
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 02 August 2021
Sec. Neurocognitive Aging and Behavior
Volume 13 - 2021 | https://doi.org/10.3389/fnagi.2021.681208
This article is part of the Research Topic Exploring Reliable Markers and Prediction Indexes for the Progression from Subjective Cognitive Decline to Cognitive Impairment View all 20 articles
Objective: Emerging evidence suggests that white matter (WM) disruption is associated with the incidence of subcortical vascular cognitive impairment (SVCI). However, our knowledge regarding this relationship in the early stage of SVCI is limited. We aimed to investigate the associations between WM disruptions and cognitive declines at the early stage of SVCI.
Method: We performed a case–control study, involving 22 cases and 19 controls. The cases were patients at the early stage of SVCI, which was defined as subcortical ischemic vascular disease with normal global cognitive measures (pre-SVCI). The controls were healthy people matched by age, sex, and education years. We assessed the differences in a battery of neuropsychological tests between the two groups, investigated the diffusion changes in 40 WM tracts among the participants via an atlas-based segmentation strategy, and compared the differences between the cases and controls by multiple linear regression analysis. We then evaluated the relationships between diffusion indices and cognitive assessment scores by Pearson’s correlation.
Results: The pre-SVCI group exhibited significant differences in the Montreal cognitive assessment (MoCA), Rey–Osterrieth Complex Figure (R-O)-copy, and Trail Making Test (TMT)-B test compared with the controls. Compared with the controls, some long associative and projective bundles, such as the right anterior corona radiata (ACR), the right inferior fronto-occipital fasciculus (IFOF), and the left external capsule (EC), were extensively damaged in cases after Bonferroni correction (p < 0.05/40). Damages to specific fibers, such as the right ACR, IFOF, and posterior thalamic radiation (PTR), exhibited significant correlations with declines in MoCA, R-O delay, and the Mini-Mental State Examination (MMSE), respectively, after Bonferroni correction (p < 0.05/14).
Conclusion: Long WM tracts, especially those in the right hemisphere, were extensively damaged in the pre-SVCI patients and correlated with declines in executive functions and spatial processing. Patients of pre-SVCI are likely at an ultra-early stage of SVCI, and there is a very high risk of this condition becoming SVCI.
Subcortical ischemic vascular disease (SIVD) is widespread among elderly individuals with asymptomatic lacunes and subcortical white matter (WM) hyperintensities (Carey et al., 2008), which is a homogeneous and the most common subtype of cerebral small vessel disease (CSVD) (Román et al., 2002; Rosenberg et al., 2014). Subcortical vascular cognitive impairment (SVCI) has a relatively insidious onset with gradual cognitive deterioration and a severe prognosis. In contrast to Alzheimer’s disease (AD), SVCI is widely considered a disease that can be prevented and improved (Sachdev et al., 2014). Therefore, early identification is crucial for preventing SIVD from developing into vascular cognitive impairment (VCI) or dementia.
Most previous studies focused on the moderate and severe stages of SVCI, including subcortical vascular mild cognitive impairment (SvMCI) and subcortical vascular dementia (SVaD). They have revealed significant brain abnormalities in SVCI, such as declines in executive function, attention, processing speed, learning and memory, and lower brain perfusion or abnormal resting-state functional connectivities (FCs) in the thalamus, temporal lobe, inferior frontal lobe, and medial prefrontal cortex (Zhang et al., 2013; Reijmer et al., 2016; Sun et al., 2016; van Leijsen et al., 2019). Among them, WM damage is attracting ever-increasing attention because the pathomechanisms of cognitive injury in SIVD seem to be most closely related to diffuse areas of WM damage with neuronal loss, demyelination, and gliosis (D’Souza et al., 2018).
However, clinicians have also encountered many patients with moderate to serious SIVD with normal global cognition measures, such as the Mini-Mental State Examination (MMSE). The diagnostic criteria for vascular cognitive disorders (VASCOG statement) (Sachdev et al., 2014) suggest that vascular brain damage can exist without any evident cognitive impairment, and such asymptomatic individuals may be at an increased risk of future decline. This stage can be referred to as the pre-stage of SVCI (pre-SVCI). Limited studies focused on these pre-SVCI stage patients. Carey et al. (2008) found that although these patients appear “normal” with normal global cognition measures, they already have poorer executive functions and processing speed based on detailed assessments in different cognitive domains. Moreover, these patients have already exhibited extensive areas of microstructural changes in WM fibers and FC of resting-state networks (Liu et al., 2019a,b). However, these studies mostly used the MMSE as the screening scale. Compared with the Montreal cognitive assessment (MoCA), the MMSE lacks sensitivity in detecting executive function mediated by the frontal lobes where SIVD is often the most prevalent (Tullberg et al., 2004; Carey et al., 2008; Dong et al., 2010, 2016). Using the MMSE alone may lead to a false-negative bias when screening cognitively normal people at the pre-SVCI stage. Therefore, the application of the MMSE and MOCA together as screening scales can more accurately distinguish “normal” patients at the pre-SVCI stage.
Diffusion tensor imaging (DTI) is a sensitive and reliable method used to detect early WM alterations (Nitkunan et al., 2008). Recent DTI studies have demonstrated that patients with cognitive impairment exhibit decreased fractional anisotropy (FA) and increased mean diffusivity (MD), and different combinations of changes in axial diffusivity (DA) and radial diffusivity (DR) of WM tracts especially those located in thalamic- and caudate-prefrontal pathways, such as the corpus callosum (CC), external capsule, and superior and anterior thalamic radiations (ATR) (Chen et al., 2015; Reijmer et al., 2016; D’Souza et al., 2018). These WMs are significantly related to the cognitive domains of executive function, attention, and processing speed. However, limited studies using DTI have been performed in pre-SVCI patients and those that have been performed indicated inconsistent results (Liu et al., 2019a,b; Du et al., 2020). While Liu et al. (2019b) thought the pre-SVCI group exhibited widespread damages in whole-brain WM skeletons, Du et al. (2020) demonstrated well-preserved rich-club organization, less nodal strength loss, and disruption of connections shown in the local connections in the preclinical stage of SVCI. Therefore, to identify pre-SVCI patients at the early stage, we used DTI, which can identify changes in WM microstructure, to perform the current study. The early identification of pre-SVCI should contribute to promoting the further longitudinal studies and the early prevention and treatment of cognitive impairment due to CSVD.
In this explorative case–control study, using a battery of neuropsychological tests and DTI, we aimed to assess changes in 40 WM tracts that can mainly contain the key WMs in the brain (Zhang et al., 2014; Chen et al., 2015) and cognitive domains, including memory, spatial processing, language, attention, and executive function, between pre-SVCI patients and healthy controls. We hypothesized that WM integrity damage and cognitive decline already exist in pre-SVCI patients.
This case–control study, with prospective recruitment of pre-SVCI cases and a healthy control group, was performed between January 2016 and January 2018 in the Department of Neurology in the China–Japan Friendship Hospital. Vascular risk factors including hypertension (HT), hypercholesterolemia (HC), coronary atherosclerotic disease (CAD), diabetes mellitus (DM), and smoking and alcohol history were collected from all patients.
The patients with pre-SVCI (case group) were defined as having SIVD on MRI with normal global cognitive measures. Two different radiologists assessed the anatomical MRI scans which contained T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR) and gave nearly the same reports. The patient will be excluded to whom the two radiologists give the different reports. The SIVD patients met the following brain imaging criteria of SIVD (Román et al., 2002): (1) Binswanger-type WM lesions: hyperintensities extending into the periventricular and deep WM, extending caps (>10 mm as measured parallel to the ventricle) or irregular halos (>10 mm with broad, irregular margins and extending into deep WM), and diffusely confluent hyperintensities (>25 mm, irregular shape) or extensive WM changes (diffuse hyperintensity without focal lesions); (2) lacunar cases: multiple lacunas (>2) in the deep gray matter and at least moderate white-matter lesions; and (3) absence of hemorrhages and cortical and/or territorial infarcts and watershed infarcts, signs of normal-pressure hydrocephalus, and specific causes of white-matter lesions. In addition, the visual Fazekas scale was used on FLAIR images to rate the severity of WM hyperintensities (WMHs) into mild (grade 1), moderate (grade 2), and severe (grade 3) WMHs (Fazekas et al., 1987).
The inclusion criteria for the pre-SVCI included (1) literate Han Chinese, education ≥ 6 years, and aged 50–80 years; (2) met with the brain imaging criteria of SIVD above (Román et al., 2002); (3) no cognitive complaints; (4) no impairments of daily life activities with clinical dementia rating (CDR) = 0.5 (Hughes et al., 1982); activities of daily living (ADL) < 23 (Salloway et al., 2004); and (5) normal cognitive screening assessments with MMSE > 26, and MoCA-Beijing version score ≥ 26 (Folstein et al., 1975; Petersen, 2004).
The healthy controls were defined as persons with no neurological and psychiatric disorders, no abnormal findings on conventional brain MRI (brain anatomical MRI was reported normal by the same two radiologists who assessed the MRI for the pre-SVCI group) (Carey et al., 2008; Liu et al., 2019a,b), and no cognitive complaints. For each case, one control was matched by age (within 2 years), sex, and years of education. All enrolled subjects underwent a clinical interview, neurologic examinations, comprehensive neuropsychological assessments, and MRI scanning.
Subjects who met the following criteria were excluded: (1) no completion of neuropsychological testing; (2) Hamilton depression scale score > 17, or anxiety; (3) new strokes within 3 months before baseline; (4) signs of large vessel disease, such as cortical and/or cortico-subcortical non-lacunar territorial infarcts and watershed infarcts or hemorrhages; and (5) leukoencephalopathy as a result of other causes, such as normal pressure hydrocephalus, multiple sclerosis, brain irradiation, and metabolic diseases.
We evaluated the cognition status of the subjects with a modified National Institute of Neurological Disorders and Stroke and Canadian Stroke Network-Canadian Stroke Network protocol (Hachinski et al., 2006; Wong et al., 2013). The following cognitive variables were included in the present analysis: (1) global cognition was measured by the MMSE and MoCA; (2) episodic memory was evaluated by the Auditory Verbal Learning Test (AVLT) and the Rey–Osterrieth Complex Figure Delay Tests (R-O delay); (3) language function was examined by the Boston Naming Test (BNT) and the Category Verbal Fluency Test; (4) visuospatial ability was assessed by the Clock Drawing Test (CDT) and the Rey–Osterrieth Complex Figure Copy Test (R-O copy); (5) executive function was assessed by the Trail Making Tests B (TMT-B) and the Stroop Test C right and time; and (6) attention was evaluated by the Digital Span Test (DST) and the Trail Making Test A (TMT-A). The evaluations of all participants were conducted by the same qualified psychologist, and each evaluation required 90 min.
The MRI data were acquired on a 3.0T Siemens MAGNETOM Prisma MRI scanner. Participants lay supine with the head snugly fixed by a belt. Foam pads were used to restrict head motion, and earplugs were used to minimize the scanner noise. Subjects were told to relax, keep their eyes closed, and remain awake. T1-weighted, sagittal 3D magnetization prepared rapid gradient echo sequences were acquired and covered the entire brain [192 sagittal slices, repetition time (TR) = 2,300 ms, echo time (TE) = 2.32 ms, slice thickness = 0.90 mm, flip angle = 8°, inversion time = 900 ms]. T2-weighted images (TR = 5000 ms, TE = 105 ms, slice thickness = 3 mm, flip angle = 150°, number of slices = 33) and T2-FLAIR images (TR = 9000 ms, TE = 81 ms, slice thickness = 3 mm, flip angle = 150°, number of slices = 25) were acquired. Two sets of DTI data scans were acquired for every subject and then averaged during the data preprocessing. DTI images covering the whole brain were acquired using a single-shot, twice-refocused, diffusion-weighted echo-planar imaging sequence with the following scan parameters: TR = 8,000 ms; TE = 60 ms; 30 diffusion-weighted directions with a b value of 1,000 s/mm2, and a single image with a b value of 0 s/mm2; slice thickness = 2 mm; no interslice gap; 75 axial slices; field of view = 282 mm2; and voxel size = 2 mm3.
All of the DTI image preprocessing and analyses described below were implemented using a pipeline tool for diffusion MRI, named “Pipeline for analyzing brain diffusion images” (PANDA) (Cui et al., 2013). A similar procedure was shown in our previous studies (Chen et al., 2015; Zhang et al., 2015). First, the DICOM files of all subjects were converted into NIfTI images using the dcm2nii tool embedded in MRI cron. Second, the brain mask was estimated, which was required for the subsequent processing steps. Third, the non-brain spaces in the raw images were removed, leading to a reduced image size, which reduced memory cost and sped up processing in subsequent steps. Fourth, each diffusion-weighted image (DWI) was coregistered to the b0 image using an affine transformation to correct the eddy-current-induced distortions and slow bulk motion-induced inter-gradient misalignment (Chen et al., 2015). The diffusion gradient directions were adjusted accordingly. Fifth, a voxel-wise calculation of the tensor matrix and the diffusion tensor metrics were yielded for each subject, including FA, MD, DA, and DR.
White matter atlases (Mori et al., 2008) (e.g., the ICBM-DTI-81 WM labels atlas and the JHU WM tractography atlas) in the standard space allow for parcelation of the WMs into multiple regions of interest (ROIs), each representing a labeled region in the atlas. In our current study, to investigate the diffusion changes in specific tracts, the ICBM-DTI-81 WM label atlas was used to parcel the WMs into 48 ROIs, and only the 40 ROIs in cerebral regions (we focused on the 40 WM tracts within the cerebrum and did not consider the other 8 ROIs within the cerebellum and brain stem) were used for the analysis. Then, the regional mean diffusion metrics including the FA, the MD, the DA, and the DR (Soares et al., 2013; Amlien and Fjell, 2014) were calculated by averaging the values within each region of the WM atlas.
We used IBM SPSS Statistics for Windows version 22.0 (IBM Corp., Armonk, NY, United States) for all statistical analyses. We assessed the normality of the data with Shapiro–Wilk tests and Q–Q plots. Independent two-sample t-tests were used to assess between-group differences for quantitative variables. The Pearson Chi-square test and Fisher exact probability test were used to compare proportions for categorical variables. Multiple linear regression analysis was used to evaluate the group differences in neuropsychological assessments and diffusion metrics including FA, MD, DR, and DA of the atlas-based ROIs. Age, gender, education years, and groups are the variables when the multiple linear regression analysis was performed. Pearson’s correlation analysis was used to calculate the correlation between diffusion metrics of atlas-based tracts with significant group effects and behavior performance (age, gender, and education years were included as covariates). For all analyses, a two-tailed p value < 0.05 was considered statistically significant. Bonferroni correction was performed in multiple comparisons of 40 atlas-based ROIs (p < 0.05/40) and correlation between diffusion metrics of atlas-based tracts with significant group effects and neuropsychological assessments (p < 0.05/14).
According to the inclusion and exclusion criteria, we ultimately enrolled 22 cases and 19 healthy controls. Figure 1 shows the participant enrollment process. The demographic and clinical characteristics of the participants are presented in Table 1. The distribution of age and education years was normal. There were no significant differences in age, sex, years of education, history of DM, CAD, HC, alcohol intake, and smoking. As expected, there was a significant difference in the history of hypertension between the two groups.
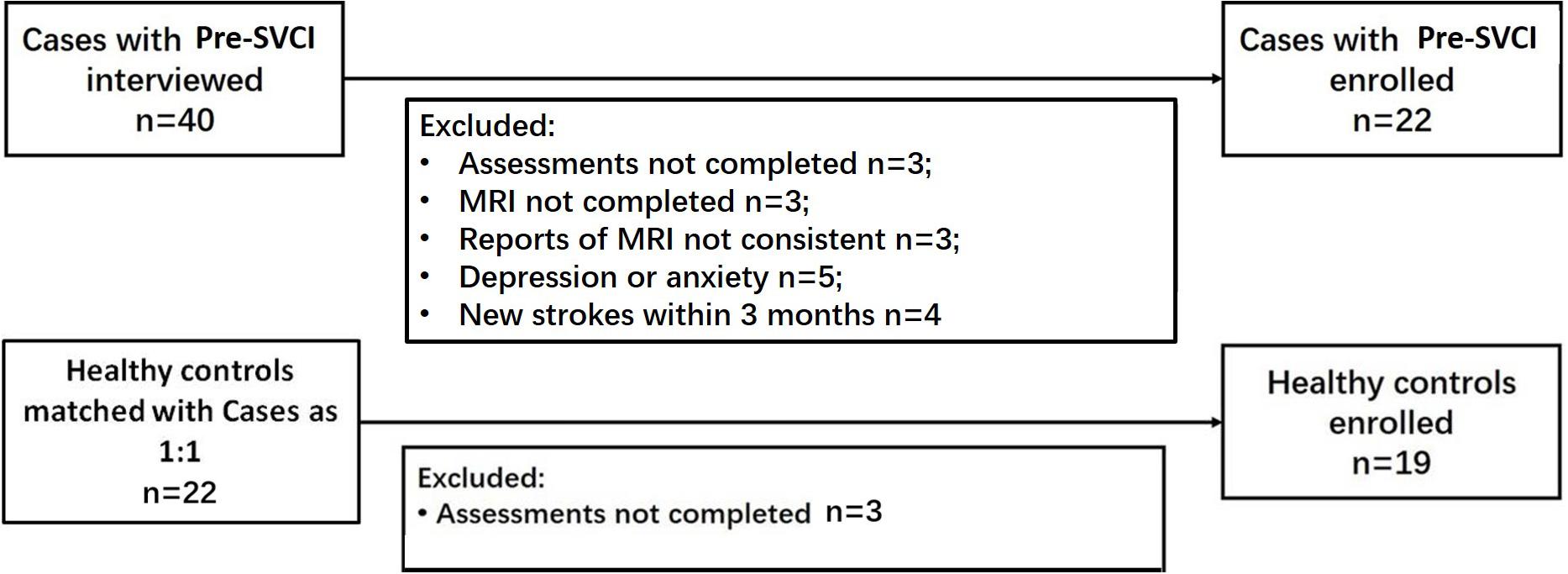
Figure 1. Flowcharts for study enrollment of case participants and control participants. Pre-SVCI is subcortical ischemic vascular disease with normal global cognition measures.
Table 2 presents the cognitive assessment results. The pre-SVCI group exhibited significant differences in the MoCA, Rey–Osterrieth Complex Figure (R-O)-copy, and TMT-B test compared with the H group.
Table 3 shows the diffusion metrics of WM tracts which are significantly different when comparing the pre-SVCI group to the H group. Compared with the control group, the pre-SVCI group exhibited significantly decreased FA in the right anterior corona radiata (ACR) and inferior fronto-occipital fasciculus (IFOF) (p < 0.05). Meanwhile, increased MD in the right side of the posterior thalamic radiation (PTR), the inferior longitudinal fasciculus (ILF), the IFOF, and the left side of the external capsule (EC) were observed (p < 0.05). The case group also exhibited increased DA on the bilateral side of the ILF, the EC, and the right side of the PTR and increased DR in the left EC and the right IFOF (p < 0.05). Among them, FA values of right ACR, MD values of left EC and right IFOF, DA values of left EC, and DR values of right IFOF are still significantly different after Bonferroni correction (p < 0.05/40) for the multiple comparisons (Figure 2).
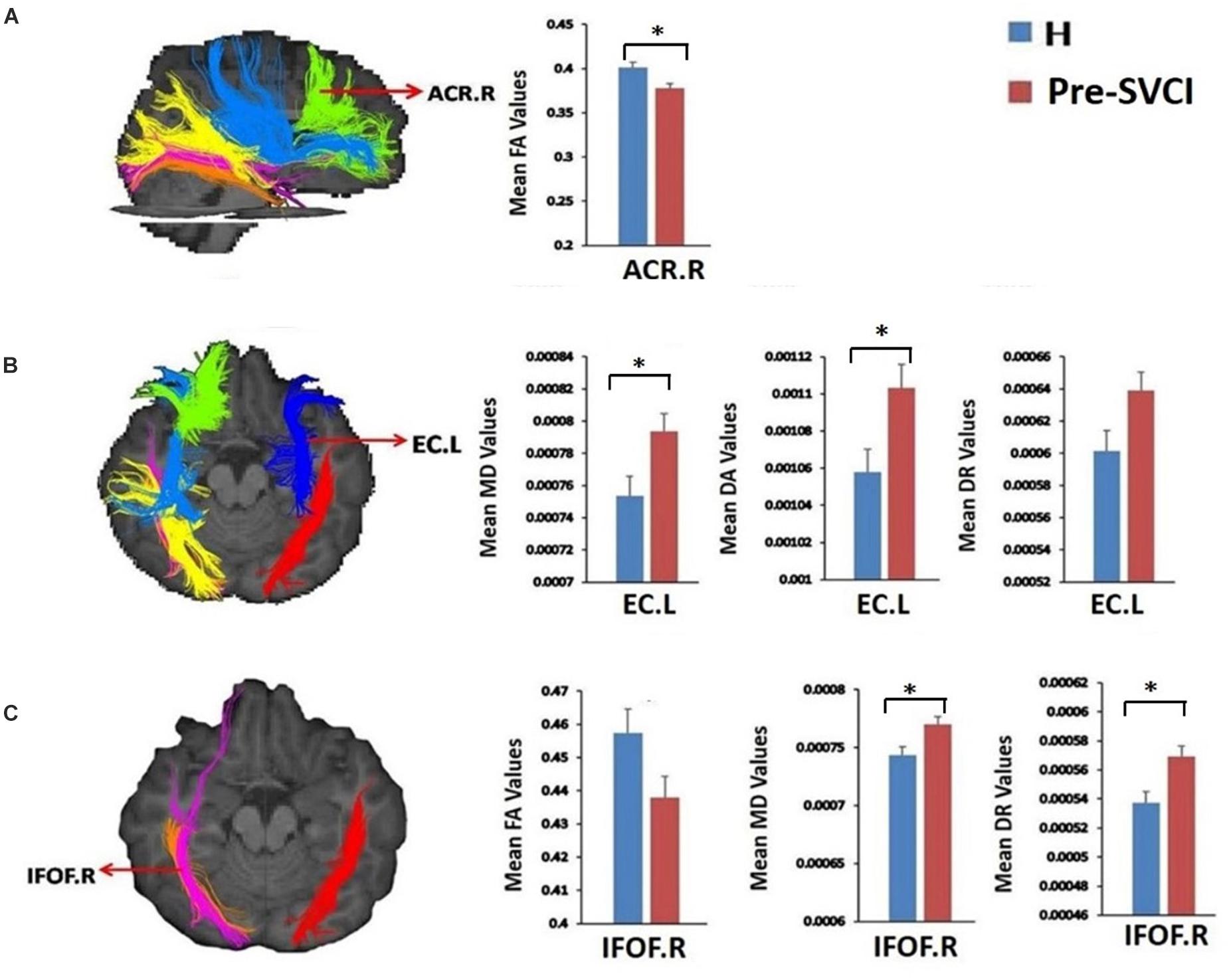
Figure 2. The tracts where diffusion metrics remained significant between the two groups after Bonferroni correction for multiple comparisons. (A) Mean FA values of ACR.R; (B) Mean MD, DA, and DR values of EC.L; (C), mean FA, MD, and DR values of IFOF.R. *is significant at p < 0.05/40 after Bonferroni correction. FA, fractional anisotropy, MD, mean diffusivity; DA, axial diffusivity; DR, radial diffusivity; pre-SVCI, subcortical ischemic vascular disease with normal global cognition measures; H, healthy; L, left; R, right; ACR, anterior corona radiata; EC, external capsule; IFOF, inferior fronto-occipital fasciculus.
We examined the relationship between the mean values of the diffusion metrics of the ROIs extracted from the significant WM regions and neuropsychological scores of the pre-SVCI group and healthy controls. In the pre-SVCI group, the mean FA values of the right ACR were significantly correlated with the MoCA (r = 0.699, p = 0.001). The R-O delay was positively correlated with the mean FA values (r = 0.600, p = 0.003) but negatively correlated with the MD values (r = −0.441, p = 0.040) of the right IFOF. Negative correlations were observed between the mean MD value of the left EC and the Category Verbal Fluency Test (CVFT) (animal) (r = −0.564, p = 0.006). Negative correlations can be seen between the mean MD value of the right PTR and the Stroop C-right and MMSE scores (r = −0.458, p = 0.032 and r = −0.607, p = 0.003, respectively). Notably, the correlations between the FA of right ACR and MoCA, the FA of right IFOF and RO-delay, and the MD of right PTR and MMSE were still significant after Bonferroni correction (p < 0.05/14) for multiple comparison (Figure 3).
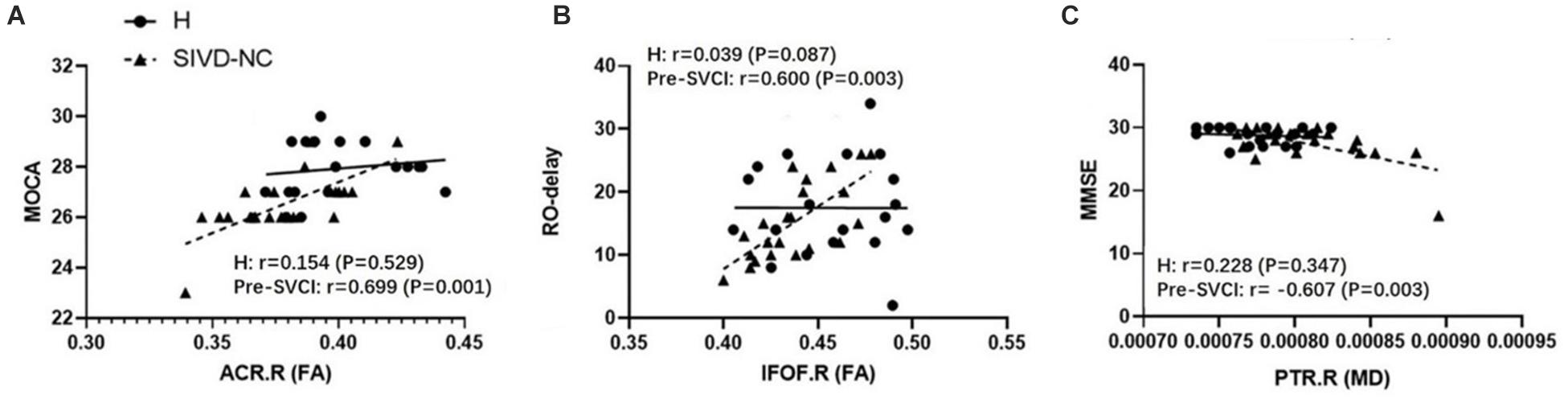
Figure 3. The significant correlations between ROI-wise diffusion metrics and behaviors in the pre-SVCI group and the H group after Bonferroni correction for multiple comparisons. (A) MoCA. (B) R-O delay. (C) MMSE. MoCA, Montreal cognitive assessment; R-O delay, Rey–Osterrieth Complex Figure delay tests; MMSE, Mini-Mental State Examination; FA, fractional anisotropy, MD, mean diffusivity; pre-SVCI, subcortical ischemic vascular disease with normal global cognition measures; H, healthy; L, left; R, right; ACR, anterior corona radiata; IFOF, inferior fronto-occipital fasciculus; PTR, posterior thalamic radiation.
In the control group, there was no negative or positive correlation between the diffusion metrics of the significant WM tracts and neuropsychological scores.
In the current study, we evaluated WM alterations and cognitive declines in the early stage of SVCI (pre-SVCI), compared with a well-matched healthy group. We made several observations. (1) Executive function and spatial processing were already declined in the pre-SVCI patients. (2) Some long associative and projective bundles were damaged in the pre-SVCI group. In particular, the right IFOF was found to have decreased FA, increased MD, and increased DR. (3) The mean values of the diffusion indices in some specific WM tracts in the pre-SVCI group were significantly correlated with neuropsychological assessments that related to executive functions or spatial processing.
According to the brain connectome theory, disruptions in the global connections between cortical and subcortical networks are partially related to damage in WM fibers (Lo et al., 2010). Thus, SVCI which is characterized by extensive cerebral WM lesions in the periventricular/deep cerebral WM (Román et al., 2002; Rosenberg et al., 2014; Tu et al., 2017), tends to result in cognitive impairment. Previous studies (Biesbroek et al., 2017; Tu et al., 2017; Liu et al., 2019b) have revealed that SVCI patients exhibit nearly global changes with decreased FA and increased MD in WM tracts especially those located in thalamic-prefrontal and caudate-prefrontal pathways. It has been proven that these two pathways are markedly related to neuropsychological assessments of executive function, attention, and processing speed in many task-related functional studies (Block et al., 2007; Müller-Oehring et al., 2015; Liu et al., 2018). Our study showed that the TMT-B assessment was significantly different between the patients and healthy controls. However, the above studies mainly focus on patients at the stages of SvMCI or SVaD and neglect the asymptomatic stage of disease when individuals suffer vascular damage without cognitive impairment.
Our study found that some types of long associative and projective WM such as right ACR, IFOF, and left EC were extensively damaged in the pre-SVCI group even given the strictest Bonferroni correction for multiple comparisons. However, Liu et al. (2019b) found that approximately all cerebral WMs were symmetrically involved in the pre-SVCI group but were less distinct than those in the SVCI group. Our different result from the previous study was mainly due to the discrepant inclusion criteria for including the pre-SVCI group. We used two screening scales, i.e., the MMSE and MoCA, both to include patients with normal global measures. The MoCA is considered more sensitive for screening VCI, which surpasses the well-known limitations of the MMSE (Dong et al., 2010). Because of the lack of sensitivity in detecting subtle cognitive changes, particularly visuospatial and executive function impairments, using the MMSE alone may lead to a false-negative bias in the recruitment of patients. In our study, the patients had a significant difference compared with the healthy controls in MoCA, but not the MMSE, further confirming the above view.
Our results demonstrated that IFOF might be one kind of WM tracts that are much more easily demyelinated in vascular disease. Notably, three of the DTI-derived indices changed with decreased FA, increased MD, and increased DR in the right IFOF. IFOF is one of the longest major associative bundles that was recognized and depicted in 2007 (Schmahmann and Pandya, 2007). It connects the occipital cortex, the superior parietal lobe, and the temporo-basal areas to the frontal cortex (Martino et al., 2010). Some DTI studies have shown that the IFOF is a probable crucial tract in reading, attention, and visual processing, especially the right IFOF in spatial attention and neglect (Catani and Thiebaut de Schotten, 2008; Urbanski et al., 2008, 2011). Consistently, based on the correlation analysis between WM impairment and cognitive decline, we found that the RO-delay, which represents long-term memory and visuospatial function, was positively correlated with the FA value of the right IFOF after the strictest Bonferroni correction, which further identified that the damage of IFOF is correlated with the decline of visual processing. The right IFOF may be impaired at the early stage because of its long course, which can easily result in myelin injury. According to our findings, the DR of IFOF.R was significantly increased. An increasing DR reflects a decline in myelin sheath integrity (Soares et al., 2013). Thus, we can further infer that long tracts, such as the IFOF, which course from the front regions to the end of the brain, are probably predamaged before the prefrontal thalamus circus. Furthermore, we found that the significantly damaged tracts in the pre-SVCI patients were mainly concentrated in the right hemisphere. The integrity of IFOF, PTR, and ATR in the right hemisphere was extensively damaged which were also correlated with the declines in visuospatial and executive functions. So far, studies (Kleinman et al., 2007; Giussani et al., 2010; Kontaxopoulou et al., 2017; Vilasboas et al., 2017; Bernard et al., 2018) have increasingly found that the non-dominant right hemisphere is responsible for primary cognitive functions such as visuospatial, intentional process, and social cognition. This is consistent with the findings in our study. But why the WMs in right hemisphere are damaged earlier than left hemisphere in SIVD patients is not clear.
There are several limitations in this study. First, although according to previous studies, we supposed that SIVD patients with normal global cognitive measures could be pre-SVCI patients, we could not clearly determine whether these pre-SVCI patients will develop into SVCI, remain unchanged, or improve as a consequence of brain plasticity or reserve capacity. This bias leads our results to underestimate the difference between the cases and the controls. Besides, as previous studies, we did not report measures of head motion for each group and just rely on registration-based correction methods that cannot eliminate the full effects of head motion on the DW images (Yendiki et al., 2014). Therefore, longitudinal follow-up studies with a large-scale and more accurate and comprehensive methods to correct head motion artifacts are needed in the future. Second, our study focused on only WM alterations, but whether cerebral blood perfusion or other elements are correlated with cognitive decline still needs further exploration. Moreover, we did not assess the degree of gray matter atrophy, which can have an impact on cognitive functions. Third, this study involved Chinese individuals, and other ethnic groups need to be further studied.
In summary, our study indicated that in SIVD patients, even with normal global cognitive measures, some types of long course tracts, especially the tracts in the right hemisphere, were damaged. Furthermore, damage to these tracts was associated with a decline in some specific cognitive domains, such as executive functions and spatial processing domains. These results indicated that pre-SVCI patients are likely at an ultra-early stage of SVCI, and there was a very high risk of this condition becoming SVCI. To prevent the progression of SVCI, longitudinal studies are needed to explore the dynamic changes from the early stage to the clinical stage of SVCI and to evaluate the value of DTI in predicting the process of SVCI.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board of China-Japan Friendship Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YQ and DP: conceptualization. XH and JZ: methodology. YQ, XH, YL, and WS: formal analysis and investigation. YQ: writing – original draft preparation. SZ: writing, review, and editing. ZZ and DP: supervision. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the Hospital Foundation of China-Japan Friendship Hospital (No. 2016-2-QN-21), the Natural Science Foundation of China (No. 81974220), and the National Key R&D Program of China (No. 2016YFC1306301).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Thanks are given to Xin Li and Yu Sun for the writing assistance.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2021.681208/full#supplementary-material
Amlien, I. K., and Fjell, A. M. (2014). Diffusion tensor imaging of white matter degeneration in Alzheimer’s disease and mild cognitive impairment. Neuroscience 276, 206–215. doi: 10.1016/j.neuroscience.2014.02.017
Bernard, F., Lemee, J. M., Ter Minassian, A., and Menei, P. (2018). Right hemisphere cognitive functions: from clinical and anatomic bases to brain mapping during awake craniotomy part I: clinical and functional anatomy. World Neurosurg. 118, 348–359. doi: 10.1016/j.wneu.2018.05.024
Biesbroek, J. M., Weaver, N. A., and Biessels, G. J. (2017). Lesion location and cognitive impact of cerebral small vessel disease. Clin. Sci. 131, 715–728. doi: 10.1042/cs20160452
Block, A. E., Dhanji, H., Thompson-Tardif, S. F., and Floresco, S. B. (2007). Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb. Cortex 17, 1625–1636. doi: 10.1093/cercor/bhl073
Carey, C. L., Kramer, J. H., Josephson, S. A., Mungas, D., Reed, B. R., Schuff, N., et al. (2008). Subcortical lacunes are associated with executive dysfunction in cognitively normal elderly. Stroke 39, 397–402. doi: 10.1161/strokeaha.107.491795
Catani, M., and Thiebaut de Schotten, M. (2008). A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44, 1105–1132. doi: 10.1016/j.cortex.2008.05.004
Chen, Y., Wang, A., Tang, J., Wei, D., Li, P., Chen, K., et al. (2015). Association of white matter integrity and cognitive functions in patients with subcortical silent lacunar infarcts. Stroke 46, 1123–1126. doi: 10.1161/strokeaha.115.008998
Cui, Z., Zhong, S., Xu, P., He, Y., and Gong, G. (2013). PANDA: a pipeline toolbox for analyzing brain diffusion images. Front. Hum. Neurosci. 7:42. doi: 10.3389/fnhum.2013.00042
D’Souza, M. M., Gorthi, S. P., Vadwala, K., Trivedi, R., Vijayakumar, C., Kaur, P., et al. (2018). Diffusion tensor tractography in cerebral small vessel disease: correlation with cognitive function. Neuroradiol. J. 31, 83–89. doi: 10.1177/1971400916682753
Dong, Y., Sharma, V. K., Chan, B. P., Venketasubramanian, N., Teoh, H. L., Seet, R. C., et al. (2010). The montreal cognitive assessment (MoCA) is superior to the mini-mental state examination (MMSE) for the detection of vascular cognitive impairment after acute stroke. J. Neurol. Sci. 299, 15–18. doi: 10.1016/j.jns.2010.08.051
Dong, Y., Xu, J., Chan, B. P., Seet, R. C., Venketasubramanian, N., Teoh, H. L., et al. (2016). The montreal cognitive assessment is superior to National Institute of Neurological Disease and Stroke-Canadian Stroke Network 5-minute protocol in predicting vascular cognitive impairment at 1 year. BMC Neurol. 16:46. doi: 10.1186/s12883-016-0570-y
Du, J., Zhu, H., Zhou, J., Lu, P., Qiu, Y., Yu, L., et al. (2020). Structural brain network disruption at preclinical stage of cognitive impairment due to cerebral small vessel disease. Neuroscience 449, 99–115. doi: 10.1016/j.neuroscience.2020.08.037
Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I., and Zimmerman, R. A. (1987). MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 149, 351–356. doi: 10.2214/ajr.149.2.351
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198.
Giussani, C., Pirillo, D., and Roux, F. E. (2010). Mirror of the soul: a cortical stimulation study on recognition of facial emotions. J. Neurosurg. 112, 520–527. doi: 10.3171/2009.5.jns081522
Hachinski, V., Iadecola, C., Petersen, R. C., Breteler, M. M., Nyenhuis, D. L., Black, S. E., et al. (2006). National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 37, 2220–2241. doi: 10.1161/01.str.0000237236.88823.47
Hughes, C. P., Berg, L., Danziger, W. L., Coben, L. A., and Martin, R. L. (1982). A new clinical scale for the staging of dementia. Br. J. Psychiatry 140, 566–572. doi: 10.1192/bjp.140.6.566
Kleinman, J. T., Sepkuty, J. P., Hillis, A. E., Lenz, F. A., Heidler-Gary, J., Gingis, L., et al. (2007). Spatial neglect during electrocortical stimulation mapping in the right hemisphere. Epilepsia 48, 2365–2368.
Kontaxopoulou, D., Beratis, I. N., Fragkiadaki, S., Pavlou, D., Yannis, G., Economou, A., et al. (2017). Incidental and intentional memory: their relation with attention and executive functions. Arch. Clin. Neuropsychol. 32, 519–532. doi: 10.1093/arclin/acx027
Liu, J., Wang, C., Diao, Q., Qin, W., Cheng, J., and Yu, C. (2018). Connection disruption underlying attention deficit in subcortical stroke. Radiology 288, 186–194. doi: 10.1148/radiol.2018171730
Liu, X., Chen, L., Cheng, R., Luo, T., Lv, F., Fang, W., et al. (2019a). Altered functional connectivity in patients with subcortical ischemic vascular disease: a resting-state fMRI study. Brain Res. 1715, 126–133. doi: 10.1016/j.brainres.2019.03.022
Liu, X., Cheng, R., Chen, L., Luo, T., Lv, F., Gong, J., et al. (2019b). Alterations of white matter integrity in subcortical ischemic vascular disease with and without cognitive impairment: a TBSS study. J. Mol. Neurosci. 67, 595–603. doi: 10.1007/s12031-019-01266-3
Lo, C. Y., Wang, P. N., Chou, K. H., Wang, J., He, Y., and Lin, C. P. (2010). Diffusion tensor tractography reveals abnormal topological organization in structural cortical networks in Alzheimer’s disease. J. Neurosci. 30, 16876–16885. doi: 10.1523/jneurosci.4136-10.2010
Martino, J., Brogna, C., Robles, S. G., Vergani, F., and Duffau, H. (2010). Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex 46, 691–699. doi: 10.1016/j.cortex.2009.07.015
Mori, S., Oishi, K., Jiang, H., Jiang, L., Li, X., Akhter, K., et al. (2008). Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40, 570–582. doi: 10.1016/j.neuroimage.2007.12.035
Müller-Oehring, E. M., Sullivan, E. V., Pfefferbaum, A., Huang, N. C., Poston, K. L., Bronte-Stewart, H. M., et al. (2015). Task-rest modulation of basal ganglia connectivity in mild to moderate Parkinson’s disease. Brain Imaging Behav. 9, 619–638. doi: 10.1007/s11682-014-9317-9
Nitkunan, A., Barrick, T. R., Charlton, R. A., Clark, C. A., and Markus, H. S. (2008). Multimodal MRI in cerebral small vessel disease: its relationship with cognition and sensitivity to change over time. Stroke 39, 1999–2005. doi: 10.1161/strokeaha.107.507475
Petersen, R. C. (2004). Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256, 183–194. doi: 10.1111/j.1365-2796.2004.01388.x
Reijmer, Y. D., Fotiadis, P., Piantoni, G., Boulouis, G., Kelly, K. E., Gurol, M. E., et al. (2016). Small vessel disease and cognitive impairment: the relevance of central network connections. Hum. Brain Mapp. 37, 2446–2454. doi: 10.1002/hbm.23186
Román, G. C., Erkinjuntti, T., Wallin, A., Pantoni, L., and Chui, H. C. (2002). Subcortical ischaemic vascular dementia. Lancet Neurol. 1, 426–436. doi: 10.1016/s1474-4422(02)00190-4
Rosenberg, G. A., Bjerke, M., and Wallin, A. (2014). Multimodal markers of inflammation in the subcortical ischemic vascular disease type of vascular cognitive impairment. Stroke 45, 1531–1538. doi: 10.1161/strokeaha.113.004534
Sachdev, P., Kalaria, R., O’Brien, J., Skoog, I., Alladi, S., Black, S. E., et al. (2014). Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis. Assoc. Disord. 28, 206–218. doi: 10.1097/wad.0000000000000034
Salloway, S., Ferris, S., Kluger, A., Goldman, R., Griesing, T., Kumar, D., et al. (2004). Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology 63, 651–657. doi: 10.1212/01.wnl.0000134664.80320.92
Schmahmann, J. D., and Pandya, D. N. (2007). The complex history of the fronto-occipital fasciculus. J. Hist. Neurosci. 16, 362–377. doi: 10.1080/09647040600620468
Soares, J. M., Marques, P., Alves, V., and Sousa, N. (2013). A hitchhiker’s guide to diffusion tensor imaging. Front. Neurosci. 7:31. doi: 10.3389/fnins.2013.00031
Sun, Y., Cao, W., Ding, W., Wang, Y., Han, X., Zhou, Y., et al. (2016). Cerebral blood flow alterations as assessed by 3D ASL in cognitive impairment in patients with subcortical vascular cognitive impairment: a marker for disease severity. Front. Aging Neurosci. 8:211. doi: 10.3389/fnagi.2016.00211
Tu, M. C., Lo, C. P., Huang, C. F., Hsu, Y. H., Huang, W. H., Deng, J. F., et al. (2017). Effectiveness of diffusion tensor imaging in differentiating early-stage subcortical ischemic vascular disease, Alzheimer’s disease and normal ageing. PLoS One 12:e0175143. doi: 10.1371/journal.pone.0175143
Tullberg, M., Fletcher, E., DeCarli, C., Mungas, D., Reed, B. R., Harvey, D. J., et al. (2004). White matter lesions impair frontal lobe function regardless of their location. Neurology 63, 246–253. doi: 10.1212/01.wnl.0000130530.55104.b5
Urbanski, M., Thiebaut de Schotten, M., Rodrigo, S., Catani, M., Oppenheim, C., Touze, E., et al. (2008). Brain networks of spatial awareness: evidence from diffusion tensor imaging tractography. J. Neurol. Neurosurg. Psychiatry 79, 598–601. doi: 10.1136/jnnp.2007.126276
Urbanski, M., Thiebaut de Schotten, M., Rodrigo, S., Oppenheim, C., Touze, E., Meder, J. F., et al. (2011). DTI-MR tractography of white matter damage in stroke patients with neglect. Exp. Brain Res. 208, 491–505. doi: 10.1007/s00221-010-2496-8
van Leijsen, E. M. C., van Uden, I. W. M., Bergkamp, M. I., van der Holst, H. M., Norris, D. G., Claassen, J., et al. (2019). Longitudinal changes in rich club organization and cognition in cerebral small vessel disease. Neuroimage Clin. 24:102048. doi: 10.1016/j.nicl.2019.102048
Vilasboas, T., Herbet, G., and Duffau, H. (2017). Challenging the myth of right nondominant hemisphere: lessons from corticosubcortical stimulation mapping in awake surgery and surgical implications. World Neurosurg. 103, 449–456. doi: 10.1016/j.wneu.2017.04.021
Wong, A., Xiong, Y. Y., Wang, D., Lin, S., Chu, W. W., Kwan, P. W., et al. (2013). The NINDS-Canadian stroke network vascular cognitive impairment neuropsychology protocols in Chinese. J. Neurol. Neurosurg. Psychiatry 84, 499–504. doi: 10.1136/jnnp-2012-304041
Yendiki, A., Koldewyn, K., Kakunoori, S., Kanwisher, N., and Fischl, B. (2014). Spurious group differences due to head motion in a diffusion MRI study. Neuroimage 88, 79–90. doi: 10.1016/j.neuroimage.2013.11.027
Zhang, J., Wang, Y., Wang, J., Zhou, X., Shu, N., Wang, Y., et al. (2014). White matter integrity disruptions associated with cognitive impairments in type 2 diabetic patients. Diabetes 63, 3596–3605. doi: 10.2337/db14-0342
Zhang, S., Chen, Y., Liu, Z., Zhang, J., Li, X., Cui, R., et al. (2015). Association of white matter integrity and cognitive functions in Chinese non-demented elderly with the APOE ϵ4 allele. J. Alzheimers Dis. 48, 781–791. doi: 10.3233/jad-150357
Keywords: subcortical vascular cognitive impairment, subcortical ischemic vascular disease, diffusion tensor imaging, white matter hyperintensities, cognitive impairment
Citation: Qiao Y, He X, Zhang J, Liang Y, Shao W, Zhang Z, Zhang S and Peng D (2021) The Associations Between White Matter Disruptions and Cognitive Decline at the Early Stage of Subcortical Vascular Cognitive Impairment: A Case–Control Study. Front. Aging Neurosci. 13:681208. doi: 10.3389/fnagi.2021.681208
Received: 16 March 2021; Accepted: 04 June 2021;
Published: 02 August 2021.
Edited by:
Jiehui Jiang, Shanghai University, ChinaReviewed by:
Hao Shu, Southeast University, ChinaCopyright © 2021 Qiao, He, Zhang, Liang, Shao, Zhang, Zhang and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dantao Peng, cGVuZ2RhbnRhbzIwMDBAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.