
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 14 June 2021
Sec. Neurocognitive Aging and Behavior
Volume 13 - 2021 | https://doi.org/10.3389/fnagi.2021.646807
This article is part of the Research TopicTwo for the Price of One – Effects and Underlying Mechanisms of Combined Motor-Cognitive Interventions on the Body and the BrainView all 11 articles
 Jiao Liu1,2,3,4†
Jiao Liu1,2,3,4† Jing Tao1,4,5†
Jing Tao1,4,5† Rui Xia1
Rui Xia1 Moyi Li1
Moyi Li1 Maomao Huang1
Maomao Huang1 Shuzhen Li1
Shuzhen Li1 Xiangli Chen6
Xiangli Chen6 Georgia Wilson3
Georgia Wilson3 Joe Park3
Joe Park3 Guohua Zheng7*
Guohua Zheng7* Lidian Chen1*
Lidian Chen1* Jian Kong3*
Jian Kong3*Mild cognitive impairment (MCI) is a common global health problem. Recently, the potential of mind-body intervention for MCI has drawn the interest of investigators. This study aims to comparatively explore the modulation effect of Baduanjin, a popular mind-body exercise, and physical exercise on the cognitive function, as well as the norepinephrine and dopamine systems using the resting state functional connectivity (rsFC) method in patients with MCI. 69 patients were randomized to the Baduanjin, brisk walking, or healthy education control group for 6 months. The Montreal Cognitive Assessment (MoCA) and magnetic resonance imaging (MRI) scans were applied at baseline and at the end of the experiment. Results showed that (1) compared to the brisk walking, the Baduanjin significantly increased MoCA scores; (2) Baduanjin significantly increased the right locus coeruleus (LC) and left ventral tegmental area (VTA) rsFC with the right insula and right amygdala compared to that of the control group; and the right anterior cingulate cortex (ACC) compared to that of the brisk walking group; (3) the increased right LC-right insula rsFC and right LC-right ACC rsFC were significantly associated with the corresponding MoCA score after 6-months of intervention; (4) both exercise groups experienced an increased effective connectivity from the right ACC to the left VTA compared to the control group; and (5) Baduanjin group experienced an increase in gray matter volume in the right ACC compared to the control group. Our results suggest that Baduanjin can significantly modulate intrinsic functional connectivity and the influence of the norepinephrine (LC) and dopamine (VTA) systems. These findings may shed light on the mechanisms of mind-body intervention and aid the development of new treatments for MCI.
Mild cognitive impairment (MCI) is a condition characterized by impaired cognitive function with a minimal impact on activities of daily living. Current pharmacologic treatments for the condition are unsatisfactory. Exercise has been recently recommended to improve cognitive function in patients with MCI (Petersen et al., 2018). Literature also suggests that mindful movement (mind-body intervention), which combines mindfulness and physical exercise, may have synergistic effects and lead to better outcomes than those achieved from either physical exercise or mindfulness meditation alone (Burgener et al., 2008).
Baduanjin is a mind-body exercise that focuses on both mindfulness and the strengthening of muscles and tendons (Liao et al., 2015). Previous studies have found that Baduanjin can improve attention, executive control function, and memory function, as well as modulate cognition-related brain function and structure. For instance, we found that compared to the education control, 12-week Baduanjin training significantly improved the memory quotient (MQ) and resting state functional connectivity of dorsal lateral prefrontal cortex (DLPFC) and hippocampus in elderly adults (Tao et al., 2016, 2017a). In another study in individuals with MCI, we found compared to the brisk walking and control groups, the Baduanjin can increased memory function as measured by Montreal Cognitive Assessment (MoCA), modulate the brain low-frequency oscillations, and increase gray matter volume of hippocampus and anterior cingulate cortex (ACC) (Tao et al., 2019).
More recently, a systematic review and meta-analysis of randomized controlled trials from 1054 participants showed that compared with conventional therapy, Baduanjin plus conventional therapy significantly improved cognitive and memory function in patients with mild cognitive impairment (Yu L. et al., 2020). In another pilot study, Xiao et al. (2020) found that community-delivered Baduanjin training program was safe for prefrail/frail older adults with the potential to improve cognitive function measured by MoCA.
Although accumulating evidence has demonstrated the potential of Baduanjin for cognition/memory improvement, its underlying mechanism remain unclear. Literature suggests that the release of stress-induced catecholamines may play an important role in cognitive processes (Zhang et al., 2016). Animal studies have found that the locus coeruleus (LC), a brain region involved in many cognitive processes, is a major node of norepinephrine (NE) release in the stress response (Liu et al., 2017). Takahashi et al. showed that compare to the healthy control, the LC contrast ratios significantly reduced in patient with Alzheimer’s disease and mild cognitive impairment (Takahashi et al., 2014). Lee et al found that older adults were associated with decline in LC functional connectivity with frontoparietal networks that coordinate attentional selectivity (Lee et al., 2018).
Dopamine is another important catecholamine and a neurotransmitter involved in the reward and motivation process. Literature suggests that the ventral tegmental area (VTA), which projects to the nucleus accumbens and ACC (Navratilova et al., 2015), plays a crucial role in the release of dopamine (Lammel et al., 2014). Accumulating literature suggest that dopamine is involved in both dementia (Koch et al., 2020) and exercise (Fan et al., 2021). For instance, a previous study found that degeneration of VTA dopaminergic neurons at pre-plaque stages contributes to memory deficits and dysfunction of reward processing in dementia (Nobili et al., 2017).
Locus coeruleus-NE and VTA-dopamine systems have many similarities in physiological effects, and both are responsive to motivationally salient events (such as reward predictors) (Ranjbar-Slamloo and Fazlali, 2020). Disturbances of both have been implicated in highly overlapping sets of clinical disorders, such as attention deficit disorder (Unsworth and Robison, 2017) and Autism (Huang et al., 2021). The interaction of these two systems may plan an important role in pathophysiology of cognitive disorders such as MCI (Aston-Jones and Cohen, 2005).
In the present study, we comparatively investigated the modulation effects of 6 months of Baduanjin exercise, compared to brisk walking and a healthy education control, on resting state functional connectivity (rsFC) of key regions of the norepinephrine (LC) and dopamine (VTA) systems. In addition, we also applied exploratory effective connectivity analysis and region-of-interest-based gray matter volume (GMV) analysis in key regions derived from the LC and VTA rsFC analysis. We hypothesized that, compared to the brisk walking and healthy education control interventions, Baduanjin exercise would significantly modulate the LC and VTA resting state functional connectivity and effective connectivity within key regions of the two systems (amygdala, prefrontal cortex, hippocampus/parahippocampus for LC (Zhang et al., 2016; Jacobs et al., 2018)) and mesolimbic and mesocortical pathways such as the ACC, medial prefrontal cortex, and hippocampus for VTA (Zhang et al., 2016; Yu S. et al., 2020). Additionally, we hypothesize that the functional connectivity changes may be associated with the cognitive function improvement.
The randomized controlled trial with three parallel groups (Baduanjin, brisk walking, and a healthy education control) was approved by the Medical Ethics Committee of the Second People’s Hospital of Fujian Province (Fuzhou, China) and was registered in the Chinese Clinical Trial Registry (ChiCTR-ICR-15005795). All subjects gave their informed consent at the initiation of study procedures, and details of these procedures can be found in our previous publication (Tao et al., 2019) in which we investigate the modulation effect of Baduanjin using the Amplitude of low-frequency fluctuations (ALFF), region-of-interest voxel-based morphometry (VBM) Analysis, and rsFC of hippocampus and ACC (based on the ALFF results). In this study, we focused on the modulation effect of Baduanjin on the LC-NE and VTA-dopamine system, which has not been previously published. Approximately half of all subjects each group who enrolled in the clinical trial were randomly selected to undergo MRI/functional MRI brain scans (n = 23 each group). This manuscript focuses on the participants with MRI data.
Inclusion criteria included: 1. Aged 60 years or older; 2. No regular physical exercise for at least half a year (exercise with a frequency of at least twice a week and 20 min per session); 3. Having a physician’s diagnosis of MCI based on the Petersen diagnostic criteria (Petersen, 2004); 4. Memory problems with MoCA score < 26 (if years of education ≤ 12, one point will be added to the patient’s score); 5. Cognitive decline in accordance with age and education; 6. Intact activities of daily living (Lawton–Brody ADL score < 18); 7. Absence of dementia (Global Deterioration Scale score at 2 or 3).
Subjects were excluded from the study if any of the following criteria were met: 1. Resistant hypertension; 2. Severe vision or hearing loss; 3. Evidence of severe psychiatric conditions (such as active suicidal ideation, schizophrenia, etc.) or Geriatric depression scale score (GDS) ≥ 10 (Scale, 1997); 4. Current severe medical conditions for which exercise is contraindicated; 5. History of alcohol or drug abuse; 6. Participation in other clinical studies; 7. Pharmacologic treatments that may interfere with cognitive function.
All participants in the study received health education every 8 weeks for 30 min per session. In these sessions, participants were educated on ways to prevent the development of MCI.
Participants in the Baduanjin and brisk walking groups received the 24-week exercise training with a frequency of 3 days/week, 60 min/day (15-min warm up, 40-min training, and 5-min cool down). Research staff members also participated in all training sessions to record the attendance of each subject.
Baduanjin training was based on the ‘Health Qigong Baduanjin Standard,’ which was enacted by the Chinese State Sports General Administration in Health Qigong Management Center of General Administration of Sport of China. (2003) and consists of 10 postures (including the preparation and ending posture) (Zheng et al., 2016). Two professional coaches with over 5 years of Baduanjin teaching experience at the Fujian University of Traditional Chinese Medicine were employed to guide participants’ training.
In the brisk walking group, professional coaches were employed to guide participants’ training. The intensity of the exercise was controlled by maintaining participants’ heart rates at 55–75% of their heart rate reserve via the Polar Heart Rate Monitor.
Subjects in the healthy education control group were required to maintain their original physical activity levels and did not receive any specific exercise interventions except for the health education.
The Chinese version of the Montreal Cognitive Assessment (MoCA-Chinese Beijing version) scale was selected as our primary clinical outcome measure to assess global cognitive function. MoCA is a brief cognitive screening instrument that was created and validated to detect MCI (about 10-min). There are 8 items (visuospatial/executive functions, naming, verbal memory registration and learning, attention, abstraction, 5-min delayed verbal memory, and orientation) with a total score of 0-30 (a higher score equates to better function). The MoCA-Chinese Beijing version demonstrated an excellent sensitivity of 90.4% and a fair specificity (31.3%) when the cut-off score was recommended 26 (Yu et al., 2012; Zheng et al., 2016). The MoCA was measured at baseline and within one week after the 24-week intervention for all participants.
The fMRI data was acquired on a 3.0-T GE scanner (General Electric, Milwaukee, WI, United States) with an eight-channel phased array head coil at baseline and at the end of the intervention. Resting state functional MRI data was acquired with the following parameters: TR = 2100 ms, TE = 30 ms, flip angle = 90°, voxel size = 3.125 mm × 3.125 mm × 3.6 mm, 42 axial slices, field of view (FOV) = 200 mm × 200 mm, time pointes = 160. High-resolution structural images (MPRAGE) were acquired with the parameters of 7° flip angle, voxel size: 1 × 1 × 1 mm3, 240 mm FOV, and 164 slices. Subjects were asked to stay awake and remain motionless during the scan with their eyes closed and ears plugged.
One-way ANOVA and Chi-square tests were applied to compare the baseline characteristics of subjects, and ANCOVA was applied to compare changes (post-treatment minus pre-treatment) in MoCA scores across all groups, adjusted for age (years), gender, education, and baseline MoCA scores using IBM SPSS Statistics software (Dimitrov and Rumrill, 2003).
Similar to our previous study (Liu et al., 2019a, c; Tao et al., 2019), the seed-to-voxel functional connectivity correlational analyses were performed using a standard pipeline in a functional connectivity toolbox (CONN1) in MATLAB. The seeds (regions of interest) of bilateral LC and VTA (left and right separately) used in the present study were applied based on previous studies. The left LC was defined as 4 × 6 × 10 mm centered at MNI coordinates −5,−34,−21, and the right LC was defined as 4 × 6 × 10 mm centered at MNI coordinates 7,−34,−21 (Naidich et al., 2009; Bär et al., 2016). The VTA was defined as a 4 mm radius sphere (MNI coordinates left VTA: −4,−15,−9; right VTA: 5,−14,−8) (Adcock et al., 2006). The images were slice-time corrected, realigned, coregistered to subjects’ respective structural images, normalized, and smoothed with a 4 mm full width half maximum (FWHM) kernel. Then, segmentation of gray matter, white matter, and cerebrospinal fluid (CSF) areas for the removal of temporal confounding factors was employed. Band-pass filtering was performed with a frequency window of 0.01 to 0.089 Hz.
To eliminate correlations caused by head motion and artifacts, we identified outlier time points in the motion parameters and global signal intensity using ART2. We treated images as outliers if the composite movement from a preceding image exceeded 0.5 mm or if the global mean intensity was greater than 3 standard deviations from the mean image intensity (Wang Z. et al., 2017). The temporal time series of the head motion matrix of outliers were put into the first-level analysis as covariates (Whitfield and Nieto, 2012). A threshold of p < 0.005 uncorrected in voxel level and p < 0.05 FDR corrected at cluster level was used for group analysis. Due to the important role of the amygdala in the modulation of emotion, stress, and mind-body intervention (Tang et al., 2015) and the small size of the area, we pre-defined the bilateral amygdala as a region of interest (ROI). For the ROI (as defined by AAL brain atlas), a threshold of voxel-wise p < 0.005 was used in data analysis. Monte Carlo simulations using the 3dFWHMx and 3dClustSim [AFNI3 released in July 2017] were applied to correct for multiple comparisons (Gollub et al., 2018).
We found the overlapped brain regions in the right ACC and right insula among the rsFC of the right LC and left VTA (see results). To further investigate the causal interaction underlying the four brain regions (overlapped right ACC, overlapped right insula, ROIs of right LC and left VTA) during intervention, we performed the effective connectivity analysis using dynamic causal modeling (Sharaev et al., 2016; Li et al., 2017). The Spectral DCM analysis was performed using DCM12 (Wellcome Trust Centre for Neuroimaging, London, United Kingdom) implemented in SPM12. We specified two connectivity models: one model including the right LC, right ACC, and right insula (named the “right LC model”), and the other model including the left VTA, right ACC, and right insula (named the “left VTA model”). Four different sub-models were specified for each model: one fully connected model with bi-directional connections between all pairs of ROIs and three models where different regions predominantly affected the others (Sharaev et al., 2016; Figures 3A1,B1). In addition, to further investigate whether there were causal interactions between the left VTA and right LC, we defined a connectivity model that included all four brain masks (named the “right LC and left VTA model”). This model included five different sub-models: one fully connected model and four models where different regions predominantly affected the others (Supplementary Figure 1A). In this study, we assumed that all participants in the same conditions (i.e., pre-Baduanjin, post-Baduanjin, pre-walking, post-walking, pre-control, post-control) used the same model (the wining model).
Time series of the four brain masks of each subject (pre- and post-treatment separately) were extracted via a General Linear Model (GLM). The 6-rigid body realignment parameters (six head motion parameters) and the signals of cerebrospinal fluid and white matter were used as constant regresses in the GLM. Random Effects Bayesian model selection (RFX-BMS) was performed to determine the best model in each condition, considering both accuracy and complexity (Stephan et al., 2009). Repeated measures analysis was used to investigate the group and time interaction of the different connectivity parameters for the “wining” model.
To investigate whether there were structural changes (gray matter volume, GMV) in the brain regions that showed significant group differences (Baduanjin vs. Control) and overlap between the rsFC of the right LC and left VTA (i.e., right amygdala, right ACC, and right insula; see Results for more information), we applied a region of interest VBM analysis (Andrew et al., 2018; Liu et al., 2019a, b; Tao et al., 2019) in SPM12. The data preprocessing for VBM analysis was similar to our previous study (Tao et al., 2017c). The T1 images of all participants were segmented into gray matter, white matter, and cerebrospinal fluid. Then, a group specific template was created after the images were normalized using the high dimensional DARTEL algorithm. Spatial smoothing was conducted with a 6 mm FWHM after the template was normalized into the standard Montreal Neurological Institute (MNI) space. A factorial design module with two factors (i.e., groups with three levels and time with two levels) was applied to explore the group differences. Age and gender were also included in the model as covariates. An absolute threshold of 0.1 was used for masking (Tao et al., 2017c). Total intracranial volume was obtained by summing up the overall volumes of gray matter, white matter, and cerebrospinal fluid. Then, we extracted the average GMV values of all voxels with the overlapping brain masks (i.e., right ACC, right amygdala, and right insula) in the three groups derived from functional connectivity analysis. ANCOVA was applied to compare changes of the three groups (post-treatment minus pre-treatment) with age and gender as covariates (Dimitrov and Rumrill, 2003).
Sixty-nine MCI patients completed the baseline behavioral test and MRI scan, and 57 subjects finished the study and were included in data analysis (Baduanjin group n = 20, brisk walking group n = 17, and health education control group n = 20). Three subjects dropped out of the Baduanjin group (one subject withdrew voluntarily, and two were unwilling to participate in the second MRI scan). Six subjects dropped out of the brisk walking group (one withdrew voluntarily, one was unwilling to participate in the second MoCA test, one was lost to follow-up, and three had poor-quality MRI data). Three subjects dropped out of the control group (one was lost to follow-up, and two were unwilling to participate in the second MoCA test). No subject reported taking additional pharmacological treatment during the training. The attendance rates (mean ± SD) for the Baduanjin and brisk walking groups were 0.842 ± 0.100 and 0.838 ± 0.116, respectively, and there were no significant differences between the two exercise groups in terms of attendance rate (p = 0.927).
Baseline characteristics and MoCA scores of participants are listed in Table 1. There were no significant differences in age, gender, education, score on the Geriatric depression scale (GDS), or MoCA scores among the three groups at baseline (three-group comparison p-Value as follows: Age: p = 0.395; Gender: p = 0.563; Education: p = 0.675; GDS: p = 0.12; pre-MoCA: p = 0.141).
ANCOVA showed a significant difference in MoCA score changes (post-treatment minus pre-treatment) among the three groups (F2,50 = 4.311; p = 0.019). Post hoc analysis showed that the Baduanjin group had a significant increase in MoCA scores compared to the health education control group (p = 0.05) and the brisk walking group (p = 0.037) after Sidak correction. There was no significant difference between the brisk walking group and the control group (p = 0.989, Table 1).
In present study, we used T2 imaging data to check whether the participants have underlying brain diseases, such as brain tumors or lesions. No obvious brain abnormalities were detected. No subject was excluded due to the head movement in the study.
Seed-to-voxel rsFC analysis using the left LC as the seed showed increased rsFC in the bilateral temporoparietal junction (TPJ), right insula, inferior frontal gyrus, supplemental motor area, and postcentral gyrus in the Baduanjin group compared to the control group. The analysis also showed increased rsFC in the right dorsolateral prefrontal cortex and decreased rsFC in the bilateral cerebellum exterior in the Baduanjin group compared to the brisk walking group. There was no other significant group difference at the threshold we set (Figures 1A1,A2,B and Table 2).
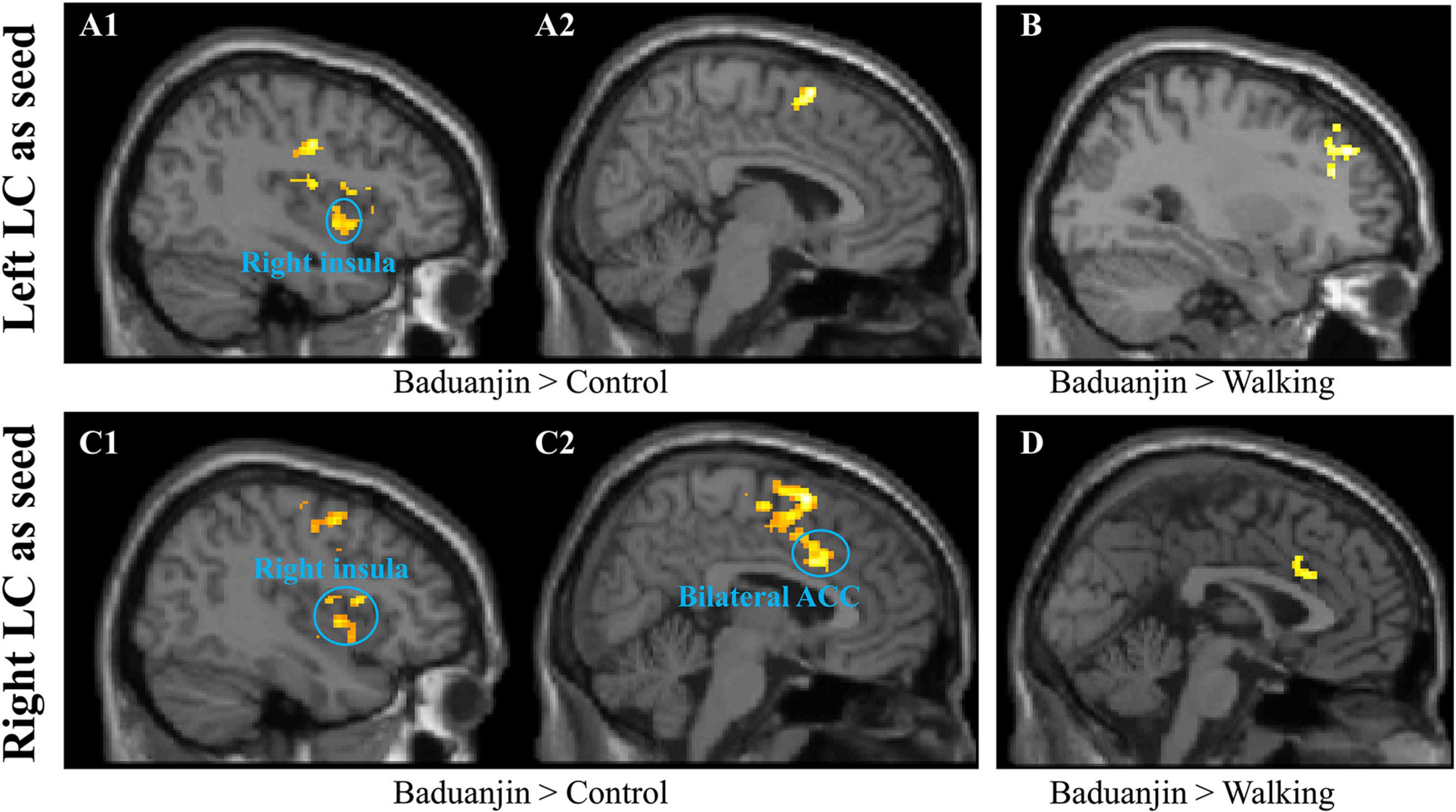
Figure 1. Resting state functional connectivity results using the LC as the seed. (A1,A2,B): using the left LC as the seed. (A1,A2), Baduanjin > Control; (B) Baduanjin > Brisk Walking. (C1,C2,D), using the right LC as the seed. (C1,C2), Baduanjin > Control; (D) Baduanjin > Brisk Walking.
Seed-to-voxel rsFC analysis using the right LC as the seed showed that Baduanjin group is associated with increased rsFC in the bilateral ACC, bilateral precentral gyrus, right insula, amygdala, TPJ, supplemental motor area, and superior temporal gyrus compared to the control group, as well as increased rsFC in the right ACC compared to the brisk walking group. Compared to the Baduanjin group, there was significantly increased rsFC in the right supplemental motor cortex, inferior occipital cortex, bilateral precentral & postcentral cortex, left middle occipital cortex, and right cerebellum in the brisk walking group. There was no other significant group difference at the threshold we set (Figures 1C1,C2,D and Table 2).
Interestingly, we found an overlapping increased right LC-right ACC rsFC in the Baduanjin group compared to the control and brisk walking groups. We also found that both the left and right LC are associated with increased rsFC with the right insula in the Baduanjin group compared to the control group. We extracted the average z-Values of the two rsFCs associated with the right LC (rsFC of right ACC-right LC and rsFC of right insula-right LC) in the three groups after treatment and performed a multiple regression analysis including age and gender as covariates. We found a significant positive association between rsFC z-Values at the right ACC and right insula (right ACC: r = 0.265, p = 0.046; right insula: r = 0.277, p = 0.037) and corresponding MoCA scores after FDR correlation across all subjects in three groups.
Seed-to-voxel analysis using the left VTA as the seed showed significantly increased rsFC in the bilateral anterior insula, putamen, caudate; right amygdala, orbital frontal gyrus, postcentral gyrus; and left nucleus accumbent and superior parietal gyrus in the Baduanjin group compared to the control group. Analysis also showed significantly decreased rsFC in the left posterior cingulate cortex in the Baduanjin group compared to the control group and increased rsFC in the bilateral ACC compared to the brisk walking group. We also found significantly decreased rsFC in the left precuneus in the brisk walking group compared to the control group. We did not find any other significant group differences above the threshold we set (Table 2).
Seed-to-voxel analysis using the right VTA as the seed showed an increased rsFC in the right TPJ in the Baduanjin group compared to the brisk walking group. There was also a decreased rsFC in the bilateral precuneus in the brisk walking group compared to the control group. No other significant group difference was found at the threshold we set (Table 2).
We found that both the right LC and left VTA are associated with increased rsFC with the right insula and right amygdala in the Baduanjin group as compared to the control group (the rsFCs of right insula-right LC/left VTA; the rsFCs of right amygdala-right LC/left VTA). We also found an overlapping brain region in the right ACC in the two rsFCs analyses (i.e., the rsFC of right LC-right ACC and the rsFC of left VTA-bilateral ACC) in the Baduanijn group compared to the brisk walking group (Figures 2A-C).
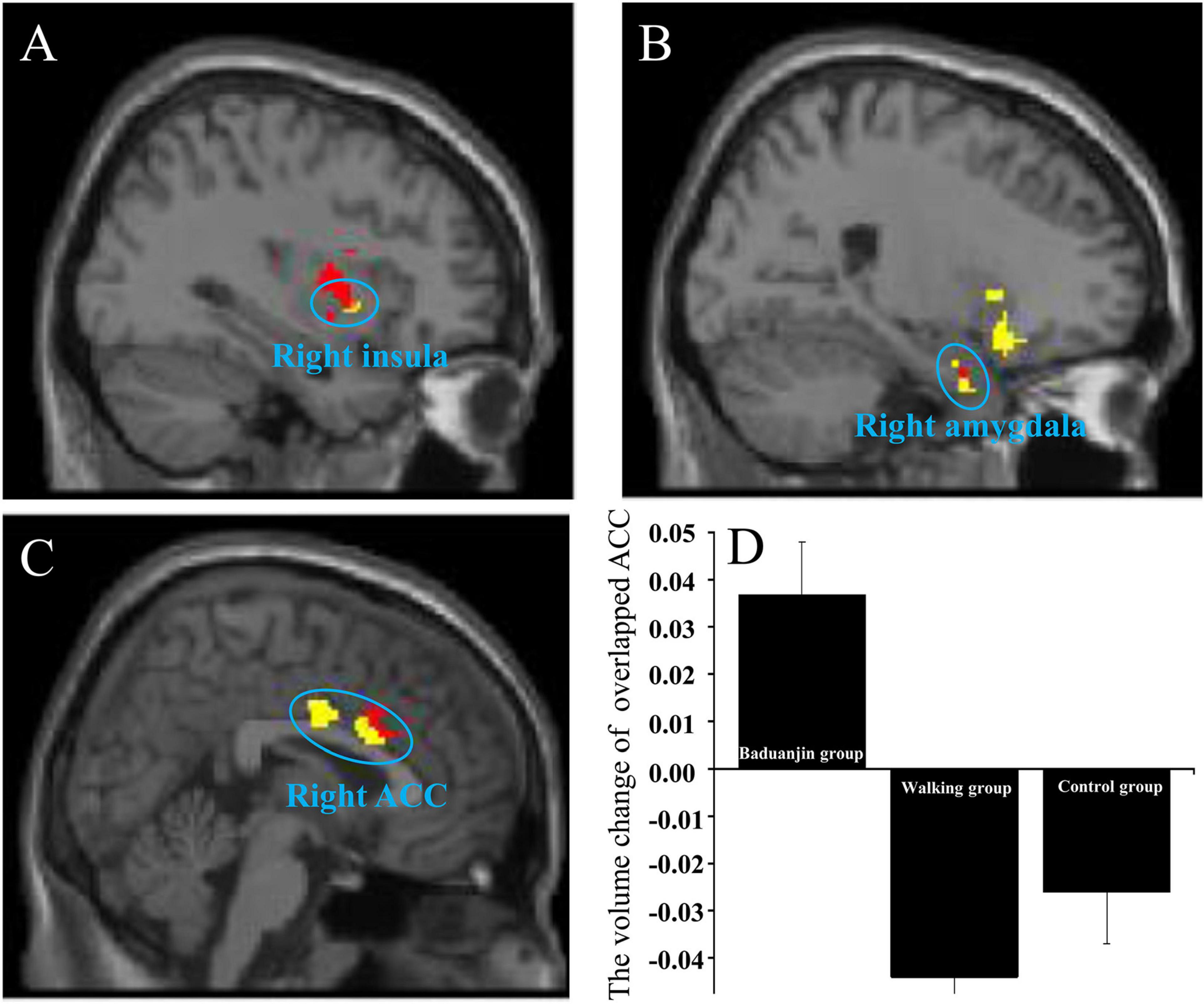
Figure 2. The increased overlapping rsFC in the Baduanjin group between the right LC and left VTA compared to the control group/walking group and the VBM analysis of the overlapping brain region. (A) the overlapping brain region in the right insula; (B) the overlapping brain region in the right amygdala; (C) the overlapping brain region in the right ACC; Red, using the right LC as the seed; yellow, using the left VTA as the seed; (D) the ROI VBM analysis in the right ACC among the three groups.
Random Effects Bayesian model analysis showed that the fully connected model was the best model at each condition (i.e., pre-Baduanjin, post-Baduanjin, pre-walking, post-walking, pre-control, post-control) in the “right LC model,” “left VTA model” (Figures 3A,B), and “right LC and left VTA model” (Supplementary Figure 1B, see the Supplementary Materials for details).
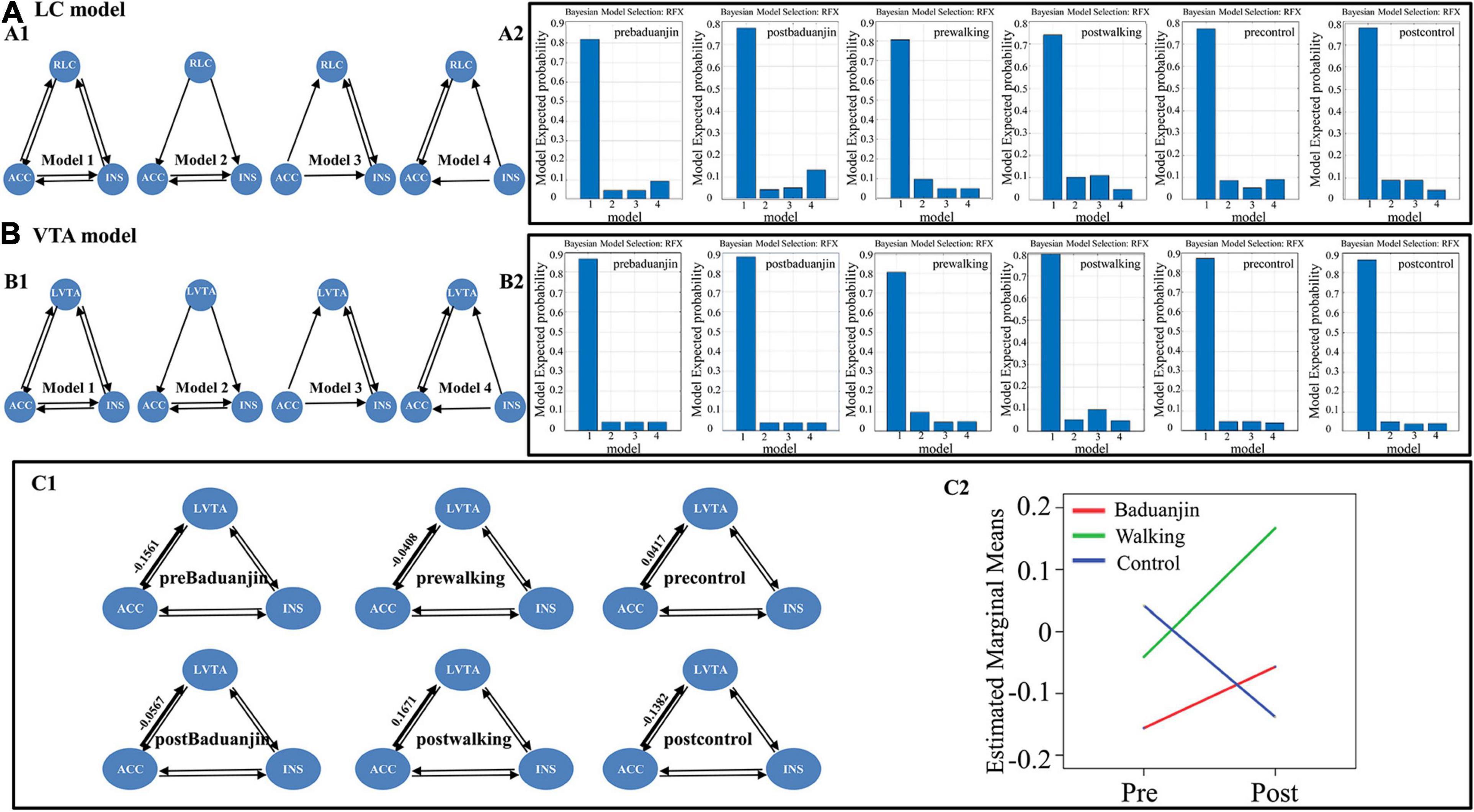
Figure 3. The “right LC model” and “left VTA model” setup, the winning sub-model at the condition level in the “right LC model” and “left VTA model,” and the group and time interaction of right ACC to left VTA effective connectivity. (A) The “right LC model” and the winning sub-model at the condition level in the “right LC model”. (A1) right LC model setup: model 1-4, sub-model of the “right LC model,” model 1-full connective model, model 2- right LC predominantly affected the others, model 3- right ACC predominantly affected the others, model 4-right insula predominantly affected the others; (A2) the winning sub-model of the “right LC model” in each condition (i.e., pre-Baduanjin, post-Baduanjin, pre-walking, post-walking, pre-control, post-control); (B) The “left VTA model” and the winning sub-model at the condition level in the “left VTA model”. (B1) Left VTA model setup: model 1-4, sub-model of “left VTA model,” model 1-full connective model, model 2-left VTA predominantly affected the others, model 3-rigth ACC predominantly affected the others, model 4-right insula predominantly affected the others; (B2) the winning sub-model of the “left VTA model” in each condition (i.e., pre-Baduanjin, post-Baduanjin, pre-walking, post-walking, pre-control, post-control). (C) Mean connection strengths and the group and time interaction of right ACC to left VTA effective connectivity. (C1) Mean connection strengths for the effective connectivity of right ACC to left VTA. (C2) The group and time interaction for the effective connectivity of right ACC to left VTA. RLC: right locus coeruleus; LVTA: left ventral tegmental area; ACC: right overlapped anterior cingulate cortex; INS: right overlapped insula.
We then performed the repeated measures analysis in each pair of endogenous connectivity (DCM.Ep.A) of the full model. No significant group and time interaction was found in the “right LC model” and “right LC and left VTA” model in all effective connectivity (see Table 3 and Supplementary Tables 1, 2; for the right LC model, the group∗time interaction P-value).
We found increased effective connectivity from the right ACC to the left VTA in the Baduanjin and brisk walking groups and decreased effective connectivity in the control group after intervention, with significant group and time interactions (p = 0.041) in the “left VTA model.” No other significant effective connectivity group and time interaction was found in the “left VTA model” (Figures 3C1, C2, Table 3, and Supplementary Table 1).
We compared VBM changes (post-treatment minus pre-treatment) at the right ACC, right insula, and right amygdala after different treatments and found a significant group difference in right ACC GMV (F2,50 = 14.076; p < 0.001). Post hoc analysis revealed increased GMV in the right ACC of the Baduanjin group compared to the control group (p < 0.001) and brisk walking group (p < 0.001), but no significant group difference between the brisk walking and control groups (p = 0.621) after Sidak correction (Figure 2D). No significant GMV difference was found in the right insula and right amygdala among groups (p > 0.05).
In the present study, we investigated the modulation effects of 6 months of Baduanjin on LC and VTA system in patients with MCI. We found that Baduanjin can significantly modulate the rsFC of the LC and VTA and increase gray matter volume of the right ACC compared to brisk walking and health education. In addition, we found a significant group and time interaction in the effective connectivity from right ACC to left VTA. Our results support our research hypothesis that Baduanjin can modulate intrinsic functional connectivity of norepinephrine (LC) and dopamine (VTA) systems.
Our findings that Baduanjin can significantly improve the cognitive function in patients with MCI are consistent with findings from a previous study that endorsed the treatment effects of mind-body exercise on MCI (Sungkarat et al., 2018), as well as our previous studies demonstrating that Baduanjin can prevent memory decline in healthy elders (Tao et al., 2017a,b,c). Interestingly, we did not find significant cognitive function improvement in the brisk walking group compared to the health education group. Some studies have suggested a positive effect of physical exercise on improving cognitive function or preventing cognitive decline (Baker et al., 2010; Brasure et al., 2018). However, a recent clinical study showed that intense aerobic exercise and strength exercise training resulted in no significant group differences in slowing cognitive impairment in people with mild to moderate dementia (Sarah et al., 2018). Further studies are needed to confirm our results.
We found that, compared to the brisk walking group, Baduanijn exercise significantly increased the rsFC between the right ACC and left VTA/right LC. The gray matter volume of the right ACC was significantly increased after 6 months of Baduanjin exercise compared to the brisk walking and health education groups. These findings are consistent with previous studies indicating the role of the ACC in cognition as well as a previous study based on the same data set in which we found increased amplitude of low-frequency fluctuations values (a measurement of brain low-frequency oscillations) in the bilateral ACC in the Baduanjin group compared to the control group (Tao et al., 2019).
Previous studies have found that ACC activation is involved in a number of cognitive processes, including decision making, model updating, and outcome-related activity (Kolling et al., 2016). Animal studies have suggested that the ACC provides prominent direct input to the LC, and that the connectivity between the LC and ACC is involved in cognitive processes like reinforcement learning (Aston-Jones and Cohen, 2005). The ACC may also be able to exert direct top-down control over the VTA (Thomas and David, 2017; Wang S. et al., 2017).
A previous study found that LC-NE and VTA-dopamine systems may interact synergistically to implement an auto-annealing reinforcement learning mechanism (Aston-Jones and Cohen, 2005). This finding aligns with our results, which showed overlapping rsFC in the ACC between the left LC and right VTA. Our results suggest that the ACC may play an important role in Baduanjin modulation by linking LC-NE and VTA- dopamine systems.
In addition, we found that the exercise groups had an increased effective connectivity from the right ACC to the left VTA and a marginally significant increase from the right ACC to the right LC (p = 0.066) compared to the control group (Table 3 and Supplementary Table 1). Effective connectivity analysis using DCM (Friston et al., 2003) can describe the causal relationships between brain regions in the functional MRI data (Friston and Penny, 2011; Sharaev et al., 2016). Specifically, effective connectivity can measure the influence that one neuronal system/brain region exerts on another (Sharaev et al., 2016). Recently, the method has been applied to investigate the modulation effect of mind-body intervention (Kong et al., 2021). Previous studies have suggested that the ACC has reciprocal anatomical connections with both the LC and VTA (Leichnetz and Astruc, 1976; Carmichael and Price, 1995). We found that the ACC has an increased causal influence on the VTA and LC (marginal significant) after exercise. This suggests that the ACC may play a major role in the modulation effect of exercise in patients with MCI.
The insula is a key member of the salience network and plays a role in regulating mental allocation (Ullsperger et al., 2010). Previous studies have suggested that anatomical connection between the LC and anterior insula may be associated with the processing of unexpected events (Dayan and Yu, 2006; Ullsperger et al., 2010). Hämmerer et al. (2018) found that older adults perform worse in a salient stimulation memory task than young adults. In addition, the insula is a dopaminergic region that functions to process stimulus salience and motivation, integrating information about task salience and regulating VTA excitability in response to reward (Rigoli et al., 2016). The above evidence suggests that the regulatory effect of the insula in cognitive function (especially in memory processing) of elders may be associated with the VTA and LC. In addition, studies have suggested that the structure and function of the insula can be significantly modulated by mindfulness training (Hodie lzel et al., 2008; Ives-Deliperi et al., 2011). We speculate that the increased rsFC between the VTA/LC and insula after Baduanjin training may represent the potential modulation of interoceptive awareness skills and salience event processing.
We also found an overlapping increased rsFC in the amygdala between the VTA and LC after Baduanjin exercise. Literature suggests that the amygdala plays a key role in the interaction of emotion and cognition (Phelps, 2006) and has a direct anatomical connection with the VTA-dopaminergic system (Thomas et al., 2018) and LC-noradrenergic system (Mohammed et al., 2016). A series of studies has suggested that stress affects the structure of the amygdala (Mitra et al., 2005; McEwen et al., 2016). Thus, Baduanjin may relieve stress and negative emotion (a key risk factor for the development of MCI) by modulating the interaction between the LC and VTA in the amygdala and further improving cognitive function.
We found increased rsFC of the LC-TPJ and VTA-TPJ in the Baduanjin group compared to both the control group and brisk walking group. The TPJ plays a key role in memory processing and social cognition (Carter and Huettel, 2013). Both the LC and TPJ are involved in executive and attentional processes (Laureiro-Martínez et al., 2015), and the functional connectivity between the TPJ and reward-related regions (such as the VTA) is involved in episodic encoding (Sugimoto et al., 2016). These results indicate that the TPJ may also be involved in the modulation effect of Baduanjin in cognition.
There are several limitations to our study. First, we did not evaluate levels of norepinephrine and dopamine directly. Further studies are needed to assess these neurotransmitter levels with neuroimaging techniques. Additionally, our sample size in this study was relatively small. Studies with larger sample sizes should be conducted to confirm our findings. Finally, we only measured global cognitive function via MoCA, and further research should be conducted to explore the treatment effects of Baduanjin on cognitive functions like memory and attention in patients with MCI.
In summary, we found that 6 months of Baduanjin exercise can significantly improve cognitive function compared to brisk walking and health education. In addition, Baduanjin can yield increases in LC and VTA rsFC with the ACC, insula, amygdala, and TPJ, increases in the effective connection of the ACC to LC/VTA, and increases in the gray matter volume of the ACC. Our findings suggest that Baduanjin may improve cognitive function in patients with MCI by modulating brain function and structure associated with the norepinephrine (LC) and dopamine (VTA) systems.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Medical Ethics Committee of the Second People’s Hospital of Fujian Province. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
GZ and LC designed the experimental. RX, ML, MH, and SL contributed to the data collection. JL, JK, and JT contributed to the data analysis. JL, JK, JT, XC, GW, and JP prepared the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (grant no. 81574045), the Collaboration Innovation Center for Rehabilitation Technology (2015003-Collaboration), the Fujian Provincial Rehabilitation Industrial Institution, and the Fujian Key Laboratory of Rehabilitation Technology.
JK has a disclosure to report (holding equity in a MNT, and pending patents to develop a new brain stimulation device).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank instructors Hongmei Yi and Qiu Lin from the Fujian University of Traditional Chinese Medicine for the Baduanjin training. The content of this manuscript has been presented (in part) at the 14th ISPRM World Congress and 53rd AAP Annual Meeting (ISPRM2020, Orlando FL, United States).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2021.646807/full#supplementary-material
Supplementary Figure 1 | The “right LC and left VTA model” setup and the winning sub-model at the condition level in the “right LC and left VTA model.” (A) model 1-5, sub-model of the “right LC and left VTA model,” model 1-full connective model, model 2-left VTA predominantly affected the others, model 3-right ACC predominantly affected the others, model 4-right insula predominantly affected the others; model 5-right LC predominantly affected the others; (B) the winning sub-model at the condition level in the “right LC and left VTA model.” RLC: right locus coeruleus; LVTA: left ventral tegmental area; ACC: right overlapped anterior cingulate cortex; INS: right overlapped insula.
Supplementary Figure 2 | The number of subjects in each condition in each sub-model in the “right LC model,” “left VTA model,” and “right LC and left VTA model.”
Supplementary Table 1 | Mean and Standard deviations of connection strengths.
Supplementary Table 2 | The group and time interaction in the effective connectivity (“right LC and left VTA” model).
MCI, mild cognitive impairment; MoCA, Montreal Cognitive Assessment; MRI, magnetic resonance imaging; LC, locus coeruleus; VTA, ventral tegmental area; rsFC, resting state functional connectivity; ACC, anterior cingulate cortex; GMV, gray matter volume; VBM Voxel-Based Morphometry; TPJ, temporoparietal junction; DLPFC, dorsolateral prefrontal cortex.
Adcock, R. A., Thangavel, A., Whitfield-Gabrieli, S., Knutson, B., and Gabrieli, J. D. E. (2006). Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron 50, 507–517. doi: 10.1016/j.neuron.2006.03.036
Andrew, S., Chelsea, K., Eric, I., Tony, L., Steven, H., Richard, H., et al. (2018). A multi-modal MRI study of the central response to inflammation in rheumatoid arthrit. Nat. Commun. 9:2243.
Aston-Jones, G., and Cohen, J. D. (2005). An interative theory of locus coeruleus norepinephrien function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450. doi: 10.1146/annurev.neuro.28.061604.135709
Baker, L., Frank, L., Foster, K., Green, P., Wilkinson, C., McTiernan, A., et al. (2010). Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch. Neurol. 67, 71–79.
Bär, K. J., De la Cruz, F., Schumann, A., Koehler, S., Sauer, H., Critchley, H., et al. (2016). Functional connectivity and network analysis of midbrain and brainstem nuclei. Neuroimage 134, 53–63. doi: 10.1016/j.neuroimage.2016.03.071
Brasure, M., Desai, P., Davila, H., Nelson, V. A., Calvert, C., Jutkowitz, E., et al. (2018). Physical activity interventions in preventing cognitive decline and alzheimer-type dementia: a systematic review. Ann. Intern. Med. 168, 30–38. doi: 10.7326/m17-1528
Burgener, S. C., Yang, Y., Gilbert, R., and Marsh-Yant, S. (2008). The effects of a multimodal intervention on outcomes of persons with early-stage dementia. Am. J. Alzheimers. Dis. Other Demen. 23, 382–394. doi: 10.1177/1533317508317527
Carmichael, S. T., and Price, J. L. (1995). Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 363, 615–641. doi: 10.1002/cne.903630408
Carter, R. M., and Huettel, S. A. (2013). A nexus model of the temporal-parietal junction. Trends Cogn. Sci. 17, 328–336. doi: 10.1016/j.tics.2013.05.007
Dayan, P., and Yu, A. J. (2006). Phasic norepinephrine: a neural interrupt signal for unexpected events. Netw. Comput. Neural Syst. 17, 335–350. doi: 10.1080/09548980601004024
Dimitrov, D., and Rumrill, P. (2003). Pretest-posttest designs and measurement of change. Work 20, 159–165.
Fan, Y., Wang, Y., Ji, W., Liu, K., and Wua, H. (2021). Exercise preconditioning ameliorates cognitive impairment and anxiety-like behavior via regulation of dopamine in ischemia rats. Physiol. Behav. 233:113353. doi: 10.1016/j.physbeh.2021.113353
Friston, K. J., Harrison, L., and Penny, W. (2003). Dynamic causal modelling. Neuroimage 19, 1273–1302. doi: 10.1016/s1053-8119(03)00202-7
Friston, K., and Penny, W. (2011). Post hoc Bayesian model selection. Neuroimage 56, 2089–2099. doi: 10.1016/j.neuroimage.2011.03.062
Gollub, R. L., Kirsch, I., Maleki, N., Wasan, A. D., Edwards, R. R., Tu, Y., et al. (2018). A functional neuroimaging study of expectancy effects on pain response in patients with Knee Osteoarthritis. J. Pain 19, 515–527. doi: 10.1016/j.jpain.2017.12.260
Hämmerer, D., Callaghan, M. F., Hopkins, A., Kosciessa, J., Betts, M., Cardenas-Blanco, A., et al. (2018). Locus coeruleus integrity in old age is selectively related to memories linked with salient negative events. Proc. Natl. Acad. Sci. 115, 2228–2233. doi: 10.1073/pnas.1712268115
Health Qigong Management Center of General Administration of Sport of China. (2003). Health Qigong-Baduanjin. Beijing: People’s Sports Publishing House of China.
Hodie lzel, B. K., Ott, U., Gard, T., Hempel, H., Weygandt, M., Morgen, K., et al. (2008). Investigation of mindfulness meditation practitioners with voxel-based morphometry. Soc. Cogn. Affect. Neurosci. 3, 55–61. doi: 10.1093/scan/nsm038
Huang, Y. T., Yu, S. Y., Wilson, G., Cheng, M., Kong, X. J., Lu, T., et al. (2021). Altered extended locus coeruleus and ventral tegmental area networks in boys with autism spectrum disorders: a resting-state functional connectivity study. Neuropsychiatric Dis. Treat. 17, 1207–1216. doi: 10.2147/ndt.s301106
Ives-Deliperi, V. L., Solms, M., and Meintjes, E. M. (2011). The neural substrates of mindfulness: an fMRI investigation. Soc. Neurosci. 6, 231–242. doi: 10.1080/17470919.2010.513495
Jacobs, H. I. L., Müller-Ehrenberg, L., Priovoulos, N., and Roebroeck, A. (2018). Curvilinear locus coeruleus functional connectivity trajectories over the adult lifespan: a 7T MRI study. Neurobiol. Aging 69, 167–176. doi: 10.1016/j.neurobiolaging.2018.05.021
Koch, G., Motta, C., Bonnì, S., Pellicciari, C., Picazio, S., Casula, E. P., et al. (2020). Effect of rotigotine vs placebo on cognitive functions among patients with mild to moderate alzheimer disease a randomized clinical trial. JAMA Netw. Open 3:e2010372. doi: 10.1001/jamanetworkopen.2020.10372
Kolling, N., Wittmann, M., Behrens, T., Boorman, E., Mars, R., and Rushworth, M. (2016). Value, search, persistence and model updating in anterior cingulate cortex. Nat. Neurosci. 19, 1280–1285. doi: 10.1038/nn.4382
Kong, J., Huang, Y., Liu, J., Yu, S., Ming, C., Chen, H., et al. (2021). Altered functional connectivity between hypothalamus and limbic system in fibromyalgia. Mol. Brain 14:7.
Lammel, S., Lim, B. K., and Malenka, R. C. (2014). Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 76, 351–359. doi: 10.1016/j.neuropharm.2013.03.019
Laureiro-Martínez, D., Brusoni, S., Canessa, N., and Zollo, M. (2015). Understanding the exploration-exploitation dilemma: an fMRI study of attention control and decision-making performance. Strateg. Manag. J. 36, 319–338. doi: 10.1002/smj.2221
Lee, T. H., Greening, S. G., Ueno, T., Clewett, D., Ponzio, A., Sakaki, M., et al. (2018). Arousal increases neural gain via the locus coeruleus-norepinephrine system in younger adults but not in older adults. Nat. Hum. Behav. 2:356. doi: 10.1038/s41562-018-0344-1
Leichnetz, G. R., and Astruc, J. (1976). The efferent projections of the medial prefrontal cortex in the squirrel monkey (Saimiri sciureus). Brain Res. 109, 455–472. doi: 10.1016/0006-8993(76)90027-5
Li, L., Li, B., Bai, Y., Liu, W., Wang, H., Leung, H. C., et al. (2017). Abnormal resting state effective connectivity within the default mode network in major depressive disorder: a spectral dynamic causal modeling study. Brain Behav. 7:e00732. doi: 10.1002/brb3.732
Liao, Y., Lin, Y., Zhang, C., Xue, L., Mao, X., Zhang, Y., et al. (2015). Intervention effect of baduanjin exercise on the fatigue state in people with fatigue-predominant subhealth: a cohort study. J. Altern. Complement. Med. 21, 554–562. doi: 10.1089/acm.2014.0395
Liu, J., Chen, D., Chen, L., Hu, K., Tu, X., Lin, Q., et al. (2019a). Modulation effects of different exercise modalities on the functional connectivity of the periaqueductal grey and ventral tegmental area in patients with knee osteoarthritis – a randomized multi-modal MRI study. Br. J. Anesth. 123, 506–518. doi: 10.1016/j.bja.2019.06.017
Liu, J., Chen, L., Tu, Y., Chen, X., Hu, K., Tu, Y., et al. (2019b). Different exercise modalities relieve pain syndrome in patients with knee osteoarthritis and modulate the dorsolateral prefrontal cortex: a multiple mode MRI study. Brain. Behav. Immun. 82, 253–263. doi: 10.1016/j.bbi.2019.08.193
Liu, J., Tao, J., Liu, W., Huang, J., Xue, X., Li, M., et al. (2019c). Different modulation effects of tai chi chuan and baduanjin on resting state functional connectivity of the default mode network in older adults. Soc. Cogn. Affect. Neurosci. 14, 217–224. doi: 10.1093/scan/nsz001
Liu, K., Marijatta, F., Hämmerer, D., Acosta-Cabronero, J., Düzel, E., and Howard, R. (2017). Magnetic resonance imaging of the human locus coeruleus: a systematic review. Neurosci. Biobehav. Rev. 83, 325–355. doi: 10.1016/j.neubiorev.2017.10.023
McEwen, B. S., Nasca, C., and Gray, J. D. (2016). Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41, 3–23. doi: 10.1038/npp.2015.171
Mitra, R., Jadhav, S., McEwen, B. S., Vyas, A., and Chattarji, S. (2005). Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. 102, 9371–9376. doi: 10.1073/pnas.0504011102
Mohammed, M., Kulasekara, K., Ootsuka, Y., and Blessing, W. W. (2016). Locus coeruleus noradrenergic innervation of the amygdala facilitates alerting-induced constriction of the rat tail artery. Am. J. Physiol. Integr. Comp. Physiol. 310, R1109–R1119.
Naidich, T. P., Duvernoy, H. M., Delman, B. N., Sorensen, A. G., Kollias, S. S., and Haacke, E. M. (2009). Duvernoy’s Atlas of the Human Brain Stem and Cerebellum: High-Field MRI, Surface Anatomy, Internal Structure, Vascularization and 3 D Sectional Anatomy. Berlin: Springer.
Navratilova, E., Atcherley, C. W., and Porreca, F. (2015). Brain circuits encoding reward from pain relief. Trends Neurosci. 38, 741–750. doi: 10.1016/j.tins.2015.09.003
Nobili, A., Latagliata, E. C., Viscomi, M. T., Cavallucci, V., Cutuli, D., Giacovazzo, G., et al. (2017). Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat. Commun. 8:14727.
Petersen, R. (2004). Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 256, 183–194. doi: 10.1111/j.1365-2796.2004.01388.x
Petersen, R. C., Lopez, O., Armstrong, M. J., Getchius, T. S. D., Ganguli, M., Gloss, D., et al. (2018). Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the american academy of neurology. Neurology 90, 126–135. doi: 10.1212/wnl.0000000000004826
Phelps, E. A. (2006). Emotion and cognition: insights from studies of the human amygdala. Annu. Rev. Psychol. 57, 27–53. doi: 10.1146/annurev.psych.56.091103.070234
Ranjbar-Slamloo, Y., and Fazlali, Z. (2020). Dopamine and noradrenaline in the brain; overlapping or dissociate functions? Front. Mol. Neurosci. 12:334.
Rigoli, F., Chew, B., Dayan, P., and Dolan, R. J. (2016). Multiple value signals in dopaminergic midbrain and their role in avoidance contexts. Neuroimage 135, 197–203. doi: 10.1016/j.neuroimage.2016.04.062
Sarah, L., Bart, S., Nicky, A., Vivien, N., Helen, C., Dipesh, M., et al. (2018). Dementia and physical activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ 361:k1675. doi: 10.1136/bmj.k1675
Sharaev, M. G., Zavyalova, V. V., Ushakov, V. L., Kartashov, S. I., and Velichkovsky, B. M. (2016). Effective connectivity within the default mode network: dynamic causal modeling of resting-state fMRI data. Front. Hum. Neurosci. 10:14.
Stephan, K. E., Penny, W. D., Daunizeau, J., Moran, R. J., and Friston, J. K. (2009). Bayesian model selection for group studies. Neuroimage 46, 1004–1017.
Sugimoto, H., Shigemune, Y., and Tsukiura, T. (2016). Competing against a familiar friend: interactive mechanism of the temporo-parietal junction with the reward-related regions during episodic encoding. Neuroimage 130, 261–272. doi: 10.1016/j.neuroimage.2016.02.020
Sungkarat, S., Boripuntakul, S., Kumfu, S., Lord, S., and Chattipakorn, N. (2018). Tai chi improves cognition and plasma BDNF in older adults with mild cognitive impairment: a randomized controlled trial. Neurorehabil. Neural Repair 32, 142–149. doi: 10.1177/1545968317753682
Takahashi, J., Shibata, T., Sasaki, M., Kudo, M., Yanezawa, H., Obara, S., et al. (2014). Detection of changes in the locus coeruleus in patients with mild cognitive impairment and Alzheimer’s disease: high-resolution fast spin-echo T1-weighted imaging. Geriatr. Gerontol. Int. 15, 334–340. doi: 10.1111/ggi.12280
Tang, Y. Y., Hölzel, B. K., and Posner, M. I. (2015). The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 16, 213–225.
Tao, J., Chen, L., Egorova, N., Liu, J., Xue, H., Wang, Q., et al. (2017a). Tai Chi Chuan and Baduanjin practice modulates functional connectivity of the cognitive control network in older adults. Sci. Rep. 7:41581.
Tao, J., Chen, L., Liu, J., Egorova, N., Xue, H., Liu, L., et al. (2017b). Tai Chi Chuan and Baduanjin mind-body training changes resting-state low-frequency fluctuations in the frontal lobe of older adults: a resting-state fMRI study. Front. Hum. Neurosci. 11:54.
Tao, J., Liu, J., Chen, X., Xia, R., Li, M., Huang, M., et al. (2019). Mind-body exercise improves cognitive function and modulates the function and structure of the hippocampus and anterior cingulate cortex in patients with mild cognitive impairment. NeuroImage Clin. 23:101834. doi: 10.1016/j.nicl.2019.101834
Tao, J., Liu, J., Egorova, N., Chen, L., Sun, S., Xue, H., et al. (2016). Increased hippocampus–medial prefrontal cortex resting state functional connectivity and memory function after Tai Chi Chuan practice in elder adults. Front. Aging Neurosci. 8:25.
Tao, J., Liu, J., Liu, L., Huang, J., Xue, H., Chen, L., et al. (2017c). Tai Chi Chuan and Baduanjin increase grey matter volume in older adults: a brain imaging study. J. Alzheimer’s Dis. 60, 389–400. doi: 10.3233/jad-170477
Thomas, E., and David, B. (2017). Neural correlate of relief in the anterior cingulate cortex and ventral tegmental area. Cell Rep. 19, 2220–2230. doi: 10.1016/j.celrep.2017.05.062
Thomas, T. S., Baimel, C., and Borgland, S. L. (2018). Opioid and hypocretin neuromodulation of VTA neuronal subpopulations. Br. J. Pharmacol. 175, 2825–2833. doi: 10.1111/bph.13993
Ullsperger, M., Harsay, H. A., Wessel, J. R., and Ridderinkhof, K. R. (2010). Conscious perception of errors and its relation to the anterior insula. Brain Struct. Funct. 214, 629–643. doi: 10.1007/s00429-010-0261-1
Unsworth, N., and Robison, M. (2017). A locus coeruleus-norepinephrine account of individual differences in working memory capacity and attention control. Psychon. Bull. Rev. 24, 1282–1311. doi: 10.3758/s13423-016-1220-5
Wang, S., Hu, S. H., Shi, Y., and Li, B. M. (2017). The roles of the anterior cingulate cortex and its dopamine receptors in self-paced cost–benefit decision making in rats. Learn. Behav. 45, 89–99. doi: 10.3758/s13420-016-0243-0
Wang, Z., Wang, X., Liu, J., Chen, J., Liu, X., Nie, G., et al. (2017). Acupuncture treatment modulates the corticostriatal reward circuitry in major depressive disorder. J. Psychiatr. Res. 84, 18–26. doi: 10.1016/j.jpsychires.2016.09.014
Whitfield, S., and Nieto, A. (2012). Conn?: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2, 125–141. doi: 10.1089/brain.2012.0073
Xiao, L., Ting, S. J. W., Jun, P. B. W., Ann, T. M., Falong, G., Chong, N. W., et al. (2020). A single-arm feasibility study of community-delivered Baduanjin (Qigong practice of the eight Brocades) training for frail older adults. Pilot Feasibility Stud. 6:105.
Yu, J., Li, J., and Huang, X. (2012). The Beijing version of the montreal cognitive assessment as a brief screening tool for mild cognitive impairment: a community-based study. BMC Psychiatry 12:156.
Yu, L., Liu, F., Nie, P., Shen, C., Chen, J., and Yao, L. (2020). Systematic review and meta-analysis of randomized controlled trials assessing the impact of Baduanjin exercise on cognition and memory in patients with mild cognitive impairment. Clin. Rehabil. 35, 492–505. doi: 10.1177/0269215520969661
Yu, S., Li, W., Shen, W., Edwards, R. R., Gollub, R. L., Wilson, G., et al. (2020). Impaired mesocorticolimbic connectivity underlies increased pain sensitivity in chronic low back pain. Neuroimage 218:116969. doi: 10.1016/j.neuroimage.2020.116969
Zhang, S., Hu, S., Chao, H. H., and Li, C. S. R. (2016). Resting-state functional connectivity of the locus coeruleus in humans: in comparison with the ventral tegmental area/substantia nigra pars compacta and the effects of age. Cereb. Cortex 26, 3413–3427. doi: 10.1093/cercor/bhv172
Keywords: Baduanjin, resting state functional connectivity, mild cognitive impairment, locus coeruleus, ventral tegmental area
Citation: Liu J, Tao J, Xia R, Li M, Huang M, Li S, Chen X, Wilson G, Park J, Zheng G, Chen L and Kong J (2021) Mind-Body Exercise Modulates Locus Coeruleus and Ventral Tegmental Area Functional Connectivity in Individuals With Mild Cognitive Impairment. Front. Aging Neurosci. 13:646807. doi: 10.3389/fnagi.2021.646807
Received: 28 December 2020; Accepted: 11 May 2021;
Published: 14 June 2021.
Edited by:
Notger G. Müller, German Center for Neurodegeneratives, Helmholtz Association of German Research Centers (HZ), GermanyReviewed by:
Henning U. Voss, Cornell University, United StatesCopyright © 2021 Liu, Tao, Xia, Li, Huang, Li, Chen, Wilson, Park, Zheng, Chen and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guohua Zheng, OTEwNjcwMDA4QHFxLmNvbQ==; Lidian Chen, MTk4NDAxMEBmanRjbS5lZHUuY24=; Jian Kong, a29uZ2pAbm1yLm1naC5oYXJ2YXJkLmVkdQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.