- 1Department of Neurosurgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Center for MRI Research, Academy for Advanced Interdisciplinary Studies, Peking University, Beijing, China
Background: Gray matter (GM) alterations in Parkinson's disease (PD) patients with rapid eye movement sleep behavior disorder (RBD) have been demonstrated in many neuroimaging studies using voxel-based morphometry (VBM). However, the inconsistent findings between studies cannot be applied to clinical practice as a neuroimaging biomarker. We performed a meta-analysis of VBM studies at a whole-brain level to investigate GM differences between PD patients with and without RBD.
Methods: A systematic search was conducted in PubMed, Embase, and Web of Science from inception to November 2019 to identify eligible VBM studies. We adopted the latest Seed-based d Mapping with Permutation of Subject Images technique to quantitatively estimate the difference of regional GM volume between PD patients with and without RBD.
Results: We included five studies comprising 105 PD patients with RBD and 140 PD patients without RBD. The pooled meta-analysis revealed that PD patients with RBD showed a significant reduction of GM volume in the right superior temporal gyrus (STG) compared with those without RBD. This result was confirmed to be robust by the jackknife sensitivity analysis.
Conclusion: Our finding shows significantly and robustly reduced GM volume in the right STG in PD patients with RBD, preliminarily suggesting the association of GM atrophy in this brain region with the occurrence of RBD in PD patients.
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder with a rising prevalence in parallel with an aging population (Zhang et al., 2005; Pringsheim et al., 2014). Though the clinical diagnosis of PD is mainly based on cardinal motor symptoms including bradykinesia, rigidity, and rest tremor (Postuma et al., 2015), non-motor symptoms, such as hyposmia, depression, sleep disorders, and constipation, have attracted increasing attention because of their negative influence on the quality of life and predictive value for disease progression (Schapira et al., 2017). The treatment of PD remains a massive challenge because there has been no reliable tool for the early detection of PD worsening. However, the non-motor symptoms of PD can occur several years before motor manifestation progression; therefore, monitoring non-motor symptoms is considered a promising method to evaluate disease risks and promote the clinical intervention of PD at an early stage.
Rapid eye movement sleep behavior disorder (RBD) is a parasomnia characterized by loss of skeletal muscle atonia and dream-enacting behaviors associated with aggression and violence during rapid eye movement sleep (St Louis and Boeve, 2017). A handful of studies have demonstrated that RBD is strongly associated with PD. The prevalence of RBD is about 30% to 50% in patients with PD (Howell and Schenck, 2015). The occurrence of RBD in PD is associated with motor function deterioration (particularly bradykinesia worsening) (Bugalho and Viana-Baptista, 2013), more severe non-motor symptoms (including anxiety, depression, sleep disorders, constipation, hallucination, and orthostatic hypotension) (Neikrug et al., 2014; Liu et al., 2017), poorer cognitive function (Huang et al., 2017; Jozwiak et al., 2017), and cerebral cortex abnormalities (Barber et al., 2017). Thus, the presence of RBD symptoms is a risk factor and a potential marker of disease progression in PD.
Previously, many magnetic resonance imaging (MRI) studies have been conducted to investigate the functional and structural brain alterations in PD with RBD in the hope of uncovering the potential pathophysiology and characteristic changes in the brain (Bourgouin et al., 2019). However, due to the limited sample size and different analytical methods, the mixed results of these studies cannot be a neuroimaging biomarker in clinical practice.
Seed-based d mapping (SDM) is a fully validated coordinate-based meta-analytic method, which can synthesize data of neuroimaging studies using functional MRI, voxel-based morphometry (VBM), diffusion tensor imaging, or positron emission tomography (Albajes-Eizagirre et al., 2019b). In the present study, we focused on regional volume changes of gray matter (GM) using VBM analysis. The current study aimed to calculate structural alterations in PD with RBD from published data to identify a robust neuroimaging biomarker. Therefore, we performed a coordinate-based meta-analysis of VBM studies at a whole-brain level to elucidate the prominent GM changes in PD patients with RBD.
Materials and Methods
The present systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. We prepared a prospective protocol of search strategy, inclusion criteria, data extraction, and methods of statistical analysis.
Literature Search
We performed a systematic and comprehensive search of the PubMed, Embase, and Web of Science from inception to November 3, 2019. For the literature search in these databases, the following terms were used in combinations: (“magnetic resonance imaging” or “MRI”) and (“Parkinson disease” or “Parkinson's disease”) and (“rapid eye movement sleep behavior disorder” or “REM sleep behavior disorder” or “RBD”). Additionally, we checked the references of relevant original articles and reviews. The language of publications was restricted to English. To avoid time-lag bias, the search process was updated in June 12, 2020, but no additional study was identified.
Inclusion Criteria
We included studies in the meta-analysis that met the following criteria: (1) was published as an original article in a peer-reviewed journal; (2) demonstrated specific diagnostic criteria of PD and RBD; (3) conducted a whole-brain analysis of GM structural alterations in a stereotactic space in three-dimensional standard coordinates; (4) reported the results of VBM analysis in PD patients with and without RBD; and (5) reported significance thresholds that were either corrected for multiple comparisons or uncorrected with spatial extent thresholds. Therefore, editorials, letters, conference abstracts, reviews, book chapters, and case reports were excluded. Two authors (C.Y. and J.C.) reviewed the studies independently.
Data Extraction
We extracted peak coordinates of abnormal brain regions from all studies eligible for meta-analysis. If the manuscript used two whole-brain statistical significance levels with and without correction for multiple comparisons, we selected the uncorrected threshold and included all peaks obtained using this uncorrected threshold (Albajes-Eizagirre et al., 2019a,b). The following data were also collected: the number of patients in each group, mean age, year of education, gender ratio, disease duration, severity, and diagnostic criteria of RBD and PD. All data were evaluated independently by two authors (C.Y. and J.C.). Any disagreement about literature search, study selection, and data extraction was resolved by consensus under the guidance of the senior authors (X.B. and R.W.).
Quality Assessment
Each study was assessed for quality and completeness using a 13-item checklist as described in previous meta-analyses (Baiano et al., 2007; Shepherd et al., 2012; Du et al., 2014). The 13 items were divided into three categories, namely, subjects, methods for image acquisition and analysis, and results and conclusions. Each item was rated 1, 0.5, or 0 if criteria were fully met, partially met, or unfulfilled, respectively (Baiano et al., 2007). The checklist was designed to rate the completeness of neuroimaging studies, providing an objective indication of rigor of included studies in a meta-analysis.
Data Analysis
We conducted the voxel-wise meta-analysis of regional differences in GM volume between PD patients with and without RBD using SDM with Permutation of Subject Images (SDM-PSI version 6.21) (Albajes-Eizagirre et al., 2019a,b). The detailed coordinate-based meta-analytic process of VBM results has been described in the software tutorial (https://www.sdmproject.com/) and previous studies (Albajes-Eizagirre et al., 2019a; Dahlgren et al., 2020). Here, we briefly summarized the SDM-PSI methods. First, the peak coordinates and t-statistics of GM differences between PD patients with and without RBD were extracted. To avoid potential bias, we ensured that the same statistical threshold was used in the whole brain in each study. If t-statistics were not presented in the publications, z-scores or p-values were converted into t-statistics by the SDM-PSI software. Second, the lower and upper bounds of potential effect sizes for all voxels were estimated by multiple imputations, and maps of GM alterations for each study were created by means of an anisotropic Gaussian kernel, which assigns higher effect sizes to the voxels more correlated with peak coordinates. Third, maximum likelihood techniques were used to estimate the most likely effect size and its standard error. The imputed dataset from each study was meta-analyzed using a random-effects model, and these imputed meta-analyzed datasets were combined using Rubin's rules. Fourth, we conducted family-wise error correction for multiple comparisons and thresholded the meta-analysis using threshold-free cluster enhancement statistics. The following SDM-PSI parameters were used: full width at half maximum (FWHM) = 20 mm, voxel size = 2 mm, imputations = 50, permutations = 1,000, threshold-free cluster enhancement family-wise error rate P = 0.05, cluster extent threshold = 10 voxels. Egger's tests and funnel plots were used to evaluate publication bias, and heterogeneity of effect sizes was assessed by I2 statistics. Besides, we performed jackknife sensitivity analyses, in which the meta-analysis was repeated after discarding one eligible study at a time to see whether the result remained statistically significant.
Results
The literature search yielded 371 publications (PubMed: 71; Embase: 200; Web of Science: 100). After screening titles and abstracts, 12 studies were reviewed in full texts. In the end, five studies were included in the current meta-analysis (Ford et al., 2013; Salsone et al., 2014; Kim et al., 2016; Lim et al., 2016; Rahayel et al., 2019). The process of study selection is presented in Figure 1.
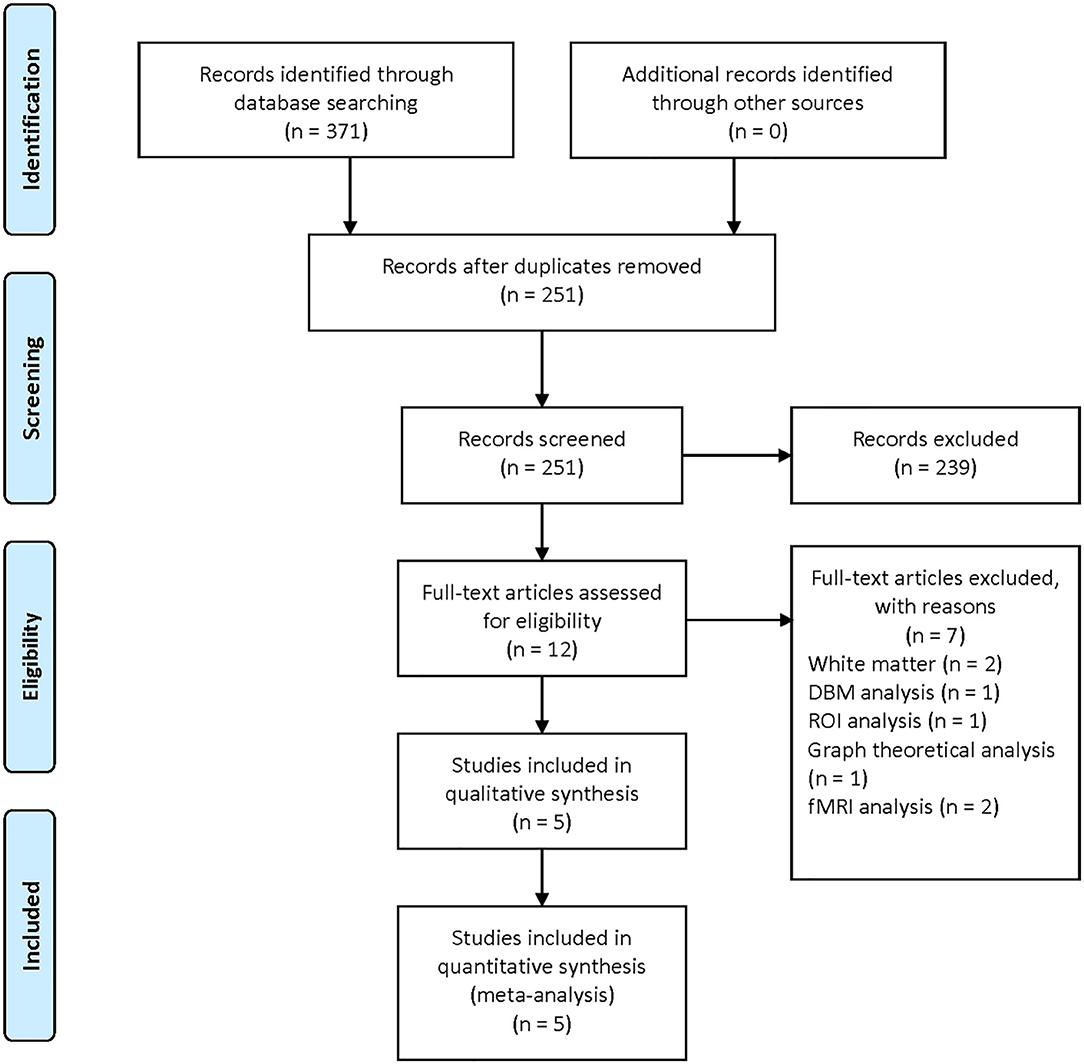
Figure 1. Flow diagram illustrating the results of the study selection and inclusion in the current meta-analysis.
Characteristics of Included Studies
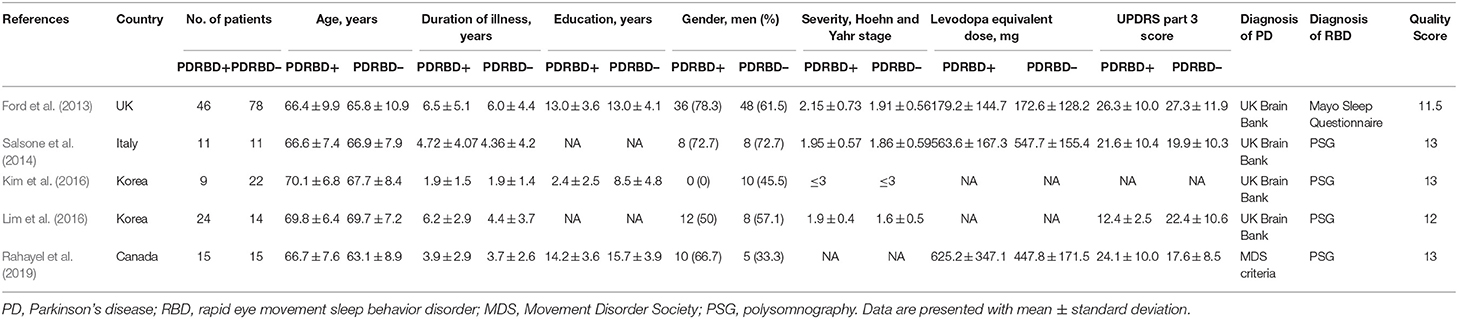
There are a total of 105 PD patients with RBD and 140 without RBD. The baseline data about age, gender, education, disease duration, and disease severity were comparable between groups in each study. The diagnosis tool of PD is UK Brain Bank in four studies from 2013 to 2016 and Movement Disorder Society clinical criteria in one study in 2019. The clinical data and quality assessment scores of five included studies are presented in Table 1. MRI parameters are demonstrated in Table 2. Statistical parameters and results of VBM analyses are shown in Table 3. The details of quality assessment in terms of subjects, methods for image acquisitions and analysis, and results and conclusions are shown in Table S1.
Table 2. Magnetic resonance imaging parameters of five included studies in the present meta-analysis.
Outcomes of Data Analysis
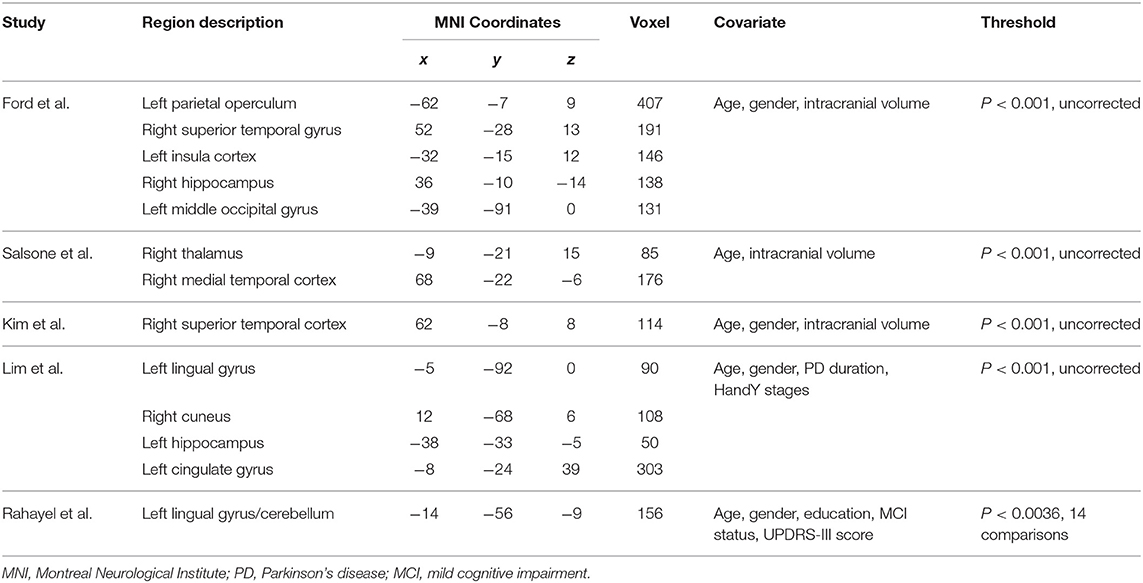
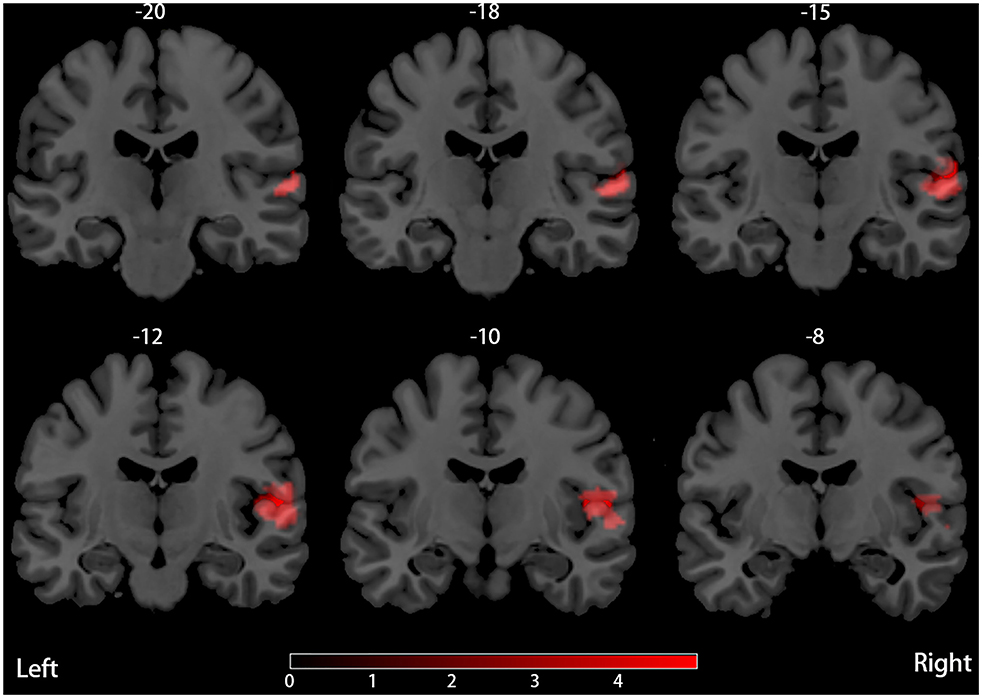
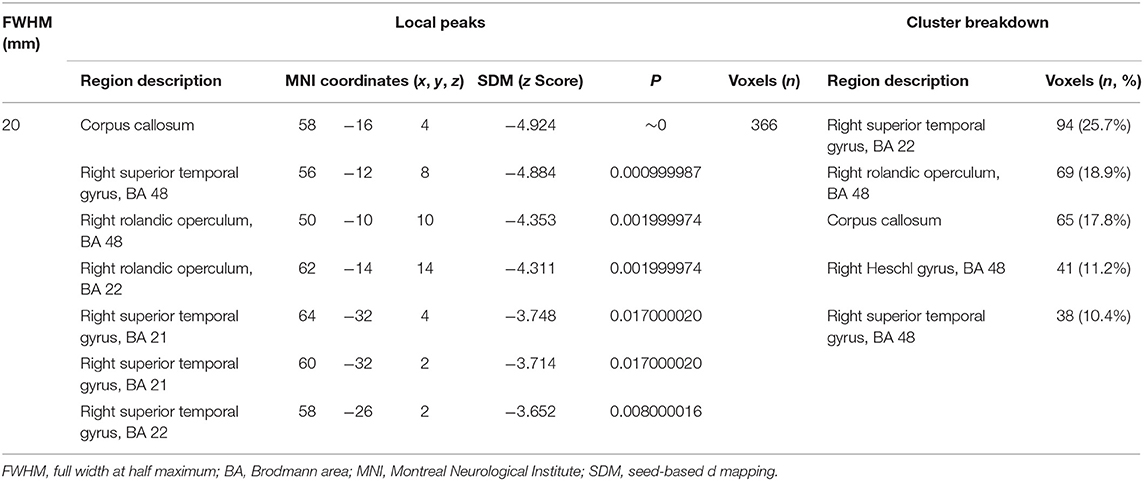
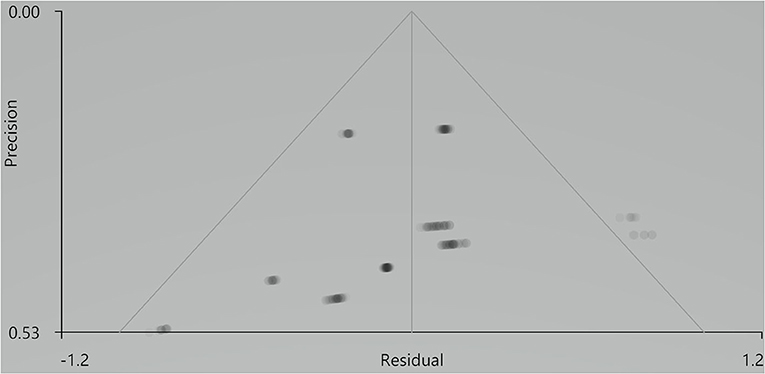
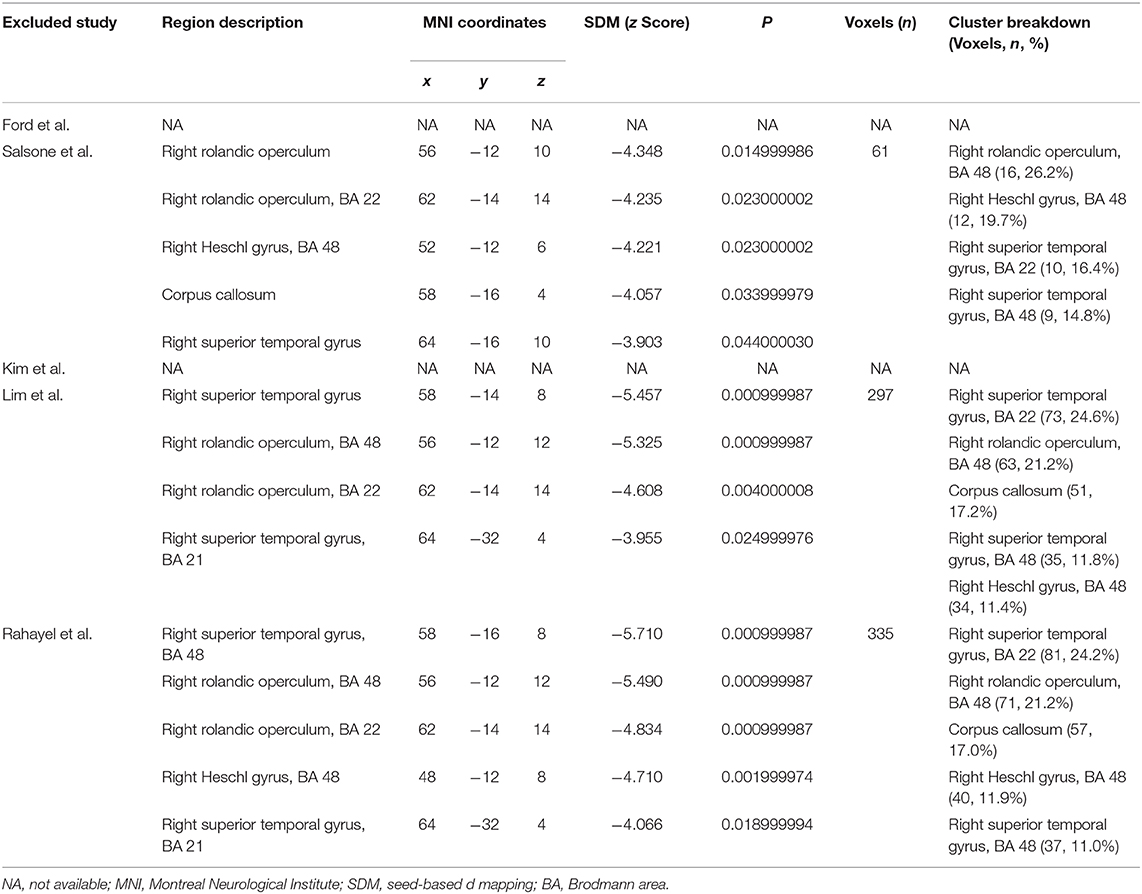
Our result demonstrated a significant GM volume reduction in the right superior temporal gyrus (STG) in Figure 2. The cluster breakdown results show which cortical areas contribute to the corresponding cluster. We included the cortical areas with more than 10% voxels of the present cluster using 20 mm FWHM in Table 4. Results of Egger's tests (bias = −0.70, t = −0.39, df = 3, P = 0.699) and funnel plots in Figure 3 revealed no significant publication bias. There was no obvious heterogeneity of effect sizes (I2 = 2.14%) in the present meta-analysis. The main findings remained largely unchanged in the jackknife sensitivity analysis (3/5), as shown in Table 5.
Discussion
Up to now, there has been no treatment method to slow or halt the neurodegenerative process in PD. The occurrence of RBD is a warning signal of worsening symptoms in PD patients (Romenets et al., 2012; Mollenhauer et al., 2016). The period between the onset of RBD symptoms and subtle cerebral structural changes is an ideal time window for prompt therapeutic intervention. Herein, we aimed to understand GM changes associated with RBD in PD using VBM.
To the best of our knowledge, this is the first neuroimaging meta-analysis to assess the difference of regional GM volume between PD patients with and without RBD based on VBM studies. The findings of our research can be summarized as follows: there is a significant reduction of GM volume in the right STG in PD patients with RBD compared with those without RBD.
As a newly emerging biomarker, neuroimaging has been extensively investigated for the early diagnosis and prognosis assessment in neurodegenerative disorders (Shimizu et al., 2018). According to human and animal studies, structural lesions in the dorsal midbrain and pons are confirmed to be responsible for idiopathic RBD (Boeve et al., 2007). However, the neuroanatomical basis of RBD in PD is considered different from idiopathic RBD (Dauvilliers et al., 2018). Thus, many studies have been performed to detect specific imaging biomarkers in PD patients before the presence of RBD using multimodal brain MRI methods (Lim et al., 2016; Ansari et al., 2017; Li et al., 2017), among which VBM using high-solution T1 imaging was the most widely used analysis method.
The neuroimaging studies in animal models showed that idiopathic RBD was attributed to selective lesions located in the pontine tegmentum, which was equivalent to the locus subcoeruleus in humans (Boeve et al., 2007). Furthermore, through VBM analysis, researchers demonstrated GM volume reduction in the anterior lobes of the right and left cerebellum, the tegmental portion of the pons, and the left parahippocampal gyrus (Hanyu et al., 2012) and increases of GM densities in both hippocampi (Scherfler et al., 2011) in patients with idiopathic RBD. In PD patients with RBD, Garcia-Lorenzo et al. (2013) showed reduced signal intensity in the coeruleus/subcoeruleus complex by neuromelanin-sensitive imaging but no evident changes in GM volume. Lim et al. (2016) analyzed the changes of GM volume at a whole-brain level, illustrating that RBD in PD may be related to decreased GM volume in the left posterior cingulate and hippocampus, but no structural abnormality was observed in the brainstem. Rahayel et al. (2019) applied VBM and deformation-based morphometry methods to analyze the regional difference in contraction or expansion between PD patients with and without RBD but still detected no significant results in the brainstem. In summary, although brainstem abnormality is found to be associated with the promotion of idiopathic RBD from experimental and clinical perspectives (Scherfler et al., 2011; Hanyu et al., 2012), there are no apparent volume changes of GM in the brainstem in PD patients with RBD compared with those without RBD.
Previous studies have demonstrated the strong association of reduced GM volume changes in the right STG with weakened spatial processing (Ellison et al., 2004; Gharabaghi et al., 2006; Shah-Basak et al., 2018), narcolepsy (Joo et al., 2009; Weng et al., 2015), impaired emotion processing to support social interactions (Muller et al., 2008; Pan et al., 2015; Van de Vliet et al., 2018; Zhang et al., 2018), violent behaviors (Zhang et al., 2019), and some psychiatric disorders (Moreira et al., 2017; Zhao et al., 2017; Wang et al., 2018). Consistent with our result, sleep disorders and nocturnal violence in PD patients with RBD can be partially explained by GM volume reduction in the right STG.
GM volume reduction in the right STG was associated with motor and non-motor manifestations, accounting for the RBD-related function impairment in PD patients. The presence of RBD in PD is associated with increased frequency of depression and falling (Romenets et al., 2012). Significant decrease in GM density was detected in the right superior temporal pole of PD patients with depression compared with that without depression (Feldmann et al., 2008). According to previous studies, STG plays a substantial role in the vestibular system and is associated with spatial information processing (Janzen et al., 2008). Consistently, Otomune et al. found that GM volume was significantly smaller in the right STG of frequent fallers than that of non-frequent fallers (Otomune et al., 2019). Besides, they found a significant linear correlation between fall frequency and GM reduction in the right STG (Otomune et al., 2019). Together, these findings supported an essential role of the right STG in the mental and postural control of PD patients with RBD. Psychosis is the most disabling non-motor complication in PD (Ffytche et al., 2017). STG is involved in processing visual and auditory information (Reale et al., 2007). Pacchetti et al. (2005) revealed that RBD increased the risk of symptomatic hallucinations and delusions in PD patients, showing the association of RBD with psychotic symptoms in PD. Additionally, there existed an inverse relationship between the severity of hallucinations and the right STG volume in early-onset schizophrenia (Matsumoto et al., 2001). Besides, a functional MRI study showed the association of reduced STG activity with auditory hallucinations (Orlov et al., 2018). Thus, our finding can partly help explain the neural substrate of association between RBD and PD psychosis. PD patients with RBD tend to develop a more rapid progression in cognition dysfunction (Fereshtehnejad et al., 2015). Reduced GM volume in the right STG is associated with poor ability to overcome misdirection (Tong et al., 2019), partly explaining its contribution to cognitive worsening in PD patients with RBD. In summary, RBD-related presentations in PD could be to some extent attributed to GM atrophy in the right STG, which can serve as a predictive tool for disease progression in PD.
The present study has some limitations that merit comment. First, polysomnography is mandatory for the diagnosis of RBD; however, the diagnosis of RBD in the research by Ford et al. was assessed by the Mayo Sleep Questionnaire, a well-validated tool for clinical screening (Ford et al., 2013). Second, the relatively small sample size of included studies limited the power of our meta-analysis using SDM-PSI methods. Third, VBM analysis is considered to be less sensitive in detecting regional abnormalities than cortical thickness analysis (Borghammer et al., 2010). Fourth, there exists heterogeneity of participants' characteristics between studies and various methodologies in VBM studies in terms of preprocessing protocols, smoothing kernels, and statistical thresholding methods.
Conclusion
The result of our meta-analysis demonstrates that PD patient with RBD is associated with reduced GM volume of the right STG, suggesting a potential imaging biomarker for disease progression in PD. In the future, more functional neuroimaging and neurobiological studies are needed to provide further insights into how regional brain areas affect sleep disorders in PD patients.
Author Contributions
CY wrote the manuscript. CY and JC performed the literature search and collected data. CY and XL performed the data analysis. XB and RW conceived of the ideas and reviewed the manuscript. All authors approved the manuscript for publication.
Funding
This work was supported by the National Key R&D Program of China (2018YFA0108602 and 2018YFA0108600); Beijing Municipal Natural Science Foundation (7182134); Chinese Academy of Medical Sciences (CAMS) Initiative for Innovative Medicine (2016-I2M-1-017); Chinese Academy of Medical Sciences (CAMS) Young Talent Award Project (2018RC320003); and Beijing Nova Program (Z181100006218003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2020.00213/full#supplementary-material
References
Albajes-Eizagirre, A., Solanes, A., Fullana, M. A., Ioannidis, J. P. A., Fusar-Poli, P., Torrent, C., et al. (2019a). Meta-analysis of voxel-based neuroimaging studies using seed-based d mapping with permutation of subject images (SDM-PSI). J. Vis. Exp. 153:841. doi: 10.3791/59841
Albajes-Eizagirre, A., Solanes, A., Vieta, E., and Radua, J. (2019b). Voxel-based meta-analysis via permutation of subject images (PSI): theory and implementation for SDM. NeuroImage 186, 174–184. doi: 10.1016/j.neuroimage.2018.10.077
Ansari, M., Rahmani, F., Dolatshahi, M., Pooyan, A., and Aarabi, M. H. (2017). Brain pathway differences between Parkinson's disease patients with and without REM sleep behavior disorder. Sleep Breath 21, 155–161. doi: 10.1007/s11325-016-1435-8
Baiano, M., David, A., Versace, A., Churchill, R., Balestrieri, M., and Brambilla, P. (2007). Anterior cingulate volumes in schizophrenia: a systematic review and a meta-analysis of MRI studies. Schizophr Res 93, 1–12. doi: 10.1016/j.schres.2007.02.012
Barber, T. R., Klein, J. C., Mackay, C. E., and Hu, M. T. M. (2017). Neuroimaging in pre-motor Parkinson's disease. Neuroimage Clin. 15, 215–227. doi: 10.1016/j.nicl.2017.04.011
Boeve, B. F., Silber, M. H., Saper, C. B., Ferman, T. J., Dickson, D. W., Parisi, J. E., et al. (2007). Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 130(Pt 11), 2770–2788. doi: 10.1093/brain/awm056
Borghammer, P., Østergaard, K., Cumming, P., Gjedde, A., Rodell, A., Hall, N., et al. (2010). A deformation-based morphometry study of patients with early-stage Parkinson's disease. Eur. J. Neurol. 17, 314–320. doi: 10.1111/j.1468-1331.2009.02807.x
Bourgouin, P.-A., Rahayel, S., Gaubert, M., Arnaldi, D., Hu, M., Heidbreder, A., et al. (2019). Neuroimaging of Rapid Eye Movement Sleep Behavior Disorder. Intern. Rev. Neurobiol. 144, 185–210. doi: 10.1016/bs.irn.2018.10.006
Bugalho, P., and Viana-Baptista, M. (2013). REM sleep behavior disorder and motor dysfunction in Parkinson's disease–a longitudinal study. Parkinsonism Relat. Disord. 19, 1084–1087. doi: 10.1016/j.parkreldis.2013.07.017
Dahlgren, K., Ferris, C., and Hamann, S. (2020). Neural correlates of successful emotional episodic encoding and retrieval: an SDM meta-analysis of neuroimaging studies. Neuropsychologia 143:107495. doi: 10.1016/j.neuropsychologia.2020.107495
Dauvilliers, Y., Schenck, C. H., Postuma, R. B., Iranzo, A., Luppi, P.-H., Plazzi, G., et al. (2018). REM sleep behaviour disorder. Nat. Rev. Disease Primers 4:19. doi: 10.1038/s41572-018-0016-5
Du, M., Liu, J., Chen, Z., Huang, X., Li, J., Kuang, W., et al. (2014). Brain grey matter volume alterations in late-life depression. J. Psychiatry Neurosci. 39, 397–406. doi: 10.1503/jpn.130275
Ellison, A., Schindler, I., Pattison, L. L., and Milner, A. D. (2004). An exploration of the role of the superior temporal gyrus in visual search and spatial perception using TMS. Brain 127(Pt 10), 2307–2315. doi: 10.1093/brain/awh244
Feldmann, A., Illes, Z., Kosztolanyi, P., Illes, E., Mike, A., Kover, F., et al. (2008). Morphometric changes of gray matter in Parkinson's disease with depression: a voxel-based morphometry study. Mov. Disord. 23, 42–46. doi: 10.1002/mds.21765
Fereshtehnejad, S.-M., Romenets, S. R., Anang, J. B. M., Latreille, V., Gagnon, J.-F., and Postuma, R. B. (2015). New clinical subtypes of parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol. 72, 863–873. doi: 10.1001/jamaneurol.2015.0703
Ffytche, D. H., Creese, B., Politis, M., Chaudhuri, K. R., Weintraub, D., Ballard, C., et al. (2017). The psychosis spectrum in Parkinson disease. Nat. Rev. Neurol. 13, 81–95. doi: 10.1038/nrneurol.2016.200
Ford, A. H., Duncan, G. W., Firbank, M. J., Yarnall, A. J., Khoo, T. K., Burn, D. J., et al. (2013). Rapid eye movement sleep behavior disorder in Parkinson's disease: magnetic resonance imaging study. Mov. Disord. 28, 832–836. doi: 10.1002/mds.25367
Garcia-Lorenzo, D., Longo-Dos Santos, C., Ewenczyk, C., Leu-Semenescu, S., Gallea, C., Quattrocchi, G., et al. (2013). The coeruleus/subcoeruleus complex in rapid eye movement sleep behaviour disorders in Parkinson's disease. Brain 136, 2120–2129. doi: 10.1093/brain/awt152
Gharabaghi, A., Fruhmann Berger, M., Tatagiba, M., and Karnath, H. O. (2006). The role of the right superior temporal gyrus in visual search-insights from intraoperative electrical stimulation. Neuropsychologia 44, 2578–2581. doi: 10.1016/j.neuropsychologia.2006.04.006
Hanyu, H., Inoue, Y., Sakurai, H., Kanetaka, H., Nakamura, M., Miyamoto, T., et al. (2012). Voxel-based magnetic resonance imaging study of structural brain changes in patients with idiopathic REM sleep behavior disorder. Parkins. Relat. Disorders 18, 136–139. doi: 10.1016/j.parkreldis.2011.08.023
Howell, M. J., and Schenck, C. H. (2015). Rapid eye movement sleep behavior disorder and neurodegenerative disease. JAMA Neurol. 72, 707–712. doi: 10.1001/jamaneurol.2014.4563
Huang, J., Zhuo, W., Zhang, Y., Sun, H., Chen, H., Zhu, P., et al. (2017). Cognitive function characteristics of parkinson's disease with sleep disorders. Parkinsons Dis. 2017:4267353. doi: 10.1155/2017/4267353
Janzen, J., Schlindwein, P., Bense, S., Bauermann, T., Vucurevic, G., Stoeter, P., et al. (2008). Neural correlates of hemispheric dominance and ipsilaterality within the vestibular system. NeuroImage 42, 1508–1518. doi: 10.1016/j.neuroimage.2008.06.026
Joo, E. Y., Tae, W. S., Kim, S. T., and Hong, S. B. (2009). Gray matter concentration abnormality in brains of narcolepsy patients. Korean. J. Radiol. 10, 552–558. doi: 10.3348/kjr.2009.10.6.552
Jozwiak, N., Postuma, R. B., Montplaisir, J., Latreille, V., Panisset, M., Chouinard, S., et al. (2017). REM sleep behavior disorder and cognitive impairment in Parkinson's Disease. Sleep 40:8. doi: 10.1093/sleep/zsx101
Kim, H. J., Im, H. K., Kim, J., Han, J. Y., de Leon, M., Deshpande, A., et al. (2016). Brain atrophy of secondary REM-sleep behavior disorder in neurodegenerative disease. J. Alzheimers Dis. 52, 1101–1109. doi: 10.3233/JAD-151197
Li, D., Huang, P., Zang, Y., Lou, Y., Cen, Z., Gu, Q., et al. (2017). Abnormal baseline brain activity in Parkinson's disease with and without REM sleep behavior disorder: a resting-state functional MRI study. J. Magn. Reson. Imaging 46, 697–703. doi: 10.1002/jmri.25571
Lim, J. S., Shin, S. A., Lee, J. Y., Nam, H., Lee, J. Y., and Kim, Y. K. (2016). Neural substrates of rapid eye movement sleep behavior disorder in Parkinson's disease. Parkinsonism Relat. Disord. 23, 31–36. doi: 10.1016/j.parkreldis.2015.11.027
Liu, Y., Zhu, X. Y., Zhang, X. J., Kuo, S. H., Ondo, W. G., and Wu, Y. C. (2017). Clinical features of Parkinson's disease with and without rapid eye movement sleep behavior disorder. Transl. Neurodegener. 6, 35. doi: 10.1186/s40035-017-0105-5
Matsumoto, H., Simmons, A., Williams, S., Hadjulis, M., Pipe, R., Murray, R., et al. (2001). Superior temporal gyrus abnormalities in early-onset schizophrenia: similarities and differences with adult-onset schizophrenia. Am. J. Psychiatry 158, 1299–1304. doi: 10.1176/appi.ajp.158.8.1299
Mollenhauer, B., Zimmermann, J., Sixel-Doering, F., Focke, N. K., Wicke, T., Ebentheuer, J., et al. (2016). Monitoring of 30 marker candidates in early Parkinson disease as progression markers. Neurology 87, 168–177. doi: 10.1212/WNL.0000000000002651
Moreira, P. S., Marques, P., Soriano-Mas, C., Magalhaes, R., Sousa, N., Soares, J. M., et al. (2017). The neural correlates of obsessive-compulsive disorder: a multimodal perspective. Transl. Psychiatry 7:e1224. doi: 10.1038/tp.2017.189
Muller, J. L., Sommer, M., Dohnel, K., Weber, T., Schmidt-Wilcke, T., and Hajak, G. (2008). Disturbed prefrontal and temporal brain function during emotion and cognition interaction in criminal psychopathy. Behav. Sci. Law 26, 131–150. doi: 10.1002/bsl.796
Neikrug, A. B., Avanzino, J. A., Liu, L., Maglione, J. E., Natarajan, L., Corey-Bloom, J., et al. (2014). Parkinson's disease and REM sleep behavior disorder result in increased non-motor symptoms. Sleep Med. 15, 959–966. doi: 10.1016/j.sleep.2014.04.009
Orlov, N. D., Giampietro, V., O'Daly, O., Lam, S.-L., Barker, G. J., Rubia, K., et al. (2018). Real-time fMRI neurofeedback to down-regulate superior temporal gyrus activity in patients with schizophrenia and auditory hallucinations: a proof-of-concept study. Transl. Psychiatry 8:46. doi: 10.1038/s41398-017-0067-5
Otomune, H., Mihara, M., Hattori, N., Fujimoto, H., Kajiyama, Y., Konaka, K., et al. (2019). Involvement of cortical dysfunction in frequent falls in patients with Parkinson's disease. Parkinsonism Relat. Disord. 64, 169–174. doi: 10.1016/j.parkreldis.2019.04.007
Pacchetti, C., Manni, R., Zangaglia, R., Mancini, F., Marchioni, E., Tassorelli, C., et al. (2005). Relationship between hallucinations, delusions, and rapid eye movement sleep behavior disorder in Parkinson's disease. Mov. Disord. 20, 1439–1448. doi: 10.1002/mds.20582
Pan, L. A., Ramos, L., Segreti, A., Brent, D. A., and Phillips, M. L. (2015). Right superior temporal gyrus volume in adolescents with a history of suicide attempt. Br. J. Psychiatry 206, 339–340. doi: 10.1192/bjp.bp.114.151316
Postuma, R. B., Berg, D., Stern, M., Poewe, W., Olanow, C. W., Oertel, W., et al. (2015). MDS clinical diagnostic criteria for Parkinson's disease. Mov. Disord. 30, 1591–1601. doi: 10.1002/mds.26424
Pringsheim, T., Jette, N., Frolkis, A., and Steeves, T. D. (2014). The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov. Disord. 29, 1583–1590. doi: 10.1002/mds.25945
Rahayel, S., Gaubert, M., Postuma, R. B., Montplaisir, J., Carrier, J., Monchi, O., et al. (2019). Brain atrophy in Parkinson's disease with polysomnography-confirmed REM sleep behavior disorder. Sleep 42:62. doi: 10.1093/sleep/zsz062
Reale, R. A., Calvert, G. A., Thesen, T., Jenison, R. L., Kawasaki, H., Oya, H., et al. (2007). Auditory-visual processing represented in the human superior temporal gyrus. Neuroscience 145, 162–184. doi: 10.1016/j.neuroscience.2006.11.036
Romenets, S. R., Gagnon, J. F., Latreille, V., Panniset, M., Chouinard, S., Montplaisir, J., et al. (2012). Rapid eye movement sleep behavior disorder and subtypes of Parkinson's disease. Mov. Disord. 27, 996–1003. doi: 10.1002/mds.25086
Salsone, M., Cerasa, A., Arabia, G., Morelli, M., Gambardella, A., Mumoli, L., et al. (2014). Reduced thalamic volume in Parkinson disease with REM sleep behavior disorder: volumetric study. Parkinsonism Relat. Disord. 20, 1004–1008. doi: 10.1016/j.parkreldis.2014.06.012
Schapira, A. H. V., Chaudhuri, K. R., and Jenner, P. (2017). Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 18, 435–450. doi: 10.1038/nrn.2017.62
Scherfler, C., Frauscher, B., Schocke, M., Iranzo, A., Gschliesser, V., Seppi, K., et al. (2011). White and gray matter abnormalities in idiopathic rapid eye movement sleep behavior disorder: a diffusion-tensor imaging and voxel-based morphometry study. Annals Neurol. 69, 400–407. doi: 10.1002/ana.22245
Shah-Basak, P. P., Chen, P., Caulfield, K., Medina, J., and Hamilton, R. H. (2018). The role of the right superior temporal gyrus in stimulus-centered spatial processing. Neuropsychologia 113, 6–13. doi: 10.1016/j.neuropsychologia.2018.03.027
Shepherd, A. M., Matheson, S. L., Laurens, K. R., Carr, V. J., and Green, M. J. (2012). Systematic meta-analysis of insula volume in schizophrenia. Biol. Psychiatry 72, 775–784. doi: 10.1016/j.biopsych.2012.04.020
Shimizu, S., Hirose, D., Hatanaka, H., Takenoshita, N., Kaneko, Y., Ogawa, Y., et al. (2018). Role of neuroimaging as a biomarker for neurodegenerative diseases. Front. Neurol. 9:265. doi: 10.3389/fneur.2018.00265
St Louis, E. K., and Boeve, B. F. (2017). REM sleep behavior disorder: diagnosis, clinical implications, and future directions. Mayo Clin. Proc. 92, 1723–1736. doi: 10.1016/j.mayocp.2017.09.007
Tong, D., Lu, P., Li, W., Yang, W., Yang, Y., Yang, D., et al. (2019). Critical thinking and regional gray matter volume interact to predict representation connection in scientific problem solving. Exp. Brain Res. 237, 2035–2044. doi: 10.1007/s00221-019-05517-y
Van de Vliet, L., Jastorff, J., Huang, Y. A., Van Paesschen, W., Vandenbulcke, M., and Van den Stock, J. (2018). Anterior temporal lobectomy impairs neural classification of body emotions in right superior temporal sulcus and reduces emotional enhancement in distributed brain areas without affecting behavioral classification. J. Neurosci. 38, 9263–9274. doi: 10.1523/JNEUROSCI.0634-18.2018
Wang, Y. M., Zou, L. Q., Xie, W. L., Yang, Z. Y., Zhu, X. Z., Cheung, E. F. C., et al. (2018). Altered grey matter volume and cortical thickness in patients with schizo-obsessive comorbidity. Psychiatry Res. Neuroimaging 276, 65–72. doi: 10.1016/j.pscychresns.2018.03.009
Weng, H. H., Chen, C. F., Tsai, Y. H., Wu, C. Y., Lee, M., Lin, Y. C., et al. (2015). Gray matter atrophy in narcolepsy: an activation likelihood estimation meta-analysis. Neurosci. Biobehav. Rev. 59, 53–63. doi: 10.1016/j.neubiorev.2015.03.009
Zhang, X., Yao, J., Lv, Y., Zhao, X., Li, Y., Sui, Y., et al. (2018). An association study on the cognitive function and the cerebral grey matter volume of patients with first-episode schizophrenia. Shanghai Arch. Psychiatry 30, 154–167.
Zhang, Y. D., Zhou, J. S., Lu, F. M., and Wang, X. P. (2019). Reduced gray matter volume in male adolescent violent offenders. PeerJ. 7:e7349. doi: 10.7717/peerj.7349
Zhang, Z. X., Roman, G. C., Hong, Z., Wu, C. B., Qu, Q. M., Huang, J. B., et al. (2005). Parkinson's disease in China: prevalence in Beijing, Xian, and Shanghai. Lancet 365, 595–597. doi: 10.1016/S0140-6736(05)70801-1
Keywords: voxel-based morphometry, gray matter, neuroimaging, sleep disorder, Parkinson's disease
Citation: Yang C, Chang J, Liang X, Bao X and Wang R (2020) Gray Matter Alterations in Parkinson's Disease With Rapid Eye Movement Sleep Behavior Disorder: A Meta-Analysis of Voxel-Based Morphometry Studies. Front. Aging Neurosci. 12:213. doi: 10.3389/fnagi.2020.00213
Received: 03 December 2019; Accepted: 17 June 2020;
Published: 12 August 2020.
Edited by:
Gjumrakch Aliev, GALLY International Biomedical Research, United StatesReviewed by:
PingLei Pan, Southeast University, ChinaAbhishek Lenka, MedStar Georgetown University Hospital, United States
Copyright © 2020 Yang, Chang, Liang, Bao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinjie Bao, YmFveGluamllMUBwdW1jaC5jbg==; Renzhi Wang, d2FuZ3J6QDEyNi5jb20=
 Chengxian Yang1
Chengxian Yang1 Xinjie Bao
Xinjie Bao Renzhi Wang
Renzhi Wang