- 1Department of Radiology, Shanghai Fifth People’s Hospital, Fudan University, Shanghai, China
- 2School of Teacher Education, Zhejiang Normal University, Jinhua, China
- 3Key Laboratory of Intelligent Education Technology and Application of Zhejiang Province, Zhejiang Normal University, Jinhua, China
- 4School of Information and Electronics Technology, Jiamusi University, Jiamusi, China
- 5School of Western Language, Heilongjiang University, Heilongjiang, China
Mild cognitive impairment (MCI) reversion refers to patients with MCI who revert from MCI to a normal cognitive state. Exploring the underlying neuromechanism of MCI reverters may contribute to providing new insights into the pathogenesis of Alzheimer’s disease and developing therapeutic interventions. Information on patients with MCI and healthy controls (HCs) was collected from the Alzheimer’s Disease Neuroimaging Initiative database. We redefined MCI reverters as patients with MCI whose logical memory scores changed from MCI to normal levels using the logical memory criteria. We explored intrinsic brain activity alterations in MCI reverters from voxel, regional, and whole-brain levels by comparing resting-state functional magnetic resonance imaging metrics of the amplitude of low-frequency of fluctuation (ALFF), the fractional amplitude of low-frequency fluctuation (fALFF), percent amplitude of fluctuation (PerAF), regional homogeneity (ReHo), and degree centrality (DC) between MCI reverters and HCs. Finally, partial correlation analyses were conducted between cognitive scale scores and resting-state functional magnetic resonance imaging metrics of brain regions, revealing significant group differences. Thirty-two patients with MCI from the Alzheimer’s Disease Neuroimaging Initiative database were identified as reverters. Thirty-seven age-, sex-, and education-matched healthy individuals were also enrolled. At the voxel level, compared with the HCs, MCI reverters had increased ALFF, fALFF, and PerAF in the frontal gyrus (including the bilateral orbital inferior frontal gyrus and left middle frontal gyrus), increased PerAF in the left fusiform gyrus, and decreased ALFF and fALFF in the right inferior cerebellum. Regarding regional and whole-brain levels, MCI reverters showed increased ReHo in the left fusiform gyrus and right median cingulate and paracingulate gyri; increased DC in the left inferior temporal gyrus and left medial superior frontal; decreased DC in the right inferior cerebellum and bilateral insular gyrus relative to HCs. Furthermore, significant correlations were found between cognitive performance and neuroimaging changes. These findings suggest that MCI reverters show significant intrinsic brain activity changes compared with HCs, potentially related to the cognitive reversion of patients with MCI. These results enhance our understanding of the underlying neuromechanism of MCI reverters and may contribute to further exploration of Alzheimer’s disease.
Introduction
Alzheimer’s disease (AD), accounting for 60–80% of all dementia cases, is an irreversible neurodegenerative disease that causes progressive problems with memory, judgment, orientation, and other functions (Dai and He, 2014). It has been reported that every 65 s someone in the United States will be diagnosed with AD, and the number of people aged ≥ 65 years with AD may increase to a projected 13.8 million by 20501. However, the pathogenesis of AD remains unclear, and no drugs or other therapeutic interventions have been proven to be effective.
With an elevated risk of progression to AD (Busse et al., 2006; Petersen et al., 2009; Belleville et al., 2011; Buschert et al., 2011), mild cognitive impairment (MCI) is generally considered a transitional state between normal cognitive functioning and dementia. Research on this mental state has recently increased. Patients with MCI may progress to dementia, maintain stability, or revert to normal (Petersen et al., 2009); thus, most studies have mainly focused on studying MCI-to-dementia or MCI-stable populations and have made considerable contributions in identifying individuals at high risk of developing dementia (Li et al., 2021; Pyun et al., 2021). However, the cognitively normal-to-MCI-to-AD trajectory is not always unidirectional. A large portion of individuals diagnosed with MCI revert to cognitively normal status when reevaluated after ≥ 1 year (up to 30–50%) (Canevelli et al., 2016; Malek-Ahmadi, 2016). Less research attention has been paid to this population, and the neuromechanism underlying cognitive reversion has not been clearly explained. Therefore, further exploration of the neural basis of MCI reverters may contribute to providing new insights into the pathogenesis of AD and developing specific pharmacologic and nonpharmacologic interventions.
Resting-state functional magnetic resonance imaging (rs-fMRI), a widely known tool for investigating brain function, is a promising approach for exploring brain activity alterations (Biswal, 2012). A series of rs-fMRI metrics are used to reflect the intrinsic brain activity from different aspects. At the voxel level, the amplitude of low-frequency fluctuation (ALFF), defined as the mean amplitude of fluctuations within the range of low frequency, directly characterizes the spontaneous activity of each voxel (Zang et al., 2007). The fractional amplitude of low-frequency fluctuation (fALFF), i.e., the ratio of ALFF within a specific low-frequency band to the total blood oxygen level-dependent (BOLD) fluctuation amplitude of the full frequency band (Zou et al., 2008), is regarded as a standardized ALFF-like metric at the single voxel level. The percent amplitude of fluctuation (PerAF), i.e., the percentage of BOLD fluctuations relative to the mean BOLD signal intensity for each time point and averaged across the whole time series (Jia et al., 2020), has better test-retest reliability in both intra-and inter-scanners. Regarding regional and whole-brain levels, regional homogeneity (ReHo) measures the functional synchronization between a given voxel and its neighboring voxels (Zang et al., 2004), and degree centrality (DC) can be used to evaluate intrinsic functional connectivity across the whole brain.
These metrics have attracted significant research interest in the neural mechanisms of individuals with MCI. Studies have identified that regions in those patients, compared with healthy controls (HCs), have decreased or increased ALFF, fALFF, PerAF, ReHo, and DC, which reflects abnormal intrinsic brain activity in widespread areas of patients with MCI (Li et al., 2017; Liu et al., 2018, 2019; Yang et al., 2018; Xiong et al., 2021). Furthermore, a longitudinal study demonstrated that ALFF and fALFF values in some brain regions changed gradually with disease progression, and these metrics were significantly correlated with neuropsychological performance (Yang et al., 2018). These results indicate that ALFF and fALFF may help to detect the underlying pathological mechanism in the AD continuum. Additionally, previous studies have detected several brain regions with higher ALFF in patients with MCI related to those exhibiting functional disruptions in AD, which may indicate a possible compensatory mechanism in the early stage of AD (Wang et al., 2015; Xing et al., 2021). These findings contribute to partly elucidating the functional abnormalities in patients with MCI. To date, the brain functional mechanism in MCI reverters remains unclear, and relatively few studies explore MCI reversion through rs-fMRI. Therefore, we aimed to perform an exploratory study to indicate the intrinsic brain activity alterations in MCI reverters from different scales by combining various rs-fMRI metrics, namely ALFF, fALFF, PerAF, ReHo, and DC.
The Alzheimer’s Disease Neuroimaging Initiative (ADNI) database is a longitudinal multisite observational study of elderly individuals with normal cognition, MCI, and AD (Veitch et al., 2021). In the present study, we obtained information on patients with MCI and healthy controls (HCs) from the ADNI. Currently, the diagnostic criteria for MCI reverters remain controversial. Generally, the most common diagnostic criteria include clinical consensus, neuropsychological tests, and a combination of neuropsychological and daily functional tests (Winblad et al., 2004; Gao et al., 2014; Canevelli et al., 2016; Malek-Ahmadi, 2016; Pandya et al., 2017). Clinical consensus is relatively easily influenced by the clinician’s subjective judgment, while the combination of neuropsychological tests and daily functional tests is associated with poor patient compliance and less sensitivity. The use of neuropsychological tests as criteria is an approach that balances sensitivity, reliability, and simplicity. ADNI applied the combination of three neuropsychological tests including clinical dementia rating (CDR), logical memory (LM), and mini-mental state examination (MMSE) as diagnostic criteria of MCI reverters (Petersen et al., 2010). Only patients with MCI that meet all these criteria can be regarded as reverters. Thomas et al. (2019a,b) demonstrated that the standard ADNI MCI criteria may result in certain rates of “false positive” and “false negative” MCI diagnoses, which may be driven by weighting the subjective CDR more heavily than the objective memory test (LM). Further analysis showed that reverters identified by LM were more reliable. These reverters were more closely resemble HCs in terms of positive AD biomarkers (cerebrospinal fluid and genetic susceptibility) and have higher global cognition than those identified by standard AD criteria. Thus, in the current study, we redefined MCI reverters in the ADNI database by applying LM criteria, which may increase the validity of our results.
In this study, we first redefined MCI reverters in the ADNI database using the LM criteria. We then assessed the intrinsic brain activity changes in these patients at the voxel, regional, and whole-brain levels by comparing ALFF, fALFF, PerAF, ReHo, and DC between the MCI-to-normal and HC groups. Finally, we examined the relationship between altered brain activity and clinical features. These results may help us to further understand the underlying neuromechanisms of cognitive reversion.
Materials and Methods
Subjects
Data used in the preparation of this article were obtained from the ADNI database2. Data collection has been conducted in accordance with the tenets of the Declaration of Helsinki and was approved by the institutional review boards of all participating sites. All subjects and their legal representatives gave written informed consent prior to data collection. We focused on the data acquired from the 3.0 Tesla Siemens Prisma MRI scanner in ADNI 3. The detailed criteria of included subjects in ADNI 3 have been described at http://adni.loni.usc.edu/wp-content/themes/freshnews-dev-v2/documents/clinical/ADNI3_Protocol.pdf. Based on a previous study (Thomas et al., 2019b), we selected 41 patients with MCI-to-normal reversion according to the following criteria: a) LM scores improved from MCI (at first scan) to normal level (at any subsequent scans); b) scores of the Mini-Mental Status Examination (MMSE) were higher than 24 when the LM ability of the patients with MCI was evaluated as normal. Additionally, 66 HCs with no significant neurologic diseases were included.
Data Acquisition
The rs-fMRI, structural MRI, and behavioral data were downloaded. We only included the MRI data of MCI reverters by the time their LM ability was evaluated as normal. The following are the two scanning methods for rs-fMRI data: basic scanning and advanced scanning (multiband scanning). The parameters of the basic scanning were as follows: axial rs-fMRI (eyes open), repetition time/echo time = 3000/30 ms, field of view = 220 × 220 mm, matrix = 64 × 64, flip angle = 90°, voxel size = 3.4 mm × 3.4 mm × 3.4 mm, thick slices = 3.4 mm, number of slices = 48, and total volume = 197. Regarding advanced scanning, the parameters were as follows: axial MB rs-fMRI (eyes open), repetition time/echo time = 607/32 ms, field of view = 220 × 220 mm, matrix = 88 × 88, flip angle = 90°, voxel size = 2.5 mm × 2.5 mm × 2.5 mm, thick slices = 2.5 mm, number of slices = 64, and total volume = 976. All structural MRI data were acquired with the following parameters: accelerated sagittal magnetization prepared-rapid gradient echo, repetition time/echo time = 2300/2.98 ms, inversion time = 900 ms, field of view = 256 × 240 mm, matrix = 256 × 240, voxel size = 1.0 mm × 1.0 mm × 1.0 mm, flip angle = 9°, thick slice = 1.0 mm, and number of slices = 208 (partial basic scanning data = 176). 19 subjects underwent advanced scanning and 50 subjects underwent basic scanning. The scanning method for each subject is shown in Supplementary Table 1. The behavioral data, including a series of cognitive scales, are presented in Supplementary Table 2.
Data Preprocessing
Data preprocessing was performed using Statistical Parametric Mapping (SPM 12)3 and the Resting-State fMRI Data Analysis Toolkit plus (RESTplus v.1.24)4 (Jia et al., 2019) and conducted using MATLAB 2018a platform5. All digital imaging and communications in medicine data were converted to neuroimaging informatics technology initiative data. To stabilize the signal of the scanner and to enable subjects to adapt to the environment (Cui et al., 2020), the first 10 volumes of each neuroimaging informatics technology initiative dataset were discarded. Slice timing was only carried out using the basic scanning data because it is not considered necessary for multiband scanning data (Glasser et al., 2013; Smith et al., 2013). All data were then realigned to correct the head motion. The realigned data were spatially normalized to the Montreal Neurological Institute space via the deformation fields derived from new segmentation of structural images and resampled to 3 mm isotropic voxels. Subsequently, we removed the linear trends and regressed nuisance covariates, including the Friston-24 motion parameters (Friston et al., 1996), white matter signals, and cerebrospinal fluid signals (Chen et al., 2018). Finally, band-pass filtering with 0.01–0.08 Hz was performed. Note that the non-filtering data would be applied in ALFF and fALFF analyses (Wu et al., 2021), because the calculation procedure of ALFF includes the filtering step (Zang et al., 2007) and the fALFF calculation needs data from whole frequency range (Zou et al., 2008). Moreover, considering that spatial smoothing could lead to an overestimation of the local inter-voxel correlations (van den Heuvel et al., 2008) and introduce a bias in computing of ReHo and DC (Zuo et al., 2012), we put the smoothing step after the metrics calculation.
Notably, the process of including subjects was shown in the Figure 1. Six MCI reverters and six HCs with excessive head motion (>3 mm translation or >3° rotation), as well as two HCs with poor normalization, were excluded from the next metric calculation. To ensure that each included site involved both MCI reverters and HCs (basic and advanced scanning data in the same site were considered as different sites in these analyses), we also excluded three MCI reverters and three HCs. Additionally, 18 HCs were excluded owing to the mismatch with MCI reverters in age, sex, education, MMSE scores, or LM scores. A list of subjects included in the final analyses is available in Supplementary Table 1.
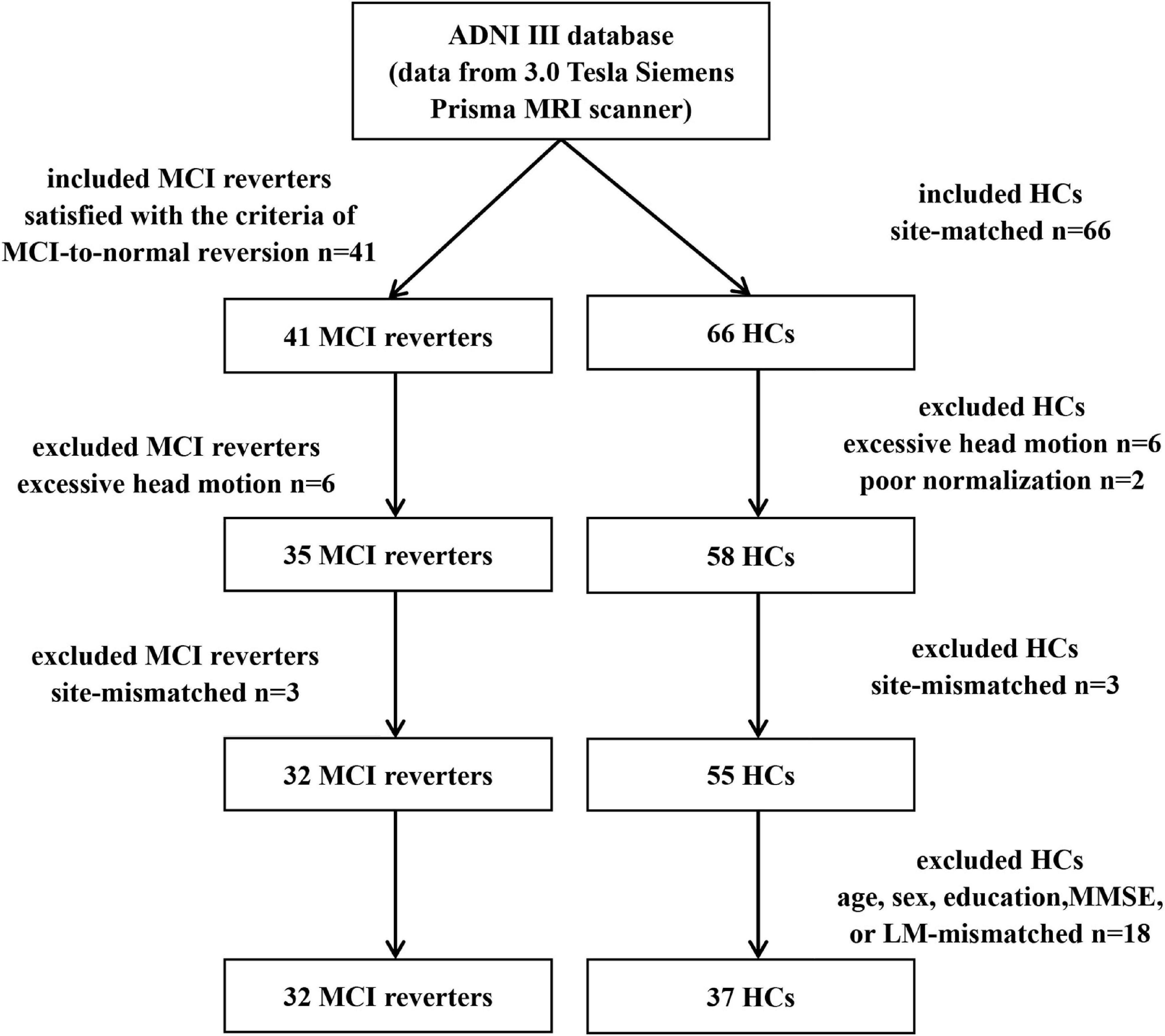
Figure 1. The process of including subjects. HCs, healthy controls; MCI, mild cognitive impairment; n, number.
Metrics Calculation
Voxel Level Metrics
ALFF, fALFF, and PerAF belong to a group of voxel-level metrics, reflecting the fluctuation amplitude of the time series for signal voxels from different aspects (Jia et al., 2020). ALFF is the averaged square root at each frequency of the power spectrum at a single voxel across 0.01–0.08 Hz. The time series of a single voxel was transformed to the frequency domain using a fast Fourier transform, and the power spectrum was obtained. The square root was calculated at each frequency of the power spectrum and the averaged square root obtained across 0.01–0.08 Hz of a single voxel was taken as the ALFF value (Zang et al., 2007). fALFF estimates the relative contribution of low-frequency (0.01–0.08 Hz) to the whole frequency range in the power spectrum. The fALFF value of a single voxel was obtained by calculating the ratio of the amplitude averaged across 0.01–0.08 Hz to that of the whole frequency range (Zou et al., 2008). PerAF is the percentage of resting-state BOLD fluctuations, relative to the mean signal intensity of a given time series. The PerAF value of a single voxel was calculated using the following formula:
where, n is the total number of volumes, Xi is the signal intensity at the ith time point, and μ is the mean value of the time series (Jia et al., 2020).
Regional Level Metric
ReHo is widely used to measure intrinsic brain activity in local regions (Zang et al., 2015; Hao et al., 2019). In this study, the ReHo calculation applied the Kendall’s coefficient of concordance to measure the similarity of the time series of a given voxel to its nearest 26 neighbors. The Kendall’s coefficient of concordance values were calculated using the following formula:
where, W is the Kendall’s coefficient of concordance value of the given voxel, Ri is the sum rank of the ith volumes, is the mean of the s, K is the number of time series within a cluster (K can be 7, 19, or 27; 27 was used in this study), and n is the number of ranks (Zang et al., 2004).
Whole-Brain Level Metric
We used DC to depict the intrinsic brain activity at the whole-brain level. We calculated Pearson’s correlation coefficients (r) of the time series from each pair of voxels in the whole-brain.
where, t is the corresponding volume, and X[t]i and X[t]j are the voxel intensities at the ith and jth voxels, respectively in the tth volume. As a result, an n × n matrix of r values was obtained, where n is the number of voxels in the whole-brain. The binarized DC value of a single voxel (i) is computed by counting the number of voxels which were correlated to the given voxel (i) above a threshold of r > 0.25 (Buckner et al., 2009).
Following this, all of the metric maps were calculated with standardization [subtracting the value from each voxel, and divided by the global mean value (Liu et al., 2017; Shan et al., 2020)] and smoothed with a with a Gaussian kernel of 4mm at full width at half-maximum (Wang et al., 2014; Li et al., 2019). We smoothed the unstandardized PerAF for subsequent statistical analyses since it is also used in group comparisons (Jia et al., 2020).
Statistical Analyses
Statistical Product and Service Solutions version (SPSS 26.0, IBM, Armonk, NY, United States) was used for statistical analyses of demographic and cognition data. Comparisons between MCI reverters and HCs were performed using a two-sample t-test for continuous variables and a chi-squared test for categorical variables. For brain intrinsic activity, statistical analyses were performed using data processing and analysis for brain imaging (DPABI 5.1)6 (Yan et al., 2016). A two-sample t-test was performed to detect differences in each metric of brain intrinsic activity between patients with MCI and HCs, regressing covariates of mean framewise displacement (Jenkinson et al., 2002) and site. The results, which remained after GRF (Gaussian Random Field) correction at two-tailed voxel p < 0.05 and a clustering level of p < 0.05, were considered to be significant. Additionally, to explore the relationship between cognitive ability and abnormal brain intrinsic activity in MCI reverters, partial correlation analyses were conducted between the cognitive scale scores and brain region metrics with significant group differences, controlling for the site. For the partial correlation analyses, we applied the two thresholds including False Discovery Rate (FDR) corrected p < 0.05 and uncorrected p < 0.05. We also performed partial correlation analysis controlling for site, age, sex, and education.
Results
Subjects
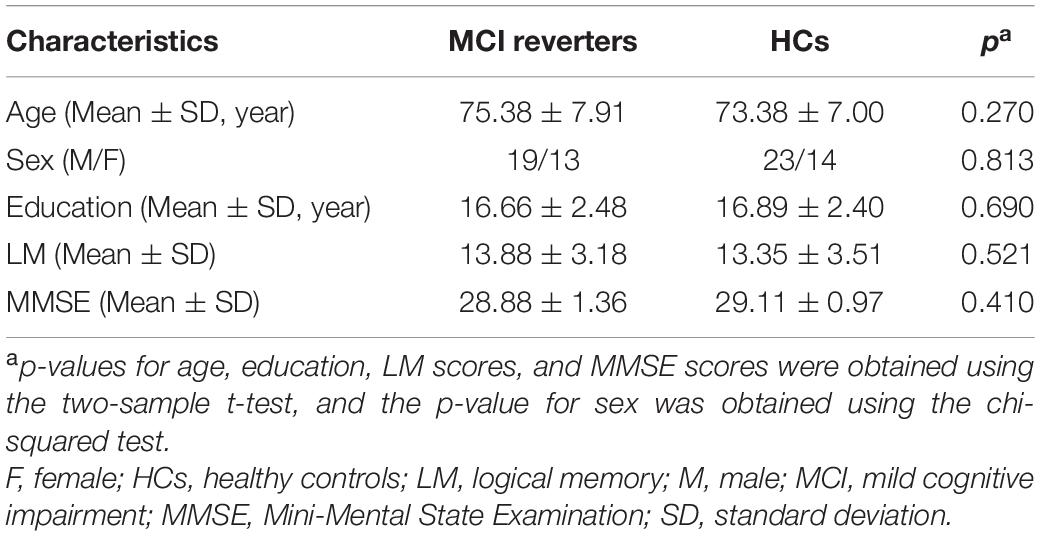
As shown in Table 1, 32 MCI reverters (19 men and 13 women; mean ± SD age, 75.38 ± 7.91 years; mean ± SD education, 16.66 ± 2.48 years; mean ± SD LM score, 13.88 ± 3.18; mean ± SD MMSE score, 28.88 ± 1.36 and 37 HCs (23 men and 14 women; mean ± SD age, 73.38 ± 7.00 years; mean ± SD education, 16.89 ± 2.40 years; mean ± SD LM score, 13.35 ± 3.51; mean ± SD MMSE score, 29.11 ± 0.97) from ADNI 3 were included in the final analysis. There were no significant group differences in age, sex, education, LM score, or MMSE score. The average reversal time of MCI reverters included in this analysis was 5.49 years.
Group Differences in Brain Intrinsic Activity
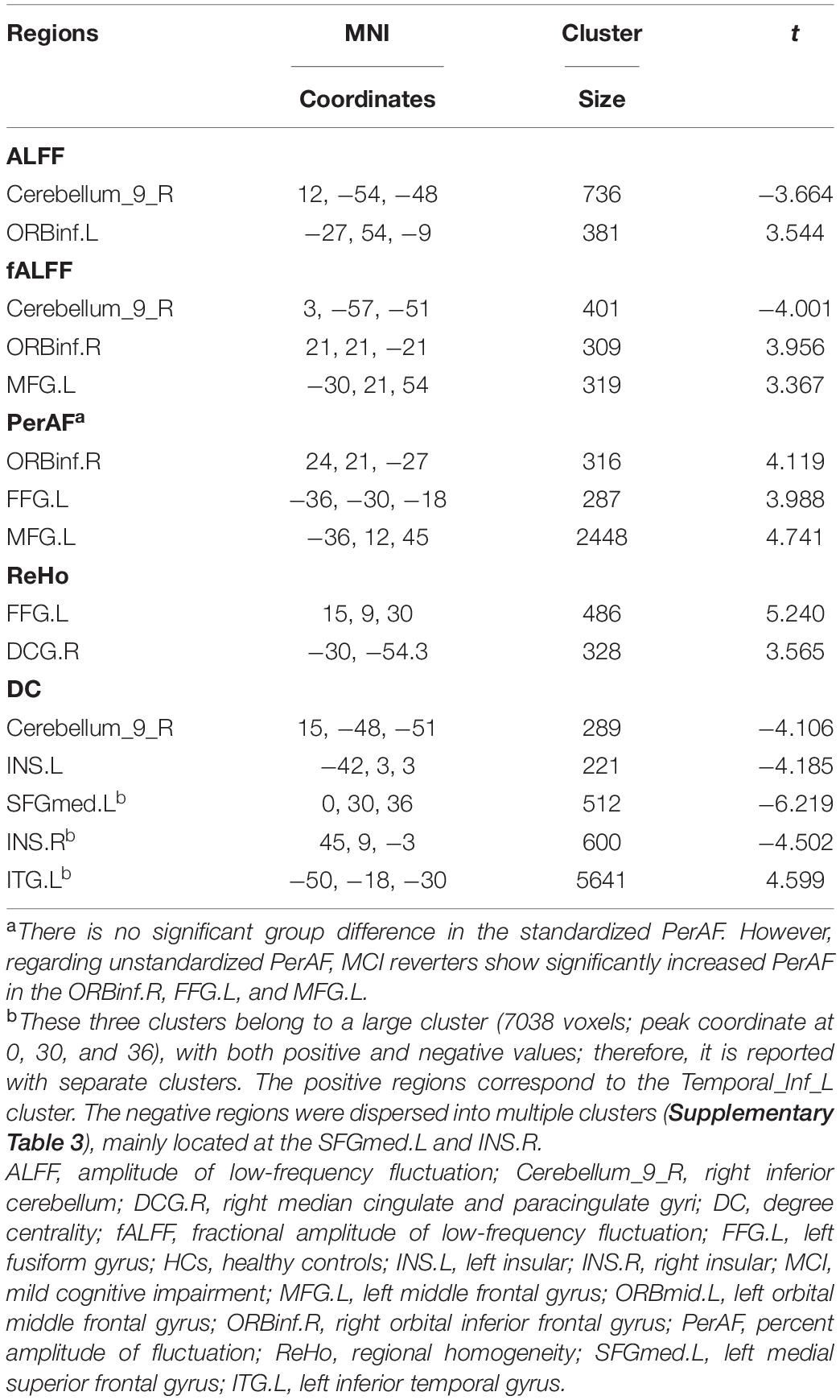
At the voxel level, MCI reverters had decreased ALFF in the right inferior cerebellum (cerebellum_9_R) and increased ALFF in the left middle inferior frontal gyrus (ORBmid.L) compared with HCs (Figure 2 and Table 2). MCI reverters exhibited decreased fALFF in the cerebellum_9_R and decreased fALFF in the right orbital inferior frontal gyrus (ORBinf.R) and left middle frontal gyrus (MFG.L) relative to HCs (Figure 3 and Table 2). Regarding PerAF, compared with HCs, MCI reverters showed increased brain intrinsic activity in the ORBinf.R, MFG.L, and left fusiform gyrus (FFG.L; Figure 4 and Table 2). At the regional level, the comparison of ReHo showed group differences between MCI reverters and HCs located in the FFG.L (t = 5.240), right median cingulate, and paracingulate gyri (DCG.R; t = 3.565; Figure 5 and Table 2). Regarding the whole-brain level, as shown in the DC comparison, MCI reverters showed decreased intrinsic brain activity in the cerebellum_9_R, bilateral insular (INS.L and INS.R) and left medial superior frontal gyrus (SFGmed.L) and increased intrinsic brain activity in the left inferior temporal gyrus (ITG.L; Figure 6, Supplementary Table 3, and Table 2).
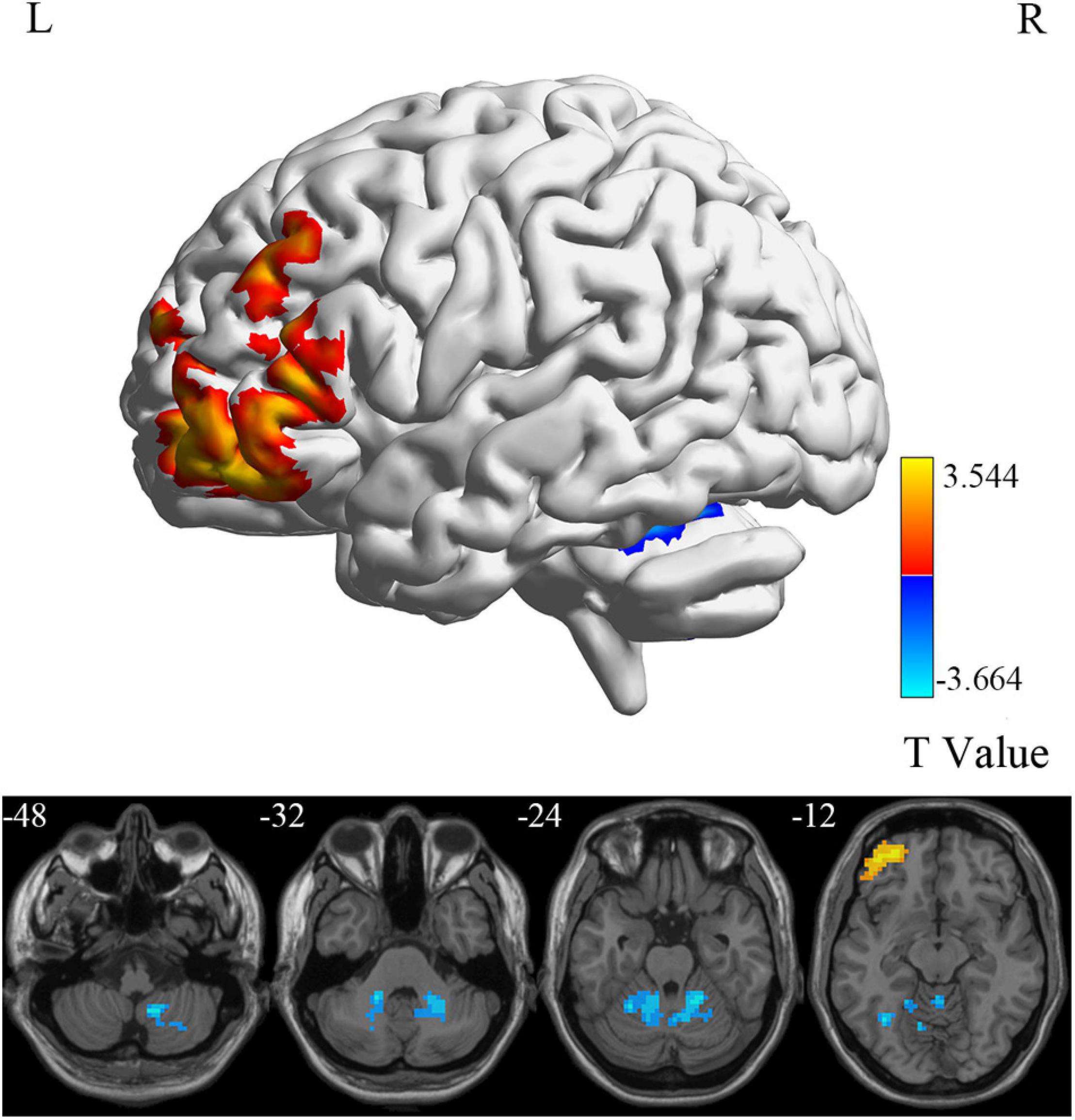
Figure 2. ALFF differences between MCI reverters and HCs. ALFF, amplitude of low-frequency fluctuation; HCs, healthy controls; L, left; MCI, mild cognitive impairment; R, right.
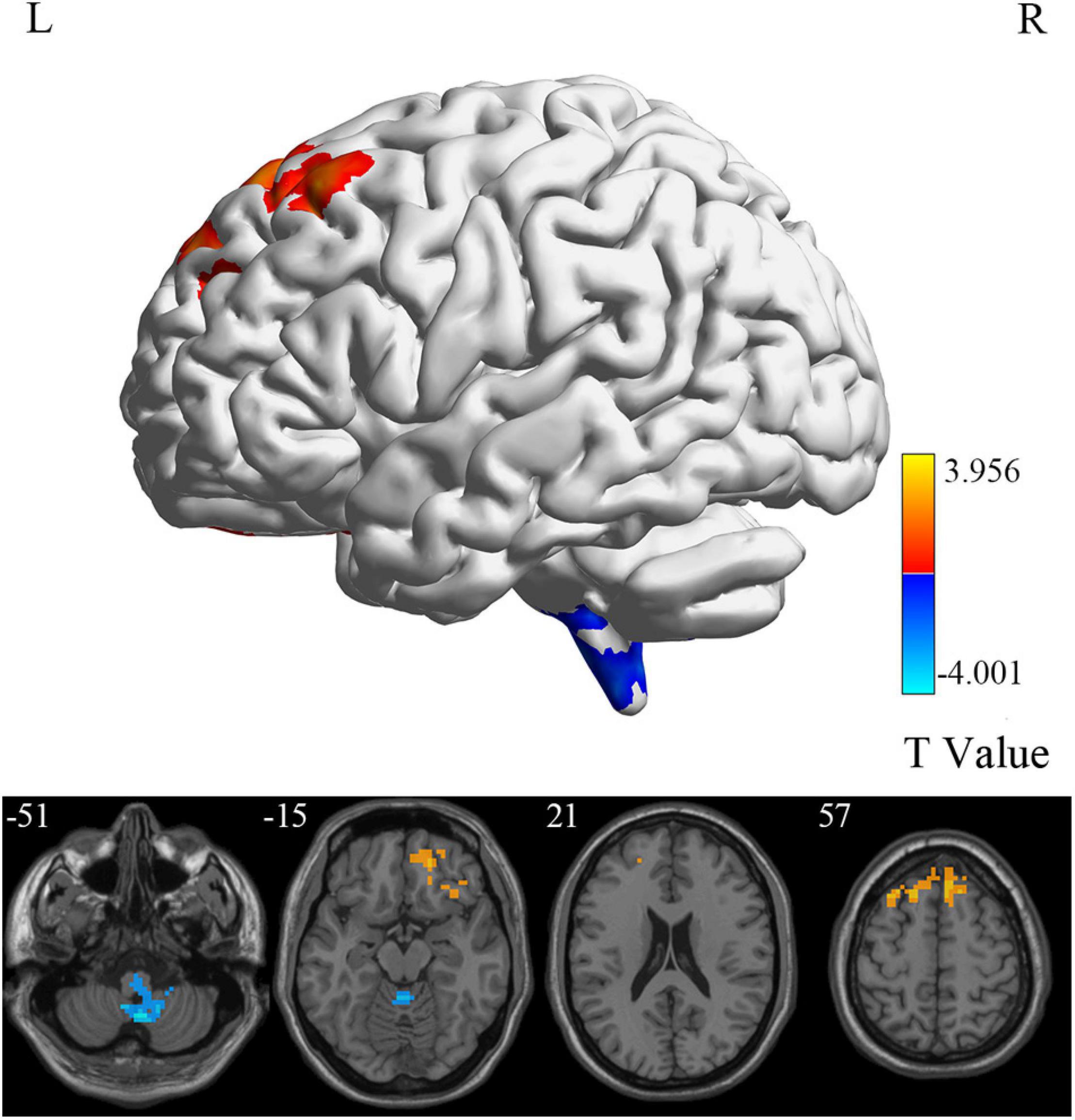
Figure 3. fALFF differences between MCI reverters and HCs. fALFF, fractional amplitude of low-frequency fluctuation; HCs, healthy controls; L, left; MCI, mild cognitive impairment; R, right.
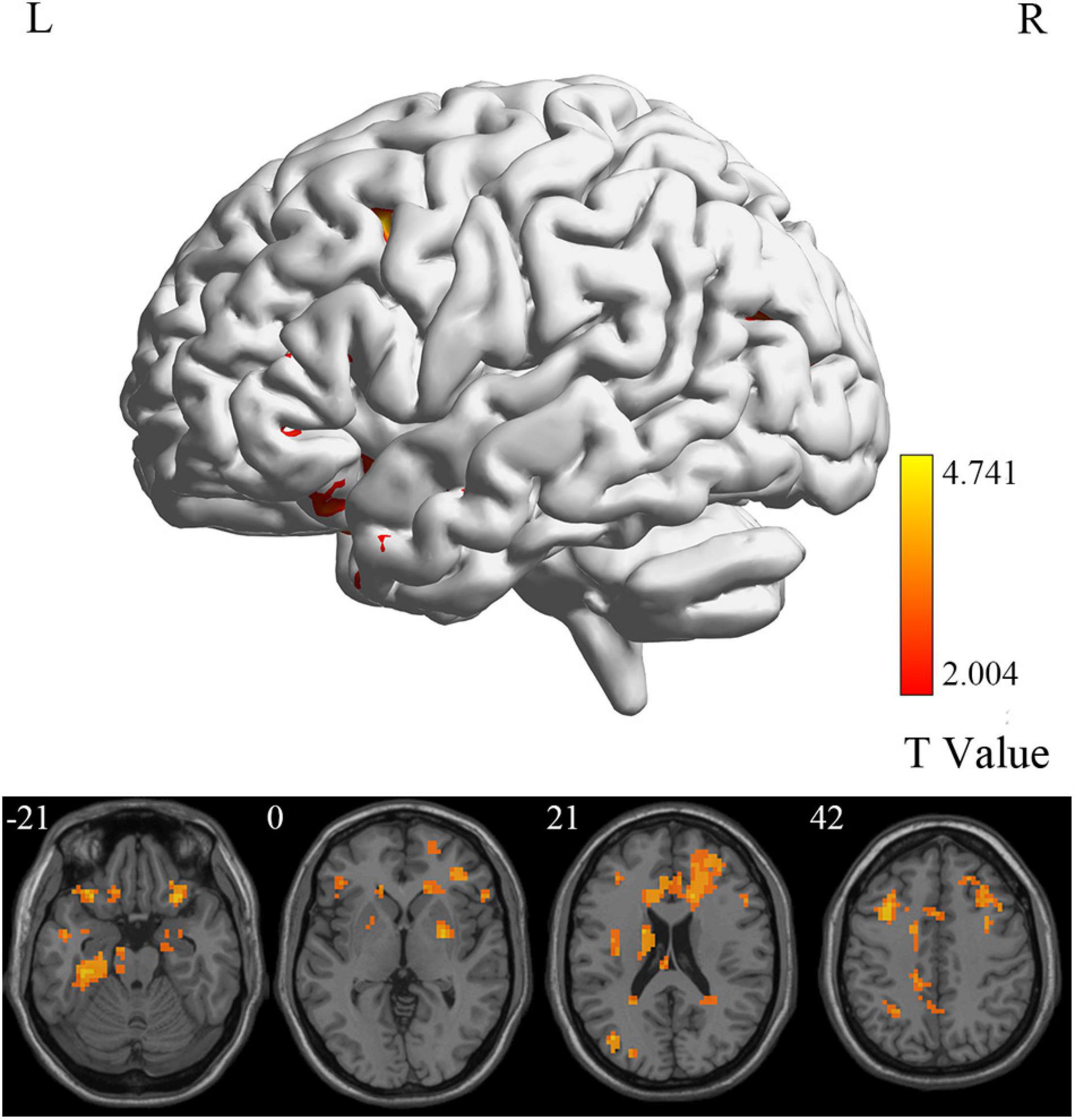
Figure 4. PerAF differences between MCI reverters and HCs. PerAF, percent amplitude of fluctuation; HCs, healthy controls; L, left; MCI, mild cognitive impairment; R, right.
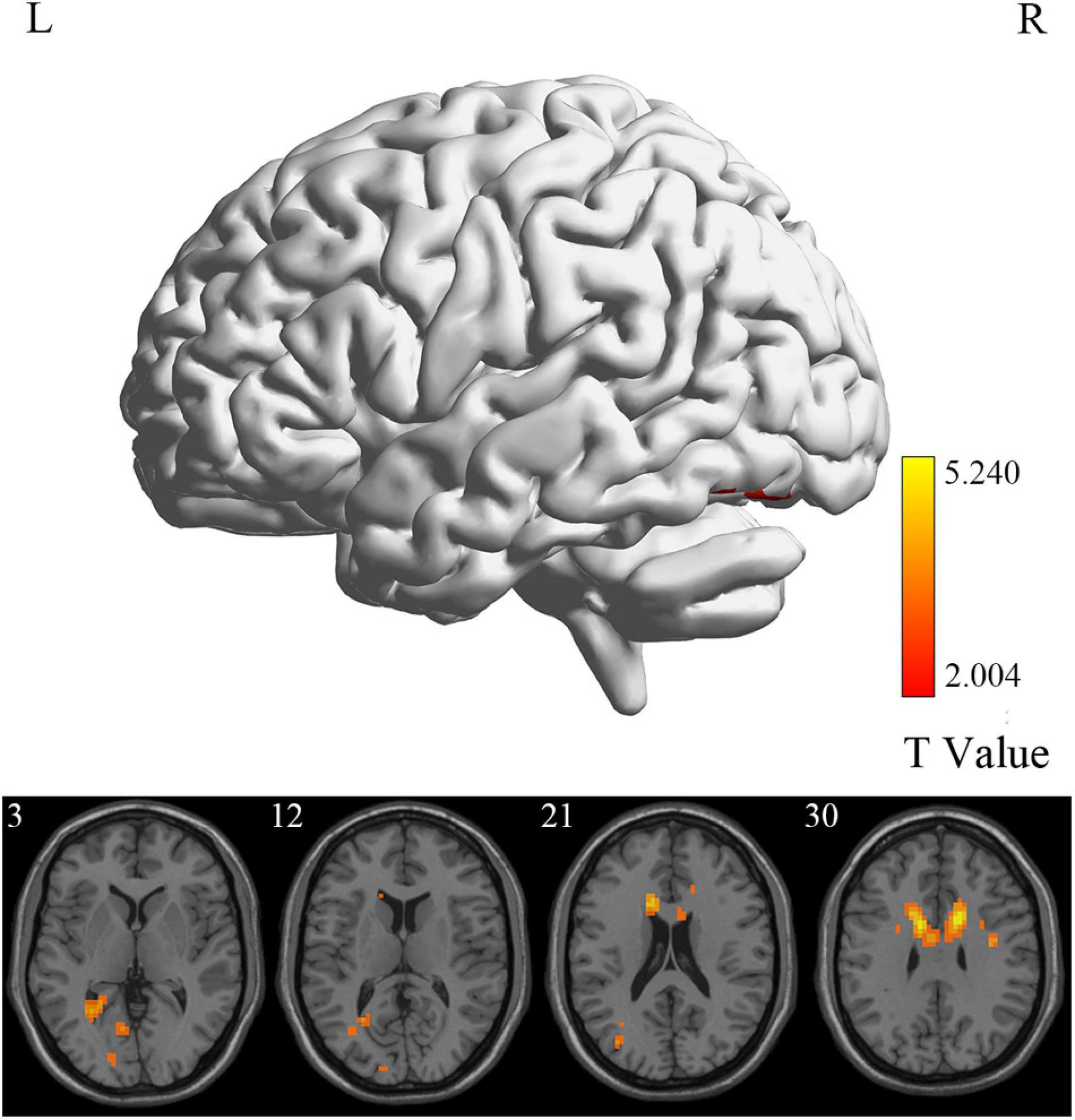
Figure 5. ReHo differences between MCI reverters and HCs. ReHo, regional homogeneity; HCs, healthy controls; L, left; MCI, mild cognitive impairment; R, right.
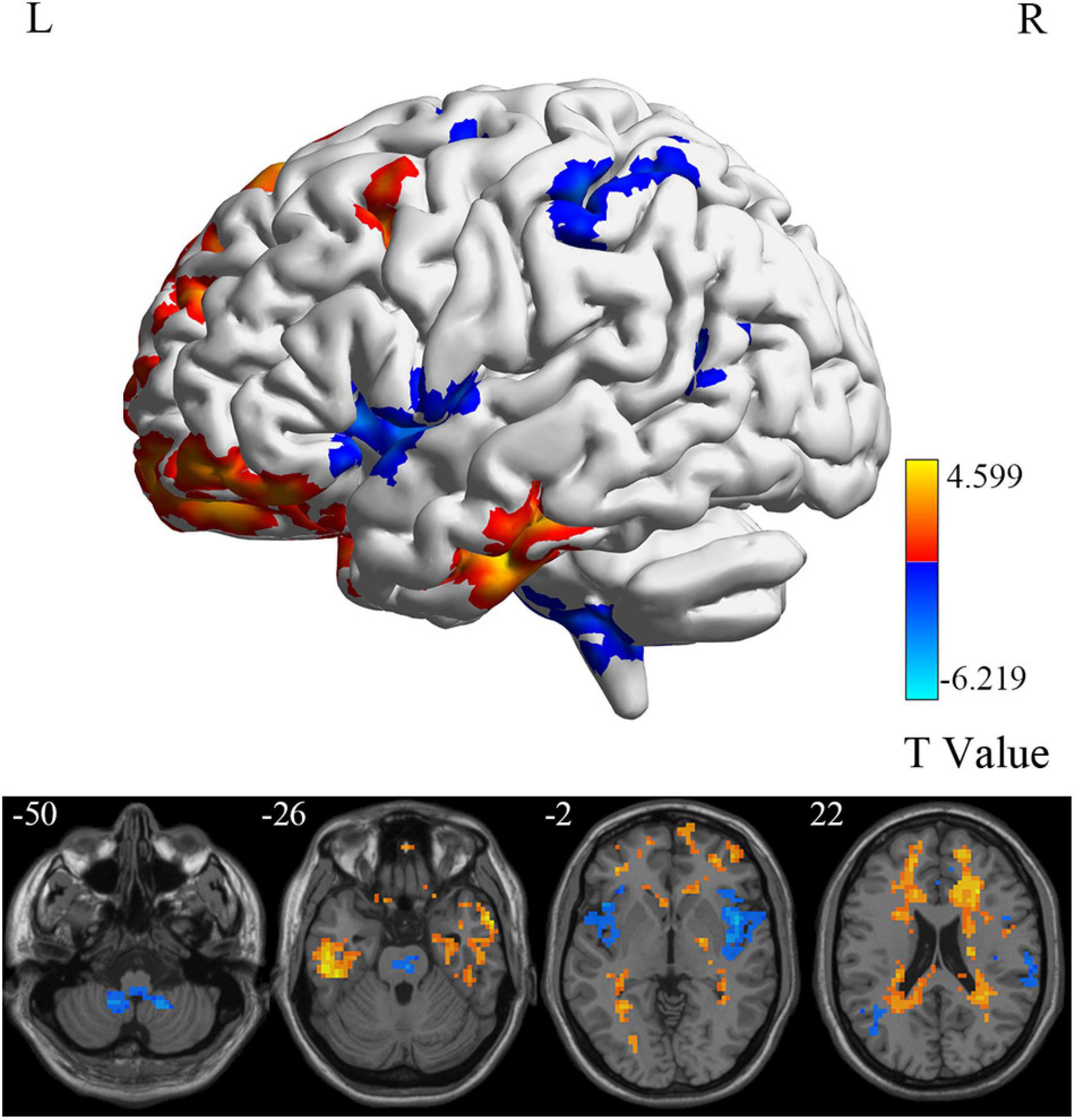
Figure 6. DC differences between MCI reverters and HCs. DC, degree centrality; HCs, healthy controls; L, left; MCI, mild cognitive impairment; R, right.
Partial Correlations
As presented in Table 3, significant partial correlations were found between cognitive scale scores and brain region metrics with significant group differences at a significance level of uncorrected p < 0.05, controlling for site. The scatter plots of significant partial correlation results (uncorrected p < 0.05) were available in the Supplementary Figures 1–9. Notably, no results remained after FDR correction. Besides, the partial correlation results controlling for site, age, sex, and education (Supplementary Table 4) were similar to the results controlling for site.
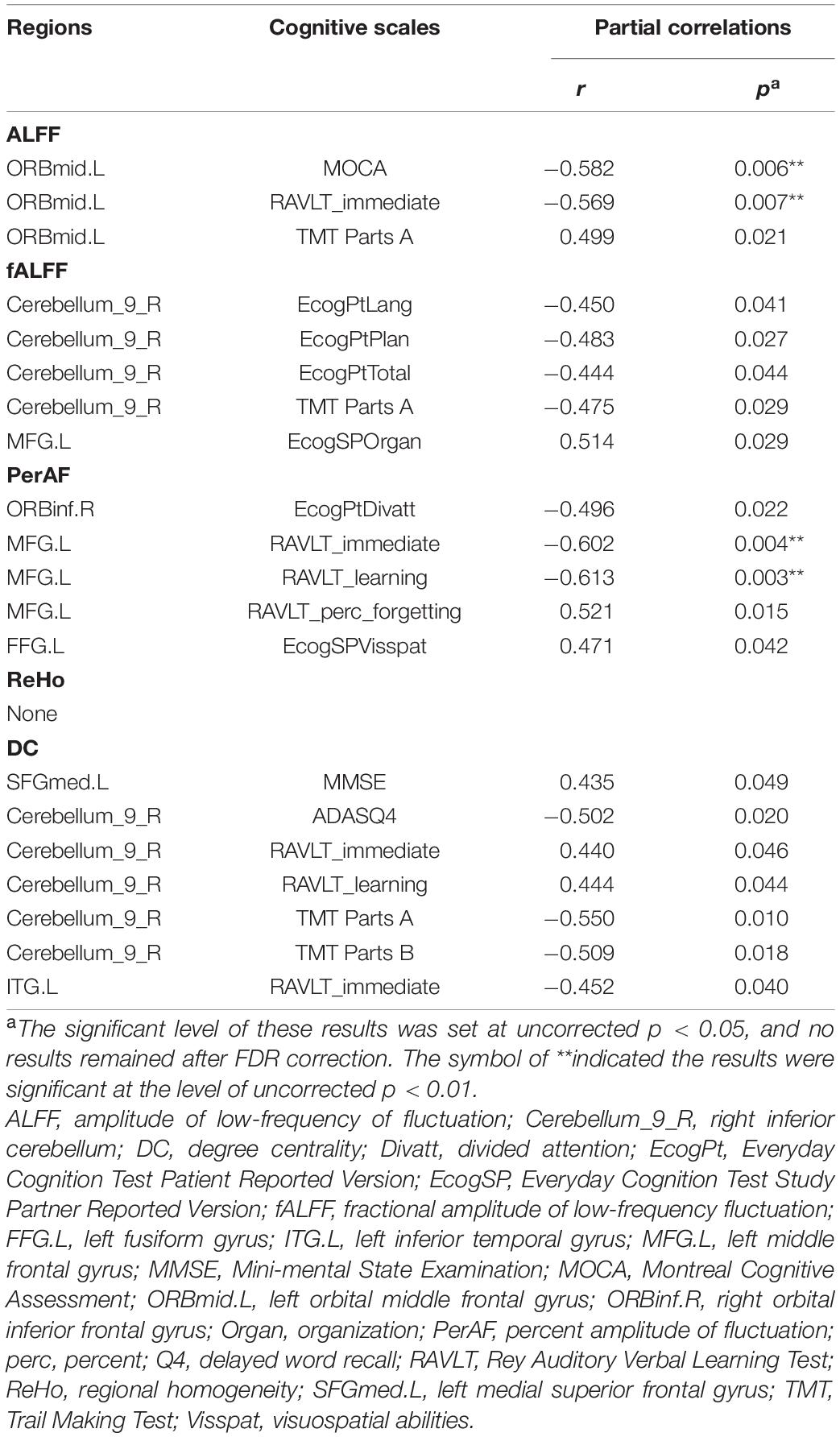
Table 3. Significant partial correlations between cognitive scale scores and brain region metrics with significant group differences.
Discussion
In this study, we identified significant intrinsic brain activity changes in MCI reverters compared to HCs on different scales. At the voxel level, MCI reverters showed increased ALFF, fALFF, and PerAF in the frontal gyrus (including ORBmid.L, ORBinf.R, and MFG.L), increased PerAF in the FFG.L and decreased ALFF and fALFF in the cerebellum_9_R compared with HCs. Regarding regional and whole-brain levels, compared to HCs, patients with MCI showed increased ReHo in the FFG.L, DCG.R; increased DC in the ITG.L and l SFGmed.L; decreased DC in the cerebellum_9_R and INS.L and INS.R.
Intrinsic Brain Activity Alteration of Mild Cognitive Impairment Reverters at the Voxel Level
ALFF reflects the degree of spontaneous neural activity (Zang et al., 2007) and fALFF is regarded as a standardized ALFF-like metric that has high sensitivity and specificity in the detection of spontaneous brain activity. It should be noted that fALFF is not as reliable as ALFF (Zou et al., 2008; Zuo et al., 2010). In this study, we found that the frontal lobe regions (including the ORBmid.L, ORBinf.R, and MFG.L) in MCI reverters showed increased ALFF and fALFF, while the cerebellum_9_R showed decreased ALFF and fALFF. The frontal lobe is thought to be involved in many high-level cognitive functions (Bae et al., 2021; Wang Y. et al., 2021). Increased frontal cortex connectivity is considered to be associated with increased cognitive control, higher IQ, and higher cognitive performance in young individuals. Therefore, we speculated that the increased neural activity in the frontal lobe regions in MCI reverters may account for the improvement of memory function.
Additionally, we found decreased ALFF and fALFF in the cerebellum_9_R of MCI reverters. According to previous studies, the cerebellum is involved in motor, balance, and cognitive functions (Wagner and Luo, 2020). Transcranial direct current stimulation is a non-invasive brain stimulation technique improving cognitive function in MCI. He et al. (2021) found that fALFF values decreased significantly in the cerebellum of patients with MCI after receiving 10 transcranial direct current stimulation sessions. Accordingly, the decreased ALFF and fALFF in the cerebellum_9_R may contribute to cognitive and motor functional improvement in patients with MCI. PerAF has higher test-retest reliability than conventional ALFF and is markedly higher than fALFF, from both intra- and inter-scanner aspects (Zhao et al., 2018; Jia et al., 2020). The MFG.L also showed decreased PerAF, which is consistent with the ALFF and fALFF results. Increased PerAF was also found in the FFG.L. We believe that the results of ALFF, fALFF, and PerAF may complement each other to increase the validity of the results.
Intrinsic Brain Activity Alteration of Mild Cognitive Impairment Reverters at the Regional and Whole-Brain Level
ReHo is a local index reflecting the local synchronization of the BOLD signal. In this study, the FFG.L and DCG.R of reverters showed increased ReHo, which represented increased local synchronization within these areas. The FFG is involved in the composition of visual regions and contributes markedly in facial recognition (Albonico and Barton, 2017; Liu et al., 2021). It has been thoroughly researched in recent years because of its abnormal structure and function in patients with mental disorders (Hwang et al., 2021; Jalbrzikowski et al., 2021). Patients with MCI have impairments in various domains, including visual-spatial dysfunction. Increased ReHo in the FFG.L is probably conducive to the process of external visual information. The DCG is involved in the composition of the cingulate gyrus, which is the key node in the default mode network and contributes considerably in information transmission and cognitive processing (Wang S. et al., 2021). The increased ReHo values observed in the DCG.R in MCI reverters may be beneficial for completing cognitive tasks.
Degree centrality is representative of the status and role of voxels in the whole-brain network (Buckner et al., 2009). In this study, the right cerebellum, INS.L and INS.R showed decreased DC, while the SFGmed.L and ITG.L showed increased DC. This suggests a reduced and increased, respectively, significance of these regions in the whole-brain. ITG.L and SFGmed.L regions are involved in memory tasks (Sidhu et al., 2013; Bae et al., 2021), and the cerebellum, INS.L, and INS.R are more related to motor and attentional processes (Wagner and Luo, 2020; Song et al., 2021). Previous studies have suggested that patients with AD may use other neurological resources to compensate for the loss of cognitive function in the early stages of the disease (Grady et al., 2003). Increased DC values in SFGmed.L and ITG.L and decreased DC values in the cerebellum and insula may imply a compensatory mechanism for maintaining memory function.
There are relatively few studies regarding MCI reverters, particularly intrinsic brain activity. Some studies have focused on patients with MCI whose cognitive function improved after treatment. He et.al (He et al., 2021) used the transcranial direct current stimulation in patients with MCI and found that the fALFF and ReHo values changed in multiple areas following tDCS. Brain regions with significant decreases in fALFF values included the INS.R, Precuneus R, and Parietal Sup R, while the FFG.L and Angular R showed significantly increased fALFF values. The brain regions with significantly increased ReHo values involved widespread frontal lobe regions. Another study (Zheng et al., 2021) has found that patients with Ginkgo biloba extract showed significantly decreased ALFF in the left parahippocampal gyrus, FFG.l, right gyrus rectus, and right superior frontal gyrus, while an increase in ALFF in the right middle temporal gyrus. Such brain activity change patterns are not consistent with our results. this may be attributed to our main focus on MCI reverters without clear treatment history. Effects of head motion during image acquisition and signal quality, as well as inconsistent image processing procedures, have also been noted as potential sources of variation.
Intrinsic Brain Activity Alteration of Mild Cognitive Impairment Reverters in the Cerebellum
Another notable finding is the abnormal brain activity in the cerebellum of MCI reverters. Traditionally, the cerebellum has been regarded as an important structure for coordinating intact motor functioning (Colloby et al., 2014). Numerous studies have recently reported that the cerebellum may also contribute to cognitive and affective processing (Grodd et al., 2005; Lin et al., 2020). There are relatively few reports on the changes of cerebellum structure and function in patients with AD. Previous studies pointed out that there is a stepwise decrease in cerebellum gray matter volume change from normal cognition to MCI and AD (Schmahmann, 2010; Tedesco et al., 2011; Tang et al., 2021) [4–6]. Another study (Toniolo et al., 2018) suggested that the cortico-cerebellum functional connectivity in MCI and AD patients were significantly disrupted with different distributions, particularly in the default mode networks and frontoparietal networks region. These results demonstrated the potential role of the cerebellum in AD progression and pathogenesis. Our study indicates that ALFF, fALFF, and DC values in the cerebellum consistently decreased in MCI reverters compared to HCs. These may be valuable and important findings, and future studies of AD should focus more on the cerebellum.
Behavioral Significance of Altered Brain Activity
Correlation analyses were conducted among the neuropsychological scales and rs-fMRI metrics. Many significant partial correlations were found between cognitive scale scores and brain region metrics with significant group differences. Among these, the strongest correlations were found between ALFF and PerAF in the MFG and Rey Auditory Verbal Learning Test scores. The MFG is a major component of the dorsolateral prefrontal cortex mediating higher-order cognitive functions such as executive attention, motor planning, decision making, and theory of mind (Lau et al., 2019; Mateu-Estivill et al., 2021). A previous study (Tang et al., 2021) has shown that AD patients had decreased functional connectivity within the medial frontal gyrus (MFG) compared to HC. Interestingly, MCI patients demonstrated increased functional connectivity within MFG and were positively correlated with memory enhancement. The increased functional connectivity in MFG in MCI patients may serve as a compensatory process. Similarly, this study showed an increased trend of ALFF and perAF values in the MFG of MCI reverters, which may also reflect a compensatory mechanism. However, its negative correlation with Rey Auditory Verbal Learning Test scores suggested that the functional improvement may not be directly subject to the recovery of MFG function. A potential effect may be mediated by the altered functional connection of MFG to the whole brain in reverters (Veitch et al., 2019; Xu et al., 2021). Future studies are required to deeply investigate this possibility and clarify the relationship between alterations in intrinsic brain activity and cognitive reversion. Besides, we found there was no correlation between Reho value and any cognitive scale. We suggested the limited sample size and the inclusion of both amnestic MCI and non-amnestic MCI patients may lead to potential bias.
However, it needs to be noted that these partial correlation results were obtained in a low threshold (uncorrected p < 0.05) and no results remained after correction (FDR corrected p < 0.05). We mainly attributed this to the small effect size, which likely led to non-significant results at a strict threshold (Jia et al., 2021).
Limitations
This study has several limitations. Firstly, since this is a cross-sectional study, we did not investigate the conversion of MCI to HCs. No baseline rs-fMRI data of MCI reverters were collected. Second, the sample size was relatively modest. In future studies, we would like to combine the rs-fMRI, structural MRI, and other biophysical data with a larger sample and reveal structural and biological substrates underlying these brain activity alterations in MCI reverters.
Conclusion
To the best of our knowledge, this is the first study to investigate the intrinsic brain activity changes in MCI reverters. We found that these patients had significantly increased brain activity in regions such as the frontal gyrus (including the ORBmid.L, ORBinf.R, MFG.L, and SFGmed.L), FFG.L, DCG.R, and ITG.L, while decreased brain activities were mainly contained in the cerebellum_9_R, INS.L and INS.R. These alterations are significantly related to cognitive reversion in patients with MCI. These results enhance our understanding of the neuro-mechanism of cognitive reversion in patients with MCI and provide new insights for developing effective interventions for AD in the future.
Data Availability Statement
The datasets analyzed for this study can be found in the ADNI database, available at: http://adni.loni.usc.edu/. The subject ID of MCI reverters included in this manuscript can be found in Supplementary Table 1.
Ethics Statement
Ethical approval was not provided for this study on human participants because data used in the preparation of this article were obtained from the ADNI. The Alzheimer’s Disease Neuroimaging Initiative (ADNI) was approved by the Institutional Review Boards of all participating sites. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
QH and XZ designed the study and protocol. YL, ZX, XL, and GH performed the literature search and collected the patient data. QW and XJ performed the image analysis and generated the figures. QH, QW, and LZ wrote the manuscript. XJ and XZ edited and revised the manuscript. All authors contributed to the manuscript and approved the submitted version.
Funding
This study was funded by the Science and Technology Commission of Shanghai Municipality under Grants 18411970300 and 201409002200, National Natural Science Foundation of China under Grant 82001898, Shanghai Municipal Health Commission under Grant 201840018, and Medical Specialty of Minhang District (Shanghai) under Grant 2020MWFC01.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Editage (http://editage.com/frontiers/) for the English language editing of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2021.788765/full#supplementary-material
Abbreviations
AD, Alzheimer’s disease; ADNI, Alzheimer’s Disease Neuroimaging Initiative; ALFF, amplitude of low-frequency of fluctuation; BOLD, blood oxygen level dependent; Cerebellum_9_R, right inferior cerebellum; DC, degree centrality; DCG.R, right median cingulate and paracingulate gyri; fALFF, fractional amplitude of low-frequency of fluctuation; FDR, false discovery rate; FFG.L, left fusiform gyrus; GRF, Gaussian Random Field; HCs, healthy controls; INS.L and INS.R, bilateral insular; ITG.L, left inferior temporal gyrus; LM, logical memory; MCI, mild cognitive impairment; MFG.L, left middle frontal gyrus; ORBinf.R, right orbital inferior frontal gyrus; ORBmid.L, left orbital middle frontal gyrus; PerAF, percent amplitude of fluctuation; ReHo, regional homogeneity; rs-fMRI, resting-state functional magnetic resonance imaging; SFGmed.L, left medial superior frontal gyrus.
Footnotes
- ^ https://www.alz.org/
- ^ http://adni.loni.usc.edu/
- ^ http://www.fil.ion.ucl.ac.uk/spm
- ^ http://restfmri.net/forum/restplus
- ^ https://ww2.mathworks.cn/products/matlab.html
- ^ http://rfmri.org/dpabi
References
Albonico, A., and Barton, J. J. S. (2017). Face perception in pure alexia: complementary contributions of the left fusiform gyrus to facial identity and facial speech processing. Cortex 96, 59–72. doi: 10.1016/j.cortex.2017.08.029
Bae, J., Jeong, H., Yoon, Y., Bae, C., Lee, H., Paik, S., et al. (2021). Parallel processing of working memory and temporal information by distinct types of cortical projection neurons. Nat. Commun. 12:4352. doi: 10.1038/s41467-021-24565-z
Belleville, S., Clément, F., Mellah, S., Gilbert, B., Fontaine, F., and Gauthier, S. (2011). Training-related brain plasticity in subjects at risk of developing Alzheimer’s disease. Brain 134(Pt 6), 1623–1634. doi: 10.1093/brain/awr037
Biswal, B. B. (2012). Resting state fMRI: a personal history. Neuroimage 62, 938–944. doi: 10.1016/j.neuroimage.2012.01.090
Buckner, R. L., Sepulcre, J., Talukdar, T., Krienen, F. M., Liu, H., Hedden, T., et al. (2009). Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J. Neurosci. 29, 1860–1873. doi: 10.1523/jneurosci.5062-08.2009
Buschert, V. C., Friese, U., Teipel, S. J., Schneider, P., Merensky, W., Rujescu, D., et al. (2011). Effects of a newly developed cognitive intervention in amnestic mild cognitive impairment and mild Alzheimer’s disease: a pilot study. J. Alzheimers Dis. 25, 679–694. doi: 10.3233/jad-2011-100999
Busse, A., Hensel, A., Gühne, U., Angermeyer, M. C., and Riedel-Heller, S. G. (2006). Mild cognitive impairment: long-term course of four clinical subtypes. Neurology 67, 2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1
Canevelli, M., Grande, G., Lacorte, E., Quarchioni, E., Cesari, M., Mariani, C., et al. (2016). Spontaneous reversion of mild cognitive impairment to normal cognition: a systematic review of literature and meta-analysis. J. Am. Med. Dir. Assoc. 17, 943–948. doi: 10.1016/j.jamda.2016.06.020
Chen, X., Lu, B., and Yan, C. G. (2018). Reproducibility of R-fMRI metrics on the impact of different strategies for multiple comparison correction and sample sizes. Hum. Brain Mapp. 39, 300–318. doi: 10.1002/hbm.23843
Colloby, S. J., O’Brien, J. T., and Taylor, J. P. (2014). Patterns of cerebellar volume loss in dementia with Lewy bodies and Alzheimer’s disease: a VBM-DARTEL study. Psychiatry Res. 223, 187–191. doi: 10.1016/j.pscychresns.2014.06.006
Cui, Q., Sheng, W., Chen, Y., Pang, Y., Lu, F., Tang, Q., et al. (2020). Dynamic changes of amplitude of low-frequency fluctuations in patients with generalized anxiety disorder. Hum. Brain Mapp. 41, 1667–1676. doi: 10.1002/hbm.24902
Dai, Z., and He, Y. J. N. B. (2014). Disrupted structural and functional brain connectomes in mild cognitive impairment and Alzheimer’s disease. Neurosci. Bull. 30, 217–232. doi: 10.1007/s12264-013-1421-0
Friston, K. J., Williams, S., Howard, R., Frackowiak, R. S., and Turner, R. (1996). Movement-related effects in fMRI time-series. Magn. Reson. Med. 35, 346–355. doi: 10.1002/mrm.1910350312
Gao, S., Unverzagt, F. W., Hall, K. S., Lane, K. A., Murrell, J. R., Hake, A. M., et al. (2014). Mild cognitive impairment, incidence, progression, and reversion: findings from a community-based cohort of elderly African Americans. Am. J. Geriatr. Psychiatry 22, 670–681. doi: 10.1016/j.jagp.2013.02.015
Glasser, M. F., Sotiropoulos, S. N., Wilson, J. A., Coalson, T. S., Fischl, B., Andersson, J. L., et al. (2013). The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124. doi: 10.1016/j.neuroimage.2013.04.127
Grady, C. L., McIntosh, A. R., Beig, S., Keightley, M. L., Burian, H., and Black, S. E. (2003). Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer’s disease. J. Neurosci. 23, 986–993. doi: 10.1523/jneurosci.23-03-00986.2003
Grodd, W., Hülsmann, E., and Ackermann, H. (2005). Functional MRI localizing in the cerebellum. Neurosurg. Clin. N. Am. 16, 77–99. doi: 10.1016/j.nec.2004.07.008
Hao, H., Chen, C., Mao, W., Xia, W., Yi, Z., Zhao, P., et al. (2019). Alterations in resting-state local functional connectivity in obsessive-compulsive disorder. J. Affect. Disord. 245, 113–119. doi: 10.1016/j.jad.2018.10.112
He, F., Li, Y., Li, C., Fan, L., Liu, T., and Wang, J. (2021). Repeated anodal high-definition transcranial direct current stimulation over the left dorsolateral prefrontal cortex in mild cognitive impairment patients increased regional homogeneity in multiple brain regions. PLoS One 16:e0256100. doi: 10.1371/journal.pone.0256100
Hwang, H. C., Kim, S. M., and Han, D. H. (2021). Different facial recognition patterns in schizophrenia and bipolar disorder assessed using a computerized emotional perception test and fMRI. J. Affect. Disord. 279, 83–88. doi: 10.1016/j.jad.2020.09.125
Jalbrzikowski, M., Hayes, R., Wood, S., Nordholm, D., Zhou, J., Fusar-Poli, P., et al. (2021). Association of structural magnetic resonance imaging measures with psychosis onset in individuals at clinical high risk for developing psychosis: an ENIGMA working group mega-analysis. JAMA Psychiatry 78, 753–766. doi: 10.1001/jamapsychiatry.2021.0638
Jenkinson, M., Bannister, P., Brady, M., and Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841. doi: 10.1016/s1053-8119(02)91132-8
Jia, X. Z., Sun, J. W., Ji, G. J., Liao, W., Lv, Y. T., Wang, J., et al. (2020). Percent amplitude of fluctuation: a simple measure for resting-state fMRI signal at single voxel level. PLoS One 15:e0227021. doi: 10.1371/journal.pone.0227021
Jia, X.-Z., Wang, J., Sun, H.-Y., Zhang, H., Liao, W., Wang, Z., et al. (2019). RESTplus: an improved toolkit for resting-state functional magnetic resonance imaging data processing. Sci. Bull. 64, 953–954. doi: 10.1016/j.scib.2019.05.008
Jia, X.-Z., Zhao, N., Dong, H.-M., Sun, J.-W., Barton, M., Burciu, R., et al. (2021). Small P values may not yield robust findings: an example using REST-meta-PD. Sci. Bull. 66, 2148–2152. doi: 10.1016/j.scib.2021.06.007
Lau, H., Shahar, S., Mohamad, M., Rajab, N. F., Yahya, H. M., Din, N. C., et al. (2019). Relationships between dietary nutrients intake and lipid levels with functional MRI dorsolateral prefrontal cortex activation. Clin. Interv. Aging 14, 43–51. doi: 10.2147/cia.S183425
Li, Y., Fang, Y., Wang, J., Zhang, H., and Hu, B. (2021). Biomarker extraction based on subspace learning for the prediction of mild cognitive impairment conversion. Biomed. Res. Int. 2021:5531940. doi: 10.1155/2021/5531940
Li, Y., Jing, B., Liu, H., Li, Y., Gao, X., Li, Y., et al. (2017). Frequency-dependent changes in the amplitude of low-frequency fluctuations in mild cognitive impairment with mild depression. J. Alzheimers Dis. 58, 1175–1187. doi: 10.3233/jad-161282
Li, Z., Li, C., Liang, Y., Wang, K., Zhang, W., Chen, R., et al. (2019). Altered functional connectivity and brain network property in pregnant women with cleft fetuses. Front. Psychol. 10:2235. doi: 10.3389/fpsyg.2019.02235
Lin, C. Y., Chen, C. H., Tom, S. E., and Kuo, S. H. (2020). Cerebellar volume is associated with cognitive decline in mild cognitive impairment: results from ADNI. Cerebellum 19, 217–225. doi: 10.1007/s12311-019-01099-1
Liu, M., Liu, C., Zheng, S., Zhao, K., and Fu, X. J. N. (2021). Reexamining the neural network involved in perception of facial expression: a meta-analysis. Neurosci. Biobehav. Rev. 131, 179–191. doi: 10.1016/j.neubiorev.2021.09.024
Liu, X., Chen, J., Shen, B., Wang, G., Li, J., Hou, H., et al. (2018). Altered intrinsic coupling between functional connectivity density and amplitude of low-frequency fluctuation in mild cognitive impairment with depressive symptoms. Neural Plast. 2018:1672708. doi: 10.1155/2018/1672708
Liu, X., Tu, Y., Zang, Y., Wu, A., Guo, Z., and He, J. (2019). Disrupted regional spontaneous neural activity in mild cognitive impairment patients with depressive symptoms: a resting-state fMRI study. Neural Plast. 2019:2981764. doi: 10.1155/2019/2981764
Liu, Y., Wang, J., Zhang, J., Wen, H., Zhang, Y., Kang, H., et al. (2017). Altered spontaneous brain activity in children with early tourette syndrome: a resting-state fMRI study. Sci. Rep. 7:4808. doi: 10.1038/s41598-017-04148-z
Malek-Ahmadi, M. (2016). Reversion from mild cognitive impairment to normal cognition: a meta-analysis. Alzheimer Dis. Assoc. Disord. 30, 324–330. doi: 10.1097/wad.0000000000000145
Mateu-Estivill, R., Forné, S., López-Sala, A., Falcón, C., Caldú, X., Sopena, J. M., et al. (2021). Functional connectivity alterations associated with literacy difficulties in early readers. Brain Imaging Behav. 15, 2109–2120. doi: 10.1007/s11682-020-00406-3
Pandya, S. Y., Lacritz, L. H., Weiner, M. F., Deschner, M., and Woon, F. L. (2017). Predictors of reversion from mild cognitive impairment to normal cognition. Dement. Geriatr. Cogn. Disord. 43, 204–214. doi: 10.1159/000456070
Petersen, R. C., Aisen, P. S., Beckett, L. A., Donohue, M. C., Gamst, A. C., Harvey, D. J., et al. (2010). Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 74, 201–209. doi: 10.1212/WNL.0b013e3181cb3e25
Petersen, R. C., Roberts, R. O., Knopman, D. S., Boeve, B. F., Geda, Y. E., Ivnik, R. J., et al. (2009). Mild cognitive impairment: ten years later. Arch. Neurol. 66, 1447–1455. doi: 10.1001/archneurol.2009.266
Pyun, J., Park, Y., Lee, K., Kim, S., Saykin, A., Nho, K., et al. (2021). Predictability of polygenic risk score for progression to dementia and its interaction with APOE ε4 in mild cognitive impairment. Transl. Neurodegener. 10:32. doi: 10.1186/s40035-021-00259-w
Schmahmann, J. D. (2010). The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol. Rev. 20, 236–260. doi: 10.1007/s11065-010-9142-x
Shan, X., Qiu, Y., Pan, P., Teng, Z., Li, S., Tang, H., et al. (2020). Disrupted regional homogeneity in drug-naive patients with bipolar disorder. Front. Psychiatry 11:825. doi: 10.3389/fpsyt.2020.00825
Sidhu, M., Stretton, J., Winston, G., Bonelli, S., Centeno, M., Vollmar, C., et al. (2013). A functional magnetic resonance imaging study mapping the episodic memory encoding network in temporal lobe epilepsy. Brain 136, 1868–1888. doi: 10.1093/brain/awt099
Smith, S. M., Beckmann, C. F., Andersson, J., Auerbach, E. J., Bijsterbosch, J., Douaud, G., et al. (2013). Resting-state fMRI in the Human Connectome Project. Neuroimage 80, 144–168. doi: 10.1016/j.neuroimage.2013.05.039
Song, Y., Xu, W., Chen, S., Hu, G., Ge, H., Xue, C., et al. (2021). Functional MRI-specific alterations in salience network in mild cognitive impairment: an ALE meta-analysis. Front. Aging Neurosci. 13:695210. doi: 10.3389/fnagi.2021.695210
Tang, F., Zhu, D., Ma, W., Yao, Q., Li, Q., and Shi, J. (2021). Differences changes in cerebellar functional connectivity between mild cognitive impairment and Alzheimer’s disease: a seed-based approach. Front. Neurol. 12:645171. doi: 10.3389/fneur.2021.645171
Tedesco, A. M., Chiricozzi, F. R., Clausi, S., Lupo, M., Molinari, M., and Leggio, M. G. (2011). The cerebellar cognitive profile. Brain 134(Pt 12), 3672–3686. doi: 10.1093/brain/awr266
Thomas, K. R., Edmonds, E. C., Eppig, J. S., Wong, C. G., Weigand, A. J., Bangen, K. J., et al. (2019a). MCI-to-normal reversion using neuropsychological criteria in the Alzheimer’s disease neuroimaging initiative. Alzheimers Dement. 15, 1322–1332. doi: 10.1016/j.jalz.2019.06.4948
Thomas, K. R., Eppig, J. S., Weigand, A. J., Edmonds, E. C., Wong, C. G., Jak, A. J., et al. (2019b). Artificially low mild cognitive impairment to normal reversion rate in the Alzheimer’s disease neuroimaging initiative. Alzheimers Dement. 15, 561–569. doi: 10.1016/j.jalz.2018.10.008
Toniolo, S., Serra, L., Olivito, G., Marra, C., Bozzali, M., and Cercignani, M. (2018). Patterns of cerebellar gray matter atrophy across Alzheimer’s disease progression. Front. Cell. Neurosci. 12:430. doi: 10.3389/fncel.2018.00430
van den Heuvel, M. P., Stam, C. J., Boersma, M., and Hulshoff Pol, H. E. (2008). Small-world and scale-free organization of voxel-based resting-state functional connectivity in the human brain. Neuroimage 43, 528–539. doi: 10.1016/j.neuroimage.2008.08.010
Veitch, D. P., Weiner, M. W., Aisen, P. S., Beckett, L. A., Cairns, N. J., Green, R. C., et al. (2019). Understanding disease progression and improving Alzheimer’s disease clinical trials: recent highlights from the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement. 15, 106–152. doi: 10.1016/j.jalz.2018.08.005
Veitch, D., Weiner, M., Aisen, P., Beckett, L., DeCarli, C., Green, R., et al. (2021). Using the Alzheimer’s disease neuroimaging initiative to improve early detection, diagnosis, and treatment of Alzheimer’s disease. Alzheimers Dement’ doi: 10.1002/alz.12422 [Epub ahead of print].
Wagner, M. J., and Luo, L. (2020). Neocortex-cerebellum circuits for cognitive processing. Trends Neurosci. 43, 42–54. doi: 10.1016/j.tins.2019.11.002
Wang, L., Li, K., Zhang, Q., Zeng, Y., Dai, W., Su, Y., et al. (2014). Short-term effects of escitalopram on regional brain function in first-episode drug-naive patients with major depressive disorder assessed by resting-state functional magnetic resonance imaging. Psychol. Med. 44, 1417–1426. doi: 10.1017/S0033291713002031
Wang, S., Kim, N., Um, Y., Kang, D., Na, H., Lee, C., et al. (2021). Default mode network dissociation linking cerebral beta amyloid retention and depression in cognitively normal older adults. Neuropsychopharmacology 46, 2180–2187. doi: 10.1038/s41386-021-01072-9
Wang, Y., Yin, X., Zhang, Z., Li, J., Zhao, W., and Guo, Z. J. N. (2021). A cortico-basal ganglia-thalamo-cortical channel underlying short-term memory. Neuron 109, 3486–3499.e7. doi: 10.1016/j.neuron.2021.08.002
Wang, Y., Zhao, X., Xu, S., Yu, L., Wang, L., Song, M., et al. (2015). Using regional homogeneity to reveal altered spontaneous activity in patients with mild cognitive impairment. Biomed. Res. Int. 2015:807093. doi: 10.1155/2015/807093
Winblad, B., Palmer, K., Kivipelto, M., Jelic, V., Fratiglioni, L., Wahlund, L. O., et al. (2004). Mild cognitive impairment–beyond controversies, towards a consensus: report of the international working group on mild cognitive impairment. J. Intern. Med. 256, 240–246. doi: 10.1111/j.1365-2796.2004.01380.x
Wu, D., Zhao, H., Gu, H., Han, B., Wang, Q., Man, X., et al. (2021). The effects of rs405509 on APOEepsilon4 non-carriers in non-demented aging. Front. Neurosci. 15:677823. doi: 10.3389/fnins.2021.677823
Xing, Y., Fu, S., Li, M., Ma, X., Liu, M., Liu, X., et al. (2021). Regional neural activity changes in Parkinson’s disease-associated mild cognitive impairment and cognitively normal patients. Neuropsychiatr. Dis. Treat 17, 2697–2706. doi: 10.2147/ndt.S323127
Xiong, J., Yu, C., Su, T., Ge, Q., Shi, W., Tang, L., et al. (2021). Altered brain network centrality in patients with mild cognitive impairment: an fMRI study using a voxel-wise degree centrality approach. Aging (Albany N. Y.) 13, 15491–15500. doi: 10.18632/aging.203105
Xu, W., Rao, J., Song, Y., Chen, S., Xue, C., Hu, G., et al. (2021). Altered functional connectivity of the basal nucleus of Meynert in subjective cognitive impairment, early mild cognitive impairment, and late mild cognitive impairment. Front. Aging Neurosci. 13:671351. doi: 10.3389/fnagi.2021.671351
Yan, C. G., Wang, X. D., Zuo, X. N., and Zang, Y. F. (2016). DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics 14, 339–351. doi: 10.1007/s12021-016-9299-4
Yang, L., Yan, Y., Wang, Y., Hu, X., Lu, J., Chan, P., et al. (2018). Gradual disturbances of the amplitude of low-frequency fluctuations (ALFF) and fractional ALFF in Alzheimer spectrum. Front. Neurosci. 12:975. doi: 10.3389/fnins.2018.00975
Zang, Y., Jiang, T., Lu, Y., He, Y., and Tian, L. (2004). Regional homogeneity approach to fMRI data analysis. Neuroimage 22, 394–400. doi: 10.1016/j.neuroimage.2003.12.030
Zang, Y. F., He, Y., Zhu, C. Z., Cao, Q. J., Sui, M. Q., Liang, M., et al. (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 29, 83–91. doi: 10.1016/j.braindev.2006.07.002
Zang, Y. F., Zuo, X. N., Milham, M., and Hallett, M. (2015). Toward a meta-analytic synthesis of the resting-state fMRI literature for clinical populations. Biomed. Res. Int. 2015:435265. doi: 10.1155/2015/435265
Zhao, N., Yuan, L. X., Jia, X. Z., Zhou, X. F., Deng, X. P., He, H. J., et al. (2018). Intra- and inter-scanner reliability of voxel-wise whole-brain analytic metrics for resting state fMRI. Front. Neuroinform. 12:54. doi: 10.3389/fninf.2018.00054
Zheng, Y., Xie, Y., Qi, M., Zhang, L., Wang, W., Zhang, W., et al. (2021). Ginkgo biloba extract is comparable with donepezil in improving functional recovery in Alzheimer’s disease: results from a multilevel characterized study based on clinical features and resting-state functional magnetic resonance imaging. Front. Pharmacol. 12:721216. doi: 10.3389/fphar.2021.721216
Zou, Q. H., Zhu, C. Z., Yang, Y., Zuo, X. N., Long, X. Y., Cao, Q. J., et al. (2008). An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J. Neurosci. Methods 172, 137–141. doi: 10.1016/j.jneumeth.2008.04.012
Zuo, X. N., Di Martino, A., Kelly, C., Shehzad, Z. E., Gee, D. G., Klein, D. F., et al. (2010). The oscillating brain: complex and reliable. Neuroimage 49, 1432–1445. doi: 10.1016/j.neuroimage.2009.09.037
Keywords: mild cognitive impairment, Alzheimer’s disease, resting-state fMRI, intrinsic brain activity, cognitive reversion
Citation: Hu Q, Wang Q, Li Y, Xie Z, Lin X, Huang G, Zhan L, Jia X and Zhao X (2022) Intrinsic Brain Activity Alterations in Patients With Mild Cognitive Impairment-to-Normal Reversion: A Resting-State Functional Magnetic Resonance Imaging Study From Voxel to Whole-Brain Level. Front. Aging Neurosci. 13:788765. doi: 10.3389/fnagi.2021.788765
Received: 03 October 2021; Accepted: 08 December 2021;
Published: 17 January 2022.
Edited by:
Jiehui Jiang, Shanghai University, ChinaReviewed by:
Yu Zheng, Nanjing Medical University, ChinaXia-an Bi, Hunan Normal University, China
Zaixu Cui, Chinese Institute for Brain Research (CIBR), China
Xiaojun Xu, Zhejiang University, China
Copyright © 2022 Hu, Wang, Li, Xie, Lin, Huang, Zhan, Jia and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xize Jia, amlheGl6ZUBmb3htYWlsLmNvbQ==; Xiaohu Zhao, eGhaaGFvOTk5QDI2My5uZXQ=
†These authors have contributed equally to this work
 Qili Hu
Qili Hu Qianqian Wang
Qianqian Wang Yunfei Li
Yunfei Li Zhou Xie
Zhou Xie Xiaomei Lin1
Xiaomei Lin1 LinLin Zhan
LinLin Zhan Xize Jia
Xize Jia