
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 06 January 2023
Sec. Parkinson’s Disease and Aging-related Movement Disorders
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.1073258
This article is part of the Research Topic Transcription Regulation - Brain Development and Homeostasis - A Finely Tuned and Orchestrated Scenario in Physiology and Pathology, volume II View all 12 articles
 Andrea Elias-Mas1,2,3
Andrea Elias-Mas1,2,3 Miriam Potrony4,5,6
Miriam Potrony4,5,6 Jaume Bague5,7
Jaume Bague5,7 David J. Cutler8
David J. Cutler8 Maria Isabel Alvarez-Mora4,5,6
Maria Isabel Alvarez-Mora4,5,6 Teresa Torres5,6
Teresa Torres5,6 Tamara Barcos4,5
Tamara Barcos4,5 Joan Anton Puig-Butille5,6,9
Joan Anton Puig-Butille5,6,9 Marta Rubio2,10
Marta Rubio2,10 Irene Madrigal4,5,6
Irene Madrigal4,5,6 Susana Puig5,6,7
Susana Puig5,6,7 Emily G. Allen8*†
Emily G. Allen8*† Laia Rodriguez-Revenga4,5,6*†
Laia Rodriguez-Revenga4,5,6*†Introduction: Fragile X-associated tremor/ataxia syndrome (FXTAS, OMIM# 300623) is a late-onset neurodegenerative disorder with reduced penetrance that appears in adult FMR1 premutation carriers (55–200 CGGs). Clinical symptoms in FXTAS patients usually begin with an action tremor. After that, different findings including ataxia, and more variably, loss of sensation in the distal lower extremities and autonomic dysfunction, may occur, and gradually progress. Cognitive deficits are also observed, and include memory problems and executive function deficits, with a gradual progression to dementia in some individuals. Aquaporin 4 (AQP4) is a commonly distributed water channel in astrocytes of the central nervous system. Changes in AQP4 activity and expression have been implicated in several central nervous system disorders. Previous studies have suggested the associations of AQP4 single nucleotide polymorphisms (SNPs) with brain-water homeostasis, and neurodegeneration disease. To date, this association has not been studied in FXTAS.
Methods: To investigate the association of AQP4 SNPs with the risk of presenting FXTAS, a total of seven common AQP4 SNPs were selected and genotyped in 95 FMR1 premutation carriers with FXTAS and in 65 FMR1 premutation carriers without FXTAS.
Results: The frequency of AQP4-haplotype was compared between groups, denoting 26 heterozygous individuals and 5 homozygotes as carriers of the minor allele in the FXTAS group and 25 heterozygous and 2 homozygotes in the no-FXTAS group. Statistical analyses showed no significant associations between AQP4 SNPs/haplotypes and development of FXTAS.
Discussion: Although AQP4 has been implicated in a wide range of brain disorders, its involvement in FXTAS remains unclear. The identification of novel genetic markers predisposing to FXTAS or modulating disease progression is critical for future research involving predictors and treatments.
The brain is a high-energy consuming organ with a high metabolic activity, producing a substantial amount of interstitial waste products. Efficient clearance of the brain’s metabolic waste is needed in order to avoid their accumulation, causing several neurological diseases (Kaur et al., 2021). Since there is a lack of conventional lymphoid circulation in the brain, the glymphatic system has been postulated as an alternative clearance for the brain waste product (Iliff et al., 2015), though evidence is still incomplete (Hladky and Barrand, 2022).
In the glymphatic system, cerebrospinal fluid (CSF) flows into the brain parenchyma within the periarterial spaces that surround the penetrating cerebral arteries, also called the perivascular spaces. Facilitated by aquaporin 4 (AQP4), CSF flows from the periarterial space into the brain interstitium and mixes with interstitial fluid, which, along with interstitial solutes, travels into the perivenous spaces, draining the fluid and its contents into the deep veins and into the basal meningeal and cervical lymphatic vessels (Hablitz and Nedergaard, 2021). AQP4 is the most abundant water channel in the brain, and, since it has a role regulating fluid exchange between perivascular spaces and the rest of the glymphatic system, it is considered the most important element in it (Nagelhus and Ottersen, 2013; Papadopoulos and Verkman, 2013; Szczygielski et al., 2021).
Alterations of glymphatic fluid circulation through AQPs variations are now emerging as central elements in the pathophysiology of different brain conditions. In fact, dysfunction of AQP4 have been implicated in the pathogenesis of many degenerative disorders, including Alzheimer’s disease (AD), vascular cognitive impairment, idiopathic normal-pressure hydrocephalus, Parkinson’s disease dementia, frontotemporal dementia and Creutzfeldt-Jakob disease (Zeppenfeld et al., 2017; Nedergaard and Goldman, 2020; Silva et al., 2021; Wang et al., 2022). Furthermore, evidence indicates that genetic variation in AQP4 modulates sleep quality and architecture, amyloid-β burden and rate and progression of cognitive decline in AD patients (Burfeind et al., 2017; Rainey-Smith et al., 2018; Ulv Larsen et al., 2020). Despite the relationship between glymphatic dysfunction and neurodegenerative diseases, dysfunction of glymphatic system has not yet been studied in Fragile X-associated tremor/ataxia syndrome (FXTAS) and its association with AQP4 genetic variants is unknown. FXTAS is a neurodegenerative disorder linked to FMR1 gene premutation carriers (55–200 CGG repeats) that is associated with cognitive loss that can evolve into dementia. Intranuclear inclusions and increased β amyloid load has been discovered in brains of patients with FXTAS (Cabal-Herrera et al., 2020; Salcedo-Arellano et al., 2021a). On the basis of these observations, we analyzed AQP4 functional variants with the aim to investigate whether AQP4 could be a new genetic risk factor for FXTAS.
The present study was conducted on genotype data from a total of 160 unrelated FMR1 premutation carriers (95 presenting FXTAS symptoms and 65 without FXTAS clinical symptoms). Participants were identified through previous fragile X syndrome research projects at Emory University (Atlanta, GA, USA), through recruitment efforts at scientific conferences, and through collaborations with other research groups. All participants were enrolled from families with members known to be affected with fragile X-associated conditions and molecularly diagnosed. Table 1 summarizes general demographics of the participants. FXTAS subjects were screened for eligibility as described in Kong et al. (2022). Briefly, case subjects were male or female premutation carriers with symptoms of tremor or ataxia before age 65, as reviewed by a neurologist. Control individuals, named as the no-FXTAS group, were male premutation carriers that reached age 68 without significant tremor or ataxia symptoms, as reviewed by a neurologist. The protocols and consent forms were approved by the Institutional Review Board at Emory University, and written informed consent was obtained from all subjects (IRB00074941).
Seven tag single nucleotide polymorphisms (SNPs) across AQP4 gene (NM_001650) were selected according to their location and known functions, based on earlier reports on their associations with clinical phenotypes (Ulv Larsen et al., 2020). The SNPs were considered for those above 15% in Utah Residents with Northern and Western European Ancestry (CEU) population according to minor allele frequency (MAF: 0.15∼0.26). These SNPs included rs162007 (Chr18:26865883, Upstream, MAF 0.16), rs162008 (Chr18:26865728, 5′UTR, MAF 0.20), rs63514 (Chr18:26863457, intron, MAF 0.17), rs335931 (Chr18:26859108, intron, MAF 0.15), rs335930 (Chr18:26856961, intron, MAF 0.23), rs335929 (Chr18:26855623, 3′UTR, MAF 0.14), rs16942851 (Chr18:26851725, downstream, MAF 0.14). The chromosome positions are based on hg38.
Whole genome sequencing was performed on samples using Illumina platforms at Hudson Alpha or Novogene as described in Kong et al. (2022). All samples were mapped using PEMapper and called using PECaller (Johnston et al., 2017). Genomic data have been uploaded to the National Institute of Mental Health (NIMH) Data Archive.1
To test the population homogeneity of the study subjects, the allele frequencies were tested against Hardy-Weinberg equilibrium (HWE) by the χ2-test. The plink v1.07 toolset was used to perform SNP association and haplotype2 (Purcell et al., 2007). The power analysis was performed using the ‘‘Quanto’’ tool.3 Statistical analyses were performed using commercially available software (SPSS SmartViewer, version 18.0; SPSS, Chicago, IL, USA). P-values < 0.05 were considered statistically significant. Association tests were corrected using the Benjamini and Hochberg step-up False Discovery Rate (FDR) for multiple comparisons.
Genotype data from 95 FXTAS and 65 no-FXTAS individuals were analyzed. Table 1 shows the demographic data of FXTAS and no-FXTAS group. Significant differences were found for the age and the CGG repeat size when comparing both groups (p < 0.0001 and p < 0.0001, respectively). Age difference can be attributed to a bias in the recruitment of no-FXTAS individuals. In order to make sure that FMR1 permutations in the no-FXTAS group did not have neurologic symptoms older men were included in this cohort. As for the CGG repeat size, the difference found might account for the CGG-repeat dependence described in clinical and neuropathologic features of FXTAS.
In agreement with HWE, there was no deviation detected in any of the analyzed SNPs (p > 0.3). All SNPs studied were in linkage disequilibrium (LD), and the pairwise LD coefficient (r2) ranged between 0.8 and 1. Table 2 shows the genotype frequency for each SNP according to the presence of FXTAS symptoms. After correction of p-values for multiple comparisons, there was no significant difference in frequencies of any of the analyzed SNPs between FXTAS and no-FXTAS group (Table 3). Adjustment for sex did not change these results (data not shown). Table 4 shows the results of the genotype association analysis between AQP4 polymorphisms and risk of FXTAS, according to different genetic inheritance models.
Fragile X-associated tremor/ataxia syndrome is a late-onset neurodegenerative disorder with reduced penetrance, meaning that not all FMR1 premutation carriers will develop it (Hagerman et al., 2001). Among FMR1 premutation carriers older than 50 years, it has been estimated that 40% of men and 16% of women will develop FXTAS symptoms, although there is significant variability in the progression of neurological dysfunction (Coffey et al., 2008; Rodriguez-Revenga et al., 2009). The description and characterization of FXTAS is of great interest, because the prevalence of FMR1 premutation in the general population is relatively high. It has been estimated that FMR1 premutation affects ∼1 out of 400 males and 1 out of 200 females (Tassone et al., 2012), leading to symptoms of FXTAS in up to 1 in 3000 men older than 50 years. Even though the FMR1 premutation is the major risk factor for FXTAS, there are still some unknown genetic, epigenetic or environmental factors that might be affecting gene penetrance. Candidate gene SNP association analysis is a commonly used approach to identify risk alleles and their association with clinical traits. In the present study we selected this method to investigate the role of AQP4 gene variants in FXTAS susceptibility. We hypothesized that AQP4 polymorphisms could play a role as risk factors for FXTAS. However, we did not find any significant difference in the distributions of alleles, genotypes, and haplotypes between FXTAS and no-FXTAS individuals, after correction for multiple testing.
A myriad of different studies point out AQP4 gene as a novel candidate gene for brain plasticity and associated with neuropsychiatric and neurodegenerative disorders. According to the human postmortem brain microarray data from the Allen Brain Atlas resources,4 AQP4 is most highly expressed in fronto-limbic and temporal cortical regions. Both neuroanatomical areas are linked to cognitive and executive processes, and its disturbance leads to the neuropsychological changes described in many different movement disorders (Robertson et al., 2016). Although indirectly, genome-wide linkage studies have repeatedly pointed out the role of AQP4 in the development of brain disorders (Dadgostar et al., 2021). Genetic variations, abnormal distribution and quantitative abnormalities of AQP4 have also been associated with several neurodegenerative disorders, such as AD, Parkinson’s disease and amyotrophic lateral sclerosis (reviewed in Mader and Brimberg, 2019). Recently the rs162008, the most prevalent genetic variant of AQP4, has been associated with a ∼15% change in AQP4 expression. In AD, genetic variations in AQP4 were shown to be associated with changes in sleep pattern and increased β-amyloid (Rainey-Smith et al., 2018), as well as to β-amyloid accumulation and disease stage progression (Burfeind et al., 2017; Chandra et al., 2021). Taking everything into account, it is implied that AQP4 distribution and regulation might have crucial role in neuronal activity and function.
Apart from intention tremor and cerebellar ataxia, core clinical features of FXTAS include executive dysfunction which may progress to dementia in some cases (Hagerman et al., 2001; Hall et al., 2016). In addition, several conditions affecting sleep quality have been frequently described among FXATS patients (Hamlin et al., 2011; Summers et al., 2014). FXTAS can coexist with other neurodegenerative disorders, such as Parkinson’s disease and AD (Aydin et al., 2020; Salcedo-Arellano et al., 2021b), suggesting a synergistic effect on the progression of disease symptoms. On the basis of these observations and the evidence of the consequences of AQP4 dysfunction in neurological conditions, we analyzed genetic variation of AQP4 gene among FXTAS patients. We compared frequency of alleles, genotypes, and haplotypes of AQP4 between FXTAs and no-FXTAS cases. Results did not find any association between any of the SNPs analyzed and the risk of developing FXTAS (Table 4). Furthermore, no association was detected when comparing frequency distribution of the two major AQP4-haplotypes. In fact, the frequency detected did not differ from the one described among CEU population (Table 5). Although no relationship between genetic variants in AQP4 gene and FXTAS was found, no association with changes in the development of the disease has been assessed due to lack of clinical information. Similarly with the AD, AQP4 SNPs have been associated with some aspects of the clinical course, such as faster cognitive decline, rather than the presence or absence of the disease (Burfeind et al., 2017).
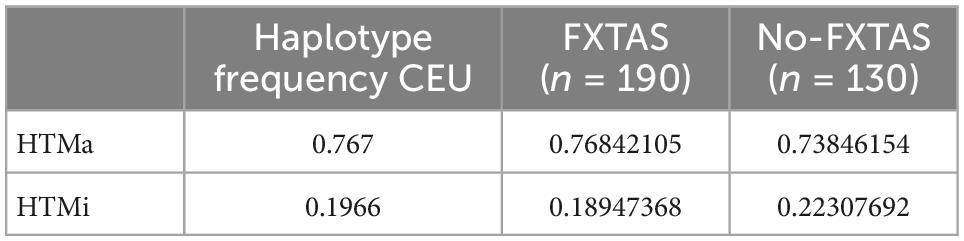
Table 5. Major allele and minor allele haplotype (HTMa and HTMi) frequencies of AQP4 functional SNPs across the CEU (Utah residents with ancestry from Northern and Western Europe) population and the FXTAS and no-FXTAS groups.
As previously described (Ulv Larsen et al., 2020), examination of the SNPs revealed two conserved haplotypes: HtMa (haplotype for the major allele) and HtMi (haplotype for the minor allele). Haplotype frequency comparison by means of dominant analysis between FXTAS and no-FXTAS group did not show significant differences (p > 0.05). Moreover, both groups showed similar haplotype frequency compared to the CEU population (Table 5).
Given the sample size this study had limited power. Post hoc power analyses showed that the power to detect the observed odds ratios for FXTAS cases vs. no-FXTAS ranged from 0.05 to 0.19.
This study has two main limitations that might be explained because of the rarity of the disorder. First the relatively small sample size. Power ranged only from 0.05 to 0.19. Second, the age differences between groups and the fact that those individuals considered as no-FXTAS may develop clinical symptoms later in life, masking differences among groups. Nonetheless, to our knowledge we are reporting for the first time, that the AQP4 SNPs (rs162007, rs162008, rs63514, rs335931, rs335930, rs335929, and rs16942851) and haplotypes were not associated with susceptibility of FXTAS in Caucasian population. Despite this lack of association, further studies are necessary to fully discard the role of AQP4 and glymphatic system in the pathology of FXTAS. There is a need to describe new evidence into how the glymphatic system functions, and how AQP4 dysfunction might take part into FXTAS disease progression.
The original contributions presented in this study are included in the article, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Institutional Review Board at Emory University (IRB00074941). The patients/participants provided their written informed consent to participate in this study.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
This work was supported by the Instituto de Salud Carlos III (ISCIII), (through the projects PI17/01067 and PI21/01085), co-funded by the European Union, and AGAUR from the Autonomous Catalan Government (2017SGR1134). The CIBER de Enfermedades Raras is an initiative of the Instituto de Salud Carlos III.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aydin, E., Schneider, A., Protic, D., Wang, J., Martínez-Cerdeño, V., Tassone, F., et al. (2020). Rapidly progressing neurocognitive disorder in a male with FXTAS and Alzheimer’s Disease. Clin. Interv. Aging 15, 285–292. doi: 10.2147/CIA.S240314
Burfeind, K., Murchison, C., Westaway, S., Simon, M., Erten-Lyons, D., Kaye, J., et al. (2017). The effects of noncoding aquaporin-4 single-nucleotide polymorphisms on cognition and functional progression of Alzheimer’s disease. Alzheimers Dement (N Y) 3, 348–359. doi: 10.1016/j.trci.2017.05.001
Cabal-Herrera, A., Tassanakijpanich, N., Salcedo-Arellano, M., and Hagerman, R. (2020). Fragile X-associated tremor/ataxia syndrome (FXTAS): Pathophysiology and clinical implications. Int. J. Mol. Sci. 21:4391. doi: 10.3390/ijms21124391
Chandra, A., Farrell, C., Wilson, H., Dervenoulas, G., De Natale, E., and Politis, M. (2021). Alzheimer’s Disease Neuroimaging Initiative. Aquaporin-4 polymorphisms predict amyloid burden and clinical outcome in the Alzheimer’s disease spectrum. Neurobiol. Aging 97, 1–9.
Coffey, S., Cook, K., Tartaglia, N., Tassone, F., Nguyen, D., Pan, R., et al. (2008). Expanded clinical phenotype of women with the FMR1 premutation. Am. J. Med. Genet. A 146A, 1009–1016. doi: 10.1002/ajmg.a.32060
Dadgostar, E., Tajiknia, V., Shamsaki, N., Naderi-Taheri, M., Aschner, M., Mirzaei, H., et al. (2021). Aquaporin 4 and brain-related disorders: Insights into its apoptosis roles. EXCLI J. 20, 983–994. doi: 10.17179/excli2021-3735
Hablitz, L., and Nedergaard, M. (2021). The glymphatic system: A novel component of fundamental neurobiology. J. Neurosci. 41, 7698–7711. doi: 10.1523/JNEUROSCI.0619-21.2021
Hagerman, R., Leehey, M., Heinrichs, W., Tassone, F., Wilson, R., Hills, J., et al. (2001). Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 57, 127–130. doi: 10.1212/wnl.57.1.12
Hall, D., Robertson, E., Shelton, A., Losh, M., Mila, M., Moreno, E., et al. (2016). Update on the clinical, radiographic, and neurobehavioral manifestations in FXTAS and FMR1 premutation carriers. Cerebellum 15, 578–586. doi: 10.1007/s12311-016-0799-4
Hamlin, A., Liu, Y., Nguyen, D., Tassone, F., Zhang, L., and Hagerman, R. (2011). Sleep apnea in fragile X premutation carriers with and without FXTAS. Am. J. Med. Genet. B Neuropsychiatr. Genet. 156B, 923–928. doi: 10.1002/ajmg.b.31237
Hladky, S., and Barrand, M. (2022). The glymphatic hypothesis: The theory and the evidence. Fluids Barriers CNS 19:9. doi: 10.1186/s12987-021-00282-z
Iliff, J., Goldman, S., and Nedergaard, M. (2015). Implications of the discovery of brain lymphatic pathways. Lancet Neurol. 14, 977–979. doi: 10.1016/S1474-4422(15)00221-5
Johnston, H., Chopra, P., Wingo, T., Patel, V., Epstein, M., Mulle, J., et al. (2017). PEMapper and PECaller provide a simplified approach to whole-genome sequencing. Proc. Natl. Acad. Sci. U.S.A. 114, E1923–E1932. doi: 10.1073/pnas.1618065114
Kaur, J., Fahmy, L., Davoodi-Bojd, E., Zhang, L., Ding, G., Hu, J., et al. (2021). Waste clearance in the brain. Front. Neuroanat. 15:665803. doi: 10.3389/fnana.2021.665803
Kong, H., Lim, J., Linsalata, A., Kang, Y., Malik, I., Allen, E., et al. (2022). Identification of PSMB5 as a genetic modifier of fragile X-associated tremor/ataxia syndrome. Proc. Natl. Acad. Sci. U.S.A. 119:e2118124119. doi: 10.1073/pnas.2118124119
Mader, S., and Brimberg, L. (2019). Aquaporin-4 water channel in the brain and its implication for health and disease. Cells 8:90. doi: 10.3390/cells8020090
Nagelhus, E., and Ottersen, O. (2013). Physiological roles of aquaporin-4 in brain. Physiol. Rev. 93, 1543–1562. doi: 10.1152/physrev.00011.2013
Nedergaard, M., and Goldman, S. (2020). Glymphatic failure as a final common pathway to dementia. Science 370, 50–56. doi: 10.1126/science.abb8739
Papadopoulos, M., and Verkman, A. (2013). Aquaporin water channels in the nervous system. Nat. Rev. Neurosci. 14, 265–277. doi: 10.1038/nrn3468
Purcell, S., Neale, B., Todd-Brown, K., Thomas, L., Ferreira, M., Bender, D., et al. (2007). PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575. doi: 10.1086/519795
Rainey-Smith, S., Mazzucchelli, G., Villemagne, V., Brown, B., Porter, T., Weinborn, M., et al. (2018). Genetic variation in Aquaporin-4 moderates the relationship between sleep and brain Aβ-amyloid burden. Transl. Psychiatry 8:47. doi: 10.1038/s41398-018-0094-x
Robertson, E., Hall, D., McAsey, A., and O’Keefe, J. (2016). Fragile X-associated tremor/ataxia syndrome: Phenotypic comparisons with other movement disorders. Clin. Neuropsychol. 30, 849–900. doi: 10.1080/13854046.2016.1202239
Rodriguez-Revenga, L., Madrigal, I., Pagonabarraga, J., Xunclà, M., Badenas, C., Kulisevsky, J., et al. (2009). Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur. J. Hum. Genet. 17, 1359–1362. doi: 10.1038/ejhg.2009.51
Salcedo-Arellano, M., Wang, J., McLennan, Y., Doan, M., Cabal-Herrera, A., Jimenez, S., et al. (2021a). Cerebral microbleeds in fragile X-associated tremor/ataxia syndrome. Mov. Disord. 36, 1935–1943. doi: 10.1002/mds.28559
Salcedo-Arellano, M., Sanchez, D., Wang, J., McLennan, Y., Clark, C., Juarez, P., et al. (2021b). Case report: Coexistence of alzheimer-type neuropathology in fragile X-Associated tremor ataxia syndrome. Front. Neurosci. 15:720253. doi: 10.3389/fnins.2021.720253
Silva, I., Silva, J., Ferreira, R., and Trigo, D. (2021). Glymphatic system, AQP4, and their implications in Alzheimer’s disease. Neurol. Res. Pract. 3:5. doi: 10.1186/s42466-021-00102-7
Summers, S., Cogswell, J., Goodrich, J., Mu, Y., Nguyen, D., Brass, S., et al. (2014). Prevalence of restless legs syndrome and sleep quality in carriers of the fragile X premutation. Clin. Genet. 86, 181–184. doi: 10.1111/cge.12249
Szczygielski, J., Kopańska, M., Wysocka, A., and Oertel, J. (2021). Cerebral microcirculation, perivascular unit, and glymphatic system: Role of Aquaporin-4 as the gatekeeper for water homeostasis. Front. Neurol. 12:767470. doi: 10.3389/fneur.2021.767470
Tassone, F., Iong, K., Tong, T., Lo, J., Gane, L., Berry-Kravis, E., et al. (2012). FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med. 4:100. doi: 10.1186/gm401
Ulv Larsen, S., Landolt, H., Berger, W., Nedergaard, M., Knudsen, G., and Holst, S. (2020). Haplotype of the astrocytic water channel AQP4 is associated with slow wave energy regulation in human NREM sleep. PLoS Biol. 18:e3000623. doi: 10.1371/journal.pbio.3000623
Wang, Y., Huang, C., Guo, Q., and Chu, H. (2022). Aquaporin-4 and cognitive disorders. Aging Dis. 13, 61–72. doi: 10.14336/AD.2021.0731
Keywords: FXTAS, AQP4, FMR1 premutation, genetic variation, glymphatic system
Citation: Elias-Mas A, Potrony M, Bague J, Cutler DJ, Alvarez-Mora MI, Torres T, Barcos T, Puig-Butille JA, Rubio M, Madrigal I, Puig S, Allen EG and Rodriguez-Revenga L (2023) Evaluation of AQP4 functional variants and its association with fragile X-associated tremor/ataxia syndrome. Front. Aging Neurosci. 14:1073258. doi: 10.3389/fnagi.2022.1073258
Received: 24 October 2022; Accepted: 19 December 2022;
Published: 06 January 2023.
Edited by:
Veronica Martinez Cerdeño, University of California, Davis, United StatesReviewed by:
Randi Jenssen Hagerman, University of California, Davis, United StatesCopyright © 2023 Elias-Mas, Potrony, Bague, Cutler, Alvarez-Mora, Torres, Barcos, Puig-Butille, Rubio, Madrigal, Puig, Allen and Rodriguez-Revenga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laia Rodriguez-Revenga,  bGJvZGlAY2xpbmljLmNhdA==; Emily G. Allen,
bGJvZGlAY2xpbmljLmNhdA==; Emily G. Allen,  ZW1ncmF2ZUBlbW9yeS5lZHU=
ZW1ncmF2ZUBlbW9yeS5lZHU=
†These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.