- 1Department of Neurology in Linköping, and Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden
- 2Gösta Ekman Laboratory, Department of Psychology, Stockholm University, Stockholm, Sweden
Olfactory impairment is a central non-motor symptom in Parkinson's disease (PD). Previous studies have demonstrated that olfactory dysfunction is associated with mental illness and impaired cognition. The frequently investigated olfactory functions are odor detection, discrimination, and identification. However, few studies have focused on odor recognition memory (ORM). ORM tasks involves episodic memory which therefore can facilitate the detection of dementia among patients with PD and consequently adjust their treatment. Thus, the aim of this systematic review is to summarize the existing research on ORM in PD. Databases and reference lists were used for data collection. Studies were included in the review if they met the eligibility criteria derived from the PICOS-framework. Quality evaluation of the studies was based on the STROBE-statement. Six studies with small samples were included in the analysis which demonstrated the scarce research on the subject. The studies targeting ORM were heterogenous and involved two main tasks: odor recognition and odor matching. The synthesis of the data demonstrated that PD patients performed significantly lower than controls on both tasks, especially on odor matching task. Only the odor recognition task exhibited a difference between patients with PD vs. Alzheimer's disease (AD). PD patients performed significantly better than AD patients. The findings based on the available limited data support the notion that odor recognition task can be of importance in identifying Parkinson's disease dementia (PDD). To investigate this hypothesis, future research needs to include larger samples of PD, PDD and AD patients executing the same odor recognition task.
Introduction
Parkinson's disease (PD) is primarily identified by the evident debilitating motor symptoms: tremor, bradykinesia, rigidity, and postural instability. In 2015 ~6 million people were reported to suffer from the disease worldwide, a number estimated to increase rapidly to 12 million by 2040 due to the increased elderly population (Dorsey et al., 2018). Nonetheless, often non-motor symptoms (NMS) as depression, cognitive decline, sleep disorder, constipation, and olfactory dysfunction are present years before diagnosis and have been significantly associated with poor quality of life (Chaudhuri et al., 2006). The prevalence of the olfactory dysfunction in PD is estimated to be 50–90% and therefore one of the most studied NMS of the disease (Fullard et al., 2017). In fact, according to Braak's theory the parkinsonian neurodegeneration begins in the olfactory bulb and spreads toward other main parts of the cerebral olfactory system (Doty, 2012). For example, patients with PD show an extensive alpha-synuclein pathology in anterior olfactory nucleus (AON), cortical nucleus of the amygdala, piriform cortex, olfactory tubercle, and entorhinal cortex. Another possible explanation for this dysfunction is the distorted levels of typically affected neurotransmitters in PD such as dopamine, acetylcholine, and serotonin (Fullard et al., 2017).
Against this background, testing olfactory function may aid identifying PD in its premotor phase and thus allowing assessment of innovative treatments that requires early detection such as stem-cell therapy. The sense of smell is frequently assessed with psychophysical tests. One of the most well-established and comprehensive smell tests is the “Sniffin Sticks” which includes measurements of odor detection, odor identification, and odor discrimination (Hummel et al., 2007). A later development of the “Sniffin Sticks” is the Test of Odor Memory (TOM) which aims to measure episodic odor recognition (Croy et al., 2015). Prior studies targeting these olfactory functions showed variation between different tasks regarding brain activity, performance among PD patients and sensitivity to the parkinsonian progression (Fullard et al., 2017). Hence, different tasks can aid in decision making regarding various clinical questions. Additionally, the variation in performance between the tasks indicate that each task put different demands on olfaction, cognition, and memory (Hedner et al., 2010).
The odor detection task aims to measure the participant's olfactory sensitivity. Therefore, it is primarily based on a bottom-up processing and assumed to be a prerequisite for higher odor functions (Boesveldt et al., 2009). Conversely, the odor identification task which requires from the participant to verbally label common odors, is associated with higher cognitive function such as semantic memory. This type of memory refers to the participant's knowledge of facts about the world. The odor discrimination task involves also higher cognitive functions, especially working memory. It requires from the participant to create olfactory representations, storage them in short-term memory and compare between the different representations (Larsson, 2002; Hedner et al., 2010).
In the episodic odor recognition task, a set of odors (olfactory targets) that the participant is asked to memorize are presented at the encoding phase. At the retrieval phase the targets are presented again intermixed with new odors (olfactory distractors). The participant is required to determine if the presented odor is a distractor or a target. The research on odor recognition is remarkably limited, nevertheless the hypothesized dominant cognitive function in this task is episodic memory (Croy et al., 2015). Episodic memory is a top-down process and refers to the intentional retrieval of information, which is associated with temporal and spatial information (Larsson, 2002).
In summary, this review focuses on odor recognition memory (ORM) in PD due to multiple reasons. Previous studies did not demonstrate a correlation between other odor tasks and classical episodic memory tasks, while the type of cognitive load in the ORM task has still not been adequately examined (Hedner et al., 2010). The integrity of episodic odor memory may be particularly sensitive for pathological disturbances as dementia (Larsson, 2002). The risk of developing cognitive impairment and dementia is high in PD, especially in the advanced phase of the disease. Previous studies showed that dementia eventually will occur in up to 83% of PD patients (Hely et al., 2008). Furthermore, some antiparkinsonian treatments (e.g., dopamine agonists and deep brain stimulation in subthalamic nucleus) have been associated with deterioration of cognitive function, making the importance of detecting de novo dementia more crucial (Witt et al., 2013). The ORM task has also been associated with a wide cerebral activity and therefore is preferable regarding detection of dementia in a multisystem disease such as PD, minimizing the risk of missing initial cognitive symptoms (Savic et al., 2000). Although the ORM task may play a crucial role in early detection of PDD and consequently influence the treatment of the disease, the research on ORM in the context of PD is remarkably limited. Therefore, the aim of this review is to summarize the existing research on ORM in PD and generate research questions for future studies.
Methods
Literature Search
The Preferred Reporting Items for Systematic Reviews (PRISMA) guided the literature search (Moher et al., 2009). To identify relevant records from 1975 and onwards, three databases: Web of Science, Medline, and PsycINFO were used from January–May 2020. Studies were also collected from other sources (e.g., studies reference lists). Since Medical Subject Headings (MeSH) which describes accurately the topic of the review had not been found, well-established and broad terms in the research field were investigated. This strategy permitted a more inclusive search and therefore suitable for an unstudied subject. The terms odor memory OR odor recognition were used in AND-combination with Parkinson's disease OR Parkinsons disease OR PD.
Inclusion and Selection Procedure
The eligibility criteria were derived from the parameters incorporated in the PICOS-framework: population, intervention, comparison, outcome, and study type (Moher et al., 2009). Studies were included if they met the following criteria:
(1) Included human subjects with PD.
(2) Explicitly intended to measure odor memory.
(3) Compared either between different olfactory functions, populations, or both.
(4) Reported results on odor memory.
(5) Designed as an empirical study.
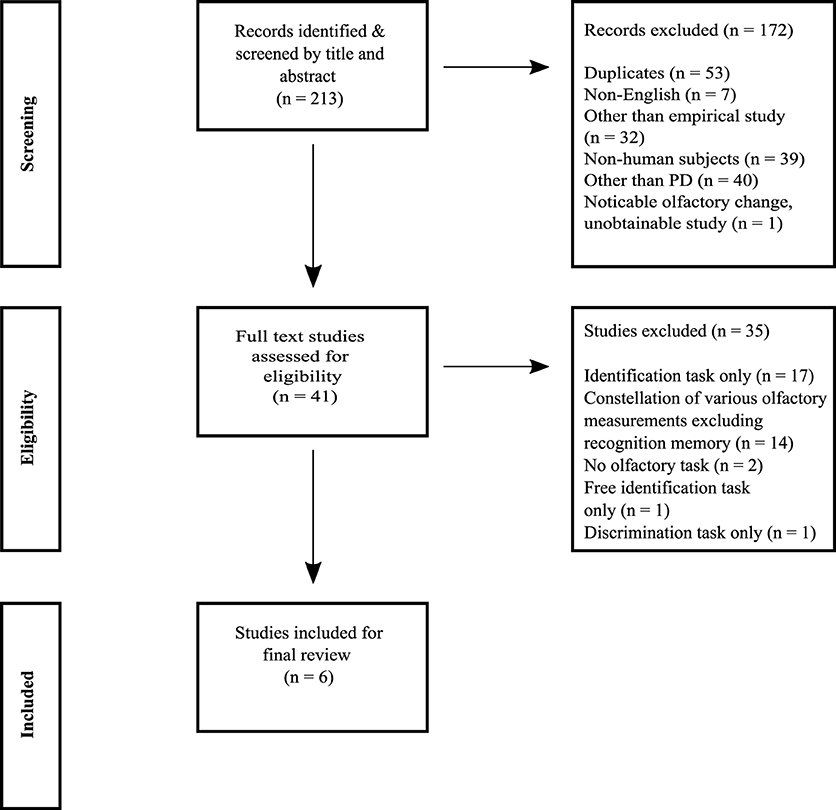
The selection process was performed in a stepwise manner and in accordance with the PRISMA flow diagram (see Figure 1). First, duplicates and records written in other languages than English were excluded by limitations in the search. The results correctness was confirmed by a manual comparison between the limited search and a search without any limitations. Second, further exclusion was performed based on descriptions in abstracts concerning study type, subject type, and focus on PD. When this information was unobtainable from the abstract the study's full text was screened. Third, to avoid biased inclusion of studies in the final analysis the remaining studies were full text appraised independently. The first and third author reviewed separately, which studies aimed to measure odor memory. Disagreements between the two authors were discussed with the second author (a professor in the field of olfaction and memory) and a consensus was made.
Data Extraction and Evaluation
The extraction of data from each eligible study was derived from the PICOS-framework (see Table 1). The evaluation of the data was based on Strengthening the Reporting of Observational studies in Epidemiology (STROBE-statement). The statement contains six domains: title and abstract, introduction (rational and objectives), methods (study design, setting, participants, variables, measurement, bias, study size, and statistical methods), results (participants, descriptive data, outcome data, main results, and other analysis), discussion (key results, limitations, interpretations, generalizability), and other information (e.g., funding) (Moher et al., 2009).
Analysis and Synthesis
The differences between patients with PD and controls in performance on ORM task were measured as effect sizes for each eligible study. When information was obtainable the same comparison was performed between PD and AD patients, due to the high risk of developing dementia in PD (Hely et al., 2008). Subsequently a synthesis of outcomes from different studies was performed to allow a broader ground for conclusions. Effect sizes were calculated in accordance with Cohen's d (d > 0.50 considered to be a medium effect size) and by scripts created in R (R Core Team, 2017).
Results
Study Selection
The literature search generated 213 potentially relevant records. A total of 172 records were excluded due to: duplications of records, other written language than English, the articles data was not based on empirical investigation, non-human research subjects (e.g., rodents, drosophila flies and electrochemical noses), focus on other populations than individuals with PD (e.g., healthy individuals, Lewy Body Dementia, Alzheimer's disease, and Schizophrenia) and unobtainability. After exclusion, the full text of 41 studies was independently appraised concerning the studies aim to measure ORM. In total, 35 (85%) of these 41 studies did not measure odor memory. The most common measurements were odor identification (n = 17, 41%) or a constellation of various odor tasks such as detection, discrimination, identification, familiarity, pleasantness excluding ORM (n = 14, 34%). Finally, six studies (14 %) postulated to measure ORM (see Figure 1).
Study Characteristics and Evaluation
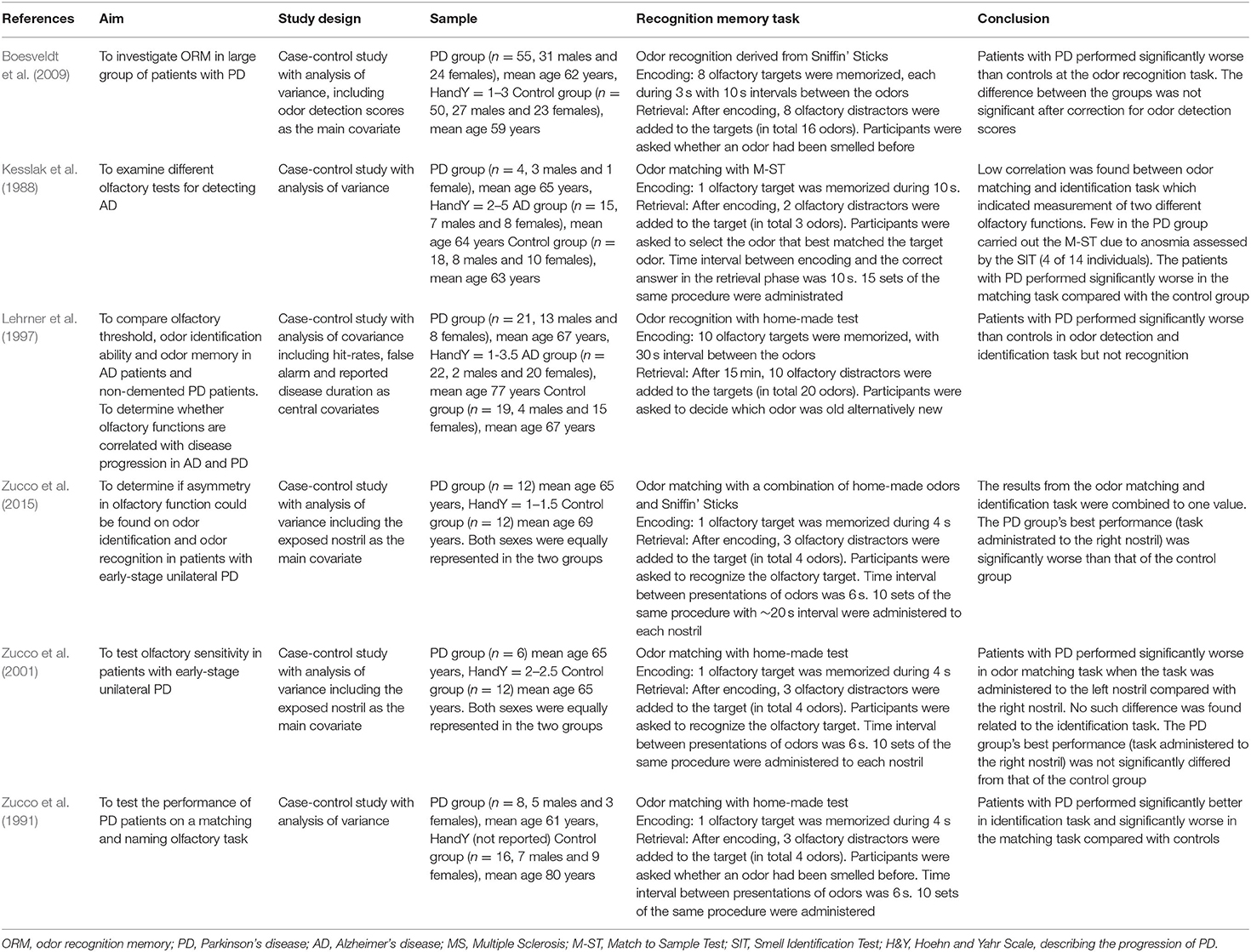
The extraction of data from each of the six eligible studies was performed in accordance with the PICOS-framework and included description of the studies aim, design, sample, recognition memory task and conclusion (see Table 1). The extracted data focused on relevant information that assumed to shed light on the review's main questions concerning ORM in PD. Therefore, information about other tasks as odor detection, discrimination and identification, and other samples as Multiple sclerosis (MS) was not extracted although included in some studies.
The first two domains in the STROBE-statement are title and abstract, as well as introduction. All studies had relevant titles and informative abstracts. In the studies introduction, the scientific background and rationale for the investigation of numerous olfactory functions were explained. All studies incorporated an ORM task in patients with PD in relation to other study populations.
The studies method is the third evaluation domain. All studies were designed as observational case-control studies and included matched control samples. This design thought to be suitable for the studies primary objectives while secondary research questions as the relation between performance in olfactory tasks and disease duration would have benefitted more from a longitudinal design (Lehrner et al., 1997). Only two studies of the six, described the recruitment of both patients and controls (Zucco et al., 1991; Boesveldt et al., 2009). Most of the studies explained the process of the ascertainment and inclusion of cases, while information on exclusion and dropout was limited. The participants age and sex were reported in all studies and significant differences between groups were observed in two studies (Zucco et al., 1991; Lehrner et al., 1997). For example, in Lehrner et al. (1997) the AD group was significantly older and contained considerably more females compared with the PD and control group. Previous studies demonstrated that these two parameters and cultural affiliation may affect the performance in olfactory tasks (Doty and Kamath, 2014). Nevertheless, none of the studies documented the participants ethnicity. Variation between studies concerning both PD duration and stage in accordance with Hoehn and Yahr Scale (H&Y) was observed. Most of the studies focused on patients in the H&Y stage 1–3.5 while one study included also patients in H&Y stage 4–5, i.e. the advanced stages of the disease (Kesslak et al., 1988). To screen for dementia, most studies used short cognitive screening tools as Mini-Mental State Examination (MMSE). Two studies did not report a cognitive screening of PD patients but postulated that the patients did not show any signs of dementia (Zucco et al., 1991, 2001). Thus, studies had varied inclusion criteria concerning cognitive performance while no study reported inclusion of patients with PDD.
In general, the recruited PD samples in the eligible study population were small ranging between four to 55 patients. Furthermore, one study partly incorporated the same participants from a previous study that also had been included in the reviewed study population (Zucco et al., 2015). Additionally, few studies (n = 2) included small AD samples (Kesslak et al., 1988; Lehrner et al., 1997). The instruments and procedures to measure ORM were documented thoroughly in all studies. Measurements were varied and divided into two main categories: odor matching (n = 4) and recognition task (n = 2). Three of the four matching tasks were administrated in the same manner while the two recognition tasks were different, especially regarding to the retention interval (see Table 1). Multiple statistical tools were used to treat the data while analysis of variance that included possible influential covariates as age, sex, disease duration etc. was the main statistical analysis in all studies.
The last three domains in the statement are results, discussion, and other information. All studies described the results concerning the odor memory recognition tasks. One of six studies did not report separately the odor matching scores but incorporate them with performance on odor identification task to one olfactory quota (Zucco et al., 2015). Discussions included key results and interpretations, but problematization of studies generalizability and limitations was scarce. Some studies commented the limited generalizability of results due to the small sample sizes (Zucco et al., 2001). None of the studies reflected on the problem with generalizability regarding varied ORM tasks and the need for standardized measurement. Only two of the six studies reported information about their funding of the research (Kesslak et al., 1988; Boesveldt et al., 2009).
Analysis and Synthesis
The effect sizes between different samples; PD, AD, and controls were calculated for each eligible study in accordance with Cohen's d. Zucco et al. (2015) was excluded from the statistical analysis due to partially inclusion of the same participants in another eligible study (Zucco et al., 2001). Furthermore, the scores in the odor matching task were not reported separately but were merged with performance in odor identification task, to one olfactory quota (Zucco et al., 2015).
Synthesis of the two studies including the odor recognition task resulted in PD sample (n = 76) and control sample (n = 69) (Lehrner et al., 1997; Boesveldt et al., 2009). The PD group performed significantly lower than controls and the difference between the groups was medium in accordance with Cohen's d (0.60). The same synthesis could not be performed in relation to the AD sample since only one study of the two included such a sample. Nevertheless, based on this one study the patients with PD (n = 76) performed better than the AD (n = 22) patients in the odor recognition task (Lehrner et al., 1997). The difference between the two groups was slightly higher (d = 0.68).
In relation to the odor matching task three studies were merged which resulted in PD sample (n = 18) and controls (n = 46) (Kesslak et al., 1988; Zucco et al., 1991, 2001). PD patients performed significantly lower than controls and the difference between the groups was noticeably large (d = 1.39). Only one study of the three, included AD patients and therefore a synthesis concerning this group was not possible. Based on that one study, no significant difference between PD and AD patients in performance on odor matching task was observed (d = 0.48) (Kesslak et al., 1988).
Discussion
The literature on ORM in PD was limited and only six eligible studies on the subject were found. The sample sizes were generally small. Differences within and between studies concerning inclusion criteria, sample size, sex ratio, age, disease stage, disease duration, and performance in cognitive tests indicated heterogenous data. This partially explained a previous meta-analysis result demonstrated that the highest inconsistency in studies was related to AD and PD patients performance in ORM tasks (I2 = 87.7%) (Rahayel et al., 2012). Additionally, variation between reviewed studies was discovered regarding the design of the ORM task. Two main task designs were identified and categorized in this review as odor matching and odor recognition task. The odor matching task resembled measurements of odor discrimination and therefore theoretically is more related to working memory. Conversely, the design of the odor recognition task included more olfactory targets per set and longer time intervals of encoding and retention suggests greater involvement of episodic memory (Larsson, 2002; Hedner et al., 2010; Croy et al., 2015).
In contrast to Rahayel et al. (2012), where odor matching and recognition were considered to measure the same olfactory function (i.e., ORM), this review investigated the tasks separately, due to discrepancies in design and hence hypothetically measuring two different olfactory functions. In line with this approach, the review's synthesis revealed variation in effect sizes. PD patients showed worse performance than controls in the odor recognition task but especially in the odor matching task. Hence, tasks targeting odor matching and odor discrimination may therefore serve as markers for identifying prodromal PD allowing early treatment. However, a moderate effect size indicating that PD patients performed better than patients with AD was observed only in relation to the odor recognition task.
These findings are in accordance with the theory that odor recognition task, which requires greater involvement of odor episodic memory, is more sensitive to signs of dementia. Consequently, the systematic review generates the hypothesis that odor recognition task may have the potential to identify patients with de novo PDD and therefore improve the choice of treatment, especially in the advanced phases of the disease. A careful differentiation between patients with PD and those with PDD is of importance since some treatments as deep brain stimulation in subthalamic nucleus (DBS-STN) have been associated with decline of cognitive function (Witt et al., 2013). Still, it is important to highlight some limitations of the present review. First, given that few studies have addressed ORM in PD and that available evidence show large heterogeneity, any generalization should be drawn with caution. Moreover, impaired olfactory sensitivity has been associated to ORM in PD and therefore the performance in ORM may be highly depended on the ability to detect odors (Boesveldt et al., 2009). Nevertheless, these two tasks demonstrate differences regarding structural and behavioral data (Savic et al., 2000). Future work should adopt longitudinal prospective designs to study ORM in different PD stages, PDD, and AD.
Author Contributions
TE conceptualized and implemented the systematic review. ND and ML evaluated studies eligibility and commented the article. All authors approved the final version of the paper and agreed to be accountable for all aspects of the work.
Funding
This study was supported by ALF grants from Region östergötland, Linköping University Hospital Research Fund and Swedish Parkinson Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Boesveldt, S., de Muinck Keizer, R. J., Wolters, E. C., and Berendse, H. W. (2009). Odor recognition memory is not independently impaired in Parkinson's disease. J. Neural Transm. 116, 575–578. doi: 10.1007/s00702-009-0208-y
Chaudhuri, K. R., Healy, D. G., and Schapira, A. H. (2006). Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol. 5, 235–245. doi: 10.1016/S1474-4422(06)70373-8
Croy, I., Zehner, C., Larsson, M., Zucco, G. M., and Hummel, T. (2015). Test-retest reliability and validity of the Sniffin' TOM odor memory test. Chem. Senses 40, 173–179. doi: 10.1093/chemse/bju069
Dorsey, E. R., Sherer, T., Okun, M. S., and Bloem, B. R. (2018). The emerging evidence of the Parkinson pandemic. J. Parkinsons. Dis. 8, S3–S8. doi: 10.3233/JPD-181474
Doty, R. L. (2012). Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol. 8, 329–339. doi: 10.1038/nrneurol.2012.80
Doty, R. L., and Kamath, V. (2014). The influences of age on olfaction: a review. Front. Psychol. 5:20. doi: 10.3389/fpsyg.2014.00020
Fullard, M. E., Morley, J. F., and Duda, J. E. (2017). Olfactory dysfunction as an early biomarker in Parkinson's disease. Neurosci. Bull. 33, 515–525. doi: 10.1007/s12264-017-0170-x
Hedner, M., Larsson, M., Arnold, N., Zucco, G. M., and Hummel, T. (2010). Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J. Clin. Exp. Neuropsychol. 32, 1062–1067. doi: 10.1080/13803391003683070
Hely, M. A., Reid, W. G., Adena, M. A., Halliday, G. M., and Morris, J. G. (2008). The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov. Disord. 23, 837–844. doi: 10.1002/mds.21956
Hummel, T., Kobal, G., Gudziol, H., and Mackay-Sim, A. (2007). Normative data for the “Sniffin' Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur. Arch. Otorhinolaryngol. 264, 237–243. doi: 10.1007/s00405-006-0173-0
Kesslak, J. P., Cotman, C. W., Chui, H. C., Van den Noort, S., Fang, H., Pfeffer, R., et al. (1988). Olfactory tests as possible probes for detecting and monitoring Alzheimer's disease. Neurobiol. Aging 9, 399–403. doi: 10.1016/S0197-4580(88)80087-3
Larsson, M. (2002). “Odor memory: a memory systems approach,” in Olfaction, Taste, and Cognition, eds C. Rouby, B. Schaal, D. Dubois, R. Gervais, and A. Holley (New York, NY: Cambridge University Press), 231–245.
Lehrner, J. P., Brucke, T., Dal-Bianco, P., Gatterer, G., and Kryspin-Exner, I. (1997). Olfactory functions in Parkinson's disease and Alzheimer's disease. Chem. Senses 22, 105–110. doi: 10.1093/chemse/22.1.105
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. doi: 10.1136/bmj.b2535
R Core Team (2017). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna. Available online at: https://www.R-project.org
Rahayel, S., Frasnelli, J., and Joubert, S. (2012). The effect of Alzheimer's disease and Parkinson's disease on olfaction: a meta-analysis. Behav. Brain Res. 231, 60–74. doi: 10.1016/j.bbr.2012.02.047
Savic, I., Gulyas, B., Larsson, M., and Roland, P. (2000). Olfactory functions are mediated by parallel and hierarchical processing. Neuron 26, 735–745. doi: 10.1016/S0896-6273(00)81209-X
Witt, K., Granert, O., Daniels, C., Volkmann, J., Falk, D., van Eimeren, T., et al. (2013). Relation of lead trajectory and electrode position to neuropsychological outcomes of subthalamic neurostimulation in Parkinson's disease: results from a randomized trial. Brain 136(Pt. 7), 2109–2119. doi: 10.1093/brain/awt151
Zucco, G., Zeni, M. T., Perrone, A., and Piccolo, I. (2001). Olfactory sensitivity in early-stage Parkinson patients affected by more marked unilateral disorder. Percept. Mot. Skills 92, 894–898. doi: 10.2466/pms.2001.92.3.894
Zucco, G. M., Rovatti, F., and Stevenson, R. J. (2015). Olfactory asymmetric dysfunction in early Parkinson patients affected by unilateral disorder. Front. Psychol. 6:1020. doi: 10.3389/fpsyg.2015.01020
Keywords: odor recognition memory, Parkinson's disease, dementia, olfaction, systematic review
Citation: Eek T, Larsson M and Dizdar N (2021) Odor Recognition Memory in Parkinson's Disease: A Systematic Review. Front. Aging Neurosci. 13:625171. doi: 10.3389/fnagi.2021.625171
Received: 02 November 2020; Accepted: 23 April 2021;
Published: 25 May 2021.
Edited by:
Shu G. Chen, Case Western Reserve University, United StatesReviewed by:
VIncenzo Donadio, IRCCS Istuto delle Scienze Neurologiche di Bologna, ItalyM. Gustavo Murer, University of Buenos Aires, Argentina
Copyright © 2021 Eek, Larsson and Dizdar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tom Eek, dG9tLmVla0ByZWdpb25vc3RlcmdvdGxhbmQuc2U=
 Tom Eek
Tom Eek Maria Larsson
Maria Larsson Nil Dizdar
Nil Dizdar