
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 10 March 2020
Sec. Neuroinflammation and Neuropathy
Volume 12 - 2020 | https://doi.org/10.3389/fnagi.2020.00054
This article is part of the Research Topic Cutting-Edge Approaches for CNS Protection and Repair: Focus on Vascular and Degenerative Disorders View all 83 articles
 Xiangnan Du1†
Xiangnan Du1† Jian Yang2†
Jian Yang2† Cuiying Liu2
Cuiying Liu2 Sainan Wang2
Sainan Wang2 Chencheng Zhang2
Chencheng Zhang2 Heng Zhao3
Heng Zhao3 Huishan Du1*
Huishan Du1* Xiaokun Geng1,2*
Xiaokun Geng1,2*Limb remote ischemic preconditioning (RIPC) has been proven to alleviate stroke injury in young rats, but its protective effect and its mechanism in aged rats are still unclear. Hypoxia-inducible factor (HIF) is one of the important markers of stroke, and its high expression plays an important role in the pathogenesis of stroke. In this study, we tested the hypothesis that RIPC could regulate the expression of HIF, leading to reduced inflammatory responses in aged rats. Stroke was induced by transient middle cerebral artery occlusion (MCAo) in aged rats, and RIPC was conducted in both hind limbs. The HIF-1α and HIF-2α mRNA and protein were examined by real-time RT-PCR and western blotting (WB). Inflammatory cytokines in the peripheral blood and brain were measured using AimPlex multiplex immunoassays. The protein levels of p-Akt, Akt, p-ERK, and ERK were examined by WB. We investigated that RIPC reduced the infarct size, improved neurological functions, and decreased the expression of HIF-1α and HIF-2α in the ischemic brain. RIPC reduced the levels of IL-1β, IL-6 and IFN-γ in the peripheral blood and the levels of IL-1β and IFN-γ in the ischemic brain 48 h post-stroke. Moreover, intraperitoneal injection of the HIF inhibitor, acriflavine hydrochloride (ACF), abolished the protection of RIPC with respect to infarct size and neurological functions and neutralized the downregulation of pro-inflammatory IL-1β, IL-6 and IFN-γ. ACF also reversed the activation of the Akt signaling pathway induced by RIPC following stroke. HIF may play a key role in RIPC, which was likely mediated by the Akt signaling pathway and systemic modulation of the inflammatory response in aged rats.
Ischemic stroke is considered to be the third most lethal and disabling disease in the world. Aging, above all risk factors of stroke, accounts for a large proportion of stroke-related mortality (Mozaffarian et al., 2015). According to the World Health Organization (WHO) statistics, twelve million people will suffer from stroke in the coming decade (Krishnamurthi et al., 2013). At present, however, no alternative neuroprotective agent has been approved by the FDA except for thrombolytic recombinant tissue plasminogen activator (tPA), which is only administrated to a small number of patients. Given the increase of the senior citizen and the aggravating of the associated stroke burden, it is urgent to find effective treatments for this disease. In recent years, remote ischemic preconditioning (RIPC) has become a feasible therapeutic strategy for stroke and has been proven to provide a protective effect on young experimental animals (Wei et al., 2012; Liu et al., 2016; Yang et al., 2018) and in clinical trials (Mi et al., 2016; Zhao S. C. et al., 2017). Nevertheless, its protective effect and its mechanism in the elderlies are still unclear.
Age is an important risk factor for pre-clinical studies on ischemic stroke, which has been largely overlooked. Previous studies have suggested that aged rats, compared with younger ones, represent a more clinically relevant model of ischemic stroke (Mayhan et al., 2008; Modrick et al., 2009). Advanced age increases the expression of immune cells including T cells, neutrophils, macrophages, and dendritic cells in middle cerebral artery occlusion (MCAo) mice, which may be responsible for the exacerbated behavioral deficits in the aged (Manwani et al., 2013). Age also stimulates the activation of macrophages and the expression of inflammatory cytokines in MCAo mice (Zhao S. C. et al., 2017). Although most experimental stroke studies have been conducted on healthy young animals, accumulating evidence points to age-related differences in gene expression and cytokine production (Kuzumaki et al., 2010; Semple et al., 2013; Orre et al., 2014; Suenaga et al., 2015); thus, applying aged animals as models may increase the clinical relevance of the experimental results.
Hypoxia-inducible factor (HIF) is the most crucial regulator of oxygen balance in mammals, and it has been shown to promote tolerance to acute ischemia (Semenza, 2012). HIF-1α and HIF-2α, as the HIF-α subunits, combine with HIF-β to regulate target gene transcription under hypoxic conditions. They share 48% identical DNA binding regions in structure, but each has its own unique biological effects. HIF can easily degrade under normal oxygen conditions within 5 min, while under hypoxia, its stability and transcriptional activity are significantly increased, which induces the expression of its downstream target genes. Because of its particularity of function, HIF is critically related to ischemic stroke (Sharp and Bernaudin, 2004; Ogle et al., 2012). Previous studies showed that suppressing the expression of basal HIF-2α, but not that of HIF-1α, was sufficient to accelerate cell death in nerve growth factor (NGF)-stimulated neurons (Lomb et al., 2009). In addition, the HIF-α subunits HIF-1α, HIF-2α and HIF-3α can be regulated by the phosphoinositide 3-kinase (PI3K) pathway in the brain of MCAo rats (Wei et al., 2017). The interaction between RIPC and HIF has been explored by some studies, where HIF was required for RIPC in protecting heart function in young animal models (Cai et al., 2013; Martin-Puig et al., 2015). Our previous study showed that HIF-1α was indispensable for RIPC against stroke by modulating inflammation in a young rat model of MCAo. Accumulating data provide indications for the roles of HIF-α in neuroprotection of RIPC against ischemia, but there is still a lack of solid evidence of its involvement in an aged model of stroke with RIPC.
After a stroke, systemic inflammation contributes to brain injury, as neuroinflammation occurs not only in the brain but also in the peripheral organs (Nguyen et al., 2016; Shim and Wong, 2016; Yamashiro et al., 2017). RIPC could reduce neuroinflammation in ischemic stroke through modulating the upregulated expression of HIF-1α (Yang et al., 2018), improving the peripheral immune cell response (Liu et al., 2016), activating the adenosine A1 receptor and affecting redox status (Hu et al., 2012), and changing the activation of signaling pathways such as Akt and ERK. All of these findings have focused on young animal models, whereas whether RIPC can regulate neuroinflammation in aged animals remains unclear.
In this study, we aimed to provide insights into the roles of HIF-1α and HIF-2α in RIPC protection in aged stroke rats and explore the regulatory mechanism of inflammatory cytokines. First, we defined the protection of RIPC on aged MCAo rats. Then, we tested the expression of HIF-1α and HIF-2α in penumbra tissue and measured the levels of multiple inflammatory cytokines in the peripheral blood and brain. Furthermore, we used the HIF inhibitor, acriflavine hydrochloride (ACF), to determine the role of HIF in modulating the protective effects of RIPC against stroke. Finally, we studied the activation of the Akt and ERK signaling pathways in this process.
All procedures in this study were conducted according to ethical standards and in accordance with the Declaration of Helsinki and with national and international guidelines and have been approved by the authors’ institutional review board. Male Sprague–Dawley (SD) rats, age-matched 19–20 months, were purchased from Vital River Laboratory Animal Technology Company Limited (Beijing, China). They were housed in a controlled room at a temperature of 24°C with a standard 12-h light/dark cycle, had free access to food and water and were randomly distributed into different groups. The number of animals in each group was 6–8.
Most pre-clinical stroke studies exclusively examined the response to stroke in young male animals. This is in part due to the difficulty of performing the surgeries, the high costs, and the poor survival rates in aged animals. In our experiment, rats were anesthetized with 3–5% isoflurane in 70% nitrous oxide and 30% oxygen and then maintained with 1–3% isoflurane. For the MCAo model, ischemia and reperfusion were established as previously described (Ren et al., 2011; Yang et al., 2018). As the aged rat weight was higher than that of younger ones, we chose a silica gel-coated nylon suture that was appropriate for rats up 450 g. The suture was withdrawn after 90 min of occlusion to allow for the re-opening of the MCA. Rat rectal temperature was maintained at 37 ± 0.5°C during the entire procedure. The cerebral blood flow (CBF) during the surgery before and after occlusion were measured by laser Doppler perfusion monitoring with a laser Doppler probe (PeriFlux System 5000, Perimed AB, Sweden) interfaced to a laptop equipped with the PeriSoft data acquisition software (PeriSoft Systems, Inc., Sweden) as previously described (Chen et al., 2018). The blood gas (PaCO2, PaO2 [mmHg] and pH) and blood sugar (mmol/L) were also examined during the surgery. The rats in the sham group were subjected to the surgical procedure without MCA occlusion.
RIPC was established as previously described (Yang et al., 2018). Briefly, 24 h before MCAo, RIPC was conducted on both hind limbs of rats anesthetized with 1–3% isoflurane by non-invasive occlusion of hind limb blood flow with a gauge bandage, in contrast to an invasive, direct femoral artery occlusion. The two hind limbs were simultaneously tied with a bandage to occlude blood circulation for 10 min and then released for 10 min to allow for reperfusion. The occlusion/reperfusion cycle was repeated three times.
Behavioral tests were conducted as described previously (Zhao et al., 2005; Yang et al., 2018). The behavioral tests were conducted to evaluate neurological functions after stroke in aged rats, including the tail hang test, home cage, and postural reflex. All behavioral tests were performed by a person who was blinded to the experimental conditions. We handled the rats for 3 days before surgery and tested their baseline on the day before surgery. All behavioral tests were then conducted 48 h after stroke onset.
For the tail hang test, the rat was hung by its tail approximately 10 cm above the floor. Stroke rats will turn to the contralateral side (left) of the ischemic hemisphere, and a head-turn of more than 90° was counted. Each time the rat was hung for no more than 5 s and each rat was hung for a total of 20 times. The percentage of head turns was calculated.
For the home cage limb test, the rat uses its forelimbs to explore the cage edge, and we counted the number of the times the ipsilateral, contralateral or both forelimbs touched the cage wall. A total of 20 contacts were recorded. The percentage of times that the ipsilateral forelimb was used was calculated using the formula: [ipsilateral + (both/2)] × 100%.
In the postural reflex test, the rat was placed on a table, its tail was held by one hand and the other hand was used to push its shoulder by nearly 20 cm three times. Non-ischemic rats gripped the table vigorously during the push, which was given a 0 score. A rat that showed less resistance and become stiff during the push was given a 1 score. A score of 2 was given if the rat had no resistance.
We also measured neurological deficits to evaluate stroke outcomes at 48 h after reperfusion using the method of the Longa scoring system, in which the scores were based on the following criteria: 0 = no deficit, 1 = failure to extend the left forepaw, 2 = circling to the left, 3 = falling to the left, 4 = failure to walk spontaneously and loss of consciousness, 5 = death.
To study the role of HIF in RIPC, a HIF inhibitor was used. Acriflavine hydrochloride (ACF, A8251, Sigma) was dissolved in phosphate-buffered saline (PBS), adjusted to a concentration of 2 mmol/L with additional PBS, and stored at 4°C. The ACF solution was given by intraperitoneal injection at a dose of 1.5 mg/kg, 24 h before stroke onset and every 24 h thereafter until euthanized (Ogle et al., 2012; Yang et al., 2018).
2,3,5-triphenyl-2H-tetrazolium chloride (TTC) staining was used for measuring the infarct size 48 h after reperfusion when the animals were euthanized. The non-ischemic hemisphere and the non-ischemic region in the ischemic hemisphere were measured, from which the infarct size was calculated according to the following formula: [(area of the non-ischemic hemisphere—area of the non-ischemic region in the ischemic hemisphere)/area of the non-ischemic hemisphere] × 100%. The detailed protocol has been described previously (Ren et al., 2009; Yang et al., 2018).
To measure HIF-1α and HIF-2α mRNA expression, total RNA was isolated from the cortical penumbra, which was collected on ice and stored at −80°C immediately after the animals were euthanized. RNA was extracted with TRIzol reagent (Cat# 15596-026, Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. The purified RNA was then reverse-transcribed into cDNA using the Reverse Transcription System (Cat# E6300S, New England BioLabs® Inc., Ipswich, MA, USA). Quantitative RT-PCR analysis of the mRNA level of HIF-1α (HIF-1α F, GATGAATCAAAAGCAGTGACGAAGG, HIF-1α R, ATGCCTTAGCAGTGGTCATTTCTTG; HIF-2α F, ATCTTCCAGCCGCTCACC, HIF-2α R, GCCCACAGACCACTTCGTA) was performed using the SYBR Green Prime Script kit (RR420A, TAKARA). GAPDH (GAPDH F, TTCCTACCCCCAATGTATCCG; GAPDH R, CCACCCTGTTGCTGTAGCCATA) was chosen as the housekeeping gene. The real-time PCR program steps were: 95°C for 5 min, 45 cycles at 95°C for 5 s, 60°C for 5 s, and 72°C for 10 s, followed by 72°C for 1 min.
The cortical penumbra was collected on ice and stored at −80°C immediately after the animals were euthanized. Protein extractions were prepared with lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 0.5% NP-40, 0.1% Triton X-100, 0.1% SDS, 1 mM PMSF, 1× protein inhibitor mix Complete Mini). Then, 20 mg of protein per lane were separated by 10% polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes for 2 h. The membranes were blocked with 5% skimmed milk for 1 h and then incubated overnight at 4°C with the primary antibody. Membranes were washed with TBST and incubated with the secondary antibody for 1 h. The signal was visualized by enhanced chemiluminescence. The primary antibodies used were anti-GAPDH (1:1000; Cat# 5174, Cell Signaling Technology, Inc., Danvers, MA, USA), anti-HIF-1α (1:1000; Cat# 14179, Cell Signaling Technology, Inc., Danvers, MA, USA), anti-HIF-2α (1:500; ab199, Abcam, Cambridge, MA, USA), anti-Akt (1:1,000, Cat# 4691, Cell Signaling Technology, Inc., Danvers, MA, USA), anti-p-Akt (1:1,000, Ser473, Cat# 4060, Cell Signaling Technology, Inc., Danvers, MA, USA), anti-ERK (1:1,000, Cat# 4695, Cell Signaling Technology, Inc., Danvers, MA, USA), and anti-p-ERK (1:1,000, Cat# 4370, Cell Signaling Technology, Inc., Danvers, MA, USA).
Peripheral blood and penumbra tissue were collected from the heart and the cortex of the ischemic brain, and an AimPlex rat inflammation assay kit (Beijing Quantobio, China) was used to detect multiple inflammatory cytokines in these samples. Six cytokines: IL-1β, IL-6, TNFα, IFN-γ, IL-4 and IL-10 were measured. The procedure was conducted according to the manufacturer’s instructions and as discussed previously (Yang et al., 2018). Briefly, 45 μl of capture bead working suspension and a 45 μl sample were sequentially shaken 750 times per minute for 60 min in the dark, followed by three washings in 100 μl washing buffer. The biotin-conjugated antibodies (25 μl) were added and the wells were sealed and shaken for 30 min in the dark. After washing three times with washing buffer, streptavidin-PE was added and they were shaken for 20 min in the dark. Finally, 100–200 μl 1 × Reading buffer was added to each well and the fluorescence signals of the samples were acquired using a flow cytometer (NovoCyte, ACEA Biosciences, Inc., San Diego, CA, USA), and the levels of the inflammatory factors were analyzed with FCAP Array 3.0 software.
Statistical analysis was performed using Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). Values are presented as means ± SEM. Differences between experimental groups were analyzed by Student’s t-test or one-way ANOVA with Newman-Keuls Multiple Comparison test as a post hoc test. A value of P < 0.05 was considered statistically significant. Before statistical analysis, data were checked for the Gaussian distribution and fitted the normal distribution.
In the previous study, we confirmed that RIPC was neuroprotective in young rats. In the current study, we used the same method we have used previously to detect the neuroprotective ability of RIPC in aged rats. The TTC staining results showed that RIPC induced a 34.64% significantly reduction of infarct size from 38.85 ± 1.15% to 25.39 ± 1.12% compared with MCAo (Figure 1A and Supplementary Figure S2), attenuated neurological scores (Figure 1B), and improved behavioral performance in the tail hang test (Figure 1B). Although there was no significant difference in the home cage test, RIPC showed a trend towards the promotion of functional recovery in aged rats. There was no significant difference in the postural reflex test (Figure 1B). Meanwhile, CBF detected during MCAo surgery had no change between MCAo and RIPC+MCAo group. It was reduced by nearly 80% of baseline during ischemia and reestablished to 78% of baseline following reperfusion (Supplementary Figure S1). There were no differences in blood gas and blood sugar between groups, either (Supplementary Tables S1, S2). These findings confirm that RIPC triggers structural and functional protection against stroke in aged rats.
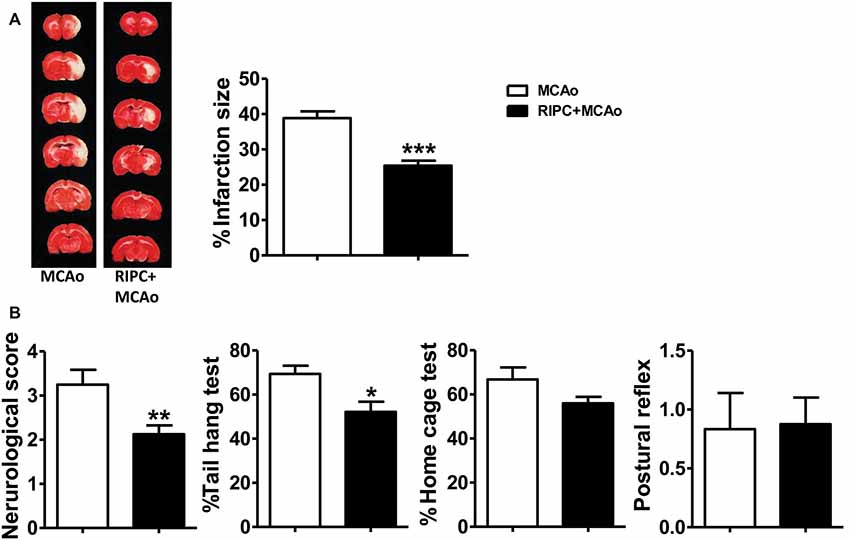
Figure 1. Remote ischemic preconditioning (RIPC) reduced infarct size and improved neurological function and the behavioral test after stroke in aged rats. (A) Representative images and infarct volume of 2,3,5-triphenyl-2H-tetrazolium chloride (TTC) staining in the MCAo and RIPC+MCAo group in aged rats. (B) Neurological score and behavior tests. Statistical analysis was performed by ANOVA. *,**,*** vs. MCAo, p < 0.05, 0.01, 0.001, respectively (N = 8–10 per group). MCAo, middle cerebral artery occlusion; RIPC, remote ischemic preconditioning.
To test whether RIPC could regulate the expression of HIF-1α and HIF-2α in the ischemic tissue in aged rats, we measured HIF-1α and HIF-2α mRNA and protein levels in the penumbra tissue. The real-time RT-PCR results showed that mRNA expression of HIF-1α and HIF-2α were more than 3-fold upregulated 48 h post-stroke in the ischemic brain (Figure 2A), whereas RIPC decreased nearly 50% of HIF-1α and 60% of HIF-2α mRNA expression (Figure 2A). Similarly, the western blotting (WB) results (Figure 2B) and the statistical data showed that RIPC attenuated the protein expression of HIF-1α and HIF-2α in the ischemic brain 48 h post-stroke (Figure 2B).
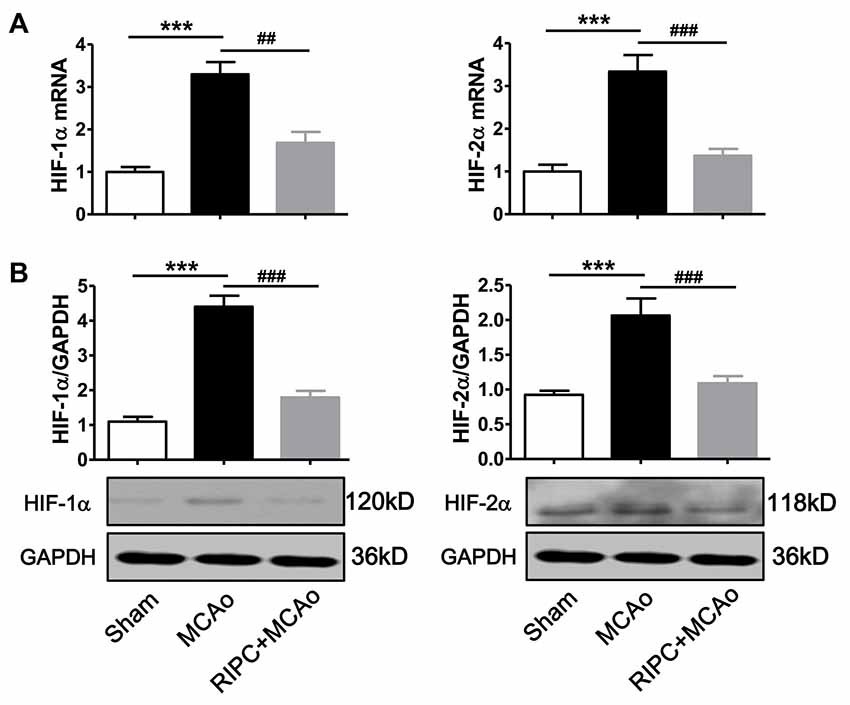
Figure 2. The expression of HIF-1α and HIF-2α in penumbra tissues. (A) The mRNA level of HIF-1α and HIF-2α was assessed by real-time RT-PCR. (B) Statistical data and representative images of western blot of HIF-1α and HIF-2α protein levels in penumbra tissues in the Sham, MCAo and RIPC+MCAo group. (N = 5–6 per group) Statistical analysis was performed by ANOVA. *** vs. Sham, p < 0.001, ##,### vs. MCAo, p < 0.01, 0.001, respectively.
To test if the inflammatory status were regulated by RIPC in aged rats after MCAo, we measured the effect of RIPC on the protein levels of the anti-inflammatory cytokines, IL-4 and IL-10, and that of the pro-inflammatory cytokines, IL-1β, IL-6, TNFα and IFN-γ in the peripheral blood and brain tissue. The results showed that MCAo increased the levels of pro-inflammatory cytokines while RIPC significantly decreased the levels of pro-inflammatory IL-1β, IL-6 and IFN-γ in the peripheral blood (Figure 3A) and that of IL-1β and IFN-γ in penumbra tissue (Figure 3B) 48 h post-stroke in aged rats. Taken together, these results suggest that RIPC reverses the increase in HIF expression caused by stroke and improves the level of inflammation in the ischemic and peripheral areas.
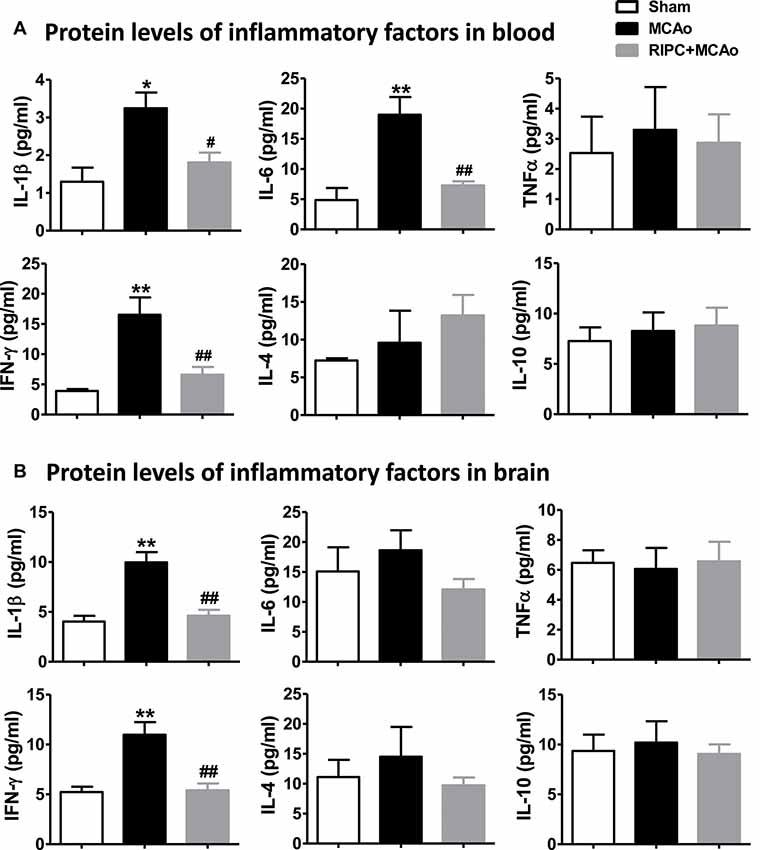
Figure 3. The effects of RIPC on inflammatory cytokines in the peripheral blood and brain. (A) Protein levels of the pro-inflammatory cytokines IL-1β, IL-6, TNFα and IFN-γ and anti-inflammatory cytokines IL-4 and IL-10, respectively, in the peripheral blood. A total of three groups were evaluated: Sham, MCAo, RIPC+MCAo. (B) Protein levels of IL-1β, IL-6, TNFα IFN-γ IL-4 and IL-10, in the ischemic brain. All samples were collected 48 h after stroke (N = 5–6 per group). *,** vs. Sham, p < 0.05, 0.01, #,## vs. MCAo, p < 0.05, 0.01, respectively.
If HIF was necessary for the brain protection of RIPC in aged rats, inhibiting HIF activity would contribute to brain injuries induced by stroke. TTC staining results showed that the HIF inhibitor, ACF, abolished the protection of RIPC which ACF induced a 27.02% increase of the infarct size from 25.39 ± 1.12% to 32.25 ± 1.65% compared with RIPC (Figure 4A and Supplementary Figure S2). In addition, ACF also abolished the protective effects of RIPC as measured by neurological scores and behavioral performance including the tail hang test (Figure 4B). The initial decrease in CBF during ischemia and the restoration of CBF during reperfusion, the changes between groups were similar. Blood gas and blood sugar values had no differences between groups (Supplementary Figure S1 and Supplementary Tables S1, S2).
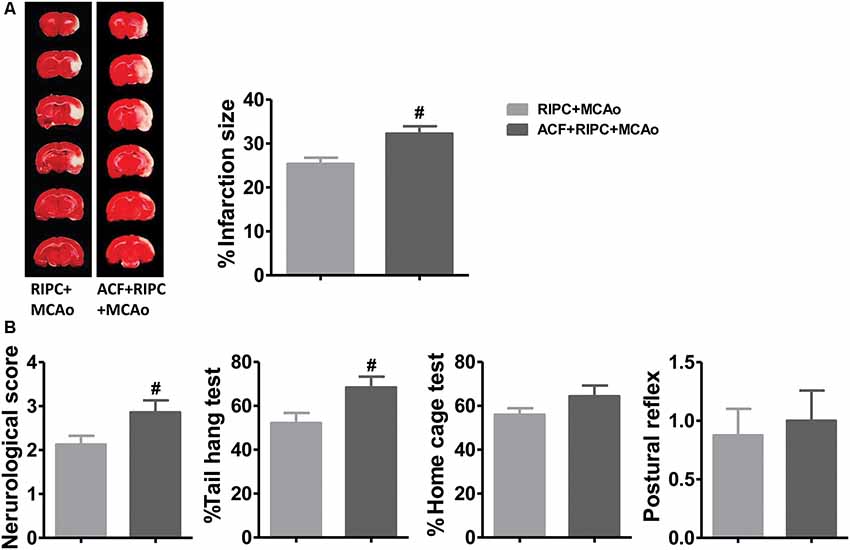
Figure 4. The effects of hypoxia-inducible factor (HIF) antagonist ACF on stroke outcomes in aged stroke animals treated with RIPC. (A) Representative images of TTC staining and the statistical results of infarct volume in the RIPC+MCAo and ACF+RIPC+MCAo group in aged rats. (B) Neurological score and behavioral tests of the tail hang test, home cage test, and postural reflex test (N = 8–10 per group). #P < 0.05, vs. RIPC+MCAo. ACF, acriflavine hydrochloride, HIF inhibitor.
After examining the antagonistic effect of the HIF inhibitor with RIPC on brain injury, we further assessed its effect on inflammatory factors in aged rats. The results of the AimPlex multiplex immunoassays showed that in the peripheral blood, the HIF inhibitor ACF had an antagonist effect on IL-6 and IFN-γ (Figure 5A). ACF+RIPC+MCAo increased the expression of IL-6 and IFN-γ at the protein level compared with the RIPC+MCAo group. In addition, ACF had an antagonist effect on IL-1β and IFN-γ in the brain (Figure 5B). These results suggest that inhibition of HIF abolished the structural and functional protection of RIPC and neutralized the downregulation of inflammatory responses.
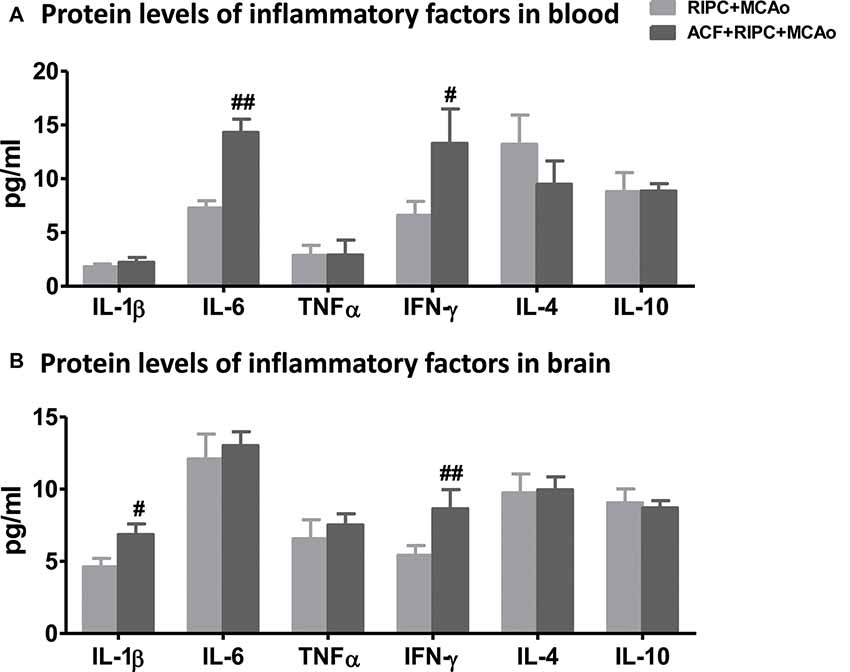
Figure 5. The effect of ACF on protein levels of inflammatory cytokines in aged stroke animals receiving RIPC. (A) Protein levels of the pro-inflammatory cytokines IL-1β, IL-6, TNFα and IFN-γ and anti-inflammatory cytokines IL-4 and IL-10 in the peripheral blood, respectively in RIPC+MCAo and ACF+RIPC+MCAo group in aged rats. (B) Protein levels of IL-1β, IL-6, TNFα, IFN-γ, IL-4 and IL-10 in the ischemic brain, respectively (N = 5–6 per group). #,##, vs. RIPC+MCAo, p < 0.05, 0.01, respectively.
Several studies have demonstrated that under ischemic conditions, the activation of the PI3k/Akt or EKR signaling pathway can be regulated by the expression of HIF, and signaling pathway activation can regulate inflammatory responses. We, therefore, investigated whether the Akt or ERK signaling pathway was involved in the action of RIPC to inhibit cerebral inflammation. Penumbra tissue was collected from the cortex of the ischemic brain. The results showed that the ratios of p-Akt/Akt (Figure 6A) and p-ERK/ERK (Figure 6B) were decreased 48 h post-stroke, whereas RIPC upregulated the expression of p-Akt not p-ERK (Figure 6). RIPC had no effect on the total expression of Akt and ERK. Moreover, ACF decreased the ratios of both p-Akt/Akt and p-ERK/ERK (Figure 6). These collective findings suggest that RIPC reverses the reduced expression of p-Akt following a stroke, while inhibition of HIF abolishes the reversion.
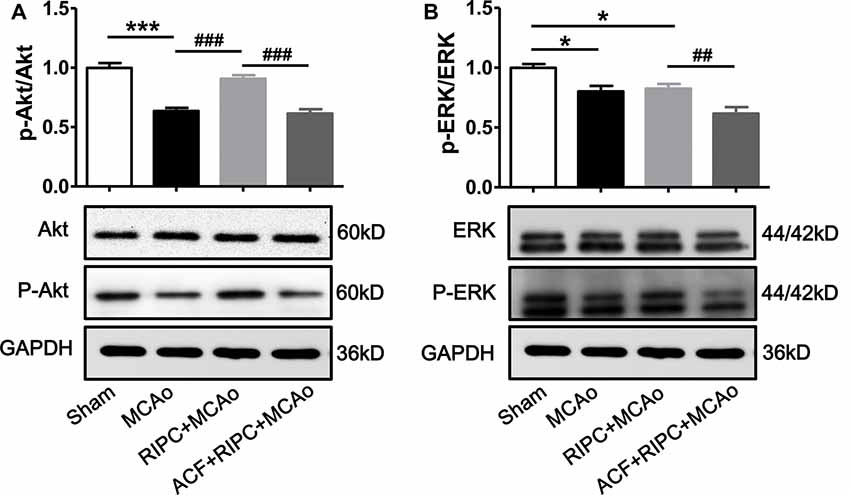
Figure 6. The effect of RIPC and ACF on the activation of Akt and ERK signaling pathway. (A) Statistical data of the ratio of p-Akt to total Akt and representative images of western blot of total Akt and p-Akt. A total of 4 groups were assessed: Sham, MCAo, RIPC+MCAo, ACF+RIPC+MCAo. (B) Statistical data of the ratio of p-ERK to total ERK and representative images of western blot of total ERK and p-ERK in penumbra tissues (N = 5–6 per group). Statistical analysis was performed by ANOVA. *,*** vs. Sham, p < 0.05, 0.001 respectively, ##,### vs. MCAo, RIPC+MCAo, p < 0.01, 0.001, respectively.
In the current study, first, we demonstrated that RIPC reduced the infarction size and improved neurological function in aged rats. Second, RIPC has been shown to decrease the expression of HIF-1α and HIF-2α in the ischemic brain and to decrease the protein levels of pro-inflammatory cytokines IL-1β and IFN-γ in both the blood and brain 48 h post-stroke. Third, the HIF-1α inhibitor, ACF, abolished the protective effects of RIPC on infarction and neurological function. Additionally, ACF inhibited the downregulation of pro-inflammatory factors induced by RIPC, including IL-6 and IFN-γ in blood and IL-1β and IFN-γ in the brain. Fourth, RIPC increased the ratio of p-Akt to total Akt, while ACF abolished the activation of the Akt signaling pathway in the brain. Collectively, these data showed that HIF-1α and HIF-2α played key roles in the protective effect of RIPC by modulating inflammatory responses likely mediated by the Akt signaling pathway in the brain and peripheral blood circulation in aged rats.
The success of most stroke models has not been well transformed into clinical practice. One potential reason for this failure is that stroke predominantly afflicts the elderly. Aging is a very important factor in stroke, and it has been found that there are many differences in stroke models between young and old animals such as infarction size, behavior, and expression and distribution of immune cells (Kuzumaki et al., 2010; Manwani et al., 2013; Orre et al., 2014; Zhao W. B. et al., 2017). In the previous study, we demonstrated that HIF-1α played a critical role in the protective effect of RIPC against stroke, which was likely achieved by modulating neuroinflammation in young rats (Yang et al., 2018). However, whether HIF plays a key role in RIPC in aged stroke is not addressed. Based on these, we chose an aged rats MCAo model and studied the protection and mechanism of RIPC post-stroke. We verified the infarction size was smaller in aged rats but there was worse neurological function than young rats. Further, our data suggested that RIPC protected against stroke not only in young rats but also in aged rats. RIPC reduced the infarction size and improved neurological function and behavior (Figure 1).
The HIF protein is a transcriptional factor that exists just a few minutes under normal oxygen levels but accumulates in hypoxic and ischemic cells (Ogle et al., 2012). Previous studies have shown that the neuroprotection against focal cerebral ischemia provided by various agents is mediated through the regulation of HIF (Doeppner et al., 2012; Wang et al., 2012; Meller and Simon, 2015). In the current study, we showed that MCAo upregulated the expression of HIF-1α and HIF-2α mRNA and protein levels in the ischemic brain of aged rats (Figure 2), which the evidence has been described in young animals in other reports (Zhong et al., 2012; Zhao et al., 2015; Zong et al., 2015). Further, we found that RIPC reversed this MCAo-induced upregulation of HIFs (Figure 2). It was suggested that RIPC improved the regional ischemia in the brain 48 h post-stroke in aged rats. Previous evidence showed that RIPC could regulate the expression of HIF, such as HIF-1α and HIF-2α, and played key roles in ischemic tissue including in the brain and heart in young experimental animals (Cai et al., 2008, 2013; Kapitsinou et al., 2014; Yang et al., 2018). To examine whether HIF also plays an important role in the neuroprotection of RIPC in aged rats, we used the heterodimer inhibitor, ACF, to inhibit HIF activity. The data showed that the injection of ACF abolished the structural and functional protection of RIPC on infarction size, neurological function and the tail hang test, one of the behavioral tests (Figure 4). These results demonstrated that HIF-1α and HIF-2α are key factors in the protection of RIPC in aged rats.
HIF is an inflammation mediator and can be stabilized by TNFα and IL-1β (Sharp and Bernaudin, 2004). It has been shown that IL-10 is the main target of HIF-1α in cardiomyocytes of circulating hypoxia-reoxygenation mice. RIPC can increase the plasma concentration of IL-10 and promote the subsequent remote activation of Akt signals in the heart (Cai et al., 2013). These findings are based on young animal models, and the changes in aged animals remain unclear. Our data showed that RIPC reduced the levels of pro-inflammatory cytokines IL-1β, IL-6 and IFN-γ in the blood, and IL-1β and IFN-γ in the brain in aged rats 48 h post-stroke (Figure 3), while the changes that were neutralized by the HIF inhibitor (Figure 5).
Several reports have identified under ischemic conditions, the activation of the Akt or EKR signaling pathway can be regulated by the expression of HIF (Hausenloy and Yellon, 2007; Cai et al., 2013; Martin-Puig et al., 2015; Meller and Simon, 2015; Ong et al., 2015). However, if HIF is directly affected by RIPC and further regulate the activity of Akt or EKR signaling pathway is unknown. Interestingly, in our study, we found that RIPC activated the Akt but not the ERK signaling pathway and that inhibition of HIF abolished the activation of Akt in aged rats following stroke (Figure 6). It suggested that HIF played key roles in RIPC reversed the reduced expression of p-Akt.
In the current study, there are several limitations. First, inhibitors of inflammatory cytokines and signaling pathways were not used. Thus, future studies should examine the infarct size when the inhibitors of inflammatory cytokines and signaling pathways are used; and come to a convincing conclusion the inflammatory responses and Akt/ERK signaling pathway is the cause of the reduced infarct size. Second, RIPC is an endogenous protective mechanism through which short-term sub-lethal ischemia in a remote organ can protect the major organ from further severe ischemia. However, due to the unpredictability of cerebral infarction, its clinical application is limited. Unlike ischemic preconditioning, ischemic postconditioning is a relatively novel concept, which follows reperfusion of a vessel and effectively inhibits inflammatory responses and delays cell death. So many researchers believe that ischemic postconditioning is more suitable for clinical transformation. However, to date, the evidence of cerebral ischemia postconditioning is limited to preclinical studies. Therefore, future studies will address the mechanism of remote ischemic postconditioning in the protection against cerebral ischemia, stimulate the clinical transformation of remote ischemic postconditioning.
Our study provided strong evidence that RIPC protected against stroke in aged rats by modulating the expression of HIF-1α and HIF-2α in ischemic tissue, not only affecting the activation of the Akt pathway but also reducing the expression of pro-inflammatory cytokines in the peripheral blood and brain.
All datasets generated for this study are included in the article/Supplementary Material.
The animal study was reviewed and approved by Capital Medical University Animal Review Committee.
XG, HD, and HZ: study concept and design. XD and JY: acquisition of data and wrote the draft. CL: made the figure and statistical analysis. SW and CZ: made a model. All authors contributed to the critical revision of the manuscript and have approved the final version of this review article.
This study was supported by the National Natural Science Foundation of China Grant No. 81701154 and Tong Zhou District Science and Technology Project Grant Nos. KJ2019CX014-31 and KJ2019CX004-03.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Xuan Liu and Haiteng Ji for their long-term feeding of experimental animals.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2020.00054/full#supplementary-material.
FIGURE S1 | Cerebral blood flow during the MCAo surgery. Cerebral blood flow was measured at four time-points, baseline, 1 and 5 min of ischemia and reperfusion in the MCAo, RIPC+MCAo and ACF+RIPC+MCAo groups. Data were normalized to baseline and expressed as percentages.
FIGURE S2 | Images of TTC staining. All the panels and representative images of TTC staining were presented. (A) MCAo group. (B) RIPC+MCAo group. (C) ACF+RIPC+MCAo group.
TABLE S1 | Arterial blood gas parameter before and immediately after MCAo.
TABLE S2 | Blood sugar value after surgery in each group.
Cai, Z. Q., Luo, W. B., Zhan, H. W., and Semenza, G. L. (2013). Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc. Natl. Acad. Sci. U S A 110, 17462–17467. doi: 10.1073/pnas.1317158110
Cai, Z. Q., Zhong, H., Bosch-Marce, M., Fox-Talbot, K., Wang, L., Wei, C. M., et al. (2008). Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1α. Cardiovasc. Res. 77, 463–470. doi: 10.1093/cvr/cvm035
Chen, C., Jiang, W., Liu, Z. J., Li, F. W., Yang, J., Zhao, Y. L., et al. (2018). Splenic responses play an important role in remote ischemic preconditioning-mediated neuroprotection against stroke. J. Neuroinflmmation 15:167. doi: 10.1186/s12974-018-1190-9
Doeppner, T. R., Mlynarczuk-Bialy, I., Kuckelkorn, U., Kaltwasser, B., Herz, J., Hasan, M. R., et al. (2012). The novel proteasome inhibitor BSc2118 protects against cerebral ischemia through HIF1A accumulation and enhanced angioneurogenesis. Brain 135, 3282–3297. doi: 10.1093/brain/aws269
Hausenloy, D. J., and Yellon, D. M. (2007). Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail. Rev. 12, 217–234. doi: 10.1007/s10741-007-9026-1
Hu, S., Dong, H. L., Zhang, H. P., Wang, S. Q., Hou, L. C., Chen, S. Y., et al. (2012). Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Res. 1459, 81–90. doi: 10.1016/j.brainres.2012.04.017
Kapitsinou, P. P., Sano, H., Michael, M., Kobayashi, H., Davidoff, O., Bian, A., et al. (2014). Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J. Clin. Invest. 124, 2396–2409. doi: 10.1172/JCI69073
Krishnamurthi, R. V., Feigin, V. L., Forouzanfar, M. H., Mensah, G. A., Connor, M., Bennett, D. A., et al. (2013). Global and regional burden of first-ever ischemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob. Health 5, e259–e281. doi: 10.1016/S2214-109X(13)70089-5
Kuzumaki, N., Ikegami, D., Imai, S., Narita, M., Tamura, R., Yajima, M., et al. (2010). Enhanced IL-1α production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice. Synapse 64, 721–728. doi: 10.1002/syn.20800
Liu, Z. J., Chen, C., Li, X. R., Ran, Y. Y., Xu, T., Zhang, Y., et al. (2016). Remote ischemic preconditioning-mediated neuroprotection against stroke is associated with significant alterations in peripheral immune responses. CNS Neurosci. Ther. 22, 43–52. doi: 10.1111/cns.12448
Lomb, D. J., Desouza, L. A., Franklin, J. L., and Freeman, R. S. (2009). Prolyl hydroxylase inhibitors depend on extracellular glucose and hypoxia-inducible factor (HIF)-2 to inhibit cell death caused by nerve growth factor (NGF) deprivation: evidence that HIF-2 has a role in NGF-promoted survival of sympathetic neurons. Mol. Pharmacol. 75, 1198–1209. doi: 10.1124/mol.108.053157
Manwani, B., Liu, F., Scranton, V., Hammond, M. D., Sansing, L. H., and McCullough, L. D. (2013). Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp. Neurol. 249, 120–131. doi: 10.1016/j.expneurol.2013.08.011
Martin-Puig, S., Tello, D., and Aragonés, J. (2015). Novel perspectives on the PHD-HIF oxygen sensing pathway in cardioprotection mediated by IPC and RIPC. Front. Physiol. 6:137. doi: 10.3389/fphys.2015.00137
Mayhan, W. G., Arrick, D. M., Sharpe, G. M., and Sun, H. (2008). Age-related alterations in reactivity of cerebral arterioles: role of oxidative stress. Microcirculation 15, 225–236. doi: 10.1080/10739680701641421
Meller, R., and Simon, R. P. (2015). A critical review of mechanisms regulating remote preconditioning-induced brain protection. J. Appl. Physiol. 119, 1135–1142. doi: 10.1152/japplphysiol.00169.2015
Mi, T. M., Yu, F., Ji, X. M., Sun, Y. X., and Qu, D. M. (2016). The interventional effect of remote ischemic preconditioning on cerebral small vessel disease: a pilot randomized clinical trial. Eur. Neurol. 76, 28–34. doi: 10.1159/000447536
Modrick, M. L., Didion, S. P., Sigmund, C. D., and Faraci, F. M. (2009). Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am. J. Physiol. Heart Circ. Physiol. 296, H1914–H1919. doi: 10.1152/ajpheart.00300.2009
Mozaffarian, D., Benjamin, E. J., Go, A. S., Arnett, D. K., Blaha, M. J., Cushman, M., et al. (2015). Heart disease and stroke statistics—2015 update: a report from the american heart association. Circulation 131, e29:e322. doi: 10.1161/CIR.0000000000000152
Nguyen, T. V., Frye, J. B., Zbesko, J. C., Stepanovic, K., Hayes, M., Urzua, A., et al. (2016). Multiplex immunoassay characterization and species comparison of inflammation in acute and non-acute ischemic infarcts in human and mouse brain tissue. Acta Neuropathol. Commun. 4:100. doi: 10.1186/s40478-016-0371-y
Ogle, M. E., Gu, X., Espinera, A. R., and Wei, L. (2012). Inhibition of prolyl hydroxylases by dimethyloxaloylglycine after stroke reduces ischemic brain injury and requires hypoxia inducible factor-1α. Neurobiol. Dis. 45, 733–742. doi: 10.1016/j.nbd.2011.10.020
Ong, S. B., Hall, A. R., Dongworth, R. K., Kalkhoran, S., Pyakurel, A., Scorrano, L., et al. (2015). Akt protects the heart against ischaemia-reperfusion injury by modulating mitochondrial morphology. Thromb. Haemost. 113, 513–521. doi: 10.1160/TH14-07-0592
Orre, M., Kamphuis, W., Osborn, L. M., Melief, J., Kooijman, L., Huitinga, I., et al. (2014). Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiol. Aging 35, 1–14. doi: 10.1016/j.neurobiolaging.2013.07.008
Ren, C. H., Gao, M. Q., Dornbos, D., Ding, Y. C., Zeng, X. W., Luo, Y. M., et al. (2011). Remote ischemic post-conditioning reduced brain damage in experimental ischemia/reperfusion injury. Neurol. Res. 33, 514–519. doi: 10.1179/016164111x13007856084241
Ren, C. C., Yan, Z. M., Wei, D. T., Gao, X. W., Chen, X. Y., and Zhao, H. (2009). Limb remote ischemic postconditioning protects against focal ischemia in rats. Brain Res. 1288, 88–94. doi: 10.1016/j.brainres.2009.07.029
Semenza, G. L. (2012). Hypoxia-inducible factors in physiology and medicine. Cell 148, 399–408. doi: 10.1016/j.cell.2012.01.021
Semple, B. D., Blomgren, K., Gimlin, K., Ferriero, D. M., and Noble-Haeusslein, L. J. (2013). Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 106–107, 1–16. doi: 10.1016/j.pneurobio.2013.04.001
Sharp, F. R., and Bernaudin, M. (2004). HIF-1 and oxygen sensing in the brain. Nat. Rev. 5, 437–448. doi: 10.1515/bc.2004.016
Shim, R., and Wong, C. H. (2016). Ischemia, immunosuppression and infection—tackling the predicaments of post-stroke complications. Int. J. Mol. Sci. 17:E64. doi: 10.3390/ijms17010064
Suenaga, J., Hu, X., Pu, H., Shi, Y., Hassan, S. H., Xu, M., et al. (2015). White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp. Neurol. 272, 109–119. doi: 10.1016/j.expneurol.2015.03.021
Wang, C. H., Wang, Z. H., Zhang, X. J., Zhang, X. L., Dong, L. P., Xing, Y. X., et al. (2012). Protection by silibinin against experimental ischemic stroke: upregulated pAkt, pmTOR, HIF-1α and Bcl-2, down-regulated Bax, NF-κB expression. Neurosci. Lett. 1, 45–50. doi: 10.1016/j.neulet.2012.08.078
Wei, Y. C., Hong, H. M., Zhang, X. Q., Lai, W. F., Wang, Y. Z., Chu, K. D., et al. (2017). Salidroside inhibits inflammation through PI3K/Akt/HIF signaling after focal cerebral ischemia in rats. Inflammation 40, 1297–1309. doi: 10.1007/s10753-017-0573-x
Wei, D. T., Ren, C. C., Chen, X. Y., and Zhao, H. (2012). The chronic protective effects of limb remote preconditioning and the underlying mechanisms involved in inflammatory factors in rat stroke. PLoS One 7:e30892. doi: 10.1371/journal.pone.0030892
Yamashiro, K., Tanaka, R., Urabe, T., Ueno, Y., Yamashiro, Y., Nomoto, K., et al. (2017). Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS One 12:e0171521. doi: 10.1371/journal.pone.0171521
Yang, J., Liu, C. Y., Du, X. N., Liu, M. L., Ji, X. M., Du, H. S., et al. (2018). Hypoxia inducible factor 1α plays a key role in remote ischemic preconditioning against stroke by modulating inflammatory responses in rats. J. Am. Heart Assoc. 7:e007589. doi: 10.1161/JAHA.117.007589
Zhao, Y., Deng, B., Li, Y., Zhou, L., Yang, L., Gou, X., et al. (2015). Electroacupuncture pretreatment attenuates cerebral ischemic injury via notch pathway-mediated up-regulation of hypoxia inducible factor-1α in rats. Cell Mol. Neurobiol. 35, 1093–1103. doi: 10.1007/s10571-015-0203-9
Zhao, W. B., Meng, R., Ma, C., Hou, B. J., Jiao, L. Q., Zhu, F. S., et al. (2017). Safety and efficacy of remote ischemic preconditioning in patients with severe carotid artery stenosis before carotid artery stenting: a proof-of-concept, randomized controlled trial. Circulation 135, 1325–1335. doi: 10.1161/circulationaha.116.024807
Zhao, H., Shimohata, T., Wang, J. Q., Sun, G., Schaal, D. W., Sapolsky, R. M., et al. (2005). Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J. Neurosci. 25, 9794–9806. doi: 10.1523/JNEUROSCI.3163-05.2005
Zhao, S. C., Wang, C., Xu, H., Wu, W. Q., Chu, Z. H. C., Ma, L. S., et al. (2017). Age-related differences in interferon regulatory factor-4 and -5 signaling in ischemic brains of mice. Acta Pharmacol. Sin. 38, 1425–1434. doi: 10.1038/aps.2017.122
Zhong, Q., Zhou, Y. F., Ye, W. B., Cai, T., Zhang, X. Q., and Deng, D. Y. (2012). Hypoxia-inducible factor 1-a-AA-modified bone marrow stem cells protect PC12 cells from hypoxia-induced apoptosis, partially through VEGF/PI3K/Akt/FoxO1 pathway. Stem Cells Dev. 21, 2703–2717. doi: 10.1089/scd.2011.0604
Keywords: cerebral ischemia, remote ischemic preconditioning, hypoxia-inducible factor, Akt signaling pathway, inflammatory cytokines
Citation: Du X, Yang J, Liu C, Wang S, Zhang C, Zhao H, Du H and Geng X (2020) Hypoxia-Inducible Factor 1α and 2α Have Beneficial Effects in Remote Ischemic Preconditioning Against Stroke by Modulating Inflammatory Responses in Aged Rats. Front. Aging Neurosci. 12:54. doi: 10.3389/fnagi.2020.00054
Received: 12 September 2019; Accepted: 18 February 2020;
Published: 10 March 2020.
Edited by:
Piotr Walczak, Johns Hopkins Medicine, United StatesReviewed by:
Carsten Culmsee, University of Marburg, GermanyCopyright © 2020 Du, Yang, Liu, Wang, Zhang, Zhao, Du and Geng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huishan Du, ZGhzMTMxMDZAMTI2LmNvbQ==; Xiaokun Geng, eGdlbmdAY2NtdS5lZHUuY24=
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.