
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 31 October 2022
Sec. Alzheimer's Disease and Related Dementias
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.1032494
Probiotics have been suggested as an effective adjuvant treatment for Alzheimer’s disease (AD) due to their modulating effect on the gut microbiota, which may affect the gut-brain axis. Therefore, we aimed to evaluate the effects of two different single-strain probiotics on cognition, physical activity, and anxiety in subjects with mild and moderate AD. Eligible patients (n = 90) with AD were randomly assigned to either of two interventions [Lactobacillus rhamnosus HA-114 (1015 CFU) or Bifidobacterium longum R0175 (1015 CFU)] or placebo group, receiving probiotic supplement twice daily for 12 weeks. The primary outcome of the study was cognitive function measured by using the two tests, namely, the Mini-Mental State Examination (MMSE) and the categorical verbal fluency test (CFT). Secondary outcomes included a performance in Activities of Daily Living (ADL), the Lawton Instrumental Activities of Daily Living (IADL) scale, and the Generalized Anxiety Disorder (GAD-7) scale. Linear mixed-effect models were used to investigate the independent effects of probiotics on clinical outcomes. After 12 weeks, MMSE significantly improved cognition (PInteraction < 0.0001), with post hoc comparisons identifying significantly more improvement in the B. longum intervention group (differences: 4.86, 95% CI: 3.91–5.81; P < 0.0001) compared with both the placebo and L. rhamnosus intervention groups (differences: 4.06, 95% CI: 3.11–5.01; P < 0.0001). There was no significant difference between the two intervention groups (differences: −0.8, 95% CI: −1.74 to 0.14; P = 0.09). In conclusion, this trial demonstrated that 12-week probiotic supplementation compared with placebo had beneficial effects on the cognition status of patients with AD.
Alzheimer’s disease (AD) is a neurodegenerative and irreversible disease with a cognitive and behavioral impairment that accounts for 60–80% of dementia cases (Porsteinsson et al., 2021). Pathologically, the accumulation of beta-amyloid plaques outside neurons and hyperphosphorylated tau (pTau) tangles inside neurons are two of several brain changes associated with AD (Kincaid et al., 2021). Currently, more than 50 million individuals are living with dementia around the world, and as the population ages, this number will be tripled to 152 million by 2050 (International AsD, 2019); AD is the seventh leading cause of death among all diseases. In addition to cognitive impairment, AD has neuropsychiatric symptoms, of which the most common are anxiety and depression. Anxiety and depression can cause major problems for caregivers, reduce the patient’s quality of life, and increase hospitalization and mortality (Mendez, 2021). AD can also impair the patient’s ability to perform activities of daily living (ADL) and increase the patient’s dependence on caregivers (Clemmensen et al., 2020). The total annual cost of AD and other types of dementia in the United States is currently $305 billion and is expected to exceed $1.1 trillion by 2050 (Porsteinsson et al., 2021). The main risk factors for AD are age, genetics, and family history. However, studies have shown that obesity, type 2 diabetes, and gut dysbiosis are also risk factors for AD (Alzheimer’s Association, 2010). Unfortunately, the approved medicine is less effective on dementia, especially AD, and cannot stop the progression of the disease (Hazar et al., 2016); besides, these drugs have negative effects on the gut microbiome and can worsen the gut microbiome in the long-term. Therefore, adjuvant therapy with prebiotics/probiotics may prevent and/or cure gut dysbiosis and, therefore, can increase the therapeutic effects of AD drugs (Hort et al., 2020). Probiotics are a group of live microorganisms that are believed to have a variety of health benefits for the host such as anti-obesity, cancer-preventing effects, reducing anxiety, and increasing lifespan. They are usually added to yogurt or taken as a dietary supplement and are often called good or friendly bacteria. In addition, studies have shown that aging can decrease good bacteria in the gut especially Bifidobacterium and Lactobacillus (Oelschlaeger, 2010; Azm et al., 2017). Yet, few studies have been conducted on the relationship between probiotic supplements and cognitive status. Recent trials have used a single-strain probiotic to determine exactly which strain is more effective (Akbari et al., 2016; Azm et al., 2017; Musa et al., 2017; Leblhuber et al., 2018; Kobayashi et al., 2019). Based on the findings, this trial was designed to evaluate the effect of two different single-strain probiotics on cognitive status in subjects with mild and moderate AD.
This was a 12-week multicenter, randomized, parallel, double-blind, and placebo-controlled clinical trial to observe the effect of probiotic supplementation on the cognitive status of patients with AD. This trial was performed in hospitals under the supervision of the Tehran University of Medical Sciences (TUMS), Iran. Our study was done according to the Declaration of Helsinki in 1975 and was also approved by the Ethics Committee of the Tehran University of Medical Sciences and registered on the Iranian Website for Registration of Clinical Trials IRCT (IRCT number: 20210513051277N1). We informed all the subjects about the risks and benefits of the study and reassured them that they could leave the study whenever they wanted. Written informed consent was received from all participants.
A total of 90 older adults with mild and moderate AD took part in this clinical trial from October 2021 to March 2022. They were assigned to three groups (one group received Lactobacillus rhamnosus HA-114, the other one received Bifidobacterium longum R0175, and the last one took a placebo). The intervention started simultaneously in October 2021, and the last observation finished in May 2022. AD was diagnosed based on the NINDS-ADRDA criteria (Mckhann, 1984) and the received criteria from the National Institute on Aging Alzheimer’s Association (Jack et al., 2011). The inclusion criteria were as follows: aged 50–90 years, ability to tolerate oral medication, and mild or moderate AD based on the Functional Assessment Staging Tool (FAST) score 4-6B.
The exclusion criteria were as follows: (1) allergy to probiotic supplements, (2) reluctance to continue cooperation, (3) drastic changes in diet, (4) inflammatory diseases that lead to long-term use (more than 2 weeks) of anti-inflammatory drugs, (5) taking antibiotics, pre-probiotic or probiotic products, or multivitamin and mineral supplements during the intervention, (6) having any kind of infection or another disease like COVID-19, (7) changing the type of drug during the intervention, and (8) enrollment in any other intervention trial.
Stratified permute block randomization was performed in two categories based on age and sex by using the site www.randomization.com. In this method, one of the letters A, B, and C was assigned to each group and randomization was done in six blocks. Within each block, patients were randomly assigned to one of the three study groups in a 1:1:1 ratio. Probiotic supplements were coded by Lallemand Company and all the investigators, research coordinators, participants, caregivers, and data analysts were kept blind to treatment allocation throughout the trial.
Eligible participants were allocated in a 1:1 ratio to receive either of two interventions [a probiotic capsule containing L. rhamnosus HA (each capsule contained 1015 CFU probiotic) or a probiotic capsule containing B. longum R0175 (each capsule contained 1015 CFU probiotic) two times a day] or the placebo (one capsule containing xylitol, maltodextrin, and malic acid two times a day). All supplements provided by Lallemand Company in Canada were similar in color, taste, smell, and size. Caregivers were guided to store the supplements in the refrigerator and gave two capsules daily to patients, one after lunch and one after dinner. The supplements were delivered to caregivers monthly. To remind the regular consumption of supplements, a message was sent to caregivers daily. Caregivers were asked to return the medicine box and consumption of less than 80% of the supplement at the end of each month was considered non-compliant.
The primary outcome was an assessment of cognitive function by using the two tests, namely, the Mini-Mental State Examination (MMSE) for illiterate patients and the categorical verbal fluency test (CFT), before and after the 12-week probiotic supplementation. Both tests were validated for the Iranian population (Seyedian et al., 2008; Clark et al., 2020).
Mini-Mental State Examination is the most widely used brief cognitive tool for evaluating a variety of cognitive disorders (Shulman et al., 2006) and it was published by Folstein et al. (1975). It is commonly used in clinical practice to assist clinicians in the diagnosis of dementia and delirium (Diniz et al., 2007). It has got 11 domains (orientation to time and place, registration of three words, calculation, language, and ability to draw a complex polygon), with a score range from 0 to 30, with higher numbers indicating better cognitive performance.
The CFT is used for assessing semantic and executive aspects of cognition (Lezak et al., 2004). It is a quick test that can be used to assess people with aphasia (Seniów et al., 2009) and neurodegenerative problems (Levy and Chelune, 2007; Arnott et al., 2010; Lepow et al., 2010). Previous research has found that dementia of AD affects CFT performance (Monsch et al., 1992; Henry et al., 2004). It usually includes two tasks, namely, category fluency (also known as semantic fluency) (Benton, 1968) and letter fluency (sometimes called phonemic fluency) (Newcombe, 1969). In the category fluency, patients are asked to say as many animal and fruit names as they can in 1 min. In the letter fluency, patients are also given 1 min to name the words beginning with the letter F. Based on the study of Shirdel et al. (2022), the letter F in English is equivalent to the letter Pe in Persian in terms of the number of words, that is why we used the letter Pe in letter fluency. The number of unique correct words was the patient’s score in each task (Benton, 1968; Levy and Chelune, 2007; Arnott et al., 2010; Lepow et al., 2010).
Performance in ADL is evaluated by the Barthel Index (BI). BI assesses the degree of independence in doing daily tasks in the elderly through 10 variables (bowel, bladder, grooming, toilet use, feeding, transfer, mobility, dressing, stairs, and bathing). How to score each question is as follows: 0: unable, 1: needs help, and 2: independent, and total score range is 0–20 and a higher score means the older person is more independent in doing daily tasks (Sinoff and Ore, 1997). This test was evaluated for the Iranian population by Hormozi et al. (2019).
We used a validated test of the Lawton Instrumental Activities of Daily Living (IADL) scale for Iranians(Mehraban et al., 2014) that was originally created by Lawton and Brody in 1969 to survey the complex ADLs essential for living within the community. It has got eight questions to assess competence in abilities such as using telephone, shopping, food preparation, housekeeping, laundry, mode of transportation, responsibility for own medications, and handling finance. IADL performance usually declines before ADL function such as eating, using the toilet, and bathing. IADL may recognize the early decline in physical or cognitive activity or both in elderly adults who seem vigorous and healthy (Lawton et al., 1969; Ward et al., 1998; Graf, 2008). Doing this test takes 10–15 min with the score ranging between 0 (low function) and 8 (high function). Each capacity is measured by the scale depending on either cognitive or physical function, even though all require a few degrees of both (Ng et al., 2006).
The Generalized Anxiety Disorder (GAD-7) scale contains seven items based on the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) to evaluate anxiety in both the primary care setting and the general population. GAD-7 was validated by Naeinian et al. (2011) in for the Iranian population (Sapra et al., 2020). Each question has got a score range from 0 to 3 (0 = not at all, 1 = several days, 2 = more than half the days, and 3 = nearly every day) with a total score between 0 and 21, with higher scores indicating more anxiety in the elderly. Total scores of 5, 10, and 15 are considered cutoff for mild, moderate, and severe anxiety (Sapra et al., 2020).
Based on a study by Kobayashi et al. (2019), the sample size was calculated with 95% confidence interval and 80% power using the following formula:
Considering 20% probable withdrawal, 30 subjects were enrolled in each group. Data were analyzed using the Statistical Package for the Social Sciences (SPSS version 26; SPSS Inc., Chicago, IL, USA). We used a one-way ANOVA for quantitative variables and χ2 test for qualitative variables. A linear mixed-effects model (using an unstructured model) was used to determine the independent effects of interventions on outcomes over time (baseline and 12 weeks) with fixed effects for intervention groups, along with a random effect for participants. When the difference was significant (P < 0.05), post hoc pairwise comparison tests were performed.
A total of 90 patients were included in the study and were randomly divided into three groups, namely, placebo (n = 30), L. rhamnosus (n = 30), and B. longum (n = 30) (Figure 1). Participants’ baseline clinical and demographic characteristics are shown in Table 1. No patients reported serious side effects while taking probiotic supplements. All the patients completed the trial and there were not any significant differences between baseline characteristics. The mean and standard deviation (SD) of MMSE, CFT, ADL, IADL, and GAD scores before and after treatment with probiotics are reported in Table 2.
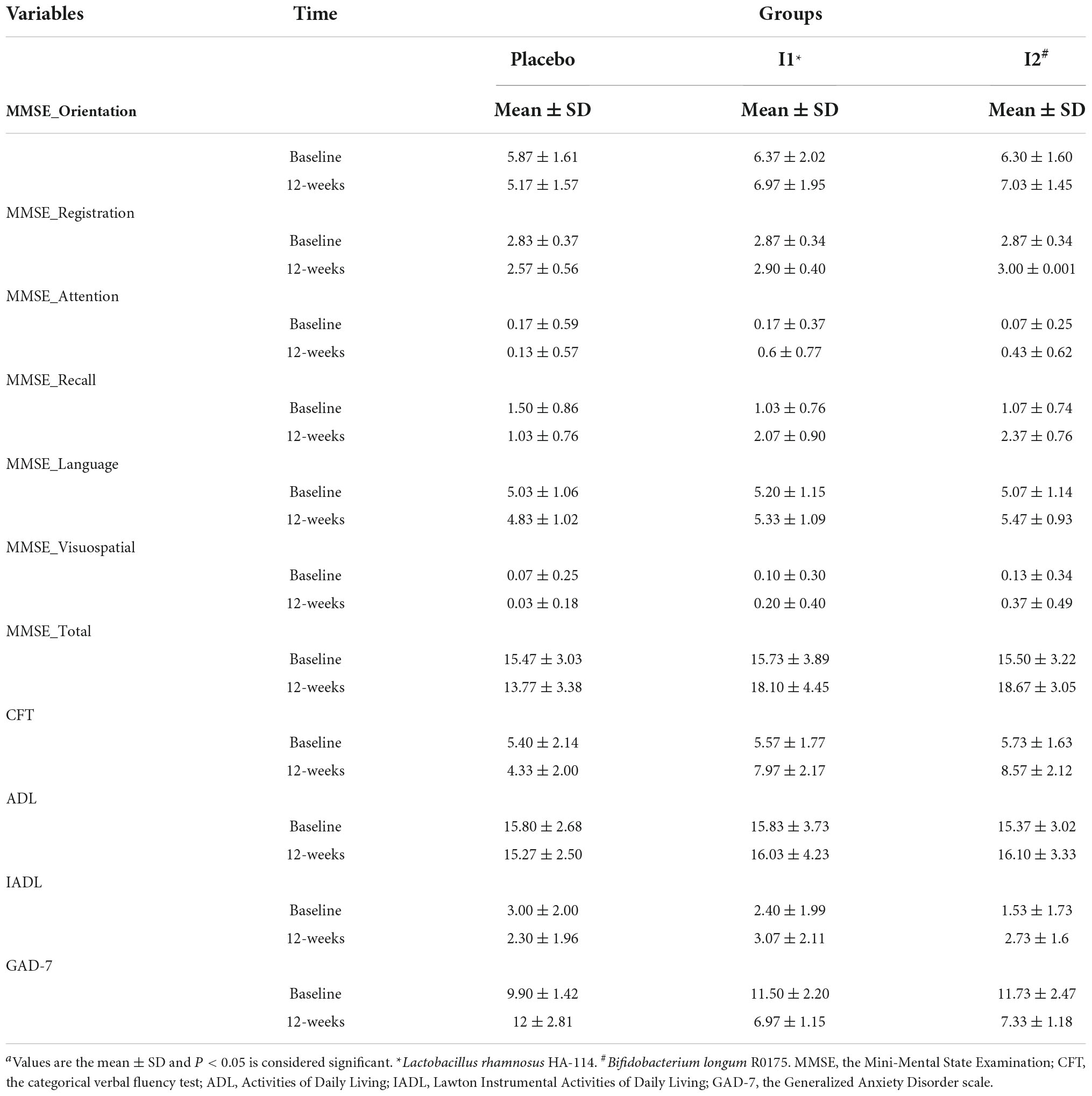
Table 2. Mini-Mental State Examination, CFT, ADL, IADL, and GAD scores of patients in the intervention groups and the placebo group over the study period.a
A significant effect of treatment for MMSE total score was found (PTime × Group < 0.0001), with post hoc comparisons identifying significantly more improvement in the B. longum intervention group (differences: 4.86, 95% CI: 3.91–5.81; P < 0.0001) compared with the placebo and L. rhamnosus intervention groups (differences: 4.06, 95% CI: 3.11–5.01; P < 0.0001). There was no significant difference between the two intervention groups (differences: −0.8, 95% CI: −1.74 to 0.14; P = 0.09). We also compared all six domains of the MMSE, and analysis showed that orientation, registration, attention, recall, language, and visuospatial domains were all significantly different between the placebo and intervention groups. The CFT was significantly improved at the end of the trial (PTime × Group < 0.0001). Post hoc comparisons indicated a significant increase in CFT score in the L. rhamnosus intervention group (differences: 3.46, 95% CI: 2.79–4.13; P < 0.0001) compared with the placebo and B. longum intervention groups (differences: 3.9, 95% CI: 3.22–4.57; P < 0.0001). There was no significant difference between the two intervention groups (differences: −0.43, 95% CI: −1.1 to 0.23; P = 0.2). More details are shown in Table 3.
The effect of probiotic supplement on ADL score was not significant (PTime × Group = 0.24). Although supplementation with B. longum compared with L. rhamnosus caused a greater increase in the ADL score, this change was not significant (differences: −0.53, 95% CI: −1.2 to 0.14; P = 0.11).
The IADL scale was demonstrated to improve significantly in the intervention groups compared to the placebo group (PTime × Group < 0.0001). Compared to the placebo group, L. rhamnosus and B. longum intervention increased IADL significantly (differences: 1.05, 95% CI: 0.49–1.61; P < 0.0001; differences: 1.5, 95% CI: 0.93–2.06; P < 0.0001, respectively). There was no significant difference between the two intervention groups (differences: −0.44, 95% CI: −0.99 to 0.09; P = 0.10).
The GAD-7 scale significantly improved after supplementation with probiotics compared with the placebo (PTime × Group < 0.0001). Within group, comparisons showed a significant improvement in the L. rhamnosus intervention group (differences: −6.44, 95% CI: −7.77 to −5.12; P < 0.0001) compared with the placebo and B. longum intervention groups (differences: −6.26, 95% CI: −7.59 to −4.93; P < 0.0001). Although supplementation with B. longum compared with L. rhamnosus caused a greater increase in the GAD score, this change was not significant (differences: −0.18, 95% CI: −1.5 to 1.13; P = 0.78).
This present trial that investigated the effect of probiotic supplementation on cognitive status in patients with mild and moderate AD indicated a significant improvement in the MMSE total score as well as CFT, IADL, and GAD-7 in response to probiotic supplementation.
Alzheimer’s disease is a progressive neurodegenerative disease with memory decline, cognition impairment, and behavioral disorders and has become a worldwide health problem in the aging population. Although the cause of this disease is not known, it seems that two toxic misfolded proteins (amyloid-β and tau proteins) cause AD by increasing oxidative stress, neuroinflammation, and synaptic impairment and, thereby, accelerate neuronal death. Sirtuin 1 (SIRT1) is a potent therapeutic strategy against neurodegenerative diseases by downregulating Rho-associated coiled-coil-containing protein kinase 1 (ROCK) expression, and promoting anti-amyloidogenic cleavage of Aβ protein precursors by α-secretase is shown to be thereby inhibiting the aggregation of toxic proteins (Mishra et al., 2021). Since the drug’s therapeutic potential is controversial and its use is not without complications and side effects, thus, the recent paradigm shifts toward natural products and their derivatives. These natural compounds are well tolerated with minimal to no side effects. They are readily available and have excellent bioavailability and some of these compounds can affect the upregulation of SIRT1 (Ghosh et al., 2020).
At present, the gastrointestinal tract with its commensal microbiome is one of the most interesting and discussed topics in neurodegenerative diseases. However, there are few trials on the effect of probiotic supplementation, especially with single strain on the brain.
Consistent with our findings, Musa et al. (2017) showed that supplementation with Lactobacillus fermentum LAB9 or Lactobacillus casei of fermented cow’s milk had a neuroprotective effect and memory improvement through attenuation of lipopolysaccharide, inhibition of acetylcholinesterase (AChE), and antioxidative activities. Besides, animal studies have shown that supplementation with Lactobacillus plantarum significantly improved learning and memory abilities and stabilized the structural and functional integrity of the biological neuron membrane (Peng et al., 2014; Mallikarjuna et al., 2016; Nimgampalle and Kuna, 2017; Athari Nik Azm et al., 2018). Kobayashi et al. (2017) indicated that administration of Bifidobacterium breve strain A1 in AD model mice can prevent cognitive dysfunction induced by amyloid-β; antioxidant and neuroprotective effects of a probiotic formulation (namely SLAB51) have also been indicated in an SD mouse model (Bonfili et al., 2018). Leblhuber et al. (2018) showed that 4 weeks of multispecies supplementation in 20 patients with AD had a neuroprotective effect through increasing neopterin and kynurenine to tryptophan ratio as well as gut bacteria composition improvement; clock drawing test (CDT) also significantly increased. Akbari et al. (2016) indicated a significant improvement in the MMSE score by using multispecies probiotic milk (2 × 109 CFU) for 12 weeks in 60 individuals with AD. Kobayashi et al. (2019) showed that supplementation with B. breve A1 > 2.0 × 1010 CFU for 12 weeks in 117 older adults with subjective memory complaints produced a significant improvement in MMSE total score due to its anti-inflammatory effect through suppressing the hippocampal gene expression of inflammation-related genes. Agahi et al. (2018) revealed that multispecies probiotic supplementation (3 × 109) for 12 weeks in 48 (25 patients with AD) subjects had no significant effect on Test Your Memory (TYM) score. In addition to the small number of participants and their old age, another possible reason is that 83.5% of patients had severe AD, making cognitive improvement more difficult.
There are some mechanisms explaining the effects of probiotic supplementation on cognition status. Studies have shown that a chronic neuroinflammatory state with the release of pro-inflammatory cytokines has been seen in patients with AD, which seems to correlate with constant accumulation of Aβ in neurons. It seems that this neuroinflammation occurs due to an alteration of the balance of gut microbiota (dysbiosis), which can adversely affect neuronal activity. In contrast, Alzheimer’s drugs can temporarily relieve the symptoms of the disease, but in the long-term, they adversely affect the gut microbiome, making the disease worse. Therefore, adjuvant treatment with probiotics may prevent or even cure intestinal dysbiosis, thus allowing the therapeutic effects of AD drugs to be more fully exploited (Jiang et al., 2017; Hort et al., 2020). Ingestion of probiotics in subjects with AD balances and modifies the gut microbiota to produce short-chain fatty acids (SCAFs) required for the integrity of the gut and blood–brain barrier (BBB) and other metabolites such as neurotransmitters and precursors like noradrenalin and tryptophan or γ-gamma-aminobutyric acid (GABA). These metabolites are essential for neuropsychiatric status in aging people (Leblhuber et al., 2018). Probiotics have also been reported to suppress oxidative stress and inflammation by increasing enzymes such as superoxide dismutase, glutathione peroxidase, and hs-CRP (Agahi et al., 2018). This trial indicated that the score of IADL improved significantly, whereas the score of ADL did not show any significant change. IADL performance usually declines before ADL functions such as eating, using the toilet, and bathing (Ward et al., 1998; Graf, 2008). It seems that significant changes in ADL scores may require long-term trials.
Despite broadening our understanding of the effect of probiotics on AD, this study had some limitations. For instance, we did not assess changes in the gut microbiota; therefore, it remained unclear what specific strains were modified by probiotic supplementation. Yet, the most important strengths of this study were its design as a three-arm study and the simultaneous assessment of two different single-strain probiotics.
This study demonstrated that 12-week probiotic supplementation compared with placebo had favorable effects on the cognitive status, anxiety, and instrumental daily activity of patients with AD; however, this supplement had no effect on ADL. We concluded that adjuvant treatment with probiotics in these patients is beneficial and can enhance drug’s efficacy, slow the disease exacerbation process, and maintain the patient’s quality of life for an extended period, but more clinical trials are needed in this respect.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was done according to the Helsinki Declaration of 1975, also approved by the Ethics Committee of Tehran University of Medical Sciences and registered on the Iranian Website for Registration of Clinical Trials IRCT (IRCT number: 20210513051277N1). The patients/participants provided their written informed consent to participate in this study.
KD was the guarantor. CA and ZV wrote the manuscript and collected the data. SS-B interpreted the data and provided professional comments. FE revised the manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by a grant from the Tehran University of Medical Sciences.
We appreciate all participants and the staff of Ziaeeian, Roozbeh, and Shariati Hospital.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Agahi, A., Hamidi, G. A., Daneshvar, R., Hamdieh, M., Soheili, M., Alinaghipour, A., et al. (2018). Does severity of Alzheimer’s disease contribute to its responsiveness to modifying gut microbiota? A double blind clinical trial. Front. Neurol. 9:662. doi: 10.3389/fneur.2018.00662
Akbari, E., Asemi, Z., Daneshvar Kakhaki, R., Bahmani, F., Kouchaki, E., Tamtaji, O. R., et al. (2016). Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer’s disease: A randomized, double-blind and controlled trial. Front. Aging Neurosci. 8:256. doi: 10.3389/fnagi.2016.00256
Alzheimer’s Association (2010). 2010 Alzheimer’s disease facts and figures. Alzheimers Dement. 6, 158–194.
Arnott, W. L., Chenery, H. J., Angwin, A. J., Murdoch, B. E., Silburn, P. A., and Copland, D. A. (2010). Decreased semantic competitive inhibition in Parkinson’s disease: Evidence from an investigation of word search performance. Int. J. Speech Lang. Pathol. 12, 437–445. doi: 10.3109/17549507.2010.492875
Athari Nik Azm, S., Djazayeri, A., Safa, M., Azami, K., Ahmadvand, B., Sabbaghziarani, F., et al. (2018). Lactobacilli and bifidobacteria ameliorate memory and learning deficits and oxidative stress in β-amyloid (1–42) injected rats. Appl. Physiol. Nutr. Metab. 43, 718–726.
Azm, S. A. N., Djazayeri, A., Safa, M., Azami, K., Djalali, M., Sharifzadeh, M., et al. (2017). Probiotics improve insulin resistance status in an experimental model of Alzheimer’s disease. Med. J. Islamic Republic Iran 31:103. doi: 10.14196/mjiri.31.103
Bonfili, L., Cecarini, V., Cuccioloni, M., Angeletti, M., Berardi, S., Scarpona, S., et al. (2018). SLAB51 probiotic formulation activates SIRT1 pathway promoting antioxidant and neuroprotective effects in an AD mouse model. Mol. Neurobiol. 55, 7987–8000. doi: 10.1007/s12035-018-0973-4
Clark, T. M., Callam, C. S., Paul, N. M., Stoltzfus, M. W., and Turner, D. (2020). Testing in the time of COVID-19: A sudden transition to unproctored online exams. J. Chem. Educ. 97, 3413–3417.
Clemmensen, F. K., Hoffmann, K., Siersma, V., Sobol, N., Beyer, N., Andersen, B. B., et al. (2020). The role of physical and cognitive function in performance of activities of daily living in patients with mild-to-moderate Alzheimer’s disease–a cross-sectional study. BMC Geriatr. 20:513. doi: 10.1186/s12877-020-01926-9
Diniz, B. S., Yassuda, M. S., Nunes, P. V., Radanovic, M., and Forlenza, O. V. (2007). Mini-mental State examination performance in mild cognitive impairment subtypes. Int. Psychogeriatr. 19, 647–656.
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Ghosh, S., Durgvanshi, S., Agarwal, S., Raghunath, M., and Sinha, J. K. (2020). Current status of drug targets and emerging therapeutic strategies in the management of Alzheimer’s disease. Curr. Neuropharmacol. 18, 883–903.
Graf, C. (2008). The Lawton instrumental activities of daily living scale. AJN Am. J. Nurs. 108, 52–62.
Hazar, N., Seddigh, L., Rampisheh, Z., and Nojomi, M. (2016). Population attributable fraction of modifiable risk factors for Alzheimer disease: A systematic review of systematic reviews. Iran. J. Neurol. 15:164.
Henry, J. D., Crawford, J. R., and Phillips, L. H. (2004). Verbal fluency performance in dementia of the Alzheimer’s type: A meta-analysis. Neuropsychologia 42, 1212–1222.
Hormozi, S., Alizadeh-Khoei, M., Sharifi, F., Taati, F., Aminalroaya, R., Fadaee, S., et al. (2019). Iranian version of barthel index: Validity and reliability in outpatients’ elderly. Int. J. Prev. Med. 10:130. doi: 10.4103/ijpvm.IJPVM_579_18
Hort, J., Valis, M., and Angelucci, F. (2020). Administration of pre/probiotics with conventional drug treatment in Alzheimer’s disease. Neural Regen. Res. 15:448. doi: 10.4103/1673-5374.266057
International AsD (2019). World Alzheimer report 2019: Attitudes to dementia. London: Alzheimer’s Disease International.
Jack, C. R. Jr., Albert, M. S., Knopman, D. S., McKhann, G. M., Sperling, R. A., Carrillo, M. C., et al. (2011). Introduction to the recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 257–262. doi: 10.1016/j.jalz.2011.03.004
Jiang, C., Li, G., Huang, P., Liu, Z., and Zhao, B. (2017). The gut microbiota and Alzheimer’s disease. J. Alzheimers Dis. 58, 1–15.
Kincaid, H. J., Nagpal, R., and Yadav, H. (2021). Diet-microbiota-brain axis in Alzheimer’s disease. Ann. Nutr.Metab. 77, 21–27. doi: 10.1159/000515700
Kobayashi, Y., Kuhara, T., Oki, M., and Xiao, J.-Z. (2019). Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: A randomised, double-blind, placebo-controlled trial. Benef. Microbes 10, 511–520. doi: 10.3920/BM2018.0170
Kobayashi, Y., Sugahara, H., Shimada, K., Mitsuyama, E., Kuhara, T., Yasuoka, A., et al. (2017). Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci. Rep. 7, 1–10. doi: 10.1038/s41598-017-13368-2
Lawton, M., Brody, E., and Médecin, U. (1969). Instrumental activities of daily living (IADL). Gerontologist 9, 179–186.
Leblhuber, F., Steiner, K., Schuetz, B., Fuchs, D., and Gostner, J. M. (2018). Probiotic supplementation in patients with Alzheimer’s dementia-an explorative intervention study. Curr. Alzheimer Res. 15, 1106–1113. doi: 10.2174/1389200219666180813144834
Lepow, L., Van Sweringen, J., Strutt, A. M., Jawaid, A., MacAdam, C., Harati, Y., et al. (2010). Frontal and temporal lobe involvement on verbal fluency measures in amyotrophic lateral sclerosis. J. Clin. Exp. Neuropsychol. 32, 913–922.
Levy, J. A., and Chelune, G. J. (2007). Cognitive-behavioral profiles of neurodegenerative dementias: Beyond Alzheimer’s disease. J. Geriatr. Psychiatry Neurol. 20, 227–238.
Lezak, M. D., Howieson, D. B., Loring, D. W., and Fischer, J. S. (2004). Neuropsychological assessment. Oxford: Oxford University Press.
Mallikarjuna, N., Praveen, K., and Yellamma, K. (2016). Role of Lactobacillus plantarum MTCC1325 in membrane-bound transport ATPases system in Alzheimer’s disease-induced rat brain. BioImpacts BI 6:203. doi: 10.15171/bi.2016.27
Mckhann, G. (1984). Report of the NINCDS-ADRDA work group under the auspices of department of health and human service task force on Alzheimer’s disease. Neurology 34, 939–944. doi: 10.1212/wnl.34.7.939
Mehraban, A. H., Soltanmohamadi, Y., Akbarfahimi, M., and Taghizadeh, G. (2014). Validity and reliability of the persian version of lawton instrumental activities of daily living scale in patients with dementia. Med. J. Islamic Republic Iran. 28:25.
Mendez, M. F. (2021). The relationship between anxiety and Alzheimer’s disease. J. Alzheimers Dis. Rep. 5, 171–177.
Mishra, P., Mittal, A. K., Kalonia, H., Madan, S., Ghosh, S., Sinha, J. K., et al. (2021). SIRT1 promotes neuronal fortification in neurodegenerative diseases through attenuation of pathological hallmarks and enhancement of cellular lifespan. Curr. Neuropharmacol. 19, 1019–1037. doi: 10.2174/1570159X18666200729111744
Monsch, A. U., Bondi, M. W., Butters, N., Salmon, D. P., Katzman, R., and Thal, L. J. (1992). Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch. Neurol. 49, 1253–1258.
Musa, N. H., Mani, V., Lim, S. M., Vidyadaran, S., Majeed, A. B. A., and Ramasamy, K. (2017). Lactobacilli-fermented cow’s milk attenuated lipopolysaccharide-induced neuroinflammation and memory impairment in vitro and in vivo. J. Dairy Res. 84, 488–495. doi: 10.1017/S0022029917000620
Naeinian, M., Shairi, M., Sharifi, M., and Hadian, M. (2011). To study reliability and validity for a brief measure for assessing generalized anxiety disorder (GAD-7).
Newcombe, F. (1969). Missile wounds of the brain: A study of psychological deficits. Oxford: Oxford University Press.
Ng, T.-P., Niti, M., Chiam, P.-C., and Kua, E.-H. (2006). Physical and cognitive domains of the instrumental activities of daily living: Validation in a multiethnic population of Asian older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 61, 726–735. doi: 10.1093/gerona/61.7.726
Nimgampalle, M., and Kuna, Y. (2017). Anti-Alzheimer properties of probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s disease induced albino rats. J. Clin. Diagn Res. JCDR 11, KC01. doi: 10.7860/JCDR/2017/26106.10428
Oelschlaeger, T. A. (2010). Mechanisms of probiotic actions–a review. Int. J. Med. Microbiol. 300, 57–62. doi: 10.1016/j.ijmm.2009.08.005
Peng, X., Meng, J., Chi, T., Liu, P., Man, C., Liu, S., et al. (2014). Lactobacillus plantarum NDC 75017 alleviates the learning and memory ability in aging rats by reducing mitochondrial dysfunction. Exp. Ther. Med. 8, 1841–1846. doi: 10.3892/etm.2014.2000
Porsteinsson, A., Isaacson, R., Knox, S., Sabbagh, M., and Rubino, I. (2021). Diagnosis of early Alzheimer’s disease: Clinical practice in 2021. J. Prev. Alzheimers Dis. 8, 371–386.
Sapra, A., Bhandari, P., Sharma, S., Chanpura, T., and Lopp, L. (2020). Using generalized anxiety disorder-2 (GAD-2) and GAD-7 in a primary care setting. Cureus 12:e8224. doi: 10.7759/cureus.8224
Seniów, J., Litwin, M., Litwin, T., Leśniak, M., and Członkowska, A. (2009). New approach to the rehabilitation of post-stroke focal cognitive syndrome: Effect of levodopa combined with speech and language therapy on functional recovery from aphasia. J. Neurol. Sci. 283, 214–218. doi: 10.1016/j.jns.2009.02.336
Seyedian, M., Falah, M., Nourouzian, M., Nejat, S., Delavar, A., and Ghasemzadeh, H. (2008)., Validity of the farsi version of mini-mental state examination. Tehran: Scientific Journal of Medical Organization of the Islamic Republic of Iran.
Shirdel, S., Esmaeeli, S., Alavi, K., Ghaemmaghami, P., and Shariat, S. V. (2022). Verbal fluency performance in normal adult population in Iran: Norms and effects of age, education, and gender. Basic Clin. Neurosci. J. 13, 129–138.
Shulman, K. I., Herrmann, N., Brodaty, H., Chiu, H., Lawlor, B., Ritchie, K., et al. (2006). IPA survey of brief cognitive screening instruments. Int. Psychogeriatr. 18, 281–294. doi: 10.1017/S1041610205002693
Sinoff, G., and Ore, L. (1997). The barthel activities of daily living index: Self-reporting versus actual performance in the old-old (≥ 75 years). J. Am. Geriatr. Soc. 45, 832–836. doi: 10.1111/j.1532-5415.1997.tb01510.x
Keywords: Alzheimer’s disease, cognition, psychological test, probiotic, oral supplementation
Citation: Akhgarjand C, Vahabi Z, Shab-Bidar S, Etesam F and Djafarian K (2022) Effects of probiotic supplements on cognition, anxiety, and physical activity in subjects with mild and moderate Alzheimer’s disease: A randomized, double-blind, and placebo-controlled study. Front. Aging Neurosci. 14:1032494. doi: 10.3389/fnagi.2022.1032494
Received: 30 August 2022; Accepted: 10 October 2022;
Published: 31 October 2022.
Edited by:
Jitendra Kumar Sinha, GloNeuro Academy, IndiaReviewed by:
Svetlana Tomic, Clinical Hospital Center Osijek, CroatiaCopyright © 2022 Akhgarjand, Vahabi, Shab-Bidar, Etesam and Djafarian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kurosh Djafarian, a2RqYWZhcmlhbkB0dW1zLmFjLmly
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.