- 1Department of Radiology, Functional and Molecular Imaging Key Laboratory of Sichuan Province, Huaxi MR Research Center (HMRRC), West China Hospital, Sichuan University, Chengdu, China
- 2Psychoradiology Research Unit of the Chinese Academy of Medical Sciences (2018RU011), West China Hospital of Sichuan University, Chengdu, China
- 3Department of Radiology, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, China
Background: Tract-based spatial statistics (TBSS) studies based on diffusion tensor imaging (DTI) have revealed extensive abnormalities in white matter (WM) fibers of Parkinson's disease (PD); however, the results were inconsistent. Therefore, a meta-analytical approach was used in this study to find the most prominent and replicable WM abnormalities of PD.
Methods: Online databases were systematically searched for all TBSS studies comparing fractional anisotropy (FA) between patients with PD and controls. Subsequently, we performed the meta-analysis using a coordinate-based meta-analytic software called seed-based d mapping. Meanwhile, meta-regression was performed to explore the potential correlation between the alteration of FA and the clinical characteristics of PD.
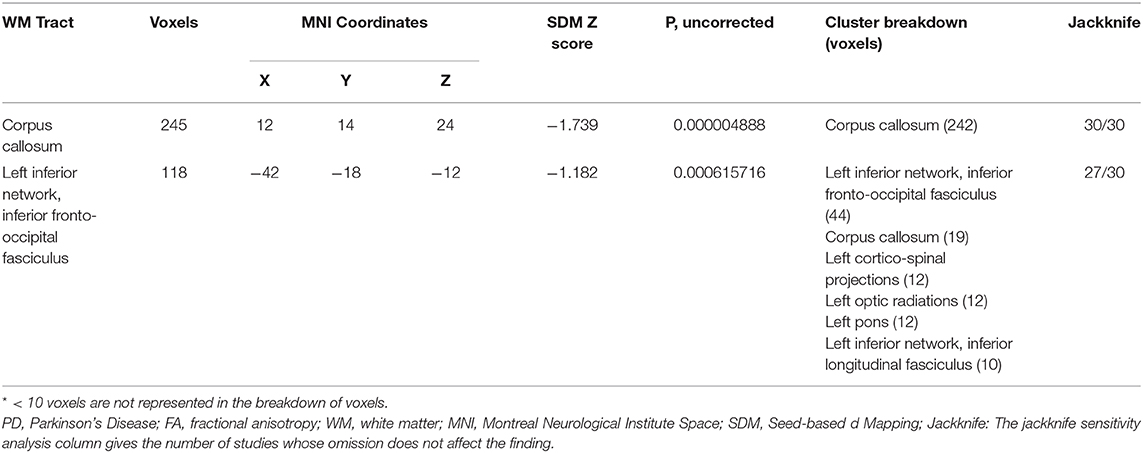
Results: Out of a total of 1,701 studies that were identified, 23 studies were included. Thirty datasets, including 915 patients (543 men) with PD and 836 healthy controls (449 men), were included in the current study. FA reduction was identified in the body of the corpus callosum (CC; 245 voxels; z = −1.739; p < 0.001) and the left inferior fronto-occipital fasciculus (IFOF) 118 voxels; z = −1.182; p < 0.001). Both CC and IFOF maintained significance in the sensitivity analysis. No increase in FA was identified, but the percentage of male patients with PD was positively associated with the value of FA in the body of the CC.
Conclusions: Although some limitations exist, DTI is regarded as a valid way to identify the pathophysiology of PD. It could be more beneficial to integrate DTI parameters with other MRI techniques to explore brain degeneration in PD.
Introduction
Parkinson's disease (PD) is a neurodegenerative disorder characterized by cardinal symptoms, including tremor, rigidity, bradykinesia, and postural instability (Tolosa et al., 2006). Although PD is defined by its motor symptoms, non-motor symptoms such as depression, sleep disturbance, autonomic dysfunction, and olfactory dysfunction are also present in patients with PD, and may even predate the emergence of the classic motor features (Berg et al., 2013). Over the past generations, the incidence and prevalence rates of PD have increased because of the general aging of the population and other factors (GBD 2016 Parkinson's Disease Collaborators, 2018). The heterogeneous symptoms and the huge burden of PD have prompted scientists to explore the neural basis of this disease to allow for both better diagnosis and intervention.
Numerous studies have sought to explain the mechanism behind PD via several different factors, e.g., genetic high risk (Chung et al., 2011; Wile et al., 2017), pathological findings of α-synuclein (Kalia, 2019), and PET with the dopamine tracer (Wile et al., 2017; Kalia, 2019). Nevertheless, these studies mostly focused on the dopamine pathway; however, patients with PD did not present with the symptoms resulting from a dopamine deficit. The development of MRI techniques offers scientists more ways, which are generally less invasive and more cost-effective, to gain insight into the neural basis of PD, bridge the cerebral changes to the clinical symptoms, and predict the progression of disease in the early stage. In numerous MRI studies, patients with PD showed extensive morphological abnormalities. A recent review summarized these abnormalities as (Sarasso et al., 2020) cortical atrophy (e.g., the caudate, the temporal/hippocampal, the frontal areas, and so on) that had accumulated with the progression of the disease and finally reached a plateau. Subsequently, a new question arose: whether these abnormalities were developed independently or resulted from the degeneration of white matter (WM) fibers. There is a hypothesis that the degeneration of WM tracts is the cause of cerebral atrophy and connectivity changes. Some studies (Lee et al., 2011; Duncan et al., 2016; Rektor et al., 2018) have suggested that changes in the WM precede changes in the gray matter (GM) in patients with PD.
Diffusion tensor imaging (DTI) is a newly developed MRI technique that can examine WM properties at a microstructural level (Basser, 1995) by encoding information about the directionality and magnitude of water molecules. Fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) are four parameters derived from DTI data to assess the WM properties (Andica et al., 2020). In the past, increasing interest in axonal analyses using DTI has deepened our understanding of the WM damage in the pathology of PD. For example, a study demonstrated that neurodegenerative microstructural alterations in patients with PD were different from normal physiological aging (Koirala et al., 2019). However, there is a great deal of inconsistency between the findings of various studies. While some studies (Kim et al., 2013; Carriere et al., 2014; Rossi et al., 2014; Duncan et al., 2016; Acosta-Cabronero et al., 2017; Georgiopoulos et al., 2017; Luo et al., 2017; Mishra et al., 2019) observed no significant differences in FA between patients with PD and healthy controls (HCs), other studies reported decreased FA in various regions, including the corpus callosum (CC; Chen Y. S. et al., 2017); left inferior fronto-occipital fasciculus (IFOF; Chen Y. S. et al., 2017); cingulum (Karagulle Kendi et al., 2008); insular (Chiang et al., 2017); thalamus (Youn et al., 2015); and the parietal, occipital, and cerebellar WM regions (Zhang et al., 2011). There have been studies reporting increased FA in the WM regions of PD, for example, the brainstem (Youn et al., 2015; Lorio et al., 2019), cerebellar, anterior corpus callosal, and inferior fronto-occipital WM (Taylor et al., 2018).
To eliminate the inconsistencies between these different studies, coordinate-based meta-analyses (Albrecht et al., 2019; Suo et al., 2020) on DTI studies were carried out, and decreases in FA were found in the CC, the left middle cerebellar peduncles (MCPs), the left IFOF, and the right inferior longitudinal fasciculus of patients with PD compared with that of HCs. These analyses combined whole-brain tract-based spatial statistics (TBSS; Smith et al., 2006) and voxel-based analyses (VBA) studies, thus attenuating the bias of region-of-interest (ROI) selection. However, VBA is inevitable with partial volume effects and misregistration errors. Compared to VBA, TBSS restricts the analysis to the center of major WM tracts and can more accurately identify WM abnormalities (Rae et al., 2012). To determine the inherent methodical differences between TBSS and VBA, a meta-analysis solely based on TBSS studies was needed.
Therefore, this study carried out a meta-analysis of only TBSS studies to identify the most robust WM microstructural abnormalities in patients with PD. Seed-based d mapping (SDM) software, a voxel-based quantitative meta-analysis approach, was performed in this study. Compared with activation likelihood estimation (ALE), another frequently used meta-analysis software, the advantage of SDM is that it considers null results. In addition, the potential relationship between clinical parameters [age, sex, the Unified Parkinson Disease Rating Scale-III (UPDRS-III) score, disease duration, the Mini-Mental State Examination (MMSE), and the levodopa equivalent daily dose (LEED)] and WM microstructural abnormalities in patients with PD was explored by regression analysis.
Materials and Methods
Study Selection
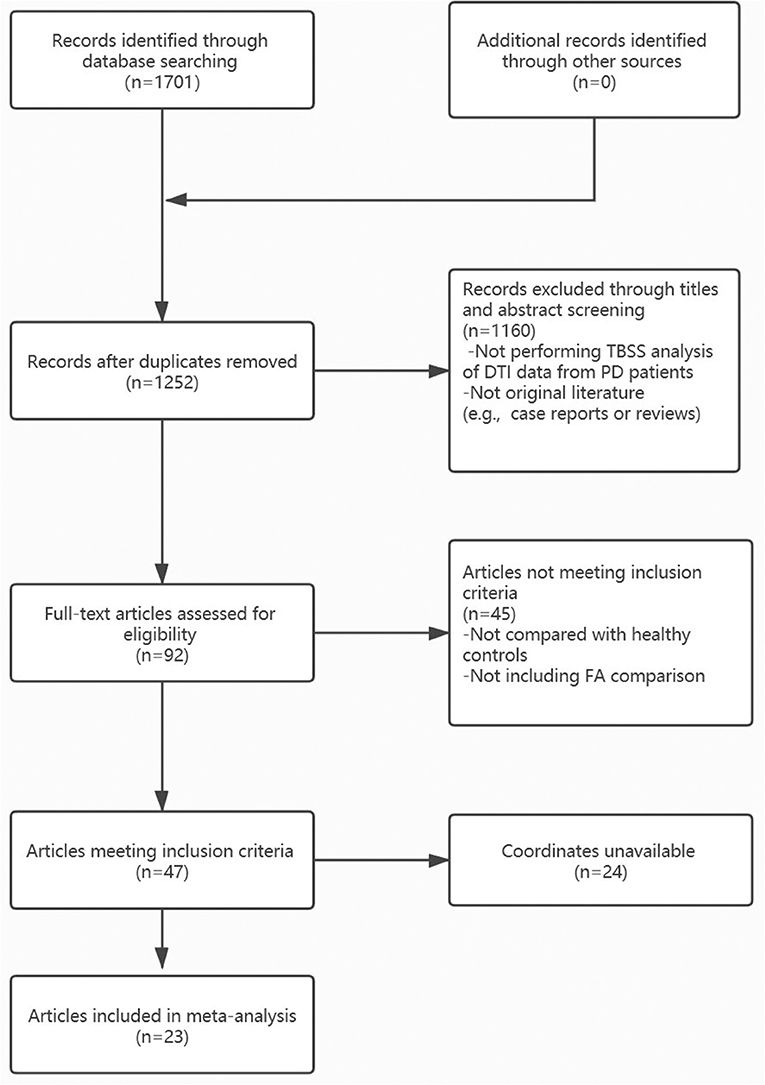
We searched for pertinent literature in PubMed, Embase, and Web of Science. The following keywords were used to identify relevant articles published up to December 2020: “Parkinson” or “Parkinson's disease”; “tract-based spatial statistical;” or “diffusion tensor” or “fractional anisotropy.” The flow diagram of the literature search is presented in Figure 1, and the inclusion/exclusion criteria are explained in the Supplementary Methods. In summary, only peer-reviewed whole-brain TBSS studies comparing WM abnormalities in patients with PD to HCs were included in this analysis, even those that report null results. The corresponding authors of studies that met all of the above inclusion criteria, but lacked global brain coordinates, were contacted to obtain additional information. Moreover, studies that used multiple independent patient samples were compared separately with the same HC groups, and the appropriate results were regarded as separate datasets. There were no overlapping samples in all included studies. All included studies were assessed and scored for quality using a 15-point checklist adapted from a previously published meta-analysis (Chen et al., 2016; Supplementary Table 1). A similar search strategy was used to explore underlying consistent MD changes in patients with PD compared to HCs. Details are presented in the Supplementary Methods.
Data Extraction
One author (CYL) searched the literature, and another author (XW) extracted demographic characteristics (sample size, age, and sex), clinical data (disease duration, UPDRS-III, MMSE, medication status, and LEED), imaging parameters, statistical thresholds, and major findings from the included studies. For studies reported with interquartile range and median, we calculated the mean and SD (Wan et al., 2014; Luo et al., 2018; http://www.math.hkbu.edu.hk/tongt/papers/median2mean.html). The three-dimensional coordinates were extracted according to the anisotropic effect-size-based algorithms AES-SDM methods (Radua et al., 2014). All information was double-checked, and decisions were reached after discussing the disagreements.
Seed-Based d Mapping Meta-Analysis and Fiber Tracking
We used AES-SDM software (version 5.15) to detect consistent FA abnormalities in patients with PD compared with HCs and used DTI-Query software (Stanford, USA) to exhibit the fibers crossing peak coordinates of FA alteration.
Following the tutorial of SDM (Radua and Mataix-Cols, 2009; Radua et al., 2014), we first recreated statistical maps and effect-sized maps of the coordinates extracted from each study (“FA,” anisotropy = 1, isotropic full width at half maximum FWHM = 20 mm, mask = “TBSS,” 10 randomizations). Then, individual study maps were entered into the meta-analysis, and the outcome was further tested in terms of sensitivity and heterogeneity, including jackknife sensitivity, heterogeneity, and publication bias analysis. The analytical parameters applied in the present study were as follows: voxel threshold p = 0.005, peak height threshold Z = 1.00, and cluster size threshold = 100 voxels, and the outcome maps were visualized using MRIcron software (University of South Carolina, USA). Subgroup analysis of studies reported with corrected results, studies with 3T scanner, and studies with a b-value of 1,000 s/mm2 were carried out. Finally, meta-regressions using a linear model were performed to detect the relationship between WM abnormalities and clinical parameters (age, sex, disease duration, UPDRS-III, MMSE, and LEED). We selected a more conservative threshold of p = 0.0005, as used in previous studies (Chen et al., 2016; Barona et al., 2019; Li et al., 2020) with peak height threshold Z = 1.00, and cluster size threshold = 10 voxels. With DTI-Query software used in previous studies (Li et al., 2012; Zhang et al., 2018), we tracked the probable fibers across the peak coordinates detected by SDM. More details of the SDM method and DTI-Query are presented in the Supplementary Methods.
Results
Included Studies and Sample Characteristics
A total of 23 whole-brain TBSS studies (Hattori et al., 2012; Kamagata et al., 2013; Kim et al., 2013; Melzer et al., 2013; Agosta et al., 2014; Carriere et al., 2014; Worker et al., 2014; Diez-Cirarda et al., 2015; Ji et al., 2015; Vercruysse et al., 2015; Vervoort et al., 2016; Wen et al., 2016; Acosta-Cabronero et al., 2017; Chen B. et al., 2017; Georgiopoulos et al., 2017; Luo et al., 2017; Firbank et al., 2018; Li et al., 2018; Minett et al., 2018; Guan et al., 2019; Quattrone et al., 2019; Inguanzo et al., 2020; Pelizzari et al., 2020) were identified using our search protocol. Six of them compared separate independent patient subgroups with the same HC groups, with details present in Table 1. Thus, a total of 30 datasets were included in our meta-analysis. Eight datasets revealed decreased FA in patients with PD in distributed regions, involving commissural fibers (e.g., CC), association fibers [e.g., IFOF and uncinate fasciculi (UF)], and projection fibers [e.g., middle cerebellar peduncle (MCP), interior capsule (IC), and anterior corona radiata (ACR)]. In addition, 22 datasets found no FA alteration in patients with PD compared to HCs. A total of 915 patients with PD (543 men, mean age 64.89 years, mean disease duration 5.65 years, mean UPDRS-III 26.83 years) and 836 matched HCs (mean age 65.19 years, males 449) were analyzed. All patients were diagnosed with PD, excluding any other Parkinsonism syndromes. The field strength of most of these studies was 3T (26/30 datasets), and the b-value was mostly 1,000 s/mm2 (21/30 datasets). The details of the demographic and clinical characteristics and study information of all included FA studies are presented in Table 1.
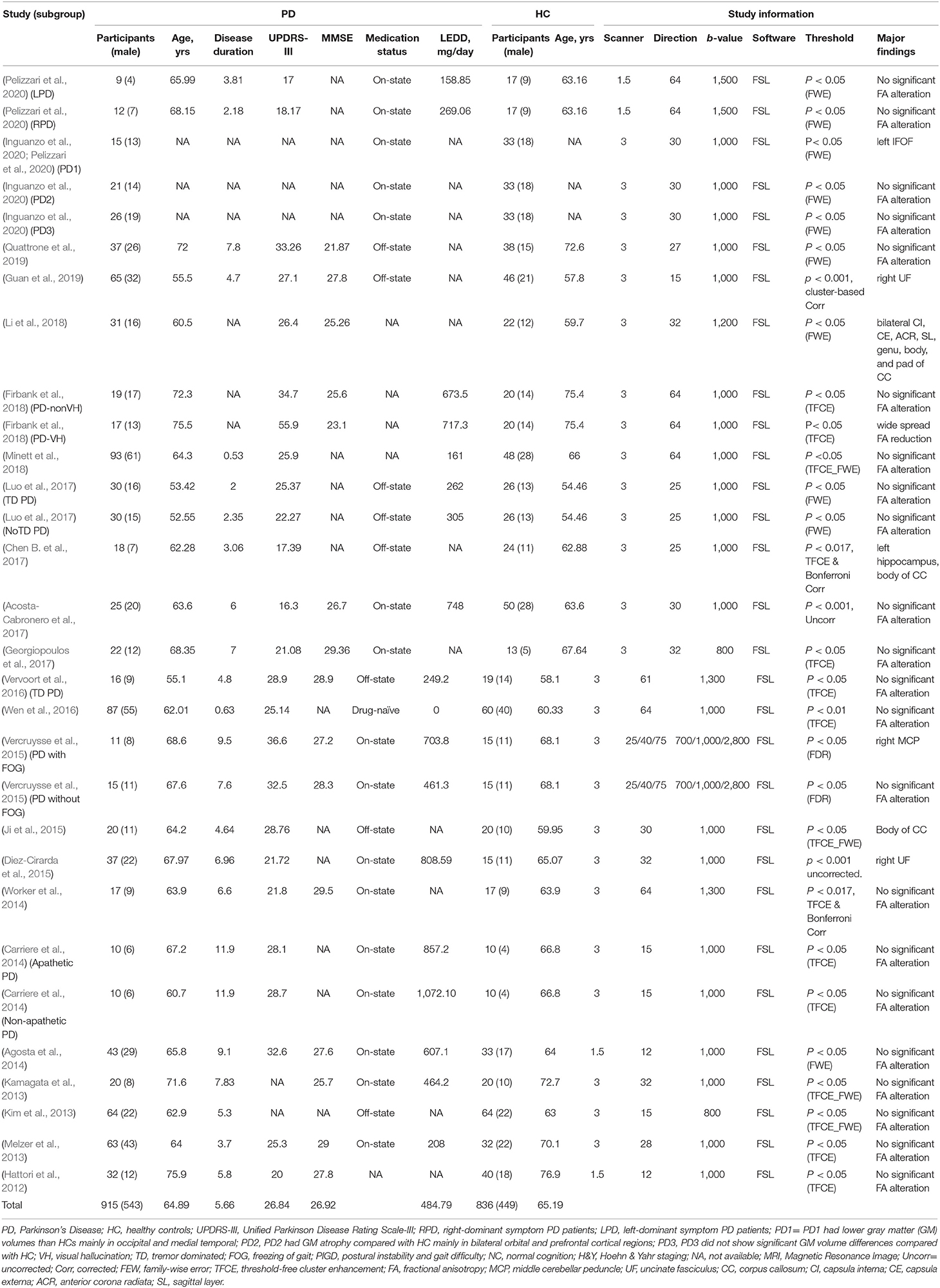
Table 1. Demographic and clinical characteristics of participants in the 23 Parkinson's disease studies (30 datasets) included in the meta-analysis.
Pooled Voxel-Based Meta-Analysis
The pooled meta-analysis of 30 datasets identified a decrease of FA in two clusters of patients with PD compared to HCs (Table 2 and Figure 2). The first cluster (245 voxels; z = −1.739; p < 0.001) peaked at the body of the CC (Montreal Neurological Institute (MNI) space: 12, 14, and 24), extending to the genu of CC. This is because the CC is interhemispheric, even if it is represented in the right hemisphere. The second cluster (118 voxels; z = −1.182; p < 0.001) included the sagittal stratum, the fornix, and the stria terminalis of the left hemisphere, peaked at the left IFOF (Montreal Neurological Institute (MNI) Space: −42, −18, −12). The main fibers likely to pass through the above peak coordinates are shown in Figure 3. No increase in FA was identified.
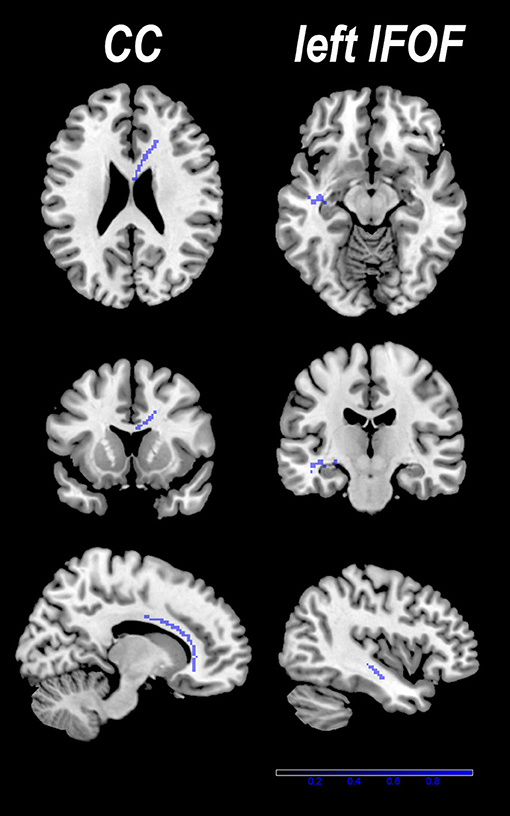
Figure 2. Two clusters with decreased fractional anisotropy (FA) were identified in patients with PD compared with healthy controls (HCs). Results are overlaid on the axial sections of the Montreal Neurological Institute standard brain in neurological convention (right is right).
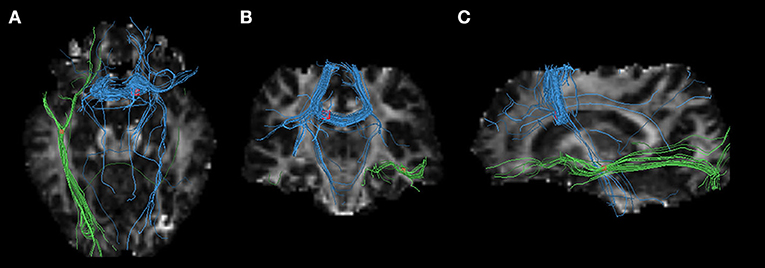
Figure 3. Three-dimensional images showing white matter tracts traversing two bounding boxes centered at were separately mapped with DTI-Query in a single normal individual. The left image (A) observed from above, the middle image (B) observed from the front, and the left side image (C) observed from the left of the brain. Tracts include the interhemispheric fibers running through the body of the CC (blue) connecting the interhemispheric MA and supplementary motor areas (SMAs), and the left inferior fronto-occipital fasciculus (IFOF) crossing left temporal lobe. Axial, coronal, and sagittal slices mapping the FA values are shown in the background for illustrative purposes.
Reliability Analysis
None of the clusters with altered FA showed statistically significant between-group heterogeneity or publication biases (Supplementary Figure 1). All clusters are highly reliable in jackknife sensitivity analysis (Supplementary Table 2), especially the body of CC, by remaining significant in all iterations. This indicates that all clusters are highly reliable.
Subgroup analysis findings of studies with corrected results and studies with 3T scanner reproduced pooled results. However, subgroup analysis of studies with a b-value of 1,000 s/mm2 failed to find any FA difference between patients with PD and HCs (Supplementary Table 3).
Result of Meta-Regression Analysis
The percentage of male patients with PD was positively associated with the FA value in the body of the CC (Figure 4). Other information on the clinical variables, including the mean age of the patients, illness duration, UPDRS-III, MMSE, and LEED is statistically non-significantly associated with FA alterations in patients with PD.
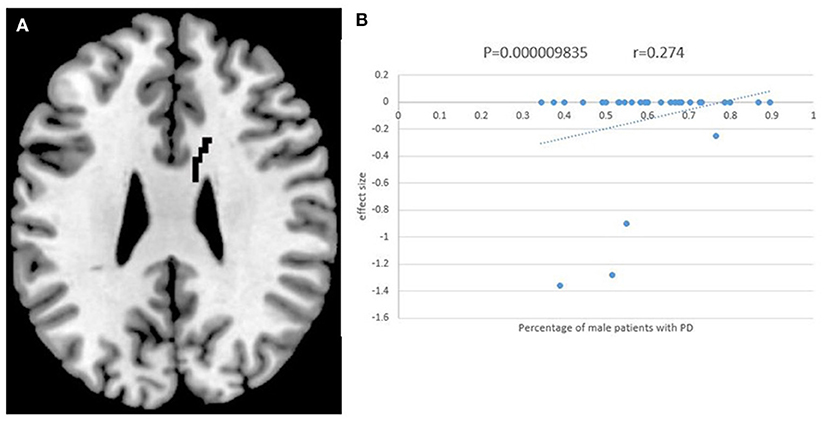
Figure 4. The results of meta-regression analysis. The percentage of male patients with PD was positively correlated with FA in the body of corpus callosum (CC). The effect sizes to create this plot were extracted from the peak of the maximum slope difference, and each study is represented as a dot. The regression line (meta-regression signed differential mapping slope) is shown.
Discussion
As far as we know, this is the first coordinate-based meta-analysis focusing on TBSS studies comparing FA in patients with PD to HCs. Our study identified lower FA mainly in the body of the CC and the left IFOF in patients with PD using the AES-SDM meta-analytical approach. Remarkably, these findings remained reproducible in jackknife sensitivity analysis and some subgroup meta-analysis, especially the body of the CC, suggesting robust disruption of the WM microstructure in those regions.
Using SDM software, Albrecht et al. (2019) identified the reduction of FA in the CC and the MCPs, and Suo et al. (2020) revealed a decrease in FA in supplementary regions including the left IFOF and the right inferior longitudinal fasciculus when merging both VBA and TBSS studies comparing FA in patients with PD to HCs. We reproduced the FA reduction of the CC and the left IFOF in patients with PD. However, some discrepancies emerged between our meta-analysis and Albrecht's findings (Albrecht et al., 2019). For instance, we failed to confirm a decrease in FA in the MCPs. There may be several reasons for such discrepancies. First, TBSS has a relative strength in the precise estimation of WM compared to the traditional VBA analysis (Smith et al., 2006), and we only included TBSS studies to avoid the inconsistency of results originating from methodological differences. Second, different samples with diverse clinical characteristics were included in our meta-analysis. The UPDRS-III score was lower in patients with PD in our sample (mean score: 26.84) than Albrecht's sample (mean score: 32.5), which might explain why we did not find WM lesions in the MCPs—a structure that mainly participates in motor coordination (Hasegawa et al., 2018).
The first cluster we identified decreased FA with a peak in the body of CC, which is consistent with many previous studies (Auning et al., 2014; Kamagata et al., 2014; Canu et al., 2015; Jiang et al., 2015; Lucas-Jiménez et al., 2016; Vervoort et al., 2016; Wang et al., 2016; Galantucci et al., 2017; Guimarães et al., 2018; Ji et al., 2019) comparing the WM diffusional properties of patients with PD to HCs. The CC plays a major role in communicating sensory, motor, and cognitive information between two cerebral hemispheres, and different parts of the CC participate in diverse cortical information communication revealed by DTI or functional MRI studies (Hofer and Frahm, 2006; Fabri et al., 2014; Courtemanche et al., 2018). Our study found decreased FA in the body and the genu of the CC, suggesting an impairment in the interhemispheric information transformation of the prefrontal lobe, motor, and supplementary motor areas (SMAs). The prefrontal cortex is a part of the limbic system and is related to higher cognitive function; apathy or reduced activation of the prefrontal lobe has been reported in patients with PD with cognitive impairment (Brück et al., 2004; Nagano-Saito et al., 2005; Trujillo et al., 2015; Gao et al., 2017). Damage to the prefrontal WM and the genu of the CC was revealed to be mainly associated with poorer intellectual or executive functions in patients with PD (Baggio et al., 2012; Hattori et al., 2012; Kamagata et al., 2013; Randver, 2018; Chondrogiorgi et al., 2019). The motor cortex generates motorial neural impulses, and functional alterations in this region are related to many motor signs in patients with PD (Burciu and Vaillancourt, 2018). The SMAs are associated with motor imagery (Iseki et al., 2008; La Fougère et al., 2010) and participates in the coupling of movement and posture during tasks (Viallet et al., 1992; Jacobs et al., 2009). Dysfunction or atrophy of the SMAs has been consistently reported in studies of patients with PD (Rosenberg-Katz et al., 2013; Herz et al., 2014; Kübel et al., 2018). In addition, transcranial magnetic stimulation (TMS) treatment (Shirota et al., 2013; Eggers et al., 2015) targeting the SMAs has been demonstrated to improve the motor function (e. g., UPSRS-III scores) of PD patients. In line with these findings, decreased FA in the body of the CC has been demonstrated to be related to poorer motor function in patients with PD (Galantucci et al., 2014), implicating the damaged interhemispheric connectivity of motor areas and SMAs in patients with PD.
However, our finding of decreased FA in the CC body in patients with PD relative to HCs was contradictory to the findings from a previous study by Wen et al. (2016) that reported increased FA in the CC. This discrepancy may be due to the disease stage of PD since, in Wen's study, these WM changes were mainly presented in the patients with PD with Hoehn & Yahr stage I, which may reflect neural compensations (Sanjari Moghaddam et al., 2020) for dopaminergic deficiency in the very early disease stage.
Furthermore, the higher percentage of male patients with PD included in our meta-analysis was associated with increased FA values in the body of the CC. It has been reported that women with PD have a higher mortality rate and faster disease progression (Bjornestad et al., 2016; Heinzel et al., 2018). Our results indicate that male patients are likely to present with a lower rate of WM degeneration. Additionally, men's WM accounted for a higher percentage of the whole-brain volume (Cosgrove et al., 2007) in a healthy population, which might contribute to this result as well.
In addition to the robust finding of decreased FA in the body of the CC, we also identified lower FA in the long fibers crossing the left temporal lobe, especially the left IFOF, in agreement with the results of previous studies (Rae et al., 2012; Gallagher et al., 2013; Theilmann et al., 2013; Lucas-Jiménez et al., 2016; Wang et al., 2016; Guimarães et al., 2018). The IFOF, a direct pathway connecting the occipital, posterior temporal, and orbitofrontal areas (Wu et al., 2016), plays an important role in visual-perceptual performance (Ashtari, 2012) and cognitive (Duncan et al., 2016) performance. Visual elaboration (Chen et al., 2013; Ekker et al., 2017) resulting from IFOF impairment might account for symptoms such as postural instability and gait difficulties (Wang et al., 2016; Tan et al., 2019) in patients with PD, and patients with predominant postural instability and gait difficulties were more likely to develop dementia in the late stage of disease (Lee et al., 2015). The current study included samples with the freezing of gait and samples with visual hallucinations, both of which have been associated with cognitive impairment in PD (Vercruysse et al., 2015; Firbank et al., 2018; Quattrone et al., 2019). The reduction of FA of WM located in the left temporal region in the present study is consistent with the lateral principle (Colosimo et al., 2010; Riederer et al., 2018) in patients with PD. Another meta-analysis (Xu et al., 2016) revealed that PD patients with mild cognitive impairment (PD-MCI) presented prominent gray matter volume (GMV) reduction in the left temporal-frontal regions, while PD patients with dementia (PDD) had bilateral reductions in those areas. The unilateral-to-bilateral development of GMV reduction in the frontal-limbic-temporal regions is a possible indicator of PD-MCI to PDD progression (Xu et al., 2016). Thus, the decreased FA in the left temporal lobe might underlie the early WM pathology of PD.
Limitations
Although this meta-analysis avoids some methodological differences by only including TBSS studies and indicates possible stable WM microstructure alterations in PD, it still has some limitations. First, the differences in scanning parameters, field strength, and MR scanner vendors between studies were impossible to eliminate by statistical means. Second, FA is a dimensional value which cannot illustrate the axonal or myelin pathology of WM (Rae et al., 2012). It might be more helpful to combine other parameters (MD, AD, and RD) with FA in a future analysis. Third, publication bias could not be avoided, although we attempted to include as many studies as possible (even if they were negative). Finally, our study uses the coordinates from the included studies rather than their original t-statistic maps, which limits the accuracy of our results. Image-based meta-analysis should be considered as an improved alternative in future studies.
Conclusion
The current quantitative meta-analysis of TBSS studies provides convergent evidence that WM disorganization exists in patients with PD and that the most robust WM abnormalities are in the body of the CC and the left IFOF, suggesting that abnormalities of interhemispheric connections and the disconnection of the left hemispheric cortical lobes might be the basis of the pathogenesis of PD. Future MRI studies could further investigate the diagnostic and prognostic value of WM microstructural abnormalities in patients with PD.
Data Availability Statement
The original contributions generated for the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
CL conceived the project and designed the protocol. XW obtained the data and analyzed the results. XW and CL wrote the main manuscript. CL and SL revised the manuscript. All authors critically reviewed the manuscript.
Funding
This study was supported by National Natural Science Foundation of China (grants 81901702 and 81761128023); grants from the Humboldt Foundation (Ref 3.5-1206715-CHN-BES); Science-Technology Support Plan Projects in Sichuan province (No. 2020YFS0117); 1.3.5 project for disciplines of excellence, Post-Doctor Research Project, West China Hospital, Sichuan University (Grant: 2018HXBH05, ZYJC18020, and ZYYC08001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2020.610962/full#supplementary-material
References
Acosta-Cabronero, J., Cardenas-Blanco, A., Betts, M. J., Butryn, M., Valdes-Herrera, J. P., Galazky, I., et al. (2017). The whole-brain pattern of magnetic susceptibility perturbations in Parkinson's disease. Brain 140, 118–131. doi: 10.1093/brain/aww278
Agosta, F., Canu, E., Stefanova, E., Sarro, L., Tomić, A., Špica, V., et al. (2014). Mild cognitive impairment in Parkinson's disease is associated with a distributed pattern of brain white matter damage. Hum. Brain Mapp. 35, 1921–1929. doi: 10.1002/hbm.22302
Albrecht, F., Ballarini, T., Neumann, J., and Schroeter, M. L. (2019). FDG-PET hypometabolism is more sensitive than MRI atrophy in Parkinson's disease: A whole-brain multimodal imaging meta-analysis. Neuroimage. Clin. 21:101594. doi: 10.1016/j.nicl.2018.11.004
Andica, C., Kamagata, K., Hatano, T., Saito, Y., Ogaki, K., Hattori, N., et al. (2020). MR biomarkers of degenerative brain disorders derived from diffusion imaging. J. Magnet. Reson. Imag. 52, 1620–1636. doi: 10.1002/jmri.27019
Ashtari, M. (2012). Anatomy and functional role of the inferior longitudinal fasciculus: a search that has just begun. Dev. Med. Child Neurol. 54, 6–7. doi: 10.1111/j.1469-8749.2011.04122.x
Auning, E., Kjærvik, V. K., Selnes, P., Aarsland, D., Haram, A., Bjørnerud, A., et al. (2014). White matter integrity and cognition in Parkinson's disease: A cross-sectional study. BMJ Open 4:e003976. doi: 10.1136/bmjopen-2013-003976
Baggio, H. C., Segura, B., Ibarretxe-Bilbao, N., Valldeoriola, F., Marti, M. J., Compta, Y., et al. (2012). Structural correlates of facial emotion recognition deficits in Parkinson's disease patients. Neuropsychologia 50, 2121–2128. doi: 10.1016/j.neuropsychologia.2012.05.020
Barona, M., Brown, M., Clark, C., Frangou, S., White, T., and Micali, N. (2019). White matter alterations in anorexia nervosa: evidence from a voxel-based meta-analysis. Neurosci. Biobehav. Rev. 100, 285–295. doi: 10.1016/j.neubiorev.2019.03.002
Basser, P. J. (1995). Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 8, 333–344. doi: 10.1002/nbm.1940080707
Berg, D., Lang, A. E., Postuma, R. B., Maetzler, W., Deuschl, G., Gasser, T., et al. (2013). Changing the research criteria for the diagnosis of Parkinson's disease: obstacles and opportunities. Lancet Neurol. 12, 514–524. doi: 10.1016/S1474-4422(13)70047-4
Bjornestad, A., Forsaa, E. B., Pedersen, K. F., Tysnes, O.-B., Larsen, J. P., and Alves, G. (2016). Risk and course of motor complications in a population-based incident Parkinson's disease cohort. Parkinsonism Relat. Disord. 22, 48–53. doi: 10.1016/j.parkreldis.2015.11.007
Brück, A., Kurki, T., Kaasinen, V., Vahlberg, T., and Rinne, J. O. (2004). Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson's disease is related to cognitive impairment. J. Neurol. Neurosurg. Psychiatr. 75, 1467–1469. doi: 10.1136/jnnp.2003.031237
Burciu, R. G., and Vaillancourt, D. E. (2018). Imaging of motor cortex physiology in Parkinson's disease. Movement Disord. 33, 1688–1699. doi: 10.1002/mds.102
Canu, E., Agosta, F., Sarasso, E., Volontè, M. A., Basaia, S., Stojkovic, T., et al. (2015). Brain structural and functional connectivity in Parkinson's disease with freezing of gait. Hum. Brain Mapp. 36, 5064–5078. doi: 10.1002/hbm.22994
Carriere, N., Besson, P., Dujardin, K., Duhamel, A., Defebvre, L., Delmaire, C., et al. (2014). Apathy in Parkinson's disease is associated with nucleus accumbens atrophy: a magnetic resonance imaging shape analysis. Movement Disord. 29, 897–903. doi: 10.1002/mds.25904
Chen, B., Fan, G., Sun, W., Shang, X., Shi, S., Wang, S., et al. (2017). Usefulness of diffusion-tensor MRI in the diagnosis of Parkinson variant of multiple system atrophy and Parkinson's disease: a valuable tool to differentiate between them? Clin. Radiol. 72, 610.e619–610.e615. doi: 10.1016/j.crad.2017.02.005
Chen, L., Hu, X., Ouyang, L., He, N., Liao, Y., Liu, Q., et al. (2016). A systematic review and meta-analysis of tract-based spatial statistics studies regarding attention-deficit/hyperactivity disorder. Neurosci. Biobehav. Rev. 68, 838–847. doi: 10.1016/j.neubiorev.2016.07.022
Chen, S. T., Geller, A. C., and Tsao, H. (2013). Update on the epidemiology of melanoma. Curr. Dermatol. Rep. 2, 24–34. doi: 10.1007/s13671-012-0035-5
Chen, Y. S., Chen, M. H., Lu, C. H., Chen, P. C., Chen, H. L., Yang, I. H., et al. (2017). Associations among cognitive functions, plasma DNA, and white matter integrity in patients with early-onset Parkinson's disease. Front. Neurosci. 11:9. doi: 10.3389/fnins.2017.00009
Chiang, P. L., Chen, H. L., Lu, C. H., Chen, P. C., Chen, M. H., Yang, I. H., et al. (2017). White matter damage and systemic inflammation in Parkinson's disease. BMC Neurosci 18:48. doi: 10.1186/s12868-017-0367-y
Chondrogiorgi, M., Astrakas, L. G., Zikou, A. K., Weis, L., Xydis, V. G., Antonini, A., et al. (2019). Multifocal alterations of white matter accompany the transition from normal cognition to dementia in Parkinson's disease patients. Brain Imaging Behav. 13, 232–240. doi: 10.1007/s11682-018-9863-7
Chung, S. J., Armasu, S. M., Biernacka, J. M., Lesnick, T. G., Rider, D. N., Lincoln, S. J., et al. (2011). Common variants in PARK loci and related genes and Parkinson's disease. Movement Disord. 26, 280–288. doi: 10.1002/mds.23376
Colosimo, C., Martínez-Martín, P., Fabbrini, G., Hauser, R. A., Merello, M., Miyasaki, J., et al. (2010). Task force report on scales to assess dyskinesia in Parkinson's disease: critique and recommendations. Movement Disord. 25, 1131–1142. doi: 10.1002/mds.23072
Cosgrove, K. P., Mazure, C. M., and Staley, J. K. (2007). Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol. Psychiatr. 62, 847–855. doi: 10.1016/j.biopsych.2007.03.001
Courtemanche, M. J., Sparrey, C. J., Song, X., Mackay, A., and D'arcy, R. C. N. (2018). Detecting white matter activity using conventional 3 Tesla fMRI: An evaluation of standard field strength and hemodynamic response function. Neuroimage 169, 145–150. doi: 10.1016/j.neuroimage.2017.12.008
Diez-Cirarda, M., Ojeda, N., Pena, J., Cabrera-Zubizarreta, A., Gomez-Beldarrain, M. A., Gomez-Esteban, J. C., et al. (2015). Neuroanatomical correlates of theory of mind deficit in Parkinson's disease: a multimodal imaging study. PLoS ONE 10:e0142234. doi: 10.1371/journal.pone.0142234
Duncan, G. W., Firbank, M. J., Yarnall, A. J., Khoo, T. K., Brooks, D. J., Barker, R. A., et al. (2016). Gray and white matter imaging: A biomarker for cognitive impairment in early Parkinson's disease? Movement Disord. 31, 103–110. doi: 10.1002/mds.26312
Eggers, C., Günther, M., Rothwell, J., Timmermann, L., and Ruge, D. (2015). Theta burst stimulation over the supplementary motor area in Parkinson's disease. J. Neurol. 262, 357–364. doi: 10.1007/s00415-014-7572-8
Ekker, M. S., Janssen, S., Seppi, K., Poewe, W., De Vries, N. M., Theelen, T., et al. (2017). Ocular and visual disorders in Parkinson's disease: common but frequently overlooked. Parkinsonism Relat. Disord. 40, 1–10. doi: 10.1016/j.parkreldis.2017.02.014
Fabri, M., Pierpaoli, C., Barbaresi, P., and Polonara, G. (2014). Functional topography of the corpus callosum investigated by DTI and fMRI. World J. Radiol. 6, 895–906. doi: 10.4329/wjr.v6.i12.895
Firbank, M. J., Parikh, J., Murphy, N., Killen, A., Allan, C. L., Collerton, D., et al. (2018). Reduced occipital GABA in Parkinson disease with visual hallucinations. Neurology 91, e675–e685. doi: 10.1212/WNL.0000000000006007
Galantucci, S., Agosta, F., Stankovic, I., Petrovic, I., Stojkovic, T., Comi, G., et al. (2014). Corpus callosum damage and motor function in Parkinson's disease (P2.006). Neurology 82:P2.006.
Galantucci, S., Agosta, F., Stefanova, E., Basaia, S., Van Den Heuvel, M. P., Stojković, T., et al. (2017). Structural brain connectome and cognitive impairment in Parkinson disease. Radiology 283, 515–525. doi: 10.1148/radiol.2016160274
Gallagher, C., Bell, B., Bendlin, B., Palotti, M., Okonkwo, O., Sodhi, A., et al. (2013). White matter microstructural integrity and executive function in parkinson's disease. J. Int. Neuropsychol. Soc. 19, 349–354. doi: 10.1017/S1355617712001373
Gao, Y., Nie, K., Huang, B., Mei, M., Guo, M., Xie, S., et al. (2017). Changes of brain structure in Parkinson's disease patients with mild cognitive impairment analyzed via VBM technology. Neurosci. Lett. 658, 121–132. doi: 10.1016/j.neulet.2017.08.028
GBD 2016 Parkinson's Disease Collaborators (2018). Global, regional, and national burden of Parkinson's disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 17, 939–953. doi: 10.1016/s1474-4422(18)30295-3
Georgiopoulos, C., Warntjes, M., Dizdar, N., Zachrisson, H., Engström, M., Haller, S., et al. (2017). Olfactory impairment in Parkinson's disease studied with diffusion tensor and magnetization transfer imaging. J. Parkinson Dis. 7, 301–311. doi: 10.3233/JPD-161060
Guan, X., Huang, P., Zeng, Q., Liu, C., Wei, H., Xuan, M., et al. (2019). Quantitative susceptibility mapping as a biomarker for evaluating white matter alterations in Parkinson's disease. Brain Imaging Behav. 13, 220–231. doi: 10.1007/s11682-018-9842-z
Guimarães, R. P., Campos, B. M., De Rezende, T. J., Piovesana, L., Azevedo, P. C., Amato-Filho, A. C., et al. (2018). Is diffusion tensor imaging a good biomarker for early Parkinson's disease? Front. Neurol. 9:626. doi: 10.3389/fneur.2018.00626
Hasegawa, T., Yamada, K., Tozawa, T., Chiyonobu, T., Tokuda, S., Nishimura, A., et al. (2018). Cerebellar peduncle injury predicts motor impairments in preterm infants: A quantitative tractography study at term-equivalent age. Brain Dev. 40, 743–752. doi: 10.1016/j.braindev.2018.04.013
Hattori, T., Orimo, S., Aoki, S., Ito, K., Abe, O., Amano, A., et al. (2012). Cognitive status correlates with white matter alteration in Parkinson's disease. Hum. Brain Mapp. 33, 727–739. doi: 10.1002/hbm.21245
Heinzel, S., Kasten, M., Behnke, S., Vollstedt, E.-J., Klein, C., Hagenah, J., et al. (2018). Age- and sex-related heterogeneity in prodromal Parkinson's disease. Movement Disord. 33, 1025–1027. doi: 10.1002/mds.27349
Herz, D. M., Eickhoff, S. B., Løkkegaard, A., and Siebner, H. R. (2014). Functional neuroimaging of motor control in Parkinson's disease: a meta-analysis. Hum. Brain Mapp. 35, 3227–3237. doi: 10.1002/hbm.22397
Hofer, S., and Frahm, J. (2006). Topography of the human corpus callosum revisited–comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 32, 989–994. doi: 10.1016/j.neuroimage.2006.05.044
Inguanzo, A., Sala-Llonch, R., Segura, B., Erostarbe, H., Abos, A., Campabadal, A., et al. (2020). Hierarchical cluster analysis of multimodal imaging data identifies brain atrophy and cognitive patterns in Parkinson's disease. Parkinsonism Relat. Disord. 82, 16–23. doi: 10.1016/j.parkreldis.2020.11.010
Iseki, K., Hanakawa, T., Shinozaki, J., Nankaku, M., and Fukuyama, H. (2008). Neural mechanisms involved in mental imagery and observation of gait. Neuroimage. 41, 1021–1031. doi: 10.1016/j.neuroimage.2008.03.010
Jacobs, J. V., Lou, J. S., Kraakevik, J. A., and Horak, F. B. (2009). The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson's disease. Neuroscience. 164, 877–885. doi: 10.1016/j.neuroscience.2009.08.002
Ji, G. J., Ren, C., Li, Y., Sun, J., Liu, T., Gao, Y., et al. (2019). Regional and network properties of white matter function in Parkinson's disease. Hum. Brain Mapp. 40, 1253–1263. doi: 10.1002/hbm.24444
Ji, L., Wang, Y., Zhu, D., Liu, W., and Shi, J. (2015). White matter differences between multiple system atrophy (parkinsonian type) and parkinson's disease: a diffusion tensor image study. Neuroscience 305, 109–116. doi: 10.1016/j.neuroscience.2015.07.060
Jiang, M. F., Shi, F., Niu, G. M., Xie, S. H., and Yu, S. Y. (2015). A novel method for evaluating brain function and microstructural changes in parkinson's disease. Neural Regene. Res. 10, 2025–2032. doi: 10.4103/1673-5374.172322
Kalia, L. V. (2019). Diagnostic biomarkers for Parkinson's disease: focus on α-synuclein in cerebrospinal fluid. Parkinsonism Relat. Disord. 59, 21–25. doi: 10.1016/j.parkreldis.2018.11.016
Kamagata, K., Motoi, Y., Tomiyama, H., Abe, O., Ito, K., Shimoji, K., et al. (2013). Relationship between cognitive impairment and white-matter alteration in Parkinson's disease with dementia: tract-based spatial statistics and tract-specific analysis. Eur. Radiol. 23, 1946–1955. doi: 10.1007/s00330-013-2775-4
Kamagata, K., Tomiyama, H., Hatano, T., Motoi, Y., Abe, O., Shimoji, K., et al. (2014). A preliminary diffusional kurtosis imaging study of Parkinson disease: comparison with conventional diffusion tensor imaging. Neuroradiology 56, 251–258. doi: 10.1007/s00234-014-1327-1
Karagulle Kendi, A. T., Lehericy, S., Luciana, M., Ugurbil, K., and Tuite, P. (2008). Altered diffusion in the frontal lobe in Parkinson disease. Am. J. Neuroradiol. 29, 501–505. doi: 10.3174/ajnr.A0850
Kim, H. J., Kim, S. J., Kim, H. S., Choi, C. G., Kim, N., Han, S., et al. (2013). Alterations of mean diffusivity in brain white matter and deep gray matter in Parkinson's disease. Neurosci. Lett. 550, 64–68. doi: 10.1016/j.neulet.2013.06.050
Koirala, N., Anwar, A. R., Ciolac, D., Glaser, M., Pintea, B., Deuschl, G., et al. (2019). Alterations in white matter network and microstructural integrity differentiate Parkinson's disease patients and healthy subjects. Front. Aging Neurosci. 11:191. doi: 10.3389/fnagi.2019.00191
Kübel, S., Stegmayer, K., Vanbellingen, T., Walther, S., and Bohlhalter, S. (2018). Deficient supplementary motor area at rest: Neural basis of limb kinetic deficits in Parkinson's disease. Hum. Brain Mapp. 39, 3691–3700. doi: 10.1002/hbm.24204
La Fougère, C., Zwergal, A., Rominger, A., Förster, S., Fesl, G., Dieterich, M., et al. (2010). Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. Neuroimage 50, 1589–1598. doi: 10.1016/j.neuroimage.2009.12.060
Lee, E.-Y., Sen, S., Eslinger, P. J., Wagner, D., Kong, L., Lewis, M. M., et al. (2015). Side of motor onset is associated with hemisphere-specific memory decline and lateralized gray matter loss in Parkinson's disease. Parkinsonism Relat. Disord. 21, 465–470. doi: 10.1016/j.parkreldis.2015.02.008
Lee, S. H., Kim, S. S., Tae, W. S., Lee, S. Y., Choi, J. W., Koh, S. B., et al. (2011). Regional volume analysis of the Parkinson disease brain in early disease stage: gray matter, white matter, striatum, and thalamus. AJNR 32, 682–687. doi: 10.3174/ajnr.A2372
Li, J., Pan, P., Huang, R., and Shang, H. (2012). A meta-analysis of voxel-based morphometry studies of white matter volume alterations in Alzheimer's disease. Neurosci. Biobehav. Rev. 36, 757–763. doi: 10.1016/j.neubiorev.2011.12.001
Li, Q., Zhao, Y., Chen, Z., Long, J., Dai, J., Huang, X., et al. (2020). Meta-analysis of cortical thickness abnormalities in medication-free patients with major depressive disorder. Neuropsychopharmacology 45, 703–712. doi: 10.1038/s41386-019-0563-9
Li, X. R., Ren, Y. D., Cao, B., and Huang, X. L. (2018). Analysis of white matter characteristics with tract-based spatial statistics according to diffusion tensor imaging in early Parkinson's disease. Neurosci. Lett. 675, 127–132. doi: 10.1016/j.neulet.2017.11.064
Lorio, S., Sambataro, F., Bertolino, A., Draganski, B., and Dukart, J. (2019). The combination of DAT-SPECT, structural and diffusion MRI predicts clinical progression in Parkinson's disease. Front. Aging Neurosci. 11:57. doi: 10.3389/fnagi.2019.00057
Lucas-Jiménez, O., Ojeda, N., Peña, J., Díez-Cirarda, M., Cabrera-Zubizarreta, A., Gómez-Esteban, J. C., et al. (2016). Altered functional connectivity in the default mode network is associated with cognitive impairment and brain anatomical changes in Parkinson's disease. Parkinsonism Relat. Disord. 33, 58–64. doi: 10.1016/j.parkreldis.2016.09.012
Luo, C. Y., Song, W., Chen, Q., Yang, J., Gong, Q. Y., and Shang, H. F. (2017). White matter microstructure damage in tremor-dominant Parkinson's disease patients. Neuroradiology 59, 691–698. doi: 10.1007/s00234-017-1846-7
Luo, D., Wan, X., Liu, J., and Tong, T. (2018). Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 27, 1785–1805. doi: 10.1177/0962280216669183
Melzer, T. R., Watts, R., Macaskill, M. R., Pitcher, T. L., Livingston, L., Keenan, R. J., et al. (2013). White matter microstructure deteriorates across cognitive stages in Parkinson disease. Neurology 80, 1841–1849. doi: 10.1212/WNL.0b013e3182929f62
Minett, T., Su, L., Mak, E., Williams, G., Firbank, M., Lawson, R. A., et al. (2018). Longitudinal diffusion tensor imaging changes in early Parkinson's disease: ICICLE-PD study. J. Neurol. 265, 1528–1539. doi: 10.1007/s00415-018-8873-0
Mishra, V. R., Sreenivasan, K. R., Zhuang, X., Yang, Z., Cordes, D., and Walsh, R. R. (2019). Influence of analytic techniques on comparing DTI-derived measurements in early stage Parkinson's disease. Heliyon 5:e01481. doi: 10.1016/j.heliyon.2019.e01481
Nagano-Saito, A., Washimi, Y., Arahata, Y., Kachi, T., Lerch, J. P., Evans, A. C., et al. (2005). Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology 64, 224–229. doi: 10.1212/01.WNL.0000149510.41793.50
Pelizzari, L., Di Tella, S., Laganà, M. M., Bergsland, N., Rossetto, F., Nemni, R., et al. (2020). White matter alterations in early Parkinson's disease: role of motor symptom lateralization. Neurol. Sci. 41, 357–364. doi: 10.1007/s10072-019-04084-y
Quattrone, A., Caligiuri, M. E., Morelli, M., Nigro, S., Vescio, B., Arabia, G., et al. (2019). Imaging counterpart of postural instability and vertical ocular dysfunction in patients with PSP: A multimodal MRI study. Parkinsonism Relat. Disord. 63, 124–130. doi: 10.1016/j.parkreldis.2019.02.022
Radua, J., Grau, M., Van Den Heuvel, O. A., Thiebaut De Schotten, M., Stein, D. J., Canales-Rodriguez, E. J., et al. (2014). Multimodal voxel-based meta-analysis of white matter abnormalities in obsessive-compulsive disorder. Neuropsychopharmacology 39, 1547–1557. doi: 10.1038/npp.2014.5
Radua, J., and Mataix-Cols, D. (2009). Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br. J. Psychiatr. 195, 393–402. doi: 10.1192/bjp.bp.108.055046
Rae, C. L., Correia, M. M., Altena, E., Hughes, L. E., Barker, R. A., and Rowe, J. B. (2012). White matter pathology in Parkinson's disease: the effect of imaging protocol differences and relevance to executive function. Neuroimage 62, 1675–1684. doi: 10.1016/j.neuroimage.2012.06.012
Randver, R. (2018). Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex to alleviate depression and cognitive impairment associated with Parkinson's disease: A review and clinical implications. J. Neurol. Sci. 393, 88–99. doi: 10.1016/j.jns.2018.08.014
Rektor, I., Svátková, A., Vojtíšek, L., Zikmundová, I., Vaníček, J., Király, A., et al. (2018). White matter alterations in Parkinson's disease with normal cognition precede grey matter atrophy. PLoS ONE 13:e0187939. doi: 10.1371/journal.pone.0187939
Riederer, P., Jellinger, K. A., Kolber, P., Hipp, G., Sian-Hülsmann, J., and Krüger, R. (2018). Lateralisation in Parkinson disease. Cell Tissue Res. 373, 297–312. doi: 10.1007/s00441-018-2832-z
Rosenberg-Katz, K., Herman, T., Jacob, Y., Giladi, N., Hendler, T., and Hausdorff, J. M. (2013). Gray matter atrophy distinguishes between Parkinson disease motor subtypes. Neurology 80, 1476–1484. doi: 10.1212/WNL.0b013e31828cfaa4
Rossi, M. E., Ruottinen, H., Saunamäki, T., Elovaara, I., and Dastidar, P. (2014). Imaging brain iron and diffusion patterns. a follow-up study of Parkinson's disease in the initial stages. Acad. Radiol. 21, 64–71. doi: 10.1016/j.acra.2013.09.018
Sanjari Moghaddam, H., Dolatshahi, M., Mohebi, F., and Aarabi, M. H. (2020). Structural white matter alterations as compensatory mechanisms in Parkinson's disease: a systematic review of diffusion tensor imaging studies. J. Neurosci. Res. 98, 1398–1416. doi: 10.1002/jnr.24617
Sarasso, E., Agosta, F., Piramide, N., and Filippi, M. (2020). Progression of grey and white matter brain damage in Parkinson's disease: a critical review of structural MRI literature. J. Neurol. doi: 10.1007/s00415-020-09863-8. [Epub ahead of print].
Shirota, Y., Ohtsu, H., Hamada, M., Enomoto, H., and Ugawa, Y. (2013). Supplementary motor area stimulation for Parkinson disease: a randomized controlled study. Neurology 80, 1400–1405. doi: 10.1212/WNL.0b013e31828c2f66
Smith, S. M., Jenkinson, M., Johansen-Berg, H., Rueckert, D., Nichols, T. E., Mackay, C. E., et al. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505. doi: 10.1016/j.neuroimage.2006.02.024
Suo, X., Lei, D., Li, W., Li, L., Dai, J., Wang, S., et al. (2020). Altered white matter microarchitecture in Parkinson's disease: a voxel-based meta-analysis of diffusion tensor imaging studies. Front. Med. doi: 10.1007/s11684-019-0725-5. [Epub ahead of print].
Tan, S. Y. Z., Keong, N. C. H., Selvan, R. M. P., Li, H., Ooi, L. Q. R., Tan, E. K., et al. (2019). Periventricular white matter abnormalities on diffusion tensor imaging of postural instability gait disorder Parkinsonism. AJNR Am. J. Neuroradiol. 40, 609–613. doi: 10.3174/ajnr.A5993
Taylor, K. I., Sambataro, F., Boess, F., Bertolino, A., and Dukart, J. (2018). Progressive decline in gray and white matter integrity in de novo Parkinson's disease: an analysis of longitudinal Parkinson progression markers initiative diffusion tensor imaging data. Front. Aging Neurosci. 10:318. doi: 10.3389/fnagi.2018.00318
Theilmann, R. J., Reed, J. D., Song, D. D., Huang, M. X., Lee, R. R., Litvan, I., et al. (2013). White-matter changes correlate with cognitive functioning in Parkinson's disease. Front. Neurol. 4:37. doi: 10.3389/fneur.2013.00037
Tolosa, E., Wenning, G., and Poewe, W. (2006). The diagnosis of Parkinson's disease. Lancet Neurol. 5, 75–86. doi: 10.1016/S1474-4422(05)70285-4
Trujillo, J. P., Gerrits, N. J. H. M., Vriend, C., Berendse, H. W., Van Den Heuvel, O. A., and Van Der Werf, Y. D. (2015). Impaired planning in Parkinson's disease is reflected by reduced brain activation and connectivity. Hum. Brain Mapp. 36, 3703–3715. doi: 10.1002/hbm.22873
Vercruysse, S., Leunissen, I., Vervoort, G., Vandenberghe, W., Swinnen, S., and Nieuwboer, A. (2015). Microstructural changes in white matter associated with freezing of gait in Parkinson's disease. Movement Disord. 30, 567–576. doi: 10.1002/mds.26130
Vervoort, G., Leunissen, I., Firbank, M., Heremans, E., Nackaerts, E., Vandenberghe, W., et al. (2016). Structural brain alterations in motor subtypes of Parkinson's disease: evidence from probabilistic tractography and shape analysis. PLoS ONE 11:e0157743. doi: 10.1371/journal.pone.0157743
Viallet, F., Massion, J., Massarino, R., and Khalil, R. (1992). Coordination between posture and movement in a bimanual load lifting task: putative role of a medial frontal region including the supplementary motor area. Experi. Brain Res. 88, 674–684. doi: 10.1007/BF00228197
Wan, X., Wang, W., Liu, J., and Tong, T. (2014). Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14:135. doi: 10.1186/1471-2288-14-135
Wang, M., Jiang, S., Yuan, Y., Zhang, L., Ding, J., Wang, J., et al. (2016). Alterations of functional and structural connectivity of freezing of gait in Parkinson's disease. J. Neurol. 263, 1583–1592. doi: 10.1007/s00415-016-8174-4
Wen, M. C., Heng, H. S., Ng, S. Y., Tan, L. C., Chan, L. L., and Tan, E. K. (2016). White matter microstructural characteristics in newly diagnosed Parkinson's disease: an unbiased whole-brain study. Sci. Rep. 6:35601. doi: 10.1038/srep35601
Wile, D. J., Agarwal, P. A., Schulzer, M., Mak, E., Dinelle, K., Shahinfard, E., et al. (2017). Serotonin and dopamine transporter PET changes in the premotor phase of LRRK2 parkinsonism: cross-sectional studies. Lancet Neurol. 16, 351–359. doi: 10.1016/S1474-4422(17)30056-X
Worker, A., Blain, C., Jarosz, J., Chaudhuri, K. R., Barker, G. J., Williams, S. C. R., et al. (2014). Diffusion tensor imaging of Parkinson's disease, multiple system atrophy and progressive supranuclear palsy: a tract-based spatial statistics study. PLoS ONE 9:e112638. doi: 10.1371/journal.pone.0112638
Wu, Y., Sun, D., Wang, Y., and Wang, Y. (2016). Subcomponents and connectivity of the inferior fronto-occipital fasciculus revealed by diffusion spectrum imaging fiber tracking. Front. Neuroanat. 10:88. doi: 10.3389/fnana.2016.00088
Xu, Y., Yang, J., Hu, X., and Shang, H. (2016). Voxel-based meta-analysis of gray matter volume reductions associated with cognitive impairment in Parkinson's disease. J. Neurol. 263, 1178–1187. doi: 10.1007/s00415-016-8122-3
Youn, J., Lee, J. M., Kwon, H., Kim, J. S., Son, T. O., and Cho, J. W. (2015). Alterations of mean diffusivity of pedunculopontine nucleus pathway in Parkinson's disease patients with freezing of gait. Parkinsonism Relat. Disord. 21, 12–17. doi: 10.1016/j.parkreldis.2014.10.003
Zhang, F., Chen, G., He, M., Dai, J., Shang, H., Gong, Q., et al. (2018). Altered white matter microarchitecture in amyotrophic lateral sclerosis: a voxel-based meta-analysis of diffusion tensor imaging. Neuroimage Clin. 19, 122–129. doi: 10.1016/j.nicl.2018.04.005
Keywords: meta-analysis, Parkinson disease, diffusion tensor imaging, tract-based spatial statistics, fractional anisotropy, seed-based d mapping
Citation: Wei X, Luo C, Li Q, Hu N, Xiao Y, Liu N, Lui S and Gong Q (2021) White Matter Abnormalities in Patients With Parkinson's Disease: A Meta-Analysis of Diffusion Tensor Imaging Using Tract-Based Spatial Statistics. Front. Aging Neurosci. 12:610962. doi: 10.3389/fnagi.2020.610962
Received: 28 September 2020; Accepted: 28 December 2020;
Published: 28 January 2021.
Edited by:
Panteleimon Giannakopoulos, Université de Genève, SwitzerlandReviewed by:
Sachchida Nand Rai, University of Allahabad, IndiaThomas Welton, National Neuroscience Institute (NNI), Singapore
Copyright © 2021 Wei, Luo, Li, Hu, Xiao, Liu, Lui and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Su Lui, bHVzdXdjdW1zQGhvdG1haWwuY29t
†These authors have contributed equally to this work
 Xia Wei
Xia Wei Chunyan Luo
Chunyan Luo Qian Li1,2,3
Qian Li1,2,3 Na Hu
Na Hu Nian Liu
Nian Liu Qiyong Gong
Qiyong Gong