
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Aging Neurosci. , 11 June 2021
Sec. Cellular and Molecular Mechanisms of Brain-aging
Volume 13 - 2021 | https://doi.org/10.3389/fnagi.2021.682633
This article is part of the Research Topic Unfolded Protein Response (UPR): An Impending Target for Multiple Neurological Disorders View all 10 articles
Immune surveillance is an essential process that safeguards the homeostasis of a healthy brain. Among the increasing diversity of immune cells present in the central nervous system (CNS), microglia have emerged as a prominent leukocyte subset with key roles in the support of brain function and in the control of neuroinflammation. In fact, impaired microglial function is associated with the development of neurodegenerative diseases, including Alzheimer’s disease (AD) and Parkinson’s disease (PD). Interestingly, these pathologies are also typified by protein aggregation and proteostasis dysfunction at the level of the endoplasmic reticulum (ER). These processes trigger activation of the unfolded protein response (UPR), which is a conserved signaling network that maintains the fidelity of the cellular proteome. Remarkably, beyond its role in protein folding, the UPR has also emerged as a key regulator of the development and function of immune cells. However, despite this evidence, the contribution of the UPR to immune cell homeostasis, immune surveillance, and neuro-inflammatory processes remains largely unexplored. In this review, we discuss the potential contribution of the UPR in brain-associated immune cells in the context of neurodegenerative diseases.
Protein homeostasis, also known as “proteostasis,” is a set of coordinated processes that govern synthesis, quality, control and localization of cellular proteins. Up to a third of protein biosynthesis takes place in the endoplasmic reticulum (ER; Brodsky and Skach, 2011) and thereby, cells possess regulatory mechanisms that maintain proteostasis in conditions that overload the folding capacity of the organelle (a process known as “ER stress”).
The main mechanism counteracting the detrimental effects of ER stress is the unfolded protein response (UPR), a signal transduction pathway that maintains the balance between the folding capacity and the secretory demand of the cell. The UPR is integrated by three ER transmembrane sensors: Inositol-Requiring Enzyme (lRE1), Protein kinase R-like ER Kinase (PERK), and Activating Transcription Factor-6 (ATF6), which are triggered by the accumulation of misfolded proteins in the ER lumen (Figure 1). The UPR transducers act in concert to activate transcription factors and cytosolic signaling modules aiming to restore proteostasis and increase ER biogenesis (Walter and Ron, 2011). Once activated, ATF6 translocates to the Golgi apparatus where it is cleaved by site-1 and site-2 proteases, releasing a transcription factor termed “ATF6-N” that controls expression of ER chaperones, ER-Associated protein degradation (ERAD) components, and lipid biosynthetic genes (Ron and Walter, 2007). PERK, on the other hand, mediates protein translation shutdown via phosphorylation of eukaryotic initiation factor-2α (p-eIF2α), which favors selective translation of mRNAs coding for proteins involved in cell survival, ER homeostasis, and antioxidant responses (Kranz et al., 2017). One of these mRNAs encodes ATF4, a transcription factor that controls the expression of the pro-apoptotic factor CHOP (C/EBP homologous protein), and GADD34 (also known as PPP1R15a), a phosphatase 1 cofactor that mediates dephosphorylation of p-eIF2α (Novoa et al., 2001; Hetz and Papa, 2018). Finally, IRE1 is the most conserved sensor of the UPR consisting of a transmembrane protein with two domains: a serine/threonine kinase domain and an endoribonuclease (RNase) domain (Grootjans et al., 2016). The IRE1 RNase domain mediates an unconventional splicing of the mRNA coding for X-box binding protein 1 (XBP1), removing a 26-nucleotide intron, followed by ligation by RtcB ligase (Hetz and Papa, 2018). XBP1 processing results in a shift in the coding reading frame, resulting in translation of the transcription factor “XBP1 spliced” (XBP1s), a master regulator of lipid biosynthesis, ER chaperones, ER biogenesis, and ERAD genes (Lee et al., 2003; Shoulders et al., 2013). Notably, ATF6 and XBP1s can also form heterodimers, amplifying the spectra of proteostatic genes particularly ERAD components (Yamamoto et al., 2007; Shoulders et al., 2013).
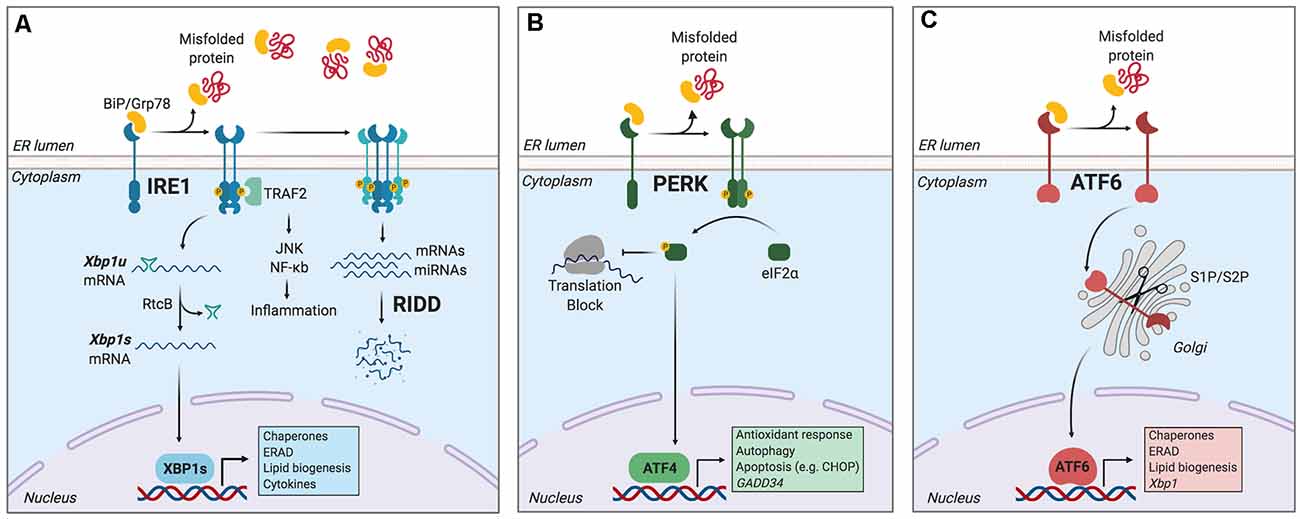
Figure 1. The unfolded protein response (UPR). Endoplasmic reticulum (ER) stress induces an adaptive response known as the unfolded protein response (UPR), which is controlled by three main ER-resident sensors: IRE1, PERK, and activating transcription factor-6 (ATF6). (A) IRE1 is activated by oligomerization and trans-phosphorylation upon binding of unfolded proteins and release of the chaperone BiP. IRE1 autophosphorylation leads to the activation of its RNase domain and the processing of the mRNA encoding for X-box binding protein 1 (XBP1s), a transcriptional factor that upregulates genes involved in protein folding and quality control, in addition to regulating ER/Golgi biogenesis and ER-mediated degradation (ERAD), lipid biogenesis and cytokine production. Additionally, IRE1 RNase also degrades a subset of specific RNAs and microRNAs, a process termed Regulated IRE1-Dependent Decay (RIDD). (B) Upon activation, PERK phosphorylates the eukaryotic initiation factor-2α (eIF2α), decreasing the synthesis of proteins and the overload of misfolded proteins at the ER. PERK phosphorylation also leads to the specific translation of ATF4, a transcription factor that promotes the expression of genes related to amino acid metabolism, antioxidant response, autophagy, and apoptosis. (C) ATF6 is activated upon release of BiP and is translocated to the Golgi, where it undergoes sequential cleavage and removal of its luminal domain. The remaining transactivation domain of ATF6 moves to the nucleus and coordinates the expression of genes encoding ER chaperones, ER-associated protein degradation (ERAD) components, and molecules involved in lipid biogenesis. Figure created with BioRender.com.
Furthermore, in contexts of prolonged ER stress or upon XBP1 deficiency, IRE1 RNase broadens its substrate repertoire and cleaves additional mRNAs/microRNAs through a process termed “Regulated IRE1-Dependent Decay” (RIDD; Lu et al., 2014; Maurel et al., 2014), which regulates mRNAs related to apoptosis and inflammation, among other processes (Maurel et al., 2014). In addition, IRE1 kinase can initiate inflammatory responses through recruitment of TRAF2 (TNF receptor-associated factor 2) protein and the transcription factor NF-κB (Urano et al., 2000; Hu et al., 2006), and it also facilitates apoptosis via mobilization of ER Ca2+ (Sprooten et al., 2019).
Remarkably, the capacity of the UPR to set the threshold between cell survival and death has associated the pathway with several neurodegenerative diseases that are typified by abnormal protein aggregation (Hetz, 2021). Nevertheless, despite this evidence, it is unclear if the UPR is selectively regulated at the level of supporting cells, nerve cells, and immune cells residing in the brain. In this review, we discuss the potential role of the UPR in controlling neuroinflammation and immunity in the central nervous system (CNS).
The advent of single-cell analysis has revolutionized our understanding of tissue immunity, demonstrating that the brain contains a broad diversity of immune cell types with roles in homeostasis, aging, and disease (Korin et al., 2017; Mrdjen et al., 2018).
Microglia is the most prominent resident macrophage of the brain parenchyma, where these cells coordinate synaptic pruning, neuron survival/plasticity, and apoptotic cell clearance (Colonna and Butovsky, 2017; Herz et al., 2017). Microglia initiate inflammation through pattern-recognition receptors (PRRs) that recognize noxious stimuli in the CNS (such as protein aggregates, cancer cells, or neurotropic viruses), and the signal for transcription of proinflammatory genes (Colonna and Butovsky, 2017; Prinz et al., 2019), via NF-κB or interferon-regulatory factors (IRFs; Reverendo et al., 2019). Relevant PRRs initiating brain inflammation include Toll-like receptors (TLR) such as TLR4 and TLR2, NOD-like receptors (NLRs), and C-type lectins including CLEC7A (Colonna and Butovsky, 2017). Additional mechanisms regulating inflammation are the signaling platforms termed “inflammasomes”, which control the secretion of interleukin-1β (IL-1β) and IL-18 (Swanson et al., 2019). In fact, the inflammasome coordinated by the intracellular sensor NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) has emerged as a critical regulator of neuroinflammation (Colonna and Butovsky, 2017). As such, microglia adapt to challenges by fine-tuning inflammation, although this response deteriorates with age contributing to neurodegeneration (Scheiblich et al., 2020).
Outside the parenchymal region, there is active immune-surveillance by border-associated macrophages (BAMs), monocytes, T cells, Natural Killer (NK) cells, NKT cells, dendritic cells (DCs), and B cells (Ransohoff and Cardona, 2010; Korin et al., 2017; Mrdjen et al., 2018). BAMs carry out scavenging/patrolling functions, whereas DCs are composed of plasmacytoid DCs (pDCs), type 1 conventional DCs (cDC1s), and type 2 conventional DCs (cDC2s; Mrdjen et al., 2018). pDCs produce type-I interferons (IFN-I) to viral infection, whereas cDC1s and cDC2s elicit long–term immunity via antigen presentation to cytotoxic CD8+ T cells and CD4+ T helper cells, respectively (Mundt et al., 2019; Cabeza-Cabrerizo et al., 2021). T cells in turn protect the brain against antigens found in neurodegenerative diseases and are critical for imprinting the functional maturation of microglia (Pasciuto et al., 2020). Notably, upon inflammation, aging, and neurodegeneration, resident immune cells become activated, and the parenchyma is infiltrated by leukocytes from the periphery, which can perpetuate inflammatory responses and propagate the progression of tissue damage (Scheiblich et al., 2020; Yang et al., 2020). This evidence indicates that the CNS contains a diverse immune microenvironment that can be targeted for intervention of neurodegenerative diseases. Finally, astrocytes are CNS glial cells that also contribute to inflammation (Giovannoni and Quintana, 2020) by expressing PRRs that trigger innate immunity, including TLR4 (Sofroniew, 2020).
Notably, although the UPR is known to control the development and function of immune cells including macrophages, DCs, B cells, eosinophils, NK cells, and T cells in physiological and pathological models (Martinon et al., 2010; Cubillos-Ruiz et al., 2015; Grootjans et al., 2016; Song et al., 2018; Dong et al., 2019), little is known about how the UPR regulates immunity in the CNS. This is a relevant area considering that neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple sclerosis (MS) display altered functions of several immune cell types, many of which are associated with disease progression (Ajami et al., 2018; Mrdjen et al., 2018; Scheiblich et al., 2020). Thus, modulating the UPR in immune cells during the progression of neurodegenerative diseases may have relevant implications for therapy.
Although most studies connecting the UPR with immunity have focused on organs outside the CNS, some of these findings could be applied to neurodegenerative diseases.
IRE1 via XBP1s controls eosinophil, DC, and plasma cell development (Reimold et al., 2001; Iwakoshi et al., 2007; Bettigole et al., 2015), which contribute to neuroinflammation in MS and Experimental Autoimmune Encephalomyelitis (EAE), the mouse model of MS (Greter et al., 2005; Wensky et al., 2005; Mundt et al., 2019; Pröbstel et al., 2020). Furthermore, DCs and plasma cells show constitutive UPR activation and display an elevated protein synthesis rate in steady state (Osorio et al., 2014; Khalsa et al., 2019; Mendes et al., 2020). In these cell types, XBP1s maintains ER architecture, whereas RIDD controls functional aspects, including antigen cross-presentation and cDC1s survival, or immunoglobulin production by plasma cells (Benhamron et al., 2014; Osorio et al., 2014; Tavernier et al., 2017). PERK signaling is also active in DCs (Mendes et al., 2020), whereas ATF6 remains understudied in immunity. These findings suggest that IRE1 and PERK signaling could be targets in neuroinflammatory pathologies involving DCs and plasma cell function, such as EAE (Greter et al., 2005; Mundt et al., 2019; Pröbstel et al., 2020).
The UPR also regulates inflammation (Bettigole and Glimcher, 2015; Grootjans et al., 2016; Flores-Santibáñez et al., 2019), by mechanisms that could be extended to neurodegeneration (Reverendo et al., 2019). Innate recognition via TLRs triggers ER stress in leukocytes and UPR components amplify the inflammatory program elicited by these receptors (Goodall et al., 2010; Martinon et al., 2010). ER stress also licenses macrophages to secrete the proinflammatory factors IL-1β, IL-6, TNF, and iNOS (Rao et al., 2014; Shenderov et al., 2014; Yang F. et al., 2018; Yang et al., 2019). Upon bacterial infection, IRE1/XBP1s promotes optimal production of IL-6 and TNF (Martinon et al., 2010; Qiu et al., 2013) and XBP1s favors the synthesis of IL-23, exacerbating psoriasis-like inflammation (Mogilenko et al., 2019). XBP1s also controls prostaglandin synthesis regulating pain behavior (Chopra et al., 2019). In addition, IRE1 RNase activates the inflammasome through degradation of the microRNA miR-17, which is a destabilizer of an NLRP3 activator termed TXNIP (Thioredoxin-Interacting Protein; Lerner et al., 2012; Bronner et al., 2015; Chen D. et al., 2018). Furthermore, IRE1 RNase regulates IFN-I production in microglia, providing a direct connection between IRE1 signaling in CNS-resident immune cells (Studencka-Turski et al., 2019). In fact, XBP1s can bind to IFNβ enhancer and promoter sequences, augmenting IFNβ production (Zeng et al., 2010; Dias-Teixeira et al., 2016). On the other hand, IRE1 kinase also activates innate immunity via NOD1/2 receptors and NF-κB (Keestra-Gounder et al., 2016). As such, IRE1 has emerged as an inflammatory regulator through its RNase and Kinase domains (Janssens et al., 2014).
Given its potential, there is interest in targeting IRE1 through the modulation of interacting regulators. Sigma-1 receptor (Sigmar1) is an ER-resident chaperone that regulates IRE1 function (Hayashi and Su, 2007; Mori et al., 2013), and that can be targeted through high-affinity agonists including fluvoxamine, an antidepressant that prevents IRE1-dependent hyperinflammation in sepsis models (Rosen et al., 2019). Thus, IRE1 can be modulated by administration of repurposed medicines.
Furthermore, PERK regulates pro-inflammatory phenotypes of macrophages by activating JAK2-STAT1 signaling pathways (Yang et al., 2019). PERK also controls IL-23 synthesis via CHOP (Goodall et al., 2010), and it promotes IL-6, CCL2, and CCL20 production in astrocytes via JAK1-STAT3 (Meares et al., 2014; Sanchez et al., 2019). PERK also promotes IFN-I production in DCs (Mendes et al., 2020), and it controls macrophage/myeloid cell function in atherosclerosis and cancer models (DeVries-Seimon et al., 2005; Erbay et al., 2009; Thevenot et al., 2014; Mohamed et al., 2020).
Although little is known about the role of ATF6 in myeloid cells, the UPR sensor regulates pro-inflammatory cytokine production by Kupffer cells in liver ischemia (Rao et al., 2014; So, 2018) and it enhances the pro-inflammatory effect of TLR4 by enhancing NF-κB signaling in macrophages (Rao et al., 2014).
Besides the documented roles in immunity, the UPR is also a hallmark of neurodegenerative diseases (Hetz and Saxena, 2017). Although studies have associated the UPR with the function of neurons and glial cells such as astrocytes and oligodendrocytes (Clayton and Popko, 2016; Godin et al., 2016; Wheeler et al., 2019), the role of the immune-associated UPR during neurodegeneration remains an emerging field. In this section, we highlight potential mechanisms by which the UPR in immune cells could influence CNS pathologies (Figure 2).
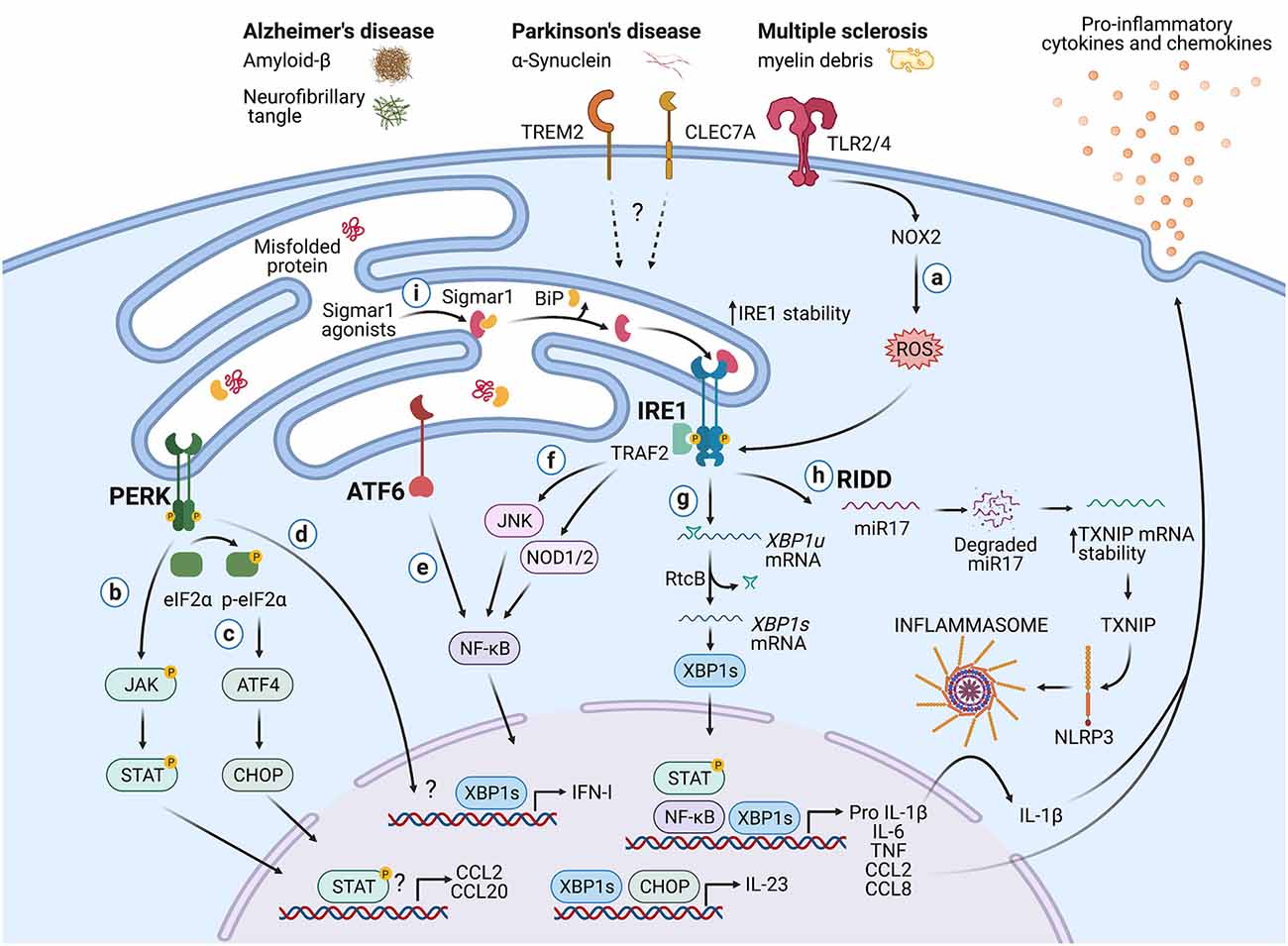
Figure 2. Potential roles of the UPR in immune cells during neurodegeneration. Protein aggregates and myelin debris can promote inflammation via triggering of innate receptors and activation of the UPR, which in turn could increase inflammation in neurodegenerative diseases mainly by enhancing the production of proinflammatory cytokines and chemokines. (a) Detection of proteins aggregates (Amyloid-β, α-synuclein, neurofibrillary tangles) and myelin debris through TLR2 and TLR4 (and probably others pattern recognition receptors) present on immune cells can activate the IRE1/XBP1s axis through reactive oxygen species (ROS) production by NOX2. (b) PERK can modulate the production of the pro-inflammatory cytokines IL-6, TNF, IL-1β, and the chemokines CCL2 and CCL20 through activation of JAK1-STAT3 and JAK2-STAT1 signaling pathways. (c) PERK also can control the synthesis of IL-23 via CHOP. (d) Additionally, PERK could control the synthesis of type I interferons. (e) ATF6 via NF-κB can enhance the production of the cytokines IL-6, TNF, and the chemokines CCL2 and CCL8. (f) The kinase domain of IRE1 can modulate the production of IL-6, TNF, and IL-1β through activation of NF-κB via JNK and NOD1/2 receptors. (g) BP1s can promote optimal production of IL-6, TNF, and type I IFNs and also favors the synthesis of IL-23. (h) IRE1 RNase via RIDD activates the NLRP3 inflammasome through degradation of the TXNIP-destabilizing microRNA miR-17, leading to IL-1β production. (i) Sigmar1 forms a complex with binding immunoglobulin protein (BiP) under normal conditions, but Sigmar1 agonists can dissociate Sigmar1 from Bip to induce its action as a chaperone protein. Sigmar1 interacts with IRE1 and stabilizes it, prolonging IRE1/XBP1s signaling. Figure created with BioRender.com.
Neurodegenerative diseases share protein misfolding, neuronal, loss and inflammation as common mechanisms (Scheiblich et al., 2020). However, evidence demonstrates that AD, PD, and MS are critically regulated by immune-signaling networks, providing a rationale for the study of immunity in these disorders. AD is an age-related disease and common cause of dementia, characterized by a decline in memory and cognitive abilities, neuronal loss, and accumulation of amyloid-β (Aβ) plaques (Holtzman et al., 2011; Efthymiou and Goate, 2017). Mutations in genes coding for amyloid precursor protein and presenilin-1/2 are identified as causes of rare familial AD (Ulland and Colonna, 2018). Nevertheless, most patients with late-onset AD do not carry familial AD mutations and instead, express genetic risk factors associated with immune-related networks, particularly in genes expressed by microglia such as TREM2, CD33, and C1R (Efthymiou and Goate, 2017; Hansen et al., 2018; Schwabe et al., 2020). On the other hand, PD is a progressive disorder characterized by selective degeneration of neuromelanin-containing neurons, especially substantia nigra dopaminergic neurons, and accumulation of α-synuclein (α-syn) aggregates (Zhang et al., 2011). Dopaminergic neuron destruction in PD is connected to genetic, environmental, and immunologic conditions (Koutsilieri et al., 2013). Finally, MS is an inflammatory demyelinating disease displaying three clinical manifestations: a pre-clinical stage; a relapsing-remitting stage, characterized by episodes of neurologic dysfunction and resolution; and a progressive stage (Baecher-Allan et al., 2018). MS pathology is typified by four pathological features: inflammation, demyelination, axonal loss, and gliosis (Constantinescu et al., 2011).
Post-mortem tissue analysis revealed that AD, PD, and MS patients display increased levels of UPR markers including CHOP, BiP, pPERK, p-eIF2α, pIRE1, and XBP1 (Hoozemans et al., 2007, 2009; Mháille et al., 2008; Wheeler et al., 2019). Interestingly, UPR activation in these pathologies is connected to immune cells. Microglia from post-mortem AD patients upregulate transcriptional signatures associated with protein folding and response to unfolded proteins (Mathys et al., 2019). Also, microglia from MS patients show increased expression of BIP, CHOP, and p-eIF2α (Mháille et al., 2008; Cunnea et al., 2011; McMahon et al., 2012), and microglia from animals with EAE also express BiP, GRP94, CHOP, and p-eIF2α (Ní Fhlathartaigh et al., 2013; Ta et al., 2016). Despite this evidence, the relevance of microglial UPR to neurodegeneration remains to be elucidated. In addition, T cells isolated from MS lesions express CHOP (Mháille et al., 2008), whereas spinal cord-infiltrating CD4+ T cells from EAE mice display ATF6 activation (Ta et al., 2016).
From an inflammatory perspective, increased levels of IL-6, TNF, and IL-1β are detected in the brain, cerebrospinal fluid, and serum of patients with AD, PD, and MS, although these responses have not been yet linked to IRE1 or PERK activation (Swardfager et al., 2010; Qin et al., 2016; Chen X. et al., 2018; Stampanoni Bassi et al., 2020). Analogous to peripheral macrophages, microglia initiate inflammation upon recognition of Aβ, α-syn, and neuromelanin through TLR2 and TLR4 (Reed-Geaghan et al., 2009; Zhang et al., 2011; Kim et al., 2013). Considering that TLR2 and TLR4 engage the IRE1/XBP1s axis to sustain the production of pro-inflammatory cytokines (Martinon et al., 2010), it is plausible that this UPR branch may initiate neuroinflammation in AD and PD.
Injections of TLR4 agonists are used as a microglial-dependent model of neuroinflammation (Qin et al., 2007; Zhao et al., 2019) and interestingly, this effect can be ameliorated by administration of the pharmacological activator of the UPR, tunicamycin (Wang et al., 2017). It is proposed that mild ER stress induces a reparative phenotype in microglia that protects against neuroinflammation (Wang et al., 2017), and mild ER stress in neurons has also shown to be protective in PD and AD models (Casas-Tinto et al., 2011; Fouillet et al., 2012; Valdés et al., 2014). As such, genetic/pharmacological manipulation of the UPR can have distinct and even opposite effects on neurodegenerative diseases depending on the cell type (Hetz and Saxena, 2017) and thus, it becomes relevant to address the UPR roles in specific cell lineages of the CNS. In fact, the importance of this topic is underscored in studies that show that selective UPR activation via the PERK axis in astrocytes results in neuronal degeneration (Smith et al., 2020).
Another regulatory node between the UPR and neurodegeneration is the NLRP3 inflammasome, which is implicated in the pathogenesis of AD, PD, and MS (Song et al., 2017; Zhou et al., 2020). Recognition of Aβ and α-syn activates the NLRP3 inflammasome in microglia (Halle et al., 2008; Codolo et al., 2013; Panicker et al., 2019), and the relevance of this complex is underscored in studies with Nlrp3−/− mice, which are protected from spatial memory loss in a model of familial AD (Heneka et al., 2013). Considering that IRE1 regulates the NLRP3 inflammasome and that IRE1 is activated in microglia from AD patients (Lerner et al., 2012; Bronner et al., 2015), it is plausible that the UPR sensor contributes to IL-1β secretion during neurodegeneration.
Perhaps the best-documented link between the UPR and neuroinflammation is in MS (Stone and Lin, 2015). Selective PERK deletion in oligodendrocytes protects against EAE (Lin et al., 2013) and XBP1 deletion in CD4+ T cells curtails the differentiation into T helper 17 (Th17) cells, delaying the onset of EAE signs (Brucklacher-Waldert et al., 2017). However, the opposite effect is noticed under ATF4 deficiency, which increases Th17 differentiation and worsens EAE (Yang X. et al., 2018). ATF6 on the other hand is required for transcription of Nos2, Ccl2, and Ccl8 in microglia in this model (Ta et al., 2016). Finally, the IRE1-XBP1s pathway promotes astrocyte-intrinsic pro-inflammatory activities during EAE, which is coordinated by the activity of Sigmar1 (Wheeler et al., 2019). XBP1s promote Nos2 and Csf2 expression in astrocytes and knock-down of Xbp1 in astrocytes ameliorates the disease (Wheeler et al., 2019), providing formal connections between IRE1 activation and CNS inflammation.
The UPR is a central proteostatic pathway coordinating immunity and inflammation, emerging as a novel therapeutic option for neurodegenerative diseases. Despite the promising advances in the field, the interplay between the UPR and immune cells in the CNS remains to be fully uncovered. Relevant questions should aim to reveal the contribution of UPR transducers and regulators in microglia and additional immune cells contributing to neuroinflammation. Consideration should be given to the aging process, which is regulated by XBP1s in C. elegans (Taylor and Dillin, 2013).
DF, AG, JB, AL, and FO provided scientific input and wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by an International Research Scholar grant from the Howard Hughes Medical Institute (HHMI#55008744), FONDECYT grant No. 1200793 (Fondo Nacional de Desarrollo Científico y Tecnológico), and ECOS-CONICYT grant (ECOS180052).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Maria Francisca Gutierrez for conceptual insights on the manuscript. We apologize to all of the authors whose work could not be referenced due to space constraints.
Ajami, B., Samusik, N., Wieghofer, P., Ho, P. P., Crotti, A., Bjornson, Z., et al. (2018). Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat. Neurosci. 21, 541–551. doi: 10.1038/s41593-018-0100-x
Baecher-Allan, C., Kaskow, B. J., and Weiner, H. L. (2018). Multiple sclerosis: mechanisms and immunotherapy. Neuron 97, 742–768. doi: 10.1016/j.neuron.2018.01.021
Benhamron, S., Hadar, R., Iwawaky, T., So, J. S., Lee, A. H., and Tirosh, B. (2014). Regulated IRE1-dependent decay participates in curtailing immunoglobulin secretion from plasma cells. Eur. J. Immunol. 44, 867–876. doi: 10.1002/eji.201343953
Bettigole, S. E., and Glimcher, L. H. (2015). Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 33, 107–138. doi: 10.1146/annurev-immunol-032414-112116
Bettigole, S. E., Lis, R., Adoro, S., Lee, A. H., Spencer, L. A., Weller, P. F., et al. (2015). The transcription factor XBP1 is selectively required for eosinophil differentiation. Nat. Immunol. 16, 829–837. doi: 10.1038/ni.3225
Brodsky, J. L., and Skach, W. R. (2011). Protein folding and quality control in the endoplasmic reticulum: recent lessons from yeast and mammalian cell systems. Curr. Opin. Cell Biol. 23, 464–475. doi: 10.1016/j.ceb.2011.05.004
Bronner, D. N., Abuaita, B. H., Chen, X., Fitzgerald, K. A., Nuñez, G., He, Y., et al. (2015). Endoplasmic reticulum stress activates the inflammasome via NLRP3- and caspase-2-driven mitochondrial damage. Immunity 43, 451–462. doi: 10.1016/j.immuni.2015.08.008
Brucklacher-Waldert, V., Ferreira, C., Stebegg, M., Fesneau, O., Innocentin, S., Marie, J. C., et al. (2017). Cellular stress in the context of an inflammatory environment supports TGF-β-independent T helper-17 differentiation. Cell Rep. 19, 2357–2370. doi: 10.1016/j.celrep.2017.05.052
Cabeza-Cabrerizo, M., Cardoso, A., Minutti, C. M., Pereira, M., and Reis e Sousa, C. (2021). Dendritic cells revisited. Annu. Rev. Immunol. 39, 131–166. doi: 10.1146/annurev-immunol-061020-053707
Casas-Tinto, S., Zhang, Y., Sanchez-Garcia, J., Gomez-Velazquez, M., Rincon-Limas, D. E., and Fernandez-Funez, P. (2011). The ER stress factor XBP1s prevents amyloid-β neurotoxicity. Hum. Mol. Genet. 20, 2144–2160. doi: 10.1093/hmg/ddr100
Chen, D., Dixon, B. J., Doycheva, D. M., Li, B., Zhang, Y., Hu, Q., et al. (2018). IRE1α inhibition decreased TXNIP/NLRP3 inflammasome activation through miR-17–5p after neonatal hypoxic-ischemic brain injury in rats. J. Neuroinflammation 15:32. doi: 10.1186/s12974-018-1077-9
Chen, X., Hu, Y., Cao, Z., Liu, Q., and Cheng, Y. (2018). Cerebrospinal fluid inflammatory cytokine aberrations in Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis: a systematic review and meta-analysis. Front. Immunol. 9:2122. doi: 10.3389/fimmu.2018.02122
Chopra, S., Giovanelli, P., Alvarado-Vazquez, P. A., Alonso, S., Song, M., Sandoval, T. A., et al. (2019). IRE1α-XBP1 signaling in leukocytes controls prostaglandin biosynthesis and pain. Science 365:eaau6499. doi: 10.1126/science.aau6499
Clayton, B. L. L., and Popko, B. (2016). Endoplasmic reticulum stress and the unfolded protein response in disorders of myelinating glia. Brain Res. 1648, 594–602. doi: 10.1016/j.brainres.2016.03.046
Codolo, G., Plotegher, N., Pozzobon, T., Brucale, M., Tessari, I., Bubacco, L., et al. (2013). Triggering of inflammasome by aggregated α-synuclein, an inflammatory response in synucleinopathies. PLoS One 8:e55375. doi: 10.1371/journal.pone.0055375
Colonna, M., and Butovsky, O. (2017). Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 35, 441–468. doi: 10.1146/annurev-immunol-051116-052358
Constantinescu, C. S., Farooqi, N., O’Brien, K., and Gran, B. (2011). Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 164, 1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x
Cubillos-Ruiz, J. R., Silberman, P. C., Rutkowski, M. R., Chopra, S., Perales-Puchalt, A., Song, M., et al. (2015). ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell 161, 1527–1538. doi: 10.1016/j.cell.2015.05.025
Cunnea, P., Mháille, A. N., McQuaid, S., Farrell, M., McMahon, J., and Fitzgerald, U. (2011). Expression profiles of endoplasmic reticulum stress-related molecules in demyelinating lesions and multiple sclerosis. Mult. Scler. 17, 808–818. doi: 10.1177/1352458511399114
DeVries-Seimon, T., Li, Y., Pin, M. Y., Stone, E., Wang, Y., Davis, R. J., et al. (2005). Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J. Cell Biol. 171, 61–73. doi: 10.1083/jcb.200502078
Dias-Teixeira, K. L., Calegari-Silva, T. C., Dos Santos, G. R. R. M., Vitorino Dos Santos, J., Lima, C., Medina, J. M., et al. (2016). The integrated endoplasmic reticulum stress response in Leishmania amazonensis macrophage infection: the role of X-box binding protein 1 transcription factor. FASEB J. 30, 1557–1565. doi: 10.1096/fj.15-281550
Dong, H., Adams, N. M., Xu, Y., Cao, J., Allan, D. S. J., Carlyle, J. R., et al. (2019). The IRE1 endoplasmic reticulum stress sensor activates natural killer cell immunity in part by regulating c-Myc. Nat. Immunol. 20, 865–878. doi: 10.1038/s41590-019-0388-z
Efthymiou, A. G., and Goate, A. M. (2017). Late onset alzheimer’s disease genetics implicates microglial pathways in disease risk. Mol. Neurodegener. 12:43. doi: 10.1186/s13024-017-0184-x
Erbay, E., Babaev, V. R., Mayers, J. R., Makowski, L., Charles, K. N., Snitow, M. E., et al. (2009). Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15, 1383–1391. doi: 10.1038/nm.2067
Flores-Santibáñez, F., Medel, B., Bernales, J. I., and Osorio, F. (2019). Understanding the role of the unfolded protein response sensor IRE1 in the biology of antigen presenting cells. Cells 8:1563. doi: 10.3390/cells8121563
Fouillet, A., Levet, C., Virgone, A., Robin, M., Dourlen, P., Rieusset, J., et al. (2012). ER stress inhibits neuronal death by promoting autophagy. Autophagy 8, 915–926. doi: 10.4161/auto.19716
Giovannoni, F., and Quintana, F. J. (2020). The role of astrocytes in CNS inflammation. Trends Immunol. 41, 805–819. doi: 10.1016/j.it.2020.07.007
Godin, J. D., Creppe, C., Laguesse, S., and Nguyen, L. (2016). Emerging roles for the unfolded protein response in the developing nervous system. Trends Neurosci. 39, 394–404. doi: 10.1016/j.tins.2016.04.002
Goodall, J. C., Wu, C., Zhang, Y., McNeill, L., Ellis, L., Saudek, V., et al. (2010). Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc. Natl. Acad. Sci. U S A 107, 17698–17703. doi: 10.1073/pnas.1011736107
Greter, M., Heppner, F. L., Lemos, M. P., Odermatt, B. M., Goebels, N., Laufer, T., et al. (2005). Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 11, 328–334. doi: 10.1038/nm1197
Grootjans, J., Kaser, A., Kaufman, R. J., and Blumberg, R. S. (2016). The unfolded protein response in immunity and inflammation. Nat. Rev. Immunol. 16, 469–484. doi: 10.1038/nri.2016.62
Halle, A., Hornung, V., Petzold, G. C., Stewart, C. R., Monks, B. G., Reinheckel, T., et al. (2008). The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 9, 857–865. doi: 10.1038/ni.1636
Hansen, D. V., Hanson, J. E., and Sheng, M. (2018). Microglia in Alzheimer’s disease. J. Cell Biol. 217, 459–472. doi: 10.1083/jcb.201709069
Hayashi, T., and Su, T. P. (2007). Sigma-1 receptor chaperones at the ER- mitochondrion interface regulate Ca2+ signaling and cell survival. Cell 131, 596–610. doi: 10.1016/j.cell.2007.08.036
Heneka, M. T., Kummer, M. P., Stutz, A., Delekate, A., Schwartz, S., Vieira-Saecker, A., et al. (2013). NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678. doi: 10.1038/nature11729
Hetz, C. (2021). Adapting the proteostasis capacity to sustain brain healthspan. Cell 184, 1545–1560. doi: 10.1016/j.cell.2021.02.007
Herz, J., Filiano, A. J., Smith, A., Yogev, N., and Kipnis, J. (2017). Myeloid cells in the central nervous system. Immunity 46, 943–956. doi: 10.1016/j.immuni.2017.06.007
Hetz, C., and Papa, F. R. (2018). The unfolded protein response and cell fate control. Mol. Cell 69, 169–181. doi: 10.1016/j.molcel.2017.06.017
Hetz, C., and Saxena, S. (2017). ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 13, 477–491. doi: 10.1038/nrneurol.2017.99
Holtzman, D. M., Morris, J. C., and Goate, A. M. (2011). Alzheimer’s disease: the challenge of the second century. Sci. Transl. Med. 3:77sr1. doi: 10.1126/scitranslmed.3002369
Hoozemans, J. J. M., van Haastert, E. S., Eikelenboom, P., de Vos, R. A. I., Rozemuller, J. M., and Scheper, W. (2007). Activation of the unfolded protein response in Parkinson’s disease. Biochem. Biophys. Res. Commun. 354, 707–711. doi: 10.1016/j.bbrc.2007.01.043
Hoozemans, J. J. M., Van Haastert, E. S., Nijholt, D. A. T., Rozemuller, A. J. M., Eikelenboom, P., and Scheper, W. (2009). The unfolded protein response is activated in pretangle neurons in Alzheimer’s disease hippocampus. Am. J. Pathol. 174, 1241–1251. doi: 10.2353/ajpath.2009.080814
Hu, P., Han, Z., Couvillon, A. D., Kaufman, R. J., and Exton, J. H. (2006). Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol. Cell. Biol. 26, 3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006
Iwakoshi, N. N., Pypaert, M., and Glimcher, L. H. (2007). The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J. Exp. Med. 204, 2267–2275. doi: 10.1084/jem.20070525
Janssens, S., Pulendran, B., and Lambrecht, B. N. (2014). Emerging functions of the unfolded protein response in immunity. Nat. Immunol. 15, 910–919. doi: 10.1038/ni.2991
Keestra-Gounder, A. M., Byndloss, M. X., Seyffert, N., Young, B. M., Chávez-Arroyo, A., Tsai, A. Y., et al. (2016). NOD1 and NOD2 signalling links ER stress with inflammation. Nature 532, 394–397. doi: 10.1038/nature17631
Khalsa, J. K., Chawla, A. S., Prabhu, S. B., Vats, M., Dhar, A., Dev, G., et al. (2019). Functionally significant metabolic differences between B and T lymphocyte lineages. Immunology 158, 104–120. doi: 10.1111/imm.13098
Kim, C., Ho, D.-H., Suk, J. E., You, S., Michael, S., Kang, J., et al. (2013). Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 4:1562. doi: 10.1038/ncomms2534
Korin, B., Ben-shaanan, T. L., Schiller, M., Dubovik, T., Azulay-debby, H., Boshnak, N. T., et al. (2017). High-dimensional, single-cell characterization of the brain’s immune compartment. Nat. Neurosci. 20, 1300–1309. doi: 10.1038/nn.4610
Koutsilieri, E., Lutz, M. B., and Scheller, C. (2013). Autoimmunity, dendritic cells and relevance for Parkinson’s disease. J. Neural Transm. 120, 75–81. doi: 10.1007/s00702-012-0842-7
Kranz, P., Neumann, F., Wolf, A., Classen, F., Pompsch, M., Ocklenburg, T., et al. (2017). PDI is an essential redox-sensitive activator of PERK during the unfolded protein response (UPR). Cell Death Dis. 8, e2986–e2986. doi: 10.1038/cddis.2017.369
Lee, A.-H., Iwakoshi, N. N., and Glimcher, L. H. (2003). XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 23, 7448–7459. doi: 10.1128/mcb.23.21.7448-7459.2003
Lerner, A. G., Upton, J. P., Praveen, P. V. K., Ghosh, R., Nakagawa, Y., Igbaria, A., et al. (2012). IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 16, 250–264. doi: 10.1016/j.cmet.2012.07.007
Lin, W., Lin, Y., Li, J., Fenstermaker, A. G., Way, S. W., Clayton, B., et al. (2013). Oligodendrocyte-specific activation of PERK signaling protects mice against experimental autoimmune encephalomyelitis. J. Neurosci. 33, 5980–5991. doi: 10.1523/JNEUROSCI.1636-12.2013
Lu, M., Lawrence, D. A., Marsters, S., Acosta-Alvear, D., Kimmig, P., Mendez, A. S., et al. (2014). Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science 345, 98–101. doi: 10.1126/science.1254312
Martinon, F., Chen, X., Lee, A.-H., and Glimcher, L. H. (2010). TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 11, 411–418. doi: 10.1038/ni.1857
Mathys, H., Davila-Velderrain, J., Peng, Z., Gao, F., Mohammadi, S., Young, J. Z., et al. (2019). Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570, 332–337. doi: 10.1038/s41586-019-1195-2
Maurel, M., Chevet, E., Tavernier, J., and Gerlo, S. (2014). Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 39, 245–254. doi: 10.1016/j.tibs.2014.02.008
McMahon, J. M., McQuaid, S., Reynolds, R., and Fitzgerald, U. F. (2012). Increased expression of ER stress- and hypoxia-associated molecules in grey matter lesions in multiple sclerosis. Mult. Scler. 18, 1437–1447. doi: 10.1177/1352458512438455
Meares, G. P., Liu, Y., Rajbhandari, R., Qin, H., Nozell, S. E., Mobley, J. A., et al. (2014). PERK-dependent activation of JAK1 and STAT3 contributes to endoplasmic reticulum stress-induced inflammation. Mol. Cell. Biol. 34, 3911–3925. doi: 10.1128/MCB.00980-14
Mendes, A., Gigan, J. P., Rodrigues, C. R., Choteau, S. A., Sanseau, D., Barros, D., et al. (2020). Proteostasis in dendritic cells is controlled by the PERK signaling axis independently of ATF4. Life Sci. Alliance 4:e202000865. doi: 10.26508/lsa.202000865
Mháille, A. N., McQuaid, S., Windebank, A., Cunnea, P., McMahon, J., Samali, A., et al. (2008). Increased expression of endoplasmic reticulum stress-related signaling pathway molecules in multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 67, 200–211. doi: 10.1097/NEN.0b013e318165b239
Mogilenko, D. A., Haas, J. T., L’homme, L., Fleury, S., Quemener, S., Levavasseur, M., et al. (2019). Metabolic and innate immune cues merge into a specific inflammatory response via the UPR. Cell 177, 1201.e19–1216.e19. doi: 10.1016/j.cell.2019.03.018
Mohamed, E., Sierra, R. A., Trillo-Tinoco, J., Cao, Y., Innamarato, P., Payne, K. K., et al. (2020). The unfolded protein response mediator PERK governs myeloid cell-driven immunosuppression in tumors through inhibition of STING signaling. Immunity 52, 668.e7–682.e7. doi: 10.1016/j.immuni.2020.03.004
Mori, T., Hayashi, T., Hayashi, E., and Su, T. P. (2013). Sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS One 8:e76941. doi: 10.1371/journal.pone.0076941
Mrdjen, D., Pavlovic, A., Hartmann, F. J., Schreiner, B., Utz, S. G., Leung, B. P., et al. (2018). High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 380.e6–395.e6. doi: 10.1016/j.immuni.2018.01.011
Mundt, S., Mrdjen, D., Utz, S. G., Greter, M., Schreiner, B., and Becher, B. (2019). Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci. Immunol. 4:eaau8380. doi: 10.1126/sciimmunol.aau8380
Ní Fhlathartaigh, M., McMahon, J., Reynolds, R., Connolly, D., Higgins, E., Counihan, T., et al. (2013). Calreticulin and other components of endoplasmic reticulum stress in rat and human inflammatory demyelination. Acta Neuropathol. Commun. 1:37. doi: 10.1186/2051-5960-1-37
Novoa, I., Zeng, H., Harding, H. P., and Ron, D. (2001). Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J. Cell Biol. 153, 1011–1022. doi: 10.1083/jcb.153.5.1011
Osorio, F., Tavernier, S. J., Hoffmann, E., Saeys, Y., Martens, L., Vetters, J., et al. (2014). The unfolded-protein-response sensor IRE-1α regulates the function of CD8α + dendritic cells. Nat. Immunol. 15, 248–257. doi: 10.1038/ni.2808
Panicker, N., Sarkar, S., Harischandra, D. S., Neal, M., Kam, T. I., Jin, H., et al. (2019). Fyn kinase regulates misfolded α-synuclein uptake and NLRP3 inflammasome activation in microglia. J. Exp. Med. 216, 1411–1430. doi: 10.1084/jem.20182191
Pasciuto, E., Burton, O. T., Roca, C. P., Fitzgerald, D. C., Dooley, J., Liston, A., et al. (2020). Microglia require CD4 T cells to complete the fetal- to-adult transition microglia require CD4 T cells to complete the fetal-to-adult transition. Cell 182, 625.e24–640.e24. doi: 10.1016/j.cell.2020.06.026
Pröbstel, A., Zhou, X., Baumann, R., Wischnewski, S., Kutza, M., Rojas, O. L., et al. (2020). Gut microbiota-specific IgA + B cells traffic to the CNS in active multiple sclerosis. Sci. Immunol. 5:eabc7191. doi: 10.1126/sciimmunol.abc7191
Prinz, M., Jung, S., and Priller, J. (2019). Microglia biology: one century of evolving concepts. Cell 179, 292–311. doi: 10.1016/j.cell.2019.08.053
Qin, L., Wu, X., Block, M. L., Liu, Y., Breese, G. R., Hong, J.-S., et al. (2007). Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55, 453–462. doi: 10.1002/glia.20467
Qin, X.-Y., Zhang, S.-P., Cao, C., Loh, Y. P., and Cheng, Y. (2016). Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: a systematic review and meta-analysis. JAMA Neurol. 73, 1316–1324. doi: 10.1001/jamaneurol.2016.2742
Qiu, Q., Zheng, Z., Chang, L., Zhao, Y. S., Tan, C., Dandekar, A., et al. (2013). Toll-like receptor-mediated IRE1α activation as a therapeutic target for inflammatory arthritis. EMBO J. 32, 2477–2490. doi: 10.1038/emboj.2013.183
Ransohoff, R. M., and Cardona, A. E. (2010). The myeloid cells of the central nervous system parenchyma. Nature 468, 253–262. doi: 10.1038/nature09615
Rao, J., Yue, S., Fu, Y., Zhu, J., Wang, X., Busuttil, R. W., et al. (2014). ATF6 mediates a pro-inflammatory synergy between ER stress and TLR activation in the pathogenesis of liver ischemia-reperfusion injury. Am. J. Transplant. 14, 1552–1561. doi: 10.1111/ajt.12711
Reed-Geaghan, E. G., Savage, J. C., Hise, A. G., and Landreth, G. E. (2009). CD14 and toll-like receptors 2 and 4 are required for fibrillar Aβ-stimulated microglial activation. J. Neurosci. 29, 11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009
Reimold, A. M., Iwakoshi, N. N., Manis, J., Vallabhajosyula, P., Szomolanyi-Tsuda, E., Gravallese, E. M., et al. (2001). Plasma cell differentiation requires the transcription factor XBP-1. Nature 412, 300–307. doi: 10.1038/35085509
Reverendo, M., Mendes, A., Argüello, R. J., Gatti, E., and Pierre, P. (2019). At the crossway of ER-stress and proinflammatory responses. FEBS J. 286, 297–310. doi: 10.1111/febs.14391
Ron, D., and Walter, P. (2007). Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529. doi: 10.1038/nrm2199
Rosen, D. A., Seki, S. M., Fernández-Castañeda, A., Beiter, R. M., Eccles, J. D., Woodfolk, J. A., et al. (2019). Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci. Transl. Med. 11:eaau5266. doi: 10.1126/scitranslmed.aau5266
Sanchez, C. L., Sims, S. G., Nowery, J. D., and Meares, G. P. (2019). Endoplasmic reticulum stress differentially modulates the IL-6 family of cytokines in murine astrocytes and macrophages. Sci. Rep. 9:14931. doi: 10.1038/s41598-019-51481-6
Scheiblich, H., Trombly, M., Ramirez, A., and Heneka, M. T. (2020). Neuroimmune connections in aging and neurodegenerative diseases. Trends Immunol. 41, 300–312. doi: 10.1016/j.it.2020.02.002
Schwabe, T., Srinivasan, K., and Rhinn, H. (2020). Shifting paradigms: the central role of microglia in Alzheimer’s disease. Neurobiol. Dis. 143:104962. doi: 10.1016/j.nbd.2020.104962
Shenderov, K., Riteau, N., Yip, R., Mayer-Barber, K. D., Oland, S., Hieny, S., et al. (2014). Cutting edge: endoplasmic reticulum stress licenses macrophages to produce mature IL-1β in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J. Immunol. 192, 2029–2033. doi: 10.4049/jimmunol.1302549
Shoulders, M. D., Ryno, L. M., Genereux, J. C., Moresco, J. J., Tu, P. G., Wu, C., et al. (2013). Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Rep. 3, 1279–1292. doi: 10.1016/j.celrep.2013.03.024
Smith, H. L., Freeman, O. J., Butcher, A. J., Holmqvist, S., Humoud, I., Schätzl, T., et al. (2020). Astrocyte unfolded protein response induces a specific reactivity state that causes non-cell-autonomous neuronal degeneration. Neuron 105, 855.e5–866.e5. doi: 10.1016/j.neuron.2019.12.014
So, J.-S. (2018). Roles of endoplasmic reticulum stress in immune responses. Mol. Cells 41, 705–716. doi: 10.14348/molcells.2018.0241
Sofroniew, M. V. (2020). Astrocyte reactivity: subtypes, states, and functions in CNS innate immunity. Trends Immunol. 41, 758–770. doi: 10.1016/j.it.2020.07.004
Song, L., Pei, L., Yao, S., Wu, Y., and Shang, Y. (2017). NLRP3 inflammasome in neurological diseases, from functions to therapies. Front. Cell. Neurosci. 11:63. doi: 10.3389/fncel.2017.00063
Song, M., Sandoval, T. A., Chae, C.-S., Chopra, S., Tan, C., Rutkowski, M. R., et al. (2018). IRE1α-XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature 562, 423–428. doi: 10.1038/s41586-018-0597-x
Sprooten, J., Agostinis, P., and Garg, A. D. (2019). “Type I interferons and dendritic cells in cancer immunotherapy,” in International Review of Cell and Molecular Biology, eds Claire. Lhuillier and Lorenzo. Galluzzi (Oxford, UK: Elsevier Inc.), 217–262.
Stampanoni Bassi, M., Iezzi, E., Drulovic, J., Pekmezovic, T., Gilio, L., Furlan, R., et al. (2020). IL-6 in the cerebrospinal fluid signals disease activity in multiple sclerosis. Front. Cell. Neurosci. 14:120. doi: 10.3389/fncel.2020.00120
Stone, S., and Lin, W. (2015). The unfolded protein response in multiple sclerosis. Front. Neurosci. 9:264. doi: 10.3389/fnins.2015.00264
Studencka-Turski, M., Çetin, G., Junker, H., Ebstein, F., and Krüger, E. (2019). Molecular insight into the IRE1α-mediated type I interferon response induced by proteasome impairment in myeloid cells of the brain. Front. Immunol. 10:2900. doi: 10.3389/fimmu.2019.02900
Swanson, K. V., Deng, M., and Ting, J. P.-Y. (2019). The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489. doi: 10.1038/s41577-019-0165-0
Swardfager, W., Lanctôt, K., Rothenburg, L., Wong, A., Cappell, J., and Herrmann, N. (2010). A meta-analysis of cytokines in Alzheimer’s disease. Biol. Psychiatry 68, 930–941. doi: 10.1016/j.biopsych.2010.06.012
Ta, H. M., Le, T. M., Ishii, H., Takarada-Iemata, M., Hattori, T., Hashida, K., et al. (2016). Atf6α deficiency suppresses microglial activation and ameliorates pathology of experimental autoimmune encephalomyelitis. J. Neurochem. 139, 1124–1137. doi: 10.1111/jnc.13714
Tavernier, S. J., Osorio, F., Vandersarren, L., Vetters, J., Vanlangenakker, N., Van Isterdael, G., et al. (2017). Regulated IRE1-dependent mRNA decay sets the threshold for dendritic cell survival. Nat. Cell Biol. 19, 698–710. doi: 10.1038/ncb3518
Taylor, R. C., and Dillin, A. (2013). XBP-1 Is a cell-nonautonomous regulator of stress resistance and longevity. Cell 153, 1435–1447. doi: 10.1016/j.cell.2013.05.042
Thevenot, P. T., Sierra, R. A., Raber, P. L., Al-Khami, A. A., Trillo-Tinoco, J., Zarreii, P., et al. (2014). The stress-response sensor chop regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity 41, 389–401. doi: 10.1016/j.immuni.2014.08.015
Ulland, T. K., and Colonna, M. (2018). TREM2—a key player in microglial biology and Alzheimer disease. Nat. Rev. Neurol. 14, 667–675. doi: 10.1038/s41582-018-0072-1
Urano, F., Wang, X., Bertolotti, A., Zhang, Y., Chung, P., Harding, H. P., et al. (2000). Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666. doi: 10.1126/science.287.5453.664
Valdés, P., Mercado, G., Vidal, R. L., Molina, C., Parsons, G., Court, F. A., et al. (2014). Control of dopaminergic neuron survival by the unfolded protein response transcription factor XBP1. Proc. Natl. Acad. Sci. U S A 111, 6804–6809. doi: 10.1073/pnas.1321845111
Walter, P., and Ron, D. (2011). The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086. doi: 10.1126/science.1209038
Wang, Y.-W., Zhou, Q., Zhang, X., Qian, Q.-Q., Xu, J.-W., Ni, P.-F., et al. (2017). Mild endoplasmic reticulum stress ameliorates lipopolysaccharide-induced neuroinflammation and cognitive impairment via regulation of microglial polarization. J. Neuroinflammation 14:233. doi: 10.1186/s12974-017-1002-7
Wensky, A. K., Furtado, G. C., Garibaldi Marcondes, M. C., Chen, S., Manfra, D., Lira, S. A., et al. (2005). IFN-γ determines distinct clinical outcomes in autoimmune encephalomyelitis. J. Immunol. 174, 1416–1423. doi: 10.4049/jimmunol.174.3.1416
Wheeler, M. A., Jaronen, M., Covacu, R., Zandee, S. E. J., Scalisi, G., Rothhammer, V., et al. (2019). Environmental control of astrocyte pathogenic activities in CNS inflammation. Cell 176, 581–596. doi: 10.1016/j.cell.2018.12.012
Yamamoto, K., Sato, T., Matsui, T., Sato, M., Okada, T., Yoshida, H., et al. (2007). Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev. Cell 13, 365–376. doi: 10.1016/j.bcp.2019.113744
Yang, F., Liu, Y., Ren, H., Zhou, G., Yuan, X., and Shi, X. (2019). ER-stress regulates macrophage polarization through pancreatic EIF-2alpha kinase. Cell. Immunol. 336, 40–47. doi: 10.1016/j.cellimm.2018.12.008
Yang, F., Wang, S., Liu, Y., Zhou, Y., Shang, L., Feng, M., et al. (2018). IRE1α aggravates ischemia reperfusion injury of fatty liver by regulating phenotypic transformation of kupffer cells. Free Radic. Biol. Med. 124, 395–407. doi: 10.1016/j.freeradbiomed.2018.06.043
Yang, Q., Wang, G., and Zhang, F. (2020). Role of peripheral immune cells-mediated inflammation on the process of neurodegenerative diseases. Front. Immunol. 11:2511. doi: 10.3389/fimmu.2020.582825
Yang, X., Xia, R., Yue, C., Zhai, W., Du, W., Yang, Q., et al. (2018). ATF4 regulates CD4 + T cell immune responses through metabolic reprogramming. Cell Rep. 23, 1754–1766. doi: 10.1016/j.celrep.2018.04.032
Zeng, L., Liu, Y.-P., Sha, H., Chen, H., Qi, L., and Smith, J. (2010). XBP-1 couples endoplasmic reticulum stress to augmented IFNβ induction via a cis-acting enhancer in macrophages. J. Immunol. 185, 2324–2330. doi: 10.4049/jimmunol.0903052
Zhang, W., Phillips, K., Wielgus, A. R., Liu, J., Albertini, A., Zucca, F. A., et al. (2011). Neuromelanin activates microglia and induces degeneration of dopaminergic neurons: implications for progression of parkinson’s disease. Neurotox. Res. 19, 63–72. doi: 10.1007/s12640-009-9140-z
Zhao, J., Bi, W., Xiao, S., Lan, X., Cheng, X., Zhang, J., et al. (2019). Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 9:5790. doi: 10.1038/s41598-019-42286-8
Keywords: UPR, neurodegeneration, microglia, inflammation, neuroinflammation, protein misfolding, ER stress, immune system
Citation: Fernández D, Geisse A, Bernales JI, Lira A and Osorio F (2021) The Unfolded Protein Response in Immune Cells as an Emerging Regulator of Neuroinflammation. Front. Aging Neurosci. 13:682633. doi: 10.3389/fnagi.2021.682633
Received: 18 March 2021; Accepted: 10 May 2021;
Published: 11 June 2021.
Edited by:
Safikur Rahman, Babasaheb Bhimrao Ambedkar Bihar University, IndiaReviewed by:
Naeem Khan, Western Michigan University, United StatesCopyright © 2021 Fernández, Geisse, Bernales, Lira and Osorio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabiola Osorio, ZmFiaW9sYW9zb3Jpb0BtZWQudWNoaWxlLmNs
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.