- 1Graduate School, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2Nursing Department, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 3Clinical College of Neurology, Neurosurgery and Neurorehabilitation, Tianjin Medical University, Tianjin, China
- 4Department of Nursing, Tianjin Huanhu Hospital, Tianjin, China
Background: Depression in Parkinson's disease (PD) is a major health concern worldwide. Recently, an increasing number of non-pharmacological interventions have been used in PD to alleviate depressive symptoms. However, it is uncertain which intervention is the best, and related evidence is limited. This network meta-analysis was performed to compare and rank non-pharmacological interventions for PD and analyze their effects on depression to provide evidence for clinicians to choose appropriate non-pharmacological management options.
Methods: The PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), PsycINFO, China National Knowledge Infrastructure (CNKI), and Wanfang databases were searched from inception to April 7, 2022. Two authors screened all studies, extracted the data, and evaluated the methodological quality. STATA software version 16.0 was used to conduct the network meta-analysis.
Results: Our network meta-analysis included 62 studies involving 3,050 participants and 35 non-pharmacological interventions. Although most non-pharmacological interventions showed non-significant effects, the surface under the cumulative ranking curve (SUCRA) values indicated that the best non-pharmacological intervention for depression was dance (82.3%), followed by LSVT-BIG therapy (77.4%), and CBT (73.6%).
Conclusion: Dance can be considered as an effective therapy for improving depression in patients with PD. In the future, more strictly designed trials are needed to verify the conclusions of this network meta-analysis.
Introduction
Parkinson's disease (PD), the second most common neurodegenerative disease, is a chronic senile disease (Hirtz et al., 2007). The prevalence of PD increases with age and affects 1% of individuals older than 60 years (Tysnes and Storstein, 2017). PD is characterized by dyskinesia; however, the non-motor symptoms (NMS) of PD have gradually attracted more attention from researchers over the past 10–20 years (Garcia-Ruiz et al., 2014). Neuropsychiatric disturbances and cognitive impairment are the main features of NMS in PD patients (Zhang et al., 2020), while depression is the most common psychiatric symptom. The prevalence of clinically significant depression in patients with PD is reported to be 40–50% (Reijnders et al., 2008). Depression often increases the incidence of disability and dysfunction in PD patients; in addition, it can affect patients' quality of life and the burdens of their caregivers. Moreover, a study by Wu et al. indicated that PD patients with depression had a higher incidence of dementia (Wu et al., 2018). Therefore, early detection and appropriate intervention are extremely important. Currently, the clinical management of depressive disorders in PD includes pharmacological and non-pharmacological treatments. However, studies on the tolerability, safety, and efficacy of antidepressant drugs in PD patients are limited (Assogna et al., 2020). Additionally, pharmacological treatments with side effects can exacerbate the motor symptoms and NMS of PD patients and lead to complications (Uhrbrand et al., 2015; Deuel and Seeberger, 2020).
Owing to the limitations of pharmacological treatments, non-pharmacological treatments have been developed and have gradually gained popularity. Many non-pharmacological treatments have been used to relieve depressive symptoms in patients with PD, and these can be roughly categorized into complementary therapies (e.g., yoga, acupuncture, auricular pressure, massage, music therapy, and dance therapy), traditional Chinese exercises (e.g., tai chi and qigong), physical exercise (e.g., aerobic exercise, resistance exercise, and balance training), virtual reality, cognitive behavioral therapy (CBT), psychotherapy, cognitive training (CT), bright light therapy (BLT), deep brain stimulation (DBS), transcranial magnetic stimulation (TMS), and transcranial direct current stimulation (tDCS) (Jin et al., 2019; Zhang et al., 2019; Deuel and Seeberger, 2020; Triegaardt et al., 2020; Chen et al., 2021; Hong et al., 2021; Huang et al., 2021). Previous studies (Troeung et al., 2014; Ryan et al., 2019; Assogna et al., 2020) have paid more attention to the effects of CBT and TMS on depression in PD patients and indicated that these two therapies are supportive for improving depression. The efficacy of other non-pharmacological treatments for depression in patients with PD remains controversial.
Previous systematic reviews have evaluated the effects of various non-pharmacological interventions on depression in individuals with PD (Cusso et al., 2016; Jin et al., 2019; Hai-Jiao et al., 2020; Triegaardt et al., 2020; Cartmill et al., 2021; Hong et al., 2021; Huang et al., 2021; Takamiya et al., 2021). However, some reviews included non-randomized controlled trials (RCTs) or lacked quantitative analyses (Cusso et al., 2016; Triegaardt et al., 2020; Cartmill et al., 2021; Takamiya et al., 2021); thus, these systematic reviews did not provide strong evidence. In addition, most RCTs compared non-pharmacological interventions with placebo, waiting list, or usual treatments, and only a few RCTs compared two different non-pharmacological interventions (Modugno et al., 2010; Kalbe et al., 2020; Schmidt et al., 2021). To our knowledge, only one systematic review has reported the efficacy of non-pharmacological interventions on depression in PD subjects (Chen et al., 2021). In that study, a population with idiopathic PD was selected, and the interventions included repetitive TMS (rTMS) and CBT. The review also included limited interventions and small sample sizes and excluded some patients with PD. Consequently, a systematic review evaluating the effects of different non-pharmacological interventions and the exploration of more effective interventions is required.
Network meta-analysis (NMA) is a general technique for comparing several interventions simultaneously (e.g., A vs. B, B vs. C) (Lu and Ades, 2004). NMA can compare multiple interventions by incorporating direct and indirect comparisons to select the best intervention based on the relative effects of different interventions from a network of evidence (Catalá-López et al., 2014). Therefore, we performed this systematic review with a NMA of RCTs to provide further evidence to clinicians when choosing appropriate non-pharmacological management options.
Methods
Search strategy
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension statement for reporting systematic reviews incorporating network meta-analyses of health care interventions (Hutton et al., 2015), we searched for randomized controlled trials (RCTs) from inception to April 7, 2022 in the following databases: PubMed, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), PsycINFO, China National Knowledge Infrastructure (CNKI), and Wanfang. A combination of Medical Subject Headings (MeSH terms or Emtree terms) and free words related to PD, non-pharmacological interventions, depression, and RCTs was used, including: (1) Parkinson's Disease, Parkinson, Idiopathic Parkinson's Disease, Lewy Body Parkinson's Disease, Parkinson's Disease, Idiopathic Parkinson's Disease, Lewy Body Parkinson's Disease, Primary Parkinsonism, Paralysis Agitans; (2) TMS, tDCS, DBS, tai chi, qigong, acupuncture, massage, song, music, dance, aromatherapy, moxibustion, exercise, CBT, psychotherapy, cognitive training, electroconvulsive therapy, transcranial magnetic stimulation, transcranial Direct Current Stimulation, cognitive behavior training, deep brain stimulation, treatment, intervention, therapy, management, rehabilitation, non-pharmacolog*, non-pharmacological; (3) depression, depressive symptoms, depressive symptom, emotional depression, depress*, central depression, clinical depression, depressive disease, depressive disorder, depressive episode, depressive illness, depressive personality disorder, depressive state, depressive syndrome, mental depression, and parental depression; and (4) randomized controlled trial, randomized, placebo. MeSH and free words were linked by “OR” in each group and searched by “AND” to link each group. In addition, we retrieved data from the U.S. National Library of Medicine Clinical Trial Registry Platform and the Chinese Clinical Trial Registry Platform for trials in progress or ready for publication. The gray literature was also considered in the search. The reference lists of the included literature and related articles were also manually searched to identify eligible studies. The search strategies for all databases are listed in Supplementary Data Sheet 1.
Eligible criteria
Eligible studies met the following criteria:
(1) Population: Adults (>18 years) diagnosed with PD according to sex, Hoehn and Yahr stage, or disease duration with no restrictions. All participants in the intervention and control groups who were stably taking antidepressants and/or anti-parkinsonian medications were also eligible.
(2) Intervention: Participants in the experimental groups received non-pharmacological interventions with no limits in frequency, duration, style, period, form, or setting.
(3) Comparison: Participants in the control groups received placebo, waiting list, or treatment as usual (TAU) options including usual care, treatment, supportive instruction, or other non-pharmacological interventions that differed from the experimental group. In our study, supportive instruction refers to simple and common advice and tests (e.g., health education, beneficial advice, “classic” game mode) provided to subjects in the control group that differ from professional psychotherapy or psychological instructions. However, original trials comparing only different approaches of the same intervention were excluded.
(4) Outcome: The primary outcome was depression, as assessed by applying validated scales.
(5) Study type: RCTs were included with no limitations in terms of language, country, and type of article (conference papers, abstracts, master theses/doctoral dissertations, and study protocols were permissible; however, reviews were excluded).
Data extraction and quality assessment
Two authors (YW, XS) independently extracted data including the first author, country, year, sample size, baseline characteristics of participants, duration of disease, Hoehn–Yahr stage, intervention details (type, frequency, and duration), comparison, and outcomes based on a predesigned form within Microsoft Excel. The methodological quality of the eligible studies was independently assessed using the Cochrane risk of bias (RoB 2.0) tool (Sterne et al., 2019) by two authors (YW, XS). The scale consists of five domains: the randomization process, deviations from the intended interventions, missing outcome data, outcome measurement, and selection of the reported result. In terms of the domain algorithm, the risk of bias for each domain was rated as low risk, some concerns, or high risk. If the assessment of risk bias in all domains was “low risk,” then the overall risk bias was considered as “low risk”; if the assessment of risk bias indicated “some concerns” in some domains and there was no “high risk” result in any domain, the overall risk bias was “some concerns”; if the assessed risk of bias was “high risk” in at least one domain, the overall risk bias was “high risk.” The results of the data extraction and quality assessment were cross-checked, and the divergences were resolved through discussion with a third author (YJ). To comprehensively compare the effects of non-pharmacological interventions on depression in PD subjects, we did not exclude medium- and low-quality studies.
Statistical analysis
The network analysis was conducted using STATA 16.0 (StataCorp, College Station, TX) and a frequentist framework with a random-effects model. For all eligible trials, post-intervention measurements were selected for comparison. Continuous variables were analyzed using standardized mean differences (SMD) with 95% percentile intervals, and the significance was set at α = 0.05. We examined the global consistency and used the node-split model to determine the local consistency. P > 0.05 indicated no significant inconsistency between direct and indirect comparisons, and in these cases, the consistency model was adopted; otherwise, the inconsistency model was used. In addition, the inconsistency of closed loops was evaluated using a loop-specific method, and a 95% confidence interval (CI) of 0 indicated no significant loop inconsistency. Each arm was included for comparison, and to discriminate the consistency between the two-arm and three-arm trials, a league table was used to perform the pairwise analysis. The league table was used to analyze the results of the comparisons among the different interventions based on a NMA. To explore the best evidence for improving depression, the SUCRA was used to summarize the ranking probability values. Funnel plots were used to visually evaluate publication bias based on the symmetry criterion (Shim et al., 2017).
Results
Study selection
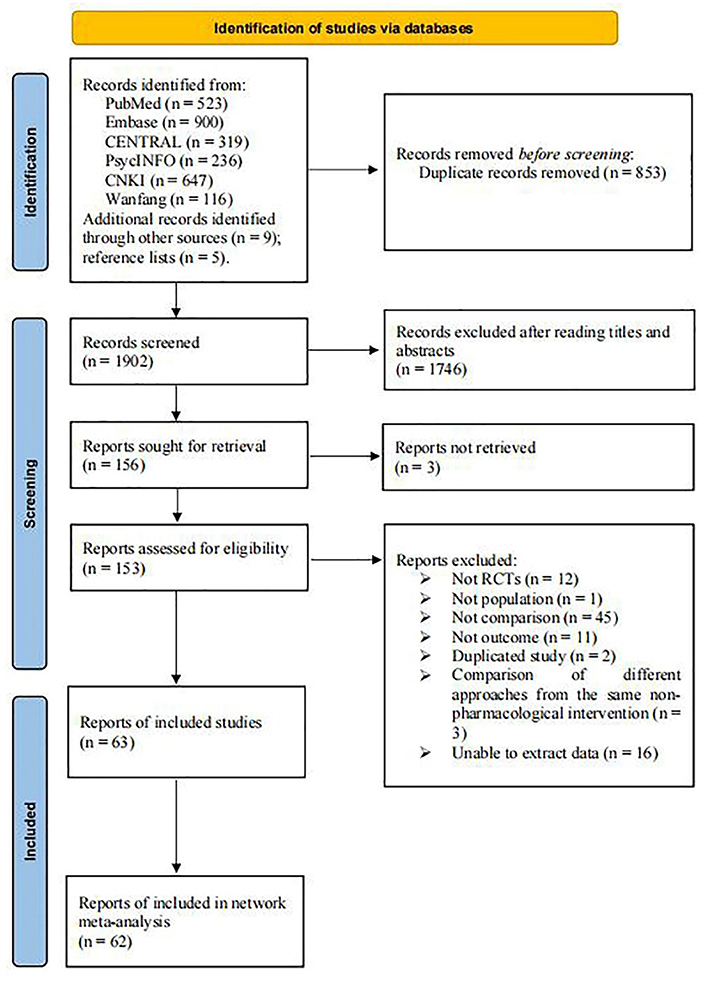
A total of 2,755 studies were identified from six databases, while 9 studies were selected from other sources. Five trials were retrieved by screening the reference lists of the included studies and related articles. After removing 853 duplicates, 1,746 articles were excluded after screening their titles and abstracts. Subsequently, 156 articles were retrieved, 153 full-text articles were evaluated, and 63 were included in the systematic review. However, one study did not meet the quantitative analysis criteria of the NMA (Manenti et al., 2018); thus, only 62 eligible RCTs were included in the NMA. Figure 1 demonstrates the process of the literature search and study selection.
Study characteristics
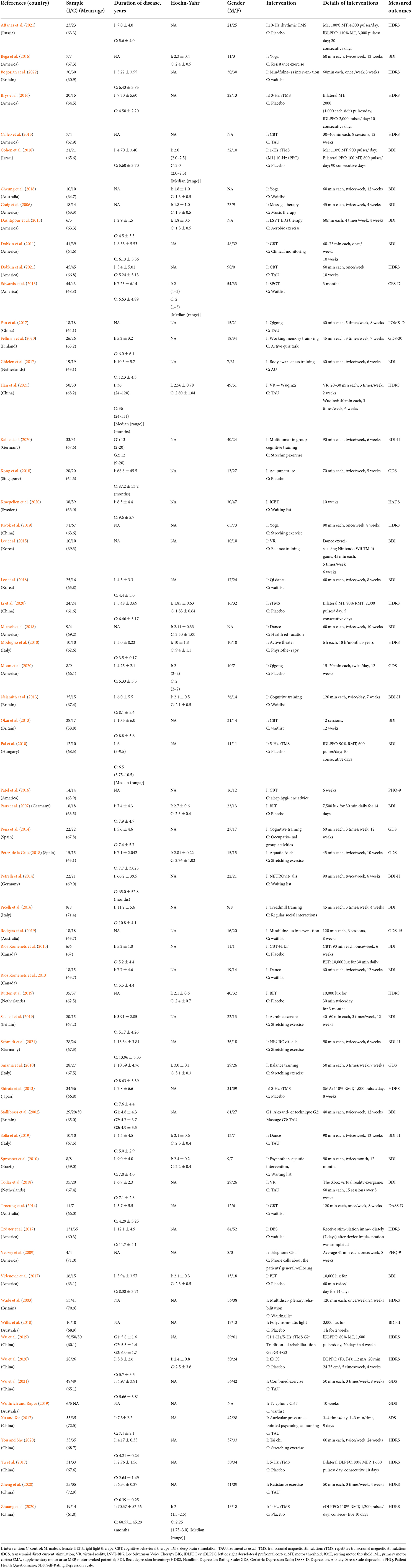
Table 1 shows the characteristics of 62 eligible RCTs (Chinese = 6, English = 56) published from 2002 to 2021 and involving 3,050 participants. In this NMA, non-pharmacological interventions included a variety of interventions such as BLT (n = 4), CBT (n = 9), dance (n = 3), massage (n = 3), music therapy (n = 1), DBS (n = 1), aerobic exercise (n = 2), resistance exercise (n = 5), balance training (n = 2), mindfulness intervention (n = 2), TMS (n = 9) including rTMS and rhythmic TMS, traditional Chinese exercise (TCE) (n = 5) including Qigong and Tai chi, tDCS (n = 1), virtual reality (VR) (n = 2), yoga (n = 3), CT (n = 7), psychotherapy (n = 3), acupuncture (n = 1), multidisciplinary rehabilitation (n = 1), and auricular pressure (n = 1). The comparison mainly consisted of placebo, waitlist, TAU including usual care and usual treatment, stretching exercises, supportive instruction (e.g., health education, sleep hygiene advice, regular social interactions, and active testing), and physiotherapy. Among all eligible studies, 60 RCTs were two-arm trials (Wade et al., 2003; Craig et al., 2006; Paus et al., 2007; Veazey et al., 2009; Modugno et al., 2010; Pal et al., 2010; Smania et al., 2010; Sproesser et al., 2010; Dobkin et al., 2011, 2021; Edwards et al., 2013; Naismith et al., 2013; Okai et al., 2013; Rios Romenets et al., 2013, 2015; Shirota et al., 2013; Peña et al., 2014; Petrelli et al., 2014; Troeung et al., 2014; Calleo et al., 2015; Dashtipour et al., 2015; Lee et al., 2015, 2018; Bega et al., 2016; Brys et al., 2016; Patel et al., 2016; Picelli et al., 2016; Fan et al., 2017; Ghielen et al., 2017; Tröster et al., 2017; Videnovic et al., 2017; Xu and Xia, 2017; Yu et al., 2017; Cheung et al., 2018; Cohen et al., 2018; Kong et al., 2018; Michels et al., 2018; Pérez-de la Cruz, 2018; Tollár et al., 2018; Willis et al., 2018; Kwok et al., 2019; Rodgers et al., 2019; Rutten et al., 2019; Sacheli et al., 2019; Solla et al., 2019; Wuthrich and Rapee, 2019; Fellman et al., 2020; Kalbe et al., 2020; Kraepelien et al., 2020; Li et al., 2020; Moon et al., 2020; Wu et al., 2020, 2021; You and She, 2020; Zheng et al., 2020; Zhuang et al., 2020; Aftanas et al., 2021; Han et al., 2021; Schmidt et al., 2021; Bogosian et al., 2022) and 2 RCTs (Stallibrass et al., 2002; Wu et al., 2019) were multi-arm trials.
Quality assessment
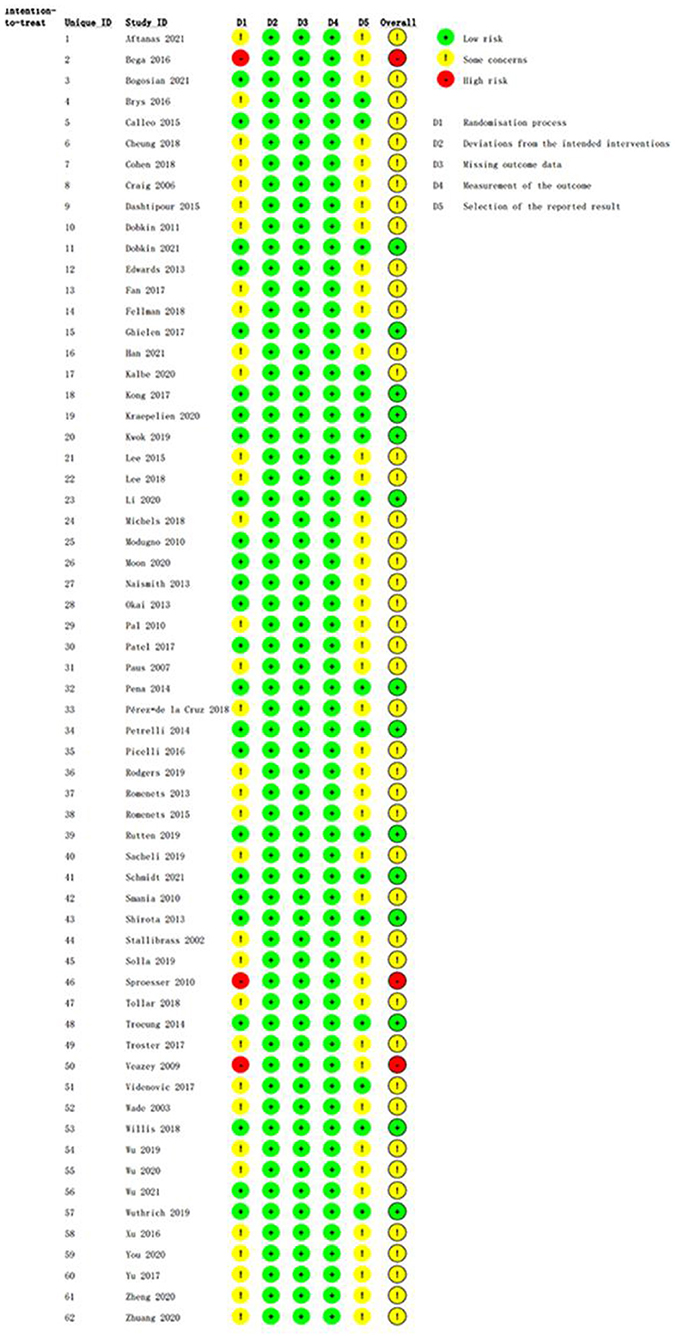
The methodological quality assessments of the eligible RCTs are shown in Figure 2, ranging from low to high risk. All eligible RCTs met the criteria for deviation from the intended interventions, missing outcome data, and outcome measurements. Three trials had obvious flaws in the domain of randomization; thus, their risk of bias was rated as high. Forty-five RCTs were rated as “some concerns” due to weaknesses in the randomization process or selection of the reported result domains.
Network meta-analysis
Figure 3 shows the network map of the different non-pharmacological interventions for depression and indicates that comparisons among CBT, TMS, BLT, and CT were common. An NMA was conducted to compare the effects of different interventions on depression in PD patients. Supplementary Table S1 shows the relative effects of the different interventions on depression. The league table shows the pairwise comparisons of 35 non-pharmacological interventions for depression in PD subjects. Compared to occupational group activities, dance (SMD: −3.23; 95% CI: −6.05–−0.41), LSVT-BIG therapy (SMD: −3.36; 95% CI: −6.27–−0.45), CBT (SMD: −2.76; 95% CI: −5.31–−0.21), aerobic exercise (SMD: −2.69; 95% CI: −5.07–−0.30), mindfulness intervention (SMD: −2.31; 95% CI: −4.06–−0.56), TCE (SMD: −2.14; 95% CI: −4.10–−0.18), DBS (SMD: −2.17; 95% CI: −4.07–−0.27), CT (SMD: −1.85; 95% CI: −3.16–−0.53), waitlist (SMD: −1.76; 95% CI: −3.26–−0.26), and physiotherapy (SMD: −1.72; 95% CI: −3.29–−0.14) all showed significant improvement effects on depression. Compared to stretching exercises, dance (SMD: −2.67; 95% CI: −4.91–−0.44), CBT (SMD: −2.2; 95% CI: −4.09–−0.31), and TCE (SMD: −1.58; 95% CI: −2.52–−0.63) showed significant improvement effects on depression. Compared to TAU, dance (SMD: −1.70; 95% CI: −3.25–−0.14) and CBT (SMD: −1.23; 95% CI: −2.22–−0.23) had significantly positive effects.
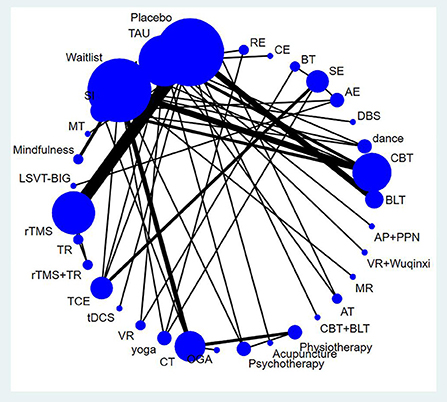
Figure 3. Network map for depression. AE, Aerobic exercise; AP+PPN, Auricular pressure and pointed psychological nursing; AT, Alexander technique; BLT, Bright light therapy; BT, Balance training; CBT, Cognitive behavior therapy; CBT + BLT, Cognitive behavioral therapy and bright light therapy; CE, Combined exercise; CM, Clinical mo- nitoring; CT, Cognitive training; DBS, Deep brain stimulation; MR, Multidisciplenary rehabilitation; MT, music therapy; OGA, Occupational group activities; RE, resistance exercise; SE, Stretching exerc-ise; SI, Supportive instruction; TAU, Treatment as usual; TCE, Traditional Chinese exercise; TMS, Transcranial magnetic stimulation; TR, Traditional rehabilitation; VR, Virtual reality; rTMS, repetitive tra- nscranial magnetic stimulation; tDCS, Transcranial direct current stimulation.
Rank probability
The SUCRA plot and values are shown in Figure 4 and Table 2, respectively. The SUCRA values and the plot revealed that the treatments' comparative efficacy in improving depression was, in order: dance >LSVT-BIG>CBT>rTMS + traditional rehabilitation>aerobic exercise>clinical monitoring>VR + Wuqinxi>tDCS>resistance exercise>mindfullness>auricular pressure and pointed psychological nursing>TMS>TCE>music therapy>acupuncture>DBS>BLT>yoga>multidisciplinary rehabilitation>massage>cognitive training>placebo>the alexander technique>CBT and BLT>combined exercise>VR>waitlist>physiotherapy>psychotherapy>traditional rehabilitation>TAU>supportive instruction>balance training>stretching exercise>occupational group activities.

Figure 4. The SUCRA plot based on cumulative probabilities of interventions. AE, Aerobic exercise; AP+PPN, Auricular pressure and pointed psychological nursing; AT, Alexander technique; BLT, Bright light therapy; BT, Balance training; CBT, Cognitive behavior therapy; CBT + BLT, Cognitive behavioral therapy and bright light therapy; CE, Combined exercise; CM, Clinical mo- nitoring; CT, Cognitive training; DBS, Deep brain stimulation; MR, Multidisciplenary rehabilitation; MT, music therapy; OGA, Occupational group activities; RE, resistance exercise; SE, Stretching exerc-ise; SI, Supportive instruction; TAU, Treatment as usual; TCE, Traditional Chinese exercise; TMS, Transcranial magnetic stimulation; TR, Traditional rehabilitation; VR, Virtual reality; rTMS, repetitive tra- nscranial magnetic stimulation; tDCS, Transcranial direct current stimulation.
Consistency analysis
The global inconsistency analysis of this NMA showed a P-value of 0.0038, indicating significant inconsistency. Moreover, the results of the node-splitting analysis indicated that the four indirect comparisons had inconsistencies (P < 0.05). The results are summarized in Supplementary Table S2. Therefore, we used an inconsistency model to perform the NMA. Considering that two triangular loops were formed by the multi-arm trials, I2 quadratic loop consistencies were examined if they were inconsistent. The 95% CI of the four closed loops included 0, indicating significant inconsistency (Figure 5). However, the lower CI was close to 0, which indicated low consistency. Although the NMA revealed an obvious overall inconsistency, the comparisons of inconsistency using node-splitting analysis and the loop-specific method both included a small number of studies and sample sizes. Therefore, the inconsistencies were not significant. Inconsistency tests can be affected by limited numbers of studies and small sample sizes, making accurate evaluation difficult. Due to the low heterogeneity of the data (τ < 0.5), a meta-regression analysis of potential effects was not performed (Turner et al., 2012).
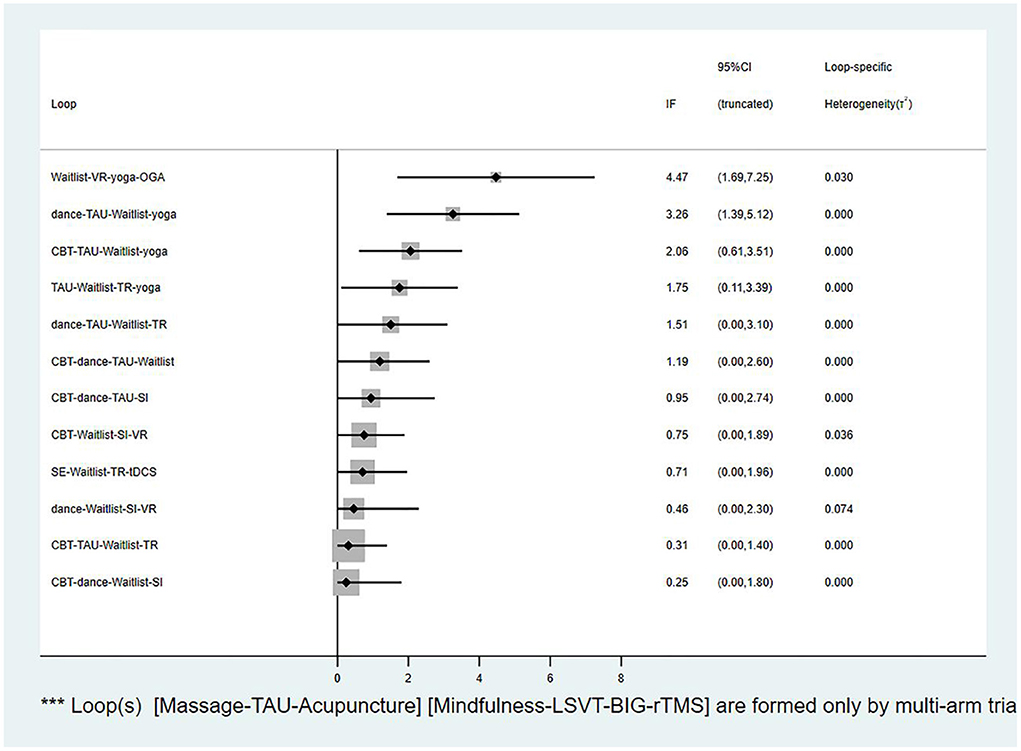
Figure 5. The loop inconsistency plot. VR, Virtual reality; OGA, Occupational group activities; TAU, Treatment as usual; CBT, Cognitive behavior therapy; TR, Traditional rehabilitation; SI, Supportive instruction; SE, Stretching exercise; tDCS, Transcranial direct current stimulation.
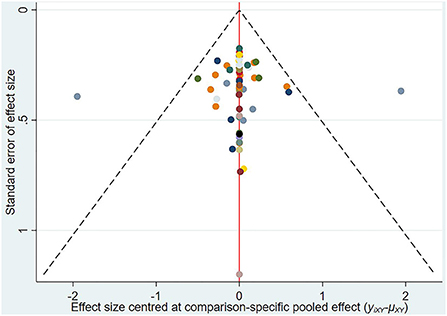
Publication analysis
A comparison-adjusted network funnel plot with a random model was constructed for the outcome (Figure 6). The funnel plot was symmetric, demonstrating that there was no significant risk of publication bias in our study.
Discussion
This NMA was conducted to synthesize existing evidence from 62 RCTs involving 35 non-pharmacological interventions for depressive symptoms using a comprehensive search. Direct and indirect comparisons were conducted to analyze the efficacy of different non-pharmacological interventions on depressive symptoms. The SUCRA values revealed that the best non-pharmacological intervention was dance, followed by LSVT-BIG therapy and CBT. Despite the lack of strong evidence, current guidelines recommend ECT, physical exercise, and psychotherapy to treat depression in PD patients (Olanow et al., 2001; Goodarzi et al., 2018). However, our NMA did not include RCTs on the effect of ECT on depression.
Although two conventional meta-analyses showed that dance had no significant effect on depression (Zhang et al., 2019; Wang et al., 2022), most original trials indicated a supportive effect of dance on depression compared to no intervention or TAU. In addition, our study demonstrated that dance was the best non-pharmacological intervention based on its high SUCRA value (82.3%). Dance is a multicomponent activity that involves moving one's muscles, maintaining one's balance, maintaining auditory, visual, and sensory reactions, memory, perception, expression, and social interactions (Kattenstroth et al., 2010). Dance can create a sense of pleasure by combining physical exercise and mental regulation, which contributes to increased compliance and continued attendance (Earhart, 2009; Hackney and Earhart, 2010; Goodarzi et al., 2018). The sense of pleasure may evoke positive emotions by stimulating basal ganglia loops and reward systems (Hackney and Earhart, 2009). Meanwhile, music used in dance can increase the release of dopamine from the ventral striatum and ventral tegmental area to alleviate depressive symptoms (Weintraub et al., 2005). However, this ranking has an underlying uncertainty and may not fully reflect reality.
LSVT-BIG therapy is a type of exercise model that focuses on training of amplitude together with sustained attention and perception of a single movement during exercise, thus enabling patients to participate physically and mentally in functional activities with great sustainability (Fox et al., 2012). Our study indicated that LSVT-BIG therapy is the second-ranked non-pharmacological intervention according to its SUCRA value (77.4%), whereas only one RCT showed a positive effect of LSVT-BIG therapy on depression compared with general exercise (Dashtipour et al., 2015). However, the small sample sizes of the studies limited the accuracy and generalization of the conclusions; therefore, more large-scale and strictly designed trials are needed. The mechanism by which LSVT-BIG therapy is effective in reducing depressive symptoms remains unclear. Additionally, current trials lack focus on the long-term effects of LSVT-BIG therapy. Thus, the maintenance of long-term effects and exploration of the underlying mechanisms should be given more attention in future studies.
CBT, as a psychotherapy, is the third-ranked non-pharmacological intervention according to its SUCRA value (73.6%), which is similar to the recommendations of the guidelines and results of conventional meta-analyses (Goodarzi et al., 2018; Zhang et al., 2020; Hong et al., 2021). CBT is a problem-oriented approach that aims to enhance a patient's coping skills. This approach involves both therapists and patients and aims to help patients overcome negative moods, dysfunctional thoughts, and behaviors by modifying their way of thinking and behaving (Farley and Koshland, 2005; Schrag et al., 2007; O'cleirigh et al., 2019; Sahranavard et al., 2019). Among the conventional meta-analyses that demonstrated that CBT had a significant positive effect on the improvement of depression (Troeung et al., 2013; Xie et al., 2015; Zhang et al., 2020; Hong et al., 2021), a meta-analysis showed that CBT had a larger effect size than antidepressant treatments (Troeung et al., 2013). To date, most trials have indicated that CBT plays a beneficial role in depression disorders in patients with PD. One review suggested that CBT is more appropriate for patients with PD without dementia, as patients with dementia are usually excluded from trials (Egan et al., 2015). In short, CBT can be considered an important approach for first-line or adjunctive treatment of depression in PD.
Interestingly, our results indicate that most non-pharmacological interventions examined in our study had no significant effect on alleviating depressive disorders. This may be due to the use of antidepressant medications and the progression of PD (Schrag et al., 2001; Bhattacharjee et al., 2018; Demarco et al., 2021). Moreover, depression in PD patients is associated with cognitive impairment. As the disease progresses, cognitive deficits negatively affect depressive symptoms (Van Uem et al., 2018). Although most included trials in our study excluded PD patients with dementia, the impact of mild cognitive impairment cannot be ignored. In addition, it is difficult for short-term non-pharmacological interventions to improve neuroanatomical degeneration (Mcdonald et al., 2003). According to their SUCRA values, some non-pharmacological interventions were ranked below placebo and waitlist. This may be due to a lack of sufficient evidence showing the efficacy of these interventions for depression in PD. Although a global inconsistency was present in our NMA, the local inconsistency was found to be weak in the node-split test, and inconsistencies were mainly due to the triangular loops from two multi-arm trials. Therefore, related results should be interpreted with caution.
Conventional meta-analyses, including RCTs, showed that yoga, BLT, rTMS, and psychotherapy significantly improved depression (Ban et al., 2021; Chen et al., 2021; Hong et al., 2021; Huang et al., 2021); however, the evidence was relatively weak. Although a previous systematic review showed that physical exercise is beneficial for depression, it did not provide a quantitative analysis of the intervention effects (Cusso et al., 2016). Guidelines and reviews suggest that ECT may be an effective therapy for depression (Bhattacharjee et al., 2018; Goodarzi et al., 2018); however, the current systematic review with meta-analysis did not identify any relevant RCTs of ECT (Takamiya et al., 2021). Therefore, our study did not compare ECT with the other non-pharmacological interventions. Recently, a systematic review and meta-analysis by Xie et al. revealed that psychodynamic psychotherapy was superior to CBT (Xie et al., 2015). According to the eligibility criteria of our study, we did not include RCTs on the effect of psychodynamic psychotherapy on depression in patients with PD. Psychotherapy in combination with non-CBT requires further high-quality evidence to explore its efficacy for treating depression in PD patients.
This NMA has several limitations. First, only RCTs that focused on PD patients with mean Hoehn–Yahr stage values of 1–3 were included in our NMA; thus, the results of this NMA may not be generalizable to all PD patients. Second, there was heterogeneity in the frequencies, durations, and periods of the non-pharmacological interventions. Third, although we comprehensively searched for non-pharmacological interventions for depression in patients with PD, the language was restricted to Chinese and English, which may have contributed to selection bias. Fourth, our NMA had obvious global inconsistencies, which may be related to the small sample size, short-term effects, discrepancies in eligibility criteria, different baseline characteristics, various measurement tools, different severities of depression, and different severities of PD. Thus, the accuracy and generalization of the conclusions are limited. Fifth, many comparisons of the interventions included only a small number of trials, which may have affected the accuracy of the conclusions. Lastly, most studies did not report concealed allocation, which may have led to selection and performance biases. Therefore, strictly designed RCTs with larger sample sizes are needed in the future.
Conclusion
To the best of our knowledge, this is the first NMA to comprehensively summarize the existing RCTs of 35 different non-pharmacological interventions used for depressive symptoms. The results showed non-significant effects of most non-pharmacological interventions on depression. According to its SUCRA values, dance may be the preferred non-pharmacological intervention for improving depression, followed by LSVT-BIG therapy and CBT. Consequently, a larger sample size and stronger high-quality trials are required to draw more reliable results regarding the efficacy of non-pharmacological interventions for depression in subjects with PD. The results of this study could provide evidence and a reference to healthcare providers and clinicians when choosing effective interventions to improve the quality of life and health status of patients with PD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YW, XS, and YJ conceived and designed the study. YW and XS searched the literature and contributed to writing of original manuscript, data acquisition and analysis, and responsible for the software. FL, QL, and YJ were responsible for revising and reviewing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.1050715/full#supplementary-material
References
Aftanas, L. I., Brack, I. V., Kulikova, K. I., Filimonova, E. A., Dzemidovich, S. S., Piradov, M. A., et al. (2021). Clinical and neurophysiological effects of the therapeutic combination of high-frequency rhythmic transcranial magnetic stimulation of the motor and frontal cortex in Parkinson's disease. Neurosci. Behav. Physiol. 51, 135–141. doi: 10.1007/s11055-021-01048-8
Assogna, F., Pellicano, C., Savini, C., Macchiusi, L., Pellicano, G. R., Alborghetti, M., et al. (2020). Drug choices and advancements for managing depression in Parkinson's disease. Curr. Neuropharmacol. 18, 277–287. doi: 10.2174/1570159X17666191016094857
Ban, M., Yue, X., Dou, P., and Zhang, P. (2021). The effects of yoga on patients with Parkinson's disease: a meta-analysis of randomized controlled trials. Behav. Neurol. 2021, 5582488. doi: 10.1155/2021/5582488
Bega, D., Stein, J., Zadikoff, C., Simuni, T., and Corcos, D. (2016). Yoga vs. resistance training in mild to moderate severity Parkinson's disease: a 12-week pilot study. J. Yoga Phys. Therap. 6, 222. doi: 10.4172/2157-7595.1000222
Bhattacharjee, S., Vadiei, N., Goldstone, L., Alrabiah, Z., and Sherman, S. J. (2018). Patterns and predictors of depression treatment among older adults with parkinson's disease and depression in ambulatory care settings in the United States. Parkinsons. Dis. 2018, 3402983. doi: 10.1155/2018/3402983
Bogosian, A., Hurt, C. S., Hindle, J. V., Mccracken, L. M., Vasconcelos E Sa, D. A., Axell, S., et al. (2022). Acceptability and feasibility of a mindfulness intervention delivered via videoconferencing for people with Parkinson's. J. Geriatr. Psychiatry Neurol. 35, 155–167. doi: 10.1177/0891988720988901
Brys, M., Fox, M. D., Agarwal, S., Biagioni, M., Dacpano, G., Kumar, P., et al. (2016). Multifocal repetitive TMS for motor and mood symptoms of Parkinson disease: a randomized trial. Neurology 87, 1907–1915. doi: 10.1212/WNL.0000000000003279
Calleo, J. S., Amspoker, A. B., Sarwar, A. I., Kunik, M. E., Jankovic, J., Marsh, L., et al. (2015). A pilot study of a cognitive-behavioral treatment for anxiety and depression in patients with Parkinson disease. J. Geriatr. Psychiatry Neurol. 28, 210–217. doi: 10.1177/0891988715588831
Cartmill, T., Skvarc, D., Bittar, R., Mcgillivray, J., Berk, M., and Byrne, L. K. (2021). Deep brain stimulation of the subthalamic nucleus in Parkinson's disease: a meta-analysis of mood effects. Neuropsychol. Rev. 31, 385–401. doi: 10.1007/s11065-020-09467-z
Catalá-López, F., Tobías, A., Cameron, C., Moher, D., and Hutton, B. (2014). Network meta-analysis for comparing treatment effects of multiple interventions: an introduction. Rheumatol. Int. 34, 1489–1496. doi: 10.1007/s00296-014-2994-2
Chen, J., He, P., Zhang, Y., Gao, Y., Qiu, Y., Li, Y., et al. (2021). Non-pharmacological treatment for Parkinson disease patients with depression: a meta-analysis of repetitive transcranial magnetic stimulation and cognitive-behavioral treatment. Int. J. Neurosci. 131, 411–424. doi: 10.1080/00207454.2020.1744591
Cheung, C., Bhimani, R., Wyman, J. F., Konczak, J., Zhang, L., Mishra, U., et al. (2018). Effects of yoga on oxidative stress, motor function, and non-motor symptoms in Parkinson's disease: a pilot randomized controlled trial. Pilot Feasibility Stud. 4, 162. doi: 10.1186/s40814-018-0355-8
Cohen, O. S., Rigbi, A., Yahalom, G., Warman-Alaluf, N., Nitsan, Z., Zangen, A., et al. (2018). Repetitive deep TMS for Parkinson disease: a 3-month double-blind, randomized sham-controlled study. J. Clin. Neurophysiol. 35, 159–165. doi: 10.1097/WNP.0000000000000455
Craig, L. H., Svircev, A., Haber, M., and Juncos, J. L. (2006). Controlled pilot study of the effects of neuromuscular therapy in patients with Parkinson's disease. Mov. Disord. 21, 2127–2133. doi: 10.1002/mds.21132
Cusso, M. E., Donald, K. J., and Khoo, T. K. (2016). The impact of physical activity on non-motor symptoms in Parkinson's disease: a systematic review. Front. Med. 3, 35. doi: 10.3389/fmed.2016.00035
Dashtipour, K., Johnson, E., Kani, C., Kani, K., Hadi, E., Ghamsary, M., et al. (2015). Effect of exercise on motor and nonmotor symptoms of Parkinson's disease. Parkinsons. Dis. 2015, 586378. doi: 10.1155/2015/586378
Demarco, E. C., Al-Hammadi, N., and Hinyard, L. (2021). Exploring treatment for depression in Parkinson's patients: a cross-sectional analysis. Int. J. Environ. Res. Public Health 18, 8596. doi: 10.3390/ijerph18168596
Deuel, L. M., and Seeberger, L. C. (2020). Complementary therapies in parkinson disease: a review of acupuncture, Tai Chi, Qi Gong, Yoga, and Cannabis. Neurotherapeutics 17, 1434–1455. doi: 10.1007/s13311-020-00900-y
Dobkin, R. D., Mann, S. L., Weintraub, D., Rodriguez, K. M., Miller, R. B., St. Hill, L., et al. (2021). Innovating Parkinson's care: a randomized controlled trial of telemedicine depression treatment. Movement Disord. 36, 2549–2558. doi: 10.1002/mds.28548
Dobkin, R. D., Menza, M., Allen, L. A., Gara, M. A., Mark, M. H., Tiu, J., et al. (2011). Cognitive-behavioral therapy for depression in Parkinson's disease: a randomized, controlled trial. Am. J. Psychiatry 168, 1066–1074. doi: 10.1176/appi.ajp.2011.10111669
Earhart, G. M. (2009). Dance as therapy for individuals with Parkinson disease. Eur. J. Phys. Rehabil. Med. 45, 231–238.
Edwards, J. D., Hauser, R. A., O'connor, M. L., Valdés, E. G., Zesiewicz, T. A., and Uc, E. Y. (2013). Randomized trial of cognitive speed of processing training in Parkinson disease. Neurology 81, 1284-1290. doi: 10.1212/WNL.0b013e3182a823ba
Egan, S. J., Laidlaw, K., and Starkstein, S. (2015). Cognitive behaviour therapy for depression and anxiety in Parkinson's disease. J. Parkinsons. Dis. 5, 443–451. doi: 10.3233/JPD-150542
Fan, J., Liu, X. L., Kong, M., Wang, X. Y., Yi, L. Y., and Zhang, Y. (2017). Effect of health qigong on mood state and cognition of patients with moderate Parkinson's disease. Chin. J. Sports Med. 36, 143-146+149.
Farley, B. G., and Koshland, G. F. (2005). Training BIG to move faster: the application of the speed-amplitude relation as a rehabilitation strategy for people with Parkinson's disease. Exp. Brain Res. 167, 462–467. doi: 10.1007/s00221-005-0179-7
Fellman, D., Salmi, J., Ritakallio, L., Ellfolk, U., Rinne, J. O., and Laine, M. (2020). Training working memory updating in Parkinson's disease: a randomised controlled trial. Neuropsychol. Rehabil. 30, 673–708. doi: 10.1080/09602011.2018.1489860
Fox, C., Ebersbach, G., Ramig, L., and Sapir, S. (2012). LSVT LOUD and LSVT BIG: behavioral treatment programs for speech and body movement in Parkinson disease. Parkinsons. Dis. 2012, 391946. doi: 10.1155/2012/391946
Garcia-Ruiz, P. J., Chaudhuri, K. R., and Martinez-Martin, P. (2014). Non-motor symptoms of Parkinson's disease A review…from the past. J. Neurol. Sci. 338, 30–33. doi: 10.1016/j.jns.2014.01.002
Ghielen, I., Van Wegen, E. E. H., Rutten, S., De Goede, C. J. T., Houniet-De Gier, M., Collette, E. H., et al. (2017). Body awareness training in the treatment of wearing-off related anxiety in patients with Parkinson's disease: results from a pilot randomized controlled trial. J. Psychosom. Res. 103, 1-8. doi: 10.1016/j.jpsychores.2017.09.008
Goodarzi, Z., Hanson, H. M., Jette, N., Patten, S., Pringsheim, T., and Holroyd-Leduc, J. (2018). Barriers and facilitators for guidelines with depression and anxiety in Parkinson's disease or dementia. Can. J. Aging 37, 185–199. doi: 10.1017/S0714980818000053
Hackney, M. E., and Earhart, G. M. (2009). Health-related quality of life and alternative forms of exercise in Parkinson disease. Parkinsonism Relat. Disord. 15, 644–648. doi: 10.1016/j.parkreldis.2009.03.003
Hackney, M. E., and Earhart, G. M. (2010). Effects of dance on gait and balance in Parkinson's disease: a comparison of partnered and nonpartnered dance movement. Neurorehabil. Neural Repair 24, 384–392. doi: 10.1177/1545968309353329
Hai-Jiao, W., Ge, T., Li-Na, Z., Deng, C., Da, X., Shan-Shan, C., et al. (2020). The efficacy of repetitive transcranial magnetic stimulation for Parkinson disease patients with depression. Int. J. Neurosci. 130, 19–27. doi: 10.1080/00207454.2018.1495632
Han, W., Dong, T., Ding, X. Y., and Yun, Z. J. (2021). Application of kinesthetic game combined with Wuqinxi in patients with Parkinson's disease. Guangxi Med. 43, 1153–1156+1160.
Hirtz, D., Thurman, D. J., Gwinn-Hardy, K., Mohamed, M., Chaudhuri, A. R., and Zalutsky, R. (2007). How common are the “common” neurologic disorders? Neurology 68, 326–337. doi: 10.1212/01.wnl.0000252807.38124.a3
Hong, C. T., Tan, S., and Huang, T. W. (2021). Psychotherapy for the treatment of anxiety and depression in patients with Parkinson disease: a meta-analysis of randomized controlled trials. J. Am. Med. Dir. Assoc. 22, 2289–2295.e2. doi: 10.1016/j.jamda.2021.03.031
Huang, H. T., Huang, T. W., and Hong, C. T. (2021). Bright light therapy for Parkinson disease: a literature review and meta-analysis of randomized controlled trials. Biology (Basel) 10, 1205. doi: 10.3390/biology10111205
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162, 777–784. doi: 10.7326/M14-2385
Jin, X., Wang, L., Liu, S., Zhu, L., Loprinzi, P. D., and Fan, X. (2019). The impact of mind-body exercises on motor function, depressive symptoms, and quality of life in Parkinson's disease: a systematic review and meta-analysis. Int. J. Environ. Res. Public Health 17, 31. doi: 10.3390/ijerph17010031
Kalbe, E., Folkerts, A. K., Ophey, A., Eggers, C., Elben, S., Dimenshteyn, K., et al. (2020). Enhancement of executive functions but not memory by multidomain group cognitive training in patients with Parkinson's disease and mild cognitive impairment: a multicenter randomized controlled trial. Parkinson's Dis. 2020, 4068706. doi: 10.1155/2020/4068706
Kattenstroth, J. C., Kolankowska, I., Kalisch, T., and Dinse, H. R. (2010). Superior sensory, motor, and cognitive performance in elderly individuals with multi-year dancing activities. Front. Aging Neurosci. 2, 31. doi: 10.3389/fnagi.2010.00031
Kong, K. H., Ng, H. L., Li, W., Ng, D. W., Tan, S. I., Tay, K. Y., et al. (2018). Acupuncture in the treatment of fatigue in Parkinson's disease: a pilot, randomized, controlled, study. Brain Behav. 8, e00897. doi: 10.1002/brb3.897
Kraepelien, M., Schibbye, R., Månsson, K., Sundström, C., Riggare, S., Andersson, G., et al. (2020). Individually tailored internet-based cognitive-behavioral therapy for daily functioning in patients with Parkinson's disease: a randomized controlled trial. J. Parkinsons. Dis. 10, 653–664. doi: 10.3233/JPD-191894
Kwok, J. Y. Y., Kwan, J. C. Y., Auyeung, M., Mok, V. C. T., Lau, C. K. Y., Choi, K. C., et al. (2019). Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. 76, 755–763. doi: 10.1001/jamaneurol.2019.0534
Lee, H. J., Kim, S. Y., Chae, Y., Kim, M. Y., Yin, C., Jung, W. S., et al. (2018). Turo (Qi Dance) program for Parkinson's disease patients: randomized, assessor blind, waiting-list control, partial crossover study. Explore (NY) 14, 216–223. doi: 10.1016/j.explore.2017.11.002
Lee, N. Y., Lee, D. K., and Song, H. S. (2015). Effect of virtual reality dance exercise on the balance, activities of daily living, and depressive disorder status of Parkinson's disease patients. J Phys Ther Sci 27, 145–147. doi: 10.1589/jpts.27.145
Li, J., Mi, T. M., Zhu, B. F., Ma, J. H., Han, C., Li, Y., et al. (2020). High-frequency repetitive transcranial magnetic stimulation over the primary motor cortex relieves musculoskeletal pain in patients with Parkinson's disease: a randomized controlled trial. Parkinsonism Relat. Disord. 80, 113–119. doi: 10.1016/j.parkreldis.2020.07.006
Lu, G., and Ades, A. E. (2004). Combination of direct and indirect evidence in mixed treatment comparisons. Stat. Med. 23, 3105–3124. doi: 10.1002/sim.1875
Manenti, R., Cotelli, M. S., Cobelli, C., Gobbi, E., Brambilla, M., Rusich, D., et al. (2018). Transcranial direct current stimulation combined with cognitive training for the treatment of Parkinson disease: a randomized, placebo-controlled study. Brain Stimul. 11, 1251–1262. doi: 10.1016/j.brs.2018.07.046
Mcdonald, W. M., Richard, I. H., and Delong, M. R. (2003). Prevalence, etiology, and treatment of depression in Parkinson's disease. Biol. Psychiatry 54, 363–375. doi: 10.1016/S0006-3223(03)00530-4
Michels, K., Dubaz, O., Hornthal, E., and Bega, D. (2018). “Dance therapy” as a psychotherapeutic movement intervention in Parkinson's disease. Complement. Ther. Med. 40, 248–252. doi: 10.1016/j.ctim.2018.07.005
Modugno, N., Iaconelli, S., Fiorlli, M., Lena, F., Kusch, I., and Mirabella, G. (2010). Active theater as a complementary therapy for Parkinson's disease rehabilitation: a pilot study. ScientificWorldJournal. 10, 2301–2313. doi: 10.1100/tsw.2010.221
Moon, S., Sarmento, C. V. M., Steinbacher, M., Smirnova, I. V., Colgrove, Y., Lai, S. M., et al. (2020). Can Qigong improve non-motor symptoms in people with Parkinson's disease - a pilot randomized controlled trial? Complement. Ther. Clin. Pract. 39, 101169. doi: 10.1016/j.ctcp.2020.101169
Naismith, S. L., Mowszowski, L., Diamond, K., and Lewis, S. J. (2013). Improving memory in Parkinson's disease: a healthy brain ageing cognitive training program. Mov. Disord. 28, 1097–1103. doi: 10.1002/mds.25457
O'cleirigh, C., Safren, S. A., Taylor, S. W., Goshe, B. M., Bedoya, C. A., Marquez, S. M., et al. (2019). Cognitive Behavioral Therapy for Trauma and Self-Care (CBT-TSC) in men who have sex with men with a history of childhood sexual abuse: a randomized controlled trial. AIDS Behav. 23, 2421–2431. doi: 10.1007/s10461-019-02482-z
Okai, D., Askey-Jones, S., Samuel, M., O'sullivan, S. S., Chaudhuri, K. R., Martin, A., et al. (2013). Trial of CBT for impulse control behaviors affecting Parkinson patients and their caregivers. Neurology 80, 792–799. doi: 10.1212/WNL.0b013e3182840678
Olanow, C. W., Watts, R. L., and Koller, W. C. (2001). An algorithm (decision tree) for the management of Parkinson's disease (2001): treatment guidelines. Neurology 56, S1–s88. doi: 10.1212/WNL.56.suppl_5.S1
Pal, E., Nagy, F., Aschermann, Z., Balazs, E., and Kovacs, N. (2010). The impact of left prefrontal repetitive transcranial magnetic stimulation on depression in Parkinson's disease: a randomized, double-blind, placebo-controlled study. Mov. Disord. 25, 2311–2317. doi: 10.1002/mds.23270
Patel, S., Ahmed, A., Foldvary-Schaefer, N., Ojo, O., Genc, G., Oravivattanakul, S., et al. (2016). A computerized cognitive behavioral therapy randomized, controlled, pilot trial for insomnia in Parkinson's disease (ACCORD-PD study). Neurology 86, 16. doi: 10.1186/s40734-017-0062-2
Paus, S., Schmitz-Hübsch, T., Wüllner, U., Vogel, A., Klockgether, T., and Abele, M. (2007). Bright light therapy in Parkinson's disease: a pilot study. Mov. Disord. 22, 1495–1498. doi: 10.1002/mds.21542
Peña, J., Ibarretxe-Bilbao, N., García-Gorostiaga, I., Gomez-Beldarrain, M. A., Díez-Cirarda, M., and Ojeda, N. (2014). Improving functional disability and cognition in Parkinson disease: randomized controlled trial. Neurology 83, 2167–2174. doi: 10.1212/WNL.0000000000001043
Pérez-de la Cruz, S. (2018). Mental health in Parkinson's disease after receiving aquatic therapy: a clinical trial. Acta Neurol. Belg. 119. doi: 10.1007/s13760-018-1034-5
Petrelli, A., Kaesberg, S., Barbe, M. T., Timmermann, L., Fink, G. R., Kessler, J., et al. (2014). Effects of cognitive training in Parkinson's disease: a randomized controlled trial. Parkinsonism Relat. Disord. 20, 1196–1202. doi: 10.1016/j.parkreldis.2014.08.023
Picelli, A., Varalta, V., Melotti, C., Zatezalo, V., Fonte, C., Amato, S., et al. (2016). Effects of treadmill training on cognitive and motor features of patients with mild to moderate Parkinson's disease: a pilot, single-blind, randomized controlled trial. Funct. Neurol. 31, 25-31. doi: 10.11138/FNeur/2016.31.1.025
Reijnders, J. S., Ehrt, U., Weber, W. E., Aarsland, D., and Leentjens, A. F. (2008). A systematic review of prevalence studies of depression in Parkinson's disease. Mov Disord. 23, 183-9; quiz 313. doi: 10.1002/mds.21803
Rios Romenets, S., Anang, J., Fereshtehnejad, S. M., Pelletier, A., and Postuma, R. (2015). Tango for treatment of motor and non-motor manifestations in Parkinson's disease: a randomized control study. Complement. Ther. Med. 23, 175–184. doi: 10.1016/j.ctim.2015.01.015
Rios Romenets, S., Creti, L., Fichten, C., Bailes, S., Libman, E., Pelletier, A., et al. (2013). Doxepin and cognitive behavioural therapy for insomnia in patients with Parkinson's disease – a randomized study. Parkinsonism Relat. Disord. 19, 670–675. doi: 10.1016/j.parkreldis.2013.03.003
Rodgers, S. H., Schütze, R., Gasson, N., Anderson, R. A., Kane, R. T., Starkstein, S., et al. (2019). Modified mindfulness-based cognitive therapy for depressive symptoms in Parkinson's disease: a pilot trial. Behav. Cogn. Psychother. 47, 446–461. doi: 10.1017/S135246581800070X
Rutten, S., Vriend, C., Smit, J. H., Berendse, H. W., Van Someren, E. J. W., Hoogendoorn, A. W., et al. (2019). Bright light therapy for depression in Parkinson disease: a randomized controlled trial. Neurology 92, e1145–e1156. doi: 10.1212/WNL.0000000000007090
Ryan, M., Eatmon, C. V., and Slevin, J. T. (2019). Drug treatment strategies for depression in Parkinson disease. Expert Opin. Pharmacother. 20, 1351–1363. doi: 10.1080/14656566.2019.1612877
Sacheli, M. A., Neva, J. L., Lakhani, B., Murray, D. K., Vafai, N., Shahinfard, E., et al. (2019). Exercise increases caudate dopamine release and ventral striatal activation in Parkinson's disease. Mov. Disord. 34, 1891-1900. doi: 10.1002/mds.27865
Sahranavard, S., Esmaeili, A., Salehiniya, H., and Behdani, S. (2019). The effectiveness of group training of cognitive behavioral therapy-based stress management on anxiety, hardiness and self-efficacy in female medical students. J. Educ. Health Promot. 8, 49. doi: 10.1051/bmdcn/2018080423
Schmidt, N., Tödt, I., Berg, D., Schlenstedt, C., Folkerts, A. K., Ophey, A., et al. (2021). Memory enhancement by multidomain group cognitive training in patients with Parkinson's disease and mild cognitive impairment: long-term effects of a multicenter randomized controlled trial. J. Neurol. 268, 4655–4666. doi: 10.1007/s00415-021-10568-9
Schrag, A., Dodel, R., Spottke, A., Bornschein, B., Siebert, U., and Quinn, N. P. (2007). Rate of clinical progression in Parkinson's disease. A prospective study. Mov. Disord. 22, 938–945. doi: 10.1002/mds.21429
Schrag, A., Jahanshahi, M., and Quinn, N. P. (2001). What contributes to depression in Parkinson's disease? Psychol. Med. 31, 65–73. doi: 10.1017/S0033291799003141
Shim, S., Yoon, B. H., Shin, I. S., and Bae, J. M. (2017). Network meta-analysis: application and practice using Stata. Epidemiol. Health 39, e2017047. doi: 10.4178/epih.e2017047
Shirota, Y., Ohtsu, H., Hamada, M., Enomoto, H., and Ugawa, Y. (2013). Supplementary motor area stimulation for Parkinson disease: a randomized controlled study. Neurology 80, 1400–1405. doi: 10.1212/WNL.0b013e31828c2f66
Smania, N., Corato, E., Tinazzi, M., Stanzani, C., Fiaschi, A., Girardi, P., et al. (2010). Effect of balance training on postural instability in patients with idiopathic Parkinson's disease. Neurorehabil. Neural Repair 24, 826–834. doi: 10.1177/1545968310376057
Solla, P., Cugusi, L., Bertoli, M., Cereatti, A., Della Croce, U., Pani, D., et al. (2019). Sardinian folk dance for individuals with Parkinson's disease: a randomized controlled pilot trial. J. Altern. Complement. Med. 25, 305–316. doi: 10.1089/acm.2018.0413
Sproesser, E., Viana, M. A., Quagliato, E. M., and De Souza, E. A. (2010). The effect of psychotherapy in patients with PD: a controlled study. Parkinsonism Relat. Disord. 16, 298–300. doi: 10.1016/j.parkreldis.2009.08.008
Stallibrass, C., Sissons, P., and Chalmers, C. (2002). Randomized controlled trial of the Alexander technique for idiopathic Parkinson's disease. Clin. Rehabil. 16, 695–708. doi: 10.1191/0269215502cr544oa
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi: 10.1136/bmj.l4898
Takamiya, A., Seki, M., Kudo, S., Yoshizaki, T., Nakahara, J., Mimura, M., et al. (2021). Electroconvulsive therapy for Parkinson's disease: a systematic review and meta-analysis. Mov. Disord. 36, 50–58. doi: 10.1002/mds.28335
Tollár, J., Nagy, F., Kovács, N., and Hortobágyi, T. (2018). A high-intensity multicomponent agility intervention improves parkinson patients' clinical and motor symptoms. Arch. Phys. Med. Rehabil. 99, 2478–2484.e1. doi: 10.1016/j.apmr.2018.05.007
Triegaardt, J., Han, T. S., Sada, C., Sharma, S., and Sharma, P. (2020). The role of virtual reality on outcomes in rehabilitation of Parkinson's disease: meta-analysis and systematic review in 1031 participants. Neurol. Sci. 41, 529–536. doi: 10.1007/s10072-019-04144-3
Troeung, L., Egan, S. J., and Gasson, N. (2013). A meta-analysis of randomised placebo-controlled treatment trials for depression and anxiety in Parkinson's disease. PLoS ONE 8, e79510. doi: 10.1371/journal.pone.0079510
Troeung, L., Egan, S. J., and Gasson, N. (2014). A waitlist-controlled trial of group cognitive behavioural therapy for depression and anxiety in Parkinson's disease. BMC Psychiatry 14, 19. doi: 10.1186/1471-244X-14-19
Tröster, A. I., Jankovic, J., Tagliati, M., Peichel, D., and Okun, M. S. (2017). Neuropsychological outcomes from constant current deep brain stimulation for Parkinson's disease. Mov. Disord. 32, 433–440. doi: 10.1002/mds.26827
Turner, R. M., Davey, J., Clarke, M. J., Thompson, S. G., and Higgins, J. P. (2012). Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int. J. Epidemiol. 41, 818–827. doi: 10.1093/ije/dys041
Tysnes, O. B., and Storstein, A. (2017). Epidemiology of Parkinson's disease. J. Neural Transm. 124, 901–905. doi: 10.1007/s00702-017-1686-y
Uhrbrand, A., Stenager, E., Pedersen, M. S., and Dalgas, U. (2015). Parkinson's disease and intensive exercise therapy–a systematic review and meta-analysis of randomized controlled trials. J. Neurol. Sci. 353, 9–19. doi: 10.1016/j.jns.2015.04.004
Van Uem, J. M. T., Cerff, B., Kampmeyer, M., Prinzen, J., Zuidema, M., Hobert, M. A., et al. (2018). The association between objectively measured physical activity, depression, cognition, and health-related quality of life in Parkinson's disease. Parkinsonism Relat. Disord. 48, 74–81. doi: 10.1016/j.parkreldis.2017.12.023
Veazey, C., Cook, K. F., Stanley, M., Lai, E. C., and Kunik, M. E. (2009). Telephone-administered cognitive behavioral therapy: a case study of anxiety and depression in Parkinson's disease. J. Clin. Psychol. Med. Settings 16, 243–253. doi: 10.1007/s10880-009-9167-6
Videnovic, A., Klerman, E. B., Wang, W., Marconi, A., Kuhta, T., and Zee, P. C. (2017). Timed light therapy for sleep and daytime sleepiness associated with parkinson disease: a randomized clinical trial. JAMA Neurol. 74, 411–418. doi: 10.1001/jamaneurol.2016.5192
Wade, D. T., Gage, H., Owen, C., Trend, P., Grossmith, C., and Kaye, J. (2003). Multidisciplinary rehabilitation for people with Parkinson's disease: a randomised controlled study. J. Neurol. Neurosurg. Psychiatry 74, 158–162. doi: 10.1136/jnnp.74.2.158
Wang, L. L., Sun, C. J., Wang, Y., Zhan, T. T., Yuan, J., Niu, C. Y., et al. (2022). Effects of dance therapy on non-motor symptoms in patients with Parkinson's disease: a systematic review and meta-analysis. Aging Clin. Exp. Res. 34, 1201–1208. doi: 10.1007/s40520-021-02030-7
Weintraub, D., Newberg, A. B., Cary, M. S., Siderowf, A. D., Moberg, P. J., Kleiner-Fisman, G., et al. (2005). Striatal dopamine transporter imaging correlates with anxiety and depression symptoms in Parkinson's disease. J. Nucl. Med. 46, 227–232.
Willis, G. L., Boda, J., and Freelance, C. B. (2018). Polychromatic light exposure as a therapeutic in the treatment and management of Parkinson's disease: a controlled exploratory trial. Front. Neurol. 9, 741. doi: 10.3389/fneur.2018.00741
Wu, P. L., Lee, M., Wu, S. L., Ho, H. H., Chang, M. H., Lin, H. S., et al. (2021). Effects of home-based exercise on motor, non-motor symptoms and health-related quality of life in Parkinson's disease patients: a randomized controlled trial. Jpn. J. Nurs. Sci. e12418. doi: 10.1111/jjns.12418
Wu, S. P., Li, X., Qi, Y. W., Wang, H., and Ma, J. J. (2020). The influence of transcranial stimulation on rapid eye movement sleep disorders among person with Parkinson's disease. Chin. J. Phys. Med. Rehabil. 42, 50–54.
Wu, S. P., Li, X., Qi, Y. W., Wang, H., Zhuang, W.S., Yang, H. Q., et al. (2019). Effects of transcranial magnetic stimulation on the motor and non-motor symptoms of Parkinson's disease. Chin. J. Phys Med Rehabil 41, 338–343.
Wu, Y. H., Chen, Y. H., Chang, M. H., and Lin, C. H. (2018). Depression in Parkinson's disease: a case-control study. PLoS ONE 13, e0192050. doi: 10.1371/journal.pone.0192050
Wuthrich, V. M., and Rapee, R. M. (2019). Telephone-delivered cognitive behavioural therapy for treating symptoms of anxiety and depression in Parkinson's disease: a pilot trial. Clin. Gerontol. 42, 444–453. doi: 10.1080/07317115.2019.1580811
Xie, C. L., Wang, X. D., Chen, J., Lin, H. Z., Chen, Y. H., Pan, J. L., et al. (2015). A systematic review and meta-analysis of cognitive behavioral and psychodynamic therapy for depression in Parkinson's disease patients. Neurol. Sci. 36, 833–843. doi: 10.1007/s10072-015-2118-0
Xu, Z., and Xia, J. (2017). Effect of auricular pressure combined with psychological nursing on anxiety and depression in patients with Parkinson's disease. Chin. Man. Rehabil. Med. 8, 73–74.
You, H., and She, J. H. (2020). Effect of taiji balance exercise group therapy on improving balance function and depression in patients with Parkinson's disease. Guizhou Med. 44, 1071–1072.
Yu, W. W., Li, Z. G., Sun, H. R., Zhang, J. B., Zhao, J. W., Cui, X. H., et al. (2017). Clinical investigation of repetitive transcranial magnetic stimulation on treating depression and sleep disorder in patients with Parkinson's disease in early stage. J. Clin. Neurol. 30, 341–345.
Zhang, Q., Hu, J., Wei, L., Jia, Y., and Jin, Y. (2019). Effects of dance therapy on cognitive and mood symptoms in people with Parkinson's disease: a systematic review and meta-analysis. Complement. Ther. Clin. Pract. 36, 12–17. doi: 10.1016/j.ctcp.2019.04.005
Zhang, Q., Yang, X., Song, H., and Jin, Y. (2020). Cognitive behavioral therapy for depression and anxiety of Parkinson's disease: a systematic review and meta-analysis. Complement. Ther. Clin. Pract. 39, 101111. doi: 10.1016/j.ctcp.2020.101111
Zheng, L. N., Zhang, L. Y., Chen, J, and Zheng, F. X. (2020). Effect of resistance exercise on sleep quality and emotional state of elderly patients with parkinson disease sleep disorder. Chin. Foreign Med. Res. 18, 175–177.
Keywords: non-pharmacological interventions, depression, network meta-analysis, Parkinson's disease (PD), randomized controlled trials
Citation: Wang Y, Sun X, Li F, Li Q and Jin Y (2022) Efficacy of non-pharmacological interventions for depression in individuals with Parkinson's disease: A systematic review and network meta-analysis. Front. Aging Neurosci. 14:1050715. doi: 10.3389/fnagi.2022.1050715
Received: 22 September 2022; Accepted: 26 October 2022;
Published: 10 November 2022.
Edited by:
Wooyoung Jang, Gangneung Asan Hospital, South KoreaReviewed by:
Robert Fekete, New York Medical College, United StatesLiang Dai, Shenzhen Hospital, Peking University, China
Copyright © 2022 Wang, Sun, Li, Li and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Jin, amlueWk2MTk2QDE2My5jb20=
 Yuxin Wang1
Yuxin Wang1 Yi Jin
Yi Jin