disorder scale (GSDS) to assess the sleep of patients with PD and found that both daytime sleepiness and sleep quantity were significantly improved with 5 mg melatonin compared to either 50 mg or placebo (Dowling et al., 2005). The difference was statistically significant (P < 0.05).
Two studies (Ahn et al., 2020; Gilat et al., 2020) reported the effect of melatonin on RBD in patients with PD. In a 12-week, randomized, double-blind, placebo-controlled study, the weekly CIRUS-RBD Questionnaire (wCIRUS-RBDQ), was used to assess the efficacy of melatonin on RBD in patients with PD; the results showed that the number of RBD events after treatment with 8 weeks of 4 mg PRM was not reduced between groups (3.4 events/week with melatonin vs. 3.6 with placebo; absolute difference: 0.2; 95% confidence interval [CI] = −3.2 to 3.6; P = 0.92) (Gilat et al., 2020). The results showed that the number of nights in which a dream enactment event occurred during RBD events was not significantly different between groups (P = 0.56). In another randomized, double-blind, placebo-controlled, multicenter trial, Ahn et al. used the RBD screening questionnaire (RBDSQ) to investigate the efficacy of PRM in patients with PD. Coincidentally, the study of Ahn et al. also showed that the RBDSQ scores after treatment with 4 weeks of 2 mg PRM did not differ from baseline in either group (Ahn et al., 2020). Results of the qualitative analysis are shown in Table 2.
PD is the second most common progressive neurodegenerative disorder. Studies have shown that mitochondrial dysfunction, abnormal protein handling, neuroinflammation, and oxidative stress play a key role in PD pathogenesis (Wood-Kaczmar et al., 2006; Michel et al., 2016). Mack et al. found that melatonin has neuroprotective, anti-inflammatory, and antioxidant properties (Mack et al., 2016). However, a series of studies have shown that the production of melatonin is reduced and the expression of melatoninergic receptors MT1 and MT2 is decreased in the substantia nigra pars compacta (Fertl et al., 1991, 1993; Bordet et al., 2003; Adi et al., 2010; Videnovic et al., 2014). Therefore, an increasing number of scholars are studying the efficacy of exogenous melatonin in patients with PD (Delucca et al., 2018; Ahn et al., 2020; Daneshvar Kakhaki et al., 2020; Gilat et al., 2020; Batla et al., 2021). Sleep disorders are one of the most common non-motor symptoms of patients with PD. However, the effect of melatonin on sleep disorders in patients with PD is unclear. Therefore, we performed a meta-analysis of RCTs and systematic review to comprehensively investigate the efficacy of melatonin in the treatment of sleep disorders in patients with PD.
Insomnia is one of the most common sleep disorders in patients with PD. Insomnia is defined as complaints of difficulty initiating sleep and/or difficulty maintaining sleep and/or early morning awakenings, according to the International Classification of Sleep Disorders Third Edition criteria (Sateia, 2014). Clinical interviews are the gold standard to determine the diagnosis of insomnia. The interview gathers information on the type of insomnia complaint, etiology, duration, and repercussions (Wallace et al., 2020). In addition to the clinical interview, some psychometrically validated scales can also be used to evaluate the subjective sleep of patients with PD, such as the PSQI scale, the Insomnia Severity Index (ISI), the Scales for Outcome in Parkinson's Disease-Sleep (SCOPA-S) and the Parkinson's Disease Sleep Scale (PDSS) (Buysse et al., 1989; Chaudhuri et al., 2002; Marinus et al., 2003; Trenkwalder et al., 2011; Suzuki et al., 2015; Wallace et al., 2020).
The PSQI scale is an effective tool for measuring subjective sleep quality. In recent expert consensus recommendations, the PSQI scale has been recommended as the main measure for global sleep and insomnia symptoms (Buysse et al., 2006). In our study, we used the PSQI scale to evaluate the subjective sleep quality of patients with PD. The scale is a self-rated questionnaire that is used to evaluate sleep quality and sleep disorders over a 1-month time interval (Buysse et al., 1989). Nineteen separate items produced seven component scores, including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction (Buysse et al., 1989). The score of each component can take the value of 0, 1, 2, or 3. The sum of the scores of the seven components is used to calculate the overall sleep quality score, which ranges from 0 to 21 (Snyder et al., 2018). The higher the score is, the worse the quality of sleep. Our meta-analysis and systematic review is the first study to evaluate the effects of melatonin on the subjective and objective sleep quality of patients with PD. Many studies have shown that melatonin can improve the subjective sleep quality of PD (Zhang et al., 2016; Fatemeh et al., 2021). A meta-analysis showed that melatonin could improve subjective sleep quality in neurodegenerative disorder patients (Zhang et al., 2016). Our study also showed that melatonin could significantly improve the subjective sleep quality of patients with PD (P = 0.001), which is consistent with previous results. To avoid false-positive results caused by random errors, we used TSA software to perform trial sequential analysis. According to the TSA results, we concluded that melatonin can improve the PSQI scores in patients with PD.
PSG is an objective sleep measure. In our study, we used PSG to objectively evaluate the sleep of patients with PD. However, PSG is not indicated in the routine assessment of adults with insomnia, and it may be necessary when comorbid sleep disorders are suspected (Wallace et al., 2020). Therefore, only a few articles use PSG to monitor objective sleep in patients with PD. Two studies objectively evaluated the efficacy of melatonin on the objective sleep quality of patients with PD using PSG. In our study, the results indicate that melatonin had a significant effect on total sleep time/time in bed (TST/TIB) and latent sleep (LS) sections of the PSG. Meanwhile, we also observed a trend of improvement in total sleep time (TST) in another study (Medeiros et al., 2007). Actigraphy is another measure of evaluating objective sleep. In Dowling et al.'s study, the authors found a statistically significant improvement in actigraphically measured total sleep time with 50 mg melatonin compared to 5 mg melatonin or placebo (Dowling et al., 2005).
Excessive daytime sleepiness is one of the most common non-motor symptoms of PD. It has been reported that most PD patients suffer from excessive daytime sleepiness (Ondo et al., 2001; Tracik and Ebersbach, 2001; Ghorayeb et al., 2007; Poryazova et al., 2010), which seriously impairs the quality of life of patients with PD (Schrempf et al., 2014). Because of sleep disorders, especially sudden sleep attacks, many activities are dangerous for patients with PD, such as driving a car or operating a machine.
The ESS scale is a widely used tool, and it has been verified as a measure of sleepiness (Walker et al., 2020). The ESS scale is an eight-item self-applicable instrument that is used to assess the propensity to fall asleep in eight mostly monotonous situations. A total score of <10 is rated as normal, a score of 10 to 12 indicates marginal sleepiness and a score higher than 12 indicates excessive sleepiness (Sandoval-Rincón et al., 2013). In our included studies, ESS scores after treatment with melatonin were not improved compared with those of the control group, regardless of the dose of melatonin and treatment duration. However, it has been questioned whether ESS was used as an evaluative instrument to measure change over time or responsiveness to treatment. Dowling's study showed that daytime sleepiness and sleep quantity significantly improved in weekly measures of the General Sleep Disturbance Scale (GSDS) daytime sleepiness subscale scores for the 5 mg melatonin treatment compared to placebo and 50 mg melatonin (Dowling et al., 2005). However, these results were not consistent in the ESS scores. The GSDS and ESS assess sleepiness across different periods and measure slightly different aspects of sleepiness, which may be explain the findings. In our study, melatonin did not improve excessive daytime sleepiness assessed with the ESS scale in patients with PD compared with the control group. However, we observed that melatonin improved GSDS daytime sleepiness subscale scores after treatment with 5 mg melatonin. Therefore, more studies that use different scales to evaluate the effect of melatonin on daytime sleepiness in patients with PD will be needed. The multiple sleep latency test (MSLT) is an objective assessment of daytime function in patients with chronic insomnia. The MSLT can also be used in research (Stepanski et al., 1989; Zhang and Zhao, 2007). Research has shown that MSLT assessment has proven useful in evaluating a broad range of patients with excessive daytime sleepiness (Roth and Ancoli-Israel, 1999).
Rapid eye movement (REM) sleep behavior disorder (RBD) is a parasomnia characterized by abnormal behaviors and loss of muscle atonia, such as vocalizations, jerks and motor behaviors during REM sleep; it is often related to REM-related dream content (Schenck and Mahowald, 2002; Zhang et al., 2020). RBD can lead to dream enactment behaviors that are often injurious to patients and their partners (Haba-Rubio et al., 2018). The prevalence of RBD is approximately 1% in adults but 20–50% in people with PD (Dauvilliers et al., 2018; Haba-Rubio et al., 2018; Hogl et al., 2018). Studies have shown that in evolving neurodegenerative disorders, idiopathic RBD (iRBD) is considered to be the first step (Iranzo et al., 2006, 2013). In a longitudinal study of 29 patients, 38% of patients with iRBD developed PD, MSA or DLB within 4 years of evaluation, and 81% developed neurodegenerative diseases after 20 years (Schenck et al., 2013). The diagnosis of RBD requires the clinical history of dream enactment behaviors (DEBs) or REM sleep-related behaviors recorded by PSG, along with REM sleep without atonia (RSWA) (St Louis and Boeve, 2017). The key to diagnosing RBD is the witnessing of dream enactment by a bed partner (Chiaro et al., 2018). Some questionnaires, such as the RBD Screening Questionnaire (RBDSQ), the REM Sleep Behavior Questionnaires-Hong-Kong (RBD-HK), the Mayo Sleep Questionnaire (MSQ), and the Innsbruck RBD Inventory, can also be used in the diagnosis of RBD (Jin et al., 2017). However, the pathogenesis of RBD is still not clear. It is speculated that the degeneration or dysfunction of the brain stem circuits controlling rapid eye movement sleep paralysis is the root cause of RBD (McKenna and Peever, 2017).
Studies have shown that melatonin has a much safer profile than clonazepam, with no reports of dependence and fewer and milder side effects (Aurora et al., 2010; Dauvilliers et al., 2018). Therefore, melatonin is considered a preferable treatment for RBD in older patients and neurodegenerative disorder patients (Kunz and Bes, 1997, 1999; Aurora et al., 2010; Kunz and Mahlberg, 2010; Dauvilliers et al., 2018). However, a few studies have investigated the efficacy of melatonin on PD-RBD. In a two-part, double-blind, placebo-controlled trial, melatonin was effective in RBD patients (Kunz and Mahlberg, 2010). Eight consecutive outpatients were included in the study, but only one was suffering from PD. Gilat recruited 30 PD participants who were confirmed to have RBD with video PSG. The 30 PD patients with RBD were randomized to 4 mg of prolonged-release melatonin or placebo, and the aggregate of RBD incidents averaged was used as the primary outcome (Gilat et al., 2020). Gilat's results showed that melatonin did not significantly improve the clinical symptoms of RBD compared to placebo (Gilat et al., 2020). In another randomized controlled study (Ahn et al., 2020), the same result was observed. Based on the available evidence, we cannot confirm that melatonin is effective for PD-RBD. Therefore, larger, multicentered RCTs are still needed to assess the effect of melatonin on PD-RBD.
Other sleep disorders are also often present in patients with PD, such as restless leg syndrome, periodic leg movements during sleep, and obstructive sleep apnea (Iranzo, 2016). However, no recent study has evaluated the efficacy of melatonin on these sleep disorders.
Melatonin is well-tolerated. One study showed that no significant side effects were reported by participants taking 20–100 mg/day melatonin orally, and no important alterations to any physiological or biochemical measures were seen (Galley et al., 2014). In previous studies, the safety of melatonin was established in patients with PD (Dowling et al., 2005; Medeiros et al., 2007). In our study, no serious adverse events occurred when the dose of melatonin was as high as 50 mg/day in patients with PD. This conclusion is consistent with previous studies.
Headache and daytime sleepiness were frequently reported and were the most common adverse reactions during melatonin treatment. In Ahn and Medeiros's studies (Medeiros et al., 2007; Ahn et al., 2020), no participants experienced adverse events. In Daneshvar's study, grade 1 side effects were reported in two PD participants in the melatonin group, including headache (n = 1) and daytime sleepiness (n = 1) (Daneshvar Kakhaki et al., 2020). In Dowling's study, one participant complained of feeling tired in the morning during treatment with 50 mg melatonin but insisted on completing the treatment (Dowling et al., 2005). In Gilat's study, because one participant felt light-headedness and morning sleepiness, the dosage of melatonin was reduced from 4 to 2 mg after 3 weeks (Gilat et al., 2020). In two studies, adverse events were reported, including headaches, fatigue, light-headedness, daytime sleepiness, dizziness, nausea, or gastrointestinal problems (Delgado-Lara et al., 2020; Gilat et al., 2020). However, these adverse events were mild and did not require further medical attention.
Our study has several major strengths: (1) This study is the first meta-analysis and systematic review that investigated the efficacy of melatonin on sleep disorders in patients with PD. (2) Previous systematic reviews and meta-analyses only discussed the effect of melatonin on subjective sleep quality. Our study is the first to evaluate the effects of melatonin on the subjective and objective sleep quality of patients with PD. However, due to data sparseness and repeated testing of the accumulated data, the accumulated meta-analysis has the risk of producing random errors. In our study, TSA was used to test whether the conclusions of the meta-analysis were sufficient.
There are several potential limitations in our study: (1) The dosage and duration of melatonin used in each included study were different. Because measures to assess the efficacy of melatonin on sleep disorders in patients with PD were different, we could not perform subgroup analysis. (2) There were only seven RCTs that met the inclusion criteria, and the sample size of each study was small.
In conclusion, the combined data from RCT studies showed that melatonin could significantly improve the subjective and objective sleep quality of patients with PD with good safety and tolerability. Melatonin could be considered an effective treatment for insomnia in patients with PD, which provides evidence for clinicians to select drugs for sleep disorders in patients with PD and provides a basis for experts to formulate guidelines. However, we also found that there was no significant improvement in PD-RBD and excessive daytime sleepiness assessed by the ESS scale. Therefore, a larger, multicentered RCT will still be needed to assess the efficacy of melatonin on sleep disorders in patients with PD.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
JY and HM designed the study. WS, MJ, and YZ carried out the literature searches, study selection, and data extraction. HM and WS assessed the quality of studies, conducted the trial sequential analysis, contributed to the analysis, and interpreted the data. HM wrote the manuscript. JY revised the manuscript. All authors contributed to the writing of this manuscript.
This work was supported by the Project of Henan Province Science and Technology (212102310216), the Key Projects of Medical Science and Technology in Henan Province (SBGJ202002099) and Medical Science and Technology Research in Henan Province (LHGJ20190560).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.784314/full#supplementary-material
Adi, N., Mash, D. C., Ali, Y., Singer, C., Shehadeh, L., and Papapetropoulos, S. (2010). Melatonin MT1 and MT2 receptor expression in Parkinson's disease. Med. Sci. Monit. 16, Br61–Br67.
Ahn, J. H., Kim, M., Park, S., Jang, W., Park, J., Oh, E., et al. (2020). Prolonged-release melatonin in Parkinson's disease patients with a poor sleep quality: a randomized trial. Parkinson. Relat. Disord. 75, 50–54. doi: 10.1016/j.parkreldis.2020.03.029
Aurora, R. N., Zak, R. S., Maganti, R. K., Auerbach, S. H., Casey, K. R., Chowdhuri, S., et al. (2010). Best practice guide for the treatment of REM sleep behavior disorder (RBD). J. Clin. Sleep Med. 6, 85–95. doi: 10.5664/jcsm.27717
Batla, A., Simeoni, S., Uchiyama, T., deMin, L., Baldwin, J., Melbourne, C., et al. (2021). Exploratory pilot study of exogenous sustained-release melatonin on nocturia in Parkinson's disease. Eur. J. Neurol. 28, 1884–1892. doi: 10.1111/ene.14774
Bordet, R., Devos, D., Brique, S., Touitou, Y., Guieu, J. D., Libersa, C., et al. (2003). Study of circadian melatonin secretion pattern at different stages of Parkinson's disease. Clin. Neuropharmacol. 26, 65–72. doi: 10.1097/00002826-200303000-00005
Buysse, D. J., Ancoli-Israel, S., Edinger, J. D., Lichstein, K. L., and Morin, C. M. (2006). Recommendations for a standard research assessment of insomnia. Sleep 29, 1155–1173. doi: 10.1093/sleep/29.9.1155
Buysse, D. J., Reynolds, C. F. III., Monk, T. H., Berman, S. R., and Kupfer, D. J. (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213. doi: 10.1016/0165-1781(89)90047-4
Chaudhuri, K. R., Pal, S., DiMarco, A., Whately-Smith, C., Bridgman, K., Mathew, R., et al. (2002). The Parkinson's disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 73, 629–635. doi: 10.1136/jnnp.73.6.629
Chiaro, G., Calandra-Buonaura, G., Cecere, A., Mignani, F., Sambati, L., Loddo, G., et al. (2018). REM sleep behavior disorder, autonomic dysfunction and synuclein-related neurodegeneration: where do we stand? Clin. Auton. Res. 28, 519–533. doi: 10.1007/s10286-017-0460-4
Daneshvar Kakhaki, R., Ostadmohammadi, V., Kouchaki, E., Aghadavod, E., Bahmani, F., Tamtaji, O. R., et al. (2020). Melatonin supplementation and the effects on clinical and metabolic status in Parkinson's disease: a randomized, double-blind, placebo-controlled trial. Clin. Neurol. Neurosurg. 195, 105878. doi: 10.1016/j.clineuro.2020.105878
Dauvilliers, Y., Schenck, C. H., Postuma, R. B., Iranzo, A., Luppi, P. H., Plazzi, G., et al. (2018). REM sleep behaviour disorder. Nat. Rev. Dis. Primers 4, 19. doi: 10.1038/s41572-018-0016-5
Delgado-Lara, D. L., Gonzalez-Enriquez, G. V., Torres-Mendoza, B. M., Gonzalez-Usigli, H., Cardenas-Bedoya, J., Macias-Islas, M. A., et al. (2020). Effect of melatonin administration on the PER1 and BMAL1 clock genes in patients with Parkinson's disease. Biomed. Pharmacother. 129, 110485. doi: 10.1016/j.biopha.2020.110485
Delucca, B. J., Richardson, R. M., and Stewart, J. T. (2018). Melatonin treatment of visual hallucinations in Parkinson disease. J. Clin. Psychopharmacol. 38, 532–534. doi: 10.1097/JCP.0000000000000930
Dowling, G. A., Mastick, J., Colling, E., Carter, J. H., Singer, C. M., and Aminoff, M. J. (2005). Melatonin for sleep disturbances in Parkinson's disease. Sleep Med. 6, 459–466. doi: 10.1016/j.sleep.2005.04.004
Fatemeh, G., Sajjad, M., Niloufar, R., Neda, S., Leila, S., and Khadijeh, M. (2021). Effect of melatonin supplementation on sleep quality: A systematic review and meta-analysis of randomized controlled trials. J. Neurol. 269, 205–216. doi: 10.1007/s00415-020-10381-w
Fertl, E., Auff, E., Doppelbauer, A., and Waldhauser, F. (1991). Circadian secretion pattern of melatonin in Parkinson's disease. J. Neural Transm. Park Dis. Dement. 3, 41–47. doi: 10.1007/BF02251135
Fertl, E., Auff, E., Doppelbauer, A., and Waldhauser, F. (1993). Circadian secretion pattern of melatonin in de novo Parkinsonian patients: evidence for phase-shifting properties of l-dopa. J. Neural Transm. Park Dis. Dement. 5, 227–234. doi: 10.1007/BF02257677
Galley, H. F., Lowes, D. A., Allen, L., Cameron, G., Aucott, L. S., and Webster, N. R. (2014). Melatonin as a potential therapy for sepsis: a phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J. Pineal Res. 56, 427–438. doi: 10.1111/jpi.12134
GBD 2016 Parkinson's Disease Collaborators (2018). Global, regional, and national burden of Parkinson's disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 17, 939–953. doi: 10.1016/S1474-4422(18)30295-3
Geoffroy, P. A., Micoulaud Franchi, J. A., Lopez, R., and Schroder, C. M. (2019). The use of melatonin in adult psychiatric disorders: expert recommendations by the French institute of medical research on sleep (SFRMS). Encephale 45, 413–423. doi: 10.1016/j.encep.2019.04.068
Ghorayeb, I., Loundou, A., Auquier, P., Dauvilliers, Y., Bioulac, B., and Tison, F. (2007). A nationwide survey of excessive daytime sleepiness in Parkinson's disease in France. Mov. Disord. 22, 1567–1572. doi: 10.1002/mds.21541
Gilat, M., Coeytaux Jackson, A., Marshall, N. S., Hammond, D., Mullins, A. E., Hall, J. M., et al. (2020). Melatonin for rapid eye movement sleep behavior disorder in Parkinson's disease: a randomised controlled trial. Mov. Disord. 35, 344–349. doi: 10.1002/mds.27886
Haba-Rubio, J., Marti-Soler, H., Tobback, N., Andries, D., Marques-Vidal, P., Vollenweider, P., et al. (2018). Clinical significance of periodic limb movements during sleep: the HypnoLaus study. Sleep Med. 41, 45–50. doi: 10.1016/j.sleep.2017.09.014
Hayes, M. T. (2019). Parkinson's disease and Parkinsonism. Am. J. Med. 132, 802–807. doi: 10.1016/j.amjmed.2019.03.001
Hogl, B., Stefani, A., and Videnovic, A. (2018). Idiopathic REM sleep behaviour disorder and neurodegeneration - an update. Nat. Rev. Neurol. 14, 40–55. doi: 10.1038/nrneurol.2017.157
Iranzo, A. (2016). Sleep in neurodegenerative diseases. Sleep Med. Clin. 11, 1–18. doi: 10.1016/j.jsmc.2015.10.011
Iranzo, A., Molinuevo, J. L., Santamaría, J., Serradell, M., Martí, M. J., Valldeoriola, F., et al. (2006). Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 5, 572–577. doi: 10.1016/S1474-4422(06)70476-8
Iranzo, A., Tolosa, E., Gelpi, E., Molinuevo, J. L., Valldeoriola, F., Serradell, M., et al. (2013). Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 12, 443–453. doi: 10.1016/S1474-4422(13)70056-5
Jin, H., Zhang, J. R., Shen, Y., and Liu, C. F. (2017). Clinical significance of REM sleep behavior disorders and other non-motor symptoms of Parkinsonism. Neurosci. Bull. 33, 576–584. doi: 10.1007/s12264-017-0164-8
Kunz, D., and Bes, F. (1997). Melatonin effects in a patient with severe REM sleep behavior disorder: case report and theoretical considerations. Neuropsychobiology 36, 211–214. doi: 10.1159/000119383
Kunz, D., and Bes, F. (1999). Melatonin as a therapy in REM sleep behavior disorder patients: an open-labeled pilot study on the possible influence of melatonin on REM-sleep regulation. Mov. Disord. 14, 507–511. doi: 10.1002/1531-8257(199905)14:3<507::AID-MDS1021>3.0.CO
Kunz, D., and Mahlberg, R. (2010). A two-part, double-blind, placebo-controlled trial of exogenous melatonin in REM sleep behaviour disorder. J. Sleep Res. 19, 591–596. doi: 10.1111/j.1365-2869.2010.00848.x
Lang, A. E., and Lozano, A. M. (1998a). Parkinson's disease. First of two parts. N. Engl. J. Med. 339, 1044–1053. doi: 10.1056/NEJM199810083391506
Lang, A. E., and Lozano, A. M. (1998b). Parkinson's disease. Second of two parts. N. Engl. J. Med. 339, 1130–1143. doi: 10.1056/NEJM199810153391607
Litvinenko, I. V., Krasakov, I. V., and Tikhomirova, O. V. (2012). [Sleep disorders in Parkinson's disease without dementia: a comparative randomized controlled study of melatonin and clonazepam]. Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova 112, 26–30.
Lyashenko, E., Levin, O. S., and Poluektov, M. G. (2015). [Melatonin in correction of REM-sleep behavior disorders in Parkinson's disease]. Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova 115, 40–43. doi: 10.17116/jnevro20151156240-43
Mack, J. M., Schamne, M. G., Sampaio, T. B., Pertile, R. A., Fernandes, P. A., Markus, R. P., et al. (2016). Melatoninergic system in Parkinson's disease: from neuroprotection to the management of motor and nonmotor symptoms. Oxid. Med. Cell Longev 2016, 3472032. doi: 10.1155/2016/3472032
Marinus, J., Visser, M., van Hilten, J. J., Lammers, G. J., and Stiggelbout, A. M. (2003). Assessment of sleep and sleepiness in Parkinson disease. Sleep 26, 1049–1054. doi: 10.1093/sleep/26.8.1049
McCall, W. V., Benca, R. M., Rosenquist, P. B., Riley, M. A., McCloud, L., Newman, J. C., et al. (2017). Hypnotic medications and suicide: risk, mechanisms, mitigation, and the FDA. Am. J. Psychiatry 174, 18–25. doi: 10.1176/appi.ajp.2016.16030336
McKenna, D., and Peever, J. (2017). Degeneration of rapid eye movement sleep circuitry underlies rapid eye movement sleep behavior disorder. Mov. Disord. 32, 636–644. doi: 10.1002/mds.27003
Medeiros, C. A., Carvalhedo de Bruin, P. F., Lopes, L. A., Magalhães, M. C., de Lourdes Seabra, M., and de Bruin, V. M. (2007). Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson's disease. A randomized, double blind, placebo-controlled study. J. Neurol. 254, 459–464. doi: 10.1007/s00415-006-0390-x
Michel, P. P., Hirsch, E. C., and Hunot, S. (2016). Understanding dopaminergic cell death pathways in Parkinson disease. Neuron 90, 675–691. doi: 10.1016/j.neuron.2016.03.038
Ondo, W. G., Dat Vuong, K., Khan, H., Atassi, F., Kwak, C., and Jankovic, J. (2001). Daytime sleepiness and other sleep disorders in Parkinson's disease. Neurology 57, 1392–1396. doi: 10.1212/WNL.57.8.1392
Palagini, L., Manni, R., Aguglia, E., Amore, M., Brugnoli, R., Bioulac, S., et al. (2021). International expert opinions and recommendations on the use of melatonin in the treatment of insomnia and circadian sleep disturbances in adult neuropsychiatric disorders. Front. Psychiatry 12, 688890. doi: 10.3389/fpsyt.2021.688890
Poryazova, R., Benninger, D., Waldvogel, D., and Bassetti, C. L. (2010). Excessive daytime sleepiness in Parkinson's disease: characteristics and determinants. Eur. Neurol. 63, 129–135. doi: 10.1159/000276402
Roth, T., and Ancoli-Israel, S. (1999). Daytime consequences and correlates of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. II. Sleep 22(Suppl 2), S354–358.
Sandoval-Rincón, M., Alcalá-Lozano, R., Herrera-Jiménez, I., and Jiménez-Genchi, A. (2013). [Validation of the Epworth sleepiness scale in Mexican population]. Gac. Med. Mex. 149, 409–416.
Sateia, M. J. (2014). International classification of sleep disorders-third edition: highlights and modifications. Chest 146, 1387–1394. doi: 10.1378/chest.14-0970
Schenck, C. H., Boeve, B. F., and Mahowald, M. W. (2013). Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 14, 744–748. doi: 10.1016/j.sleep.2012.10.009
Schenck, C. H., and Mahowald, M. W. (2002). REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep 25, 120–138. doi: 10.1093/sleep/25.2.120
Schrempf, W., Brandt, M. D., Storch, A., and Reichmann, H. (2014). Sleep disorders in Parkinson's disease. J. Parkinsons Dis. 4, 211–221. doi: 10.3233/JPD-130301
Snyder, E., Cai, B., DeMuro, C., Morrison, M. F., and Ball, W. (2018). A new single-item sleep quality scale: results of psychometric evaluation in patients with chronic primary insomnia and depression. J. Clin. Sleep Med. 14, 1849–1857. doi: 10.5664/jcsm.7478
St Louis, E. K., and Boeve, B. F. (2017). REM sleep behavior disorder: diagnosis, clinical implications, and future directions. Mayo Clin. Proc. 92, 1723–1736. doi: 10.1016/j.mayocp.2017.09.007
Stepanski, E., Koshorek, G., Zorick, F., Glinn, M., Roehrs, T., and Roth, T. (1989). Characteristics of individuals who do or do not seek treatment for chronic insomnia. Psychosomatics 30, 421–427. doi: 10.1016/S0033-3182(89)72248-9
Suzuki, K., Miyamoto, T., Miyamoto, M., Suzuki, S., Numao, A., Watanabe, Y., et al. (2015). Evaluation of cutoff scores for the Parkinson's disease sleep scale-2. Acta Neurol. Scand. 131, 426–430. doi: 10.1111/ane.12347
Tracik, F., and Ebersbach, G. (2001). Sudden daytime sleep onset in Parkinson's disease: polysomnographic recordings. Mov. Disord. 16, 500–506. doi: 10.1002/mds.1083
Trenkwalder, C., Kohnen, R., Högl, B., Metta, V., Sixel-Döring, F., Frauscher, B., et al. (2011). Parkinson's disease sleep scale–validation of the revised version PDSS-2. Mov. Disord. 26, 644–652. doi: 10.1002/mds.23476
Tysnes, O. B., and Storstein, A. (2017). Epidemiology of Parkinson's disease. J. Neural Transm. 124, 901–905. doi: 10.1007/s00702-017-1686-y
Videnovic, A., Noble, C., Reid, K. J., Peng, J., Turek, F. W., Marconi, A., et al. (2014). Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol. 71, 463–469. doi: 10.1001/jamaneurol.2013.6239
Walker, N. A., Sunderram, J., Zhang, P., Lu, S. E., and Scharf, M. T. (2020). Clinical utility of the Epworth sleepiness scale. Sleep Breath 24, 1759–1765. doi: 10.1007/s11325-020-02015-2
Wallace, D. M., Wohlgemuth, W. K., Trotti, L. M., Amara, A. W., Malaty, I. A., Factor, S. A., et al. (2020). Practical evaluation and management of insomnia in Parkinson's disease: a review. Mov. Disord. Clin. Pract. 7, 250–266. doi: 10.1002/mdc3.12899
Wood-Kaczmar, A., Gandhi, S., and Wood, N. W. (2006). Understanding the molecular causes of Parkinson's disease. Trends Mol. Med. 12, 521–528. doi: 10.1016/j.molmed.2006.09.007
Zhang, F., Niu, L., Liu, X., Liu, Y., Li, S., Yu, H., et al. (2020). Rapid eye movement sleep behavior disorder and neurodegenerative diseases: an update. Aging Dis. 11, 315–326. doi: 10.14336/AD.2019.0324
Zhang, L., and Zhao, Z. X. (2007). Objective and subjective measures for sleep disorders. Neurosci. Bull. 23, 236–240. doi: 10.1007/s12264-007-0035-9
Keywords: melatonin, Parkinson's disease, sleep disorders, meta-analysis, systematic review
Citation: Ma H, Yan J, Sun W, Jiang M and Zhang Y (2022) Melatonin Treatment for Sleep Disorders in Parkinson's Disease: A Meta-Analysis and Systematic Review. Front. Aging Neurosci. 14:784314. doi: 10.3389/fnagi.2022.784314
Received: 27 September 2021; Accepted: 11 January 2022;
Published: 04 February 2022.
Edited by:
Parnetti Lucilla, University of Perugia, ItalyReviewed by:
Maria Paola Mogavero, Scientific Clinical Institute Maugeri (ICS Maugeri), ItalyCopyright © 2022 Ma, Yan, Sun, Jiang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junqiang Yan, eWFuanFAaGF1c3QuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.