
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Aging Neurosci. , 03 November 2021
Sec. Alzheimer's Disease and Related Dementias
Volume 13 - 2021 | https://doi.org/10.3389/fnagi.2021.766587
This article is part of the Research Topic Cholesterol and Neurodegenerative Diseases: Pressing Questions and How to Address Them View all 11 articles
Neurodegenerative diseases, namely Alzheimer’s (AD), Parkinson’s (PD), and Huntington’s disease (HD) together with amyotrophic lateral sclerosis (ALS) and multiple sclerosis (MS), devastate millions of lives per year worldwide and impose an increasing socio-economic burden across nations. Consequently, these diseases occupy a considerable portion of biomedical research aiming to understand mechanisms of neurodegeneration and to develop efficient treatments. A potential culprit is cholesterol serving as an essential component of cellular membranes, as a cofactor of signaling pathways, and as a precursor for oxysterols and hormones. This article uncovers the workforce studying research on neurodegeneration and cholesterol using the TeamTree analysis. This new bibliometric approach reveals the history and dynamics of the teams and exposes key players based on citation-independent metrics. The team-centered view reveals the players on an important field of biomedical research.
Neurodegenerative disorders devastate millions of lives worldwide and impose an increasing socio-economic burden (Kalia and Lang, 2015; Feigin et al., 2017; Erkkinen et al., 2018; El-Hayek et al., 2019). Research within the last decades has helped to clarify the mechanisms underlying each disease and suggested new therapeutic approaches (Fu et al., 2018; Ga et al., 2018; Jucker and Walker, 2018; Reich et al., 2018; Lassmann, 2019; Savelieff et al., 2019; Schwartz et al., 2021). A decisive step is the identification of molecular culprits that provoke or contribute to the dysfunction and degeneration of neurons. In the case of AD, research focused on three targets: hyperphosphorylated forms of tau protein, proteolytic fragments of amyloid precursor protein, and specific variants of apolipoprotein E (Long and Holtzman, 2019). A prime target for PD-related research has been alpha synuclein (Rocha et al., 2018), but other genes, as well as environmental factors, have come under scrutiny (Deng et al., 2018; Bandres-Ciga et al., 2020; Blauwendraat et al., 2020). In the case of amyotrophic lateral sclerosis (ALS), superoxide dismutase 1 has been investigated intensely as it was the first gene shown to be mutated in familial forms of the disease (Rosen et al., 1993). TAR DNA binding protein-43 (TDP-43) has become a target for ALS- and frontotemporal dementia-related research, as it was identified as a major component of ubiquitin-positive inclusions (Neumann et al., 2006). Since then, other genes have come under study as disease-causing alleles were identified in familial forms of ALS (Chia et al., 2018; Mejzini et al., 2019). Huntingtin has been at the center of attention as the long-sought gene bearing Huntington’s disease (HD)-causing mutations (The Huntington’s Disease Collaborative Research Group, 1993). Repeat expansions similar to those induced by the Huntingtin alleles cause neurodegeneration in numerous diseases including ALS and frontotemporal dementia by combinations of distinct molecular mechanisms (Malik et al., 2021; Schwartz et al., 2021). Research on multiple sclerosis (MS) has focused on immune and glial cells since chronic inflammation and demyelination are known pathologic changes preceding neurodegeneration (Faissner et al., 2019; Lassmann, 2019; Voet et al., 2019).
Why should cholesterol play a role in these diseases? Cholesterol is one of the most widely known and most studied biological molecules due to its involvement in cardiovascular and other diseases (Goldstein and Brown, 2015; Tall and Yvan-Charvet, 2015; Gliozzi et al., 2021) and due to its functions as a component of membranes in eukaryotic cells (Yeagle, 1985), as a cofactor of signaling pathways and as a precursor for steroid hormones (Miller and Auchus, 2011; Prabhu et al., 2016). Notably, cholesterol is also converted to biologically active oxysterols by specific enzymes or by autoxidation (Mutemberezi et al., 2016; Wang et al., 2021). Given the diverse functions of cholesterol, its cellular homeostasis relies on a multitude of proteins and mechanisms (Ikonen, 2008; Luo et al., 2020). In the brain, cholesterol represents a major building block due to the diversity and sheer mass of membraneous structures. This includes highly branched axons and dendrites of neurons (Elston and Fujita, 2014), fine perisynaptic processes of astrocytes (Oberheim et al., 2009), countless synaptic vesicles (Binotti et al., 2021), and the multi-layered myelin sheaths surrounding axons (Schmitt et al., 2015). Based on these considerations, disturbances of cholesterol homeostasis seem likely to cause neuronal dysfunction and degeneration. The mechanisms of cholesterol homeostasis in brain cells are probably distinct from those operating in the rest of the body (Dietschy, 2009; Pfrieger and Ungerer, 2011; Zhang and Liu, 2015; Mahley, 2016; Moutinho et al., 2016; Yoon et al., 2016; Hussain et al., 2019). Possible implications of cholesterol and derived molecules in neurodegenerative diseases have been reviewed elsewhere (Martín et al., 2014; Zarrouk et al., 2014; Leoni and Caccia, 2015; Doria et al., 2016; Arenas et al., 2017; Chang et al., 2017; Testa et al., 2018; Zarrouk et al., 2018; Adorni et al., 2019; Griffiths and Wang, 2019; Hussain et al., 2019; Jeong et al., 2019; Jin et al., 2019; Loera-Valencia et al., 2019; Petrov and Pikuleva, 2019; Segatto et al., 2019; Blauwendraat et al., 2020; González-Guevara et al., 2020; McFarlane and Kędziora-Kornatowska, 2020; Sáiz-Vazquez et al., 2020; Dai et al., 2021; Duong et al., 2021; Feringa and van der Kant, 2021; García-Sanz et al., 2021; Pikuleva and Cartier, 2021; Samant and Gupta, 2021). This article shows the workforce driving research in the field using original research articles obtained from MEDLINE (Table 1) and a new bibliometric approach (Pfrieger, 2021; https://github.com/fw-pfrieger/TeamTree). Bibliometric analyses of other aspects can be found elsewhere (Guido et al., 2015; Barboza and Ghisi, 2018; Zhang et al., 2020; Du et al., 2021; Li et al., 2021; Rizzi et al., 2021). Articles related to Niemann-Pick type C disease were excluded from the analysis as this rare lysosomal storage disorder is directly linked to perturbed cholesterol transport (Loftus et al., 1997; Naureckiene et al., 2000; Vanier, 2010).
The earliest publications date back to the 1950s when three groups investigated the cholesterol content in tissues and body fluids of patients with dementia (Mori and Barucci, 1951; Scanu et al., 1955) and MS (Chiavacci and Sperry, 1952; Poser and Curran, 1958). The number of articles published per year remained relatively low until the 1990s and increased thereafter. Since 2000, the annual count of articles has grown linearly reaching around 300 articles per year in 2020 (Figure 1A). The number of authors listed on the article byline grew in parallel, however at a much stronger pace reaching more than 2,000 per year within the last years (Figure 1B). The strong expansion of the workforce was due to an increasing number of authors per article (Figure 1C). Notably, the expansion of the field was mainly driven by authors contributing single articles, as their number grew steadily. The balance of authors publishing in the field for more than 1 year has become negative within the last years, but the number of authors leaving the field within the last years is inherently inaccurate (Figure 1D).
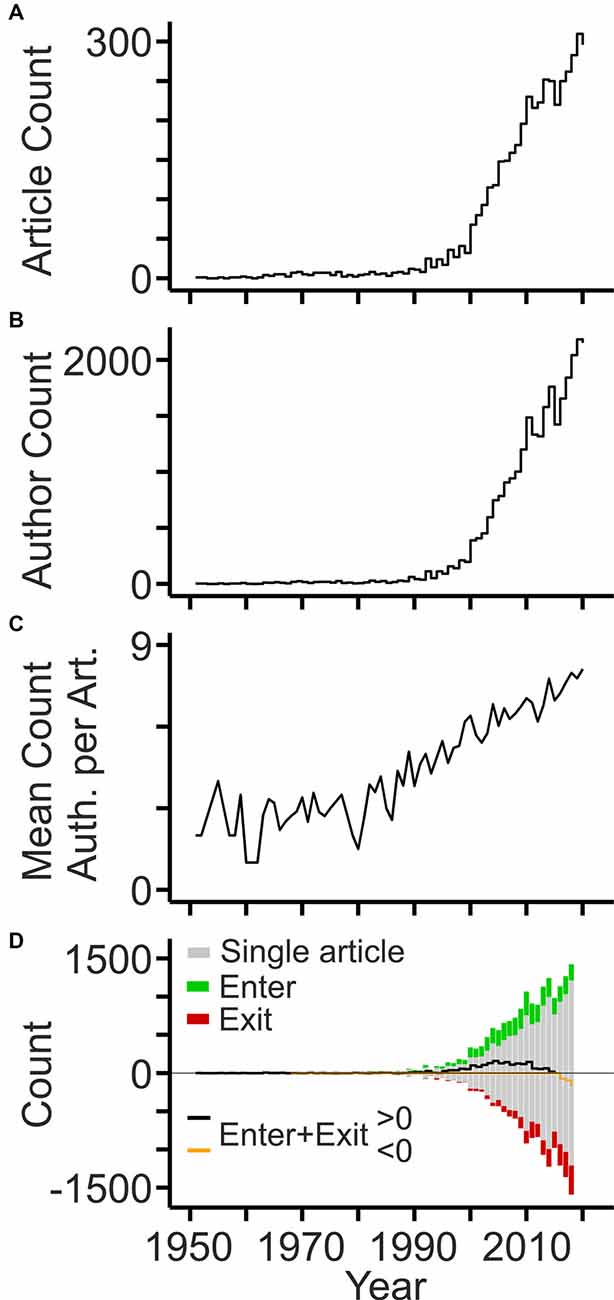
Figure 1. Development of the workforce. (A) Annual counts of original articles related to cholesterol and neurodegeneration (PubMed query shown in Table 1). (B) Annual counts of authors contributing to the field per year. (C) Mean number of authors listed on article bylines per year. (D) Annual counts of authors entering (green bars) and exiting (red bars) the field per year based on the first and last year of publication, respectively. Black and orange lines indicate the sum of annual author counts. Gray bars indicate the number of authors contributing single articles to the field (shown as negative and positive values).
More information about the workforce can be drawn by analyzing the authors on specific positions of the article byline, which indicate the roles and contributions of authors (Claxton, 2005; Marušić et al., 2011). A total of ~3,100 authors was listed on the last byline position of articles identifying these authors as principal investigators in the field. This corresponds to 10% of the total workforce. The development of the field with respect to these contributors is shown in Figure 2A using TeamTree graphs. In this type of scatterplot, the years of publication are plotted against a chronologic index assigned to each author (Pfrieger, 2021). The number of last authors entering the field per year has grown steadily during the last two decades (Figure 2B). The total publication counts of individual last authors reached up to 21 articles, but the large majority (81%) contributed single articles (Figure 2C) as observed for the entire workforce (Figure 1D). Ranking authors by PCs identified the top contributors among the last authors (Figure 2D).
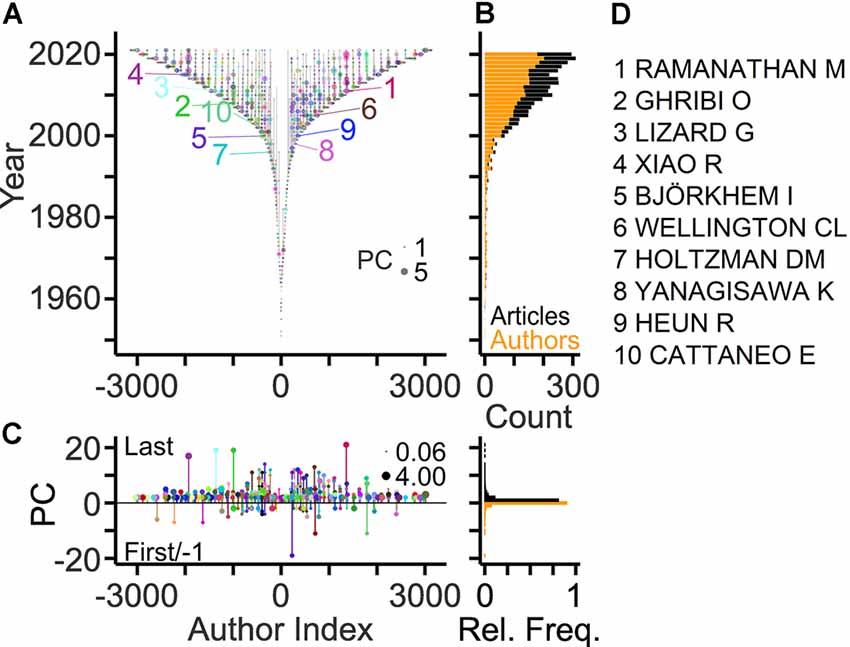
Figure 2. Publication records of last authors. (A) TeamTree graph showing the publication records of the last authors contributing to the field. Circles connected by vertical gray lines represent for each author the years of publications as the last author plotted against a chronologic author index with alternating signs and author-specific colors to enhance visibility. Circle area indicates publication count (PC) per year. Numbers indicate authors with 10 largest PCs (names indicated in panel D). (B) Number of authors entering the field per year (orange) and of articles (black) published per year. (C) Left, PCs per author indicating last and first author articles by positive and negative values, respectively. Circle area indicates the average number of publications per year. Right, relative frequency distributions of PC values shown on the left. (D) Names of authors with largest PCs in the field.
Genealogical relations in a field can be derived from the last and first authors on article bylines representing ancestor and offspring, respectively (Pfrieger, 2021). Figure 3A shows family relations among authors highlighting those with the largest offspring counts. About 10% of last authors published previously as first authors thus qualifying as offspring, and 7% of last authors qualified as ancestors (Figure 3B). These ancestors generated up to four offspring authors and published up to 10 articles with their offspring (Figure 3C). Overall, the field comprised 192 families with up to six members spanning maximally four generations (Figures 3D,E). The large majority of families (91%) had only two members. Ranking by OCs revealed the most prolific authors and their families in the field (Figures 3F,G).
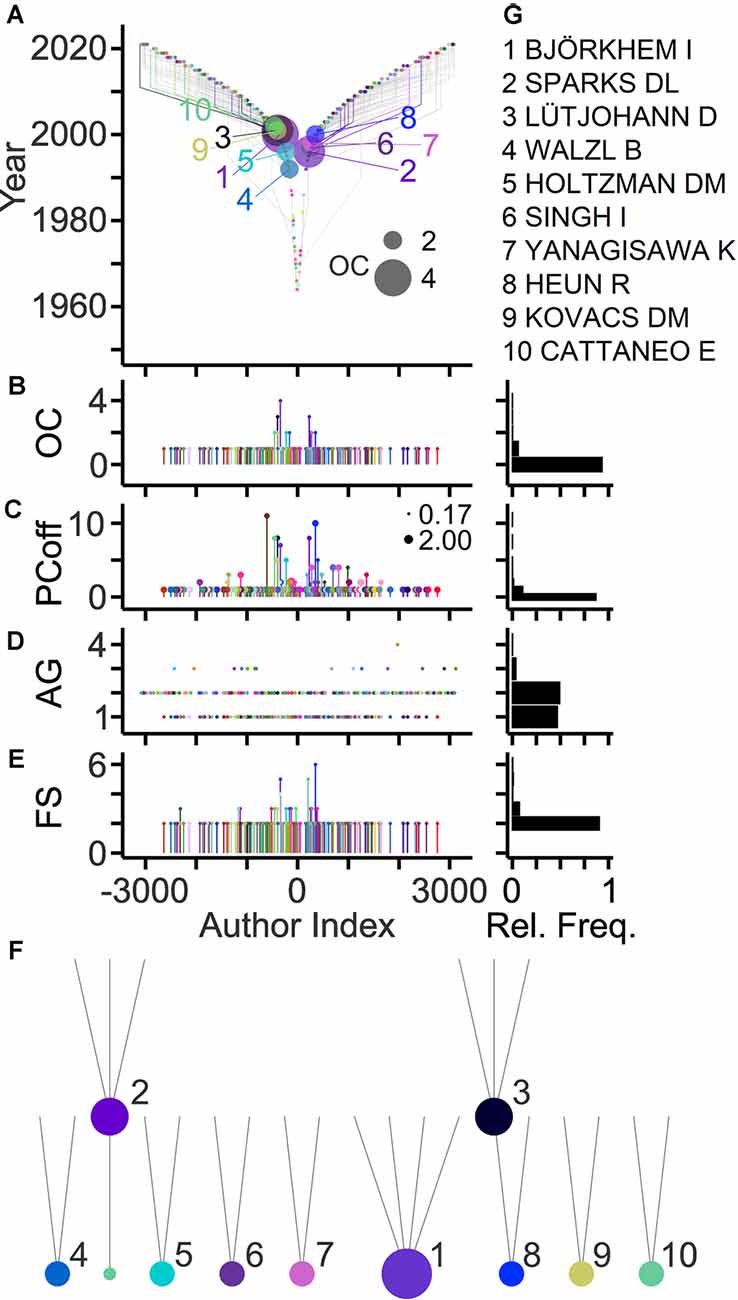
Figure 3. Genealogic relations in the field. (A) TeamTree graph showing genealogic relations among authors. Circles and gray lines indicate ancestor-offspring connections based on first author-last author pairs on article bylines. Connections of authors with the 10 largest offspring count (OC) values are shown in color (names indicated in panel G). Circle area indicates OC value. The signs of author indices of offspring and of ancestors were adjusted to the first-generation ancestor. (B–E) Quantitative data showing for individual last authors, the number of offspring (B) the number of articles published together with offspring (PCoff; circle area indicates average PC per year) (C), the generation of authors (AG) starting with AG = 1 for first ancestors (D) and the family size (FS) of individual first-generation ancestors comprising all offspring across subsequent generations (E). (F,G) Family trees (F) and names (G) of authors with 10 largest OC values (indicated by circle area).
Collaborative connections can be delineated based on middle and last byline positions (Newman, 2001; Pfrieger, 2021). Figure 4 exposes collaborations between authors contributing to the field. In total, 43% of the authors established collaborations with maximally 46 other authors and published up to 77 collaborative articles as last and co-author, respectively (Figures 4B,C). Ranking authors based on collaboration counts revealed the most strongly connected teams in the field and their networks (Figures 4D,E).
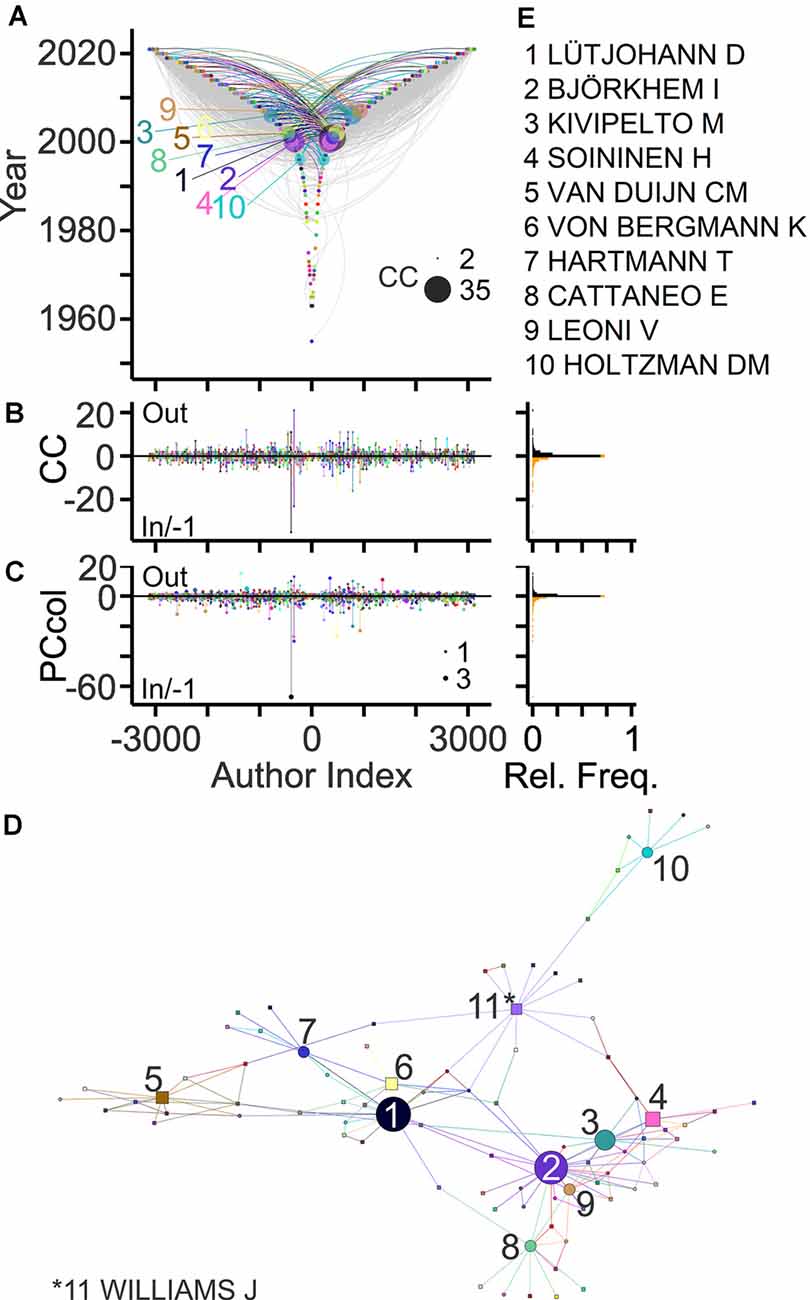
Figure 4. Collaborative connections in the field. (A) TeamTree graph showing collaborations between last authors (out; negative author index) and (non-first) co-authors (in; positive author index) on article bylines. For out- and in-degree connections an author lists other authors as co-authors and an author is listed as a co-author, respectively. Connections of authors with 10 highest connection count (CC) values (in+out) are shown in color (names indicated in panel E). Circle areas indicate CCout and CCin values of these authors. (B,C) Left, counts of collaborators (B) and of collaborative articles (C) per author. Circle area indicates PCannu. Right, relative frequency distributions of parameters shown on the left. (D,E) Network (D) and names (E) of authors with 10 largest CC values. Symbol areas (D) indicate CC values normalized to the maximum. Circles and rectangles represent family and non-family authors, respectively.
An important goal of bibliometric analyses is to estimate the contribution of individual authors. The “key players” may serve as experts, key opinion leaders, referees, and collaborators. Different indicators of scientific production have been explored including PCs, citations, invitations, grants, and honors (Hicks et al., 2015; Schimanski and Alperin, 2018; Braithwaite et al., 2019). Original articles represent an accessible primary basis to estimate the contribution of an author. A new approach takes into account publication record, offspring generation, and collaborative connections, and delivers a new citation-independent parameter named TeamTree product (TTP; Pfrieger, 2021). Based on this parameter, key players studying neurodegenerative diseases and cholesterol are exposed in Figure 5. Due to the high selectivity, only a small fraction of authors (5%) reached TTP values above zero. Notably, TTP values of authors were strongly correlated with citation-dependent measures such as the total number of citations or the H index (Figure 5C).
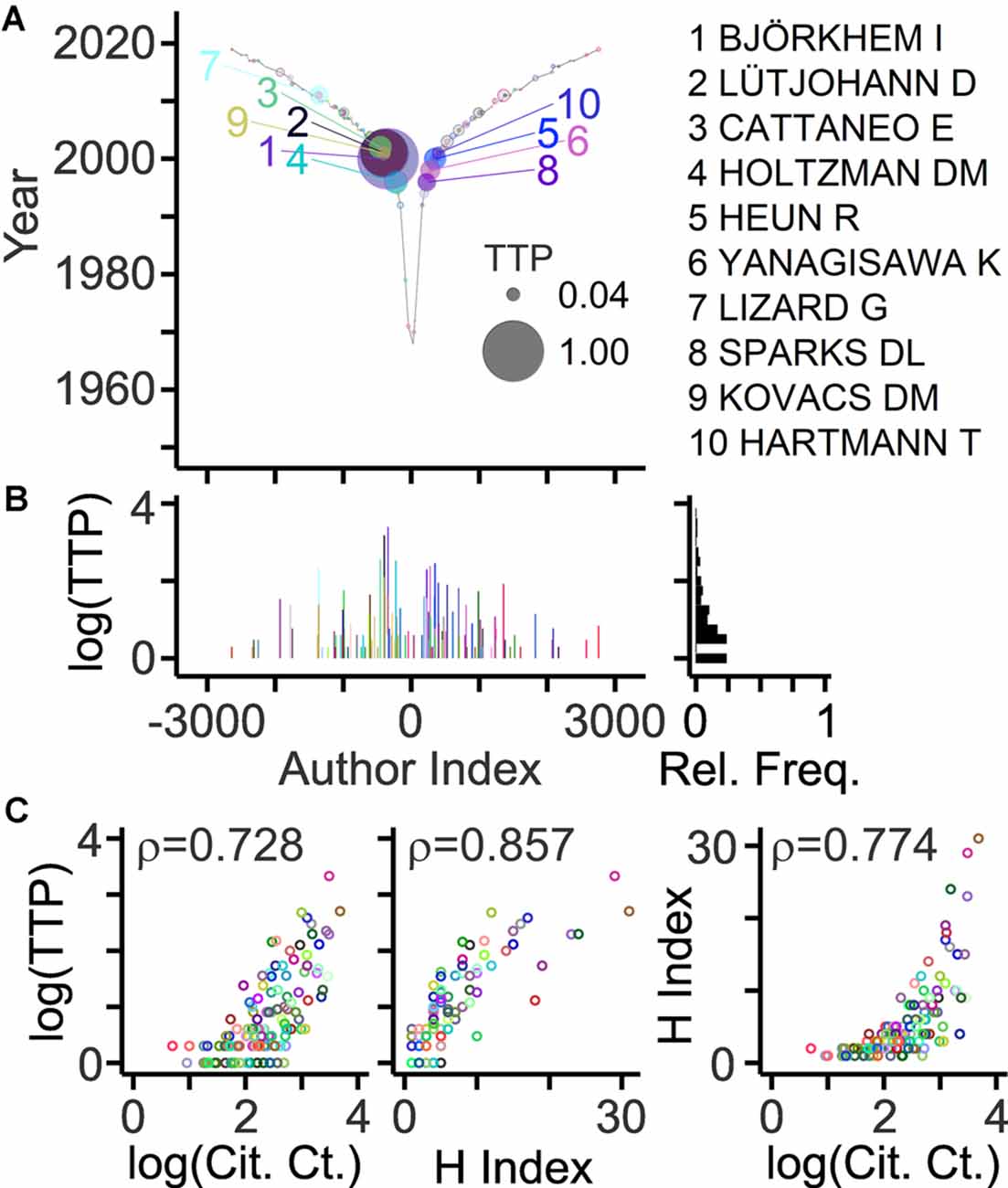
Figure 5. Author ranking based on the TeamTree product. (A) Graph showing the TeamTree product (TTP) of individual last authors in the field represented by their author indices. This new metric takes into account publication records, offspring training and mentorship, and collaborative connections. Numerically, it represents the product of PC (last author articles) × OC × CC. Circle sizes indicate TTP values normalized to the maximum. Colored circles and numbers indicate authors with 10 highest values. Their names are shown on the right. Gray circles with colored border indicate authors with TTP values above zero. (B) Log10(TTP) values and their relative frequency distribution. (C) Scatterplots with circles representing individual authors (indicated by color; different from panels A,B) with their TTP values (log10) plotted against the total number of citing articles (left; Cit. Ct.; log10 values) and their H indices (middle) and with their H indices plotted against the total number of citing articles (right; log10 values). Numbers represent correlation coefficients [Spearman’s rho values; two-sided test; n = 126; S = 90,803 (left)/47,558 (middle)/75,414 (right); p < 10−10]. Citation-related parameters were calculated from bibliographic records obtained by a Web of Science query (Clarivate Analytics).
To gain deeper insight, diseases with the largest numbers of publications were analyzed separately (Table 1). Notably, AD-related research produced half of the articles published in the field (Table 1). Overall, the fields showed marked differences with respect to length and growth pattern: MS has the longest and most continuous publication record (Figure 6). Except for two articles published in the 1960s, research on AD and cholesterol started in the 1980s. The subsequent growth of this field was probably triggered by discoveries that the epsilon allele of apolipoprotein E (Corder et al., 1993; Poirier et al., 1993; Rebeck et al., 1993; Saunders et al., 1993; Strittmatter et al., 1993) and high blood levels of cholesterol raise the risk of sporadic AD (Kivipelto et al., 2001). Parallel studies revealed connections between cholesterol and beta amyloid (Hartmann et al., 1994; Bodovitz and Klein, 1996; Avdulov et al., 1997; Howland et al., 1998; Simons et al., 1998; Refolo et al., 2000; Fassbender et al., 2001; Kojro et al., 2001; Puglielli et al., 2001; Runz et al., 2002; Wahrle et al., 2002) and between statins and AD (Wolozin et al., 2000; Refolo et al., 2001). The other disease fields are characterized by intermittent publication activity starting in the 1960s (HD) and 1970 (PD, ALS) and a more continuous development since 2000 (Figure 6). In the case of HD, pioneering studies showing links to cholesterol synthesis were published at the beginning of the 2000s (Sipione et al., 2002; Valenza et al., 2005). In all fields, the workforce grew more strongly than the number of publications (Figure 6) due to the increasing number of authors per article (Figure 1C). The ratios of author counts to publication counts were very similar across fields (6.6 ± 0.5; mean ± standard deviation; n = 5).
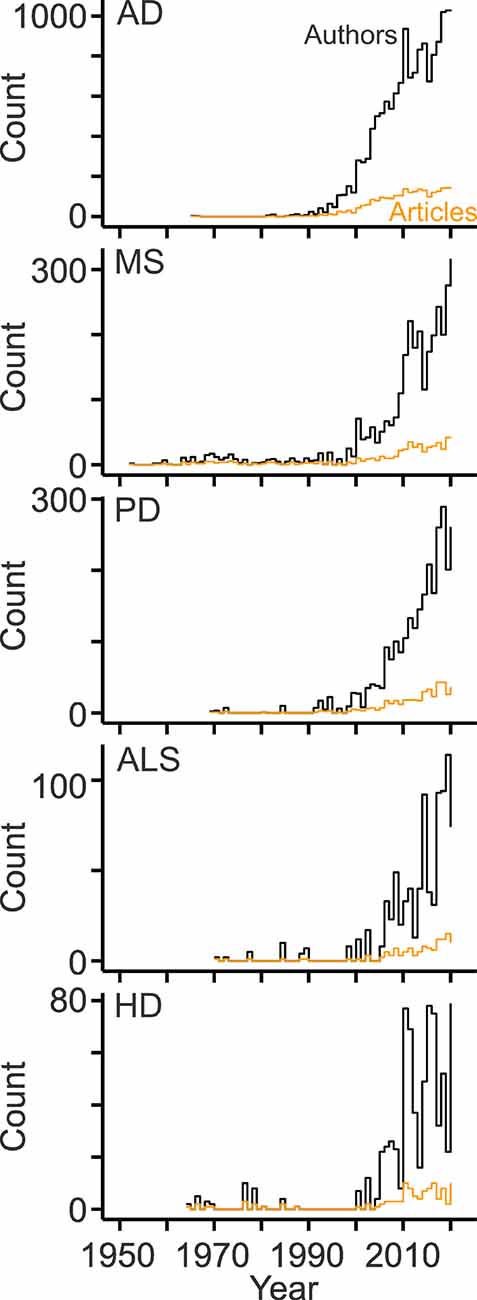
Figure 6. Development of the disease-specific workforce. Line plots showing counts of original articles (orange) and of the contributing authors (black) per year related to cholesterol and the indicated diseases. AD, Alzheimer’s disease; MS, multiple sclerosis; PD, Parkinson’s disease; ALS, amyotrophic lateral sclerosis; HD, Huntington’s disease.
In each field, most authors contributed single articles with their fractions ranging from the lowest value in AD to the highest in ALS (Figure 7A). Inversely, the AD and ALS fields showed the highest and lowest fraction of authors involved in collaborations, respectively (Figure 7A). Authors with family ties represented a minority of the workforce with disease-specific fractions between 3% and 13% (Figure 7A). The analysis also revealed relatively little overlap among the workforce of each disease. Only 6% of authors (146 out of 2,379) contributed articles to more than one field (Figure 7B) and established up to six connections among them with AD and PD showing the largest workforce overlap (Figure 7C).
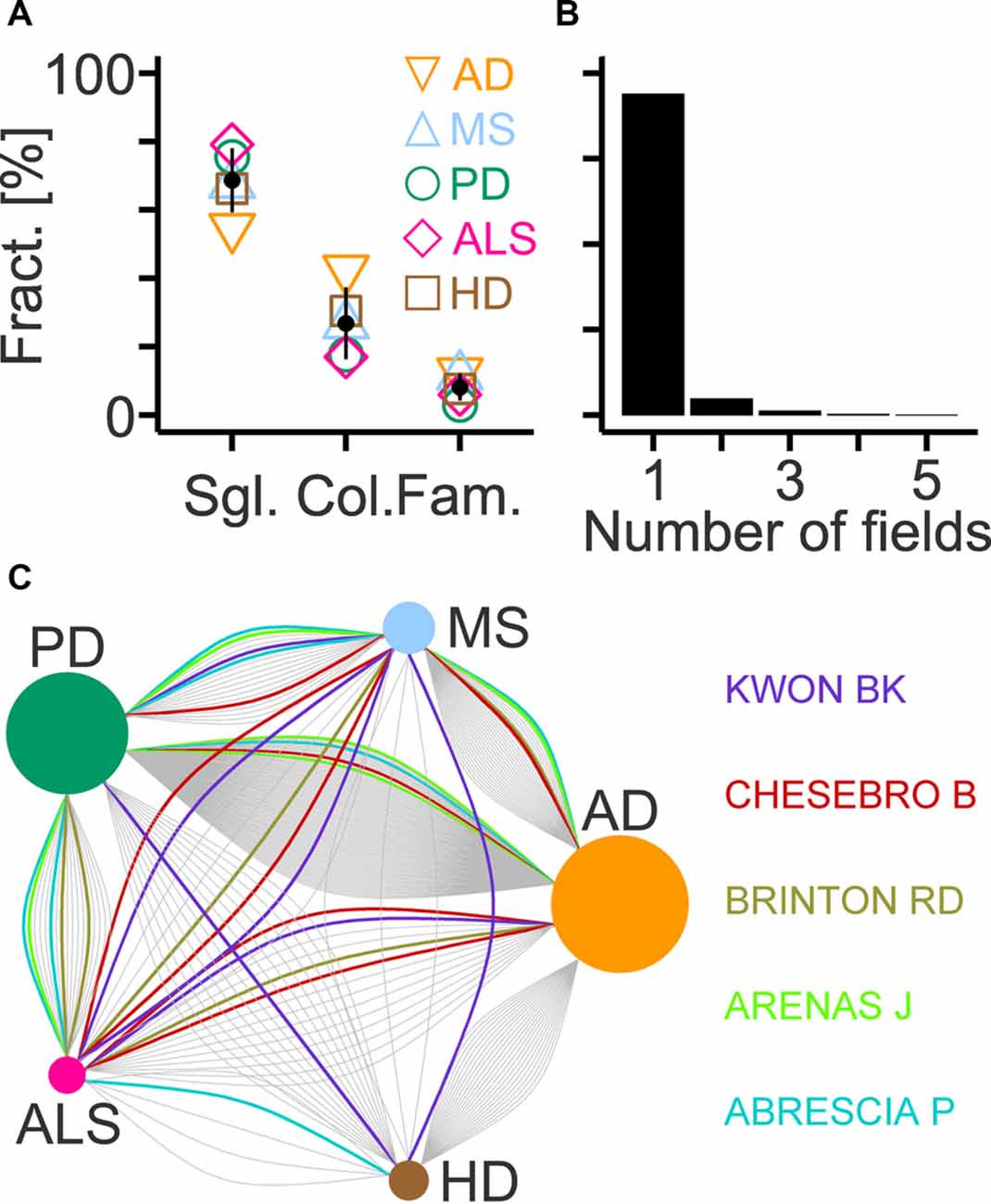
Figure 7. Workforce composition and overlap across selected diseases. (A) Fractions of authors contributing single articles compared to the total workforce (Sgl.), of collaborating authors among last authors (Col.) and of authors with family ties among last authors (Fam.) in indicated fields (AD, Alzheimer’s disease; MS, multiple sclerosis; PD, Parkinson’s disease; ALS, amyotrophic lateral sclerosis; HD, Huntington’s disease). Black circles and lines indicate mean and standard deviation (n = 5), respectively. (B) Histogram showing the fraction of last authors that contributed articles to the indicated number of fields. (C) Diagram showing connections between two diseases that are established by last authors contributing to both fields. Names and colored lines indicate the last authors with the highest number of connections (n = 6). Circle size represents the number of connections normalized to the maximum (AD; 160 links).
TeamTree graphs illustrate the workforce that studies links between cholesterol and the selected diseases (Table 1; Figure 8). Not surprisingly key players of the AD field dominate the global rankings (Figures 2–5, 8). The analysis shows further that OCs are particularly sensitive to the size of the field. In those with the lowest number of articles and the smallest workforce (PD, ALS, HD), authors produced maximally one offspring indicating that this parameter requires a critical mass of authors (Figure 8). The TTP values reveal distinct disease-specific origins of the top 10 contributors. Notably, in the AD field, these authors entered the field within one decade starting in the 1990s, whereas, in other fields, these contributors entered after the year 2000 (Figure 8).
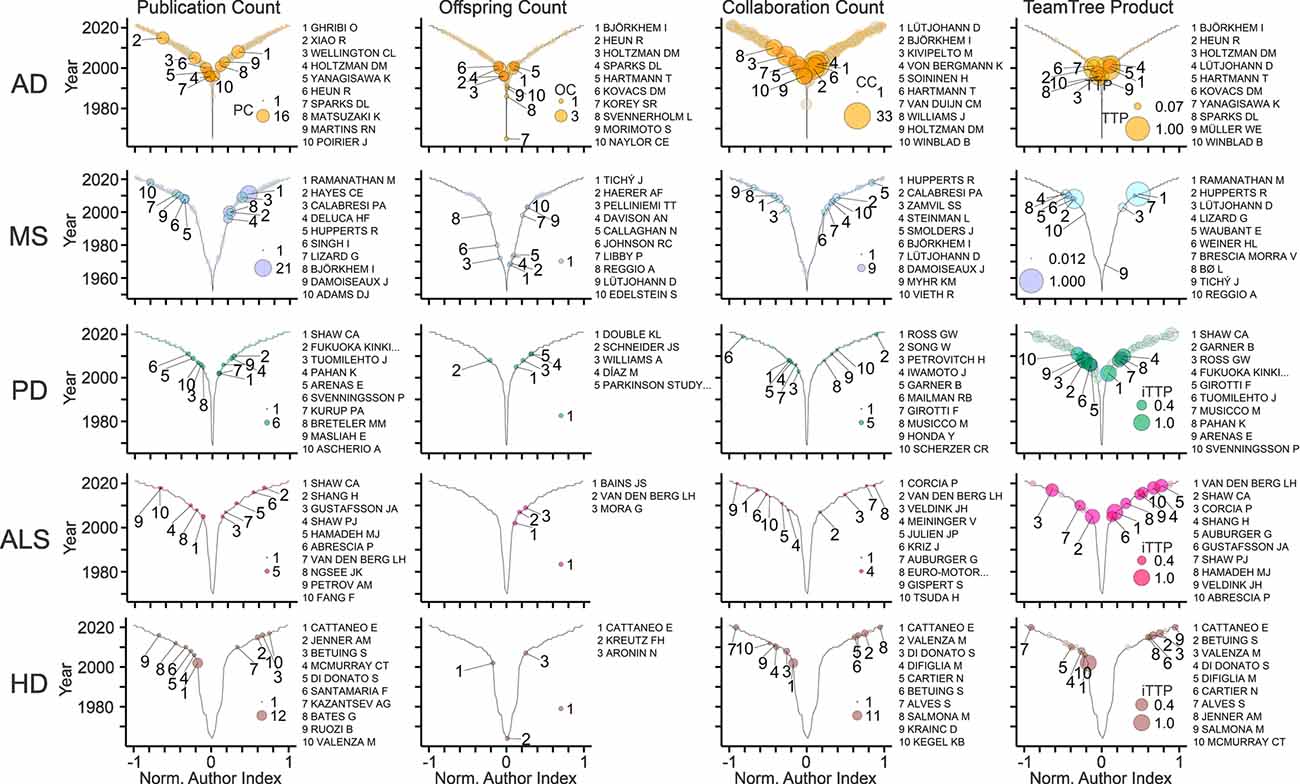
Figure 8. In-depth view on the field-specific workforce. TeamTree graphs showing counts of publications (PC), offspring (OC), collaborative connections (CC), and the TeamTree product (TTP) in the indicated fields (AD, Alzheimer’s disease; MS, multiple sclerosis; PD, Parkinson’s disease; ALS, amyotrophic lateral sclerosis; HD, Huntington’s disease) together with names of authors with the 10 largest values for each parameter. Note that for PD, ALS and HD, TTP values were replaced by an inclusive version of this measure (iTTP). For iTTP, zero counts of OC or CC values are set to one to include authors without offspring or lacking collaborators in the TTP-based ranking.
The new bibliometric analysis provides a detailed view of the development and structure of the workforce driving research on cholesterol and neurodegenerative diseases and complements content-specific summaries. The analysis revealed that the field started in the 1950s and remained relatively small until the 1990s. Except for MS, all fields showed intermittent publications, but a strong growth since 2000. The continuous expansion of the workforce during this period was mainly driven by authors contributing single articles although their contribution varied among the diseases analyzed. More than half of the articles are related to AD, therefore, the family ties, collaborative connections, and key players of this field dominate the overall picture. The analysis has caveats. A key challenge for this and other bibliometric approaches are ambiguous author names, as distinct authors can share the same name precluding correct evaluation (Smalheiser and Torvik, 2009). Evaluation of contributions based on single metrics such as TTP values is context-dependent, unsuited to evaluate junior scientists, and insensitive to ground-breaking contributions from small teams or from teams that contribute only briefly to a field.
FWP designed the review, performed literature queries, wrote and validated the code, analyzed the bibliographic records, prepared figures, and wrote the manuscript.
This work did not receive specific funding. APC were paid by the author’s institutional funding from the Centre National de la Recherche Scientifique.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The author would like to thank Dr. V. Pallottini for helpful comments on the manuscript.
Adorni, M. P., Ruscica, M., Ferri, N., Bernini, F., and Zimetti, F. (2019). Proprotein convertase subtilisin/kexin type 9, brain cholesterol homeostasis and potential implication for Alzheimer’s disease. Front. Aging Neurosci. 11:120. doi: 10.3389/fnagi.2019.00120
Arenas, F., Garcia-Ruiz, C., and Fernandez-Checa, J. C. (2017). Intracellular cholesterol trafficking and impact in neurodegeneration. Front. Mol. Neurosci. 10:382. doi: 10.3389/fnmol.2017.00382
Avdulov, N. A., Chochina, S. V., Igbavboa, U., Warden, C. S., Vassiliev, A. V., and Wood, W. G. (1997). Lipid binding to amyloid β-peptide aggregates: preferential binding of cholesterol as compared with phosphatidylcholine and fatty acids. J. Neurochem. 69, 1746–1752. doi: 10.1046/j.1471-4159.1997.69041746.x
Bandres-Ciga, S., Diez-Fairen, M., Kim, J. J., and Singleton, A. B. (2020). Genetics of Parkinson’s disease: an introspection of its journey towards precision medicine. Neurobiol. Dis. 137:104782. doi: 10.1016/j.nbd.2020.104782
Barboza, L. A., and Ghisi, N. C. (2018). Evaluating the current state of the art of Huntington disease research: a scientometric analysis. Braz. J. Med. Biol. Res. 51:e6299. doi: 10.1590/1414-431X20176299
Binotti, B., Jahn, R., and Pérez-Lara, Á. (2021). An overview of the synaptic vesicle lipid composition. Arch. Biochem. Biophys. 709:108966. doi: 10.1016/j.abb.2021.108966
Blauwendraat, C., Nalls, M. A., and Singleton, A. B. (2020). The genetic architecture of Parkinson’s disease. Lancet Neurol. 19, 170–178. doi: 10.1016/S1474-4422(19)30287-X
Bodovitz, S., and Klein, W. L. (1996). Cholesterol modulates α-secretase cleavage of amyloid precursor protein. J. Biol. Chem. 271, 4436–4440. doi: 10.1074/jbc.271.8.4436
Braithwaite, J., Herkes, J., Churruca, K., Long, J. C., Pomare, C., Boyling, C., et al. (2019). Comprehensive Researcher Achievement Model (CRAM): a framework for measuring researcher achievement, impact and influence derived from a systematic literature review of metrics and models. BMJ Open 9:e025320. doi: 10.1136/bmjopen-2018-025320
Chang, T. Y., Yamauchi, Y., Hasan, M. T., and Chang, C. (2017). Cellular cholesterol homeostasis and Alzheimer’s disease. J. Lipid Res. 58, 2239–2254. doi: 10.1194/jlr.R075630
Chia, R., Chiò, A., and Traynor, B. J. (2018). Novel genes associated with amyotrophic lateral sclerosis: diagnostic and clinical implications. Lancet Neurol. 17, 94–102. doi: 10.1016/S1474-4422(17)30401-5
Chiavacci, L. V., and Sperry, W. M. (1952). Concentration of cholesterol and of lipid phosphorus in blood serum in multiple sclerosis. AMA Arch. Neurol. Psychiatry 68, 37–42. doi: 10.1001/archneurpsyc.1952.02320190043003
Claxton, L. D. (2005). Scientific authorship: part 2. History, recurring issues, practices, and guidelines. Mutat. Res. 589, 31–45. doi: 10.1016/j.mrrev.2004.07.002
Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., Small, G. W., et al. (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923. doi: 10.1126/science.8346443
Dai, L., Zou, L., Meng, L., Qiang, G., Yan, M., and Zhang, Z. (2021). Cholesterol metabolism in neurodegenerative diseases: molecular mechanisms and therapeutic targets. Mol. Neurobiol. 58, 2183–2201. doi: 10.1007/s12035-020-02232-6
Deng, H., Wang, P., and Jankovic, J. (2018). The genetics of Parkinson disease. Ageing Res. Rev. 42, 72–85. doi: 10.1016/j.arr.2017.12.007
Dietschy, J. M. (2009). Central nervous system: cholesterol turnover, brain development and neurodegeneration. Biol. Chem. 390, 287–293. doi: 10.1515/BC.2009.035
Doria, M., Maugest, L., Moreau, T., Lizard, G., and Vejux, A. (2016). Contribution of cholesterol and oxysterols to the pathophysiology of Parkinson’s disease. Free Radic. Biol. Med. 101, 393–400. doi: 10.1016/j.freeradbiomed.2016.10.008
Du, Y.-H., Yang, R.-Y., Wang, Q., Wang, L.-Y., Liang, L.-C., Zhu, L., et al. (2021). Bibliometric analysis study on the mechanisms of brain energy metabolism disorders in Alzheimer’s disease from 2000 to 2020. Front. Neurol. 12:670220. doi: 10.3389/fneur.2021.670220
Duong, M. T., Nasrallah, I. M., Wolk, D. A., Chang, C. C. Y., and Chang, T. Y. (2021). Cholesterol, atherosclerosis, and APOE in vascular contributions to cognitive impairment and dementia (VCID): potential mechanisms and therapy. Front. Aging Neurosci. 13:647990. doi: 10.3389/fnagi.2021.647990
El-Hayek, Y. H., Wiley, R. E., Khoury, C. P., Daya, R. P., Ballard, C., Evans, A. R., et al. (2019). Tip of the iceberg: assessing the global socioeconomic costs of Alzheimer’s disease and related dementias and strategic implications for stakeholders. J. Alzheimers Dis. 70, 323–341. doi: 10.3233/JAD-190426
Elston, G. N., and Fujita, I. (2014). Pyramidal cell development: postnatal spinogenesis, dendritic growth, axon growth, and electrophysiology. Front. Neuroanat. 8:78. doi: 10.3389/fnana.2014.00078
Erkkinen, M. G., Kim, M. O., and Geschwind, M. D. (2018). Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 10:a033118. doi: 10.1101/cshperspect.a033118
Faissner, S., Plemel, J. R., Gold, R., and Yong, V. W. (2019). Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov. 18, 905–922. doi: 10.1038/s41573-019-0035-2
Fassbender, K., Simons, M., Bergmann, C., Stroick, M., Lutjohann, D., Keller, P., et al. (2001). Simvastatin strongly reduces levels of Alzheimer’s disease β -amyloid peptides Aβ 42 and Aβ 40 in vitro and in vivo. Proc. Natl. Acad. Sci. U S A 98, 5856–5861. doi: 10.1073/pnas.081620098
Feigin, V. L., Abajobir, A. A., Abate, K. H., Abd-Allah, F., Abdulle, A. M., Abera, S. F., et al. (2017). Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 16, 877–897. doi: 10.1016/S1474-4422(17)30299-5
Feringa, F. M., and van der Kant, R. (2021). Cholesterol and Alzheimer’s disease; from risk genes to pathological effects. Front. Aging Neurosci. 13:690372. doi: 10.3389/fnagi.2021.690372
Fu, H. J., Hardy, J., and Duff, K. E. (2018). Selective vulnerability in neurodegenerative diseases. Nat. Neurosci. 21, 1350–1358. doi: 10.1038/s41593-018-0221-2
Ga, L., Cookson, M. R., Petrucelli, L., and La Spade, A. R. (2018). Converging pathways in neurodegeneration, from genetics to mechanisms. Nat. Neurosci. 21, 1300–1309. doi: 10.1038/s41593-018-0237-7
García-Sanz, P., Aerts, J., and Moratalla, R. (2021). The role of cholesterol in α-synuclein and lewy body pathology in GBA1 Parkinson’s disease. Mov. Disord. 36, 1070–1085. doi: 10.1002/mds.28396
Gliozzi, M., Musolino, V., Bosco, F., Scicchitano, M., Scarano, F., Nucera, S., et al. (2021). Cholesterol homeostasis: researching a dialogue between the brain and peripheral tissues. Pharmacol. Res. 163:105215. doi: 10.1016/j.phrs.2020.105215
Goldstein, J. L., and Brown, M. S. (2015). A century of cholesterol and coronaries: from plaques to genes to statins. Cell 161, 161–172. doi: 10.1016/j.cell.2015.01.036
González-Guevara, E., Cárdenas, G., Pérez-Severiano, F., and Martínez-Lazcano, J. C. (2020). Dysregulated brain cholesterol metabolism is linked to neuroinflammation in Huntington’s disease. Mov. Disord. 35, 1113–1127. doi: 10.1002/mds.28089
Griffiths, W. J., and Wang, Y. (2019). Oxysterol research: a brief review. Biochem. Soc. Trans. 47, 517–526. doi: 10.1042/BST20180135
Guido, D., Morandi, G., Palluzzi, F., and Borroni, B. (2015). Telling the story of frontotemporal dementia by bibliometric analysis. J. Alzheimers Dis. 48, 703–709. doi: 10.3233/JAD-150275
Hartmann, H., Eckert, A., and Müller, W. E. (1994). Apolipoprotein E and cholesterol affect neuronal calcium signalling: the possible relationship to β-amyloid neurotoxicity. Biochem. Biophys. Res. Commun. 200, 1185–1192. doi: 10.1006/bbrc.1994.1576
Hicks, D., Wouters, P., Waltman, L., De Rijcke, S., and Rafols, I. (2015). The Leiden Manifesto for research metrics. Nature 520, 429–431. doi: 10.1038/520429a
Howland, D. S., Trusko, S. P., Savage, M. J., Reaume, A. G., Lang, D. M., Hirsch, J. D., et al. (1998). Modulation of secreted β-amyloid precursor protein and amyloid β-peptide in brain by cholesterol. J. Biol. Chem. 273, 16576–16582. doi: 10.1074/jbc.273.26.16576
Hussain, G., Wang, J., Rasul, A., Anwar, H., Imran, A., Qasim, M., et al. (2019). Role of cholesterol and sphingolipids in brain development and neurological diseases. Lipids Health Dis. 18:26. doi: 10.1186/s12944-019-0965-z
Ikonen, E. (2008). Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 9, 125–138. doi: 10.1038/nrm2336
Jeong, W., Lee, H., Cho, S., and Seo, J. (2019). ApoE4-induced cholesterol dysregulation and its brain cell type-specific implications in the pathogenesis of Alzheimer’s disease. Mol. Cells 42, 739–746. doi: 10.14348/molcells.2019.0200
Jin, U., Park, S. J., and Park, S. M. (2019). Cholesterol metabolism in the brain and its association with Parkinson’s disease. Exp. Neurobiol. 28, 554–567. doi: 10.5607/en.2019.28.5.554
Jucker, M., and Walker, L. C. (2018). Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases. Nat. Neurosci. 21, 1341–1349. doi: 10.1038/s41593-018-0238-6
Kalia, L. V., and Lang, A. E. (2015). Parkinson’s disease. Lancet 386, 896–912. doi: 10.1016/S0140-6736(14)61393-3
Kivipelto, M., Helkala, E. L., Laakso, M. P., Hanninen, T., Hallikainen, M., Alhainen, K., et al. (2001). Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. Br. Med. J. 322, 1447–1451. doi: 10.1136/bmj.322.7300.1447
Kojro, E., Gimpl, G., Lammich, S., Marz, W., and Fahrenholz, F. (2001). Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the α -secretase ADAM 10. Proc. Natl. Acad. Sci. U S A 98, 5815–5820. doi: 10.1073/pnas.081612998
Lassmann, H. (2019). Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front. Immunol. 9:3116. doi: 10.3389/fimmu.2018.03116
Leoni, V., and Caccia, C. (2015). The impairment of cholesterol metabolism in Huntington disease. Biochim. Biophys. Acta 1851, 1095–1105. doi: 10.1016/j.bbalip.2014.12.018
Li, Y., Fang, R., Liu, Z., Jiang, L., Zhang, J., Li, H., et al. (2021). The association between toxic pesticide environmental exposure and Alzheimer’s disease: a scientometric and visualization analysis. Chemosphere 263:128238. doi: 10.1016/j.chemosphere.2020.128238
Loera-Valencia, R., Goikolea, J., Parrado-Fernandez, C., Merino-Serrais, P., and Maioli, S. (2019). Alterations in cholesterol metabolism as a risk factor for developing Alzheimer’s disease: potential novel targets for treatment. J. Steroid Biochem. Mol. Biol. 190, 104–114. doi: 10.1016/j.jsbmb.2019.03.003
Loftus, S. K., Morris, J. A., Carstea, E. D., Gu, J. Z., Cummings, C., Brown, A., et al. (1997). Murine model of niemann-pick C disease: mutation in a cholesterol homeostasis gene. Science 277, 232–235. doi: 10.1126/science.277.5323.232
Long, J. M., and Holtzman, D. M. (2019). Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179, 312–339. doi: 10.1016/j.cell.2019.09.001
Luo, J., Yang, H. Y., and Song, B.-L. (2020). Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 21, 225–245. doi: 10.1038/s41580-019-0190-7
Mahley, R. W. (2016). Central nervous system lipoproteins: apoe and regulation of cholesterol metabolism. Arterioscler. Thromb. Vasc. Biol. 36, 1305–1315. doi: 10.1161/ATVBAHA.116.307023
Malik, I., Kelley, C. P., Wang, E. T., and Todd, P. K. (2021). Molecular mechanisms underlying nucleotide repeat expansion disorders. Nat. Rev. Mol. Cell Biol. 22, 589–607. doi: 10.1038/s41580-021-00382-6
Martín, M. G., Pfrieger, F., and Dotti, C. G. (2014). Cholesterol in brain disease: sometimes determinant and frequently implicated. EMBO Rep. 15, 1036–1052. doi: 10.15252/embr.201439225
Marušić, A., Bošnjak, L., and Jerončić, A. (2011). A systematic review of research on the meaning, ethics and practices of authorship across scholarly disciplines. PLoS One 6:e23477. doi: 10.1371/journal.pone.0023477
McFarlane, O., and Kędziora-Kornatowska, K. (2020). Cholesterol and dementia: a long and complicated relationship. Curr. Aging Sci. 13, 42–51. doi: 10.2174/1874609812666190917155400
Mejzini, R., Flynn, L. L., Pitout, L. L., Fletcher, S., Wilton, S. D., and Akkari, P. A. (2019). ALS genetics, mechanisms, and therapeutics: where are we now? Front. Neurosci. 13:1310. doi: 10.3389/fnins.2019.01310
Miller, W. L., and Auchus, R. J. (2011). The molecular biology, biochemistry ,and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32, 81–151. doi: 10.1210/er.2010-0013
Mori, F., and Barucci, M. (1951). [Cholesterol content of the adrenals in dementia]. Boll. Soc. Ital. Biol. Sper. 27, 1029–1030.
Moutinho, M., Nunes, M. J., and Rodrigues, E. (2016). Cholesterol 24-hydroxylase: brain cholesterol metabolism and beyond. Biochim. Biophys. Acta 1861, 1911–1920. doi: 10.1016/j.bbalip.2016.09.011
Mutemberezi, V., Guillemot-Legris, O., and Muccioli, G. G. (2016). Oxysterols: from cholesterol metabolites to key mediators. Prog. Lipid Res. 64, 152–169. doi: 10.1016/j.plipres.2016.09.002
Naureckiene, S., Sleat, D., Lackland, H., Fensom, A., Vanier, M. T., Wattiaux, R., et al. (2000). Identification of HE1 as the second gene of niemann-pick C disease. Science 290, 2298–2301. doi: 10.1126/science.290.5500.2298
Neumann, M., Sampathu, D. M., Kwong, L. K., Truax, A. C., Micsenyi, M. C., Chou, T. T., et al. (2006). Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133. doi: 10.1126/science.1134108
Newman, M. E. (2001). The structure of scientific collaboration networks. Proc. Natl. Acad. Sci. U S A 98, 404–409. doi: 10.1073/pnas.021544898
Oberheim, N. A., Takano, T., Han, X., He, W., Lin, J. H., Wang, F., et al. (2009). Uniquely hominid features of adult human astrocytes. J. Neurosci. 29, 3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009
Petrov, A. M., and Pikuleva, I. A. (2019). Cholesterol 24-hydroxylation by CYP46A1: benefits of modulation for brain diseases. Neurotherapeutics 16, 635–648. doi: 10.1007/s13311-019-00731-6
Pfrieger, F. W. (2021). TeamTree analysis: a new approach to evaluate scientific production. PLoS One 16:e0253847. doi: 10.1371/journal.pone.0253847
Pfrieger, F. W., and Ungerer, N. (2011). Cholesterol metabolism in neurons and astrocytes. Prog. Lipid Res. 50, 357–371. doi: 10.1016/j.plipres.2011.06.002
Pikuleva, I. A., and Cartier, N. (2021). Cholesterol hydroxylating cytochrome P450 46A1: from mechanisms of action to clinical applications. Front. Aging Neurosci. 13:696778. doi: 10.3389/fnagi.2021.696778
Poirier, J., Davignon, J., Bouthillier, D., Kogan, S., Bertrand, P., and Gauthier, S. (1993). Apolipoprotein E polymorphism and Alzheimer’s disease. Lancet 342, 697–699. doi: 10.1016/0140-6736(93)91705-q
Poser, C. M., and Curran, G. L. (1958). Cerebrospinal fluid free cholesterol as index of activity of multiple sclerosis and allied diseases. AMA Arch. Neurol. Psychiatry 80, 304–313. doi: 10.1001/archneurpsyc.1958.02340090040005
Prabhu, A. V., Luu, W. N., Li, D. F., Sharpe, L. J., and Brown, A. J. (2016). DHCR7: a vital enzyme switch between cholesterol and vitamin D production. Prog. Lipid Res. 64, 138–151. doi: 10.1016/j.plipres.2016.09.003
Puglielli, L., Konopka, G., Pack-Chung, E., Ingano, L. A., Berezovska, O., Hyman, B. T., et al. (2001). Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid β-peptide. Nat. Cell Biol. 3, 905–912. doi: 10.1038/ncb1001-905
Rebeck, G. W., Reiter, J. S., Strickland, D. K., and Hyman, B. T. (1993). Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron 11, 575–580. doi: 10.1016/0896-6273(93)90070-8
Refolo, L. M., Pappolla, M. A., Lafrancois, J., Malester, B., Schmidt, S. D., Thomas-Bryant, T., et al. (2001). A cholesterol-lowering drug reduces β-amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 8, 890–899. doi: 10.1006/nbdi.2001.0422
Refolo, L. M., Pappolla, M. A., Malester, B., Lafrancois, J., Bryant-Thomas, T., Wang, R., et al. (2000). Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol. Dis. 7, 321–331. doi: 10.1006/nbdi.2000.0304
Reich, D. S., Lucchinetti, C. F., and Calabresi, P. A. (2018). Multiple sclerosis. N. Engl. J. Med. 378, 169–180. doi: 10.1056/NEJMra1401483
Rizzi, L., Aventurato, Í. K., and Balthazar, M. L. F. (2021). Neuroimaging research on dementia in brazil in the last decade: scientometric analysis, challenges, and peculiarities. Front. Neurol. 12:640525. doi: 10.3389/fneur.2021.640525
Rocha, E. M., De Miranda, B., and Sanders, L. H. (2018). α-synuclein: pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 109, 249–257. doi: 10.1016/j.nbd.2017.04.004
Rosen, D. R., Siddique, T., Patterson, D., Figlewicz, D. A., Sapp, P., Hentati, A., et al. (1993). Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62. doi: 10.1038/362059a0
Runz, H., Rietdorf, J., Tomic, I., De Bernard, M., Beyreuther, K., Pepperkok, R., et al. (2002). Inhibition of intracellular cholesterol transport alters presenilin localization and amyloid precursor protein processing in neuronal cells. J. Neurosci. 22, 1679–1689. doi: 10.1523/JNEUROSCI.22-05-01679.2002
Sáiz-Vazquez, O., Puente-Martinez, A., Ubillos-Landa, S., Pacheco-Bonrostro, J., and Santabarbara, J. (2020). Cholesterol and Alzheimer’s disease risk: a meta-meta-analysis. Brain Sci. 10:386. doi: 10.3390/brainsci10060386
Samant, N. P., and Gupta, G. L. (2021). Novel therapeutic strategies for Alzheimer’s disease targeting brain cholesterol homeostasis. Eur J. Neurosci. 53, 673–686. doi: 10.1111/ejn.14949
Saunders, A. M., Strittmatter, W. J., Schmechel, D., George-Hyslop, P. H., Pericak-Vance, M. A., Joo, S. H., et al. (1993). Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 43, 1467–1472. doi: 10.1212/wnl.43.8.1467
Savelieff, M. G., Nam, G., Kang, J., Lee, H. J., Lee, M., and Lim, M. H. (2019). Development of multifunctional molecules as potential therapeutic candidates for Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis in the last decade. Chem. Rev. 119, 1221–1322. doi: 10.1021/acs.chemrev.8b00138
Scanu, A., Sinisi, C., Mancini, M., and Schiano, S. (1955). [Cholesterol and lipoproteins in the humoral picture of senile and atherosclerotic dementia]. Osp. Psichiatr. 23, 183–194.
Schimanski, L. A., and Alperin, J. P. (2018). The evaluation of scholarship in academic promotion and tenure processes: past, present, and future. F1000Res. 7, 1605–1605. doi: 10.12688/f1000research.16493.1
Schmitt, S., Castelvetri, L. C., and Simons, M. (2015). Metabolism and functions of lipids in myelin. Biochim. Biophys. Acta 1851, 999–1005. doi: 10.1016/j.bbalip.2014.12.016
Schwartz, J. L., Jones, K. L., and Yeo, G. W. (2021). Repeat RNA expansion disorders of the nervous system: post-transcriptional mechanisms and therapeutic strategies. Crit. Rev Biochem. Mol. Biol. 56, 31–53. doi: 10.1080/10409238.2020.1841726
Segatto, M., Tonini, C., Pfrieger, F. W., Trezza, V., and Pallottini, V. (2019). Loss of mevalonate/cholesterol homeostasis in the brain: a focus on autism spectrum disorder and rett syndrome. Int. J. Mol. Sci. 20:3317. doi: 10.3390/ijms20133317
Simons, M., Keller, P., De Strooper, B., Beyreuther, K., Dotti, C. G., and Simons, K. (1998). Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons. Proc. Natl. Acad. Sci. U S A 95, 6460–6464. doi: 10.1073/pnas.95.11.6460
Sipione, S., Rigamonti, D., Valenza, M., Zuccato, C., Conti, L., Pritchard, J., et al. (2002). Early transcriptional profiles in huntingtin-inducible striatal cells by microarray analyses. Hum. Mol. Genet. 11, 1953–1965. doi: 10.1093/hmg/11.17.1953
Smalheiser, N. R., and Torvik, V. I. (2009). Author name disambiguation. Annu. Rev. Inf. Sci. Technol. 43, 287–313. doi: 10.1002/aris.2009.1440430113
Strittmatter, W. J., Saunders, A. M., Schmechel, D., Pericak-Vance, M., Enghild, J., Salvesen, G. S., et al. (1993). Apolipoprotein E: high-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. U S A 90, 1977–1981. doi: 10.1073/pnas.90.5.1977
Tall, A. R., and Yvan-Charvet, L. (2015). Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 15, 104–116. doi: 10.1038/nri3793
Testa, G., Rossin, D., Poli, G., Biasi, F., and Leonarduzzi, G. (2018). Implication of oxysterols in chronic inflammatory human diseases. Biochimie 153, 220–231. doi: 10.1016/j.biochi.2018.06.006
The Huntington’s Disease Collaborative Research Group. (1993). A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s disease collaborative research group. Cell 72, 971–983. doi: 10.1016/0092-8674(93)90585-e
Valenza, M., Rigamonti, D., Goffredo, D., Zuccato, C., Fenu, S., Jamot, L., et al. (2005). Dysfunction of the cholesterol biosynthetic pathway in Huntington’s disease. J. Neurosci. 25, 9932–9939. doi: 10.1523/JNEUROSCI.3355-05.2005
Vanier, M. T. (2010). Niemann-Pick disease type C. Orphanet. J. Rare Dis. 5:16. doi: 10.1186/1750-1172-5-16
Voet, S., Prinz, M., and Van Loo, G. (2019). Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol. Med. 25, 112–123. doi: 10.1016/j.molmed.2018.11.005
Wahrle, S., Das, P., Nyborg, A. C., Mclendon, C., Shoji, M., Kawarabayashi, T., et al. (2002). Cholesterol-dependent γ-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol. Dis. 9, 11–23. doi: 10.1006/nbdi.2001.0470
Wang, Y., Yutuc, E., and Griffiths, W. J. (2021). Neuro-oxysterols and neuro-sterols as ligands to nuclear receptors, GPCRs, ligand-gated ion channels and other protein receptors. Br. J. Pharmacol. 178, 3176–3193. doi: 10.1111/bph.15191
Wolozin, B., Kellman, W., Ruosseau, P., Celesia, G. G., and Siegel, G. (2000). Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch. Neurol. 57, 1439–1443. doi: 10.1001/archneur.57.10.1439
Yeagle, P. L. (1985). Cholesterol and the cell membrane. Biochim. Biophys. Acta 822, 267–287. doi: 10.1016/0304-4157(85)90011-5
Yoon, H., Flores, L. F., and Kim, J. (2016). MicroRNAs in brain cholesterol metabolism and their implications for Alzheimer’s disease. Biochim. Biophys. Acta 1861, 2139–2147. doi: 10.1016/j.bbalip.2016.04.020
Zarrouk, A., Debbabi, M., Bezine, M., Karym, E. M., Badreddine, A., Rouaud, O., et al. (2018). Lipid biomarkers in Alzheimer’s disease. Curr. Alzheimer Res. 15, 303–312. doi: 10.2174/1567205014666170505101426
Zarrouk, A., Vejux, A., Mackrill, J., O’callaghan, Y., Hammami, M., O’brien, N., et al. (2014). Involvement of oxysterols in age-related diseases and ageing processes. Ageing Res. Rev. 18, 148–162. doi: 10.1016/j.arr.2014.09.006
Zhang, J., and Liu, Q. (2015). Cholesterol metabolism and homeostasis in the brain. Protein Cell 6, 254–264. doi: 10.1007/s13238-014-0131-3
Keywords: neurologic disease, bibliometric analyses, scientific impact, sterol, research evaluation, informetric, scientometric, key opinion leader
Citation: Pfrieger FW (2021) Neurodegenerative Diseases and Cholesterol: Seeing the Field Through the Players. Front. Aging Neurosci. 13:766587. doi: 10.3389/fnagi.2021.766587
Received: 29 August 2021; Accepted: 14 October 2021;
Published: 03 November 2021.
Edited by:
Irina A. Pikuleva, Case Western Reserve University, United StatesReviewed by:
Elena Cattaneo, University of Milan, ItalyCopyright © 2021 Pfrieger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frank W. Pfrieger, ZnctcGZyaWVnZXJAZ214LmRl; ZnJhbmsucGZyaWVnZXJAdW5pc3RyYS5mcg== orcid.org/0000-0001-7085-1431
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.