- 1Department of Psychiatry, Tri-Service General Hospital, School of Medicine, National Defense Medical Center, Taipei, Taiwan
- 2Department of Psychiatry, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
- 3Department of Medical Research, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
- 4School of Public Health, National Defense Medical Center, Taipei, Taiwan
- 5Graduate Institute of Life Sciences, National Defense Medical Center, Taipei, Taiwan
- 6Taiwanese Injury Prevention and Safety Promotion Association, Taipei, Taiwan
- 7Department of Emergency Medicine, Tri-Service General Hospital, School of Medicine, National Defense Medical Center, Taipei, Taiwan
- 8Department of General Surgery, Tri-Service General Hospital, School of Medicine, National Defense Medical Center, Taipei, Taiwan
- 9Student Counseling Center, National Defense Medical Center, Taipei, Taiwan
Background: To explore the association between leptospirosis, the risk of dementia, and the potential protective role of antibiotic treatment.
Methods: We conducted a retrospective cohort nationwide, population-based study, from Taiwan’s National Health Insurance Research Database (NHIRD). We enrolled 1,428 subjects aged 50 years or above, in the index year of 2000, which included those retrieved from the NHIRD record. Dementia diagnosis and incidence over 16 years follow-up was retrieved from the NHIRD records. The Fine and Gray survival analysis was used to determine the risk of dementia, and the results were presented as a sub-distribution hazard ratio (SHR) with a 95% confidence interval.
Results: In the study period, 43 of the 357 leptospirosis patients developed dementia, as compared to 103 of the control group (930.90 vs. 732.49 per 105 person-years). By the Fine and Gray survival analysis, the leptospirosis was associated with the risk of dementia, and the adjusted SHR was 1.357 (95% confidence interval [CI]: 1.213–1.519, P < 0.001), across 16-year of the follow-up period. To exclude the protopathic bias, the sensitivity analysis was conducted. This analysis revealed that the leptospirosis was associated with the increased risk of dementia, even after excluding the dementia diagnosis within the first year (adjusted SHR = 1.246, 95%CI: 1.114–1.395, P < 0.001) or within the first 5 years (adjusted SHR = 1.079, 95%CI: 1.023–1.152, P = 0.028), antibiotic treatment for leptospirosis was associated with the reduced risk of dementia (P = 0.001).
Conclusion: Leptospirosis was associated with an increased risk for dementia, and antibiotic treatment was associated with a reduced risk. Further research will be necessary to explore the underlying mechanisms of this association.
Introduction
In Taiwan, 4–8% of those aged 65 years or over have dementia (Sun et al., 2014), which is a heavy burden for the patients, their caregivers, and the community. Alzheimer’s dementia (AD) is the most common type of dementia, which is a progressive condition that principally affects the elderly. Even though the underlying etiology of AD is not known, there are extensive beta-amyloid (Aβ) deposits in the brain of individuals with no signs of cognitive impairment (Rodrigue et al., 2009). In addition, Aβ may play a role in response to several types of infection (Soscia et al., 2010; Moir et al., 2018). Several recent studies have focused on the possibility that infectious agents might be predisposed to the AD development (Itzhaki et al., 2016). Research has focused on pathogens, such as herpes viruses, yeasts, or bacteria, notably including spirochetes (Miklossy, 2011).
The spirochetes pathogens are generally acquired by the exposure to wild animal secretions or arthropod bites, which are the most prevalent spirochetosis worldwide, particularly in wet tropical and subtropical regions (Levett, 2001). A broad spectrum of clinical manifestations may occur in humans, ranging from subclinical infections and self-limited anicteric febrile illnesses to the severe and potentially fatal icteric disease including Weil’s disease. Antibiotics, especially penicillin, are the mainstay of treatment for suspected or confirmed cases of leptospirosis, and treatment with the appropriate antibiotics should be initiated immediately (Center for Disease Control and Prevention [CDCP], 2018). Although some infected individuals display fulminant disease, it is suspected that the chronic carriage in others can remain subclinical. The treatment of leptospirosis differs depending on the severity and duration of the symptoms at the time of presentation, and patients with mild, flu-like symptoms may require only symptomatic treatment.
A possible relationship between Leptospira infection and neuropsychiatric disorders has been previously discussed, including dementia, depression, mania, and psychosis (Marshall and Scrimgeour, 1978; Semiz et al., 2005; Chiu et al., 2019). Importantly, it was recently reported that patients with a history of leptospirosis were at a moderately increased risk of developing dementia (Chiu et al., 2019). In addition, since the allelic variants of Apolipoprotein E4 (APOE4) allele are a risk factor not only for AD (Strittmatter et al., 1993) but also for a disease caused by the herpes simplex virus (HSV)-1 (Burgos et al., 2006), human immunodeficiency virus (HIV)-1 (Burt et al., 2008), and bacteria including Chlamydia pneumoniae (Gerard et al., 2005), we hypothesize that there might be a link between leptospirosis infections and dementia. For this reason, we have conducted a retrospective cohort study so as to investigate the association between leptospirosis and dementia, and the role of the antibiotic treatment in the risk of dementia in the leptospirosis group.
Materials and Methods
Data Sources
The National Health Insurance (NHI) Program was launched in Taiwan in 1995, and as of June 2009, includes contracts with 97% of medical providers, up to 23 million beneficiaries, and covers more than 99% of the entire population (Ho Chan, 2010). Several previous studies have documented the details of this program (Chen et al., 2020; Tzeng et al., 2020; Wan et al., 2020). We used the Taiwan NHI Research Database (NHIRD) to investigate the association between leptospirosis diagnosis and dementia over a 16-year period (2000–2015) for all outpatients and hospitalizations recorded in the NHIRD.
Diagnosis of leptospirosis was performed according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) (Chinese Hospital Association [CHA], 2000). Typical clinical findings indicative of leptospirosis were confirmed in all cases by a culture of Leptospira spp. and/or serology (Chiu et al., 2019). For the culture, blood and urine collected during the first 10 days of the disease were sent for microbiological analysis. For the serological diagnosis, paired acute and convalescent sera were analyzed by a microscopic agglutination test (MAT). The MAT titer is obtained by incubating serial dilutions of a patient’s serum with different Leptospira serovars; the serovar that reacts most strongly is suggested to be the infecting serovar. A positive laboratory diagnosis of leptospirosis required one of the following two criteria: (i) positive culture isolation, and/or (ii) a fourfold rise in MAT titer between the acute phase and the convalescent phase and a titer ≥1:400 in a single serum. Laboratory studies were performed at the Taiwan Centers for Disease Control [TCDC] (2017).
Dementia diagnosis was performed by board-certified neurologists or psychiatrists according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition and its text-revised edition (American Psychiatric Association [APA], 1994, 2000). Licensed medical records technicians review and verify the diagnostic coding before claiming reimbursements (Chen et al., 1995). The NHI Administration randomly reviews the records of 1 in 100 ambulatory care visits and 1 in 20 in-patient claims to verify the accuracy of the diagnoses (Ministry of Justice, 2019). Several studies have demonstrated the accuracy and validity of the diagnoses in the NHIRD (Cheng et al., 2011; Liang et al., 2011; Chou et al., 2013).
Study Design and Sampled Participants
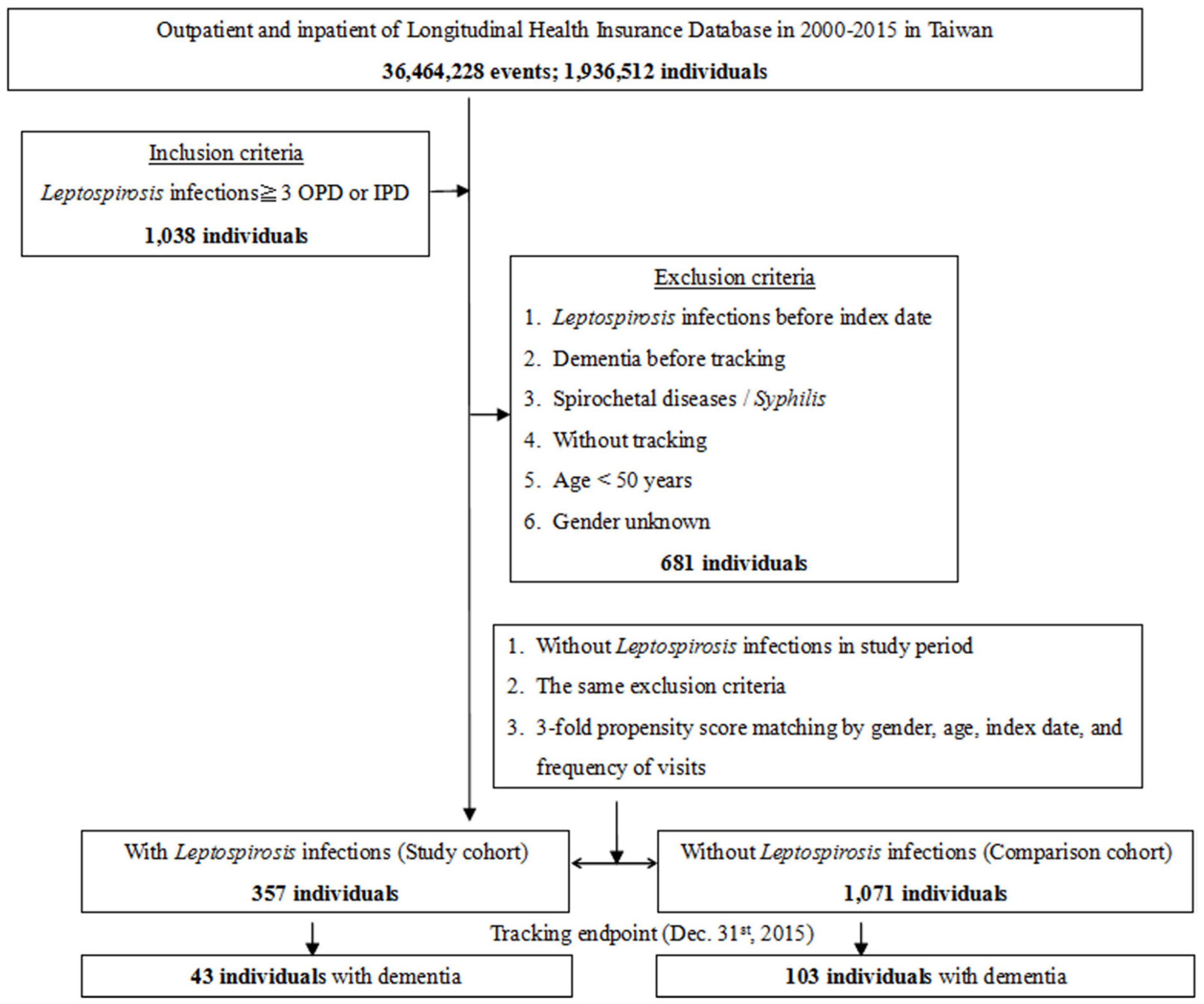
This study was of a retrospective matched-cohort design. Patients with a history of leptospirosis, spirochetal disease, or syphilis, dementia diagnosis before the index date, who were aged <50 years, or where relevant information was missing were excluded from this study. Patients with leptospirosis were selected from January 1 to December 31, 2000, according to ICD-9-CM code 100 (100.0, 100.8, or 100.9). This included 357 patients first diagnosed with leptospirosis during the index year. A control group (n = 1038) matched for age and sex with no diagnosis of leptospirosis was also selected for study (Figure 1).
Covariates included gender, age group (50–64, ≥65 years), marital status, years of education, geographical area of residence (north, center, south, and east of Taiwan), urbanization level of residence area (levels 1 to 4), level of hospital as medical centers, regional and local hospitals, and insurance premiums (in New Taiwan Dollars [NT$]; <18 000, 18 000–34 999, ≥35 000). The urbanization level of residence was defined according to the total population and indicators of the level of development (Chiu et al., 2014). The comorbidities included diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease, obesity, cancer, depressive disorder, bipolar disorder, anxiety disorder, alcohol usage disorder, substance usage disorder, sleep disorder, septicemia (ICD-9-CM codes listed in Supplementary Table 1). We also used the Charlson Comorbidity Index (CCI, scores of 0, 1, 2, 3, ≧4), which is the most widely used comorbidity index in the literature (Charlson et al., 1987; de Groot et al., 2003).
The antibiotics used in the treatment for the leptospirosis includeβ-lactams, cephalosporins, and doxycycline, and the data on the usage of these antibiotics were collected. The data of the defined daily dose (DDD) were obtained from the WHO Collaborating Centre for Drug Statistics Methodology1, and the duration of the usage of antibiotics was calculated by dividing the cumulative dosages by the DDD of the antibiotics. The duration of the antibiotic treatment is usually 7 days (Charan et al., 2013; Day, 2021), therefore, we divided the treatment durations as 7, 8–14, 15–21, 22–28, and >28 days, respectively.
Outcome Measures
All the study participants were followed from the index date until the onset of dementia including Alzheimer’s dementia, vascular dementia, and other degenerative dementias, withdrawal from the NHI program, or the end of 2015. To ensure accuracy, each patient diagnosed with dementia was required to have made at least three outpatient visits during 1 year within the study period (Chiu et al., 2018).
Statistical Analysis
All analyses were performed using the SPSS software version 22 (SPSS Inc., Chicago, IL, United States). χ2 and t-tests were used to evaluate the distributions of the categorical and continuous variables, respectively. The Fisher exact test for categorical variables was used to statistically examine the differences between the two cohorts. The Fine and Gray survival analysis was used to determine the risk of dementia, and the results were presented as a sub-distribution hazard ratio (SHR) with a 95% confidence interval (CI). The difference in the incidence of dementia between the study and control groups was estimated using the Kaplan–Meier method with log-rank test. A two-tailed P-value of <0.05 was considered so as to indicate the statistical significance.
Results
Sample Characteristics
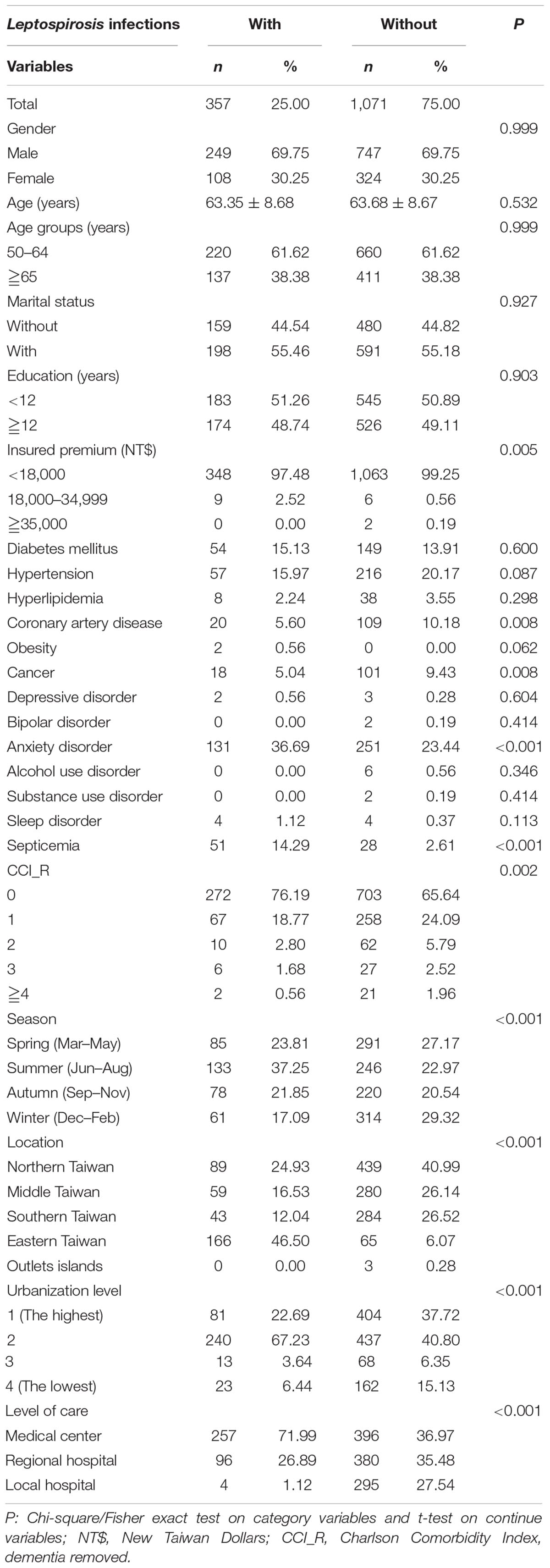
The flowchart for enrollment of the leptospirosis patients and the controls is as presented in Figure 1. A total of 1428 patients were enrolled including 357 subjects with leptospirosis and 1071 controls without leptospirosis. The ratio of leptospirosis cohort and the control cohort was 1:3. These two cohorts were matched for age, sex, and index year (Figure 1 and Table 1). The prevalence of leptospirosis was 18.44 per 105 (357 from an eligible population of 1 936 512) during the 16 years of follow-up; none of the control group received a diagnosis of leptospirosis during the study period. There were no differences between the groups in sex, age, marital status, or insurance premiums. In the leptospirosis cohort, there was a higher percentage of anxiety and septicemia and a lower percentage of coronary artery disease and cancer.
Kaplan–Meier Model for the Cumulative Incidence of Dementia
In the overall study period, 43 of the 357 leptospirosis patients (930.90 per 105 person-years) developed dementia as compared to 103 of the control group (n = 1071, 732.49 per 105 person-years); the Kaplan–Meier analysis indicated that the difference was statistically significant (log-rank, P < 0.001, Figure 2).
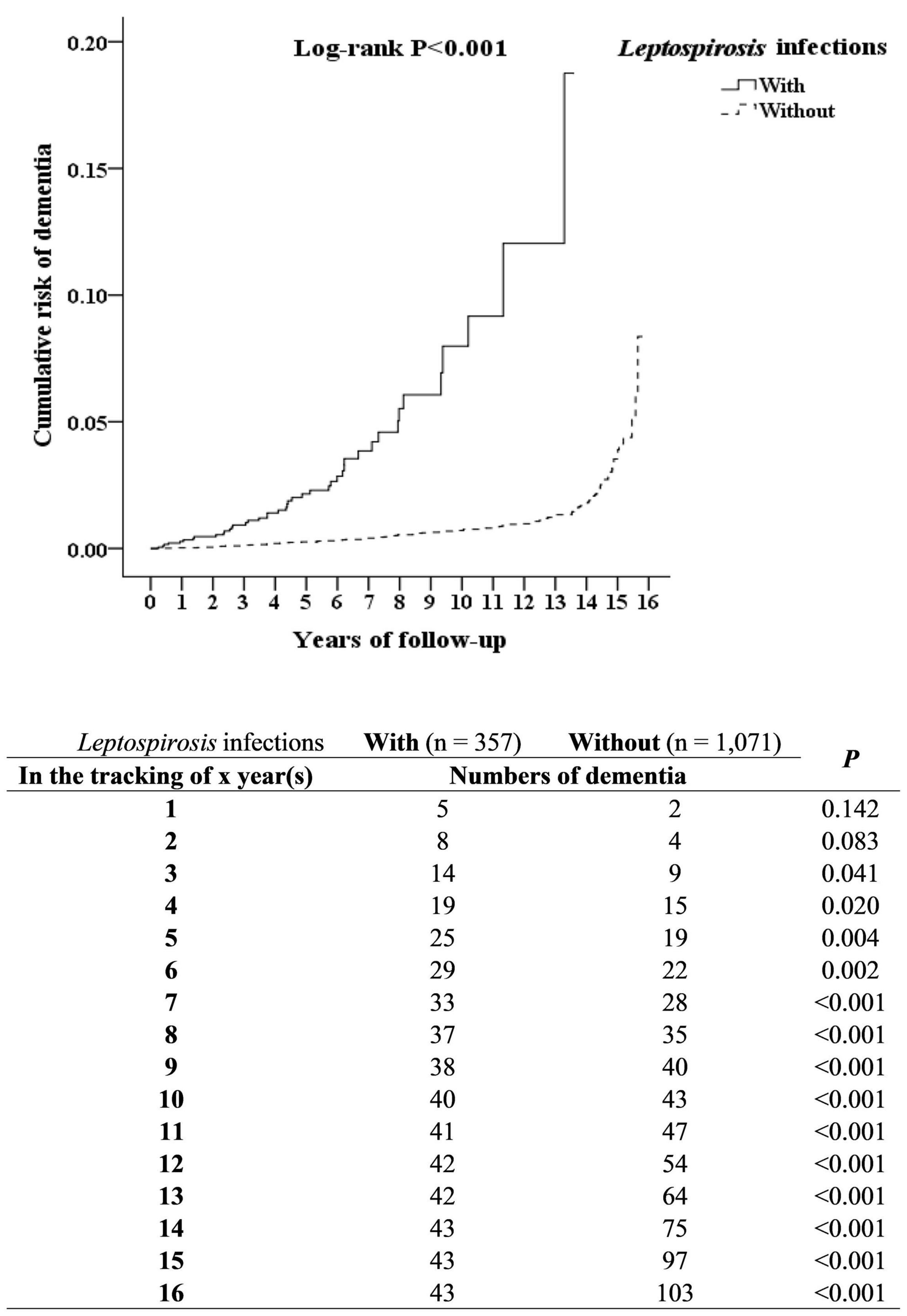
Figure 2. Kaplan–Meier for cumulative incidence of dementia aged 50 and over stratified by Leptospirosis infections with log-rank test.
Hazard Ratio Analysis of Dementia in Patients With Leptospirosis
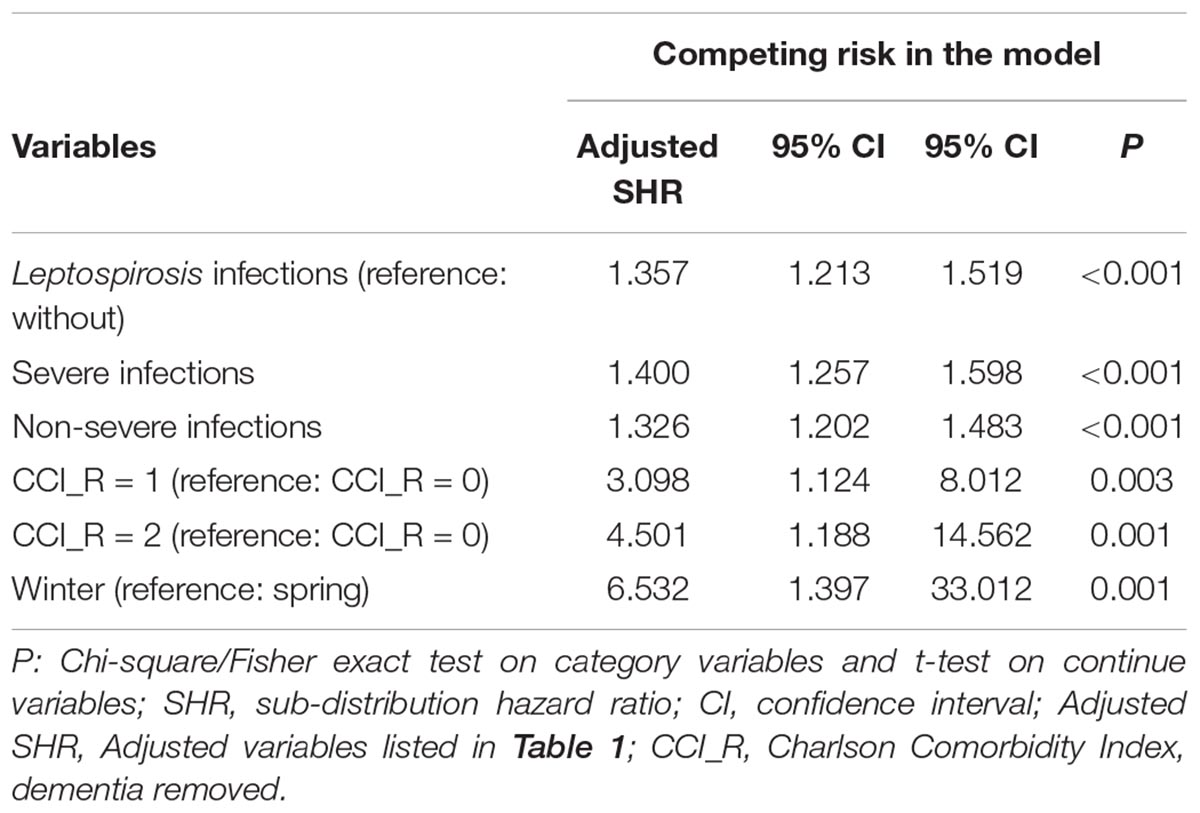
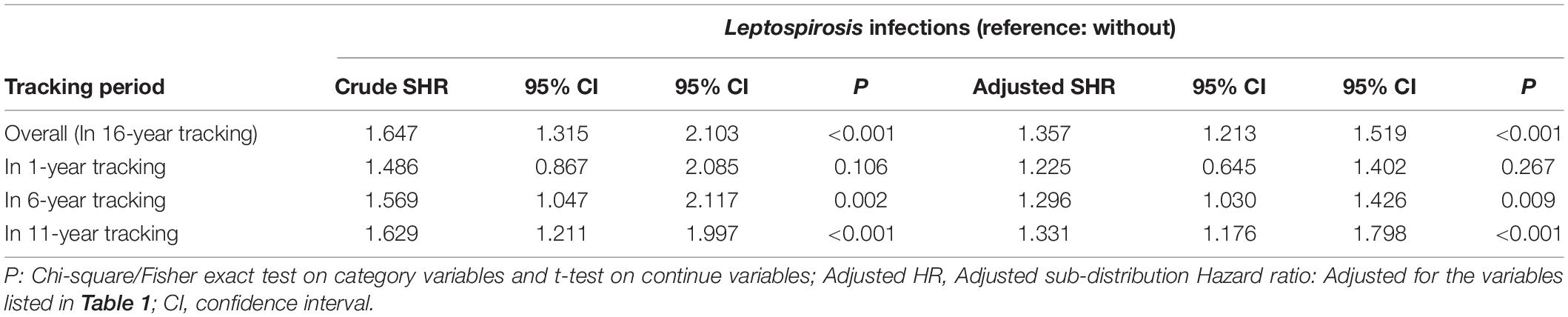
Fine and Gray’s competing risk model analysis revealed that the leptospirosis group was more likely to develop dementia (crude SHR = 1.350; 95% CI 1.211–1.496; P < 0.001). After adjustment for sex, age, monthly insurance premiums, urbanization level, geographic region, and comorbidities, the adjusted SHR over the 16-year period was 1.357 (95% CI 1.213–1.519; P < 0.001) (Table 2).
The Risk and Sensitivity Analysis of Different Types of Dementia After Leptospirosis
As shown in Table 3, leptospirosis was associated with different types of dementia, including AD (adjusted SHR = 1.300 [95% CI: 1.162–1.455, P < 0.001]), VaD (adjusted SHR = 1.243 [95% CI: 1.111–1.391, P < 0.001]), or other degenerative dementia (adjusted SHR = 1.459 [95% CI: 1.305–1.634, P < 0.001]), respectively.
To exclude the protopathic bias, the sensitivity analysis was conducted. This analysis revealed that the leptospirosis was associated with the increased risk of dementia, even after excluding dementia diagnosis within the first year (adjusted SHR = 1.246, 95% CI: 1.114–1.395, P < 0.001) or within the first 5 years (adjusted SHR = 1.079, 95% CI: 1.023–1.152, P = 0.028). However, after excluding the diagnosis within the first and first 5 years, leptospirosis was associated with AD and other degenerative dementia, but not VaD.
Subgroup Analysis for the Risk of Dementia After Leptospirosis
As shown in Table 4, the subgroup analysis revealed that the differential risk stratified by sex, age, marital status, educational level, monthly insurance premiums, comorbidities, urbanization and region of residence, CCI score, season, and level of medical care (possible exceptions include higher insured premiums). The subgroup analysis found that the leptospirosis patients with nearly all the covariates were associated with a higher risk of dementia, with the exceptions of insured premium of NT$18,000–34,999 and NT$ ≧35,000. In addition, the patients with or without most of these comorbidities were associated with a higher risk of dementia, with the exceptions of obesity, depression, bipolar disorder, alcohol usage disorder, other substance usage disorder, sleep disorder, and CCI as 3 and ≧4.
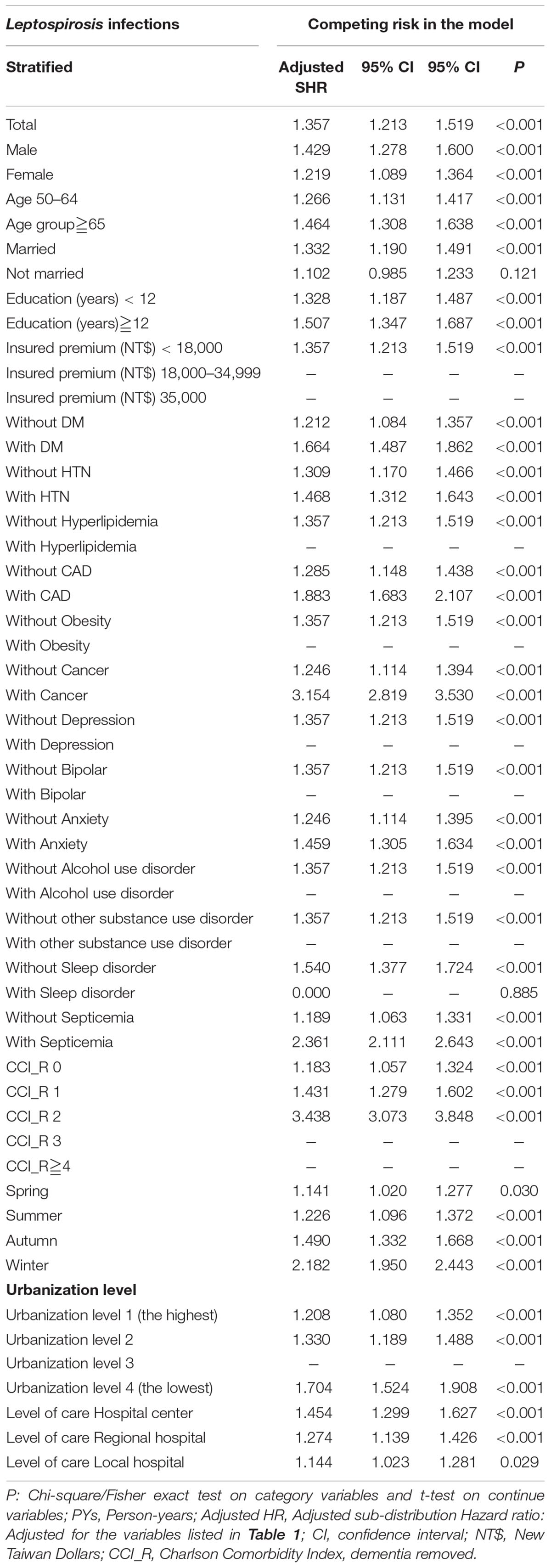
Table 4. Subgroup analysis stratified by variables listed in the table by using Fine and Gray’s competing risk model.
Effect of Leptospirosis Antibiotic Treatment and Risk of Dementia
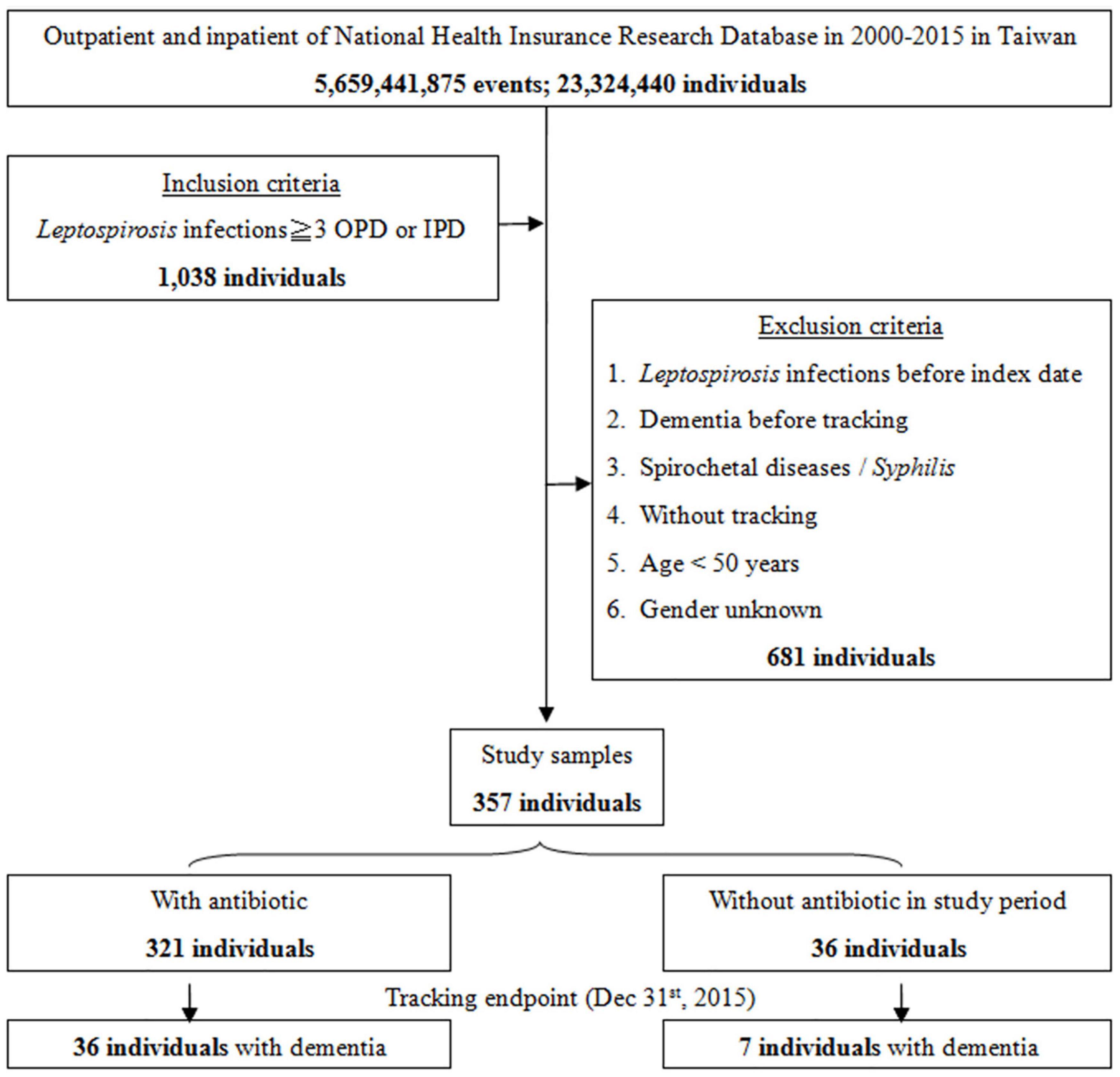
Although the majority of patients diagnosed with leptospirosis were recorded as having received antibiotic treatment (β-lactams, cephalosporins, and doxycycline), a small number were not treated. Figure 3 shows the flowchart of the study sample selection with or without antibiotic treatment. These subgroups differed in their risk of developing dementia. Of the leptospirosis patients with antibiotic treatment, 36 of 321 (916.52 per 105 person-years) developed dementia compared to seven of 36 (938.04 per 105 person-years) patients without antibiotic treatment; the difference was statistically significant in the Kaplan–Meier analysis (log-rank, P < 0.001; Figure 4). The risk of dementia development was lower in patients treated for a longer time or with higher antibiotic dosages (adjusted SHR = 0.685, 95%CI: 0.534–0.950, P = 0.001, Table 5).
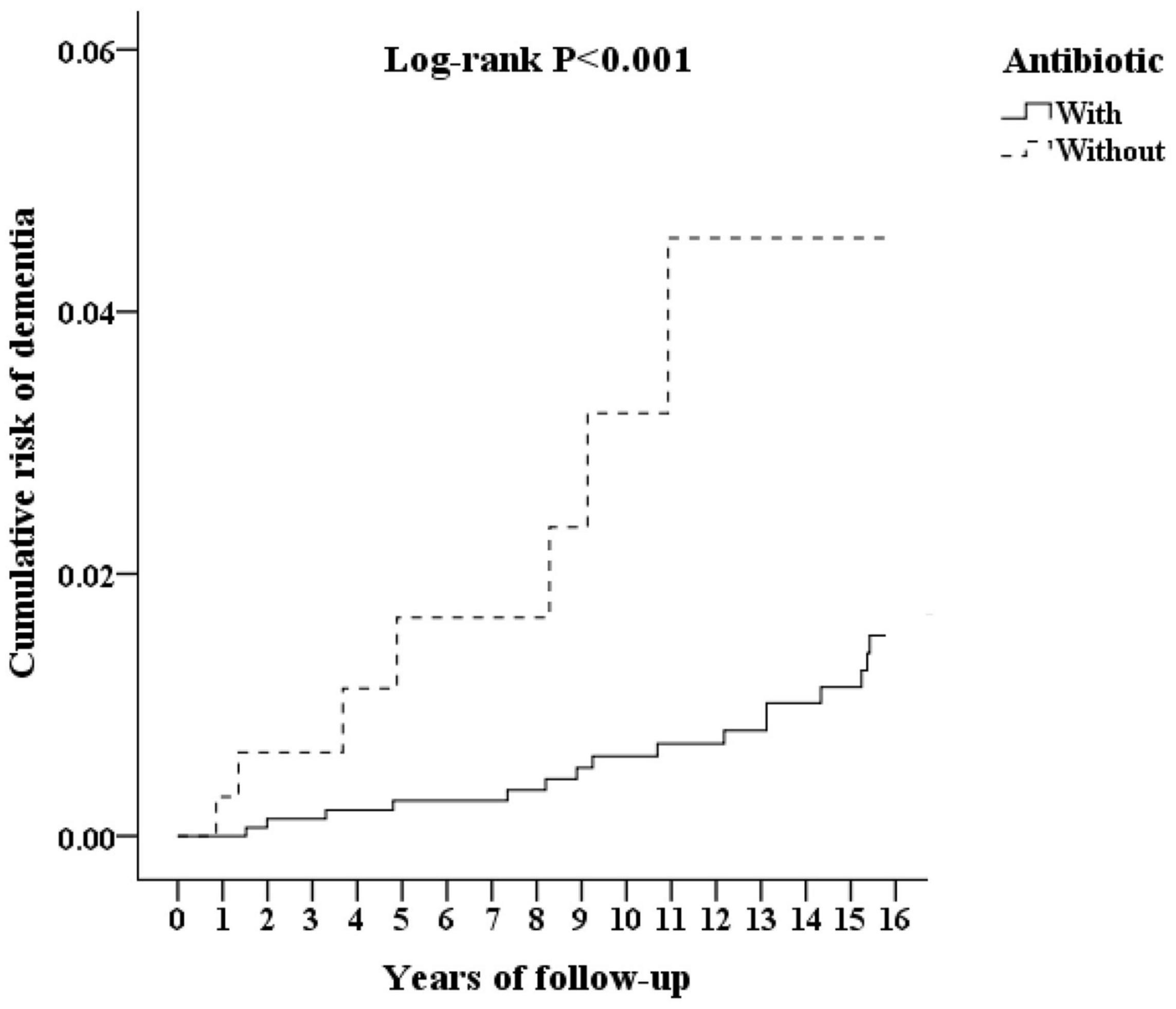
Figure 4. Kaplan–Meier for cumulative incidence of dementia aged 50 and over stratified by Leptospirosis infections with or without antibiotics, with log-rank test.
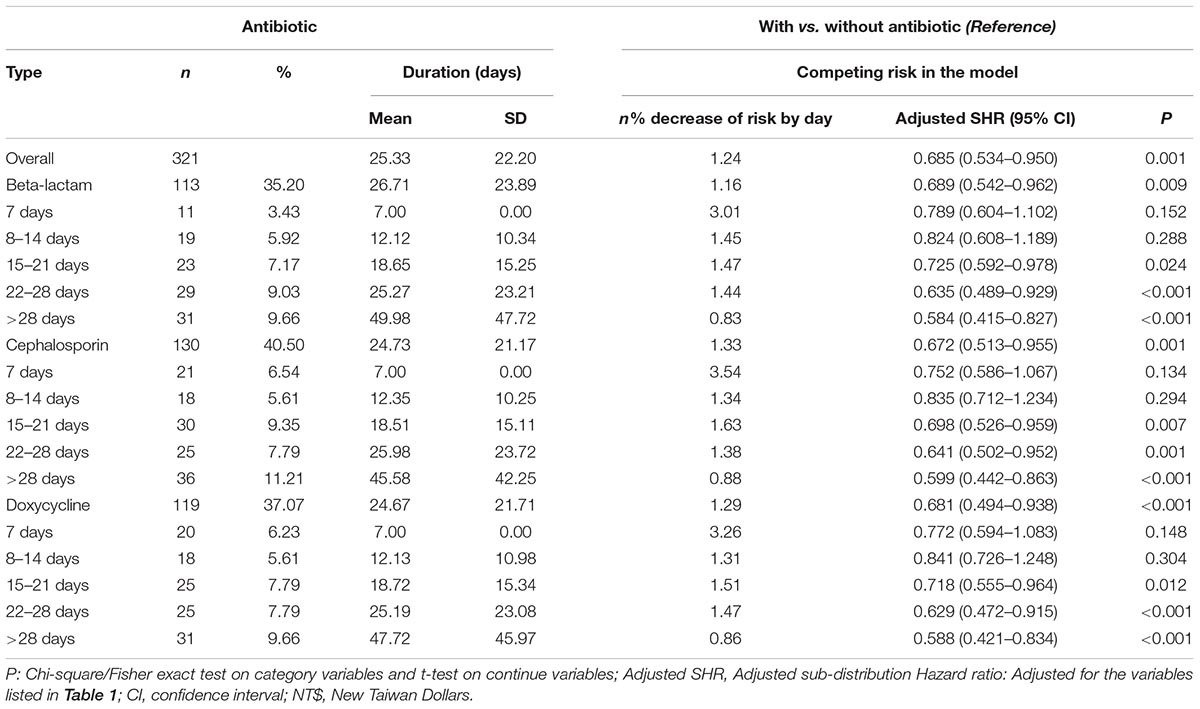
Table 5. Percentage, duration, percentage of decrease of risk by days, and adjusted sub-distribution hazard ratios of antibiotic among Leptospirosis infections patients.
Time-Dependence of Risk of Dementia Development
Patients with a diagnosis of leptospirosis showed an overall increased risk of dementia development over the 16-year follow-up period (adjusted SHR = 1.357). However, the SHR evolved with time. The greatest differential was in the years immediately following the leptospirosis development, and the cumulative unnormalized relative incidences of dementia development were 3.96 at 6 years, 2.61 at 11 years, and 1.25 at 16 years. Cumulative SHR values adjusted for sex, age, monthly insurance premium, urbanization level, geographic region, and comorbidity were 1.296 at 6 years, to 1.331 at 11 years, to 1.357 at 16 years. Linear regression predicts that the SHR for dementia development between the two groups declines to 1.0 at 18–19 years following the leptospirosis development (Table 6).
The Interaction Term Analysis Between Anxiety and Leptospirosis
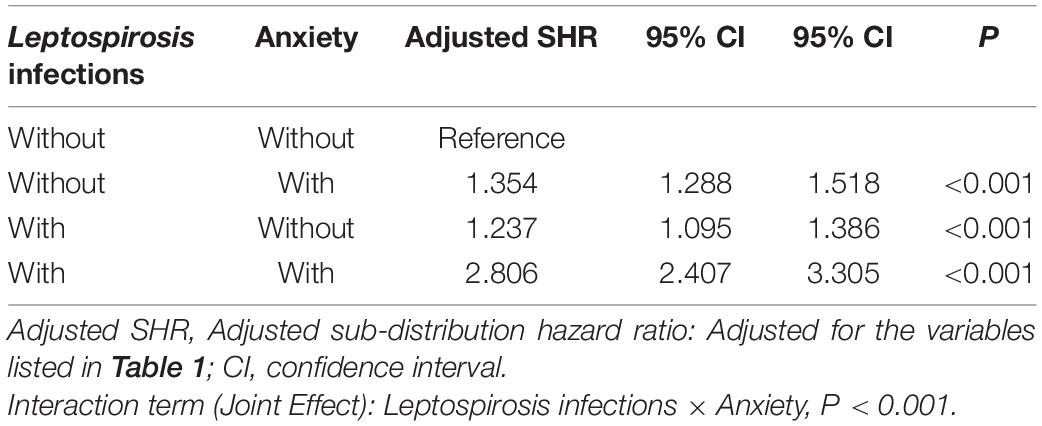
Table 7 shows the subgroup analysis for the factors of dementia using the Fine and Gray’s competing risk model. The adjusted SHR of leptospirosis with anxiety was 2.806 (95% CI: 2.407–3.305, P < 0.001) and the adjusted SHR of leptospirosis without anxiety was 1.237 (95% CI: 1.095–1.386, P < 00.001) The P-values of the interaction term analysis of anxiety × leptospirosis was 0.001 in the non-competing risk model and <0.001 in the competing risk model.
Discussion
Association Between Leptospirosis, Antibiotic Treatment, and the Risk of Dementia
We have reported that the individuals with a previous diagnosis of leptospirosis had an increased risk of developing any type of dementia, including AD and VaD (P < 0.001). When individuals with a diagnosis of dementia within the first year or the first 5 years were excluded, the leptospirosis patients were still associated with an increased risk of AD and related dementias. The results of our study (adjusted SHR = 1.357; 95% CI 1.213–1.519; P < 0.001; 16 years follow-up) are comparable to the conclusions of an earlier study on leptospirosis patients (adjusted SHR = 1.89; 95% CI 1.72–2.08; 10 years follow-up) (Chiu et al., 2019). In addition, leptospirosis was associated with AD, VaD, and other degenerative dementia. We also reported that treatment of the leptospirosis patients with antibiotic medications, such as β-lactams, cephalosporin, and doxycycline, for the first time, was associated with a reduced risk of dementia (adjusted SHR = 0.685) in a dose-dependent manner (P = 0.001).
The adjusted SHR increased from the sixth year and, as the Linear regression predicts, declined after the 16-years of follow-up (Table 6). This finding might hint that the risk of dementia would be higher between six and 16 years after the leptospirosis.
In the leptospirosis groups, there was a significant higher incidence of anxiety disorders (N = 131, 36.69%) as compared to the control group (N = 251, 23.44%). As previously reported, anxiety has been associated with all types of dementia (Santabarbara et al., 2020). Thus, we have conducted an interaction term analysis, which revealed an interaction between anxiety and leptospirosis in the contribution of the risk of dementia (Table 7).
Possible Mechanisms of Association Between Leptospirosis and the Risk of Dementia
The underlying mechanisms of the association between leptospirosis infections and dementia remain unclear. Nonetheless, inflammatory changes in the brain have been reported in studies on the pathogenesis of dementia (Daulatzai, 2016; Stefaniak and O’Brien, 2016). Previous researchers have found that infectious diseases, such as hepatitis C, viral infection, Helicobacter pylori infection, cytomegalovirus infections, chronic osteomyelitis, or even sepsis, were associated with an increased risk of dementia (Barnes et al., 2015; Kao et al., 2015). Other antibiotic-susceptible bacterial species were widely present in both the control and AD brain (Branton et al., 2013; Emery et al., 2017). In 2019, Chiu first reported that patients with leptospirosis were associated with higher risks of dementia, pointing out the role of endothelial damage and vascular hypo-perfusion in subsequent chronic diseases such as dementia (Chiu et al., 2019).
Leptospira may cause cytokine storm, endothelial damage, vascular hypo-perfusion, and subsequent organ failure (Cagliero et al., 2018). AD is the most common cause of dementia. The major hypothesis on the etiology of AD is the amyloid cascade hypothesis (Barage and Sonawane, 2015). In addition, there is also growing evidence indicating that vascular dysfunction plays a pivotal role in the development of AD, and several vascular risk factors, such as hypertension, atherosclerosis, hyperlipidemia, and stroke, are associated with AD-type dementia (Launer et al., 2000; Fratiglioni et al., 2007). The significant association between vascular diseases and AD suggests that dementia associated with AD may be caused by vascular mechanisms.
However, the specific role of spirochetes is uncertain. For Leptospira spp., only 1.4% of United States army soldiers deployed in disease-endemic countries, and 2.5% of United States veterinarians, were reported to be seropositive (Lettieri et al., 2004; Whitney et al., 2009). In the United Kingdom, fewer than 100 cases of acute leptospirosis were recorded each year (Forbes et al., 2012). In addition, serological testing may not detect many individuals infected with Borrelia spp. (Lloyd and Hawkins, 2018). Several studies have found that, in the countries closer to the equator where Leptospira spp. seroprevalence rates closer to 40% (Gonwong et al., 2017; Garba et al., 2018; Sohail et al., 2018). Therefore, the role of the difference of seroprevalence of the Leptospira spp. in different countries might well need to be further studied.
Possible Mechanisms of Association Between Antibiotics and the Lower Risk of Dementia in Patients With Leptospirosis
We also found that the antibiotic treatment was associated with a lower risk of dementia in patients with leptospirosis infections, and the adjusted SHR was 0.685. The leptospirosis-infected subjects treated with the antibiotics of beta-lactam, cephalosporin, and doxycycline showed a decreased risk of dementia, when compared to the group without antibiotic medications. Moreover, once the treatment duration was more than 14 days, the risk of dementia decreased subsequently with the treatment duration prolonged.
The role of the antibiotic treatment of leptospirosis infections for the prevention of dementia has not, as yet, been studied. The association between antibiotic usage and Alzheimer’s disease had only been studied in animal models before. Ceftriaxone may restore the glial glutamate transporter and further ameliorate tau pathology, and the cognitive decline in Alzheimer’s disease (Zumkehr et al., 2015). Neuroinflammation is a chronic event whose perpetuation leads to the continuous release of pro-inflammatory cytokines, promoting neuronal cell death and gross brain atrophy. Doxycycline emerged as a promising preventive strategy in prion diseases and gave compelling pre-clinical results in mouse models of AD against Aβ oligomers and neuroinflammation (Balducci and Forloni, 2019). Our present study might be the first to report on the role of antibiotic medication treatment in attenuating the risk of developing dementia for patients with leptospirosis infections in a nationwide, population-based study. However, in the leptospirosis group, only 10.1% (36 in 357) did not receive antibiotic treatment. This suggests that further study is needed so as to clarify whether antibiotic usage plays a role in reducing the risk of dementia for leptospirosis-infected patients.
Strengths of This Study
The present study has several strengths: First, we used Taiwan’s NHIRD, which is a valuable resource to cover a nationwide population, to address this issue. Second, one previous study has demonstrated the accuracy and validity of several diagnoses of psychiatric disorders in the NHIRD, including major depressive disorder, schizophrenia, and dementia (Wu et al., 2020). Besides, the in-hospital licensed medical records technicians and NHI Administration would have verified the diagnoses in the claims dataset (Hsieh et al., 2019; Ministry of Justice, 2019), for the diagnosis. Third, previous studies have also demonstrated the concordance between Taiwan’s National Health Survey and the NHIRD on a variety of diagnoses (Wu et al., 2014), medication usage (Wu et al., 2014), and health system utilization (Yu et al., 2009; Wu et al., 2014).
Limitations of This Study
This study has several limitations. First, regarding the antibiotic treatment, only 10.1% (36 of 357) of the leptospirosis group did not receive antibiotic treatment, and of these only seven went on to develop dementia. Although our results point to statistical significance, further investigations with a larger number of subjects will be essential to clarify whether the antibiotic treatment truly plays a role in reducing the risk of dementia for the leptospirosis-infected patients. Second, patients with dementia were identified using the insurance claims data, but data on severity or stage were not available, and we could only estimate the treatment durations of each antibiotic medication by dividing the cumulative doses of individual medications by the defined daily dose. Third, other confounding factors such as genetics and dietary factors are also not included in the NHIRD. Because no imaging findings or other laboratory data are included in the NHIRD, we relied on the professional diagnosis of dementia by board-certified psychiatrists or neurologists according to the ICD-9-CM codes from the NHIRD, and the leptospirosis cases that were presented as mild symptoms may not have been recorded and were thus not enrolled in this study. Nevertheless, because our analysis is based on a large cohort of patients (1428 subjects: 357 leptospirosis and 1071 controls) as well as internationally recognized diagnostic codes, and furthermore corroborates previous work on leptospirosis and AD risk (Chiu et al., 2019), our central results may be reliable – and raise three important issues concerning causation and the pathogen(s) that might be involved.
Conclusion
Patients with leptospirosis infections have an increased risk of developing dementia, and the antibiotic treatment was associated with a diminished risk of dementia. These findings might well be considered as an indicator to clinicians caring for patients with leptospirosis infections. Further research will be necessary so as to explore the underlying mechanism(s) of this association.
Data Availability Statement
The datasets on the study population that were obtained from the NHIRD (http://nhird.nhri.org.tw/en/index.html) are maintained in the NHIRD (http://nhird.nhri.org.tw/). The National Health Research Institutes (NHRI) is a nonprofit foundation established by the government. Only citizens of Taiwan who fulfill the requirements for conducting research projects are eligible to apply for the NHIRD. The use of the NHIRD is limited to research purposes only. Applicants must follow the Computer-Processed Personal Data Protection Act (https://dep.mohw.gov.tw/dos/lp-2506-113.html) and the related regulations of the National Health Insurance Administration and NHRI, and an agreement must be signed by the applicant and their supervisor upon application submission. All applications are reviewed for approval of data release.
Ethics Statement
Since the identifiable database of individuals included in the NHIRD were all encrypted in order to protect individual privacy, the NHI Administration has given general approval for their data to be used in this research. Because the NHIRD has the advantage of providing a large-scale, longitudinal, reliable dataset, leading to extensive use for population-based researches in Taiwan, the Institutional Review Board of Tri-Service General Hospital was aware of this and approved the research to proceed, and also agreed that the benefit justified waiving the need for individual written informed consent in such a study (IRB No. 1-106-05-169).
Author Contributions
P-CC and N-ST contributed to the study concept and design. W-CC, C-HC, and N-ST contributed to the acquisition of data. W-CC, C-HC, C-KH, H-ML, P-CC, and N-ST contributed to the analysis and interpretation of data. P-CC contributed to the drafting of the manuscript. N-ST contributed to the critical revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Medical Affairs Bureau, the Ministry of Defense of Taiwan (MAB-107-084 and MND-MAB-D-111-075), the Tri-Service General Hospital Research Foundation (TSGH-C108-003, TSGHC108-027, TSGH-C108-151, TSGH-B-109-010, TSGH-B-111-018, and TSGH-D-111-121), and the Taoyuan Armed Forces General Hospital (TYAFGH-A-110-020).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We appreciate Taiwan’s Health and Welfare Data Science Center and Ministry of Health and Welfare (HWDC, MOHW) for providing the National Health Research Database. We also appreciate Richard Lathe for his help in the writing of this article.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.771486/full#supplementary-material
Footnotes
References
American Psychiatric Association [APA] (1994). Diagnostic and Statistical Manual of Mental Disorders, (DSM-IV), 4th Edn. Virginia, VA: American Psychiatric Association.
American Psychiatric Association [APA] (2000). Diagnostic and Statistical Manual of Mental Disorders, Text Revision (DSM-IV-TR), 4th Edn. Virginia, VA: American Psychiatric Association.
Balducci, C., and Forloni, G. (2019). Doxycycline for Alzheimer’s Disease: fighting β-Amyloid oligomers and neuroinflammation. Front. Pharmacol. 10:738. doi: 10.3389/fphar.2019.00738
Barage, S. H., and Sonawane, K. D. (2015). Amyloid cascade hypothesis: pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides 52, 1–18. doi: 10.1016/j.npep.2015.06.008
Barnes, L. L., Capuano, A. W., Aiello, A. E., Turner, A. D., Yolken, R. H., Torrey, E. F., et al. (2015). Cytomegalovirus infection and risk of Alzheimer disease in older black and white individuals. J. Infect. Dis. 211, 230–237. doi: 10.1093/infdis/jiu437
Branton, W. G., Ellestad, K. K., Maingat, F., Wheatley, B. M., Rud, E., Warren, R. L., et al. (2013). Brain microbial populations in HIV/AIDS: alpha-proteobacteria predominate independent of host immune status. PLoS One 8:e54673. doi: 10.1371/journal.pone.0054673
Burgos, J. S., Ramirez, C., Sastre, I., and Valdivieso, F. (2006). Effect of apolipoprotein E on the cerebral load of latent herpes simplex virus type 1 DNA. J. Virol. 80, 5383–5387. doi: 10.1128/jvi.00006-06
Burt, T. D., Agan, B. K., Marconi, V. C., He, W., Kulkarni, H., Mold, J. E., et al. (2008). Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression. Proc. Natl. Acad. Sci. U.S.A. 105, 8718–8723. doi: 10.1073/pnas.0803526105
Cagliero, J., Villanueva, S., and Matsui, M. (2018). Leptospirosis pathophysiology: into the storm of cytokines. Front. Cell Infect. Microbiol. 8:204. doi: 10.3389/fcimb.2018.00204
Center for Disease Control and Prevention [CDCP] (2018). Leptospirosis Fact Sheet for Clinicians [Online]. Available online at: https://www.cdc.gov/leptospirosis/pdf/fs-leptospirosis-clinicians-eng-508.pdf (accessed January 10, 2022).
Charan, J., Saxena, D., Mulla, S., and Yadav, P. (2013). Antibiotics for the treatment of leptospirosis: systematic review and meta-analysis of controlled trials. Int. J. Prev. Med. 4, 501–510.
Charlson, M. E., Pompei, P., Ales, K. L., and MacKenzie, C. R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40, 373–383.
Chen, H. L., Lea, Y. P., Young, S. C., and Wu, C. Y. (1995). A summary report of concurrent peer review of inpatient medical records in a medical center. Taiwan J. Public Health 14, 103–110.
Chen, T. Y., Huang, C. H., Chung, C. H., Mao, W. C., Yeh, C. B., Yang, C. C. H., et al. (2020). Sex and age differences in the association between anxiety disorders and narcolepsy: a nationwide population-based case control study. J. Affect. Disord. 264, 130–137. doi: 10.1016/j.jad.2019.12.010
Cheng, C. L., Kao, Y. H., Lin, S. J., Lee, C. H., and Lai, M. L. (2011). Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol. Drug Saf. 20, 236–242. doi: 10.1002/pds.2087
Chinese Hospital Association [CHA] (2000). ICD-9-CM English-Chinese Dictionary. Taipei: Chinese Hospital Association Press.
Chiu, C. H., Chen, P. C., Wang, Y. C., Lin, C. L., Lee, F. Y., Wu, C. C., et al. (2019). Risk of dementia in patients with leptospirosis: a nationwide cohort analysis. Int. J. Environ. Res. Public Health 16:3168. doi: 10.3390/ijerph16173168
Chiu, M. L., Cheng, C. F., Liang, W. M., Lin, P. T., Wu, T. N., and Chen, C. Y. (2018). The temporal relationship between selected mental disorders and substance-related disorders: a nationwide population-based cohort study. Psychiatry J. 2018:5697103. doi: 10.1155/2018/5697103
Chiu, W. C., Tsan, Y. T., Tsai, S. L., Chang, C. J., Wang, J. D., Chen, P. C., et al. (2014). Hepatitis C viral infection and the risk of dementia. Eur. J. Neurol. 21, 1068–e1059. doi: 10.1111/ene.12317
Chou, I. C., Lin, H. C., Lin, C. C., Sung, F. C., and Kao, C. H. (2013). Tourette syndrome and risk of depression: a population-based cohort study in Taiwan. J. Dev. Behav. Pediatr. 34, 181–185. doi: 10.1097/DBP.0b013e3182829f2b
Daulatzai, M. A. (2016). Fundamental role of pan-inflammation and oxidative-nitrosative pathways in neuropathogenesis of Alzheimer’s disease. Am. J. Neurodegener. Dis. 5, 1–28.
Day, N. (2021). Up to Date: Leptospirosis: Treatment and Prevention-Antimicrobial Therapy [Online]. Available online at: https://www.uptodate.com/contents/leptospirosis-treatment-and-prevention#H7863015 (accessed January 12, 2022).
de Groot, V., Beckerman, H., Lankhorst, G. J., and Bouter, L. M. (2003). How to measure comorbidity. a critical review of available methods. J. Clin. Epidemiol. 56, 221–229.
Emery, D. C., Shoemark, D. K., Batstone, T. E., Waterfall, C. M., Coghill, J. A., Cerajewska, T. L., et al. (2017). 16S rRNA next generation sequencing analysis shows bacteria in Alzheimer’s post-mortem brain. Front. Aging Neurosci. 9:195. doi: 10.3389/fnagi.2017.00195
Forbes, A. E., Zochowski, W. J., Dubrey, S. W., and Sivaprakasam, V. (2012). Leptospirosis and Weil’s disease in the UK. QJM 105, 1151–1162. doi: 10.1093/qjmed/hcs145
Fratiglioni, L., Winblad, B., and von Strauss, E. (2007). Prevention of Alzheimer’s disease and dementia. Major findings from the Kungsholmen Project. Physiol. Behav. 92, 98–104. doi: 10.1016/j.physbeh.2007.05.059
Garba, B., Bahaman, A. R., Bejo, S. K., Zakaria, Z., Mutalib, A. R., and Bande, F. (2018). Major epidemiological factors associated with leptospirosis in Malaysia. Acta Trop. 178, 242–247. doi: 10.1016/j.actatropica.2017.12.010
Gerard, H. C., Wildt, K. L., Whittum-Hudson, J. A., Lai, Z., Ager, J., and Hudson, A. P. (2005). The load of Chlamydia pneumoniae in the Alzheimer’s brain varies with APOE genotype. Microb. Pathog. 39, 19–26. doi: 10.1016/j.micpath.2005.05.002
Gonwong, S., Chuenchitra, T., Khantapura, P., Islam, D., Ruamsap, N., Swierczewski, B. E., et al. (2017). Nationwide seroprevalence of leptospirosis among young Thai Men, 2007-2008. Am. J. Trop. Med. Hyg. 97, 1682–1685. doi: 10.4269/ajtmh.17-0163
Ho Chan, W. S. (2010). Taiwan’s healthcare report 2010. Epma J. 1, 563–585. doi: 10.1007/s13167-010-0056-8
Hsieh, C. Y., Su, C. C., Shao, S. C., Sung, S. F., Lin, S. J., Kao Yang, Y. H., et al. (2019). Taiwan’s national health insurance research database: past and future. Clin. Epidemiol. 11, 349–358. doi: 10.2147/clep.S196293
Itzhaki, R. F., Lathe, R., Balin, B. J., Ball, M. J., Bearer, E. L., Braak, H., et al. (2016). Microbes and Alzheimer’s disease. J. Alzheimers Dis. 51, 979–984. doi: 10.3233/jad-160152
Kao, L. T., Sheu, J. J., Lin, H. C., Tsai, M. C., and Chung, S. D. (2015). Association between sepsis and dementia. J. Clin. Neurosci. 22, 1430–1433. doi: 10.1016/j.jocn.2015.02.035
Launer, L. J., Ross, G. W., Petrovitch, H., Masaki, K., Foley, D., White, L. R., et al. (2000). Midlife blood pressure and dementia: the Honolulu–Asia aging study. Neurobiol. Aging 21, 49–55. doi: 10.1016/s0197-4580(00)00096-8
Lettieri, C., Moon, J., Hickey, P., Gray, M., Berg, B., and Hospenthal, D. (2004). Prevalence of leptospira antibodies in U.S. Army blood bank donors in Hawaii. Mil. Med. 169, 687–690. doi: 10.7205/milmed.169.9.687
Levett, P. N. (2001). Leptospirosis. Clin. Microbiol. Rev. 14, 296–326. doi: 10.1128/cmr.14.2.296-326.2001
Liang, J. A., Sun, L. M., Muo, C. H., Sung, F. C., Chang, S. N., and Kao, C. H. (2011). The analysis of depression and subsequent cancer risk in Taiwan. Cancer Epidemiol. Biomarkers Prev. 20, 473–475. doi: 10.1158/1055-9965.epi-10-1280
Lloyd, V. K., and Hawkins, R. G. (2018). Under-detection of Lyme disease in Canada. Healthcare (Basel) 6:125. doi: 10.3390/healthcare6040125
Marshall, R. B., and Scrimgeour, G. (1978). Schizophreniform psychosis associated with leptospirosis. N. Z. Med. J. 88, 212–213.
Miklossy, J. (2011). Alzheimer’s disease - a neurospirochetosis. Analysis of the evidence following Koch’s and Hill’s criteria. J. Neuroinflamm. 8:90. doi: 10.1186/1742-2094-8-90
Ministry of Justice (2019). National Health Insurance Reimbursement Regulations [Online]. Available online at: https://law.moj.gov.tw/LawClass/LawAll.aspx?pcode=L0060035 (accessed December 18, 2019).
Moir, R. D., Lathe, R., and Tanzi, R. E. (2018). The antimicrobial protection hypothesis of Alzheimer’s disease. Alzheimers Dement 14, 1602–1614. doi: 10.1016/j.jalz.2018.06.3040
Rodrigue, K. M., Kennedy, K. M., and Park, D. C. (2009). Beta-amyloid deposition and the aging brain. Neuropsychol. Rev. 19, 436–450. doi: 10.1007/s11065-009-9118-x
Santabarbara, J., Lipnicki, D. M., Olaya, B., Villagrasa, B., Bueno-Notivol, J., Nuez, L., et al. (2020). Does anxiety increase the risk of all-cause Dementia? An updated meta-analysis of prospective cohort studies. J. Clin. Med. 9:1791. doi: 10.3390/jcm9061791
Semiz, U. B., Turhan, V., Basoglu, C., Oner, O., Ebrinc, S., and Cetin, M. (2005). Leptospirosis presenting with mania and psychosis: four consecutive cases seen in a military hospital in Turkey. Int. J. Psychiatry Med. 35, 299–305. doi: 10.2190/0kux-je1j-hv6k-3r9j
Sohail, M. L., Khan, M. S., Ijaz, M., Naseer, O., Fatima, Z., Ahmad, A. S., et al. (2018). Seroprevalence and risk factor analysis of human leptospirosis in distinct climatic regions of Pakistan. Acta Trop. 181, 79–83. doi: 10.1016/j.actatropica.2018.01.021
Soscia, S. J., Kirby, J. E., Washicosky, K. J., Tucker, S. M., Ingelsson, M., Hyman, B., et al. (2010). The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One 5:e9505. doi: 10.1371/journal.pone.0009505
Stefaniak, J., and O’Brien, J. (2016). Imaging of neuroinflammation in dementia: a review. J. Neurol. Neurosurg. Psychiatry 87, 21–28. doi: 10.1136/jnnp-2015-311336
Strittmatter, W. J., Saunders, A. M., Schmechel, D., Pericak-Vance, M., Enghild, J., Salvesen, G. S., et al. (1993). Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 90, 1977–1981. doi: 10.1073/pnas.90.5.1977
Sun, Y., Lee, H. J., Yang, S. C., Chen, T. F., Lin, K. N., Lin, C. C., et al. (2014). A nationwide survey of mild cognitive impairment and dementia, including very mild dementia, in Taiwan. PLoS One 9:e100303. doi: 10.1371/journal.pone.0100303
Taiwan Centers for Disease Control [TCDC] (2017). Leptospirosis [Online]. Available online at: https://www.cdc.gov.tw/En/Category/ListContent/bg0g_VU_Ysrgkes_KRUDgQ?uaid=CCSE04tqavhEr7r7mVUj6Q (accessed January 12, 2022).
Tzeng, N. S., Chien, W. C., Chung, C. H., Chang, H. A., Kao, L. C., and Liu, Y. P. (2020). Association between amphetamine-related disorders and Dementia-A nationwide cohort study in Taiwan. Ann. Clin. Transl. Neurol. 7, 1284–1295.
Wan, F. J., Chien, W. C., Chung, C. H., Yang, Y. J., and Tzeng, N. S. (2020). Association between traumatic spinal cord injury and affective and other psychiatric disorders-A nationwide cohort study and effects of rehabilitation therapies. J. Affect. Disord. 265, 381–388. doi: 10.1016/j.jad.2020.01.063
Whitney, E. A., Ailes, E., Myers, L. M., Saliki, J. T., and Berkelman, R. L. (2009). Prevalence of and risk factors for serum antibodies against Leptospira serovars in US veterinarians. J. Am. Vet. Med. Assoc. 234, 938–944. doi: 10.2460/javma.234.7.938
Wu, C. S., Kuo, C. J., Su, C. H., Wang, S. H., and Dai, H. J. (2020). Using text mining to extract depressive symptoms and to validate the diagnosis of major depressive disorder from electronic health records. J. Affect. Disord. 260, 617–623. doi: 10.1016/j.jad.2019.09.044
Wu, C. S., Lai, M. S., Gau, S. S., Wang, S. C., and Tsai, H. J. (2014). Concordance between patient self-reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS One 9:e112257. doi: 10.1371/journal.pone.0112257
Yu, S. T., Chang, H. Y., Lin, M. C., and Lin, Y. H. (2009). Agreement between self-reported and health insurance claims on utilization of health care: a population study. J. Clin. Epidemiol. 62, 1316–1322. doi: 10.1016/j.jclinepi.2009.01.016
Zumkehr, J., Rodriguez-Ortiz, C. J., Cheng, D., Kieu, Z., Wai, T., Hawkins, C., et al. (2015). Ceftriaxone ameliorates tau pathology and cognitive decline via restoration of glial glutamate transporter in a mouse model of Alzheimer’s disease. Neurobiol. Aging 36, 2260–2271. doi: 10.1016/j.neurobiolaging.2015.04.005
Keywords: leptospirosis, antibiotic, dementia, time-dependent, risk
Citation: Chao P-C, Chien W-C, Chung C-H, Huang C-K, Li H-M and Tzeng N-S (2022) Association Between Antibiotic Treatment of Leptospirosis Infections and Reduced Risk of Dementia: A Nationwide, Cohort Study in Taiwan. Front. Aging Neurosci. 14:771486. doi: 10.3389/fnagi.2022.771486
Received: 06 September 2021; Accepted: 14 February 2022;
Published: 23 March 2022.
Edited by:
Omar Yaxmehen Bello-Chavolla, Instituto Nacional de Geriatría, MexicoReviewed by:
Chia-Fen Tsai, Taipei Veterans General Hospital, TaiwanLay Khoon Too, The University of Sydney, Australia
David Wang, National Institutes of Health (NIH), United States
Copyright © 2022 Chao, Chien, Chung, Huang, Li and Tzeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nian-Sheng Tzeng, cGllcnJlbnNAbWFpbC5uZG1jdHNnaC5lZHUudHc=
 Pei-Chun Chao1,2
Pei-Chun Chao1,2 Wu-Chien Chien
Wu-Chien Chien Chi-Hsiang Chung
Chi-Hsiang Chung Nian-Sheng Tzeng
Nian-Sheng Tzeng