- 1Key Laboratory of Neuromolecular Biology, The First Affiliated Hospital of Henan University of Science and Technology, Luoyang, China
- 2Department of Neurology, The First Affiliated Hospital, College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China
- 3School of Nursing, The First Affiliated Hospital of Henan University of Science and Technology, Luoyang, China
To review the therapeutic effects of drugs on REM sleep behavior disorder (RBD) in Parkinson's disease (PD) by searching the MEDLINE/PubMed, Embase, Cochrane, and CBM databases. According to the inclusion and exclusion criteria, studies were included after excluding duplicate data. We evaluated the safety and efficacy of pharmacological intervention to improve RBD in patients with Parkinson's disease (PD-RBD). This systematic review mainly describes the drugs that can be used to treat PD-RBD patients. The results have shown that melatonin can be used as the first-line drug for PD-RBD, and clonazepam provides significant improvement on PD-RBD, androtigotine can be used as an alternative drug. However, further large-scale clinical trial studies are still needed to provide the best guidelines for the pharmacological treatment of PD-RBD.
Introduction
Parkinson's disease (PD) is the second most common progressive neurodegenerative disease in elderly individuals over the age of 65 (Sherer et al., 2012). In addition to the typical motor symptoms, there are also non-motor symptoms such as constipation, dysphagia, cognitive impairment, and sleep disorders (McDonald et al., 2018) which seriously affect patients' quality of life (Chaudhuri and Schapira, 2009). Sleep disorders in PD mainly include insomnia, excessive daytime sleepiness (EDS), restless legs syndrome (RLS), and rapid eye movement (REM) sleep behavior disorder (RBD) (Chahine et al., 2017; Stefani and Hogl, 2021).
RBD is a sleep disorder characterized by dreams and physical activities during REM sleep. Most RBD patients have dream-related Violent behavior, which often results to injure themself or others. RBD can be divided into idiopathic RBD (iRBD) and secondary RBD (sRBD) according to different causes. The iRBD appears as an independent symptom without other accompanying symptoms; sRBD includes drug-induced, symptomatic and neurodegenerative diseases related RBD. IRBD is the most reliable clinical marker for pro-synucleinopathy, such as Parkinson's disease (PD) (Hogl et al., 2018). Patients may eventually develop neurodegenerative diseases after a few years or decades, and the risk of occurrence ranges from more than 30% at 5 years to more than 90% at14 years. Idiopathic RBD can be used as a pre-exercise biomarker for PD and about 20% of RBD occurred before PD and about 20% of cases had both Parkinson's disease and RBD and more than 50% of RBD occurred for several years after the clinically manifest of Parkinson's disease (Diaconu et al., 2021).
The incidence of RBD in PD patients is ~20–50% (Sixel-Doring et al., 2011; Romenets et al., 2012; Bugalho and Viana-Baptista, 2013). The main symptoms can vary from simple muscle tension to complex behavioral disorders (Gagnon et al., 2002; Schenck and Mahowald, 2002). Patients often yell, laugh and even have violent behavior in their sleep, which is usually discovered by their bed partners. Patients can often remember their vivid dreams and dream enactment when they wake up (Sforza et al., 1997; Olson et al., 2000). PD-RBD is generally observed and diagnosed by polysomnography (PSG) (Duchna, 2006). PD patients with RBD not only suffer from a decline in sleep quality, but also easily cause injuries to the patients themselves and their bed partners, increasing the risk of intimacy interruption and bed partner injuries (Postuma et al., 2012; Schenck et al., 2013a).
RBD not only affects sleep quality but also cognitive function in PD patients (Nomura et al., 2013a; Yarnall et al., 2013). Research by Schenck et al. showed that RBD is related to cognitive decline, and more than 80% of elderly patients with RBD develop Parkinson's disease or dementia (Schenck et al., 2013b). Compared with PD patients without RBD, the cognitive dysfunction (especially delayed memory function) of PD patients with RBD is more prominent (Zhang J. R. et al., 2016). A recent clinical study confirmed that there is a significant correlation between sleep efficiency and overall cognitive ability in patients with PD (Sobreira et al., 2019). Therefore, the clinical treatment of patients has become important and needed. This article mainly collects all relevant studies to analyse and evaluate the effectiveness and safety of drug interventions, and puts forward some suggestions and questions.
Materials and Methods
Search Strategy and Selection Criteria
This study used the search generators available in each database to search all relevant literature up to January 1, 2020 in the MEDLINE/PubMed, Embase, Cochrane, and CMB databases. The search method was based on the following terms: “Parkinson's disease” and synonyms and “rapid eye movement sleep behavior disorder” and related terms; the search commonly used acronyms for these phrases, and duplicate studies were excluded.
Study Selection
The search results were independently evaluated by two reviewers, and differences were resolved through discussion. The inclusion criteria included the following: 1. Studies with crossover trials or open-label designs; 2. Studies that involved patients with Parkinson's disease; 3. The study targeted RBD among sleep disorders; 4. The treatment involved a single drug; and 5. Experiments were performed in vivo. The exclusion criteria were as follows: 1. Duplicate studies; 2. Studies that involved patients with diagnosis other than Parkinson's disease; 3. The studies sleep events related to PD other than RBD; 4. The treatment involved non-drug therapy or non-monotherapy; 5. Experiments were performed in vitro. In theory, we followed the PRISMA guidelines (Liberati et al., 2009) for a systematic review. However, due to the qualitative and non-quantitative research in this study, we did not further conduct bias risk assessment and data extraction synthesis.
Results
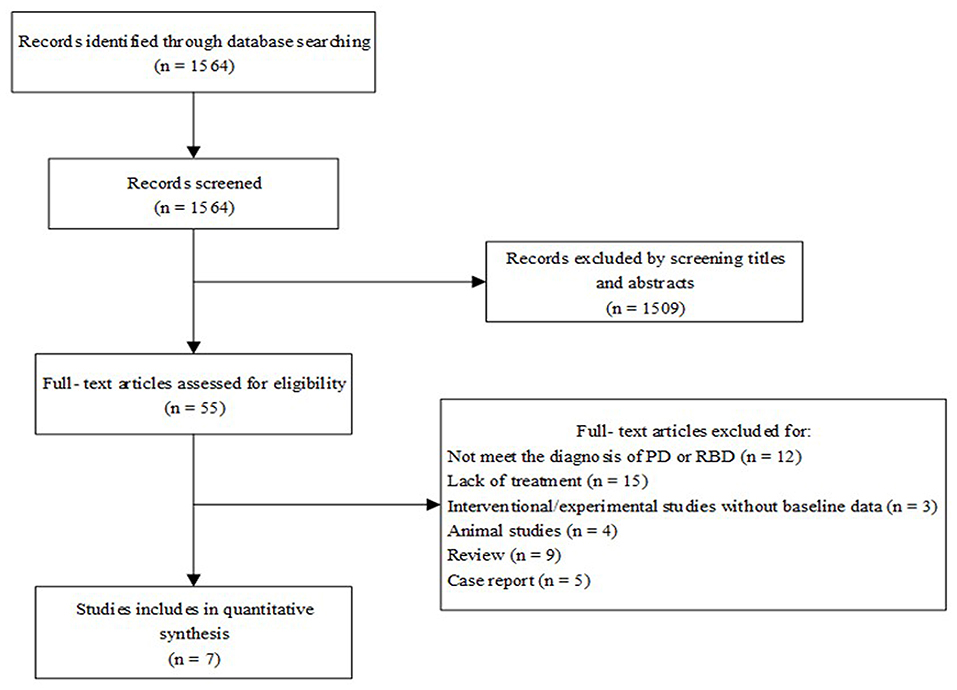
Literature searches were conducted based on PRISMA's preferred reporting project guidelines (Figure 1) (Liberati et al., 2009). After duplicate studies were excluded, resulting in 1,564 articles. Based on the study's inclusion criteria, 55 related clinical drug studies were selected, of which seven clinical trials met the criteria.
Clonazepam
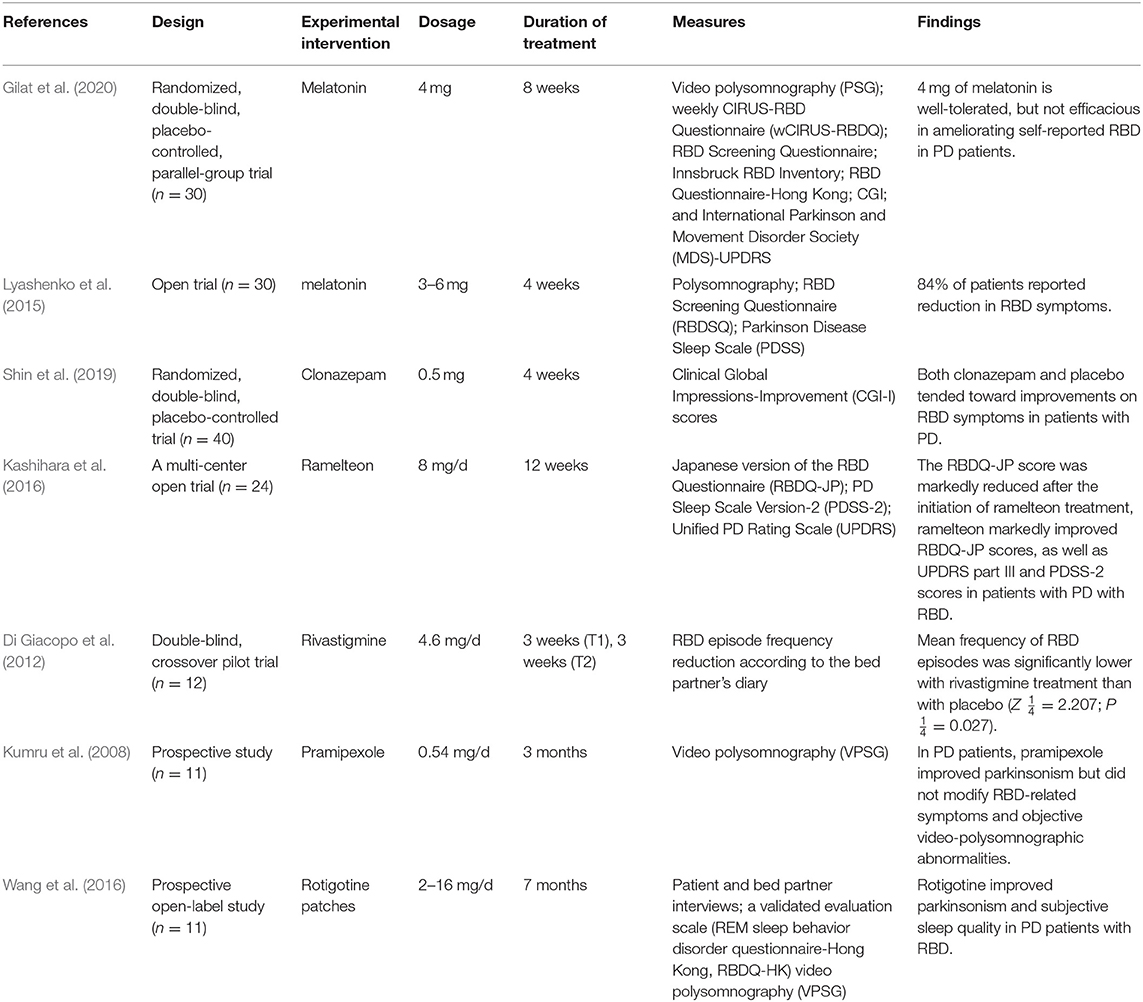
Clonazepam has long been considered the first-line treatment for PD-RBD (Sforza et al., 1997; Olson et al., 2000; Schenck and Mahowald, 2002; Aurora et al., 2010) and has been widely used clinically (Seppi and Ray Chaudhuri, 2019). However, evidence for its effectiveness is based only on case reports and follow-up studies (Aurora et al., 2010; Li et al., 2016), and evidence for treatment in randomized controlled clinical trials is lacking. In 2013, the International RBD Study Group (IRBD-SG) published a consensus statement on the design of controlled clinical studies for RBD (Schenck et al., 2013a). Shin compared the overall clinical impression improvement score at the fourth week of using 0.5 mg clonazepam and placebo, and the results showed no significant difference (p = 0.253) (Shin et al., 2019). However, this experiment was mainly evaluated by the Clinical Global Impressions-Improvement (CGI-I) score, without further clarification with PSG. At the same time, research shows side effects (morning sedation, confusion, dizziness, and falls) which may limit the effectiveness of clonazepam, especially in the elderly and/or RBD that coexists with obvious neurodegenerative diseases (Anderson and Shneerson, 2009). The result remains to be demonstrated by more experiments.
Melatonin
Clinical evidence has indicated that melatonin can be an effective adjuvant therapy for RBD in PD patients (Aurora et al., 2010). A study showed that after 4 weeks of treatment with 3–6 mg melatonin, RBD symptoms in 84% of PD patients significant improvement (Lyashenko et al., 2015). A randomized controlled study including 30 PD patients also showed that the extended release of 4 mg of melatonin did not significantly reduce PD-RBD symptoms (P = 0.92) (Gilat et al., 2020), but the study was mainly based on the Movement Disorder Society (MDS)-UPDRS questionnaire score, and the small sample couldn't detect group differences on secondary outcomes, and there were differences in the primary data at baseline level ADDIN EN.CITE (Gilat et al., 2020). In a multi-site, double-blind, placebo-controlled, crossover trial, 50 mg of melatonin improved sleep quality better than 5 mg (Dowling et al., 2005). Ramelteon, a melatonin receptor agonist, can significantly improve the RBD symptoms of PD patients compared with control group in a Multicenter Open Trial (P < 0.05) (Kashihara et al., 2016). But, the study mainly used the Japanese version of the RBD screening questionnaire to diagnose RBD, and did not use PSG, therefore, it had limitations. In another case report, PD-RBD patient was treated with ramelteon (8 mg/day before sleeping), PSG monitoring found that RBD symptoms were significantly improved (Nomura et al., 2013b). A meta-analysis in 2016 further demonstrated that melatonin showed significant improvements on sleep disorders in neurodegenerative diseases through nine randomized controlled trial (Zhang W. et al., 2016). The results of the latest 4-week randomized, double-blind, placebo-controlled pilot study showed that there was no difference between the iRBD patients receiving sustained-release melatonin and the placebo group. Although the study subjects were not PD-RBD, they didn't effect on results (Jun et al., 2019).
Rotigotine
Wang et al. (2016) studied rotigotine transdermal patches for 7 months in PD-RBD patients through interviews with PD patients themselves and their families, the REM Sleep Behavior Disorder Questionnaire (RBDQ-HK) and video polysomnography (VPSG) measurements, and VPSG analysis showed that total sleep time (TST) and stage 1% were increased, and the PLMS index decreased (Wang et al., 2016). The results suggested that rotigotine can improve symptoms of RBD of PD patients. Pierantozzi et al. also designed a randomized, double-blind, placebo-controlled parallel experiment, and they found that rotigotine could significantly improve sleep efficiency of patients with PD through PSG, but the study lacked the definition of RBD (Pierantozzi et al., 2016).
Rivastigmine
A double-blind crossover trial study (Di Giacopo et al., 2012) using rivastigmine at 4.6 mg/d showed a significant reduction in the frequency of RBD episodes recorded by bed partners (P = 0.027). However, four patients underwent multiple PSG tests, and REM sleep without atonia (RSWA) showed no significant changes (Di Giacopo et al., 2012).
Pramipexole
In a prospective study, through bed partner recording and PSG monitoring, patients' PD symptoms improved, but RBD was not significantly improved (P > 0.05) (Kumru et al., 2008). This study was more accurate because of the combined subjective and objective detection methods, but the sample size was insufficient.
In addition, we briefly summarize the 55 articles in the query that involved drugs for treating sleep disorders but did not conform to the inclusion criteria, such as individual case reports or non-PD-RBD patients. This study provides additional information regarding these drug treatments as follows.
Levodopa
Ozekmekçi et al. used levodopa at 460.3 and 320.3 mg/d in PD patients with RBD. Studies have shown that dopamine can improve the scores of UPDRS (Unified Parkinson's Disease rate Scale; Ozekmekçi et al., 2005). However, Wailke et al. did not find any improvement in REM sleep among PD patients' sleep status after taking 200 mg levodopa/carbidopa controlled-release tablet (CR) by PSG (P = 0.615) (Wailke et al., 2011). Tan et al.'s case report showed that RBD preceded PD in all three cases, and the three patients significantly improved their RBD after levodopa use, but without polygraph detection (Tan et al., 1996). There was a significant difference in subjective sleep symptoms (P = 0.082), six patients with PD-RBD received intestinal levodopa infusion after 6 months treatment (Zibetti et al., 2017).
Cannabinoids
Cannabinoids (CBD) can also improve sleep quality and reduce sleep disorders (Kuhathasan et al., 2019). Clinical studies have shown that cannabinoids were beneficial for sleep disorders of PD patients, and the mechanism may be related to the distribution of cannabinoid receptors in the structure of the basal ganglia (Buhmann et al., 2019). In an observational study of four PD patients receiving CBD treatment, it was found that the frequency of RBD in patients was rapidly and significantly reduced, and there were no side effects (Chagas et al., 2014).
Memantine
In a randomized controlled study (Larsson et al., 2010), sleep scale evaluation scores showed that 20 mg/d memantine reduced REM sleep behavior disorder that may occur in PD patients, but the specificity of PD-RBD was unclear.
Discussion and Conclusions
We hereby conducted a systematic review of all relevant drug clinical trials to evaluate the safety and efficacy of drug treatment for PD-RBD. The latest consensus guidelines for the clinical management of PD non-motor symptoms published in 2020 mentioned that there is currently a lack of RCT (Randomized Controlled Trial) studies for PD-RBD treatment, and that clonazepam or/and melatonin can be used to treat PD-RBD (帕金森病非运动症状管理专家共识, 2020).
The results showed that melatonin and clonazepam can improve PD-RBD as current first-line drugs. Rotigotine can be used as an alternative or a monotherapy for the above two drugs, but more definitive clinical trial evidence is lacking. Askenasy thought that changes in dopamine receptor sensitivity in the substantia nigra and striatum during REM sleep may be responsible for sleep disorders (Askenasy, 1993). In addition, serotonin significantly decreased in the brains of PD patients, and levodopa activated dopamine receptors and reduced serotonin levels (Askenasy and Yahr, 1985), which may lead to improvement of PD-RBD, so dopamine therapy is promising to become an alternative to clonazepam. Rotigotine, a non-ergot dopamine agonist, has been demonstrated to be effective both an early monotherapy in PD (Watts et al., 2007) and adjuvant therapy for levodopa in advanced PD (Poewe et al., 2007). The mechanism of rotigotine to improve RBD may be through activation of dopamine D 1 receptors involved in the regulation of REM sleep (Wang et al., 2016). Santiago Pérez-Lloret concluded in his study that sleep changes in PD patients may be disrupted by the melatonin system, which can be associated with low levels of melatonin MT1 and MT2 receptor densities in the substantia nigra and amygdala in PD (Perez-Lloret and Cardinali, 2021). Clonazepam can improve RBD by activating glycine and GABA transport pathway (Brooks and Peever, 2011).
There is no reliable evidence to consider rivastigmine, memantine, cannabinoid, and other drugs, but it is still possible to consider them as adjuvant or even alternative drugs in the absence of significantly improved with first-line drug treatment. Cannabinoids (CBD) may regulate the changes in glutamic acid and other chemicals caused by decreased dopamine levels (Giacoppo et al., 2014; Gomez-Galvez et al., 2016; Stampanoni Bassi et al., 2017). Rivastigmine, as an acetylcholinesterase inhibitor, may be used in PD-RBD and acts on the cholinergic pathway in the context of pontine bulbar degeneration (Braak et al., 2003; Boeve et al., 2007). Videnovic et al. concluded that donepezil and quetiapine/clozapine can improve the symptoms of RBD, but these are individual case studies, and a large number of clinical studies are needed (During and Miglis, 2019).
In this study, according to the seven included literature studies, the results of a single trial show that (1) clonazepam and melatonin can currently be used as first-line drugs for the treatment of PD-RBD; (2) rotigotine can become a substitute for the above two drugs; and (3) rivastigmine, memantine, and cannabinoids may be effective for RBD in PD patients. According to the results of this systematic review, these drugs can improve the symptoms of PD-RBD and have been used clinically (Table 1). However, due to the insufficient sample size of these trials and the defects in evaluation methods, large-scale clinical trials are still needed for further confirmation.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
AL and JW were involved in the execution. RS and JYang were involved in the statistical analysis and manuscript preparation. HM and JH were involved in analyzing the data. JYan was involved in the research project (conception, design, writing, and organization). All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Project of Henan Province Science and Technology (202102310216) and the Key projects of medical science and technology in Henan Province (SBGJ202002099).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anderson, K. N., and Shneerson, J. M. (2009). Drug treatment of REM sleep behavior disorder: the use of drug therapies other than clonazepam. J. Clin. Sleep Med. 5, 235–239. doi: 10.5664/jcsm.27492
Askenasy, J. J. (1993). Sleep in Parkinson's disease. Acta Neurol. Scand. 87, 167–170. doi: 10.1111/j.1600-0404.1993.tb04095.x
Askenasy, J. J., and Yahr, M. D. (1985). Reversal of sleep disturbance in Parkinson's disease by antiparkinsonian therapy: a preliminary study. Neurology 35, 527–532. doi: 10.1212/WNL.35.4.527
Aurora, R. N., Zak, R. S., Maganti, R. K., Auerbach, S. H., Casey, K. R., Chowdhuri, S., et al. (2010). Best practice guide for the treatment of REM sleep behavior disorder (RBD). J. Clin. Sleep Med. 6, 85–95. doi: 10.5664/jcsm.27717
Boeve, B. F., Silber, M. H., Saper, C. B., Ferman, T. J., Dickson, D. W., Parisi, J. E., et al. (2007). Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 130, 2770–2788. doi: 10.1093/brain/awm056
Braak, H., Del Tredici, K., Rub, U., de Vos, R. A., Jansen Steur, E. N., and Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 24, 197–211. doi: 10.1016/S0197-4580(02)00065-9
Brooks, P. L., and Peever, J. H. (2011). Impaired GABA and glycine transmission triggers cardinal features of rapid eye movement sleep behavior disorder in mice. J. Neurosci. 31, 7111–7121. doi: 10.1523/JNEUROSCI.0347-11.2011
Bugalho, P., and Viana-Baptista, M. (2013). REM sleep behavior disorder and motor dysfunction in Parkinson's disease–a longitudinal study. Parkinsonism Relat. Disord. 19, 1084–1087. doi: 10.1016/j.parkreldis.2013.07.017
Buhmann, C., Mainka, T., Ebersbach, G., and Gandor, F. (2019). Evidence for the use of cannabinoids in Parkinson's disease. J. Neural Transm. 126, 913–924. doi: 10.1007/s00702-019-02018-8
Chagas, M. H., Eckeli, A. L., Zuardi, A. W. M. A, Pena-Pereira, M. A., Sobreira-Neto, Sobreira, E. T., et al. (2014). Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson's disease patients: a case series. J. Clin. Pharm. Ther. 39, 564–566. doi: 10.1111/jcpt.12179
Chahine, L. M., Amara, A. W., and Videnovic, A. (2017). A systematic review of the literature on disorders of sleep and wakefulness in Parkinson's disease from 2005 to 2015. Sleep Med. Rev. 35, 33–50. doi: 10.1016/j.smrv.2016.08.001
Chaudhuri, K. R., and Schapira, A. H. (2009). Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 8, 464–474. doi: 10.1016/S1474-4422(09)70068-7
Di Giacopo, R., Fasano, A., Quaranta, D., Marca, G. D., Bove, F., and Bentivoglio, A. R. (2012). Rivastigmine as alternative treatment for refractory REM behavior disorder in Parkinson's disease. Mov. Disord. 27, 559–561. doi: 10.1002/mds.24909
Diaconu, S., Falup-Pecurariu, O., Tint, D., and Falup-Pecurariu, C. (2021). REM sleep behaviour disorder in Parkinson's disease (Review). Exp. Ther. Med. 22:812. doi: 10.3892/etm.2021.10244
Dowling, G. A., Mastick, J., Colling, E., Carter, J. H., Singer, C. M., and Aminoff, M. J. (2005). Melatonin for sleep disturbances in Parkinson's disease. Sleep Med. 6, 459–466. doi: 10.1016/j.sleep.2005.04.004
Duchna, H. W. (2006). Sleep-related breathing disorders–a second edition of the International Classification of Sleep Disorders (ICSD-2) of the American Academy of Sleep Medicine (AASM). Pneumologie 60, 568–575. doi: 10.1055/s-2006-944248
During, E. H., and Miglis, M. G. (2019). Clinical trials in REM sleep behavior disorder: an urgent need for better evidence. Sleep Med. 63, 1–2. doi: 10.1016/j.sleep.2019.06.001
Gagnon, J. F., Bedard, M. A., Fantini, M. L., Petit, D., Panisset, M., Rompre, S., et al. (2002). REM sleep behavior disorder and REM sleep without atonia in Parkinson's disease. Neurology 59, 585–589. doi: 10.1212/WNL.59.4.585
Giacoppo, S., Mandolino, G., Galuppo, M., Bramanti, P., and Mazzon, E. (2014). Cannabinoids: new promising agents in the treatment of neurological diseases. Molecules 19, 18781–18816. doi: 10.3390/molecules191118781
Gilat, M. A, Coeytaux Jackson, Marshall, N. S., Hammond, D., Mullins, A. E., Hall, J. M., et al. (2020). Melatonin for rapid eye movement sleep behavior disorder in Parkinson's disease: a randomised controlled trial. Mov. Disord. 35, 344–349. doi: 10.1002/mds.27886
Gomez-Galvez, Y., Palomo-Garo, C., Fernandez-Ruiz, J., and Garcia, C. (2016). Potential of the cannabinoid CB(2) receptor as a pharmacological target against inflammation in Parkinson's disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 64, 200–208. doi: 10.1016/j.pnpbp.2015.03.017
Hogl, B., Stefani, A., and Videnovic, A. (2018). Idiopathic REM sleep behaviour disorder and neurodegeneration - an update. Nat. Rev. Neurol. 14, 40–55. doi: 10.1038/nrneurol.2017.157
Jun, J. S., Kim, R., Byun, J. I., Kim, T. J., Lim, J. A., Sunwoo, J. S., et al. (2019). Prolonged-release melatonin in patients with idiopathic REM sleep behavior disorder. Ann. Clin. Transl. Neurol. 6, 716–722. doi: 10.1002/acn3.753
Kashihara, K., Nomura, T., Maeda, T., Tsuboi, Y., Mishima, T., Takigawa, H., et al. (2016). Beneficial effects of ramelteon on rapid eye movement sleep behavior disorder associated with Parkinson's disease - results of a multicenter open trial. Intern. Med. 55, 231–236. doi: 10.2169/internalmedicine.55.5464
Kuhathasan, N., Dufort, A., MacKillop, J., Gottschalk, R., Minuzzi, L., and Frey, B. N. (2019). The use of cannabinoids for sleep: a critical review on clinical trials. Exp. Clin. Psychopharmacol. 27, 383–401. doi: 10.1037/pha0000285
Kumru, H., Iranzo, A., Carrasco, E., Valldeoriola, F., Marti, M. J., Santamaria, J., et al. (2008). Lack of effects of pramipexole on REM sleep behavior disorder in Parkinson disease. Sleep 31, 1418–1421. doi: 10.5665/sleep/31.10.1418
Larsson, V., Aarsland, D., Ballard, C., Minthon, L., and Londos, E. (2010). The effect of memantine on sleep behaviour in dementia with Lewy bodies and Parkinson's disease dementia. Int. J. Geriatr. Psychiatry 25, 1030–1038. doi: 10.1002/gps.2506
Li, S. X., Lam, S. P., Zhang, J., Yu, M. W., Chan, J. W., Liu, Y., et al. (2016). A prospective, naturalistic follow-up study of treatment outcomes with clonazepam in rapid eye movement sleep behavior disorder. Sleep Med. 21, 114–120. doi: 10.1016/j.sleep.2015.12.020
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gotzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann. Intern. Med. 151, W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136
Lyashenko, C. A. C., Levin, O. S., and Poluektov, M. G. (2015). Melatonin in correction of REM-sleep behavior disorders in Parkinson's disease. Zh Nevrol Psikhiatr Im S S Korsakova 115, 40–43. doi: 10.17116/jnevro20151156240-43
McDonald, C., Gordon, G., Hand, A., Walker, R. W., and Fisher, J. M. (2018). 200 years of Parkinson's disease: what have we learnt from James Parkinson? Age Ageing 47, 209–214. doi: 10.1093/ageing/afx196
Nomura, T., Inoue, Y., Kagimura, T., and Nakashima, K. (2013a). Clinical significance of REM sleep behavior disorder in Parkinson's disease. Sleep Med. 14, 131–135. doi: 10.1016/j.sleep.2012.10.011
Nomura, T., Kawase, S., Watanabe, Y., and Nakashima, K. (2013b). Use of ramelteon for the treatment of secondary REM sleep behavior disorder. Intern. Med. 52, 2123–2126. doi: 10.2169/internalmedicine.52.9179
Olson, E. J., Boeve, B. F., and Silber, M. H. (2000). Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain 123, 331–339. doi: 10.1093/brain/123.2.331
Ozekmekçi, S., Apaydin, H., and Kiliç, E. (2005). Clinical features of 35 patients with Parkinson's disease displaying REM behavior disorder. Clin. Neurol. Neurosurg. 107, 306–309. doi: 10.1016/j.clineuro.2004.09.021
Perez-Lloret, S., and Cardinali, D. P. (2021). Melatonin as a chronobiotic and cytoprotective agent in Parkinson's disease. Front. Pharmacol. 12:650597. doi: 10.3389/fphar.2021.650597
Pierantozzi, M., Placidi, F., Liguori, C., Albanese, M., Imbriani, P., Marciani, M. G., et al. (2016). Rotigotine may improve sleep architecture in Parkinson's disease: a double-blind, randomized, placebo-controlled polysomnographic study. Sleep Med. 21, 140–144. doi: 10.1016/j.sleep.2016.01.016
Poewe, W. H., Rascol, O., Quinn, N., Tolosa, E., Oertel, W. H., Martignoni, E., et al. (2007). Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson's disease: a double-blind, double-dummy, randomised controlled trial. Lancet Neurol. 6, 513–520. doi: 10.1016/S1474-4422(07)70108-4
Postuma, R. B., Bertrand, J. A., Montplaisir, J., Desjardins, C., Vendette, M., Rios Romenets, S., et al. (2012). Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson's disease: a prospective study. Mov. Disord. 27, 720–726. doi: 10.1002/mds.24939
Romenets, S. R., Gagnon, J. F., Latreille, V., Panniset, M., Chouinard, S., Montplaisir, J., et al. (2012). Rapid eye movement sleep behavior disorder and subtypes of Parkinson's disease. Mov. Disord. 27, 996–1003. doi: 10.1002/mds.25086
Schenck, C. H., Boeve, B. F., and Mahowald, M. W. (2013b). Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 14, 744–748. doi: 10.1016/j.sleep.2012.10.009
Schenck, C. H., and Mahowald, M. W. (2002). REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep 25, 120–138. doi: 10.1093/sleep/25.2.120
Schenck, C. H., Montplaisir, J. Y., Frauscher, B., Hogl, B., Gagnon, J. F., Postuma, R., et al. (2013a). Rapid eye movement sleep behavior disorder: devising controlled active treatment studies for symptomatic and neuroprotective therapy–a consensus statement from the International Rapid Eye Movement Sleep Behavior Disorder Study Group. Sleep Med. 14, 795–806. doi: 10.1016/j.sleep.2013.02.016
Seppi, K., and Ray Chaudhuri, K. (2019). Update on treatments for nonmotor symptoms of Parkinson's disease-an evidence-based medicine review. Mov. Disord. 34, 180–198. doi: 10.1002/mds.27602
Sforza, E., Krieger, J., and Petiau, C. (1997). REM sleep behavior disorder: clinical and physiopathological findings. Sleep Med. Rev. 1, 57–69. doi: 10.1016/S1087-0792(97)90006-X
Sherer, T. B., Chowdhury, S., Peabody, K., and Brooks, D. W. (2012). Overcoming obstacles in Parkinson's disease. Mov. Disord. 27, 1606–1611. doi: 10.1002/mds.25260
Shin, C., Park, H., Lee, W. W., Kim, H. J., Kim, H. J., and Jeon, B. (2019). Clonazepam for probable REM sleep behavior disorder in Parkinson's disease: a randomized placebo-controlled trial. J. Neurol. Sci. 401, 81–86. doi: 10.1016/j.jns.2019.04.029
Sixel-Doring, F., Trautmann, E., Mollenhauer, B., and Trenkwalder, C. (2011). Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology 77, 1048–1054. doi: 10.1212/WNL.0b013e31822e560e
Sobreira, E. S. T, Sobreira-Neto, M. A., Pena-Pereira, M. A., Chagas, M. H. N, Fernandes, R. M. F., Eckeli, A. L., et al. (2019). Global cognitive performance is associated with sleep efficiency measured by polysomnography in patients with Parkinson's disease. Psychiatry Clin. Neurosci. 73, 248–253. doi: 10.1111/pcn.12819
Stampanoni Bassi, M., Sancesario, A., Morace, R., Centonze, D., and Iezzi, E. (2017). Cannabinoids in Parkinson's disease. Cannabis. Cannabinoid. Res. 2, 21–29. doi: 10.1089/can.2017.0002
Stefani, A., and Hogl, B. (2021). Sleep disorders in Parkinson disease. Sleep Med. Clin. 16, 323–334. doi: 10.1016/j.jsmc.2021.03.001
Tan, A., Salgado, M., and Fahn, S. (1996). Rapid eye movement sleep behavior disorder preceding Parkinson's disease with therapeutic response to levodopa. Mov. Disord. 11, 214–216. doi: 10.1002/mds.870110216
Wailke, S., Herzog, J., Witt, K., Deuschl, G., and Volkmann, J. (2011). Effect of controlled-release levodopa on the microstructure of sleep in Parkinson's disease. Eur. J. Neurol. 18, 590–596. doi: 10.1111/j.1468-1331.2010.03213.x
Wang, Y., Yang, Y., Wu, H., Lan, D., Chen, Y., and Zhao, Z. (2016). Effects of rotigotine on REM sleep behavior disorder in Parkinson disease. J. Clin. Sleep Med. 12, 1403–1409. doi: 10.5664/jcsm.6200
Watts, R. L., Jankovic, J., Waters, C., Rajput, A., Boroojerdi, B., and Rao, J. (2007). Randomized, blind, controlled trial of transdermal rotigotine in early Parkinson disease. Neurology 68, 272–276. doi: 10.1212/01.wnl.0000252355.79284.22
Yarnall, A. J., Rochester, L., and Burn, D. J. (2013). Mild cognitive impairment in Parkinson's disease. Age Ageing 42, 567–576. doi: 10.1093/ageing/aft085
Zhang, J. R., Chen, J., Yang, Z. J., Zhang, H. J., Fu, Y. T., Shen, Y., et al. (2016). Rapid eye movement sleep behavior disorder symptoms correlate with domains of cognitive impairment in Parkinson's disease. Chin. Med. J. 129, 379–385. doi: 10.4103/0366-6999.176077
Zhang, W., Chen, X. Y., Su, S. W., Jia, Q. Z., Ding, T., Zhu, Z. N., et al. (2016). Exogenous melatonin for sleep disorders in neurodegenerative diseases: a meta-analysis of randomized clinical trials. Neurol. Sci. 37, 57–65. doi: 10.1007/s10072-015-2357-0
Zibetti, M., Romagnolo, A., Merola, A., Priano, L., Montanaro, E., Angrisano, S., et al. (2017). A polysomnographic study in parkinsonian patients treated with intestinal levodopa infusion. J. Neurol. 264, 1085–1090. doi: 10.1007/s00415-017-8491-2
Keywords: Parkinson's disease, rapid eye movement, drugs, systematic review, sleep
Citation: Yan J, Liu A, Huang J, Wu J, Shen R, Ma H and Yang J (2021) Pharmacological Interventions for REM Sleep Behavior Disorder in Parkinson's Disease: A Systematic Review. Front. Aging Neurosci. 13:709878. doi: 10.3389/fnagi.2021.709878
Received: 14 May 2021; Accepted: 21 July 2021;
Published: 13 August 2021.
Edited by:
Oscar Arias-Carrion, Hospital General Dr. Manuel Gea Gonzalez, MexicoReviewed by:
Paola Imbriani, University of Rome Tor Vergata, ItalyMilton Cesar Biagioni, UCB Pharma, Belgium
Copyright © 2021 Yan, Liu, Huang, Wu, Shen, Ma and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junqiang Yan, eWFuanEyMDA2MjAwNyYjeDAwMDQwOzEyNi5jb20=; Jianxue Yang, ODM1Mzc4Njk3JiN4MDAwNDA7cXEuY29t
 Junqiang Yan
Junqiang Yan Anran Liu
Anran Liu Jiarui Huang2
Jiarui Huang2