- Department of Physiology, Keio University School of Medicine, Tokyo, Japan
Alzheimer’s disease (AD) is an aging-dependent neurodegenerative disease that impairs cognitive function. Although the main pathologies of AD are the aggregation of amyloid-beta (Aβ) and phosphorylated Tau protein, the mechanisms that lead to these pathologies and their effects are believed to be heterogeneous among patients. Many epidemiological studies have suggested that sex is involved in disease prevalence and progression. The reduction of sex hormones contributes to the pathogenesis of AD, especially in females, suggesting that the supplementation of sex hormones could be a therapeutic intervention for AD. However, interventional studies have revealed that hormone therapy is beneficial under limited conditions in certain populations with specific administration methods. Thus, this suggests the importance of identifying crucial factors that determine hormonal effects in patients with AD. Based on these factors, it is necessary to decide which patients will receive the intervention before starting it. However, the long observational period and many uncontrollable environmental factors in clinical trials made it difficult to identify such factors, except for the APOE ε4 allele. Induced pluripotent stem cells (iPSCs) derived from patients can differentiate into neurons and recapitulate some aspects of AD pathogenesis. This in vitro model allows us to control non-cell autonomous factors, including the amount of Aβ aggregates and sex hormones. Hence, iPSCs provide opportunities to investigate sex-dependent pathogenesis and predict a suitable population for clinical trials of hormone treatment.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease associated with cognitive decline over time and has the highest prevalence compared to other types of dementia worldwide (Mayeux and Stern, 2012; Alzheimer’s Association, 2021). Although several clinical trials targeting the main components of AD neuropathology, such as amyloid-beta (Aβ) peptide and phosphorylated Tau (pTau) protein have failed to achieve good outcomes (Mehta et al., 2017; Mullane and Williams, 2018; Yiannopoulou et al., 2019), it is believed that the heterogeneous nature of the disease mechanism among patients with AD determines the response to the drug (Devi and Scheltens, 2018). Even in the only successful clinical trial of aducanumab, the heterogeneity of disease progression is reported to be the reason that in one cohort, they did not show statistically significant drug effects (Knopman et al., 2021). Sex has been reported to affect the pathogenesis of neurodegenerative diseases, such as Parkinson’s disease (PD) (Wooten et al., 2004) and AD (Dubal, 2020). Females have a higher risk of developing AD than males, while males have a higher risk of developing PD. However, male patients with AD have a higher prevalence of mild cognitive impairment (MCI) and faster progression to AD, which is associated with a higher mortality rate than females (Barnes et al., 2003; Mielke, 2018; Strand et al., 2018; Davis et al., 2020; Dubal, 2020). A recent study suggested that higher expression of KDM6A, which is the escape of X chromosome inactivation, contributes to greater resilience to AD in females (Davis et al., 2020). However, this mechanism of cell-autonomous effects in AD is different from that in PD. The expression level of the SRY gene on the Y chromosome regulates the response to pathogens related to PD (Lee et al., 2019). These sex differences may make it difficult to interpret the negative results of clinical trials for AD.
In terms of non-cell-autonomous causes, since most patients with AD are often late-onset, when the level of sex steroid hormones is decreased in both males (testosterone) and females (estrogen and progesterone), sex steroid hormones are suggested to contribute to the pathogenesis of the disease. Therefore, hormone replacement therapy (HRT), which is often used in patients with decreased hormone levels, has also been suggested as a potential intervention for patients with AD. However, many previous randomized clinical trials (RCTs) on the effects of sex hormones on cognitive function and AD development showed varying results between cohorts/studies. In addition, the discrepancy in the results between clinical and preclinical trials was also an issue. In particular, because mice maintain hormone levels throughout their life span, mouse models of AD have some limitations in studying sex differences and hormonal effects on AD pathogenesis (Dubal et al., 2012). In addition, Aβ aggregates have been reported to induce apoptotic changes in human neurons but not in mouse neurons (Espuny-Camacho et al., 2017). These results indicate the need for developing a robust AD model of human cells capable of recapitulating both autonomous and non-autonomous effects, especially for the investigation of sex effects on AD. This perspective aimed to summarize the results and problems of previous clinical studies using sex hormones for AD patients and discuss the potential of next generation in vitro AD models, especially induced pluripotent stem cells (iPSCs), together with our preliminary results using iPSC-derived neurons.
Past Clinical Trials Investigating Sex Steroid Hormone Therapy Effects on Alzheimer’s Disease
The neuropathology of AD usually starts developing over 10 − 20 years before the onset of symptoms and progresses with age. Therefore, it is suspected that aging factors, including the reduction of sex hormone levels, contribute to AD development. Many epidemiological studies have suggested that AD has a high prevalence in menopausal elderly females, whose levels of sex hormones, including estrogens and progesterone, have decreased compared with levels at reproductive age (Santoro and Sutton-Tyrrell, 2011). Likewise, biologically active testosterone decreases with age in males. This decrease may also contribute to AD development in males (Moffat, 2005; Lv et al., 2016). In AD mouse models that had undergone gonadectomy, it was also found that treatment with sex steroid hormones could slow the progression of Aβ accumulation, decrease Tau hyperphosphorylation, and improve working memory (Yue et al., 2005; Carroll et al., 2007). Many clinical trials have attempted to reveal the beneficial effects of sex hormones on AD, mainly estrogen and progesterone, in menopausal females with AD. However, clinical studies have shown mixed results, and the association between sex hormones and AD remains elusive.
Observational Studies of Hormone Replacement Therapy in Alzheimer’s Disease
Early observational studies showed a compelling beneficial effect of estrogen on AD in cognitive tests and positron emission tomography scans using bolus injected [15O] water. Honjo et al. (1989) treated female patients with AD with oral conjugated estrogen (estrone sulfate as the main component) and suggested an improvement in cognitive function after 6-weeks of treatment (Honjo et al., 1989). Henderson et al. (1996) compared female patients with AD who received estrogen replacement therapy with female and male patients with AD who did not receive estrogen replacement therapy. They showed that female patients with AD who received estrogen showed significantly better performance on cognitive function tests than female and male patients who did not receive estrogen (Henderson et al., 1996). In addition, Resnick et al. (1997) showed that female patients who received HRT had better cognitive function tests, especially those related to visual memory (Resnick et al., 1997). A subsequent study also showed that HRT users had better regional cerebral blood flow (rCBF) in the right parahippocampal gyrus, right precuneus, and right frontal regions during memory tasks, suggesting better activation of the brain areas that are severely affected in patients with AD (Resnick et al., 1998).
Randomized Clinical Trials of Hormone Replacement Therapy in Alzheimer’s Disease
Based on the positive results of the observational studies, RCTs were launched. However, the RCTs showed various results, including positive, partially positive, and negative effects of HRT (Table 1). Some of the first RCTs indicated that HRT had no effect on AD or adversely increased the risk of developing AD, contrary to expectations. Henderson et al. (2000) showed that the treatment of conjugated equine estrogens (CEE) for 4 weeks in postmenopausal female patients with mild to moderate AD did not result in significant improvement in cognitive function compared to the placebo group (Henderson et al., 2000). Mulnard et al. (2000) treated menopausal female patients with AD with high and low doses of CEE for 1 year, a longer period than previous studies, and found that CEE did not affect attenuating AD (Mulnard et al., 2000). One large clinical cohort, the Women’s Health Initiative Memory Study that included more than 4,000 menopausal female patients who were receiving CEE and/or medroxyprogesterone acetate for more than 4 years, also revealed negative or even adverse effects of hormone treatment. Treatment with estrogen and progesterone has been reported to have adverse effects that may promote the development of AD. Thus, the opposed estrogen therapy, which is a form of estrogen therapy supplemented with progesterone to prevent the adverse effects of excessive estrogen alone, such as enhancing the risk of endometrial cancer development, was less beneficial in preventing the development of AD (Shumaker et al., 2003; Craig et al., 2005).
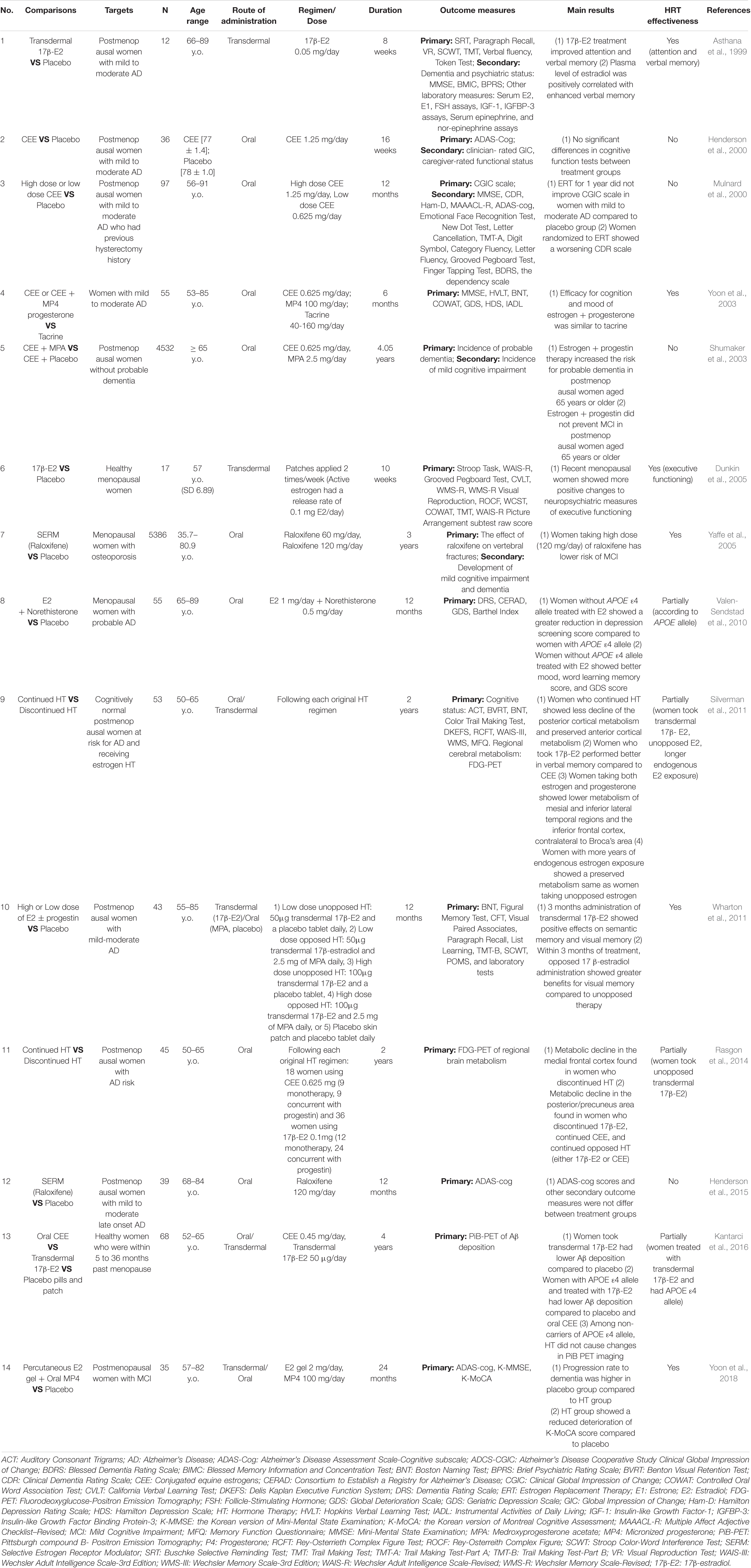
Table 1. Summary of selected randomized clinical trials (RCTs) of the hormone replacement therapy (HRT) effects on cognitive function among postmenopausal women of AD or non-AD group.
Due to the negative results of RCTs during the early 2000s, the following RCTs focused more on the stratification of patient characteristics (e.g., age, cognitive status, APOE genotype, duration after menopause), along with the formulation of drugs and the route of administration (e.g., oral CEE, transdermal 17β-estradiol). These RCTs were finally able to assert the beneficial effects of estrogen in human clinical trials. Valen-Sendstad et al. (2010) showed that females without the APOE ε4 allele (a major genetic risk for AD) received estradiol and norethisterone, they had better mood and depression than females with the APOE ε4 allele (Valen-Sendstad et al., 2010). Silverman et al. (2011) compared the continued and discontinued HRT effects among cognitively normal postmenopausal females with a family history of AD and showed the following results: (i) 17β-estradiol had a better effect than CEE; (ii) unopposed estrogen therapy preserved more cortical metabolism compared to the opposed one; and (iii) females with longer endogenous estrogen exposure showed relatively preserved metabolism of specific brain areas more than females with shorter endogenous estrogen exposure (Silverman et al., 2011). Moreover, the Kronos Early Estrogen Prevention study, which included only recent postmenopausal females, concluded that 17β-estradiol could lower Aβ deposition, especially in those with APOE ε4 among the recent menopausal female population (Kantarci et al., 2016). In addition to conventional transdermal 17β-estradiol or oral CEE, selective estrogen receptor modulators as another form of medication are also being investigated for their effects on improving cognitive function. A study by Wang et al. (2020) showed that PhytoSERM [an estrogen receptor beta (ERβ) modulator comprised of genistein, daidzein, and S-equol] improved cognitive function, especially for verbal learning and executive function (Wang et al., 2020).
Similar to estrogen and progesterone, testosterone levels also decrease with age, and low testosterone levels contribute to cognitive decline in elderly males (Moffat, 2005; Lv et al., 2016). Although only a few RCTs have evaluated the effect of testosterone on cognitive function, studies of short-term testosterone replacement therapy in elderly patients with MCI or AD found improvement in cognitive function after treatment, especially for spatial and verbal memory (Tan and Pu, 2003; Cherrier et al., 2005), while long-term therapy of testosterone showed only modest or no significant improvement (Asih et al., 2015; Huang et al., 2016; Wahjoepramono et al., 2016). Meanwhile, Huang et al. (2015) reported no significant cognitive improvement after testosterone administration, even among females with low testosterone levels (Huang et al., 2015).
Next Generation in vitro Models of AD and Future Utilization for the Study of Sex Difference
Alzheimer’s Disease Modeling Using Induced Pluripotent Stem Cells
After the establishment of iPSCs technology by the group of Shinya Yamanaka (Takahashi and Yamanaka, 2006; Takahashi et al., 2007), human cell models of many diseases, including neurological disorders, have been generated using iPSCs (Okano and Yamanaka, 2014; reviewed in Imaizumi and Okano, 2014; Rowe and Daley, 2019; Sharma et al., 2020). For AD, iPSCs were first established from fibroblasts obtained from patients with familial Alzheimer’s disease (fAD) with mutations in PS1 (A246E) and PS2 (N141l) (Yagi et al., 2011), and the iPSCs were differentiated into neurons. These neurons induced by iPSCs recapitulated the phenotypes of AD, such as increased Aβ42 levels and responded to AD targeting drugs, such as γ-secretase inhibitors and modulators. The following models of fAD with different mutations also showed similar phenotypes (reviewed in Zhang et al., 2016). In addition to the models of familial AD cases, a sporadic AD model of iPSC-derived neurons was established in 2012, in which increased Aβ levels, pTau protein, and other AD phenotypes were observed (Israel et al., 2012). Recent studies using iPSCs focused on sAD risk genes that increase the risk of developing AD, such as SORL1 (Young et al., 2015) and APOE. However, since the comparison of iPSCs from mutation carrier and non-carrier cannot exclude the effects of other SNPs that are different between the mutation carrier and non-carrier, using genome-editing technology like CRISPR/Cas9 allows us to introduce only the mutation of interest and examine the effects. Knupp et al. (2020) generated the SORL1 knockout iPSCs using CRISPR/Cas9 and could conclude that the loss of SORL1 induced early endosome enlargement (Knupp et al., 2020). Furthermore, another study utilized CRISPR/Cas9 to establish APOE ε3/ε3 and APOE ε4/ε4 hiPSC lines that have equal genomic background except for the APOE gene. Then, they investigated functions of APOE genotypes on diverse cell types including neurons, astrocytes, and microglia-like cells (Lin et al., 2018). Since the AD models established from iPSCs can reflect the phenotypes of the disease and respond to drugs, this tool can be used to investigate both cell-autonomous and non-cell-autonomous effects arising from sex differences within the AD population.
Induced Pluripotent Stem Cell-Derived Neuronal Models Recapitulated Alzheimer’s Disease Pathology and Responded to Estradiol Treatment
In our analyses of the neurons differentiated from human iPSCs of healthy female donor (1210B2 line) and female donor of fAD (APP V717L line) (Figure 1A) (Kondo et al., 2013; Nakagawa et al., 2014), secreted Aβ peptides were measured by enzyme-linked immunosorbent assay. Neurons from fAD donor increased Aβ42/40, indicating that iPSC-derived neurons recapitulated the disease pathophysiology of AD (Figure 1B) (Jack et al., 2013; Lee et al., 2020). Furthermore, iPSC-derived neurons of the fAD donor increased the frequency of Ca imaging using Fluo-8 compared to healthy control (Figure 1C). This finding was reminiscent of the finding of Zott et al. (2019) that neuronal hyperactivation in AD mouse models is caused by Aβ-induced accumulation of perisynaptic glutamate (Zott et al., 2019). These neurons derived from iPSCs also responded to short-time exposure to 17β-estradiol. Exposure to 17β-estradiol for 15 min increased the Ca oscillation (Figure 1D). Previously, Zhang et al. (2010) also performed Ca imaging on human and mouse embryonic stem cell (ESC)-derived neurons after treatment with 17β-estradiol and showed increased neuronal firing (Zhang et al., 2010). Increased activation of neurons after acute treatment with estradiol is suggested to be mediated by the stimulation of L-type Ca2+ channels, which activate the MAPK/ERK pathway and promote the firing of neurons as part of the rapid signaling cascade (Sheldahl et al., 2008; Vega-Vela et al., 2017; Albert-Gascó et al., 2020). Shum et al. (2015) also treated iPSC-derived neurons from healthy donors with 17β-estradiol for 24 h and showed increased dendritic branching (Shum et al., 2015), suggesting promotion of neuronal microstructure by estrogen in the iPSC-derived models. The increased neuronal branching may explain the preserved cognitive function by HRT, as shown in previous RCTs. Further experiments such as the treatment of 17β-estradiol before/after Aβ treatment in iPSC-derived neurons will tell us whether 17β-estradiol protects neurons against oxidative stressor. Another study differentiated neurons from the iPSCs of the autism individual revealed an increased expression of androgen receptor and brain-derived neurotrophic factor after testosterone treatment, suggesting that the iPSC-derived neurons also respond to testosterone treatment (Adhya et al., 2018). These evidence suggest that the in vitro model using iPSCs responds to sex steroid hormone and has potential for future study of sex differences, including the effects of hormone therapy, among AD patients.
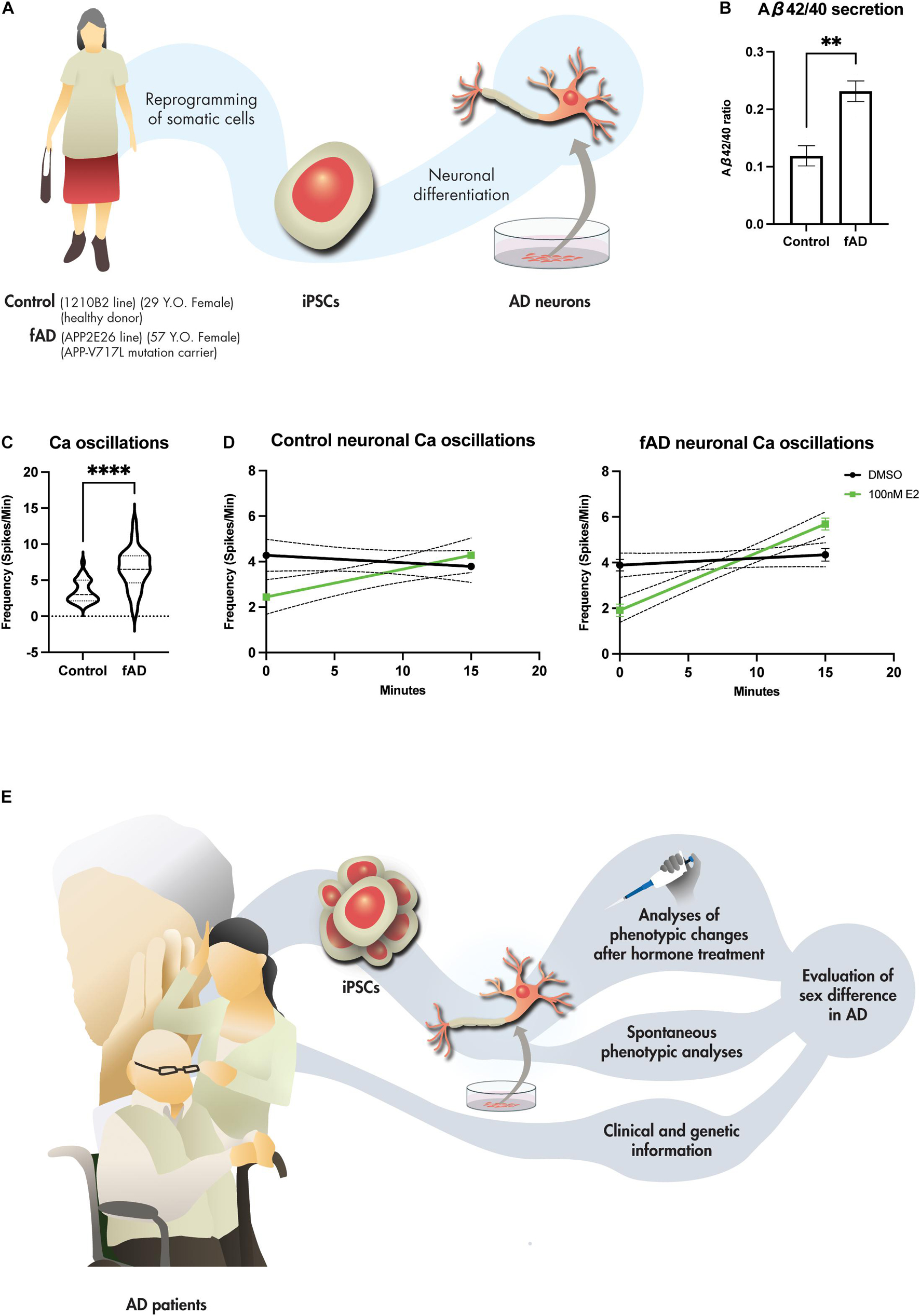
Figure 1. iPSC-derived neurons for the future study of sex difference in AD. (A) Generation of iPSC-derived neurons from female healthy (1210B2 line) and fAD (APP2E26 line) donors. (B) Aβ42/40 ratio of the iPSC-derived neurons of healthy (n = 4) and fAD (n = 4) donors measured at 45 div. Bars, mean ± SEM. **p < 0.01 (unpaired t-test). (C) Neuronal hyperactivation seen in fAD-derived neurons measured by Ca imaging using Fluo-8 indicator after 45 div (n = 32 versus 32). ****p < 0.001 (Mann Whitney test). (D) 17β-estradiol (E2) responses of iPSC-derived neurons measured by Ca imaging using Fluo-8 indicator at 45 div of 1210B2 line and APP2E26 line before (0 min) and after (15 min) treatment with 100 nM E2 (n = 45 versus 45). p = 0.3331 (1210B2, DMSO); p = 0.0010 (1210B2, 100 nM E2); p = 0.2289 (APP2E26, DMSO); p < 0.0001 (APP2E26, 100 nM E2) (simple linear regression). (E) Stratification and investigation of the hormone therapy effects in AD utilizing iPSC-derived neuronal models.
Advantages of Induced Pluripotent Stem Cell-Derived In vitro Models for the Study of Neurodegenerative Diseases
Induced pluripotent stem cells (iPSCs) can hold the genetic information of the donors, thus allowing in vitro study of diseases with the donor’s genetic background (Stadtfeld and Hochedlinger, 2010). Non-cell-autonomous factors, such as the exposure time to hormones and the amount of Aβ aggregates, can also be controlled. Moreover, the iPSC-derived disease model can recapitulate the phenotypes of individual patients and can be used for disease stratification and drug screening of incurable neurodegenerative diseases. Previously, stratification and drug screening for sporadic disease were performed in amyotrophic lateral sclerosis (ALS). Motor neurons differentiated from patients with ALS-derived iPSCs showed multiple phenotypes, such as neurite retraction, lactate dehydrogenase leakage, increased cleaved caspase-3, and abnormal protein aggregation. These phenotypes in vitro matched the clinical manifestations and could stratify the disease (Fujimori et al., 2018). The study further identified ropinirole, a well-known medication for PD, as a drug candidate that could be used to treat patients with ALS. This in vitro study finally led to a successful clinical trial of ropinirole in patients with ALS (Morimoto et al., 2019; Okano et al., 2020; Keio University School of Medicine, 2021). The success of ALS indicated the possibility of stratification and prediction of drug response for patients with AD using iPSC models derived from patients along with their clinical information. Additionally, one recent study also generated the human iPSCs-derived model of microglia and confirmed that female sex and APOE genotype drive the microglial transcription profiles, thus indicating another important aspect of sex difference and APOE genotype in AD (Moser et al., 2021).
The cell-autonomous effect of different sexes on cell models can be observed through several readouts under pathogen-free conditions, while non-cell-autonomous phenotypic changes can be observed by exposing the cells to pathogens or drugs. Furthermore, genetic information (especially AD risk genes) and clinical information of the patients can be included in the stratification of the disease. The effects of hormone therapy in patients can be predicted by phenotypic changes based on the results of the iPSC model. Finally, stratification for hormone treatment using iPSCs from individual participants would yield a robust simulation before the clinical trial, although the cost and time for the generation of iPSCs and subsequent analysis of cell models from individual participants will be a barrier (Figure 1E). Currently, the development of the 3D model such as brain organoids has advanced the disease modeling using iPSCs and were able to recapitulate the patient’s pathophysiology (Chiaradia and Lancaster, 2020). For example, the 3D brain organoid could recapitulate the amyloid aggregation which 2D model could not (Gonzalez et al., 2018). In 2D culture, the secreted Aβ is released into the medium and cannot be concentrated high enough to aggregate. However, in 3D culture, the secreted Aβ can be constrained in the extracellular space of the brain organoids and can increase the concentration high enough to aggregate. Furthermore, the utilization of 3D organoid in combination with the co-culture with other CNS cells/structure like microglia and blood vessels would yield a better modeling of disease and can be used for observation of the hormone therapy in the future.
Nevertheless, the iPSC technology has some limitations such as the genetic alterations that could occur during the reprogramming of the somatic cells to the iPSCs. Even though new reprogramming methods using the non-integrating tools like Sendai virus and episomal vectors caused less damages than the first generation of the reprogramming with lentivirus (Kang et al., 2015), the genetic alterations can still occur. Thus, many of the tests such as karyotyping, genotyping, stem cell markers’ expressions are usually required to confirm the quality of the established iPSCs before using for the modeling of diseases. In addition to neurons differentiated from iPSCs, human neurons can also be induced directly from somatic cells, such as fibroblasts (Victor et al., 2018). Directly induced neurons (iNs) obtained from the fibroblasts preserved the aging status of the donors, while the aging status of the donor was erased in iPSCs. The iNs of sporadic AD patients could also reflect AD phenotypes, such as uncomplicated dendrites, reduced synapses, epigenetic erosion, and increased DNA damage (Mertens et al., 2021). Thus, AD models of iN cells can also be used to evaluate aging-dependent sex effects and could provide insights into sex and age-related changes in AD.
Conclusion
At the human level, early observational studies of estrogen and progesterone replacement therapy have suggested that sex hormones are beneficial to postmenopausal female patients with AD. However, some of the RCTs showed negative results or adverse effects. Factors such as patient characteristics (e.g., age, APOE genotype, duration after menopause), formulation of therapeutic hormones, and route of administration were suggested to be associated with the positive/partially positive/negative outcomes of each study. These results suggest that hormone therapy could only be beneficial in certain populations (Maki, 2013). More recent RCTs showed positive effects of HRT in specific groups of AD patients after stratification of participants by age, duration of exposure to endogenous estrogen, APOE genotype, and formula of estrogen. Therefore, the stratification of the patients and the adjustment of the regimen before starting the trial are crucial steps to predict the precise outcomes of hormonal therapy on cognitive function.
In this regard, in vitro models, such as iPSCs that can recapitulate human disease development of individual donors, is a promising tool for studies that aim to stratify diseases with heterogeneity, including AD. Since the unknown factors that affect the outcomes of hormone treatment may still exist, the uncontrollable environmental factors in clinical trials may mask the beneficial effects of drugs. The iPSC-derived models can foster the discovery of such factors and redesign clinical trials. Although clinical studies at the human level need to be conducted, human cell models can provide significant evidence before proceeding to clinical trials. Our phenotypic analyses of human iPSC-derived neuronal models from AD and non-AD donors showed that the models could recapitulate AD pathology and respond to 17β-estradiol treatment. Hence, this platform of in vitro models using iPSCs provides opportunities to search for new potential factors that are crucial for the stratification of AD. This will allow us to search for a new treatment strategy in the future.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Keio University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SS involved in the study design, performing experiments, data analyses, and drafting the original manuscript. SM supervised the study design, experiments, data analyses, and revised the manuscript. HO involved in the critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research project was supported by the Grants-in-Aid for Scientific Research (KAKENHI, #21J21244 to SS and #21K06376 to SM), the Keio Global Research Institute from Keio University (to SM and HO), the Japan Agency for Medical Research and Development (AMED) [The Acceleration Program for Intractable Disease Research Utilizing Disease-specific iPS Cells to HO (JP21bm0804003)], and the Keio University Doctorate Student Grant-in-Aid Program from Ushioda Memorial Fund 2021 (to SS).
Conflict of Interest
HO is a founder scientist and a Scientific Advisory Board Member for SanBio Co., Ltd. and K Pharma Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Ronnakrit Rojyindeelert (Experience Designer, Science Museum of Minnesota, United States) for the graphical supports. fAD iPSC (APP2E26 line) was generously provided by Haruhisa Inoue (CiRA, Kyoto University, Japan). SS is DC1 research fellow of the Japan Society for the Promotion of Science (JSPS), scholar of Otsuka Toshimi Scholarship Foundation (fiscal year 2020), and scholar of Koizumi Memorial Graduate School Special Scholarship of Keio University (fiscal year 2021).
References
Adhya, D., Annuario, E., Lancaster, M. A., Price, J., Baron-Cohen, S., and Srivastava, D. P. (2018). Understanding the role of steroids in typical and atypical brain development: advantages of using a “brain in a dish” approach. J. Neuroendocrinol. 30:e12547. doi: 10.1111/jne.12547
Albert-Gascó, H., Ros-Bernal, F., Castillo-Gómez, E., and Olucha-Bordonau, F. E. (2020). ERK signaling in developing cognitive and emotional function and its effect on pathological and neurodegenerative processes. Int. J. Mol. Sci. 21:4471. doi: 10.3390/ijms21124471
Alzheimer’s Association (2021). 2021 Alzheimer’s disease facts and figures. Alzheimers Dement 17, 327–406. doi: 10.1002/alz.12328
Asih, P. R., Wahjoepramono, E. J., Aniwiyanti, V., Wijaya, L. K., de Ruyck, K., Taddei, K., et al. (2015). Testosterone replacement therapy in older male subjective memory complainers: double-blind randomized crossover placebo-controlled clinical trial of physiological assessment and safety. CNS Neurol. Disord. Drug Targets 14, 576–586. doi: 10.2174/1871527314666150429112112
Asthana, S., Craft, S., Baker, L. D., Raskind, M. A., Birnbaum, R. S., Lofgreen, C. P., et al. (1999). Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology 24, 657–677. doi: 10.1016/s0306-4530(99)00020-7
Barnes, L. L., Wilson, R. S., Schneider, J. A., Bienias, J. L., Evans, D. A., and Bennett, D. A. (2003). Gender, cognitive decline, and risk of AD in older persons. Neurology 60, 1777–1781. doi: 10.1212/01.wnl.0000065892.67099.2a
Carroll, J. C., Rosario, E. R., Chang, L., Stanczyk, F. Z., Oddo, S., LaFerla, F. M., et al. (2007). Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J. Neurosci. 27, 13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007
Cherrier, M. M., Matsumoto, A. M., Amory, J. K., Asthana, S., Bremner, W., Peskind, E. R., et al. (2005). Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology 64, 2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1
Chiaradia, I., and Lancaster, M. A. (2020). Brain organoids for the study of human neurobiology at the interface of in vitro and in vivo. Nat. Neurosci. 23, 1496–1508. doi: 10.1038/s41593-020-00730-3
Craig, M. C., Maki, P. M., and Murphy, D. G. (2005). The Women’s Health Initiative Memory Study: findings and implications for treatment. Lancet. Neurol. 4, 190–194. doi: 10.1016/S1474-4422(05)01016-1
Davis, E. J., Broestl, L., Abdulai-Saiku, S., Worden, K., Bonham, L. W., Minones-Moyano, E., et al. (2020). A second X chromosome contributes to resilience in a mouse model of Alzheimer’s disease. Sci. Transl. Med. 12:eaaz5677. doi: 10.1126/scitranslmed.aaz5677
Devi, G., and Scheltens, P. (2018). Heterogeneity of Alzheimer’s disease: consequence for drug trials? Alzheimers Res. Ther. 10:122. doi: 10.1186/s13195-018-0455-y
Dubal, D. B. (2020). Sex difference in Alzheimer’s disease: An updated, balanced and emerging perspective on differing vulnerabilities. Handb. Clin. Neurol. 175, 261–273. doi: 10.1016/B978-0-444-64123-6.00018-7
Dubal, D. B., Broestl, L., and Worden, K. (2012). Sex and gonadal hormones in mouse models of Alzheimer’s disease: what is relevant to the human condition? Biol. Sex Differ. 3:24. doi: 10.1186/2042-6410-3-24
Dunkin, J., Rasgon, N., Wagner-Steh, K., David, S., Altshuler, L., and Rapkin, A. (2005). Reproductive events modify the effects of estrogen replacement therapy on cognition in healthy postmenopausal women. Psychoneuroendocrinology 30, 284–296. doi: 10.1016/j.psyneuen.2004.09.002
Espuny-Camacho, I., Arranz, A. M., Fiers, M., Snellinx, A., Ando, K., Munck, S., et al. (2017). Hallmarks of Alzheimer’s disease in stem-cell-derived human neurons transplanted into mouse brain. Neuron 93, 1066–1081. doi: 10.1016/j.neuron.2017.02.001
Fujimori, K., Ishikawa, M., Otomo, A., Atsuta, N., Nakamura, R., Akiyama, T., et al. (2018). Modeling sporadic ALS in iPSC-derived motor neurons identifies a potential therapeutic agent. Nat. Med. 24, 1579–1589. doi: 10.1038/s41591-018-0140-5
Gonzalez, C., Armijo, E., Bravo-Alegria, J., Becerra-Calixto, A., Mays, C. E., and Soto, C. (2018). Modeling amyloid beta and tau pathology in human cerebral organoids. Mol. Psychiatry 23, 2363–2374. doi: 10.1038/s41380-018-0229-8
Henderson, V. W., Ala, T., Sainani, K. L., Bernstein, A. L., Stephenson, B. S., Rosen, A. C., et al. (2015). Raloxifene for women with Alzheimer disease: A randomized controlled pilot trial. Neurology 85, 1937–1944. doi: 10.1212/WNL.0000000000002171
Henderson, V. W., Paganini-Hill, A., Miller, B. L., Elble, R. J., Reyes, P. F., Shoupe, D., et al. (2000). Estrogen for Alzheimer’s disease in women: randomized, double-blind, placebo-controlled trial. Neurology 54, 295–301. doi: 10.1212/wnl.54.2.295
Henderson, V. W., Watt, L., and Buckwalter, J. G. (1996). Cognitive skills associated with estrogen replacement in women with Alzheimer’s disease. Psychoneuroendocrinology 21, 421–430. doi: 10.1016/0306-4530(95)00060-7
Honjo, H., Ogino, Y., Naitoh, K., Urabe, M., Kitawaki, J., Yasuda, J., et al. (1989). In vivo effects by estrone sulfate on the central nervous system-senile dementia (Alzheimer’s type). J. Steroid Biochem. 34, 521–525. doi: 10.1016/0022-4731(89)90137-4
Huang, G., Wharton, W., Bhasin, S., Harman, S. M., Pencina, K. M., Tsitouras, P., et al. (2016). Effects of long-term testosterone administration on cognition in older men with low or low-to-normal testosterone concentrations: a prespecified secondary analysis of data from the randomised, double-blind, placebo-controlled TEAAM trial. Lancet Diab. Endocrinol. 4, 657–665. doi: 10.1016/S2213-8587(16)30102-4
Huang, G., Wharton, W., Travison, T. G., Ho, M. H., Gleason, C., Asthana, S., et al. (2015). Effects of testosterone administration on cognitive function in hysterectomized women with low testosterone levels: a dose-response randomized trial. J. Endocrinol. Invest. 38, 455–461. doi: 10.1007/s40618-014-0213-3
Imaizumi, Y., and Okano, H. (2014). Modeling human neurological disorders with induced pluripotent stem cells. J. Neurochem. 129, 388–399. doi: 10.1111/jnc.12625
Israel, M. A., Yuan, S. H., Bardy, C., Reyna, S. M., Mu, Y., Herrera, C., et al. (2012). Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature 482, 216–220. doi: 10.1038/nature10821
Jack, C. R. Jr., Barrio, J. R., and Kepe, V. (2013). Cerebral amyloid PET imaging in Alzheimer’s disease. Acta Neuropathol. 126, 643–657. doi: 10.1007/s00401-013-1185-7
Kang, X., Yu, Q., Huang, Y., Song, B., Chen, Y., Gao, X., et al. (2015). Effects of Integrating and non-integrating reprogramming methods on copy number variation and genomic stability of human induced pluripotent stem cells. PLoS One 10:e0131128. doi: 10.1371/journal.pone.0131128
Kantarci, K., Lowe, V. J., Lesnick, T. G., Tosakulwong, N., Bailey, K. R., Fields, J. A., et al. (2016). Early Postmenopausal Transdermal 17β-Estradiol Therapy and Amyloid-β Deposition. J. Alzheimers Dis. 53, 547–556. doi: 10.3233/JAD-160258
Keio University School of Medicine (2021). Ropinirole hydrochloride may attenuate the disease progression of ALS. Available online at: https://www.keio.ac.jp/en/press-releases/2021/May/20/49-80091/ (accessed August 12, 2021)
Knopman, D. S., Jones, D. T., and Greicius, M. D. (2021). Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen. December 2019. Alzheimers Dement 17, 696–701. doi: 10.1002/alz.12213
Knupp, A., Mishra, S., Martinez, R., Braggin, J. E., Szabo, M., Kinoshita, C., et al. (2020). Depletion of the AD Risk Gene SORL1 selectively impairs neuronal endosomal traffic independent of amyloidogenic APP Processing. Cell Rep. 1:107719. doi: 10.1016/j.celrep.2020.107719
Kondo, T., Asai, M., Tsukita, K., Kutoku, Y., Ohsawa, Y., Sunada, Y., et al. (2013). Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness. Cell Stem Cell 12, 487–496. doi: 10.1016/j.stem.2013.01.009
Lee, J., Pinares-Garcia, P., Loke, H., Ham, S., Vilain, E., and Harley, V. R. (2019). Sex-specific neuroprotection by inhibition of the Y-chromosome gene, SRY, in experimental Parkinson’s disease. Proc. Natl. Acad. Sci. USA 116, 16577–16582. doi: 10.1073/pnas.1900406116
Lee, S. H., Kang, J., Ho, A., Watanabe, H., Bolshakov, V. Y., and Shen, J. (2020). APP Family regulates neuronal excitability and synaptic plasticity but not neuronal survival. Neuron 108, 676–690. doi: 10.1016/j.neuron.2020.08.011
Lin, Y. T., Seo, J., Gao, F., Feldman, H. M., Wen, H. L., Penney, J., et al. (2018). APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s Disease Phenotypes in Human iPSC-derived brain cell types. Neuron 98, 1141–1154. doi: 10.1016/j.neuron.2018.05.008
Lv, W., Du, N., Liu, Y., Fan, X., Wang, Y., Jia, X., et al. (2016). Low Testosterone Level and Risk of Alzheimer’s disease in the elderly men: a systematic review and meta-analysis. Mol. Neurobiol. 53, 2679–2684. doi: 10.1007/s12035-015-9315-y
Maki, P. (2013). Is timing everything? New insights into why the effect of estrogen therapy on memory might be age dependent. Endocrinology 154, 2570–2572. doi: 10.1210/en.2013-1598
Mayeux, R., and Stern, Y. (2012). Epidemiology of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2:a006239. doi: 10.1101/cshperspect.a006239
Mehta, D., Jackson, R., Paul, G., Shi, J., and Sabbagh, M. (2017). Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010-2015. Expert Opin. Investig. Drugs 26, 735–739. doi: 10.1080/13543784.2017.1323868
Mertens, J., Herdy, J. R., Traxler, L., Schafer, S. T., Schlachetzki, J. C. M., Bohnke, L., et al. (2021). Age-dependent instability of mature neuronal fate in induced neurons from Alzheimer’s patients. Cell Stem Cell 2021:4. doi: 10.1016/j.stem.2021.04.004
Mielke, M. M. (2018). Sex and gender differences in Alzheimer’s Disease Dementia. Psychiatr. Times 35, 14–17.
Moffat, S. D. (2005). Effects of testosterone on cognitive and brain aging in elderly men. Ann. NY Acad. Sci. 1055, 80–92. doi: 10.1196/annals.1323.014
Morimoto, S., Takahashi, S., Fukushima, K., Saya, H., Suzuki, N., Aoki, M., et al. (2019). Ropinirole hydrochloride remedy for amyotrophic lateral sclerosis - Protocol for a randomized, double-blind, placebo-controlled, single-center, and open-label continuation phase I/IIa clinical trial (ROPALS trial). Regen. Ther. 11, 143–166. doi: 10.1016/j.reth.2019.07.002
Moser, V. A., Workman, M. J., Hurwitz, S. J., Lipman, R. M., Pike, C. J., and Svendsen, C. N. (2021). Microglial transcription profiles in mouse and in human are driven by APOE4 and sex. Isience 2021:3238. doi: 10.1016/j.isci.2021.103238
Mullane, K., and Williams, M. (2018). Alzheimer’s disease (AD) therapeutics - 2: Beyond amyloid - Re-defining AD and its causality to discover effective therapeutics. Biochem. Pharmacol. 158, 376–401. doi: 10.1016/j.bcp.2018.09.027
Mulnard, R. A., Cotman, C. W., Kawas, C., van Dyck, C. H., Sano, M., Doody, R., et al. (2000). Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer’s Disease Cooperative Study. JAMA 283, 1007–1015. doi: 10.1001/jama.283.8.1007
Nakagawa, M., Taniguchi, Y., Senda, S., Takizawa, N., Ichisaka, T., Asano, K., et al. (2014). A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci. Rep. 4:3594. doi: 10.1038/srep03594
Okano, H., and Yamanaka, S. (2014). iPS cell technologies: significance and applications to CNS regeneration and disease. Mol. Brain 7:22. doi: 10.1186/1756-6606-7-22
Okano, H., Yasuda, D., Fujimori, K., Morimoto, S., and Takahashi, S. (2020). Ropinirole, a New ALS drug candidate developed using iPSCs. Trends Pharmacol. Sci. 41, 99–109. doi: 10.1016/j.tips.2019.12.002
Rasgon, N. L., Geist, C. L., Kenna, H. A., Wroolie, T. E., Williams, K. E., and Silverman, D. H. (2014). Prospective randomized trial to assess effects of continuing hormone therapy on cerebral function in postmenopausal women at risk for dementia. PLoS One 9:e89095. doi: 10.1371/journal.pone.0089095
Resnick, S. M., Maki, P. M., Golski, S., Kraut, M. A., and Zonderman, A. B. (1998). Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm. Behav. 34, 171–182. doi: 10.1006/hbeh.1998.1476
Resnick, S. M., Metter, E. J., and Zonderman, A. B. (1997). Estrogen replacement therapy and longitudinal decline in visual memory. A possible protective effect? Neurology 49, 1491–1497. doi: 10.1212/wnl.49.6.1491
Rowe, R. G., and Daley, G. Q. (2019). Induced pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Genet. 20, 377–388. doi: 10.1038/s41576-019-0100-z
Santoro, N., and Sutton-Tyrrell, K. (2011). The SWAN song: study of women’s health across the Nation’s recurring themes. Obstet. Gynecol. Clin. North Am. 38, 417–423. doi: 10.1016/j.ogc.2011.05.001
Sharma, A., Sances, S., Workman, M. J., and Svendsen, C. N. (2020). Multi-lineage Human iPSC-derived platforms for disease modeling and drug discovery. Cell Stem Cell 26, 309–329. doi: 10.1016/j.stem.2020.02.011
Sheldahl, L. C., Shapiro, R. A., Bryant, D. N., Koerner, I. P., and Dorsa, D. M. (2008). Estrogen induces rapid translocation of estrogen receptor beta, but not estrogen receptor alpha, to the neuronal plasma membrane. Neuroscience 153, 751–761. doi: 10.1016/j.neuroscience.2008.02.035
Shum, C., Macedo, S. C., Warre-Cornish, K., Cocks, G., Price, J., and Srivastava, D. P. (2015). Utilizing induced pluripotent stem cells (iPSCs) to understand the actions of estrogens in human neurons. Horm. Behav. 74, 228–242. doi: 10.1016/j.yhbeh.2015.06.014
Shumaker, S. A., Legault, C., Rapp, S. R., Thal, L., Wallace, R. B., Ockene, J. K., et al. (2003). Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 289, 2651–2662. doi: 10.1001/jama.289.20.2651
Silverman, D. H., Geist, C. L., Kenna, H. A., Williams, K., Wroolie, T., Powers, B., et al. (2011). Differences in regional brain metabolism associated with specific formulations of hormone therapy in postmenopausal women at risk for AD. Psychoneuroendocrinology 36, 502–513. doi: 10.1016/j.psyneuen.2010.08.002
Stadtfeld, M., and Hochedlinger, K. (2010). Induced pluripotency: history, mechanisms, and applications. Genes Dev. 24, 2239–2263. doi: 10.1101/gad.1963910
Strand, B. H., Knapskog, A. B., Persson, K., Edwin, T. H., Amland, R., Mjørud, M., et al. (2018). Survival and years of life lost in various aetiologies of dementia, mild cognitive impairment (MCI) and subjective cognitive decline (SCD) in Norway. PLoS One 13:e0204436. doi: 10.1371/journal.pone.0204436
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. doi: 10.1016/j.cell.2007.11.019
Takahashi, K., and Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. doi: 10.1016/j.cell.2006.07.024
Tan, R. S., and Pu, S. J. (2003). A pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer’s disease. Aging Male 6, 13–17.
Valen-Sendstad, A., Engedal, K., and Stray-Pedersen, B. (2010). ADACT Study Group, Strobel C, Barnett L, Meyer N, Nurminemi M. Effects of hormone therapy on depressive symptoms and cognitive functions in women with Alzheimer disease: a 12 month randomized, double-blind, placebo-controlled study of low-dose estradiol and norethisterone. Am. J. Geriatr. Psychiatry 18, 11–20. doi: 10.1097/JGP.0b013e3181beaaf4
Vega-Vela, N. E., Osorio, D., Avila-Rodriguez, M., Gonzalez, J., García-Segura, L. M., Echeverria, V., et al. (2017). Type calcium channels modulation by estradiol. Mol. Neurobiol. 54, 4996–5007. doi: 10.1007/s12035-016-0045-6
Victor, M. B., Richner, M., Olsen, H. E., Lee, S. W., Monteys, A. M., Ma, C., et al. (2018). Striatal neurons directly converted from Huntington’s disease patient fibroblasts recapitulate age-associated disease phenotypes. Nat. Neurosci. 21, 341–352. doi: 10.1038/s41593-018-0075-7
Wahjoepramono, E. J., Asih, P. R., Aniwiyanti, V., Taddei, K., Dhaliwal, S. S., Fuller, S. J., et al. (2016). The effects of testosterone supplementation on cognitive functioning in older men. CNS Neurol. Disord. Drug Targets 15, 337–343. doi: 10.2174/1871527315666151110125704
Wang, Y., Hernandez, G., Mack, W. J., Schneider, L. S., Yin, F., and Brinton, R. D. (2020). Retrospective analysis of phytoSERM for management of menopause-associated vasomotor symptoms and cognitive decline: a pilot study on pharmacogenomic effects of mitochondrial haplogroup and APOE genotype on therapeutic efficacy. Menopause 27, 57–65. doi: 10.1097/GME.0000000000001418
Wharton, W., Baker, L. D., Gleason, C. E., Dowling, M., Barnet, J. H., Johnson, S., et al. (2011). Short-term hormone therapy with transdermal estradiol improves cognition for postmenopausal women with Alzheimer’s disease: results of a randomized controlled trial. J. Alzheimers Dis. 26, 495–505. doi: 10.3233/JAD-2011-110341
Wooten, G. F., Currie, L. J., Bovbjerg, V. E., Lee, J. K., and Patrie, J. (2004). Are men at greater risk for Parkinson’s disease than women? J. Neurol. Neurosurg. Psychiatry 75, 637–639. doi: 10.1136/jnnp.2003.020982
Yaffe, K., Krueger, K., Cummings, S. R., Blackwell, T., Henderson, V. W., Sarkar, S., et al. (2005). Effect of raloxifene on prevention of dementia and cognitive impairment in older women: the Multiple Outcomes of Raloxifene Evaluation (MORE) randomized trial. Am. J. Psychiatry 162, 683–690. doi: 10.1176/appi.ajp.162.4.683
Yagi, T., Ito, D., Okada, Y., Akamatsu, W., Nihei, Y., Yoshizaki, T., et al. (2011). Modeling familial Alzheimer’s disease with induced pluripotent stem cells. Hum. Mol. Genet. 20, 4530–4539. doi: 10.1093/hmg/ddr394
Yiannopoulou, K. G., Anastasiou, A. I., Zachariou, V., and Pelidou, S. H. (2019). Reasons for failed trials of disease-modifying treatments for alzheimer disease and their contribution in recent research. Biomedicines 7:97. doi: 10.3390/biomedicines7040097
Yoon, B. K., Chin, J., Kim, J. W., Shin, M. H., Ahn, S., Lee, D. Y., et al. (2018). Menopausal hormone therapy and mild cognitive impairment: a randomized, placebo-controlled trial. Menopause 25, 870–876. doi: 10.1097/GME.0000000000001140
Yoon, B. K., Kim, D. K., Kang, Y., Kim, J. W., Shin, M. H., and Na, D. L. (2003). Hormone replacement therapy in postmenopausal women with Alzheimer’s disease: a randomized, prospective study. Fertil. Steril. 79, 274–280. doi: 10.1016/s0015-0282(02)04666-6
Young, J. E., Boulanger-Weill, J., Williams, D. A., Woodruff, G., Buen, F., Revilla, A. C., et al. (2015). Elucidating molecular phenotypes caused by the SORL1 Alzheimer’s disease genetic risk factor using human induced pluripotent stem cells. Cell Stem Cell 16, 373–385. doi: 10.1016/j.stem.2015.02.004
Yue, X., Lu, M., Lancaster, T., Cao, P., Honda, S., Staufenbiel, M., et al. (2005). Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer’s disease animal model. Proc. Natl. Acad. Sci. USA 102, 19198–19203. doi: 10.1073/pnas.0505203102
Zhang, L., Blackman, B. E., Schonemann, M. D., Zogovic-Kapsalis, T., Pan, X., Tagliaferri, M., et al. (2010). Estrogen receptor beta-selective agonists stimulate calcium oscillations in human and mouse embryonic stem cell-derived neurons. PLoS One 5:e11791. doi: 10.1371/journal.pone.0011791
Zhang, W., Jiao, B., Zhou, M., Zhou, T., and Shen, L. (2016). Modeling Alzheimer’s disease with induced pluripotent stem cells: current challenges and future concerns. Stem Cells Int. 2016:7828049. doi: 10.1155/2016/7828049
Keywords: Alzheimer’s disease, sex difference, hormone therapy, iPSCs (induced pluripotent stem cells), in vitro model
Citation: Supakul S, Okano H and Maeda S (2021) Utilization of Human Induced Pluripotent Stem Cells-Derived In vitro Models for the Future Study of Sex Differences in Alzheimer’s Disease. Front. Aging Neurosci. 13:768948. doi: 10.3389/fnagi.2021.768948
Received: 01 September 2021; Accepted: 15 October 2021;
Published: 04 November 2021.
Edited by:
Yang (Ted) D. Teng, Harvard Medical School, United StatesReviewed by:
Henning Ulrich, University of São Paulo, BrazilMarcin Majka, Jagiellonian University Medical College, Poland
Copyright © 2021 Supakul, Okano and Maeda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sumihiro Maeda, c3VtaWhpcm8ubWFlZGFAa2Vpby5qcA==
 Sopak Supakul
Sopak Supakul Hideyuki Okano
Hideyuki Okano Sumihiro Maeda
Sumihiro Maeda