- 1Department of Neurology, First Affiliated Hospital of China Medical University, Shenyang, Liaoning, China
- 2Department of Neurology, Zhongshan Hospital, Fudan University, Shanghai, China
- 3Department of Rehabilitation, Fourth Affiliated Hospital of China Medical University, Shenyang, Liaoning, China
- 4Department of General Surgery, Sheng Jing Hospital of China Medical University, Shenyang, Liaoning, China
Background: Depressive symptoms play an essential role in cognition decline, while the benefit and acceptability of treatments for depressive symptoms in cognitive impairment are still unknown.
Objective: To comprehensively evaluate the comparative efficacy and acceptability of treatments for depressive symptoms in cognitive impairment based on the quantitative Bayesian network meta-analysis method (NMA).
Method: We searched MEDLINE, Embase, the Cochrane Library, CINAHL, and PsycINFO from inception until August 2022 to identify randomized clinical trials (RCTs) evaluating treatments for depressive symptoms in cognitive impairment. Efficacy was evaluated by the Cornell Scale for Depression in Dementia (CSDD), the Hamilton Depression Rating Scale (HDRS), and the Geriatric Depression Scale (GDS) for depression; the Neuropsychiatric Inventory (NPI) and the Cohen–Mansfeld Agitation Inventory (CMAI) for behavior; and the Mini-Mental State Examination (MMSE) for cognition. Safety was evaluated by total adverse events (AEs), serious AEs, diarrhea, headache, and nausea.
Results: In this study, 13,043 participants from 107 RCTs were included, involving 28 treatments and the discontinuation of antidepressants. On CSDD, aerobic exercise (MD −4.51, 95%CrI −8.60 to −0.37), aripiprazole (MD −1.85, 95%CrI −3.66 to −0.02), behavioral training (MD −1.14, 95%CrI −2.04 to −0.34), electrical current stimulation (MD −3.30, 95%CrI −5.94 to −0.73), massage (MD −12.67, 95%CrI −14.71 to −10.59), music therapy (MD −2.63, 95%CrI −4.72 to −0.58), and reminiscence therapy (MD −2.34, 95%CrI −3.51 to −1.25) significantly outperformed the placebo. On MMSE, cognitive stimulation therapy (MD 1.42, 95%CrI 0.49 to 2.39), electrical current stimulation (MD 4.08, 95%CrI 1.07 to 7.11), and reminiscence therapy (MD 1.31, 95%CrI 0.04 to 2.91) significantly outperformed the placebo. Additionally, no treatments showed a significantly higher risk than the placebo.
Conclusion: Our NMAs indicated that non-pharmacological interventions were more efficacious and safe than pharmacological treatments for reducing depressive symptoms as well as improving cognitive impairment. Electrical current stimulation, aerobic exercise, and reminiscence therapy could be first recommended considering their beneficial performance on both depression and cognition. Hence, non-pharmacological treatments deserve more attention and extensive application and should at least be considered as an alternative or assistance in clinical settings.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021239621, identifier: CRD42021239621.
Introduction
Dementia is a common debilitating disorder affecting an estimated population of 55 million worldwide, and there are nearly 10 million new cases every year (World Health Organization, 2021). Apart from cognition decline, depressive symptoms are closely associated with faster progression of the disease and a huge burden on caregivers (Diniz et al., 2013; Vaughan et al., 2015), affecting up to 63% of the patients with cognitive impairment (Solfrizzi et al., 2007).
Depressive symptoms play an essential role in the occurrence and prognosis of cognition impairment. First, it may be an independent risk factor for cognitive impairment (Bennett and Thomas, 2014), which means that the risk of developing dementia is two-fold in elderly people with a history of depression (Saczynski et al., 2010; Byers and Yaffe, 2011). Second, depressive symptoms can accelerate the deterioration of cognitive impairment and behavioral disturbance, resulting in increased morbidity and mortality (Rapp et al., 2011a,b). Third, viewed as a prodrome of dementia, it could be a reaction or a psychological response to the disease (Kessing, 2012; Bennett and Thomas, 2014; Baruch et al., 2019). Moreover, depressed patients with cognitive impairment are more likely to experience recurrent depression compared to those with simple depression (Hall and Reynolds-Iii, 2014). Hence, alleviation of depressive symptoms is of paramount importance to delay the course of the disease and improve the quality of life of patients.
Pharmacological approaches for depression remain the mainstay of treating depressive symptoms in cognitive impairment (Kessing et al., 2007), though they may not be necessarily effective and tolerable (Bingham et al., 2019). Recent reviews provided conflicting results on the benefits of antidepressants and even claimed minimal or no effect on depression symptoms, cognitive function, or activities of daily living (Orgeta et al., 2017; Dudas et al., 2018). Some also indicated that patients on antidepressants were more likely to suffer from side effects (Farina et al., 2017; Dudas et al., 2018; Baruch et al., 2019). Meanwhile, a few meta-analyses claimed the beneficial effects of non-pharmacological treatments for depressive symptoms in cognitive impairment, though most of them only focused on limited interventions and were of unsatisfactory quality (Woods et al., 2018; Bennett et al., 2019; Li H. C. et al., 2019; Li X. et al., 2019; Zafra-Tanaka et al., 2019). There is still a lack of comprehensive assessment of both pharmacological and non-pharmacological treatments for depressive symptoms in cognitive impairment.
The traditional pairwise meta-analysis method can only compare two interventions at a time utilizing direct evidence, which would provide limited insights when there are no head-to-head clinical trials. Given the complexity of this targeted issue, network meta-analysis (NMA) is adopted to face this challenge, as it is capable of fully utilizing both direct and indirect evidence and presenting a comparative hierarchy of efficacy and acceptability. As a powerful and reliable method, NMA has been widely applied to explore the potential evidence (Mutz et al., 2019; Zhou et al., 2020).
Hence, we conducted this systematic review and NMA to evaluate all available treatments for depressive symptoms in cognitive impairment, aiming to provide comparative evidence and quantitative hierarchies on both efficacy and acceptability.
Methods
We performed a series of NMAs using the Bayesian model, which strictly conformed to the principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for reporting systematic reviews incorporating NMA of health interventions (Supplementary material 1) (Hutton et al., 2015). We registered our work in the PROSPERO database (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021239621, identifier: CRD42021239621).
Eligibility criteria
Participants
Participants were diagnosed with mild cognitive impairment (MCI) or various types of dementia according to corresponding criteria. According to the ADNI definition, cognitive impairment is found to be consistent with amnestic MCI (Petersen, 2004; Albert et al., 2011). Dementia was defined by the study authors on the basis of diagnostic criteria such as the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) for dementia (American Psychiatric Association, 2013), the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS–ADRDA) criteria for Alzheimer's disease (AD) (McKhann et al., 1984), etc. There were no restrictions on sex, age, ethnicity, nationality, or duration of disease.
Interventions
All available treatments including both pharmacological and non-pharmacological treatments of depressive symptoms in cognitive impairment were carefully considered. Accordingly, we mainly searched several fields of pharmacological and non-pharmacological therapies, such as antidepressants, antipsychotics, N-Methyl-d-aspartate receptor antagonists (NMDA), analgesics, hormones, cognitive stimulation therapy, non-invasive brain stimulation, psychological treatments, multidomain interventions, and so on. Specific potential pharmacological and non-pharmacological treatments that we searched for are listed in Table 1.
Comparators
Placebo, usual care or therapy, and any other corresponding pharmacological or non-pharmacological interventions were eligible.
Outcomes
After a comprehensive investigation of all the scales evaluating symptoms of cognition impairment, we finally selected the Cornell Scale for Depression in Dementia (CSDD) (Alexopoulos et al., 1988), the Hamilton Depression Rating Scale (HDRS) (Endicott et al., 1981), and the Geriatric Depression Scale (GDS) (Yesavage, 1988) to evaluate the alleviation of depressive symptoms; the Neuropsychiatric Inventory (NPI) (Cummings et al., 1994) and the Cohen–Mansfeld Agitation Inventory (CMAI) (Finkel et al., 1992) to appraise the psychiatric condition; and the Mini-Mental State Examination (MMSE) (Folstein et al., 1983) to access the change of cognition impairment. Among the overall adverse events (AEs), we selected the risk of total AEs, diarrhea, headaches, nausea, and severe AEs as secondary outcomes of acceptability because of their highest occurrence. The data that we extracted were the results of the intent-to-treat population using the last observation carried forward method, but some were unavailable.
Information source and literature search
The systematic literature search was performed utilizing databases of MEDLINE, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health (CINAHL), and PsycINFO. The search strategy was characteristically designed for each of the five databases by combining free text, Medical Subject Heading, and EMTREE terms, among others (Supplementary material 2). The search covered English-language articles from inception until August 2022. Each database and registration platform would be retrieved again before completing the NMAs in case of omitting any newly published works. The unpublished studies were retrieved via conference proceedings, clinical trial registries, and author contact. Only potential studies for inclusion were scanned carefully from the reference lists of included studies and related reviews.
Data collection and analysis
Study selection
We only included high-quality randomized controlled trials (RCTs) in English that appraised the efficacy or acceptability of any pharmacological or non-pharmacological intervention treating depressive symptoms in cognitive impairment. Following the eligibility criteria elucidated above, the evaluation and screening of articles were performed by two reviewers independently. When there is any controversy after elaborate discussion, a third reviewer then intervened to make the final decision. After deleting the duplicates, they screened the titles and abstracts of the left literature to select the ones that were worthy of being reviewed in full text. Based on such a rigorous and scientific review, the finally included RCTs were identified.
Data extraction and quality
Baseline characteristics of the included studies and potential effect modifiers were widely abstracted, including age, sex constituent ratio of patients, duration of treatment, the sample size of trials, the dosage of treatments, baseline scores of MMSE, CSDD, GDS, and NPI, and completed ratio of participants. Then, primary outcomes of efficacy and secondary outcomes of acceptability were extracted, carefully considering the methods of drug delivery, schedules of drug administration, the context of non-pharmacological treatment, etc.
Risk of bias assessment
The risk of bias of included RCTs was strictly evaluated according to the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins et al., 2019), which appraises six aspects including random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias. This evaluation was done by two reviewers independently to assign a level of risk of bias (high risk, unclear risk, and low risk) for each item, and when there was any controversy, a third reviewer would give the final assessment. Overall, RCTs included in our NMAs had a relatively acceptable low risk of biases across different parameters scored. Additionally, the non-pharmacological treatment could not realize the concealment of patients, thus causing minimal bias which is usually allowed.
Outcome measurement
The primary outcomes were described by the changed scores of the scales, which belong to continuous data and were computed as mean difference (MD) and 95% credibility intervals (CrI). The secondary outcomes of acceptability were described by the number of adverse events that occurred, which belong to discontinuous data, and were computed as the hazard ratio (HR) and 95% CrIs.
Data synthesis and statistical analysis
Our systematic review and NMAs were done across all types of dementia and MCI to derive the overall efficacy and acceptability of comprehensive therapies for depressive symptoms in cognitive impairment. Initially, we summarized and examined baseline data of characteristics of the involved RCTs and patients to access the clinical and methodological heterogeneity. Additionally, the geometry of the network of comparisons across trials was connected to make sure each included RCT would be involved in our NMAs. Then, traditional pairwise meta-analyses were done to anticipate the heterogeneity and publication bias among the RCTs before NMAs. The heterogeneity was assessed by I2 statistic, and the publication bias was judged by funnel plots.
Next, NMAs were conducted within a Bayesian hierarchical model framework to estimate all the included valuable treatments. We adopted the random-effect model in our NMAs because it could be the most appropriate and advisable methodology in consideration of between-study heterogeneities (Mills et al., 2013). Specifically, models were applied using four chains of Markov Chain Monte Carlo estimation running for 100,000 iterations with thinning of 10, and the first 20,000 iterations were discarded as burn-in after visual inspection of the mixing chains. The convergence was estimated by visually examining the iteration plot and the potential scale reduction factor. Overall, the process above was performed in R version 4.0.4.
Additionally, we carefully considered transitivity and similarity, based on which assumption was made by comparing the distribution of studies and baseline characteristics of participants and by examining potential effect modifiers such as age, the timing of exposure, and the risk of bias. Besides, since a large number of treatments may lead to unavailable cases, the common within-network between-study variance (τ2) across comparisons was presumed for all comparisons in the entire networks. As for consistency, the design-by-treatment interaction model was adapted to examine the consistency of NMAs. If the inconsistency was tracked without identifying any discrepancy to blame, we then appraised the local inconsistency of each network loop using a loop-specific method (Veroniki et al., 2013). Furthermore, additional analyses were done to enhance the scientificity and preciseness of this NMA, such as sensitivity analysis and subgroup analysis.
Results
Literature search and description of studies
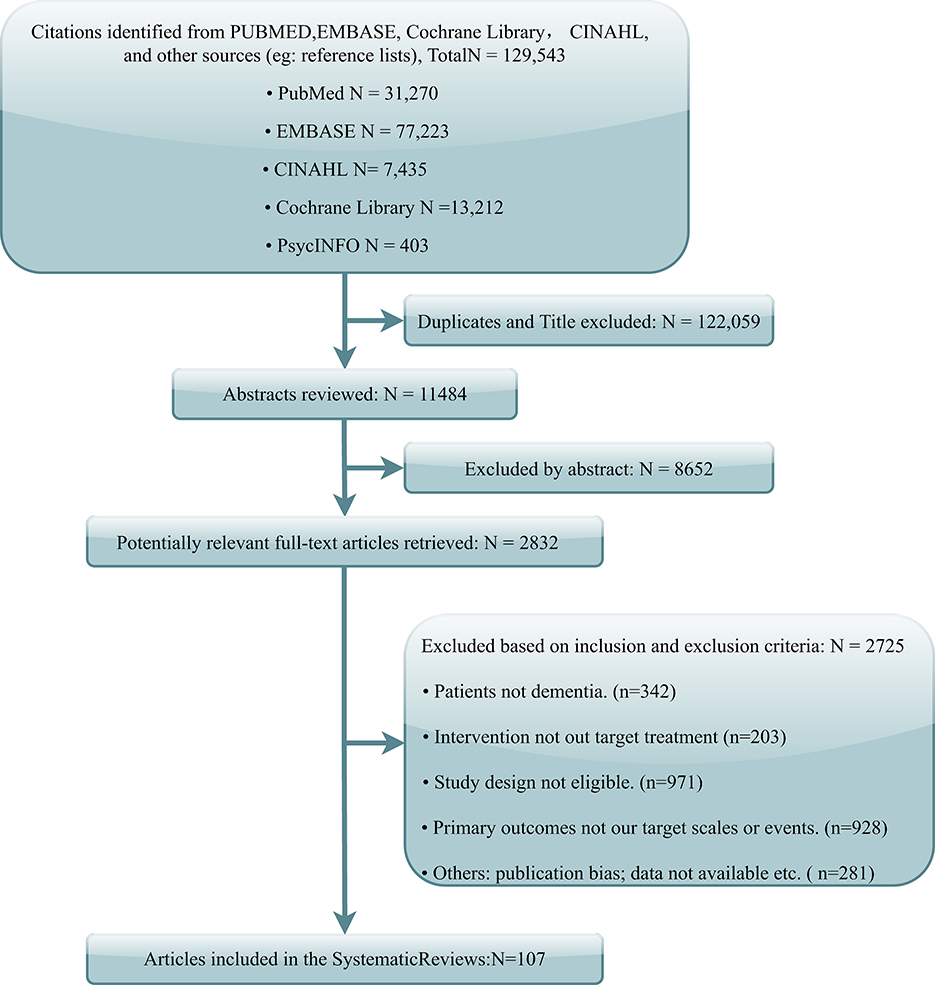
The literature search yielded 129,543 potentially relevant records. After the deletion of duplicates and titles, 11,484 abstracts were screened, and 2,832 articles were left for full-text review. Finally, 107 articles were identified referring to the inclusion criteria (Figure 1). Notably, one study fulfilled all criteria but was excluded from the NMA because of the apparent publication bias displayed in funnel plots and recognized by three reviewers (Rodriguez-Mansilla et al., 2015).
Characteristics of the studies
In this study, 13,043 participants from 107 RCTs were included, involving 13 pharmacological treatments, 15 non-pharmacological treatments, and the discontinuation of antidepressants. The weighted network plots of efficacy and safety are described in Figures 2A,B. The characteristics of these RCTs are displayed in Supplementary material 3.
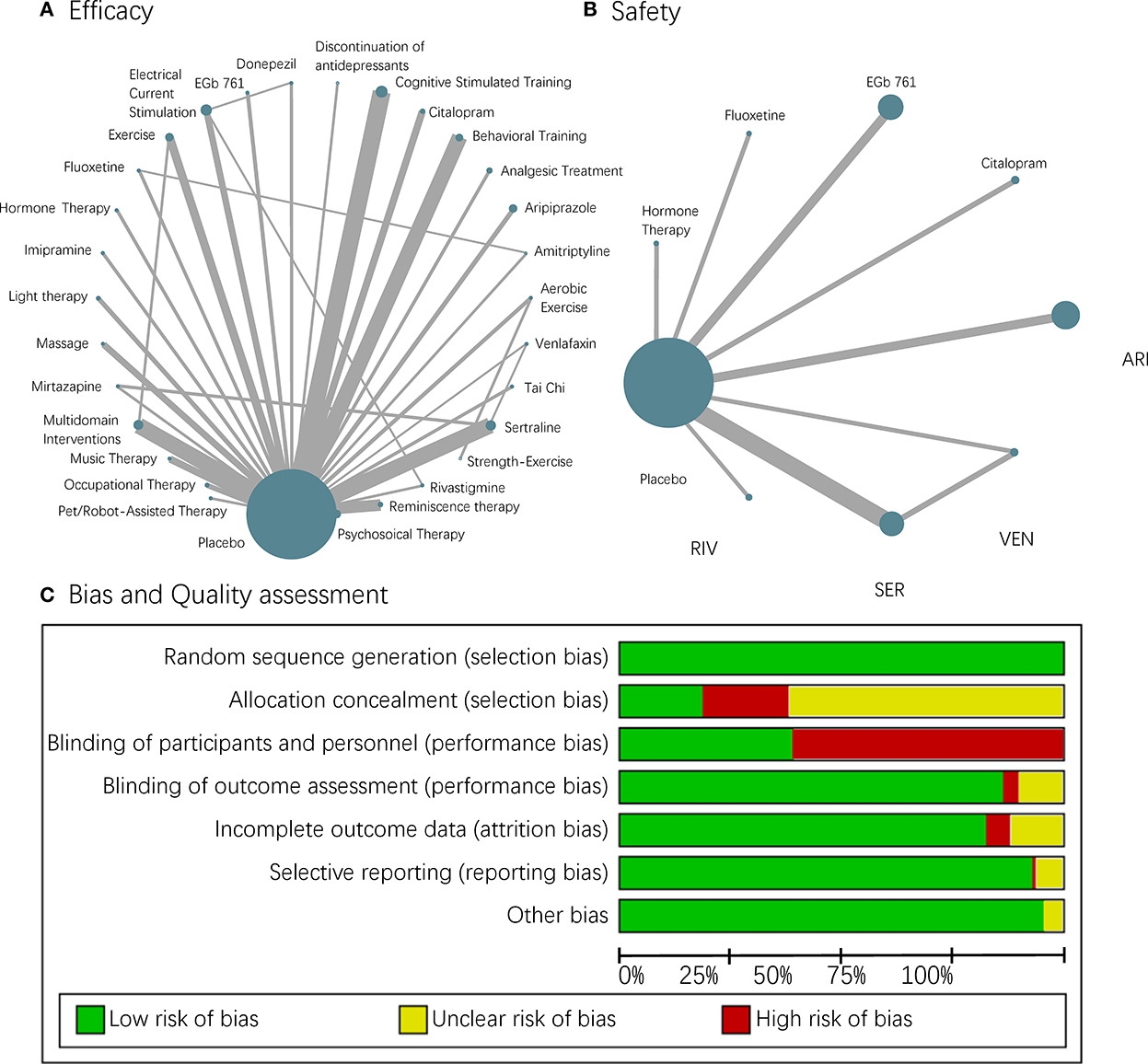
Figure 2. A network plot and risk of bias. (A,B) Each node in the network geometry represents a kind of intervention. Nodes will be linked by a line when the treatments are directly comparable with “head-to-head trails.” The size of the nodes corresponds to the number of participants receiving that intervention, and the width of the lines is proportional to the number of RCTs this comparison included. (C) Risk of bias chart of studies included in the quantitative analysis.
Risk of bias
The risk of bias within each study included in our NMAs was acceptable (Figure 2C), though the blinding of participants and personnel in non-pharmacological interventions was impossible and appeared to cause a high risk. Funnel plots were also employed to evaluate the publication bias, and they were all visualized as being symmetric generally (Supplementary material 4). The heterogeneity described by I2 was small, and the overall stability of NMAs revealed from sensitivity analysis was quite well, except for a minimal hesitation on MMSE (Supplementary material 5). Therefore, we further did a subgroup analysis of AD to explore the potential effect of diagnosis and gained generally consistent results with the whole group (Supplementary materials 6–8).
Grading the evidence
We used the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach to grade the quality of underlying evidence and the strength of recommendations in this study. Our GRADE judgments focused on six aspects, including within-study bias, reporting bias, indirectness, imprecision, heterogeneity, and incoherence, and finally led to a good confidence rating. Additionally, we performed this evaluation using CINeMA, which is recommended for assessing confidence in the results of a network meta-analysis (Nikolakopoulou et al., 2020) (Supplementary material 9).
Efficacy
Depression
The NMA on CSDD was performed across 20 treatments and the discontinuation of antidepressants, based on 46 RCTs with 5,143 patients. The results showed that seven treatments showed significant differences superior to placebo including aerobic exercise (MD −4.51, 95%CrI −8.60 to −0.37), aripiprazole (MD −1.86, 95%CrI −3.66 to −0.02), behavioral training (MD −1.14, 95%CrI −2.04 to −0.34), electrical current stimulation (MD −3.30, 95%CrI −5.94 to −0.73), massage (MD −12.67, 95%CrI −14.71 to −10.59), music therapy (MD −2.63, 95%CrI −4.72 to −0.58), and reminiscence therapy (MD −2.34, 95%CrI −3.51 to −1.25). Massage outperformed all the other 21 interventions. Interventions such as aerobic exercise, behavioral training, and electrical current stimulation also demonstrated significant efficacy, while the venlafaxine behaved quite unsatisfactorily. Antidepressants that were discontinued displayed no significant harm. Specific results of efficacy and elaborate rank of the hierarchy are shown in Figure 3A and Supplementary materials 10A, 11A.
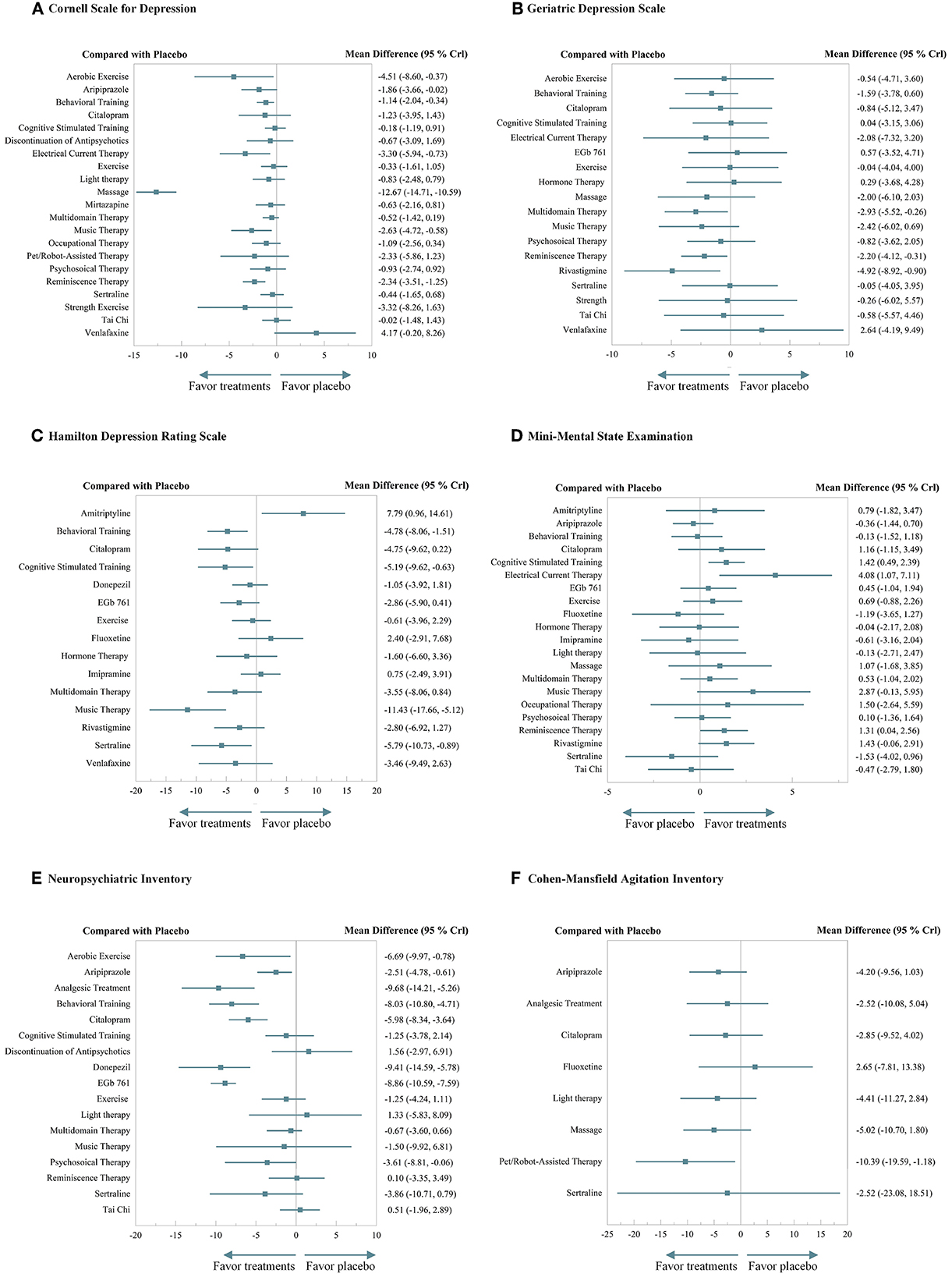
Figure 3. A forest plot of efficacy. (A) Cornell Scale for Depression. (B) Geriatric Depression Scale. (C) Hamilton Depression Rating Scale. (D) Mini-Mental State Examination. (E) Neuropsychiatric Inventory. (F) Cohen–Mansfeld Agitation Inventory.
The NMA on GDS was performed across 18 treatments based on 31 RCTs with 2,872 patients. The results demonstrated that multidomain interventions (MD −2.93, 95%CrI −5.52 to −0.26), reminiscence therapy (MD −2.20, 95%CrI −4.12 to −0.31), and rivastigmine (MD −4.92, 95%CrI −8.92 to −0.90) significantly outperformed the placebo, and other interventions also demonstrated an effective tendency over placebo, though without significance. Specific results of efficacy and elaborate rank of the hierarchy are shown in Figure 3B and Supplementary materials 10B, 11B.
The NMA on HDRS was performed across 15 treatments based on 19 RCTs with 1,591 patients. The results indicated that behavioral training (MD −4.78, 95%CrI −8.06 to −1.51), cognitive stimulation therapy (MD −5.19, 95%CrI −9.62 to −0.63), music therapy (MD −11.43, 95%CrI −17.66 to −5.12), and sertraline (MD −5.79, 95%CrI −10.73 to −0.89) significantly outperformed the placebo. Music therapy outperformed nine other treatments with significant differences, while amitriptyline performed disappointingly. Specific results of efficacy and elaborate rank of the hierarchy are shown in Figure 3C and Supplementary materials 10C, 11C.
Cognition
The NMA on MMSE was performed across 21 treatments based on 53 RCTs with 5,995 patients. The results indicated that cognitive stimulation therapy (MD 1.42, 95%CrI 0.49 to 2.39), electrical current stimulation (MD 4.08, 95%CrI 1.07 to 7.11), and reminiscence therapy (MD 1.31, 95%CrI 0.04 to 2.56) significantly outperformed the placebo. Especially, electrical current stimulation significantly outperformed the other 10 treatments, while sertraline performed inferior to the three treatments. Specific results of efficacy and elaborate rank of the hierarchy are shown in Figure 3D and Supplementary materials 10D, 11D.
Behavior
The NMA on NPI was performed across 17 treatments based on 33 RCTs with 6,524 patients. The results suggested that aerobic exercise (MD −6.69, 95%CrI −9.97 to −0.78), aripiprazole (MD −2.51, 95%CrI −4.78 to −0.61), analgesic treatment (MD −9.68, 95%CrI −14.21 to −5.26), behavioral training (MD −8.03, 95%CrI −10.80 to −4.71), citalopram (MD −5.98, 95%CrI −8.34 to −3.64), donepezil (MD −9.41, 95%CrI −14.59 to −5.78), EGb761 (MD −8.86, 95%CrI −10.59 to −7.59), and psychosocial therapy (MD −3.61, 95%CrI −8.81 to −0.06) significantly outperformed the placebo. Citalopram significantly outperformed five treatments. Analgesic treatment, behavioral training, and EGb761 also demonstrated beneficial significance over others. Besides, the discontinuation of antidepressants did not show significant harm. Specific results of efficacy and elaborate rank of the hierarchy are shown in Figure 3E and Supplementary materials 10E, 11E.
The NMA on CMAI was performed across 8 treatments based on 11 RCTs with 1,508 patients. The results demonstrated that pet/robot-assisted therapy (MD −10.39, 95%CrI −19.59 to −1.18) significantly outperformed the placebo. Specific results of efficacy and elaborate rank of the hierarchy are shown in Figure 3F and Supplementary materials 10F, 11F.
Acceptability
The reported data on adverse events from the included RCTs were carefully considered based on eight pharmacological treatments. NMAs were conducted over the incidence of total adverse events and the four most common and important adverse events, including headache, nausea, diarrhea, and serious adverse events. Overall, none of the treatments showed any significance compared to the placebo, while most of them had a riskier tendency than the placebo. In terms of total adverse events, severe AEs, and nausea, venlafaxine seemed to be the most likely to cause adverse events. EGB 761 and citalopram performed well usually, with relatively lower risk. Specific results of efficacy and elaborate rank of the hierarchy are shown in Figure 4 and Supplementary materials 12, 13.

Figure 4. A forest plot of acceptability. (A) Total adverse events. (B) Severe adverse events. (C) Diarrhea. (D) Headache. (E) Nausea.
Discussion
Our NMAs are based on 107 RCTs, including 13,043 patients with depressive symptoms in cognitive impairment, who were randomly assigned to 13 pharmacological treatments, 15 non-pharmacological treatments, and the discontinuation of antidepressants. We evaluated all available and high-quality treatments for depressive symptoms in cognitive impairment concerning four aspects of depression, cognition, behavior, and acceptability, providing comparative evidence and quantitative hierarchies using the NMA method. Our objective was to quantitatively determine if all the most common 28 treatments helped patients with depressive symptoms in cognitive impairment and if the discontinuation of antidepressants did significantly harm patients.
Overall, our results suggested that non-pharmacological treatments could be beneficial in reducing symptoms of depression and may be even more efficacious than pharmacological ones. On depression, NMA on CSDD revealed that six treatments could provide more benefits than control care or placebo, including aerobic exercise, behavioral training, electrical current stimulation, massage, music therapy, and reminiscence therapy. However, no pharmacological treatments presented any statistical significance superior to a placebo on depression, some of which even had a tendency of aggravation, such as venlafaxine and amitriptyline. On cognition, non-pharmacological interventions such as cognitive stimulation therapy, electrical current stimulation, and reminiscence therapy outperformed the usual care and pharmacological ones. On behavior, non-pharmacological interventions also behaved quite well, especially for the pet/robot-assisted therapy on agitation and aerobic exercise on total neuropsychiatric symptoms. Moreover, NMAs of acceptability revealed that most pharmacological treatments were associated with a riskier tendency of adverse events, indicating potential poor acceptability. Hence, our analysis suggested that non-pharmacological treatments could provide more benefits and cause less risk, which should be applied more widely and at least considered as an alternative in clinical settings.
Specifically, among the non-pharmacological treatments, electrical current stimulation, aerobic exercise, and reminiscence therapy are quite recommended due to their satisfactory performance in both depression and cognition. When considering the behavioral problem, aerobic exercise may be the first choice due to its beneficial performance in all aspects. Although massage demonstrated significant efficacy over usual care, there should be hesitation in interpretation due to the limited data and asymmetric funnel plot. As for the pharmacological treatments, aripiprazole and citalopram may own the most possibility to have some benefits on depression, which also showed significant improvements on NPI and no harm to MMSE. We also noted that the efficacy of pharmacological treatments on the alleviation of neuropsychiatric symptoms according to NMA on NPI should be claimed, which reminds us that there may be a combined treatment when there are severe neuropsychiatric problems. Besides, no significant harm caused by the discontinuation of antidepressants was observed for both depressive and neuropsychiatric symptoms. A clinical treatment strategy should be designed concerning the specific situation of physical and mental health of patients to figure out the most beneficial simple or combined interventions. In addition, we called for more RCTs on combined interventions to help us better assess and design the clinical treatment strategy.
Pursuing the credibility of evidence, the bias of risk accessed by the Cochrane risk of bias tool implies relatively low bias, though some unclear bias may come from the blinding inadequacy due to the methodological shortcoming that non-pharmacological approaches are unable to realize double-blind. No important discrepancies across the direct comparisons in the distribution of study characteristics were observed after attempting to examine the potential effect modifiers on transitivity. Funnel plots were evaluated visually and believed to be symmetrical after excluding one; thus, publication bias is unlikely with our comprehensive search strategy. Subgroup analysis of AD was done to explore the potential heterogeneity from diagnosis and delivered consistent conclusions. Besides, we appraise the network inconsistency through node-split modeling.
It should be highlighted that we have several strengths. First, as there is a lack of evidence, this study may be the first attempt to quantitatively synthesize the efficacy and safety of treatments for depressive symptoms in cognitive impairment by the NMA method. The included RCTs were concerned with both pharmacological and non-pharmacological approaches, all of which strictly followed the inclusion/exclusion criteria with high quality and low bias. Second, based on the NMA method, not only the head-to-head studies but also the indirect comparisons were comprehensively analyzed, which gave rise to comparative evaluation and derived hierarchies. Third, we performed NMAs on three aspects of efficacy, including depression, cognition, and behavior, utilizing six professional scales, aiming at providing a more specific evaluation and description of the benefits and harms. In addition, acceptability was carefully assessed to rise some attention to clinical prescriptions. Fourth, our conclusions were based on a substantial number of patients and RCTs compared with the previous knowledge syntheses, giving rise to a great guarantee of precision and credibility.
Our analysis also has limitations. First, for some treatments, the paucity of reported RCTs may limit the comprehensiveness and power of this analysis. Second, to maintain the precision of conclusions, the review was restricted to high-quality trials of a single intervention, given that most combined ones were inconsistent and lacked enough data. Third, since the number of patients and comprehensive interventions are quite large, some biases may be inevitable, such as the discrepancies in duration and gender radio, though we have tried our best to appraise and avoid them. Fourth, since different scales may have incompatible abilities in evaluation owing to their intrinsic characteristics, the divergences of their results should be interpreted modestly.
Conclusion
Our NMAs indicated that non-pharmacological interventions were more efficacious and safe than pharmacological treatments for treating depressive symptoms in cognitive impairment. Specifically, electrical current stimulation, aerobic exercise, and reminiscence therapy were quite recommended considering their beneficial performance on both depression and cognition. Additionally, since there were some limitations, we expect to update and revise our NMAs furthermore.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
HL, LQ, and BJ developed the concept and design of the study. BJ and BZ conducted systematic literature searches, extracted the data, and rated the risk of bias and methodological quality criteria of included studies, under the supervision of YX. LQ and BJ analyzed and interpreted the data. BJ wrote the first draft of the manuscript. All authors have contributed to the further revision and have approved the final manuscript.
Funding
This work was supported by Scientific Research Funding Project of Education Department of Liaoning Province (JC2019014) and Natural Science Foundation of Liaoning Province of China (2020-MS-156).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.1037414/full#supplementary-material
Supplementary material 1. PRISMA NMA checklist.
Supplementary material 2. Searching strategies of the current network meta-analysis.
Supplementary material 3. Baseline characteristics of the included randomized controlled trails.
Supplementary material 4. Comparison-adjusted funnel plot. The red vertical line represents the null hypothesis that independent effect size estimates do not differ from the comparison-specific pooled estimates.
Supplementary material 5. Sensitivity and heterogeneity analysis. When the difference in DIC between the random-effect and fixed-effect models is close, the models could be viewed as stable and reliable. When the I2% is <28%, we can conclude that the heterogeneity is relatively minor.
Supplementary material 6. A subgroup analysis of Alzheimer's disease (forest plot of efficacy).
Supplementary material 7. A subgroup analysis of Alzheimer's disease (league tables of efficacy). The diagonal gives the different treatments examined in the network meta-analyses. The efficacy data are given as mean difference (MD) with a 95% credible interval (CrI), in which each cell indicates the values for a specific contrast between the treatments.
Supplementary material 8. A subgroup analysis of Alzheimer's disease (relative ranks of efficacy). The derived hierarchies (relative ranks) are described from the most to the least effective and the most effective to the least.
Supplementary material 9. Grades of Recommendation, Assessment, Development, and Evaluation (GRADE).
Supplementary material 10. League tables of efficacy. The diagonal gives the different treatments examined in the network meta-analyses. The efficacy data are given as mean difference (MD) with a 95% credible interval (CrI), in which each cell indicates the values for a specific contrast between the treatments.
Supplementary material 11. Relative ranks of efficacy. The derived hierarchies (relative ranks) are described from the most to the least effective and the most effective to the least.
Supplementary material 12. League tables of acceptability. The diagonal gives the different treatments examined in the network meta-analyses. The efficacy data are given as hazard ratio (HR) with a 95% credible interval (CrI), in which each cell indicates the values for a specific contrast between the treatments.
Supplementary material 13. Relative ranks of acceptability. The derived hierarchies (relative ranks) are described from the most to the least effective and the most acceptable to the least.
References
Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers. Dement. 7, 270–9. doi: 10.1016/j.jalz.2011.03.008
Alexopoulos, G. S., Abrams, R. C., Young, R. C., and Shamoian, C. A. (1988). Cornell scale for depression in dementia. Biol. Psychiatry. 23, 271–84. doi: 10.1016/0006-3223(88)90038-8
American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Washington, DC: American Psychiatric Publishing. doi: 10.1176/appi.books.9780890425596
Baruch, N., Burgess, J., Pillai, M., and Allan, C. L. (2019). Treatment for depression comorbid with dementia. Evid. Based Ment. Health 22, 167–71. doi: 10.1136/ebmental-2019-300113
Bennett, S., Laver, K., Voigt-Radloff, S., Letts, L., Clemson, L., Graff, M., et al. (2019). Occupational therapy for people with dementia and their family carers provided at home: a systematic review and meta-analysis. BMJ Open. 9, e026308. doi: 10.1136/bmjopen-2018-026308
Bennett, S., and Thomas, A. J. (2014). Depression and dementia: cause, consequence or coincidence? Maturitas 79, 184–90. doi: 10.1016/j.maturitas.2014.05.009
Bingham, K. S., Flint, A. J., and Mulsant, B. H. (2019). Management of late-life depression in the context of cognitive impairment: a review of the recent literature. Curr. Psychiatry Rep. 21, 74. doi: 10.1007/s11920-019-1047-7
Byers, A. L., and Yaffe, K. (2011). Depression and risk of developing dementia. Nat. Rev. Neurol. 7, 323. doi: 10.1038/nrneurol.2011.60
Cummings, J. L., Mega, M., Gray, K., Rosenberg-Thompson, S., Carusi, D. A., Gornbein, J., et al. (1994). The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 44, 2308. doi: 10.1212/WNL.44.12.2308
Diniz, B. S., Butters, M. A., Albert, S. M., Dew, M. A., and Reynolds, C. F. (2013). 3rd. Late-life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta-analysis of community-based cohort studies. Br. J. Psychiatry. 202, 329–35. doi: 10.1192/bjp.bp.112.118307
Dudas, R., Malouf, R., McCleery, J., and Dening, T. (2018). Antidepressants for treating depression in dementia. Cochrane Database Syst. Rev. 8, CD003944. doi: 10.1002/14651858.CD003944.pub2
Endicott, J., Cohen, J., Nee, J., Fleiss, J., and Sarantakos, S. (1981). Hamilton Depression Rating Scale: extracted from regular and change versions of the Schedule for Affective Disorders and Schizophrenia. Arch. Gen. Psychiatry. 38, 98–103. doi: 10.1001/archpsyc.1981.01780260100011
Farina, N., Morrell, L., and Banerjee, S. (2017). What is the therapeutic value of antidepressants in dementia? A narrative review. Int. J. Geriatr. Psychiatry. 32, 32–49. doi: 10.1002/gps.4566
Finkel, S. I., Lyons, J. S., and Anderson, R. L. (1992). Reliability and validity of the Cohen–Mansfield agitation inventory in institutionalized elderly. Int. J. Geriatr. Psychiatry. 7, 487–90. doi: 10.1002/gps.930070706
Folstein, M. F., Robins, L. N., and Helzer, J. E. (1983). The mini-mental state examination. Arch. Gen. Psychiatry 40, 812. doi: 10.1001/archpsyc.1983.01790060110016
Hall, C. A., and Reynolds-Iii, C. F. (2014). Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas 79, 147–52. doi: 10.1016/j.maturitas.2014.05.026
Higgins, J. P., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., et al. (2019). Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: John Wiley and Sons. doi: 10.1002/9781119536604
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162, 777–84. doi: 10.7326/M14-2385
Kessing, L. V. (2012). Depression and the risk for dementia. Curr. Opin. Psychiatry 25, 457–61. doi: 10.1097/YCO.0b013e328356c368
Kessing, L. V., Harhoff, M., and Andersen, P. K. (2007). Treatment with antidepressants in patients with dementia-a nationwide register-based study. Int. Psychogeriatr. 19, 902. doi: 10.1017/S1041610206004376
Li, H. C., Wang, H. H., Lu, C. Y., Chen, T. B., Lin, Y. H., Lee, I., et al. (2019). The effect of music therapy on reducing depression in people with dementia: a systematic review and meta-analysis. Geriatr. Nurs. 40, 510–6. doi: 10.1016/j.gerinurse.2019.03.017
Li, X., Guo, R., Wei, Z., Jia, J., and Wei, C. (2019). Effectiveness of exercise programs on patients with dementia: a systematic review and meta-analysis of randomized controlled trials. Biomed Res. Int. 2019, 2308475. doi: 10.1155/2019/2308475
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., Stadlan, E. M., et al. (1984). Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34, 939–44. doi: 10.1212/WNL.34.7.939
Mills, E. J., Thorlund, K., and Ioannidis, J. P. A. (2013). Demystifying trial networks and network meta-analysis. BMJ. 346, f2914. doi: 10.1136/bmj.f2914
Mutz, J., Vipulananthan, V., Carter, B., Hurlemann, R., Fu, C. H. Y., Young, A. H., et al. (2019). Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: systematic review and network meta-analysis. BMJ. 364, l1079. doi: 10.1136/bmj.l1079
Nikolakopoulou, A., Higgins, J. P. T., Papakonstantinou, T., Chaimani, A., Del Giovane, C., Egger, M., et al. (2020). CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 17, e1003082. doi: 10.1371/journal.pmed.1003082
Orgeta, V., Tabet, N., Nilforooshan, R., and Howard, R. (2017). Efficacy of antidepressants for depression in Alzheimer's disease: systematic review and meta-analysis. J. Alzheimers. Dis. 58, 725–33. doi: 10.3233/JAD-161247
Petersen, R. C. (2004). Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256, 183–94. doi: 10.1111/j.1365-2796.2004.01388.x
Rapp, M. A., Schnaider-Beeri, M., Wysocki, M., Guerrero-Berroa, E., Grossman, H. T., Heinz, A., et al. (2011a). Cognitive decline in patients with dementia as a function of depression. Am. J. Geriatr. Psychiatry. 19, 357–63. doi: 10.1097/JGP.0b013e3181e898d0
Rapp, M. A., Schnaider-Beeri, M., Wysocki, M., Guerrero-Berroa, E., Grossman, H. T., Heinz, A., et al. (2011b). Cognitive decline in patients with dementia as a function of depression. Am. J. Geriatr. Psychiatry 19, 357–63.
Rodriguez-Mansilla, J., Gonzalez Lopez-Arza, M. V., Varela-Donoso, E., Montanero-Fernandez, J., Gonzalez Sanchez, B., Garrido-Ardila, E. M., et al. (2015). The effects of ear acupressure, massage therapy and no therapy on symptoms of dementia: a randomized controlled trial. Clin. Rehabil. 29, 683–93. doi: 10.1177/0269215514554240
Saczynski, J. S., Beiser, A., Seshadri, S., Auerbach, S., Wolf, P., Au, R., et al. (2010). Depressive symptoms and risk of dementia: the Framingham Heart Study. Neurology 75, 35–41. doi: 10.1212/WNL.0b013e3181e62138
Solfrizzi, V., D'Introno, A., Colacicco, A. M., Capurso, C., Del Parigi, A., Caselli, R. J., et al. (2007). Incident occurrence of depressive symptoms among patientswith mild cognitive impairment - the Italian longitudinal study on aging. Dement. Geriatr. Cogn. Disord. 24, 55–64. doi: 10.1159/000103632
Vaughan, L., Corbin, A. L., and Goveas, J. S. (2015). Depression and frailty in later life: a systematic review. Clin. Interv. Aging 10, 1947–58. doi: 10.2147/CIA.S69632
Veroniki, A. A., Vasiliadis, H. S., Higgins, J. P. T., and Salanti, G. (2013). Evaluation of inconsistency in networks of interventions. Int. J. Epidemiol. 42, 332–45. doi: 10.1093/ije/dys222
Woods, B., O'Philbin, L., Farrell, E. M., Spector, A. E., and Orrell, M. (2018). Reminiscence therapy for dementia. Cochrane Database Syst. Rev. 3, CD001120. doi: 10.1002/14651858.CD001120.pub3
World Health Organization. (2021). Dementia. Geneva: WHO. Available online at: https://wwwwhoint/news-room/fact-sheets/detail/dementia (accessed December 2, 2022).
Zafra-Tanaka, J. H., Pacheco-Barrios, K., Tellez, W. A., and Taype-Rondan, A. (2019). Effects of dog-assisted therapy in adults with dementia: a systematic review and meta-analysis. BMC Psychiatry 19, 41. doi: 10.1186/s12888-018-2009-z
Zhou, X., Teng, T., Zhang, Y., Del Giovane, C., Furukawa, T. A., Weisz, J. R., et al. (2020). Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: a systematic review and network meta-analysis. Lancet Psychiatry 7, 581–601. doi: 10.1016/S2215-0366(20)30137-1
Keywords: depressive symptoms, cognitive impairment, network meta-analysis, non-pharmacological (NON-Mesh), pharmacological
Citation: Jin B, Xv Y, Zhang B, Qiao L and Liu H (2022) Comparative efficacy and acceptability of treatments for depressive symptoms in cognitive impairment: A systematic review and Bayesian network meta-analysis. Front. Aging Neurosci. 14:1037414. doi: 10.3389/fnagi.2022.1037414
Received: 05 September 2022; Accepted: 18 November 2022;
Published: 12 December 2022.
Edited by:
Eddy A. Van Der Zee, University of Groningen, NetherlandsReviewed by:
Yingshi Zhang, Shenyang Pharmaceutical University, ChinaMartien Kas, University of Groningen, Netherlands
Copyright © 2022 Jin, Xv, Zhang, Qiao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Qiao, cWlhb2xlaV9jbXUyaEAxNjMuY29t; Huayan Liu, bGl1aHVheWFuY211QDE2My5jb20=
 Boru Jin
Boru Jin Yunting Xv3
Yunting Xv3 Bixuan Zhang
Bixuan Zhang Huayan Liu
Huayan Liu