
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 19 January 2023
Sec. Parkinson’s Disease and Aging-related Movement Disorders
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.1062964
This article is part of the Research Topic Disease-Modifying Targets and Strategies for Alzheimer's Disease and Parkinson's Disease View all 9 articles
 Jiang-ting Li
Jiang-ting Li Yi Qu
Yi Qu Hong-ling Gao
Hong-ling Gao Jing-yi Li
Jing-yi Li Qi-xiong Qin
Qi-xiong Qin Dan-lei Wang
Dan-lei Wang Jing-wei Zhao
Jing-wei Zhao Zhi-juan Mao
Zhi-juan Mao Zhe Min
Zhe Min Yong-jie Xiong*
Yong-jie Xiong* Zheng Xue*
Zheng Xue*Backgrounds: Apathy is common in Parkinson’s disease (PD) but difficult to identify. Growing evidence suggests that abnormal iron metabolism is associated with apathy in PD. We aimed to investigate the clinical features and iron metabolism of apathetic patients with PD, and construct a nomogram for predicting apathy in PD.
Methods: Data of 201 patients with PD were analyzed. Demographic data, Apathy Scale (AS) assessments, and serum iron metabolism parameters were obtained. Spearman correlations were used to assess relationships between AS scores and iron metabolism parameters, separately for male and female patients. Additionally, a nomograph for detecting apathetic patients with PD was built based on the results of logistic regression analysis.
Results: The serum transferrin (TRF, p < 0.0024) concentration and total iron binding capacity (TIBC, p < 0.0024) were lower in the apathetic group after Bonferroni correction, and they were negatively associated with AS scores in male participants with PD (TRF, r = −0.27, p = 0.010; TIBC, r = −0.259, p = 0.014). The nomogram was developed by incorporating the following five parameters: age, sex, serum iron concentration, TIBC and Hamilton Depression Rating Scale (HAMD) scores, which showed good discrimination and calibration, with a consistency index of 0.799 (95% confidence interval = 0.732–0.865).
Conclusion: Abnormal iron metabolism may contribute to apathy in PD, especially among men. TIBC levels in combination with HAMD scores can be effectively used for the prediction of apathetic patients with PD.
Parkinson’s disease (PD) represents a progressive neurodegenerative disorder associated with a spectrum of motor and non-motor symptoms (Bloem et al., 2021). Therein, apathy is one of the most common neuropsychiatric disturbances in PD, with a prevalence of nearly 40% (den Brok et al., 2015). It can be defined as a syndrome of primary motivational loss, which cannot be attributed to emotional distress, cognitive impairment, or diminished level of consciousness (Marin, 1991). Increasing studies have shown that apathy may be a sign of PD worsening as the disease progresses (den Brok et al., 2015; Pagonabarraga et al., 2015). Apathy can be a predictor of dementia and cognitive decline over time in PD (Dujardin et al., 2009), and it is associated with more severe motor symptoms (Liu et al., 2017), lower health-related quality of life (Skorvanek et al., 2013), and heavier caregiver burden (Leroi et al., 2012; Skorvanek et al., 2013; Eglit et al., 2021).
Abnormal iron metabolism might not only contribute to the pathogenesis of PD (Dexter et al., 1989; Riederer et al., 1989; Ward et al., 2014), but also be involved in apathy (Wang et al., 2016). Neuropathological studies have shown the accumulation of iron in the substantia nigra (SN) of patients with PD, and the iron concentrations in the region increase with disease severity (Dexter et al., 1989; Riederer et al., 1989). High concentrations of iron detected in brain could induce neurotoxicity, leading to the death of dopaminergic neurons (Ward et al., 2014). Many studies have indicated that the disturbance of iron metabolism plays a crucial role in non-motor symptoms in PD, including sleep disorder (Hu et al., 2015), restless legs syndrome (Li et al., 2019), depression and anxiety (Xu et al., 2018). However, only a few studies have focused on the relationship between iron metabolism and apathy in PD. A 2016 study found that iron levels in the cerebrospinal fluid (CSF) of patients with PD were positively correlated with the Apathy Scale (AS) scores, and iron overload may be involved in apathy in PD via oxidative stress (Wang et al., 2016). Therefore, there is an urgent call for similar studies with larger sample sizes to explore the clinical significance of iron in apathy in PD.
Although awareness of the prevalence of apathy and its effect on clinical outcomes for patients have increased recently, the nature of apathy and the subjectivity of this neuropsychiatric symptom make the accurate diagnosis difficult (Pagonabarraga et al., 2015). Therefore, we aim to determine the features of apathy and its association with iron metabolism in the Chinese PD population, and construct a simple and useful nomogram to predict the occurrence of apathy in PD.
A total of 201 patients with PD participated in the study. They were inpatients or outpatients at Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology from March 2015 to January 2022. The diagnosis of PD was defined according to the Movement Disorders Society Clinical Diagnostic Criteria (Postuma et al., 2015). Exclusion criteria were as follows: (a)patients were diagnosed with atypical Parkinsonian syndrome or secondary Parkinsonism; (b) patients had anemia, or were on treatment for anemia, such as supplemental iron, folate; (c) patients had a history of digestive-tract diseases (gastritis, gastric ulcer, hepatosis and others); and (d) patients had acute or chronic infections, tumor or serious systemic disease. Informed consent was obtained from all patients, and the study was approved by the Medical Ethics Committee of Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology.
Demographic data, including age, sex, education years, age of onset, disease duration, family history of PD and antiparkinsonian drugs, were collected, and the levodopa-equivalent daily dose (LEDD) was calculated for each participant (Tomlinson et al., 2010). Meanwhile, fasting venous blood (5 ml) was sampled and centrifuged at 4°C for 10 min (3,000 r/min). And the supernatant was separated and stored at −80°C for detection. Iron metabolism indicators, including serum iron (SI), serum ferritin (SF), transferrin (TRF), unsaturated iron-binding capacity (UIBC), total iron binding capacity (TIBC), transferrin saturation (TSAT) and soluble transferrin receptor (sTfR), were examined using the fully automated analyzer (Roche/Hitachi Cobas c 701/702, Roche Diagnostics GmbH).
The symptom of apathy was evaluated using the AS, which was classified as “recommended” to assess apathy in PD (Leentjens et al., 2008). The AS consists of 14 questions with four response choices to each item, corresponding to 0–3 points. The total scores range from 0 to 42 points, with higher scores indicating more severe apathy. Participants with a sum score ≥14 were classified as having a clinically meaningful apathy (Starkstein et al., 1992).
The clinical assessments (motor and non-motor symptoms) of each subject were completed by neurological specialists. On the one hand, the severity and stage of PD were measured using the modified Hoehn and Yahr scale (Goetz et al., 2004), and the motor function was assessed using the Movement Disorder Society-sponsored Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part II and the MDS-UPDRS Part III (Goetz et al., 2008). On the other hand, each patient underwent a comprehensive non-motor symptoms examination: China-Modified Mini-Mental State Examination (CM-MMSE; Xu et al., 2003), Parkinson’s Disease Sleep Scale (PDSS; Chaudhuri et al., 2002). Hamilton Depression Rating Scale (HAMD; Hamilton, 1960), Hamilton Anxiety Rating Scale (HAMA; Hamilton, 1959), Scale for Outcomes in PD for Autonomic Symptoms (SCOPA-AUT; Visser et al., 2004) and Non-Motor Symptoms Scale (NMSS; Chaudhuri et al., 2007).
Continuous variables were described as means and standard deviations (SD), and categorical variables were described as counts and percentages. Data comparing two groups were performed using Student’s t-test, Mann–Whitney U test or chi-squared test. The Bonferroni method was applied for multiple testing. The univariate correlation analysis was performed using the Spearman rank correlations. In order to screen the predictive factors of apathy in PD, factors with p < 0.10 in the univariate analysis were enrolled in the multivariate stepwise logistic regression analysis with two exceptions. Age and sex were also included since they have been previously associated with apathy (Pedersen et al., 2009, 2010; Pagonabarraga et al., 2015), and the exit criteria was set at p > 0.10 for regression analysis. Finally, five selected predictors were used to construct a logistic regression model and then integrated into the nomogram for predicting the occurrence of apathy in PD. The discrimination ability of the model was evaluated by the consistency index (C-index) and the area under the curve (AUC) of a receiver operating curve (ROC), which ranges from 0.5 (discrimination no better than chance) to 1 (perfect discrimination). The calibration of the prediction model was conducted using a calibration plot, which estimates how close the nomogram predicted risk is to the actual observed risk. Both internal validation and calibration were performed by bootstrapping with 1,000 resamples.
All statistical analyses were performed with SPSS (version 26.0), R software (version 4.2.0) and GraphPad Prism (version 8.0). Statistical significance was defined as a two-tailed p < 0.05.
The demographic and clinical characteristics are summarized in Table 1. Briefly, 201 patients aged 59.27 years (SD = 10.72; 102 males) were enrolled in the study. A total of 99 patients (49.25%) reported apathy, while 102 patients (50.75%) reported non-apathy. Compared with non-apathetic patients, apathetic participants had higher scores of the MDS-UPDRS Part II (p = 0.045). There was no significant difference in age, sex, education years, disease onset, disease duration, family history of PD, LEDD, Hoehn-Yahr stage or MDS-UPDRS Part III scores between these two groups.
Both TRF concentration (p < 0.0024) and TIBC levels (p < 0.0024) were lower in the apathetic group than in the non-apathetic group after Bonferroni correction (Table 2; Figure 1). No significant changes were observed in the levels of SI, SF, UIBC, TSAT or sTfR between the two groups. When stratified by gender, lower TRF concentration (r = −0.27, p = 0.010) and TIBC (r = −0.259, p = 0.014) were significantly associated with lower AS scores in male participants with PD (Figure 2). However, no correlations were not found for female patients (TRF, r = −0.109, p = 0.333; TIBC, r = −0.116, p = 0.302).
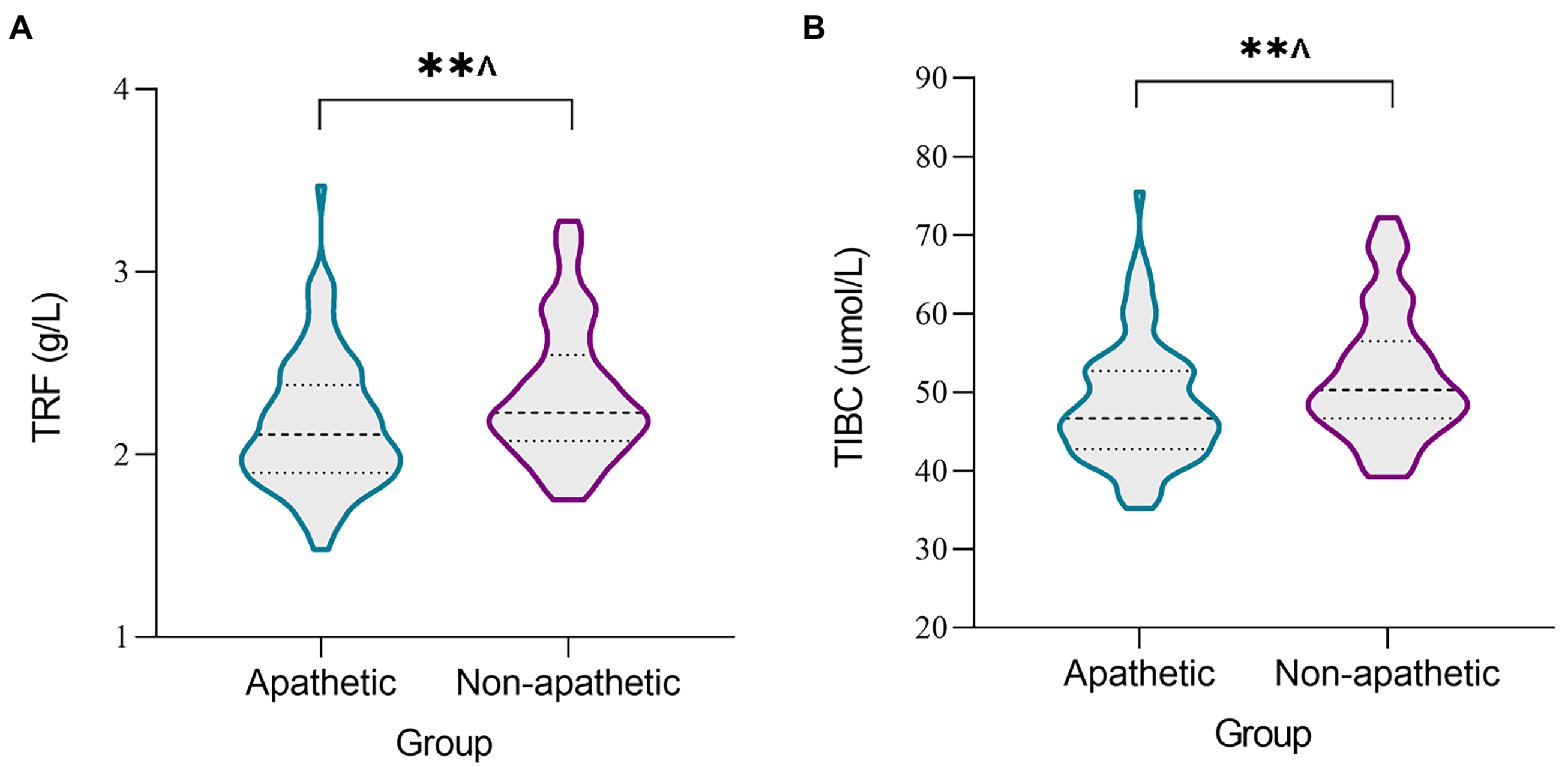
Figure 1. The levels of iron metabolism in serum between apathetic and non-apathetic groups. The serum concentrations of TRF (A) and TIBC (B) were lower in the apathetic group than those in the non-apathetic group. TIBC, total iron binding capacity; TRF, transferrin. **p < 0.01. ^The p-value was survived after Bonferroni correction (p < 0.05/21 = 0.0024).
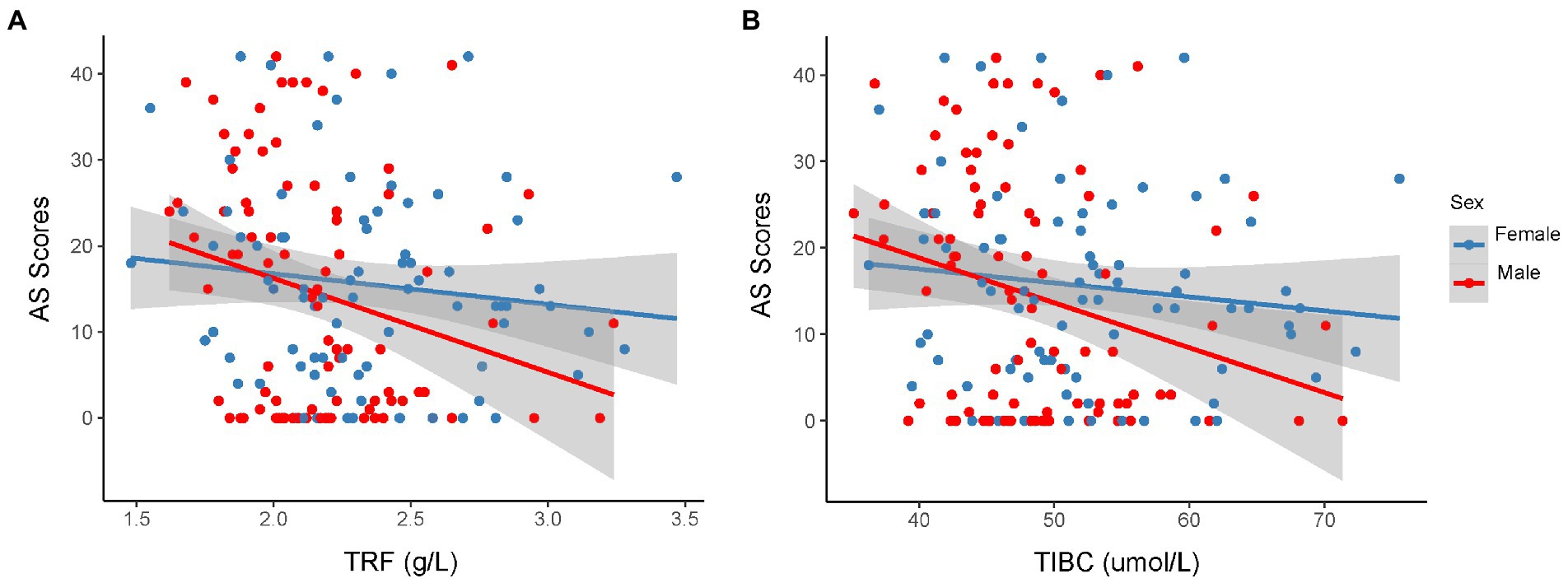
Figure 2. Correlation between AS scores and serum TRF (A) or TIBC (B) in the different sexual patients with PD. Spearman correlation showed a weak relationship between AS score and serum TRF concentration (r = −0.27, p = 0.010), TIBC (r = −0.259, p = 0.014) in male participants with PD. However, there were no significant associations between AS score and the level of TRF (r = −0.109, p = 0.333) or TIBC (r = −0.116, p = 0.302) for female patients. AS, Apathy Scale; PD, Parkinson’s disease; TIBC, total iron binding capacity; TRF, transferrin.
The mean (SD) AS scores for the apathetic and the non-apathetic patients were 25.21 (8.33) and 4.52 (4.57), respectively (p < 0.001). Overall, Patients with apathy showed more severe non-motor symptoms, including cognitive function, sleep disturbance, depression, anxiety and autonomic dysfunction (Table 1). Apathetic patients had significantly lower scores for the CM-MMSE (p = 0.039) and PDSS (p = 0.003), and higher scores for the HAMD, HAMA, SCOPA-AUT, and NMSS (all p < 0.05) compared to non-apathetic patients. For cognitive domains, apathetic patients showed poorer performance on executive function (p = 0.009), attention and calculation abilities (p = 0.030) than non-apathetic patients (Supplementary Table 1).
The correlation between other variables and AS scores are shown in Supplementary Table 2. The stepwise logistic regression analysis indicated that the concentration of SI (OR = 0.943, 95% CI = 0.884–1.007, p = 0.079), TIBC (OR = 0.935, 95% CI = 0.891–0.981, p = 0.006) and HAMD (OR = 1.165, 95% CI = 1.102–1.232, p < 0.001) scores can be identified as the risk factors for apathy in PD (Supplementary Table 3). After taking the influence of age and sex into account, the final logistic model incorporated five predictors (age, sex, SI concentration, TIBC, and HAMD scores) and was developed as a nomogram that is represented in Figure 3. For each patient, higher total points indicated a higher risk of apathy. The red line and red dots on the graph show an example of using the nomograph to predict the probability of apathy for a given patient with PD.
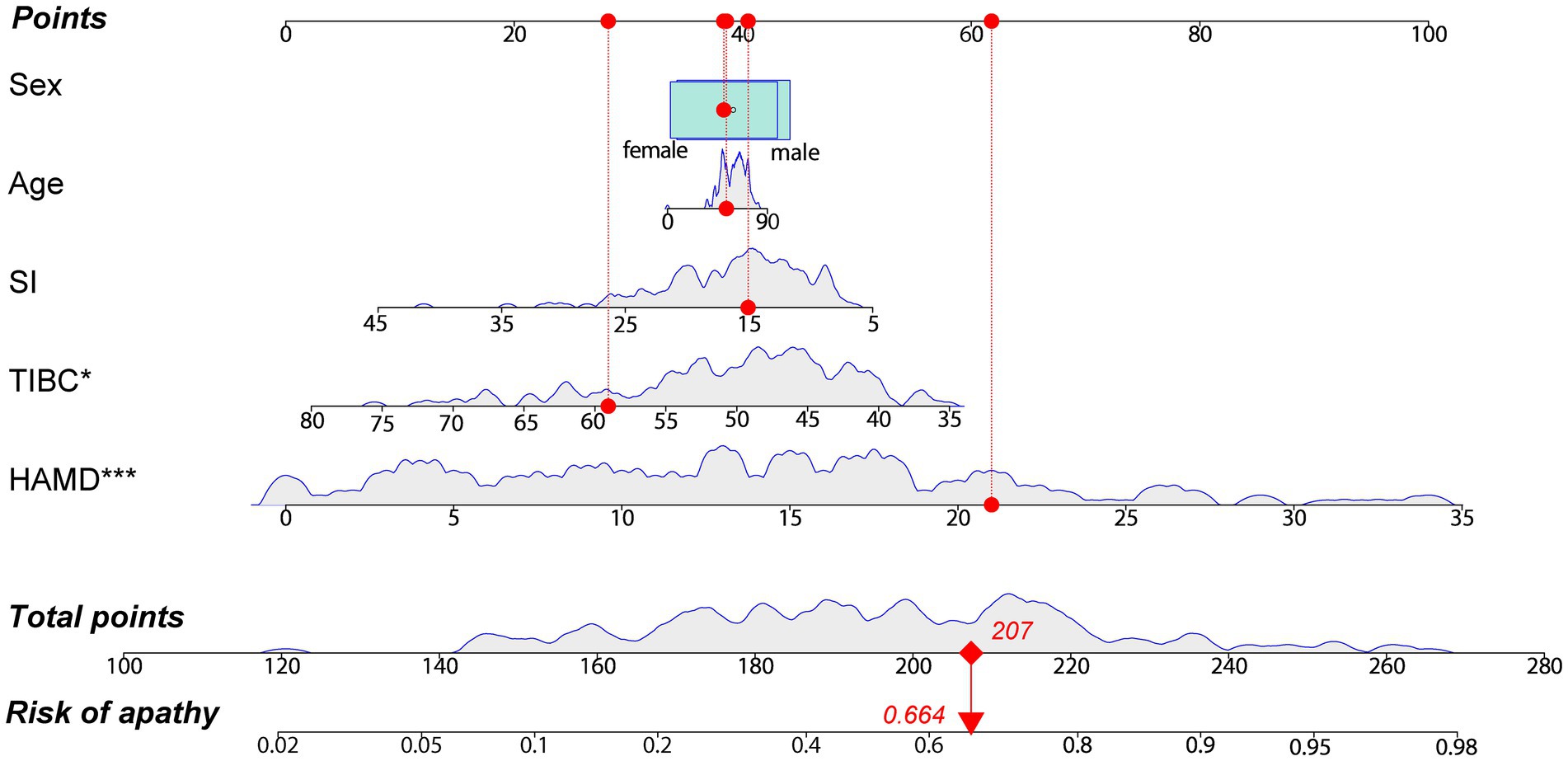
Figure 3. A constructed nomogram for the prediction of a patient with apathy. The nomogram was developed by incorporating the following five parameters: age (years), sex (male, female), SI concentration (μmol/L), TIBC (μmol/L) and HAMD scores. For each patient, a value of related variable is situated on the variable axis of the nomogram, and a vertical upward line determines the points calculated for each variable. Sum the points for each variable and draw a line from the total points axis to determine apathy probabilities. The red line and dots depict an example for predicting the probability of having apathy for a 53 years old female with PD, who has a SI level of 15.06 μmol/L, a TIBC level of 59.06 μmol/L and HAMD scores of 21. HAMD, Hamilton Depression Rating Scale; SI, serum iron; TIBC, total iron binding capacity. *p < 0.05, ***p < 0.001.
Furthermore, the internal validation was performed by using the bootstrap method with 1,000 resamples to validate the performance of the nomogram. And the calibration curve (Figure 4A) indicated a preferable consistency between the predicted model and the ideal model. The C-index was 0.799 (95% CI = 0.732–0.865), and the correspondent ROC curve and its AUC were provided in Figure 4B, reflecting a good discrimination ability of the nomogram.
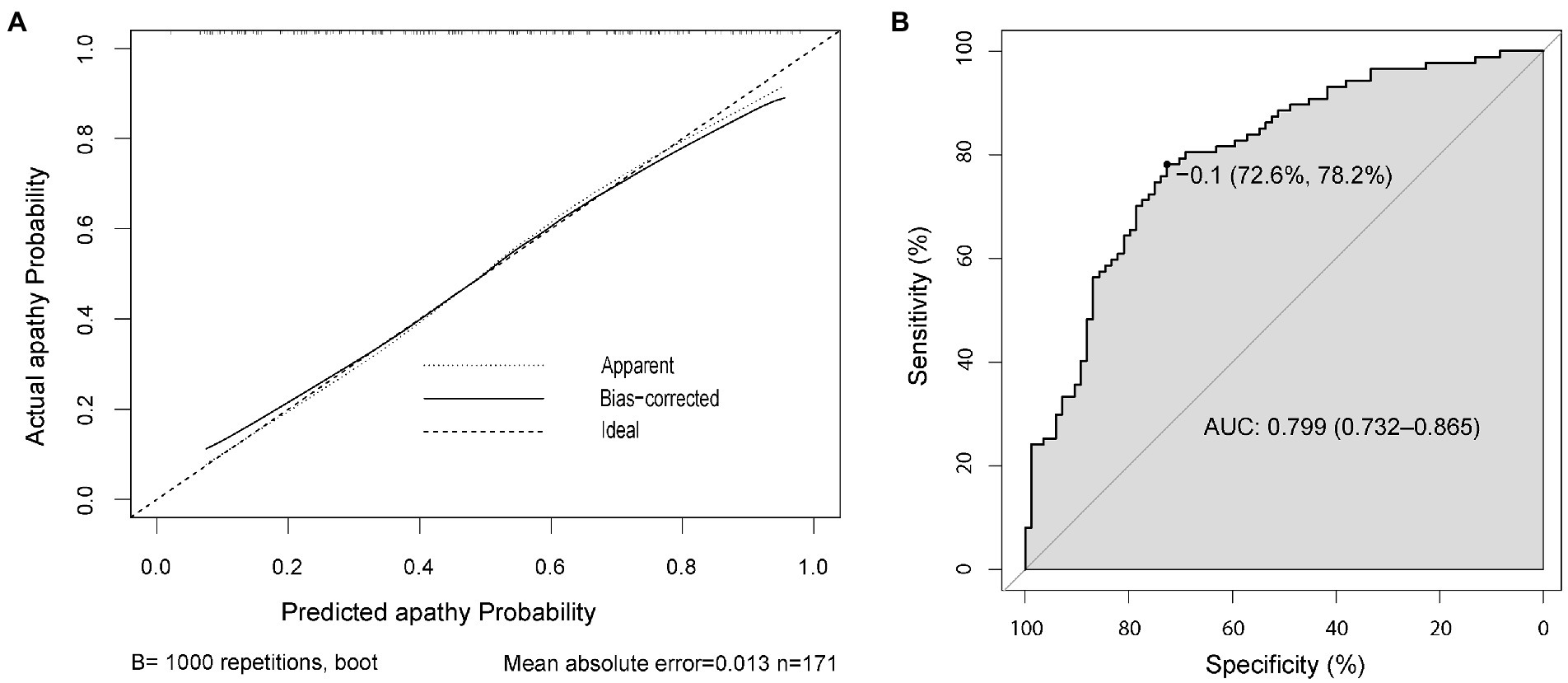
Figure 4. The calibration and accuracy of the nomogram. Calibration curve for predicting the probability of apathetic patients with PD (A). ROC curve of the predictive model showing an AUC of 0.799 (95% CI = 0.732–0.865) (B). AUC, area under the curve; CI, confidence interval; PD, Parkinson’s disease; ROC, receiver operating characteristic.
Apathy was common in our Chinese cohort, which is associated with more severe motor and non-motor symptoms. To the best of our knowledge, lower serum TRF levels and TIBC were observed in apathetic patients with PD for the first time, and both were negatively associated with AS scores in male patients with PD. Therefore, the disturbance of iron metabolism may contribute to apathy in PD, especially among male patients. More importantly, we developed an objective and practical nomogram to assist clinicians in screening apathetic patients with PD.
About 49.25% of the 201 patients were apathetic in our study, which is similar to the findings of a Chinese study on 145 patients assessed by AS (Wang et al., 2016). The subjective motor experiences of daily living in apathetic patients were impaired, implying that these patients exhibit a more severe neurodegenerative pattern progression (den Brok et al., 2015; Pagonabarraga et al., 2015). Also, dopamine ascending pathways were reported to play critical roles in the pathophysiology of apathy in PD (Levy and Czernecki, 2006).
We also found that apathy in PD was related to worse cognitive function, which is consistent with previous studies (Liu et al., 2017; Brown et al., 2019). Neuroimaging studies have demonstrated that cognitive decline in apathetic patients with PD is strongly linked with significant gray and white matter loss in the frontal lobe areas and the insular region (Alzahrani et al., 2016). In addition, apathetic patients with PD had more severe depression and anxiety, worse sleep quality and more severe autonomic dysfunction than non-apathetic patients. These aspects may probably account for the poorer quality of life experienced by apathetic compared to non-apathetic patients, as previously reported (Skorvanek et al., 2013).
As far as we known, the difference in serum TRF concentration or TIBC between PD participants with and without apathy has not been reported yet. TRF is a glycoprotein that circulates iron in a soluble, non-toxic form and delivers it to other body tissues (Gkouvatsos et al., 2012), including the brain (Moos et al., 2007). Another biomarker, TIBC, is regarded as a parameter to evaluate the maximal capacity of serum to transport iron, which is proportional to the concentration of TRF in serum (Kasvosve and Delanghe, 2002). Due to the decreased TRF concentration, a lack of iron load to transferrin may allow non-transferrin-bound iron enters the pool of labile iron (Ashraf et al., 2020), leading to enhanced harmful lipid peroxide production. It was considered one of the reasons contributing to the death of dopaminergic neurons in PD (Ma et al., 2021). Furthermore, similar research has also revealed that iron level in the CSF is significantly higher and positively correlated with·OH level in apathetic patients with PD. The authors also speculated excessive iron may be involved in apathy via oxidative stress (Wang et al., 2016). However, the association between central and peripheral iron in PD has not been fully elucidated (Costa-Mallen et al., 2017; Ashraf et al., 2020). Costa-Mallen et al. (2017) found decreased iron in circulation in favor of iron accumulation in the substantia nigra pars compacta (SNpc). In this investigation, levels of TRF and TIBC were significantly lower in apathetic patients than that in non-apathetic patients, based on which, we speculated that the decrease in serum TRF level in apathetic patients may result from the accumulation of iron in the brain, suggesting that disrupted iron metabolism may be involved in apathy in PD.
In male patients with PD, further analyses indicated that AS scores increased with decreased TRF concentration or TIBC. Recently, there has been increased interest in sex-related differences in PD or brain iron metabolism. An imaging study conducted on healthy individuals showed a selective lower total subcortical brain iron levels in middle-aged women compared to men and young women (Persson et al., 2015). Previous studies have also demonstrated the regulatory role of estrogen in brain iron metabolism (Sohrabji, 2007; Grubić Kezele and Ćurko-Cofek, 2020). Tzu-Yun et al. found that estradiol via different estrogen receptors could diminish the iron overload-induced autophagy and injury in female and male mice, respectively (Chen et al., 2019). Furthermore, the results by Wang and colleagues suggested that the striatum of male mice was more sensitive to iron accumulation than that of female mice. This trend was similar to those previously observed in clinical studies, which showed that men have a much higher risk of developing PD than women (Grubić Kezele and Ćurko-Cofek, 2020), and they are more likely to be apathetic (Pagonabarraga et al., 2015). Sex-associated differences are common among patients with PD, and we believe that this would be valuable for a future study.
The other particular strength of our study is that we developed a novel practical model to identify patients with PD who had a high risk of apathy. Based on standard deviation along the nomogram, both TIBC concentration and HAMD scores were important predictors, while age, sex and SI concentration do not appear to play a role in predicting apathy in this model. Our findings revealed that patients with a lower TIBC level were at a higher risk of apathy. Meanwhile, a trend towards decreased serum iron was found in apathetic patients although this difference did not reach statistical significance. Lower serum iron may also be linked with apathy. Patients with iron deficiency anemia could present with apathy (Bourre, 2006). And a study on psychiatric patients also found that oral iron treatment could bring about a reduction in apathy and the likelihood of resorting to psychiatric admission (Kassir, 2017). TRF delivers iron to the brain from the blood by binding to TRF receptors at the blood–brain barrier (Ma et al., 2021). As discussed previously, the relationship between peripheral and central iron in PD is still unclear and some points of view are contradictory. A previous study showed that lower serum iron in patients with PD may result from progressive iron deposition in the SNpc (Costa-Mallen et al., 2017). Therefore, we speculated that lower TIBC and the trend towards decreased serum iron in apathetic patients might provide indirect evidence for brain iron overload. And the specific mechanism may involve the regulation of TRF receptors expression on the cells of the blood–brain barrier (Ma et al., 2021). However, both iron deficiency in peripheral and central systems were simultaneously observed in patients with PD by Piao et al. (2017). In summary, the disturbance of iron metabolism is definitively involved in the pathogenesis of PD, and may also play a role in apathy although further investigation is required.
In addition to TIBC level, patients with severe depression were also more likely to be apathetic, which is consistent with the study from Brown et al. (2019). A 4-year prospective cohort study demonstrated that a high Hamilton Depression Rating Scale (HDRS) score was a predictor of apathy in PD (Ou et al., 2020), which may result from shared pathophysiological mechanisms. Maillet et al. found that the serotonergic alteration within the bilateral ventral striatum and the right caudate nucleus may play a role in apathy as well as depression (Maillet et al., 2016). Moreover, it is worth noting that we did not confuse apathy with depression, although there is a large overlap between apathy and depression (Pagonabarraga et al., 2015). The results from the nomogram should be viewed with caution, that means when patients with PD have both depression and iron metabolism abnormality, clinicians need to be alert to the possibility of apathy.
Since apathy is frequently associated with greater cognitive decline, more severe motor symptoms and heavier caregiver burden (Pagonabarraga et al., 2015), it is a need for earlier intervention. But there is no standard treatment for apathy. Acetylcholinesterase inhibitors and dopamine agonists were reported to improve apathy in PD (Seppi et al., 2019). Based on our study, we believed that maintaining iron homeostasis could be a potential therapeutic target for apathy in PD. Notably, the results should be interpreted with caution and there is much work needed to be carried out.
Several limitations of our study should be acknowledged. Firstly, this is a single-center study evaluating Chinese patients, which needs multicenter clinical datasets to perform the external validation of the nomogram. Secondly, further longitudinal studies are warranted to determine predictors of apathy. Thirdly, cognitive function of each participant was evaluated only by CM-MMSE, and more cognitive tests such as the Montreal Cognitive Assessment should be used in future studies. Finally, the analysis presented here has not incorporated radiological findings of patients, which will help to further explore the neural structures and levels of iron in the brain related to apathetic patients with PD.
In summary, apathy in PD is related to more severe motor and non-motor impairment. Lower serum TRF concentration and TIBC were first reported in apathetic patients with PD, and both were negatively associated with AS scores in male patients with PD. These findings suggest that abnormal iron metabolism may serve as an underlying mechanism of apathy in PD. TIBC level in combination with HAMD scores could be effectively used for the prediction of apathy in PD.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology. The patients/participants provided their written informed consent to participate in this study.
ZX, Y-jX, and J-tL contributed to study design. J-tL, YQ, H-lG, J-yL, Q-xQ, D-lW, and J-wZ contributed to data acquisition. J-tL, Z-jM, ZM, Y-jX, and ZX analyzed and extracted the data. J-tL, YQ, H-lG, Y-jX, and ZX wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (grant number 81771376).
We are grateful to patients for participating in this research and to the physicians and nurses for assisting with recruitment and clinical evaluations of the participants.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.1062964/full#supplementary-material
Alzahrani, H., Antonini, A., and Venneri, A. (2016). Apathy in mild Parkinson's disease:neuropsychological and neuroimaging evidence. J. Parkinsons Dis. 6, 821–832. doi: 10.3233/JPD-160809
Ashraf, A., Ashton, N. J., Chatterjee, P., Goozee, K., Shen, K., Fripp, J., et al. (2020). Plasma transferrinand hemopexin are associated with altered Aβ uptake and cognitive decline in Alzheimer's disease pathology. Alzheimers Res. Ther. 12:72. doi: 10.1186/s13195-020-00634-1
Bloem, B. R., Okun, M. S., and Klein, C. (2021). Parkinson's disease. Lancet 397, 2284–2303. doi: 10.1016/S0140-6736(21)00218-X
Bourre, J. M. (2006). Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 1: micronutrients. J. Nutr. Health Aging 10, 377–385. doi: 10.1063/1.4913145
Brown, D. S., Barrett, M. J., Flanigan, J. L., and Sperling, S. A. (2019). Clinical and demographic correlates of apathy in Parkinson's disease. J. Neurol. 266, 507–514. doi: 10.1007/s00415-018-9166-3
Chaudhuri, K. R., Martinez-Martin, P., Brown, R. G., Sethi, K., Stocchi, F., Odin, P., et al. (2007). The metric properties of a novel non-motor symptoms scale for Parkinson's disease: results from an international pilot study. Mov. Disord. 22, 1901–1911. doi: 10.1002/mds.21596
Chaudhuri, K. R., Pal, S., DiMarco, A., Whately-Smith, C., Bridgman, K., Mathew, R., et al. (2002). The Parkinson's disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 73, 629–635. doi: 10.1136/jnnp.73.6.629
Chen, T.-Y., Lin, C.-L., Wang, L.-F., Tsai, K.-L., Lin, J.-Y., and Hsu, C. (2019). Targeting GPER1 to suppress autophagy as a male-specific therapeutic strategy for iron-induced striatal injury. Sci. Rep. 9:6661. doi: 10.1038/s41598-019-43244-0
Costa-Mallen, P., Gatenby, C., Friend, S., Maravilla, K. R., Hu, S.-C., Cain, K. C., et al. (2017). Brain iron concentrations in regions of interest and relation with serum iron levels in Parkinson disease. J. Neurol. Sci. 378, 38–44. doi: 10.1016/j.jns.2017.04.035
den Brok, M. G. H. E., van Dalen, J. W., van Gool, W. A., Moll van Charante, E. P., de Bie, R. M. A., and Richard, E. (2015). Apathy in Parkinson's disease: a systematic review and meta-analysis. Mov. Disord. 30, 759–769. doi: 10.1002/mds.26208
Dexter, D. T., Wells, F. R., Lees, A. J., Agid, F., Agid, Y., Jenner, P., et al. (1989). Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson's disease. J. Neurochem. 52, 1830–1836. doi: 10.1111/j.1471-4159.1989.tb07264.x
Dujardin, K., Sockeel, P., Delliaux, M., Destée, A., and Defebvre, L. (2009). Apathy may herald cognitive decline and dementia in Parkinson's disease. Mov. Disord. 24, 2391–2397. doi: 10.1002/mds.22843
Eglit, G. M. L., Lopez, F., Schiehser, D. M., Pirogovsky-Turk, E., Litvan, I., Lessig, S., et al. (2021). Delineation of apathy subgroups in Parkinson's disease: differences in clinical presentation, functional ability, health-related quality of life, and caregiver burden. Mov. Disord. Clin. Pract. 8, 92–99. doi: 10.1002/mdc3.13127
Gkouvatsos, K., Papanikolaou, G., and Pantopoulos, K. (2012). Regulation of iron transport and the role of transferrin. Biochim. Biophys. Acta 1820, 188–202. doi: 10.1016/j.bbagen.2011.10.013
Goetz, C. G., Poewe, W., Rascol, O., Sampaio, C., Stebbins, G. T., Counsell, C., et al. (2004). Movement Disorder Society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov. Disord. 19, 1020–1028. doi: 10.1002/mds.20213
Goetz, C. G., Tilley, B. C., Shaftman, S. R., Stebbins, G. T., Fahn, S., Martinez-Martin, P., et al. (2008). Movement Disorder Society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170. doi: 10.1002/mds.22340
Grubić Kezele, T., and Ćurko-Cofek, B. (2020). Age-related changes and sex-related differences in brain iron metabolism. Nutrients 12:2601. doi: 10.3390/nu12092601
Hamilton, M. (1959). The assessment of anxiety states by rating. Br. J. Med. Psychol. 32, 50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x
Hamilton, M. (1960). A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. doi: 10.1136/jnnp.23.1.56
Hu, Y., Yu, S.-Y., Zuo, L.-J., Piao, Y.-S., Cao, C.-J., Wang, F., et al. (2015). Investigation on abnormal iron metabolism and related inflammation in Parkinson disease patients with probable RBD. PLoS One 10:e0138997. doi: 10.1371/journal.pone.0138997
Kassir, A. (2017). Iron deficiency: a diagnostic and therapeutic perspective in psychiatry. Encéphale 43, 85–89. doi: 10.1016/j.encep.2016.08.002
Kasvosve, I., and Delanghe, J. (2002). Total iron binding capacity and transferrin concentration in the assessment of iron status. Clin. Chem. Lab. Med. 40, 1014–1018. doi: 10.1515/CCLM.2002.176
Leentjens, A. F., Dujardin, K., Marsh, L., Martinez-Martin, P., Richard, I. H., Starkstein, S. E., et al. (2008). Apathy and anhedonia rating scales in Parkinson's disease: critique and recommendations. Mov. Disord. 23, 2004–2014. doi: 10.1002/mds.22229
Leroi, I., Harbishettar, V., Andrews, M., McDonald, K., Byrne, E. J., and Burns, A. (2012). Carer burden in apathy and impulse control disorders in Parkinson's disease. Int. J. Geriatr. Psychiatry 27, 160–166. doi: 10.1002/gps.2704
Levy, R., and Czernecki, V. (2006). Apathy and the basal ganglia. J. Neurol. 253 Suppl 7, VII54–VII61. doi: 10.1007/s00415-006-7012-5
Li, K., Liu, B., Wang, F., Bao, J., Wu, C., Huang, X., et al. (2019). Decreased serum ferritin may be associated with increased restless legs syndrome in Parkinson's disease (PD): a meta-analysis for the diagnosis of RLS in PD patients. Int. J. Neurosci. 129, 995–1003. doi: 10.1080/00207454.2019.1608200
Liu, H., Ou, R., Wei, Q., Hou, Y., Zhang, L., Cao, B., et al. (2017). Apathy in drug-naïve patients with Parkinson's disease. Parkinsonism Relat. Disord. 44, 28–32. doi: 10.1016/j.parkreldis.2017.08.008
Ma, L., Gholam Azad, M., Dharmasivam, M., Richardson, V., Quinn, R. J., Feng, Y., et al. (2021). Parkinson's disease: alterations in iron and redox biology as a key to unlock therapeutic strategies. Redox Biol. 41:101896. doi: 10.1016/j.redox.2021.101896
Maillet, A., Krack, P., Lhommée, E., Météreau, E., Klinger, H., Favre, E., et al. (2016). The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson's disease. Brain J. Neurol. 139, 2486–2502. doi: 10.1093/brain/aww162
Marin, R. S. (1991). Apathy: a neuropsychiatric syndrome. J. Neuropsychiatry Clin. Neurosci. 3, 243–254. doi: 10.1176/jnp.3.3.243
Moos, T., Rosengren Nielsen, T., Skjørringe, T., and Morgan, E. H. (2007). Iron trafficking inside the brain. J. Neurochem. 103, 1730–1740. doi: 10.1111/j.1471-4159.2007.04976.x
Ou, R., Lin, J., Liu, K., Jiang, Z., Wei, Q., Hou, Y., et al. (2020). Evolution of apathy in early Parkinson's disease: a 4-years prospective cohort study. Front. Aging Neurosci. 12:620762. doi: 10.3389/fnagi.2020.620762
Pagonabarraga, J., Kulisevsky, J., Strafella, A. P., and Krack, P. (2015). Apathy in Parkinson's disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 14, 518–531. doi: 10.1016/S1474-4422(15)00019-8
Pedersen, K. F., Alves, G., Brønnick, K., Aarsland, D., Tysnes, O.-B., and Larsen, J. P. (2010). Apathy in drug-naïve patients with incident Parkinson's disease: the Norwegian ParkWest study. J. Neurol. 257, 217–223. doi: 10.1007/s00415-009-5297-x
Pedersen, K. F., Larsen, J. P., Alves, G., and Aarsland, D. (2009). Prevalence and clinical correlates of apathy in Parkinson's disease: a community-based study. Parkinsonism Relat. Disord. 15, 295–299. doi: 10.1016/j.parkreldis.2008.07.006
Persson, N., Wu, J., Zhang, Q., Liu, T., Shen, J., Bao, R., et al. (2015). Age and sex related differences in subcortical brain iron concentrations among healthy adults. NeuroImage 122, 385–398. doi: 10.1016/j.neuroimage.2015.07.050
Piao, Y.-S., Lian, T.-H., Hu, Y., Zuo, L.-J., Guo, P., Yu, S.-Y., et al. (2017). Restless legs syndrome in Parkinson disease: clinical characteristics, abnormal iron metabolism and altered neurotransmitters. Sci. Rep. 7:10547. doi: 10.1038/s41598-017-10593-7
Postuma, R. B., Berg, D., Stern, M., Poewe, W., Olanow, C. W., Oertel, W., et al. (2015). MDS clinical diagnostic criteria for Parkinson's disease. Mov. Disord. 30, 1591–1601. doi: 10.1002/mds.26424
Riederer, P., Sofic, E., Rausch, W. D., Schmidt, B., Reynolds, G. P., Jellinger, K., et al. (1989). Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J. Neurochem. 52, 515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x
Seppi, K., Ray Chaudhuri, K., Coelho, M., Fox, S. H., Katzenschlager, R., Perez Lloret, S., et al. (2019). Update on treatments for nonmotor symptoms of Parkinson's disease-an evidence-based medicine review. Mov. Disord. 34, 180–198. doi: 10.1002/mds.27602
Skorvanek, M., Rosenberger, J., Gdovinova, Z., Nagyova, I., Saeedian, R. G., Groothoff, J. W., et al. (2013). Apathy in elderly nondemented patients with Parkinson's disease: clinical determinants and relationship to quality of life. J. Geriatr. Psychiatry Neurol. 26, 237–243. doi: 10.1177/0891988713500587
Sohrabji, F. (2007). Guarding the blood-brain barrier: a role for estrogen in the etiology of neurodegenerative disease. Gene Expr. 13, 311–319. doi: 10.3727/000000006781510723
Starkstein, S. E., Mayberg, H. S., Preziosi, T. J., Andrezejewski, P., Leiguarda, R., and Robinson, R. G. (1992). Reliability, validity, and clinical correlates of apathy in Parkinson's disease. J. Neuropsychiatry Clin. Neurosci. 4, 134–139. doi: 10.1176/jnp.4.2.134
Tomlinson, C. L., Stowe, R., Patel, S., Rick, C., Gray, R., and Clarke, C. E. (2010). Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov. Disord. 25, 2649–2653. doi: 10.1002/mds.23429
Visser, M., Marinus, J., Stiggelbout, A. M., and Van Hilten, J. J. (2004). Assessment of autonomic dysfunction in Parkinson's disease: the SCOPA-AUT. Mov. Disord. 19, 1306–1312. doi: 10.1002/mds.20153
Wang, F., Yu, S.-Y., Zuo, L.-J., Cao, C.-J., Hu, Y., Chen, Z.-J., et al. (2016). Excessive iron and α-Synuclein oligomer in brain are relevant to pure apathy in Parkinson disease. J. Geriatr. Psychiatry Neurol. 29, 187–194. doi: 10.1177/0891988716632918
Ward, R. J., Zucca, F. A., Duyn, J. H., Crichton, R. R., and Zecca, L. (2014). The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 13, 1045–1060. doi: 10.1016/S1474-4422(14)70117-6
Xu, G., Meyer, J. S., Huang, Y., Du, F., Chowdhury, M., and Quach, M. (2003). Adapting mini-mental state examination for dementia screening among illiterate or minimally educated elderly Chinese. Int. J. Geriatr. Psychiatry 18, 609–616. doi: 10.1002/gps.890
Keywords: Parkinson’s disease, apathy, iron metabolism, transferrin, nomogram
Citation: Li J-t, Qu Y, Gao H-l, Li J-y, Qin Q-x, Wang D-l, Zhao J-w, Mao Z-j, Min Z, Xiong Y-j and Xue Z (2023) A nomogram based on iron metabolism can help identify apathy in patients with Parkinson’s disease. Front. Aging Neurosci. 14:1062964. doi: 10.3389/fnagi.2022.1062964
Received: 06 October 2022; Accepted: 23 December 2022;
Published: 19 January 2023.
Edited by:
Xiaoya Gao, Southern Medical University, ChinaReviewed by:
Anubhuti Dixit, Amity University, IndiaCopyright © 2023 Li, Qu, Gao, Li, Qin, Wang, Zhao, Mao, Min, Xiong and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-jie Xiong,  eW9uZ2ppZS54aW9uZ0Bmb3htYWlsLmNvbQ==; Zheng Xue,
eW9uZ2ppZS54aW9uZ0Bmb3htYWlsLmNvbQ==; Zheng Xue,  eHVlemhlbmdAaHVzdC5lZHUuY24=
eHVlemhlbmdAaHVzdC5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.