
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 11 January 2022
Sec. Neurocognitive Aging and Behavior
Volume 13 - 2021 | https://doi.org/10.3389/fnagi.2021.787654
This article is part of the Research TopicThe Impact of Age-Related Changes in Brain Network Organization and Sleep on MemoryView all 8 articles
Oscillatory neural activity during sleep, such as that in the delta and sigma bands, is important for motor learning consolidation. This activity is reduced with typical aging, and this reduction may contribute to aging-related declines in motor learning consolidation. Evidence suggests that brain regions involved in motor learning contribute to oscillatory neural activity during subsequent sleep. However, aging-related differences in regional contributions to sleep oscillatory activity following motor learning are unclear. To characterize these differences, we estimated the cortical sources of consolidation-related oscillatory activity using individual anatomical information in young and older adults during non-rapid eye movement sleep after motor learning and analyzed them in light of cortical thickness and pre-sleep functional brain activation. High-density electroencephalogram was recorded from young and older adults during a midday nap, following completion of a functional magnetic resonance imaged serial reaction time task as part of a larger experimental protocol. Sleep delta activity was reduced with age in a left-weighted motor cortical network, including premotor cortex, primary motor cortex, supplementary motor area, and pre-supplementary motor area, as well as non-motor regions in parietal, temporal, occipital, and cingulate cortices. Sleep theta activity was reduced with age in a similar left-weighted motor network, and in non-motor prefrontal and middle cingulate regions. Sleep sigma activity was reduced with age in left primary motor cortex, in a non-motor right-weighted prefrontal-temporal network, and in cingulate regions. Cortical thinning mediated aging-related sigma reductions in lateral orbitofrontal cortex and frontal pole, and partially mediated delta reductions in parahippocampal, fusiform, and lingual gyri. Putamen, caudate, and inferior parietal cortex activation prior to sleep predicted frontal and motor cortical contributions to sleep delta and theta activity in an age-moderated fashion, reflecting negative relationships in young adults and positive or absent relationships in older adults. Overall, these results support the local sleep hypothesis that brain regions active during learning contribute to consolidation-related neural activity during subsequent sleep and demonstrate that sleep oscillatory activity in these regions is reduced with aging.
Sleep is important for consolidating motor sequence learning. Performance improves over a night of sleep or a nap, but not an equivalent interval of wakefulness (Fischer et al., 2002; Walker et al., 2002; Backhaus and Junghanns, 2006; Korman et al., 2007; Doyon et al., 2009b; Debas et al., 2010). Non-rapid eye movement (NREM) sleep features, including oscillatory neural activity in the delta frequency band (0.5–4 Hz; e.g., slow waves, K-complexes), in the theta frequency band (4–8 Hz), and in the sigma frequency band (12–16 Hz; e.g., sleep spindles), have been linked to motor learning consolidation during sleep (Nishida and Walker, 2007; Tucker and Fishbein, 2009; Barakat et al., 2012; Albouy et al., 2013; Tamaki et al., 2013; Fitzroy et al., 2021a).
Consolidation involves synaptic changes in learning-related cortical circuits, and learning induces local, use-dependent changes in sleep oscillatory neural activity apparent in the scalp electroencephalogram (EEG) (“local sleep”; e.g., Huber et al., 2004). Thus, learning-related circuits are expected to contribute to subsequent consolidation-related sleep oscillatory activity. Indeed, source estimation, a method of identifying the neural generators of the scalp-recorded EEG and near-scalp-recorded magnetoencephalogram (MEG), suggests that regions involved in learning contribute to subsequent delta and sigma activity. For example, increased slow wave activity is observed in premotor cortex during NREM sleep following visuomotor learning (Murphy et al., 2011), and increased slow sigma activity is observed in visual cortex during NREM sleep following visual perceptual learning (Bang et al., 2014).
With regard to motor sequence learning, MEG source activity in consolidation-related frequency bands is increased in multiple motor cortical areas during sleep following an explicit sequential finger tapping task, relative to pre-task control sleep (Tamaki et al., 2013). Specifically, motor sequence learning led to increased NREM delta in premotor and primary motor cortices, supplementary motor area (SMA), and pre-SMA; increased NREM theta in primary motor cortex, SMA, and pre-SMA; and increased NREM fast sigma activity in SMA (Tamaki et al., 2013). Moreover, the training-related increases in delta and fast sigma activity in SMA correlated with performance improvements on the motor task over the nap, suggesting that this activity contributed to motor memory consolidation (Tamaki et al., 2013). Together, these results suggest the neural activity during learning prior to sleep has a direct impact on both the set of generators contributing to consolidation-related neural activity during sleep, and the magnitude of activity within these generators.
Aging is associated with reduced consolidation-related sleep oscillatory activity, which may lead to impaired consolidation of motor sequence learning in older adults (e.g., Wilson et al., 2012; Fogel et al., 2014). Delta, theta, and sigma activity measured at the scalp all decline over the adult lifespan (Dijk et al., 1989; Landolt et al., 1996; Carrier et al., 2001; Gaudreau et al., 2001; Nicolas et al., 2001; Crowley et al., 2002; Peters et al., 2014; Schwarz et al., 2017), in a manner that varies topographically across the scalp. Delta declines are broad but largest medial, theta declines are broad but largest medial frontocentral, and sigma declines are focal over only medial frontocentral scalp (Landolt and Borbély, 2001; Münch et al., 2004; Robillard et al., 2010; Carrier et al., 2011; Martin et al., 2013; Mander et al., 2014; Sprecher et al., 2016; Fitzroy et al., 2021b). These decline patterns lead to aging-related increases in the relative frontality of delta, and in the relative laterality of sigma (Sprecher et al., 2016; Fitzroy et al., 2021b).
The topographic variability of aging-related declines in sleep oscillatory activity presumably reflects differential effects of aging on sleep oscillatory activity across brain regions. Aging is also associated with greater fluctuation in the estimated sources of delta during the transition from wake to sleep, further suggesting aging-related differences in the generators of sleep-related neural activity (Tsuno et al., 2002). However, aging-related changes in the estimated sources of sleep oscillatory activity have not been previously investigated. Given that aging-related changes in cortical structure (Mander et al., 2013; Dubé et al., 2015; Varga et al., 2016; Fogel et al., 2017; Latreille et al., 2019; Fitzroy et al., 2021b) and brain activation during learning (Fitzroy et al., 2021a) are associated with changes in the scalp EEG during sleep, it is likely that these factors contribute to changes in the generators of the underlying activity during sleep.
The nature of relationships between aging-related changes in cortical structure and changes in the scalp EEG during sleep suggests changes in the underlying source activity with age. Gray matter atrophy mediates aging-related changes in the magnitude and scalp topography of delta and theta activity in a region-specific manner (Mander et al., 2013; Dubé et al., 2015; Varga et al., 2016; Latreille et al., 2019; Fitzroy et al., 2021b). Gray matter volume also has age-moderated effects on the magnitude and scalp topography of sigma activity in a region-specific manner (Fogel et al., 2017; Fitzroy et al., 2021b). The location of the atrophied brain region and the scalp topography of the influenced sleep oscillatory activity often correspond. For example, gray matter volume loss in frontal medial cortex predicts and mediates declines in delta over frontal scalp (Varga et al., 2016; Fitzroy et al., 2021b), and occipital cortical thinning mediates declines in theta over occipital scalp (Latreille et al., 2019). However, EEG recorded at a specific scalp location is not necessarily a reflection of neural activity under that scalp location. Rather, it is a weighted combination of activity from all generators across the brain (e.g., Luck, 2014). Assessing whether the mediation of aging-related changes in scalp-recorded sleep EEG by gray matter atrophy represents reduced direct contributions of the atrophied regions to sleep oscillatory activity therefore requires estimation of the underlying sources of the scalp EEG. In the present study, we examine the relationship between aging-related differences in estimated cortical sources of scalp-recorded sleep EEG and cortical thickness in the same region to address this question.
In addition to changes in cortical structure, aging-related differences in brain activation during learning are associated with aspects of subsequent sleep EEG, and may therefore drive differences in the underlying source activity. For example, hippocampal activation during motor sequence learning predicts the relative frontality of sigma power during subsequent sleep (Fitzroy et al., 2021a). The rate and magnitude of motor sequence learning is reduced with age, at least under complex and/or explicit task conditions (Curran, 1997; Howard and Howard, 2001; Feeney et al., 2002; Bennett et al., 2007; Rieckmann and Bäckman, 2009), which leads to altered patterns of brain activation during motor sequence learning with age (Aizenstein et al., 2006; Rieckmann et al., 2010; King et al., 2013, 2017b; Fogel et al., 2014; Fitzroy et al., 2021a; but see Daselaar et al., 2003). Aging-related activity reductions are observed in striatal and frontal and motor cortical regions (Aizenstein et al., 2006; Fitzroy et al., 2021a), while aging-related activity increases are sometimes observed in medial temporal and prefrontal areas (Rieckmann et al., 2010; Fogel et al., 2014). Additionally, variability across studies in the brain regions for which gray matter atrophy influences sleep oscillatory activity suggests that task-related brain activity prior to sleep contributes to these relationships. Studies examining sleep after motor sequence learning (Fogel et al., 2017; Fitzroy et al., 2021b) report relationships between gray matter atrophy in motor learning related regions and sleep oscillatory activity that are not observed in studies examining sleep after no specific learning task (Dubé et al., 2015; Latreille et al., 2019). Together, these findings suggest aging-related differences in motor sequence learning lead to differences in source activity during subsequent sleep.
We recently observed relationships between aging-related declines in gray matter volume and changes in the magnitude and topography of sleep delta, theta, and sigma activity at the scalp (Fitzroy et al., 2021b). Additionally, in a subset of these participants, we observed aging-related reductions in striato-cortical network activity during learning of a serial reaction time task (SRTT), along with relationships between brain activity during learning and the topography of sigma activity during subsequent sleep (Fitzroy et al., 2021a). Here, using anatomically informed minimum-norm EEG source estimation and automated cortical thickness measurement in this same cohort, we sought to characterize how aging-related changes in gray matter and pre-sleep learning-related brain activity contributed to aging-related changes in the cortical sources of sleep oscillatory activity.
We hypothesized that the estimated sources of sleep oscillatory activity following motor sequence learning would reflect brain regions active prior to sleep in both young and older adults, and that aging-related declines in estimated source activity would reflect cortical thinning and aging-related differences in brain activity prior to sleep. Specifically, based on prior investigations of estimated source activity in young adults (Tamaki et al., 2013; Brancaccio et al., 2020), we predicted delta, theta, and sigma activity during a midday nap following the SRTT would contain contributions from motor cortical regions in all participants. Additionally, based on known aging-related changes in scalp-recorded sleep EEG magnitude and topography (e.g., Sprecher et al., 2016; Fitzroy et al., 2021b), we predicted source activity would be reduced in older relative to young adults in a region-dependent manner. Moreover, based on prior evidence that gray matter atrophy mediates aging-related declines in scalp-recorded sleep oscillatory activity (Mander et al., 2013; Dubé et al., 2015; Varga et al., 2016; Fogel et al., 2017; Latreille et al., 2019; Fitzroy et al., 2021b), we predicted aging-related thinning in individual cortical regions would mediate aging-related reductions in sleep oscillatory activity within those regions, especially for frontal medial and primary motor cortices. Lastly, based on our prior findings relating brain activation during motor sequence learning to the scalp topography of sleep oscillatory activity in this cohort (Fitzroy et al., 2021a), we predicted aging-related differences in pre-sleep activation of motor sequence learning brain regions would contribute to aging-related differences in source activity during sleep, above and beyond the contributions of cortical thinning.
All data were collected as part of a larger study examining relationships between aging-related differences in brain activity during learning and subsequent sleep-dependent consolidation (Fitzroy et al., 2021a,b). In the current analyses, data were included from 17 young adults (8 female) between 18 and 31 years old (M = 22.71, SD = 3.51), and 18 older adults (8 female) between 58 and 75 years old (M = 65.39, SD = 5.80). These are the same 35 participants analyzed in Fitzroy et al. (2021a). Participants were excluded during recruitment based on the following criteria: left-handedness; self-reported presence of neurological, psychiatric, cardiac, or sleep disorders; use of medications or supplements affecting sleep; excessive napping, caffeine, or alcohol consumption; habitual bedtime after 12 PM or wake time before 5 AM; and implanted metal or other contraindications for the magnetic resonance imaging (MRI) environment.
All older adults were free of self-reported cognitive decline, and standardized cognitive assessments were administered for 14 of the older adults within the 2 years following participation. Nine older adults completed the Mini-Mental State Exam (MMSE; Folstein et al., 1975), with all scoring above the typical threshold (24; Mitchell, 2009) for detecting cognitive impairment (score range: 27–30). Six older adults completed the Telephone Interview for Cognitive Status (TICS; Brandt et al., 1988), with all scoring within the normal cognitive status range (≥30). As reported in Fitzroy et al. (2021a), young and older adults did not differ on depressive symptoms (Beck Depression Inventory [BDI]; Beck et al., 1996), typical daytime sleepiness (Epworth Sleepiness Scale [ESS]; Johns, 1991), or habitual sleep quality (Pittsburgh Sleep Quality Index [PSQI]; Buysse et al., 1989), but did differ in chronotype (Morningness-Eveningness Questionnaire [MEQ]; Horne and Ostberg, 1976), with older adults typically morning types and young adults typically intermediate types.
All procedures were approved by the Institutional Review Board at the University of Massachusetts Amherst, and written informed consent was obtained from all participants. Participants completed two experimental sessions separated by 1 week (2 weeks for one young adult); sessions were identical other than whether participants were given a nap opportunity or asked to stay awake during a midday interval. Participants were instructed to get quality sleep the night before experimental sessions, and to abstain from caffeine and alcohol consumption the night before and day of experimental sessions.
For each experimental session, participants arrived at the lab at approximately 9 AM, provided informed consent, were screened for MRI contraindications, and were given instructions for the SRTT. Participants then practiced random blocks of the SRTT in a mock MRI scanner, completed the Stanford Sleepiness Scale (SSS; Shahid et al., 2012), were positioned in the real MRI scanner, and underwent a high-resolution T1-weighted structural brain scan. After the T1-weighted scan, participants performed the SRTT while functional MRI (fMRI) images were collected. After completing the morning scan session, participants took a short break including 30 min for lunch in the lab, then had high-density polysomnography (HD-PSG) equipment applied. HD-PSG was recorded during a 2-h nap or wake opportunity (order counterbalanced; order did not affect nap sleep microstructure, see Supplementary Material) from 1 to 3 PM in a private bedroom, which was darkened for the nap opportunity. At 3 PM participants were awakened (nap condition), had the HD-PSG cap and electrodes removed, and were given time to clean up. Participants then performed a second random-only SRTT practice in the mock scanner, completed a second SSS, and returned to the real MRI scanner for a high-resolution diffusion-weighted structural brain scan and to again perform the SRTT during functional imaging. After completing the afternoon scan session, standardized questionnaires were administered to assess sleep habits, sleep quality, chronotype, and self-assessment of task performance.
Participants performed an explicit variant of the SRTT, in which they were instructed that visual cue sequences would contain a pattern on some blocks and that they should learn that pattern, but not directly informed what the pattern was. Cues were presented on a computer screen positioned behind the scanner bore, made visible to the participant via an angled mirror mounted to the head coil directly in front of their eyes. Movements were cued when one of four horizontally arranged boxes filled white. Participants were instructed to respond to cues quickly and accurately by pressing the button corresponding to the cue location on a four-button MRI-compatible response pad (Current Designs 932). All responses were made using the non-dominant (left) hand. Cues appeared at a regular interval (1000 ms) either randomly with constraint (random blocks), or according to a regular repeating eight-item pattern (sequence blocks) that did not contain repeats, trills, or runs of three or more (3-1-4-2-1-3-2-4 or 2-3-1-2-4-1-3-4; session order counterbalanced across participants), with 40 cues in each block. Participants completed 12 blocks, organized in six pairs of alternating block types (sequence, random), with a 30 s rest period between the third and fourth block-pairs. Block type was indicated on the screen at the start of each block. For full task design details, see Fitzroy et al. (2021a).
HD-PSG data were acquired with reference to FCz during the nap and wake intervals using a custom 129-channel cap (Easycap, Herrsching, Germany) and BrainAmp MR plus amplifiers (Brain Products GmbH, Gilching, Germany). The cap layout consisted of 123 scalp EEG electrodes placed at 10–10 and intermediary locations, four electrooculogram (EOG) electrodes placed beside and below the eyes, and two electromyogram (EMG) electrodes placed over the zygomatic major and mylohyoid muscles. Data were recorded using a hardware bandpass of 0.1–1000 Hz and digitized at 500 Hz using BrainVision Recorder (Brain Products GmbH, Gilching, Germany). Scalp impedances were reduced below 20 kΩ using high-chloride abrasive gel before the nap/wake interval. The wake session was a control condition for our previously reported analyses of sleep-dependent memory consolidation (Fitzroy et al., 2021a), and thus HD-PSG data from the wake session are not analyzed here.
To identify sleep stages, HD-PSG data recorded during the nap interval were offline-filtered (0.3–35 Hz EEG, 10–70 Hz EMG), re-referenced to the contralateral mastoid, and visually scored in 30 s epochs according to American Academy of Sleep Medicine criteria (Iber et al., 2007) using the Hume toolbox (Saletin and Greer, 2015). As originally reported in Fitzroy et al. (2021a), young and older adults did not significantly differ in sleep period time, or in minutes spent in NREM1, NREM2, NREM3, or REM (Supplementary Table 1). Additionally, we calculated minutes of wake after sleep onset (WASO) and a sleep fragmentation index for each participant. Following (Morrell et al., 2012), the sleep fragmentation index was calculated as the total number of shifts from NREM2, NREM3, or REM sleep to either NREM1 or wake over the entire session, divided by the number of hours spent asleep during the session. Young and older adults did not differ in minutes of WASO or in sleep fragmentation (Supplementary Table 1).
To quantify oscillatory neural activity in typical sleep frequency bands at the scalp, the original unfiltered HD-PSG data recorded during the nap were re-referenced to the averaged mastoid, bad channels were visually identified and interpolated, and amplitude envelopes were extracted in the delta (0.5–4 Hz), theta (4–8 Hz), and sigma (12–16 Hz) frequency bands using the filter-Hilbert method (Freeman, 2004). EEG data were filtered using band-specific filter designs: delta using a 2nd order Butterworth IIR filter, theta using a Chebyshev Type II filter requiring ≥20 dB attenuation in the stopband (outside 4–9 Hz) and ≤2 dB attenuation in the passband (4.5–8 Hz), and sigma using a 164th order FIR filter after demeaning the data. Artifactual sections of filtered data were identified automatically for each frequency band and electrode using simple voltage thresholds (delta: ±250 μV; theta/sigma: ±75 μV). Amplitude envelopes were derived at each electrode as the magnitude of the complex analytic signal produced by the Hilbert transform of the bandpass-filtered EEG. Artifact-free regions of the amplitude envelopes recorded during the first 60 min of NREM2 or NREM3 sleep were averaged into non-overlapping 20 s epochs, then averaged again across epochs to give a single amplitude measure per electrode per participant. Scalp EEG preprocessing was completed using a combination of EEGLAB (Delorme and Makeig, 2004), ERPLAB (Lopez-Calderon and Luck, 2014), Fieldtrip (Oostenveld et al., 2011), and in-house MATLAB software (PSGpower1).
Whole-brain images were collected with a Siemens 3T MAGNETOM Skyra scanner (Siemens Healthcare, Erlangen, Germany) and a 20-channel head coil. High-resolution T1-weighted structural images were collected in the sagittal plane using a three-dimensional magnetization-prepared rapid gradient echo (MPRAGE) sequence (TR = 1810 ms, TE = 2.26 ms, TI = 915 ms, FA = 9°, 224 slices, FoV = 224 × 256 × 256 mm2, voxel size 0.8 × 0.797 × 0.797 mm3). During functional scans, automated scout images were acquired and shimming procedures were performed to optimize field homogeneity, then 372 blood oxygenation level dependent (BOLD) fMRI images were acquired using an interleaved T2*-weighted echo-planar imaging (EPI) sequence (TR = 1500 ms, TE = 30.0 ms, FA = 73°, 60 slices, slice acceleration factor = 3, FoV = 68 × 68 mm2, voxel size 3.0 × 3.0 × 2.4 mm3).
Cortical reconstruction based on the T1-weighted structural images of each individual was performed in FreeSurfer (v6.0.02), using the automated ‘recon-all’ processing stream. Processing included motion correction and averaging (Reuter et al., 2010) of the T1-weighted structural images from the nap and wake sessions, removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Segonne et al., 2004), automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (Fischl et al., 2002, 2004a), intensity normalization (Sled et al., 1998), tessellation of the gray/white matter boundary, automated topology correction (Fischl et al., 2001; Segonne et al., 2007), and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Dale and Sereno, 1993; Dale et al., 1999; Fischl and Dale, 2000). The completed cortical models were registered to a spherical atlas based on individual cortical folding patterns to match cortical geometry across subjects (Fischl et al., 1999a,b), and parcellated into regions with respect to gyral and sulcal structure (Fischl et al., 2004b; Desikan et al., 2006). Cortical thickness was calculated as the closest distance from the gray/white boundary to the gray/CSF boundary at each vertex on the tessellated surface (Fischl and Dale, 2000). The procedures for automated measurement of cortical thickness have been validated against manual measurements (Kuperberg et al., 2003; Salat et al., 2004) and histological analysis (Rosas et al., 2002). FreeSurfer morphometric procedures have been demonstrated to show good test-retest reliability across scanner manufacturers and field strengths (Han et al., 2006; Jovicich et al., 2006; Reuter et al., 2012).
For full details regarding functional image preprocessing, quantification, and analysis, the reader is referred to Fitzroy et al. (2021a). Briefly, after standard image preprocessing steps, we modeled our random and sequence conditions using individual event-related task regressors for each participant and scan session (pre-nap, post-nap) that maximized the contrast between random and sequence blocks by excluding ambiguous random cues (i.e., trills, runs of three or more, transitional probabilities > 0.33), and were matched in included length to equate signal-to-noise ratio across conditions. We then generated whole-brain images of the sequence > random contrast for each participant and scan session. To reduce dimensionality and facilitate comparisons with source estimate measures, beta weights from the whole-brain contrast images were extracted and averaged separately within seven ROIs using the region of interest extraction (REX) toolbox3 : premotor cortex, inferior parietal cortex, hippocampus, parahippocampal gyrus, caudate, putamen, and cerebellum. The premotor cortex and cerebellum ROIs were defined as 10 mm radius spheres, centered on the peak activations of the within-group whole-brain conjunction analysis of activation for sequence vs. random (Fitzroy et al., 2021a). All other ROIs were anatomically defined using the Wake Forest University PickAtlas (Maldjian et al., 2003).
The cortical sources of sleep oscillatory activity were estimated separately for each participant and frequency band using depth-weighted minimum-norm estimation (MNE) as implemented in Brainstorm (Hämäläinen and Ilmoniemi, 1994; Tadel et al., 2011, 2019; Gramfort et al., 2014), using individual anatomically accurate forward models based on the cortical reconstructions derived from individual participants’ T1-weighted scans. The anatomically accurate forward models were generated using OpenMEEG (Gramfort et al., 2010), and consisted of three non-intersecting boundary element layers (scalp, outer skull, inner skull) and the cortical surface source space (modeled with 15,002 vertices). Sources of the time-averaged amplitude envelope scalp maps were estimated using dipole orientations constrained to be perpendicular to the cortical sheet (Lin et al., 2006), without modeling noise covariance. The absolute value of each resultant current density map was smoothed using an 8 mm kernel, then projected to standard ICBM152 space using the spherical atlas registration generated during FreeSurfer processing to facilitate across-participant comparisons.
Estimated cortical sources of sleep oscillatory activity were assessed separately for each frequency band and age group using one-tailed one-sample t-tests within each ROI of the Desikan-Killiany atlas as implemented in FreeSurfer (Desikan et al., 2006; Figure 1 and Supplementary Table 2), evaluated against the Bonferroni-corrected α = 0.00071. Visual inspection of the full cortex results suggested that rostral and caudal divisions of the superior frontal gyrus contributed differently across frequency bands. To investigate this possibility, and motivated by prior work demonstrating functional subregions of superior frontal gyrus (Li et al., 2013), the superior frontal gyrus ROI was split into rostral and caudal subsections using the ‘subdivide scout’ tool in Brainstorm (Figure 1). Aging-related differences in estimated cortical contributions to sleep oscillatory activity were assessed separately for each frequency band using a Monte Carlo permutation approach (5000 iterations) based on the two-tailed independent-samples t-test. The permutation procedure was first performed vertex-wise over the entire cortex (e.g., Figure 2, bottom), with significance evaluated against α = 0.025 to reflect the two-tailed nature of the underlying t-tests. Then, to facilitate comparisons with other measures, the permutation procedure was repeated region-wise after averaging estimated source activity within each ROI of the modified Desikan-Killiany atlas. Region-wise statistical significance was evaluated against α = 0.025 while controlling for false discovery rate (FDR) using the Benjamini–Hochberg step-up procedure (Benjamini and Hochberg, 1995), based on the total number of investigated ROIs (70).
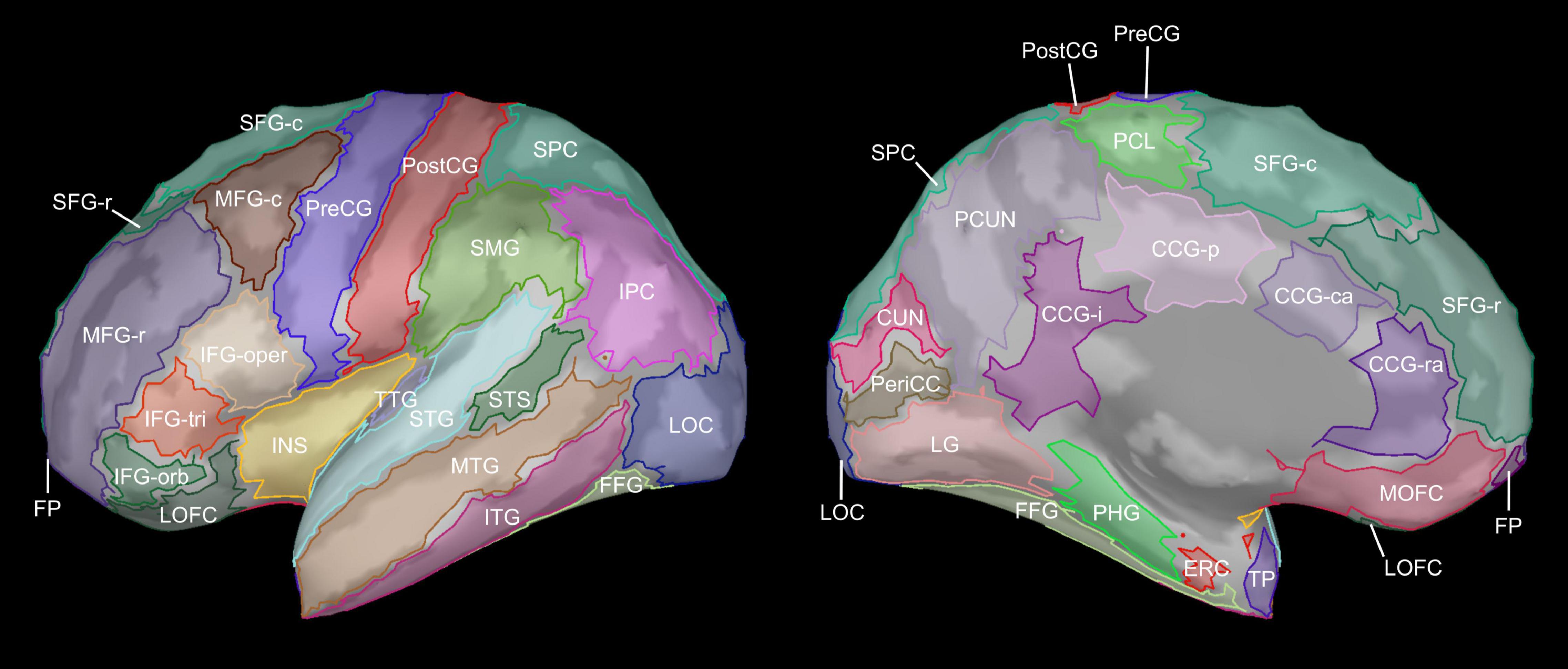
Figure 1. Cortical regions of interest. Cortical regions of interests (ROIs) are shown on the lateral and medial surfaces of the inflated left hemisphere. Bilateral cortical ROIs were defined using the Desikan-Killiany atlas (Desikan et al., 2006) as implemented in FreeSurfer, modified to split the superior frontal gyrus into rostral and caudal subdivisions. CCG-ra, cingulate gyrus (rostral anterior); CCG-ca, cingulate gyrus (caudal anterior); CCG-p, cingulate gyrus (posterior); CCG-i, cingulate gyrus (isthmus); CUN, cuneus; ERC, entorhinal cortex; FFG, fusiform gyrus; FP, frontal pole; IFG-oper, inferior frontal gyrus (pars opercularis); IFG-orb, inferior frontal gyrus (pars orbitalis); IFG-tri, inferior frontal gyrus (pars triangularis); INS, insula; IPC, inferior parietal cortex; ITG, inferior temporal gyrus; LG, lingual gyrus; LOC, lateral occipital cortex; LOFC, lateral orbitofrontal cortex; MFG-r, middle frontal gyrus (rostral); MFG-c, middle frontal gyrus (caudal); MOFC, medial orbitofrontal cortex; MTG, middle temporal gyrus; PCL, paracentral lobule; PCUN, precuneus; PeriCC, pericalcarine cortex; PHG, parahippocampal gyrus; PostCG, postcentral gyrus; PreCG, precentral gyrus; SFG-r, superior frontal gyrus (rostral); SFG-c, superior frontal gyrus (caudal); SMG, supramarginal gyrus; SPC, superior parietal cortex; STG, superior temporal gyrus; STS, superior temporal sulcus (banks); TP, temporal pole; TTG, transverse temporal gyrus.
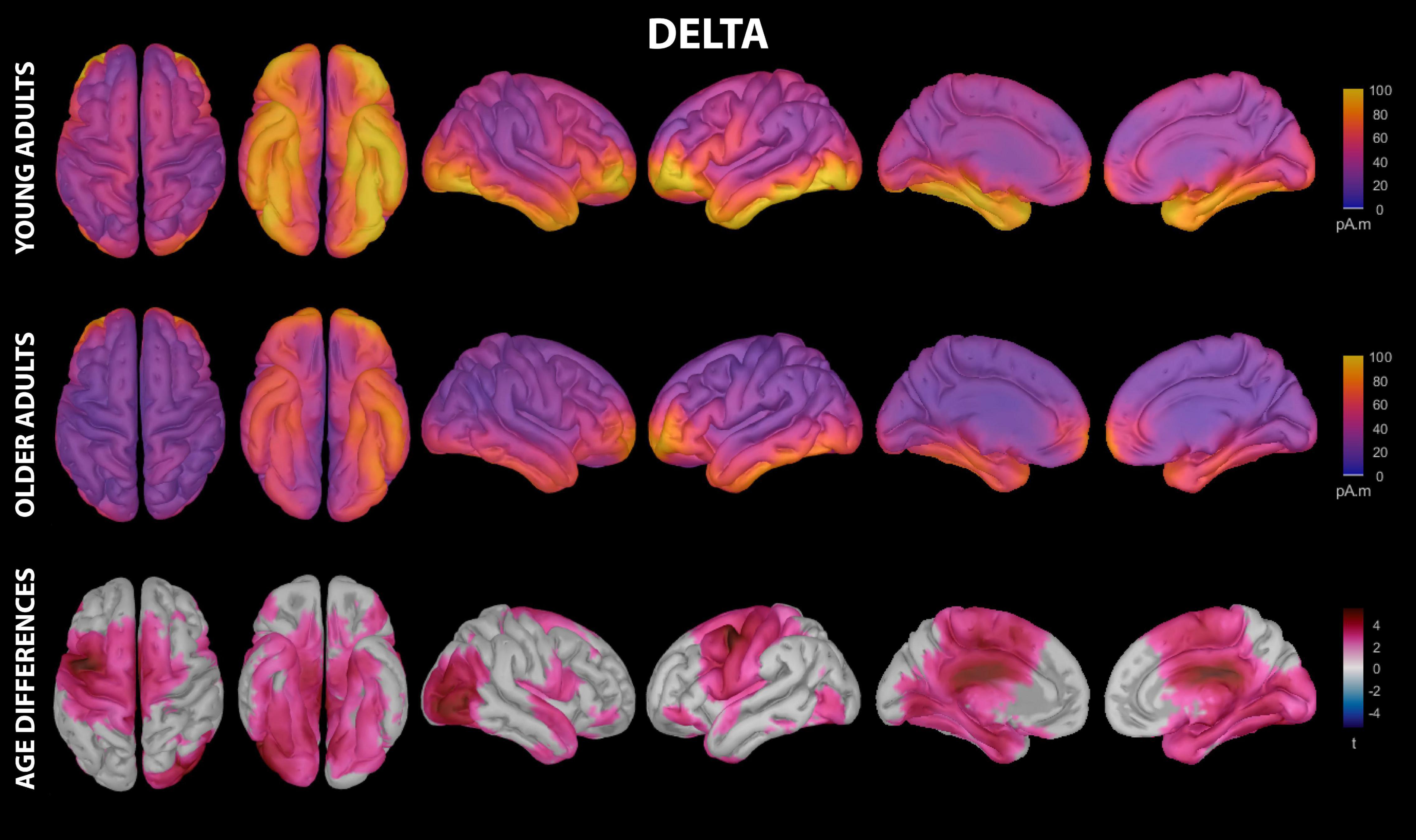
Figure 2. Estimated cortical sources of nap delta activity. Grand average current density maps for the estimated sources of delta activity during the first 60 min of N2/N3 sleep are shown for young adults (top row) and older adults (middle row). The results of the vertex-wise permutation test comparing estimated sources of delta activity between young and older adults are shown as the test statistic (t) map (bottom row), masked using α = 0.025 to reflect the two-tailed nature of the underlying t-tests.
To test the hypothesis that aging-related reductions in cortical activity during sleep are driven by aging-related gray matter atrophy, we performed a set of model-based simple mediation analyses (Imai et al., 2010; Tingley et al., 2014) examining whether aging-related reductions in estimated delta, theta, or sigma source activity within a given cortical region were mediated by aging-related cortical thinning within the same region. We restricted these analyses to only ROIs within which significant aging-related differences in estimated source activity were observed in the frequency band under consideration. For each simple mediation analysis, we estimated the magnitude and reliability of the total effect (TE), average causal mediation effect (ACME), and average direct effect (ADE) using a bootstrap procedure with 1000 iterations. Though it is debated whether a significant TE is a necessary criterion for mediation (Baron and Kenny, 1986; MacKinnon et al., 2000; Agler and De Boeck, 2017), in the interest of conservatism we only assessed potential mediation when a significant TE of age predicting estimated source activity was observed for a given ROI and frequency band. Because we had specific directional hypotheses of positive relationships between cortical thinning and source activity reduction within each ROI, the cortical thinning mediation analyses only considered potential positive relationships, and were therefore evaluated against α = 0.05. For significant mediation results (pTE < 0.05 and pACME < 0.05), the proportion of the TE of age on estimated source activity mediated by cortical thinning was calculated as (ACME + ADE)/ACME.
To test the hypothesis that brain activity prior to sleep influences the set of generators contributing to sleep oscillatory activity, and the magnitude of activity within those generators, we performed a series of regression-based analyses examining the relationships between pre-nap functional activity and estimated source activity during the nap. Specifically, we analyzed relationships between sequence-block-specific pre-nap functional activation within the seven motor sequence learning ROIs examined in Fitzroy et al. (2021a), and estimated source activity within each ROI of the modified Desikan-Killiany atlas for which significant aging-related differences in estimated source activity were observed in the frequency band under consideration. For all regression analyses investigating potential contributions of pre-nap brain activity to estimated source activity during the nap in a given ROI, we included cortical thickness in that same ROI as a covariate to ensure observed effects were not explainable by aging-related differences in cortical structure. Notably, and in contrast to our investigations of the contributions of cortical thickness to estimated source activity, we did not restrict our investigations of the contributions of pre-nap brain activity within a given region to estimated source activity during the nap in only that same region. This is because motor sequence learning follows a progression through multiple subcortical and cortical regions across learning and consolidation (e.g., Doyon et al., 2009a; King et al., 2017a), and it is therefore feasible that pre-sleep activity in one brain region could lead to activity during sleep in another downstream brain region.
For each potential relationship of pre-nap brain activation (PreActivity) to estimated source activity during the nap (EstSource), we first tested whether age moderated the relationship using interaction models of the general form EstSourceROI = β0 + β1(CorticalThicknessROI) + β2(Age) + β3(PreActivity) + β4(Age*PreActivity). When an interaction with age was present, the predictive power of brain activity prior to sleep was assessed separately in young and older adults using the reduced model structure EstSourceROI = β0 + β1(CorticalThicknessROI) + β2(PreActivity). Coefficient statistical significance in the interaction and reduced models was evaluated against α = 0.05 while controlling for FDR within each source ROI using the Benjamini–Hochberg step-up procedure (Benjamini and Hochberg, 1995), to account for multiple pre-nap brain activation predictors.
Second, we tested whether aging-related differences in estimated source activity during the nap were mediated by aging-related changes in pre-nap brain activation using model-based simple mediation analysis, similar to that performed for cortical thinning, while covarying for cortical thickness in the same ROI. Because we did not have specific directional hypotheses regarding relationships between pre-nap functional activation and during-nap estimated source activity, the functional activation mediation analyses considered both potential positive and negative relationships and were therefore evaluated against α = 0.025. For significant mediation results (pTE < 0.025 and pACME < 0.025), the proportion of the TE of age on estimated source activity during the nap mediated by pre-nap functional activation was calculated as (ACME + ADE)/ACME.
Delta activity during the nap had significant contributions from all investigated regions of cortex in both young and older adults, including motor cortical regions (Figure 2 and Supplementary Table 2). Activation peaks were evident in multiple frontal regions (middle frontal gyrus, inferior frontal gyrus, lateral orbitofrontal cortex, frontal pole), temporal regions (entorhinal cortex, parahippocampal gyrus, temporal pole, fusiform gyrus, middle temporal gyrus, inferior temporal gyrus), and lateral occipital cortex in both groups (Figure 2 and Supplementary Table 2).
Young adults had increased contributions to delta activity during the nap from several cortical regions compared to older adults, which can be conceptually grouped into four clusters (Figures 2, 3 and Supplementary Table 3): (1) a left-weighted motor cortical network consisting of caudal superior frontal gyrus (i.e., premotor cortex, SMA, pre-SMA), left caudal middle frontal gyrus (i.e., premotor cortex), left inferior frontal gyrus (pars opercularis and pars orbitalis), precentral gyrus (i.e., primary motor cortex), paracentral lobule, left postcentral gyrus, and left precuneus, (2) a right-weighted occipitoparietal network consisting of right inferior parietal cortex, lingual gyrus, right pericalcarine cortex, right cuneus, and right lateral occipital cortex, (3) an assembly of medial and lateral temporal cortical regions consisting of entorhinal cortex, right parahippocampal gyrus, right temporal pole, fusiform gyrus, right superior temporal gyrus, and right inferior temporal gyrus, and (4) cingulate gyrus (left caudal anterior, posterior, and isthmus divisions). No cortical regions were estimated to contribute more to delta during the nap in older adults than in young adults.
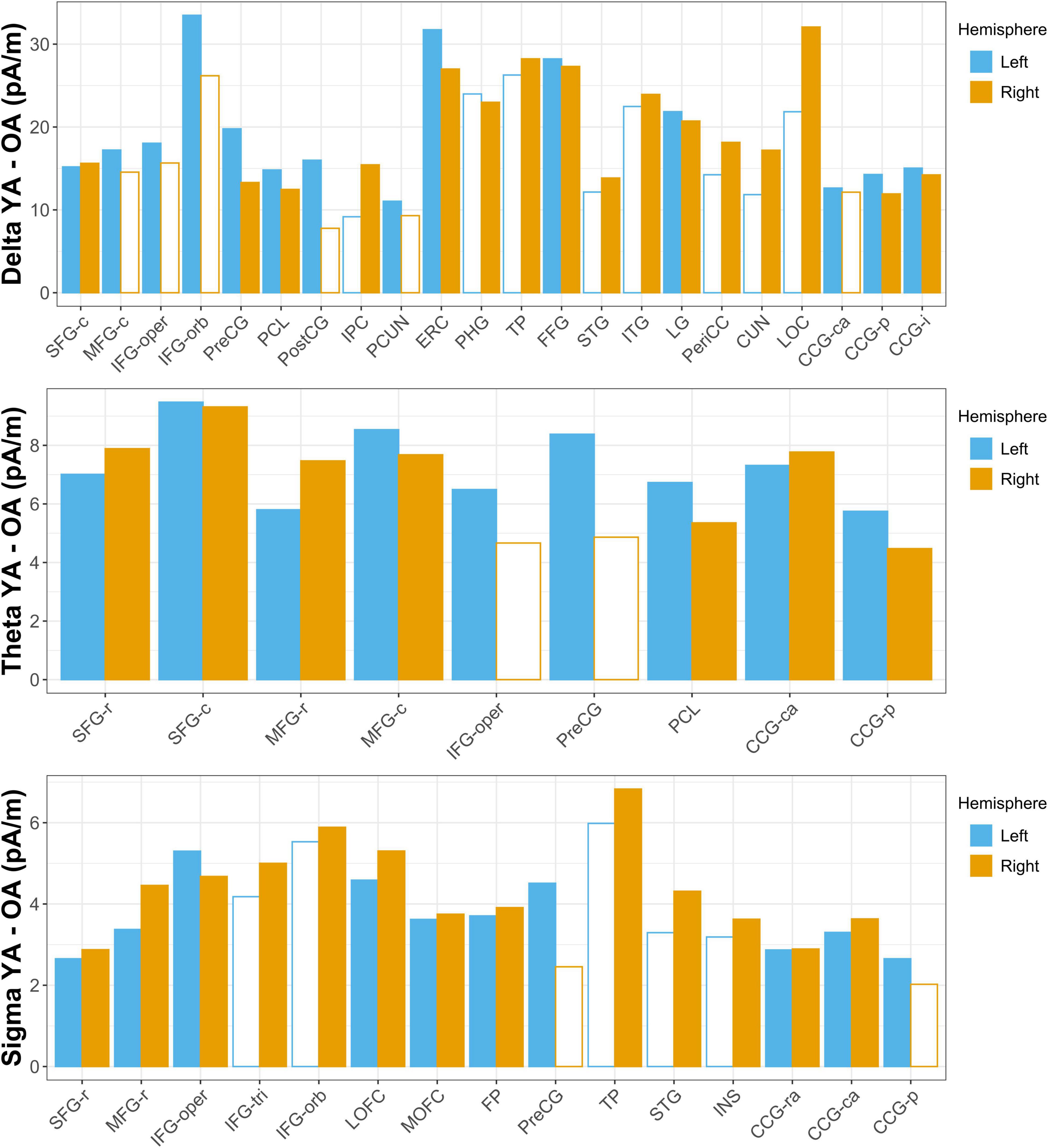
Figure 3. Aging-related differences in estimated source activity. Mean current density differences (young adult [YA] – older adult [OA]) are shown for all ROIs in which the region-wise permutation test revealed a significant effect of age group. Hemispheres in which the aging effect did not reach significance are indicated with empty bars; ROIs for which the aging effect did not reach significance in either hemisphere are omitted for clarity. Abbreviations are the same as those used in Figure 1.
Theta activity during the nap had significant contributions from all investigated regions of cortex in both young and older adults, including motor cortical regions (Figure 4 and Supplementary Table 2). Activation peaks were evident in multiple temporal regions (entorhinal cortex, parahippocampal gyrus, temporal pole, fusiform gyrus, inferior temporal gyrus) and occipital regions (lingual gyrus, pericalcarine cortex, cuneus, lateral occipital cortex) in both groups (Figure 4 and Supplementary Table 2).
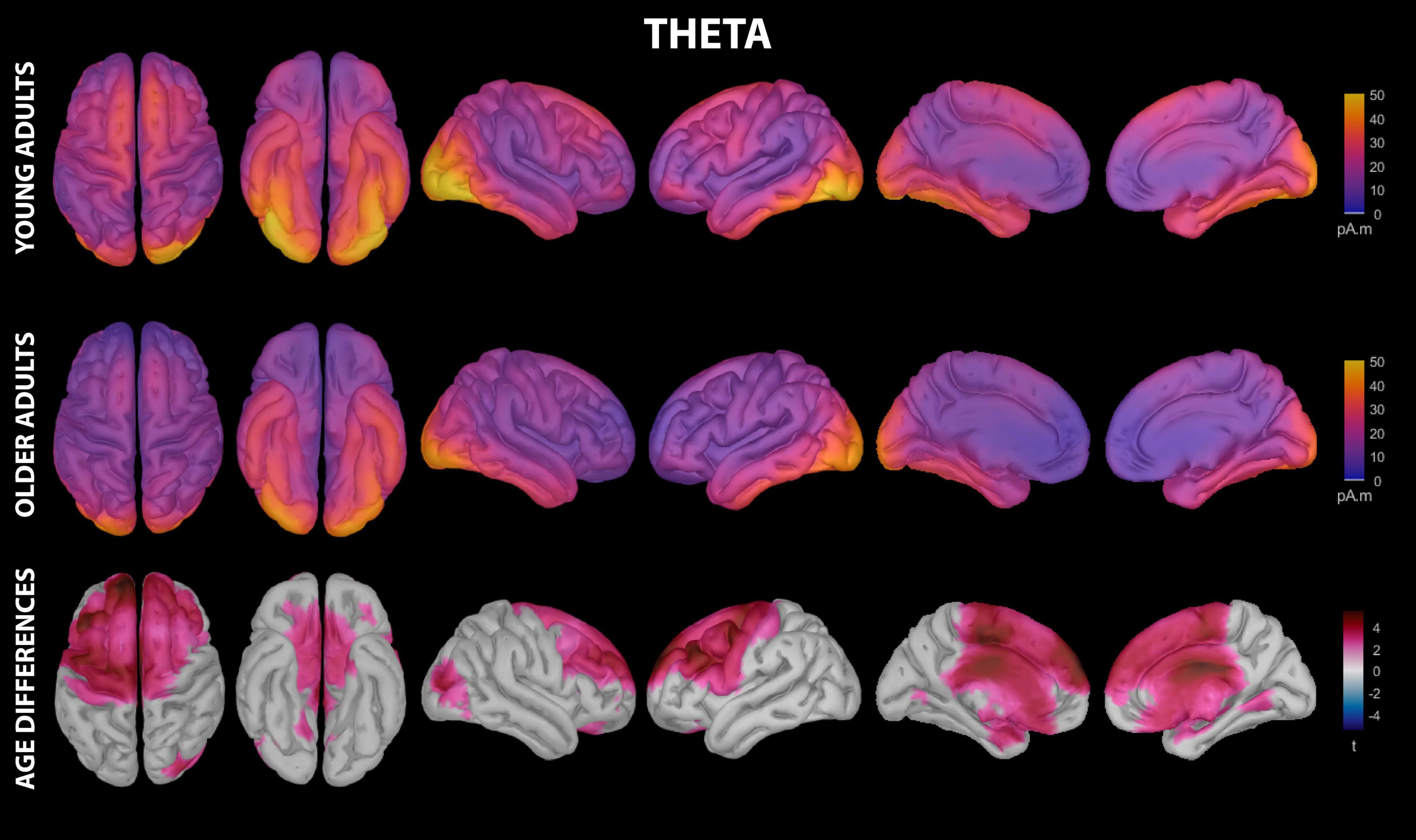
Figure 4. Estimated cortical sources of nap theta activity. Grand average current density maps for the estimated sources of theta activity during the first 60 min of N2/N3 sleep are shown for young adults (top row) and older adults (middle row). The results of the vertex-wise permutation test comparing estimated sources of theta activity between young and older adults are shown as the test statistic (t) map (bottom row), masked using α = 0.025 to reflect the two-tailed nature of the underlying t-tests.
Young adults had increased contributions to theta activity during the nap from multiple cortical regions compared to older adults, which can be conceptually grouped into three clusters (Figures 3, 4 and Supplementary Table 4): (1) a left-weighted motor cortical network consisting of caudal superior frontal gyrus, caudal middle frontal gyrus, left inferior frontal gyrus (pars opercularis), left precentral gyrus, and paracentral lobule, (2) a prefrontal network consisting of rostral superior frontal gyrus and rostral middle frontal gyrus, and (3) cingulate gyrus (caudal anterior and posterior divisions). No cortical regions were estimated to contribute more to theta during the nap in older adults than in young adults.
Sigma activity during the nap had significant contributions from all investigated regions of cortex in both young and older adults, including motor cortical regions (Figure 5 and Supplementary Table 2). Activation peaks were evident in multiple temporal regions (entorhinal cortex, parahippocampal gyrus, fusiform gyrus, inferior temporal gyrus) and occipital regions (lingual gyrus, pericalcarine cortex, lateral occipital cortex) in both groups (Figure 5 and Supplementary Table 2).
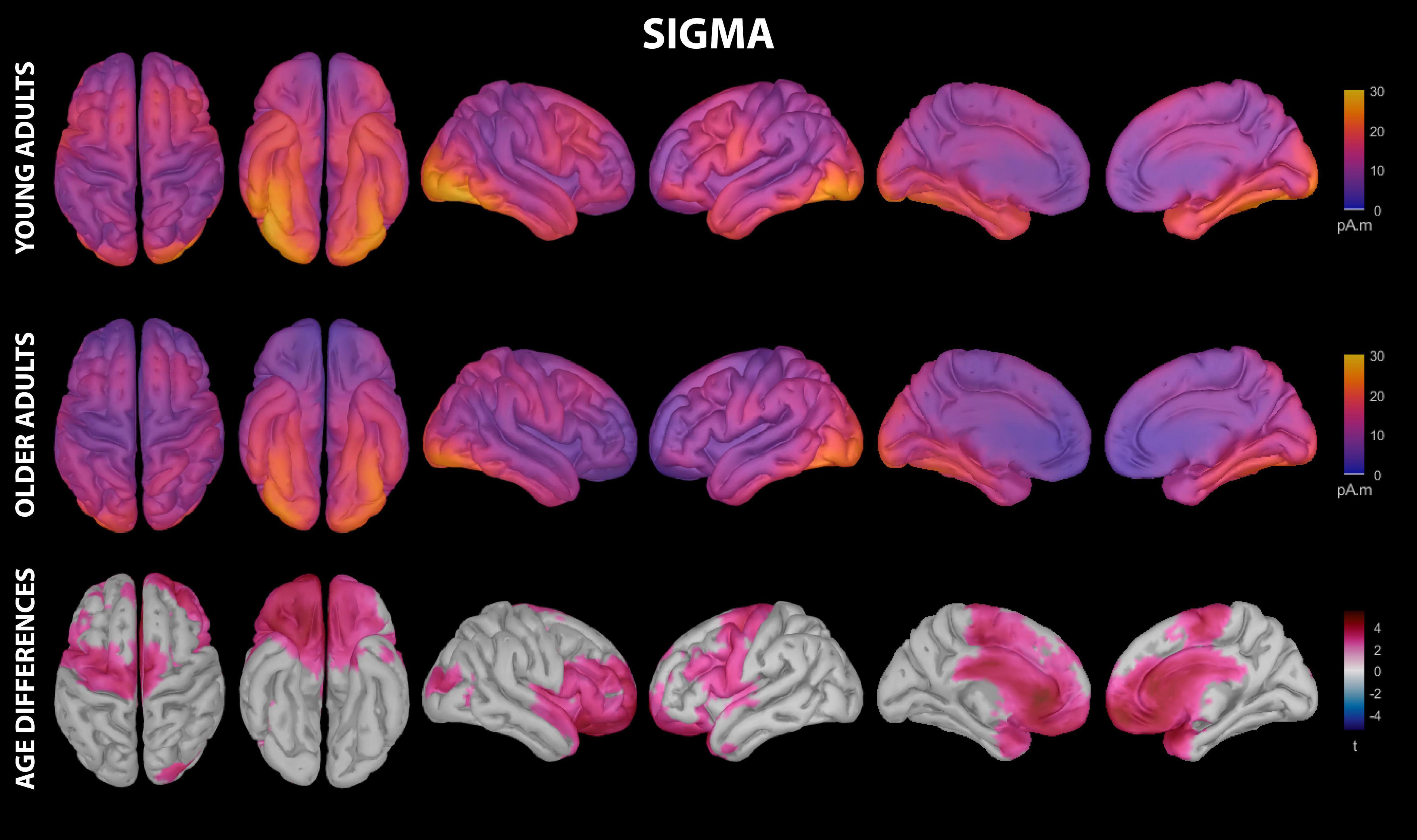
Figure 5. Estimated cortical sources of nap sigma activity. Grand average current density maps for the estimated sources of sigma activity during the first 60 min of N2/N3 sleep are shown for young adults (top row) and older adults (middle row). The results of the vertex-wise permutation test comparing estimated sources of sigma activity between young and older adults are shown as the test statistic (t) map (bottom row), masked using α = 0.025 to reflect the two-tailed nature of the underlying t-tests.
Young adults had increased contributions to sigma activity during the nap from multiple cortical regions compared to older adults, which can be conceptually grouped into three clusters (Figures 3, 5 and Supplementary Table 5): (1) left precentral gyrus, (2) a right-weighted prefrontal-temporal network consisting of rostral superior frontal gyrus, rostral middle frontal gyrus, inferior frontal gyrus (pars opercularis, right pars triangularis, right pars orbitalis), lateral orbitofrontal cortex, medial orbitofrontal cortex, frontal pole, right temporal pole, right superior temporal gyrus, and right insula, and 3) cingulate gyrus (rostral anterior, caudal anterior, and left posterior divisions). No cortical regions were estimated to contribute more to sigma during the nap in older adults than in young adults.
Mean cortical thickness was, on average, lower in older adults than young adults in all investigated regions of cortex (Supplementary Table 2). Moreover, this reduction was largest in frontal regions (Supplementary Table 2), and was significant at the Bonferonni-corrected threshold (α = 0.00071) for all cortical ROIs except an occipital cluster containing lingual gyrus, pericalcarine cortex, lateral occipital cortex, and right cuneus. This cortical thinning mediated aging-related declines in estimated sigma activity within left lateral orbitofrontal cortex and frontal pole, and partially mediated aging-related declines in estimated delta activity in right parahippocampal gyrus, fusiform gyrus, and right lingual gyrus (Table 1).
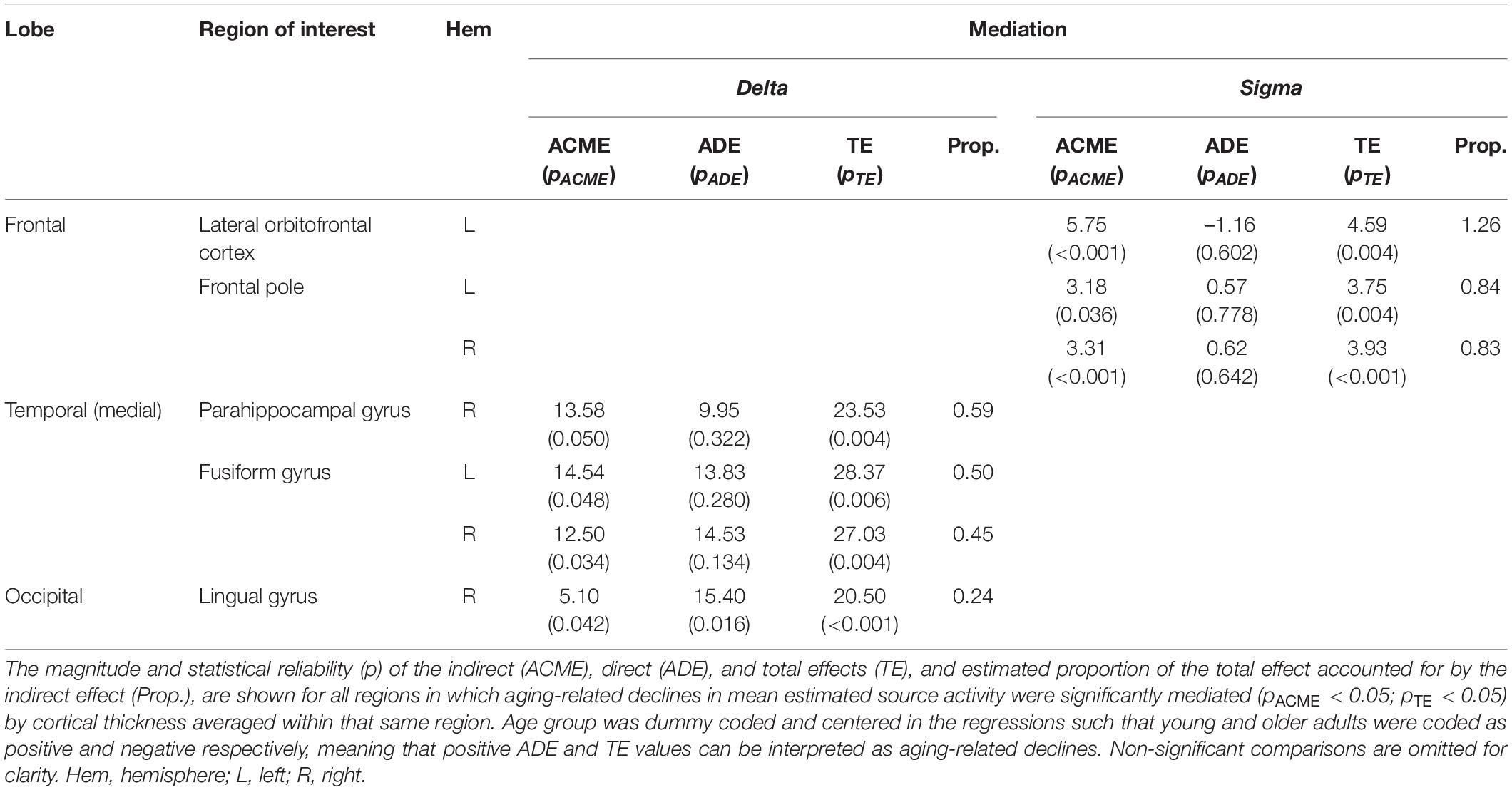
Table 1. Mediation of aging-related differences in estimated source activity by same-region cortical thickness.
Functional activation in the striatum during pre-nap learning related to estimated delta and theta source activity during the nap in multiple frontal and medial cortical regions, in a manner that was moderated by age. Specifically, age moderated the relationships between putamen activation and delta activity in right caudal superior frontal gyrus and left precuneus; between putamen activation and theta activity in caudal superior frontal gyrus, caudal middle frontal gyrus, and cingulate gyrus (caudal anterior and posterior divisions); and between right caudate activation and theta activity in caudal superior frontal gyrus (Table 2). These interactive effects of age and pre-nap striatum activation reflected a general pattern of negative relationships between pre-nap striatum activation and estimated source activity during the nap in young adults, and positive or absent relationships between these measures in older adults (Table 2). Similarly, age moderated the relationship between pre-nap functional activation in left inferior parietal cortex and delta activity during the nap in right caudal superior frontal gyrus, reflecting a negative relationship between these measures in young adults and a marginal positive relationship between these measures in older adults (Table 2). Aging-related differences in estimated source activity during the nap were not mediated by functional activation prior to the nap for any frequency band, source ROI, or functional activation ROI, when covarying for source ROI cortical thickness (ps > 0.05).
Here we demonstrate that sleep oscillatory neural activity following motor sequence learning contains contributions from multiple cortical regions, including those involved in motor sequence learning, in both young and older adults. Further, we demonstrate that these cortical contributions are reduced in older adults in a region-specific manner, and that these aging-related reductions in estimated source activity reflect aging-related changes in both brain structure and pre-sleep brain activation.
Based on prior source estimation work in young adults (Tamaki et al., 2013; Brancaccio et al., 2020) and evidence that motor learning regions are active during the SRTT in young and older adults (e.g., King et al., 2017b), we predicted that sleep oscillatory activity following motor sequence learning would contain contributions from multiple cortical regions, including motor cortices (premotor cortex, primary motor cortex, pre-SMA, SMA), in both young and older adults. Consistent with this prediction, we observed significant estimated contributions to delta, theta, and sigma activity during sleep after motor sequence learning from all regions of the modified Desikan-Killiany atlas, including caudal superior frontal gyrus, caudal middle frontal gyrus, precentral gyrus, and paracentral lobule (which collectively encompass premotor cortex, primary motor cortex, pre-SMA, and SMA; Nieuwenhuys et al., 2008).
Although local activation peaks were evident in these motor cortical regions, especially in young adults, motor cortical regions were not the largest contributors to sleep oscillatory activity following motor sequence learning. Rather, global activation peaks were evident in other cortical regions in a frequency-specific manner. The largest contributions to delta were in prefrontal and ventral temporal regions, whereas the largest contributions to theta and sigma were in ventral temporal and occipital regions. Regarding delta and theta, our pattern of EEG-estimated source activity in a nap after motor sequence learning is markedly similar to that previously reported by Brancaccio et al. (2020), who used similar source estimation techniques on overnight sleep (relative to wake) MEG data after no specific learning task. This converging evidence across overnight MEG and nap EEG data, and across sleep following varied learning task requirements, suggests that the contributions of frontal and temporal regions to sleep delta activity, and of temporal and occipital regions to sleep theta activity, are a basic aspect of sleep physiology not related to sleep pressure, circadian influences, pre-sleep brain activation, or the specific neural populations assessed by the EEG or MEG techniques.
Regarding sigma, our pattern of estimated source activity diverges from that of Brancaccio et al. (2020), who report increased frontal and medial cortical contributions but decreased occipital contributions for sleep sigma relative to wake sigma. We posit this difference reflects the contrast with wake MEG employed by Brancaccio et al. (2020). Delta and theta band activity are substantially increased overall during sleep relative to wake, whereas sigma band activity is more comparable between the sleep and wake states (e.g., Sazgar and Young, 2019). Contrasting against wake will therefore have a larger overall impact on sleep neural activity in the sigma band than in the delta and theta bands, which explains the frequency-dependent pattern of correspondence between our activation maps and those of Brancaccio et al. (2020).
Based on prior examinations of aging-related changes in sleep oscillatory activity at the scalp (e.g., Sprecher et al., 2016; Fitzroy et al., 2021b), we predicted that estimated source activity in the delta, theta, and sigma frequency bands during sleep would be reduced in older relative to young adults in a region-dependent manner. Moreover, given prior evidence of aging-related reductions in frontal and motor cortical activation during motor sequence learning (Aizenstein et al., 2006; Fitzroy et al., 2021a), we predicted that aging-related reductions in estimated source activity would be larger in frontal and motor cortical regions. Broadly speaking, our results are in line with these predictions: aging-related reductions in estimated source activity were observed, varied across brain regions, and were evident in motor regions predicted to be active during the SRTT. Additionally, reductions were observed in non-motor regions which also would likely have been active during the SRTT. In this way, our results are consistent with the local sleep hypothesis that neural activity during sleep reflects neural activation prior to sleep. The specific regional distributions of aging-related differences in estimated source activity differed across frequency bands, which we consider separately below.
Aging-related declines in estimated delta source activity were observed in multiple motor cortical regions implicated in the SRTT. Reduced activity was observed in the premotor cortex, primary motor cortex, SMA, and pre-SMA regions previously shown to contribute to motor-learning-related sleep delta activity in young adults (Tamaki et al., 2013), as well as the premotor and SMA regions that show increased sequence-specific activation during SRTT performance in the present cohort (Fitzroy et al., 2021a). Reduced delta activity was also observed in caudal anterior and posterior cingulate regions, which are anatomically interconnected with premotor and primary motor cortices (Beckmann et al., 2009).
Interestingly, while the post-motor-learning sleep delta activity in motor regions reported by Tamaki et al. (2013) was either bilateral or contralateral to the task-performing hand, in the present study aging-related reductions in post-motor-learning delta in motor regions were larger or only present ipsilateral to the task-performing hand. Tamaki et al. (2013) used a within-subjects design, comparing post-motor-learning sleep to control sleep to isolate the areas of largest learning-related increase. Our opposite reduction pattern with regard to hemisphere could then represent a relative preservation in older adults of delta contributions from motor cortical regions in which post-motor-learning activation is highest. Alternatively, these hemisphericity differences may reflect differences between brain activity during motor sequence learning that requires sequence detection and identification like the SRTT, and that which does not like the explicit sequential finger tapping task employed by Tamaki et al. (2013).
Additionally, we observed aging-related delta reductions in several cortical regions which have not been explicitly shown to contribute to delta activity during post-motor-learning sleep, but that could be associated with aspects of performing a visuomotor SRTT. Delta contributions were reduced from medial temporal and parietal regions associated with memory encoding, retrieval, and sleep-dependent consolidation (Nielsen et al., 2005; Cavanna and Trimble, 2006; Aminoff et al., 2013; Navarro Schröder et al., 2015), from occipitoparietal, medial temporal, and lateral temporal regions associated with visual and object processing (Wang et al., 1999; Grill-Spector et al., 2001; Herlin et al., 2021), from parietal regions associated with spatial attention (Numssen et al., 2021), from frontal regions associated with processing rule-governed structure in temporal sequences (Tyler et al., 2011), and from auditory and somatosensory regions, potentially reflecting the loud, uncomfortable MRI scanner environment (Bushnell et al., 1999; Gaab et al., 2006). Given that all of these regions are plausibly associated with SRTT performance, aging-related reductions in delta contributions from them may reflect lower overall activation of task-relevant regions in older adults during the task, and therefore reduced activity in these regions in subsequent sleep. Alternatively, given that ventral frontal and temporal regions have also been implicated in delta generation following no specific learning task (Brancaccio et al., 2020), it is possible that aging-related differences in delta contributions from these regions reflect a general (i.e., not task specific) aging-related reduction in delta activity. Future work investigating aging-related differences in delta activation following different learning tasks is required to separate these interpretations.
Similar to delta, older adults showed reduced estimated theta source activity in a left-weighted cluster of motor cortical regions, which included the primary motor cortex and SMA regions in which Tamaki et al. (2013) reported training-related increases in theta activity, as well as the premotor cortex and pre-SMA regions in which training-related increases did not reach significance in Tamaki et al. (2013). Older adults also showed reduced theta activity in the same cingulate regions that showed aging-related delta reductions, and which are anatomically interconnected with premotor and primary motor cortices.
In further similarity to delta, our age-related reduction in primary motor cortex theta activity was ipsilateral only, whereas that observed by Tamaki et al. (2013) was contralateral only. These theta contributions from additional motor regions relative to Tamaki et al. (2013), and opposite hemisphericity, could again reflect either the within- vs. between-subjects designs of the two studies, or possible differences in theta generators following implicit vs. explicit motor sequence learning. However, despite these differences, both our theta findings and those of Tamaki et al. (2013) are consistent with the hypothesis that cortical regions involved in motor sequence learning contribute to neural activity during subsequent sleep.
Unlike delta, older adults additionally showed large reductions in estimated theta activity in rostral superior frontal and rostral middle frontal gyri. Rostral superior frontal cortex is suggested to work together with the anatomically interconnected left pars opercularis, which also showed reduced theta activity in older adults, in the execution of cognitive manipulations (Li et al., 2013). Similarly, rostral middle frontal cortex is implicated in executive functioning and working memory processes (Michalski et al., 2017). These prefrontal, non-motor cortical regions are likely to have been active during the SRTT. Aging-related reductions in theta contributions from these regions are therefore consistent with lower overall activation of task-relevant regions in older adults prior to the nap, and reduced activity in these regions during subsequent sleep. Moreover, previous investigations of cortical contributions to sleep theta activity following no specific learning task do not show large contributions of rostral superior frontal or rostral middle frontal gyri (Brancaccio et al., 2020), suggesting that the aging-related difference in the present study is related to the SRTT prior to sleep. However, this interpretation remains speculative until aging-related differences in theta generators during sleep following different learning tasks are directly compared.
Older adults showed reduced estimated sigma source activity in left (ipsilateral) precentral gyrus, corresponding to primary motor cortex, and the anatomically interconnected left posterior cingulate gyrus. This differs from the regions previously shown to contribute to sleep sigma following motor sequence learning in young adults by Tamaki et al. (2013), who report increased fast sigma activity in contralateral SMA and, to a lesser extent, premotor and primary motor cortices.
This variability between studies likely reflects differences in the depth of motor sequence encoding in young adults prior to sleep. In the study by Tamaki et al. (2013), young adults’ performance on the motor sequence task improved over post-training sleep, and improvements correlated with SMA fast sigma activity during sleep. Young adults in the present study, however, were overtrained during the pre-sleep motor sequence task, achieving a cortical motor sequence memory representation and ceiling behavioral performance during the task (Fitzroy et al., 2021a). It is consistent then that sleep sigma activity in SMA correlated with performance improvement in Tamaki et al. (2013), but was not elevated in our young adults, who did not show performance improvement over sleep. However, the aging-related reduction in primary motor cortex sigma activity in the present study suggests that consolidation-related sigma activity did occur during sleep in the young adults, which is consistent with the automatization-related decreases in motor cortex activation during SRTT performance observed over sleep in these young adults (Fitzroy et al., 2021a).
In addition to primary motor cortex, older adults showed reduced sigma activity in a right-weighted cluster of prefrontal and temporal regions implicated in multiple cognitive aspects of the SRTT: cognitive manipulations (Li et al., 2013), attention and inhibition (Japee et al., 2015; Hartwigsen et al., 2019), integrating temporal sequences during decision making (Nogueira et al., 2017), processing higher-order relations (Hartogsveld et al., 2018), and visual object processing (Herlin et al., 2021). Older adults also showed reduced sigma contributions from cingulate regions associated with motor task performance and reward processing (Stevens et al., 2011), and auditory regions. The potential relevance of these non-motor regions’ function to cognitive aspects of the SRTT suggests that the aging-related reductions in sigma activity in these regions may reflect reduced engagement of these regions during the SRTT in older adults, who performed worse on the task (Fitzroy et al., 2021a). However, as with delta and theta activity, confirming this interpretation requires future work comparing aging-related differences in sleep sigma generators following different types of learning. Lastly, older adults showed reduced sigma contributions from right insula, in which reduced gray matter volume has previously been associated with a dominance of fast (vs. slow) spindle activity (Saletin et al., 2013). This reduction likely therefore reflects reduced insular gray matter volume in our older adults (Fitzroy et al., 2021b).
Based on prior evidence that gray matter atrophy mediates aging-related declines in sleep oscillatory activity at the scalp (Mander et al., 2013; Dubé et al., 2015; Fogel et al., 2017; Latreille et al., 2019; Fitzroy et al., 2021b), we predicted that cortical thinning in individual regions would mediate aging-related reductions in those regions’ contributions to sleep delta, theta, and sigma activity. We additionally predicted that this mediation would be stronger for frontal medial and primary motor cortices, within which gray matter volume loss has been shown to mediate aging-related changes in scalp EEG in a superset of the present cohort (Fitzroy et al., 2021b). Instead, we observed a constrained set of regions within which aging-related reductions in estimated delta and sigma source activity were mediated by same-region cortical thinning. Sigma activity reductions were mediated by cortical thinning in prefrontal regions (left lateral orbitofrontal cortex, frontal pole), whereas delta activity reductions were partially mediated by cortical thinning in medial temporal and occipital regions (right parahippocampal gyrus, fusiform gyrus, right lingual gyrus). The result that aging-related differences in estimated motor cortex source activity were not mediated by cortical thinning suggests that these differences reflect motor cortical activation prior to sleep, rather than aging-related structural brain differences.
Notably, aging-related reductions in estimated frontal medial cortex delta activity were not mediated by cortical thinning, despite prior evidence that frontal medial cortex gray matter loss (volume and thickness) mediates aging-related reductions in scalp-recorded delta activity (Mander et al., 2013; Latreille et al., 2019; Fitzroy et al., 2021b). This result indicates that the mediating role of frontal medial cortex atrophy in aging-related sleep delta reductions at the scalp is not a result of reduced delta activity in frontal medial cortex with aging. Rather, this likely points to the role of frontal medial cortex as a structural and functional network hub (van den Heuvel and Sporns, 2013; Fernández, 2017). From this view, frontal medial cortex plays a coordinating role in delta activity generation, such that atrophy in this region impairs regulation of downstream regions that are the actual delta generators. Additionally, primary motor cortical thickness did not mediate aging-related reductions in primary motor cortical source activity, despite gray matter volume in this region mediating sleep delta and theta reductions at the scalp in a superset of this cohort (Fitzroy et al., 2021b). This result may suggest that the mediation of aging-related scalp delta and theta reductions by primary motor cortex gray matter volume is more driven by gyral surface area than by cortical thickness, as gray matter volume reflects both of these distinct measures, and is particularly influenced by surface area in precentral gyrus (Panizzon et al., 2009; Kong et al., 2015).
We predicted that aging-related differences in pre-sleep activation of motor sequence learning brain regions would contribute to aging-related differences in the estimated sources of oscillatory activity during sleep, above and beyond the contributions of cortical thinning. Consistent with this prediction, we observed a number of age-moderated effects of pre-nap putamen activation on region-specific source activity in the theta, and to a lesser extent delta, frequency bands, while controlling for same-region cortical thickness. We observed similar age-moderated effects of pre-nap caudate activation on superior frontal gyrus theta, and of pre-nap inferior parietal cortex activation on superior frontal gyrus delta.
In all cases, these interactive effects consisted of robust negative relationships in young adults, and positive or absent relationships in older adults. This may reflect differences in the depth of encoding reached in young and older adults prior to sleep. In young adults, ceiling behavioral performance (indicating a high depth of encoding) was achieved during learning, and accompanied by reductions over sleep in SRTT activation of frontal and motor cortical regions. Such reductions are proposed to reflect increases in cortical efficiency (Fitzroy et al., 2021a). Putamen activation increases with practice across the slow phase of motor sequence learning (Hikosaka et al., 1999; Penhune and Steele, 2012; Albouy et al., 2015). The negative relationships between pre-nap putamen activation and cortical source activity during the nap in young adults could therefore reflect higher cortical efficiency during the nap in young adults who progressed farther through slow learning prior to sleep. Conversely in older adults, behavioral improvement was observed over sleep (indicating a low to moderate depth of pre-sleep encoding), and accompanied by increases over sleep in SRTT activation of frontal and motor cortical regions. Increased putamen activation in older adults could therefore reflect further progress through slow learning prior to the nap, and as a result, higher consolidation-related activity in frontal and motor cortical regions during sleep.
Notably, although the restriction of observed aging-related differences in estimated source activity to task-related regions supports the local sleep hypothesis that regions active prior to sleep will be active during sleep, we did not observe any relationships between functional activity in a region prior to the nap and source activity in that same region during the nap. We posit this result reflects a fundamental difference between our fMRI-based and EEG-based neuroimaging approaches. Our fMRI-based measures are necessarily defined as a contrast between two conditions, specifically random and sequence blocks of the SRTT. Low-level aspects of the SRTT, such as visual stimulation and response preparation, were the same between random and sequence blocks, meaning that brain activation related to these aspects is absent from our fMRI contrast. Conversely, our estimated source activity measures do not require an explicit contrast, and therefore reflect local sleep effects due to all aspects of pre-sleep cognitive activity, not just those specific to sequence learning. Thus, while the sequence-specificity of our fMRI contrast is advantageous for understanding during-task activation unto itself, it likely reduces the predictive power of the fMRI-based measures for the source activity measures. Our observation of multiple predictive relationships between pre-sleep putamen activity and cortical activity during sleep then likely reflects a particularly pivotal role of the putamen in aging-related differences in motor sequence learning and consolidation, which is consistent with prior work examining neural activity during post-motor sequence learning sleep with simultaneous fMRI and EEG in young adults (Vahdat et al., 2017).
The regional specificity of the observed aging-related differences in source activity, along with their limited attribution to cortical thinning and the observed influences of some pre-sleep brain activity on source activity, suggests that the aging-related differences in source activity are related to the performance of the SRTT prior to sleep. However, our study design did not include a control sleep condition following either a different learning task, or no specific learning task. Comparing aging-related differences in source activity following motor sequence learning versus other types of learning, or no specific learning task, would give more direct evidence that the aging effects were attributable to pre-sleep motor sequence learning. Our work therefore provides preliminary support for this hypothesis, and should be followed up with work directly comparing aging-related source activity differences during sleep following different learning tasks.
Additionally, in the interest of keeping the overall number of comparisons low, we constrained our investigation of cortical thinning influences to only same-region mediation, and our investigation of pre-sleep brain activity influences to only functional ROIs which we have previously examined in this dataset (Fitzroy et al., 2021a). Similarly, we limited all investigations of influences on aging-related differences in source activity to regions in which the main effect of age was significant. As a result, our methods would have missed second-order or higher effects of cortical thinning on downstream generators of sleep oscillatory activity, potential effects of pre-sleep activation in non-motor cortical regions on sleep oscillatory activity, and crossover interaction effects with age that were unaccompanied by an age main effect. We accept this as a necessary tradeoff to limit the potential for type I error.
Lastly, although we employed state of the art techniques for EEG source estimation, we did not model noise covariance of the EEG. Unlike MEG, it is not possible to collect a room noise sample using EEG equipment disconnected from the participant. Best practices are to therefore use an appropriate baseline in the EEG recording as a model of noise covariance (Tadel et al., 2019). This is difficult for source estimation of sleep EEG however, as there is effectively no in vivo baseline for sleep; sleep is already the most quiescent typical brain state. Some studies have used eyes-closed wake EEG recordings as a baseline condition, but this method has conceptual issues. Waking EEG activity in the sigma band includes alpha and beta band activity, which reflects attention and other cognitive factors (e.g., Engel and Fries, 2010) and is variable over time. Subtracting this variable wake activity from the sleep EEG would introduce it as epiphenomenal inverted signals. Future work estimating sleep EEG sources could consider using a bioelectrically appropriate phantom to collect a room noise sample prior to recording, which could be weighted by participant-specific impedance measures to approximate recording noise. Our source estimation techniques were also limited to the cortical surface, despite a priori evidence that subcortical structures are involved in motor sequence learning and likely contribute to subsequent sleep neural activity. Future work should investigate the source contributions of deep structures, as analytic techniques for extracting this information from scalp-recorded EEG (e.g., Attal et al., 2009) continue to improve.
Aging-related reductions in estimated sleep delta, theta, and sigma source activity following motor sequence learning are observed in several motor and other cortical regions associated with aspects of motor sequence learning, in a manner consistent with the local sleep hypothesis (Huber et al., 2004). These aging-related differences are largely unexplained by cortical thinning, which explains aging-related differences in only a small number of cortical generators of delta and sigma activity. Pre-sleep sequence-learning-specific functional activation in the striatum and inferior parietal cortex have age-moderated effects on delta and theta contributions from multiple frontal and motor cortical regions, providing additional evidence that pre-sleep neural activity influences neural activity during sleep. Taken together, these results support the local sleep hypothesis. Moreover, these results identify putamen, caudate, and inferior parietal cortex as brain regions that may be amenable to targeted training paradigms to enhance motor sequence learning and subsequent sleep-dependent consolidation in older adults.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board at the University of Massachusetts Amherst. The patients/participants provided their written informed consent to participate in this study.
AF: conceptualization, methodology, software, formal analysis, investigation, data curation, writing – original draft, and visualization. BJ: conceptualization, methodology, and writing – original draft. KK: investigation, data curation, and writing – review and editing. JS: data curation and writing – review and editing. RS: conceptualization, supervision, project administration, funding acquisition, and writing – review and editing. All authors: contributed to the article and approved the submitted version.
This work was supported by National Institutes of Health (National Institute on Aging) award R01 AG040133 (PI: RS).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to acknowledge Phuong Bui, Aazam Najeebi, and Sarah Spagnolo for assistance with aspects of the work presented in this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2021.787654/full#supplementary-material
Agler, R., and De Boeck, P. (2017). On the Interpretation and Use of Mediation: Multiple Perspectives on Mediation Analysis. Front. Psychol. 8:01984. doi: 10.3389/fpsyg.2017.01984
Aizenstein, H. J., Butters, M. A., Clark, K. A., Figurski, J. L., Andrew Stenger, V., Nebes, R. D., et al. (2006). Prefrontal and striatal activation in elderly subjects during concurrent implicit and explicit sequence learning. Neurobiol. Aging 27, 741–751. doi: 10.1016/j.neurobiolaging.2005.03.017
Albouy, G., Fogel, S., King, B. R., Laventure, S., Benali, H., Karni, A., et al. (2015). Maintaining vs. enhancing motor sequence memories: Respective roles of striatal and hippocampal systems. NeuroImage 108, 423–434. doi: 10.1016/j.neurobiolaging.2005.03.017
Albouy, G., Fogel, S., Pottiez, H., Nguyen, V. A., Ray, L., Lungu, O., et al. (2013). Daytime Sleep Enhances Consolidation of the Spatial but Not Motoric Representation of Motor Sequence Memory. PLoS One 8:e52805. doi: 10.1371/journal.pone.0052805
Aminoff, E. M., Kveraga, K., and Bar, M. (2013). The role of the parahippocampal cortex in cognition. Trends Cognit. Sci. 17, 379–390. doi: 10.1016/j.tics.2013.06.009
Attal, Y., Bhattacharjee, M., Yelnik, J., Cottereau, B., Lefèvre, J., Okada, Y., et al. (2009). Modelling and detecting deep brain activity with MEG and EEG. IRBM 30, 133–138. doi: 10.1016/j.irbm.2009.01.005
Backhaus, J., and Junghanns, K. (2006). Daytime naps improve procedural motor memory. Sleep Med. 7, 508–512. doi: 10.1016/j.sleep.2006.04.002
Bang, J. W., Khalilzadeh, O., Hämäläinen, M., Watanabe, T., and Sasaki, Y. (2014). Location specific sleep spindle activity in the early visual areas and perceptual learning. Vis. Res. 99, 162–171. doi: 10.1016/j.visres.2013.12.014
Barakat, M., Carrier, J., Debas, K., Lungu, O., Fogel, S., Vandewalle, G., et al. (2012). Sleep spindles predict neural and behavioral changes in motor sequence consolidation. Hum. Brain Mapp. 34, 2918–2928. doi: 10.1002/hbm.22116
Baron, R. M., and Kenny, D. A. (1986). The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Personal. Soc. Psychol. 51, 1173–1182. doi: 10.1037//0022-3514.51.6.1173
Beck, A. T., Steer, R. A., and Brown, G. K. (1996). Beck depression inventory-II. San Antonio 78, 490–498.
Beckmann, M., Johansen-Berg, H., and Rushworth, M. F. S. (2009). Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J. Neurosci. 29, 1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Statist. Soc. Ser. B 57, 289–300.
Bennett, I. J., Howard, J. H., and Howard, D. V. (2007). Age-Related Differences in Implicit Learning of Subtle Third-Order Sequential Structure. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 62, 98–103. doi: 10.1093/geronb/62.2.P98
Brancaccio, A., Tabarelli, D., Bigica, M., and Baldauf, D. (2020). Cortical source localization of sleep-stage specific oscillatory activity. Sci. Rep. 10:6976. doi: 10.1038/s41598-020-63933-5
Brandt, J., Spencer, M., and Folstein, M. (1988). The Telephone Interview for Cognitive Status. Neuropsychiatry Neuropsychol. Behav. Neurol. 1, 111–117.
Bushnell, M. C., Duncan, G. H., Hofbauer, R. K., Ha, B., Chen, J.-I., and Carrier, B. (1999). Pain perception: Is there a role for primary somatosensory cortex? Proc. Natl. Acad. Sci. 96, 7705–7709. doi: 10.1073/pnas.96.14.7705
Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R., and Kupfer, D. J. (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213. doi: 10.1016/0165-1781(89)90047-4
Carrier, J., Land, S., Buysse, D. J., Kupfer, D. J., and Monk, T. H. (2001). The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20-60 years old). Psychophysiology 38, 232–242.
Carrier, J., Viens, I., Poirier, G., Robillard, R., Lafortune, M., Vandewalle, G., et al. (2011). Sleep slow wave changes during the middle years of life. Eur. J. Neurosci. 33, 758–766. doi: 10.1111/j.1460-9568.2010.07543.x
Cavanna, A. E., and Trimble, M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129, 564–583. doi: 10.1093/brain/awl004
Crowley, K., Trinder, J., Kim, Y., Carrington, M., and Colrain, I. M. (2002). The effects of normal aging on sleep spindle and K-complex production. Clin. Neurophysiol. 113, 1615–1622. doi: 10.1016/S1388-2457(02)00237-7
Curran, T. (1997). Effects of aging on implicit sequence learning: Accounting for sequence structure and explicit knowledge. Psychol. Res. 60, 24–41. doi: 10.1007/BF00419678
Dale, A. M., and Sereno, M. I. (1993). Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. J. Cognit. Neurosci. 5, 162–176. doi: 10.1162/jocn.1993.5.2.162
Dale, A. M., Fischl, B., and Sereno, M. I. (1999). Cortical Surface-Based Analysis: I. Segmentation and Surface Reconstruction. NeuroImage 9, 179–194. doi: 10.1006/nimg.1998.0395
Daselaar, S. M., Rombouts, S. A. R. B., Veltman, D. J., Raaijmakers, J. G. W., and Jonker, C. (2003). Similar network activated by young and old adults during the acquisition of a motor sequence. Neurobiol. Aging 24, 1013–1019. doi: 10.1016/S0197-4580(03)00030-7
Debas, K., Carrier, J., Orban, P., Barakat, M., Lungu, O., Vandewalle, G., et al. (2010). Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc. Natl. Acad. Sci. 107, 17839–17844. doi: 10.1073/pnas.1013176107
Delorme, A., and Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21.
Desikan, R. S., Ségonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021
Dijk, D.-J., Beersma, D. G. M., and van den Hoofdakker, R. H. (1989). All night spectral analysis of EEG sleep in young adult and middle-aged male subjects. Neurobiol. Aging 10, 677–682. doi: 10.1016/0197-4580(89)90004-3
Doyon, J., Korman, M., Morin, A., Dostie, V., Hadj Tahar, A., Benali, H., et al. (2009b). Contribution of night and day sleep vs. Simple passage of time to the consolidation of motor sequence and visuomotor adaptation learning. Exp. Brain Res. 195, 15–26. doi: 10.1007/s00221-009-1748-y
Doyon, J., Bellec, P., Amsel, R., Penhune, V., Monchi, O., Carrier, J., et al. (2009a). Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav. Brain Res. 199, 61–75. doi: 10.1016/j.bbr.2008.11.012
Dubé, J., Lafortune, M., Bedetti, C., Bouchard, M., Gagnon, J. F., Doyon, J., et al. (2015). Cortical thinning explains changes in sleep slow waves during adulthood. J. Neurosci. 35, 7795–7807. doi: 10.1523/JNEUROSCI.3956-14.2015
Engel, A. K., and Fries, P. (2010). Beta-band oscillations—Signalling the status quo? Curr. Opin. Neurobiol. 20, 156–165. doi: 10.1016/j.conb.2010.02.015
Feeney, J. J., Howard, J. H., and Howard, D. V. (2002). Implicit learning of higher order sequences in middle age. Psychol. Aging 17, 351–355.
Fernández, G. (2017). “The Medial Prefrontal Cortex is a Critical Hub in the Declarative Memory System,” in Cognitive Neuroscience of Memory Consolidation, eds N. Axmacher and B. Rasch (Berlin: Springer International Publishing), 45–56. doi: 10.1007/978-3-319-45066-7_3
Fischer, S., Hallschmid, M., Elsner, A. L., and Born, J. (2002). Sleep forms memory for finger skills. Proc. Natl. Acad. Sci. 99, 11987–11991. doi: 10.1073/pnas.182178199
Fischl, B., and Dale, A. M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. 97, 11050–11055. doi: 10.1073/pnas.200033797
Fischl, B., Liu, A., and Dale, A. M. (2001). Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Med. Imaging 20, 70–80.
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. doi: 10.1016/s0896-6273(02)00569-x
Fischl, B., Salat, D. H., Kouwe, A. J. W., van der, Makris, N., Ségonne, F., et al. (2004a). Sequence-independent segmentation of magnetic resonance images. NeuroImage 23, (Suppl. 1) S69–S84. doi: 10.1016/j.neuroimage.2004.07.016
Fischl, B., van der Kouwe, A., Destrieux, C., Halgren, E., Ségonne, F., Salat, D. H., et al. (2004b). Automatically Parcellating the Human Cerebral Cortex. Cereb. Cortex 14, 11–22. doi: 10.1093/cercor/bhg087
Fischl, B., Sereno, M. I., and Dale, A. (1999a). Cortical Surface-Based Analysis: II: Inflation, Flattening, and a Surface-Based Coordinate System. NeuroImage 9, 195–207.
Fischl, B., Sereno, M. I., Tootell, R. B. H., and Dale, A. M. (1999b). High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 8, 272–284.
Fitzroy, A. B., Kainec, K. A., Seo, J., and Spencer, R. M. C. (2021a). Encoding and consolidation of motor sequence learning in young and older adults. Neurobiol. Learn. Mem. 185:107508. doi: 10.1016/j.nlm.2021.107508
Fitzroy, A. B., Kainec, K. A., and Spencer, R. M. C. (2021b). Ageing-related changes in nap neuroscillatory activity are mediated and moderated by grey matter volume. Eur. J. Neurosci. 54, 7332–7354. doi: 10.1111/ejn.15468
Fogel, S., Albouy, G., Vien, C., Popovicci, R., King, B. R., Hoge, R., et al. (2014). FMRI and sleep correlates of the age-related impairment in motor memory consolidation. Hum. Brain Mapp. 35, 3625–3645. doi: 10.1002/hbm.22426
Fogel, S., Vien, C., Karni, A., Benali, H., Carrier, J., and Doyon, J. (2017). Sleep spindles: A physiological marker of age-related changes in gray matter in brain regions supporting motor skill memory consolidation. Neurobiol. Aging 49, 154–164. doi: 10.1016/j.neurobiolaging.2016.10.009
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Freeman, W. J. (2004). Origin, structure, and role of background EEG activity. Part 1. Analytic amplitude. Clin. Neurophysiol. 115, 2077–2088.
Gaab, N., Gabrieli, J. D. E., and Glover, G. H. (2006). Assessing the influence of scanner background noise on auditory processing. II. An fMRI study comparing auditory processing in the absence and presence of recorded scanner noise using a sparse design. Hum. Brain Mapp. 28, 721–732. doi: 10.1002/hbm.20299
Gaudreau, H., Carrier, J., and Montplaisir, J. (2001). Age-related modifications of NREM sleep EEG: From childhood to middle age. J. Sleep Res. 10, 165–172. doi: 10.1046/j.1365-2869.2001.00252.x
Gramfort, A., Luessi, M., Larson, E., Engemann, D., Strohmeier, D., Brodbeck, C., et al. (2014). MNE software for processing MEG and EEG data. NeuroImage 86, 446–460. doi: 10.1016/j.neuroimage.2013.10.027
Gramfort, A., Papadopoulo, T., Olivi, E., and Clerc, M. (2010). OpenMEEG: Opensource software for quasistatic bioelectromagnetics. BioMed. Engine. OnLine 9:45. doi: 10.1186/1475-925X-9-45
Grill-Spector, K., Kourtzi, Z., and Kanwisher, N. (2001). The lateral occipital complex and its role in object recognition. Vis. Res. 41, 1409–1422. doi: 10.1016/S0042-6989(01)00073-6
Hämäläinen, M. S., and Ilmoniemi, R. J. (1994). Interpreting magnetic fields of the brain: Minimum norm estimates. Med. Biol. Engine. Comput. 32, 35–42. doi: 10.1007/BF02512476
Han, X., Jovicich, J., Salat, D., van der Kouwe, A., Quinn, B., Czanner, S., et al. (2006). Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage 32, 180–194.
Hartogsveld, B., Bramson, B., Vijayakumar, S., van Campen, A. D., Marques, J. P., Roelofs, K., et al. (2018). Lateral frontal pole and relational processing: Activation patterns and connectivity profile. Behav. Brain Res. 355, 2–11. doi: 10.1016/j.bbr.2017.08.003
Hartwigsen, G., Neef, N. E., Camilleri, J. A., Margulies, D. S., and Eickhoff, S. B. (2019). Functional Segregation of the Right Inferior Frontal Gyrus: Evidence From Coactivation-Based Parcellation. Cereb. Cortex 29, 1532–1546. doi: 10.1093/cercor/bhy049
Herlin, B., Navarro, V., and Dupont, S. (2021). The temporal pole: From anatomy to function—A literature appraisal. J. Chem. Neuroanat. 113:101925. doi: 10.1016/j.jchemneu.2021.101925
Hikosaka, O., Nakahara, H., Rand, M. K., Sakai, K., Lu, X., Nakamura, K., et al. (1999). Parallel neural networks for learning sequential procedures. Trends Neurosci. 22, 464–471. doi: 10.1016/S0166-2236(99)01439-3
Horne, J. A., and Ostberg, O. (1976). A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 4, 97–110.
Howard, D. V., and Howard, J. H. (2001). When it does hurt to try: Adult age differences in the effects of instructions on implicit pattern learning. Psychonomic Bull. Rev. 8, 798–805. doi: 10.3758/bf03196220
Huber, R., Felice Ghilardi, M., Massimini, M., and Tononi, G. (2004). Local sleep and learning. Nature 430, 78–81. doi: 10.1038/nature02663
Iber, C., Ancoli-Israel, S., Chesson, A. L., and Quan, S. F. (2007). The AASM manual for the scoring of sleep and associated events: Rules, terminology, and technical specifications. Westchester, IL: American Academy of Sleep Medicine.
Imai, K., Keele, L., and Tingley, D. (2010). A general approach to causal mediation analysis. Psychol. Methods 15, 309–334. doi: 10.1037/a0020761
Japee, S., Holiday, K., Satyshur, M. D., Mukai, I., and Ungerleider, L. G. (2015). A role of right middle frontal gyrus in reorienting of attention: A case study. Front. Syst. Neurosci. 9:23. doi: 10.3389/fnsys.2015.00023
Johns, M. W. (1991). A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep 14, 540–545. doi: 10.1093/sleep/14.6.540
Jovicich, J., Czanner, S., Greve, D., Haley, E., Kouwe, A., van der, et al. (2006). Reliability in multi-site structural MRI studies: Effects of gradient non-linearity correction on phantom and human data. NeuroImage 30, 436–443. doi: 10.1016/j.neuroimage.2005.09.046
King, B. R., Fogel, S. M., Albouy, G., and Doyon, J. (2013). Neural correlates of the age-related changes in motor sequence learning and motor adaptation in older adults. Front. Hum. Neurosci. 7:142. doi: 10.3389/fnhum.2013.00142
King, B. R., Saucier, P., Albouy, G., Fogel, S. M., Rumpf, J.-J., Klann, J., et al. (2017b). Cerebral Activation During Initial Motor Learning Forecasts Subsequent Sleep-Facilitated Memory Consolidation in Older Adults. Cereb. Cortex 27, 1588–1601. doi: 10.1093/cercor/bhv347
King, B. R., Hoedlmoser, K., Hirschauer, F., Dolfen, N., and Albouy, G. (2017a). Sleeping on the motor engram: The multifaceted nature of sleep-related motor memory consolidation. Neurosci. Biobehav. Rev. 80, 1–22. doi: 10.1016/j.neubiorev.2017.04.026
Kong, L., Herold, C. J., Zöllner, F., Salat, D. H., Lässer, M. M., Schmid, L. A., et al. (2015). Comparison of grey matter volume and thickness for analysing cortical changes in chronic schizophrenia: A matter of surface area, grey/white matter intensity contrast, and curvature. Psychiatry Res. Neuroimaging 231, 176–183. doi: 10.1016/j.pscychresns.2014.12.004
Korman, M., Doyon, J., Doljansky, J., Carrier, J., Dagan, Y., and Karni, A. (2007). Daytime sleep condenses the time course of motor memory consolidation. Nat. Neurosci. 10, 1206–1213. doi: 10.1038/nn1959
Kuperberg, G. R., Broome, M., McGuire, P. K., David, A. S., Eddy, M., Ozawa, F., et al. (2003). Regionally localized thinning of the cerebral cortex in Schizophrenia. Arch. General Psychiatry 60, 878–888.
Landolt, H.-P., and Borbély, A. A. (2001). Age-dependent changes in sleep EEG topography. Clin. Neurophysiol. 112, 369–377. doi: 10.1016/S1388-2457(00)00542-3
Landolt, H.-P., Dijk, D.-J., Achermann, P., and Borbély, A. A. (1996). Effect of age on the sleep EEG: Slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 738, 205–212. doi: 10.1016/S0006-8993(96)00770-6
Latreille, V., Gaubert, M., Dubé, J., Lina, J.-M., Gagnon, J.-F., and Carrier, J. (2019). Age-related cortical signatures of human sleep electroencephalography. Neurobiol. Aging 76, 106–114. doi: 10.1016/j.neurobiolaging.2018.12.012
Li, W., Qin, W., Liu, H., Fan, L., Wang, J., Jiang, T., et al. (2013). Subregions of the human superior frontal gyrus and their connections. NeuroImage 78, 46–58. doi: 10.1016/j.neuroimage.2013.04.011
Lin, F., Belliveau, J. W., Dale, A. M., and Hämäläinen, M. S. (2006). Distributed current estimates using cortical orientation constraints. Hum. Brain Mapp. 27, 1–13. doi: 10.1002/hbm.20155
Lopez-Calderon, J., and Luck, S. J. (2014). ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front. Hum. Neurosci. 8:213. doi: 10.3389/fnhum.2014.00213
Luck, S. J. (2014). An Introduction to the Event-Related Potential Technique (Second). Cambridge, MA: MIT Press.
MacKinnon, D. P., Krull, J. L., and Lockwood, C. M. (2000). Equivalence of the mediation, confounding and suppression effect. Prevent. Sci. 1, 173–181. doi: 10.1023/a:1026595011371
Maldjian, J. A., Laurienti, P. J., Kraft, R. A., and Burdette, J. H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239. doi: 10.1016/S1053-8119(03)00169-1
Mander, B. A., Rao, V., Lu, B., Saletin, J. M., Ancoli-Israel, S., Jagust, W. J., et al. (2014). Impaired prefrontal sleep spindle regulation of hippocampal-dependent learning in older adults. Cereb. Cortex 24, 3301–3309. doi: 10.1093/cercor/bht188
Mander, B. A., Rao, V., Lu, B., Saletin, J. M., Lindquist, J. R., Ancoli-Israel, S., et al. (2013). Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat. Neurosci. 16, 357–364. doi: 10.1038/nn.3324
Martin, N., Lafortune, M., Godbout, J., Barakat, M., Robillard, R., Poirier, G., et al. (2013). Topography of age-related changes in sleep spindles. Neurobiol. Aging 34, 468–476. doi: 10.1016/j.neurobiolaging.2012.05.020
Michalski, L. J., Demers, C. H., Baranger, D. A. A., Barch, D. M., Harms, M. P., Burgess, G. C., et al. (2017). Perceived stress is associated with increased rostral middle frontal gyrus cortical thickness: A family-based and discordant-sibling investigation. Genes Brain Behav. 16, 781–789. doi: 10.1111/gbb.12404
Mitchell, A. J. (2009). A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J. Psychiatric Res. 43, 411–431. doi: 10.1016/j.jpsychires.2008.04.014
Morrell, M. J., Finn, L., Kim, H., Peppard, P. E., Safwan Badr, M., and Young, T. (2012). Sleep Fragmentation, Awake Blood Pressure, and Sleep-Disordered Breathing in a Population-based Study. Am. J. Respirat. Crit. Care Med. 2012:9904008. doi: 10.1164/ajrccm.162.6.9904008
Münch, M., Knoblauch, V., Blatter, K., Schröder, C., Schnitzler, C., Kräuchi, K., et al. (2004). The frontal predominance in human EEG delta activity after sleep loss decreases with age. Eur. J. Neurosci. 20, 1402–1410. doi: 10.1111/j.1460-9568.2004.03580.x
Murphy, M., Huber, R., Esser, S., Riedner, B. A., Massimini, M., Ferrarelli, F., et al. (2011). The cortical topography of local sleep. Curr. Top. Med. Chem. 11, 2438–2446. doi: 10.2174/156802611797470303
Navarro Schröder, T., Haak, K. V., Zaragoza Jimenez, N. I., Beckmann, C. F., and Doeller, C. F. (2015). Functional topography of the human entorhinal cortex. ELife 4:e06738. doi: 10.7554/eLife.06738
Nicolas, A., Petit, D., Rompré, S., and Montplaisir, J. (2001). Sleep spindle characteristics in healthy subjects of different age groups. Clin. Neurophysiol. 112, 521–527. doi: 10.1016/S1388-2457(00)00556-3
Nielsen, F. Å, Balslev, D., and Hansen, L. K. (2005). Mining the posterior cingulate: Segregation between memory and pain components. NeuroImage 27, 520–532. doi: 10.1016/j.neuroimage.2005.04.034
Nieuwenhuys, R., Voogd, J., and van Huijzen, C. (eds) (2008). “Telencephalon: Neocortex,” in The Human Central Nervous System, (Berlin: Springer), 491–679. doi: 10.1007/978-3-540-34686-9_15
Nishida, M., and Walker, M. P. (2007). Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One 2:e341. doi: 10.1371/journal.pone.0000341
Nogueira, R., Abolafia, J. M., Drugowitsch, J., Balaguer-Ballester, E., Sanchez-Vives, M. V., and Moreno-Bote, R. (2017). Lateral orbitofrontal cortex anticipates choices and integrates prior with current information. Nat. Commun. 8:14823. doi: 10.1038/ncomms14823
Numssen, O., Bzdok, D., and Hartwigsen, G. (2021). Functional specialization within the inferior parietal lobes across cognitive domains. ELife 10:e63591. doi: 10.7554/eLife.63591
Oostenveld, R., Fries, P., Maris, E., and Schoffelen, J. M. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computat. Intellig. Neurosci. 2011:156869. doi: 10.1155/2011/156869
Panizzon, M. S., Fennema-Notestine, C., Eyler, L. T., Jernigan, T. L., Prom-Wormley, E., Neale, M., et al. (2009). Distinct Genetic Influences on Cortical Surface Area and Cortical Thickness. Cereb. Cortex 19, 2728–2735. doi: 10.1093/cercor/bhp026
Penhune, V. B., and Steele, C. J. (2012). Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav. Brain Res. 226, 579–591. doi: 10.1016/j.bbr.2011.09.044
Peters, K. R., Ray, L. B., Fogel, S., Smith, V., and Smith, C. T. (2014). Age differences in the variability and distribution of sleep spindle and rapid eye movement densities. PLoS One 9:e91047. doi: 10.1371/journal.pone.0091047
Reuter, M., Rosas, H. D., and Fischl, B. (2010). Highly Accurate Inverse Consistent Registration: A Robust Approach. NeuroImage 53, 1181–1196. doi: 10.1016/j.neuroimage.2010.07.020
Reuter, M., Schmansky, N. J., Rosas, H. D., and Fischl, B. (2012). Within-Subject Template Estimation for Unbiased Longitudinal Image Analysis. NeuroImage 61, 1402–1418. doi: 10.1016/j.neuroimage.2012.02.084
Rieckmann, A., and Bäckman, L. (2009). Implicit learning in aging: Extant patterns and new directions. Neuropsychol. Rev. 19, 490–503. doi: 10.1007/s11065-009-9117-y
Rieckmann, A., Fischer, H., and Bäckman, L. (2010). Activation in striatum and medial temporal lobe during sequence learning in younger and older adults: Relations to performance. NeuroImage 50, 1303–1312. doi: 10.1016/j.neuroimage.2010.01.015
Robillard, R., Massicotte-Marquez, J., Kawinska, A., Paquet, J., Frenette, S., and Carrier, J. (2010). Topography of homeostatic sleep pressure dissipation across the night in young and middle-aged men and women. J. Sleep Res. 19, 455–465. doi: 10.1111/j.1365-2869.2010.00820.x
Rosas, H. D., Liu, A. K., Hersch, S., Glessner, M., Ferrante, R. J., Salat, D. H., et al. (2002). Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology 58, 695–701.
Salat, D., Buckner, R. L., Snyder, A. Z., Greve, D. N., Desikan, R. S., Busa, E., et al. (2004). Thinning of the cerebral cortex in aging. Cereb. Cortex 14, 721–730.
Saletin, J. M., and Greer, S. (2015). Hume Software Package (previously sleepSMG): Open-Source MATLAB User Interface for Scoring Sleep [MATLAB]. San Francisco, CA: GitHub.
Saletin, J. M., van der Helm, E., and Walker, M. P. (2013). Structural brain correlates of human sleep oscillations. NeuroImage 83, 658–668. doi: 10.1016/j.neuroimage.2013.06.021
Sazgar, M., and Young, M. G. (2019). “Normal EEG Awake and Sleep,” in Absolute Epilepsy and EEG Rotation Review: Essentials for Trainees, eds M. Sazgar and M. G. Young (Berlin: Springer International Publishing), 127–139. doi: 10.1007/978-3-030-03511-2_6
Schwarz, J. F. A., Åkerstedt, T., Lindberg, E., Gruber, G., Fischer, H., and Theorell-Haglöw, J. (2017). Age affects sleep microstructure more than sleep macrostructure. J. Sleep Res. 26, 277–287. doi: 10.1111/jsr.12478
Segonne, F., Dale, A. M., Busa, E., Glessner, M., Salat, D., Hahn, H. K., et al. (2004). A hybrid approach to the skull stripping problem in MRI. NeuroImage 22, 1060–1075. doi: 10.1016/j.neuroimage.2004.03.032
Segonne, F., Pacheco, J., and Fischl, B. (2007). Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans. Med. Imaging 26, 518–529.
Shahid, A., Wilkinson, K., Marcu, S., and Shapiro, C. M. (2012). “Stanford Sleepiness Scale (SSS),” in STOP, THAT and One Hundred Other Sleep Scales, eds A. Shahid, K. Wilkinson, S. Marcu, and C. M. Shapiro (Berlin: Springer), 369–370. doi: 10.1007/978-1-4419-9893-4_91
Sled, J. G., Zijdenbos, A. P., and Evans, A. C. (1998). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging 17, 87–97.
Sprecher, K. E., Riedner, B. A., Smith, R. F., Tononi, G., Davidson, R. J., and Benca, R. M. (2016). High resolution topography of age-related changes in non-rapid eye movement sleep electroencephalography. PLoS One 11:e0149770. doi: 10.1371/journal.pone.0149770
Stevens, F. L., Hurley, R. A., Taber, K. H., Hurley, R. A., Hayman, L. A., and Taber, K. H. (2011). Anterior Cingulate Cortex: Unique Role in Cognition and Emotion. J. Neuropsych. Clin. Neurosciences 23, 121–125. doi: 10.1176/jnp.23.2.jnp121
Tadel, F., Baillet, S., Mosher, J. C., Pantazis, D., and Leahy, R. M. (2011). Brainstorm: A User-Friendly Application for MEG/EEG Analysis. Computat. Intellig. Neurosci. 2011:879716. doi: 10.1155/2011/879716
Tadel, F., Bock, E., Niso, G., Mosher, J. C., Cousineau, M., Pantazis, D., et al. (2019). MEG/EEG Group Analysis With Brainstorm. Front. Neurosci. 13:76. doi: 10.3389/fnins.2019.00076
Tamaki, M., Huang, T.-R., Yotsumoto, Y., Hämäläinen, M., Lin, F.-H., Náñez, J. E., et al. (2013). Enhanced spontaneous oscillations in the supplementary motor area are associated with sleep-dependent offline learning of finger-tapping motor-sequence task. J. Neurosci. 33, 13894–13902. doi: 10.1523/JNEUROSCI.1198-13.2013
Tingley, D., Yamamoto, T., Hirose, K., Keele, L., and Imai, K. (2014). mediation: R package for causal mediation analysis. J. Statist. Softw. 59:i05. doi: 10.18637/jss.v059.i05
Tsuno, N., Shigeta, M., Hyoki, K., Kinoshita, T., Ushijima, S., Faber, P. L., et al. (2002). Spatial organization of EEG activity from alertness to sleep stage 2 in old and younger subjects. J. Sleep Res. 11, 43–51. doi: 10.1046/j.1365-2869.2002.00288.x
Tucker, M. A., and Fishbein, W. (2009). The impact of sleep duration and subject intelligence on declarative and motor memory performance: How much is enough? J. Sleep Res. 18, 304–312. doi: 10.1111/j.1365-2869.2009.00740.x
Tyler, L. K., Marslen-Wilson, W. D., Randall, B., Wright, P., Devereux, B. J., Zhuang, J., et al. (2011). Left inferior frontal cortex and syntax: Function, structure and behaviour in patients with left hemisphere damage. Brain Neurol. 134, 415–431. doi: 10.1093/brain/awq369
Vahdat, S., Fogel, S., Benali, H., and Doyon, J. (2017). Network-wide reorganization of procedural memory during NREM sleep revealed by fMRI. ELife 6:e24987. doi: 10.7554/eLife.24987
van den Heuvel, M. P., and Sporns, O. (2013). Network hubs in the human brain. Trends Cognit. Sci. 17, 683–696. doi: 10.1016/j.tics.2013.09.012
Varga, A. W., Ducca, E. L., Kishi, A., Fischer, E., Parekh, A., Koushyk, V., et al. (2016). Effects of Aging on Slow Wave Sleep Dynamics and Human Spatial Navigational Memory Consolidation. Neurobiol. Aging 42, 142–149. doi: 10.1016/j.neurobiolaging.2016.03.008
Walker, M. P., Brakefield, T., Morgan, A., Hobson, J. A., and Stickgold, R. (2002). Practice with Sleep Makes Perfect: Sleep-Dependent Motor Skill Learning. Neuron 35, 205–211. doi: 10.1016/S0896-6273(02)00746-8
Wang, J., Zhou, T., Qiu, M., Du, A., Cai, K., Wang, Z., et al. (1999). Relationship between ventral stream for object vision and dorsal stream for spatial vision: An fMRI+ERP study. Hum. Brain Mapp. 8, 170–181.
Keywords: aging, sleep, EEG, source estimation, MNE, cortical thickness, motor sequence learning, fMRI
Citation: Fitzroy AB, Jones BJ, Kainec KA, Seo J and Spencer RMC (2022) Aging-Related Changes in Cortical Sources of Sleep Oscillatory Neural Activity Following Motor Learning Reflect Contributions of Cortical Thickness and Pre-sleep Functional Activity. Front. Aging Neurosci. 13:787654. doi: 10.3389/fnagi.2021.787654
Received: 01 October 2021; Accepted: 25 November 2021;
Published: 11 January 2022.
Edited by:
Alison Mary, Université Libre de Bruxelles, BelgiumReviewed by:
Giulio Bernardi, IMT School for Advanced Studies Lucca, ItalyCopyright © 2022 Fitzroy, Jones, Kainec, Seo and Spencer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rebecca M. C. Spencer, cnNwZW5jZXJAdW1hc3MuZWR1
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.