
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 10 February 2021
Sec. Alzheimer's Disease and Related Dementias
Volume 13 - 2021 | https://doi.org/10.3389/fnagi.2021.630409
This article is part of the Research Topic Metabolic Alterations in Neurodegenerative Disorders View all 13 articles
 Zhaoyang Tang1†
Zhaoyang Tang1† Xueyu Chen1†
Xueyu Chen1† Wenran Zhang1†
Wenran Zhang1† Xiangfu Sun2
Xiangfu Sun2 Qingzhi Hou1
Qingzhi Hou1 Yuejin Li1
Yuejin Li1 Xia Feng1
Xia Feng1 Yanru Chen1
Yanru Chen1 Jian Lv1
Jian Lv1 Long Ji1*
Long Ji1* Guoyong Ding1*
Guoyong Ding1* Dong Li1,3*
Dong Li1,3*Background: Dementia, as a global public health problem, is becoming increasingly serious. As a precursor of dementia, mild cognitive impairment (MCI) plays an important role in the diagnosis and prevention of dementia. Recent studies have found a correlation between gamma-glutamyl transferase (GGT) levels and cognitive function in men. The relationship between GGT levels and cognitive function in women remains unclear because GGT activity and expression differ between the sexes.
Method: We recruited a total of 2,943 Chinese women from Jidong and Taian in 2019. We grouped the participants according to GGT levels, diagnosed MCI using the Montreal Cognitive Assessment (MOCA) scale, and modeled the study outcomes using logistic regression to explore the relationship between GGT level and MCI. We also analyzed the interaction of obesity, sleep duration, and hyperuricemia with GGT in the development of MCI.
Results: The prevalence of MCI increased with increasing GGT level, from the lowest quartile to the highest quartile of GGT: 8.4% (66/786), 14.2% (119/840), 17.6% (108/613), and 21.4% (151/704), respectively. At the same time, as GGT levels increased, so did the risk of MCI. In the fully adjusted model, compared with those for participants in the lowest GGT quartiles, the odds ratios (ORs), and 95% confidence intervals (CIs) for MCI for participants in the second, third, and fourth GGT quartiles were 1.49 (1.04–2.12), 1.53(1.06–2.21), and 1.88 (1.33–2.65), respectively. The risk of developing MCI was further increased in people with high GGT levels who were obese (OR = 1.96, 95% CI: 1.39–2.76, P < 0.001), slept less (OR = 1.91, 95% CI: 1.35–2.71, P < 0.001), had high levels of uric acid (OR = 1.55, 95% CI: 1.03–2.32, P < 0.001), or after menopause (OR = 2.92, 95% CI: 2.07–4.12, P < 0.001).
Conclusion: We found that MCI is more common in women with elevated GGT levels, so GGT could be a potential diagnostic marker for MCI. Meanwhile, our findings indicated that women with high GGT levels had an increased risk of MCI when they were obese, sleep deprived, had high serum uric acid (SUA) levels or underwent menopause.
With the aging of the population, dementia has become a major global public health problem and caused a huge disease burden (WHO, 2017). The global prevalence of dementia is expected to rise from 30 million in 2010 to 106 million in 2050 (Brookmeyer et al., 2007). Mild cognitive impairment (MCI) refers to the clinical condition between normal aging and dementia in which persons experience memory loss to a greater extent than one would expect for their age, yet they do not meet currently accepted criteria for clinically probable dementia (Petersen, 2001). Therefore, MCI is regarded as the prodromal stage of dementia; when people have MCI, their rate of conversion to dementia will be considerably accelerated compared with that of healthy age-matched individuals (Petersen, 2000; Thompson and Hodges, 2002; Ding et al., 2015).
Gamma-glutamyl transferase (GGT) is routinely used in clinical practice as an indicator of potential hepatic or biliary diseases, as it is mainly found in the liver and plays an important role in maintaining the intracellular concentration of glutathione (Kristenson et al., 1985; Forman et al., 2009; Kunutsor, 2016). Recently, the serum level of GGT has been reported to be associated with vascular diseases, which are considered to have pro-oxidant and proinflammatory properties (Emdin et al., 2005; Kunutsor et al., 2014, 2015; Jeon et al., 2020). However, inflammation and oxidative stress are the most likely pathways leading to cognitive impairment (Zafrilla et al., 2006; Gackowski et al., 2008). Therefore, GGT levels might be associated with an increased risk of dementia. A previous cohort study showed that serum GGT was log-linearly associated with the risk of dementia, and the risk of dementia increases with increasing GGT level in the male population (Kunutsor and Laukkanen, 2016). Another longitudinal study of older adults also showed that increased GGT levels are associated with cognitive decline at the end of life (Praetorius Bjork and Johansson, 2018). However, the result of a population-wide genetic study could not confirm a causal effect of GGT on the risk of dementia (Kunutsor et al., 2018). Therefore, the relationship between GGT and cognitive impairment is far from clear, especially among female individuals, among whom there is a lack of related research.
A systematic review of 75 studies in 2013 showed that the prevalence of dementia was significantly higher in women, with a prevalence rate that was 1.65 times higher among women than men (Chan et al., 2013); as a result, women are more likely to suffer from cognitive impairment. At the same time, an ovariectomized mouse model found that GGT activity might be related to estrogen (Zarida et al., 1994), which suggests that women not only face a higher risk of dementia but also are more sensitive to GGT activity.
In summary, owing to the sex-specific expression of GGT and the unknown relationship between GGT and MCI (Schiele et al., 1977), we collected data on GGT levels, cognitive function and general condition information from 2,943 women from two different Chinese cities to explore the association between GGT and MCI in the female population.
In 2019, a total of 8,166 participants from Jidong (n = 6,154) and Taian (n = 2,012), including 4,655 male participants and 3,511 female participants, underwent health checks. Among the 3,511 female participants, individuals with missing data on GGT, body mass index (BMI), sleep duration, serum uric acid (SUA), Montreal Cognitive Assessment (MOCA) score, or important confounders (n = 355, 10.1%); heavy alcoholism (defined as 30 g/day, n = 25, 0.7%); hepatobiliary and pancreatic diseases including cancer and viral hepatitis (n = 119, 3.3%); and a history of dementia (n = 6, 0.2%) were excluded from this study. In our study, participants with severe cognitive impairment (defined as a chief complaint of memory impairment and a MOCA score <18, n = 66, 1.9%) were excluded, even if they were not diagnosed with dementia. Eventually, a total of 2,943 female participants were included in our study (Figure 1).
The study was conducted in accordance with the guiding principles of the Helsinki Declaration and approved by the Ethics Committee of Jidong Oilfield Inc. Medical Centers and Taian Traffic Hospital. Written informed consent was obtained from all participants.
Previous studies have found that the MOCA scale is more sensitive in MCI screening than other scales, and the specificity is not significantly different from that of other scales (Dong et al., 2010, 2012; Kasten et al., 2010). Therefore, we decided to use the MOCA scale to evaluate MCI. In this study, using the Chinese version of the MOCA scale and cutoffs for the Chinese population. The MOCA scale is a rapid tool for detecting MCI, which is assessed from different cognitive domains, they include attention, memory, executive function, language, visuospatial ability, abstract thinking, numeracy, and orientation. Each MOCA is limited to 10 min, with a maximum score of 30. We defined no subjective cognitive decline and a MOCA score ≥26 as normal (Dong et al., 2010, 2012; Kasten et al., 2010), and complaints of memory loss and a MOCA score of more than 18 and <26 points as MCI (Nasreddine et al., 2005). Considering the effect of education on cognitive function, those with 12 years or less of education had 1 point added to their scores. The MOCA scale was administered by specially trained investigators.
Blood samples were collected by venipuncture from the large antecubital veins in the morning after overnight fasting. All blood samples were stored in vacuum tubes containing ethylenediaminetetraacetic acid (EDTA). GGT and SUA levels were determined using an autoanalyzer (Hitachi 747; Hitachi, Tokyo, Japan) with the kinetic and uricase-peroxidase methods, respectively, in the laboratories of Jidong Oilfield Hospital and Taian Traffic Hospital.
A series of standardized questionnaires, clinical examinations, and laboratory tests were used to collect basic information (Zhang et al., 2013). A standardized questionnaire was administered by well-trained interviewers to collect subjects' information. Demographic variables, including age, sex, education level and histories of hypertension, diabetes mellitus, dyslipidemia and hepatobiliary, and pancreatic diseases, were collected through the questionnaire. We also asked them about their menstrual history to see if they had gone through menopause and their age at menopause. According to self-reported information, alcohol consumption was classified as heavy drinker (alcohol consumption ≥30 g/day), mild-moderate drinker (alcohol consumption <30 g/day) or abstainer (alcohol consumption = 0 g/day) (Yoo et al., 2020), and smoking status was classified as non-smoker or having quit more than 1 year ago or current smoker or having quit <1 year ago. BMI was defined based on the measured height and weight and calculated as weight (kg)/height (m2). Education was categorized into illiterate or primary school, middle school, or university or above. The Pittsburgh Sleep Quality Index Questionnaire was used to measure sleep quality and duration (Mollayeva et al., 2016). Sleep duration was classified as <6, 6–7, 7–8, and >8 h, and adequate sleep or insufficient sleep were defined based on whether the sleep duration was greater than the median of 7 h (Papandreou et al., 2019). Hypertension was defined as the use of antihypertensive drugs, a self-reported history, diastolic blood pressure ≥90 mmHg, or systolic blood pressure ≥140 mmHg. Diabetes mellitus was defined as current treatment with insulin or an oral hypoglycemic agent, presence of a history of diabetes, or fasting blood glucose level ≥7.0 mmol/L (126 mg/dL). Dyslipidemia was defined as current use of lipid-lowering therapy, a self-reported history, or serum levels of triglyceride (TG) ≥1.7 mmol/L, total cholesterol (TC) ≥5.18 mmol/L, high-density lipoprotein (HDL) <1.04 mmol/L, or low-density lipoprotein (LDL) ≥3.37 mmol/L (Lv et al., 2016).
The Kolmogorov-Smirnov test was used to measure the normality of the distribution of the test variables. Continuous variables with normal distributions were expressed as the mean ± standard deviation (SD). Continuous variables that did not have a normal distribution were represented by the mean and interquartile range. Categorical variables were presented as frequencies and percentages. We compared the baseline characteristics of different serum GGT quartile groups using one-way ANOVA or the Kruskal-Wallis rank-sum test for normally or non-normally distributed continuous variables, respectively, and the chi-square test or Fisher's exact test for categorical variables. We also compared the baseline characteristics of groups with and without MCI using Student's t-test or the Mann-Whitney U-test for normally or non-normally distributed continuous variables, respectively, and the chi-square test or Fisher's exact test for categorical variables. Logistic regression model was used to evaluate the association between risk factors (GGT, BMI, sleep duration, SUA, and estrogen level) and MCI by calculating odds ratios (ORs) with 95% confidence intervals (CIs). The 25th, 50th, and 75th percentiles of GGT nodes were selected by using the restricted cubic spline method, and the confounding factors were adjusted by using the 25th percentile (12 U/L) as the reference. A dose-response diagram was also drawn by the GGT levels as horizontal coordinates, and the corresponding OR values as vertical coordinates. Nomogram was based on the results of logistic regression analysis. We used the postestimation Wald test in the multivariable-adjusted logistic model to obtain an omnibus P-value for interaction, the models were adjusted for age, education, smoking status, alcohol consumption, hypertension, hyperlipidemia, and diabetes mellitus.
The statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA), and the significance level was set as P < 0.05.
A total of 2,943 participants from Jidong (2,821) and Taian (122) eventually enrolled in our study (Figure 1). We found no statistically significant differences between the populations from the two centers in terms of GGT, sleep duration, BMI, smoking status, education level, hypertension, diabetes mellitus, dyslipidemia, SUA, total cholesterol, triglycerides, HDL-C, LDL-C, and estrogen level (P > 0.05). Although there was a significant difference in the age of potential subjects from the two centers, there was no significant difference in the ages of the MCI patients from the two centers (Supplementary Table 1). Therefore, we combined the patients from the two centers for the analysis.
We compared baseline characteristics by different quartiles of serum GGT activity (Table 1). There were 14 factors that were significantly associated with serum GGT levels, including age, sleep duration, BMI, smoking status, education level, hypertension, diabetes mellitus, dyslipidemia, SUA, total cholesterol, triglycerides, HDL-C, LDL-C, and estrogen level (P < 0.05). We considered all of these possible confounding variables when evaluating the independent relationship between GGT and MCI.
At the same time, baseline characteristics were compared in women participants with and without MCI. Significant differences were found in age, sleep duration, BMI, smoking status, education level, hypertension, diabetes mellitus, dyslipidemia, triglycerides, SUA, LDL-C, GGT, and estrogen level (P < 0.05). Supplementary Table 2 clearly shows the association between baseline characteristics and MCI.
The prevalence of MCI in the participants increased with increasing GGT level, being 8.4, 14.2, 17.6, and 21.4% in the lowest to highest quartiles of GGT, respectively (Table 1).
For the participants in the present study, compared to the lowest quartile, the highest quartile of GGT exhibited a positive association with MCI risk (OR = 1.88, 95% CI: 1.33–2.65, P < 0.001) in the fully adjusted model. At the same time, we found that as GGT levels increased, the ORs and 95% CIs for MCI of the participants in the second, third, and fourth GGT quartiles increased, respectively, compared with those of the participants in the lowest GGT quartile. Moreover, in all three models of the participants, the P for trend across quartiles was <0.001 (Figure 2). The dose-response relationship between GGT level and risk of MCI was analyzed by using the restricted cubic spline regression model. And the risk of MCI increased with increasing GGT level when the GGT level was below 35 μ/l, and when the GGT level was higher than 35 U/l, the risk of MCI remained high but decreased slightly with increasing GGT level (Figure 3).
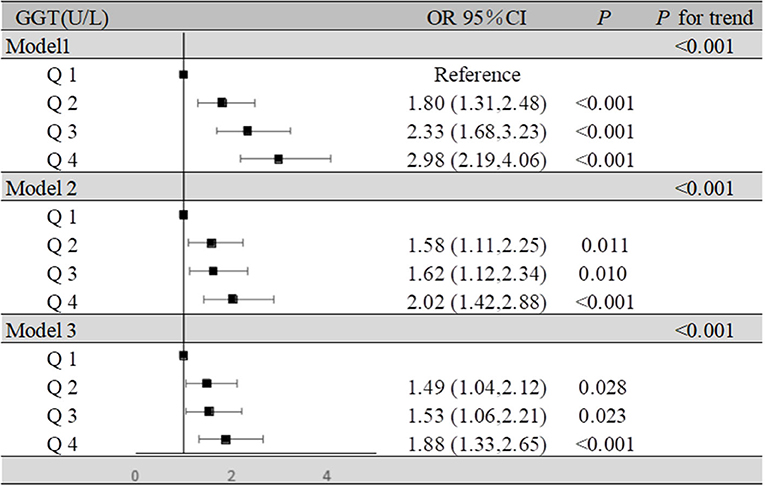
Figure 2. Association of GGT levels and MCI in the participants. Model 1: unadjusted. Model 2: adjusted for age, BMI, education, sleep duration, smoking status, and alcohol consumption. Model 3: adjusted for age, BMI, education, sleep duration, smoking status, alcohol consumption, hypertension, hyperlipidemia, diabetes mellitus, serum uric acid, and estrogen level.
In our study, obesity, insufficient sleep, hyperuricemia, and menopause all increased the risk of MCI of the participants. The OR and 95% CI for MCI due to obesity was 1.32 (1.04–1.69) in the fully adjusted model. Likewise, insufficient sleep also increased the risk of MCI, and the OR and 95% CI for MCI due to insufficient sleep was 1.33 (1.05–1.69) in the fully adjusted model. For hyperuricemia, the OR and 95% CI for MCI was 1.34 (1.01–1.77) in the fully adjusted model. Reduced estrogen levels also affect the development of MCI in postmenopausal women, the OR and 95% CI for MCI due to menopause was 2.28 (1.78–2.91) in the fully adjusted model (Supplementary Table 3). We also found that obesity, insufficient sleep, hyperuricemia, and menopause were significant risk factors for MCI according to the nomogram based on the logistic regression results for all factors (Supplementary Figure 1).
We summarized the results of the analysis on the effects of the interactions between serum GGT and the presence of obesity, insufficient sleep, and hyperuricemia on the risk of MCI in Figure 4. Through interaction analysis, we found that women with high GGT levels had an increased risk of MCI when they were obese (OR = 1.96 for obese women with Q4 GGT compared to nonobese women in Q1-Q3 GGT, P for interaction = 0.032), lacked sleep (OR = 1.91 for women who slept less and had Q4 GGT compared to women who slept more and had Q1-Q3 GGT, P for interaction = 0.001), had a high level of SUA (OR = 1.55 for women with high UA levels and Q4 GGT compared to women with low UA levels and Q1-Q3 GGT, P for interaction = 0.037) or after menopause (OR = 2.92 for postmenopausal women and Q4 GGT compared to premenopausal women and Q1-Q3 GGT, P for interaction <0.001). Considering women with high GGT levels, when they were obese, lack of sleep, had high levels of uric acid or after menopause, we obtained higher OR values than that we did for women with high GGT levels alone (OR = 1.19, 95% CI: 1.07–1.33). So obesity, lack of sleep, hyperuricemia, and menopause all had subadditive effects on the increased risk of MCI associated with GGT (Supplementary Table 3, Figure 4).
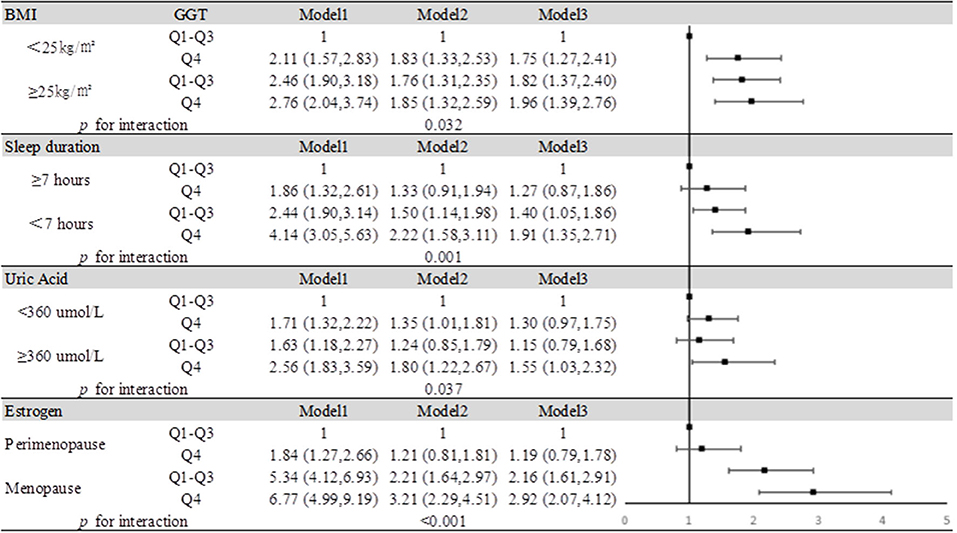
Figure 4. Impact of serum GGT level on MCI according to BMI, sleep duration, uric acid, and estrogen. Model 1: unadjusted. Model 2: adjusted for age, education, smoking status, and alcohol consumption. Model 3: adjusted for age, education, smoking status, alcohol consumption, hypertension, hyperlipidemia, and diabetes mellitus.
In our study, we found that GGT might be an independent risk factor for MCI in the female population, and as GGT levels increased, so did the risk of MCI. At the same time, we also found that women with high GGT levels were at increased risk of developing MCI when they were obese, lacked sleep, had high levels of uric acid or after menopause. BMI, sleep duration and uric acid may interact with GGT in the development of MCI.
Our study found an association between higher GGT and higher risk of cognitive impairment in the female population, which is consistent with previous studies in the male population (Kunutsor and Laukkanen, 2016). A study of GGT and Parkinson's disease is also consistent with our finding, that GGT may contribute to neurodegenerative disease in the female population (Yoo et al., 2020). However, contrary to our findings, a genetic study found no clear link between GGT and cognitive function (Kunutsor et al., 2018), possibly because that study did not include a subgroup analysis based on sex, which is essential because of the finding from previous study that estrogen may affect GGT activity in the body (Zarida et al., 1994).
The potential proinflammatory and pro-oxidative effects of GGT might be an important cause of the increased risk of MCI related to GGT (Kunutsor et al., 2014, 2015). Additionally, GGT levels were directly involved in atheromatous plaque formation, which has also been implicated as an underlying link in the pathogenesis of cognitive impairment (Breteler, 2000; Paolicchi et al., 2004; Franzini et al., 2009).
Through the analysis of the baseline data, we found that the differences in age, education, sleep duration, BMI, hypertension, dyslipidemia, diabetes, SUA, triglycerides. LDL-C, and estrogen decline were statistically significant not only in the MCI and non-MCI groups (Supplementary Table 2), but also in the different GGT level groups (Table 1). However, previous studies found that only BMI (Carter et al., 2019; Cho et al., 2019), sleep (D'Rozario et al., 2020; Zhang et al., 2020), uric acid (Zhang et al., 2015; Ya et al., 2017; Tana et al., 2018) and estrogen level (D'Rozario et al., 2020; Zhang et al., 2020) affected both GGT and MCI. Moreover, after we used logistic regression to establish the nomogram, we found that obesity, lack of sleep, high uric acid levels, and menopause were risk factors for MCI. Therefore, the effects of the interaction of obesity, lack of sleep, high uric acid levels, and menopause with GGT on MCI was analyzed by demographic, lifestyle, and biochemical parameters. Interestingly, when we constructed nomograms, we found that mild-moderate alcohol consumption may have a weak protective effect on MCI, consistent with previous research on the relationship between alcohol consumption and Alzheimer's disease (AD) (Venkataraman et al., 2016).
Direct release in the context of hepatocyte injury is not the sole cause of serum GGT elevation. GGT elevation may also be due to factors such as obesity related to serum GGT activities that occur and lead to increased expression or decreased breakdown. A previous study showed that the level of GGT expression in obese people may be different from that in normal people (Carter et al., 2019). Moreover, obesity also plays an important role in neurodegenerative diseases by inducing insulin resistance (Pugazhenthi et al., 2017). These findings make it possible for BMI and GGT to play a synergistic role in the development of MCI.
Sleep may cause changes in GGT levels because of its interaction with obesity, and a recent nematode model study demonstrated a synergistic effect between insomnia and obesity (Grubbs et al., 2020; Zhang et al., 2020). Therefore, lack of sleep may also lead to GGT elevation. In the same way, lack of sleep is a major cause of cognitive impairment. In summary, the mechanism of sleep deprivation affecting MCI may be partially enhanced by GGT.
The levels of serum UA, an important natural antioxidant, may parallel the levels of serum GGT due to their relationship with oxidative stress, as reported in the Chinese population, especially in Chinese females (Zhang et al., 2015; Ya et al., 2017). Large cohort studies completed in recent years have found that high uric acid levels may increase the risk of cognitive impairment, possibly because uric acid is more damaging to vascular tissue than it is productive of antioxidant effects (Latourte et al., 2018; Singh and Cleveland, 2018). Therefore, the synergistic effect of GGT and SUA in MCI may be due to their effect on the risk of cardiovascular disease. In addition, a previous study found that increased SUA in women increases the risk of non-alcoholic fatty liver disease and that fatty liver disease development leads to increased GGT secretion, which might be an important reason for the parallel relationship between GGT and SUA levels (Chen et al., 2019).
In animal study, GGT levels have been found to increase significantly after ovariectomy, suggesting that estrogen levels have a significant effect on GGT expression (Zarida et al., 1994). At the same time, studies have shown that endogenous estrogens are associated with better cognitive performance, and our study found that a decrease in estrogens and an increase in GGT both affect MCI, this is of positive significance for the study of cognitive impairment in postmenopausal women (Fink et al., 2018; Shimizu et al., 2019).
In our study, we found that MCI is more common in women with elevated GGT levels, so GGT could be a potential diagnostic marker for MCI. Meanwhile, our findings indicated that women with high GGT levels had an increased risk of MCI when they were overweight, sleep deprived, or had high uric acid levels.
Since we performed a cross-sectional study, we could not refute the conclusion reached in previous studies that there is no causal link between GGT and MCI (Kunutsor et al., 2018). However, there has been no previous research into the relationship between GGT and cognitive function in the female population, so our results are significant in terms of the diagnosis and early indication of MCI in the female population. In addition, because our study only included Chinese women, the results may not be generalizable to the entire population. However, at the same time, our study was a two-center study that integrated analysis of different populations, and the conclusions are more representative and extrapolatable in Chinese female population.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was conducted in accordance with the guiding principles of the Helsinki Declaration and approved by the Ethics Committee of Oilfield Inc. Medical Centers and Taian Traffic Hospital. Written informed consent was obtained from all participants.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (81973138) and National Key Research and Development Project of China (2017YFE0118800).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2021.630409/full#supplementary-material
Breteler, M. (2000). Vascular risk factors for Alzheimer's disease: an epidemiologic perspective. Neurobiol. Aging 21, 153–160. doi: 10.1016/S0197-4580(99)00110-4
Brookmeyer, R., Johnson, E., Ziegler-Graham, K., and Arrighi, H. (2007). Forecasting the global burden of Alzheimer's disease. Alzheimer's Dementia 3, 186–191. doi: 10.1016/j.jalz.2007.04.381
Carter, A., Borges, M., Benn, M., Tybjaerg-Hansen, A., Davey Smith, G., Nordestgaard, B., et al. (2019). Combined association of body mass index and alcohol consumption with biomarkers for liver injury and incidence of liver disease: a mendelian randomization study. JAMA Network Open 2:e190305. doi: 10.1001/jamanetworkopen.2019.0305
Chan, K., Wang, W., Wu, J., Liu, L., Theodoratou, E., Car, J., et al. (2013). Epidemiology of Alzheimer's disease and other forms of dementia in China, 1990-2010: a systematic review and analysis. Lancet 381, 2016–2023. doi: 10.1016/S0140-6736(13)60221-4
Chen, Y., Huang, Q., Ai, P., Liu, H., Chen, X., Xu, X., et al. (2019). Association between serum uric acid and non-alcoholic fatty liver disease according to different menstrual status groups. Canad. J. Gastroenterol. Hepatol. 2019:2763093. doi: 10.1155/2019/2763093
Cho, G., Hwang, S., Lee, K., Choi, K., Hyun Baik, S., Kim, T., et al. (2019). Association between waist circumference and dementia in older persons: a nationwide population-based study. Obesity 27, 1883–1891. doi: 10.1002/oby.22609
Ding, D., Zhao, Q., Guo, Q., Meng, H., Wang, B., Luo, J., et al. (2015). Prevalence of mild cognitive impairment in an urban community in China: a cross-sectional analysis of the Shanghai Aging Study. Alzheimer's Dementia 11, 300–309 e302. doi: 10.1016/j.jalz.2013.11.002
Dong, Y., Lee, W., Basri, N., Collinson, S., Merchant, R., Venketasubramanian, N., et al. (2012). The montreal cognitive assessment is superior to the Mini-Mental State Examination in detecting patients at higher risk of dementia. Int. Psychogeriat. 24, 1749–1755. doi: 10.1017/S1041610212001068
Dong, Y., Sharma, V., Chan, B., Venketasubramanian, N., Teoh, H., Seet, R., et al. (2010). The Montreal Cognitive Assessment (MoCA). is superior to the Mini-Mental State Examination (MMSE). for the detection of vascular cognitive impairment after acute stroke. J. Neurol. Sci. 299, 15–18. doi: 10.1016/j.jns.2010.08.051
D'Rozario, A., Chapman, J., Phillips, C., Palmer, J., Hoyos, C., Mowszowski, L., et al. (2020). Objective measurement of sleep in mild cognitive impairment: a systematic review and meta-analysis. Sleep Med. Rev. 52:101308. doi: 10.1016/j.smrv.2020.101308
Emdin, M., Pompella, A., and Paolicchi, A. (2005). Gamma-glutamyltransferase, atherosclerosis, and cardiovascular disease: triggering oxidative stress within the plaque. Circulation 112, 2078–2080. doi: 10.1161/CIRCULATIONAHA.105.571919
Fink, H., Jutkowitz, E., McCarten, J., Hemmy, L., Butler, M., Davila, H., et al. (2018). Pharmacologic interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-Type dementia: a systematic review. Ann. Internal Med. 168, 39–51. doi: 10.7326/M17-1529
Forman, H., Zhang, H., and Rinna, A. (2009). Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 30, 1–12. doi: 10.1016/j.mam.2008.08.006
Franzini, M., Corti, A., Martinelli, B., Del Corso, A., Emdin, M., Parenti, G., et al. (2009). Gamma-glutamyltransferase activity in human atherosclerotic plaques–biochemical similarities with the circulating enzyme. Atherosclerosis 202, 119–127. doi: 10.1016/j.atherosclerosis.2008.03.023
Gackowski, D., Rozalski, R., Siomek, A., Dziaman, T., Nicpon, K., Klimarczyk, M., et al. (2008). Oxidative stress and oxidative DNA damage is characteristic for mixed Alzheimer disease/vascular dementia. J. Neurol. Sci. 266, 57–62. doi: 10.1016/j.jns.2007.08.041
Grubbs, J., Lopes, L., van der Linden, A., and Raizen, D. (2020). A salt-induced kinase is required for the metabolic regulation of sleep. PLoS Biol. 18:e3000220. doi: 10.1371/journal.pbio.3000220
Jeon, J., Kim, D., Kim, W., Choi, D., Jung, K., and Jang, S. (2020). Dose-response relationship between gamma-glutamyltransferase and the risk of atherosclerotic cardiovascular diseases in Korean adults. Atherosclerosis 292, 152–159. doi: 10.1016/j.atherosclerosis.2019.11.004
Kasten, M., Bruggemann, N., Schmidt, A., and Klein, C. (2010). Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 75:478. doi: 10.1212/WNL.0b013e3181e7948a
Kristenson, H., Hood, B., Peterson, B., and Trell, E. (1985). Prevention of alcohol-related problems in urban middle-aged males. Alcohol 2, 545–549. doi: 10.1016/0741-8329(85)90132-6
Kunutsor, S. (2016). Gamma-glutamyltransferase-friend or foe within? Liver Int. 36, 1723–1734. doi: 10.1111/liv.13221
Kunutsor, S., Apekey, T., and Khan, H. (2014). Liver enzymes and risk of cardiovascular disease in the general population: a meta-analysis of prospective cohort studies. Atherosclerosis 236, 7–17. doi: 10.1016/j.atherosclerosis.2014.06.006
Kunutsor, S., Bakker, S., Kootstra-Ros, J., Gansevoort, R., and Dullaart, R. (2015). Circulating gamma glutamyltransferase and prediction of cardiovascular disease. Atherosclerosis 238, 356–364. doi: 10.1016/j.atherosclerosis.2014.12.045
Kunutsor, S., and Laukkanen, J. (2016). Gamma glutamyltransferase and risk of future dementia in middle-aged to older Finnish men: a new prospective cohort study. Alzheimer's Dementia 12, 931–941. doi: 10.1016/j.jalz.2016.03.003
Kunutsor, S., Laukkanen, J., and Burgess, S. (2018). Genetically elevated gamma-glutamyltransferase and Alzheimer's disease. Exp. Gerontol. 106, 61–66. doi: 10.1016/j.exger.2018.03.001
Latourte, A., Soumare, A., Bardin, T., Perez-Ruiz, F., Debette, S., and Richette, P. (2018). Uric acid and incident dementia over 12 years of follow-up: a population-based cohort study. Ann. Rheumatic Dis. 77, 328–335. doi: 10.1136/annrheumdis-2016-210767
Lv, H., Yang, X., Zhou, Y., Wu, J., Liu, H., Wang, Y., et al. (2016). Parity and serum lipid levels: a cross-sectional study in chinese female adults. Sci. Rep. 6:33831. doi: 10.1038/srep33831
Mollayeva, T., Thurairajah, P., Burton, K., Mollayeva, S., Shapiro, C., and Colantonio, A. (2016). The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med. Rev. 25, 52–73. doi: 10.1016/j.smrv.2015.01.009
Nasreddine, Z., Phillips, N., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Paolicchi, A., Emdin, M., Ghliozeni, E., Ciancia, E., Passino, C., Popoff, G., et al. (2004). Images in cardiovascular medicine. human atherosclerotic plaques contain gamma-glutamyl transpeptidase enzyme activity. Circulation 109:1440. doi: 10.1161/01.CIR.0000120558.41356.E6
Papandreou, C., Babio, N., Diaz-Lopez, A., Martinez-Gonzalez, M., Becerra-Tomas, N., Corella, D., et al. (2019). Sleep duration is inversely associated with serum uric acid concentrations and uric acid to creatinine ratio in an elderly mediterranean population at high cardiovascular risk. Nutrients 11:761. doi: 10.3390/nu11040761
Petersen, R. (2000). Aging, mild cognitive impairment, and Alzheimer's disease. Neurol. Clin. 18, 789–806. doi: 10.1016/S0733-8619(05)70226-7
Petersen, R., Doody, R., Kurz, A., Mohs, R., Morris, J., Rabins, P., et al. (2001). Current concepts in mild cognitive impairment. Arch. Neurol. 58, 1985–1992. doi: 10.1001/archneur.58.12.1985
Praetorius Bjork, M., and Johansson, B. (2018). Gamma-Glutamyltransferase (GGT). as a biomarker of cognitive decline at the end of life: contrasting age and time to death trajectories. Int. Psychogeriat. 30, 981–990. doi: 10.1017/S1041610217002393
Pugazhenthi, S., Qin, L., and Reddy, P. (2017). Common neurodegenerative pathways in obesity, diabetes, and Alzheimer's disease. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 1037–1045. doi: 10.1016/j.bbadis.2016.04.017
Schiele, F., Guilmin, A., Detienne, H., and Siest, G. (1977). Gamma-glutamyltransferase activity in plasma: statistical distributions, individual variations, and reference intervals. Clin. Chem. 23, 1023–1028. doi: 10.1093/clinchem/23.6.1023
Shimizu, Y., Sawada, N., Iwasaki, M., Shikimoto, R., Nozaki, S., Mimura, M., et al. (2019). Reproductive history and risk of cognitive impairment in Japanese women. Maturitas 128, 22–28. doi: 10.1016/j.maturitas.2019.06.012
Singh, J., and Cleveland, J. (2018). Gout and dementia in the elderly: a cohort study of Medicare claims. BMC Geriat. 18:281. doi: 10.1186/s12877-018-0975-0
Tana, C., Ticinesi, A., Prati, B., Nouvenne, A., and Meschi, T. (2018). Uric acid and cognitive function in older individuals. Nutrients 10:975. doi: 10.3390/nu10080975
Thompson, S., and Hodges, J. (2002). Mild cognitive impairment: a clinically useful but currently ill-defined concept? Neurocase 8, 405–410. doi: 10.1076/neur.8.5.405.16182
Venkataraman, A., Kalk, N., Sewell, G., Ritchie, C W., and Lingford-Hughes, A. (2016). Alcohol and Alzheimer's disease does alcohol dependence contribute to beta-amyloid deposition,neuroinflammation and neurodegeneration in Alzheimer's disease. Alcohol Alcohol. 52:158. doi: 10.1093/alcalc/agw101
Ya, Z., Fei, L., Yue, Z., Dan, L., Neng-Bo, L., Yi, L., et al. (2017). Association between serum gamma-glutamyl transferase and serum uric acid levels in Chinese females: a cross-sectional study. Endocrine Res. 42, 296–301. doi: 10.1080/07435800.2017.1300809
Yoo, D., Kim, R., Jung, Y., Han, K., Shin, C., and Lee, J. (2020). Serum gamma-glutamyltransferase activity and Parkinson's disease risk in men and women. Sci. Rep. 10:1258. doi: 10.1038/s41598-020-58306-x
Zafrilla, P., Mulero, J., Xandri, J., Santo, E., Caravaca, G., and Morillas, J. (2006). Oxidative stress in Alzheimer patients in different stages of the disease. Curr. Med. Chem. 13, 1075–1083. doi: 10.2174/092986706776360978
Zarida, H., Wan Zurinah, W., Zanariah, J., Michael, L., and Khalid, B. (1994). Effect of ovariectomy and sex hormones replacement on tumour marker enzymes under administration of chemical carcinogens in the rat. Exp. Toxicol. Pathol. 46, 31–36. doi: 10.1016/S0940-2993(11)80010-2
Zhang, J., Xiang, G., Xiang, L., and Dong, J. (2015). Serum gamma-glutamyl transferase is associated with the elevated uric acid levels in normotensive Chinese adults. Clin. Chim. Acta 441, 122–126. doi: 10.1016/j.cca.2014.12.031
Zhang, L., Zhang, X., Meng, H., Li, Y., Han, T., and Wang, C. (2020). Obstructive sleep apnea and liver injury in severely obese patients with nonalcoholic fatty liver disease. Sleep Breath. 24, 1515–1521. doi: 10.1007/s11325-020-02018-z
Keywords: mild cognitive impairment, female population, diagnostic marker, cross-sectional study, gamma-glutamyl transferase
Citation: Tang Z, Chen X, Zhang W, Sun X, Hou Q, Li Y, Feng X, Chen Y, Lv J, Ji L, Ding G and Li D (2021) Association Between Gamma-Glutamyl Transferase and Mild Cognitive Impairment in Chinese Women. Front. Aging Neurosci. 13:630409. doi: 10.3389/fnagi.2021.630409
Received: 17 November 2020; Accepted: 08 January 2021;
Published: 10 February 2021.
Edited by:
Carlos J. Rodriguez-Ortiz, University of California, Irvine, United StatesReviewed by:
Venkata Saroja Voruganti, University of North Carolina at Chapel Hill, United StatesCopyright © 2021 Tang, Chen, Zhang, Sun, Hou, Li, Feng, Chen, Lv, Ji, Ding and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Long Ji, bGppQHNkZm11LmVkdS5jbg==; Guoyong Ding, ZGd5MTUzQDEyNi5jb20=; Dong Li, dHNtY2RvbmdsaUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.