
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Aging Neurosci. , 09 November 2020
Sec. Parkinson’s Disease and Aging-related Movement Disorders
Volume 12 - 2020 | https://doi.org/10.3389/fnagi.2020.592751
This article is part of the Research Topic Novel Technologies to Measure and Modulate the Supraspinal Control of Standing and Walking in Older Adults View all 9 articles
 Lars I. E. Oddsson1,2,3*
Lars I. E. Oddsson1,2,3* Teresa Bisson2,4
Teresa Bisson2,4 Helen S. Cohen5
Helen S. Cohen5 Laura Jacobs1
Laura Jacobs1 Mohammad Khoshnoodi6
Mohammad Khoshnoodi6 Doris Kung5
Doris Kung5 Lewis A. Lipsitz7,8,9
Lewis A. Lipsitz7,8,9 Brad Manor7,8,9
Brad Manor7,8,9 Patricia McCracken10
Patricia McCracken10 Yvonne Rumsey1
Yvonne Rumsey1 Diane M. Wrisley11
Diane M. Wrisley11 Sara R. Koehler-McNicholas2,10
Sara R. Koehler-McNicholas2,10Background: Sensory peripheral neuropathy (PN) is associated with gait, balance problems and high fall risk. The walk2Wellness trial investigates effects of long-term, home-based daily use of a wearable sensory prosthesis on gait function, balance, quality of life and fall rates in PN patients. The device (Walkasins®, RxFunction Inc., MN, United States) partially substitutes lost nerve function related to plantar sensation providing directional tactile cues reflecting plantar pressure measurements during standing and walking. We tested the null hypothesis that the Functional Gait Assessment (FGA) score would remain unchanged after 10 weeks of use.
Methods: Participants had PN with lost plantar sensation, gait and balance problems, an FGA score < 23 (high fall risk), and ability to sense tactile stimuli above the ankle. Clinical outcomes included FGA, Gait Speed, Timed Up&Go (TUG) and 4-Stage Balance Test. Patient-reported outcomes included Activities-Specific Balance Confidence (ABC) scale, Vestibular Disorders Activities of Daily Living Scale, PROMIS participation and satisfaction scores, pain rating, and falls. Evaluations were performed at baseline and after 2, 6, and 10 weeks. Subjects were not made aware of changes in outcomes. No additional balance interventions were allowed.
Results: Forty-five participants of 52 enrolled across four sites completed in-clinic assessments. FGA scores improved from 15.0 to 19.1 (p < 0.0001), normal and fast gait speed from 0.86 m/s to 0.95 m/s (p < 0.0001) and 1.24 m/s to 1.33 m/s (p = 0.002), respectively, and TUG from 13.8 s to 12.5 s (p = 0.012). Four-Stage Balance Test did not improve. Several patient-reported outcomes were normal at baseline and remained largely unchanged. Interestingly, subjects with baseline ABC scores lower than 67% (high fall risk cut-off) increased their ABC scores (49.9% to 59.3%, p = 0.01), whereas subjects with ABC scores above 67% showed a decrease (76.6% to 71.8%, p = 0.019). Subjects who reported falls in the prior 6 months (n = 25) showed a decrease in the number of fall-risk factors (5.1 to 4.3, p = 0.023) and a decrease in fall rate (13.8 to 7.4 falls/1000 days, p = 0.014). Four pre-study non-fallers (n = 20) fell during the 10 weeks.
Conclusion: A wearable sensory prosthesis presents a new way to treat gait and balance problems and manage falls in high fall-risk patients with PN.
Trial registration: ClinicalTrials.gov (#NCT03538756).
Falls are a widely recognized problem in the elderly (Ganz and Latham, 2020). About 29% of community-dwelling adults 65 years or older fall once annually and 10% fall at least twice annually (Ganz et al., 2007; Bergen et al., 2016). Data from the Centers for Disease Control (CDC) indicate that medical treatment was required by 37.5% of individuals who fell in 2014 (Bergen et al., 2016). Sterling et al. (2001) reported that 30% of falls in the elderly result in serious injury. In 2015, medical cost related to fatal and nonfatal falls was approximately $50.0 billion (Florence et al., 2018). Overall, falls are associated with poor health, shortened survival (Jónsdóttir and Ruthig, 2020), reduced quality of life, and a fear of falling (Lawrence et al., 1998; Scheffer et al., 2008). Sensory peripheral neuropathy (PN) is associated with poor balance and is an independent risk factor for falls (Richardson and Hurvitz, 1995), regardless if the etiology is idiopathic (Riskowski et al., 2012), due to diabetes (Mustapa et al., 2016; Vinik et al., 2017), or chemotherapy (Winters-Stone et al., 2017). The prevalence of PN in the US population for those over age 40 has been reported to be nearly 15% (Gregg et al., 2004). The importance of sensory information from plantar cutaneous mechanoreceptors for balance control has been shown in healthy individuals (Meyer et al., 2004a, b), with loss of such information in patients with PN likely leading to problems with gait and balance function and increased risk of falls (Menz et al., 2004; DeMott et al., 2007; Dixon et al., 2017; Lipsitz et al., 2018). The occurrence of fall-related injuries is up to 15 times higher in patients with diabetic PN than in healthy individuals (Cavanagh et al., 1992). Furthermore, the prevalence of polyneuropathy has been reported to be almost four times higher in persons older than 60 years and to independently contribute to functional impairments including difficulty walking and tendency to fall (Hoffman et al., 2015). Persons with polyneuropathy are more likely to fall and more often incur fall-related injuries (Hanewinckel et al., 2017). In a prospective study, 65% of older individuals with PN fell during a 1-year period and 30% reported an injury from a fall (DeMott et al., 2007). In addition, low gait speed is a risk factor for falls (Studenski et al., 2003; Montero-Odasso et al., 2005), an important indicator of frailty (Kim et al., 2019) and a predictor of survival (Studenski et al., 2011). Although gait speed declines with healthy aging (Buracchio et al., 2010), the decline in individuals with progressive sensory loss may be four times as high (Lipsitz et al., 2018). Interestingly, interventions designed to improve gait speed may also increase survival (Hardy et al., 2007).
Clinical treatment of gait and balance problems related to PN is mainly limited to the use of canes, walkers, physical therapy interventions and balance exercises (Richardson et al., 2001; Ganz and Latham, 2020) including Tai-Chi (Li and Manor, 2010; Manor et al., 2014; Quigley et al., 2014). Long-term use of bilateral ankle foot orthoses in elderly individuals with a history of falls showed positive changes in certain in-clinic static sway measures (Wang et al., 2019a), although long-term benefits related to fall rates and gait function appear limited (Wang et al., 2019b).
Several review studies support the hypothesis that strength and balance training interventions can improve balance and reduce fall risk and falls in patients with PN (Ites et al., 2011; Tofthagen et al., 2012; Streckmann et al., 2014). The training, however, should be specific to balance (Bulat et al., 2007; Oddsson et al., 2007; Halvarsson et al., 2011; Akbari et al., 2012) because strength and/or endurance training in patients with PN appears to have less impact on balance (Streckmann et al., 2014). In addition, unless balance activities, including Tai Chi or balance therapies are conducted with sufficient intensity, frequency (Lipsitz et al., 2019) and specificity, benefits may be limited or absent (Kruse et al., 2010; Lipsitz et al., 2018, 2019) leading to mixed outcomes. Furthermore, continued exercise is required to maintain benefits long-term (Wolf et al., 2001; Halvarsson et al., 2013; Melzer and Oddsson, 2013), although some improvements last up to 6 months (Allet et al., 2010). Guidelines regarding physical activity for older adults with mobility problems recommend a minimum of activity at least twice a week (Chodzko-Zajko et al., 2009). Some studies on patients with diabetic PN following a period of balance training 2–3 times/week over 6–12 weeks did show improved balance and reduced fall risk (Morrison et al., 2010, 2018). However, there currently are no specific guidelines regarding frequency of balance exercises and even three times a week may be insufficient to see an improvement in balance function (Kruse et al., 2010). Consequently, there is a need for additional solutions to help improve gait and balance function in patients with PN.
A growing body of literature on various sensory substitution and augmentation technologies suggest novel ways of improving gait and balance function in different populations of patients. The concept of sensory substitution related to brain plasticity was laid out by Bach-y-Rita and colleagues, initially for vision and the vestibular system (Bach-y-Rita et al., 1969; Bach-y-Rita, 2004) and other sensory systems (Tyler et al., 2003; Bach-y-Rita, 2004). Recent efforts include wearable systems showing benefits to patients with vestibular loss (Hegeman et al., 2005; Wall et al., 2009; Basta et al., 2011; Yamanaka et al., 2016), PN (Wall et al., 2012; Wrisley et al., 2018, 2020) and Parkinson’s Disease (Rossi-Izquierdo et al., 2013; Lee et al., 2015). Combining wearable neurostimulation with balance therapy has shown benefits in patients with multiple sclerosis (Leonard et al., 2017), cerebellar ataxia (Cakrt et al., 2012), stroke (Badke et al., 2011) traumatic brain injury (Ptito et al., 2020) and in-home balance therapy (Bao et al., 2018).
In a randomized crossover trial, a recent study further supported findings from an earlier pilot study (Wall et al., 2012) and demonstrated meaningful short-term, in-clinic improvements in Functional Gait Assessment (FGA) scores and gait speed in subjects with PN using a wearable sensory prosthesis (Koehler-McNicholas et al., 2019). The device (Walkasins®, RxFunction Inc., MN, United States, Figure 1) is an external lower limb sensory prosthesis designed to replace lost nerve function used for detection and signaling of foot pressure sensation in patients with PN. A Leg Unit placed around the lower leg provides gentle and brief (150 ms) directional tactile stimuli (in the form of low-intensity vibrations) at four locations that indicate anteroposterior and mediolateral excursion of the center of pressure under the foot as measured with a thin (∼2 mm) instrumented Foot Pad placed in the shoe. The device has different functional modes for standing and walking activities. In standing, it signals out of balance events, i.e., when the center of pressure drifts away in a specific direction from a mid-foot in-balance zone where no feedback is provided. During walking the device signals heel strike and toe-off events. During walking and standing activities, the subject’s nervous system senses these new tactile cues that may help improve their gait and balance (cf. Koehler-McNicholas et al., 2019).

Figure 1. Picture of the Walkasins sensory prosthesis device showing the pressure sensor embedded Foot Pad that is placed in the subject’s shoe and connected to the Leg Unit that houses an embedded microprocessor with software, supporting electronics, a rechargeable battery, and four tactile stimulators placed around the lower leg. The system is worn bilaterally. Leg Unit and left Foot Pad shown
Currently, effects of long-term daily use of Walkasins on clinical outcomes are unknown. The multi-site clinical trial, walk2Wellness, (NCT #03538756)1 investigates long-term, home-based use of Walkasins on clinical and patient-reported outcomes of balance and gait function, quality of life, physical activity, social participation, pain and fall rates. Data from the primary endpoint of the study at 10 weeks are reported here. We tested the null hypothesis that the FGA score as measured at baseline would remain unchanged after 10 weeks of use (cf. Beninato et al., 2014; Koehler-McNicholas et al., 2019). Additionally, we compared baseline data with assessments conducted after 2 and 6 weeks of device use. Early data from the trial were presented in abstract form (Oddsson et al., 2019, 2020a).
Human subject testing was approved according to the Declaration of Helsinki by Advarra IRB (formerly Quorum Review IRB), serving as the Institutional Review Board (IRB) of record for three of the participating sites under the study protocol for walk2Wellness: Long-term Use Effects of Walkasins® Wearable Sensory Prosthesis on Gait Function, Balance-Confidence, and Social Participation. The three sites include Baylor College of Medicine, Houston, TX; Hebrew SeniorLife, a Harvard Medical School Affiliate, Boston, MA; and M Health Fairview, Minneapolis, MN. Advarra IRB determined that Walkasins are a non-significant risk device because they do not meet the criteria of a significant risk device according to U.S. Food and Drug Administration regulations. The IRB Subcommittee, the Subcommittee on Research Safety, and the Research and Development Committee of the Minneapolis VA Health Care System (MVAHCS) also approved the trial. The study is registered on ClinicalTrials.gov (#NCT03538756). At the time this study began, Walkasins were available only for research purposes. All data from the trial was collected and stored using REDCap Cloud, a 21 CFR Part 11 compliant Electronic Data Capture system (Encinitas, CA, United States).
Inclusion criteria for the study were similar to Koehler-McNicholas et al. (2019): age 21–90 years; male or female; a formal diagnosis of sensory PN prior to participating in the study as indicated by subject’s medical record or a signed letter by a physician; self-reported problems with balance; ability for transfers or ambulation on level surfaces at fixed cadence as assessed by trained study personnel; a Functional Gait Assessment (FGA) score < 23, the cut-off score for high fall risk (Wrisley and Kumar, 2010); ability to understand and provide informed consent; foot size to allow the Walkasins device to function properly, and ability to complete all functional outcome measures without the use of an assistive device to ensure sufficient motor function. Subjects could use an assistive device at their own discretion during the trial. Subjects were excluded from participation if they were unable to perceive tactile stimuli from the Walkasins leg unit or used an ankle-foot orthosis for ambulation that prevented donning of the device. Subjects with any of the following conditions were also excluded: acute thrombophlebitis; deep vein thrombosis; untreated lymphedema; a lesion of any kind, swelling, infection, inflamed area of skin, or eruptions on the lower leg near placement of the device; foot or ankle fractures; or severe peripheral vascular disease. In addition, subjects with any musculoskeletal or other neurological conditions that would prohibit use of the device, as determined by a clinician, were excluded. Due to risk of overloading the pressure sensor Foot Pad, subjects weighing over 136 kg (300lbs) were excluded from participation. Furthermore, subjects were prohibited from initiating any balance training (e.g., Tai-Chi etc.) or balance-related therapy during the 10 weeks of the trial. Subjects were not systematically provided information about changes in any outcomes scores or changes in their performance throughout the 10 weeks of the trial, and study personnel did not monitor outcomes during the study. Potential subjects responded to announcements that specifically targeted individuals with PN and balance problems, or they were referred by clinicians who were familiar with the study and believed them to be good candidates for the trial.
All participants signed IRB-approved consent forms prior to the initiation of study activities. Following the informed consent process, a study team member tested the subjects on both legs to determine whether they could feel the four different stimuli locations from the Walkasins Leg Unit (Figure 1). Two subjects who were unable to perceive the stimuli from the Walkasins Leg Unit due to proximal progression of their neuropathy (Figure 1) were excluded from participation in the study. Enrolled subjects were fitted with Walkasins on both feet by a study team member trained in the process. The Foot Pad was matched to size and carefully placed and fitted into the shoe making sure there were no pressure points and ensuring the shoe was not overly tight due to potential ulcer risk in these patients. Participants were encouraged to use loose fitting comfortable shoes. Participants completed a demographics and health screening questionnaire to assess common health issues related to neurological, musculoskeletal, cardiopulmonary disorders, and other systemic diseases along with information on their history of falls over the past 6 and 12 months and regular use of an assistive device (Koehler-McNicholas et al., 2019). Falls were defined according to the World Health Organization: “an event which results in a person coming to rest inadvertently on the ground or floor or other lower level”. Subjects enrolled in the study were instructed to wear the device as much as possible throughout their daily activities, indoors as well as outdoors. At each follow-up visit, participants were asked about changes in their health status and any falls and adverse events they experienced since the previous visit. During the baseline visit, participants also provided a list of their medications (medication name, indication, dose, and frequency), which was updated over the course of the study. Medications are a known risk factor for falling, based on side effects of medication use or drug interactions (Woolcott et al., 2009).
Subjects then completed the Activities-Specific Balance Confidence (ABC) Questionnaire, which measures levels of balance confidence in elderly persons. The ABC asks the question “How confident are you that you will not lose your balance or become unsteady” when performing 16 different tasks (Powell and Myers, 1995). Subjects rated themselves on a scale from 0 to 100, and an average score was calculated across the 16 responses. An ABC score below 67% has been associated with high fall risk (Lajoie and Gallagher, 2004). In addition, subjects completed the Vestibular Activities of Daily Living Scale (VADL) (Cohen et al., 2000), which evaluates self-reported effects of vertigo and balance disorders on independence in everyday activities of daily living that are relevant for individuals living in the community. Individuals rate their level of functional ability for basic and instrumental activities of daily living on a scale from 1 (independent) to 10 (dependent), which incorporates the use of assistive devices.
Following completion of the questionnaires, a study team member performed tactile and vibration sensation testing to document loss of sensation. Loss of sensation was tested with the Weinstein Enhanced Sensory Test (WEST) monofilament foot test (0.5g, 2g, 10g, 50g, and 200g) applied perpendicular to the skin at four test sites on the plantar surface of the foot, including the first, third, and fifth metatarsal heads as well as the great toe. Study personnel began testing with the 10g filament and used a smaller filament if the subject was sensate and a larger filament if the subject was insensate. The smallest filament the subject was able to feel was recorded (if none were felt this result was recorded as “none”). Vibration sensation was assessed with a Rydel-Seiffer tuning fork, which is a 128Hz tuning fork with end weights that convert the tuning fork from 128 to 64 Hz. The weights are scaled allowing a score 0–8 (lower scores indicating less sensation), allowing reliable quantitative vibratory testing. Scores were read from the black triangle and rounded to the nearest whole number. Vibration values ≤ 4 are categorized as abnormal at the first metatarsal joint (Kästenbauer et al., 2004). The tuning fork was applied firmly and perpendicular to the lateral aspect of the first metatarsophalangeal, lateral malleolus, and patella testing sites. The monofilament and vibration tests were repeated at the 10-week visit.
Upon completion of the monofilament and vibration sensation testing, subjects performed a series of functional outcome measures while wearing the device turned off (baseline). Tests of clinical outcomes were repeated at weeks 2, 6, and 10 with the device turned on. Subjects could rest as needed during the clinical assessments. For study purposes the clinical outcomes were standardized and performed by study personnel who were trained by one of the investigators (DW). Observation visits were conducted periodically during the study to ensure standardization among the sites.
The FGA (Wrisley et al., 2004) is the recommended clinical outcome measure for walking balance based on current physical therapy Clinical Practice Guidelines for outcome measures for adults with neurologic conditions (Moore et al., 2018). It is a reliable and valid measure of gait function related to postural stability and has been shown to be effective in classifying fall risk in older adults and predicting unexplained falls in community-dwelling older adults (scores ≤ 22/30) (Wrisley et al., 2004; Wrisley and Kumar, 2010). It has also been validated in multiple neurological conditions (stroke, Parkinson’s, vestibular conditions) (Lin et al., 2010; Leddy et al., 2011) and has less floor and ceiling effects than the similar Dynamic Gait Index (Lin et al., 2010). The FGA includes 10 different items that challenge gait balance where each item is scored from 0 to 3 (3 = normal, 2 = mild impairment, 1 = moderate impairment, 0 = severe impairment) with a maximum score of 30. An increase of ≥ 4 points is considered the Minimal Clinically Important Difference (MCID) for community-dwelling elderly individuals (Beninato et al., 2014). Subjects whose baseline FGA score was 23 or higher were excluded from further participation in the study. Subjects completed the FGA in a large open area with a 6-m (20-ft) walkway marked with tape according to published recommendations (Wrisley et al., 2004).
The 10m-walk (Perera et al., 2006) is the recommended clinical outcome measure for walking speed based on current physical therapy Clinical Practice Guidelines for outcome measures for adults with neurologic conditions (Moore et al., 2018). It is routinely used in rehabilitation and has excellent reliability in multiple neurologic conditions (chronic stroke, traumatic brain injury, Parkinson’s) (Hiengkaew et al., 2012). Gait speed has been found to be an important predictor of survival in older adults (Hardy et al., 2007), further emphasizing its importance as a clinical outcomes measure. Gait speed was assessed during the middle 6 meters of a 10-meter-long pathway to allow for acceleration and deceleration, completed in one trial under two conditions: 1) walk at normal speed and 2) walk as fast as possible. An increase by 0.05 m/s is deemed “small meaningful” and 0.10 m/s as “substantial” (Perera et al., 2006). These are considered the MCID in the geriatric population (Perera et al., 2006).
The TUG (Mathias et al., 1986) is part of the CDC recommended STEADI test protocol for balance function (CDC, 2017). It is commonly used in rehabilitation and has excellent validity and reliability for elderly adults and has been shown to be effective in classifying community dwelling adults at risk for falls (Podsiadlo and Richardson, 1991; Shumway-Cook et al., 2000; Bischoff et al., 2003; CDC, 2017). From a seated position in a standard armchair, the subject is asked to do the following: 1) stand up from the chair, 2) walk at normal pace around a tape mark on the floor 10 feet from the chair, 3) turn, 4) walk back to the chair at a normal pace, and 5) sit down again. Subjects were provided one practice trial that was not recorded followed by the recorded timed trial. The tester recorded the time from the command “Go” until the subject’s buttocks returned to the chair when sitting. We used > 12s as a cut-off for high fall risk (Bischoff et al., 2003; CDC, 2017). The Minimal Detectable Change (MDC) for older adults with type 2 diabetes has been reported to be 1s (Alfonso-Rosa et al., 2014).
The 4-Stage Balance Test is part of the Centers for Disease Control and Prevention (CDC)-recommended STEADI test protocol for balance function (CDC, 2017). It includes four gradually more challenging postures the subject is exposed to: 1) stand with feet side by side, 2) stand with feet in semi-tandem stance, 3) stand with feet in tandem stance, and 4) stand on one leg. Subjects were allowed upper extremity support to obtain the position and passed each level if they were able to hold the stance unsupported for 10 s. The assessment ended when subjects were unable to hold a stance for 10 s. The times for each position held was recorded and summed as a measure of overall performance. A fail of stances 1, 2, or 3 (i.e., total time < 30s) indicates high risk of falling (CDC, 2017).
As part of the baseline visit, subjects performed a standardized set of balance activities, once while wearing the device turned off and once while wearing it turned on (Koehler-McNicholas et al., 2019). Activities lasted approximately 10 min and included standing (two-leg standing, tandem standing, and one-legged standing) and walking (walking straight, turning right and left) at both normal and fast speeds. Activities were repeated with the eyes closed. During standing exercises subjects were challenged to explore their base of support in both mediolateral and anteroposterior directions and to notice the pattern of tactile stimuli when the device was turned on. During walking activities subjects were instructed to notice the pattern of tactile stimuli when the foot was in contact with the ground, how it matched their pace of walking, and the flow of tactile stimuli from step to step. Subjects were not instructed how to respond to the tactile stimuli from Walkasins instead, the activities focused on orientation and familiarization with the device.
At the baseline visit and at each follow-up visit at weeks 2, 6, and 10, subjects also completed the five subject-reported outcome measures described below:
The PHQ-9 (Kroenke et al., 2001) is a concise, self-administered tool for assessing depression. Commonly used for screening and diagnosis of depression, the PHQ-9 incorporates depression criteria according to the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) with other leading major depressive symptoms.
The PROMIS Pain Interference instrument (Askew et al., 2016) measures the self-reported impact of pain on relevant aspects of a person’s life within the past 7 days. Items capture the extent to which pain hinders social, cognitive, emotional, physical, and recreational activities. The Pain Interference short form is a global scale rather than disease specific.
The PROMIS Pain Intensity instrument assesses reported average pain intensity on a scale from 0 to 10 with higher scores indicating greater levels of pains. The Pain Intensity short form is global rather than disease specific.
The PROMIS Ability to Participate in Social Roles and Activities instrument (Hahn et al., 2016b) assesses the individual’s perceived ability to perform usual social roles and activities. The measure does not use a designated time frame (e.g., over the past 7 days), and higher scores represent fewer limitations (e.g., I have trouble doing all of my regular leisure activities with others).
The PROMIS Satisfaction with Social Roles and Activities (Hahn et al., 2014, 2016a) is a self-reported instrument to assess satisfaction with the ability to perform usual social roles and activities (e.g., “I am satisfied with my ability to do things for my family”).
PROMIS scores are presented as T-scores, a standardized score with a mean of 50 (representing average for the US population) and a standard deviation of 10.
At the 2 and 10-week visits, subjects completed a 10-question survey to collect information concerning their experience with the device. Subjects rated aspects of Walkasins use (e.g., donning and doffing, charging, etc.) on a 7-point Likert scale ranging from “Very Easy” to “Very Hard”. Subjects also rated their overall satisfaction with the device and were able to provide additional comments and feedback regarding their experience with the device. Between visits, subjects were asked to document their use of Walkasins on a calendar by marking the days they wore their Walkasins and for how many hours. The subject calendar was also used to facilitate documentation of falls that occurred over the course of the study (Hannan et al., 2010). Subjects were asked to return their calendars at their next study visit.
Sample size estimation was based on data from the recent study of a similar population of subjects (Koehler-McNicholas et al., 2019), showing a baseline average FGA score of 15.2 and standard deviation 4.8. The data was normally distributed according to the Shapiro-Wilk’s test. To detect a mean difference in pre- and post-FGA score ≥ 4 points, the Minimal Clinically Important Difference for community dwelling elderly individuals (Beninato et al., 2014), required at least 20 subjects using a significance level of 0.01 and a power of 0.8. Accounting for an expected ∼20% drop-out rate (National Heart Lung and Blood Institute, 2020) target enrollment was set at 25 subjects (20/0.8 = 25). Sample size calculation was performed according to Dupont and Plummer (1990) using their power and sample size program (Dupont and Plummer, 1998). Multiple sites were engaged in the trial to expand geographical, ethnical, and clinical variation in the data initially allowing each site to recruit up to 25 subjects. Due to the COVID-19 pandemic, the trial was interrupted and continued enrollment as well as in-clinic testing was halted. At this time, sufficient overall statistical power based on the sample size calculation above has been achieved following enrollment of 52 subjects, well over the 20 subjects required to achieve statistical significance to reject the null hypothesis. Collection of participant-reported outcomes has continued through phone calls and the longer-term outcomes assessments as originally planned (at 26 and 52 weeks) are expected to continue. These long-term data will be reported in a separate manuscript.
Descriptive statistics were calculated and presented as mean and standard deviation of the mean. Variables were tested for normality using the Shapiro-Wilk’s test. The two-proportion Z-test was used to compare proportion-based measures. A post-hoc analysis was performed to compare subjects who reported falls in the previous 6 months (Pre-Fallers, n = 30) to those who did not (Pre-NonFallers, n = 22). Their baseline characteristics were compared using a t-test for independent samples or a Mann-Whitney U test if data was not normally distributed based on a Shapiro-Wilk’s test. Repeated measures analysis of variance (ANOVA) was performed for outcomes measured across the four assessment events, baseline, 2, 6, and 10 weeks for all subjects. Huyhn and Feldt correction was implemented for violations of sphericity. If the ANOVA was significant (p < 0.05), three pairwise comparisons were made using dependent t-tests between the baseline assessments and each of the 2, 6 and 10-week assessments. If the ANOVA was non-significant, no further comparisons were made. A Bonferroni’s adjustment of significance levels for correlated measures was applied, ranging from p < 0.0167 (0.05/3 for three comparisons) for a full correction (non-correlated measures, r = 0) and p < 0.05 for perfectly correlated measures (r = 1) (Uitenbroek, 1997). Effect sizes were calculated using Cohen’s drm according to recommendations by Lakens (2013) and were interpreted according to Cohen (1988) with 0.2 representing a small effect, 0.5 a medium effect, and 0.8 a large effect. Ninety-five percent confidence intervals of effect sizes were estimated according to Algina (Algina et al., 2005). Statistical analysis was performed using the Analysis-ToolPak module in Microsoft Excel 2016 and the Real Statistics Resource Pack software, release 6.8 (Zaiontz, 2020). Table 1 shows baseline characteristics of subjects enrolled in the study from the four different clinical sites. Subject data were pooled for the continued analysis presented here.
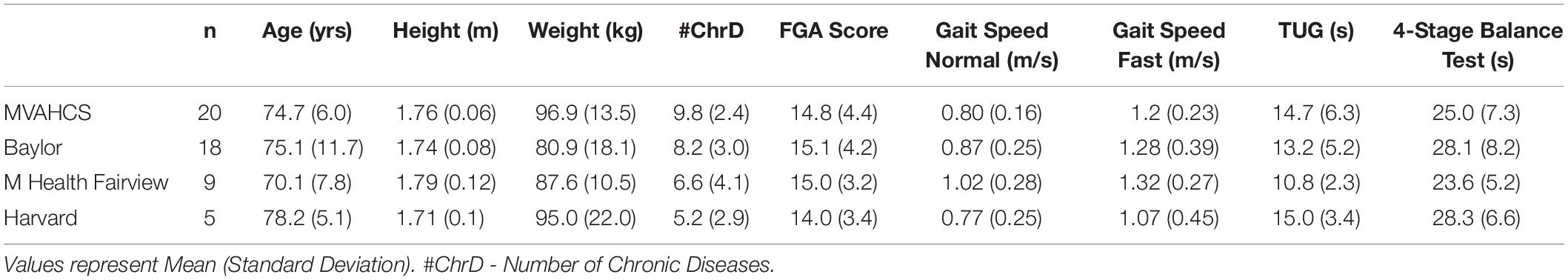
Table 1. Baseline characteristics of subjects from the four different clinical sites enrolled in the study.
Datasets from the current study are available upon request. The raw data supporting the conclusions of this article will be made available to qualified researchers, without undue reservation.
The flow chart for the study is shown in Figure 2. Sixty-seven subjects were assessed for eligibility across the four participating sites. Baseline characteristics for subjects enrolled in the study are shown in Table 1. First enrollment occurred at the MVAHCS on 10/22/2018, followed by M Health Fairview/University of Minnesota on 10/23/2018, Baylor College of Medicine on 12/20/2018 and Marcus Institute/Harvard Medical School on 09/19/2019. The last enrollment occurred at Baylor College of Medicine on 01/10/2020. Subjects across the four sites were pooled for the continued analysis presented here. Fifteen subjects were excluded from participation of which five had an FGA score higher than 22; two were unable to sense tactile stimuli from the device; two had neurological conditions that prevented device use; two were planning to start physical therapy treatment, one declined to participate and three for other reasons. Fifty-two subjects were enrolled for baseline assessment and allocated for the intervention. A total of seven subjects discontinued participation, four subjects at the 2-week assessment (two related to device use, one due to transportation issues, and one due to an unrelated adverse event, Figure 2), one ahead of the 6-week assessment and two ahead of the 10-week assessment (due to device and study issues), respectively (Figure 2). A total of 45 subjects (87%) completed all in-clinic assessments from baseline to the 10-week endpoint.
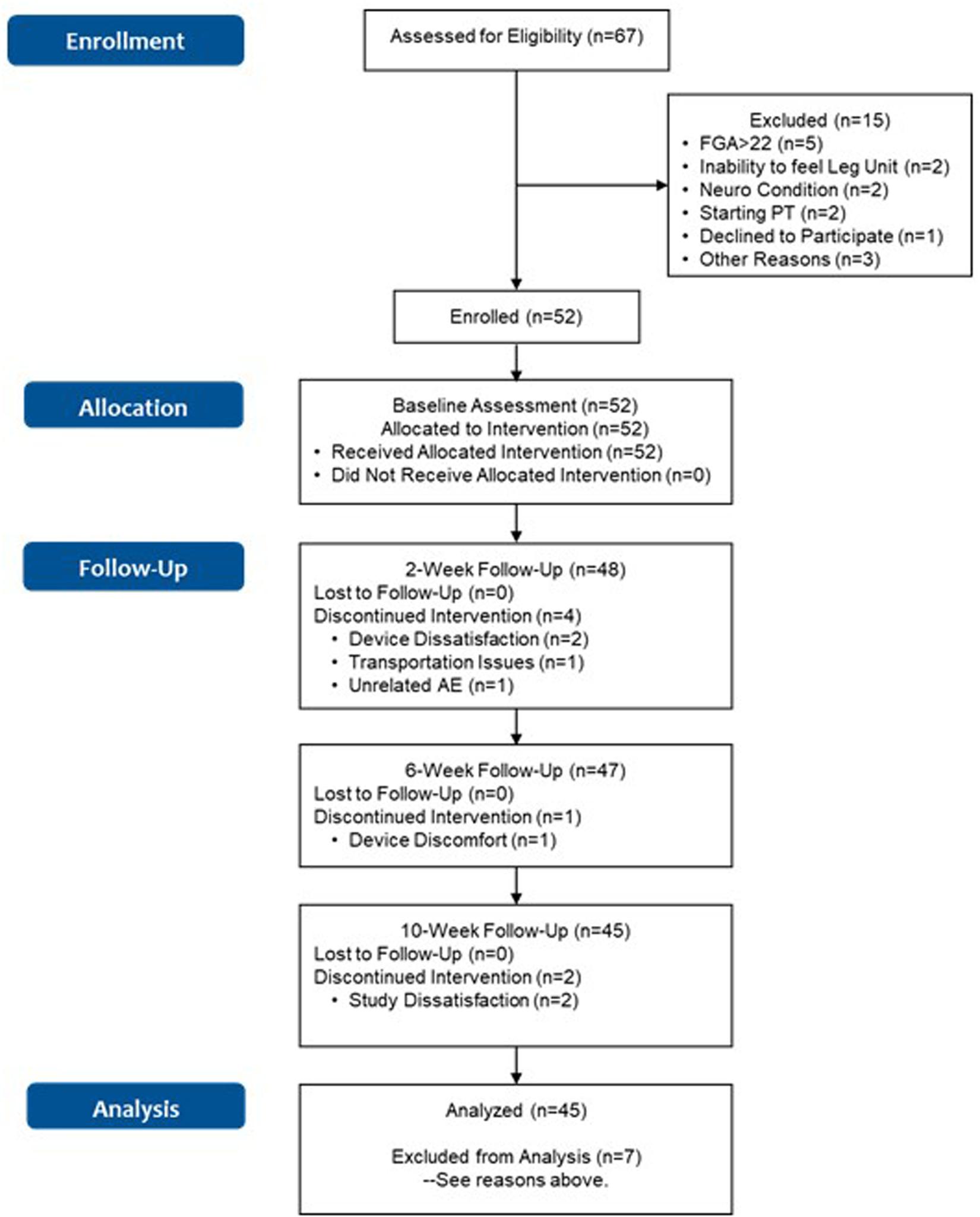
Figure 2. Flowchart of the study. Discontinuation due to “Study Dissatisfaction” was related to refusal to do the functional assessments, and dislike of answering questions in patient reported outcomes.
Table 2 shows baseline characteristics of all enrolled participants (n = 52), then separately for subjects who reported having fallen in the 6 months preceding the study (Pre-Faller, n = 30) and those who did not report a fall (Pre-NonFaller, n = 22). Overall, participants were elderly (74.4 ± 8.7 yrs.), overweight (BMI > 25) and mostly male (79%). A majority used an assistive device (54%) and had fallen in the previous year (71%) or in the past 6 months (58%). Furthermore, a majority showed high fall risk based on low 4-Stage Balance Test outcomes (63% of subjects < 30s) (CDC, 2017) or low ABC scores (56% of subjects scored < 67%) (Lajoie and Gallagher, 2004). Twenty-five percent of participants had a normal gait speed less than 0.7 m/s (Montero-Odasso et al., 2005) and half of the participants performed the TUG slower than 12 s (CDC, 2017), the commonly used thresholds for high fall risk. The Pre-Faller group had a higher number of fall-risk factors as compared to the Pre-NonFaller group (5.3 ± 1.0, vs. 3.5 ± 1.3, respectively, p < 0.0001). The baseline FGA score was statistically significantly lower in Pre-Faller (13.5 ± 3.7) as compared to Pre-NonFaller (16.7 ± 3.6, p = 0.004). There was no difference in normal gait speed between the two groups (p = 0.12), although fast gait speed was higher in the Pre-NonFaller (1.41 ± 0.35 m/s) as compared to the Pre-Faller subjects (1.13 ± 0.34 m/s, p = 0.006). Furthermore, TUG times were significantly slower in the Pre-Faller group compared to the Pre-NonFaller (14.7 ± 6.3s and 12.0 ± 2.9, respectively, p = 0.049).
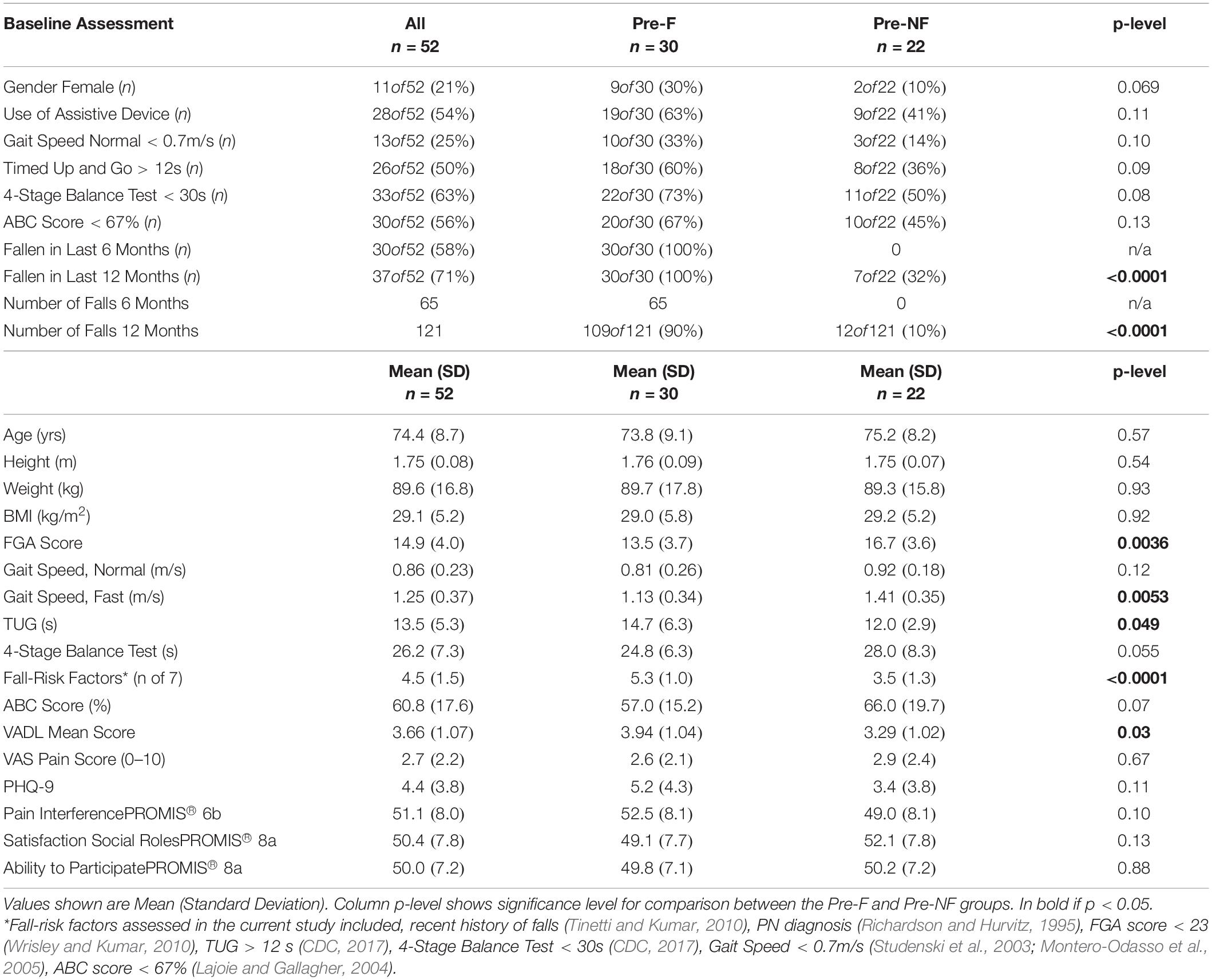
Table 2. Baseline characteristics of subjects enrolled in the study (n = 52), then separately for subjects who reported having fallen in the past 6 months (Pre-F) and those who did not (Pre-NF).
The ABC score was higher in the Pre-NonFaller compared to the Pre-Faller group, although the difference was not statistically significant (66.0 ± 19.7 vs. 57.0 ± 15.2, respectively, p = 0.07). The VADL score was marginally lower in the Pre-NonFaller group (3.29 ± 1.02, vs. 3.94 ± 1.04, p = 0.03). Pain scores were similar for the two groups (2.6 ± 2.1 vs. 2.9 ± 2.4). The PHQ-9 score was slightly higher in the Pre-Faller group, although the difference was not statistically significant (p = 0.11). PROMIS outcome scores for “Pain Interference,” “Satisfaction with Social Roles,” and “Ability to Participate” (Table 2) showed mean values around 50 for both groups, which is considered average for the US population (Askew et al., 2016; Hahn et al., 2016a, b). Any differences were well within 10, which is one standard deviation of these measures in the US population (Table 2; Askew et al., 2016; Hahn et al., 2016a, b).
Table 3 shows self-reported chronic conditions and medication use for subjects enrolled in the study. On average, subjects reported having 8.2 ± 3.3 chronic conditions. All subjects had a diagnosis of PN either in their medical chart or provided in a letter signed by their physician. Most subjects reported having neuropathic pain in their feet (73%) as well hypertension (63%) and half of participants reported having chronic back pain. All subjects reported having difficulty with walking and balance. The Pre-Faller group reported a higher incidence of cancer as a chronic condition than the Pre-NonFaller group (43% vs. 14%, respectively, p = 0.03). Falls in the 12 months preceding study participation were reported by all the Pre-Faller participants (i.e., reporting falls over the past 12 and 6 months) versus by seven of the 22 in the Pre-NonFaller group (i.e., reporting falls over the past 12 but not 6 months) (p < 0.0001). Ninety percent of the falls reported in the 7–12 months preceding the study were reported by the Pre-Faller participants (p < 0.0001). Overall, participants reported taking one non-prescription medication and eight (median) prescription medications of which three are known to cause potential balance issues and increase the risk of falling (Woolcott et al., 2009). Medication use was similar between the Pre-Faller and Pre-NonFaller groups (Table 3).
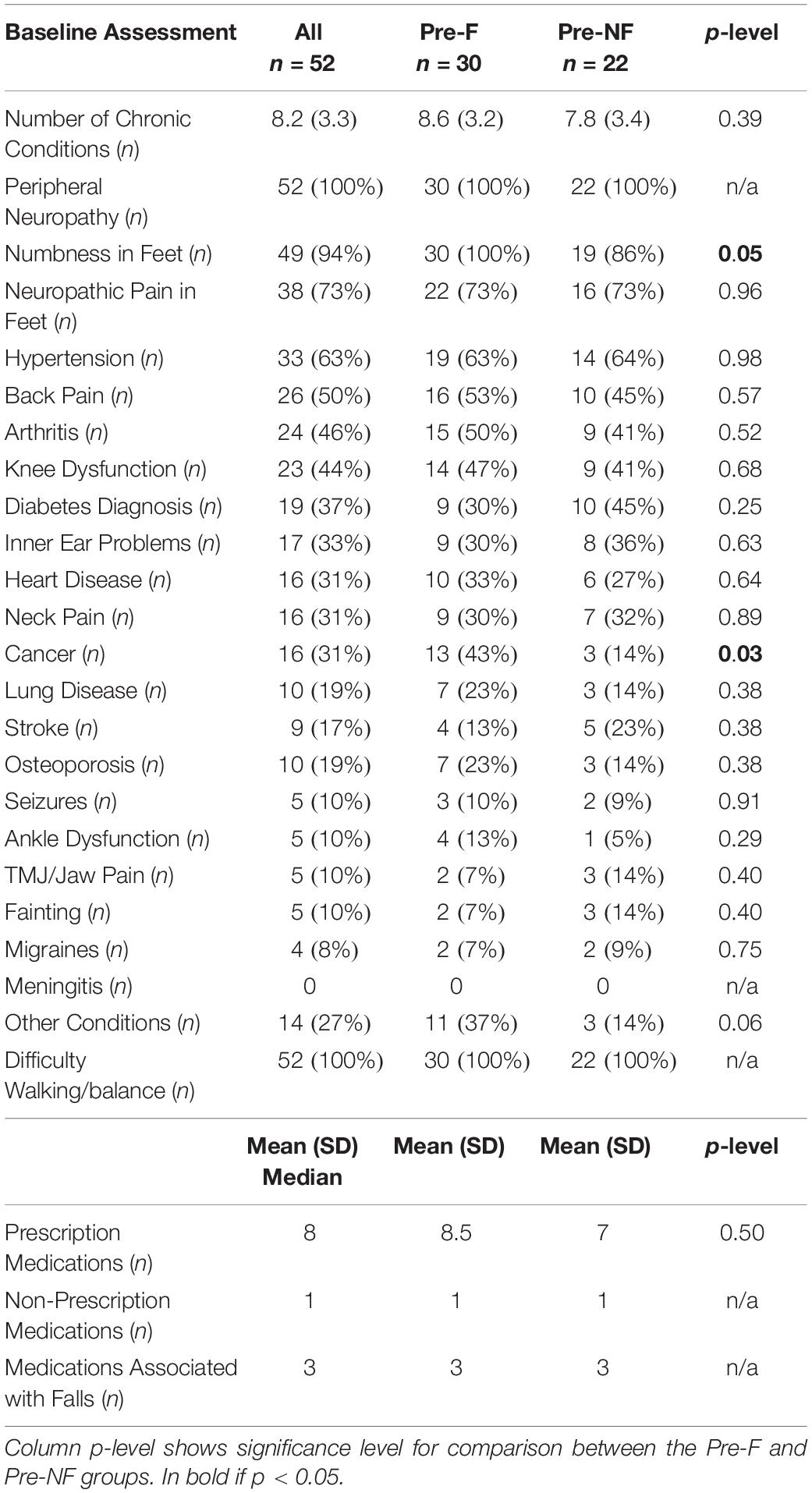
Table 3. Self-reported chronic conditions as well as medication use for subjects enrolled in the study.
Table 4 shows clinical outcomes for the 45 subjects who completed assessments at baseline, 2, 6 and 10 weeks. Across all subjects, one-way repeated measures ANOVA conducted separately for each outcome showed statistically significant differences across the assessment events for all subjects and all clinical outcomes (0.00001 < p < 0.01) except for the 4-Stage Balance Test, which was not statistically significant (p = 0.23, Table 4 ANOVA column). Further pairwise comparisons following statistically significant ANOVA showed statistically significant differences between the baseline assessment and the 2, 6, and 10-week assessments, respectively, for the FGA score and normal gait speed and between baseline and 6 and 10 weeks, respectively, for fast gait speed and the TUG scores. Cohen’s drm effect size calculated between the baseline and 10-week primary endpoint was large for FGA (0.92, FGA change from 15.0 to 19.1) and small to medium for normal gait speed (0.42, 0.86m/s to 0.95m/s), fast gait speed (0.27, 1.24 m/s to 1.33 m/s) and the TUG (0.28, 13.8 s to 12.5 s, Table 4).
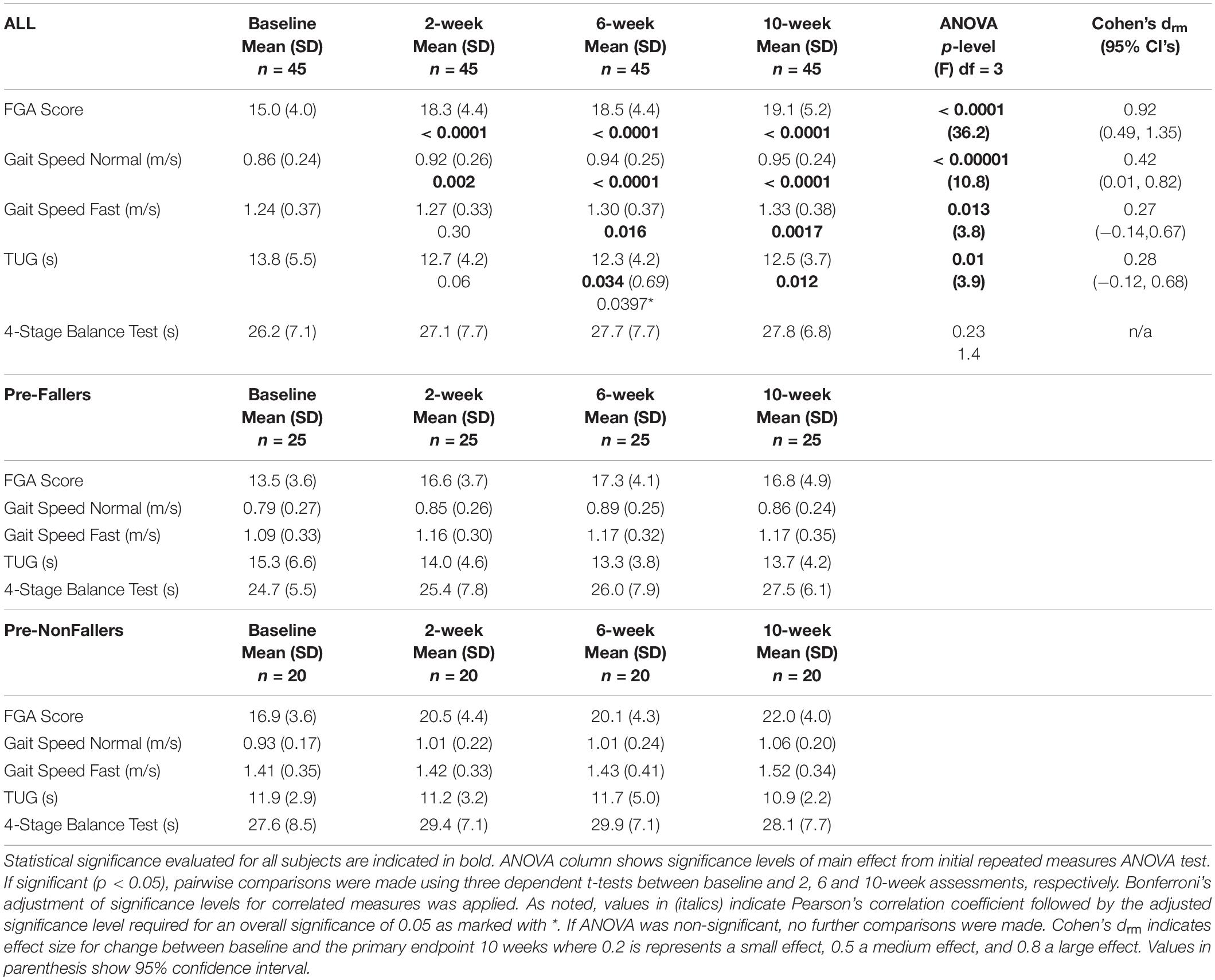
Table 4. Clinical outcomes for the 45 subjects completing all assessments for baseline, 2, 6, and 10 weeks as well as the subgroups of Pre-Fallers and Pre-NonFallers.
Both the Pre-Faller and Pre-NonFaller groups increased FGA scores from baseline to the 2-, 6-, and 10-week assessments, respectively. The Pre-Faller group had lower FGA scores both at baseline as compared to the Pre-NonFaller (13.5 vs. 16.9) and at the 10-week assessment (16.8 vs. 22.0). Similarly, normal gait speed increased in both groups while fast gait speed only increased in the Pre-Faller group from 1.09 m/s at baseline to 1.17 m/s at 10 weeks (Table 4). A statistical comparison was not performed due to the post-hoc nature of these observations.
Patient reported outcomes for the 45 subjects who completed all assessments are shown in Table 5 as well as for the Pre-Faller and Pre-NonFaller groups. For all subjects, there was an overall significant ANOVA for the VADL score (p = 0.044) although none of the pairwise comparisons reached statistical significance following Bonferroni correction (0.053 < p < 0.99). There were no other statistically significant differences across all subjects during the 10-week period.
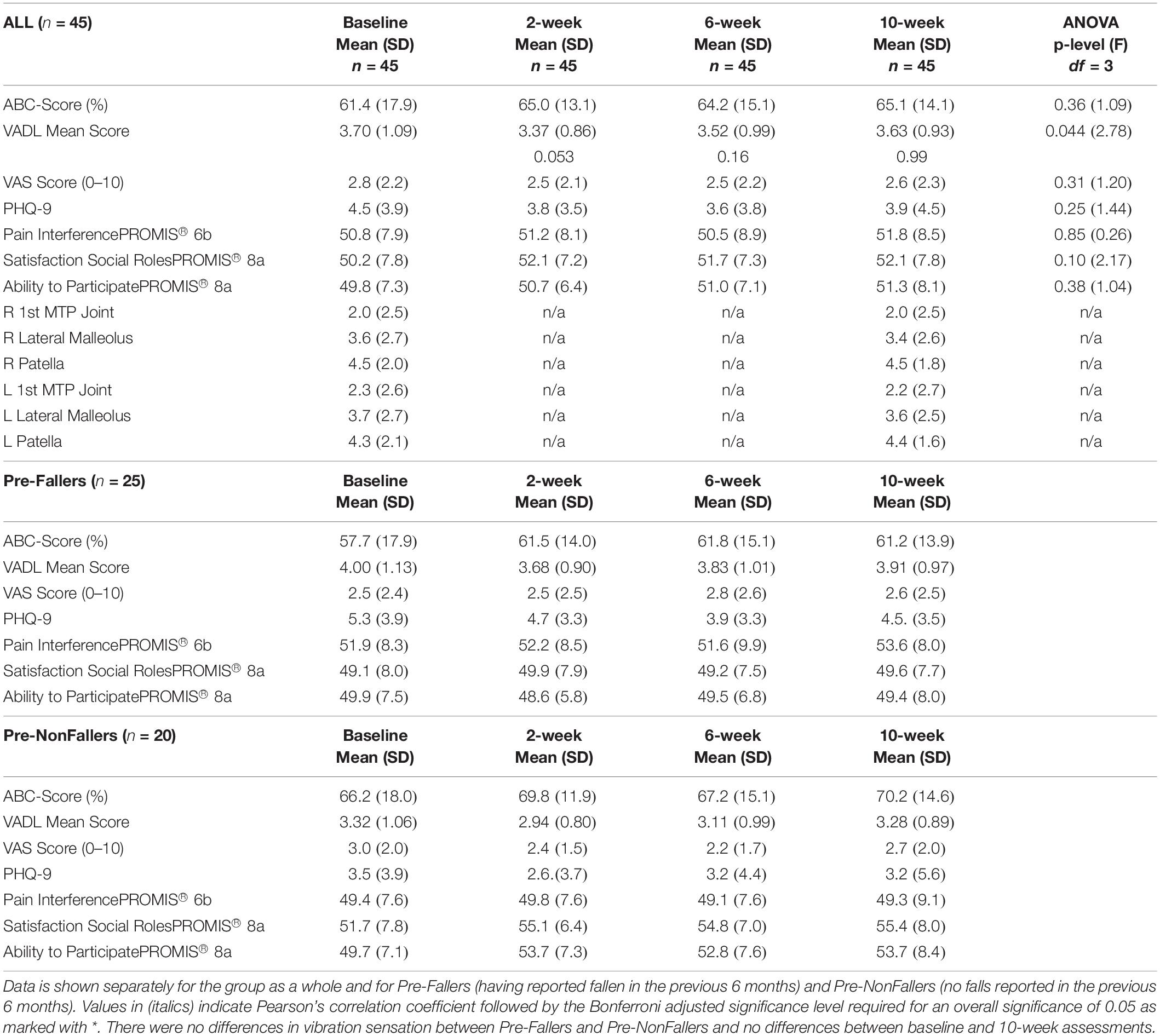
Table 5. Results from participant-reported outcomes and Rydel-Seiffer vibration sensation screening for the 45 subjects who completed all assessments.
Table 5 shows data from vibration sensation testing using the Rydel-Seiffer graduated tuning fork for all subjects. There were no differences in vibration sensation between Pre-Faller and Pre-NonFaller and no differences between baseline and 10-week assessments. Vibration sensitivity showed bilateral symmetry across the three sites tested, right and left patella, lateral malleolus and first metatarsophalangeal joints, respectively. There was a gradual proximal to distal decrease in vibration sensitivity across the three sites from 4.3 to 4.4 proximally and 2.0 distally based on the 0–8 Rydel-Seiffer scale (Table 5). Similarly, there was bilateral symmetry for the WEST monofilament sensitivity test (not shown in Table). At baseline, the median for the monofilament test was 50g for both feet and across all four anatomical test sites, the first, third, and fifth metatarsal heads as well as the great toe. This was the same at the 10-week assessment except for the first metatarsals of both feet where the median monofilament increased to 200g.
Any changes in patient reported outcomes over time in the Pre-Faller and Pre-NonFaller subgroups were small, well within one standard deviation (Table 5). A consistent change after baseline appeared for the Pre-NonFaller group in the PROMIS “Ability to Participate” increasing from 49.7 ± 7.1 at baseline to 53.7 ± 8.4, 52.8 ± 7.6 and 53.7 ± 7.3 at 2, 6 and 10 weeks, respectively (Table 5).
Subjects reported using the device on average 5.5 ± 1.4 days/week (range 3.5–7.0) for a total of 36.1 ± 14.9 h/week (range 5.3–56.0). Over 80% of subjects reported using the device more than 21 h/week and 60% more than 36 h/week. Sixteen subjects (36%) rated their overall satisfaction with the device as “Very Satisfied”, 13 (29%) as “Satisfied”, 8 (18%) were “Somewhat Satisfied”, 6 (13%) were “Neutral” and 1 (2%) subject each were “Somewhat Dissatisfied” and “Dissatisfied”, respectively. No subjects rated their overall satisfaction as “Very Dissatisfied”. Table 6 shows results from the user experience survey of subjects rating their experience with activities related to the device on a scale from 1 “Very Hard” to 7, “Very Easy”. Overall, average ratings for putting on Walkasins was 5.4, taking off 6.1, charging them 6.2, cleaning 5.2 and learning to use them 6.0. See Table 6 for further details.
Parameters related to falls and fall risk assessed at baseline and throughout the 10 weeks are shown in Table 7. Overall, after 10 weeks, 13 of the 45 subjects had achieved FGA scores higher than 22, the cut-off for normal fall risk (Wrisley and Kumar, 2010). Four of these subjects were part of the Pre-Faller subgroup and nine were from the Pre-NonFaller group. The 45 subjects who reached 10 weeks reported a total of 62 falls in the 6 months prior to the study. During the 10-week study period, 17 falls were reported, 13 of which occurred in the Pre-Faller group and four in the Pre-NonFaller group (Table 7). No falls that occurred in-study were related to device use. Three falls led to injuries that required medical attention. A trip on a cord led to a dislocated finger joint treated with a splint in Urgent Care, a trip over a groove in the driveway resulted in minor cuts and scrapes, an X-ray in the Emergency Room showed no fractures; and finally another subject was putting on socks, lost balance and fell backwards leading to Emergency Room visit where an X-ray showed a fractured wrist requiring minor surgery. Sixteen of the 25 Pre-Fallers who reached 10 weeks participation did not fall during the 10-week period of the trial. There was a non-significant decrease in fall rate across all subjects (from 7.7 to 5.4 falls/1000 patient days, p = 0.27). In the Pre-Faller group, there was a 46% statistically significant decrease in fall rate (from 13.8 to 7.4 falls per 1000 patient days, p = 0.014). The Pre-NonFaller group showed a smaller non-significant increase in fall rate (from 0 to 2.9 falls per 1000 patient days, p = 0.125). This increase was based on one fall each by four subjects who had not fallen in the prior 6 months (Table 7). There was a statistically significant decrease in the number of fall risk factors (Table 7) from baseline to 10 weeks across all subjects (from 4.2 to 3.8 fall risk factors, p = 0.047). This decrease was larger and statistically significant in the Pre-Faller group (from 5.1 to 4.3 fall risk factors, p = 0.023). There was no change in the number of fall risk factors in the Pre-NonFaller group (p = 0.76, Table 7).
Results from this multi-site clinical trial supported our a priori hypothesis that patients with gait and balance problems due to sensory PN and high risk of falls would show clinically meaningful improvements of gait and dynamic balance function after 10 weeks of using a wearable sensory prosthesis, confirming previously demonstrated in-clinic findings in a randomized controlled cross-over trial (Koehler-McNicholas et al., 2019). Overall, the mean FGA score improved from 15.0 at baseline to 19.1 at 10 weeks across all subjects (Table 4), a change that is beyond the MCID for the FGA (Beninato et al., 2014). Thirteen subjects reached normal fall risk status showing an FGA score higher than 22 (Wrisley and Kumar, 2010) after 10 weeks of device use. Both normal and fast gait speed improved overall by 0.09m/s, beyond what it considered small meaningful (0.05m/s) and near a substantial change (0.10m/s) for older adults (Perera et al., 2006). Across all subjects, TUG improved from 13.8 s to 12.5 s, which is beyond the MDC for older adults with type 2 diabetes (Alfonso-Rosa et al., 2014). Effect sizes ranged from large for FGA scores (Cohen’s drm, 0.92) to small for fast gait speed and TUG (0.27 and 0.28, respectively). The severity of patients’ PN as indirectly indicated by the vibratory sensation threshold, did not change over the 10-week period. Consequently, plantar sensation was still absent suggesting new sensory information from the device may have provided relevant input to improve function.
Although both Pre-NonFallers and Pre-Fallers showed improvements, changes appeared larger in the Pre-NonFaller group for FGA and normal gait speed. Fast gait speeds at baseline (1.41 m/s) and 10 weeks (1.52 m/s) in the Pre-NonFaller group are considered in the high range of elderly community ambulators (Middleton et al., 2015) suggesting a potential ceiling effect in ability to improve further.
Interestingly, the 4-Stage Balance measure, an indicator of static balance, did not improve significantly over the 10-week period although our recent in-clinic study (Koehler-McNicholas et al., 2019) found a statistically significant improvement in this measure in a group of PN patients. One important difference between these two cohorts may be a slightly lower baseline static balance performance in the first cohort, a mean of 22.2 s versus 26.2 s in the current one. The first group improved their in-clinic performance to a mean of 27.6 s (p < 0.001) while the current cohort improved to a nearly identical 27.8 s (n.s.). It may be that further improvement of static balance performance would require some additional training challenge in addition to device use (e.g., Romberg and sharpened Romberg types of activities) and simply wearing the device daily does not sufficiently challenge the static balance ability as assessed by the 4-Stage Balance Test.
To our knowledge, this is the first trial where a cohort of subjects with gait and balance problems related to PN have worn a sensory prosthetic device of this kind in the community and on a regular basis. Other wearable neuromodulation technologies have been used as a treatment modality in a home setting, although typically only worn in conjunction with a home therapy program for balance and mobility in different categories of subjects, e.g., multiple sclerosis (Leonard et al., 2017) and healthy elderly (Bao et al., 2018). A recent case study of a patient with PN wearing Walkasins for a year (Wrisley et al., 2018, 2020), found dramatic improvements in gait and balance outcomes when daily continuous device use was combined with balance therapy. Prior to using Walkasins, the patient had received balance therapy twice a week for over 5 months by an expert physical therapist (DW) and had plateaued in his improvements (Wrisley et al., 2018, 2020). In the current study, in an attempt to isolate the effect of long-term device use on clinical outcomes, subjects were instructed to wear the device as much as possible and were not allowed to participate in any additional balance training or therapy. Furthermore, they were not systematically informed of any changes in their outcomes or performance, which they would normally receive in regular clinical care. Still, improvements in clinical outcomes were similar to those reported after various balance intervention programs conducted over comparable time periods up to 12 weeks in similar patient populations (Shumway-Cook et al., 1997; Wolf et al., 2001; Li et al., 2005; Manor et al., 2014), or even longer up to 6 months and nearly a year (Wolf et al., 2003; Li et al., 2005; Li and Manor, 2010).
It may be particularly difficult to enhance gait and balance function in individuals with PN using physical therapy or balance training activities alone, possibly because these interventions do not replace lost somatosensory input. Furthermore, effects of a training or therapy program on gait and balance function are likely due to different mechanisms than the use of a sensory substitution device. Interestingly, two systematic reviews of interventions specifically for patients with diabetic PN gave lower extremity strengthening a fair recommendation while other interventions showed insufficient evidence to increase function (Ites et al., 2011; Tofthagen et al., 2012). More recently, improvement in function in patients with PN following specific task-oriented training has been shown (Salsabili et al., 2015).
For training/therapy programs to be effective, well known principles of training and exercise physiology must be adhered to (Oddsson et al., 2007) ensuring that sensorimotor systems are sufficiently challenged to adapt and improve their capabilities leading to improved muscle function and neuromotor coordination. However, patients with PN have lost important cutaneous afferent systems that could be affected by such interventions. As a result, improvements related to use of Walkasins seen in the current study are most likely due to participants receiving new tactile balance stimuli that are relevant for gait and balance and therefore become integrated into their neuromotor control and movement repertoire. Subjects in the current study received hundreds of such stimuli per hour from the device during their regular standing and walking activities throughout the day.
Consequently, we hypothesize that any balance-related therapy or training activity in conjunction with wearing the device would provide an additive effect to overall function and balance outcomes. This hypothesis is supported by our recent case study (Wrisley et al., 2018, 2020) as well as our previous in-clinic study where subjects following a baseline assessment were randomized to either wearing the device turned on or turned off while performing a brief 10–15 min standardized balance activity session with a physical therapist (Koehler-McNicholas et al., 2019). Ten of 15 subjects in the on group increased their FGA scores by at least 4 points compared to five of 16 in the off group (p < 0.05). Furthermore, seven of 15 subjects in the on group increased gait speed by > 0.13m/s compared to 3 of 16 in the off group (p < 0.05) (Koehler-McNicholas et al., 2019).
Gait speed is a powerful indicator of overall health and survival in the elderly population and improving gait speed is an important therapeutic goal. Based on a large population study, Studenski et al. (2011) found that gait speed, age, and gender predicted survival as well as factors related to chronic conditions, smoking history, blood pressure, and hospitalization. In fact, improvement in gait speed by 0.10 m/s was found to predict better survival in older adults (Hardy et al., 2007). Furthermore, while each decrease in gait speed by 0.10m/s has been associated with longer hospital stays and higher healthcare costs, a similar amount of increase per year in gait speed has been shown to be predictive of shorter hospital stays and a reduction in healthcare cost (Purser et al., 2005), further emphasizing the importance of gait speed as a relevant indicator of health and vitality (Middleton et al., 2015).
Healthy aging is associated with an annual decrease in gait speed by 0.013 m/s (Buracchio et al., 2010). However, it is well know that individuals with PN walk slower than their healthy counterparts (Menz et al., 2004; Lipsitz et al., 2018), likely as an adapted strategy to maintain balance (Dingwell et al., 2000). In fact, subjects with peripheral sensory loss (Lipsitz et al., 2018) who were consistently impaired over 5 years showed a decline in gait speed of 0.23 m/s over that time period, i.e., 0.046 m/s/yr., more than 3.5 times higher than reported by Buracchio in healthy aging (Buracchio et al., 2010). Our current population of subjects with PN, who appear similar to the “impaired” category of community-dwelling older individuals in Lipsitz et al. (2018), showed an increase in gait speed of 0.09 m/s following 10 weeks of use of the Walkasins device, corresponding to an annual rate of 0.47 m/s. Interestingly, the Pre-Faller group appeared to increase both normal and fast gait speeds while the Pre-NonFaller group appeared to only increase their normal gait speed, possibly due to a ceiling effect in fast gait speed (Middleton et al., 2015).
Although specific mechanisms of action of sensory substitution and augmentation stimulation is currently unknown, sensory re-weighting has some support in the current literature, i.e., the brain gradually increases use of new sensory balance cues to enhance performance (For a review see (Sienko et al., 2018). Specifically, subjects performing in-home balance training while receiving vibrotactile sensory augmentation to the trunk (Bao et al., 2018) showed an increased reliance on vestibular inputs as indicated by functional Magnetic Resonance Imaging of brain areas that process somatosensory, visual, and vestibular information (Noohi et al., 2017; Sienko et al., 2018).
Consequently, the improvement in gait speed and function from wearing Walkasins may hypothetically be interpreted from our understanding of the sensorimotor control of human locomotion and balance. When subjects with PN perform gait and balance activities, sensory information related to foot pressure is either completely absent or at least distorted and, therefore, likely non-veridical; and it is unlikely that remaining balance-related somatosensory information can sufficiently compensate, leading to decreased stability and increased risk of balance loss. Plantar cutaneous sensory information is important for standing balance (Meyer et al., 2004b; Strzalkowski et al., 2018), gait stability (Zehr et al., 2014), for signaling stance limb placement and withdrawal to facilitate phase-dependent modulation of controlling reflex responses (Zehr and Stein, 1999), and when responding to balance perturbations (Meyer et al., 2004a). Hlavacka et al. (1995) stated that an internal representation of the body vertical requires integration of somatosensory and vestibular inputs, later emphasized by Bronstein who concluded that somatosensory information has a “prominent role” in verticality perception, which is crucial for optimal balance control (Bronstein, 1999). Marsden et al. (2003) further concluded that the processing of vestibular information is influenced by load-related afferent feedback for control of balance.
The integration of somatosensory and vestibular information appears of particular importance for gait function during the double support phase following foot placement during walking (Bent et al., 2004), which is near the events when Walkasins provides tactile stimuli. Although of lesser fidelity than intact plantar sensation, the Walkasins device may hypothetically provide sufficient and relevant sensory information that is veridical both during standing and walking activities by signaling out of balance events during standing as well as indicating stance and swing phases of gait, which can help improve gait and balance function. Furthermore, these tactile stimuli are provided just proximally to the original sensory loss and mainly along the same dermatomes representing the plantar surface of the foot possibly making it intuitive to integrate into functional behavior (Koehler-McNicholas et al., 2019) by providing relevant sensory information to spinal central pattern generators for locomotion (Guertin, 2012). Further research into these hypothetical mechanisms is warranted to better understand how wearable sensory prosthetic devices may help improve function.
Although we noticed an encouraging decrease in fall rate as well as in the number of fall-risk factors in the Pre-faller subgroup (Table 7), a 10-week time period with a fairly small number of subjects is too short to draw any final conclusions related to prevention of falls. Interestingly, studies have found that effects of improved clinical gait and balance outcomes may lead to a delayed effect of fall reduction, which was reported following 6 months of Tai Chi (Li et al., 2004, 2005) and also observed by Wolf et al. (1996, 2003).
There appeared to be none or only small changes in the self-report measures throughout the 10 weeks of the trial (Table 5). Although this may seem counterintuitive, Richardson et al. (2001) reported significant improvements in clinical balance outcomes, but non-significant improvement in the ABC score following a strength and balance intervention for patients with PN. Similarly, an exercise intervention study for older adults found discrepancies between balance and ABC score improvements suggesting that the relationship between balance confidence and functional performance may not be well understood (Cyarto et al., 2008). In the current study, a lack of overall improvement in the ABC score may also be influenced by subject expectations, the short duration of study, or the time of the year as some subjects were enrolled in the winter months during snowy and icy conditions. Furthermore, results of the ABC balance confidence scores may also be viewed in the context of the subjects not being systematically informed of changes in clinical outcomes during the study, nor receiving any organized encouragement reflecting their performance and altering their appraisal of their own abilities (Hadjistavropoulos et al., 2011).
Interestingly, upon further investigation we observed differences between the overall pattern of improvement in clinical outcomes versus self-reported measures of balance confidence as illustrated in Figure 3. Clinical outcomes at 10 weeks showed similar improvement across the full range of baseline scores, indicated by a regression line between baseline and 10-week scores being near parallel with the line of unity as illustrated with the FGA scores in Figure 3A. However, this was not the case for the balance confidence ABC-score as seen in Figure 3B (similar observations were made for the VADL scores, not shown here). As can be seen, the regression line between baseline and 10-week ABC scores intersects the line of unity and it has a slope of 0.47 (Figure 3B). Interestingly, the two lines intersect at the baseline value 67%, the published cut-off value for high fall risk (Lajoie and Gallagher, 2004).
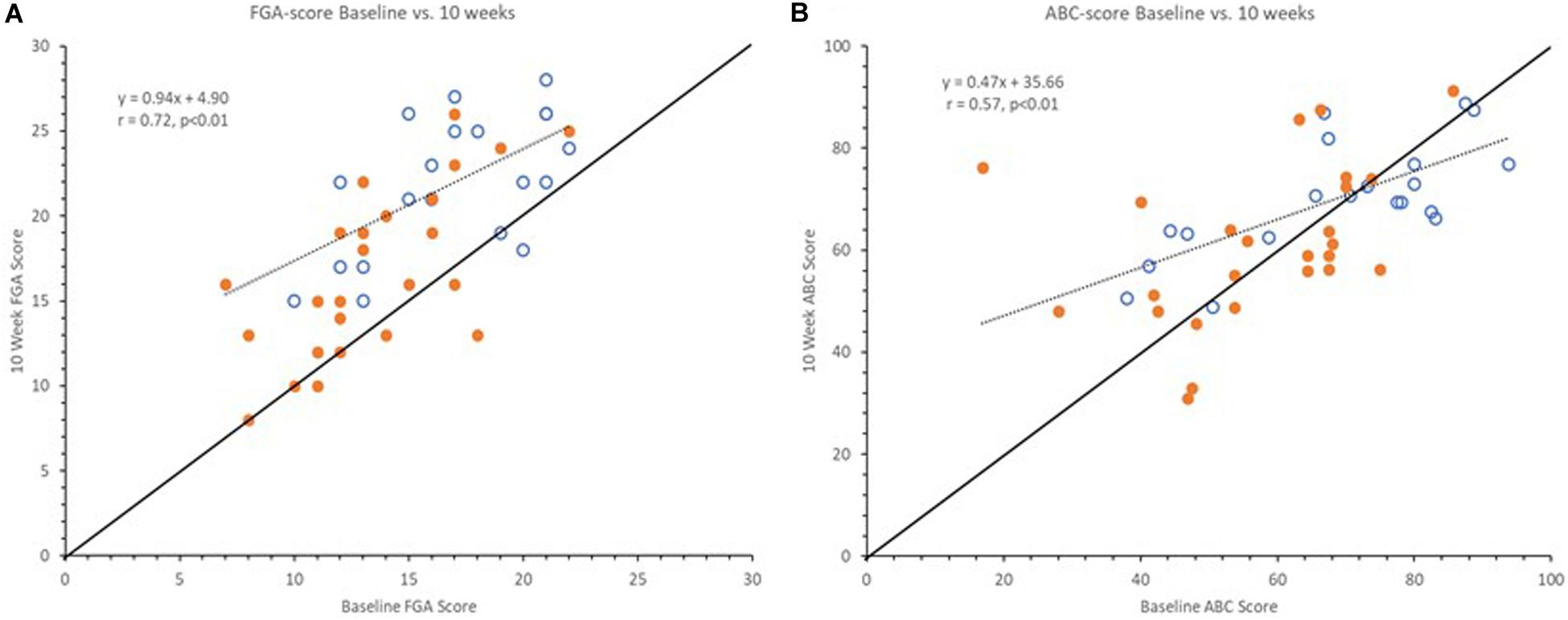
Figure 3. Graphs showing baseline vs. 10-week FGA (A) and ABC scores (B). Open markers represent Pre-Fallers and closed markers Pre-NonFallers. Markers above line of identity indicate higher scores at 10-week assessment. Notice line of regression for FGA scores is near parallel to line of identity indicating a similar increase across all baseline FGA scores. For ABC scores the line of regression intersects the line of identify near 67% indicating an increase for lower baseline ABC scores and a decrease for higher baseline ABC scores. Two markers in panel (A) are not visible since two pairs of subjects had the same pre- and post-study FGA values, (16, 21) and (21, 26).
Subjects enrolled in the current study were all at high fall risk based on their FGA score ≤ 22 (Wrisley and Kumar, 2010) as well as a diagnosis of PN (Richardson and Hurvitz, 1995). Based on additional outcomes and baseline characteristics, subjects had on average more than four fall risk factors (Tables 1, 2, 7). Consequently, it could be argued that study participants with multiple impairments related to balance maybe “should not” have a balance confidence ABC score above 67% and those who report such scores are either overly confident and/or simply unaware of their true balance capabilities (not a “Realistic Appraisal of One’s Own Abilities”, (Hadjistavropoulos et al., 2011)). Compellingly, subjects with greater balance confidence at baseline actually showed a decrease in their balance confidence during the study (from 76.5 ± 8.1 to 71.8 ± 9.9, p = 0.02) while subjects with a baseline ABC score < 67% (in the range of high-fall risk) increased their balance confidence scores (49.9 ± 12.5% to 59.3 ± 15.1%, p = 0.01). In fact, this finding appears to align with the multifactorial causation model for falls and fear proposed by Hadjistavropoulos et al. (2011). Their model incorporated “Realistic Appraisal of One’s Own Abilities” as a feature that interacts with “Falls Efficacy” (balance confidence) and “Balance Performance” (in our case FGA). The authors discuss how this information can be used to guide clinicians toward suitable rehabilitation and stated that “The extent to which balance confidence reflects realistic appraisals has not been adequately researched” (Hadjistavropoulos et al., 2011). We propose that a ratio between a falls efficacy and balance performance measure could be used to indicate an “Appraisal of One’s Own Abilities” and guide the clinician to the most suitable rehabilitation.
If we consider a simple ratio between ABC and FGA scores representing “amount” of balance confidence per FGA point, someone with 100% balance confidence and the maximum FGA score of 30 would have a ratio of 3.3. A ratio between the established cut-offs for high fall risk for ABC and FGA scores, 67% and 22, respectively, represents a ratio of 3.0. At baseline in the current trial, the overly confident subjects had a ratio of 5.1 ± 1.3, versus 3.6 ± 1.1 for the low confidence subjects. After 10 weeks of device use, with increased FGA scores across the board, the ratio was 3.8 ± 1.4 for the overly confident subjects versus 3.4 ± 1.0 for the lower confidence subjects. It appears the overly confident subjects may have “normalized” their self-perception of their balance ability while the lower confidence subjects increased their ABC score proportionally to their improved FGA score and maintained a similar self-confidence to FGA ratio. Since the FGA and ABC capture different constructs related to balance, it would be important for clinicians to be aware of the potential discrepancy between a patient’s self-perception and actual functional performance when developing a plan of care targeting gait, balance function and fall prevention.
The T-scores for baseline PROMIS measures (Pain Interference, Satisfaction Social Roles, Ability to Participate) were all close to 50, which was unexpected since it is considered the average for the US population (Hahn et al., 2014, 2016a, b; Askew et al., 2016). We had expected these outcomes to deviate significantly in this complex clinical population. Consequently, any major changes in these measures should not be expected although a small increase in PROMIS Ability to Participate score, which remained through 10 weeks, was seen for the Pre-NonFaller sub-group.
Some additional trends in the patient reported outcomes of interest for further research were observed, especially some differences between the Pre-Faller and Pre-NonFaller group. The Pre-Faller group had a PHQ-9 score of 5.3 at baseline, > 5 considered mild depression (Kroenke et al., 2001), which decreased to 3.9 at 6 weeks and reached 4.5 at 10 weeks. Although such changes are of minimal clinical relevance (Kroenke et al., 2001), it may be of interest to investigate individuals with higher initial PHQ-9 scores to better understand this observation. Finally, while VAS Pain scores were overall in the range of mild pain (≤ 3) and remained steady throughout 10 weeks, it may be of interest to further investigate individuals with higher initial pain levels, especially those with neuropathy-related foot pain by using a more disease-specific pain rating scale.
There are several limitations to this trial. It is not blinded, lacks a control group and a placebo treatment. Unfortunately, it is not feasible to blind subjects from treatment in the current study since being able to feel the tactile stimuli from the device is an inclusion criterion. Using some form of random pattern stimuli as a sham may seem possible (Basta et al., 2011), although it is not known if such stimuli may have an effect of their own and it would not help address the question whether using the device as currently designed, according to principles of sensorimotor control of balance and gait, has an effect on gait and balance function. Consequently, the best placebo treatment would likely be wearing a device that is turned off. However, without using some form of deceit claiming the device is working although it cannot be felt, it would likely be difficult to recruit participants for such research and/or to ensure long-term compliance. In addition, incorporating a minimal stimulation amplitude as a sham, assuming it has no effect may be incorrect since studies implementing stochastic resonance using subsensory mechanical noise have demonstrated improvement in balance (Lipsitz et al., 2015). Furthermore, using a randomized control cross-over design, we recently demonstrated in-clinic improvements in clinical outcomes when the Walkasins device was worn and turned on as compared to turned off (Koehler-McNicholas et al., 2019). Consequently, we felt comfortable incorporating a single treatment arm design knowing the in-clinic effects. Furthermore, any placebo effects were likely decreased by not systematically informing subjects about any changes in outcomes and minimizing encouragement during interactions with subjects that could affect expectation and beliefs in the treatment (Finniss et al., 2010; Enck et al., 2013; Coste and Montel, 2017), and prohibiting any additional balance training/therapy intervention during the 10 weeks of the trial.
If the effects in this study were placebo our findings should align with research findings on the placebo arm of randomized, placebo-controlled trials (Wartolowska et al., 2016). A systematic review of temporal changes in the placebo arm across 47 surgical randomized control trials found that effects size of subjective outcomes was large (0.64), while effect size of objective clinical outcomes was small (0.11) (Wartolowska et al., 2016). Furthermore, major differences in placebo-effect sizes have been reported with subject-reported self-perception effects being larger than observer-based ratings (Rief et al., 2009). On the contrary, effect sizes in the current study were large for the clinical outcomes and small for the self-reported outcomes, supporting the interpretation that effects were due to device use and not placebo. Further support of this view includes relatively high subject compliance and reported device use and, a low subject dropout rate of 13.5% as well as the sustained duration and continued gradual improvement in clinical outcomes throughout the 10-week period. However, conclusive causality cannot be determined due to the limitations of the single-arm study design.
Although subjects were instructed to use the device as much as possible throughout their regular daily activities, the range of reported device use was large. However, the intent was to not impose changes in activity levels, but rather just add the device to regular daily routines. Considering the large range of health issues in this cohort of patients, the variability in device use may simply reflect variability in common daily activity levels in this population of individuals. Subjects who were mostly inactive throughout the day, may have reported less device use. Enrollment of mostly male subjects is a weakness, which is partly due to nearly half of the subjects being Veterans, who especially in this older generation are predominantly male. Strengths of this trial include involvement of multiple sites across different geographies with different assessors at different clinics limiting confounding balance interventions, and the use of standardized objective clinical outcome measures.
A wearable sensory prosthesis may provide a new way to treat gait and balance problems and manage falls in high fall-risk patients with PN. Longer term data would be required to further investigate actual decreases in falls.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved according to the Declaration of Helsinki by Advarra IRB (formerly Quorum Review IRB), serving as the Institutional Review Board (IRB) of record for three of the participating sites under the study protocol. The three sites include Baylor College of Medicine, Houston, TX; Hebrew SeniorLife, a Harvard Medical School Affiliate, Boston, MA; and M Health Fairview, Minneapolis, MN. The IRB Subcommittee, the Subcommittee on Research Safety, and the Research and Development Committee of the Minneapolis VA Health Care System (MVAHCS) also approved the trial. The study is registered on ClinicalTrials.gov (#NCT03538756). All necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived. The patients/participants provided their written informed consent to participate in this study.
LO was involved in conception, study design, data analysis, database development, and wrote the first draft. TB performed data acquisition. HC was involved in design and data analysis. LJ performed training and monitoring, data analysis, and drafted early sections. MK was involved in design. DK was involved in design and data acquisition. LL and BM were involved in study design. PM performed data acquisition. YR developed the REDCap Cloud database, training, monitoring, data analysis and drafted early sections. DW was involved in conception, design and outcomes standardization training. SK-M was part of conception, design, data acquisition, and analysis. All authors contributed to data interpretation, critical review and manuscript revisions, read, and approved version to be submitted.
The walk2Wellness trial is completely funded by RxFunction Inc. Participating sites have received funding from RxFunction to conduct research activities related to the trial.
The authors declare that this study received funding from RxFunction Inc. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
We thank Lori Danzl, Alexandria Lloyd, Christine Olney (Minneapolis Department of Veterans Affairs Health Care System, Minneapolis, MN), Jackie Geiser, Tien Dat Nguyen, Delorianne Sander (M Health Fairview, Minneapolis, MN), Nathan Silver (Baylor College of Medicine, Houston, TX), Ikechukwu Iloputaife, Wanting Yu (Hinda and Arthur Marcus Institute for Aging Research, Hebrew SeniorLife, Roslindale, MA) for providing study coordination, subject recruitment and data gathering activities. We also thank Amy Gravely, MA for input on statistical analysis and Annette Xenopoulos-Oddsson (University of Minnesota, MN), Dr. Lee Newcomer and Dr. Steven Stern for reviewing drafts of the manuscript. The manuscript was published as a MedRxiv preprint (Oddsson et al., 2020b).
Akbari, M., Jafari, H., Moshashaee, A., and Forugh, B. (2012). Do diabetic neuropathy patients benefit from balance training? J. Rehabil. Res. Dev. 49, 333–338. doi: 10.1682/jrrd.2010.10.0197
Alfonso-Rosa, R. M., Del Pozo-Cruz, B., Del Pozo-Cruz, J., Sañudo, B., and Rogers, M. E. (2014). Test-retest reliability and minimal detectable change scores for fitness assessment in older adults with type 2 diabetes. Rehabil. Nurs. 39, 260–268. doi: 10.1002/rnj.111
Algina, J., Keselman, H. J., and Penfield, R. D. (2005). Effect sizes and their intervals: the two-level repeated measures case. Educ. Psych. Meas. 65, 241–258. doi: 10.1177/0013164404268675
Allet, L., Armand, S., de Bie, R. A., Golay, A., Monnin, D., Aminian, K., et al. (2010). The gait and balance of patients with diabetes can be improved: a randomised controlled trial. Diabetologia 53, 458–466. doi: 10.1007/s00125-009-1592-4
Askew, R. L., Cook, K. F., Revicki, D. A., Cella, D., and Amtmann, D. (2016). Evidence from diverse clinical populations supported clinical validity of PROMIS pain interference and pain behavior. J. Clin. Epidemiol. 73, 103–111. doi: 10.1016/j.jclinepi.2015.08.035
Bach-y-Rita, P. (2004). Tactile sensory substitution studies. Ann. N. Y. Acad. Sci. 1013, 83–91. doi: 10.1196/annals.1305.006
Bach-y-Rita, P., Collins, C. C., Saunders, F. A., White, B., and Scadden, L. (1969). Vision substitution by tactile image projection. Trans. Pac. Coast Otoophthalmol. Soc. Annu. Meet. 50, 83–91.
Badke, M. B., Sherman, J., Boyne, P., Page, S., and Dunning, K. (2011). Tongue-based biofeedback for balance in stroke: results of an 8-week pilot study. Arch. Phys. Med. Rehabil. 92, 1364–1370. doi: 10.1016/j.apmr.2011.03.030
Bao, T., Carender, W. J., Kinnaird, C., Barone, V. J., Peethambaran, G., Whitney, S. L., et al. (2018). Effects of long-term balance training with vibrotactile sensory augmentation among community-dwelling healthy older adults: a randomized preliminary study. J. Neuroeng. Rehabil. 15:5. doi: 10.1186/s12984-017-0339-6
Basta, D., Rossi-Izquierdo, M., Soto-Varela, A., Greters, M. E., Bittar, R. S., Steinhagen-Thiessen, E., et al. (2011). Efficacy of a vibrotactile neurofeedback training in stance and gait conditions for the treatment of balance deficits: a double-blind, placebo-controlled multicenter study. Otol. Neurotol. 32, 1492–1499. doi: 10.1097/MAO.0b013e31823827ec
Beninato, M., Fernandes, A., and Plummer, L. S. (2014). Minimal clinically important difference of the functional gait assessment in older adults. Phys. Ther. 94, 1594–1603. doi: 10.2522/ptj.20130596
Bent, L. R., Inglis, J. T., and McFadyen, B. J. (2004). When is vestibular information important during walking? J. Neurophysiol. 92, 1269–1275. doi: 10.1152/jn.01260.2003
Bergen, G., Stevens, M. R., and Burns, E. R. (2016). Falls and fall injuries among adults aged ≥ 65 years - United States, 2014. Morb. Mortal. Wkly. Rep. 65, 993–998. doi: 10.15585/mmwr.mm6537a2
Bischoff, H. A., Stähelin, H. B., Monsch, A. U., Iversen, M. D., Weyh, A., von Dechend, M., et al. (2003). Identifying a cut-off point for normal mobility: a comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age. Ageing 32, 315–320. doi: 10.1093/ageing/32.3.315
Bronstein, A. M. (1999). The interaction of otolith and proprioceptive information in the perception of verticality. The effects of labyrinthine and CNS disease. Ann. N.Y. Acad. Sci. 871, 324–333. doi: 10.1111/j.1749-6632.1999.tb09195.x
Bulat, T., Hart-Hughes, S., Ahmed, S., Quigley, P., Palacios, P., Werner, D. C., et al. (2007). Effect of a group-based exercise program on balance in elderly. Clin. Interv. Aging 2, 655–660. doi: 10.2147/cia.s204
Buracchio, T., Dodge, H. H., Howieson, D., Wasserman, D., and Kaye, J. (2010). The trajectory of gait speed preceding mild cognitive impairment. Arch. Neurol. 67, 980–986. doi: 10.1001/archneurol.2010.159
Cakrt, O., Vyhnálek, M., Slabı, K., Funda, T., Vuillerme, N., Koláø, P., et al. (2012). Balance rehabilitation therapy by tongue electrotactile biofeedback in patients with degenerative cerebellar disease. NeuroRehabilitation 31, 429–434. doi: 10.3233/NRE-2012-00813
Cavanagh, P. R., Derr, J. A., Ulbrecht, J. S., Maser, R. E., and Orchard, T. J. (1992). “Problems with gait and posture in neuropathic patients with insulin-dependent diabetes mellitus. Diabet. Med. 9, 469–474.
CDC (2017). STEADI - Older Adult Fall Prevention. Centers for Disease Control and Prevention. Available online at: https://www.cdc.gov/steadi/materials.html (accessed May 5, 2018)
Chodzko-Zajko, W. J., Proctor, D. N., Fiatarone Singh, M. A., Minson, C. T., Nigg, C. R., Salem, G. J., et al. (2009). American college of sports medicine position stand, Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 41, 1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c
Cohen, H. S., Kimball, K. T., and Adams, A. S. (2000). Application of the vestibular disorders activities of daily living scale. Laryngoscope 110, 1204–1209. doi: 10.1097/00005537-200007000-00026
Coste, J., and Montel, S. (2017). Placebo-related effects: a meta-narrative review of conceptualization, mechanisms and their relevance in rheumatology. Rheumatology 56, 334–343. doi: 10.1093/rheumatology/kew274
Cyarto, E. V., Brown, W. J., Marshall, A. L., and Trost, S. G. (2008). Comparative effects of home- and group-based exercise on balance confidence and balance ability in older adults: cluster randomized trial. Gerontology 54, 272–280. doi: 10.1159/000155653
DeMott, T. K., Richardson, J. K., Thies, S. B., and Ashton-Miller, J. A. (2007). Falls and gait characteristics among older persons with peripheral neuropathy. Am. J. Phys. Med. Rehabil. 86, 125–132. doi: 10.1097/PHM.0b013e31802ee1d1
Dingwell, J. B., Cusumano, J. P., Sternad, D., and Cavanagh, P. R. (2000). Slower speeds in patients with diabetic neuropathy lead to improved local dynamic stability of continuous overground walking. J. Biomech. 33, 1269–1277. doi: 10.1016/s0021-9290(00)00092-0
Dixon, C. J., Knight, T., Binns, E., Ihaka, B., and O’Brien, D. (2017). Clinical measures of balance in people with type two diabetes: a systematic literature review. Gait Posture 58, 325–332. doi: 10.1016/j.gaitpost.2017.08.022
Dupont, W. D., and Plummer, W. D. (1990). Power and sample size calculations, a review and computer program. Cont. Clin. Trials 11, 116–128. doi: 10.1016/0197-2456(90)90005-m
Dupont, W. D., and Plummer, W. D. (1998). PS power and sample size program available for free on the internet. Control Clin. Trials 18:274. doi: 10.1016/S0197-2456(97)00074-3
Enck, P., Bingel, U., Schedlowski, M., and Rief, W. (2013). The placebo response in medicine: minimize, maximize or personalize? Nat. Rev. Drug Discov. 12, 191–204. doi: 10.1038/nrd3923
Finniss, D. G., Kaptchuk, T. J., Miller, F., and Benedetti, F. (2010). Biological, clinical, and ethical advances of placebo effects. Lancet 375, 686–695. doi: 10.1016/S0140-6736(09)61706-2
Florence, C. S., Bergen, G., Atherly, A., Burns, E., Stevens, J., and Drake, C. (2018). “Medical Costs of Fatal and nonfatal falls in older adults. J. Am. Geriatr. Soc. 66, 693–698. doi: 10.1111/jgs.15304
Ganz, D. A., Bao, Y., Shekelle, P. G., and Rubenstein, L. Z. (2007). Will my patient fall? JAMA 297, 77–86. doi: 10.1001/jama.297.1.77
Ganz, D. A., and Latham, N. K. (2020). Prevention of Falls in Community-Dwelling Older Adults. N. Engl. J. Med. 382, 734–743. doi: 10.1056/NEJMcp1903252
Gregg, E. W., Sorlie, P., Paulose-Ram, R., Gu, Q., Eberhardt, M. S., Wolz, M., et al. (2004). “Prevalence of lower-extremity disease in the US adult population > = 40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care 27, 1591–1597. doi: 10.2337/diacare.27.7.1591
Guertin, P. A. (2012). Central pattern generator for locomotion: anatomical, physiological, and pathophysiological considerations. Front. Neurol. 3:183. doi: 10.3389/fneur.2012.00183
Hadjistavropoulos, T., Delbaere, K., and Fitzgerald, T. D. (2011). Reconceptualizing the role of fear of falling and balance confidence in fall risk. J Aging Health 23, 3–23. doi: 10.1177/0898264310378039
Hahn, E. A., Beaumont, J. L., Pilkonis, P. A., Garcia, S. F., Magasi, S., DeWalt, D. A., et al. (2016a). The PROMIS satisfaction with social participation measures demonstrated responsiveness in diverse clinical populations. J. Clin. Epidemiol. 73, 135–141. doi: 10.1016/j.jclinepi.2015.08.034
Hahn, E. A., DeWalt, D. A., Bode, R. K., Garcia, S. F., DeVellis, R. F., Correia, H., et al. (2014). New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychol. 33, 490–499. doi: 10.1037/hea0000055
Hahn, E. A., Kallen, M. A., Jensen, R. E., Potosky, A. L., Moinpour, C. M., Ramirez, M., et al. (2016b). Measuring social function in diverse cancer populations: evaluation of measurement equivalence of the Patient Reported Outcomes Measurement Information System. Psychol. Test Assess. Model 58, 403–421.
Halvarsson, A., Franzén, E., Farén, E., Olsson, E., Oddsson, L., and Ståhle, A. (2013). Long-term effects of new progressive group balance training for elderly people with increased risk of falling - a randomized controlled trial. Clin. Rehabil. 27, 450–458. doi: 10.1177/0269215512462908
Halvarsson, A., Oddsson, L., Olsson, E., Farén, E., Pettersson, A., and Ståhle, A. (2011). Effects of new, individually adjusted, progressive balance group training for elderly people with fear of falling and tend to fall: a randomized controlled trial. Clin. Rehabil. 25, 1021–1031. doi: 10.1177/0269215511411937
Hanewinckel, R., Drenthen, J., Verlinden, V. J. A., Darweesh, S. K. L., van der Geest, J. N., Hofman, A., et al. (2017). Polyneuropathy relates to impairment in daily activities, worse gait, and fall-related injuries. Neurology 89, 76–83. doi: 10.1212/WNL.0000000000004067
Hannan, M. T., Gagnon, M. M., Aneja, J., Jones, R. N., Cupples, L. A., Lipsitz, L. A., et al. (2010). Optimizing the tracking of falls in studies of older participants: comparison of quarterly telephone recall with monthly falls calendars in the MOBILIZE Boston Study. Am. J. Epidemiol. 171, 1031–1036. doi: 10.1093/aje/kwq024
Hardy, S. E., Perera, S., Roumani, Y. F., Chandler, J. M., and Studenski, S. A. (2007). Improvement in usual gait speed predicts better survival in older adults. J. Am. Geriatr. Soc. 55, 1727–1734. doi: 10.1111/j.1532-5415.2007.01413.x
Hegeman, J., Honegger, F., Kupper, M., and Allum, J. H. (2005). The balance control of bilateral peripheral vestibular loss subjects and its improvement with auditory prosthetic feedback. J. Vestib. Res. 15, 109–117.
Hiengkaew, V., Jitaree, K., and Chaiyawat, P. (2012). Minimal detectable changes of the Berg Balance Scale, Fugl-Meyer Assessment Scale, Timed “Up & Go. Test, gait speeds, and 2-minute walk test in individuals with chronic stroke with different degrees of ankle plantarflexor tone. Arch. Phys. Med. Rehabil. 93, 1201–1208. doi: 10.1016/j.apmr.2012.01.014
Hlavacka, F., Krizková, M., and Horak, F. B. (1995). Modification of human postural response to leg muscle vibration by electrical vestibular stimulation. Neurosci. Lett. 189, 9–12. doi: 10.1016/0304-3940(95)11436-z
Hoffman, E. M., Staff, N. P., Robb, J. M., St Sauver, J. L., Dyck, P. J., and Klein, C. J. (2015). Impairments and comorbidities of polyneuropathy revealed by population-based analyses. Neurology 84, 1644–1651. doi: 10.1212/WNL.0000000000001492
Ites, K. I., Anderson, E. J., Cahill, M. L., Kearney, J. A., Post, E. C., and Gilchrist, L. S. (2011). Balance interventions for diabetic peripheral neuropathy: a systematic review. J. Geriatr. Phys. Ther. 34, 109–116. doi: 10.1519/JPT.0b013e318212659a
Jónsdóttir, H. L., and Ruthig, J. C. (2020). A longitudinal study of the negative impact of falls on health, well-being, and survival in later life: the protective role of perceived control. Aging Ment. Health 1–7. doi: 10.1080/13607863.2020.1725736
Kästenbauer, T., Sauseng, S., Brath, H., Abrahamian, H., and Irsigler, K. (2004). The value of the Rydel-Seiffer tuning fork as a predictor of diabetic polyneuropathy compared with a neurothesiometer. Diabet. Med. 21, 563–567. doi: 10.1111/j.1464-5491.2004.01205.x
Kim, D. H., Glynn, R. J., Avorn, J., Lipsitz, L. A., Rockwood, K., Pawar, A., et al. (2019). Validation of a claims-based frailty index against physical performance and adverse health outcomes in the health and retirement study. J. Gerontol. A Biol. Sci. Med. Sci. 74, 1271–1276. doi: 10.1093/gerona/gly197
Koehler-McNicholas, S. R., Danzl, L., Cataldo, A. Y., and Oddsson, L. I. E. (2019). Neuromodulation to improve gait and balance function using a sensory neuroprosthesis in people who report insensate feet - A randomized control cross-over study. PLoS One 14:e0216212. doi: 10.1371/journal.pone.0216212
Kroenke, K., Spitzer, R. L., and Williams, J. B. (2001). The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16, 606–613. doi: 10.1046/j.1525-1497.2001.016009606.x
Kruse, R. L., Lemaster, J. W., and Madsen, R. W. (2010). Fall and balance outcomes after an intervention to promote leg strength, balance, and walking in people with diabetic peripheral neuropathy: “feet first” randomized controlled trial. Phys. Ther. 90, 1568–1579. doi: 10.2522/ptj.20090362
Lajoie, Y., and Gallagher, S. P. (2004). “Predicting falls within the elderly community: comparison of postural sway, reaction time, the Berg balance scale and the Activities-specific Balance Confidence (ABC) scale for comparing fallers and non-fallers.”Arch. Gerontol. Geriatr. 38, 11–26. doi: 10.1016/s0167-4943(03)00082-7
Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol. 4:863. doi: 10.3389/fpsyg.2013.00863
Lawrence, R. H., Tennstedt, S. L., Kasten, L. E., Shih, J., Howland, J., and Jette, A. M. (1998). Intensity and correlates of fear of falling and hurting oneself in the next year: baseline findings from a Roybal Center fear of falling intervention. J. Aging Health 10, 267–286. doi: 10.1177/089826439801000301
Leddy, A. L., Crowner, B. E., and Earhart, G. M. (2011). Functional gait assessment and balance evaluation system test: reliability, validity, sensitivity, and specificity for identifying individuals with Parkinson disease who fall. Phys. Ther. 91, 102–113. doi: 10.2522/ptj.20100113
Lee, B. C., Thrasher, T. A., Fisher, S. P., and Layne, C. S. (2015). The effects of different sensory augmentation on weight-shifting balance exercises in Parkinson’s disease and healthy elderly people: a proof-of-concept study. J. Neuroeng. Rehabil. 12:75. doi: 10.1186/s12984-015-0064-y
Leonard, G., Lapierre, Y., Chen, J. K., Wardini, R., Crane, J., and Ptito, A. (2017). Noninvasive tongue stimulation combined with intensive cognitive and physical rehabilitation induces neuroplastic changes in patients with multiple sclerosis: a multimodal neuroimaging study. Mult. Scler J. Exp. Transl. Clin. 3:2055217317690561. doi: 10.1177/2055217317690561
Li, F., Harmer, P., Fisher, K. J., and McAuley, E. (2004). Tai Chi: improving functional balance and predicting subsequent falls in older persons. Med. Sci. Sports Exerc. 36, 2046–2052. doi: 10.1249/01.mss.0000147590.54632.e7
Li, F., Harmer, P., Fisher, K. J., McAuley, E., Chaumeton, N., Eckstrom, E., et al. (2005). Tai Chi and fall reductions in older adults: a randomized controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 60, 187–194. doi: 10.1093/gerona/60.2.187
Li, L., and Manor, B. (2010). Long term Tai Chi exercise improves physical performance among people with peripheral neuropathy. Am. J. Chin. Med. 38, 449–459. doi: 10.1142/S0192415X1000797X
Lin, J. H., Hsu, M. J., Hsu, H. W., Wu, H. C., and Hsieh, C. L. (2010). Psychometric comparisons of 3 functional ambulation measures for patients with stroke. Stroke 41, 2021–2025. doi: 10.1161/STROKEAHA.110.589739
Lipsitz, L. A., Lough, M., Niemi, J., Travison, T., Howlett, H., and Manor, B. (2015). A shoe insole delivering subsensory vibratory noise improves balance and gait in healthy elderly people. Arch. Phys. Med. Rehabil. 96, 432–439. doi: 10.1016/j.apmr.2014.10.004
Lipsitz, L. A., Macklin, E. A., Travison, T. G., Manor, B., Gagnon, P., Tsai, T., et al. (2019). A cluster randomized trial of tai Chi vs health education in subsidized housing: the MI-WiSH Study. J. Am. Geriatr. Soc. 67, 1812–1819. doi: 10.1111/jgs.15986
Lipsitz, L. A., Manor, B., Habtemariam, D., Iloputaife, I., Zhou, J., and Travison, T. G. (2018). The pace and prognosis of peripheral sensory loss in advanced age: association with gait speed and falls. BMC Geriatr. 18:274. doi: 10.1186/s12877-018-0970-5
Manor, B., Lough, M., Gagnon, M. M., Cupples, A., Wayne, P. M., and Lipsitz, L. A. (2014). Functional benefits of tai chi training in senior housing facilities. J. Am. Geriatr. Soc. 62, 1484–1489. doi: 10.1111/jgs.12946
Marsden, J. F., Blakey, G., and Day, B. L. (2003). “Modulation of human vestibular-evoked postural responses by alterations in load. J. Physiol. 548(Pt. 3), 949–953. doi: 10.1113/jphysiol.2002.029991
Mathias, S., Nayak, U. S., and Isaacs, B. (1986). Balance in elderly patients: the “get-up and go” test. Arch. Phys. Med. Rehabil. 67, 387–389.
Melzer, I., and Oddsson, L. I. (2013). Improving balance control and self-reported lower extremity function in community-dwelling older adults: a randomized control trial. Clin. Rehabil. 27, 195–206. doi: 10.1177/0269215512450295
Menz, H. B., Lord, S. R., St George, R., and Fitzpatrick, R. C. (2004). Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch. Phys. Med. Rehabil. 85, 245–252. doi: 10.1016/j.apmr.2003.06.015
Meyer, P. F., Oddsson, L. I., and De Luca, C. J. (2004a). Reduced plantar sensitivity alters postural responses to lateral perturbations of balance. Exp. Brain Res. 157, 526–536. doi: 10.1007/s00221-004-1868-3
Meyer, P. F., Oddsson, L. I., and De Luca, C. J. (2004b). The role of plantar cutaneous sensation in unperturbed stance. Exp. Brain Res. 156, 505–512. doi: 10.1007/s00221-003-1804-y
Middleton, A., Fritz, S. L., and Lusardi, M. (2015). Walking speed: the functional vital sign. J Aging Phys Act 23, 314–322. doi: 10.1123/japa.2013-0236
Montero-Odasso, M., Schapira, M., Soriano, E. R., Varela, M., Kaplan, R., Camera, L. A., et al. (2005). Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J. Gerontol. A Biol. Sci. Med. Sci. 60, 1304–1309. doi: 10.1093/gerona/60.10.1304
Moore, J. L., Potter, K., Blankshain, K., Kaplan, S. L., O’Dwyer, L. C., and Sullivan, J. E. (2018). A core set of outcome measures for adults with neurologic conditions undergoing rehabilitation: a clinical practice guideline. J. Neurol. Phys. Ther. 42, 174–220. doi: 10.1097/NPT.0000000000000229
Morrison, S., Colberg, S. R., Mariano, M., Parson, H. K., and Vinik, A. I. (2010). Balance training reduces falls risk in older individuals with type 2 diabetes. Diabetes Care 33, 748–750. doi: 10.2337/dc09-1699
Morrison, S., Simmons, R., Colberg, S. R., Parson, H. K., and Vinik, A. I. (2018). Supervised balance training and wii fit-based exercises lower falls risk in older adults with type 2 diabetes. J. Am. Med. Dir. Assoc. 19, 185.e7–185.e13. doi: 10.1016/j.jamda.2017.11.004
Mustapa, A., Justine, M., Mohd Mustafah, N., Jamil, N., and Manaf, H. (2016). Postural control and gait performance in the diabetic peripheral neuropathy: a systematic review. Biomed. Res. Int. 2016:9305025. doi: 10.1155/2016/9305025
National Heart Lung and Blood Institute. (2020). Study Quality Assessment Tools. Available online at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed July 7, 2020).
Noohi, F., Kinnaird, C., DeDios, Y., Kofman, I. S., Wood, S., Bloomberg, J., et al. (2017). Functional brain activation in response to a clinical vestibular test correlates with balance. Front. Syst. Neurosci. 11:11. doi: 10.3389/fnsys.2017.00011
Oddsson, L., Bisson, T., Cohen, H. S., Koehler-McNicholas, S., Kung, D., and Wrisley, D. (2019). Slips and Falls and Record Snow in Minnesota: walk2wellness Clinical Trial Update. AAPM&R Annual Assembly, San Antonio, Texas. Available online at: https://pmrjabstracts.org/abstract/slips-and-falls-and-record-snow-in-minnesota-walk2wellness-clinical-trial-update/. (accessed October 25, 2020).
Oddsson, L. I. E., Bisson, T., Cohen, H., Iloputaife, I., Koehler-McNicholas, S., Kung, D., et al. (2020a). A wearable sensory neuroprosthesis to improve gait and balance function in persons with sensory peripheral neuropathy – the walk2Wellness Trial (1170). Neurology 94(15 Suppl.):1170.
Oddsson, L. I. E., Bisson, T., Cohen, H. S., Jacobs, L., Khoshnoodi, M., Kung, D., et al. (2020b). Gait and balance function improves after 10 weeks of using a wearable sensory neuroprosthesis in persons with peripheral neuropathy and high fall risk - the walk2wellness trial. medRxiv[Preprint]. doi: 10.1101/2020.08.02.20166231
Oddsson, L. I. E., Boissy, P., and Melzer, I. (2007). How to improve gait and balance function in elderly individuals—compliance with principles of training. Eur. Rev. Aging Phys. Act 4, 15–23. doi: 10.1007/s11556-007-0019-9
Perera, S., Mody, S. H., Woodman, R. C., and Studenski, S. A. (2006). Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 54, 743–749. doi: 10.1111/j.1532-5415.2006.00701.x
Podsiadlo, D., and Richardson, S. (1991). The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 39, 142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x
Powell, L. E., and Myers, A. M. (1995). The activities-specific balance confidence (ABC) scale. J. Gerontol. A Biol. Sci. Med. Sci. 50A, M28–M34.
Ptito, A., Papa, L., Gregory, K., Folmer, R. L., Walker, W. C., Prabhakaran, V., et al. (2020). A prospective, multicenter study to assess the safety and efficacy of translingual neurostimulation plus physical therapy for the treatment of a chronic balance deficit due to mild-to-moderate traumatic brain injury. Neuromodulation doi: 10.1111/ner.13159 [Epub ahead of print].
Purser, J. L., Weinberger, M., Cohen, H. J., Pieper, C. F., Morey, M. C., Li, T., et al. (2005). Walking speed predicts health status and hospital costs for frail elderly male veterans. J. Rehabil. Res. Dev. 42, 535–546. doi: 10.1682/jrrd.2004.07.0087
Quigley, P. A., Bulat, T., Schulz, B., Friedman, Y., Hart-Hughes, S., Richardson, J. K., et al. (2014). Exercise interventions, gait, and balance in older subjects with distal symmetric polyneuropathy: a three-group randomized clinical trial. Am. J. Phys. Med. Rehabil 93, 1–12. doi: 10.1097/PHM.0000000000000052
Richardson, J. K., and Hurvitz, E. A. (1995). “Peripheral neuropathy: a true risk factor for falls. J. Gerontol. A Biol. Sci. Med. Sci. 50, M211–M215.
Richardson, J. K., Sandman, D., and Vela, S. (2001). A focused exercise regimen improves clinical measures of balance in patients with peripheral neuropathy. Arch. Phys. Med. Rehabil. 82, 205–209. doi: 10.1053/apmr.2001.19742
Rief, W., Nestoriuc, Y., Weiss, S., Welzel, E., Barsky, A. J., and Hofmann, S. G. (2009). Meta-analysis of the placebo response in antidepressant trials. J. Affect. Disord. 118, 1–8. doi: 10.1016/j.jad.2009.01.029
Riskowski, J. L., Quach, L., Manor, B., Menz, H. B., Lipsitz, L. A., and Hannan, M. T. (2012). Idiopathic peripheral neuropathy increases fall risk in a population-based cohort study of older adults. J. Foot Ank. Res. 5:19. doi: 10.1186/1757-1146-5-S1-P19
Rossi-Izquierdo, M., Ernst, A., Soto-Varela, A., Santos-Pérez, S., Faraldo-García, A., Sesar-Ignacio, A., et al. (2013). Vibrotactile neurofeedback balance training in patients with Parkinson’s disease: reducing the number of falls. Gait Posture 37, 195–200. doi: 10.1016/j.gaitpost.2012.07.002
Salsabili, H., Bahrpeyma, F., and Esteki, A. (2015). The effects of task-oriented motor training on gait characteristics of patients with type 2 diabetes neuropathy. J. Diabetes Metab. Disord. 15:14. doi: 10.1186/s40200-016-0236-8
Scheffer, A. C., Schuurmans, M. J., van Dijk, N., van der Hooft, T., and de Rooij, S. E. (2008). Fear of falling: measurement strategy, prevalence, risk factors and consequences among older persons. Age. Ageing 37, 19–24. doi: 10.1093/ageing/afm169
Shumway-Cook, A., Brauer, S., and Woollacott, M. (2000). Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys. Ther. 80, 896–903. doi: 10.1093/ptj/80.9.896
Shumway-Cook, A., Gruber, W., Baldwin, M., and Liao, S. (1997). The effect of multidimensional exercises on balance, mobility, and fall risk in community-dwelling older adults. Phys. Ther. 77, 46–57. doi: 10.1093/ptj/77.1.46
Sienko, K. H., Seidler, R. D., Carender, W. J., Goodworth, A. D., Whitney, S. L., and Peterka, R. J. (2018). Potential mechanisms of sensory augmentation systems on human balance control. Front. Neurol. 9:944. doi: 10.3389/fneur.2018.00944
Sterling, D. A., O’Connor, J. A., and Bonadies, J. (2001). Geriatric falls: injury severity is high and disproportionate to mechanism. J. Trauma 50, 116–119. doi: 10.1097/00005373-200101000-00021
Streckmann, F., Zopf, E. M., Lehmann, H. C., May, K., Rizza, J., Zimmer, P., et al. (2014). Exercise intervention studies in patients with peripheral neuropathy: a systematic review. Sports Med. 44, 1289–1304. doi: 10.1007/s40279-014-0207-5
Strzalkowski, N. D. J., Peters, R. M., Inglis, J. T., and Bent, L. R. (2018). Cutaneous afferent innervation of the human foot sole: what can we learn from single-unit recordings? J. Neurophysiol. 120, 1233–1246. doi: 10.1152/jn.00848.2017
Studenski, S., Perera, S., Patel, K., Rosano, C., Faulkner, K., Inzitari, M., et al. (2011). Gait speed and survival in older adults. JAMA 305, 50–58. doi: 10.1001/jama.2010.1923
Studenski, S., Perera, S., Wallace, D., Chandler, J. M., Duncan, P. W., Rooney, E., et al. (2003). Physical performance measures in the clinical setting. J. Am. Geriatr. Soc. 51, 314–322. doi: 10.1046/j.1532-5415.2003.51104.x
Tinetti, M. E., and Kumar, C. (2010). The patient who falls: “It’s always a trade-off”. JAMA 303, 258–266. doi: 10.1001/jama.2009.2024
Tofthagen, C., Visovsky, C., and Berry, D. L. (2012). Strength and balance training for adults with peripheral neuropathy and high risk of fall: current evidence and implications for future research. Oncol. Nurs. Forum 39, E416–E424. doi: 10.1188/12.ONF.E416-E424
Tyler, M., Danilov, Y., and Bach-Y-Rita, P. (2003). Closing an open-loop control system: vestibular substitution through the tongue. J. Integr. Neurosci. 2, 159–164. doi: 10.1142/s0219635203000263
Uitenbroek, D. G. (1997). SISA-Binomial. Available online at: https://www.quantitativeskills.com/sisa/calculations/bonfer.htm (accessed June 10, 2020).
Vinik, A. I., Camacho, P., Reddy, S., Valencia, W. M., Trence, D., Matsumoto, A. M., et al. (2017). Aging, diabetes, and falls. Endocr. Pract. 23, 1117–1139. doi: 10.4158/EP171794.RA
Wall, C., Wrisley, D., and Oddsson, L. (2012). Vibrotactile feedback of mediolateral trunk tilt or foot pressure increases locomotor performance in healthy older adults–a pilot study. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2012, 6145–6148. doi: 10.1109/EMBC.2012.6347396
Wall, C., Wrisley, D. M., and Statler, K. D. (2009). Vibrotactile tilt feedback improves dynamic gait index: a fall risk indicator in older adults. Gait Posture 30, 16–21. doi: 10.1016/j.gaitpost.2009.02.019
Wang, C., Goel, R., Rahemi, H., Zhang, Q., Lepow, B., and Najafi, B. (2019a). Effectiveness of daily use of bilateral custom-made ankle-foot orthoses on balance, fear of falling, and physical activity in older adults: a randomized controlled trial. Gerontology 65, 299–307. doi: 10.1159/000494114
Wang, C., Goel, R., Zhang, Q., Lepow, B., and Najafi, B. (2019b). Daily use of bilateral custom-made ankle-foot orthoses for fall prevention in older adults: a randomized controlled trial. J. Am. Geriatr. Soc. 67, 1656–1661. doi: 10.1111/jgs.15929
Wartolowska, K. A., Feakins, B. G., Collins, G. S., Cook, J., Judge, A., Rombach, I., et al. (2016). The magnitude and temporal changes of response in the placebo arm of surgical randomized controlled trials: a systematic review and meta-analysis. Trials 17:589. doi: 10.1186/s13063-016-1720-7
Winters-Stone, K. M., Horak, F., Jacobs, P. G., Trubowitz, P., Dieckmann, N. F., Stoyles, S., et al. (2017). Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J. Clin. Oncol. 35, 2604–2612. doi: 10.1200/JCO.2016.71.3552
Wolf, B., Feys, H., De Weerdt, van der Meer, J., Noom, M., and Aufdemkampe, G. (2001). Effect of a physical therapeutic intervention for balance problems in the elderly: a single-blind, randomized, controlled multicentre trial. Clin. Rehabil. 15, 624–636. doi: 10.1191/0269215501cr456oa
Wolf, S. L., Barnhart, H. X., Kutner, N. G., McNeely, E., Coogler, C., and Xu, T. (1996). Reducing frailty and falls in older persons: an investigation of Tai Chi and computerized balance training. Atlanta FICSIT Group. Frailty and Injuries: cooperative studies of intervention techniques. J. Am. Geriatr. Soc. 44, 489–497. doi: 10.1111/j.1532-5415.1996.tb01432.x
Wolf, S. L., Sattin, R. W., Kutner, M., O’Grady, M., Greenspan, A. I., and Gregor, R. J. (2003). Intense tai chi exercise training and fall occurrences in older, transitionally frail adults: a randomized, controlled trial. J. Am. Geriatr. Soc. 51, 1693–1701. doi: 10.1046/j.1532-5415.2003.51552.x
Woolcott, J. C., Richardson, K. J., Wiens, M. O., Patel, B., Marin, J., Khan, K. M., et al. (2009). Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch. Intern. Med. 169, 1952–1960. doi: 10.1001/archinternmed.2009.357
Wrisley, D., McLean, G., Hill, J., and Oddsson, L. (2020). Long-term use of a sensory neuroprosthesis improves function in a patient with peripheral neuropathy: a case report. Preprints 2020080435. doi: 10.20944/preprints202008.0435.v1
Wrisley, D., McLean, G., and Oddsson, L. (2018). “The Use of a Wearable Sensory Prosthesis to Improve Gait and Balance in a Patient with Peripheral Neuropathy,” in Proceedings of the APTA Combined Sections Meeting, New Orleans.
Wrisley, D. M., and Kumar, N. A. (2010). Functional gait assessment: concurrent, discriminative, and predictive validity in community-dwelling older adults. Phys. Ther. 90, 761–773. doi: 10.2522/ptj.20090069
Wrisley, D. M., Marchetti, G. F., Kuharsky, D. K., and Whitney, S. L. (2004). Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Phys. Ther. 84, 906–918. doi: 10.1093/ptj/84.10.906
Yamanaka, T., Sawai, Y., Murai, T., Nishimura, T., and Kitahara, T. (2016). Long-term effects of electrotactile sensory substitution therapy on balance disorders. Neuroreport 27, 744–748. doi: 10.1097/WNR.0000000000000606
Zaiontz, C. (2020). Real Statistics R:esource Pack (Release 6.8). Available online at: http://www.real-statistics.com/ (accessed May 12, 2020).
Zehr, E. P., Nakajima, T., Barss, T., Klarner, T., Miklosovic, S., Mezzarane, R. A., et al. (2014). Cutaneous stimulation of discrete regions of the sole during locomotion produces “sensory steering” of the foot. BMC Sports Sci. Med. Rehabil. 6:33. doi: 10.1186/2052-1847-6-33
Keywords: peripheral neuropathy, falls, neuroprosthesis, balance, gait speed, neuromodulation, sensory substitution, sensory prosthesis
Citation: Oddsson LIE, Bisson T, Cohen HS, Jacobs L, Khoshnoodi M, Kung D, Lipsitz LA, Manor B, McCracken P, Rumsey Y, Wrisley DM and Koehler-McNicholas SR (2020) The Effects of a Wearable Sensory Prosthesis on Gait and Balance Function After 10 Weeks of Use in Persons With Peripheral Neuropathy and High Fall Risk – The walk2Wellness Trial. Front. Aging Neurosci. 12:592751. doi: 10.3389/fnagi.2020.592751
Received: 08 August 2020; Accepted: 22 October 2020;
Published: 09 November 2020.
Edited by:
Federico Ranieri, University of Verona, ItalyReviewed by:
Alexander Stamenkovic, Virginia Commonwealth University, United StatesCopyright © 2020 Oddsson, Bisson, Cohen, Jacobs, Khoshnoodi, Kung, Lipsitz, Manor, McCracken, Rumsey, Wrisley and Koehler-McNicholas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lars I. E. Oddsson, bG9kZHNzb25AdW1uLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.