
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 29 September 2022
Sec. Alzheimer's Disease and Related Dementias
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.1014305
This article is part of the Research TopicPresent and Future of Biological Fluid Biomarkers in DementiaView all 10 articles
Alzheimer’s disease (AD) is a severe neurodegenerative brain disorder. The determination of beta-amyloid (Aβ)-40, –42, total tau, and phospho-tau-181 (pTau181) in cerebrospinal fluid (CSF) using Lumipulse technology has been established as biomarkers for AD in recent years. As CSF collection is an invasive procedure, one aims to find biomarkers in blood or other human fluids, such as saliva. In the present study, we aim to measure these markers in human saliva. Using Salivettes, we collected saliva samples from healthy controls (n = 27), patients with AD dementia (n = 44), mild cognitive impairment (MCI) (n = 45), depression (n = 31), and 21 blinded samples, all older than 60 years. Lumipulse technology with a G600II was used to detect all four biomarkers. Our data show that the levels of total protein were highly variable and thus biomarker levels were corrected to 1 mg/ml of total protein. Saliva Aβ−40 and –42 were not detectable, because it was not recovered from the Salivettes. However, saliva total tau (577 ± 134 pg/mg, n = 22) and phospho-tau-181 (9.7 ± 1.3 pg/mg, n = 21) could be analyzed by Lumipulse technology. Saliva total tau levels were significantly decreased in patients with AD (≤ 300 pg/mg protein), while pTau181 levels (≥ 18 pg/mg protein) were significantly enhanced in patients with MCI compared to controls. Laboratory diagnosis with a cut-off of ≥ 18 pg/mg protein pTau181 (for MCI) and ≤ 300 pg/mg protein tau (for AD) for blinded samples could diagnose MCI and AD with an accuracy of 71.4%. Despite these initial promising results, the findings must be replicated in larger cohorts, and several technical problems due to saliva processing must be solved and Salivettes should not be used.
The concept of Alzheimer’s disease (AD) has extensively changed over the past years and there is increasing evidence for a long preclinical disease stage with hardly any clinical and cognitive impairment but detectable microstructural changes in the brain and metabolic changes in body fluids. Following a preclinical phase, AD patients merge to mild cognitive impairment stage (MCI) and finally convert to clinically manifest AD dementia (Sperling et al., 2011). Within the last century, the mean life expectancy of humans has increased from around 40 to approximately 80 years. As age is the main risk factor for AD, the number of patients suffering from AD will dramatically increase within the next 50 years so about 80 million patients with AD in the dementia stage can be expected worldwide by 2050. These enormously high numbers of presumed patients with AD dementia call for further establishment of reliable diagnostic surrogate markers for diagnosing and monitoring disease progression and therapy. A valid and easily accessible diagnostic procedure should be the basis for treatment. Definitive diagnosis of AD requires both a clinical diagnosis and post-mortem detection of AD pathologies. A probable diagnosis of AD or prediction of imminent conversion from MCI to AD dementia can be made based on clinical criteria, laboratory tests, neuroimaging, and neuropsychological evaluation with moderate certainty depending on available diagnostic possibilities.
A promising area of research for laboratory diagnosis of AD is the analysis of cerebrospinal fluid (CSF), where the measurement of beta-amyloid (Aβ) with 40 or 42 amino acids, total tau, and phospho-tau-181 can distinguish patients with AD from healthy subjects with high specificity and sensitivity (Blennow, 2005; Blennow et al., 2010; Humpel, 2011). Further on, numerous studies have found abnormalities of the noradrenergic system with higher levels of CSF norepinephrine (NE) in patients with AD (Sheline et al., 1998), and lower levels of plasma NE in patients with depression (Anand and Charney, 2000). The latter is a well-known risk factor for AD (Defrancesco et al., 2017). Unfortunately, the use of CSF biomarkers is limited by invasive collection. Non-invasive methods for the detection of cerebral Aβ and tau, e.g., by positron emission tomography have the disadvantage of very high costs and lack of availability.
Thus, there is a need to discover biomarkers in other human biological fluids, such as blood, urine, or saliva, which are easily obtainable and allow collecting a high number of samples. In 2008, Li et al. (2008) showed a relationship between saliva levels of 3-methoxy-4-hydroxyphenylglycol (sMHPG) - a NE metabolite, and mental health in the elderly general population. In the same year, Boston et al. (2008) developed a simple laboratory test to measure acetylcholinesterase as a possible biomarker for AD in saliva. Another pilot study reports on the discovery of diagnostic biomarkers in saliva of patients with AD using 1H-NMR-based metabolomics (Yilmaz et al., 2017). Recently we (Marksteiner et al., 2019) have shown for the first time using targeted metabolomics that acyl-alkyl phosphatidylcholines (sum of PCae C34:1-2; PCae C36:1-2-3; PCaeC38:1-3; PCae C40:2-3) are significantly reduced in saliva of patients with AD dementia compared to healthy controls. Thus, saliva could serve as a very powerful and easily accessible human fluid to detect biomarkers of AD (Huan et al., 2018; Ashton et al., 2019; François et al., 2019; Gleerup et al., 2019; Liang and Lu, 2019).
Regarding the expression of the Aβ and tau AD biomarkers in saliva, only very few data have been published and some are not clear and controversial. In 2010, Bermejo-Pareja et al. (2010) reported for the first time that saliva Aβ−42 could become a potential biomarker, which was recently reproduced by others (Lee et al., 2017; Sabbagh et al., 2018; Gleerup et al., 2019). There is preliminary evidence that also tau is found in saliva. In 2011, Shi et al. (2011) reported the presence of tau and phospho-tau in saliva confirmed by mass-spectrometry. However, there are many discrepancies and so far the role of tau in saliva is not clear. Some authors found that Aβ−42 is enhanced in saliva (Bermejo-Pareja et al., 2010), while others could not detect it (Shi et al., 2011). Some authors show that saliva tau is not associated with AD (Ashton et al., 2018), while others see an increase in early AD (Bermejo-Pareja et al., 2010). In CSF, normal levels of phospho-tau-181 (< 60 pg/ml) are markedly lower than those of total tau (< 500 pg/ml). In saliva, tau levels are low (20 pg/ml) but others found 3–5x higher phospho-tau levels (Lau et al., 2015; Tvarijonaviciute et al., 2020). Meanwhile, tau saliva has also been detected in relapsing-remitting multiple sclerosis (Mirzaii-Dizgah et al., 2020) and traumatic brain injury (Olczak et al., 2019).
Tau is a microtubule-associated protein and physiologically stabilizes and regulates axonal transport (Liu and Götz, 2013; Spillantini and Goedert, 2013; Nisbet et al., 2015; Wang and Mandelkow, 2016). However, the physiological roles of tau seem to be more complex and by far not fully explored. Full-length tau (2N4R) exists also in truncated forms (1N4R, 0N4R, 2N3R, 1N3R, and 0N3R) and tau has more than 40 possible phosphorylation sites (Grundke-Iqbal et al., 1986; Hanger et al., 2009). The phosphorylation is regulated by several kinases and phosphatases and it is extremely important to identify these critical phosphorylation sites in the tau protein, as they are either therapeutic or diagnostic targets (Liu and Götz, 2013; Spillantini and Goedert, 2013; Wang and Mandelkow, 2016). In our laboratory, we have extensive routine experience in the analysis of tau and pTau181 in CSF using Lumipulse (Blasko et al., 2006; Lederer et al., 2016), but the functional role of tau in saliva is completely unknown.
In this study, we aim to analyze Aβ–42 and –40, total tau, and pTau181 in saliva collected with Salivettes. We investigate whether there are disease-specific changes in cognitively healthy subjects, patients with MCI, AD dementia, or depression using the well-established Lumipulse technology. The purpose of the study was to determine possible biomarkers for diagnosing clinical and preclinical stages of AD in saliva.
Cognitively healthy subjects and patients suffering from AD, MCI, and depression were recruited at the state hospital Hall/Tirol, as reported in detail in several previous studies (Marksteiner et al., 2019). The following groups were included in this study: healthy controls (group 1, n = 27), patients with MCI (group 2, n = 45), patients with AD dementia (group 3, n = 44), and depressive patients without marked cognitive impairment (group 4, n = 31). For the blinded study another 21 saliva samples from healthy controls and patients with AD dementia or MCI aged older than 60 years were collected at the memory clinic of the University Clinic of Psychiatry Innsbruck in July 2022. All patients completed a clinical and neuropsychological assessment including subtests of the “Consortium to Establish a Registry for Alzheimer’s Disease” (CERAD) battery (Rosen et al., 1984) as well as the MMSE (Folstein et al., 1975). The MMSE served as a measure for the severity of cognitive impairment. The 15 items version geriatric depression scale (GDS-15) was used to assess depressive symptoms. Cerebral neuroimaging (magnetic resonance imaging, MRI) was performed to evaluate cortical atrophy, cerebrovascular pathology, and to exclude other brain pathologies. Probable AD dementia was diagnosed according to the current NINCDS-ARDRA (National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association) criteria. MCI was diagnosed according to the criteria of Petersen et al. (2001). A general blood examination was part of the routine diagnostic procedure. Exclusion criteria for healthy subjects, patients with MCI, and AD dementia included (1) another primary neurological or mental disorder, (2) any kind of metabolic decompensation or any sign of peripheral inflammation (e.g., rheumatic disease), (3) long-term alcohol or drug abuse, (4) or any current, clinically significant cardiovascular disease, (5) or current intake of medication that could influence saliva production or saliva composition (e.g., anticholinergic medication, cyclosporine, immunosuppressives, bronchodilators, anti-inflammatory drugs). The study was approved by the ethics committee of the Medical University of Innsbruck. All subjects and/or their caregivers enrolled in the study gave their informed consent. In preliminary experiments, we analyzed tau and Aβ saliva levels in healthy volunteers aged from 5 to 90 years.
While the total volume of saliva varies per individual, the average daily production ranges from 1 to 2 liters per day. Normal stimulated saliva flow is 1–2 ml per minute and rates below 0.6 ml/min are considered low. Collection of saliva is done in the early morning and all subjects were asked to refrain from eating, drinking, smoking, or using oral hygiene prior to saliva collection (at least for 8 h). We routinely used a Salivette (Sarstedt; Nr. 51.1534, Cotton). Patients were asked to have the cotton in the mouth for exactly 2 min in order to measure salivary flow. Our data showed that we collected 1.52 ± 0.04 ml/2 min (n = 5) using our Salivette. The samples were sent to the laboratory at room temperature within 4 h, centrifuged (3,000 × g 5 min), the volume was measured and recorded and saliva was aliquoted (500 μl) and frozen at –80°C until analysis (for all samples from Table 1; stored from 2016 to 2022). For the blinded study, saliva of 21 patients was collected in July 2022 and analyzed within 4 h after collection (without freezing).
Total protein was determined in undiluted saliva using the Bradford assay. NE was measured using HPLC-EC as described by us (Hutter et al., 1996).
Levels of Aβ–40, Aβ–42, total tau, and pTau181 were measured using automated Lumipulse enzymatic light emitting technology (Fujirebio G600II).1 The Lumipulse assay is an automated robotic platform (Figure 1A), using an enzymatic light emitting system. This system gives very fast and accurate values within 35 min. The single racks are placed in the system (Figure 1B) and each unit contains a triple-tube with the antibodies and magnetic beads (Figure 1C). For every analyte, a standard curve is generated (Figure 1D) and for every analyte also quality controls are run in parallel (Figure 1E). The background levels are very low (16 ± 3 pg/ml, n = 3), and every quality control (e.g., tau Level 1 290 = 292 ± 0.6 pg/ml, n = 3) or standard (e.g., full-length 2N4R tau 100 = 103 ± 4 pg/ml, n = 3) gives a very accurate reproducible value with very low variance (Figure 1F). As a control, a CSF sample (1,209 ± 8 pg/ml, analyzed as triplicate) is shown from a patient with tauopathy (Figure 1F). As an additional control, a wild-type mouse brain was analyzed giving very high tau levels of 847,000 ± 160,000 pg/mg total protein (n = 3) or a wild-type mouse male salivary gland gives very low tau levels of 201 ± 21 tau pg/mg protein (n = 3) (Figure 1F).
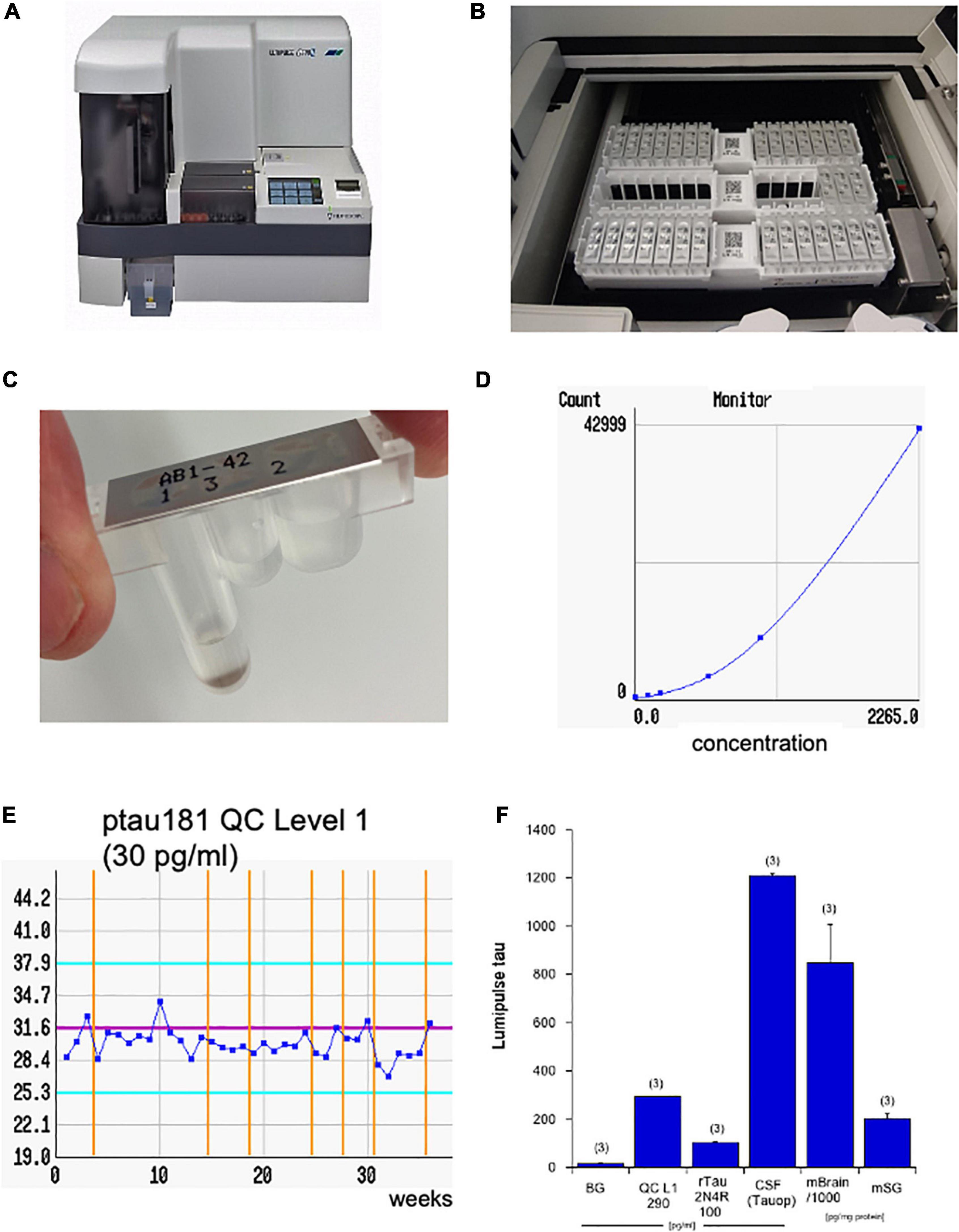
Figure 1. The four AD biomarkers were analyzed using the Lumipulse G600II (A). The principle is an enzymatic reaction, where the antibodies and magnetic beads are stored in three different tubes within a single unit located on a rack with 14 units (B,C). The computer generates a standard curve (D; e.g., for tau) and quality controls (QCs; e.g., for phospho-tau-181, 30 pg/ml) to guarantee an accurate analysis over weeks (E). Values are generated from standards and display a very low background (BG) versus the tau quality level 1 (290 pg/ml), a recombinant 2N4R tau standard (100 pg/ml), as well as a cerebrospinal fluid (CSF) sample of a patient with tauopathy (Tauop) (F). As a control tau levels are also shown in a mouse brain (mBrain) or in a mouse male salivary gland (mSG). The later ones are corrected for total protein and are given as pg/mg. Note that the brain contains extremely high tau values and are presented as 1,000× less in the diagram. Values are given as mean ± SEM, with n = 3 different samples.
Statistical analysis was performed by one-way ANOVA with a subsequent Fisher LSD post-hoc test where p ≤ 0.05 was significant. To determine differences between male and female participants (age-study), a student’s t-test was performed.
In the present study, 147 subjects were included, which were grouped in healthy controls (n = 27, group 1), patients with MCI (n = 45, group 2), patients with AD dementia (n = 44, group 3), and patients with depression (n = 31, group 4) (Table 1). In all groups nearly 40% were male participant, only in the depressive group there were less male participant (Table 1). The mean age of the patients was 71 years, and only patients with AD dementia were slightly older (Table 1). For healthy subjects, the MMSE score was 29.6 ± 0.1 and was significantly lower in the MCI group and even lower in the AD dementia group (Table 1). Except in the depression group, the GDS score was lower than 5 (Table 1).
In fresh saliva, the protein concentration was 1,558 ± 341 μg/ml in controls and was not significantly different from the other groups (Table 1). However, the variability of saliva total protein levels was high ranging from 0.16 mg/ml to 8.3 mg/ml (Table 1). This variability was also seen when saliva was analyzed by HPLC-EC detection. Norepinephrine (NE) concentration was determined as a measure of sympathetic neuronal activity indicating stress. Figure 2A shows a blank chromatogram and Figure 2B shows that a NE standard elutes at approx. 5 min. The detection limit was 100 pg on the column. The chromatogram for fresh saliva (diluted to 1 mg/ml) was very heterogeneous ranging from a few peaks (Figure 2C) to a very high protein load, where NE could not be detected (Figure 2D). The NE levels in control saliva were 7.5 ± 4.2 ng/ml and were not different in MCI, but significantly reduced in AD saliva and there was a tendency to be increased in depressive patients (Table 1).
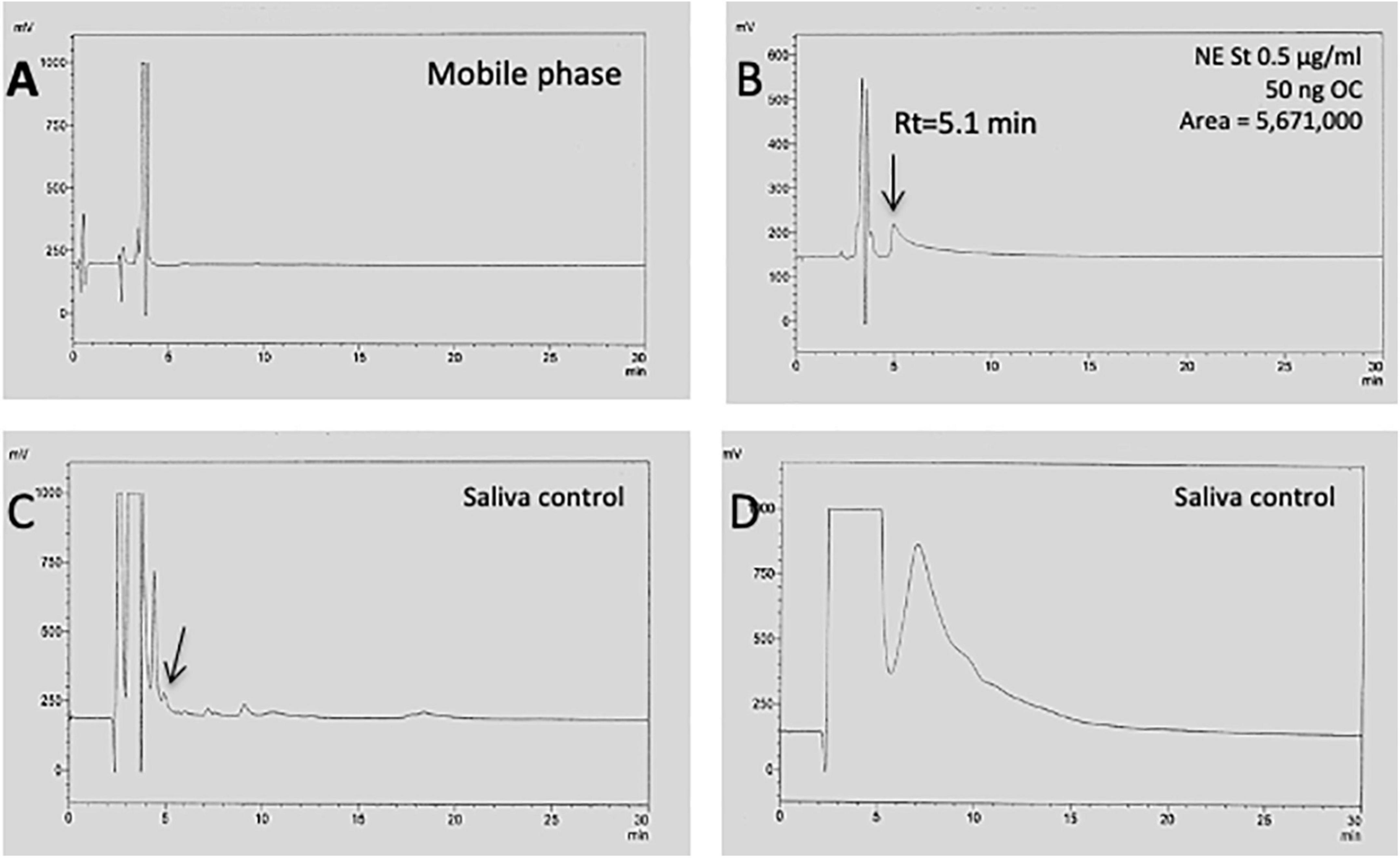
Figure 2. HPLC chromatogram of saliva to detect norepinephrine. When only the mobile phase was injected onto the HPLC, an injection peak was seen but only a very low background (A). Injection of a norepinephrine (NE) standard (0.5 μg/ml) shows that NE elutes at approx. 5 min, giving a single peak (B). Injection of pure saliva (1 mg/ml) shows a chromatogram with only a few peaks (C), but also a very heterogeneous chromatogram, with a high peak load during the first 5 min, where NE could not be detected (D).
All four AD biomarkers were analyzed using the robotic-automated enzymatic Lumipulse assay (Figure 1). In order to exclude the heterogeneity of saliva samples, all values were corrected to 1 mg/ml total protein and are given as pg/mg total protein.
Beta-amyloid-40 was below the detection limit (5 pg/ml) in all cases but only two values were detectable in controls (5 and 20 pg/ml) and one value in depression (13 pg/ml). Beta-amyloid-42 was below the detection limit (9 pg/ml) in all cases, and only two values were detectable in controls (19 and 21 pg/ml). In order to exclude a stability problem over freezing at –80°C, saliva of seven healthy control individuals was tested fresh within 3 h and all seven samples of beta-amyloid-42 and –40 were below the detection level. In order to rule out that beta-amyloid is captured by the cotton in the Salivettes, we performed a recovery experiment. Our data show, indeed, that the recovery of beta-amyloid-40 from the Salivettes was very low (6.5 ± 3% recovery, n = 6). However, when native saliva was collected (only spitting in tubes, without Salivettes), the levels of beta-amyloid were clearly above the detection level: 89.5 ± 12.1 pg/ml (n = 4) Aβ(40) and 26.3 ± 5.1 pg/ml (n = 4) Aβ(42).
Total tau in saliva was 577 ± 134 pg/mg in healthy controls (Table 1). Tau levels did not differ significantly between male and female participants in controls (Figure 3). Total tau saliva levels were significantly reduced in patients with AD dementia (Table 1), but not in patients with MCI or depression (Table 1). This effect was markedly pronounced in female patients with AD dementia but not in male patients with AD dementia (Figure 3). The saliva levels of phospho-tau-181 were 9.7 ± 1.3 (n = 21) pg/mg in healthy controls (Table 1). The phospho-tau-181 saliva levels were significantly enhanced in patients with MCI (Table 1), but not in patients with depression (Table 1). There was a tendency for an increase in patients with AD dementia saliva (Table 1). The ratio tau/pTau181 did not provide a statistical significance. In a preliminary experiment, we analyzed saliva from healthy volunteer controls at an age between 5 and 90 years and found that saliva tau levels did not show an age-dependent effect (data not shown).

Figure 3. Total tau saliva levels in male and female controls and patients with Alzheimer’s disease dementia (AD). Saliva was collected, analyzed for total tau by Lumipulse, and corrected for the protein. Values are given as mean ± SEM and the values in parenthesis give the number of analyzed patients. Statistical analysis was performed by one-way ANOVA with a subsequent Fisher LSD post-hoc test (*p ≤ 0.05).
In order to verify our values, we performed a blinded study (see Table 2). In this study, we collected 21 samples and determined a laboratory diagnosis with a cut-off of ≥ 18 pg/mg pTau181 (for MCI) and ≤ 300 pg/mg tau (for AD dementia). Out of these 21 samples, we excluded three with very high tau levels, one with very low protein, two with very high protein, and one after breakfast (Table 2). Out of the remaining 14 samples, we could diagnose 10 samples correctly (= 71.4%).
In this present study, we could show that total tau and pTau181 can be reliably detected in human saliva, while Aβ-42 and –40 were below the detection limit. A significant increase of pTau181 is shown for patients with MCI and a decrease of total tau for patients with AD dementia, which could make them useful as biomarkers, especially in combination with the very sensitive Lumipulse technology.
Saliva is a human fluid that is easily applicable, without any ethical concerns, but is still underrecognized as a source of AD biomarkers. The saliva proteome contains approx a total of 2,300 proteins and 27% are identical to plasma proteins (Loo et al., 2010). Saliva is the fluid that bathes the mouth and oral cavity and is made and secreted from the salivary glands (parotid, submandibular, sublingual, and minor salivary glands) which are directly innervated via cranial nerves (facial nerve, glossopharyngeal nerve). Furthermore, it consists of non-salivary components gingival crevicular fluid, nasal and bronchial secretions, serum and blood derivatives from wounds, desquamated epithelial linings, food components, and micro-organisms in the oral cavity (Kaufman and Lambster, 2002; Loo et al., 2010). Whole saliva is composed of water, peptides, and proteins, including hormones and enzymes, sugars, lipids, and electrolytes. The saliva metabolome appears to be comparable to the human serum and CSF metabolome in terms of chemical complexity and the number of compounds (Dame et al., 2015). This is consistent with the data from previous studies that showed that compounds found in human saliva are usually found in human blood, albeit at different concentrations (Takeda et al., 2009). Our data clearly show that saliva displays a very high variability even if it was collected the same way and also within the same group. First, the total protein content varied between 9,000 and 160 μg/ml. Second, when the saliva was applied onto an HPLC with EC detection (+ 0.55V), then the chromatogram was also very heterogeneous, sometimes with a few clear peaks, sometimes with peaks detectable, and sometimes there appeared a broad smear within the first 6 min, which did not allow to detect NE (rt = 5 min). This clearly shows that saliva can be a heterogenous fluid and this must be considered when saliva should be used as a fluid for biomarker detection.
As mentioned, there were some studies showing that saliva Aβ−42 could be a potential biomarker for AD and is enhanced in saliva (Bermejo-Pareja et al., 2010; Lee et al., 2017; Sabbagh et al., 2018; Gleerup et al., 2019), while others could not even detect it (Shi et al., 2011). There is also a report showing that amyloid-precursor protein (APP), the precursor protein of Aβ-42, was found to be expressed in human salivary epithelial cells (Oh and Turner, 2006). Our results showed that Aβ was close or below to the detection limit in most of the saliva samples. During the revision, we performed additional experiments and found that beta-amyloid cannot be recovered from the Salivettes and binds probably to the cotton. This was unfortunate, as the whole study was performed with Salivettes. We showed in a subsequent additional experiment with healthy volunteers, that, indeed, Aβ-40 and –42 can be detected in native saliva (spitting directly into tubes), and thus we recommend not to use Salivettes for such biomarker studies.
In the present study, we show that saliva total tau and pTau181 are detectable and altered in patients with AD dementia and MCI. We found lowest levels of total tau in patients with AD dementia and significantly or slightly increased levels of pTau181 in patients with MCI and AD dementia. Our results are in agreement with those of Lau et al. (2015) who found a higher expression of phospho-tau in patients with AD compared to controls. In contrast to Pekeles et al. (2018), we found no significant changes in the tau/pTau181 ratio in our study sample. Our study, however, could show for the first time that tau-markers are altered already in MCI, which is associated with increased risk for conversion to AD dementia.
Therefore, our results might make an important contribution to the current understanding of biomarkers in the early clinical stages of AD. In support of this, our blinded study was able to differentiate between MCI and AD dementia with an accuracy of over 70%. However, other authors argued that a large variation in the AD salivary tau levels limits the utility of tau as a clinical biomarker. We agree with this assumption, but note that prior studies showed high heterogeneity in saliva collection and analyzing methods. Our present study shows that the very sensitive Lumipulse technology is very potent to determine saliva total tau and pTau181 in fresh and frozen samples.
While total tau and phosphorylated tau in CSF originate from the brain, the origin of tau in saliva is not yet sufficiently clear. It has been suggested, that tau could be secreted from the innervating cranial nerves into the salivary glands (Kim et al., 2010), is derived from ultrafiltration from blood, or comes directly from epithelial cells. Supporting the latter option, tau mRNA was reported to be highly expressed in salivary glands (Conrad et al., 2002). This can lead to the assumption that tau comes directly from the salivary glands, which is also supported by our preliminary data with mouse salivary glands. Our data show that tau is, indeed, present in human saliva, and it was surprising to see that Lumipulse detects relatively high levels of tau (300–700 pg/ml) which are comparable to the tau levels in CSF. In addition, we detected some saliva samples, where tau was in the far ng/ml range, which means 1,000 × higher tau levels. This underlines that Lumipulse assay is very sensitive and accurate and we trust that the assay detects real tau; however, we cannot completely exclude some forms of small measuring inaccuracies. It needs to be noted that the Lumipulse technology has been validated for CSF and not saliva. Moreover, all the extreme tau levels could be related to patients with recent inflammation of salivary glands or bleedings into the mouth. More work is definitely necessary to investigate this issue.
In a preliminary experiment, we aimed to analyze saliva by Western blot (using a pool of 50 ml saliva from a healthy volunteer), but the detection levels were extremely low, and the data suggested tau fragments of approx. 30 kDa (data not shown). This needs a better investigation with more sensitive methods, but could fully agree with the very important work from Meredith et al. (2013). They show that multiple N-terminal and mid-domain fragments of tau were detected in pooled CSF with apparent sizes ranging from 20 kDa to approx. 40 kD. The pattern of tau fragments in AD and control samples was similar. They (Meredith et al., 2013) also did not detect full-length tau and C-terminal fragments in their CSF samples. Definitely, more work is necessary to study the metabolism and stability of the tau protein and the functional role of smaller fragmented tau forms.
The blinded verification study already highlights several limits of the study and of saliva as a source of human fluid biomarkers. (1) First, we found that the saliva protein showed a strong heterogeneity from very high to very low protein values. Neither the laboratory nor the clinicians are able to verify that the saliva collection was done very accurately. Possible bias could be the consumption of food before saliva collection or that patients had the Salivette for less than 2 min in the mouth. (2) Second, we found some samples with very high saliva tau, in the far ng/ml range. This is surprising and should be verified by other methods. We could correlate some high saliva tau levels with acute brain injury or inflammation of the glands. Alternatively, we cannot exclude bleeding of the teeth or periodontitis, and thus a dentist could give advice in future studies. (3) Unfortunately, the use of Salivettes was not optimal as beta-amyloid could not be recovered. Indeed, there is no international consensus on saliva collection procedure, stability, origin, inflammatory processes in the mouth, and salivary flow of salivary glands. This all needs to be tested in future studies. (4) And finally, in saliva, a functional role of tau is still largely unexplored. It will be necessary to conduct more research in humans, or in mouse models, including tau transgenic mice.
In conclusion, our data show that total tau and pTau181 are reproducible and detectable in saliva and total tau is decreased in AD dementia, while pTau181 is increased in MCI. We suggest that the very sensitive Lumipulse technology could become an interesting tool to establish saliva tau detection in AD. However, saliva is a heterogeneous fluid and the collection process needs to be established and verified in order to provide stable values. We recommend not using Salivettes for saliva sample collection.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was approved by the Ethics Committee of Medical University of Innsbruck. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
CH designed the study, wrote the manuscript, and acquired funding. JM collected saliva samples and diagnosed patients. MD collected saliva samples and diagnosed patients for the blinded study. All authors contributed to the article and approved the submitted version.
We thank Karin Albrecht and Anna Draxl for their excellent technical help.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Anand, A., and Charney, D. S. (2000). Norepinephrine dysfunction in depression. J. Clin. Psychiatry 61 Suppl 10, 16–24.
Ashton, N. J., Ide, M., Schöll, M., Blennow, K., Lovestone, S., Hye, A., et al. (2018). No association of salivary total tau concentration with Alzheimer’s disease. Neurobiol. Aging 70, 125–127. doi: 10.1016/j.neurobiolaging.2018.06.014
Ashton, N. J., Ide, M., Zetterberg, H., and Blennow, K. (2019). Salivary biomarkers for Alzheimer’s disease and related disorders. Neurol. Ther. 8, 83–94. doi: 10.1007/s40120-019-00168-1
Bermejo-Pareja, F., Antequera, D., Vargas, T., Molina, J. A., and Carro, E. (2010). Saliva levels of abeta1-42 as potential biomarker of Alzheimer’s disease: A pilot study. BMC Neurol. 10:108. doi: 10.1186/1471-2377-10-108
Blasko, I., Lederer, W., Oberbauer, H., Walch, T., Kemmler, G., Hinterhuber, H., et al. (2006). Measurement of thirteen biological markers in CSF of patients with Alzheimer’s disease and other dementias. Dement. Geriatr. Cogn. Disord. 21, 9–15. doi: 10.1159/000089137
Blennow, K. (2005). CSF biomarkers for Alzheimer’s disease: Use in early diagnosis and evaluation of drug treatment. Expert Rev. Mol. Diagn. 5, 661–672. doi: 10.1586/14737159.5.5.661
Blennow, K., Hampel, H., Weiner, M., and Zetterberg, H. (2010). Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 6, 131–144. doi: 10.1038/nrneurol.2010.4
Boston, P. F., Gopalkaje, K., Manning, L., Middleton, L., and Loxley, M. (2008). Developing a simple laboratory test for Alzheimer’s disease: Measuring acetylcholinesterase in saliva - a pilot study. Int. J. Geriatr. Psychiatry 23, 439–440. doi: 10.1002/gps.1882
Conrad, C., Vianna, C., Freeman, M., and Davies, P. (2002). A polymorphic gene nested within an intron of the tau gene: Implications for Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 99, 7751–7756. doi: 10.1073/pnas.112194599
Dame, Z. T., Aziat, F., Mandal, R., Krishnanurthy, R., Bouatra, S., Borzouie, S., et al. (2015). The human saliva metabolome. Metabolomics 11, 1864–1883. doi: 10.1007/s11306-015-0840-5
Defrancesco, M., Marksteiner, J., Kemmler, G., Fleischhacker, W. W., Blasko, I., and Deisenhammer, E. A. (2017). Severity of depression impacts imminent conversion from mild cognitive impairment to Alzheimer’s disease. J. Alzheimers Dis. 59, 1439–1448. doi: 10.3233/JAD-161135
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
François, M., Bull, C. F., Fenech, M. F., and Leifert, W. R. (2019). Current state of saliva biomarkers for aging and Alzheimer’s disease. Curr. Alzheimer Res. 16, 56–66. doi: 10.2174/1567205015666181022094924
Gleerup, H. S., Hasselbalch, S. G., and Simonsen, A. H. (2019). Biomarkers for Alzheimer’s disease in saliva: A systematic review. Dis. Markers 2019:11. doi: 10.1155/2019/4761054
Grundke-Iqbal, I., Iqbal, K., Tung, Y. C., Quinlan, M., Wisniewski, H. M., and Binder, L. I. (1986). Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. U.S.A. 83, 4913–4917. doi: 10.1073/pnas.83.13.4913
Hanger, D. P., Anderton, B. H., and Noble, W. (2009). Tau phosphorylation: The therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 15, 112–119. doi: 10.1016/j.molmed.2009.01.003
Huan, T., Tran, T., Zheng, J., Sapkota, S., MacDonald, S. W., Camicioli, R., et al. (2018). Analyses of saliva detect novel biomarkers of Alzheimer’s disease. Metabolomics 65, 1401–1416. doi: 10.3233/JAD-180711
Humpel, C. (2011). Identifying and validating biomarkers for diagnosing Alzheimer’s disease. Trends Biotechnol. 29, 26–32. doi: 10.1016/j.tibtech.2010.09.007
Hutter, P., Johansson, M., Saria, A., and Humpel, C. (1996). Acute and chronic noradrenergic regulation of neurotrophin mRNA expression in rat hippocampus: Evidence from lesions and organotypic cultures. Neuroscience 70, 15–29. doi: 10.1016/0306-4522(95)00346-k
Kaufman, E., and Lambster, I. B. (2002). The diagnostic application of saliva - a review. Crit. Rev. Oral Biol. Med. 13, 197–212.
Kim, W., Lee, S., Jung, C., Ahmed, A., Lee, G., and Hall, G. F. (2010). Interneuronal transfer of human tau between Lamprey central neurons in situ. J. Alzheimers Dis. 19, 647–664. doi: 10.3233/JAD-2010-1273
Lau, H. C., Lee, I. K., Ko, P. W., Lee, H. W., Huh, J. S., Cho, W. J., et al. (2015). Non-invasive screening for Alzheimer’s disease by sensing salivary sugar using drosophila cells expressing gustatory receptor (Gr5a) immobilized on an extended gate ion-sensitive field-effect transistor (EG-ISFET) biosensor. PLoS One 10:e0117810. doi: 10.1371/journal.pone.0117810
Lederer, W., Alomar Dominguez, C., Popovscaia, M., Putz, G., and Humpel, C. (2016). Cerebrospinal fluid levels of tau and phospho-tau-181 proteins during pregnancy. Pregnancy Hypertens. 6, 384–387. doi: 10.1016/j.preghy.2016.08.243
Lee, M., Gio, J. P., Kennedy, K., McGeer, E. G., and McGeer, P. L. (2017). A method for diagnosing Alzheimer’s disease based on salivary amyloid-beta protein 42 levels. J. Alzheimers Dis. 55, 1175–1182. doi: 10.3233/JAD-160748
Li, G. Y., Watanabe, I., Kunitake, Y., Sugataka, K., Muraoka, T., Kojima, N., et al. (2008). Relationship between saliva level of 3-methoxy-4-hydroxyphenylglycol and mental health in the elderly general population. Psychiatry Clin. Neurosci. 62, 562–567. doi: 10.1111/j.1440-1819.2008.01850.x
Liang, D., and Lu, H. (2019). Salivary biological biomarkers for Alzheimer’s disease. Arch. Oral Biol. 105, 5–12. doi: 10.1016/j.archoralbio.2019.06.004
Liu, C., and Götz, J. (2013). How it all started: Tau and protein phosphatase 2A. J. Alzheimers Dis. 37, 483–494. doi: 10.3233/JAD-130503
Loo, J. A., Yan, W., Ramachandran, P., and Wong, D. T. (2010). Comparative human salivary and plasma proteomes. J. Dent. Res. 89, 1016–1023. doi: 10.1177/0022034510380414
Marksteiner, J., Oberacher, H., and Humpel, C. (2019). Acyl-alkyl-phosphatidlycholines are decreased in saliva of patients with Alzheimer’s disease as identified by targeted metabolomics. J. Alzheimers Dis. 68, 583–589. doi: 10.3233/JAD-181278
Meredith, J. E. Jr., Sankaranarayanan, S., Guss, V., Lanzetti, A. J., Berisha, F., Neely, R. J., et al. (2013). Characterization of novel CSF Tau and ptau biomarkers for Alzheimer’s disease. PLoS One 8:e76523. doi: 10.1371/journal.pone.0076523
Mirzaii-Dizgah, M. H., Mirzaii-Dizgah, M. R., and Mirzaii-Dizgah, I. (2020). Serum and saliva total tau protein as a marker for relapsing-remitting multiple sclerosis. Med. Hypotheses 135:109476. doi: 10.1016/j.mehy.2019.109476
Nisbet, R., Polanco, J. C., Ittner, L. M., and Götz, J. (2015). Tau aggregation and its interplay with amyloid-beta. Acta Neuropathol. 129, 207–220. doi: 10.1007/s00401-014-1371-2
Oh, Y. S., and Turner, R. J. (2006). Effect of gamma-secretase inhibitors on muscarinic receptor-mediated calcium signaling in human salivary epithelial cells. Am. J. Physiol. Cell Physiol. 291, C76–C82. doi: 10.1152/ajpcell.00508.2005
Olczak, M., Poniatowski, ŁA., Niderla-Bielińska, J., Kwiatkowska, M., Chutorański, D., Tarka, S., et al. (2019). Concentration of microtubule associated protein tau (MAPT) in urine and saliva as a potential biomarker of traumatic brain injury in relationship with blood-brain barrier disruption in postmortem examination. T. Forensic Sci. Int. 301, 28–36. doi: 10.1016/j.forsciint.2019.05.010
Pekeles, H., Qureshi, H. Y., Paudel, H. K., Schipper, H. M., Gornistky, M., and Chertkow, H. (2018). Development and validation of a salivary tau biomarker in Alzheimer’s disease. Alzheimers Dement 11, 53–60. doi: 10.1016/j.dadm.2018.03.003
Petersen, R. C., Doody, R., Kurz, A., Mohs, R. C., Morris, J. C., Rabins, P. V., et al. (2001). Current concepts in mild cognitive impairment. Arch. Neurol. 58, 1985–1992. doi: 10.1001/archneur.58.12.1985
Rosen, W. G., Mohs, R. C., and Davis, K. L. (1984). A new rating scale for Alzheimer’s disease. Am. J. Psychiatry 141, 1356–1364. doi: 10.1176/ajp.141.11.1356
Sabbagh, M. N., Shi, J., Lee, M., Arnold, L., Al-Hasan, Y., Heim, J., et al. (2018). Salivary beta amyloid protein levels are detectable and differentiate patients with Alzheimer’s disease dementia from normal controls: Preliminary findings. BMC Neurol. 18:155. doi: 10.1186/s12883-018-1160-y
Sheline, Y. I., Miller, K., Bardgett, M. E., and Csernansky, J. G. (1998). Higher cerebrospinal fluid MHPG in subjects with dementia of the Alzheimer type. Relationship with cognitive dysfunction. Am. J. Geriatr. Psychiatry 6, 155–161.
Shi, M., Sui, Y. T., Peskind, E. R., Li, G., Hwang, H., Devic, I., et al. (2011). Salivary tau species are potential biomarkers of Alzheimer’s disease. J. Alzheimers Dis. 27, 299–305. doi: 10.3233/JAD-2011-110731
Sperling, R. A., Aisen, P. S., Beckett, L. A., Bennett, D. A., Craft, S., Fagan, A. M., et al. (2011). Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 280–292. doi: 10.1016/j.jalz.2011.03.003
Spillantini, M. G., and Goedert, M. (2013). Tau pathology and neurodegeneration. Lancet Neurol. 12, 609–622. doi: 10.1016/S1474-4422(13)70090-5
Takeda, I., Stretch, C., Barnaby, P., Bhatnager, K., Rankin, K., Fu, H., et al. (2009). Understanding the human salivary metabolome. NMR Biomed. 22, 577–584.
Tvarijonaviciute, A., Camora, C., Ceron, J. J., Bravo-Cantero, A. F., Pardon-Marin, L., Valverdem, S., et al. (2020). Salivary biomarkers in Alzheimer’s disease. Clin. Oral Investig. 24, 3437–3444. doi: 10.1007/s00784-020-03214-7
Keywords: Alzheimer’s disease, saliva, biomarker, tau, beta-amyloid
Citation: Marksteiner J, Defrancesco M and Humpel C (2022) Saliva tau and phospho-tau-181 measured by Lumipulse in patients with Alzheimer’s disease. Front. Aging Neurosci. 14:1014305. doi: 10.3389/fnagi.2022.1014305
Received: 08 August 2022; Accepted: 01 September 2022;
Published: 29 September 2022.
Edited by:
Javier Frontiñan-Rubio, University of Castilla-La Mancha, SpainReviewed by:
Anja Hviid Simonsen, Copenhagen University Hospital, DenmarkCopyright © 2022 Marksteiner, Defrancesco and Humpel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian Humpel, Y2hyaXN0aWFuLmh1bXBlbEBpLW1lZC5hYy5hdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.