
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 10 December 2021
Sec. Neurocognitive Aging and Behavior
Volume 13 - 2021 | https://doi.org/10.3389/fnagi.2021.763264
This article is part of the Research Topic The Impact of Age-Related Changes in Brain Network Organization and Sleep on Memory View all 8 articles
 Omonigho M. Bubu1,2*
Omonigho M. Bubu1,2* Ellita T. Williams2
Ellita T. Williams2 Ogie Q. Umasabor-Bubu3
Ogie Q. Umasabor-Bubu3 Sonya S. Kaur4
Sonya S. Kaur4 Arlener D. Turner5
Arlener D. Turner5 Judite Blanc5
Judite Blanc5 Jaime Ramos Cejudo1
Jaime Ramos Cejudo1 Anna E. Mullins6
Anna E. Mullins6 Ankit Parekh6
Ankit Parekh6 Korey Kam6
Korey Kam6 Zainab T. Osakwe7
Zainab T. Osakwe7 Ann W. Nguyen8
Ann W. Nguyen8 Antoine R. Trammell9
Antoine R. Trammell9 Alfred K. Mbah10
Alfred K. Mbah10 Mony de Leon11
Mony de Leon11 David M. Rapoport6
David M. Rapoport6 Indu Ayappa6
Indu Ayappa6 Gbenga Ogedegbe2
Gbenga Ogedegbe2 Girardin Jean-Louis1,5
Girardin Jean-Louis1,5 Arjun V. Masurkar12
Arjun V. Masurkar12 Andrew W. Varga6
Andrew W. Varga6 Ricardo S. Osorio1,13*
Ricardo S. Osorio1,13*
Background: To determine whether sleep disturbance (SD) and vascular-risk interact to promote Alzheimer’s disease (AD) stage-progression in normal, community-dwelling older adults and evaluate their combined risk beyond that of established AD biomarkers.
Methods: Longitudinal data from the National Alzheimer’s Coordinating Center Uniform-Dataset. SD data (i.e., SD+ vs. SD-), as characterized by the Neuropsychiatric Inventory-Questionnaire, were derived from 10,600 participants at baseline, with at-least one follow-up visit. A subset (n = 361) had baseline cerebrospinal fluid (CSF) biomarkers and MRI data. The Framingham heart study general cardiovascular disease (FHS-CVD) risk-score was used to quantify vascular risk. Amnestic mild cognitive impairment (aMCI) diagnosis during follow-up characterized AD stage-progression. Logistic mixed-effects models with random intercept and slope examined the interaction of SD and vascular risk on prospective aMCI diagnosis.
Results: Of the 10,600 participants, 1,017 (9.6%) reported SD and 6,572 (62%) were female. The overall mean (SD) age was 70.5 (6.5), and follow-up time was 5.1 (2.7) years. SD and the FHS-CVD risk-score were each associated with incident aMCI (aOR: 1.42 and aOR: 2.11, p < 0.01 for both). The interaction of SD and FHS-CVD risk-score with time was significant (aOR: 2.87, p < 0.01), suggesting a synergistic effect. SD and FHS-CVD risk-score estimates remained significantly associated with incident aMCI even after adjusting for CSF (Aβ, T-tau, P-tau) and hippocampal volume (n = 361) (aOR: 2.55, p < 0.01), and approximated risk-estimates of each biomarker in the sample where data was available.
Conclusions: Clinical measures of sleep and vascular risk may complement current AD biomarkers in assessing risk of cognitive decline in older adults.
Mounting evidence recognizes Alzheimer’s disease (AD) as a multifactorial and heterogeneous disease with multiple contributors to its pathophysiology, including disturbed sleep (Bubu et al., 2017) and vascular risk factors (VRFs) (Gottesman et al., 2017). In cognitively normal individuals, disturbed sleep and VRFs have each been associated with brain Aβ deposition, tau aggregation, and neurodegeneration (Bangen et al., 2015; Branger et al., 2016; Lucey et al., 2019). All three are robust predictors and markers of future cognitive decline and the development of AD. However, identifying these biological markers requires the use of expensive and invasive methods like PET imaging and lumbar puncture for cerebrospinal fluid (CSF) collection. Therefore, it is imperative to improve the clinical characterization of the preclinical stages and identify additional risk factors and non-invasive, cost-effective surrogates of established AD biomarkers.
On the one hand and relevant to this study, our group and others have shown that self-reported sleep disturbances (SD) are associated with amyloid and tau pathology (Spira et al., 2013; Bubu et al., 2020). Objectively, changes in sleep characteristics, such as decreased non-rapid eye movement sleep (NREM) slow-wave activity (SWA), have been proposed as markers for amyloid and tau pathology (Winer et al., 2019). Poor sleep quality and sleep-EEG alterations also co-occur in mild cognitive impairment (MCI) and AD (Hita-Yanez et al., 2013). SDs can also predict a decline in simple measures of memory and global cognitive functioning in cognitively normal older adults at 1 year of follow-up (Potvin et al., 2012). Recently, we showed that reduced spindle density during NREM stage 2 sleep may represent an early dysfunction related to tau, possibly reflecting axonal damage or altered neuronal tau secretion (Kam et al., 2019). On the other hand, neuropathological studies indicate that vascular brain changes frequently co-occur with AD pathology and may lower the threshold for cognitive impairment (Esiri et al., 1999). Importantly, VRFs including hypertension are associated with lower brain glucose metabolism (Langbaum et al., 2012) and also higher Aβ(Langbaum et al., 2012; Gottesman et al., 2017) and tau burden (Langbaum et al., 2012; Gottesman et al., 2017). VRFs also act synergistically with Aβ burden to promote cognitive decline (Rabin et al., 2018). The combined impact of Aβ burden and cerebrovascular pathology has generally demonstrated additive effects on cognition (Marchant et al., 2013). Of significance to our study hypothesis is the fact that SD and VRFs commonly co-occur (Gottlieb et al., 2006; Gangwisch et al., 2007), as normal sleep significantly affects the cardiovascular system, with varying levels of autonomic regulation occurring during the different sleep stages (Legramante and Galante, 2005). Large epidemiologic studies also show self-reported SD associated with incident and prevalent VRFs including hypertension and diabetes (Gottlieb et al., 2006; Gangwisch et al., 2007). However, many patients with SD do not exhibit daytime symptoms and/or attribute sleep problems to cardiometabolic outcomes. Since multiple VRFs often coexist (Genest and Cohn, 1995) and incrementally increase the risk for AD (Luchsinger et al., 2005), the co-occurrence of VRFs and SD would be an important target for successful prevention strategies for AD. Furthermore, sleep and VRFs may serve as additional physiologic risks that may complement current (and future) biomarkers in assessing the risk of cognitive decline in older adults.
Using clinical setting data from a publicly available national repository, we examined whether SD was associated with prospective cognitive decline in a large cohort of cognitively normal older adults. We also examined time effects as it relates to the progression and conversion rates across SD groups. Second, we determined whether SD’s effect on prospective cognitive decline would be additive or synergistic with a well-validated, multivariable measure of vascular risk. Third, we evaluated the unique influence of the combined SD/VRFs effect beyond that of the commonly used AD biomarkers, including levels of CSF-Aβ, T-tau, P-tau, and hippocampal volume.
Prospective longitudinal study utilizing data derived from the National Alzheimer’s Coordinating Center (NACC) uniform data set (UDS) covering the period from September 2005 to December 2018. NACC UDS is a data resource funded by the National Institute of Aging (NIA) and located at the University of Washington. The origin and development of the UDS are described elsewhere (Besser et al., 2018). Briefly, the UDS is a data repository containing deidentified clinical research data collected by the 33 NIA funded Alzheimer’s Disease Research Centers (ADRC). These deidentified data are made available to researchers by the NACC via a formal request to the NACC Steering Committee through the NACC website1. Each NACC individual site conducted its procedures in compliance with appropriate local laws, guidelines, and institutional review boards.
Data were derived from 10,600 participants, aged between 65 and 99 years who were cognitively normal at baseline, with at least one completed UDS follow-up visit. Participants were required to have baseline medical data to quantify vascular risk using the Framingham Heart Study general cardiovascular disease (FHS-CVD) risk score. This group of participant data was used to examine the independent and combined effects of baseline SD and VRFs on prospective cognitive decline. A subset of the participants (n = 361) had baseline AD CSF biomarkers (i.e., CSF-Aβ, CSF P-tau, and CSF T-tau) and structural brain MRI data with at least one UDS follow-up visit. These individuals completed both lumbar puncture and MRI protocols. Data from this subset were used to evaluate the unique influence of the SD/FHS-CVD risk score combined risk on prospective cognitive decline beyond that of the established AD biomarkers.
The NACC UDS repository lacks objective sleep measures, like polysomnography or actigraphy data, and other validated sleep questionnaires. NACC UDS only recently started collecting sleep disorders data. As such, the Neuropsychiatric Inventory Questionnaire (NPI-Q) (Cummings et al., 1994), which has been validated to evaluate psychopathology in dementia, was used to characterize SD. A trained health professional, via informant interview, administers the NPI-Q. Data includes an informant report, also covering severity, on the patient’s behaviors during the preceding month. Our focus was on the nighttime behavior disturbance item. Refer to Cummings et al. (1994) for specific wording of other NPI-Q items. Informant response to the question “Does the patient awaken you at night, rise too early in the morning, or take excessive naps during the day?” was categorized as positive (i.e., yes) or negative (i.e., no) for nighttime SD. Since this NPI-Q item characterizes nighttime behavioral disturbance and could potentially be conflated with other psychiatric conditions, especially if interpreted within the contexts of the participants’ possible neuropsychiatric/behavioral problems, we controlled for the possible effects of other NPI-Q reported psychiatric symptoms, such as hallucinations, delusions, apathy, and agitation in our analysis.
Vascular risk was quantified using the FHS-CVD risk score (D’Agostino et al., 2008), which provides a 10-year probability of future cardiovascular events (defined as coronary death, myocardial infarction, coronary insufficiency, angina, ischemic stroke, hemorrhagic stroke, transient ischemic attack, peripheral artery disease, and heart failure). Using baseline data, the FHS-CVD risk score was calculated. This score represents a weighted sum of age, sex, antihypertensive treatment (yes or no), systolic blood pressure (millimeters of mercury), body mass index, history of diabetes (yes or no), and current cigarette smoking status (yes or no). Higher scores represent a greater risk of cardiovascular events. In this sample, scores ranged from 5% to 89%. Similar to a previous study (Rabin et al., 2018), for stratified analyses and visualization purposes, participants were split into tertiles [at a FHSCVD risk score of 19% (lowest), 38% (middle), and 56% (highest)].
For this analysis, CSF values were available from a subset of clinically normal UDS participants (n = 361) from a small number of centers. ADRCs use different lab assay kits which provide ranges for amyloid beta and tau that are scaled completely differently for each brand of kit, have different ranges, and different cut-off values for positivity. Therefore, we utilized CSF data that were measured using the same assay method (i.e., ELISA) and standardized the data with center or batch-wise rescaling of the coefficient of variation from 20 to 10%, prior to combining the data for analyses. Site-by-site analyses limited the data and there was significant variation in the effect sizes though all in the same direction. Rescaling was performed using linear regression controlling for center-ID, age-at-baseline, sex, APOE4-status, and years-of-education with a reference batch. Very few participants have longitudinal CSF data; as such, NACC strongly cautioned using them for longitudinal analysis. CSF data used for this analysis were obtained only from baseline visits between September 2005 and October 2017.
Only a small subset of the clinically normal UDS participants had both structural MRI and CSF data available (n = 1,607); however, for this analysis, we excluded subjects whose CSF assay method was not ELISA and whose MRI data was not T1-weighted, leaving us with 361 subjects. Cortical reconstruction and volumetric segmentation were performed with the Freesurfer image analysis suite, which is documented and freely available for download online2. Data from this subset were used to evaluate the unique influence of the SD/FHS-CVD risk score combined risk on prospective cognitive decline beyond that of established AD biomarkers. Participants included in this analysis underwent high-resolution T1-weighted anatomical imaging to measure hippocampal volume. Hippocampal volumes were adjusted for total intracranial volumes by including total intracranial volumes as a covariate in the analytic models (Jack et al., 1989). Clinical UDS data were generally restricted to scans that were within ± 6 months from a UDS visit.
Participants at the ADRCs undergo detailed clinical and neuropsychological evaluation encompassing global cognitive functioning, processing speed, attention, working memory, executive functions, and episodic memory. Composite Z-scores are calculated for each cognitive domain, and a team of multidisciplinary clinicians (including neurologists and neuropsychologists) makes a clinical consensus diagnosis. Cognitive normal and MCI subjects scored between 24 and 30 on the mini-mental state examination (MMSE) whereas AD subjects scored between 20 and 26. Cognitive normal and MCI participants had global clinical dementia rating (CDR) scores of 0 and 0.5, respectively. At baseline, all participants had MMSE scores greater than 27, a CDR of 0, had ≤ 5 on the shorter version of the geriatric depression scale (Yesavage et al., 1982), and had a consensus clinical diagnosis of cognitively normal. For this analysis, our primary outcome was an incident amnestic MCI diagnosis via a consensus diagnosis using established clinical diagnostic criteria (McKhann et al., 1984) during UDS follow-up. As a secondary clinical outcome, we also investigated MCI as a global outcome (i.e., including both amnestic and non-amnestic MCI).
Covariates were selected a priori and included age, sex, BMI, education, and apolipoprotein E (APOE) ε4 status that was determined by the presence of at least one ε4 allele, clinical history of diabetes, hypertension, smoking, marital status, living arrangement, NPI-Q assessed comorbidity, and informant characteristics.
National Alzheimer’s Coordinating Center database has unbalanced data with an unequal number of measurements for each study participant. As such, our analyses included cox, multivariate analysis of covariance (MANCOVA), and multilevel mixed-effects regression models with normal errors (Harville, 1977; Laird and Ware, 1982; Jennrich and Schluchter, 1986) as they provide a flexible and valuable tool for analyzing such unbalanced longitudinal data. More importantly, they incorporate all the available information in the data and can reduce or even eliminate any bias resulting from an analysis confined to the complete cases (Ten Have et al., 1998).
Cox proportional hazards regression models estimated the effect of SD on the relative hazard of progression from being cognitively normal at baseline to an incident mild cognitive impairment (MCI)/amnestic mild cognitive impairment (aMCI) diagnosis at a UDS follow-up. Mean and median time to incident MCI/aMCI were also calculated. This analysis considered competing risks for death/attrition and controlled for age-at-baseline, sex, APOE4-status, years-of-education, clinical history of diabetes, hypertension, smoking, marital status, living arrangement, NPI-Q-assessed comorbidity (including other neuropsychiatric symptoms such as hallucinations, delusions, agitation, and apathy) and informant characteristics.
Multivariate ANOVA was conducted to test whether significant differences in mean conversion rates to aMCI existed between SD groups when the previous time-point was compared to the next. In the MANCOVA analysis, time was treated as discrete. To investigate the additive or synergistic associations of SD and cardiovascular burden with incident aMCI, logistic (i.e., non-linear) mixed-effects models with random intercept and slope were used to assess associations between SD, FHS-CVD risk score, and prospective cognitive decline, controlling for covariates/potential confounders, center-ID, and their interactions with time (operationalized as years from baseline for each participant). We examined interactions of SD with time and FHS-CVD risk score with time in a single model (model 1: incident aMCI (Yes vs. No) ≈ SD × time + FHS-CVD risk score × time + covariates × time). Next, we added an interaction term between SD, FHS-CVD risk score, and time to examine whether these two factors increase the likelihood of clinical diagnosis of incident aMCI at a UDS follow-up, beyond their separate effects (i.e., synergistic effect model: Incident aMCI (Yes vs. No) ≈ SD × FHS-CVD risk score × time + covariates × time). In the non-linear mixed-effects model, time was treated as continuous. We then conducted stratified analyses, splitting participants into tertiles based on FHSCVD risk score, comparing the risk of incident aMCI among the highest and middle tertile relative to the lowest tertile. Lastly, we examined the unique influence of the SD/FHS-CVD risk score combined risk on incident aMCI beyond that of hippocampal volume, CSF Aβ42, CSF Tau, and CSF PTau. We did this by evaluating the relative association of each biomarker with prospective cognitive decline by including all biomarkers within a single model (model: Incident aMCI (Yes vs. No) ≈ SD × FHS-CVD risk score × time + CSF-Aβ42 × time + hippocampal volume × time + CSF-Tau × time + CSF-PTau × time + covariates × time). For all analyses, we also tried to account for center differences statistical wise by including the center ID in the adjusted model. Statistical analyses were performed using SAS (version 9.4; SAS Institute Inc., Cary, NC, United States). For descriptive data P-values ≤0.05 were considered significant. For the MANCOVA, Cox models and mixed effects analyses, we controlled for family wise error and P-values ≤0.01, ≤0.017 and 0.025 were considered significant respectively.
Deidentified or anonymized data are made available to researchers by the NACC via a formal request to the NACC Steering Committee through the NACC website3. Data from this study will be shared with qualified investigators upon request.
Figure 1 shows the flowchart for the selection of subjects in the study. Table 1 shows the demographic and clinical characteristics of study participants at baseline. Of the 10,600 participants, 1,017 (9.6%) reported SD and were classified as SD+. The overall mean (SD) age was 75.5 (6.5). The mean (SD) ages of SD+ and SD- were 76 (7.3) and 75 (5.7) years, respectively. The overall mean (SD) follow-up time was 5.1 (2.7) years. The mean (SD) follow-up time was 5.2 (2.6) and 4.9 (2.7) for SD+ and SD- groups, respectively. Overall, female participants represented 62% of the sample with 60 and 62% representation in the SD+ and SD- groups, respectively. Participants in the SD groups did not significantly differ in age, ApoE4 status, BMI, and the FHS-CVD risk score. Of the 361 with AD biomarker data, 35 (9.7%) were SD+. In this group, SD- had significantly higher education and greater hippocampal volume relative to SD+. CSF amyloid and tau levels were similar for both SD groups.
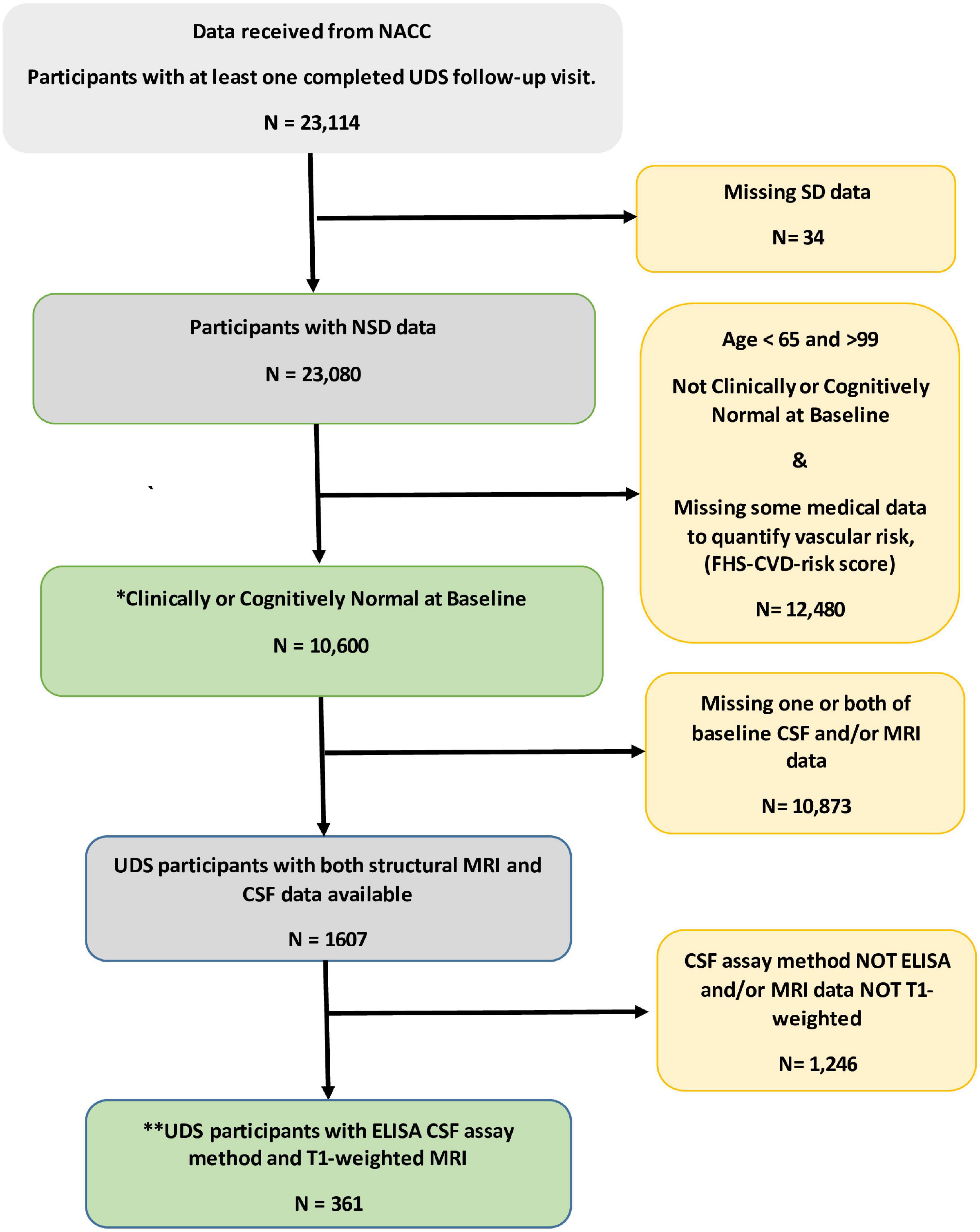
Figure 1. Flowchart for the selection of subjects in the study. * This group of participant data was used to examine independent and combined effects of sleep disturbance (SD) and vascular risk factors on prospect cognitive decline. **Data from this subset were used to evaluate the unique influence of the SD/FHS-CVD risk score combined risk on prospective cognitive decline beyond that of established AD biomarkers. CSF, Cerebrospinal fluid; ELISA, enzyme linked immunosorbent assay; MRI, magnetic resonant imaging; NACC, National Alzheimer’s Coordinating Center; SD, sleep disturbance; UDS, uniform dataset.
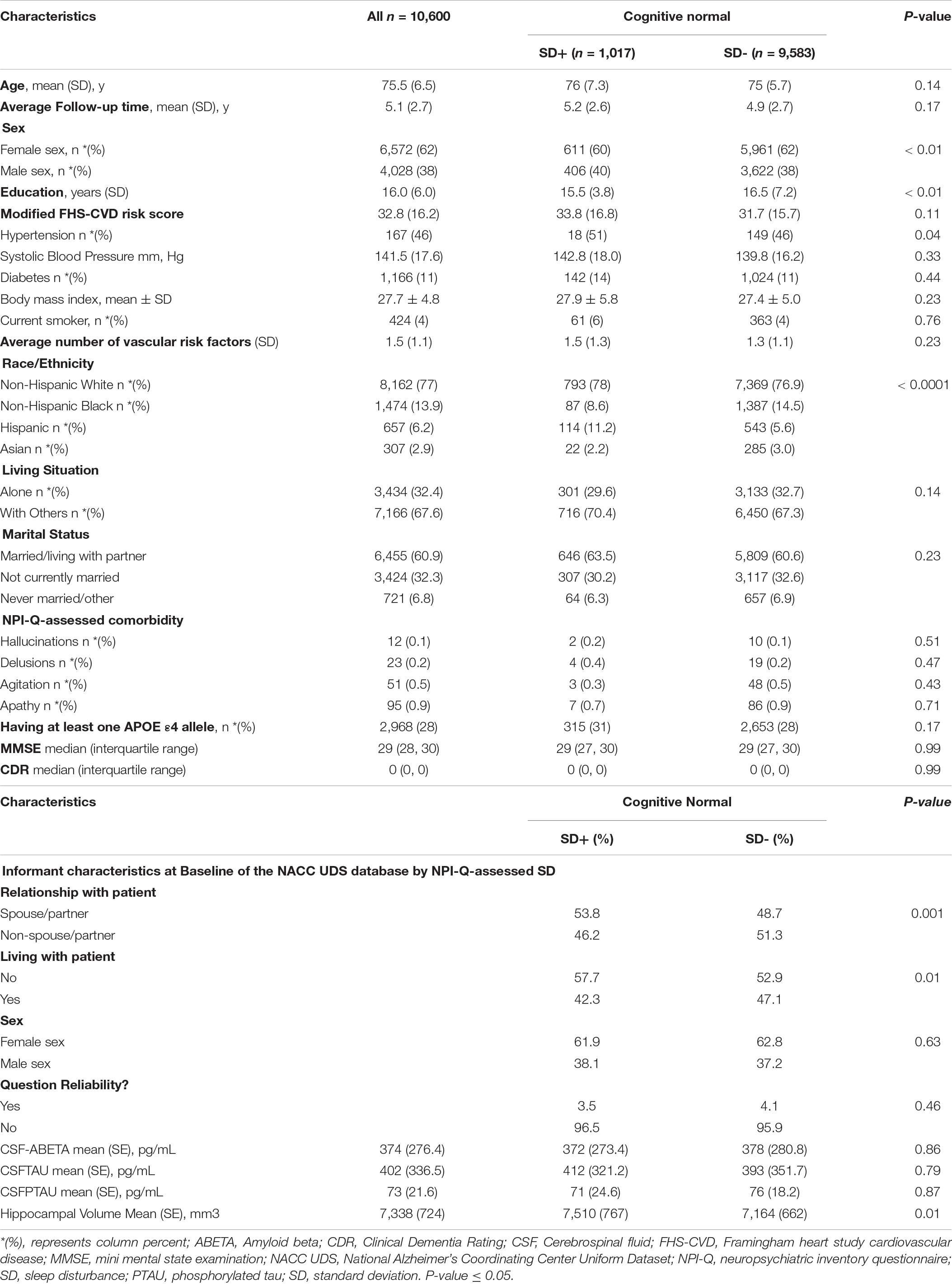
Table 1. Baseline characteristics of cognitive normal study participants by NPI-Q-assessed sleep disturbance (SD), NACC UDS data.
One of the objectives of the analyses was to examine whether being clinically diagnosed as cognitively normal and having SD at baseline was associated with an incident aMCI, and secondarily a MCI (aMCI + non-amnestic MCI) diagnosis at a UDS follow-up. For the aMCI outcome, 195/1,017 (19%) SD positive subjects converted to aMCI relative to 958/9,583 (10%) SD negative subjects. For the general MCI (aMCI + non-amnestic MCI) outcome, 301/1,017 (30%) SD positive subjects converted to MCI relative to 1,607/9,583 (17%) SD negative subjects. We also wanted to determine time effects as it relates to progression and conversion rates across SD groups. Compared with SD- participants, SD+ participants had a significantly shorter time-to-progression to aMCI [mean ± SD (median) 4.2 ± 0.5 (4.0) years vs. 5.1 ± 0.4 (4.8) years, p < 0.001] and had an increased hazard risk of developing aMCI [adjusted hazard ratio (aHR): 2.49, 95% confidence interval (CI):1.18–3.81, p < 0.01] (Table 2A). Similar results were obtained with just an incident MCI (non-amnestic + amnestic) outcome (Table 2B). Compared with SD- participants, SD+ participants had a significantly shorter time-to-progression to MCI [mean ± SD (median) 4.0 ± 0.3 (3.8) years vs. 4.9 ± 0.4 (4.6) years, p < 0.001] and had an increased hazard risk of developing MCI [adjusted hazard ratio (aHR): 2.37, 95% confidence interval (CI):1.15–3.59, p < 0.01].
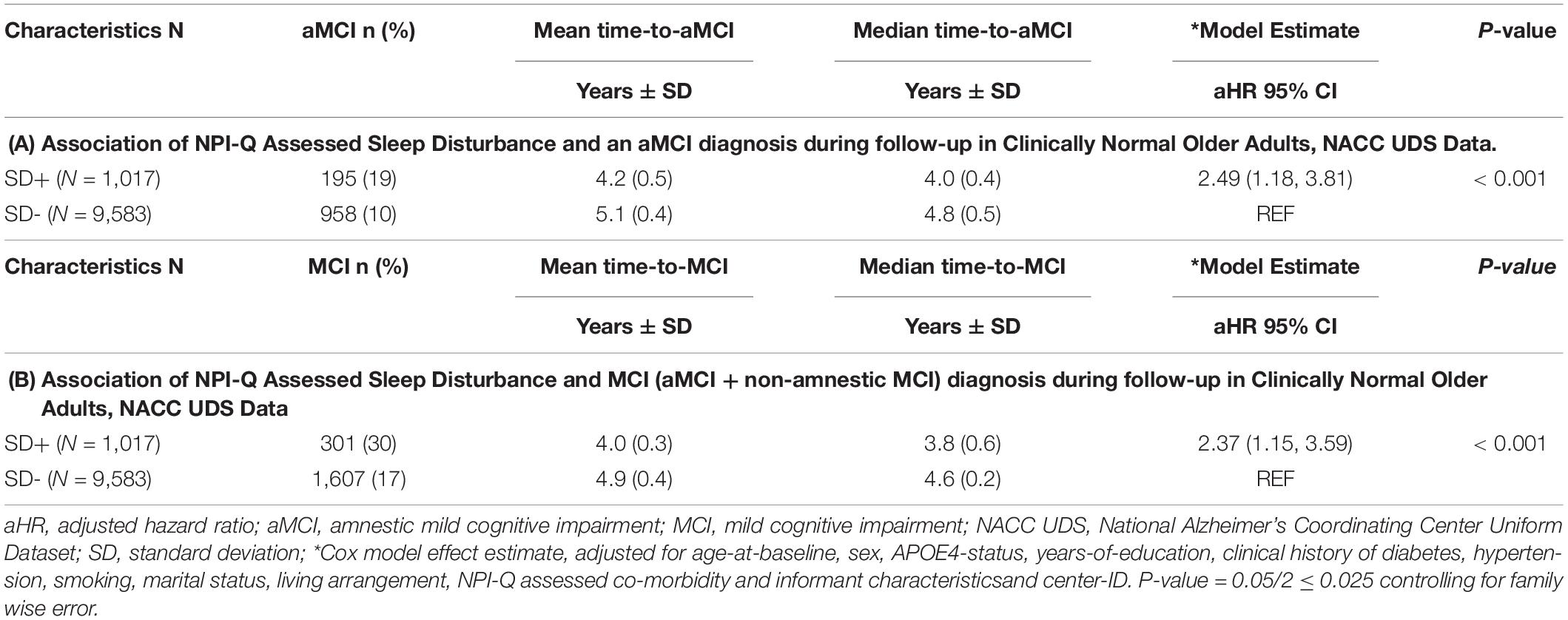
Table 2. Association of NPI-Q assessed sleep disturbance (SD) and an MCI diagnosis during follow-up in clinically normal older adults, NACC UDS data.
Table 3 shows MANCOVA results testing the differences in the mean incident aMCI conversion rates over time, based on SD status. The Pillai’s trace test values presented examine time-point*SD effect and provide the exact F statistics for the time trend of mean incident aMCI conversion rates across SD groups. The time-point effect provides the exact F statistics for the mean incident aMCI conversion rates over time. Other statistical interpretations are delineated in the Table 3 footnote. The data showed that the time trend of incident aMCI conversion rates differed across SD groups (Pillai’s trace test, p < 0.01 for all). Across all subjects, mean incident aMCI conversion rates increased significantly over time (Pillai’s trace test, p < 0.001 for all). For the comparisons of repeated measures, there were significant differences in mean incident aMCI conversion rates across the SD groups, with SD+ generally having higher conversion rates than SD-, when the previous time-point was compared with the next (p ≤ 0.01 for all).
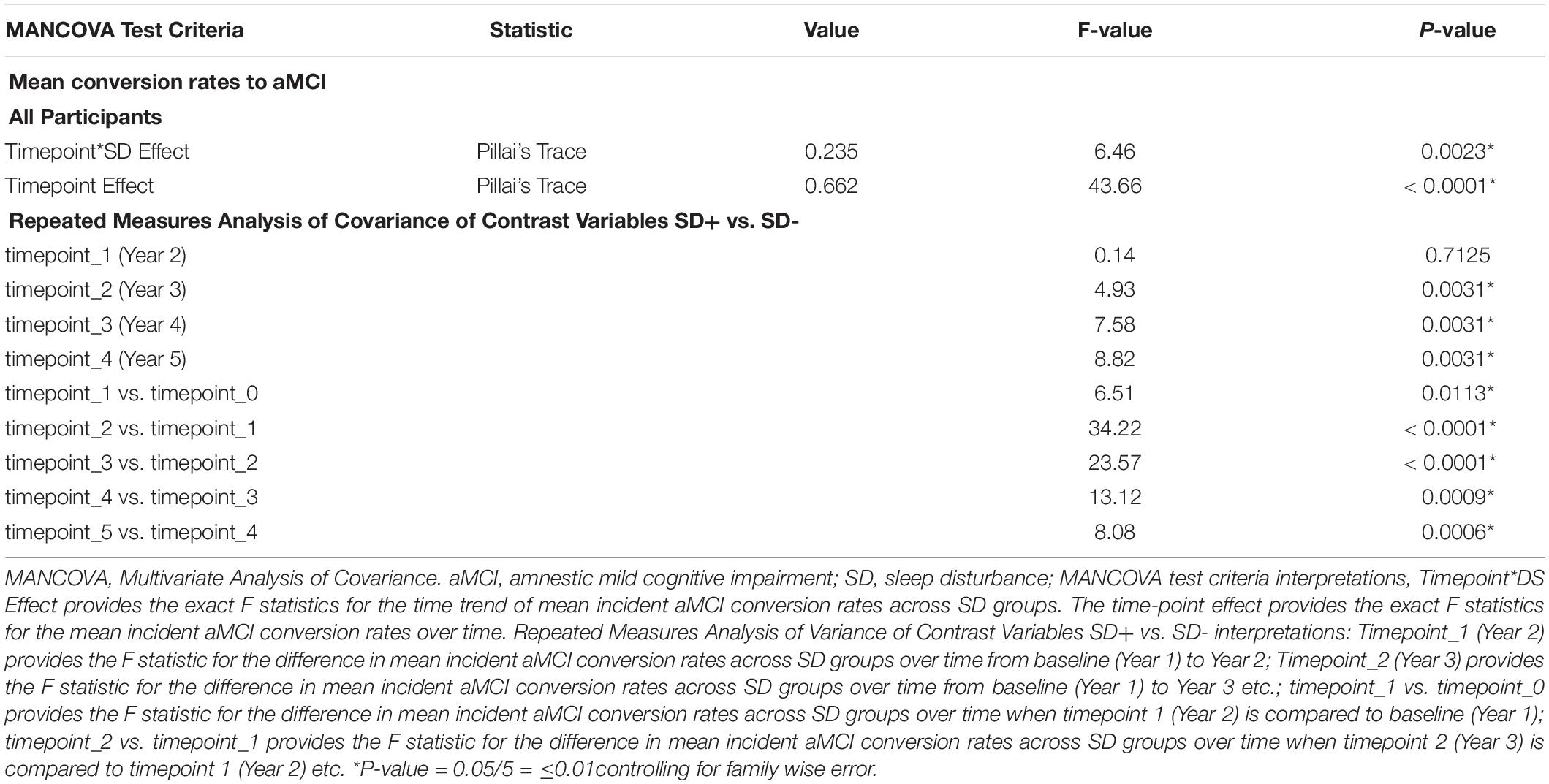
Table 3. Multivariate ANCOVA results testing mean aMCI change conversion rates in time trend, groups and time points, NACC UDS data.
A second objective was to determine whether the presence of SD at baseline and an elevated FHS-CVD risk score were additive or synergistic in their associations with an incident aMCI diagnosis at a UDS follow-up. We examined interactions of SD with time and FHS-CVD risk score with time in a single model (i.e., Model 1, Table 4). Both SD and having a higher FHS-CVD risk score were associated with an incident aMCI diagnosis at a UDS follow-up. Since age and sex are incorporated into the FHS-CVD risk score, we conducted sensitivity analyses omitting age and sex as covariates and these yielded similar results (e.g., SD’s significant effect on incident aMCI changed from aOR:1.42 to 1.55, p < 0.003). The model (i.e., Model 2, Table 4) that included the interaction between SD and FHS-CVD risk score (i.e., the synergistic effect model) yielded a significant interaction term suggesting that SD and an elevated FHS-CVD risk score together increased the likelihood of having an incident aMCI at a UDS follow-up beyond their individual effects. For strata specific effects, we stratified FHS-CVD risk score into tertiles. Participants with SD and in the highest and middle tertiles of the FHS-CVD risk score were significantly more likely to develop incident aMCI during UDS follow-up, compared with participants without SD in the lowest FHS-CVD risk score tertile (OR: 2.82, 95%CI, 1.23–4.35; p < 0.003 and OR: 2.38, 95%CI 1.17–3.59; p < 0.001, respectively; as shown in Model 2, Table 4). Overall, the findings were similar for the MCI (non-amnestic + amnestic) outcome; however, with attenuated but significant effects seen for SD and stronger effects seen for FHS-CVD risk and the interaction between SD and FHS-CVD risk score (i.e., the synergistic effect model, as shown in Models 1 and 2, Table 5).
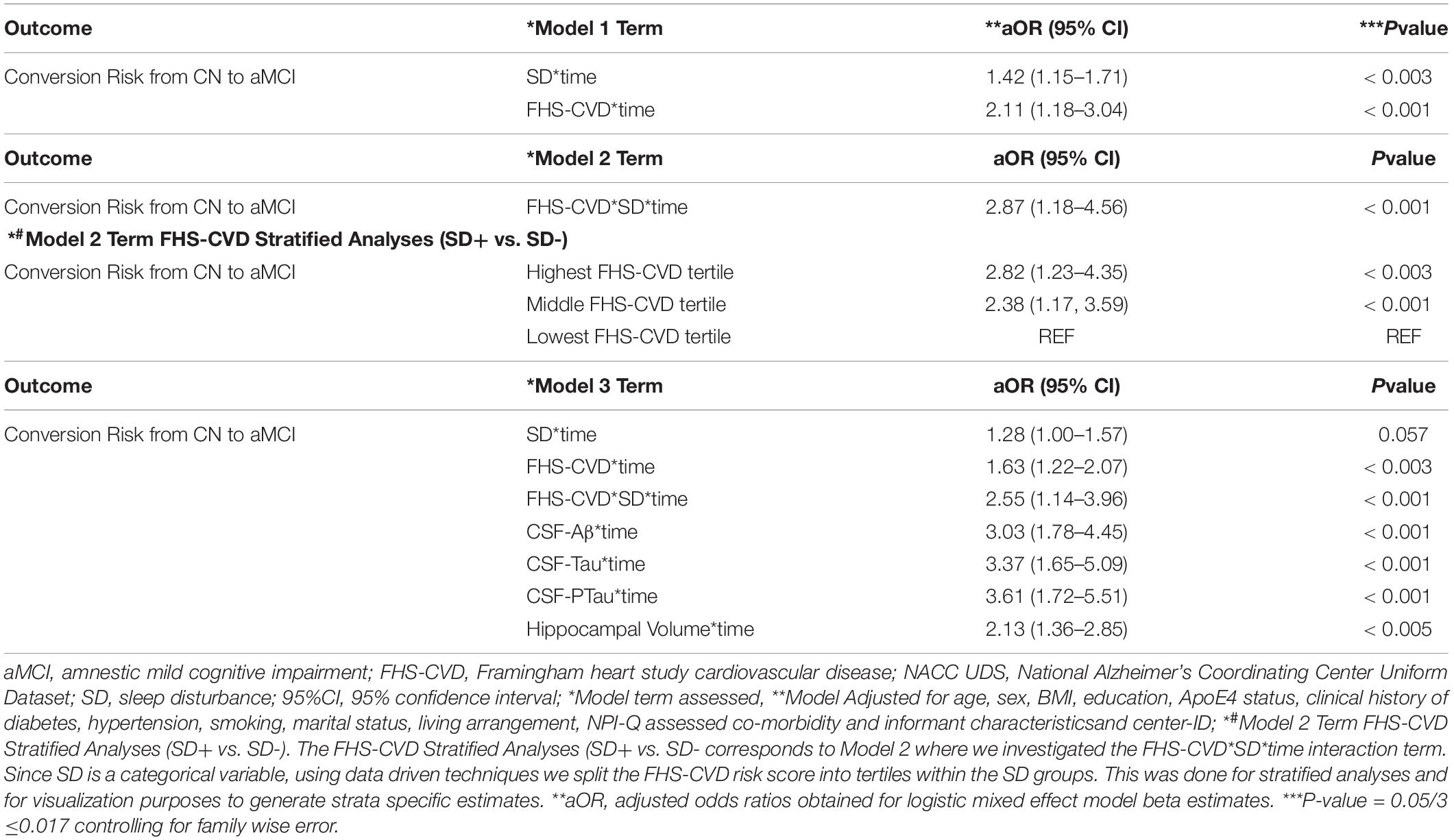
Table 4. Interactive associations of NPI-Q assessed sleep disturbance (SD) and vascular risk with an aMCI diagnosis during follow-up in clinically normal older adults, NACC UDS data.
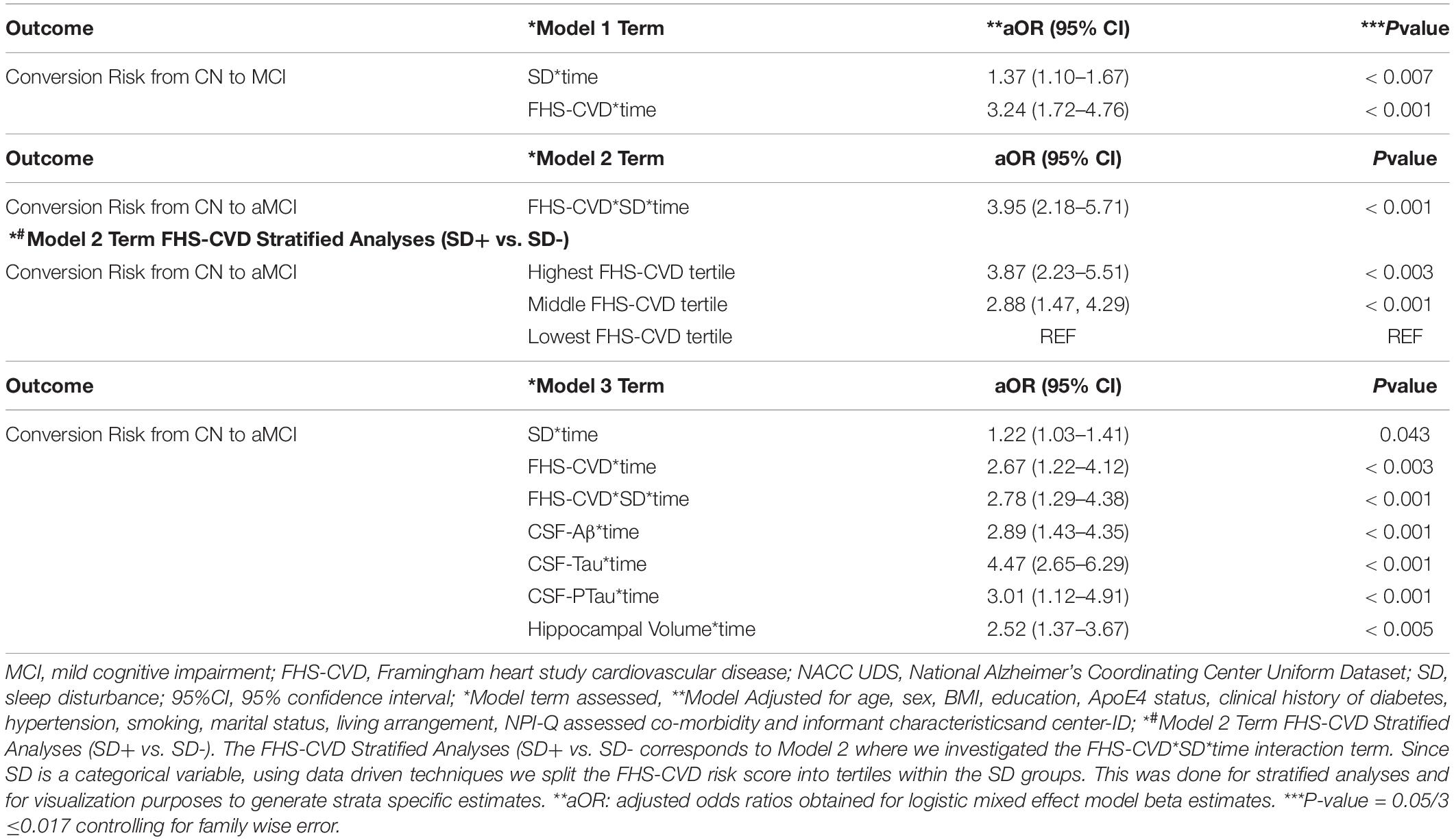
Table 5. Interactive associations of NPI-Q assessed sleep disturbance (SD) and vascular risk with an MCI (aMCI + non-amnestic MCI) diagnosis during follow-up in clinically normal older adults, NACC UDS data.
Our third objective was to evaluate the unique influence of the SD/FHS-CVD risk score combined risk on an incident aMCI diagnosis at a UDS follow-up beyond that of commonly used AD biomarkers, including levels of CSF-Aβ, CSF-Tau, and CSF-PTau and hippocampal volume. We did this by evaluating the relative association of each biomarker with an incident MCI diagnosis at a UDS follow-up by including all biomarkers within a single model (Model 3, Table 4). SD’s effect on an incident aMCI diagnosis at a UDS follow-up was attenuated (aOR: 1.28, 95%CI, 1.00–1.57; p = 0.057) when all AD biomarkers were included in the model.
However, the FHS-CVD risk score and the interaction between SD and FHS-CVD risk score remained significantly associated with an incident aMCI diagnosis at a UDS follow-up even after including these AD biomarkers in the model (OR: 1.63, 95%CI, 1.22–2.07; p < 0.003 and OR: 2.55, 95%CI, 1.14–3.96; p < 0.001, respectively). Overall, the findings were similar for the MCI (non-amnestic + amnestic) outcome, as shown in Model 3, Table 5. More importantly, the SD and FHS-CVD risk score synergism approximated the risk estimates of each molecular AD biomarker, even though they were significantly different (Figure 2).
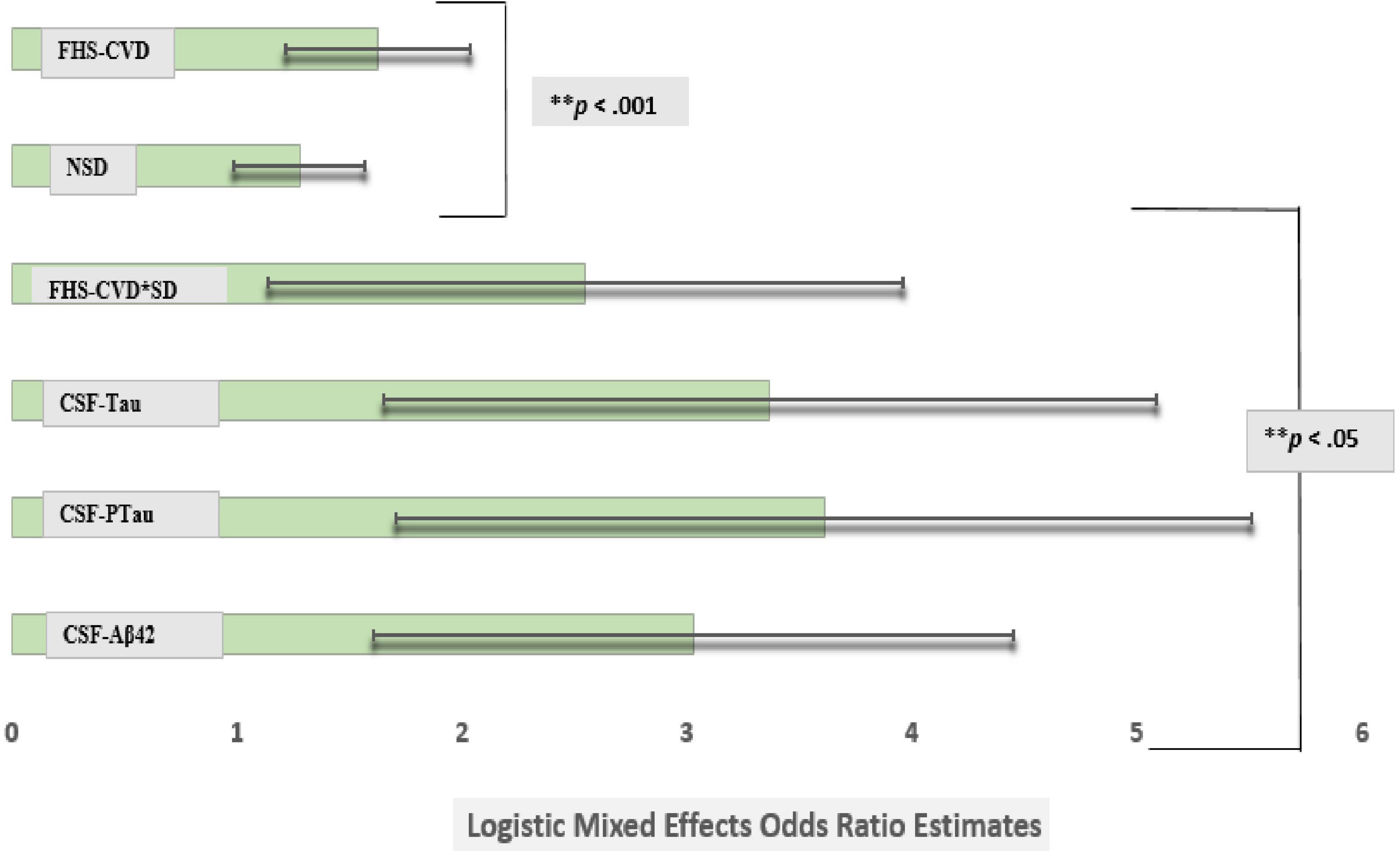
Figure 2. Comparison of logistic mixed effects model estimates of CSF Aβ42, CSF Tau, CSF PTau, SD, and vascular risk on the risk of conversion from cognitively normal to amnestic mild cognitive impairment (MCI). Sleep disturbance’s (SD) effect on an incident amnestic MCI diagnosis at a UDS follow-up was attenuated after adjusting for commonly used Alzheimer’s disease markers in the same model (model 3). However, the Framingham Heart Study-general cardiovascular disease (FHS-CVD) risk score and the interaction between SD and FHS-CVD risk score remained significantly associated with an incident MCI diagnosis at a uniform dataset (UDS) follow-up even after including these molecular biomarkers in the model. More importantly, the SD and FHS-CVD risk score synergism approximated the risk estimates of each molecular AD biomarker though significantly different. **Multiple comparisons: FHS-CVD*SD vs. CSF-Aβ42, FHS-CVD*SD vs. CSF-Tau and FHS-CVD*SD vs. CSF-PTau (p < 0.01 for all).
We examined whether SD was independently and with VRFs interactively associated with prospective cognitive decline i.e., incident aMCI diagnosis, in clinically normal older adults. Our results indicate that SD was associated with an increased hazard risk of an incident aMCI. More importantly and novel is the finding that having reported SD at baseline led to 9–12 months significantly shorter time-to an incident aMCI diagnosis at a UDS follow-up, compared with not having reported SD at baseline. In addition, SD and FHS-CVD risk score were each associated with an incident aMCI when entered together into a single model. These findings underscore the importance of both sleep and vascular risk as contributors to cognitive decline in clinically normal older adults. The interaction between SD and the FHS-CVD risk score in association with prospective cognitive decline was synergistic. Stratified results showed a dose-response finding such that participants with SD and in the highest and middle tertiles of the FHS-CVD risk score were significantly more likely to develop incident aMCI during UDS follow-up, compared with participants without SD in the lowest FHS-CVD risk score tertile. Notably, the synergistic effect of SD and the FHS-CVD risk score remained strongly associated with prospective cognitive decline after adjusting for commonly used AD biomarkers, including levels of CSF-Aβ, CSF-Tau, CSF-Ptau, and hippocampal volume, and approximated the effects of these commonly used imaging biomarkers, thus suggesting their possible use as complementing the existing AD pathology markers in assessing the risk of prospective cognitive decline.
Our results show that SD is associated with an increased hazard risk of an incident aMCI diagnosis, which is in line with results from previous prospective studies examining whether self-reported sleep problems in individuals who were not demented at baseline are early independent markers of risk for developing AD or dementia over time (Sterniczuk et al., 2013; Virta et al., 2013). Metaanalysis has shown that multiple markers of SD are linked to an increased risk for all-cause dementia in older adults (Xu et al., 2020). SDs are also associated with AD pathology, in cognitively normal, late middle-aged, and older adults including Aβ and tau aggregation and regional brain atrophy (Spira et al., 2013; Branger et al., 2016; Lucey et al., 2019). The prevailing thought regarding the relationship between SD and AD is that of a bidirectional relationship (Wang and Holtzman, 2020). Our study supports the notion that SDs may precede clinical AD onset. Possible mechanistic pathways linking sleep as a risk factor for AD include the diurnal nature of the sleep-wake cycle and its effects on extracellular metabolism of Aβ and tau in the CSF and in brain interstitial fluid (ISF) as these fluctuate diurnally, with soluble Aβ levels higher during wakefulness, and lower during sleep (Kang et al., 2009). Studies in humans have demonstrated a 25–30% increase in soluble Aβ in CSF via increased overnight Aβ production in acute sleep-deprived individuals relative to sleeping controls (Lucey et al., 2018). In addition, Holth et al. (2019) recently showed that ISF tau also fluctuates diurnally with sleep deprivation increasing tau levels in human CSF and mouse brain ISF. Acceleration of the spreading of tau protein aggregates was seen in specific brain networks because of chronic sleep deprivation in a tau-seeding model (Holth et al., 2019). The role of sleep in amyloid pathogenesis is bolstered further with sleep deprivation studies in humans and mice showing that overnight sleep deprivation in healthy young adults significantly increased morning Aβ38, Aβ40, and Aβ42 levels in the CSF by 25–30% relative to a night of normal sleep (Lucey et al., 2018). Other possible mechanisms linking sleep as a risk factor for AD include reduced SWA (Varga et al., 2016), impaired glymphatic clearance during sleep (Xie et al., 2013), and chronic inflammation (Irwin and Vitiello, 2019).
Our results showing SD predicting a shorter progression time-to-aMCI are novel and in line with our work in patients with cognitive normal obstructive sleep apnea (OSA), and the most common is sleep disorder in the elderly. In these studies, cognitively normal OSA+ participants progressed to MCI 6–18 months earlier than OSA- participants did, depending on their amyloid and tau burden (Osorio et al., 2015; Bubu et al., 2020). In addition, we have shown that cognitively normal OSA+ subjects experience a faster annual increase in florbetapir uptake and decrease in CSF Aβ42 levels, and also increases in CSF T-tau and P-tau compared with OSA- participants (Bubu et al., 2019). More importantly, SD and FHS-CVD risk score were each associated with incident aMCI when entered together into a single model and further showed a synergistic interaction between these two factors in promoting cognitive decline, even when the model included commonly used established AD biomarkers. Our FHS-CVD risk score finding is in line with a recent study demonstrating FHS-CVD risk score associations with prospective cognitive decline (Rabin et al., 2018). VRFs are associated with lower FDG-PET (Langbaum et al., 2012), more cerebrovascular disease (CVD) (Bangen et al., 2015), higher cerebral Aβ burden (Langbaum et al., 2012; Gottesman et al., 2017), and higher tau burden (Langbaum et al., 2012; Gottesman et al., 2017), and they act synergistically with Aβ burden to promote cognitive decline (Rabin et al., 2018). The combined impact of Aβ burden and increased WMH have generally been additive on cognition (Marchant et al., 2013), thus possibly suggesting that the FHS-CVD risk score may detect other features of vascular burden not reflected by infarcts and/or WMH (Rabin et al., 2018). Possible mechanisms linking VRFs to AD risk include atherosclerosis-induced brain hypoperfusion and its impact on neurodegeneration, formation of neuritic plaques in both the hippocampus and neocortex, and hippocampal neurofibrillary tangles (Petrovitch et al., 2000). VRFs can lead to disruption of the endothelium and the blood–brain barrier and induce cerebral amyloidosis affecting cholinergic neurotransmission, which plays a critical role in normal cognition, particularly in the domains of attention, emotion, and memory (Román and Kalaria, 2006). There is also evidence that vascular protective factors may attenuate pathological processes that ultimately modify the risk of AD (Scarmeas et al., 2009).
The observed synergy between SD and FHS-CVD risk score in our study is novel and clinically relevant since a substantial number of sleep-disturbed patients have co-occurring VRFs. Possible synergistic mechanisms may include impaired cardiovascular autonomic regulation by SD (Legramante and Galante, 2005), increased sympathetic activation and oxidative stress, combined induction of cerebral amyloidosis, reduced SWA (Varga et al., 2016), and chronic inflammation (Irwin and Vitiello, 2019), by both disturbed sleep and VRFs. Many cerebrovascular changes are not well visualized on MRI (Smith et al., 2012), since conventional neuroimaging captures only a portion of the total CVD burden. Moreover, in assessing AD risk, SDs, characterized by shorter sleep duration and poor sleep quality in community-dwelling older adults are detectable using validated sleep questionnaires (Spira et al., 2013). Therefore, both sleep and VRFs may represent clinical non-invasive complements to the more invasive methods of assessing cognitive decline risk.
This study has its limitations; as such, results from this study are best interpreted within the context of the study sample. The NACC UDS sample consists of a convenience sampling of patients and research participants at academic ADRCs. Recruitment patterns may vary across the 33 centers. Our population included clinically normal participants at baseline followed for an incident MCI/aMCI diagnosis during a UDS follow-up. Fortunately, ADRCs employ the standardization of the clinical consensus diagnosis across the centers. The NACC database consists of mostly a white sample as such, this may affect generalizability of our findings. It is likely that sleep problems and cardiovascular outcomes are causally related; however, we ensured that there was no temporal ordering of both SD and cardio VRFs as they were obtained at baseline. As our covariates were selected a priori, we did not adjust for possible treatments for cardiovascular risks or disorders. However, any sort of treatment would have resulted in an attenuation of our risk estimates. The utilization of the NPI-Q to measure SD is a limitation as its inclusion in NACC UDS was because of its validity in obtaining neuropsychiatric and behavioral symptoms/syndromes’ data associated with dementia (Cummings et al., 1994). The NPI-Q sleep item conflates three separate sleep components including nocturnal awakenings, waking up too early in the morning, and excessive daytime napping behavior into one. While we argue that a positive answer to any of the three components reflects poor nighttime sleep or the presence of a sleep disorder, it cannot differentiate between possibilities (e.g., insomnia vs. hypersomnia vs. circadian rhythm abnormality), does not capture any information about difficulties in sleep initiation, which seems to be one of the most important aspects of sleep-related problems in later life that has implications for health, and negative answers may still be elicited in individuals with OSA. Notably, the NPI-Q has been validated in patients with AD (Cummings et al., 1994). Moreover, studies exist that have utilized this measure in assessing SDs as a risk factor in the elderly and in patients with AD (Bliwise et al., 2011). More importantly, the direction of the findings is in line with the existing literature suggesting SD as a risk factor for AD (Spira et al., 2013; Branger et al., 2016; Lucey et al., 2019). Another limitation is that the UDS had no data on household sleeping arrangements, thereby making it impossible to know whether some informants misreported SD, especially if they did not sleep in the same bedroom as the patients. Notably less than half of the informants lived with the participant, thus depending on the informant characteristic (e.g., coresidence, relationship with the patient—spouse, children, friend, paid caregiver), the response to this item is subject to varying degrees of reporting bias, which may include both over- and underreporting, especially given that the item may be conflated with neuropsychiatric problems. However, if this occurred, it would most likely have resulted in an aggregate underreporting of SD that would have resulted in an attenuation of our risk estimates. Furthermore, informants’ reliability was extremely high and we controlled for informants’ characteristics in our models. Another limitation is concerning CSF and MRI measures. To guard against differences in CSF assays and MRI imaging, we utilized CSF data measured using the same assay method (i.e., ELISA) and MRI data from same high-resolution T1-weighted anatomical imaging scans. We also tried to account for center differences statistical wise by including the center-ID in the adjusted models. Lastly, many NACC UDS participants have some level of advanced education, and may therefore have some level of cognitive reserve. However, we expect this to have attenuated our estimates. Our results suggest that even with some level of reserve, vascular risk can interact with sleep to accelerate cognitive decline.
In summary, our results suggest that SD and vascular risk are strongly associated with prospective cognitive decline, both alone and in synergism beyond commonly used biofluid and imaging AD biomarkers, in cognitively normal older adults. We, therefore, propose a framework for additional research. Examples include: (a) using objective measures to examine whether sleep-vascular risk synergism related to cognitive decline is independent of Aβ and tau pathology and (b) determine specific sleep and vascular disorders’ effects on slow wave and rapid eye movement sleep and their mediating role in increasing Aβ and tau accumulation. More importantly, these less invasive clinical measures of sleep and vascular risk may actually serve as possible complements of the more invasive biofluid and imaging biomarkers in assessing the risk of prospective cognitive decline in cognitively normal older adults. The relevance of these findings is bolstered by epidemiological studies suggesting that an approximate combined 20–23% [15% for sleep problems/disorders (Bubu et al., 2017) and 5–8% for hypertension (Barnes and Yaffe, 2011)] of AD may be prevented should interventions be implemented to reduce sleep problems/disorder, and hypertension.
Publicly available datasets were analyzed in this study. This data can be found here: https://naccdata.org/requesting-data/nacc-data.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
OB, EW, OU-B, AKM, and RO contributed to the conception and design of the study. OB, EW, OU-B, ADT, AKM, JC, and JB contributed to the acquisition and analysis of data. OB, EW, OU-B, SK, ADT, JC, AEM, AP, KK, JB, ZO, AN, ART, ML, DR, IA, GO, GJ-L, AV, AVM, and RO drafted a significant portion of the manuscript, tables, or figures. All authors contributed to the article and approved the submitted version.
This study was supported by the National Institute of Aging and the National Heart Lung and Blood Institute at the National Institutes of Health [K23AG068534, L30-AG064670, CIRAD P30AG059303 Pilot, P30AG066512 NYU ADRC Developmental Project, R25HL105444, R01HL118624, R21AG049348, R21AG055002, R01AG056031, R01AG022374, R21AG059179, R01AG056682, R01AG056531, K07AG05268503, and K25HL151912], the American Academy of Sleep Medicine Foundation [BS-231-20 and BS-233-20], and the Alzheimer’s Association [AARGD-21-848397]. The NACC database is funded by NIA/NIH grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 [principal investigator (PI) Eric Reiman], P30 AG013846 (PI Neil Kowall), P30 AG062428-01 (PI James Leverenz), P50 AG008702 (PI Scott Small), P50 AG025688 (PI Allan Levey), P50 AG047266 (PI Todd Golde), P30 AG010133 (PI Andrew Saykin), P50 AG005146 (PI Marilyn Albert), P30 AG062421-01 (PI Bradley Hyman), P30 AG062422-01 (PI Ronald Petersen), P50 AG005138 (PI Mary Sano), P30 AG008051 (PI Thomas Wisniewski), P30 AG013854 (PI Robert Vassar), P30 AG008017 (PI Jeffrey Kaye), P30 AG010161 (PI David Bennett), P50 AG047366 (PI Victor Henderson), P30 AG010129 (PI Charles DeCarli), P50 AG016573 (PI Frank LaFerla), P30 AG062429-01 (PI James Brewer), P50 AG023501 (PI Bruce Miller), P30 AG035982 (PI Russell Swerdlow), P30 AG028383 (PI Linda Van Eldik), P30 AG053760 (PI Henry Paulson), P30 AG010124 (PI John Trojanowski), P50 AG005133 (PI Oscar Lopez), P50 AG005142 (PI Helena Chui), P30 AG012300 (PI Roger Rosenberg), P30 AG049638 (PI Suzanne Craft), P50 AG005136 (PI Thomas Grabowski), P30 AG062715-01 (PI Sanjay Asthana), P50 AG005681 (PI John Morris), and P50 AG047270 (PI Stephen Strittmatter). The funders had no role in the conception or preparation of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank the services of the National Alzheimer’s Coordinating Center, all the Alzheimer’s Disease Research Centers, and all the study participants.
Bangen, K. J., Nation, D. A., Delano-Wood, L., Weissberger, G. H., Hansen, L. A., Galasko, D. R., et al. (2015). Aggregate effects of vascular risk factors on cerebrovascular changes in autopsy-confirmed Alzheimer’s disease. Alzheimer’s Dement. 11, 394.e1–403.e1. doi: 10.1016/j.jalz.2013.12.025
Barnes, D. E., and Yaffe, K. (2011). The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 10, 819–828. doi: 10.1016/S1474-4422(11)70072-2
Besser, L., Kukull, W., Knopman, D. S., Chui, H., Galasko, D., Weintraub, S., et al. (2018). Version 3 of the National Alzheimer’s Coordinating Center’s uniform data set. Alzheimer Dis. Assoc. Disord. 32, 351–358. doi: 10.1097/WAD.0000000000000279
Bliwise, D. L., Mercaldo, N. D., Avidan, A. Y., Boeve, B. F., Greer, S. A., and Kukull, W. A. (2011). Sleep disturbance in dementia with Lewy bodies and Alzheimer’s disease: a multicenter analysis. Dement. Geriatr. Cogn. Disord. 31, 239–246. doi: 10.1159/000326238
Branger, P., Arenaza-Urquijo, E. M., Tomadesso, C., Mézenge, F., André, C., de Flores, R., et al. (2016). Relationships between sleep quality and brain volume, metabolism, and amyloid deposition in late adulthood. Neurobiol. Aging 41, 107–114. doi: 10.1016/j.neurobiolaging.2016.02.009
Bubu, O. M., Brannick, M., Mortimer, J., Umasabor-Bubu, O., Sebastião, Y. V., Wen, Y., et al. (2017). Sleep, Cognitive impairment, and Alzheimer’s disease: a systematic review and meta-analysis. Sleep 40:zsw032. doi: 10.1093/sleep/zsw032
Bubu, O. M., Pirraglia, E., Andrade, A. G., Sharma, R. A., Gimenez-Badia, S., Umasabor-Bubu, O. Q., et al. (2019). Obstructive sleep apnea and longitudinal Alzheimer’s disease biomarker changes. Sleep 42:zsz048. doi: 10.1093/sleep/zsz048
Bubu, O. M., Umasabor-Bubu, O. Q., Turner, A. D., Turner, A. D., Parekh, A., Mullins, A. E., et al. (2020). Self-reported obstructive sleep apnea, amyloid and tau burden, and Alzheimer’s disease time-dependent progression. Alzheimer’s Dement. 17, 226–245. 10.1002/alz.12184. doi: 10.1002/alz.12184
Cummings, J. L., Mega, M., Gray, K., Rosenberg-Thompson, S., Carusi, D. A., and Gornbein, J. (1994). The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314. doi: 10.1212/WNL.44.12.2308
D’Agostino, Sr, Vasan, R. S., Pencina, M. J., Wolf, P. A., Cobain, M., Massaro, J. M., et al. (2008). General cardiovascular risk profile for use in primary care: the framingham heart study. Circulation 117, 743–753. doi: 10.1161/CIRCULATIONAHA.107.699579
Esiri, M. M., Nagy, Z., Smith, M. Z., Barnetson, L., and Smith, A. D. (1999). Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer’s disease. Lancet 354, 919–920. doi: 10.1016/S0140-6736(99)02355-7
Gangwisch, J. E., Heymsfield, S. B., Boden-Albala, B., Buijs, R. M., Kreier, F., Pickering, T. G., et al. (2007). Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep 30, 1667–1673. doi: 10.1093/sleep/30.12.1667
Genest, J. Jr., and Cohn, J. S. (1995). Clustering of cardiovascular risk factors: targeting high-risk individuals. Am. J. Cardiol. 76, 8A–20A. doi: 10.1016/S0002-9149(05)80010-4
Gottesman, R. F., Albert, M. S., Alonso, A., Coker, L. H., Coresh, J., Davis, S. M., et al. (2017). Associations between midlife vascular risk factors and 25-Year incident dementia in the atherosclerosis risk in communities (ARIC) cohort. JAMA Neurol. 74, 1246–1254. doi: 10.1001/jamaneurol.2017.1658
Gottlieb, D. J., Redline, S., Nieto, F. J., Baldwin, C. M., Newman, A. B., Resnick, H. E., et al. (2006). Association of usual sleep duration with hypertension: the sleep heart health study. Sleep 29, 1009–1014. doi: 10.1093/sleep/29.8.1009
Harville, D. A. (1977). Maximum likelihood approaches to variance component estimation and to related problems. J. Am. Stat. Assoc. 72, 339–340. doi: 10.2307/2286798
Hita-Yanez, E., Atienza, M., and Cantero, J. L. (2013). Polysomnographic and subjective sleep markers of mild cognitive impairment. Sleep 36, 1327–1334. doi: 10.5665/sleep.2956
Holth, J. K., Fritschi, S. K., Wang, C., Pedersen, N. P., Cirrito, J. R., Mahan, T. E., et al. (2019). The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363, 880–884. doi: 10.1126/science.aav2546
Irwin, M. R., and Vitiello, M. V. (2019). Implications of sleep disturbance and inflammation for Alzheimer’s disease dementia. Lancet Neurol. 18, 296–306. doi: 10.1016/S1474-4422(18)30450-2
Jack, C. R. Jr., Twomey, C. K., Zinsmeister, A. R., Sharbrough, F. W., Petersen, R. C., and Cascino, G. D. (1989). Anterior temporal lobes and hippocampal formations: normative volumetric measurements from MR images in young adults. Radiology 172, 549–554. doi: 10.1148/radiology.172.2.2748838
Jennrich, R. I., and Schluchter, M. D. (1986). Unbalanced repeated-measures models with structured covariance matrices. Biometrics 42, 805–820. doi: 10.2307/2530695
Kam, K., Parekh, A., Sharma, R. A., Andrade, A., Lewin, M., Castillo, B., et al. (2019). Sleep oscillation-specific associations with Alzheimer’s disease CSF biomarkers: novel roles for sleep spindles and tau. Mol. Neurodegen. 14:10. doi: 10.1186/s13024-019-0309-5
Kang, J. E., Lim, M. M., Bateman, R. J., Lee, J. J., Smyth, L. P., Cirrito, J. R., et al. (2009). Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 326, 1005–1007. doi: 10.1126/science.1180962
Laird, N. M., and Ware, J. H. (1982). Random-effects models for longitudinal data. Biometrics 38, 963–974. doi: 10.2307/2529876
Langbaum, J. B., Chen, K., Launer, L. J., Fleisher, A. S., Lee, W., Liu, X., et al. (2012). Blood pressure is associated with higher brain amyloid burden and lower glucose metabolism in healthy late middle-age persons. Neurobiol. Aging 33, e811–e829. doi: 10.1016/j.neurobiolaging.2011.06.020
Legramante, J. M., and Galante, A. (2005). Sleep and hypertension: a challenge for the autonomic regulation of the cardiovascular system. Circulation 112, 786–788. doi: 10.1161/CIRCULATIONAHA.105.555714
Lucey, B. P., Hicks, T. J., McLeland, J. S., Toedebusch, C. D., Boyd, J., Elbert, D. L., et al. (2018). Effect of sleep on overnight cerebrospinal fluid amyloid β kinetics. Ann. Neurol. 83, 197–204. doi: 10.1002/ana.25117
Lucey, B. P., McCullough, A., Landsness, E. C., Toedebusch, C. D., McLeland, J. S., Zaza, A. M., et al. (2019). Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease. Sci. Transl. Med. 11:eaau6550. doi: 10.1126/scitranslmed.aau6550
Luchsinger, J. A., Reitz, C., Honig, L. S., Tang, M. X., Shea, S., and Mayeux, R. (2005). Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology 65, 545–551. doi: 10.1212/01.wnl.0000172914.08967.dc
Marchant, N. L., Reed, B. R., Sanossian, N., Madison, C. M., Kriger, S., Dhada, R., et al. (2013). The aging brain and cognition: contribution of vascular injury and abeta to mild cognitive dysfunction. JAMA Neurol. 70, 488–495. doi: 10.1001/2013.jamaneurol.405
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., and Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s Disease. Neurology 34, 939–944. doi: 10.1212/WNL.34.7.939
Osorio, R. S., Gumb, T., Pirraglia, E., Varga, A. W., Lu, S. E., Lim, J., et al. (2015). Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 84, 1964–1971. doi: 10.1212/WNL.0000000000001566
Petrovitch, H., White, L. R., Izmirilian, G., Ross, G. W., Havlik, R. J., Markesbery, W., et al. (2000). Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia aging Study. Neurobiol. Aging 21, 57–62. doi: 10.1016/S0197-4580(00)00106-8
Potvin, O., Lorrain, D., Forget, H., Dubé, M., Grenier, S., Préville, M., et al. (2012). Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep 35, 491–499. doi: 10.5665/sleep.1732
Rabin, J. S., Schultz, A. P., Hedden, T., Viswanathan, A., Marshall, G. A., Kilpatrick, E., et al. (2018). Interactive associations of vascular risk and beta-amyloid burden with cognitive decline in clinically normal elderly individuals: findings from the harvard aging brain study. JAMA Neurol. 75, 1124–1131. doi: 10.1001/jamaneurol.2018.1123
Román, G. C., and Kalaria, R. N. (2006). Vascular determinants of cholinergic deficits in Alzheimer disease and vascular dementia. Neurobiol. Aging 27, 1769–1785. doi: 10.1016/j.neurobiolaging.2005.10.004
Scarmeas, N., Luchsinger, J. A., Schupf, N., Brickman, A. M., Cosentino, S., Tang, M. X., et al. (2009). Physical activity, diet, and risk of Alzheimer disease. Jama 302, 627–637. doi: 10.1001/jama.2009.1144
Smith, E. E., Schneider, J. A., Wardlaw, J. M., and Greenberg, S. M. (2012). Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 11, 272–282. doi: 10.1016/S1474-4422(11)70307-6
Spira, A. P., Gamaldo, A. A., An, Y., Wu, M. N., Simonsick, E. M., Bilgel, M., et al. (2013). Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol. 70, 1537–1543. doi: 10.1001/jamaneurol.2013.4258
Sterniczuk, R., Theou, O., Rusak, B., and Rockwood, K. (2013). Sleep disturbance is associated with incident dementia and mortality. Curr. Alzheimer Res. 10, 767–775. doi: 10.2174/15672050113109990134
Ten Have, T. R., Kunselman, A. R., Pulkstenis, E. P., and Landis, J. R. (1998). Mixed effects logistic regression models for longitudinal binary response data with informative drop-out. Biometrics 54, 367–383. doi: 10.2307/2534023
Varga, A. W., Wohlleber, M. E., Gimenez, S., Romero, S., Alonso, J. F., Ducca, E. L., et al. (2016). Reduced slow-wave sleep is associated with high cerebrospinal Fluid Abeta42 levels in cognitively normal elderly. Sleep 39, 2041–2048. doi: 10.5665/sleep.6240
Virta, J. J., Heikkila, K., Perola, M., Koskenvuo, M., Räihä, I., Rinne, J. O., et al. (2013). Midlife sleep characteristics associated with late life cognitive function. Sleep 36, 1533–1541. doi: 10.5665/sleep.3052
Wang, C., and Holtzman, D. M. (2020). Bidirectional relationship between sleep and Alzheimer’s disease: role of amyloid, tau, and other factors. Neuropsychopharmacology 45, 104–120. doi: 10.1038/s41386-019-0478-5
Winer, J. R., Mander, B. A., Helfrich, R. F., Maass, A., Harrison, T. M., Baker, S. L., et al. (2019). Sleep as a potential biomarker of tau and beta-amyloid burden in the human brain. J. Neurosci. 39, 6315–6324. doi: 10.1523/JNEUROSCI.0503-19.2019
Xie, L., Kang, H., Xu, Q., Chen, M. J., Liao, Y., Thiyagarajan, M., et al. (2013). Sleep drives metabolite clearance from the adult brain. Science 342, 373–377. doi: 10.1126/science.1241224
Xu, W., Tan, C. C., Zou, J. J., Cao, X. P., and Tan, L. (2020). Sleep problems and risk of all-cause cognitive decline or dementia: an updated systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 91, 236–244. doi: 10.1136/jnnp-2019-321896
Keywords: sleep disturbance, Alzheimer’s disease, amnestic mild cognitive impairment, cardiovascular disease, biomarkers
Citation: Bubu OM, Williams ET, Umasabor-Bubu OQ, Kaur SS, Turner AD, Blanc J, Cejudo JR, Mullins AE, Parekh A, Kam K, Osakwe ZT, Nguyen AW, Trammell AR, Mbah AK, de Leon M, Rapoport DM, Ayappa I, Ogedegbe G, Jean-Louis G, Masurkar AV, Varga AW and Osorio RS (2021) Interactive Associations of Neuropsychiatry Inventory-Questionnaire Assessed Sleep Disturbance and Vascular Risk on Alzheimer’s Disease Stage Progression in Clinically Normal Older Adults. Front. Aging Neurosci. 13:763264. doi: 10.3389/fnagi.2021.763264
Received: 23 August 2021; Accepted: 08 November 2021;
Published: 10 December 2021.
Edited by:
Christine Bastin, University of Liège, BelgiumReviewed by:
Lucas Ronat, Centre de Recherche de l’Institut Universitaire de Gériatrie de Montréal, CanadaCopyright © 2021 Bubu, Williams, Umasabor-Bubu, Kaur, Turner, Blanc, Cejudo, Mullins, Parekh, Kam, Osakwe, Nguyen, Trammell, Mbah, de Leon, Rapoport, Ayappa, Ogedegbe, Jean-Louis, Masurkar, Varga and Osorio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Omonigho M. Bubu, b21vbmlnaG8uYnVidUBueXVsYW5nb25lLm9yZw==; Ricardo S. Osorio, cmljYXJkby5vc29yaW9Abnl1bGFuZ29uZS5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.